Abstract
Apolipoprotein (apo) E4 isoform, a major risk factor for Alzheimer’s disease (AD), is more susceptible to proteolysis than apoE2 and apoE3 isoforms. ApoE4 fragments have been found in AD patients’ brain. In the present study, we examined the effect of full-length apoE4 and apoE4 fragments apoE4[Δ(186-299)] and apoE4[Δ(166-299)] on inflammation in human neuroblastoma SK-N-SH and human astrocytoma SW-1783 cells. Western blot and zymography analysis showed that treatment of SK-N-SH cells with apoE4[Δ(186-299)], but not full-length apoE4 or the shorter apoE4[Δ(166-299)] fragment, leads to increased extracellular levels of matrix metalloproteinase 9 (MMP9) and tissue inhibitor of metalloproteinase 1 (TIMP1). Real-time PCR showed that interleukin (IL)-1β gene expression is also increased in SK-N-SH cells treated with apoE4[Δ(186-299)]. Treatment of SK-N-SH cells with IL-1β leads to increased MMP9 and TIMP1 extracellular levels, suggesting that the induction of IL-1β may be the mechanism by which apoE4[Δ(186-299)] regulates MMP9 and TIMP1 levels in these cells. In contrast to SK-N-SH cells, treatment of SW-1783 cells with apoE4[Δ(186-299)] and to a lesser extent with apoE4, leads to increased TIMP1 extracellular levels without affecting MMP9 levels, Additionally, apoE4[Δ(186-299)] leads to decreased IL-10 gene expression in SK-N-SH cells, while both apoE4 and apoE4[Δ(186-299)] lead to decreased TNFα gene expression without affecting IL-1β and IL-10 gene expression in SW-1783 cells. Overall, our findings indicate that a specific apoE4 fragment (apoE4[Δ(186-299)]), with molecular mass similar that of apoE4 fragments detected in AD patients’ brain, can influence the level of inflammatory molecules in brain cell lines. It is possible that these phenomena contribute to AD pathogenesis.
Keywords: apolipoprotein E, Alzheimer’s disease, apolipoprotein E4 fragments, cytokines, matrix metalloproteinase 9, neuroinflammation
Apolipoprotein E (apoE) is a major protein of the lipoprotein transport system that plays critical roles in atherosclerosis, dyslipidemia and Alzheimer’s disease (AD) (Mahley et al., 2006; Zannis et al., 2004). ApoE contains 299 residues and has three common isoforms (apoE2, apoE3, apoE4) in the general population (Zannis et al., 1982; Zannis et al., 2004). ApoE4 is a major genetic risk factor for AD since 40% of all patients have at least one ε4 allele (Corder et al., 1993) and it increases the occurrence and lowers the age of onset of sporadic AD (Corder et al., 1993; Myers et al., 1996).
Several studies have suggested that the production, oligomerization and extra- or intra-neuronal deposition of β-amyloid peptide (Aβ), along with intracellular neurofibrillary tangle formation play central roles in AD (Ballatore et al., 2007; Bayer and Wirths, 2008; Bayer and Wirths, 2011; Haass and Selkoe, 2007; LaFerla et al., 2007). Accumulating evidence suggests that inflammation may also play an important role in the initiation or progression of AD (Frank-Cannon et al., 2009; Glass et al., 2010; Heneka et al., 2010; Johnston et al., 2011; McGeer and McGeer, 2010; Wyss-Coray, 2006). It has been hypothesized that chronic activation of inflammatory pathways may be either the consequence of or the driving force for AD pathogenesis (Frank-Cannon et al., 2009; Glass et al., 2010; Heneka et al., 2010; Johnston et al., 2011; McGeer and McGeer, 2010; Wyss-Coray, 2006). It is also possible that some aspects of inflammation may be protective against AD or that the disease process inhibits beneficial inflammatory responses (Frank-Cannon et al., 2009; Glass et al., 2010; Heneka et al., 2010; Johnston et al., 2011; McGeer and McGeer, 2010; Wyss-Coray, 2006).
A series of inflammatory mediators, including complement system components, cytokines, chemokines, cycloxygenase and acute phase proteins, as well as matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) are produced by microglia, astrocytes and neurons and contribute to neuroinflammatory responses in AD (Candelario-Jalil et al., 2009; Gardner and Ghorpade, 2003; Glass et al., 2010; Heneka et al., 2010; Wyss-Coray, 2006). Matrix metalloproteinase 9 (MMP9) is a major MMP that has been identified in neuroinflammation (Candelario-Jalil et al., 2009). MMP9 is expressed and released by neurons, astrocytes and microglia and its expression is regulated, among other factors, by cytokines (Candelario-Jalil et al., 2009). MMP9 has been reported to cleave Aβ fibrils in vitro as well as compact plaques in situ, thus contributing to the ongoing clearance of plaques from amyloid-laden brains (Yan et al., 2006). However, in another study, it was proposed that even if MMP9 degrades Aβ, it may also randomly and non-selectively destroy the extracellular matrix and brain cell membranes leading to neuronal dysfunction and Aβ-induced cognitive impairment in mice (Mizoguchi et al., 2009). MMP9 has been found in neurons, neurofibrillary tangles and senile/amyloid plaques in postmortem AD brains (Asahina et al., 2001), as well as in the plasma of AD patients (Lorenzl et al., 2003b). Plasma MMP9 levels were not significantly different between the apoE subgroups (Lorenzl et al., 2003b). Whether alterations in MMP9 levels observed in the end stages of AD also occur in early stages of the disease is unknown. Analysis of MMP9 activity in postmortem frontal cortex tissue samples showed greater activity in subjects with AD and mild cognitive impairment, a precursor syndrome to AD, compared to controls, indicating that MMP9 changes may underlie the pathogenesis of cognitive deficits in mild cognitive impairment and AD (Bruno et al., 2009). The activity of MMP9 is inhibited by the tissue inhibitor of metalloproteinase-1 (TIMP1) (Gardner and Ghorpade, 2003). TIMP1 is expressed in neurons and astrocytes and similarly to MMP9, its expression is regulated by cytokines (Gardner and Ghorpade, 2003). TIMP1 has been proposed to have neuroprotective effects, although its exact functions in brain remain unknown (Dzwonek et al., 2004; Gardner and Ghorpade, 2003). An imbalance between MMP9 and TIMP1 has been implicated in several inflammatory diseases of the central nervous system (Gardner and Ghorpade, 2003). It was shown that neuronal TIMP1 release accompanies astrocytic MMP9 secretion and enhances astrocyte proliferation induced by the neurotoxic Aβ 25-35 fragment, possibly as a defensive mechanism against Aβ deposition (Hernandez-Guillamon et al., 2009). In postmortem AD brains TIMP1 was found in neuritic senile/amyloid plaques and neurofibrillary tangles (Peress et al., 1995). Analysis of cerebrospinal fluid (CSF) showed elevated TIMP1 levels in AD patients compared to controls (Lorenzl et al., 2003a). In contrast, in a more recent study the CSF TIMP1 levels were found to be reduced in AD patients compared to cognitive healthy individuals, while the MMP9/TIMP1 ratio was elevated (Stomrud et al., 2010) In that study, the CSF MMP9 and TIMP1 levels or MMP9/TIMP1 ratio did not correlate with the apoE genotype in AD patients (Stomrud et al., 2010). However, in the same study it was shown that healthy elderly individuals with at least one apoE4 allele had higher levels of CSF MMP9 compared to the non-carriers (Stomrud et al., 2010). Overall, all the above studies indicate that changes in MMP9 or TIMP1 levers, or MMP9/TIMP1 ratio may be associated with the pathogenesis of AD.
Cytokines associated with MMP9/TIMP1 expression, as well as AD and neuroinflammation, include interleukin-1β (IL-1β), tumor-necrosis factor α (TNFα) and interleukin-10 (IL-10) (Candelario-Jalil et al., 2009; Gardner and Ghorpade, 2003; Glass et al., 2010; Heneka et al., 2010; Johnston et al., 2011; Remarque et al., 2001; Shaftel et al., 2008; Strle et al., 2001; Wyss-Coray, 2006). IL10 has been proposed to play a regulatory role by maintaining an anti-inflammatory environment in the CNS (Strle et al., 2001), while increased levels of IL-10 have also been associated with attenuation of AD-like neuropathology following immunotherapy in mice (Koronyo-Hamaoui et al., 2009). In contrast, IL-1β and TNFα have been implicated in AD pathogenesis (Heneka et al., 2010; Shaftel et al., 2008). However, some studies reported that TNFα protected neurons and modulated neurotransmission (Heneka et al., 2010; Stellwagen and Malenka, 2006) and also suggested that IL-1β should not be regarded as having purely detrimental role in AD, but instead it might be a factor modulating the balance between detrimental and beneficial processes in brain (Frank-Cannon et al., 2009; Shaftel et al., 2008).
