Abstract
Background
Previous Cochrane reviews have considered the use of cholinesterase inhibitors in both Parkinson's disease with dementia (PDD) and dementia with Lewy bodies (DLB). The clinical features of DLB and PDD have much in common and are distinguished primarily on the basis of whether or not parkinsonism precedes dementia by more than a year. Patients with both conditions have particularly severe deficits in cortical levels of the neurotransmitter acetylcholine. Therefore, blocking its breakdown using cholinesterase inhibitors may lead to clinical improvement.
Objectives
To assess the efficacy, safety and tolerability of cholinesterase inhibitors in patients with dementia with Lewy bodies (DLB), Parkinson’s disease with dementia (PDD), and cognitive impairment in Parkinson’s disease falling short of dementia (CIND‐PD) (considered as separate phenomena and also grouped together as Lewy body disease).
Search methods
The trials were identified from a search of ALOIS, the Specialised Register of the Cochrane Dementia and Cognitive Improvement Group (on 30 August 2011) using the search terms Lewy, Parkinson, PDD, DLB, LBD. This register consists of records from major healthcare databases (MEDLINE, EMBASE, PsycINFO, CINAHL) and many ongoing trial databases and is updated regularly.
Reference lists of relevant studies were searched for additional trials.
Selection criteria
Randomised, double‐blind, placebo‐controlled trials assessing the efficacy of treatment with cholinesterase inhibitors in DLB, PDD and CIND‐PD.
Data collection and analysis
Data were extracted from published reports by one review author (MR). The data for each 'condition' (that is DLB, PDD or CIND‐PD) were considered separately and, where possible, also pooled. Statistical analysis was conducted using Review Manager 5.0.
Main results
Six trials met the inclusion criteria for this review, in which a total of 1236 participants were randomised. Four of the trials were of a parallel group design and two cross‐over trials were included. Four of the trials included participants with a diagnosis of PDD (Aarsland 2002a; Dubois 2007; Emre 2004; Ravina 2005), of which Dubois 2007 remains unpublished (n = 550). Leroi 2004 included patients with cognitive impairment and Parkinson's disease (both with and without dementia). Patients with DLB were included in only one of the trials (McKeith 2000).
For global assessment, three trials comparing cholinesterase inhibitor treatment to placebo in PDD (Aarsland 2002a; Emre 2004; Ravina 2005) reported a difference in the Alzheimer's Disease Cooperative Study‐Clinical Global Impression of Change (ADCS‐CGIC) score of ‐0.38, favouring the cholinesterase inhibitors (95% confidence interval (CI) ‐0.56 to ‐0.24, P < 0.0001). A clinically meaningful improvement was observed in 19.8% of patients receiving cholinesterase inhibitors, compared to 14.5% of those in the placebo group.
For cognitive function, a pooled estimate of the effect of cholinesterase inhibitors on cognitive function measures was consistent with the presence of a therapeutic benefit (standardised mean difference (SMD) ‐0.34, 95% CI ‐0.46 to ‐0.23, P < 0.00001). There was evidence of a positive effect of cholinesterase inhibitors on the Mini‐Mental State Examination (MMSE) in patients with PDD (weighted mean difference (WMD) 1.09, 95% CI 0.45 to 1.73, P = 0.0008) and in the single PDD and CIND‐PD trial (WMD 1.05, 95% CI 0.42 to 1.68, P = 0.01) but not in the single DLB trial.
For behavioural disturbance, analysis of the pooled continuous data relating to behavioural disturbance rating scales favoured treatment with cholinesterase inhibitors (SMD ‐0.20, 95% CI ‐0.36 to ‐0.04, P = 0.01).
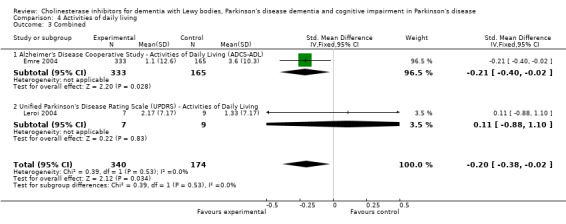
For activities of daily living, combined data for the ADCS and the Unified Parkinson's Disease Rating Scale (UPDRS) activities of daily living rating scales favoured treatment with cholinesterase inhibitors (SMD ‐0.20, 95% CI ‐0.38 to ‐0.02, P = 0.03).
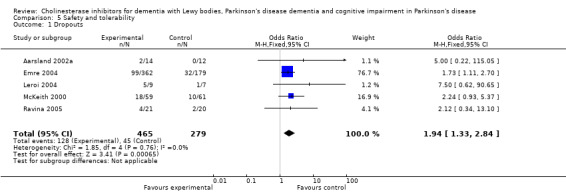
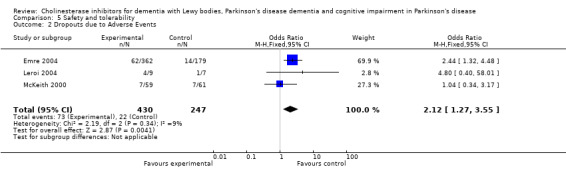
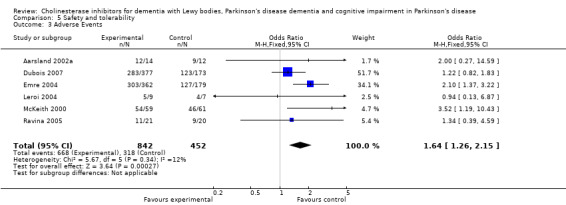
For safety and tolerability, those taking a cholinesterase inhibitor were more likely to experience an adverse event (318/452 versus 668/842; odds ratio (OR) 1.64, 95% CI 1.26 to 2.15, P = 0.0003) and to drop out (128/465 versus 45/279; OR 1.94, 95% CI 1.33 to 2.84, P = 0.0006). Adverse events were more common amongst those taking rivastigmine (357/421 versus 173/240; OR 2.28, 95% CI 1.53 to 3.38, P < 0.0001) but not those taking donepezil (311/421 versus 145/212; OR 1.24, 95% CI 0.86 to 1.80, P = 0.25). Parkinsonian symptoms in particular tremor (64/739 versus 12/352; OR 2.71, 95% CI 1.44 to 5.09, P = 0.002), but not falls (P = 0.39), were reported more commonly in the treatment group but this did not have a significant impact on the UPDRS (total and motor) scores (P = 0.71). Fewer deaths occurred in the treatment group than in the placebo group (4/465 versus 9/279; OR 0.28, 95% CI 0.09 to 0.84, P = 0.03).
Authors' conclusions
The currently available evidence supports the use of cholinesterase inhibitors in patients with PDD, with a positive impact on global assessment, cognitive function, behavioural disturbance and activities of daily living rating scales. However, almost half of the trial data, which could potentially change this conclusion, have not been made public. The effect in DLB remains unclear. There is no current disaggregated evidence to support their use in CIND‐PD.
Keywords: Humans, Cholinesterase Inhibitors, Cholinesterase Inhibitors/adverse effects, Cholinesterase Inhibitors/therapeutic use, Cognition Disorders, Cognition Disorders/drug therapy, Cognition Disorders/etiology, Dementia, Dementia/drug therapy, Dementia/etiology, Donepezil, Indans, Indans/adverse effects, Indans/therapeutic use, Lewy Body Disease, Lewy Body Disease/drug therapy, Neuroprotective Agents, Neuroprotective Agents/adverse effects, Neuroprotective Agents/therapeutic use, Parkinson Disease, Parkinson Disease/complications, Phenylcarbamates, Phenylcarbamates/adverse effects, Phenylcarbamates/therapeutic use, Piperidines, Piperidines/adverse effects, Piperidines/therapeutic use, Randomized Controlled Trials as Topic, Rivastigmine
Plain language summary
Cholinesterase inhibitors are beneficial for people with Parkinson's disease and dementia
The clinical features of dementia with Lewy bodies (DLB) and Parkinson's disease with dementia (PDD) have much in common. As patients with DLB and PDD have particularly severe deficits in cortical levels of the neurotransmitter acetylcholine, blocking its breakdown using a group of chemicals known as cholinesterase inhibitors may lead to clinical improvement. Six trials showed a statistically significant improvement in global assessment, cognitive function, behavioural disturbance and activities of daily living rating scales in PDD and cognitive impairment in Parkinson's disease (CIND‐PD) patients treated with cholinesterase inhibitors. There was no current disaggregated evidence to support their use in CIND‐PD. In a single trial, no statistically significant improvement was observed in patients with DLB who were treated with cholinesterase inhibitors and further trials are necessary to clarify the effect of cholinesterase inhibitors in this patient group.
Background
'When you've seen one patient with dementia, you've seen one patient with dementia'. This commonplace observation about the wide heterogeneity in the clinical presentation of dementia raises the possibility that there may be useful diagnostic subdivisions. The most common cause of dementia is Alzheimer's disease but there are several others, of which dementia with Lewy bodies is arguably the second most common.
Lewy bodies are the defining pathological feature of idiopathic Parkinson's disease. These inclusion bodies are found in the cytoplasm of cells of a wide variety of subcortical nuclei, including those of monoaminergic neurons. They are more likely to occur in cortical neurons in patients with Parkinson's disease when the patients also have dementia. A defining constituent is fibrillar aggregates of alpha‐synuclein, a presynaptic protein involved in vesicle formation (Lee 2006). One current theory about why Lewy bodies form is that the cellular mechanisms for degrading and disposing of intracellular protein fragments (proteasomes) are dysfunctional (Olanow 2006). In epidemiological studies, up to 30% of those people with dementia have Lewy bodies (Zaccai 2005). The rate of dementia in clinical Parkinson's disease (24% to 31%) (Aarsland 2005) is at least two to five times that expected in age matched controls. Longitudinal studies suggest that most patients with Parkinson's disease who survive will eventually develop dementia (Aarsland 2003).
Scope of this review
Previous Cochrane reviews have considered the use of cholinesterase inhibitors in both Parkinson's disease with dementia (PDD) (Maidment 2006) and dementia with Lewy bodies (DLB) (Wild 2003). The clinical features of DLB and PDD have much in common. There is some convergence of opinion that DLB and PDD may be the same condition, but the matter is not fully resolved because DLB and PDD have slightly different neuropathological correlates (Ballard 2006; Burn 2006; McKeith 2005).
The diagnosis of PDD rests on the occurrence of formally diagnosed Parkinson's disease followed at least 12 months later by dementia (with no other apparent cause identified). Most patients with Parkinson's disease have at least subtle deficits in neuropsychological function, typically affecting visuospatial and sometimes executive function. In many cases this does not cause problems and is only apparent on detailed specialist evaluation. Cognitive impairment that is clinically significant typically involves more clear‐cut deficits in these areas but also tends to affect attention. These are the three areas of function (visuospatial, executive, attention) prominently affected early in patients labelled DLB. Memory function may be affected late in the process. Dementia is more likely to occur in those in whom the Parkinson's disease develops later, and tends to be of the postural instability‐gait disorder subtype and to be associated with visual hallucinations when treated with L‐dopamine (L‐DOPA). The development of dementia associated with Parkinson's disease increases caregiver distress, nursing home requirements and mortality, twofold. It also reduces quality of life (Bedard 2003; Burn 2003). Similarly, parkinsonism in Alzheimer's disease increases the cost of care (Bostrom 2006).
The formal distinction between PDD and early cognitive impairment in Parkinson's disease rests on the definition of dementia. Dementia is defined as occurring when cognitive impairment is of a severity or type such that it interferes with day‐to‐day occupational and social functioning. However, it is particularly difficult to judge reliably whether any impairment in function in Parkinson's disease is due to cognitive decline or alternatively to the motor, mood or personality changes which may occur. In this review, we have therefore also included a further group of patients, patients who have Parkinson's disease and who have clinically significant cognitive impairment but in whom the diagnosis of dementia has not been formally established. This is analogous to the 'Cognitive Impairment, Not Dementia (CIND)' category and will be termed 'CIND‐PD'. This approach is consistent with the current version of the Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV‐R) criteria (294.1x).
The diagnosis of 'probable DLB' is more complex than that for PDD. It depends on the presence of two of: persistent visual hallucinations; fluctuations in cognitive and functional ability; and parkinsonism. If parkinsonian symptoms are part of the picture, dementia should have occurred within 12 months of the onset of the parkinsonian symptoms. Additionally, 'probable DLB' can be diagnosed if just one of these original features is present plus one of the following: severe sensitivity to neuroleptics; rapid eye movement (REM) sleep behaviour disorder; or evidence of striatal dopamine transporter protein loss on neuroimaging (McKeith 2005).
Other symptoms that support the diagnosis but are of less clear‐cut diagnostic value are repeated falls, syncope, transient disturbances of consciousness, severe autonomic dysfunction (for example orthostatic hypotension), urinary incontinence, systematised delusions, non‐visual hallucinations, depression, relative preservation of medial temporal lobe structures on a computed tomography (CT) or magnetic resonance imaging (MRI) scan, generalized low uptake on single‐photon emission CT (SPECT) or a positron emission tomography (PET) perfusion scan with reduced occipital activity, abnormal (low uptake) [123I]meta‐iodobenzylguanidine (MIBG) myocardial scintigraphy and prominent slow wave activity on electroencephalography (EEG) with temporal lobe transient sharp waves. Olfactory function may also be impaired (Williams 2009).
The distinction between PDD and DLB was introduced in 1995 (McKeith 1996). It was recognised at the time that these conditions had much in common, and that the cut‐off period of 12 months was arbitrary. The distinction was in part driven by the fact that, in some health systems, patients who develop Parkinson's disease first tend to see neurologists whereas those who develop cognitive impairment first tend to see psychiatrists. It also reflected in the fact that regulators can only issue licenses for drugs where a claim is made for the drug in a clearly defined (and accepted) condition. In the third revision of the consensus statement (McKeith 2005), the DLB consortium has suggested that a generic term such as Lewy body disease (LB disease) may be helpful when PDD and DLB are considered together, but that in clinical situations the terms PDD and DLB should be retained as they differentiate between whether symptoms of dementia occur before or after those of Parkinson's disease.
By considering the results of treatment trials for PDD, DLB and CIND‐PD, both separately and together, it may be possible to see whether there is any difference in the response to cholinesterase inhibitors in these conditions.
Rationale for cholinesterase inhibitors
Lewy bodies occur in the dopamine‐producing cells of the substantia nigra, where their presence is associated with the movement problems of Parkinson's disease. However, alpha‐synuclein aggregation occurs in many other brain areas too and the extent of this may correlate with dementia (Braak 2006). A broad correlation can also be made between the areas affected and specific clinical symptoms: cholinergic deficits and attention or memory (Nakano 1984); serotonergic deficits and depression (Jellinger 1994); dopaminergic deficits and visuospatial or executive symptoms (Dubois 1997); and cortical LBs and executive function impairment. In his original description, Frederick Lewy actually put more emphasis on the occurrence of LBs in the large cells of the substantia innominata (now named the nucleus basalis of Myenert). We now know that these cells synthesise acetylcholine and project widely to cortical areas. This nucleus is also affected by the neurofibrillary tangles of Alzheimer's disease. Patients with DLB or PDD have particularly severe deficits in cortical levels of acetylcholine and its enzyme for synthesis, even exceeding the deficits of patients with just Alzheimer's disease (AD) pathology (Perry 1994). The key neuropathological defect that is targeted by cholinesterase inhibitors is therefore present in AD, PDD and DLB. Moreover, the lower cholinergic functioning in DLB and PDD may indicate a greater potential improvement from these drugs than that seen in AD. Since there are fewer neurofibrillary tangles and neuritic plaques and less neuronal loss in DLB than AD (Lippa 1998), it is possible that cortical neurons in DLB are more viable than those in AD and could be more responsive to cholinergic stimulation. Similarly, because those patients with visual hallucinations and more profound deficits in attention tend to have worse cholinergic deficits, the presence of this symptom may be a predictor of treatment response.
