Abstract
Immunotherapy using autologous T-cells has emerged to be a powerful treatment option for patients with metastatic melanoma. These include the adoptive transfer of autologous tumor-infiltrating lymphocytes (TIL), T-cells transduced with high-affinity T-cell receptors (TCR) against major melanosomal tumor antigens, and T cells transduced with chimeric antigen receptors (CAR) composed of hybrid immunoglobulin light chains with endo-domains of T-cell signaling molecules. Among these and other options for T-cell therapy, TIL together with high-dose IL-2 has had the longest clinical history with multiple clinical trials in centers across the world consistently demonstrating durable clinical response rates near 50% or more. A distinct advantage of TIL therapy making it still the T-cell therapy of choice is the broad nature of the T-cell recognition against both defined as well as un-defined tumors antigens against all possible MHC, rather than the single specificity and limited MHC coverage of the newer TCR and CAR transduction technologies. In the past decade, significant inroads have been made in defining the phenotypes of T cells in TIL mediating tumor regression. CD8+ T cells are emerging to be critical, although the exact subset of CD8+ T cells exhibiting the highest clinical activity in terms of memory and effector markers is still controversial. We present a model in which both effector-memory and more differentiated effector T cells ultimately may need to cooperate to mediate long-term tumor control in responding patients. Although TIL therapy has shown great potential to treat metastatic melanoma, a number of issues have emerged that need to be addressed to bring it more into the mainstream of melanoma care. First, we have a reached the point where a pivotal phase II or phase III trials are needed in an attempt to gain regulatory approval of TIL as standard-of-care. Second, improvements in how we expand TIL for therapy are needed, that minimize the time the T-cells are in culture and improve the memory and effector characteristics of the T cells for longer persistence and enhanced anti-tumor activity in vivo. Third, there is a critical need to identify surrogate and predictive biomarkers in order to better select suitable patients for TIL therapy in order to improve response rate and duration. Overall, the outlook for TIL therapy for melanoma is very bright. We predict that TIL will indeed emerge to become an approved treatment in the upcoming years through pivotal clinical trials. Moreover, new approaches combining TIL with targeted signaling pathway drugs, such as mutant B-RAF inhibitors, and synergistic immunomodulatory interventions enhancing T-cell costimulation and preventing negative regulation, should further increase therapeutic efficacy and durable complete response rates.
Both passive and active immunotherapies for melanoma have come a long way from being a dream of academicians a few decades ago to real proven clinical successes. In fact, the tables are turning with immunotherapy emerging to be one of the most powerful approaches to treat metastatic melanoma, as evidenced by the recent FDA approval of the human monocloncal antibody anti-CTLA-4 (ipilimumab) as a single-agent therapy for Stage IV disease (1, 2). Anti-CTLA-4 interrupts engagement of a key inhibitory signaling pathway or checkpoint during T-cell activation and “takes the brakes off” of T cells resulting in enhanced proliferation and effector activity against a large array of auto-antigens, including tumor antigens (3, 4). In addition to anti-CTLA-4, a number of other immunotherapy approaches have been steadily developed and improved over the last few decades with incremental successes. These include IL-2 therapy (5), peptide vaccines together with IL-2 (6), and adoptive T-cell therapy using autologous expanded tumor-infiltrating lymphocytes (TIL) (7–9). In addition, other monoclonal antibody therapies further activating anti-tumor T cell and NK cell activity using blocking antibodies to inhibitory signaling receptors (e.g., PD-1) or activating costimulatory receptors (e.g., CD137/4-1BB) are also being tested now in the clinic with some success (10, 11). Moreover, rational combinations of these immunotherapies with chemotherapies (12) and targeted therapies against the MAPK pathway (13–15) are now making an entry into the clinic. These are capitalizing on the new-found mechanisms of chemotherapies and targeted therapies in facilitating immune responses by activating innate immunity (e.g., “immunogenic chemotherapy”) (12, 16, 17) or affecting the expression of immunoregulatory factors in the tumor microenvironment.
To date, one of the most powerful immunotherapies against metastatic melanoma has been adoptive T-cell therapy (ACT) using autologous ex vivo-expanded TIL adoptively transferred back into patients. Adoptive transfer of TIL for the treatment of human metastatic melanoma was initially described in 1988 (18) and has since yielded dramatic results since these early days with >50% clinical responses many of which are lasting for years in recent clinical trials (7–9, 19, 20). More recently, other forms of ACT using gene modified T cells are now being developed and tested clinically. These include T-cells propagated from peripheral blood mononuclear cells (PBMCs) expressing cloned recombinant TCR αβ chains recognizing epitopes from shared tumor associated antigens (TAAs) (21, 22), or expressing chimeric antigen receptors (CAR) composed of immunoglobulin variable regions recognizing tumor antigens fused to signaling domains of the TCR ζ chain and costimulatory molecules, such as CD28 and CD137/4-1BB (23, 24). The pace of research in autologous T-cell-based therapies for melanoma has increased dramatically over the last decade with new target antigens and increased numbers of clinical trials testing both TIL and TCR- or CAR- transduced T cells.
Here, we provide an overview of the different forms of autologous T-cell therapies being developed for melanoma. We then focus in detail on TIL adoptive cell therapy and its increasing clinical success, as well as critical outstanding biological and clinical issues that need to be resolved so that it can be adopted as a mainstream treatment option for melanoma. We also discuss the issue of surrogate and predictive biomarkers for TIL immunotherapy and what possible approaches can be taken to select patients for TIL therapy. Lastly, we briefly discuss the future of TIL therapy and new synergistic approaches with targeted therapeutic agents (e.g., kinase inhibitors) and immunomodulatory drugs as well as gene transduction of the T cells to improve response rates.
Overview of different forms of T-cell based therapies for melanoma
A number of different strategies have been employed to propagate TAA-specific T cells either from patient-derived PBMC or by transduction of activated peripheral blood T cells with TAA-reactive TCR genes or CARs specific for melanoma cell surface antigens. Table 1 summarizes these different forms of ACT that are being investigated for melanoma, a brief description of the method, the antigens targeted, and the status of clinical development. The first form of therapy that has been most extensively studied both pre-clinically and in clinical trials is autologous TIL expanded ex vivo from tumor fragments or single cell enzymatic digests of melanoma metastases (25, 26). TIL therapy capitalizes on the polyclonal nature of the T-cell infiltrates in melanomas and the recognition of multiple TAA, some of which are shared melanoma/melanocyte differentiation antigens, such as gp100, MART-1, TRP-2, tyrosinase, and NY-ESO-1, as well as reactivities against a host of unknown antigens. In fact, recent data on screening for TAA-specificities of CD8+ T-cells in TIL using peptide-loaded HLA multimers (27) has found that the only a minority of TIL respond against defined melanoma/melanocyte differentiation antigens, while the vast majority of the CD8+ T cells (>90% of TIL) seem to specific against hitherto unknown antigens, presumably epitopes from mutated self proteins (e.g., mutated signaling and housekeeping genes) that would not be subject to central tolerance during T-cell differentiation (28). This is a key benefit of TIL therapy over other forms ACT. Below, we will describe in more detail the TIL therapy protocol and current issues and improvements in TIL therapy being developed. Here, we will describe the other forms of melanoma ACT shown in Table 1 that have been largely developed as alternatives to TIL therapy, mainly to address situations where access to TIL is not possible (e.g., no resectable tumor or inability to expand TIL to adequate numbers for adoptive transfer).
Table 1.
Summary of Different Forms of Autologous T-cell Therapies for Metastatic Melanoma and the Phase of Clinical Development
| Strategy for ACT | Brief Description | Targets | Clinical Status |
|---|---|---|---|
| Tumor infiltrating lymphocytes (TIL) | Isolation of T lymphocytes from primary or secondary tumors followed by in vitro expansion with IL-2 | Polyclonal | Clinical (phase II) Dudley et al., 2005 and 2008, J Clin Oncol (8, 63) Dudley et al., 2010, Clin Can Res (74) Rosenberg et al., 2011, Clin Can Res (20) Radvanyi et al., 2010, J. Immunother (9) Besser et al., 2010, Clin Can Res (7) |
| Antigen-expanded CD8+ or CD4+ T cells | In vitro reactivation and expansion of T lymphocytes specifically recognizing tumor-associated antigen (TAA) | MART-1, Tyrosinase, gp100, NY-ESO-1, or polyclonal |
Clinical (phase I/II) Yee et al., 2002, Proc Natl Acad Sci (31) Mitchell et al., 2002, J Clin Oncol (30) Mackensen et al., 2006, J Clin Oncol (29) Hunder et al., 2008, N Engl J Med (32) Verdegaal et al., 2011, Cancer Immunol Immunother (33) Butler et al., 2011, Sci Transl Med (35) |
| Engineered T cell receptor (TCR) expression in lymphocytes | Genetic modification of T cells for expression of second TCR (human or mouse) directed against TAA | MART-1, gp100, p53, and NY-ESO-1 |
Clinical (phase I/II) Morgan et al., 2006, Science (21) Johnson et al., 2009, Blood (37) Robbins et al., 2011, J Clin Oncol (38) |
| Chimeric antigen receptor (CAR) expression in lymphocytes | Genetic modification of T cells for expression of a chimeric receptor partly constituted of TAA-specific antibody and CD3/co-stimulatory molecule trans-membrane and cytoplasmic domains | Ganglioside GD2, GD3, and HMW-MAA (MCSP-1) |
Pre-clinical Yvon et al., 2009, Clin Can Res (24) Lo et al., 2010, Clin Can Res (48) Burns et al., 2010, Can Res (51) |
Activation and expansion of antigen-specific T cells from PBMC
The first alternative strategy that has been investigated is to expand TAA-specific CD8+ and/or CD4+ T cell clones or polyclonal T cells in vitro by multiple antigenic stimulation of autologous PBMC. A number of Phase I and Phase II clinical trials with CD8+ and CD4+ T cells and clones specific for MART-1 and gp100 have been completed. However, in all cases, only modest results have been obtained with typically low response rates (<10%), many of which are mixed responses, and only anecdotal observations of durable remissions is a few select patients (29–31). For example, an interesting study reported long term ongoing response in refractory metastatic in one melanoma patient following ACT of NY-ESO-1-specific CD4+ T cell clones (32). Although the CD4+ T-cell clone did not persist in vivo, an anti-tumor response directed against TAA other than NY-ESO-1 mediated by endogenous CD8+ T cells was found in this patient which may be attributed to the release of other TAA (called antigen spreading) after limited destruction of tumor deposits, or provision of cytokines to endogenously-activated CD8+ T cells, by the infused CD4+ T cells. Unfortunately, the other eight patients of this cohort did not respond to treatment (32). Recently, a phase I/II clinical trial infusion polyclonal tumor-specific CD4+ and CD8+ T cells generated in vitro with repeat stimulation of irradiated autologous tumor cells has reported one complete regression, one partial response, and three patients with stable disease out of 10 patients (33). Despite some promising results, the expansion of TAA-specific T cells from largely naïve PBMC populations is a time-consuming and labor-intensive process. Another major drawback is is that generally only low antigen-specific T-cell frequencies (5% or less) can be generated after multiple rounds of stimulation and expansion. Many of these cells are also of low avidity. Moreover, the multiple rounds of T-cell stimulation to reach antigen-specific T-cell frequencies high enough to be of any therapeutic benefit leads to T-cell differentiation and loss of memory characteristics needed for long-term persistence in vivo. However, recently a newer approach to generate melanoma antigen-specific T cells for adoptive transfer through multiple stimulation of patient-derived PBMC using artificial antigen-presenting cells (aAPC) expressing HLA-A0201 and costimulatory molecules and membrane-bound cytokines may improve upon these limitations. Here, the K562 erythroleukemia cell line has been adopted as an aAPC due to its lack of HLA-A and HLA-B expression, while retaining antigen processing and presentation machinery (34). Using K562 aAPC expressing HLA-A0201 and a blend of costimulatory molecules (CD54, CD58, CD80, CD86, CD83) MART-1 peptide-specific CD8+ T-cells have been expanded from PBMC using IL-2 and IL-15. A sub-population of these cells had a central memory T-cell phenotype and could persist for 4 months or more. Although no clinical response was found after T-cell infusion, subsequent treatment with anti-CTLA-4 antibody (ipilimumab) in some of these patients lead to partial responses in 3/5 of the antibody-treated patients (35). This represents a new paradigm in T-cell therapy for melanoma where patients receive an infusion of T-cells enriched for TAA-specificity which can re-circulate and persist for long periods of time and can be then further activated in vivo against the tumors by immunomodulatory co-therapies, such as anti-CTLA-4. This “boosting” effect can lead to more consistent clinical responses. Another benefit of this approach is that patients do not need to be lymphodepleted prior to the T-cell infusion as with TIL therapy (35, 36).
Transduction of T cells with TAA-specific TCR genes
Generation of autologous T cell modified to express exogenous TAA-specific TCR presents a lot of advantages. As mentioned before, TIL are not always accessible and do not always expand to sufficient numbers from tumor samples (at least 5–10 billion cells) needed for therapy. On the opposite side, engineering T lymphocytes isolated from peripheral blood by transduction with a retrovirus encoding for TCR can give rapid access to a great number of TAA-specific cells for transfer. The first clinical trial however used an HLA-A0201-restricted MART-1 specific TCR with lower affinity, isolated from a patient who received successful TIL ACT, which had a clinical response rate of only of 13% (21). However, this was succeeded by a second trial using a higher affinity MART-1 TCR which led to a more impressive 30% clinical response rate with many patients experiencing autoimmune vitilago and other autoimmune complications (37). TCR transduction has also been used to target NY-ESO-1 in a recent clinical trial in which 5 out of 11 melanoma patients showed objective melanoma regression, with 2 complete responses (38). Since these initial clinical trials, additional TCR receptors restricted to HLA-A0201 recognizing epitopes from other tumor antigens in melanoma have been isolated and cloned, including TCRs directed against gp100, and p53, but clinical responses are yet to be completed (39).