A large number of studies have examined the association of apoE4 with sporadic AD. These studies suggested that apoE4 is involved in modulation of plaque formation and clearance of Aβ, promotes intraneuronal accumulation of Aβ, affects cholesterol homeostasis, alters phosphorylation of tau and formation of neurofibrillary tangles, disrupts cytoskeleton structure, impairs cholinergic signal transduction and causes dysregulation of various signaling pathways (Cedazo-Minguez, 2007; Dafnis et al., 2010; Kim et al., 2009; Mahley et al., 2006). Thus, it is possible that apoE4 has pleiotropic functions and several parallel pathways may contribute to the pathogenic role of apoE4 in AD. It is also possible that some of the above pathological processes are early events, while others follow subsequently in AD pathogenesis.
ApoE is subject to proteolytic cleavage and apoE4 is much more susceptible to proteolysis than apoE3 (Harris et al., 2003; Huang et al., 2001). Analysis of brain lysates from nondemented normal subjects (apoE3/3) and AD patients (apoE4/2, apoE4/4, apoE4/3) showed the presence of 25-30 and 14-22 kDa carboxy-terminal truncated apoE fragments in the detergent-solubilized pellets from AD brains, which were enriched in plaques and neurofibrillary tangles, but not in the pellets of normal brains (Huang et al., 2001). A recent study also showed that apoE4 undergoes more cleavage than apoE3 in AD patients’ brains (Jones et al., 2011). In that study, 21-33 kDa carboxy-terminal truncated apoE fragments were observed in the brain homogenates (Jones et al., 2011). In addition, analysis of brain homogenates from transgenic mice expressing apoE3 or apoE4 or both apoE3 and apoE4 in neurons showed the presence of 29 and 14-20 kDa carboxy-terminal truncated fragments in apoE4/3 mice, mostly 29 kDa fragment in apoE3 mice and mostly 14-20 kDa fragments in apoE4 mice (Brecht et al., 2004). The primary proteolytic cleavage site of apoE has been proposed to be either at residues 268/272 (Harris et al., 2003) or close to residue 187 (Wellnitz et al., 2005) or after residue 160 (Cho et al., 2001). Furthermore, it was proposed that the 29kDa fragment generated from cleavage at residues 268/272 of apoE4 is rapidly cleaved to generate 14-20 kDa fragments, whereas the 29 kDa fragment generated from apoE3 appears to be resistant to further cleavage (Brecht et al., 2004; Harris et al., 2003).
Previous findings by us and others indicated that not all truncated apoE4 fragments exert the same biological effects and that specific apoE4 fragments may be involved in distinct processes associated with AD pathogenesis (Brecht et al., 2004; Chang et al., 2005; Dafnis et al., 2010; Harris et al., 2003). Interestingly, it was reported that the presence of 14-20 kDa apoE4 fragments in transgenic mice expressing apoE4 in neurons was observed at 1 month of age, while the accumulation of phosphorylated tau started at 5 months of age, indicating that apoE4 fragmentation is an early event in the pathogenesis of AD (Brecht et al., 2004).
In the current study we addressed the question whether truncated apoE4 forms have an effect on neuroinflammatory response. Our hypothesis was that if an early pathogenetic event in AD brain, such as apoE4 fragmentation, can facilitate the induction of neuroinflammatory response, this could provide support for a causative role of inflammation in AD. Furthermore, while previous studies showed that short carboxy-terminal truncated apoE4 fragments are bioactive (Brecht et al., 2004), it is not know which exact fragments are bioactive and what is their function. We examined the effect of two truncated apoE4 forms, apoE4[Δ(186-299)] (designated thereafter as apoE4-185) and apoE4[Δ(166-299)] (designated thereafter as apoE4-165) on extracellular levels of MMP9 and TIMP1, as well as levels of various cytokines (IL-1β, TNFα and IL-10) in human neuroblastoma SK-N-SH and human astrocytoma SW-1783 cells. ApoE4-185 is similar to the fragment generated by proteolysis close to residue 187 of apoE4 in neuroblastoma cells Neuro2α (Wellnitz et al., 2005). In addition, it has a molecular weight of 21kDa that falls within the range of molecular weight of carboxy-terminal truncated apoE reported to be present in detergent-solubilized pellets from AD patients’ brains (14-22kDa) (Huang et al., 2001) or in AD brain homogenates (21-33 kDa) (Jones et al., 2011). ApoE4-165 contains the four-helix bundle (residues 24-164) of the amino-terminal domain of apoE determined by both x-ray crystallography and NMR (Chen et al., 2011; Wilson et al., 1991) and also has molecular weight of 19kDa that falls within the range of molecular weight (14-20kDa) of carboxy-terminal truncated apoE found in brain homogenates of AD patients (apoE4/3) and apoE4 transgenic mice expressing apoE in neurons (Brecht et al., 2004). We found that treatment of SK-N-SH cells with lipid-free apoE4-185, but not with full-length apoE4 or apoE4-165, leads to increased extracellular levels of MMP9 and TIMP1. In contrast, treatment of SW-1783 cells with either lipid-free apoE4 or apoE4-185 does not affect the extracellular levels of MMP9, but leads to increased extracellular levels of TIMP1, with apoE4-185 resulting in higher TIMP1 levels than apoE4. Furthermore, we found that apoE4-185 promotes the expression of IL-1β gene in SK-N-SH cells, but not in SW-1783 cells. Treatment of SK-N-SH cells with IL-1β also leads to increased extracellular levels of MMP9 and TIMP1, indicating that the apoE4-185 fragment increases MMP9 and TIMP1 levels by inducing IL-1β gene expression in these cells. TNFα gene expression was not affected in SK-N-SH cells treated by apoE4 or apoE4-185, but was decreased in SW-1783 cells treated either by apoE4 or apoE4-185. Additionally, we observed that IL-10 gene expression is decreased in SK-N-SH cells, but not in SW-1783 cells, treated with apoE4-185. Our results indicate that a specific apoE4 fragment can affect the levels of inflammatory molecules in brain cell lines. This finding suggests that fragmented apoE4 may be involved in inflammatory responses in brain, an event that has been associated with AD.
Experimental procedures
Materials
Fetal bovine serum (FBS) and IL-1β were purchased from Sigma Aldrich Corp. (St. Louis, MO, USA). Leibovitz’s L-15 medium and opti-MEM I medium were from Invitrogen (Carlsbad, CA, USA). Minimum Essential Medium (Eagle), L-glutamine, non-essential amino acids, sodium pyruvate, and sodium bicarbonate were from Biochrom AG (Germany). Dextran sulfate and epoxy-activated Sepharose 6B were obtained from GE Healthcare (Sweden). LipofectAMIN-2000 was purchased from Invitrogen. Phenylmethylsulphonyl fluoride (PMSF) and the complete protease inhibitor cocktail were purchased from Sigma. Dc Protein Assay Kit was from Bio-Rad (Hercules, CA, USA). All other reagents were purchased from Sigma Aldrich Corp., Bio-Rad, Fisher Scientific (Germany), or other standard commercial sources.
Production and purification of apoE using the adenovirus system
All plasmids and recombinant adenoviruses containing the wild-type and mutated human apoE4 genes were constructed as described previously (Li et al., 2003). Human astrocytoma SW-1783, cells, (HTB13 cells, ATCC, Rockville, MD, USA) grown to 80% confluence in Leibovitz’s L-15 medium containing 10% (v/v) FBS in roller bottles, were infected with adenoviruses expressing wild-type or mutant apoE4 forms at a multiplicity of infection of 20. Twenty-four hours post-infection, the cells were washed twice with serum-free medium and then pre-incubated in serum-free medium for 30 min. Fresh serum-free medium then was added for 24 h. After the end of this period the medium was harvested and fresh serum-free medium was added to the cells. The harvests were repeated approximately 8-10 times. ApoE was purified from the culture medium of adenovirus-infected HTB-13 cells as described previously (Chroni et al., 2008). The purification scheme involved dextran sulfate Sepharose column fractionation (Chroni et al., 2008).
Cell cultures and transfection
Human neuroblastoma SK-N-SH cells (ATCC, Rockville, MD, USA) were cultured in Eagle medium supplemented with 2mM L-glutamine, 0.1mM non-essential amino acids, 1mM sodium pyruvate, 1.5g/l sodium bicarbonate , 10% (v/v) FBS and antibiotics.
Studies in untransfected SK-N-SH and SW-1783 cells
cells were plated on 24-well plates at a density of 1×105 cells/well. After 4 h the cells were incubated with 400μl serum-free medium in the presence or absence of various concentrations of lipid-free WT or truncated apoE4 forms or IL-1β for 24 h. The culture medium was then collected, complete protease inhibitor cocktail was added and centrifuged in a microcentrifuge for 5 min. The cells were washed twice with ice-cold phosphate buffered saline (PBS), lysed at 4 0C with lysis buffer (20 mM Tris-HCl pH 7.4, 140 mM NaCl, 10 mM NaF, 1mM PMSF, 1 mM Na3VO4, 1% Triton X-100 containing complete protease inhibitor cocktail) and centrifuged at 12,500 rpm in a microcentrifuge for 30 min at 40C. After centrifugation, supernatants were collected and their protein concentration was determined using the Dc Protein assay.
