Abstract
The administration of recombinant Factor VIII (FVIII) is the first line therapy for Hemophilia A (HA), but 25–35% of patients develop an inhibitory antibody response. In general, the presence of aggregates contributes to unwanted immunogenic responses against therapeutic proteins. FVIII has been shown to form both native-like and non-native aggregates. Previously, we showed that non-native aggregates of FVIII are less immunogenic compared to the native protein. Here we investigated the effect of native-like aggregates of FVIII on immunogenicity in HA and von Willebrand Factor knockout (vWF−/−) mice. Mice immunized with native-like aggregates showed significantly higher inhibitory antibody titers compared to animals that received native FVIII. Following re-stimulation in vitro with native FVIII, the activation of CD4+ T cells isolated from mice immunized with native-like aggregates is ~4 fold higher than mice immunized with the native protein. Furthermore, this is associated with increases in the secretion of pro-inflammatory cytokines IL-6 and IL-17 in the native-like aggregate treatment group. The results indicate that the native-like aggregates of FVIII are more immunogenic than native FVIII for both the B cell and T cell responses.
Keywords: Factor VIII, Protein Aggregation, Immunogenicity, Native-like aggregates, von Willebrand Factor, Immunology
INTRODUCTION
Factor VIII is a critical protein in the blood coagulation cascade and its deficiency causes Hemophilia A, a bleeding disorder1. Administration of recombinant FVIII as a replacement is the first line of therapy2,3. Clinical complications arise from the development of inhibitory antibodies against FVIII in 25–35% of severe hemophilia A patients, increasing the frequency of life threatening bleeding episodes4–6.
Aggregation and its impact on immunogenicity have been well studied7–9. It is recognized that the presence of protein aggregates can increase immunogenicity, although little is known about the relative contribution of various types of aggregates10. There are two types of non-covalent aggregates that are commonly observed. Non-native aggregates are those in which the monomeric unit of the protein is partially unfolded11. Conversely, native-like aggregates are made up of self-assembled monomeric units that retain several conformational characteristics of native protein12. Recent studies have demonstrated that non-native aggregates may induce potent antibody responses, but these are not expected to include a large population of inhibitory antibodies because the native structure of the protein is lost upon aggregation10,13. In contrast, native-like aggregates have also been shown to be highly immunogenic and capable of producing inhibitory antibodies14,15. Inhibitory antibodies frequently target regions of macromolecular interactions that are sensitive to conformational changes. Hermeling and coworkers demonstrated that antibodies against recombinant human interferon alpha in transgenic mice induced a breaking of central tolerance, and that the magnitude of the inhibitory response was directly correlated with an increase in the percentage of native-like aggregates15.
The aggregation of FVIII is well studied and has been shown to form both native-like and non-native aggregates16–18. Thermal stress of FVIII at elevated temperatures led to the formation of non-native aggregates that were stabilized by intermolecular beta-strands16,19. Previously we showed that these non-native aggregates were less immunogenic than native FVIII, but acted as a unique antigen18. Extensive characterization of the stability of FVIII demonstrated that a small conformational fluctuation of the native state is sufficient to form aggregates16–20. Grillo et al. have shown that incubation of FVIII at 37 °C leads to the formation of soluble aggregates, initiated by a small transition in the tertiary structure16. Far-UV circular dichroism (CD) and fluorescence analysis of the species formed at 37 °C did not show significant conformational changes compared to the native state. The role of these native-like aggregates of FVIII in eliciting immune responses is not known. Here, we investigate the effect of native-like aggregates of FVIII on immunogenicity in two preclinical animal models: Hemophilia A (HA) and von Willebrand Factor deficient (vWF−/−) mice. We found that the native-like FVIII aggregates are significantly more immunogenic than the non-aggregated monomeric form of FVIII. The enhanced immunogenicity of the FVIII aggregates was manifested by an increase in T cell activation, anti-FVIII antibody production, and the secretion of pro-inflammatory cytokines. Thus the presence of native-like aggregates will likely have a negative effect upon the therapeutic efficacy and safety of FVIII in the treatment of HA patients.
MATERIALS AND METHODS
MATERIALS
Full length recombinant FVIII was obtained from Baxter Health Care Corporation (Carlsbad, CA). IgG-free bovine serum albumin (BSA) and diethanolamine were purchased from Sigma (St. Louis, MO). Normal coagulation control plasma and FVIII-deficient plasma were purchased from Trinity Biotech (County Wicklow, Ireland). The monoclonal antibody ESH8 and aPTT reagents were purchased from American Diagnostica Inc. (Greenwich, CT, USA). The Coamatic FVIII kit from DiaPharma Group (West Chester, OH) was used to determine FVIII activity in plasma samples. The Dynal CD4+ negative isolation kit, RPMI-1640 culture medium, penicillin, streptomycin, L-glutamine, 2-mercaptoethanol, and polymyxin-B were all obtained from Invitrogen Inc., (Carlsbad, CA). Recombinant murine Granulocyte Macrophage Colony-Stimulating Factor (rmGMCSF) was obtained from Peprotech (Rocky Hill, NJ) and heat-inactivated Fetal Bovine Serum was purchased from Lonza (Basel, Switzerland). 3H-thymidine was obtained from Perkin Elmer, Inc. (Boston, MA). All buffer salts and solvents were purchased from Fisher Scientific, (Fair Lawn, NJ). Cytokines (IL-6, IL-17) were obtained from R&D Systems Inc. (Minneapolis, MN).
Preparation of Aggregates
Native-like aggregates of FVIII were prepared by the method of Grillo and co-workers16. Briefly, native FVIII in Tris buffer (25 mM Tris, 5 mM CaCl2 and 300 mM NaCl, pH 7.0) was incubated at 37°C for 6 and 24 hr to form native-like aggregates and are referred as N6 Agg-FVIII and N24 Agg-FVIII, respectively. Non-native aggregates of FVIII were prepared by incubating FVIII in Tris buffer at 80 °C for 2 min to yield 100% Agg-FVIII 18. This degraded FVIII was then mixed with native FVIII in a 1:20 or 1:5 ratio to form 5% (5% Agg-FVIII) and 20% (20% Agg-FVIII) aggregated formulations. Native FVIII, N6 Agg-FVIII, N24 Agg-FVIII, 5% Agg-FVIII, and 20% Agg-FVIII were then used for immunogenicity studies in HA and vWF−/− mice. For all samples sterile pyrogen free buffer was used. All samples were handled under aseptic condition.
Endotoxin Test
All FVIII formulations were tested for the presence of endotoxin using the Endosafe Endochrome-K® kit (Charles River, MA) to confirm they were below the detection limit of 0.05 EU/mL.
Characterization of Aggregates
The conformational changes associated with aggregation were analyzed by far-UV circular dichroism (CD) and intrinsic fluorescence spectroscopy as previously described18,19. Far-UV CD spectra were obtained from 206–240 nm using a J-815 CD spectrophotometer (Jasco, Easton, MD). Fluorescent emission was measured from 300–400 nm following excitation at 265 nm on a PTI Quantamaster fluorescence spectrophotometer (Photon Technology International, Birmingham, NJ). Native FVIII, N6 Agg-FVIII, N24 Agg-FVIII, and 100% Agg-FVIII were analyzed. The presence of aggregates was also confirmed with size exclusion chromatography (SEC) on an Aglient 1100 series HPLC (Agilent Technologies, Santa Clara, CA)21. Briefly, 25 µl of FVIII preparations (40 µg/mL) were injected into a Biosep-SEC-S-4000 4.6×300mm column (Phenomenex, Torrance, CA) maintained at 20 °C. Isocratic elution was run at 0.5 mL/min with Tris buffer and monitored with an Agilent G1321A fluorescent detector set at 285 nm emission/335 nm excitation and an Agilent G1315B diode array detector set at 215 nm. Percentage of aggregate composition was determined by comparing the area of the aggregate peak to the total area of FVIII.
Animals
A colony of mice that are homozygous null due to the targeted deletion in exons four and five of the vWF gene was established using a breeding pairs (JAX Strain B6.129S2-vWFtm1Wgr/J) from the original colony (Jackson Lab, Bar Harbour, ME). These mice do not have any circulating levels of vWF and 8–10% of normal FVIII activity, representing severe von Willebrand disease type 3 with secondary mild HA4,22. A colony of HA mice (C57BL/6J) with a targeted deletion in exon 16 of the FVIII gene was established from breeding pairs provided by Dr. Kazazian and Dr. Sarkar from University of Pennsylvania, PA23. These mice produce no functional FVIII and represent a severe HA mouse model23. Since, the immune response produced by these mice is qualitatively similar to that observed in humans, this model is routinely used to investigate the relative immunogenicity of different FVIII preparations24,25. Mice between 20–25 g and 8–12 weeks old were used for the immunogenicity studies. All animal handling was performed in accordance with the guidelines of institutional animal care and use committees (IACUC) at the University at Buffalo.