Although TCR engineering and ACT with TCR-transduced T cells is a promising approach, it has a number of limitations in comparison with TIL. First, the TCR recognizes a specific peptide presented by only specific MHC class I molecule, and at present, only HLA-A0201 has been targeted. This limits the therapy to only about 30–35% of melanoma patients that express at least one HLA-A0201 allele. Others are in the pipeline, but require identification of new epitopes restricted by other less prevalent HLA-A sub-types in the human population. Second, transfer of modified T cells recognizing only one specific TAA opens the door to immuno-selection and tumor escape by antigen loss. In addition, there are concerns about bystander autoimmune manifestations with the high affinity TCRs in these transduced T cells due to the expression of many shared TAA on normal cells; although these are expressed at lower levels on normal cells, the high affinity of the TCRs can facilitate significant binding to low abundance epitopes on these cells (40). Finally, although it was only reported in animals, mispairing of new TCR alpha and beta chains with endogenous TCR chains can occur leading to completely unknown new specificities some of which may be reactive against non-tumor self-antigens (40). To avoid these and the other shortcomings of TCR transduction technology and the issue of complication MHC restriction, some researches have been began to focus on gene modification of T cell for expression of chimeric antigen receptors (CARs) recognizing tumor-specific or tumor-associated cell surface proteins.
Chimeric antigen receptor (CAR) technology
The first generation of CAR consisted of a junction between the heavy and light chain of a monoclonal antibody with variable domains, combined with trans-membrane and cytoplasmic tail of the ζ chain of the CD3 complex. Engineering of T cells with such construct was aimed to avoid MHC restriction and tumor evasion because of MHC down regulation. This first generation led to poor proliferation of T cells, with triggering of ζ chain signaling alone being insufficient to activate the cells (41, 42). These observations led to the addition of the endo-domain of the CD28 T-cell co-stimulatory molecule to CAR constructs along with the ζ chain to mimic the two-signal model of T cell activation (43, 44). Addition of other co-stimulatory molecule endo-domains molecules from CD134 (OX40) and CD137 (4-1BB) to the CAR contructs, combined have CD28 further improved T cell activation (45). Although clinical trails in hematologic cancer such as advance leukemia and lymphoma have lately produced promising results regarding anti-tumor potential and persistence (23, 46), adoptive transfer of CAR-modified T cells for melanoma is still in preclinical stages of development. Some of the targets of CAR-transduced T cells for melanoma therapy include the gangliosides GD2, GD3 over-expressed in 50–80% of metastatic melanomas and the proteoglycan MCSP-1 (HMW-MAA) that is specifically expressed in >90 of melanomas (47–50). In mouse models, a second generation CAR directed against GD2 combined with CD28 and CD134 endo-domains have significantly improved survival of mice following transfer (24) and a study using anti-GD3 CAR combined with CD28 has lead to a complete response in 50% of following in mice with established melanoma tumors (48). IL-2 administration was critical in both cases. Recently, a clinical-grade CAR directed against MCSP-1 has been generated and tested in vitro and should soon be tested in a clinical trial (51). Data on clinical trials of adoptive transfer of CAR transduced T-cells in melanoma are on the horizon and it will be exciting to see how they perform in the clinic and whether any unexpected toxicities arise.
Overview of current the TIL therapy protocol for melanoma and recent clinical trials
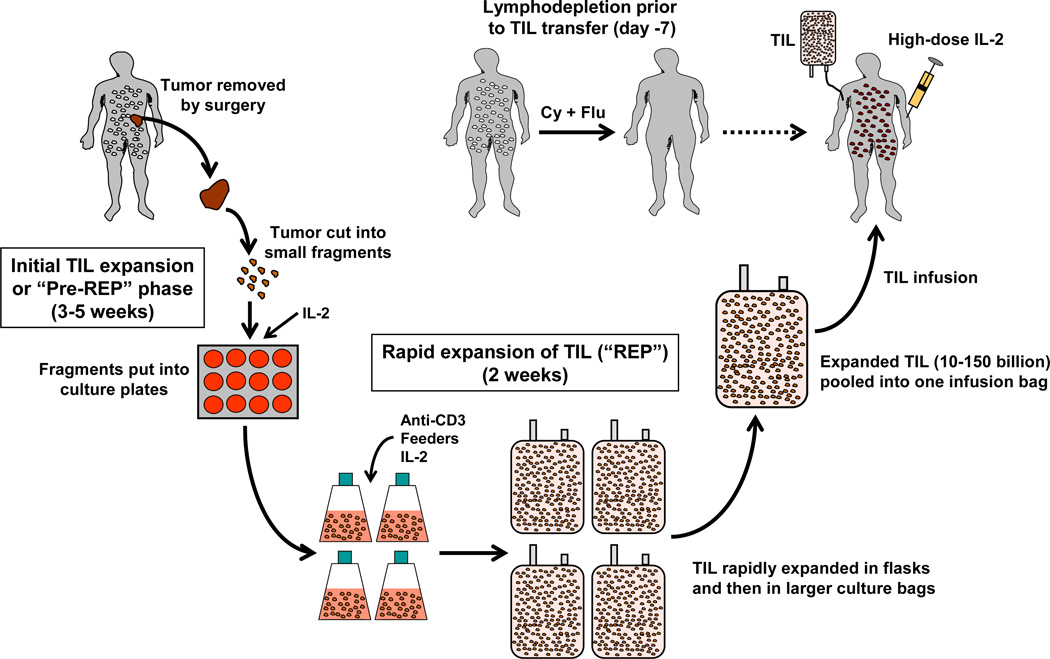
The steps in TIL expansion and treatment are summarized in Figure 1. Expansion of TIL ex vivo for ACT is done in two stages. The first stage involves the initial expansion of TIL from the tumor fragments or digested tumor cell suspensions over a 5-week period of time in culture medium with IL-2 (52–54). Medium exchanges with fresh IL-2 are performed regularly to ensure continued T-cell division and survival during this time. This first stage yields a product (“pre-REP” TIL) that is then used generate a final TIL infusion product following a “rapid expansion protocol” (REP) (52, 55). These pre-REP TIL can be cryopreserved at this stage in the process for later secondary expansion in the REP and patient treatment, or can be used immediately. The REP involves activating the TIL through the CD3 complex using anti-CD3 mAb in the presence of a 200:1 ratio of irradiated (5,000 cGy) PBMC feeder cells obtained from the patient (autologous feeders), or more often from normal healthy donors (allogeneic feeders). Two days after initiation of the REP 6,000 U/ml IL-2 (final concentration) is added to drive rapid cell division in the activated TIL (52, 56, 57). The TIL are then expanded for another 12 days and diluted as needed with 1:1 culture medium with more IL-2 (52, 56, 57).
Figure 1. Schematic representation of the process of TIL expansion and TIL therapy for metastatic melanoma starting from tumor fragments.
Suitable tumors from eligible stage IIIc-IV patients are resected and taken to the lab under sterile conditions where they are cut up into small 3–5 mm2 fragments and placed in culture plates or small culture flasks with growth medium and high-dose (HD) IL-2. The TIL are initially expanded for 3–5 weeks during this “pre-rapid expansion protocol” (pre-REP) phase to at least 50 × 106 cells. The cells are then subjected to a rapid expansion protocol (REP) over two weeks by stimulating the T cells using anti-CD3 in the presence of PBMC feeders cells and IL-2. The expanded TIL (now billions of cells) are washed, pooled, and infused into the patient followed by one or two cycles of HD IL-2 therapy. Before TIL transfer, the patient is treated with a preparative regimen using cyclophosphamide (Cy) and fludaribine (Flu) that transiently depletes host lymphocytes “making room” for the infused TIL and removing cytokine sinks and regulatory T cells in order to facilitate TIL persistence.
A typical REP results in 1,000-fold to 2,000 fold expansion of TIL during the 14-day culture period. The TIL are harvested, concentrated, and infused intravenously into the patient followed by further treatment with high-dose IL-2 to drive further TIL survival and expansion in vivo (52, 56, 57). Prior to infusing the TIL back into the patient, the patient is lymphodepleted using a cocktail of drugs to deplete all T and B cells. IL-2 is systemically administered in order to help support the transferred TIL following lymphodepletion and TIL infusion (5, 58, 59). Treatment with IL-2 after TIL infusion supports the persistence of the infused TIL in vivo which is then critical for the short-term survival of the TIL and inducing tumor regression (60–62).
A critical aspect that has greatly contributed to the success of ACT using autologous TIL is the ability to lymphodeplete the host prior to infusing the T cells. Metastatic melanoma patients were initially treated with TIL and IL-2 without lymphodepletion, resulting in a response rate of 39% (8, 63–65). However, a series of seminal Phase II clinical trials in humans conducted by Dudley et al. at the National Cancer Institute (NCI; Bethesda, MD, USA) in 2002 utilized a preparative lymphodepleting chemotherapy regimen using cyclophosphamide and fludarabine in melanoma patients prior to infusing the TIL and IL-2 resulting in an unprecedented 50% response rate (8, 63, 64, 66, 67). Lymphodepletion prior to TIL infusion resulted in improved TIL persistence, elimination of lymphocytes that could compete with the infused TIL for homeostatic cytokines, such as Interleukin-7 (IL-7) and Interleukin-15 (IL-15) (8, 63, 64, 66, 67). Lymphodepletion also eliminates endogenous CD4+Foxp3+ T regulatory cells (Tregs) that can inhibit effector T-cell function and the continued proliferation of infused TIL in vivo after adoptive transfer (68–70). Although utilizing lymphodepletion with cyclophosphamide and fludaribine for melanoma patients treated with ACT and TIL has resulted in impressive clinical responses, an issue that may occur is de novo T-cell recovery, particularly the re-appearance of Tregs after lymphodepletion that could interfere with anti-tumor activity of the transferred TIL. In order to address this, total-body irradiation (TBI) plus cyclophosphamide and fludaribine lymphodepletion has been tested (20, 63). Two TBI plus chemotherapy preparative regimens for TIL have been tested in the clinic at the NCI; one involving TBI of 2 Gy, and the other using a more intensive TBI of 12 Gy (8, 20, 57, 63, 71). The results demonstrated a significant enhancement of objective clinical response rate to 72% with the 12 Gy TBI plus chemotherapy preparative regimen together with a striking 40% rate of complete response (8, 20, 57, 63, 71). Recently, the NCI group summarized their 10-year experience with Phase II TIL therapy trials using different preparative regimens. Overall, these clinical trials have achieved a response rate of 51%, and 13% rate of durable and complete regression continuing past 5 years (20, 25).
These positive results have spawned a number of additional centers to develop TIL therapy programs. Different clinical centers are now utilizing TIL therapy to treat metastatic melanoma patients and exhibiting promising results. At MD Anderson Cancer Center (Houston, TX, USA), we are currently conducting a phase II trial of TIL therapy in metastatic melanoma patients that have failed first-line and second-line therapies (9). We have treated over 40 patients and have also achieved a 47% overall response rate (9). These responses have been durable with the majority of responding patients having relapse-free survival over 12 months. The Sheba Cancer Center (Jerusalem, Israel) has demonstrated an overall response rate of 50% using TIL therapy in their clinical trials (7, 72). Overall, these impressive response rates have demonstrated that TIL therapy is a very powerful regimen to treat metastatic melanoma patients*. The next step for TIL therapy for melanoma would be to conduct a randomized Phase III trial and finally seek FDA-approval as licensed standard-of-care therapy in select patients.
One critical point to make at this point is that all TIL adoptive cell therapy clinical trials performed up until this point have not been based on an “intent-to-treat” scenario in which all patients who undergo tumor resection for TIL growth are considered in determining overall response rates, regardless of whether TIL expansion is successful or not. So far, only patients actually receiving TIL therapy have been counted. This has been an acceptable approach, however, some oncologists are still asking for an evaluation of the efficacy of TIL therapy in melanoma using an intent-to-treat scenario based on TIL expansion success rates being factored into the analysis. This sounds reasonable, but on closer analysis, it is not straight-forward and may in fact yield erroneous interpretations due to a number of factors. The main issue is how we define what successful TIL expansion is in the first place.
A survey of different centers that have expanded TIL both pre-clinically and for clinical applications has found that different criteria have been used to decide what defines a successful pre-REP growth phase (see Fig. 1) for taking the process to the full-scale cell expansion in the REP for patient therapy (9, 72, 74–76). Although the exact cut-offs have varied, in most cases at least 50 × 106 cells are needed after the pre-REP stage to initiate a sufficient number of cultures for the REP stage to yield a final cell number in the range of 10–150 billion for infusion. Using this criterion, the historical overall success rates for initial TIL expansion from cultured tumor fragments have been between 60–70% (8, 52, 75). We have used this 50 × 106 minimal cell threshold as well in the TIL therapy trials at MD Anderson Cancer Center in order to make a positive decision to proceed with further large-scale TIL expansion using the REP for each patient (9). We recently, reported a pre-REP TIL expansion rate of 63% using this cut-off (77).
TIL expansion methods have improved in the past few years and success rates for initial TIL out-growth from tumor fragments have improved recently to >75%. In addition, as discussed later on, newer methods to initially expand TIL from enzymatic digests of tumors yielding cell suspensions have further improved the initial pre-REP TIL expansion success rates to >80% (7, 74, 75). Thus, it should be possible to attempt clinical trials using an intent-to-treat approach at calculating clinical response in the near future.
TIL therapy for melanoma and T-cell biology: Defining and optimizing active T-cell subsets
What subset of T cells mediating tumor regression?