Studies in SK-N-SH and SW-1783 cells following transfection with an MMP-9 expression plasmid
Twenty-four hours before transfection, the cells were plated on 100mm Petri dishes at a density of 1.5×106 cells/dish in culture medium without antibiotics. The next day, cells at about 90% confluence were transfected with a pcDNA3.1 plasmid encoding human MMP9 or vector alone (mock) using the Lipofectamine 2000 reagent. The DNA-Lipofectamin 2000 complexes were formed in serum-free Opti-MEM I according to manufacturer’s instructions. Six hours after DNA-Lipofectamine 2000 addition the cells were removed from the dish with the use of trypsin/EDTA and plated on 24-well plates at a density of 1×105 cells/well in serum-free medium without antibiotics. Cells were incubated for 4 h to allow attachment to the plate and then incubated with 400 μl serum-free medium in the presence or absence of 1 μM lipid-free WT or truncated apoE4 forms for 24 h. At the end of this period culture media and cell lysates were collected as described above.
Western blotting
Culture media were subjected to 10% SDS-PAGE and the resolved proteins were transferred to nitrocellulose membrane for Western blotting. The volume of the culture medium loaded per well of the gel was adjusted to correspond to equal cell protein content, determined using the Dc Protein assay. The calculated total cell protein content was not found to vary significantly between wells in each experiment. Pro-MMP9 and active-MMP9 were detected using a rabbit polyclonal anti-human MMP9 antibody (AB19016, Chemicon/Millipore, Billerica, MA, USA) and a goat anti-rabbit IgG coupled to horseradish peroxidise (HRP) (Chemicon/Millipore). TIMP1 was detected using the mouse monoclonal anti-human TIMP1 antibody 7-6C1 (Chemicon/Millipore, Billerica, MA, USA) and a goat anti-mouse IgG coupled to horseradish peroxidise (HRP) (Chemicon/Millipore). For densitometric analysis, immunoreactive bands were scanned and quantified using the Image J image analysis software (NIH) (Abramoff et al., 2004). The results are presented as mean ± SD arbitrary units (AU).
Zymography
Gelatin zymography was performed as previously described (Anderson et al., 1996). Briefly, culture media, normalised for cell protein content, were subjected to SDS-PAGE under non-reducing conditions in 10% polyacrylamide gels containing 0.1% gelatin. After electrophoresis, SDS was removed from the gel by washing in 50 mM Tris–HCl pH 7.5, 5 mM CaCl2, 1 μM ZnCl2 and 2.5% Triton X-100 and the gel was incubated in the same buffer without Triton X-100 for 48 h at 37 °C. Following staining with Coomassie Brilliant Blue R-250 for 45 min and de-staining in water, gelatin-degrading enzymes were identified by their ability to clear the gelatinous substrate at their respective molecular mass. For densitometric analysis, clear bands were scanned and quantified using the Image J image analysis software (NIH) (Abramoff et al., 2004).
RNA Isolation and quantitative real-time PCR (qRT-PCR)
For total RNA isolation, cells were removed from the dish by pipetting and washed twice with PBS. RNA was isolated with the RNeasy Plus Micro Kit (Qiagen, Valencia, CA, USA). The RNA samples were then reverse transcribed using the GoScript Reverse Transcription System (Promega, Madison, WI, USA). qRT-PCR was done using the Mx3000P qPCR system (Stratagene, La Jolla, CA, USA). The sequences of the primer pairs used for amplification of human TNF-α (Zhang et al., 2003), IL-1β (Locati et al., 2002), IL-10 (Nagaeva et al., 2002) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Zhang et al., 2003) have been described previously. qRT-PCR reactions were carried out using cDNA template equivalent to 15 ng (for TNF-α, IL-1β, GAPDH amplification) or 100 ng (for IL-10, GAPDH amplification) reverse-transcribed RNA, primers at a final concentration of 200 nM (IL-1β, GAPDH) or 300 nM (TNF-α, IL-10) and SYBR Fast Universal qPCR kit (KAPA Biosystems, Woburn, MA, USA) in a total volume of 20 μL. Amplification conditions consisted of initial denaturation at 95°C for 3 min, followed by 41 cycles of denaturation at 95°C for 3 s, annealing at 64°C for 20 s and elongation at 72°C for 10 s. Each sample was run in triplicate. The analysis was carried out using the MxPro-Mx3000P v4.10 software (Stratagene). The relative expression ratio of a target gene (X = TNF-α, IL-1β or IL-10) expressed in a sample (WT or truncated apoE4 treated cells) versus a control (untreated cells) in comparison to a reference gene (R = GAPDH) is calculated as described (Pfaffl, 2001) using the equation:
where E is the qRT-PCR efficiency of the target or reference gene transcript; ΔCTX is the difference of threshold cycles (CT) of control minus the CT of sample of the target gene (CTX control – CTX sample) and ΔCTR is the difference of CT of control minus the CT of sample of the reference gene (CTR control – CTR sample). E values were calculated as described previously (Rasmussen, 2001) according to E = 10[-1/slope], where slope was calculated from the graph of CT versus log(ng of cDNA) for the target or reference gene transcript.
Statistical analysis
All results are reported as mean ± SD. Data obtained from cells treated with apoE4 forms or IL-1β were compared with data from untreated cells (control) using unpaired, two-tailed t tests. p < 0.05 was considered significant.
Results
Effect of truncated apoE4 forms on MMP9 levels in SK-N-SH cells
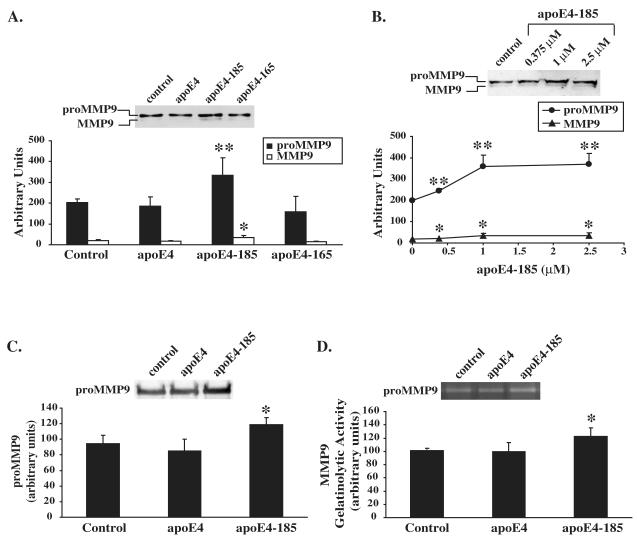
Human neuroblastoma SK-N-SH cells were incubated in the presence or absence of 1 μM lipid-free WT apoE4, apoE4-185 and apoE4-165 for 24 h. Treatment of cells with WT apoE4 or apoE4-165 had no effect in both pro-MMP9 and active MMP9 forms levels secreted by cells as assessed by Western blotting (Figure 1A). In contrast incubation of SK-N-SH cells with apoE4-185 led to a 65% increase of levels of both pro-MMP9 and active MMP9 forms secreted by cells as compared to untreated cells (Figure 1A). The concentration of apoE4 forms used, 1 μM, is similar to the reported apoE concentration (1-1.7 μM) in human plasma (Siest et al., 2000) and apoE concentrations (0.9 μM, 1.5 μM ) used to study the effect of recombinant apoE on neuroinflammatory responses of brain cells (Chen et al., 2005; Guo et al., 2004). A lower concentration of apoE4-185, 0.375 μM, was also used. This concentration is within the range of the reported apoE concentrations (0.365-0.396 μM) in human CSF (Kay et al., 2003; Wang et al., 2010). In addition, a higher concentration of apoE4-185 (2.5 μM) was used. Treatment of SK-N-SH cells with increasing concentrations of apoE4-185 showed that even at the lowest concentration of apoE used (0.375 μM) there was a significant increase (24% and 19%, respectively) in the levels of both pro-MMP9 and active MMP9 forms secreted by cells as compared to untreated cells (Figure 1B). Treatment of SK-N-SH cells with 2.5 μM apoE4-185 resulted in similar increase of pro-MMP9 and active MMP9 levels as the treatment with 1 μM apoE4-185 (Figure 1B).
Figure 1A-C. Effect of WT and carboxy-terminal truncated apoE4 forms on MMP9 levels in SK-N-SH cells.
