Abstract
Functional magnetic resonance imaging (fMRI) has helped to elucidate the neurobiological bases of psychiatric and neurodevelopmental disorders by localizing etiologically-relevant aberrations in brain function. Functional MRI also has shown great promise to help understand potential mechanisms of action of effective treatments for a range of psychiatric and neurodevelopmental disorders, including mood and anxiety disorders, schizophrenia, and autism. However, the use of fMRI to probe intervention effects in psychiatry is associated with unique methodological considerations, including the psychometric properties of repeated fMRI scans, how to assess potential relations between the effects of an intervention on symptoms and on specific brain activation patterns, and how to best make causal inferences about intervention effects on brain function. Additionally, the study of treatment effects in neurodevelopmental disorders presents additional unique challenges related to brain maturation, analysis methods, and the potential for motion artifacts. We review these methodological considerations and provide recommendations for best practices for each of these topics.
Keywords: clinical trials, fMRI, functional magnetic resonance imaging, neurodevelopmental disorders, psychiatry
INTRODUCTION
Randomized controlled clinical trials in psychiatry are designed to evaluate the efficacy of an intervention [1, 2]. Such trials may rely on assessments of symptom severity, global functioning, and neurocognitive function as outcome measures. With the recent advent of functional brain imaging techniques, the opportunity exists to investigate the potential neurobiological mechanisms of action of interventions by comparing brain scans acquired before and after a particular treatment (or set of treatments). The use of cognitive neuroscience techniques, such as functional magnetic resonance imaging (fMRI), to address treatment effects represents a shift to a mechanistic approach to understanding not only disease states but also treatment effects and has the potential to reveal heretofore unknown neurobiological mechanisms of treatment effects. The evaluation of treatments via functional brain imaging more closely models early drug development and basic science approaches to screening new drug therapies and thus may promote swifter transition of agents to clinical trails [3]. However, the use of fMRI in evaluating treatment effects in psychiatry also involves a number of unique methodological considerations. Our purpose here is to outline important considerations to guide design decisions in the context of particular research questions. We have incorporated general experimental and methodological principles derived from our own fMRI intervention studies [4–7] to guide investigations of treatment effects in psychiatric and neurodevelopmental disorders via fMRI.
General Methodological Concerns
Psychometric Properties of Repeated fMRI Scans
Although a small handful of studies have examined pretreatment fMRI scans as predictors of treatment response [8], the most common neuroimaging clinical trial design in psychiatry involves the collection of symptom and fMRI data from a patient group at least twice, once before the initiation of treatment and once after treatment course. Although far less common, additional fMRI scans may also be collected mid-way through the course of treatment as well as after treatment termination to investigate the timecourse of effects on brain activation. All of the above contexts require the collection of multiple fMRI scans from patients receiving treatment. Given the longitudinal nature of intervention studies, brain imaging methods for evaluating treatment mechanisms must meet the same psychometric properties as traditional “paper-and-pencil” outcome measures, including high test–retest reliability [9], limited practice effects, and sensitivity to change [10]. Despite methodological advances in ways to assess the test–retest reliability of functional brain imaging data [11–13], only recently have the psychometrics of repeated fMRI assessments been evaluated, with initial results suggesting high reliability in some studies [14–16] and low reliability in other studies [17–20].
Recommendations
Cognitive tasks, data acquisition parameters, and analysis methods must be selected that have adequate test–retest stability prior to use in clinical trials [21]. Researchers should also consider that stability estimates may be highly variable even in non-clinical contexts, are likely poorer in clinical contexts, and may be differentially affected by various treatment approaches. We recommend assessing test–retest stability in both basic and clinical samples prior to implementing a neuroimaging clinical trial. We also recommend tasks that have been demonstrated to robustly activate relevant neurocircuitry by multiple investigative teams across sites. Though not directly related to the psychometrics of properties of repeated fMRI scans, we further recommend stock pulse sequences available on commonly used MR scanners to facilitate multi-site studies and the use of widely accepted fMRI analysis methods [16, 22, 23] to promote validation and replication across sites.