The findings from the melanoma ACT trials by Besser et al. (7) as well as the one at the MD Anderson Cancer Center (9) have revealed a positive correlation between a higher number of infused CD8+ TIL and positive clinical response in a majority of Stage IIIc/IV metastatic melanoma patients. The clinical data from NCI, however, was less conclusive (8, 63, 75). CD8+ CTL are critical effector cells in anti-tumor responses, and are known to mediate the antigen-specific cytolysis of tumor cells through the action of granzymes (e.g., granzyme B), that are serine proteases that cleave at aspartate residues in the substrate, and perforin (78, 79). Perforin forms pores in the plasma membrane of target cells, which allows granzymes to enter the target cell which activate caspases (e.g., caspase 3) and eventually lead to apoptosis in the target tumor cells (78). The Fas-Fas ligand interaction may also be important for the tumor cell killing (80), although the extent this pathway contributes to tumor killing is not known. Although CD8+ T cells are merging to be the dominant cell type active in TIL adoptive cell therapy, CD4+ T cells can not simply be dismissed. We and others have consistently found that a minority of patients that have a CD4+ T-cell dominated TIL product for adoptive transfer (about 8–10%) nevertheless undergo dramatic clinical responses and even complete remission of all their tumors (81, 82) (Radvanyi et al., unpublished data). However, in the majority of cases, a CD4+ T-cell dominated TIL product is a harbinger of bad news. Thus, a critical question that has emerged is what is special about the CD4+ T cells in the TIL in these few responding patients? CD4+ T cells are known to play a central role in orchestrating many elements of the adaptive immunity (83). They participate not only priming CD8+ T cells but can also induce tumor eradication by a number of direct and indirect mechanisms through direct killing of MHC class II+ tumor cells, the activation dendritic cells cross-presenting TAA, the activation of tumor cytotoxic macrophages and eosinophil, and supporting the survival of transferred CD8+ CTL via lymphokines (84). CD4+ T cells in some healthy donors have been reported to also express cytolytic molecules typically associated with CD8+ cytotoxic T lymphocyte (CTL), such as granzyme B and perforin, upon chronic viral infections (85). Recent data suggest that indeed a possible pathway of differentiation of a subset of CD4+ T-cells into highly tumor cytolytic cells expressing the transcription factor Eomesodermin (Eomes) that drives granzyme and perforin expression (10). It is possible that such a subset of “cytotoxic” CD4+ T cells may exist in the TIL of some patients (86). Moreover, costimulation through CD134 and CD137 may help drive the expansion of these Eomes+ “killer” CD4+ T cells against tumor targets (86).
Although CD8+ and CD4+ αβ TCR+ T cells are the dominant types of lymphocytes in expanded TIL used for therapy, other minor sub-populations of lymphocytes are present to varying degrees, including NK (CD8+CD56+ and CD16+ cells) and innate-type T cells expressing the γδ TCR (87–90). In fact, NK cells usually expand along with T cells with IL-2 alone during the initial stages of TIL expansion, but are then mostly lost during the REP due to the conditions used that greatly favor T-cell activation (anti-CD3 stimulation). It would be interesting to develop improved methods for NK cell expansion from TIL and test whether NK cell-enriched cell therapy products would have some activity in melanoma or can be used as synergistic cell therapy products with T-cell-enriched TIL products. A similar question exists for the role of γδ T cells, as these cells can be powerful cytolytic cells against tumors recognizing MHC-unrestricted cell surface entities such as phosphoantigens and NK cell receptor ligands such as MICA and MICB (91).
Relationship between CD8+ T cell differentiation and clinical response
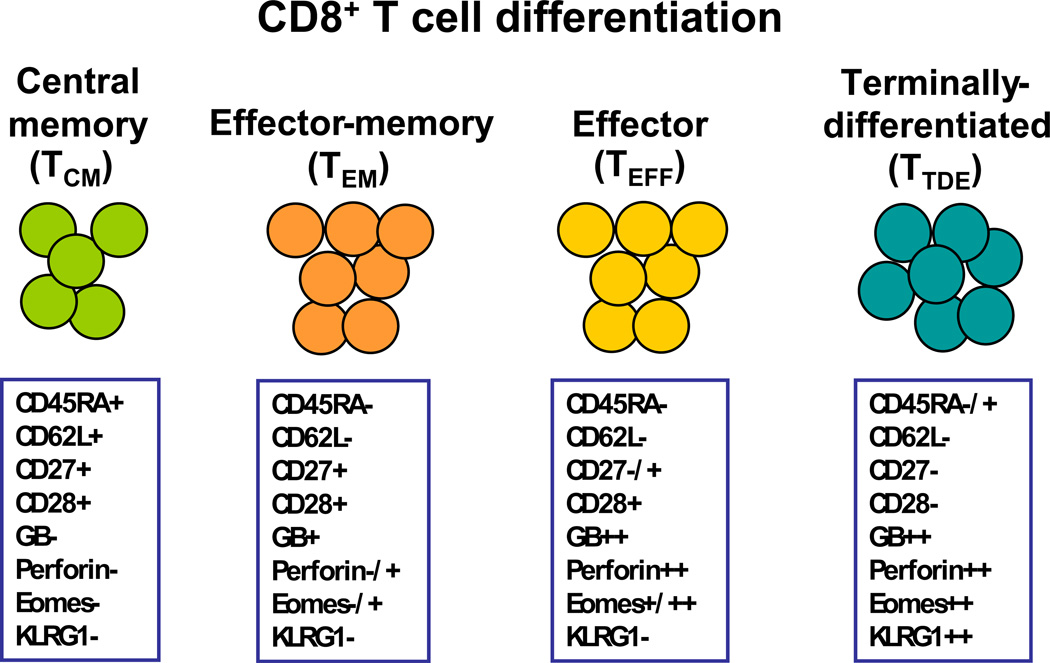
CD8+ T cells have been the subject of the most intense research in melanoma ACT. A matter of controversy has been which differentiation stage of CD8+ T cells contributes more to the immediate tumor killing as well as the long term control of tumor growth. Figure 2 summarizes the key cell surface and intracellular phenotypic markers of the different stages of CD8+ T-cell differentiation found in melanoma TIL (92–94). The majority of melanoma CD8+ T-cells in TIL are less-differentiated effector-memory (TEM) and more-differentiated CD8+ effector (TEFF) cells and terminally-differentiated effector cells (TTDE). CD8+ TEM cells are capable of proliferation, have longer telomere length, and have some capacity for self-renewal (92–94). However, they have a limited capacity as cytolytic effector cells, as they express granzymes, but little or no perforin and other critical killing proteins, such as granulysin (95). On the other hand, more differentiated CD8+ effector (TEFF) cells and terminally-differentiated CD8+ CTL (TTDE), which express much higher amounts of granzymes and perforin, have been reported as the most potent cells for overcoming CTL resistance mechanisms and inducing effective cytotoxic killing of tumor cells (96–99). However, these CD8+ T cells have shorter telomeres and less T-cell costimulatory molecules causing them to be less able to undergo cell division following TCR stimulation than TEM cells (100). They are also more sensitive to activation-induced cell death (AICD) and cannot traffic to lymph nodes and it is doubtful that they mediate long-term memory response against tumors during ACT. Only very few central memory CD8+ T-cells (TCM) are found TIL, especially after the extensive expansion used to generate TIL infusion products. CD8+ TCM can traffic to lymph nodes, where they may encounter activated antigen-presenting cells (e.g., dendritic cells) presenting TAA either derived from vaccination with TAA or from released de novo from tumors (101). Melanoma TAA-specific TCM have been shown in mouse models to be superior T cells for ACT, it must noted this occurs only when a concurrent cancer vaccine with the specific TAA is given following cell transfer (101). Thus, TCM may not be useful for ACT without concurrent vaccination with TAA since they have limited tissue/tumor homing ability and no anti-tumor effector activity unless re-stimulated by mature APC.
Figure 2. Stages of CD8+ T-cell differentiation found in melanoma CD8+ T-cell in TIL and phenotypic markers assessed by flow cytometry used to delineate these stages.
CD8+ T cells in TIL can be found having markers of central memory (TCM), effector-memory (TEM), effector (TEFF) or terminally-differentiated effector (TTDE) stages. These stages and markers are based on previous work on anti-viral CD8+ T-cell response and the states of CTL differentiation associated with the control of both acute and chronic viral infections in humans. These markers are only a guide to identify these functional memory or effector stages and may not always fit into these defined categories. In addition, the expression of these subset markers may be transiently down-modulated, permanently lost, or re-expressed under different cytokine or in different tissue niches in vivo. Nevertheless, understanding the changes in these effector and memory markers in TIL is very useful in biomarker studies to identify clinically active T-cell subsets. Melanoma TIL expanded for therapy are a mixture of mostly TEM, TEFF, TTDE (that have lost CD28 expression). Relatively very few TCM are found (<5%). Initially, NK cells are found in initial TIL expansions (pre-REP), but these are lost after the REP which selectively activates the T cells. Abbreviations: GB, granzyme B; Perf, perforin.
Currently, relatively little is known on what states of CD8+ T-cell differentiation in transferred TIL are optimal in ACT for melanoma. One of the confounding problems in the field is that the markers used to define TEM and TEFF and TTDE in vitro after long-term expansion or ex vivo after short-term culture may not reflect the true memory and proliferative capacity of the cells in vivo and, moreover, these markers can be re-expressed or lost under different cytokine conditions in context of TCR re-stimulation (81, 102). Nevertheless, these current differentiation/memory markers have been used in an attempt to gain an understanding into this question, but the data has been inconsistent and confusing. For example, clinical trials at the NCI have suggested that less-differentiated TEM cells expressing CD27 are associated with clinical response to TIL therapy (20, 102). These studies however only show a positive correlation of this parameter when expressed as total CD8+CD27+ infused, but not as a percentage of the CD8+ T cells infused (20, 102). Thus, higher numbers of CD8+ T cells infused may account for this correlation. In addition, earlier data from TIL-treated patients associated TIL persistence in vivo with clinical response and the CD8+ T cells found to correlate with this longer persistence expressed CD28 and not CD27 (61, 103, 104). This is inconsistent with the role of CD27+ cells mediating the response. To confuse matters more, in an ongoing TIL therapy clinical trial at MD Anderson Cancer Center in which we have treated over 40 patients, we have found that more differentiated CD8+CD27− T cells (TEFF, as shown in Figure 2) were highly correlated with objective clinical response to TIL, while the correlation with CD8+ TEM did not reach statistical significance (9) (Radvanyi et al., unpublished data). These contrasting results indicate that further studies with the existing markers as well as newer differentiation markers (such as differential chemokine receptor expression) (105), together with functional studies, and comprehensive tracking of the original TEM, TEFF, and TCM clonotypes using TCR Vβ CDR3 region sequencing in adoptively transferred TIL persisting in vivo will be needed to resolve this issue.
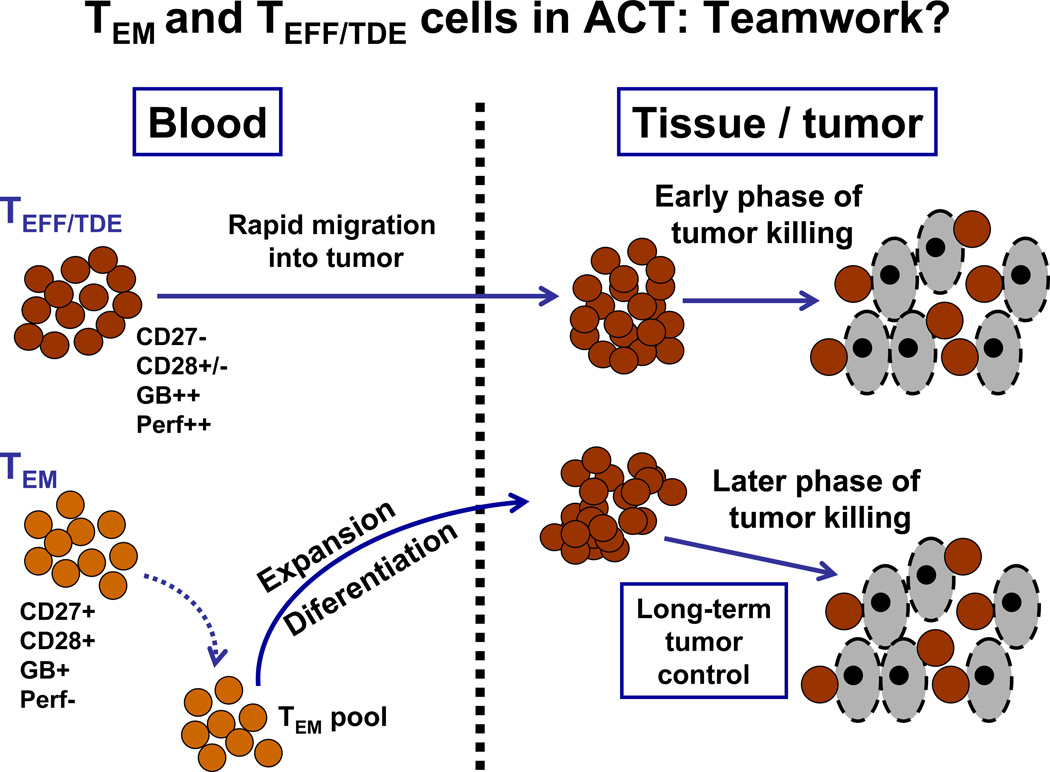
An alternative approach in thinking of this problem about what states of CD8+ T-cell differentiation are critical is a non-mutually exclusive view that hypothesizes that a cooperation between different CD8+ memory and effector subsets, as defined in Figure 2, are needed to reach the full potential of TIL therapy for melanoma. Thus, one way to reconcile the data so far on CD8+ T-cell states in TIL related to clinical response is that cooperation between TEM and TEFF CD8+ TIL is needed for maximal and durable tumor control. According to this view, TEFF, which highly express granzymes and perforin, yet are shorter-lived, provide immediate control of tumor growth, while “younger” and less-differentiated TEM cells, having the capacity to divide and replenish a pool of TEFF after adoptive transfer, maintain more long-term tumor control. This model, illustrated in Figure 3, opens up a new conceptual framework for TIL therapy that, if proven correct, suggests that adoptively transferring different types of TIL products enriched in CD8+ T cells with appropriate effector and memory phenotype at different times into patients may be a new and exciting approach for melanoma ACT.
Figure 3. Model for how CD8+ T-cells at different stages of CTL differentiation can synergize or cooperate in controlling tumors following TIL adoptive transfer into patients.