A, SK-N-SH cells were incubated in the absence (control) or presence of 1 μM lipid-free WT apoE4, apoE4-185 or apoE4-165 for 24 h. Secreted MMP9 levels were measured by immunoblotting as described under “Experimental procedures”. Western blots were scanned and quantified by ImageJ (lower panel). Values represent the means ± SD of six experiments performed in duplicate or triplicate. *, p = 0.01 vs. control. **, p < 0.005 vs. control. B, SK-N-SH cells were incubated in the presence of increasing concentrations of apoE4-185, as indicated, for 24 h. Secreted MMP9 levels were measured by immunoblotting. Western blots were scanned and quantified by ImageJ (lower panel). Values represent the means ± SD of four measurements. *, p < 0.05 vs. control. **, p < 0.005 vs. control. C, D, SK-N-SH cells transiently transfected with proMMP9 were incubated in the absence (control) or presence of 1 μM lipid-free WT apoE4 or apoE4-185 for 24 h. Secreted MMP9 levels were measured by immunoblotting (C) and enzymatic activity was assessed by gelatin zymography (D) as described under “Experimental procedures”. Western blots and zymograms were scanned and quantified by ImageJ (lower panel). Values represent the means ± SD of three experiments performed in duplicate. *, p < 0.05 vs. control.
Since the enzymatic activity of MMP9 released from SK-N-SH cells could be barely detected by gelatin zymography, to confirm the effect of apoE4-185 on MMP9 secretion we transfected the SK-N-SH cells with proMMP9 cDNA. Incubation of MMP9 over-expressing SK-N-SH cells with 1 μM apoE4-185 resulted in an increase of secreted proMMP9 levels, although in a lesser extent compared to non MMP9 over-expressing cells, as assessed by western blotting (25% increase compared to untreated cells, Figure 1C) and gelatin zymography (22% increase compared to untreated cells, Figure 1D). WT apoE4 had no effect on secreted proMM9 as assessed by western blotting and gelatin zymography (Figure 1C, D).
Effect of truncated apoE4 forms on TIMP1 levels in SK-N-SH cells
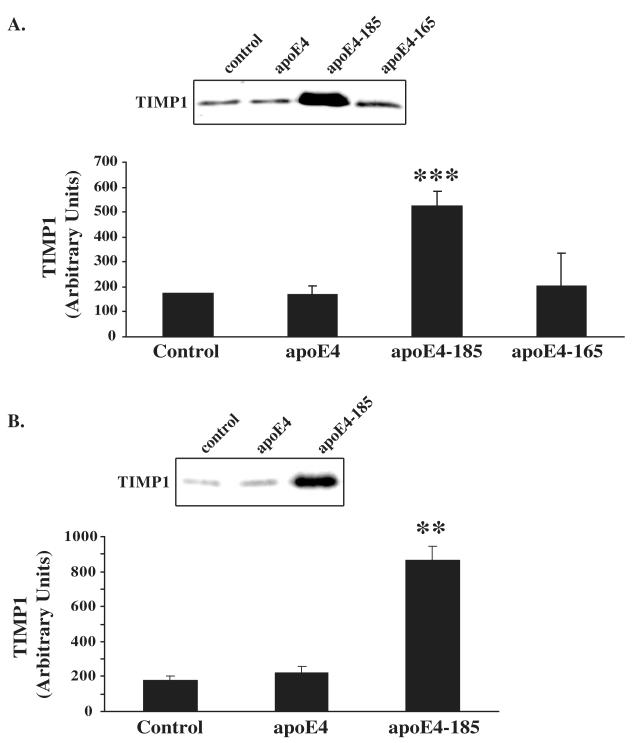
Since apoE4-185 increases the levels of MMP9 we asked whether apoE4-185 affects also the levels of TIMP1. For this purpose, we incubated SK-N-SH cells in the presence or absence of 1 μM WT apoE4, apoE4-185 and apoE4-165 for 24 h and measured the TIMP1 levels in cell medium by western blotting. As shown in Figure 2A, WT apoE4 or apoE4-165 had no effect on secreted TIMP1 levels. In contrast, incubation with apoE4-185 led to a 200% increase of secreted TIMP1 levels as compared to untreated cells (Figure 2). Incubation of SK-N-SH cells with lower concentration of apoE4-185 (0.375 μM) also led to an increase (110%) of secreted TIMP1 levels as compared to untreated cells (data not shown). In addition, incubation of SK-N-SH cells transfected with a proMMP9-expressing plasmid with 1μM apoE4-185 increased the secreted TIMP1 levels (393% increase compared to untreated cells, Figure 2B). Our findings show that apoE4 fragment apoE4-185 affects the balance of extracellular MMP9 and TIMP1 in human neuroblastoma SK-N-SH cells.
Figure 2. Effect of WT and carboxy-terminal truncated apoE4 forms on TIMP1 levels in SK-N-SH cells.
A, SK-N-SH cells were incubated in the absence (control) or presence of 1 μM lipid-free WT apoE4, apoE4-185 or apoE4-165 for 24 h. Secreted TIMP1 levels were measured by immunoblotting as described under “Experimental procedures”. Western blots were scanned and quantified by ImageJ (lower panel). Values represent the means ± SD of four experiments performed in duplicate or triplicate. ***, p < 0.0001 vs. control. B, SK-N-SH cells transiently transfected with proMMP9 were incubated in the absence (control) or presence of 1 μM lipid-free WT apoE4 or apoE4-185 for 24 h. Secreted TIMP1 levels were measured by immunoblotting as described under “Experimental procedures”. Western blots were scanned and quantified by ImageJ (lower panel). Values represent the means ± SD of three experiments performed in duplicate. **, p < 0.01 vs. control.
Effect of IL-1β on MMP9 and TIMP1 levels in SK-N-SH cells
Various cytokines were demonstrated to affect the balance between matrix metalloproteinases and their tissue inhibitors (Candelario-Jalil et al., 2009; Gardner and Ghorpade, 2003). Among these cytokines, IL-1β has been shown to induce the expression of MMP9 and TIMP1 in brain cells (Candelario-Jalil et al., 2009; Crocker et al., 2006; Gardner and Ghorpade, 2003; Gottschall and Yu, 1995; Suryadevara et al., 2003). Thus, we examined whether incubation of SK-N-SH cells with IL-1β (0.1-1 ng/mL) results also in increase of extracellular MMP9 and TIMP1 levels. The concentrations of IL-1β used are within the range of IL-1β concentrations used to study its effect on MMP9 and TIMP1 expression in brain cells (Crocker et al., 2006; Gottschall and Yu, 1995; Suryadevara et al., 2003), but below the concentration (10ng/mL) reported to induce neuronal death (Downen et al., 1999). As shown in Figure 3A, B, incubation of cells with increasing concentrations of IL-1β resulted in 34-43%, 70-96% and 40-105% increase of extracellular proMMP9, active MMP9 and TIMP1 levels, respectively.
Figure 3A, B. Effect of IL-1β on MMP9 and TIMP1 levels in SK-N-SH cells.
SK-N-SH cells were incubated in the absence (control) or presence of increasing concentrations of IL-1β, as indicated, for 24 h. Secreted MMP9 (A) and TIMP1 (B) levels were measured by immunoblotting as described under “Experimental procedures”. Western blots were scanned and quantified by ImageJ (lower panel). Values represent the means ± SD of three experiments performed in duplicate. *, p < 0.05 vs. control. **, p < 0.01 vs. control. ***, p < 0.005 vs. control.
Effect of truncated apoE4 form apoE4-185 on cytokine mRNA levels in SK-N-SH cells
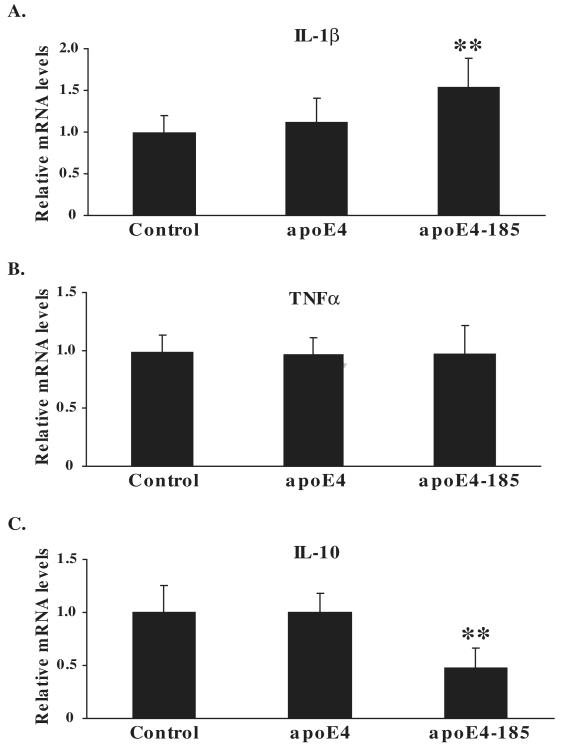
Since apoE4-185 increased the extracellular levels of MMP9 and TIMP1 in SK-N-SH cells and IL-1β also increased the levels of MMP9 and TIMP1, we next asked whether apoE4-185 affects the cellular expression of IL-1β. We also examined whether apoE4-185 affects the expression of other cytokines such as TNFα and IL-10 that, along with IL-1β, have been associated with changes in MMP9/TIMP1 expression, (Bugno et al., 1999; Crocker et al., 2006; Gottschall and Yu, 1995; Koronyo-Hamaoui et al., 2009; Suryadevara et al., 2003). We incubated SK-N-SH cells in the presence or absence of 1 μM WT apoE4 or apoE4-185 for 24 h and measured the various cytokines mRNA levels by qRT-PCR. The analysis showed that the IL-1β gene expression was increased by 55% in cells treated with apoE4-185 compared to untreated cells or cells treated with WT apoE4 (Figure 4A). This finding suggest that treatment of SK-N-SH cells with apoE4-185 increases the levels of IL-1β, which subsequently may lead to increase of extracellular MMP9 and TIMP1 levels.