Immunogenicity Studies
Immunogenicity studies were carried out in HA and vWF−/− mice4,18. Briefly, groups of mice (n=8) received 4 weekly subcutaneous (s.c.) injections of FVIII or FVIII-aggregates (10 IU/injection). Each antigen dose consisted of 2 µg of total protein in 100 µL of sterile Tris buffer. Two weeks after the last injection, plasma samples were collected. Groups of mice that received saline injections served as a control group. Plasma samples were stored at −80 °C until analyses.
Measurement of Total Titers
Total antibody titers were determined by a standard antibody capture ELISA as described earlier4,18,26. Briefly, 96-well plates were coated with rFVIII (2.5 µg/ mL in carbonate buffer) overnight then blocked with 1% bovine serum albumin (blocking buffer). Samples were diluted (1:100–1:40000) with blocking buffer. Standard concentrations (12.5–150 ng/mL) of ESH8 antibody (recognizing 2248–2285 within the C2 domain of the light chain) were analyzed on each plate to account for plate-to-plate variability. Sample and ESH8 antibody standards were incubated at 37 °C for 1 hr. A 1:1000 dilution of goat antimouse-Ig (IgG + IgM+ H+L)-alkaline phosphatase conjugate in blocking buffer was added at room temperature for 1 hr. Detection was made with 100 µL of 1 mg/mL p-nitrophenyl phosphate solution in diethanolamine buffer (1 M diethanolamine and 0.5 mM MgCl2) for 30 min at room temperature then quenched with 100 µL 3N NaOH solution. Optical density was measured at 405 nm using a SpectraMax (Molecular Devices) plate reader. A linear regression of the plot of absorbance of various dilutions (1:100 to 1:40,000) versus log of dilution was used to calculate the dilution that gave an optical density equal to the half of the difference between the maximum and minimum predicted absorbance. The dilution so obtained was the antibody titer for the sample. To avoid variation between plates a plate specific parameter was used as described earlier26. Plates were washed between each step prior to detection and all samples were analyzed in triplicate.
Measurement of Inhibitory Titers
Inhibitory antibody titers were determined using the Nijmegen modification of the Bethesda assay4,27. Briefly, a calibration curve was constructed using clotting times determined from various dilutions (1:2 to 1:1,024) of normal coagulation control plasma. Serial dilutions (1:8 to 1:20,000) of mouse plasma samples were prepared in human FVIII-deficient plasma. 100 µl of each diluted plasma sample was mixed with an equal volume of normal human plasma and incubated at 37°C for 2 hr. Residual FVIII activity was determined at the end of the incubation using the activated partial thromboplastin time (aPTT) assay and plotted against the log of dilution. The linear portion of the curve was isolated and linear regression performed. Inhibitory titers, the dilution at which 50% of FVIII activity remains, were expressed in Bethesda Units/ml. To minimize the interference from endogenous FVIII on antibody measurement, plasma samples from vWF−/− mice were heat inactivated (90 minutes at 58°C). This heat treatment neutralizes FVIII but does not affect the heat stable immunoglobulin28. Heating does not release the inhibitors already complexed to FVIII. Heat inactivation of plasma samples from HA mice was not necessary as these animals lack endogenous FVIII. However, selected plasma samples from HA mice were reanalyzed after heat inactivation to confirm the titer values were unaffected by the procedure.
T cell Proliferation
T cell proliferation was determined by 3H-thymidine incorporation into splenocytes from immunized animals as described earlier4. Briefly, groups of vWF−/− mice (n=6) received 4 weekly s.c. injections of normal or aggregated FVIII formulations (2 µg/injection). Two weeks after the last injection animals were sacrificed and spleens were collected and homogenized. Splenic T cells were isolated from the uniform suspension with the Dynal CD4+ negative isolation kit (Invitrogen Inc., Carlsbad, CA). Separately, dendritic cells (DCs) were isolated from bone marrow of vWF−/− mice (n=4) as described previously29. DCs were treated with FVIII antigen (2 µg/ml) for 24 hours at 37 °C and 5% CO2. Splenic T cells (2×105 cells/well) from individual mice were cultured in 96-well plates along with naïve or FVIII treated murine DCs (7×104 cells/well) in T cell media (RPMI-1640 containing L-glutamine, penicillin/streptomycin, sodium pyruvate, 2-mercaptoethanol, FBS, polymyxin-B, and 0.22 µm filtered). T cells plated naïve DCs in the absence of antigen served as the negative control. T cells exposed to Concanavalin A (Con A) served as the positive control. After 72 hr, 3H-thymidine (1 µCi/well) was added and the cultures were harvested after 16 hr of incubation using a plate harvester onto a Uni-filter-96® GF/C plate (Perkin Elmer, Boston, MA). The mean counts from quadruplicate wells were measured using a TopCount scintillation counter (Perkin Elmer, Boston, MA). To normalize the data and facilitate comparison between experiments, the activation of T cells was expressed as the stimulation index (SI). SI is the ratio of 3H-thymidine incorporation in the presence and absence of FVIII antigen.