Study Design
Cognitive Task Selection
fMRI relies on cognitive tasks to engage relevant brain circuitry. Although it may be tempting to transfer behavioral tasks sensitive to treatment effects outside the scanner to fMRI contexts, this must be done with caution [24]. Tasks must be selected based on prior research suggesting functional deficits in specific brain circuits in the disorder of interest and hypothesized to be impacted by the treatment under study. In most contexts there is no consensus regarding “gold standard” cognitive neuroscience tasks to address treatment effects. This lack of consensus impedes cross-site validation and replication.
Recommendations
Working groups should establish optimal tasks to assess treatment effects via fMRI in different disease and treatment contexts. One notable example of this approach is the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia (CNTRICS) initiative, which has disseminated recommendations regarding cognitive paradigms to best measure relevant constructs in the context of schizophrenia clinical trials [25]. We recommend similar initiatives for other psychiatric and neurodevelopmental disorders to facilitate neuroimaging intervention trials.
Selection of Comparison Groups
Evaluating the effects of novel interventions using fMRI may be accomplished via different designs that largely parallel methods used in traditional clinical trials [26]. It is well known that clinical trials produce improvement in symptom measures with nonspecific origins (i.e., “trial effects”). Such effects may be due to active treatment effects, placebo effects (e.g., expectancy effects, nonspecific effects of receiving treatment), Hawthorne effects (i.e., improvements due to being in a research study), and observer effects (i.e., improvements due to being observed) [27]. Further, it is well established that even inactive placebo treatments may impact global functioning as well as fMRI outcomes [28–31].
The following designs options each have unique strengths and weaknesses for establishing potential treatment mechanisms of action.
Comparison of pre- and posttreatment scans in a single group of patients receiving a treatment (i.e., a single-group design) [32].
Comparison of pre- and posttreatment scans in a patient group to a single brain scan in a control group [33].
Comparison of pre- and posttreatment scans in a treatment group to repeated scans in a control group [5].
Comparison of pre- and posttreatment scans in a patient group receiving an active treatment to repeated scans in a patient group receiving no treatment (i.e., wait-list control design) [34].
Double-blind comparison of pre- and posttreatment scans in a patient group receiving an active treatment to repeated scans in patient group receiving a placebo treatment [35].
Comparison of pre- and posttreatment scans in patients groups receiving different active pharmacologic (i.e., an active medication comparator) or behavioral (i.e., a behavioral comparator) treatments either randomly assigned [36] or based on patient preference [37] (may be conducted either as double-blind or open-label). The goal of this approach is to determine if the effects on brain function of the different treatments are equivalent or distinct.
Recommendations
The single-group design (#1 above) confounds treatment effects with a number of other nonspecific factors and is thus recommended only for initial pilot studies. Comparison of pre- and post-treatment scans in a patient group to a single brain scan from a control group (#2 above) is not recommended because time, practice, and placebo effects are not modeled. Comparisons of pre- and posttreatment scans in a patient group to repeated scans in a control group (#3 above) allows for modeling the effects of repeated scans; however, this approach does not account for placebo effects or differential fMRI stability in patient and control groups. Thus, when adequate samples are available, the use of two patient arms (#4, #5, and #6 above) is optimal. Wait-list comparisons (#4 above) do not allow for assessment of placebo effects on brain function, which are know to be robust [38]. Thus, a double-blind placebo comparison design (#5 above) is recommended because it adequately controls for placebo response and allows for modeling fMRI stability in a patient sample. Additionally, comparisons of two active treatments (#6 above), preferably with relatively similar rates of side effects, allows for a comparison of the relative specificity of different treatments on brain function. It should be noted that when resources allow, approaches #5 and #6 may include a true control group (i.e., disorder-free) as well to establish that both patient groups replicate patterns of differences from controls at pretreatment baseline scans. Although other, more complex approaches are possible (e.g., crossover designs, nested case-control studies, discontinuation designs, etc.), they are generally not used in neuroimaging clinical trials because of their complexity and associated costs.