Figure 4A-C. Cytokine mRNA levels in SK-N-SH cells incubated with WT apoE4 and fragment apoE4-185.
SK-N-SH cells were incubated in the absence (control) or presence of 1 μM lipid-free WT apoE4 or apoE4-185 for 24 h. The relative mRNA expression of IL-1β (A), TNFα (B) and IL-10 (C) were measured by qRT-PCR as described under “Experimental procedures”. Values represent the means ± SD of four experiments performed in duplicate or triplicate. **, p < 0.005 vs. control.
As shown in Figure 4B there was no change in TNFα gene expression in SK-N-SH cells incubated with WT apoE4 or apoE4-185 compared to the untreated cells. In contrast, it was found that IL-10 gene expression was decreased by 53% in cells treated apoE4-185 compared to untreated cells or cells treated with WT apoE4 (Figure 4C). Overall, our data indicate that the apoE4 fragment apoE4-185 is associated with changes in extracellular levels or mRNA expression of proteins involved in inflammatory response in human neuroblastoma SK-N-SH cells.
Effect of apoE4 forms on MMP9 and TIMP1 levels in SW-1783 cells
To study the effect of apoE4-185 on MMP9 and TIMP1 in another brain-derived cell line, we performed a similar set of analyses as those described above using human astrocytoma SW-1783 cells. As shown in Figure 5A in SW-1783 cells we could detect only the pro-MMP9 form, which was not affected either by WT apoE4 or apoE4-185 as assessed by Western blotting. Additionally, incubation of MMP9 over-expressing SW-1783 cells with WT apoE4 or apoE4-185 had no effect on secreted proMM9 as assessed by western blotting and gelatin zymography (Figure 5B, C). In contrast, incubation of SW-1783 cells with WT apoE4 resulted in increase of secreted TIMP1 levels by 33% as compared to untreated cells, while apoE4-185 resulted in greater increase (81%) of TIMP1 levels (Figure 5D). Overall, apoE4-185 seems to increase TIMP1 extracellular levels and affect the MMP9/TIMP1 balance in both SK-N-SH neuroblastoma and SW-1783 astrocytoma cells.
Figure 5A-D. Effect of WT apoE4 and fragment apoE4-185 on MMP9 and TIMP1 levels in SW-1783 cells.
A, SW-1783 cells were incubated in the absence (control) or presence of 1 μM lipid-free WT apoE4 or apoE4-185 for 24 h. Secreted MMP9 levels were measured by immunoblotting as described under “Experimental procedures”. Western blots were scanned and quantified by ImageJ (lower panel). B, C, SW-1783 cells transiently transfected with proMMP9 were incubated in the absence (control) or presence of 1 μM lipid-free WT apoE4 or apoE4-185 for 24 h. Secreted MMP9 levels were measured by immunoblotting (B) and enzymatic activity was assessed by gelatin zymography (C) as described under “Experimental procedures”. Western blots and zymograms were scanned and quantified by ImageJ (lower panel). D, SW-1783 cells were incubated in the absence (control) or presence of 1 μM lipid-free WT apoE4 or apoE4-185 for 24 h. Secreted TIMP1 levels were measured by immunoblotting as described under “Experimental procedures”. Western blots were scanned and quantified by ImageJ (lower panel).Values represent the means ± SD of three experiments performed in duplicate or triplicate. **, p < 0.005 vs. control. ***, p < 0.0001 vs. control. *, p< 0.05 between apoE4 and apoE-185 treated.
Effect of apoE4 forms on cytokine mRNA levels in SW-1783 cells
Various studies have shown that TIMP1 levels can be affected by various cytokines (Gardner and Ghorpade, 2003) and our data in SK-N-SH cells showed that apoE4-185 increases both the TIMP1 protein levels and IL-1β mRNA expression and that IL-1β increases also the TIMP1 levels. Thus, we next asked whether apoE4 and apoE4-185 affect the cellular expression of various cytokines (IL-1β, TNFα and IL-10) in SW-1783 cells. As shown in Figure 6A, C there was no change in IL-1β and IL-10 gene expression in SW-1783 cells incubated with WT apoE4 or apoE4-185 compared to untreated cells. In contrast, it was found that TNFα gene expression was decreased by 29-33% in cells treated either with apoE4 or apoE4-185 compared to untreated cells (Figure 6B). Overall, our data indicate that apoE4 forms may be associated with the inflammatory response in human neuroblastoma SW-1783 cells. WT apoE4 and apoE4-185 seem to exert different effects on cytokines in SK-N-SH neuroblastoma and SW-1783 astrocytoma cells.
Figure 6A-C. Cytokine mRNA levels in SW-1783 cells incubated with WT apoE4 and fragment apoE4-185.
SW-1783 cells were incubated in the absence (control) or presence of 1 μM lipid-free WT apoE4 or apoE4-185 for 24 h. The relative mRNA expression of IL-1β (A), TNFα (B) and IL-10 (C) were measured by qRT-PCR as described under “Experimental procedures”. Values represent the means ± SD of three experiments performed in duplicate or triplicate. *, p< 0.05 vs. control. **, p < 0.01 vs. control.
Discussion
Various neurodegenerative CNS disorders, including AD, have been reported to be associated with neuroinflammation and elevated levels of several inflammatory mediators (Frank-Cannon et al., 2009; Glass et al., 2010; Heneka et al., 2010; Johnston et al., 2011; McGeer and McGeer, 2010; Wyss-Coray, 2006). ApoE4 is considered a major risk factor for AD, but its exact role in the pathogenesis of AD has not been elucidated. ApoE4 carboxy-terminal truncated proteolytic fragments accumulate in brains of AD patients (Brecht et al., 2004; Harris et al., 2003; Huang et al., 2001; Jones et al., 2011) and mice expressing apoE4 in neurons (Brecht et al., 2004), but not in mice expressing apoE4 in astrocytes (Brecht et al., 2004). Furthermore, it has been suggested that apoE proteolysis occurs in the secretory and not in the internalization pathway in neurons (Brecht et al., 2004). In the current study we determined the effect of two lipid-free truncated apoE4 forms, apoE4-185 and apoE4-165, on inflammation in human neuroblastoma SK-N-SH cells. We chose to study the effect of lipid-free than lipidated apoE4 fragments, since previous studies showed that the carboxy-terminal truncated apoE4 forms apoE-185 and apoE4-165 (at ~6μM) fail to bind and solubilize DMPC multilamellar vesicles ((Li et al., 2003) and unpublished data). In addition, the ATP-binding cassette transporter (ABCA1) mediated cholesterol efflux was essentially abrogated in the presence of apoE-185 at concentration of 1μM (Vedhachalam et al., 2007; Vezeridis et al., 2011). Similar results were observed for apoE4-165 (unpublished data). Therefore, it is not expected carboxy-terminal truncated apoE4 forms that lack residues 230-299 (Li et al., 2003; Vedhachalam et al., 2007; Vezeridis et al., 2011), at the low apoE concentrations reported for CSF and used here (less than or equal to 1μM), be able to form lipoprotein particles in the brain in vivo. Regarding WT apoE4, it can accept lipids from cells and may be partially lipidated (Kypreos et al, 2007; Vezerdis et al, 2011). Thus, exogenously added apoE4 behaves similarly to apoE4 secreted from brain cells.
Our data showed that the apoE4 fragment apoE4-185 leads to increased extracellular levels of MMP9 (65%) and TIMP1 (200%) in SK-N-SH cells. Although it is difficult to accurately compare the relative increase of those two proteins based on western blotting quantification, these results still suggest that the TIMP1 increase may be more than sufficient to inhibit the activated form of MMP9 secreted from these cells. ApoE4-185 also leads to increased expression of IL-1β, but not TNFα and decreased expression of IL-10 in SK-N-SH cells. Since IL-1β leads to increased extracellular levels of MMP9 and TIMP1 in neuroblastoma SK-N-SH cells, it is possible that apoE4-185 increases the MMP9 and TIMP1 levels by inducing the IL-1β gene expression in these cells. In another brain-derived cell line tested, the human astrocytoma SW-1783 cell line, both apoE4 and apoE4-185 lead to increased TIMP1 extracellular levels without any effect on MMP9. However, in these cells apoE4-185 induces a higher increase in TIMP1 levels than apoE4 indicating that in both SW-1783 and SK-N-SH cells apoE4-185 may lead in inhibition of MMP9. On the other hand, since apoE4-185 has no effect on IL-1β and IL-10 expression, while it reduces TNFα expression (similarly as WT apoE4) in SW-1783 cells, it is suggested that apoE4-185 induces the increase in TIMP1 levels by a different mechanism in the two cell lines.