The model reconciles a number of contrasting results on phenotypic analysis on TIL subsets and their correlation with clinical response from a number of groups. It proposes that both TEM and TEFF and TTDE may all ultimately play a critical role in adoptive cell therapy at different times to control tumor growth. The TIL need to become cytolytic effector cells to kill tumor cells requiring differentiation into TEFF and TTDE cells. However, TEM cell are long-lived, but have little cytolytic potential. The TEFF and TTDE (more oligoclonal) may rapidly home into tumors and orchestrate an early wave of tumor killing. These cells are relatively short-lived and can be replaced by TEM (more polyclonal) that have a higher intrinsic proliferative capacity and can differentiate later into TEFF and TTDE and mediate later waves of tumor killing. In addition to TEM and TEFF/TTDE cells, endogenous T cells re-emerging in the patients after recovery from the transient lymphodepletion may also play a role at these later times by recognizing tumor antigens initially released from earlier waves of tumor killing. Overall, this model explains data on TCR clonotype analysis during TIL therapy where different clonotypes that predominate in the infused decrease and other less frequent clonotypes increase, while other clonotypes are lost altogether to be replaced by TCR clonotypes later on that were not detected in the infused TIL (Radvanyi et al., unpublished data).
Consequences of TIL expansion needed for therapy: Loss of critical costimulatory molecules and loss of proliferative capacity
As mentioned in the above discussion, one of the key problems that may limit tumor regression and long-term durable clinical responses in ACT is the persistence of TIL following infusion (61, 104). Long-term TIL persistence has been correlated with accumulation in vivo of T cells from the infused TIL population having a TEM phenotype, characterized by the expression (or re-expression) of CD27 and CD28 (61). However, the factors regulating or facilitating TIL survival and persistence are not known. The current approach for ACT in melanoma involves a secondary expansion step in which TIL numbers are increased by 1000- to 3000-fold a REP (19, 54) (see also Figure 1). The REP not only expands TIL (up to 100 billion or more), but also drives further T cell differentiation and phenotypic changes that can affect the survival and proliferative capacity of T cells in vivo shortly after infusion (26, 54). This extensive cell division process, together with the types of cytokines (IL-2) and signaling pathways used to drive proliferation also largely determines the states of CD8+ and CD4+ T-cell differentiation (TEM, TEFF, TTDE, and TCM) found at the end of the process.
We previously reported that post-REP CD8+ TIL were hyporesponsive to melanoma antigen re-stimulation, as shown by resistance to proliferation and increased apoptosis (54). We found that this hypo-responsiveness was associated with a profound loss of CD28 in many in post-REP CD8+ T cells relative to the pre-REP TIL, while CD27 levels were unaffected (53, 54). A permanent loss of CD28 in CD8+ T cells in humans is a key factor leading to T-cell senescence during aging (106–109). Thus, it is possible that many of the TAA-specific CD8+ T cells are driven to senescence during the REP. In support of this concept, we found that in the MART-1/Melan-A-specific CD8+ T-cell population in post-REP TIL, only the few remaining CD28+ T cells retained proliferative capacity in response to MART-1/Melan-A peptide re-stimulation (54). This is in line with data from ACT patients showing that CD28+ T cells in the adoptively-transferred TIL had longer persistence T cells (81). We speculate that the reason for the loss of CD28 on a majority of CD8+ TIL is the continued exposure of the T-cells TIL to IL-2, a cytokine that is known to simultaneously promote T-cell proliferation and differentiation (110). In addition, pro-inflammatory cytokines, such as TNF-α, that are induced during the REP induce a loss of CD28 gene expression (111). Another consequence from the repeated cell division and TCR stimulation occurring during TIL expansion is the chronic up-regulation of negative T-cell costimulatory molecules, such as Programmed Death-1 (PD-1) (112). PD-1 signaling inhibits T-cell activation and proliferation, and can facilitate T-cell apoptosis (113). The role of PD-1 signaling during TIL therapy for melanoma, and whether expression of PD-1 on CD8+ TIL makes any difference however still needs to be investigated, especially since the frequency of TAA-specific T cells may be higher in the CD8+PD-1+ subset (114).
Can TIL expansion methods be improved to yield a better balance of effector and memory T cells?
The induction of terminally-differentiated T-cell phenotyes, coupled with the loss of proliferative capacity, during current TIL expansion protocols has caused us to re-think the way we grow TIL by developing approaches that generate T-cells with the right balance or effector and memory properties to improve therapeutic efficacy. There is a need to develop new culture and T-cell selection conditions that allow for attributes of memory T-cells for persistence (TEM/TCM characteristics with CD28 and CD27 expression) together with cytotoxic T-cell capacity or the ability to readily differentiate into more active cytolytic cells.
“Young TIL”
Recently, one approach that has been developed in an effort to speed up the process and avoid terminal differentiation of T cells is to limit the time TIL are initially expanded from excised tumors with high-dose IL-2. Usually it takes between 4 to 5 weeks to expand enough TIL from tumor fragments (about 50 million) with IL-2 to proceed with the next step of culture which is the REP generating the final TIL infusion product (26, 56). The new approach, called “young TIL”, reduces this initial expansion period in IL-2 down to a few weeks before the cells are subjected to the REP (72, 74, 115). In the “young TIL” protocol, TIL are immediately isolated by enzymatic digestion of tumors and expanded rather than having to wait for them to migrate out of tumor fragments in culture. This results in more TIL being immediately accessible for expansion and a shorter time needed to reach minimal numbers needed for the REP as well as higher success rates (>80%) in reaching these minimal cell numbers needed from accrued patients (7, 72, 74). The “minimally-cultured” TIL have been reported to have longer telomeres and express higher levels of CD27 and CD28 compare to TIL expanded from fragments (115). Clinical trial results with this “young TIL” approach have been promising, but clinical response rates (around 50%) have not improved over the previous longer-term culture methods (7, 72, 74). Thus, additional factors seem to be involved that need to be addressed to further improve response rates.
Alternative cytokines for TIL expansion besides IL-2
In order for the ACT to achieve its highest possible efficacy against melanoma, we believe new approaches must both maintain the TEM features of CD8+ TIL as well as expand more differentiated cytolytic TEFF cells that express high levels of granzymes and perforin, as depicted in our model in Figure 3. Another approach to reaching this optimal balance between TEM and TEFF is the use of alternative cytokine combinations without IL-2 or with lower levels of IL-2 to drive TIL expansion. In this regard, a number of cytokines, such as IL-15 and IL-21 have been found to drive CD8+ T-cell proliferation and effector function, yet better maintain their memory properties (54, 116, 117). This is based on work in murine models showing that these cytokines helped maintain CD28 expression and prevented the premature terminal differentiation (TTDE phenotype in Fig. 2) of T cells after antigenic stimulation of CD8+ T cells in vivo (110). We and others have reported a synergy between IL-21 and IL-15, where IL-15 promotes TIL proliferation while IL-21 maintains the memory features of CD8+ TIL by promoting continued CD28 expression (54, 117, 118). Therefore, rapid expansion of TIL using IL-15 together with IL-21 may be one new approach to create an optimal TIL product.
Other approaches suggested by others include using TGF-β which has been shown to augment the expansion of CD8+ T cells during the REP and may preserve memory functioning (119) or activators of Wnt/β-catenin signaling pathway (e.g, Wnt-3A, or pharmacologic inhibitor of glycogen synthase kinase-3β (GSK-3β), such as lithium chloride) to arrest CD8+ differentiation during the REP (120, 121). IL-21 was also reported to have Wnt-like effect in maintaining memory features of CD8+ T cells (116, 122). Although TGF-β has a beneficial effect of improve CD8/CD4 ratio in the final REP product, the major concern with it is that it is a potently immunosuppressive cytokine that also suppresses granzyme and perforin expression in CD8+ T cells (123, 124). Although the Wnt pathway agonists enhance the memory features and survival mechanisms of the CD8+ T cells from peripheral blood and tumor environment, they unfortunately do not facilitate T-cell expansion to the same extent as seen in the conventional IL-2 based REP (Forget, M.A., et al., unpublished data). It is possible that fewer cells with more TCM or long-term surviving TEM can be active, but in context of a patient with rapidly progressing tumors and high tumor burden receiving TIL, high numbers of more differentiated TEFF and TDE cells would also be needed. One idea to get out of these conundrums is to generate “split” cell products using different protocols for rapid expansion from the TIL initially expanded from tumors. For example, one set of TIL would undergo rapid expansion (REP) using the traditional IL-2-based protocol used currently (Figure 1), while the other part of the original TIL culture would be expanded with Wnt pathway agonist, Wnt-3A (120) or with IL-15 plus IL-21 (54) or other factors that enhance memory T cell expansion without inducing effector cell differentiation. At the end of expansion, the TIL product from those two REP conditions would be pooled together and infused into the same patient, who would then receive sufficient number of CD8+ TIL that have both TEM and TEFF populations, based on our model of cooperation between these two subsets in short-term and long-term control of tumor growth.
Furthermore, manipulating critical costimulatory pathways, especially through TNF-receptor (TNF-R) family members that operate largely on TEM, TEFF, and TTDE populations may be an additional mechanism to generating improved TIL products for infusion. One potential pathway to target in this respect is CD137, highly induced on CD8+ T cells after TCR stimulation (53, 125). Moreover, CD137 can still be induced even in CD8+ TIL that have lost CD28 expression after TCR stimulation and cab mediate potent costimulatory signals in these cells protecting them from AICD (53, 126). Enhancing costimulation through CD137 during TIL expansion, especially during the REP may be beneficial. This can be done by providing agonistic monoclonal antibodies to the culture or using APC that express the ligand for CD137. Indeed, we have found that addition of a fully human agonistic anti-CD137 monoclonal antibody at the beginning of the REP preserved CD28 expression while enhancing granzyme B and perforin expression in CD8+ TIL (53). Additional costimulation via CD134/OX40 may also be beneficial based on provocative work in mouse models showing that synergistic activation of CD137 and CD134 promoted the accumulation of “super-effector” CD8+ T cells expressing highly cytolytic features yet expressed the IL-7 receptor and were capable of long-term survival in response to IL-7 (127). The effects of these TNF-R family members on preserving telomere length in TIL will should also be determined, given the demonstrated role of these pathways in inducing telomerase in activated T cells (128). Thus, it will be of immense interest for the ACT field to manipulate these TNF-R pathway members to generate more highly cytotoxic anti-tumor T cells that can also persist long-term in patients by maintaining CD28 and turning on homeostatic cytokine signaling pathways such as IL-7.
Artificial antigen-presenting cells (aAPC) to provide TCR stimulation with the right balance of costimulation and cytokines for TIL expansion
Another way to improve TIL culture and generate more favorable T-cell phenotypes for transfer currently being investigated is the use of aAPC expressing defined costimulatory and cytokine molecules. These aAPC are also a good alternative to the “feeder problem” in TIL expansion, where high numbers of either autologous or allogeneic normal donor PBMC (at least a 100 to 1 ratio of PBMC to TIL) are needed to facilitate TIL expansion using anti-CD3 and IL-2 in the REP (52). These feeder products require patient or normal donor leukopheresis and pooling of more than one product to ensure that an adequate numbers of PBMC with optimal activity are obtained. This not only has logistical problems, but is also expensive.
Currently, the most popular aAPC is based on the human K562 erythroleukemia cell line that by itself lacks endogenous MHC class I (HLA-A and HLA-B) expression (34, 129). It has been classically used as an NK cell target in assays, but due to the robustness of these cells in culture and the ease with which they can be genetically manipulated while maintaining a stable phenotype in culture, K562 cells have been adopted as viable alternative to PBMC feeders and other APC, such as EBV-transduced B cells, for T-cell activation and expansion protocols. Different forms of the K562 aAPC have now been engineered that stably express co-stimulatory molecules, such as CD86, CD137L or CD83 (34, 129–131). Clinical grade modified K562 have already been generated and used to expand MART-1 specific CD8+ T cells in a recent ACT clinical trial that showed some reasonable degree of efficacy (35, 36). The CD8 T cells showed long term persistence and memory phenotype after transfer (35, 36). Modified K562 have also been used to generate long lived multiple TAA-specific CD4+ T cells (132, 133). The use of K562 for initial growth of melanoma TIL (pre-REP) was also recently exploited and was reported to skewed TIL phenotype to a lesser differentiated state, with expression of CD27, CD28 and CD62L (134, 135). K562 aAPC expressing CD64 (high-affinity Fc receptor), CD86, and CD137 ligand (4-1BB-L) have been tested recently the TIL REP protocol. However, they did not quite succeed in generating an equivalent fold expansion as the traditional REP with PBMC feeder cells (134). This could represent a challenge in the context of expanding enough TIL for transfer suggesting that improved methods using aAPC or different forms of aAPC may be needed to maximize TIL expansion. One approach that is being investigated is to mix K562 aAPC with smaller numbers of PBMC feeder cells in the REP thereby reducing the need for these expensive PBMC feeder products. One clear advantage that aAPC may have however, especially given the provision of potent CD137 costimulation through the expression of CD137-L, is that they have been found to favor the activation and expansion CD8+ T cells in TIL that has been correlated with objective clinical responses (9, 53, 74). The K562 aAPC can also be engineered to express other costimulatory molecules and either secreted or membrane-bound (cell surface) cytokines, such as IL-15, IL-7, IL-12, and IL-21, to provide an optimal blend of costimulaory and cytokine signals for TIL expansion. These results open the path towards exploiting aAPC as a replacement for feeder and as tools to manipulate TIL phenotype.