Cytokine Analysis
The supernatant media obtained from cells incubated for 72 hr under conditions identical to those described in the T cell proliferation studies (before 3H-thymidine incorporation) were used for analysis of cytokine secretion. The cell-containing plates were centrifuged at 300 × g for 10 min at 4 °C. The supernatant was collected and stored at −80 °C until analysis. Supernatants were analyzed for the pro-inflammatory cytokines Interleukin-6 (IL-6) and Interleukin-17 (IL-17) with ELISA kits (R&D systems, Minneapolis, MN).
Statistical Analysis
Statistical Analysis was conducted using Minitab 16 (Minitab Inc, State College, PA). Significant differences (p<0.05) were tested using a non parametric two-way Mann-Whitney test or Kruskal-Wallis ANOVA with Dunn’s post hoc analysis as appropriate.
RESULTS
Preparation and characterization of Aggregates
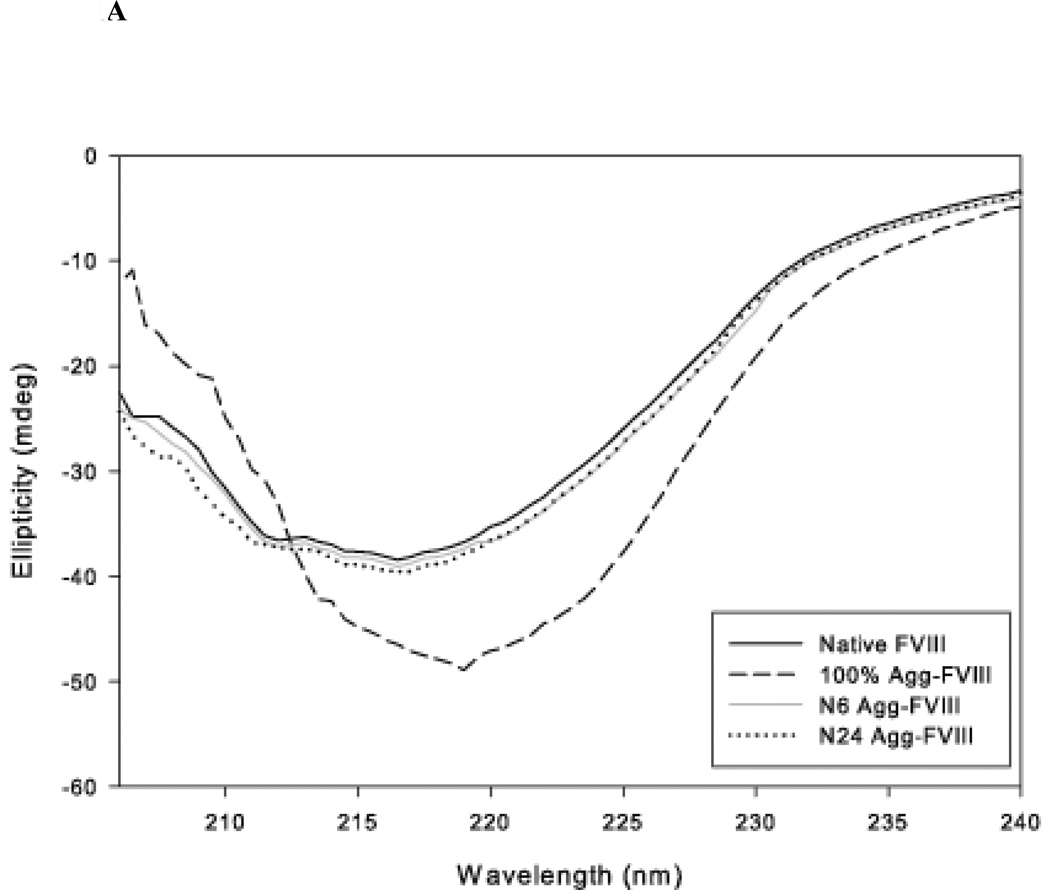
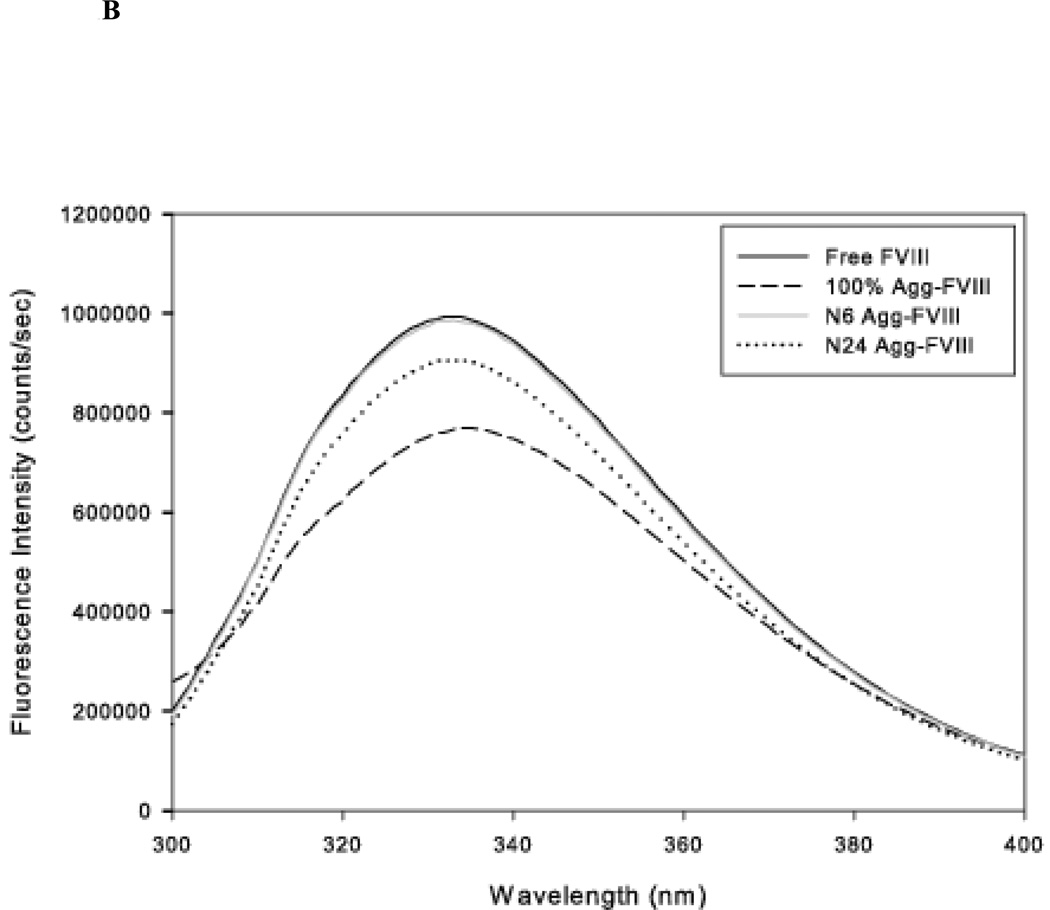
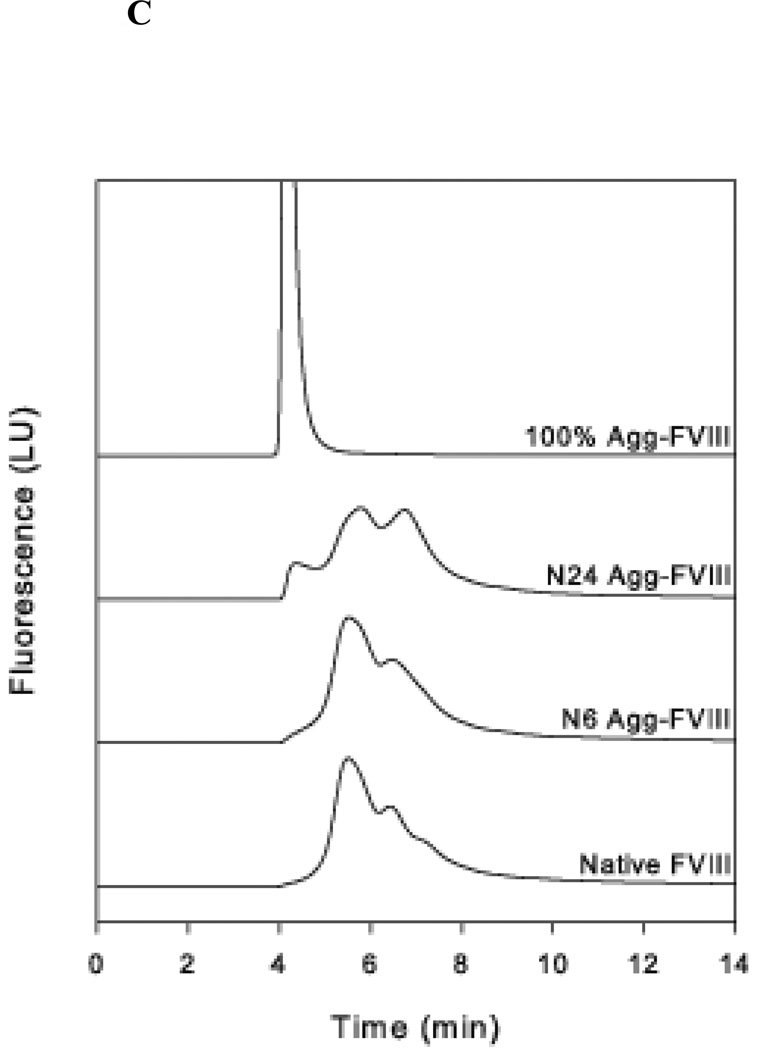
The aggregation and stability of FVIII is well characterized. Grillo et al. have shown that thermal stress at 37 °C induces minor tertiary structural changes in FVIII that led to the formation of soluble aggregates in which the monomeric unit retained substantial native-like structure16. Conformational analysis using far-UV CD and intrinsic fluorescence suggested that major conformational changes are not required for the formation of these aggregates. Further increases in temperature led to the formation of non-native aggregates that were stabilized by intermolecular beta strands. Native-like and non-native Factor VIII aggregates were prepared as described by Grillo et al. The conformation of the protein was analyzed using far-UV CD spectroscopy and intrinsic fluorescence (Figure 1A, 1B)16,18,19. The formation of aggregates was investigated by SEC (Figure 1C).
Figure 1.
A: The representative far UV-CD spectrum of rFVIII and aggregated rFVIII (80µg/100µL) in Tris buffer (300 mM NaCl, 25 mM Tris and 5 mM CaCl2, pH 7.0). The CD spectra were acquired multiple times with multiple samples and representative spectra are shown.
B: The representative fluorescence emission spectra of fractionated native and aggregate (80µg/mL) species in Tris buffer (25 mM Tris, 300 mM NaCl, 5 mM CaCl2, pH 7.0). The samples were excited at 280 nm. The fluorescence spectra were acquired multiple times with multiple samples and representative spectra are shown.
C: Size exclusion studies of native, native-like aggregates, and non-native aggregates of FVIII. Fluorescent intensity was monitored at 285ex/335em following separation on a Biosep-SEC-4000-S column run with a 0.5 mL/min Tris buffer elution.
The CD spectrum of native FVIII shows a broad negative band at 215 nm suggesting that it is comprised primarily of β-sheets (Figure 1A). This observation is consistent with the 3D structure of the protein as elucidated by scattering and molecular modeling studies30. FVIII was also analyzed following 6 and 24 hr incubations at 37 °C. Thermal stress did not produce any significant changes in spectral characteristics indicating that there are no significant alterations in the secondary structure of the protein. Incubation at higher temperatures resulted in the formation of non-native aggregates. Far-UV CD spectra of the sample incubated at 80 °C showed a positive band at 205 nm and a small bathochromic shift of the broad negative band at 215 nm suggesting the formation of intermolecular beta strands. This is consistent with previous FTIR spectroscopy that found signatures for intermolecular beta strands following exposure of FVIII to similar temperatures16.
The samples were also analyzed using fluorescence spectroscopy. The fluorescence emission maxima of monomeric FVIII were observed at approximately 333 nm, consistent with previous observations (Figure 1B)19. The fluorescence spectrum of FVIII following 6 hr of incubation at 37 °C did not show any change either in intensity or peak position, suggesting no significant change in the tertiary structure of the protein. In contrast, prolonged incubation at 37 °C for 24 hr showed a small reduction in intensity but no change in λmax. The small drop in intensity may be due to the formation of aggregates and is consistent with the observation that fractionated protein aggregates showed a decrease in intensity. The fluorescent intensity of the non-native aggregates generated by incubating at 80 °C showed a substantial loss in intensity that is accompanied by a small red shift. Overall the data suggest that incubation of FVIII at 37 °C for 24 hr produces native-like aggregates, but incubation at higher temperatures results in non-native aggregates of FVIII. These results are consistent with previous reports16–18.