Previous studies have shown that changes in MMP9 and/or TIMP1, IL-1β and IL-10 levels are associated with neuroinflammation and possibly with AD pathogenesis (Asahina et al., 2001; Benzing et al., 1999; Dzwonek et al., 2004; Frank-Cannon et al., 2009; Gardner and Ghorpade, 2003; Kettlun et al., 2003; Koronyo-Hamaoui et al., 2009; Remarque et al., 2001; Shaftel et al., 2008; Strle et al., 2001). Thus, the apoE4-185 induced MMP9/TIMP1 imbalance in both SK-N-SH and SW-1783 cells and IL-1β increase and IL-10 decrease in SK-N-SH cells suggest that specific apoE4 proteolytic fragments may affect inflammatory processes in brain cells and may participate in neuroinflammation and AD. Whether apoE4-185 is driving AD pathogenic processes or elicits responses that could be beneficial for the disease is not clear and necessitates in vivo studies.
Interestingly, the striking differences between the ability of apoE4-185 to affect the MMP9 and TIMP1 levels in SK-N-SH cells, compared to the apoE4-165 fragment and WT apoE4 can be correlated to structural differences between the apoE4 forms. ApoE in the lipid-free state is folded into two independent structural domains (Wetterau et al., 1988). Digestion with thrombin produces a 22 kDa amino-terminal fragment (residues 1 to 191) and a 10 kDa carboxy-terminal fragment (residues 216 to 299) (Wetterau et al., 1988). X-ray crystallographic analysis of the apoE amino-terminal domain (residues 1-191) has revealed a four-helix bundle spanning residues 24-164 that segregates the hydrophobic core of the four helices from the solvent (Wilson et al., 1994; Wilson et al., 1991). Unfolding of this amino-terminal domain is thought to constitute a necessary conformational change for lipid binding and apoE function (Wilson et al., 1994; Wilson et al., 1991). In a previous study, we characterized the two apoE4 truncated forms by biophysical techniques and discovered that apoE4-185 is stabilized compared to apoE4-165 and WT apoE4 (Chroni et al., 2008). Furthermore, apoE4-185 contains less solvent-exposed hydrophobic sites compared to apoE4-165 and WT apoE4 (Chroni et al., 2008). Overall, the results described here are consistent with our previous study suggesting that apoE4 carboxy-terminal truncations can have complex effects on the stability and dynamics of the remaining fragment (Chroni et al., 2008). Additionally, in another previous study we showed that the apoE4 fragment apoE4-165, but not apoE4-185 or WT apoE4, can promote the uptake and intracellular accumulation of Aβ42 and lead to increased formation of reactive oxygen species in neuroblastoma cells (Dafnis et al., 2010). It is therefore possible that not all apoE4 fragments found in AD patient’s brains are equally bioactive and specific fragments can affect different processes of pathogenesis of the disease.
Apolipoprotein E4 has been previously associated with brain inflammation. AD patients that carry the ε4 allele demonstrated increased microglial activation compared with AD patients not carrying this allele (Egensperger et al., 1998). ApoE4 transgenic mice had been shown to have greater systemic and brain elevations of cytokines TNFα and IL-6 compared to apoE3 transgenic mice (Lynch et al., 2003). Furthermore, exogenous addition of apoE4 in adult microglia and neonatal mixed glia cultured from rat brain cortex stimulated to a greater extent the expression of IL-1β compared to apoE3 (Chen et al., 2005; Guo et al., 2004). In the current study we showed that an apoE4 truncated form, the apoE4-185, promotes the increase of IL-1β and decrease of IL-10 mRNA in human neuroblastoma SK-N-SH cells. This result may suggest a link between apoE4 proteolysis, an early or preceding event in AD pathogenesis, and inflammatory response elicited by neurons. It is thus possible that inflammation may have a causative role in AD, although it could still accelerate the disease if it is activated at later stages of the disease. Further studies are necessary to provide a better understanding of inflammatory pathways contribution in AD.
In summary, our findings may provide an association between two molecular events, the proteolysis of apoE4 and neuroinflammation, both of which have been correlated with AD pathogenesis. The apoE4 truncated form apoE4-185 increases the expression of IL-1β that possibly promotes MMP9/TIMP1 imbalance, and also decreases the expression of IL-10 in SK-N-SH neuroblastoma cells. In addition, apoE4-185 also induces a MMP9/TIMP1 imbalance in SW-1783 astrocytoma cells. We therefore propose that apoE4 proteolysis and specific short apoE4 proteolytic fragments produced in the brain of AD patients may induce or sustain inflammatory responses in brain cells.
Highlights.
We examined the effect of apoE4 fragments on inflammation in brain cell lines.
ApoE4[Δ(186-299)] promotes MMP9/TIMP1 imbalance by inducing IL-1β in SK-N-SH cells.
ApoE4[Δ(186-299)] decreases IL-10 gene expression in SK-N-SH cells.
ApoE4[Δ(186-299)] promotes MMP9/TIMP1 imbalance in SW-1783 cells.
Specific apoE4 proteolytic fragments may affect the neuroinflammatory responses.
Acknowledgements
Funding for this work was provided by the General Secretariat of Research and Technology of Greece (Synergasia 09SYN-12-897 to A.C.) and by the National Institutes of Health (HL68216 to V.Z.).
Abbreviations
- Aβ
β-amyloid peptide
- AD
Alzheimer’s disease
- apoE
apolipoprotein E
- apoE4-185
apoE4[Δ(186-299)]
- apoE4-165
apoE4[Δ(166-299)]
- AU
arbitrary units
- CT
threshold cycles
- CSF
cerebrospinal fluid
- DMEM
Dulbecco’s Modified Eagle’s Medium
- Eagle
Minimum Essential Medium
- EDTA
Ethylenediaminetetraacetic acid
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HRP
horseradish peroxidase
- IL-1β
interleukin-1β
- IL-10
interleukin-10
- MMP9
matrix metalloproteinase 9
- PBS
phosphate buffered saline
- PMSF
phenylmethylsulphonyl fluoride
- qRT-PCR
quantitative real-time polymerase chain reaction
- TIMP1
tissue inhibitor of metalloproteinase 1
- TNFα
tumor-necrosis factor α
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Anderson SS, Wu K, Nagase H, Stettler-Stevenson WG, Kim Y, Tsilibary EC. Effect of matrix glycation on expression of type IV collagen, MMP-2, MMP-9 and TIMP-1 by human mesangial cells. Cell Adhes Commun. 1996;4:89–101. doi: 10.3109/15419069609010765. [DOI] [PubMed] [Google Scholar]
- Asahina M, Yoshiyama Y, Hattori T. Expression of matrix metalloproteinase-9 and urinary-type plasminogen activator in Alzheimer’s disease brain. Clin Neuropathol. 2001;20:60–63. [PubMed] [Google Scholar]
- Ballatore C, Lee VM, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nat Rev Neurosci. 2007;8:663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- Bayer TA, Wirths O. Review on the APP/PS1KI mouse model: intraneuronal Abeta accumulation triggers axonopathy, neuron loss and working memory impairment. Genes Brain Behav. 2008;7(Suppl 1):6–11. doi: 10.1111/j.1601-183X.2007.00372.x. [DOI] [PubMed] [Google Scholar]
- Bayer TA, Wirths O. Intraneuronal Abeta as a trigger for neuron loss: can this be translated into human pathology? Biochem Soc Trans. 2011;39:857–861. doi: 10.1042/BST0390857. [DOI] [PubMed] [Google Scholar]
- Benzing WC, Wujek JR, Ward EK, Shaffer D, Ashe KH, Younkin SG, Brunden KR. Evidence for glial-mediated inflammation in aged APP(SW) transgenic mice. Neurobiol Aging. 1999;20:581–589. doi: 10.1016/s0197-4580(99)00065-2. [DOI] [PubMed] [Google Scholar]