The combination of psychotic features and parkinsonism which occurs in DLB and PDD can be particularly difficult to manage. Antipsychotic drugs used to treat hallucinations, delusions and agitation can dramatically worsen cognitive and extrapyramidal symptoms and may lead to severe, and even fatal, neuroleptic sensitivity (McKeith 1992). Conversely, L‐DOPA treatment of parkinsonism can exacerbate the psychosis. Given that anticholinergic agents are effective in reducing symptoms of tremor in Parkinson's disease, there are theoretical reasons why 'pro‐cholinergic' interventions such as cholinesterase inhibitors, which act to reduce breakdown of acetylcholine, might worsen the motor symptoms of Parkinson's disease (Thomas 2005).
Drug licensing
To date, rivastigmine is the only cholinesterase inhibitor that is licensed for the treatment of mild to moderate dementia in Alzheimer's disease and Parkinson's disease in the UK (Medicines and Healthcare products Regulatory Agency) and the USA (Federal Drug Authority). The use of donepezil and galantamine is only licensed in mild to moderate Alzkeimer's disease.
Objectives
To assess the efficacy, safety and tolerability of cholinesterase inhibitors in patients with dementia with Lewy bodies (DLB), Parkinson's disease with dementia (PDD) and cognitive impairment in Parkinson's disease (considered as separate phenomena and also grouped together as Lewy body (LB) disease).
Methods
Criteria for considering studies for this review
Types of studies
Randomised, double‐blind, placebo‐controlled trials assessing the efficacy of treatment with cholinesterase inhibitors in DLB, PDD and cognitive impairment in Parkinson's disease (CIND‐PD).
Types of participants
All patients with either DLB, PDD or CIND‐PD. Coexisting Alzheimer's disease was not an exclusion criterion.
Types of interventions
Any studies comparing one of the current cholinesterase inhibitors (donepezil, galantamine, rivastigmine, tacrine) at any dose, taken over any length of time, against placebo.
Types of outcome measures
Outcome measures included the following. 1. Neuropsychiatric features (e.g. psychiatric symptoms, behavioural features); subgroup analysis of those with and without visual hallucinations. 2. Cognitive function; subgroup analysis of those with and without attentional deficits. 3. Activities of daily living. 4. Global assessments. 5. Quality of life (e.g. including maintenance of social functioning). 6. Effect on carers. 7. Institutionalization. 8. Effect on parkinsonian features (e.g. tremor, rigidity). 9. Acceptability of treatment, as indicated by patient or carer assessment or by measurement of withdrawal from trials, or both. 10. Safety, as measured by severity and frequency of side effects and adverse events. 11. Deaths, including deaths during trials and time to death. 12. Heath economics.
Search methods for identification of studies
Electronic searches
We searched ALOIS (www.medicine.ox.ac.uk/alois), the Cochrane Dementia and Cognitive Improvement Group Specialised Register (on 30 August 2011). The search terms used were: PDD, parkinson, LBD, DLB, lewy.
ALOIS is maintained by the Trials Search Co‐ordinator of the Cochrane Dementia and Cognitive Improvement Group and contains studies in the areas of dementia prevention, dementia treatment and cognitive enhancement in healthy people. The studies are identified from the following.
Monthly searches of a number of major healthcare databases: MEDLINE, EMBASE, CINAHL, PsycINFO and LILACS.
Monthly searches of a number of trial registers: ISRCTN; UMIN (Japan's Trial Register); the WHO International Clinical Trials Registry Platform portal (which covers ClinicalTrials.gov; ISRCTN; the Chinese Clinical Trials Register; the German Clinical Trials Register; the Iranian Registry of Clinical Trials; the Netherlands National Trials Register plus others).
A quarterly search of The Cochrane Library’s Central Register of Controlled Trials (CENTRAL).
Six‐monthly searches of a number of grey literature sources: ISI Web of Knowledge Conference Proceedings; Index to Theses; Australasian Digital Theses.
To view a list of all sources searched for ALOIS see About ALOIS on the ALOIS website.
Details of the search strategies used for the retrieval of reports of trials from the healthcare databases, CENTRAL and conference proceedings can be viewed in the ‘methods used in reviews’ section within the editorial information about the Dementia and Cognitive Improvement Group.
Additional searches were performed in many of the sources listed above, to cover the timeframe from the last searches performed for ALOIS, to ensure that the search for the review was as up‐to‐date and as comprehensive as possible. The search strategies used can be seen in Appendix 1.
The latest search (August 2011) retrieved a total of 240 results. After a first assessment and de‐duplication of these results the authors were left with 50 references to further assess.
Searching other resources
Reference lists of relevant studies were searched for additional trials.
Data collection and analysis
Selection of studies
Two review authors (MR, RMcS) independently selected trials for relevance using the defined criteria in the current Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008).
Asessment of methodological quality
Review authors (MR, RMcS) independently assessed the quality of the trials according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions. Where the review authors (MR, RMcS) identified bias and agreed that it was significant, trials were excluded from further analysis; reasons for such exclusion were given.
Data extraction
Data were extracted from the published reports (MR). Any uncertainty over inclusion or exclusion of a trial, methodological quality or data extraction were settled by discussion with a second review author (RMcS) who had previously extracted data in an earlier draft of this review.
The summary statistics required for each trial and each outcome for continuous data was the mean change from baseline, the standard error of the mean change, and the number of patients for each treatment group at each assessment. Where changes from baseline were not reported, the mean, standard deviation and the number of patients for each treatment group at the final time point were extracted, if available. Results from the donepezil groups in a study which compared two doses of donepezil were combined (Dubois 2007). For binary data the numbers in each treatment group and the numbers experiencing the outcome of interest were sought. The baseline assessment was defined as the latest available assessment prior to randomisation, but no longer than two months earlier. Data from titration phases prior to the randomised phase, or from open label follow‐up periods, were not used to assess safety or efficacy because patients were not randomised or treatments concealed.
Analysis plan
Data for trials in each 'condition' (that is PDD, DLB, CIND‐PD) were considered separately for each outcome measure. Data for the conditions were also combined. It was intended that data for the three conditions would be considered both separately and combined. Results were also analysed according to the cholinesterase used and the duration of the trial. Results were examined to establish whether any heterogeneity was explicable on the basis of the condition. Where there was no heterogeneity, then the focus in the text was determined by the number and quality of trials. Where the heterogeneity of results was high, as indicated by I² > 40%, this was reported in the text.
Results
Description of studies
Six trials met the inclusion criteria for this review and 1236 participants were randomised in total. Four of the trials were of a parallel group design and two were cross‐over trials.
Participants
All participants were aged 18 years and over, with both males and females included in all of the trials. Four of the trials included participants with a diagnosis of Parkinson's disease with dementia (PDD) (Aarsland 2002a; Dubois 2007; Emre 2004; Ravina 2005), of which Dubois 2007 remains unpublished. Leroi 2004 included patients with either PDD or cognitive impairment in Parkinson's disease (CIND‐PD). Patients with dementia with Lewy bodies (DLB) were included in only one of the trials (McKeith 2000).
Setting
The trials were all conducted in the outpatient population. Three of the trials were multi‐centre studies (Dubois 2007; Emre 2004; McKeith 2000). Two of the trials took place in the USA (Leroi 2004; Ravina 2005) with the remaining trial taking place in Norway (Aarsland 2002a).
Intervention
Two of the trials compared the use of oral rivastigmine, up to 12 mg daily, with the use of placebo (Emre 2004; McKeith 2000). The remaining trials compared the use of oral donepezil to oral placebo. Three of the trials (Aarsland 2002a; Leroi 2004; Ravina 2005) studied donepezil at the highest tolerated dose (up to 10 mg daily). Dubois 2007 compared the use of donepezil at two different doses (either 5 mg or 10 mg) to placebo.
Duration
Four of the trials were 18 weeks or more in duration (Dubois 2007; Emre 2004; Leroi 2004; McKeith 2000). The remaining two trials lasted 10 weeks (Aarsland 2002a; Ravina 2005).
Outcome measures
Global assessment
The Alzheimer's Disease Cooperative Study Clinician's Global Impression of Change (ADCS‐CGIC) scale (Schneider 1997) is a 7‐point scale providing a global rating of patient function in four areas: general, cognitive, behaviour and activities of daily living. Assessments should be performed by the same clinician with input from the patient and the caregiver.
Cognitive function
Mini‐Mental State Examination (MMSE) (Folstein 1975) evaluates cognition in five areas: orientation, immediate recall, attention and calculation, delayed recall, and language. Test scores range from 0 (severe impairment) to 30 (normal).
The cognitive part of the Alzheimer's Disease Assessment Scale (ADAS‐Cog) (Rosen 1984) comprises 11 individual sections testing spoken language, recall of test instructions, word finding difficulty, following commands, naming objects, construction drawing, ideational praxis, orientation, word recall and word recognition. The maximum score is 70, with higher scores representing greater impairment.
The Mattis Dementia Rating Scale (MDRS) (Mattis 1988) assesses cognitive function on five subscales: attention, initiation‐perseveration, construction, conceptualisation and memory.
The Cognitive Drug Research (CDR) Computerized Assessment System (Simpson 1991) power of attention tests evaluate simple and complex reaction times and digit vigilance. Scores are measured in milliseconds with higher scores indicating a worse performance.
The Delis‐Kaplan Executive Function System (D‐KEFS) Verbal Fluency test (Delis 2001) requires patients to produce as many words starting with a particular letter as they can in one minute. Higher scores indicate better performance.
The Ten Point Clock‐Drawing test (Manos 1994) is used as a measure of spatial dysfunction and neglect. Scores range from 0 to 10 with higher results indicating better performance.
Brief Test of Attention (BTA) (Schretlen 1997) is an auditory perception task that measures divided attention in the verbal‐linguistic system. Raw scores range from 0 (severe impairment) to 20 (normal).
The Trail Making Test (TMT) (Reitan 1958) tests visual attention and task switching. It is divided into two parts: part A, containing only numbers; and part B, in which the participant must alternate between numbers and letters. The time to complete the test is used as the performance measure.
The Verbal Fluency test (Barr 1996) assesses the efficiency of verbal retrieval, short‐term memory and cognitive flexibility by asking the participant to name as many animals as he or she can in 60 seconds.
Hopkins Verbal Learning Test (Brandt 2001) is a brief verbal learning and memory test.
Developmental Test of Visual‐Motor Integration (VMI) (Beery 1989) consists of copying 24 geometric forms. A higher score indicates a better performance.
Behavioural disturbance
The 10‐item Neuropsychiatric Inventory (NPI) (Cummings 1994) is a relatively brief interview that assesses 10 types of behavioural disturbance: delusions, hallucinations, dysphoria, anxiety, agitation or aggression, euphoria, disinhibition, irritability or lability, apathy, and aberrant motor behaviour. Scores range from 0 (normal) to 120 (severely disturbed).
The Brief Psychiatric Rating Scale (BPRS) (Overall 1962) is used to measure psychiatric symptoms such as depression, anxiety, hallucinations, and unusual behaviour. Each symptom is rated 1 to 7 according to severity.
Activities of daily living (ADL)
The Alzheimer's Disease Cooperative Study Activities of Daily Living inventory (ADCS‐ADL) (Galasko 1997) is a scale for basic and complex abilities that has been validated in patients with dementia. The highest score is 78 and implies no impairment.
Unified Parkinson's Disease Rating Scale (UPDRS) ‐ Activities of Daily Living is a subscale of the UPDRS (see below).
Safety and tolerability
The Unified Parkinson's Disease Rating Scale (UPDRS) (Fahn 1987) is used to follow the longitudinal course of Parkinson's disease. It is divided into five sections: evaluation of mentation, behaviour and mood; self evaluation of activities of daily living; clinician‐scored motor evaluation; severity of Parkinson's disease (Hoehn and Yahr); and the Schwab and England ADL scale. Higher scores imply more severe disease.
Risk of bias in included studies
Allocation
Aarsland 2002a and McKeith 2000 provided details of adequate sequence generation and concealment. Emre 2004 provided good details of sequence generation but did not specify methods used to maintain concealment of allocation, whilst Leroi 2004 did not discuss the randomisation procedures followed. Neither Dubois 2007 nor Ravina 2005 provided any details of the allocation procedure.
Blinding
All the trials described the use of 'double‐blind' methods but none of them described how they were achieved.
Reporting of withdrawals or dropouts
Dubois 2007 did not disclose whether any of the participants withdrew or dropped out during the study. All other trials reported the numbers of withdrawals and dropouts but only McKeith 2000 included all of these in the final analysis. Aarsland 2002a, Emre 2004 and Leroi 2004 only included in the efficacy analysis participants that received at least one dose of the study medication and had at least one measurement at baseline and at one other time point, using the last observation carried forward (LOCF). Emre 2004 did not include a supplementary table reporting the details of completer analysis. It was therefore impossible to assess the impact of retrieval of dropouts on the validity of the LOCF‐intention‐to‐treat (ITT) analysis. One study (Ravina 2005) specified that participants had to have at least one visit in the second period to be included in the efficacy analysis. Three participants were included in the safety but not the efficacy analysis as data from both periods were required for the cross‐over analysis.
Selective reporting
Aarsland 2002a only published primary outcomes of the study and the publication of the secondary outcome measures is still pending. The results of one trial (Dubois 2007) are only available in poster format and, due to the very limited details provided, only one of the efficacy variables could be included in this meta‐analysis. Despite numerous attempts to contact the authors, no further details of the trial have been made available.
Other sources of bias
Two trials (Aarsland 2002a; Ravina 2005) were cross‐over in design and were considered in accordance with the guidance of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). Although dementia is a neuro‐degenerative condition, the duration of the trials was considered to be too short for any significant disease progression to have occurred in that period. Neither of the two studies demonstrated a significant carry‐over effect between the two phases of the trial.
Effects of interventions
1. Effect of cholinesterase inhibitors on dementia with Lewy bodies, Parkinson's disease dementia and cognitive impairment in Parkinson's disease.
| Dementia with Lewy bodies (DLB) | Parkinson's disease with dementia (PDD) | Parkinson's Disease with dementia (PDD) and cognitive impairment in Parkinson's disease (CIND‐PD) | Pooled results for DLB/PDD/CIND‐PD | |
| Global assessment | N/A |
Favours treatment (SMD ‐0.38, 95% CI ‐0.56, ‐0.24, P<0.00001) |
N/A | N/A |
| Cognitive function |
No effect (SMD ‐0.29, 95% CI ‐0.65, 0.07, P=0.12) |
Favours treatment (SMD ‐0.36, 95% CI ‐0.48, ‐0.23, P<0.00001) |
Favours treatment (SMD ‐0.36, 95% CI ‐0.48, ‐0.23, P<0.00001) |
Favours treatment (SMD ‐0.34, 95% CI ‐0.46, ‐0.23, P<0.00001) |
|
Behavioural disturbance |
No effect (SMD ‐0.24, 95% CI ‐0.60, 0.12, P=0.19) |
Favours treatment (SMD ‐0.18, 95% CI ‐0.36, ‐0.01, P=0.04) |
Favours treatment (SMD ‐0.19, 95% CI ‐0.36, ‐0.01, P=0.04) |
Favours treatment (SMD ‐0.20, 95% CI ‐0.36, ‐0.04, P=0.01) |
| Activities of daily living | N/A |
Favours treatment (SMD ‐0.21, 95% CI ‐0.40, ‐0.02, P=0.03) |
Favours treatment (SMD ‐0.20, 95% CI ‐0.39, ‐0.02, P=0.03) |
N/A |
Global assessment
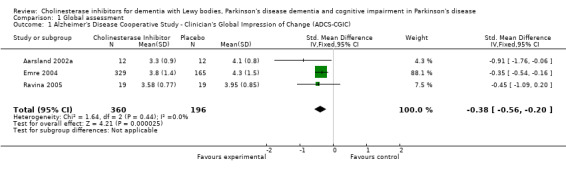
Three trials comparing cholinesterase inhibitor treatment to placebo in PDD (Aarsland 2002a; Emre 2004; Ravina 2005) reported a difference in the ADCS‐CGIC score of ‐0.38, favouring the cholinesterase inhibitors (95% CI ‐0.56 to ‐0.24, P < 0.0001) (Figure 1). A therapeutic benefit of cholinesterase inhibitors was observed irrespective of the agent used and the duration of the trial (weighted mean difference (WMD) ‐0.62, 95% CI ‐1.13 to ‐0.10, P = 0.02 for donepezil used for 10 weeks or less; and WMD ‐0.35, 95% CI ‐0.54 to ‐0.16, P = 0.0003 for rivastigmine used for 18 weeks or more).