Other improvements to TIL therapy: Combination therapies and genetically-modified TIL to improve persistence and anti-tumor activity in vivo
Combination therapies/immunomodulators
Although ACT using autologous tumor infiltrating lymphocytes (TIL) has proven itself as one of the most efficacious treatments for metastatic melanoma to date (20), the current regimens have reached a plateau in terms of clinical response rates (about 50% with the cyclophosphamide plus fludaribine) (20). Although a combination of TBI together cyclophosphamide plus fludaribine pre-conditioning regimen as a pre-conditioning regimen has increased response rates further (20, 63), this approach is too toxic and requires a stem cell back-up each time precluding its widespread application. Alternatively, it is still possible to further increase clinical response rate of ACT by combining it with other types of available therapies for metastatic melanoma. One potential avenue is in combining TIL therapy with B-RAF inhibitors, such as the recently FDA-approved drug Vemurafenib (Zelboraf™) (136, 137). The rationale behind such combination lies in the idea that as melanoma cells experience apoptosis associated with inactivation of an oncogene such as mutated V600E B-RAF, they release tumor-associated antigens, such as tissue differentiation antigens, cancer/testis antigens, or products of mutated genes expressed by the transformed cells (14). Other potentially immune activating signals released by apoptotic tumor cells include calreticulin, the S100 family of proteins, and the high mobility group box 1 protein (HMGB1), which are released by tumor cells undergoing secondary necrosis (12, 17). The consequent activation of APC and antigen presentation could further stimulate adoptively transferred TIL. Thus, the administration of adoptively transferred TIL in combination with BRAF inhibitor will likely synergistically enhance the efficacies of both immunotherapy and oncogene-targeted therapy. Importantly, we and others have found that Vemurafenib showed no adverse impact on T-cell function in vitro and in vivo (14) (Hong et al., unpublished data). However, the combination of TIL therapy with MEK inhibitors may not be optimal as others have found that such targeted agents negatively impact T-cell functions (14). As targeted therapy for melanoma does not usually lead to a durable clinical response due to the emergence of drug-resistant clones, it remains an exciting possibility whether combining it with TIL therapy will ameliorate such shortcomings by inducing a long-term, polyclonal T-cell response against tumors via antigen spreading. Based on the pioneering work of Zitvogel et al. on “immunogenic” chemotherapy with agents such as oxaloplatin, in which tumor immunogenicity is enhanced following chemotherapy-induced apoptosis or necrosis of tumor cells (12), there is now also a strong rationale for combining TIL therapy with these select chemotherapeutic against, although the effect of a specific chemotherapeutic agent on T-cell functions will need to be determined before attempting such combinations.
Enhancing TIL function in vivo
Another avenue to improving TIL therapy for melanoma is by directly enhancing TIL function in vivo. Combining TIL therapy with blocking antibodies in vivo against negative costimulatory molecules, such as PD-1 (138) that can be highly expressed on TAA-specific T-cells, represents a promising approach. One of the ligands for PD-1, B7-H1, is frequently expressed on melanoma cells, resulting in tumor-induced immune suppression that has been correlated to poor clinical outcome (113, 139–141). PD-1 is highly expressed on endogenous melanoma antigen-specific T cells, can be found on T cells induced by melanoma peptide vaccination, and is also found at high levels on TIL from melanoma patients (141). A blocking antibody against PD-1, MDX-1106 (Bristol Myers Squibb) is a fully humanized IgG4 antibody, and has been tested in a phase I dose escalation trial of 39 patients with solid tumors, including those with metastatic melanoma (10, 141). A high clinical response rate with little toxicity was found; this has now led to ongoing phase II clinical trials as a single agent (10). However, with TIL therapy showing a high rate of effectiveness, and the constitutive expression of PD-1 on many TAA-reactive TIL, especially CD8+ T cells, a combination therapy of TIL plus anti-PD-1 may be synergistic. In this regard, we have found that anti-PD-1 blockade in vitro significantly enhanced the proliferation of melanoma tumor antigen-specific CD8+ TIL when re-stimulated with their cognate antigen (Radvanyi et al., unpublished data). Combination therapy with anti-CTLA-4 (ipilimumab) may also be option. In contrast, augmenting positive T-cell costimulatory pathways, especially through TNF-R family members, such as CD137 and CD134 using agonistic antibodies or fusion proteins of their ligands, is another approach. These agents can be infused into patients following TIL adoptive transfer, or can also be used in vitro during TIL expansion to augment costimulatory pathways and generate T cells with improved effector and memory properties. CD137 costimulation especially has emerged to be a powerful costimulatory pathway in maintaining the survival of transferred T cells in vivo (53, 126). For example, in recent landmark clinical trial using CAR-transduced T cells recognizing CD19 in CLL patients, inclusion of a CD137 signaling endo-domain was found to be critical in maintaining T-cell persistence and anti-tumor cell activity (23).
Genetic modification of TIL by viral transduction
Improving TIL migration into tumors
One of the most important aspects of immunotherapy is whether TAA-specific T cells actually migrate into the tumor site. Early studies of TIL labeled with Indium-111 found that many intravenously infused T cells can indeed traffic to tumor sites in the majority of patients (81%), but this represented only a fraction of the infused cells (142, 143). Thus, getting more of the infused cells to home to tumor sites is still a critical problem. One possible way to solve this issue is to make use of T-cell chemotactic molecules (chemokines) produced by tumor cells and tumor-associated stromal cells to enhance TIL migration into the tumor site. For example, the chemokine CXCL1 is produced by a large proportion of melanomas, but its receptor, CXCR2, is not usually expressed by T cells (144). Our group has investigated the possibility of exploiting this as a way of improving T-cell homing into melanoma tumors. In a murine melanoma tumor model, adoptively transferred TCR transgenic T cells recognizing gp100 (pmel T cells) exhibited a much higher level of migration towards CXCL1-expressing gp100+ tumor cells both in vitro and into tumors in vivo after genetically modifying them to express CXCR2 constitutively (145). We are currently in the process of testing this new approach in an upcoming TIL therapy trial clinical trial at MD Anderson Cancer Center. Effector T cells generally express lower levels of different tissue/tumor-homing chemokine receptors, such as CCR2, CCR4, and CCD5, than myeloid cells and regulatory T cells (146). Genetically modifying TIL to express higher levels of these chemokine receptors to increase avidity and facilitate improved migration into tumors could be another approach. An elegant study recently found that a key chemokine inducing CD8+ T-cell migration into the core of tumors, CCL2, can be highly nitrotyrosinylated due to the accumulation of reactive nitrogen species in tumors (146). This decreased the affinity and avidity of binding to CCR2 on T cells, while not affecting binding to myeloid cells that express much higher levels of CCR2. Thus, enhancing CCR2 expression in T cells using gene transduction may be a good approach to overcome this problem.
Improving TIL survival and function in vivo by gene transduction
TGF-β is an immunosuppressive cytokine that is produced by about 50% of melanomas (147). TGF-β is known to repress the expression of factors in CD8+ T cells, such as IFN-γ, granzyme A, granzyme B, perforin, and FasL, that are important for tumor killing (123). T-cells transduced with a gene encoding a dominant-negative form of the TGF-β receptor have increased resistance to TGF-β-mediated inhibition, as shown by their ability to continue to proliferate and secrete cytokines in response to antigen in the presence of TGF-β (148). Another approach to this problem has been to modify the T cells to produce the cytokine IL-12 which also yields resistance to TGF-β and promotes IFN-γ production by T cells (149). Recently, Zhang et al developed a new approach in which T cells from peripheral blood expressing a TCR recognizing gp100 were transduced with an inducible IL-12 vector under the control of NFAT (Nuclear Factor of Activated T cells) promoter (150). In this approach, T cells induce expression of the IL-12 gene only upon TCR triggering by the tumor antigen. They were able to demonstrate regression of murine B16 melanoma upon adoptive transfer of such genetically modified pmel transgenic T cells without the toxicity that systemic administration of IL-12 can induce (150).
Direct genetic manipulation of the cellular apoptotic machinery is also being investigated to increase T-cell survival after adoptive transfer of TIL. Over-expression of either of the antiapoptotic molecules B-cell lymphoma 2, bcl-2 (151, 152) or bcl-xL (153) has been found to increase survival of activated T cells in vitro and improve anti-tumor efficacy in vivo. Other possibilities to improve TIL survival and persistence after adoptive transfer include transduction of CD28 or CD137 ligand (4-1BBL) genes into the expanding T cells to prevent loss of CD28 and maintain responsiveness to antigenic re-stimulation and provide direct CD137 costimulation between T cells. The otherwise senescent T-cells that are transduced with CD28 may be able to up-regulate telomerase activity to improve their proliferative potential (109). Furthermore, T-cells stimulated with aAPCs expressing CD137 ligand can provide costimulation and survival signal to T cells and maintain CD28 expression (134, 154).
Surrogate and predictive biomarkers in TIL therapy: Can we select patients for TIL therapy?
In our push to develop “personalized” treatment options for our patients, another critical issue facing TIL therapy, as well as other forms of immunotherapies and targeted therapies, is improved patient selection. Despite the promising results obtained with TIL therapy, the current accepted therapy regimen using the cyclophosphamide and fludaribine pre-conditioning seems to have reached a peak, not being able to push past the 50% response rate barrier. The combined myeloablative pre-conditioning using TBI together with chemotherapy-based lymphodepletion has resulted in increased response rates, as pointed out earlier, this regimen is considered much too toxic. Even if this alternative pre-conditioning regimen with TBI could be considered, we are still plagued by the fact that overall TIL therapy is still a complex and lengthy procedure requiring 5–7 weeks for expansion of T cells to sufficient numbers (billions) for therapy and that TIL from some patients fail to expand to sufficient numbers. The lengthy wait time has resulted in a significant fraction of would-be-treated patients falling out due to rapid disease progression and other clinical issues related to a decrease in overall performance status, or simply anxiety and determination to start or continue on therapy without having to wait all this time. Other options, such as B-RAF inhibitor drug therapy or ipilimumab for eligible patients has become an option (2, 136), but many patients considered for TIL therapy may have previously failed these and other regimens and have exhausted most other treatment options.
These critical issues point to a need to identify patients who will respond to TIL that can be used as a selection tool. The rational integration of biomarkers in selecting patients for TIL therapy would be a huge step in catapulting TIL therapy into the mainstream as a treatment option much earlier, especially considering the growing widespread use of B-RAF and other MAPK pathway inhibitors, ipilimumab, and the upcoming clinical development of anti-PD-1 blocking therapies. As pointed out earlier, currently we still know relatively little about the types of T cells in TIL mediating tumor regression and there are no reliable biomarkers to select melanoma patients for TIL therapy and other forms of ACT. Whether a patient responds to TIL therapy, as well as other immunotherapies, such as IL-2 and ipilimumab, ultimately involves an array of factors that include the tumor microenvironment regulating the interplay between the infused T cells, systemic inflammatory factors in cancer patients, and the genetic make-up of the individual (e.g., polymorphisms in genes regulating the immune response) that may “hard-wire” the way the immune system responds to challenges such as infection and cancer. Studies on biomarkers in immunotherapy will need to rationally combine these different interacting host features to develop assays predictive of response.
So far major clinical features of the patients, including age, sex, site of disease, sub-stage of metastatic disease (III, IV-M1b, M1b, M1c), and tumor burden at time of TIL transfer, as well as type of prior therapy have not been found to be predictive of clinical response to TIL therapy (20, 75) (Radvanyi et al., unpublished data). In addition, a number of TIL clinical trials have not been able to correlate the degree of tumor reactivity of TIL against autologous or HLA-matched melanoma cell lines in vitro early in the expansion process with clinical response using traditional approaches such as IFN-γ secretion assays (7, 20, 75). Thus, assays anti-tumor reactivity in vitro may not be a good biomarker approach. Although, this may reflect the shortcoming of our assays, it also suggests that ultimately a number of T-cell phenotypic and host features may over-ride TIL antigen specificity. In addition, mutational status (B-RAF, NRAS or wild-type) has not correlated to TIL therapy clinical response (Radvanyi et al., unpublished data). Thus, other immune and molecular parameters need to be identified as surrogate markers of clinical response to TIL therapy and as predictive markers to pre-select patients for TIL therapy. These markers can be derived from a number other sources that more subtly reflect tumor progression, immunoreactivity against the tumor, and other inflammatory and immunosuppressive risk factors. A plausible conceptual framework that can be used to drive a search for biomarkers for TIL therapy is that both the functional attributes of the TIL, as measured by specific T-cell phenotypic features, together with specific immunosuppressive features in the tumor microenvironment and the systemic inflammatory environment in patients all interact to determine the outcome of TIL therapy. Specific analytes in TIL, blood, and tumor samples from patients that measure these relative states have already been identified individually as prognostic factors in melanoma and surrogate biomarkers in TIL therapy. These include flow cytometry markers for T-cell subsets and states of T-cell differentiation in infused TIL (e.g., CD8+ versus CD4+ T cells and effector-memory states), intratumoral biomarkers by immunohistochemistry for T-cell infiltration and subsets and immunosuppressive molecules and cells (e.g., macrophages, neutrophils, inducible nitric oxide synthase, PD-L1/B7H1 and activated STAT3), systemic markers of “bad” inflammation (e.g., high IL-12p40, CRP, and IFN-γ) and tumor progression (VEGF, LDH, S100 proteins) that drive tumor growth (146, 155–160). This approach is not a “one size fits all” scenario, but rather that a combination of biomarkers in a “fingerprint” established before patient treatment may predict responders from non-responders.
One place to start is to look at T-cell subsets during TIL expansion and whether the types and numbers of T cells able to be expanded ex vivo can be predicted by looking at samples of the tumors used to expand TIL. This would include looking at the degree of CD8+ and CD4+ T-cell infiltration (intratumoral and peritumoral) in relation to immunomodulatory factors such as PD-1 and PD-L1 expression. In TIL therapy trials at MD Anderson Cancer Center, we have found that a higher frequency and number of infused CD8+ T cells in TIL is highly correlated with clinical response (9). Future studies are further dissecting the CD8+ subset to find more discrete subpopulations that are better associated with response. It would be important to determine whether specific tumor microenvironmental features (e.g., degree of CD8+ infiltration) and/or systemic inflammatory features at the time of tumor harvest for TIL can ultimately predict whether these optimal T cells are expanded. This would need to be a critical first step in biomarker studies identifying a predictive fingerprint for patient selection.
TIL: Ready for prime time?