- Brecht WJ, Harris FM, Chang S, Tesseur I, Yu GQ, Xu Q, Dee FJ, Wyss-Coray T, Buttini M, Mucke L, Mahley RW, Huang Y. Neuron-specific apolipoprotein e4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J Neurosci. 2004;24:2527–2534. doi: 10.1523/JNEUROSCI.4315-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno MA, Mufson EJ, Wuu J, Cuello AC. Increased matrix metalloproteinase 9 activity in mild cognitive impairment. J Neuropathol Exp Neurol. 2009;68:1309–1318. doi: 10.1097/NEN.0b013e3181c22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugno M, Witek B, Bereta J, Bereta M, Edwards DR, Kordula T. Reprogramming of TIMP-1 and TIMP-3 expression profiles in brain microvascular endothelial cells and astrocytes in response to proinflammatory cytokines. FEBS Lett. 1999;448:9–14. doi: 10.1016/s0014-5793(99)00323-3. [DOI] [PubMed] [Google Scholar]
- Candelario-Jalil E, Yang Y, Rosenberg GA. Diverse roles of matrix metalloproteinases and tissue inhibitors of metalloproteinases in neuroinflammation and cerebral ischemia. Neuroscience. 2009;158:983–994. doi: 10.1016/j.neuroscience.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedazo-Minguez A. Apolipoprotein E and Alzheimer’s disease: molecular mechanisms and therapeutic opportunities. J Cell Mol Med. 2007;11:1227–1238. doi: 10.1111/j.1582-4934.2007.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, ran Ma T, Miranda RD, Balestra ME, Mahley RW, Huang Y. Lipid- and receptor-binding regions of apolipoprotein E4 fragments act in concert to cause mitochondrial dysfunction and neurotoxicity. Proc Natl Acad Sci U S A. 2005;102:18694–18699. doi: 10.1073/pnas.0508254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li Q, Wang J. Topology of human apolipoprotein E3 uniquely regulates its diverse biological functions. Proc Natl Acad Sci U S A. 2011;108:14813–14818. doi: 10.1073/pnas.1106420108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Averett NT, Manelli A, LaDu MJ, May W, Ard MD. Isoform-specific effects of apolipoprotein E on secretion of inflammatory mediators in adult rat microglia. J Alzheimers Dis. 2005;7:25–35. doi: 10.3233/jad-2005-7104. [DOI] [PubMed] [Google Scholar]
- Cho HS, Hyman BT, Greenberg SM, Rebeck GW. Quantitation of apoE domains in Alzheimer disease brain suggests a role for apoE in Abeta aggregation. J Neuropathol Exp Neurol. 2001;60:342–349. doi: 10.1093/jnen/60.4.342. [DOI] [PubMed] [Google Scholar]
- Chroni A, Pyrpassopoulos S, Thanassoulas A, Nounesis G, Zannis VI, Stratikos E. Biophysical analysis of progressive C-terminal truncations of human apolipoprotein E4: insights into secondary structure and unfolding properties. Biochemistry. 2008;47:9071–9080. doi: 10.1021/bi800469r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Crocker SJ, Milner R, Pham-Mitchell N, Campbell IL. Cell and agonist-specific regulation of genes for matrix metalloproteinases and their tissue inhibitors by primary glial cells. J Neurochem. 2006;98:812–823. doi: 10.1111/j.1471-4159.2006.03927.x. [DOI] [PubMed] [Google Scholar]
- Dafnis I, Stratikos E, Tzinia A, Tsilibary EC, Zannis VI, Chroni A. An apolipoprotein E4 fragment can promote intracellular accumulation of amyloid peptide beta 42. J Neurochem. 2010;115:873–884. doi: 10.1111/j.1471-4159.2010.06756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downen M, Amaral TD, Hua LL, Zhao ML, Lee SC. Neuronal death in cytokine-activated primary human brain cell culture: role of tumor necrosis factor-alpha. Glia. 1999;28:114–127. [PubMed] [Google Scholar]
- Dzwonek J, Rylski M, Kaczmarek L. Matrix metalloproteinases and their endogenous inhibitors in neuronal physiology of the adult brain. FEBS Lett. 2004;567:129–135. doi: 10.1016/j.febslet.2004.03.070. [DOI] [PubMed] [Google Scholar]
- Egensperger R, Kosel S, von Eitzen U, Graeber MB. Microglial activation in Alzheimer disease: Association with APOE genotype. Brain Pathol. 1998;8:439–447. doi: 10.1111/j.1750-3639.1998.tb00166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener. 2009;4:47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner J, Ghorpade A. Tissue inhibitor of metalloproteinase (TIMP)-1: the TIMPed balance of matrix metalloproteinases in the central nervous system. J Neurosci Res. 2003;74:801–806. doi: 10.1002/jnr.10835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschall PE, Yu X. Cytokines regulate gelatinase A and B (matrix metalloproteinase 2 and 9) activity in cultured rat astrocytes. J Neurochem. 1995;64:1513–1520. doi: 10.1046/j.1471-4159.1995.64041513.x. [DOI] [PubMed] [Google Scholar]
- Guo L, LaDu MJ, Van Eldik LJ. A dual role for apolipoprotein e in neuroinflammation: anti- and pro-inflammatory activity. J Mol Neurosci. 2004;23:205–212. doi: 10.1385/JMN:23:3:205. [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Harris FM, Brecht WJ, Xu Q, Tesseur I, Kekonius L, Wyss-Coray T, Fish JD, Masliah E, Hopkins PC, Scearce-Levie K, Weisgraber KH, Mucke L, Mahley RW, Huang Y. Carboxyl-terminal-truncated apolipoprotein E4 causes Alzheimer’s disease-like neurodegeneration and behavioral deficits in transgenic mice. Proc Natl Acad Sci U S A. 2003;100:10966–10971. doi: 10.1073/pnas.1434398100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, O’Banion MK, Terwel D, Kummer MP. Neuroinflammatory processes in Alzheimer’s disease. J Neural Transm. 2010;117:919–947. doi: 10.1007/s00702-010-0438-z. [DOI] [PubMed] [Google Scholar]
- Hernandez-Guillamon M, Delgado P, Ortega L, Pares M, Rosell A, Garcia-Bonilla L, Fernandez-Cadenas I, Borrell-Pages M, Boada M, Montaner J. Neuronal TIMP-1 release accompanies astrocytic MMP-9 secretion and enhances astrocyte proliferation induced by beta-amyloid 25-35 fragment. J Neurosci Res. 2009;87:2115–2125. doi: 10.1002/jnr.22034. [DOI] [PubMed] [Google Scholar]
- Huang Y, Liu XQ, Wyss-Coray T, Brecht WJ, Sanan DA, Mahley RW. Apolipoprotein E fragments present in Alzheimer’s disease brains induce neurofibrillary tangle-like intracellular inclusions in neurons. Proc Natl Acad Sci U S A. 2001;98:8838–8843. doi: 10.1073/pnas.151254698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston H, Boutin H, Allan SM. Assessing the contribution of inflammation in models of Alzheimer’s disease. Biochem Soc Trans. 2011;39:886–890. doi: 10.1042/BST0390886. [DOI] [PubMed] [Google Scholar]
- Jones PB, Adams KW, Rozkalne A, Spires-Jones TL, Hshieh TT, Hashimoto T, von Armin CA, Mielke M, Bacskai BJ, Hyman BT. Apolipoprotein E: isoform specific differences in tertiary structure and interaction with amyloid-beta in human Alzheimer brain. PLoS ONE. 2011;6:e14586. doi: 10.1371/journal.pone.0014586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay AD, Petzold A, Kerr M, Keir G, Thompson EJ, Nicoll JA. Cerebrospinal fluid apolipoprotein E concentration decreases after traumatic brain injury. J Neurotrauma. 2003;20:243–250. doi: 10.1089/089771503321532824. [DOI] [PubMed] [Google Scholar]
- Kettlun AM, Cartier L, Garcia L, Collados L, Vasquez F, Ramirez E, Valenzuela MA. TIMPs and MMPs expression in CSF from patients with TSP/HAM. Life Sci. 2003;72:2863–2876. doi: 10.1016/s0024-3205(03)00146-2. [DOI] [PubMed] [Google Scholar]
- Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koronyo-Hamaoui M, Ko MK, Koronyo Y, Azoulay D, Seksenyan A, Kunis G, Pham M, Bakhsheshian J, Rogeri P, Black KL, Farkas DL, Schwartz M. Attenuation of AD-like neuropathology by harnessing peripheral immune cells: local elevation of IL-10 and MMP-9. J Neurochem. 2009;111:1409–1424. doi: 10.1111/j.1471-4159.2009.06402.x. [DOI] [PubMed] [Google Scholar]
- Kypreos KE, Zannis VI. Pathway of biogenesis of apolipoprotein E - containing HDL in vivo with the participation of ABCA1 and LCAT. Biochem J. 2007;403:359–367. doi: 10.1042/BJ20061048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- Li X, Kypreos K, Zanni EE, Zannis V. Domains of apoE required for binding to apoE receptor 2 and to phospholipids: Implications for the functions of apoE in the brain. Biochemistry. 2003;42:10406–10417. doi: 10.1021/bi027093c. [DOI] [PubMed] [Google Scholar]