1.
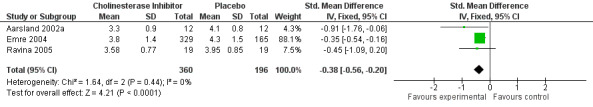
Forest plot of comparison: 1 Global assessment, outcome: 1.1 Alzheimer's Disease Cooperative Study ‐ Clinician's Global Impression of Change (ADCS‐CGIC).
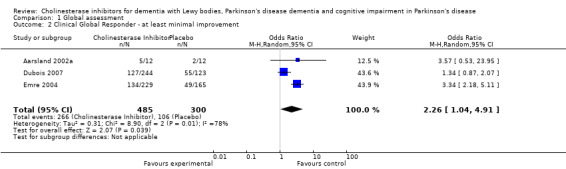
Three trials reported response rates (Aarsland 2002a; Dubois 2007; Emre 2004). These favoured the cholinesterase inhibitor (OR 2.26, 95% CI 1.04 to 4.91, P = 0.04) but with high heterogeneity (I2 = 78%).
Cognitive function
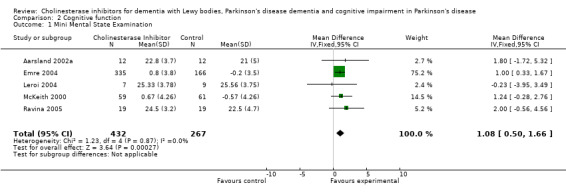
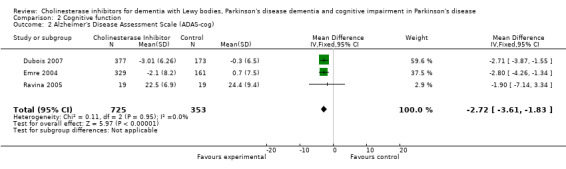
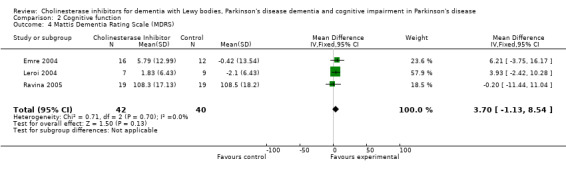
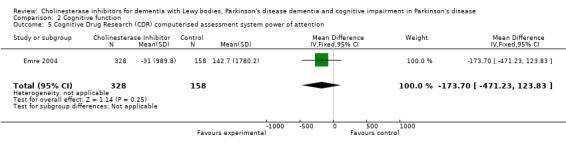
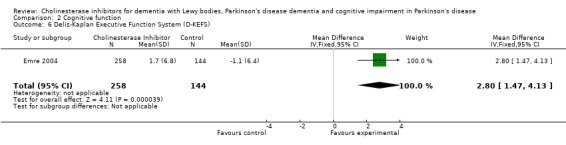
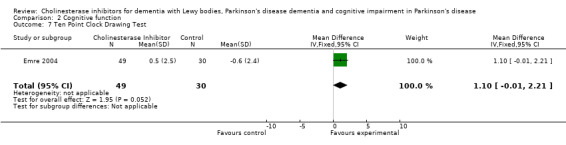
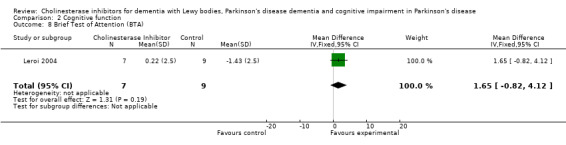
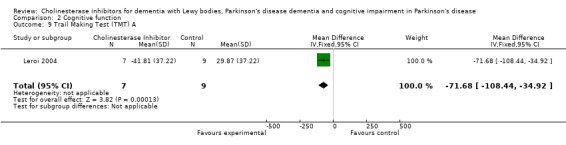
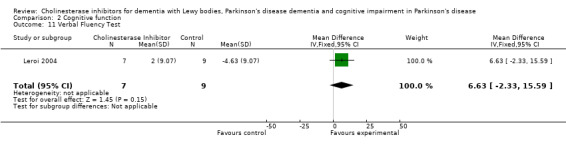
Although there was no statistically significant difference in the MMSE between the control and treatment groups for patients with DLB (McKeith 2000) (WMD 1.24, 95% CI 0.28 to 2.76, P = 0.11), a beneficial treatment effect was seen in PDD patients (Aarsland 2002a; Emre 2004; Ravina 2005) (WMD 1.09, 95% CI 0.45 to 1.73, P = 0.0008) and in PDD and CIND‐PD patients (Aarsland 2002a; Emre 2004; Leroi 2004; Ravina 2005) (WMD 1.05, 95% CI 0.42 to 1.68, P = 0.001). Pooling all available data showed an improvement in the MMSE favouring treatment with cholinesterase inhibitors (WMD 1.08, 95% CI 0.50 to 1.66, P = 0.0003). Furthermore, cholinesterase inhibitors led to an improvement in cognitive function in patients with PDD, as measured by the ADAS‐Cog (Dubois 2007; Emre 2004; Ravina 2005) (WMD ‐2.72, 95% CI ‐3.61 to ‐1.83, P < 0.00001) and the Delis‐Kaplan Executive Function System (Emre 2004) (WMD 2.80, 95% CI 1.47 to 4.13, P < 0.0001). There was also a statistically significant improvement in the Trail Making Test A in patients with PDD or CIND‐PD (Leroi 2004) (WMD ‐71.68, 95% CI ‐108.44 to ‐34.92, P = 0.0001). Cholinesterase inhibitor use had no statistically significant impact on the Mattis Dementia Rating Scale (MDRS) when used in patients with PDD alone (Emre 2004; Ravina 2005) (WMD 3.39, CI 95% ‐4.06 to 10.84, P = 0.37) or in patients with PDD or CIND‐PD (Emre 2004; Leroi 2004; Ravina 2005) (WMD 3.70, 95% CI ‐1.13 to 8.54, P = 0.30). No significant difference between the two groups was observed using the Cognitive Drug Research Computerized Assessment system power of attention scale (Emre 2004) (WMD ‐173.70, 95% CI ‐471.23 to 123.83, P = 0.25), the Ten Point Clock‐Drawing Test (Emre 2004) (WMD 1.10, 95% CI ‐0.01 to 2.21, P = 0.05), Brief Test of Attention (Leroi 2004) (WMD 1.65, 95% CI ‐0.82 to 4.12, P = 0.19), Trail Making Test B (Leroi 2004) (WMD ‐87.24, 95% CI ‐202.89 to 28.41, P = 0.14), Verbal Fluency Test (Leroi 2004) (WMD 6.63, 95% CI ‐2.33 to 15.59, P = 0.15), Hopkins Verbal Learning Test (Leroi 2004) (WMD 1.72, 95% CI ‐2.93 to 6.37, P = 0.47) and the Developmental Test of Visual‐Motor Integration (Leroi 2004) (WMD 0.03, 95% CI ‐3.28 to 3.34, P = 0.99).
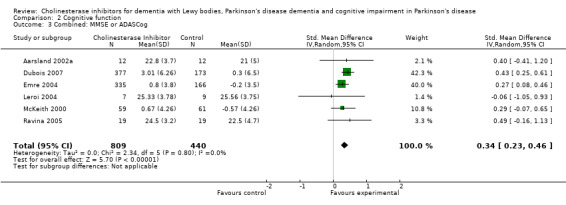
In an overall assessment of the cognitive function domain, combining MMSE scores where available, and ADASCog scores where not, the pooled estimate of the effect of cholinesterase inhibitors on cognitive function measures was consistent with the presence of a therapeutic benefit (standardised mean difference (SMD) ‐0.34, 95% CI ‐0.46 to ‐0.23, P < 0.00001) (Figure 2). The beneficial effect of cholinesterase inhibitors on cognitive function was observed in both the donepezil and rivastigmine groups (SMD ‐0.42, 95% CI ‐0.58 to ‐0.25, P < 0.00001; SMD ‐0.27, 95% CI ‐0.44 to ‐0.11, P < 0.001, respectively).
2.
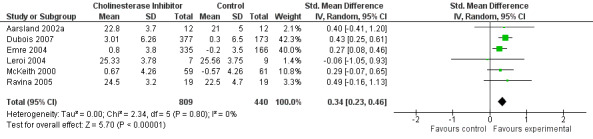
Forest plot of comparison: 2 Cognitive function, outcome: 2.3 Combined: MMSE or ADASCog.
Behavioural disturbance
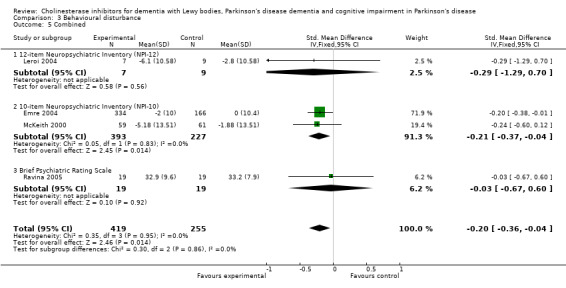
Analysis of the pooled continuous data relating to behavioural disturbance rating scales once again favoured treatment with cholinesterase inhibitors (SMD ‐0.20, 95% CI ‐0.36 to ‐0.04, P = 0.01) (Figure 3). This effect was only seen in trials using rivastigmine (SMD ‐0.21, 95% CI ‐0.36 to ‐0.06, P = 0.006) and those lasting 18 weeks or longer (SMD ‐0.21, 95% CI ‐0.36 to ‐0.06, P = 0.005). Breakdown of the individual rating scales did not reveal any effect of the treatment on the Brief Psychiatric Rating Scale (Ravina 2005) (WMD ‐0.30, 95% CI ‐5.89 to 5.25, P = 0.92) or the 12‐item Neuropsychiatric Inventory (NPI) (Leroi 2004) (WMD ‐3.30, 95% CI ‐13.75 to 7.15, P = 0.54). Patients with DLB failed to improve their NPI‐4 (McKeith 2000) (WMD ‐1.65, 95% CI ‐4.33 to 1.03, P = 0.23) or NPI‐10 (McKeith 2000) (WMD ‐3.30, 95% CI ‐8.14 to 1.54, P = 0.18) scores on active treatment. Emre 2004 showed an improvement of ‐2.00 (95% CI ‐3.91 to ‐0.09, P = 0.04) in NPI‐10 in PDD patients treated with cholinesterase inhibitors. Hallucinations were less frequently reported in the active treatment group than the placebo group, however this was not statistically significant (Dubois 2007; Emre 2004) (46/739 versus 33/352; odds ratio (OR) 0.64, 95% CI 0.40 to 1.02, P = 0.06). There was a risk of bias due to selective reporting of this outcome, which was not available from the large Dubois 2007 study or Aarsland 2002a.
3.
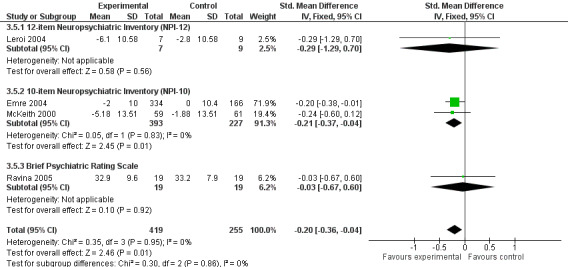
Forest plot of comparison: 3 Behavioural disturbance, outcome: 3.5 Combined.
Activities of daily living
There was an improvement in the Alzheimer's Disease Cooperative Study activities of daily living rating scale (Emre 2004) (WMD 2.50, 95% CI 0.43 to 4.57, P = 0.02), with no difference observed using the UPDRS activities of daily living rating scale (Leroi 2004) (WMD 0.84, 95% CI ‐6.24 to 7.92, P = 0.82). Combined data favoured treatment with cholinesterase inhibitors (SMD ‐0.20, 95% CI ‐0.38 to ‐0.02, P = 0.03).
Safety and tolerability
Both the total number of dropouts and the number of dropouts due to adverse events were significantly higher in the treatment group as compared to the patients receiving placebo (128/465 versus 45/279; OR 1.94, 95% CI 1.33 to 2.84, P = 0.0006 and 73/430 versus 22/247; OR 2.12, 95% CI 1.27 to 3.55, P = 0.004). The placebo group experienced significantly fewer adverse events (668/842 versus 318/452; OR 1.64, 95% CI 1.26 to 2.15, P = 0.0003), although the number of adverse events that were judged to be severe was not significantly different between the two groups (21/73 versus 15/73; OR 1.60, 95% CI 0.68 to 3.81, P = 0.28). Interestingly, the increase in the number of dropouts and adverse events in the treatment group were significant in studies using rivastigmine (117/421 versus 42/240; OR 1.82, 95% CI 1.22 to 2.71, P = 0.003 and 357/421 versus 173/240; OR 2.28, 95% CI 1.53 to 3.38, P < 0.0001, respectively) but not in the studies using donepezil (11/44 versus 3/39; OR 3.64, 95% CI 0.99 to 13.46, P = 0.05 and 311/421 versus 145/212; OR 1.24, 95% CI 0.86 to 1.80, P = 0.25, respectively). Parkinsonian symptoms were reported more commonly in the treatment group (139/739 versus 40/352; OR 1.88, 95% CI 1.28 to 2.75, P = 0.001), however this did not have a significant impact on the UPDRS (total and motor) scores (SMD ‐0.07, 95% CI ‐0.42 to 0.29, P = 0.71) (Figure 4). Although tremor was more commonly reported in the treatment groups (64/739 versus 12/352; OR 2.71, 95% CI 1.44 to 5.09, P = 0.002), the same was not true of falls (43/739 versus 16/352; OR 1.29, 95% CI 0.72 to 2.33, P = 0.39). Fewer deaths occurred in the treatment group when compared to the placebo group (4/465 versus 9/279; OR 0.28, 95% CI 0.09 to 0.84, P = 0.03).
4.
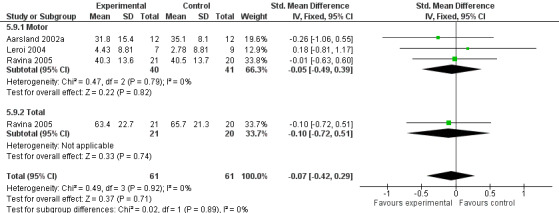
Forest plot of comparison: 5 Safety and tolerability, outcome: 5.9 Unified Parkinson's Disease Rating Scale (UPDRS).
Discussion
We identified six trials to help us assess the efficacy, safety and tolerability of cholinesterase inhibitors in dementia with Lewy bodies (DLB), Parkinson's disease with dementia (PDD) and cognitive impairment in Parkinson's disease (CIND‐PD). Where possible, we considered outcome measures for the separate diseases as well as combining the data available to estimate the general effect of cholinesterase inhibitors on Lewy body disease.