Molecular sieve chromatography and dynamic light scattering studies performed on samples subjected to similar thermal stress showed about 10% aggregates were formed after 24 hr of incubation whereas 6 hr of incubation did not induce any detectable aggregate formation16. SEC analysis was performed to determine the size distribution of aggregates. Native FVIII showed a broad peak that eluted around 5.5 min (Figure 1C). The observed pattern is consistent with the heterogeneous molecular weight of the heavy chain, in particular B domain following intracellular, post-translational processing21. 100% aggregated factor FVIII elutes in the void volume as a peak around 4.17 min. A small change in convexity at 4.37 min emerges following the 6 hr 37 °C incubation. For native FVIII, this small peak at 4.37 min represented less than 0.5% of the total area. This initial appearance of aggregates may be due to a slow nucleation process. However, when the incubation period was extended to 24 hours, this shoulder emerged as a distinct peak consistent with elution of the completely aggregated species. This peak represented 11% of the total area and was in line with previous observations. Profiles generated by UV absorption at 215 nm were similar to the fluorescent data (data not shown). The exclusion limit of the column is 1500kDa. As the native-like aggregate peak occurs 0.20 min later than the peak observed for the non-native aggregates, this suggests that the native-like aggregates have a molecular weight lower than the exclusion limit of the column. Based on a molecular weight of 330 kDa for rFVIII, we speculate that the native-like aggregates may be comprised of less than five monomers. These studies suggest that incubation at 37 °C generates aggregates with native-like conformational states.
Impact of Aggregation on the Immunogenicity of FVIII
The impact of aggregates on FVIII immunogenicity was investigated in HA and vWF−/− animal models. HA mice are routinely used to evaluate the relative immunogenicity of FVIII preparations since these mice are not immunologically tolerant of FVIII and produce immune responses to this molecule that are qualitatively similar to those observed in humans31,32. Human and murine FVIII also share a high sequence homology (~90%) in conserved regions of the FVIII molecule33. We previously showed that the administration of non-native aggregates of FVIII elicited much lower antibody responses in HA mice than native FVIII18. However, the impact of native-like aggregates has not been studied. To determine the immunogenic potential of native-like aggregates, antibody responses were evaluated in the HA mouse model.
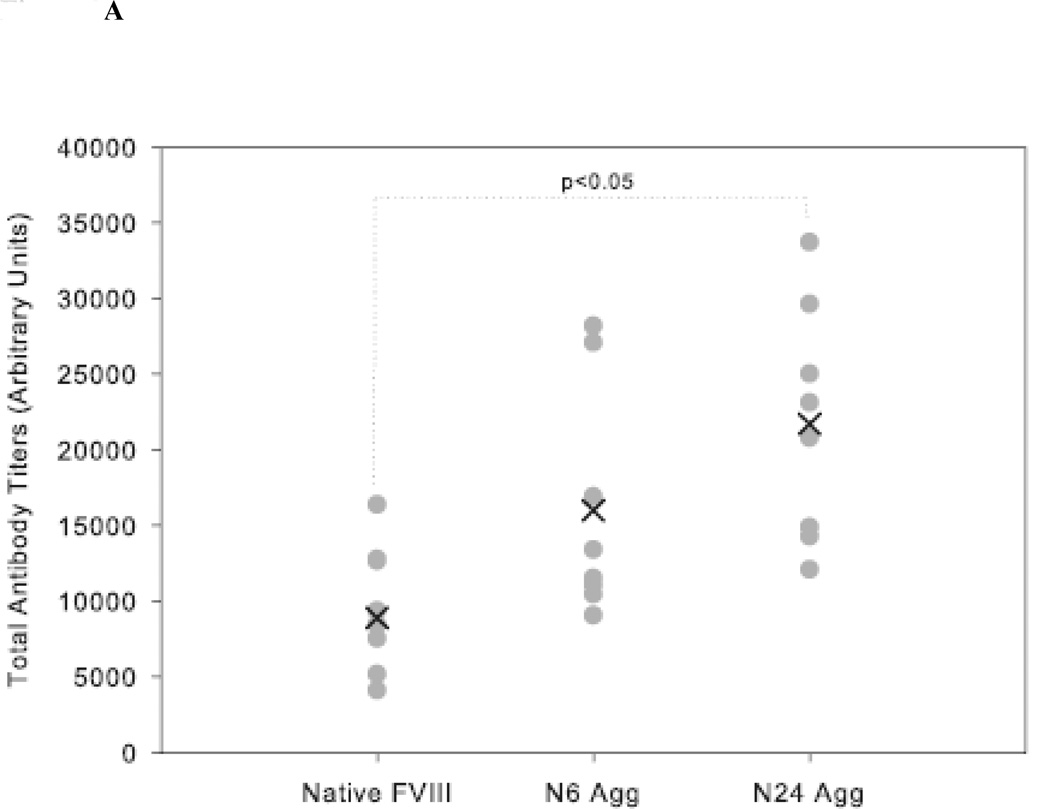
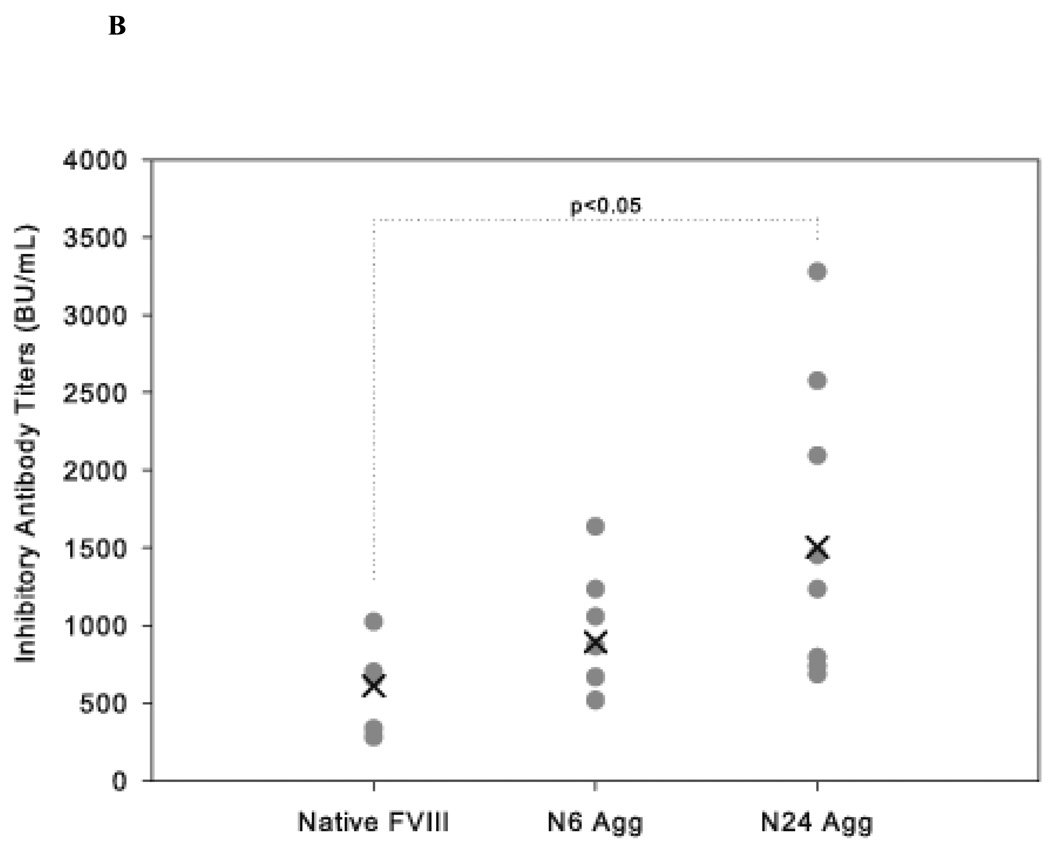
HA mice received 4 weekly injections of either native FVIII, N6 Agg-FVIII, or N24 Agg-FVIII. Plasma was collected to analyze both the total (Figure 2A) and inhibitory (Figure 2B) anti-FVIII antibody response. Mice that received N24 Agg-FVIII generated a total anti-FVIII titer of 21700 ± 2723 (mean ± S.E.; n=8), more than two-fold higher than for mice that received native FVIII with a total titer of 9566 ± 1467 (mean ± S.E.; n=8). Similar results were observed for inhibitory antibody titers. Mice that received N24 Agg-FVIII had more than a two-fold higher mean inhibitory titer of 1503 ± 315 BU/mL (mean ± S.E.; n=9) than mice that received native FVIII of 608 ± 111 BU/mL (mean ± S.E.; n=6). N6 Agg-FVIII showed a similar trend for both total and inhibitory titers, but fell short of significance.
Figure 2.