Effects of Psychopharmacologic Agents on the BOLD Response
Quantitative fMRI
One of the major obstacles to the application of blood oxygen level dependent (BOLD) fMRI to assess effects of pharmacologic interventions is that BOLD fMRI is not a quantitative metric. In other words, BOLD fMRI as typically implemented provides measures of relative changes in brain function across conditions, but does not provide activation metrics in meaningful absolute units. To date, BOLD imaging largely remains a qualitative assessment of brain function, and the relevant signal changes can be affected by the combined results of neuronal activity, cerebral blood flow (CBF), cerebral blood volume (CBV), and cerebral oxygen metabolism (CMRO2). Variables such as caffeine, nicotine, pharmacologic agents, and varying severity of disease states can drastically impact resting brain perfusion and thus the hemodynamic properties and BOLD response in the brain [39, 40].
Recommendations
Researchers have investigated quantitative relations between blood oxygenation and brain metabolism over the past decade and have suggested using calibrated fMRI to achieve improved quantitative correlation with neuronal activity [41–44]. Specifically, calibrated fMRI relies on complementary measurements of CBF and CBV changes to calibrate common BOLD activation so that a specific and quantitative measurement of the regional energy metabolism in CMRO2, which is directly proportional to neuronal activity, can be obtained. Recent reports using arterial spin labeling (ASL) techniques further suggest that dynamic measurements of CBF changes can be used to calibrate the BOLD signal [45, 46]. However, calibrated fMRI that collects dynamic BOLD and perfusion timecourses can often be time-consuming and impractical in human experiments. As such, simplified approaches that acquire only the baseline perfusion signal to normalize the BOLD signal changes may be a practical solution.
In experiments where high temporal resolution is not critical, a simultaneous acquisition of dynamic perfusion and BOLD contrasts may be adopted to achieve improved quantification of the BOLD signal. However, in contexts where high temporal resolution is required, or when the baseline perfusion level varies at a much slower temporal scale, infrequent sampling of baseline perfusion (e.g., using ASL) can be introduced among the BOLD timecourse acquisition, such that these baseline perfusion levels can be incorporated into functional analysis to remove their impact on the BOLD signal. As a result, improved correlation between the BOLD signals and underlying neuronal activity can be obtained [45].
Interpretation and Possible Confounds
Etiologic Relevance
It may be tempting to infer etiologic relevance of a particular brain region or network for a disorder based on data indicating changes in activation in that region after treatment. However, it is critical to recognize that observing changes in brain activation due to a given treatment for a psychiatric condition does not necessarily suggest etiologic relevance of the brain region for the disorder or even for treatment effects. Put more simply, intervention effects do not necessarily indicate a causal mechanism of disease development or treatment effects. To illustrate, although aspirin relieves muscle pain, muscle pain is not caused by a lack of aspirin. Similarly, although a given intervention may change activation within a given brain network, atypical activation of that same network may not necessarily be etiologically relevant for a given disorder.
Recommendations
Inferring etiology on the basis of treatment effects must be done with caution and only on the basis of a compelling theoretical framework and auxiliary supporting data. Inferences of causality are bolstered by sequentially demonstrating abnormal brain activation in patients relative to controls as well as normalization of brain activation following treatment (i.e., a discontinuation design). In disorders where full remission is possible, it is critical to establish normalization of brain activation patterns in individuals with a history of the disorders (i.e., trait effects) as well as in individuals at risk for the disorders but currently asymptomatic (e.g., prodromal individuals or first-degree relatives of affected patients) [47]. Given these cautions, however, observing theoretically predicted changes in brain function in response to treatment may corroborate and substantiate a nomological network articulating a theory of disease etiology1.