Currently available evidence suggests that cholinesterase inhibitors improve global assessment measures in patients with PDD, with no data for DLB and CIND‐PD being available. As there was no evidence of a positive impact of cholinesterase inhibitors on cognitive function and behavioural disturbance rating scales in patients with DLB, the overall response in favour of using cholinesterase inhibitors is likely to be due to the effect seen in patients with PDD. As only one of the six trials included in this meta‐analysis randomised patients with DLB, the over‐representation of patents with PDD could have a substantial effect on the overall effects. The effect of cholinesterase inhibitors on measures of activities of daily living was not assessed in the DLB population but was statistically significant in the very small trial which included both patients with PDD and CIND‐PD.
The trials that were included provided evidence that cholinesterase inhibitors were not as well tolerated as placebo, with significantly more adverse effects and dropouts seen in the active treatment group. Reassuringly, the frequency of severe adverse effects was the same in both groups. Indeed, death rates were lower amongst those taking the active drug than placebo, though this is based on small numbers. Although parkinsonian symptoms, and tremor in particular, were reported more frequently as adverse effects in patients receiving cholinesterase inhibitors, this did not seem to have an impact on the Parkinson's disease severity rating scales.
An important limitation of the current review lies in the incomplete public presentation of data from the important Dubois 2007 study, which was sponsored by Pfizer. The study was completed prior to current US legislation on trial registration.
Authors' conclusions
Implications for practice.
The currently available evidence supports the use of cholinesterase inhibitors in patient with PDD and CIND‐PD, with a positive impact on global assessment, cognitive function, behavioural disturbance and activities of daily living rating scales. The effect of cholinesterase inhibitors on patients with DLB has only been investigated in one small study and, therefore, evidence for their use in this patient group is less clear.
Implications for research.
Patients with DLB were under‐represented in this meta‐analysis and further randomised evidence is required to reduce the uncertainty of the effects that cholinesterase inhibitors have in this patient group. A large trial of donepezil in patients with CIND‐PD was due to commence in 2012 (Burn 2009).
Feedback
Evidence supporting cholinesterase use in patients with PDD, 22 April 2014
Summary
Dear Rolinski et al.,
I am writing to you regarding the Cochrane meta‐analysis of Cholinesterase inhibitors for dementia with lewy bodies (DLB), Parkinson's disease dementia (PDD) and cognitive impairment in Parkinson's disease (CIND‐PD).
The first issue I wanted to address was regarding the conclusion of the available evidence supporting the use of cholinesterase inhibitors in patients with PDD. These results were driven primarily by Emre 2004, which used last observation carried forward for any missing data. This study had a significant dropout rate compared to placebo (99 and 32, respectively). In the end, only 72.7% of patients in the treatment group completed the study. When a study uses the last observation carried forward in a patient population with a progressively worsening disease state, you tend to reflect a better response in the treatment arm than actual response. You conclude that cholinesterase inhibitors improve global assessment, cognitive function, behavioural disturbance and activities of daily living rating scales in PDD. However, this could likely be due to initial assessments of the treatment group and subsequently, a significant portion of the treatment group dropped out due to significantly more adverse effects compared to placebo. On the other hand, most of the placebo group stayed throughout the trial, then declined clinically due to a progressively worsening condition and was eventually captured in the results. The use of last observation carried forward in a dementia population likely favours more toxic drugs like cholinesterase inhibitors over placebo and can also introduce bias which may exaggerate the effectiveness of cholinesterase inhibitors.
The next issue I wanted to address was in regards to the assessment tools used to measure cognitive function. Cognitive function was mainly measured with the Mini‐Mental State Examination (MMSE) and Alzheimer's disease Assessment Scale (ADAS‐COG) in these trials. There was a statistically significant mean difference in MMSE score of 1.08 and ADAS‐COG of ‐2.72, both favouring cholinesterase inhibitors. However, a statistically significant difference may not necessarily mean clinical significance. A cohort study published in 2012 analysed the ADAS‐COG tool and found a difference of 3.1‐3.8 points to be clinically significant. Also, clinical guidelines and most studies use a change of 4 points or more in the ADAS‐COG to be considered clinically significant. As for the MMSE, a change of 3 points or more is considered clinically significant. A reader that is unfamiliar with the ADAS‐COG and MMSE might be misled into thinking that cholinesterase inhibitors would have a clinically meaningful impact on cognitive function.
These results were driven primarily by Emre 2004 and since this study had limitations such as last observation carried forward, as mentioned above, and having a non‐clinically relevant change in the assessment tools, I feel that the conclusion made by Rolinski et al. had a very strong recommendation favouring the use of anticholinesterase inhibitors. The issues addressed above were not addressed in the Cochrane review and may mislead others into thinking that the anticholinesterase inhibitors are more effective than they actually are. Thank you for your time and I look forward to hearing from you.
Reply
We completely agree that imputing missing data using the “last observation carried forward” (LOCF) approach may introduce bias into the analysis, with attrition secondary to the side effects of the study medication. However, we feel that we have already addressed this issue in the review and disagree with the conclusions that have been drawn in the feedback received. Firstly, the use of LOCF is discussed in the “Risk of bias in included studies” that can be found on page seven of the review. Further assessment of potential bias is included in the description of included studies that starts on page 18 of the review. Here, in the case of Leroi et al., 2004, the risk of bias was assessed to be high, owing to a high dropout rate and the use of LOCF. In the Emre study, 362 subjects were randomised to rivastigmine, and 263 continued on drug to the end. Three hundred and twenty nine subjects had a ADASCog score in the primary LOCF analysis, with the authors stating: ‘Patients who discontinued treatment prematurely were encouraged to attend assessments at predefined times; when available, the results of these assessments were used for efficacy analyses’. However, it is not stated what the extent of this ‘retrieved dropout’ data was. Therefore, we do not know to what extent the data reflect genuine ITT. The authors stated they performed sensitivity analyses, one with LOCF and one where only complete datasets were included, with results consistent with the findings presented in their publication. If the data for the Emre completers had been presented (e.g. in an online supplementary table) then this could have been assessed further in our review. Moreover, in relation to the Dubois study, we have repeatedly stated that the fact that the study data is not available is problematic.
LOCF is particularly problematic when there is differential dropout and marked decline in cognitive function in the placebo group. However, in this study, the decline in placebo was small whereas those on rivastigmine tended to improve. Importantly, Emre and colleagues also performed further analyses looking at clinically relevant changes. They showed that patients receiving treatment with cholinesterase inhibitors were more likely to have a moderate or marked improvement and were less likely to have a moderate or marked worsening in their cognitive function, when compared to the placebo group using the Alzheimer’s Disease Cooperative Study‐Clinician’s Global Impression of Change (ADCS‐CGIC).
We consider that the 3 point MMSE criteria is excessively stringent, and prefer the Howard et al (DOI: 10.1002/gps.2607) criterion of 1.4 MMSE point for MCID, but acknowledge that this is still higher than the mean 1.08 achieved here. (We were unaware of the Schrag 2012 study – thank you for drawing our attention to this – but this was published after our review). However, in determining the MCID, there is controversy over whether ANY significant difference in the Global Impression of Change is sufficient to justify a claim of clinical utility. It is our belief that, when supported by other measures (as is the case with multiple outcomes in Emre) there is sufficient evidence to claim clinical utility. This is based on the clinical experience that the rating of global change over 6 months in patients with dementia, whose condition fluctuates and declines, is prone to all sorts of influence that would militate against showing an effect.
Ultimately, the wording of the conclusion of the Abstract reflects a judgment based on the balance of the evidence. We do not propose to change the key sentence ‘The currently available evidence supports the use of cholinesterase inhibitors in patients with PDD, with a positive impact on global assessment, cognitive function, behavioural disturbance and activities of daily living rating scales’.
However, in order to contextualise this further, we propose to add to the abstract:
a) The known sample size of Dubois (N=550) and a comment in the Author’s Conclusion that: ‘Almost half the trial data, which could potentially change this conclusion, have not been made public’.
b) A comment that 80% of people who take RVS had no clinically meaningful improvement in Emre (Main Results Para2).
Moreover, add the following to ‘Reporting of withdrawals or dropouts’: “Emre did not include a supplementary table reporting the details of completer analysis. It is therefore impossible to assess the impact of retrieval of dropouts on the validity of the LOCF‐ITT analysis”.
Contributors
Mr Melvin Lau, Author of feedback letter
Dr Michal Rolinski, Review author
Dr Chris Fox, Review author
Dr Ian Maidment, Review author
Dr Rupert McShane, Review author
What's new
| Date | Event | Description |
|---|---|---|
| 4 July 2014 | Feedback has been incorporated | Minor changes made to the abstract based on feedback received. |
History
Protocol first published: Issue 2, 2007 Review first published: Issue 3, 2012
| Date | Event | Description |
|---|---|---|
| 30 August 2011 | Amended | A pre‐publication search was performed for this review on 30 August 2011 to ensure the review was as up‐to‐date as possible before publication. |
| 8 February 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors gratefully acknowledge the contributions of the consumer editor, Margaret Bullard. We are also grateful to David Beversdorf for sharing with us the data from his study.
Appendices
Appendix 1. Search: August 2011 (pre‐publication search)
|
Source |
Search strategy | Hits retrieved |
| 1. ALOIS (www.medicine.ox.ac.uk/alois) | PDD OR "parkinson disease" OR DLB OR lewy [Added to ALOIS since January 2010] | 21 |
| 2. MEDLINE In‐Process and other non‐indexed citations and MEDLINE 1950‐present (OvidSP) | 1. exp Cholinesterase Inhibitors/ 2. "cholinesterase inhibitor*".mp. 3. Galantamine/ 4. (galantamine or galanthamine).mp. 5. (reminyl* or Nivalin* or Razadyne*).mp. 6. donepezil.mp. 7. (Aricept* or E2020).mp. 8. rivastigmine.mp. 9. (Exelon* or "SDZ ENA 713").mp. 10. Tacrine/ 11. (tacrine or cognex*).mp. 12. or/1‐11 13. DLB.mp. 14. LBD.mp. 15. "lewy* bod*".mp. 16. Lewy Bodies/ 17. PDD.mp. 18. "parkinson* disease dement*".mp. 19. Parkinson Disease/ 20. (cognit* and (PD or parkinson*)).mp. 21. PD‐CIND.mp. 22. (CIND and parkinson*).mp. 23. or/13‐22 24. 12 and 23 25. randomized controlled trial.pt. 26. controlled clinical trial.pt. 27. randomized.ab. 28. placebo.ab. 29. drug therapy.fs. 30. randomly.ab. 31. trial.ab. 32. groups.ab. 33. or/25‐32 34. (animals not (humans and animals)).sh. 35. 33 not 34 36. 24 and 35 37. (2010* or 2011*).ed. 38. 36 and 37 |
43 |
| 3. EMBASE 1980‐2011 week 34 (OvidSP) |
1. cholinesterase inhibitor/ 2. "cholinesterase inhibitor*".mp. 3. galantamine/ 4. (galantamine or galanthamine).mp. 5. (reminyl* or Nivalin* or Razadyne*).mp. 6. donepezil/ 7. donepezil.mp. 8. (Aricept* or E2020).mp. 9. rivastigmine/ 10. rivastigmine.mp. 11. (Exelon* or "SDZ ENA 713").mp. 12. (tacrine or cognex*).mp. 13. or/1‐12 14. DLB.mp. 15. LBD.mp. 16. "lewy* bod*".mp. 17. exp Lewy body/ 18. PDD.mp. 19. "parkinson* disease dement*".mp. 20. Parkinson disease/ 21. (cognit* and (PD or parkinson*)).mp. 22. PD‐CIND.mp. 23. (CIND and parkinson*).mp. 24. or/14‐23 25. 13 and 24 26. randomized controlled trial/ 27. controlled clinical trial/ 28. random*.ti,ab. 29. "control group".ab. 30. trial.ab. 31. or/26‐30 32. 25 and 31 33. (2010* or 2011*).em. 34. 32 and 33 |
|
| 4. PsycINFO 1806‐August week 4 2011 (OvidSP) |
1. exp Cholinesterase Inhibitors/ 2. ("cholinesterase inhibitor*" or CHoI).mp. 3. exp Galanthamine/ 4. (galantamine or galanthamine).mp. 5. (reminyl* or Nivalin* or Razadyne*).mp. 6. donepezil.mp. 7. (Aricept* or E2020).mp. 8. rivastigmine.mp. 9. (Exelon* or "SDZ ENA 713").mp. 10. (tacrine or cognex*).mp. 11. or/1‐10 12. DLB.mp. 13. LBD.mp. 14. "lewy* bod*".mp. 15. exp Dementia with Lewy Bodies/ 16. PDD.mp. 17. "parkinson* disease dement*".mp. 18. exp Parkinsons Disease/ 19. (cognit* and (PD or parkinson*)).mp. 20. PD‐CIND.mp. 21. (CIND and parkinson*).mp. 22. or/12‐21 23. 11 and 22 24. exp Clinical Trials/ 25. random*.ti,ab. 26. placebo*.ab. 27. "control group".ab. 28. ("single‐blind*" or "double‐blind*").ti,ab. 29. or/24‐28 30. 23 and 29 31. (2010* or 2011*).up. 32. 30 and 31 |
15 |
| 5. CINAHL (EBSCOhost) | S1 (MH "Dementia+") S2 (MH "Delirium") or (MH "Delirium, Dementia, Amnestic, Cognitive Disorders") S3 (MH "Wernicke's Encephalopathy") S4 TX dement* S5 TX alzheimer* S6 TX lewy* N2 bod* S7 TX deliri* S8 TX chronic N2 cerebrovascular S9 TX "organic brain disease" or "organic brain syndrome" S10 TX "normal pressure hydrocephalus" and "shunt*" S11 TX "benign senescent forgetfulness" S12 TX cerebr* N2 deteriorat* S13 TX cerebral* N2 insufficient* S14 TX pick* N2 disease S15 TX creutzfeldt or jcd or cjd S16 TX huntington* S17 TX binswanger* S18 TX korsako* S19 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 S20 TX "cognit* impair*" S21 TX "cognit* defect*" S22 (MH "Cognition Disorders+") S23 TX MCI S24 TX ACMI S25 TX ARCD S26 TX SMC S27 TX CIND S28 TX BSF S29 TX AAMI S30 AB MD S31 AB LCD S32 AB QD OR "questionable dementia" S33 TX AACD S34 TX MNCD S35 TX "N‐MCI" or "A‐MCI" or "M‐MCI" S36 TX "preclinical AD" S37 TX "pre‐clinical AD" S38 TX "preclinical alzheimer*" or "pre‐clinical alzheimer*" S39 TX aMCI OR MCIa S40 TX "CDR 0.5" or "clinical dementia rating scale 0.5" S41 TX "GDS 3" OR "stage 3 GDS" S42 TX "global deterioration scale" AND "stage 3" S43 TX "Benign senescent forgetfulness" S44 TX "mild neurocognit* disorder*" S45 TX prodrom* N2 dement* S46 TX "age‐related symptom*" S47 TX cognit* N2 deficit* S48 TX cognit* N2 deteriorat* S49 TX cognit* N2 declin* S50 TX cognit* N2 degenerat* S51 TX cognit* N2 complain* S52 TX cognit* N2 disturb* S53 TX cognit* N2 disorder* S54 TX memory N2 episod* or TX memory N2 los* or TX memory N2 impair* or TX memory N2 complain* S55 TX memory N2 disturb* or TX memory N2 disorder* or TX cerebr* N2 impair* or TX cerebr* N2 los* S56 TX cerebr* N2 complain* or TX cerebr* N2 deteriorat* or TX cerebr* N2 disorder* or TX cerebr* N2 disturb* S57 TX mental* N2 declin* or TX mental* N2 los* or TX mental* N2 impair* or TX mental* N2 deteriorat* S58 TX "pre‐clinical dementia" or TX "preclinical dementia" S59 S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 or S48 or S49 or S50 or S51 or S52 or S53 or S54 or S55 or S56 or S57 or S58 S60 S19 or S59 |
|
| 6. ISI Web of Knowledge – all databases [includes: Web of Science (1945‐present); BIOSIS Previews (1926‐present); MEDLINE (1950‐present); Journal Citation Reports] | Topic=("lew* bod*" OR PDD OR (parkinson* AND cognit*)) AND Topic=(donepezil OR aricept* OR E2020 OR rivastigmin* OR Exelon* OR "SDZ ENA 713" OR galantamin* OR galanthamin* OR reminyl* OR Nivalin* OR Razadyne* OR tacrine OR cognex*) AND Topic=(randomly OR placebo OR groups OR trial OR RCT OR randomized OR randomised) AND Year Published=(2010‐2011) Timespan=All Years. Search language=English Lemmatization=On |
33 |
| 7. LILACS (BIREME) | donepezil OR rivastigmine OR galantamine OR galanthamine OR tacrine [Words] and lewy OR PDD OR parkinson OR parkinsons OR parkinson's [Words] | 1 |
| 8. CENTRAL (The Cochrane Library) (Issue 3 of 4, 2011) | #1 MeSH descriptor Cholinesterase Inhibitors explode all trees #2 "cholinesterase inhibitor*" #3 MeSH descriptor Galantamine explode all trees #4 galantamine OR galanthamine #5 reminyl* or Nivalin* or Razadyne* #6 donepezil #7 Aricept* #8 rivastigmine #9 rivastigmin #10 Exelon* #11 MeSH descriptor Tacrine explode all trees #12 cognex* #13 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12) #14 "lew* bod*" OR DLB OR LBD #15 “Parkinson* disease” OR PDD #16 #14 OR #15 #17 #16 AND #13 |
3 |
| 9. Clinicaltrials.gov (www.clinicaltrials.gov) | cognition | Interventional Studies | lewy body OR lewy bodies OR PDD OR parkinson disease | Adult, Senior | received from 01/01/2010 to 08/30/2011 | 11 |
| 10. ICTRP Search Portal (http://apps.who.int/trialsearch) [includes: Australian New Zealand Clinical Trials Registry; ClinicalTrilas.gov; ISRCTN; Chinese Clinical Trial Registry; Clinical Trials Registry – India; Clinical Research Information Service – Republic of Korea; German Clinical Trials Register; Iranian Registry of Clinical Trials; Japan Primary Registries Network; Pan African Clinical Trial Registry; Sri Lanka Clinical Trials Registry; The Netherlands National Trial Register] | (parkinson OR parkinsons OR parkinsons OR PDD OR lewy OR LBD OR DLB) AND (donepezil OR rivastigmine OR galantamine OR tacrine OR aricept OR E2020) AND (01/01/2010 to 30/08/2011) | 40 |
| TOTAL before de‐duplication | 240 | |
| TOTAL after de‐duplication and first assessment | 50 | |
Data and analyses
Comparison 1. Global assessment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Alzheimer's Disease Cooperative Study ‐ Clinician's Global Impression of Change (ADCS‐CGIC) | 3 | 556 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.56, ‐0.20] |
| 2 Clinical Global Responder ‐ at least minimal improvement | 3 | 785 | Odds Ratio (M‐H, Random, 95% CI) | 2.26 [1.04, 4.91] |