The mean of inhibitory titers (cross) and individual (closed circles) antibody titers against FVIII in HA mice immunized via s.c. administration of native and native-like aggregates of FVIII. (A) Total anti-FVIII titers as determined by ELISA. (B) Inhibitory anti-FVIII titers as determined by the Nijmegen modification of the Bethesda assay. Significant differences are shown as determined by Kruskal-Wallis ANOVA with Dunn’s post hoc.
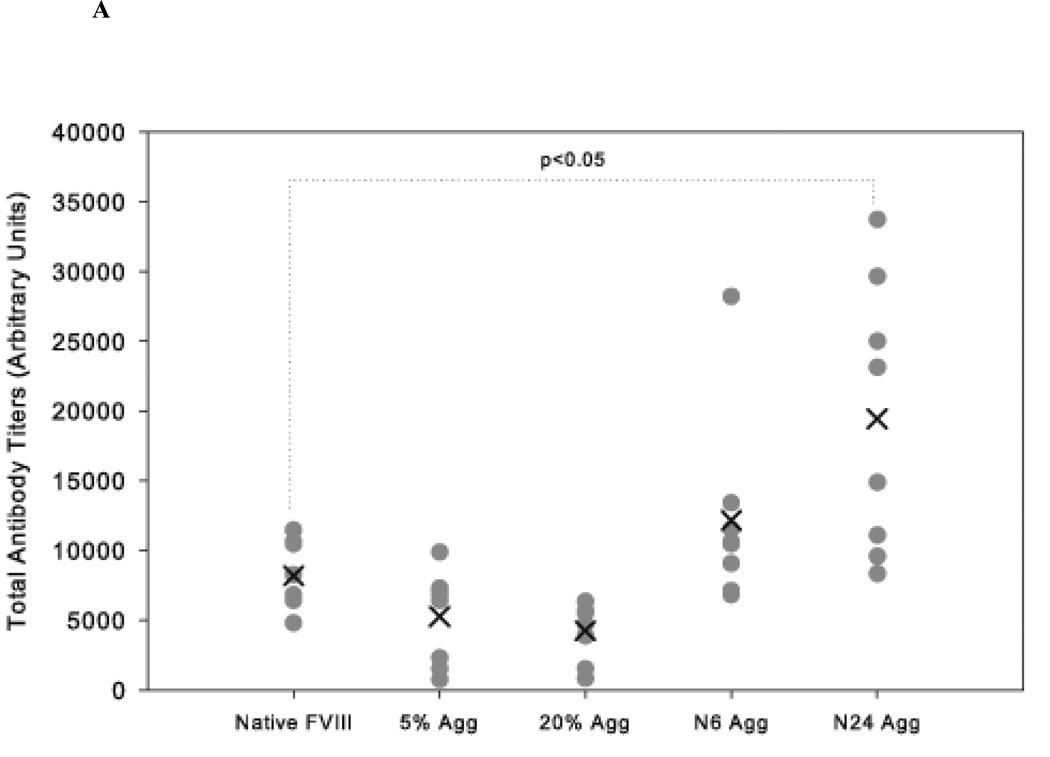
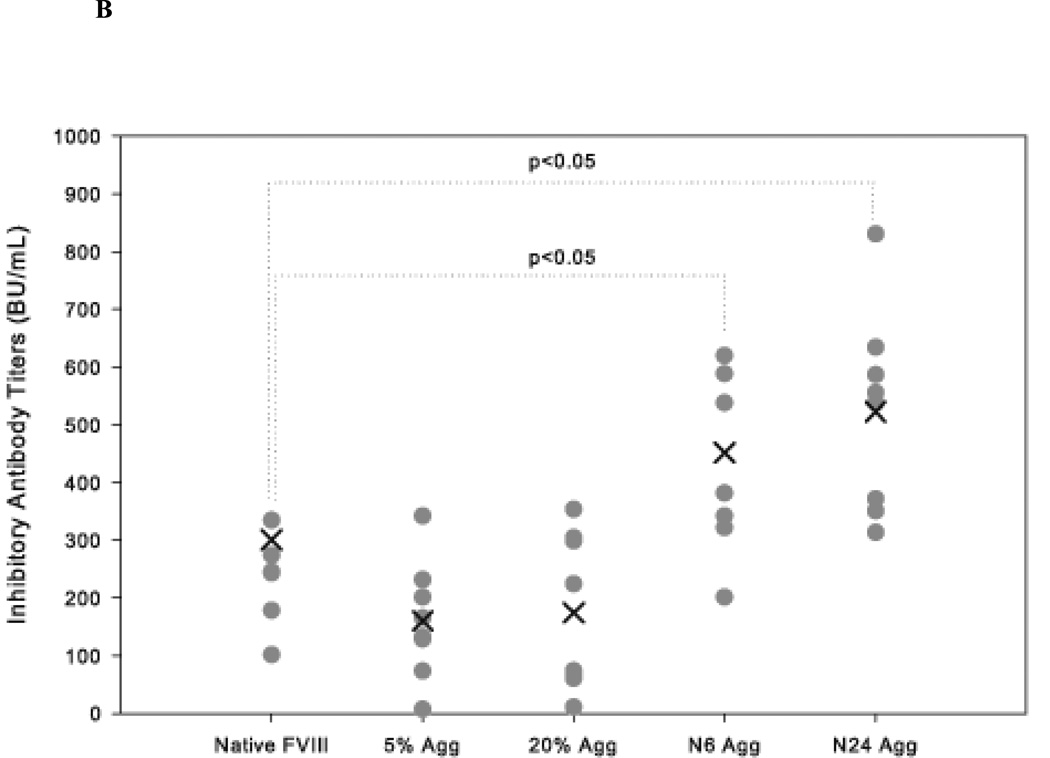
The effect of native-like and non-native aggregates was also studied in vWF−/− mice. vWF is a carrier protein that is essential for the plasma survival of FVIII. In the absence of vWF, the endogenous concentration of FVIII drops to 8–10% of normal34,35. Thus, vWF −/− mice show hemophilia-like characteristics in that they reflect FVIII deficiency22,24. Similar to that observed in HA mice, native-like aggregate treatment (N24 Agg-FVIII) showed significantly higher (p<0.05) mean total antibody titers 19425 ± 3443 (S.E.; n=8) compared to animals that received free FVIII 8192 ± 853 (S.E.; n=8) (Figure 3A). Similar observations were made for neutralizing titers as well (Figure 3B). In these mice the inhibitory titers elicited by both N6 Agg-FVIII 452 ± 57 BU/mL (mean ± S.E.; n=8) and N24 Agg-FVIII 523 ± 61 BU/mL (mean ± S.E.; n=8) were significantly increased compared to native FVIII 236 ± 28 BU/mL (mean ± S.E.; n=8). For the groups of mice which received 5% Agg-FVIII and 20% Agg-FVIII, the mean inhibitory were comparable to each other and lower than the native FVIII group, consistent with our previous findings in HA mice18. No inhibitory titers were observed following saline treatment for either HA or vWF−/− mice. These results suggest that the presence of native-like aggregates in FVIII protein formulations is more immunogenic than non-native aggregates.
Figure 3.
The mean of inhibitory titers (cross) and individual (closed circles) antibody titers against FVIII in vWF−/− mice immunized via s.c. administration of native, native-like aggregates, and non-native aggregates of FVIII. (A) Total anti-FVIII titers as determined by ELISA. (B) Inhibitory anti-FVIII titers as determined by the Nijmegen modification of the Bethesda assay. Significant differences are shown as determined by Kruskal-Wallis ANOVA with Dunn’s post hoc.
An interesting observation is that in both animal models, N6 Agg-FVIII was more immunogenic than native FVIII even though CD and fluorescence studies showed no conformational changes from native state and molecular sieve chromatography and dynamic light scattering detected minimal aggregate development. The magnitude of immune response did not correlate with amount of aggregates detected by SEC.