Given the weight of accumulating successful Phase II clinical trials and improvements in our understanding of the T cell subsets mediating tumor regression, together with technological improvements in more rapidly and efficiently expanding TIL, we would argue that the answer to the above question is a resounding yes. Indeed, TIL therapy has reached a tipping point, where we need to decide whether or not it should be integrated into mainstream melanoma care. The key next step will be to perform a series of pivotal non-randomized Phase II or randomized Phase III clinical trials with the aim of regulatory approval as a treatment for metastatic melanoma. Recently, the National Institutes of Health (NIH) in the USA has recognized this need and through Cancer Therapy and Evaluation Program (CTEP) has convened a committee to summarize the current state of TIL therapy, identify the key clinical, technical, and regulatory issues to be resolved, and suggest different possible clinical trial designs aimed at finally gaining FDA approval of TIL therapy as standard-of-care. Key members of this CTEP committee recently summarized their findings and developed a roadmap to proceed (84). This has sparked an increased interest in TIL therapy and we expect that a pivotal clinical trial will be completed within the next 5 years that should answer the question once and for all.
A number of different clinical trial designs can be used towards FDA approval. The first one would be a non-randomized Phase II approach to gain more rapid approval through FDA 21CFR601 Sub-part E guidelines; this would be followed immediately by a confirmatory Phase III clinical trial. This clinical trial would probably need at least 100–120 patients and would need to demonstrate a clearly increased durable progression-free survival over a critical threshold over current therapies (these may include ipilimimuab, IL-2, or B-RAF inhibitors in the case of mutant B-RAF melanoma patients). Patients failing these therapies would be accrued in an “intent-to-treat” scenario in which all patients would be included in the denominator (i.e., whether or not they had successful TIL expansion or not from tumor samples and ultimately were treated with TIL). Such a design would also need to reach a critical clinical response rate (e.g., 30%) based on immune-related response criteria (irRC) or RECIST (161–163). A second possible design to obtain regulatory approval may be a Phase III randomized clinical trial in which TIL is expanded from tumor fragments or digests and banked. Patients would then be randomized to receive either HD IL-2 alone (control arm) or TIL plus HD IL-2 (treatment arm). Those randomized to get TIL will have their cells rapidly expanded (REP) to generate an infusion product from the banked “pre-REP” cells and treated. Here, the denominator would take into consideration only those patients that had successful initial TIL outgrowth from their resected tumors. A clear progression-free survival end-point would be used with possibly overall survival. However, a cross-over design, with patients progressing on the control arm receiving TIL plus IL-2, would be the most appropriate strategy. The control arm in this trial could also be composed of patients receiving B-RAF inhibitor or ipilimumab with the same end-points.
Whatever the design of future pivotal trials, we envision that TIL adoptive cell therapy has a bright future, especially with newer approaches that will couple it with synergistic drug therapies and immunomodulators boosting TAA release, T-cell persistence, and anti-tumor effector function. Although other potentially competing technologies have emerged, such as TCR transduction and CAR technology, at present these can only target a single TAA or a limited array of TAA. Many TAA are still unknown and many more will be discovered as a result of full exome sequencing efforts that will reveal mutated self proteins that are recognized by endogenous T-cells residing in TIL populations. This ability to recognize a panoply of unknown TAA (as well as major shared melanoma antigens such as MART-1) ultimately still place TIL at the top of pedestal in terms of T-cell therapy choices for melanoma. In fact, recent studies using large libraries of bar-coded peptide-loaded multimers covering a number of different shared TAA specificities on major HLA subtypes by Schumacher and colleagues in Holland has found that the majority of TIL specificities in the CD8+ subset is still unknown, likely composed of cells specific for mutated self-antigens (28). Together with the development of new biomarkers for improved patient selection, TIL therapy has a very bright future indeed.
ACKNOWLEDGEMENTS
We would like to thank all the individuals who have contributed to the success of adoptive T-cell therapy for melanoma, especially members of MD Anderson Cancer Center (Houston, TX) who have contributed to the success of the TIL therapy program, including members of the surgical staff, melanoma medical oncology nurses and physicians and pathology staff. We especially thank the hard working members of our “TIL Lab” who have tirelessly worked over the years in expanded TIL for our patients (Rhamatu Mansaray, Orenthial, J. Fulbright, Christopher Toth, Renjith Ramachandran, Seth Wardell, Audrey Gonzalez, Kathryn Bushnell, and Marissa Gonzalez). We also thank Drs. Mark Dudley, Steve Rosenberg, Ena Wang and Franco Marincola (National Cancer Institute, Bethesda, MD) for helpful discussions. Additional consultation with Dr. Laurence Cooper (Pediatric Department, MD Anderson Cancer Center) is also greatly appreciated. Work in the laboratory of the authors was supported by NIH research grants CA111999 (P. Hwu), CA093459-DRP21 (L. Radvanyi), and grants from the Melanoma Research Alliance (L. Radvanyi and P. Hwu), the Dr. Miriam and Sheldon Adelson Medical Research Foundation/AMRF (P. Hwu and L. Radvanyi), and the Gillson Longenbough Foundation (P. Hwu).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The authors declare no competing financial interests.
This conclusion does not consider a recent TIL adoptive cell therapy clinical trial by a group in Uppsala, Sweden, reporting only a 21% clinical response rate (73). This group treated 24 patients with relatively few TIL cells (median of 5.4 × 106 cells) below the range considered to be clinically active (>10 × 109) (9, 72). Moreover, they used low-dose IL-2 therapy following TIL infusion rather than high-dose IL-2 (73). Nevertheless, under the circumstances, a 21% clinical response rate is promising considering this attenuated regimen.
REFERENCES
- 1.Trial watch: ipilimumab success in melanoma provides boost for cancer immunotherapy. Nat Rev Drug Discov. 2010;9(8):584. doi: 10.1038/nrd3245. [DOI] [PubMed] [Google Scholar]
- 2.Hoos A, Ibrahim R, Korman A, Abdallah K, Berman D, Shahabi V, et al. Development of ipilimumab: contribution to a new paradigm for cancer immunotherapy. Semin Oncol. 2011;37(5):533–546. doi: 10.1053/j.seminoncol.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 3.Callahan MK, Wolchok JD, Allison JP. Anti-CTLA-4 antibody therapy: immune monitoring during clinical development of a novel immunotherapy. Semin Oncol. 2011;37(5):473–484. doi: 10.1053/j.seminoncol.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6 Suppl 1:S11–S14. [PubMed] [Google Scholar]
- 6.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364(22):2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Hershkovitz L, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16(9):2646–2655. doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 8.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23(10):2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radvanyi LG, Bernatchez C, Zhang M, Miller P, Glass M, Papadopoulos N, et al. Adoptive T-cell therapy for metastatic melanoma: The MD Anderson experience. J Immunother. 2010;33(8):863. [Google Scholar]
- 10.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer--preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol. 2010;37(5):430–439. doi: 10.1053/j.seminoncol.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8(1):59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 13.Sumimoto H, Imabayashi F, Iwata T, Kawakami Y. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med. 2006;203(7):1651–1656. doi: 10.1084/jem.20051848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70(13):5213–5219. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- 15.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364(26):2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Locher C, Conforti R, Aymeric L, Ma Y, Yamazaki T, Rusakiewicz S, et al. Desirable cell death during anticancer chemotherapy. Ann N Y Acad Sci. 2010;1209:99–108. doi: 10.1111/j.1749-6632.2010.05763.x. [DOI] [PubMed] [Google Scholar]
- 17.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13(1):54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 18.Yang JC, Rosenberg SA. Current approaches to the adoptive immunotherapy of cancer. Adv Exp Med Biol. 1988;233:459–467. doi: 10.1007/978-1-4899-5037-6_50. [DOI] [PubMed] [Google Scholar]
- 19.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298(5594):850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17(13):4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morgan RA, Dudley ME, Yu YY, Zheng Z, Robbins PF, Theoret MR, et al. High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. J Immunol. 2003;171(6):3287–3295. doi: 10.4049/jimmunol.171.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalos M, Levine BL, Porter DL, Katz S, Grupp SA, Bagg A, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3(95):95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yvon E, Del Vecchio M, Savoldo B, Hoyos V, Dutour A, Anichini A, et al. Immunotherapy of metastatic melanoma using genetically engineered GD2-specific T cells. Clin Cancer Res. 2009;15(18):5852–5860. doi: 10.1158/1078-0432.CCR-08-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dudley ME. Adoptive cell therapy for patients with melanoma. J Cancer. 2011;2:360–362. doi: 10.7150/jca.2.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8(4):299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hadrup SR, Bakker AH, Shu CJ, Andersen RS, van Veluw J, Hombrink P, et al. Parallel detection of antigen-specific T-cell responses by multidimensional encoding of MHC multimers. Nat Methods. 2009;6(7):520–526. doi: 10.1038/nmeth.1345. [DOI] [PubMed] [Google Scholar]
- 28.Heemskerk B, Shu CJ, Kvistborg PK, van Rooij N, Besser MJ, Schachter J, et al. Dissecting the effects of TIL therapy in melanoma patients. J Immunother. 2011;34(9):677. [Google Scholar]
- 29.Mackensen A, Meidenbauer N, Vogl S, Laumer M, Berger J, Andreesen R. Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J Clin Oncol. 2006;24(31):5060–5069. doi: 10.1200/JCO.2006.07.1100. [DOI] [PubMed] [Google Scholar]
- 30.Mitchell MS, Darrah D, Yeung D, Halpern S, Wallace A, Voland J, et al. Phase I trial of adoptive immunotherapy with cytolytic T lymphocytes immunized against a tyrosinase epitope. J Clin Oncol. 2002;20(4):1075–1086. doi: 10.1200/JCO.2002.20.4.1075. [DOI] [PubMed] [Google Scholar]
- 31.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99(25):16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358(25):2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verdegaal EM, Visser M, Ramwadhdoebe TH, van der Minne CE, van Steijn JA, Kapiteijn E, et al. Successful treatment of metastatic melanoma by adoptive transfer of blood-derived polyclonal tumor-specific CD4+ and CD8+ T cells in combination with low-dose interferon-alpha. Cancer Immunol Immunother. 2011;60(7):953–963. doi: 10.1007/s00262-011-1004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suhoski MM, Golovina TN, Aqui NA, Tai VC, Varela-Rohena A, Milone MC, et al. Engineering artificial antigen-presenting cells to express a diverse array of co-stimulatory molecules. Mol Ther. 2007;15(5):981–988. doi: 10.1038/mt.sj.6300134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butler MO, Friedlander P, Milstein MI, Mooney MM, Metzler G, Murray AP, et al. Establishment of antitumor memory in humans using in vitro-educated CD8+ T cells. Sci Transl Med. 2011;3(80):80ra34. doi: 10.1126/scitranslmed.3002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butler MO, Lee JS, Ansen S, Neuberg D, Hodi FS, Murray AP, et al. Long-lived antitumor CD8+ lymphocytes for adoptive therapy generated using an artificial antigen-presenting cell. Clin Cancer Res. 2007;13(6):1857–1867. doi: 10.1158/1078-0432.CCR-06-1905. [DOI] [PubMed] [Google Scholar]
- 37.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114(3):535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29(7):917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Davis JL, Theoret MR, Zheng Z, Lamers CH, Rosenberg SA, Morgan RA. Development of human anti-murine T-cell receptor antibodies in both responding and nonresponding patients enrolled in TCR gene therapy trials. Clin Cancer Res. 2010;16(23):5852–5861. doi: 10.1158/1078-0432.CCR-10-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bendle GM, Linnemann C, Hooijkaas AI, Bies L, de Witte MA, Jorritsma A, et al. Lethal graft-versus-host disease in mouse models of T cell receptor gene therapy. Nat Med. 2010;16(5):565–570. doi: 10.1038/nm.2128. [DOI] [PubMed] [Google Scholar]
- 41.Brocker T, Karjalainen K. Signals through T cell receptor-zeta chain alone are insufficient to prime resting T lymphocytes. J Exp Med. 1995;181(5):1653–1659. doi: 10.1084/jem.181.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramos CA, Dotti G. Chimeric antigen receptor (CAR)-engineered lymphocytes for cancer therapy. Expert Opin Biol Ther. 2011;11(7):855–873. doi: 10.1517/14712598.2011.573476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hombach A, Wieczarkowiecz A, Marquardt T, Heuser C, Usai L, Pohl C, et al. Tumor-specific T cell activation by recombinant immunoreceptors: CD3 zeta signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3 zeta signaling receptor molecule. J Immunol. 2001;167(11):6123–6131. doi: 10.4049/jimmunol.167.11.6123. [DOI] [PubMed] [Google Scholar]