1.1. Analysis.
Comparison 1 Global assessment, Outcome 1 Alzheimer's Disease Cooperative Study ‐ Clinician's Global Impression of Change (ADCS‐CGIC).
1.2. Analysis.
Comparison 1 Global assessment, Outcome 2 Clinical Global Responder ‐ at least minimal improvement.
Comparison 2. Cognitive function.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mini Mental State Examination | 5 | 699 | Mean Difference (IV, Fixed, 95% CI) | 1.08 [0.50, 1.66] |
| 2 Alzheimer's Disease Assessment Scale (ADAS‐cog) | 3 | 1078 | Mean Difference (IV, Fixed, 95% CI) | ‐2.72 [‐3.61, ‐1.83] |
| 3 Combined: MMSE or ADASCog | 6 | 1249 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [0.23, 0.46] |
| 4 Mattis Dementia Rating Scale (MDRS) | 3 | 82 | Mean Difference (IV, Fixed, 95% CI) | 3.70 [‐1.13, 8.54] |
| 5 Cognitive Drug Research (CDR) computerised assessment system power of attention | 1 | 486 | Mean Difference (IV, Fixed, 95% CI) | ‐173.7 [‐471.23, 123.83] |
| 6 Delis‐Kaplan Executive Function System (D‐KEFS) | 1 | 402 | Mean Difference (IV, Fixed, 95% CI) | 2.8 [1.47, 4.13] |
| 7 Ten Point Clock Drawing Test | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | 1.1 [‐0.01, 2.21] |
| 8 Brief Test of Attention (BTA) | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 1.65 [‐0.82, 4.12] |
| 9 Trail Making Test (TMT) A | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐71.68 [‐108.44, ‐34.92] |
| 10 Trail Making Test (TMT) B | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐87.24 [‐202.89, 28.41] |
| 11 Verbal Fluency Test | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 6.63 [‐2.33, 15.59] |
| 12 Hopkins Verbal Learning Test | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 1.72 [‐2.93, 6.37] |
| 13 Developmental Test of Visual‐Motor Integration (VMI) | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐3.28, 3.34] |
2.1. Analysis.
Comparison 2 Cognitive function, Outcome 1 Mini Mental State Examination.
2.2. Analysis.
Comparison 2 Cognitive function, Outcome 2 Alzheimer's Disease Assessment Scale (ADAS‐cog).
2.3. Analysis.
Comparison 2 Cognitive function, Outcome 3 Combined: MMSE or ADASCog.
2.4. Analysis.
Comparison 2 Cognitive function, Outcome 4 Mattis Dementia Rating Scale (MDRS).
2.5. Analysis.
Comparison 2 Cognitive function, Outcome 5 Cognitive Drug Research (CDR) computerised assessment system power of attention.
2.6. Analysis.
Comparison 2 Cognitive function, Outcome 6 Delis‐Kaplan Executive Function System (D‐KEFS).
2.7. Analysis.
Comparison 2 Cognitive function, Outcome 7 Ten Point Clock Drawing Test.
2.8. Analysis.
Comparison 2 Cognitive function, Outcome 8 Brief Test of Attention (BTA).
2.9. Analysis.
Comparison 2 Cognitive function, Outcome 9 Trail Making Test (TMT) A.
2.10. Analysis.

Comparison 2 Cognitive function, Outcome 10 Trail Making Test (TMT) B.
2.11. Analysis.
Comparison 2 Cognitive function, Outcome 11 Verbal Fluency Test.
2.12. Analysis.
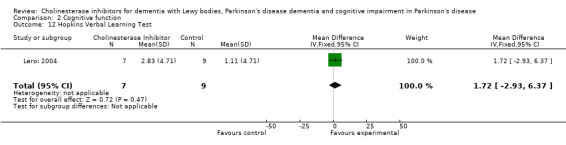
Comparison 2 Cognitive function, Outcome 12 Hopkins Verbal Learning Test.
2.13. Analysis.
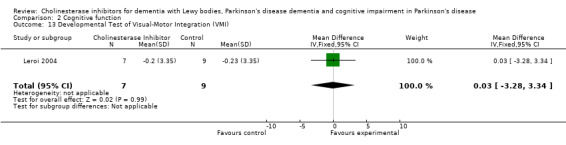
Comparison 2 Cognitive function, Outcome 13 Developmental Test of Visual‐Motor Integration (VMI).
Comparison 3. Behavioural disturbance.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 12‐item Neuropsychiatric Inventory (NPI‐12) | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐3.3 [‐13.75, 7.15] |
| 2 10‐item Neuropsychiatric Inventory (NPI‐10) | 2 | 620 | Mean Difference (IV, Fixed, 95% CI) | ‐2.18 [‐3.95, ‐0.40] |
| 3 4‐item Neuropsychiatric Inventory (NPI‐4) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐1.66 [‐4.34, 1.02] |
| 4 Brief Psychiatric Rating Scale | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐0.30 [‐5.89, 5.29] |
| 5 Combined | 4 | 674 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.36, ‐0.04] |
| 5.1 12‐item Neuropsychiatric Inventory (NPI‐12) | 1 | 16 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.29 [‐1.29, 0.70] |
| 5.2 10‐item Neuropsychiatric Inventory (NPI‐10) | 2 | 620 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.37, ‐0.04] |
| 5.3 Brief Psychiatric Rating Scale | 1 | 38 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.67, 0.60] |
3.1. Analysis.
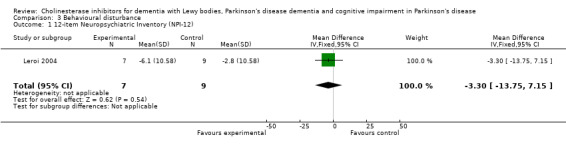
Comparison 3 Behavioural disturbance, Outcome 1 12‐item Neuropsychiatric Inventory (NPI‐12).
3.2. Analysis.
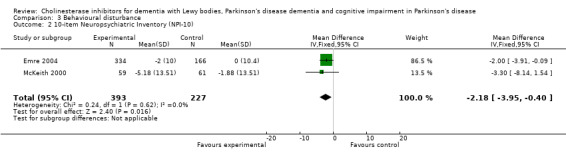
Comparison 3 Behavioural disturbance, Outcome 2 10‐item Neuropsychiatric Inventory (NPI‐10).
3.3. Analysis.
Comparison 3 Behavioural disturbance, Outcome 3 4‐item Neuropsychiatric Inventory (NPI‐4).
3.4. Analysis.
Comparison 3 Behavioural disturbance, Outcome 4 Brief Psychiatric Rating Scale.
3.5. Analysis.
Comparison 3 Behavioural disturbance, Outcome 5 Combined.
Comparison 4. Activities of daily living.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Alzheimer's Disease Cooperative Study ‐ Activities of Daily Living (ADCS‐ADL) | 1 | 498 | Mean Difference (IV, Fixed, 95% CI) | 2.5 [0.43, 4.57] |
| 2 Unified Parkinson's Disease Rating Scale (UPDRS) ‐ Activities of Daily Living | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 0.84 [‐6.24, 7.92] |
| 3 Combined | 2 | 514 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.38, ‐0.02] |
| 3.1 Alzheimer's Disease Cooperative Study ‐ Activities of Daily Living (ADCS‐ADL) | 1 | 498 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.40, ‐0.02] |
| 3.2 Unified Parkinson's Disease Rating Scale (UPDRS) ‐ Activities of Daily Living | 1 | 16 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.88, 1.10] |
4.1. Analysis.

Comparison 4 Activities of daily living, Outcome 1 Alzheimer's Disease Cooperative Study ‐ Activities of Daily Living (ADCS‐ADL).
4.2. Analysis.
Comparison 4 Activities of daily living, Outcome 2 Unified Parkinson's Disease Rating Scale (UPDRS) ‐ Activities of Daily Living.
4.3. Analysis.
Comparison 4 Activities of daily living, Outcome 3 Combined.
Comparison 5. Safety and tolerability.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dropouts | 5 | 744 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.33, 2.84] |
| 2 Dropouts due to Adverse Events | 3 | 677 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.12 [1.27, 3.55] |
| 3 Adverse Events | 6 | 1294 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.26, 2.15] |
| 4 Severe Adverse Events | 2 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.68, 3.81] |
| 5 Parkinsonian symptoms reported as adverse effects | 2 | 1091 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.28, 2.75] |
| 6 Tremor | 2 | 1091 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.71 [1.44, 5.09] |
| 7 Falls | 2 | 1091 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.72, 2.33] |
| 8 Hallucinations | 2 | 1091 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.40, 1.02] |
| 9 Unified Parkinson's Disease Rating Scale (UPDRS) | 3 | 122 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.42, 0.29] |
| 9.1 Motor | 3 | 81 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.05 [‐0.49, 0.39] |
| 9.2 Total | 1 | 41 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.72, 0.51] |
| 10 Deaths | 5 | 744 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.09, 0.84] |
5.1. Analysis.
Comparison 5 Safety and tolerability, Outcome 1 Dropouts.
5.2. Analysis.
Comparison 5 Safety and tolerability, Outcome 2 Dropouts due to Adverse Events.
5.3. Analysis.
Comparison 5 Safety and tolerability, Outcome 3 Adverse Events.
5.4. Analysis.
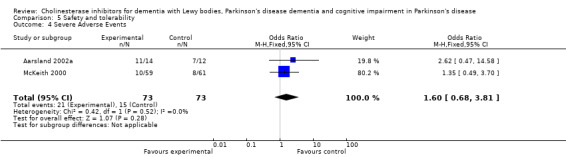
Comparison 5 Safety and tolerability, Outcome 4 Severe Adverse Events.
5.5. Analysis.
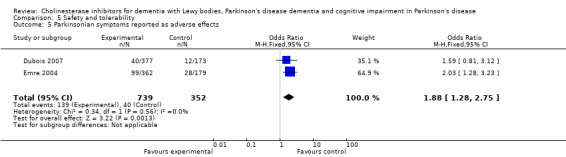
Comparison 5 Safety and tolerability, Outcome 5 Parkinsonian symptoms reported as adverse effects.
5.6. Analysis.
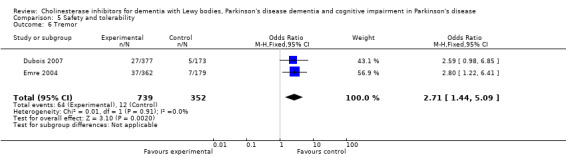
Comparison 5 Safety and tolerability, Outcome 6 Tremor.
5.7. Analysis.
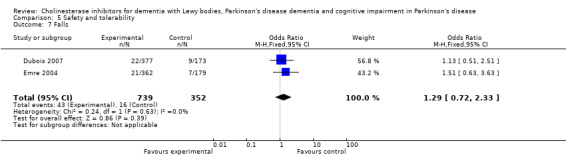
Comparison 5 Safety and tolerability, Outcome 7 Falls.
5.8. Analysis.
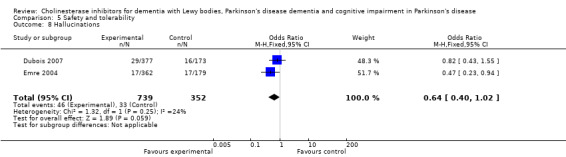
Comparison 5 Safety and tolerability, Outcome 8 Hallucinations.
5.9. Analysis.
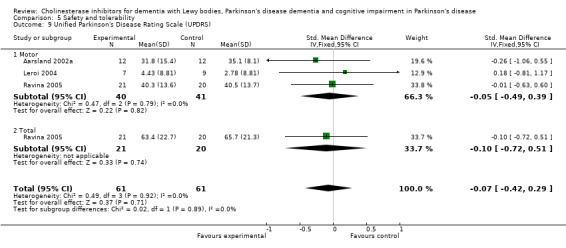
Comparison 5 Safety and tolerability, Outcome 9 Unified Parkinson's Disease Rating Scale (UPDRS).