Impact of Aggregates on T-Cell Proliferation
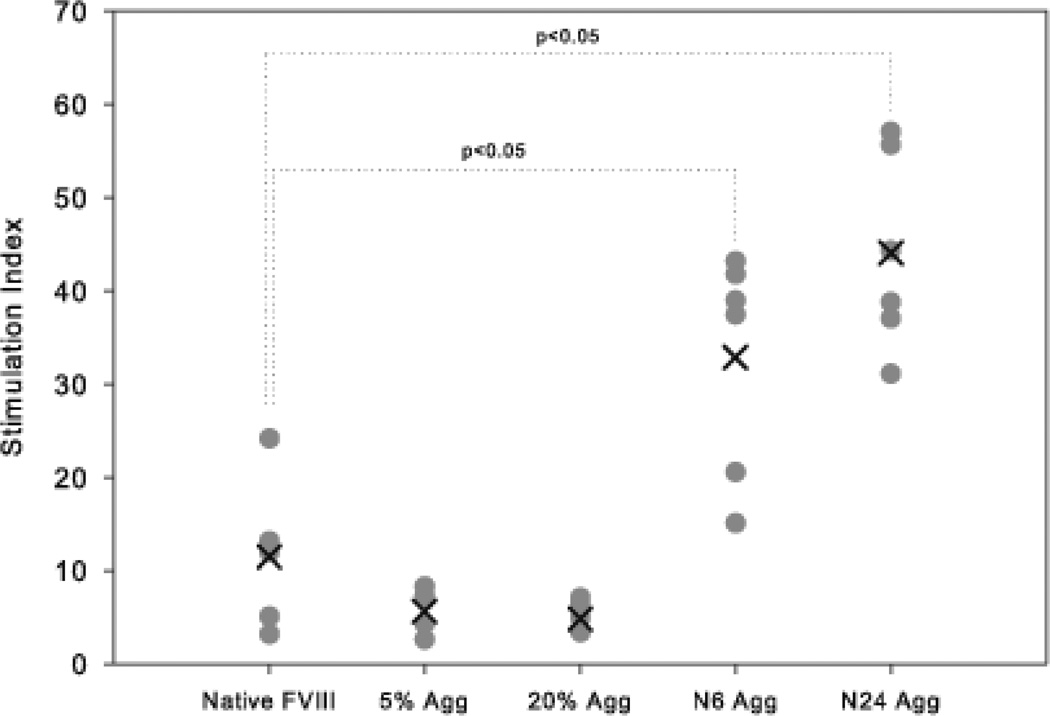
While it was clear that the native-like aggregates of FVIII provoked a greater B cell response than native FVIII as determined by inhibitory titers, it was of interest to determine if mechanistically this included a greater T cell based response as well. To address this question, the generation and proliferation of CD4+ T cells were studied using a 3H-thymidine T cell proliferation assay. T cells derived from vWF−/− mice immunized with either native FVIII or FVIII aggregates were assayed in vitro for their response to native FVIII. Following restimulation with native FVIII, the T cells derived from mice immunized with the native-like aggregates showed a significantly increased stimulation index compared to T cells of mice immunized with native FVIII (Figure 4). For splenic T cells harvested from mice immunized with N6 Agg-FVIII, the mean SI of 32.8 ± 4.9 (S.E.; n=6) was significantly higher (p>0.05, Mann–Whitney nonparametric test) than for mice immunized with native FVIII 11.5 ± 3.7 (S.E.; n=6). Similarly, for the group that received N24 Agg-FVIII, the mean SI of 44.0 ± 4.3 (S.E.; n=6) was ~4 fold higher compared to the native FVIII group. The mean SI for both non-native aggregate treatments groups was lower than native FVIII with 5.6 ± 0.9 (S.E.; n=6) for 5% Agg-FVIII and 4.8 ± 0.7 (S.E.; n=6) for 20% Agg-FVIII. We conclude that immunization of mice with the native-like FVIII aggregates induces a greater T cell response to in vitro re-stimulation than either the native FVIII or the non-native aggregated FVIII in vWF−/− mice.
Figure 4.
The stimulation indices of different splenocytes from mice immunized with different preparations of FVIII. The mean stimulation index (cross) and individual (filled circles) stimulation indices from individual spleens (n=6) of different treatment groups as determined by 3H-thymidine incorporation T cell proliferation assay in vWF−/− mice.
Impact of Aggregates on Pro-inflammatory Cytokine Secretion
The activation of T cells requires a co-stimulatory signal provided by cytokine secretion36. T cells release pro-inflammatory cytokines such as IL-6 and IL-17 that trigger an immune response37. To investigate whether native-like aggregates induce production of pro-inflammatory cytokines, the secretion of IL-6 and IL-17 induced by CD4+ T cells obtained from spleens of animals that received native or aggregated FVIII was determined using by ELISA. The concentration of pro-inflammatory cytokines was much higher in the groups treated with native-like aggregates when compared to the groups treated with native FVIII or non-native aggregates. The trends observed for both inhibitory titers and T cell proliferation continued for cytokine secretion (Table 1). N24 Agg-FVIII significantly increased IL-6 secretion, but N6 Agg-FVIII did not produce a significant increase, likely due to the limited sample size (n=3). IL-17 production was significantly increased by both N6 Agg-FVIII and N24 Agg-FVIII as well as significantly decreased by both 5% Agg-FVIII and 20% Agg-FVIII. These cytokines are key elements in modulating immune responses and their elevated levels suggest that native-like aggregates are more immunogenic than non-native aggregates or native FVIII.
Table 1.
Cytokine secretion by immunizing formulation after FVIII rechallenge in vWF−/− Mice
| Cytokine Secreted |
Factor VIII Formulation | ||||
|---|---|---|---|---|---|
| Native FVIII |
5% Agg FVIII |
20% Agg FVIII |
N6 Agg FVIII |
N24 Agg FVIII |
|
| [IL-6] (pg/mL) | 414 ± 34 | 318 ± 4 | 277 ± 55 | 593 ± 78 | 642 ± 48* |
| [IL-17] (pg/mL) | 358 ± 37 | 105 ± 12** | 93 ± 25** | 575 ± 77** | 605 ± 74** |
p<0.05 compared to IL-6 concentration of Native FVIII by Kruskal-Wallis ANOVA with Dunn’s post hoc analysis
p<0.05 compared to IL-17 concentration of Native FVIII by Kruskal-Wallis ANOVA with Dunn’s post hoc analysis
DISCUSSION
Immunogenic responses against therapeutic proteins are not only an important safety issue, but can also influence efficacy. Antibodies may bind to proteins altering pharamacokinetics or interact with critical epitopes directly neutralizing activity38,39. The causes of immunogenicity can be classified as product, treatment and patient related issues40,41. Product-related factors include protein sequence, presence of immunodominant epitopes, aggregation, the effects of glycosylation on protein degradation, exposure of antigenic sites and solubility of the protein13,40,42–45. In particular, the presence of native-like aggregates has been shown to be highly immunogenic14. Our previous work has shown that non-native aggregates of FVIII generated by heat denaturation were less immunogenic than the native monomer18. FVIII is also prone to form native-like aggregates and we hypothesized that these highly immunogenic species would lead to an inhibitory immune response16. A high incidence of inhibitor development has been observed in patients that received a plasma derived FVIII pasteurized at 63 °C for 10 hours46. It is possible that the increases in immunogenicity resulted from the generation of subvisible particles or native-like aggregate species that could not be distinguished from the native protein by the analytical techniques employed in those experiments. We generated native-like aggregates and administered the formulations to HA mice that produce no active, endogenous FVIII and lack innate tolerance, as well as vWF−/− mice that have reduced FVIII concentrations but an innate tolerance to the protein.
The results showed that native-like aggregates elicited a higher neutralizing antibody response in both of these animal models (Figure 2, Figure 3). Similar conclusions have been drawn from previous studies of other therapeutic proteins. Native-like aggregates of both of erythropoietin alpha and recombinant human interferon alpha are highly immunogenic15,47. Native-like aggregates retain more of the conformational epitopes recognized by the immune system than non-native aggregates. In addition to the retention of higher order structure, native-like aggregates often present antigens in a repetitive manner that enhances immune responses. Even subtle changes such as those observed with N6 Agg-FVIII, which produced no conformational changes detected by biophysical techniques and displayed only a slight trend towards an aggregated species by SEC, were capable of inducing an enhanced immune response. It is possible that several factors could contribute to this observation, including the presence of sub-visible particles. The size of the aggregates may also be a critical factor in eliciting immunogenicity and longer incubation periods could also lead to the formation of larger aggregate complexes. The current study design does not address the role of aggregate size on immunogenicity, but it is nonetheless an important issue. However, the results demonstrate that native-like aggregates are more immunogenic than monomeric native FVIII or non-native aggregates of FVIII.
The mechanism of immune responses against protein aggregates has been the subject of several investigations. It is known that B cells process repetitive antigens in aggregates by cross linking B cell receptors and intracellular signaling pathways involving Bruton’s tyrosine kinase/Tec Kinase48. This is sufficient to initiate the proliferation of B cells to produce antibodies independent of T cells, but antigen processing and presentation could also involve T cell help40 and this aspect of T-cell help for aggregate mediated immunogenicity has received very little attention49. Since the immune response against FVIII is a T cell based response, we investigated the role of T cells in the immunogenicity of native-like aggregates. Mice were immunized with different formulations to generate CD4+ T cell clones. These splenic CD4+ T cells were isolated and re-stimulated with native FVIII. Clinically this study design is akin to a patient who develops inhibitory titers and effector T cell clones upon exposure to native-like aggregates present in a therapeutic preparation, and the subsequent reactivation of those clones with further dosing. T cell clones isolated from mice that received native-like aggregates were activated at significantly higher levels compared to clones from mice receiving monomeric, native FVIII. This could be due to several contributing factors including: (i) native-like aggregates of FVIII generate a greater CD4+ memory T cell response than the other forms of FVIII, (ii) high avidity/affinity T cell clones are generated following the administration of native-like aggregates that proliferate in an efficient manner in response to FVIII epitopes50, and/or (iii) native-like aggregates present neo or cryptic epitopes to antigen presenting cells (such as dendritic and B cells) that can then react with monomeric FVIII25,51.