- 44.Maher J, Brentjens RJ, Gunset G, Riviere I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002;20(1):70–75. doi: 10.1038/nbt0102-70. [DOI] [PubMed] [Google Scholar]
- 45.Finney HM, Akbar AN, Lawson AD. Activation of resting human primary T cells with chimeric receptors: costimulation from CD28, inducible costimulator, CD134, and CD137 in series with signals from the TCR zeta chain. J Immunol. 2004;172(1):104–113. doi: 10.4049/jimmunol.172.1.104. [DOI] [PubMed] [Google Scholar]
- 46.Savoldo B, Ramos CA, Liu E, Mims MP, Keating MJ, Carrum G, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest. 2011;121(5):1822–1826. doi: 10.1172/JCI46110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Erfurt C, Muller E, Emmerling S, Klotz C, Hertl M, Schuler G, et al. Melanoma-associated chondroitin sulphate proteoglycan as a new target antigen for CD4+ T cells in melanoma patients. Int J Cancer. 2009;124(10):2341–2346. doi: 10.1002/ijc.24235. [DOI] [PubMed] [Google Scholar]
- 48.Lo AS, Ma Q, Liu DL, Junghans RP. Anti-GD3 chimeric sFv-CD28/T-cell receptor zeta designer T cells for treatment of metastatic melanoma and other neuroectodermal tumors. Clin Cancer Res. 2010;16(10):2769–2780. doi: 10.1158/1078-0432.CCR-10-0043. [DOI] [PubMed] [Google Scholar]
- 49.Nishi H, Inoue Y, Kageshita T, Takata M, Ihn H. The expression of human high molecular weight melanoma-associated antigen in acral lentiginous melanoma. Biosci Trends. 2010;4(2):86–89. [PubMed] [Google Scholar]
- 50.Timar J, Ladanyi A, Lapis K, Moczar M. Differential expression of proteoglycans on the surface of human melanoma cells characterized by altered experimental metastatic potential. Am J Pathol. 1992;141(2):467–474. [PMC free article] [PubMed] [Google Scholar]
- 51.Burns WR, Zhao Y, Frankel TL, Hinrichs CS, Zheng Z, Xu H, et al. A high molecular weight melanoma-associated antigen-specific chimeric antigen receptor redirects lymphocytes to target human melanomas. Cancer Res. 2010;70(8):3027–3033. doi: 10.1158/0008-5472.CAN-09-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26(4):332–342. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hernandez-Chacon JA, Li Y, Wu RC, Bernatchez C, Wang Y, Weber JS, et al. Costimulation through the CD137/4-1BB pathway protects human melanoma tumor-infiltrating lymphocytes from activation-induced cell death and enhances antitumor effector function. J Immunother. 2011;34(3):236–250. doi: 10.1097/CJI.0b013e318209e7ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y, Liu S, Hernandez J, Vence L, Hwu P, Radvanyi L. MART-1-specific melanoma tumor-infiltrating lymphocytes maintaining CD28 expression have improved survival and expansion capability following antigenic restimulation in vitro. J Immunol. 2010;184(1):452–465. doi: 10.4049/jimmunol.0901101. [DOI] [PubMed] [Google Scholar]
- 55.Riddell SR, Greenberg PD. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. J Immunol Methods. 1990;128(2):189–201. doi: 10.1016/0022-1759(90)90210-m. [DOI] [PubMed] [Google Scholar]
- 56.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3(9):666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dudley ME, Rosenberg SA. Adoptive cell transfer therapy. Semin Oncol. 2007;34(6):524–531. doi: 10.1053/j.seminoncol.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou J, Dudley ME, Rosenberg SA, Robbins PF. Selective growth, in vitro and in vivo, of individual T cell clones from tumor-infiltrating lymphocytes obtained from patients with melanoma. J Immunol. 2004;173(12):7622–7629. doi: 10.4049/jimmunol.173.12.7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Phan GQ, Attia P, Steinberg SM, White DE, Rosenberg SA. Factors associated with response to high-dose interleukin-2 in patients with metastatic melanoma. J Clin Oncol. 2001;19(15):3477–3482. doi: 10.1200/JCO.2001.19.15.3477. [DOI] [PubMed] [Google Scholar]
- 60.Huang J, Khong HT, Dudley ME, El-Gamil M, Li YF, Rosenberg SA, et al. Survival, persistence, and progressive differentiation of adoptively transferred tumor-reactive T cells associated with tumor regression. J Immunother. 2005;28(3):258–267. doi: 10.1097/01.cji.0000158855.92792.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, et al. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173(12):7125–7130. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rosenberg SA, Dudley ME. Cancer regression in patients with metastatic melanoma after the transfer of autologous antitumor lymphocytes. Proc Natl Acad Sci U S A. 2004;101 Suppl 2:14639–14645. doi: 10.1073/pnas.0405730101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26(32):5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klebanoff CA, Khong HT, Antony PA, Palmer DC, Restifo NP. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 2005;26(2):111–117. doi: 10.1016/j.it.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang LX, Shu S, Plautz GE. Host lymphodepletion augments T cell adoptive immunotherapy through enhanced intratumoral proliferation of effector cells. Cancer Res. 2005;65(20):9547–9554. doi: 10.1158/0008-5472.CAN-05-1175. [DOI] [PubMed] [Google Scholar]
- 66.Lake RA, Robinson BW. Immunotherapy and chemotherapy--a practical partnership. Nat Rev Cancer. 2005;5(5):397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 67.Wallen H, Thompson JA, Reilly JZ, Rodmyre RM, Cao J, Yee C. Fludarabine modulates immune response and extends in vivo survival of adoptively transferred CD8 T cells in patients with metastatic melanoma. PLoS One. 2009;4(3):e4749. doi: 10.1371/journal.pone.0004749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poehlein CH, Haley DP, Walker EB, Fox BA. Depletion of tumor-induced Treg prior to reconstitution rescues enhanced priming of tumor-specific, therapeutic effector T cells in lymphopenic hosts. Eur J Immunol. 2009;39(11):3121–3133. doi: 10.1002/eji.200939453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Powell DJ, Jr, de Vries CR, Allen T, Ahmadzadeh M, Rosenberg SA. Inability to mediate prolonged reduction of regulatory T Cells after transfer of autologous CD25-depleted PBMC and interleukin-2 after lymphodepleting chemotherapy. J Immunother. 2007;30(4):438–447. doi: 10.1097/CJI.0b013e3180600ff9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Turk MJ, Guevara-Patino JA, Rizzuto GA, Engelhorn ME, Sakaguchi S, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200(6):771–782. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21(2):233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Schallmach E, et al. Minimally cultured or selected autologous tumor-infiltrating lymphocytes after a lympho-depleting chemotherapy regimen in metastatic melanoma patients. J Immunother. 2009;32(4):415–423. doi: 10.1097/CJI.0b013e31819c8bda. [DOI] [PubMed] [Google Scholar]
- 73.Ullenhag GJ, Sadeghi AM, Carlsson B, Ahlstrom H, Mosavi F, Wagenius G, et al. Adoptive T-cell therapy for malignant melanoma patients with TILs obtained by ultrasound-guided needle biopsy. Cancer Immunol Immunother. 2011 doi: 10.1007/s00262-011-1182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dudley ME, Gross CA, Langhan MM, Garcia MR, Sherry RM, Yang JC, et al. CD8+ enriched "young" tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin Cancer Res. 2010;16(24):6122–6131. doi: 10.1158/1078-0432.CCR-10-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goff SL, Smith FO, Klapper JA, Sherry R, Wunderlich JR, Steinberg SM, et al. Tumor infiltrating lymphocyte therapy for metastatic melanoma: analysis of tumors resected for TIL. J Immunother. 2010;33(8):840–847. doi: 10.1097/CJI.0b013e3181f05b91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nguyen LT, Yen PH, Nie J, Liadis N, Ghazarian D, Al-Habeeb A, et al. Expansion and characterization of human melanoma tumor-infiltrating lymphocytes (TILs) PLoS One. 2011;5(11):e13940. doi: 10.1371/journal.pone.0013940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Joseph RW, Peddareddigari VR, Liu P, Miller PW, Overwijk WW, Bekele NB, et al. Impact of clinical and pathologic features on tumor-infiltrating lymphocyte expansion from surgically excised melanoma metastases for adoptive T-cell therapy. Clin Cancer Res. 2011;17(14):4882–4891. doi: 10.1158/1078-0432.CCR-10-2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Trapani JA. Target cell apoptosis induced by cytotoxic T cells and natural killer cells involves synergy between the pore-forming protein, perforin, and the serine protease, granzyme B. Aust N Z J Med. 1995;25(6):793–799. doi: 10.1111/j.1445-5994.1995.tb02883.x. [DOI] [PubMed] [Google Scholar]
- 79.Young JD, Hengartner H, Podack ER, Cohn ZA. Purification and characterization of a cytolytic pore-forming protein from granules of cloned lymphocytes with natural killer activity. Cell. 1986;44(6):849–859. doi: 10.1016/0092-8674(86)90007-3. [DOI] [PubMed] [Google Scholar]
- 80.Andersen MH, Schrama D, Thor Straten P, Becker JC. Cytotoxic T cells. J Invest Dermatol. 2006;126(1):32–41. doi: 10.1038/sj.jid.5700001. [DOI] [PubMed] [Google Scholar]
- 81.Powell DJ, Jr, Dudley ME, Robbins PF, Rosenberg SA. Transition of late-stage effector T cells to CD27+ CD28+ tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood. 2005;105(1):241–250. doi: 10.1182/blood-2004-06-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prieto PA, Durflinger KH, Wunderlich JR, Rosenberg SA, Dudley ME. Enrichment of CD8+ cells from melanoma tumor-infiltrating lymphocyte cultures reveals tumor reactivity for use in adoptive cell therapy. J Immunother. 2010;33(5):547–556. doi: 10.1097/CJI.0b013e3181d367bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Muranski P, Restifo NP. Adoptive immunotherapy of cancer using CD4(+) T cells. Curr Opin Immunol. 2009;21(2):200–208. doi: 10.1016/j.coi.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weber J, Atkins M, Hwu P, Radvanyi L, Sznol M, Yee C. White paper on adoptive cell therapy for cancer with tumor-infiltrating lymphocytes: a report of the CTEP subcommittee on adoptive cell therapy. Clin Cancer Res. 2011;17(7):1664–1673. doi: 10.1158/1078-0432.CCR-10-2272. [DOI] [PubMed] [Google Scholar]
- 85.Appay V, Zaunders JJ, Papagno L, Sutton J, Jaramillo A, Waters A, et al. Characterization of CD4(+) CTLs ex vivo. J Immunol. 2002;168(11):5954–5958. doi: 10.4049/jimmunol.168.11.5954. [DOI] [PubMed] [Google Scholar]
- 86.Qui HZ, Hagymasi AT, Bandyopadhyay S, St Rose MC, Ramanarasimhaiah R, Menoret A, et al. CD134 plus CD137 dual costimulation induces Eomesodermin in CD4 T cells to program cytotoxic Th1 differentiation. J Immunol. 2011;187(7):3555–3564. doi: 10.4049/jimmunol.1101244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maccalli C, Nonaka D, Piris A, Pende D, Rivoltini L, Castelli C, et al. NKG2D-mediated antitumor activity by tumor-infiltrating lymphocytes and antigen-specific T-cell clones isolated from melanoma patients. Clin Cancer Res. 2007;13(24):7459–7468. doi: 10.1158/1078-0432.CCR-07-1166. [DOI] [PubMed] [Google Scholar]
- 88.Bialasiewicz AA, Ma JX, Richard G. Alpha/beta- and gamma/delta TCR(+) lymphocyte infiltration in necrotising choroidal melanomas. Br J Ophthalmol. 1999;83(9):1069–1073. doi: 10.1136/bjo.83.9.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nanno M, Seki H, Mathioudakis G, Suzuki R, Itoh K, Ioannides CG, et al. Gamma/delta T cell antigen receptors expressed on tumor-infiltrating lymphocytes from patients with solid tumors. Eur J Immunol. 1992;22(3):679–687. doi: 10.1002/eji.1830220310. [DOI] [PubMed] [Google Scholar]
- 90.Yazdi AS, Morstedt K, Puchta U, Ghoreschi K, Flaig MJ, Rocken M, et al. Heterogeneity of T-cell clones infiltrating primary malignant melanomas. J Invest Dermatol. 2006;126(2):393–398. doi: 10.1038/sj.jid.5700082. [DOI] [PubMed] [Google Scholar]
- 91.Morita CT, Jin C, Sarikonda G, Wang H. Nonpeptide antigens, presentation mechanisms, and immunological memory of human Vgamma2Vdelta2 T cells: discriminating friend from foe through the recognition of prenyl pyrophosphate antigens. Immunol Rev. 2007;215:59–76. doi: 10.1111/j.1600-065X.2006.00479.x. [DOI] [PubMed] [Google Scholar]
- 92.Appay V, Dunbar PR, Callan M, Klenerman P, Gillespie GM, Papagno L, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8(4):379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 93.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 94.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78(11):5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chavez-Galan L, Arenas-Del Angel MC, Zenteno E, Chavez R, Lascurain R. Cell death mechanisms induced by cytotoxic lymphocytes. Cell Mol Immunol. 2009;6(1):15–25. doi: 10.1038/cmi.2009.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Van den Hove LE, Van Gool SW, Vandenberghe P, Boogaerts MA, Ceuppens JL. CD57+/CD28− T cells in untreated hemato-oncological patients are expanded and display a Th1-type cytokine secretion profile, ex vivo cytolytic activity and enhanced tendency to apoptosis. Leukemia. 1998;12(10):1573–1582. doi: 10.1038/sj.leu.2401146. [DOI] [PubMed] [Google Scholar]
- 97.Ohkawa T, Seki S, Dobashi H, Koike Y, Habu Y, Ami K, et al. Systematic characterization of human CD8+ T cells with natural killer cell markers in comparison with natural killer cells and normal CD8+ T cells. Immunology. 2001;103(3):281–290. doi: 10.1046/j.1365-2567.2001.01248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pittet MJ, Speiser DE, Valmori D, Cerottini JC, Romero P. Cutting edge: cytolytic effector function in human circulating CD8+ T cells closely correlates with CD56 surface expression. J Immunol. 2000;164(3):1148–1152. doi: 10.4049/jimmunol.164.3.1148. [DOI] [PubMed] [Google Scholar]