5.10. Analysis.
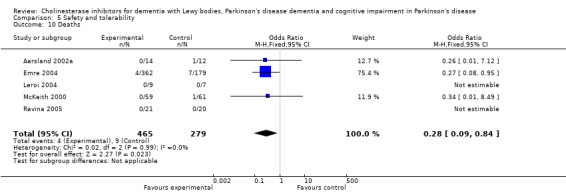
Comparison 5 Safety and tolerability, Outcome 10 Deaths.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aarsland 2002a.
| Methods | Randomised, single centre, double‐blind, cross‐over, placebo‐controlled Duration: 10 weeks | |
| Participants | Country: Norway
No. of centres: 1
Diagnosis: definite or probable PD as per Larsen (Clinical diagnosis of Parkinson's disease. Proposal of diagnostic subgroups classified at different levels of confidence) AND dementia due to PD by DSM‐IV criteria
Inclusions: age 41‐95 years, mild‐severe Parkinsonism (Hoehn and Yahr stage <5), clinical evidence of decline in memory AND at least one other category of cognitive function (starting at least 1 year after onset of parkinsonism), MMSE 16‐26, on stable regimen anti‐Parkinsonian mediation for at least 1 month immediately preceding study and throughout its duration, patient accompanied by care‐giver (= informant)
Exclusions: brain disease except PD, other severe medical disorders, use of anticholinergic drugs or psychtropic drugs with anticholinergic effects, use benzodiazepine medication (except short‐acting) in 24 hours before testing Number of patients: 14 |
|
| Interventions | Route: oral Treatment: donepezil started at 5mg once daily for 6 weeks and increased to 10mg daily for 4 weeks if tolerated | |
| Outcomes | Primary outcome measures: mini‐mental state examination (MMSE), the clinician's interview based global impression of change (CIBIC+), the motor subscale of the unified Parkinson's disease rating scale (UPDRS) Secondary measures: Neuropsychiatric Inventory (NPI) and the severity of parkinsonism (rated by patient and informer) |
|
| Notes | Details of secondary outcome measures are currently unpublished | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a randomisation list was computer generated according to a random block design" |
| Allocation concealment (selection bias) | Low risk | Quote: "the principal investigator was given a sealed envelope containing the individual treatment regimens of each patient" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | 'The initial dose was donepezil 5 mg or identically appearing placebo tablets taken once a day in the evening. The dose was increased to 10 mg after six weeks if well tolerated.' |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Imputation of missing data using LOCF. Too small to be able to say whether there was differential dropout in drug arm. The lack of a published behavioural outcome increases the risk that there is selective reporting bias |
| Other bias | High risk | Small pilot studies such as this are inherently at high risk of bias |
Dubois 2007.
| Methods | Randomised, multi‐centre, double‐blind, placebo‐controlled, 3‐arm parallel group Duration: 24 weeks |
|
| Participants | Countries: Details not available No. of centres: Details not available Diagnosis: PD by UK Brain Bank Criteria AND dementia by DSM‐IV Inclusions: Mild‐moderately severe dementia, MMSE 10‐26, present at least 1 year after onset of PD Exclusions: Details not available Number of patients: 550 (377 on active treatment) |
|
| Interventions | Route: oral Treatment: donepezil 5mg or 10mg for 24 weeks |
|
| Outcomes | Primary outcome measures: Alzheimer's Disease Assessment Scale ‐ Cognitive subscale (ADAS‐Cog); Alzheimer's Disease Cooperative Study ‐ Clinician's Global Impression of Change (ADCS‐CGIC) Secondary outcome measures: executive function tests, working memory, attention and visuospatial function tests |
|
| Notes | Tolerability and safety assessed Treatment‐related motor impairment assessed by motor subscale of UPDSRS Treatment‐by‐country interaction investigated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of randomisation process available |
| Allocation concealment (selection bias) | Unclear risk | No details of available |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details available |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Only available as a poster. High risk of selective reporting of data, especially given that remains unpublished |
Emre 2004.
| Methods | Randomised, multicentre, double‐blind, placebo‐controlled Duration: 24 weeks | |
| Participants | Countries: Austria, Belgium, Canada, France, Germany, Italy, Holland, Norway, Portugal, Spain, Turkey, UK No. of centres: not stated Diagnosis: PD by UK Parkinson's Disease Society Brain Bank criteria; Dementia by DSM‐IV (dementia due to Parkinson's disease code 294.1) Inclusions: MMSE 10 to 24; onset of symptoms of dementia more than 2 years after diagnosis of PD; regular caregiver Exclusions: primary neurodegenerative disease other than PD or dementia; history major depression; presence of active uncontrolled seizure disorder; disability or unstable disease unrelated to PD; hypersensitivity rivastigmine or similar drugs; use cholinesterase inhibitor or anticholinergic drug Number of patients (Randomised/ITT/completers): rivastigmine: 362/329/263; placebo:179/161/147 | |
| Interventions | Route: oral Treatment: rivastigmine commenced at 1.5mg twice daily and increased according to tolerability by 3mg daily at intervals of at least 4 weeks over a 16 week period | |
| Outcomes | Primary outcome measures: Intention‐to‐treat analysis with LOCF Alzheimer's Disease Assessment Scale ‐ Cognitive subscale (ADAS‐Cog); Alzheimer's Disease Cooperative Study ‐ Clinician's Global Impression of Change (ADCS‐CGIC) Secondary measures: Mini‐Mental State Examination (MMSE); Alzheimer's Disease Cooperative Study ‐ Activities of Daily Living (ADCS‐ADL); Neuropsychiatric Inventory (NPI); Cognitive Drug Research (CDR) Computerized Assessment System power of attention tests; Delis‐Kaplan Executive Function System (D‐KEFS) Verbal Fluency test; Ten Point Clock‐Drawing test; Unified Parkinson's Disease Rating Scale (UPDRS) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "automated random assignment of treatment was performed with the use of a validated system, managed by Novartis Drug Supply Management" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis with LOCF. About half those who discontinued drug continued to attend for ITT evaluations, thereby reducing the attrition bias towards a positive effect of drug which would be associated with the greater discontinuation rate amongst those taking drug which occurred |
| Other bias | Low risk | Comment: The study appears to be free of other sources of bias |
Leroi 2004.
| Methods | Randomised, multicentre, double‐blind, placebo‐controlled Duration: 18 weeks | |
| Participants | Country: USA
No. of centres: 2
Diagnosis: PD by UK Parkinson's Disease Brain Bank Criteria AND either dementia or cognitive impairment secondary to PD by DSM IV
Inclusions: on stable regimens of anti‐Parkinsonian medications
Exclusions: MMSE<10, substance abuse or dependence (by DSM IV criteria), severe cardiac disease, severe renal disease, severe vascular disease, non‐ambulatory, known inability to tolerate donepezil Number of patient: 16 (9 on active treatment) |
|
| Interventions | Route: oral Treatment: donepezil started at 2.5mg daily for 5 days then 5mg daily for 30 days then 7.5 mg daily for 5 days then 10mg daily for 91 days (total 18 weeks), study medication could be reduced in 2.5 mg decrements in response to adverse effects | |
| Outcomes | LOCF analysis. Primary outcome measure: Mini‐Mental State Examination (MMSE); Dementia Rating Scale (DRS) ‐ total score, attention subscore, initiation‐perseveration subscore, conceptualisation subscore, memory subscore; Brief Test of Attention (BTA);Trail Making Test‐Part A and B (TMT‐A and TMT‐B); Verbal Fluency; Hopkins Verbal Learning Test‐Revised (HVLT); Developmental Test of Visual‐motor Integration (VMI) Secondary measures: Neuropsychiatric Inventory (NPI); Cornell Scale for Depression in Dementia (CSDD); UPDRS‐Activities of Daily Living (ADL) and Complications of Therapy subscales Safety measures: Unified Parkinson's Disease Rating Scale (UPDRS) motor subscale; Hoehn and Yahr stage |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "the pharmacy maintained the randomisation code" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The high dropout rate (>30%), which was due to more adverse effects in the drug arm, and LOCF analysis, combine to create a high risk of bias towards finding a positive effect of drug |
| Other bias | High risk | This was a small pilot study. Such studies are inherently at risk of bias |
McKeith 2000.
| Methods | Randomised, multicentre, double‐blind, placebo‐controlled Duration: 23 weeks ‐ 20 weeks treatment followed by 3 weeks 'rest' |
|
| Participants | Countries: Spain, UK and Italy No. of centres: details not available Diagnosis: clinical diagnosis of probable Lewy body dementia Inclusion: MMSE >9, regular caregiver Exclusion: severe extrapyramidal symptoms, asthma, taking neuroleptics, anticholinergics, selegiline or similar drugs Number of patients (Randomised/ITT/Completers): RVS: 59/n1/41 Placebo: 61/n2/51 (n1+n2=117) |
|
| Interventions | Route: oral Treatment: rivastigmine started at 1.5 mg twice daily, titrated to 6mg twice daily (or maximum tolerated) over 8 weeks maximum |
|
| Outcomes | ITT Neuropsychiatric Inventory, 10 and 4 item versions (NPI‐10 and NPI‐4); speed of response to selected tests; Clinician's Global Impression of Change (ADCS‐CGIC); Mini‐Mental State Examination (MMSE); Unified Parkinson's Disease Rating Scale (UPDRS) | |
| Notes | Data on speed of response was not used in this Cochrane review, as we viewed it as a proxy measure rather than a direct measure of a clinically important feature of DLB We were unable to include the UPDRS, as data was not presented in the published paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation list was computer generated with a proprietary computer application, according to a randomised block design" |
| Allocation concealment (selection bias) | Low risk | Quote: "envelopes were to be opened only in case of emergency and were collected after unblinding and verified for code breaks" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The presentation of ITT, LOCF and OC data is commendable. However, it is not reported how many of those in the ITT dataset were providing data for the final time point despite having discontinued medication and whether there was an imbalance in this. It is not clear whether outcome reporting was selective |
| Other bias | Low risk | Comment: The study appears to be free of other sources of bias |
Ravina 2005.
| Methods | Randomised, multicentre, double‐blind, crossover, placebo‐controlled Duration: 10 weeks | |
| Participants | Country: USA No. of centres: 4 Diagnosis: PD by Movement Disorders Society Scientific issues Committee Parkinsonian disorders (diagnosis made by movement disorder specialists) Inclusions: age >40 years, mild‐moderate dementia according to DSM IV criteria for dementia AND MMSE 17‐26 Exclusions: clinical diagnosis of DLB, other causes of dementia (e.g. stoke), use of cholinergic or anticholinergic agents except amantadine or tolterodine within 2 weeks before screening, medical conditions or uncontrolled psychosis which were thought by investigator to interfere with the safe conduct of study, pregnancy or lactation Number of patients: 22 | |
| Interventions | Route: oral Treatment: donepezil started at 5mg once daily and increased according to tolerability to donepezil 5mg twice daily after 3 weeks, then continued at that dose (if tolerated, otherwise reduced back to 5mg once daily) for 6 weeks | |
| Outcomes | Primary outcome measure: Alzheimer's Disease Assessment Scale‐Cognitive Subscale (ADAS‐Cog) Secondary measures: Mini‐Mental State Examination (MMSE); Mattis Dementia Rating Scale (MDRS); Brief Psychiatric Rating Scale (BPRS); Unified Parkinson's Disease Rating Scale (UPDRS); Clinician's Global Impression of Change (ADCS‐CGIC) |
|
| Notes | Data from first 10 week period was analysed for this review BPRS is a measure of psychosis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Drug distribution to the sites and randomisation were performed by the University of Pennsylvania Investigational Drug Services Unit. Subjects were randomised in blocks of four to receive either donepezil in period I and placebo in period II or placebo in period I and donepezil in period II.' |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | 'matching placebo' |
| Incomplete outcome data (attrition bias) All outcomes | High risk | LOCF imputation of missing data |
| Other bias | High risk | This was a very small study. Such studies are at inherent risk of bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aarsland 2002 | Open label study |
| Adler 2011 | Non‐interventional trial |
| Anand 2000 | Conference presentation. Review, not a clinical trial |
| Barone 2008 | Only hyperhomocysteinemic Parkinson's disease dementia patients included |
| Barone 2010 | Open label study |
| Bergman 2002 | Open label study |
| Bergman 2003 | Open label study; trial of people with Alzheimer's disease, not Parkinson's |
| Beversdorf 2004 | Data kindly provided by the author, judged unsuitable for meta‐analysis as a small double cross‐over study without a wash‐out period and no carry‐over effect analysis |
| Brashear 2004 | Data limited, details not available |
| Chung 2010 | Trial of people with Parkinson's disease with advanced postural instability |
| Cummings 2010 | Trial of people with Alzheimer's disease, not Parkinson's |
| De Deyn 2011 | Open label study |
| Fabbrini 2002 | Open label study |
| Fogelson 2003 | Open label study; non‐standard outcome measures |
| Foy 2000 | Diagnostic criteria outside specification |
| Fujita 2010 | Case report |
| Giladi 2003 | Open label study |
| Gustavsson 2009 | Open label study |
| Hutchinson 1996 | Open label study |
| Korczyn 2001 | Open label study |
| Lanctot 2000 | Case series |
| Linsarazo 2005 | Open label study |
| Litvinenko 2008 | Open label study |
| McKeith 2000a | Open label exploratory trial; 20 weeks active treatment then 6 weeks of withdrawal |
| McLaren 2003 | Open label study |
| Minett 2003 | Open label study |
| Mori 2006 | Open label study |
| Olin 2010a | Open label study, rivastigmine and memantine used |
| Pakrasi 2006 | Open label study |
| Reading 2001 | Open label study |
| Rektorova 2004 | Open label study |
| Rosengarten 2010 | Investigation of pathophysiological changes |
| Samuel 2000 | Lewy body dementia patients compared with Alzheimer's disease patients regarding response to donepezil |
| Satoh 2010 | Investigation of pathophysiological changes |
| Thomas 2005 | Open label study |
| Touchon 2006a | Retrospective study |
| Touchon 2006b | as Touchon 2006a |
| Van Laar 2001 | Open label study |
| Vasile 2010a | Comparative efficacy of cholinesterase inhibitors, no placebo group |
| Vasile 2010b | Comparative efficacy of cholinesterase inhibitors, no placebo group |
| Walker 2000b | Investigation of pathophysiological changes |
| Werber 2001 | Open label study |
| Wilcock 2000 | Project abandoned |
Characteristics of ongoing studies [ordered by study ID]
Anon 2004a.
| Trial name or title | Donepezil for dementia in Parkinson's disease: A randomised double blinded placebo controlled crossover trial. |
| Methods | RCT |
| Participants | N = 28 Country = USA Duration = 26 weeks |
| Interventions | Donepezil + Placebos |
| Outcomes | ‐ADAS/cog ‐cognitive function ‐activities of daily living ‐mood ‐quality of life ‐side effects ‐motor performance |
| Starting date | February 2002 |
| Contact information | |
| Notes | Study ID numbers 020115; 02‐N‐0115//NLM identifier NCT00030979 |
Anon 2007a.
| Trial name or title | Double‐blind study of E2020 in patients with dementia with Lewy bodies ‐ Phase II |
| Methods | RCT |
| Participants | N = 160 Country = Japan Duration = 12 weeks |
| Interventions | Donepezil (E2020), Dosage of drug, Placebo |
| Outcomes | Primary outcome measures: Cognitive function, psychiatric symptoms, and global clinical function, burden on caregiver at 12 weeks |
| Starting date | November 2007 |
| Contact information | |
| Notes | Study ID(s) and Acronym(s): NCT00543855 // E2020‐J081‐431 |
Anon 2011.
| Trial name or title | A study of E2020 in patients with dementia with Lewy bodies (DLB), followed by a long‐term extension phase ‐ Phase III |
| Methods | RTC |
| Participants | N = 141 Country = Japan Duration = 52 weeks |
| Interventions | Donepezil (E2020), Dosage of drug, Placebo |
| Outcomes | Primary Outcome Measures: Change from baseline in Mini‐Mental State Examination and Neuropsychiatric Inventory after 12 weeks |
| Starting date | February 2011 |
| Contact information | |
| Notes | Study ID(s) and Acronym(s): NCT01278407 // E2020‐J081‐341 |
Burn 2009.