FVIII is efficiently taken-up and processed by antigen presenting cells. The glycosylation pattern in FVIII has been shown to promote uptake by antigen presenting cells via C-type lectins and result in efficient presentation to T cells for activation21. The presence of native-like aggregates can promote further uptake using Toll-like receptors. Cross talk between C-type lectin receptors and TLR could also provide higher immune responses52. These cellular interactions are supported by the enhanced secretion of the pro-inflammatory cytokines IL-6 and IL-17 (Table 1).
Based on conformational studies, it was demonstrated that minor conformational changes initiating aggregation involve the lipid binding domain20. Amino acid residues 2191–2210, 2241–2290, 2291–2330 all constitute CD4+ T cell epitopes present in this same aggregation prone region53,54. More detailed studies are warranted to investigate whether the minor conformational changes associated with the aggregation process expose neo or cryptic epitopes that lead to generation of efficient T cell clones against native FVIII, or enhance the presentation of recognized epitopes.
Overall, native-like FVIII aggregates produced a greater immune response than both native FVIII and non-native FVIII aggregates. This increase in antibody titers may be due to both B cell and T cell responses and is accompanied by elevated secretion of the pro-inflammatory cytokines IL-6 and IL-17. Our results suggest that it is not just the presence or absence of aggregates, but whether those species are native-like or non-native that can play a critical role in eliciting immunogenic responses.
Acknowledgements
This work was supported by a grant from the National Institutes of Health (R01 HL-70227) to SVB. Dipak S. Pisal received a pre-doctoral fellowship from Pfizer Inc. We would like to thank Dr. William J. Jusko, Department of Pharmaceutical Sciences, University at Buffalo, for letting us use the plate harvester and Top-count scintillation counter instruments in his lab and Nancy Pyszczynski for her technical help with these instruments. We would also like to thank Dr. Marilyn Morris, Department of Pharmaceutical Sciences, University at Buffalo, for the use of her HPLC SEC system.
References
- 1.Ewenstein BM, Collins P, Tarantino MD, Negrier C, Blanchette V, Shapiro AD, Baker D, Spotts G, Sensel M, Yi SE, Gomperts ED. Hemophilia therapy innovation: development of an advanced category recombinant factor VIII by a plasma/albumin-free method. Proceedings of a Special Symposium at the XIXth Congress of the International Society on Thrombosis and Haemostasis; July 12–18, 2003; Semin Hematol; Birmingham, UK. pp. 1–16. discussion 16–18. [DOI] [PubMed] [Google Scholar]
- 2.Kempton CL, White GC., 2nd. How we treat a hemophilia A patient with a factor VIII inhibitor. Blood. 2009;113(1):11–17. doi: 10.1182/blood-2008-06-160432. [DOI] [PubMed] [Google Scholar]
- 3.Gouw SC, van der Bom JG, Auerswald G, Ettinghausen CE, Tedgard U, van den Berg HM. Recombinant versus plasma-derived factor VIII products and the development of inhibitors in previously untreated patients with severe hemophilia A: the CANAL cohort study. Blood. 2007;109(11):4693–4697. doi: 10.1182/blood-2006-11-056317. [DOI] [PubMed] [Google Scholar]
- 4.Pisal DS, Balu-Iyer SV. Phospholipid binding lowers immunogenicity of human recombinant factor VIII in von Willebrand factor knockout mice. Thromb Haemost. 2011;105(6):1115–1118. doi: 10.1160/TH10-09-0628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pisal DS, Balu-Iyer SV. Phospholipid binding improves plasma survival of factor VIII. Thromb Haemost. 2010;104(5):1073–1075. doi: 10.1160/TH10-06-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lacroix-Desmazes S, Navarrete AM, Andre S, Bayry J, Kaveri SV, Dasgupta S. Dynamics of factor VIII interactions determine its immunologic fate in hemophilia A. Blood. 2008;112(2):240–249. doi: 10.1182/blood-2008-02-124941. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Roberts CJ. Lumry-Eyring nucleated-polymerization model of protein aggregation kinetics. 2. Competing growth via condensation and chain polymerization. J Phys Chem B. 2009;113(19):7020–7032. doi: 10.1021/jp8083088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh SK, Bisen PS. Adjuvanticity of stealth liposomes on the immunogenicity of synthetic gp41 epitope of HIV-1. Vaccine. 2006;24(19):4161–4166. doi: 10.1016/j.vaccine.2006.02.047. [DOI] [PubMed] [Google Scholar]
- 9.Van Beers MM, Gilli F, Schellekens H, Randolph TW, Jiskoot W. Immunogenicity of recombinant human interferon beta interacting with particles of glass, metal, and polystyrene. J Pharm Sci. 2011 doi: 10.1002/jps.22744. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg AS. Effects of protein aggregates: an immunologic perspective. The AAPS journal. 2006;8(3):E501–E507. doi: 10.1208/aapsj080359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ludwig DB, Webb JN, Fernandez C, Carpenter JF, Randolph TW. Quaternary conformational stability: The effect of reversible self-association on the fibrillation of two insulin analogs. Biotechnol Bioeng. 2011 doi: 10.1002/bit.23188. [DOI] [PubMed] [Google Scholar]
- 12.Roberts CJ. Non-native protein aggregation kinetics. Biotechnol Bioeng. 2007;98(5):927–938. doi: 10.1002/bit.21627. [DOI] [PubMed] [Google Scholar]
- 13.Fradkin AH, Carpenter JF, Randolph TW. Glass particles as an adjuvant: a model for adverse immunogenicity of therapeutic proteins. Journal of pharmaceutical sciences. 2011;100(11):4953–4964. doi: 10.1002/jps.22683. [DOI] [PubMed] [Google Scholar]
- 14.Carpenter JF, Randolph TW, Jiskoot W, Crommelin DJ, Middaugh CR, Winter G, Fan YX, Kirshner S, Verthelyi D, Kozlowski S, Clouse KA, Swann PG, Rosenberg A, Cherney B. Overlooking subvisible particles in therapeutic protein products: gaps that may compromise product quality. J Pharm Sci. 2009;98(4):1201–1205. doi: 10.1002/jps.21530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hermeling S, Schellekens H, Maas C, Gebbink MF, Crommelin DJ, Jiskoot W. Antibody response to aggregated human interferon alpha2b in wild-type and transgenic immune tolerant mice depends on type and level of aggregation. J Pharm Sci. 2006;95(5):1084–1096. doi: 10.1002/jps.20599. [DOI] [PubMed] [Google Scholar]
- 16.Grillo AO, Edwards KL, Kashi RS, Shipley KM, Hu L, Besman MJ, Middaugh CR. Conformational origin of the aggregation of recombinant human factor VIII. Biochemistry. 2001;40(2):586–595. doi: 10.1021/bi001547t. [DOI] [PubMed] [Google Scholar]
- 17.Ramani K, Purohit V, Middaugh CR, Balasubramanian SV. Aggregation kinetics of recombinant human FVIII (rFVIII) Journal of pharmaceutical sciences. 2005;94(9):2023–2029. doi: 10.1002/jps.20432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Purohit VS, Middaugh CR, Balasubramanian SV. Influence of aggregation on immunogenicity of recombinant human Factor VIII in hemophilia A mice. Journal of pharmaceutical sciences. 2006;95(2):358–371. doi: 10.1002/jps.20529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miclea RD, Purohit VS, Balu-Iyer SV. O-phospho-L-serine, multi-functional excipient for B domain deleted recombinant factor VIII. The AAPS journal. 2007;9(2):E251–E259. doi: 10.1208/aapsj0902028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramani K, Purohit VS, Miclea RD, Middaugh CR, Balasubramanian SV. Lipid binding region (2303–2332) is involved in aggregation of recombinant human FVIII (rFVIII) J Pharm Sci. 2005;94(6):1288–1299. doi: 10.1002/jps.20340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kosloski MP, Miclea RD, Balu-Iyer SV. Role of glycosylation in conformational stability, activity, macromolecular interaction and immunogenicity of recombinant human factor VIII. The AAPS journal. 2009;11(3):424–431. doi: 10.1208/s12248-009-9119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denis C, Methia N, Frenette PS, Rayburn H, Ullman-Cullere M, Hynes RO, Wagner DD. A mouse model of severe von Willebrand disease: defects in hemostasis and thrombosis. Proc Natl Acad Sci U S A. 1998;95(16):9524–9529. doi: 10.1073/pnas.95.16.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bi L, Lawler AM, Antonarakis SE, High KA, Gearhart JD, Kazazian HH., Jr. Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat Genet. 1995;10(1):119–121. doi: 10.1038/ng0595-119. [DOI] [PubMed] [Google Scholar]