Intervention Effects on Symptoms Versus Brain Activation
Neuroimaging clinical trials involve collection of both symptom and fMRI data. Given the widespread heterogeneity in response to psychiatric treatments [48, 49], it is not surprising that not all patients experience significant symptom reductions even in the context of widely used and effective treatments. Additionally, given the wide interindividuals differences in task-related neural activation patterns [50], it is similarly not surprising that changes in regional brain activation patterns are variable even in the context of patients with similar symptom profiles and similar profiles of symptom reductions. Thus, researchers must consider the nearly ubiquitous scenario of symptoms changes in the absence of changes in brain function or brain function changes in the absence of changes in symptoms. Additionally, psychiatric treatments may change patterns of behavior during fMRI task performance [51], resulting in yet another dissociation that must be resolved to optimally interpret neuroimaging treatment trials.
Recommendations
Investigators must specify prior to trial initiation whether symptom profiles, behavioral performance, or neuroimaging measures are of primary interest. Generally, traditional clinical trials require large samples across multiple sites and thus are not optimally suited to examine the effects of interventions on brain activation because methods for multi-site fMRI studies are still evolving [16]. In contexts where clinical efficacy is of primary concern (e.g., phase III clinical trials), neuroimaging measures may not be warranted. We thus recommend that in most contexts fMRI intervention studies examine the effects of interventions that have been previously established as effective in traditional clinical trials. This approach allows for a priori hypotheses concerning intervention effects on brain imaging metrics.
Alternatively, in certain contexts brain imaging may be used in an experimental manner to test the effects of novel treatments. In this framework, fMRI tasks may be used to evaluate changes in neural activation or connectivity associated with changes in symptom severity. fMRI tasks capable of indexing symptom change can then be used to help develop treatments that are designed to target neural circuitry changes associated with symptom change. One example of this is research on the effects of instructional treatment for dyslexia that has yielded information about novel targets for interventions [52–55].
Heterogeneity of Treatment Response
In nearly all contexts, the effects of psychiatric interventions are highly heterogenous. [48, 49] Thus, a primary motivating factor in neuroimaging clinical trials is to link changes in brain function with variability in treatment response.
There are two primary analytic approaches to address heterogenous treatment response:
Comparison of responders versus nonresponders: Symptom-based cutoff scores determining treatment responder status may be established a priori and then treatment-related changes in brain activation may be compared between responders and nonresponders. However, this strategy may have relatively low-statistical power to detect effects because of the sample sizes of responder and/or nonresponders subgroups. Additionally, patients with nearly equivalent treatment response may be categorized to different responder groups (i.e., one as a responder and one as a nonresponder).
Covariation of treatment response: An alternative analytic approach is to covary treatment response as a continuous variable in the analysis of neuroimaging data. This approach addresses parametric linkages between changes in regional brain function and the magnitude of symptom changes. In many contexts, the covariation approach will yield greater statistical power because the dimensional nature of the clinical outcome measure is maintained.
Treatment Effects on fMRI Task Performance
Interventions designed to ameliorate core symptoms of a disorder may produce improved neurocognitive function that in turn improves fMRI task performance (e.g., accuracy, reaction time, or eye gaze patterns).
Recommendations
It contexts where fMRI task performance is not related to the putative effects of an intervention, task performance should be equated (if possible) [51] or modeled during fMRI data analyses [56]. However, caution is warranted because improved task performance may be related to intervention effects in unforeseen ways, and thus group matching or covariation may attenuate power to detect treatment effects on brain activation.
Psychopharmacologic Side Effects and Dosing
Recommendations
Side effects are common in psychopharmacologic trials, even amongst patients receiving blinded placebo treatments [27]. Side effects that impact attention (e.g., dizziness, drowsiness) may affect task-related brain activation as well. Additionally, there may be differential effects on brain activation of differing medication dosages, even in the context of similar clinical benefits.
Recommendations
If sample sizes allow, secondary analyses should be conducted that subgroup patients on the basis of particular side effects profiles or by final medication dosage or that analyze only the subgroup of patients without significant side effects or with the same final medication dosage.