| Trial name or title | Multi‐centre UK study of the acetylcholinesterase inhibitor donepezil in early dementia associated with Parkinson's disease (MUSTARDD‐PD) |
| Methods | ‐ RCT ‐ 1. Donepezil: Experimental ‐ 5mg of donepezil for the first 8 weeks raising to 10mg thereafter if patient adjusted to 5mg dose. 10mg does continues for the remainder of the study; 2. Placebo: Placebo comparator ‐ patient commences medication to match appearance of 5mg donepezil for first 8 weeks then 10mg for the remainder of the study |
| Participants | N = 500 Country = UK Duration = 24 months |
| Interventions | Donepezil, Placebo |
| Outcomes | Primary outcome measures: To demonstrate the superiority of donepezil over placebo in improving cognitive function, neuropsychiatric burden and functional ability in people with Parkinson's disease and mild dementia after 24 months of treatment Secondary outcome measures: To demonstrate the superiority of donepezil over placebo in improving patient and carer quality of life and to establish the cost‐effectiveness of donepezil |
| Starting date | March 2010 |
| Contact information | |
| Notes | Study ID(s) and Acronym(s): NCT01014858 // MUSTARDD‐PD // 5137 // 08/13/14 |
Kurlan 2003.
| Trial name or title | Treatment of agitation/psychosis in dementia/parkinsonism |
| Methods | |
| Participants | N = Unknown Country = USA Duration = Unknown |
| Interventions | Donepezil, Quetiapine, Dosage of drug |
| Outcomes | Unknown |
| Starting date | |
| Contact information | Kurlan R Treatment of agitation/psychosis in dementia/parkinsonism Alzheimer's Disease Education and Referral Center (ADEAR) 2003 |
| Notes |
Marion 2003.
| Trial name or title | An open 24 week prospective, randomised, double‐blind placebo controlled parallel group study of efficacy, tolerability and safety of 3‐12mg/day of Exelon and Exelon (rivastigmine) capsules in patients with Parkinson's disease dementia |
| Methods | |
| Participants | N = 10 Country = UK Duration = 24 weeks |
| Interventions | Rivastigmine + Dosage of Drug + Placebos |
| Outcomes | Unclear |
| Starting date | |
| Contact information | Marie‐Helene.Marion@stgeorges.nhs.uk |
| Notes |
Contributions of authors
MR extracted the data, did the analysis and wrote the current draft. CF and IM contributed to previous versions of the review and commented on the current draft. RM wrote the first draft of this new title, was responsible for design and contributed to data extraction, interpretation, analysis and redrafting.
Sources of support
Internal sources
Oxfordshire and Buckinghamshire Mental Health Partnership NHS Trust, UK.
Nuffield Department of Medicine, University of Oxford, UK.
External sources
No sources of support supplied
Declarations of interest
RMcS has received financial support to attend conferences from Eisai, Shire and Novartis, all marketers of cholinesterase inhibitors, more than five years ago. Within the last two years, his institution has received funding for his activities as a local PL for a Novartis study of rivastigmine patch versus tablets in Parkinson's disease dementia.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Aarsland 2002a {published data only}
- Aarsland D, Laake K, Larsen JP, Janvin C. Donepezil for cognitive impairment in Parkinson's disease: a randomized control trial. Journal of Neurology, Neurosurgery, and Psychiatry 2002;72:708‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dubois 2007 {unpublished data only}
- Dubois B, Tolosa E, Kulisevsky J, Reichman H, Jones R, Burn D, et al. Efficacy and safety of donepezil in the treatment of Parkinson's disease patients with dementia. Alzheimer's and Parkinson's Disease Congress, Salzburg. 2007.
Emre 2004 {published data only}
- Burn D, Emre M, McKeith I. Reponse to rivastigmine in patients with and without visual hallucinations in dementia associated with Parkinson's disease (EXPRESS study group). 57th Annual meeting American Academy of Neurology. April 2005. [http:www.abstracts2view.com/aan/]
- Burn D, Emre M, McKeith I, Deyn PP, Aarsland D, Hsu C, et al. Effects of rivastigmine in patients with and without visual hallucinations in dementia associated with Parkinson's disease. Movement Disorders 2006; Vol. 21, issue 11:1899‐907. [DOI] [PubMed]
- Dujardin K, Devos D, Duhem S, Destee A, Marie R‐M, Durif F, et al. Utility of the Mattis dementia rating scale to assess the efficacy of rivastigmine in dementia associated with Parkinson's disease. Journal of Neurology 2006; Vol. 253, issue 9:1154‐9. [DOI] [PubMed]
- Emre M, Aarsland D, Albanese A, Byrne EJ, Deuschl G, DeDeyn PP, et al. Rivastigmine for dementia associated with Parkinson's disease. New England Journal of Medicine 2004;351:2509‐18. [DOI] [PubMed] [Google Scholar]
- Emre M, Onofrj M, Tekin S, Quarg P, Lane R, Express Study Group. Benefits of rivastigmine in Parkinson's disease dementia: Results from the EXPRESS study. Neurobiology of Aging 2004;25 Suppl 2:19. [Google Scholar]
- Lees AJ. An extension study to investigate the effect of Exelon (rivastigmine) on Parkinson's disease dementia. National Research Register 2003.
- Olin JT, Aarsland D, Meng X. Rivastigmine in the treatment of dementia associated with Parkinson's disease (PDD): subscale analysis of activities of daily living (ADL). Annals of Neurology. Conference: 134th American Neurological Association, ANA Meeting Baltimore, MD United States. October 2009. Conference Publication 2009; Vol. 66:S45.
- Olin JT, Aarsland D, Meng X. Rivastigmine in the treatment of dementia associated with Parkinson's disease: effects on activities of daily living. Dementia and Geriatric Cognitive Disorders 2010; Vol. 29, issue 6:510‐5. [DOI] [PubMed]
- Poewe W, EXPRESS Study Group. Long‐term benefits of rivastigmine in dementia associated with Parkinson's disease: An open‐label extension study. 57th Annual Meeting of the American Academy of Neurology, Miami Beach. April 2005.
- Schmitt F, A, Aarsland D, Bronnick K, S, Xiangyi Meng Tekin S, et al. Evaluating rivastigmine in mild‐to‐moderate Parkinsons disease dementia using ADAS‐cog items. American Journal of Alzheimer's Disease and other Dementias 2010; Vol. 25, issue 5:407‐13. [DOI] [PMC free article] [PubMed]
- Schmitt F, Farlow M, Olin J. Effects of rivastigmine on executive function in parkinson's disease dementia: Results from a 24‐week placebo‐controlled clinical trial. Annals of Neurology. Conference: 134th American Neurological Association, ANA Meeting Baltimore, MD United States. October 2009. Conference Publication 2009; Vol. 66 Suppl:48.
- Schmitt FA, Aarsland D, Bronnick KS, Olin JT, Meng X. Evaluating cognitive effects of oral rivastigmine using subscales and items of the ADAS‐cog in patients with mild to moderate Parkinson's disease dementia. American Journal of Geriatric Psychiatry. Conference: 2010 AAGP Annual Meeting Savannah, GA United States. March 2010. Conference Publication 2010; Vol. 18, issue 3 Suppl 1:79.
- Schmitt FA, Aarsland DAG, Bronnick KS, Olin JT, Meng X. Evaluating cognitive effects of oral rivastigmine using subscales and items of the Adas‐Cog in patients with mild to moderate Parkinson's disease dementia. Neurology 2010; Vol. 74, issue 9:A270.
- Schmitt FA, Farlow MR, Meng X, Tekin S, Olin JT. Efficacy of rivastigmine on executive function in patients with Parkinson's disease dementia. CNS Neuroscience & Therapeutics 2010; Vol. 16, issue 6:330‐6. [DOI] [PMC free article] [PubMed]
- Wesnes KA, McKeith I, Edgar C, Emre M, Lane R. Benefits of rivastigmine on attention in dementia associated with Parkinson disease. Neurology 2005;65:1654‐6. [DOI] [PubMed] [Google Scholar]
Leroi 2004 {published data only}
- Leroi I, Brandt J, Reich SG, Lyketsos CG, Grill S, Thompson R, Marsh L. Randomized placebo‐controlled trial of donepezil in cognitive impairment in Parkinson's disease. International Journal of Geriatric Psychiatry 2004;19(1):1‐8. [DOI] [PubMed] [Google Scholar]
McKeith 2000 {published data only}
- Byrne J. Treatment of Lewy body dementia with Exelon. National Research Register 2000. [MEDLINE: ]
- Ser T. Efficacy of cholinesterase inhibitors in lewy body dementia. Sixth International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy; 5‐8 April 2000, Stockholm, Sweden 2000:51.
- Ser T, McKeith I, Anand R, Cicin‐Sain A, Ferrara R, Spiegel R. Dementia with Lewy bodies: findings from an international multicentre study. International Journal of Geriatric Psychiatry 2000;15(11):1034‐45. [DOI] [PubMed] [Google Scholar]
- McKeith I, Ser T, Anand R, et al. Rivastigmine provides symptomatic benefit in dementia with Lewy bodies: findings from a placebo‐controlled international multicenter study. Neurology 2000e; Vol. 54 Suppl 3:A450.
- McKeith I, Ser T, Spano P, Emre M, Wesnes K, Anand R, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double‐blind, placebo‐controlled international study. Lancet 2000f; Vol. 356, issue 9247:2031‐6. [DOI] [PubMed]
- Rossor MN. A prospective, multicentre, randomised double blind placebo controlled exploratory study to evaluate the safety, tolerability and efficacy of a new drug in patients suffering from probable dementia with Lewy bodies. National Research Register http://www.update‐software.com/NRR 1999.
- Wesnes KA, McKeith IG, Ferrara R, Emre M, Ser T, Spano PF, et al. Effects of rivastigmine on cognitive function in dementia with Lewy bodies: a randomised placebo‐controlled international study using the Cognitive Drug Research computerized assessment system. Dementia and Geriatric Cognitive Disorders 2002;13:183‐92. [DOI] [PubMed] [Google Scholar]
Ravina 2005 {published data only}
- Ravina B, Putt M, Siderow A, Farrar JT, Gillespie M, Crawley A, et al. Donepezil for dementia in Parkinson's disease: a randomised, double‐blind, placebo controlled, crossover study. Journal of Neurology, Neurosurgery, and Psychiatry 2005;76(7):934‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Aarsland 2002 {published data only}
- Aarsland D. Galantamine for Parkinson's disease with dementia. European Neuropsychopharmacology 2002;12 Suppl:378‐9. [Google Scholar]
- Aarsland D, Hutchinson M, Larsen JP. Cognitive, psychiatric and motor response to galantamine in Parkinson's disease with dementia. International Journal of Geriatric Psychiatry 2003;18(10):937‐41. [DOI] [PubMed] [Google Scholar]
Adler 2011 {published data only}
- Adler G, Christenn M, Bektas M, Ko‐Inoshishi Y, Kupsch A, Scholz E, et al. Rivastigmine treatment in Parkinson's disease dementia: short‐term cholinergic effects are correlated with six‐month treatment response. Alzheimer's and Dementia. Conference: Alzheimer's Association International Conference, AAIC 11 Paris France. 16‐21 July 2011. Conference Publication 2011; Vol. 7 Suppl 1, issue 4:776.
Anand 2000 {published data only}
- Anand R, Enz A, Novartis Pharmaceuticals Corporation. Effects of rivastigmine extend beyond symptomatic treatment in patients with Alzheimer's disease(AD): new clinical studies. Sixth International Stockholm/Springfield Symposium on Advances in Alzheimer Therapy; April 5‐8, 2000; Stockholm, Sweden 2000:29.
Barone 2008 {published data only}
Barone 2010 {published data only}
- Barone P, Deyn P, Emre M, Kulisevsky J, Poewe W, Pourcher E, et al. Baseline data from a long‐term safety study of rivastigmine capsules and patch in mild to moderate Parkinson's disease dementia. Movement Disorders. Conference: 14th International Congress of Parkinson's Disease and Movement Disorders Buenos Aires Argentina. 13‐17 June 2010. Conference Publication 2010; Vol. 25 Suppl:486‐7.
Bergman 2002 {published data only}
- Bergman J, Lerner V. Successful use of donepezil for the treatment of psychotic symptoms in patients with Parkinson's disease. Clinical Neuropharmacology 2002;25(2):107‐10. [DOI] [PubMed] [Google Scholar]
Bergman 2003 {published data only}
- Bergman J, Brettholz I, Shneidman M, Lerner V. Donepezil as add‐on treatment of psychotic symptoms in patients with dementia of the Alzheimer's type. Clinical Neuropharmacology 2003;26(2):88‐92. [DOI] [PubMed] [Google Scholar]
Beversdorf 2004 {published data only}
- Beversdorf DQ, Warner JL, Davis RA, Sharma UK, Nagaraja HN, Scharre DW. Donepezil in the treatment of dementia with Lewy bodies. American Journal of Geriatric Psychiatry 2004;12(5):542‐4. [DOI] [PubMed] [Google Scholar]
Brashear 2004 {published data only}
- Brashear A, Kuhn ER, Lane KA, Farlow MR, Unverzagt FW. A double‐blind placebo‐controlled trial of donepezil in patients with Parkinson's disease and related dementia. Neurology 2004;62(7 Suppl):5. [Google Scholar]
Chung 2010 {published data only}
- Chung KA, Lobb BM, Nutt JG, Horak FB. Effects of a central cholinesterase inhibitor on reducing falls in Parkinson disease. Neurology 2010; Vol. 75, issue 14:1263‐9. [DOI] [PMC free article] [PubMed]
Cummings 2010 {published data only}
De Deyn 2011 {published data only}
- Deyn P, Poewe W, Barone P, Emre M, Kulisevsky J, Pourcher E, et al. Results of a long‐term safety study of rivastigmine capsules and patch in patients with mild to moderate dementia associated with Parkinson's disease. Neurodegenerative Diseases. Conference: 10th International Conference AD/PD ‐ Alzheimer's and Parkinson's Diseases: Advances, Concepts and New Challenges Barcelona Spain. 9‐13 March 2011. Conference Publication 2011; Vol. 8.
Fabbrini 2002 {published data only}
- Fabbrini G, Barbanti P, Aurilia C, Pauletti C, Lenzi GL, Meco G. Donepezil in the treatment of hallucinations and delusions in Parkinson's disease. Neurological Sciences 2002;23:41‐3. [DOI] [PubMed] [Google Scholar]
Fogelson 2003 {published data only}
- Fogelson N, Kogan E, Korczyn AD, Giladi N, Shabtai H, Neufeld MY. Effects of rivastigmine on the quantitative EEG in demented parkinsonian patients. Acta Neurologica Scandinavica 2003;107(4):252‐5. [DOI] [PubMed] [Google Scholar]
Foy 2000 {published data only}
- Foy C. The non‐dopaminergic neuropharmacology of idiopathic Parkinson's Disease. Thesis submitted in fulfilment for a Doctor of Philosophy, Section of Clinical Neurology, University of Sheffield (accessed from the British Library) 2000.
- Grunewald RA. Investigation of the effects of rivastigmine on cognition in Parkinson's disease. National Research Register 1999.