- 24.Behrmann M, Pasi J, Saint-Remy JM, Kotitschke R, Kloft M. Von Willebrand factor modulates factor VIII immunogenicity: comparative study of different factor VIII concentrates in a haemophilia A mouse model. Thromb Haemost. 2002;88(2):221–229. [PubMed] [Google Scholar]
- 25.Qian J, Borovok M, Bi L, Kazazian HH, Jr., Hoyer LW. Inhibitor antibody development and T cell response to human factor VIII in murine hemophilia A. Thromb Haemost. 1999;81(2):240–244. [PubMed] [Google Scholar]
- 26.Purohit VS, Ramani K, Sarkar R, Kazazian HH, Jr., Balasubramanian SV. Lower inhibitor development in hemophilia A mice following administration of recombinant factor VIII-O-phospho-L-serine complex. J Biol Chem. 2005;280(18):17593–17600. doi: 10.1074/jbc.M500163200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng A, Straubinger RM, Balu-Iyer SV. Phosphatidylinositol containing lipidic particles reduces immunogenicity and catabolism of factor VIII in hemophilia a mice. The AAPS journal. 2010;12(3):473–481. doi: 10.1208/s12248-010-9207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verbruggen B, van Heerde WL, Laros-van Gorkom BA. Improvements in factor VIII inhibitor detection: From Bethesda to Nijmegen. Semin Thromb Hemost. 2009;35(8):752–759. doi: 10.1055/s-0029-1245107. [DOI] [PubMed] [Google Scholar]
- 29.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223(1):77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 30.Ngo JC, Huang M, Roth DA, Furie BC, Furie B. Crystal structure of human factor VIII: implications for the formation of the factor IXa-factor VIIIa complex. Structure. 2008;16(4):597–606. doi: 10.1016/j.str.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Reipert BM, Schoppmann A, Schwarz HP. A caution on the use of murine hemophilia models for comparative immunogenicity studies of FVIII products with different protein compositions. Thrombosis and Haemostasis. 2003;89(6):1110–1112. [PubMed] [Google Scholar]
- 32.Reipert BM, Ahmad RU, Turecek PL, Schwarz HP. Characterization of antibodies induced by human factor VIII in a murine knockout model of hemophilia A. Thromb Haemost. 2000;84(5):826–832. [PubMed] [Google Scholar]
- 33.Elder B, Lakich D, Gitschier J. Sequence of the murine factor VIII cDNA. Genomics. 1993;16(2):374–379. doi: 10.1006/geno.1993.1200. [DOI] [PubMed] [Google Scholar]
- 34.Jacquemin M. Factor VIII-von Willebrand factor binding defects in autosomal recessive von Willebrand disease type Normandy and in mild hemophilia A. New insights into factor VIII-von Willebrand factor interactions. Acta Haematol. 2009;121(2–3):102–105. doi: 10.1159/000214849. [DOI] [PubMed] [Google Scholar]
- 35.Altieri DC, Capitanio AM, Mannucci PM. von Willebrand factor contaminating porcine factor VIII concentrate (Hyate:C) causes platelet aggregation. Br J Haematol. 1986;63(4):703–711. doi: 10.1111/j.1365-2141.1986.tb07554.x. [DOI] [PubMed] [Google Scholar]
- 36.Chirino AJ, Ary ML, Marshall SA. Minimizing the immunogenicity of protein therapeutics. Drug Discov Today. 2004;9(2):82–90. doi: 10.1016/S1359-6446(03)02953-2. [DOI] [PubMed] [Google Scholar]
- 37.Oukka M. Th17 cells in immunity and autoimmunity. Ann Rheum Dis. 2008;67(Suppl 3):iii26–iii29. doi: 10.1136/ard.2008.098004. [DOI] [PubMed] [Google Scholar]
- 38.Musso R. Efficacy and safety of recombinant factor VIII products in patients with hemophilia A. Drugs Today (Barc) 2008;44(10):735–750. doi: 10.1358/dot.2008.44.10.1284765. [DOI] [PubMed] [Google Scholar]
- 39.Pisal DS, Kosloski MP, Balu-Iyer SV. Delivery of Therapeutic Proteins. Journal of Pharmaceutical Sciences. 2010;99(6):2557–2575. doi: 10.1002/jps.22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kessler M, Goldsmith D, Schellekens H. Immunogenicity of biopharmaceuticals. Nephrol Dial Transplant. 2006;21(Suppl 5):v9–v12. doi: 10.1093/ndt/gfl476. [DOI] [PubMed] [Google Scholar]
- 41.Barbosa MD, Celis E. Immunogenicity of protein therapeutics and the interplay between tolerance and antibody responses. Drug Discov Today. 2007;12(15–16):674–681. doi: 10.1016/j.drudis.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Maas C, Hermeling S, Bouma B, Jiskoot W, Gebbink MF. A role for protein misfolding in immunogenicity of biopharmaceuticals. J Biol Chem. 2007;282(4):2229–2236. doi: 10.1074/jbc.M605984200. [DOI] [PubMed] [Google Scholar]
- 43.Bai S, Manning MC, Randolph TW, Carpenter JF. Aggregation of recombinant human botulinum protein antigen serotype C in varying solution conditions: implications of conformational stability for aggregation kinetics. J Pharm Sci. 2011;100(3):836–848. doi: 10.1002/jps.22345. [DOI] [PubMed] [Google Scholar]
- 44.Barnard JG, Singh S, Randolph TW, Carpenter JF. Subvisible particle counting provides a sensitive method of detecting and quantifying aggregation of monoclonal antibody caused by freeze-thawing: insights into the roles of particles in the protein aggregation pathway. J Pharm Sci. 2011;100(2):492–503. doi: 10.1002/jps.22305. [DOI] [PubMed] [Google Scholar]
- 45.Carpenter JF, Randolph TW, Jiskoot W, Crommelin DJ, Middaugh CR, Winter G. Potential inaccurate quantitation and sizing of protein aggregates by size exclusion chromatography: essential need to use orthogonal methods to assure the quality of therapeutic protein products. J Pharm Sci. 2010;99(5):2200–2208. doi: 10.1002/jps.21989. [DOI] [PubMed] [Google Scholar]
- 46.Kumar S, Singh SK, Wang X, Rup B, Gill D. Coupling of aggregation and immunogenicity in biotherapeutics: T- and B-cell immune epitopes may contain aggregation-prone regions. Pharmaceutical research. 2011;28(5):949–961. doi: 10.1007/s11095-011-0414-9. [DOI] [PubMed] [Google Scholar]
- 47.Hermeling S, Schellekens H, Crommelin DJ, Jiskoot W. Micelle-associated protein in epoetin formulations: aA risk factor for immunogenicity? Pharm Res. 2003;20(12):1903–1907. doi: 10.1023/b:pham.0000008034.61317.02. [DOI] [PubMed] [Google Scholar]
- 48.Fluckiger A-C, Li Z, Kato RM, Wahl MI, Ochs HD, Longnecker R, Kinet J-P, Witte ON, Scharenberg AM, Rawlings DJ. Btk/Tec kinases regulate sustained increases in intracellular Ca2+ following B-cell receptor activation. EMBO J. 1998;17(7):1973–1985. doi: 10.1093/emboj/17.7.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh SK. Impact of product-related factors on immunogenicity of biotherapeutics. J Pharm Sci. 2011;100(2):354–387. doi: 10.1002/jps.22276. [DOI] [PubMed] [Google Scholar]
- 50.Hayball JD, Robinson BW, Lake RA. CD4+ T cells cross-compete for MHC class II-restricted peptide antigen complexes on the surface of antigen presenting cells. Immunol Cell Biol. 2004;82(2):103–111. doi: 10.1046/j.0818-9641.2004.01233.x. [DOI] [PubMed] [Google Scholar]
- 51.De Groot AS, Scott DW. Immunogenicity of protein therapeutics. Trends Immunol. 2007;28(11):482–490. doi: 10.1016/j.it.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 52.Vos QLA, Wu ZQ, Snapper CM, Mond JJ. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunological Reviews. 2000;176(1):154–170. doi: 10.1034/j.1600-065x.2000.00607.x. [DOI] [PubMed] [Google Scholar]
- 53.Pratt KP, Qian J, Ellaban E, Okita DK, Diethelm-Okita BM, Conti-Fine B, Scott DW. Immunodominant T-cell epitopes in the factor VIII C2 domain are located within an inhibitory antibody binding site. Thromb Haemost. 2004;92(3):522–528. doi: 10.1160/TH03-12-0755. [DOI] [PubMed] [Google Scholar]
- 54.Reding MT, Okita DK, Diethelm-Okita BM, Anderson TA, Conti-Fine BM. Human CD4+ T-cell epitope repertoire on the C2 domain of coagulation factor VIII. J Thromb Haemost. 2003;1(8):1777–1784. doi: 10.1046/j.1538-7836.2003.00251.x. [DOI] [PubMed] [Google Scholar]