- 99.Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, et al. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369(6475):31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 100.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2(4):251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 101.Klebanoff CA, Gattinoni L, Palmer DC, Muranski P, Ji Y, Hinrichs CS, et al. Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clin Cancer Res. 2011;17(16):5343–5352. doi: 10.1158/1078-0432.CCR-11-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang J, Kerstann KW, Ahmadzadeh M, Li YF, El-Gamil M, Rosenberg SA, et al. Modulation by IL-2 of CD70 and CD27 expression on CD8+ T cells: importance for the therapeutic effectiveness of cell transfer immunotherapy. J Immunol. 2006;176(12):7726–7735. doi: 10.4049/jimmunol.176.12.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou J, Dudley ME, Rosenberg SA, Robbins PF. Persistence of multiple tumor-specific T-cell clones is associated with complete tumor regression in a melanoma patient receiving adoptive cell transfer therapy. J Immunother. 2005;28(1):53–62. doi: 10.1097/00002371-200501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005;175(10):7046–7052. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim TK, St John LS, Wieder ED, Khalili J, Ma Q, Komanduri KV. Human late memory CD8+ T cells have a distinct cytokine signature characterized by CC chemokine production without IL-2 production. J Immunol. 2009;183(10):6167–6174. doi: 10.4049/jimmunol.0902068. [DOI] [PubMed] [Google Scholar]
- 106.Baerlocher GM, Mak J, Tien T, Lansdorp PM. Telomere length measurement by fluorescence in situ hybridization and flow cytometry: tips and pitfalls. Cytometry. 2002;47(2):89–99. doi: 10.1002/cyto.10053. [DOI] [PubMed] [Google Scholar]
- 107.Effros RB, Dagarag M, Spaulding C, Man J. The role of CD8+ T-cell replicative senescence in human aging. Immunol Rev. 2005;205:147–157. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 108.McElhaney JE, Effros RB. Immunosenescence: what does it mean to health outcomes in older adults? Curr Opin Immunol. 2009;21(4):418–424. doi: 10.1016/j.coi.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Parish ST, Wu JE, Effros RB. Sustained CD28 expression delays multiple features of replicative senescence in human CD8 T lymphocytes. J Clin Immunol. 2010;30(6):798–805. doi: 10.1007/s10875-010-9449-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mueller K, Schweier O, Pircher H. Efficacy of IL-2- versus IL-15-stimulated CD8 T cells in adoptive immunotherapy. Eur J Immunol. 2008;38(10):2874–2885. doi: 10.1002/eji.200838426. [DOI] [PubMed] [Google Scholar]
- 111.Lewis DE, Merched-Sauvage M, Goronzy JJ, Weyand CM, Vallejo AN. Tumor necrosis factor-alpha and CD80 modulate CD28 expression through a similar mechanism of T-cell receptor-independent inhibition of transcription. J Biol Chem. 2004;279(28):29130–29138. doi: 10.1074/jbc.M402194200. [DOI] [PubMed] [Google Scholar]
- 112.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, et al. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207(10):2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Inozume T, Hanada K, Wang QJ, Ahmadzadeh M, Wunderlich JR, Rosenberg SA, et al. Selection of CD8+PD-1+ lymphocytes in fresh human melanomas enriches for tumor-reactive T cells. J Immunother. 2010;33(9):956–964. doi: 10.1097/CJI.0b013e3181fad2b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tran KQ, Zhou J, Durflinger KH, Langhan MM, Shelton TE, Wunderlich JR, et al. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J Immunother. 2008;31(8):742–751. doi: 10.1097/CJI.0b013e31818403d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, et al. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111(11):5326–5333. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201(1):139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li Y, Bleakley M, Yee C. IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell response. J Immunol. 2005;175(4):2261–2269. doi: 10.4049/jimmunol.175.4.2261. [DOI] [PubMed] [Google Scholar]
- 119.Liu S, Etto T, Rodriguez-Cruz T, Li Y, Wu C, Fulbright OJ, et al. TGF-beta1 induces preferential rapid expansion and persistence of tumor antigen-specific CD8+ T cells for adoptive immunotherapy. J Immunother. 2010;33(4):371–381. doi: 10.1097/CJI.0b013e3181cd1180. [DOI] [PubMed] [Google Scholar]
- 120.Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15(7):808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gattinoni L, Ji Y, Restifo NP. Wnt/beta-catenin signaling in T-cell immunity and cancer immunotherapy. Clin Cancer Res. 2010;16(19):4695–4701. doi: 10.1158/1078-0432.CCR-10-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324(5934):1572–1576. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8(5):369–380. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 124.Smyth MJ, Strobl SL, Young HA, Ortaldo JR, Ochoa AC. Regulation of lymphokine-activated killer activity and pore-forming protein gene expression in human peripheral blood CD8+ T lymphocytes. Inhibition by transforming growth factor-beta. J Immunol. 1991;146(10):3289–3297. [PubMed] [Google Scholar]
- 125.Zhang H, Snyder KM, Suhoski MM, Maus MV, Kapoor V, June CH, et al. 4-1BB is superior to CD28 costimulation for generating CD8+ cytotoxic lymphocytes for adoptive immunotherapy. J Immunol. 2007;179(7):4910–4918. doi: 10.4049/jimmunol.179.7.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Guinn BA, Bertram EM, DeBenedette MA, Berinstein NL, Watts TH. 4-1BBL enhances anti-tumor responses in the presence or absence of CD28 but CD28 is required for protective immunity against parental tumors. Cell Immunol. 2001;210(1):56–65. doi: 10.1006/cimm.2001.1804. [DOI] [PubMed] [Google Scholar]
- 127.Lee SJ, Rossi RJ, Lee SK, Croft M, Kwon BS, Mittler RS, et al. CD134 Costimulation Couples the CD137 Pathway to Induce Production of Supereffector CD8 T Cells That Become IL-7 Dependent. J Immunol. 2007;179(4):2203–2214. doi: 10.4049/jimmunol.179.4.2203. [DOI] [PubMed] [Google Scholar]
- 128.Wang C, Lin GH, McPherson AJ, Watts TH. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunol Rev. 2009;229(1):192–215. doi: 10.1111/j.1600-065X.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 129.Paulos CM, Suhoski MM, Plesa G, Jiang T, Basu S, Golovina TN, et al. Adoptive immunotherapy: good habits instilled at youth have long-term benefits. Immunol Res. 2008;42(1–3):182–196. doi: 10.1007/s12026-008-8070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hirano N, Butler MO, Xia Z, Ansen S, von Bergwelt-Baildon MS, Neuberg D, et al. Engagement of CD83 ligand induces prolonged expansion of CD8+ T cells and preferential enrichment for antigen specificity. Blood. 2006;107(4):1528–1536. doi: 10.1182/blood-2005-05-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Maus MV, Thomas AK, Leonard DG, Allman D, Addya K, Schlienger K, et al. Ex vivo expansion of polyclonal and antigen-specific cytotoxic T lymphocytes by artificial APCs expressing ligands for the T-cell receptor, CD28 and 4-1BB. Nat Biotechnol. 2002;20(2):143–148. doi: 10.1038/nbt0202-143. [DOI] [PubMed] [Google Scholar]
- 132.Butler MO, Ansen S, Tanaka M, Imataki O, Berezovskaya A, Mooney MM, et al. A panel of human cell-based artificial APC enables the expansion of long-lived antigen-specific CD4+ T cells restricted by prevalent HLA-DR alleles. Int Immunol. 2011;22(11):863–873. doi: 10.1093/intimm/dxq440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tanaka M, Butler MO, Ansen S, Imataki O, Berezovskaya A, Nadler LM, et al. Induction of HLA-DP4-restricted anti-survivin Th1 and Th2 responses using an artificial antigen-presenting cell. Clin Cancer Res. 2011;17(16):5392–5401. doi: 10.1158/1078-0432.CCR-10-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Friedman KM, Devillier LE, Feldman SA, Rosenberg SA, Dudley ME. Augmented lymphocyte expansion from solid tumors with engineered cells for costimulatory enhancement. J Immunother. 2011;34(9):651–661. doi: 10.1097/CJI.0b013e31823284c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ye Q, Loisiou M, Levine BL, Suhoski MM, Riley JL, June CH, et al. Engineered artificial antigen presenting cells facilitate direct and efficient expansion of tumor infiltrating lymphocytes. J Transl Med. 2011;9:131. doi: 10.1186/1479-5876-9-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Flaherty KT, Yasothan U, Kirkpatrick P. Vemurafenib. Nat Rev Drug Discov. 2011;10(11):811–812. doi: 10.1038/nrd3579. [DOI] [PubMed] [Google Scholar]
- 137.Ribas A, Flaherty KT. BRAF targeted therapy changes the treatment paradigm in melanoma. Nat Rev Clin Oncol. 2011;8(7):426–433. doi: 10.1038/nrclinonc.2011.69. [DOI] [PubMed] [Google Scholar]
- 138.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gadiot J, Hooijkaas AI, Kaiser AD, van Tinteren H, van Boven H, Blank C. Overall survival and PD-L1 expression in metastasized malignant melanoma. Cancer. 2011;117(10):2192–2201. doi: 10.1002/cncr.25747. [DOI] [PubMed] [Google Scholar]
- 140.Wong RM, Scotland RR, Lau RL, Wang C, Korman AJ, Kast WM, et al. Programmed death-1 blockade enhances expansion and functional capacity of human melanoma antigen-specific CTLs. Int Immunol. 2007;19(10):1223–1234. doi: 10.1093/intimm/dxm091. [DOI] [PubMed] [Google Scholar]
- 141.Weber J. Immunotherapy for melanoma. Curr Opin Oncol. 2011;23(2):163–169. doi: 10.1097/CCO.0b013e3283436e79. [DOI] [PubMed] [Google Scholar]
- 142.Griffith KD, Read EJ, Carrasquillo JA, Carter CS, Yang JC, Fisher B, et al. In vivo distribution of adoptively transferred indium-111-labeled tumor infiltrating lymphocytes and peripheral blood lymphocytes in patients with metastatic melanoma. J Natl Cancer Inst. 1989;81(22):1709–1717. doi: 10.1093/jnci/81.22.1709. [DOI] [PubMed] [Google Scholar]
- 143.Pockaj BA, Sherry RM, Wei JP, Yannelli JR, Carter CS, Leitman SF, et al. Localization of 111indium-labeled tumor infiltrating lymphocytes to tumor in patients receiving adoptive immunotherapy. Augmentation with cyclophosphamide and correlation with response. Cancer. 1994;73(6):1731–1737. doi: 10.1002/1097-0142(19940315)73:6<1731::aid-cncr2820730630>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 144.Kershaw MH, Teng MW, Smyth MJ, Darcy PK. Supernatural T cells: genetic modification of T cells for cancer therapy. Nat Rev Immunol. 2005;5(12):928–940. doi: 10.1038/nri1729. [DOI] [PubMed] [Google Scholar]
- 145.Peng W, Ye Y, Rabinovich BA, Liu C, Lou Y, Zhang M, et al. Transduction of tumor-specific T cells with CXCR2 chemokine receptor improves migration to tumor and antitumor immune responses. Clin Cancer Res. 2010;16(22):5458–5468. doi: 10.1158/1078-0432.CCR-10-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208(10):1949–1962. doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bennicelli JL, Guerry Dt. Production of multiple cytokines by cultured human melanomas. Exp Dermatol. 1993;2(4):186–190. doi: 10.1111/j.1600-0625.1993.tb00031.x. [DOI] [PubMed] [Google Scholar]
- 148.Bollard CM, Rossig C, Calonge MJ, Huls MH, Wagner HJ, Massague J, et al. Adapting a transforming growth factor beta-related tumor protection strategy to enhance antitumor immunity. Blood. 2002;99(9):3179–3187. doi: 10.1182/blood.v99.9.3179. [DOI] [PubMed] [Google Scholar]
- 149.Wagner HJ, Bollard CM, Vigouroux S, Huls MH, Anderson R, Prentice HG, et al. A strategy for treatment of Epstein-Barr virus-positive Hodgkin's disease by targeting interleukin 12 to the tumor environment using tumor antigen-specific T cells. Cancer Gene Ther. 2004;11(2):81–91. doi: 10.1038/sj.cgt.7700664. [DOI] [PubMed] [Google Scholar]
- 150.Zhang L, Kerkar SP, Yu Z, Zheng Z, Yang S, Restifo NP, et al. Improving adoptive T cell therapy by targeting and controlling IL-12 expression to the tumor environment. Mol Ther. 2011;19(4):751–759. doi: 10.1038/mt.2010.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Charo J, Finkelstein SE, Grewal N, Restifo NP, Robbins PF, Rosenberg SA. Bcl-2 overexpression enhances tumor-specific T-cell survival. Cancer Res. 2005;65(5):2001–2008. doi: 10.1158/0008-5472.CAN-04-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lin CM, Wang FH. Selective modification of antigen-specific CD4(+) T cells by retroviral-mediated gene transfer and in vitro sensitization with dendritic cells. Clin Immunol. 2002;104(1):58–66. doi: 10.1006/clim.2002.5229. [DOI] [PubMed] [Google Scholar]
- 153.Eaton D, Gilham DE, O'Neill A, Hawkins RE. Retroviral transduction of human peripheral blood lymphocytes with Bcl-X(L) promotes in vitro lymphocyte survival in pro-apoptotic conditions. Gene Ther. 2002;9(8):527–535. doi: 10.1038/sj.gt.3301685. [DOI] [PubMed] [Google Scholar]
- 154.Habib-Agahi M, Jaberipour M, Searle PF. 4-1BBL costimulation retrieves CD28 expression in activated T cells. Cell Immunol. 2009;256(1–2):39–46. doi: 10.1016/j.cellimm.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 155.Ekmekcioglu S, Ellerhorst JA, Prieto VG, Johnson MM, Broemeling LD, Grimm EA. Tumor iNOS predicts poor survival for stage III melanoma patients. Int J Cancer. 2006;119(4):861–866. doi: 10.1002/ijc.21767. [DOI] [PubMed] [Google Scholar]
- 156.Allin KH, Nordestgaard BG. Elevated C-reactive protein in the diagnosis, prognosis, and cause of cancer. Crit Rev Clin Lab Sci. 2011;48(4):155–170. doi: 10.3109/10408363.2011.599831. [DOI] [PubMed] [Google Scholar]
- 157.Chun YS, Wang Y, Vo AH, Lee JA. Elevated interleukin-12 is a plasma marker of poor prognosis in stage III melanoma patients. American Association of Cancer Research Annual Meeting; 2008 April 12–16; San Diego, CA. 2008. [Google Scholar]
- 158.Chun YS, Wang Y, Wang DY, Lee JE. S100B levels provide independent and complementary prognostic information to LDH levels in melanoma patients. American Society of Clinical Oncology Annual Meeting; 2008; Chicago, IL. 2008. [Google Scholar]
- 159.Tarhini AA, Stuckert J, Lee S, Sander C, Kirkwood JM. Prognostic significance of serum S100B protein in high-risk surgically resected melanoma patients participating in Intergroup Trial ECOG 1694. J Clin Oncol. 2009;27(1):38–44. doi: 10.1200/JCO.2008.17.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sabatino M, Kim-Schulze S, Panelli MC, Stroncek D, Wang E, Taback B, et al. Serum vascular endothelial growth factor and fibronectin predict clinical response to high-dose interleukin-2 therapy. J Clin Oncol. 2009;27(16):2645–2652. doi: 10.1200/JCO.2008.19.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 162.Hoos A, Eggermont AM, Janetzki S, Hodi FS, Ibrahim R, Anderson A, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102(18):1388–1397. doi: 10.1093/jnci/djq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]