Fujita 2010 {published data only}
Giladi 2003 {published data only}
- Giladi N, Shabtai H, Gurevich T, Benbunan B, Anca M, Korczyn AD. Rivastigmine for dementia in patients with Parkinson's disease. Acta Neurologica Scandinavica 2003;108:368‐73. [DOI] [PubMed] [Google Scholar]
Gustavsson 2009 {published data only}
Hutchinson 1996 {published data only}
- Hutchinson M, Fazzini E. Cholinesterase inhibition in Parkinson's disease. Journal of Neurology, Neurosurgery, and Psychiatry 1996;61:324‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Korczyn 2001 {published data only}
- Korczyn AD, Shabati H, Benbunan B, Gurevich T, Anca M, Sidis S, Giladi N. The effect of treatment with rivastigmine (Exelon) on cognitive functions of patients with dementia and Parkinson's disease. Parkinsonism & Related Disorders 2001;7 Suppl:61. [Google Scholar]
Lanctot 2000 {published data only}
- Lanctot KL, Hermann N. Donepezil for behavioural disorders associated with Lewy bodies: a case series. International Journal of Geriatric Psychiatry 2000;15:183‐8. [DOI] [PubMed] [Google Scholar]
Linsarazo 2005 {published data only}
- Linsarozo G, Lasa A, Blercom N. Efficacy and safety of donepezil in cognitive impairment in Parkinson's disease: a pilot study. Clinical Neuropharmacology 2005;28(4):176‐8. [DOI] [PubMed] [Google Scholar]
Litvinenko 2008 {published data only}
McKeith 2000a {published data only}
- McKeith IG. A pilot study into the effects of donepezil on cognitive impairment and neuropsychiatric features in patients with dementia with Lewy bodies and Parkinson's disease. National Research Register 2000a.
McLaren 2003 {published data only}
- McLaren AT, Allen J, Murray A, Ballard CG, Kenny RA. Cardiovascular effects of donepezil in patients with dementia. Dementia and Geriatric Cognitive Disorders 2003;15:183‐8. [DOI] [PubMed] [Google Scholar]
Minett 2003 {published data only}
- Minett TS, Thomas A, Wilkinson LM, Daniel SL, Sanders J, Richardson J, et al. What happens when donepezil is suddenly withdrawn? An open label trial in dementia with Lewy bodies and Parkinson's disease with dementia. International Journal of Geriatric Psychiatry 2003;18(11):988‐93. [DOI] [PubMed] [Google Scholar]
Mori 2006 {published data only}
- Mori S, Mori E, Iseki E, Kosaka K. Efficacy and safety of donepezil in patients with dementia with Lewy bodies: preliminary findings from an open‐label study. Psychiatry and Clinical Neurosciences 2006;60(2):190‐5. [DOI] [PubMed] [Google Scholar]
Olin 2010a {published data only}
- Olin JT, Bhatnagar V, Reyes P, Koumaras B, Meng X, et al. Safety and tolerability of rivastigmine capsule with memantine in patients with probable Alzheimer's disease: A 26‐week, open‐label, prospective trial (Study ENA713B US32). International Journal of Geriatric Psychiatry 2010; Vol. 25, issue 4:419‐26. [DOI] [PubMed]
Pakrasi 2006 {published data only}
- Pakrasi S, Thomas A, Mosimann UP, Cousins DA, Burn DJ, O'Brien JT, McKeith IG. Cholinesterase inhibitors in advanced dementia with Lewy bodies: increase or stop?. International Journal of Geriatric Psychiatry 2006;21(8):719‐21. [DOI] [PubMed] [Google Scholar]
Reading 2001 {published data only}
- Reading PJ, Luce AK, McKeith IG. Rivastigmine in the treatment of Parkinson's psychosis and cognitive impairment: preliminary findings from an open trial. Movement Disorders 2001;16:1171‐95. [DOI] [PubMed] [Google Scholar]
Rektorova 2004 {published data only}
- Rektorova I. Effect of donepezil on dementia in Parkinson's disease and Alzheimer's disease: a pilot study [Ucinek donepezilu na demenci u parkinonovy nemoci a alzheimerovy nemoci. pilotni studie]. Ceska a Slovenska Neurologie a Neurchirurgie 2004;67(5):359‐63. [Google Scholar]
Rosengarten 2010 {published data only}
Samuel 2000 {published data only}
- Samuel W, Caligiuri M, Galasko D, Lacro J, Marini M, McClure FS, et al. Better cognitive and psychopathologic response to donepezil in patients prospectively diagnosed as dementia with Lewy bodies: A preliminary study. International Journal of Geriatric Psychiatry 2000; Vol. 15, issue 9:794‐802. [DOI] [PubMed]
Satoh 2010 {published data only}
Thomas 2005 {published data only}
- Thomas AJ, Burns A, Rowan EN, Littlewood E, Newby J, Cousins D, et al. A comparison of the efficacy of donepezil in Parkinson's disease with dementia and dementia with Lewy bodies. International Journal of Geriatric Psychiatry 2005;20(10):938‐44. [DOI] [PubMed] [Google Scholar]
Touchon 2006a {published data only}
- Touchon J, Bergman H, Bullock R, Rapatz G, Nagel J, Lane R. Response to rivastigmine or donepezil in Alzheimer's patients with symptoms suggestive of concomitant Lewy body pathology. Current Medical Research and Opinion 2006;22(1):49. [DOI] [PubMed] [Google Scholar]
Touchon 2006b {published data only}
- Touchon J, Bergman H, Bullock R. Erratum: Response to rivastigmine or donepezil in Alheimer's patients with symptoms suggestive of concomitant Lewy body pathology. Current Medical Research and Opinion 2006;22(1):45. [DOI] [PubMed] [Google Scholar]
Van Laar 2001 {published data only}
- Laar T, Vries JJ, Nakhosteen A, Leenders KL. Rivastigmine as anti‐psychotic treatment in patients with Parkinson's disease. Parkinsonism & Related Disorders 2001;7 Suppl:73. [Google Scholar]
Vasile 2010a {published data only}
- Vasile D, Vasiliu O, Vasile ML, Terpan M, Grigorescu G, Bogdan V, et al. Comparative efficacy of cholinesterase inhibitors in dementia associated with Parkinson's disease. European Neuropsychopharmacology. Conference: 23rd European College of Neuropsychopharmacology, ECNP Congress Amsterdam, Netherlands. 28 August to 1 September 2010. Conference Publication 2010; Vol. 20 Suppl:553‐4.
Vasile 2010b {published data only}
- Vasile D, Vasiliu O. Management of cognitive symptoms in dementia associated with Parkinson's disease. Proceedings of the World Medical Conference 2010:284‐6.
Walker 2000b {published data only}
- Walker Z. Lewy body dementia: The investigation of pre and post synaptic dopaminergic receptors with SPET. NRR 2000. [MEDLINE: ]
Werber 2001 {published data only}
- Werber EA, Rabey JM. The beneficial effect of cholinesterase inhibitors on patients suffering from Parkinson's disease and dementia. Journal of Neural Transmission 2001;108(11):1319‐25. [DOI] [PubMed] [Google Scholar]
Wilcock 2000 {published data only}
- Wilcock GK. A double‐blind, placebo‐controlled, randomised, parallel group phase IIa study of the efficacy and safety of a novel therapy in the treatment of behavioural and psychological symptoms in subjects with dementia with Lewy bodies. National Research Register 2000e.
References to ongoing studies
Anon 2004a {published data only}
- Anon. Donepezil to treat dementia in Parkinson's disease. ClinicalTrials.gov 2004.
Anon 2007a {published data only}
- Anon. Double‐blind study of E2020 in patients with dementia with Lewy bodies ‐ Phase II. ClinicalTrials.gov 2007.
Anon 2011 {published data only}
- Anon. A study of E2020 in patients with dementia with Lewy bodies (DLB), followed by a long‐term extension phase ‐ Phase III. ClinicalTrials.gov 2011.
Burn 2009 {published data only}
- Burn DJ. Multi‐centre UK study of the acetylcholinesterase inhibitor donepezil in early dementia associated with Parkinson's disease (MUSTARDD‐PD). ClinicalTrials.gov: http://clinicaltrials.gov/ct2/show/NCT01014858 2009.
Kurlan 2003 {published data only}
- Kuran R. Treatment of agitation/psychosis in dementia/parkinsonism. Alzheimer's Disease Education and Referral Centre (ADEAR) 2003.
Marion 2003 {published data only}
- Marion MH. An open 24 week prospective, randomised, double‐blind placebo controlled parallel group study of efficacy, tolerability and safety of 3‐12mg/day of Exelon and Exelon (rivastigmine) capsules in patients with Parkinson's disease dementia. National Research Register 2003.
Additional references
Aarsland 2003
- Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh‐Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8‐year prospective study. Archives of Neurology 2003;60:387‐92. [DOI] [PubMed] [Google Scholar]
Aarsland 2005
- Aarsland D, Zaccai J, Brayne C. A systematic review of prevalence studies of dementia in Parkinson's disease. Movement Disorders 2005;20:1255‐63. [DOI] [PubMed] [Google Scholar]
Ballard 2006
- Ballard C, Ziabreva I, Perry R, Larsen JP, O'Brien J, McKeith I, et al. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology 2006;67(11):1931‐4. [DOI] [PubMed] [Google Scholar]
Barr 1996
- Barr A, Brandt J. Word‐list generation deficits in dementia. Journal of Clinical and Experimental Neuropsychology 1996;18:810‐22. [DOI] [PubMed] [Google Scholar]
Bedard 2003
- Bedard MA, Agid Y, Chouinard S, et al. Mental and Behavioural dysfunction in movement disorders. Totowa, NJ: Humana Press, 2003. [Google Scholar]
Beery 1989
- Beery KE. Revised Administration, Scoring and Teaching Manual for the Developmental Test of Visual‐Motor Integration. Cleveland: Modern Curriculum Press, 1989. [Google Scholar]
Bostrom 2006
Braak 2006
- Braak H, Rub U, Tredici K. Cognitive decline correlates with neuropathological stage in Parkinson's disease. Journal of the Neurological Sciences 2006;248(1‐2):255‐8. [DOI] [PubMed] [Google Scholar]
Brandt 2001
- Brandt J, Benedict RHB. Hopkins Verbal Learning Test‐Revised, Professional Manual. Odessa, FL: Psychological Assessment Resources, Inc., 2001. [Google Scholar]
Burn 2003
- Burn DJ, McKeith IG. Current treatment of dementia with Lewy bodies and dementia associated with Parkinson's disease. Movement Disorders 2003;18 Suppl 6:72‐9. [DOI] [PubMed] [Google Scholar]
Burn 2006
- Burn DJ. Cortical Lewy body disease and Parkinson's disease dementia. Current Opinions in Neurology 2006;19(6):572‐9. [DOI] [PubMed] [Google Scholar]
Cummings 1994
- Cummings JL, Mega M, Gray K, Rosenburg‐Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308‐13. [DOI] [PubMed] [Google Scholar]
Delis 2001
- Delis DC, Kaplan E, Kramer JH. Delis‐Kaplan executive function system. San Antonio, Tex.: Psychological Corporation, 2001. [Google Scholar]
Dubois 1997
- Dubois B, Pillon B. Cognitive deficits in Parkinson's disease. Journal of Neurology 1997;244:2‐8. [DOI] [PubMed] [Google Scholar]
Fahn 1987
- Fahn S, Elton RS, Members of the UPDRS Develpment Committee. Unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Goldstein M editor(s). Recent Developments in Parkinson's Disease II. New York: Macmillan, 1987:153‐63. [Google Scholar]
Folstein 1975
- Folstein NF, Folstein SE, McHugh PR. Mini‐Mental State: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12:189‐98. [DOI] [PubMed] [Google Scholar]
Galasko 1997
- Galasko D, Bennett D, Sano M, et al. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer Disease and Associated Disorders 1997;11 Suppl 2:533‐9. [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons, 2008. [Google Scholar]
Jellinger 1994
- Jellinger KA, Bancher CH, Fischer P. Neuropathological correlates of mental dysfunction in Parkinson's disease. In: Wolters EC, Scheltens PH editor(s). Mental Dysfunction in Parkinson's Disease. Dordrecht: ICG Printing, 1994:141‐61. [Google Scholar]
Lee 2006
- Lee VM, Trojanowski JQ. Mechanisms of Parkinson's disease linked to pathological alpha‐synuclein: new targets for drug discovery. Neuron 2006;52(1):33‐8. [DOI] [PubMed] [Google Scholar]
Lippa 1998
- Lippa CF, Johnson R, Smith TW. The medial temporal lobe in dementia with Lewy bodies: a comparative study with Alzheimer's disease. Annals of Neurology 1998;43(1):102‐6. [DOI] [PubMed] [Google Scholar]
Maidment 2006
- Maidment I, Fox C, Boustani M. Cholinesterase inhibitors for Parkinson's disease dementia. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD004747.pub2] [DOI] [PubMed] [Google Scholar]
Manos 1994
- Manos PJ, Wu R. The ten point clock drawing test: a quick screen and grading method for cognitive impairment in medical and surgical patients. International Journal of Psychiatry in Medicine 1994;24:229‐44. [DOI] [PubMed] [Google Scholar]
Mattis 1988
- Mattis S. Dementia Rating Scale (DRS) Professional Manual. Odessa, FL: Psychological Assessment Resources, Inc., 1988. [Google Scholar]
McKeith 1992
- McKeith I, Fairbairn A, Perry R, Thompson P, Perry E. Neuroleptic sensitivity in patients with senile dementia of Lewy body type. BMJ 1992;305:673‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
McKeith 1996
- McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology 1996;47:1113‐24. [DOI] [PubMed] [Google Scholar]
McKeith 2005
- McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies. Third report of the DLB consortium. Neurology 2005;65:1863‐72. [DOI] [PubMed] [Google Scholar]
Nakano 1984
- Nakano I, Hirano A. Parkinson's disease: neuron loss in the nucleus basalis without concomitant Alzheimer's disease. Annals of Neurology 1984;15:415‐8. [DOI] [PubMed] [Google Scholar]
Olanow 2006
- Olanow CW, McNaught KS. Ubiquitin‐proteasome system and Parkinson's disease. Movement Disorders 2006;21(11):1806‐23. [DOI] [PubMed] [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10:790‐812. [Google Scholar]
Perry 1994
- Perry EK, Haroutunian V, Davis KL, et al. Neocortical cholinergic activities differentiate Lewy body dementia from classical Alzheimer's disease. NeuroReport 1994;5(7):747‐9. [DOI] [PubMed] [Google Scholar]
Reitan 1958
- Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills 1958;8:271‐6. [Google Scholar]
Rosen 1984
- Rosen WG, Mohs RC, Davis K. A new rating scale for Alzheimer's disease. American Journal of Psychiatry 1984;141:1356‐64. [DOI] [PubMed] [Google Scholar]
Schneider 1997
- Schneider LS, Olin JT, Doody RS, et al. Validity and reliability of the Alzheimer's Disease Cooperative Study ‐ Clinical Global Impression of Change. Alzheimer Disease and Associated Disorders 1997;11 Suppl 2:22‐32. [DOI] [PubMed] [Google Scholar]
Schretlen 1997
- Schretlen D. Brief Test of Attention Professional Manual. Odessa, FL: Psychological Assessment Resources, Inc., 1997. [Google Scholar]
Simpson 1991
- Simpson PM, Surmon DJ, Wesnes KA, Wilcock GK. The cognitive assessment system for the demented patients: a validation study. International Journal of Geriatric Psychiatry 1991;6:95‐102. [Google Scholar]
Wild 2003
- Wild R, Pettit T, Burns A. Cholinesterase inhibitors for dementia with Lewy bodies. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD003672] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2009
- Williams SS, Williams J, Combrinck M, Christie S, Smith AD, McShane R. Olfactory impairment is more marked in patients with mild dementia with Lewy bodies than those with mild Alzheimer disease. Journal of Neurology, Neurosurgery, and Psychiatry 2009;80(6):667‐70. [DOI] [PubMed] [Google Scholar]
Zaccai 2005
- Zaccai J, McCracken C, Brayne C. A systematic review of prevalence and incidence studies of dementia with Lewy bodies. Age and Ageing 2005;34:561‐6. [DOI] [PubMed] [Google Scholar]