- Locati M, Deuschle U, Massardi ML, Martinez FO, Sironi M, Sozzani S, Bartfai T, Mantovani A. Analysis of the gene expression profile activated by the CC chemokine ligand 5/RANTES and by lipopolysaccharide in human monocytes. J Immunol. 2002;168:3557–3562. doi: 10.4049/jimmunol.168.7.3557. [DOI] [PubMed] [Google Scholar]
- Lorenzl S, Albers DS, LeWitt PA, Chirichigno JW, Hilgenberg SL, Cudkowicz ME, Beal MF. Tissue inhibitors of matrix metalloproteinases are elevated in cerebrospinal fluid of neurodegenerative diseases. J Neurol Sci. 2003a;207:71–76. doi: 10.1016/s0022-510x(02)00398-2. [DOI] [PubMed] [Google Scholar]
- Lorenzl S, Albers DS, Relkin N, Ngyuen T, Hilgenberg SL, Chirichigno J, Cudkowicz ME, Beal MF. Increased plasma levels of matrix metalloproteinase-9 in patients with Alzheimer’s disease. Neurochem Int. 2003b;43:191–196. doi: 10.1016/s0197-0186(03)00004-4. [DOI] [PubMed] [Google Scholar]
- Lynch JR, Tang W, Wang H, Vitek MP, Bennett ER, Sullivan PM, Warner DS, Laskowitz DT. APOE genotype and an ApoE-mimetic peptide modify the systemic and central nervous system inflammatory response. J Biol Chem. 2003;278:48529–48533. doi: 10.1074/jbc.M306923200. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103:5644–5651. doi: 10.1073/pnas.0600549103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL. Neuroinflammation in Alzheimer’s disease and mild cognitive impairment: a field in its infancy. J Alzheimers Dis. 2010;19:355–361. doi: 10.3233/JAD-2010-1219. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Takuma K, Fukuzaki E, Ibi D, Someya E, Akazawa KH, Alkam T, Tsunekawa H, Mouri A, Noda Y, Nabeshima T, Yamada K. Matrix metalloprotease-9 inhibition improves amyloid beta-mediated cognitive impairment and neurotoxicity in mice. J Pharmacol Exp Ther. 2009;331:14–22. doi: 10.1124/jpet.109.154724. [DOI] [PubMed] [Google Scholar]
- Myers RH, Schaefer EJ, Wilson PW, D’Agostino R, Ordovas JM, Espino A, Au R, White RF, Knoefel JE, Cobb JL, McNulty KA, Beiser A, Wolf PA. Apolipoprotein E epsilon4 association with dementia in a population-based study: The Framingham study. Neurology. 1996;46:673–677. doi: 10.1212/wnl.46.3.673. [DOI] [PubMed] [Google Scholar]
- Nagaeva O, Jonsson L, Mincheva-Nilsson L. Dominant IL-10 and TGF-beta mRNA expression in gammadeltaT cells of human early pregnancy decidua suggests immunoregulatory potential. Am J Reprod Immunol. 2002;48:9–17. doi: 10.1034/j.1600-0897.2002.01131.x. [DOI] [PubMed] [Google Scholar]
- Peress N, Perillo E, Zucker S. Localization of tissue inhibitor of matrix metalloproteinases in Alzheimer’s disease and normal brain. J Neuropathol Exp Neurol. 1995;54:16–22. doi: 10.1097/00005072-199501000-00002. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen R. In: Quantification on the LightCycler. In: Rapid Cycle Real-time PCR, Methods and Applications. Meuer S, Wittwer C, Nakagawara K, editors. Spronger Press; Heidelberg: 2001. pp. 21–34. [Google Scholar]
- Remarque EJ, Bollen EL, Weverling-Rijnsburger AW, Laterveer JC, Blauw GJ, Westendorp RG. Patients with Alzheimer’s disease display a pro-inflammatory phenotype. Exp Gerontol. 2001;36:171–176. doi: 10.1016/s0531-5565(00)00176-5. [DOI] [PubMed] [Google Scholar]
- Shaftel SS, Griffin WS, O’Banion MK. The role of interleukin-1 in neuroinflammation and Alzheimer disease: an evolving perspective. J Neuroinflammation. 2008;5:7. doi: 10.1186/1742-2094-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siest G, Bertrand P, Qin B, Herbeth B, Serot JM, Masana L, Ribalta J, Passmore AP, Evans A, Ferrari M, Franceschi M, Shepherd J, Cuchel M, Beisiegel U, Zuchowsky K, Rukavina AS, Sertic J, Stojanov M, Kostic V, Mitrevski A, Petrova V, Sass C, Merched A, Salonen JT, Tiret L, Visvikis S. Apolipoprotein E polymorphism and serum concentration in Alzheimer’s disease in nine European centres: the ApoEurope study. ApoEurope group. Clin Chem Lab Med. 2000;38:721–730. doi: 10.1515/CCLM.2000.102. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Stomrud E, Bjorkqvist M, Janciauskiene S, Minthon L, Hansson O. Alterations of matrix metalloproteinases in the healthy elderly with increased risk of prodromal Alzheimer’s disease. Alzheimers Res Ther. 2010;2:20. doi: 10.1186/alzrt44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strle K, Zhou JH, Shen WH, Broussard SR, Johnson RW, Freund GG, Dantzer R, Kelley KW. Interleukin-10 in the brain. Crit Rev Immunol. 2001;21:427–449. [PubMed] [Google Scholar]
- Suryadevara R, Holter S, Borgmann K, Persidsky R, Labenz-Zink C, Persidsky Y, Gendelman HE, Wu L, Ghorpade A. Regulation of tissue inhibitor of metalloproteinase-1 by astrocytes: links to HIV-1 dementia. Glia. 2003;44:47–56. doi: 10.1002/glia.10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedhachalam C, Narayanaswami V, Neto N, Forte TM, Phillips MC, Lund-Katz S, Bielicki JK. The C-terminal lipid-binding domain of apolipoprotein E is a highly efficient mediator of ABCA1-dependent cholesterol efflux that promotes the assembly of high-density lipoproteins. Biochemistry. 2007;46:2583–2593. doi: 10.1021/bi602407r. [DOI] [PubMed] [Google Scholar]
- Vezeridis AM, Chroni A, Zannis VI. Domains of apoE4 required for the biogenesis of apoE-containing HDL. Ann Med. 2011;43:302–311. doi: 10.3109/07853890.2010.549143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Han Y, Chen D, Xiao Z, Xi Z, Xiao F, Wang X. Cerebrospinal fluid apolipoprotein E concentration decreases after seizure. Seizure. 2010;19:79–83. doi: 10.1016/j.seizure.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Wellnitz S, Friedlein A, Bonanni C, Anquez V, Goepfert F, Loetscher H, Adessi C, Czech C. A 13 kDa carboxy-terminal fragment of ApoE stabilizes Abeta hexamers. J Neurochem. 2005;94:1351–1360. doi: 10.1111/j.1471-4159.2005.03295.x. [DOI] [PubMed] [Google Scholar]
- Wetterau JR, Aggerbeck LP, Rall SC, Jr., Weisgraber KH. Human apolipoprotein E3 in aqueous solution. I. Evidence for two structural domains. J Biol Chem. 1988;263:6240–6248. [PubMed] [Google Scholar]
- Wilson C, Mau T, Weisgraber KH, Wardell MR, Mahley RW, Agard DA. Salt bridge relay triggers defective LDL receptor binding by a mutant apolipoprotein. Structure. 1994;2:713–718. doi: 10.1016/s0969-2126(00)00072-1. [DOI] [PubMed] [Google Scholar]
- Wilson C, Wardell MR, Weisgraber KH, Mahley RW, Agard DA. Three-dimensional structure of the LDL receptor-binding domain of human apolipoprotein E. Science. 1991;252:1817–1822. doi: 10.1126/science.2063194. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response? Nat Med. 2006;12:1005–1015. doi: 10.1038/nm1484. [DOI] [PubMed] [Google Scholar]
- Yan P, Hu X, Song H, Yin K, Bateman RJ, Cirrito JR, Xiao Q, Hsu FF, Turk JW, Xu J, Hsu CY, Holtzman DM, Lee JM. Matrix metalloproteinase-9 degrades amyloid-beta fibrils in vitro and compact plaques in situ. J Biol Chem. 2006;281:24566–24574. doi: 10.1074/jbc.M602440200. [DOI] [PubMed] [Google Scholar]
- Zannis VI, Breslow JL, Utermann G, Mahley RW, Weisgraber KH, Havel RJ, Goldstein JL, Brown MS, Schonfeld G, Hazzard WR, Blum C. Proposed nomenclature of apoE isoproteins, apoE genotypes, and phenotypes. J Lipid Res. 1982;23:911–914. [PubMed] [Google Scholar]
- Zannis VI, Kypreos KE, Chroni A, Kardassis D, Zanni EE. In: Lipoproteins and atherogenesis. In: Molecular Mechanisms of Atherosclerosis. Loscalzo J, editor. Taylor & Francis; Abington, UK: 2004. pp. 111–174. [Google Scholar]
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]