Considerations for fMRI Intervention Studies in Neurodevelopmental Disorders
Excessive Motion Artifacts
As with all neuroimaging studies, data quality in neuroimaging clinical trials is paramount. Data quality may be compromised by noise intensity spikes, ghosting, flow artifact, susceptibility artifacts, physiological noise (breathing, heartbeat), and participant motion. Participant motion is a critical consideration in the context of neurodevelopmental disorders (e.g., autism, Fragile X syndrome) that are, by definition, present in childhood and, in many contexts, are characterized by either movement disorders and/or repetitive behaviors [57]. Additionally, when scanning young children, the presence of anxiety increases the likelihood of participant motion. Finally, certain treatments, including neuroleptic agents, may produce extrapyramidal side effects that increase head motion [58]. Thus, issues of data quality related to participant motion are particularly critical in fMRI clinical trials involving pediatric samples with neurodevelopmental disorders.
Recommendations
Minimizing participant motion is vital because task-related changes in BOLD signals are relatively small compared to motion-related changes in BOLD signal [59], and mathematical corrections for head motion are imperfect [60]. Further, signal changes due to motion may correlate with functional BOLD signal changes resulting in activation patterns that spuriously appear to be a function of the fMRI task [61]. Measures of heart rate and respiration should be collected and modeled during data analysis [62, 63] (see Figure 1). Controlling for head motion is critical, via bite bars, semiflexible face masks, head-conforming foam, or pillows. As in all pediatric and clinical studies, scanner acclimation involving a mock scanner is essential (these methods have been detailed elsewhere [64, 65]), and there are established behavioral paradigms to promote decreased motion during scanning [66, 67]. These considerations are particularly salient in the context of neurodevelopmental disorders where anxiety is prevalent and may systematically attenuate with repeated scan sessions. Additionally, self-report measures of anxiety collected at pre- and posttreatment scans may be used to assess for differences in anxiety levels that may be statistically modeled during data analysis [68], though this approach may be problematic in treatments designed to reduce anxiety or in disorders where self-reports are of questionable validity.
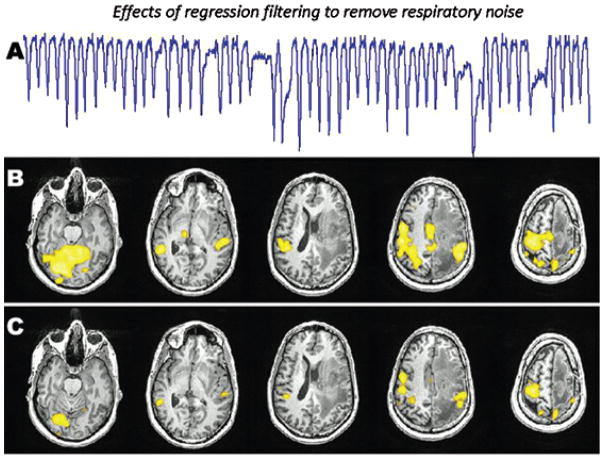
FIGURE 1.
Functional MRI (fMRI) activation maps depicting the effects of regression filtering to remove respiratory noise. The fMRI task is a simple hand sensorimotor task. This patient had a large left frontal glioblastoma and irregularity in his respiration pattern during the scan (A) that caused significant artifacts in the motor activation map (B). These artifacts were filtered out to produce a more specific motor function map (C). The noise removal was done using regression filtering implemented in fScan [63] based on the RETROICOR method of Glover and colleagues [90].
Effects of Development on Intervention Effects
As discussed in a preceding section, dissociations may exist between the effects of an intervention on symptom profiles, regional brain activation, and task-related behavior. These potential confound are compounded in the context of pediatric neuroimaging treatment trials, where relations between symptoms, behavior, and brain activation may be variable across development. Additionally, such interactive effects may be heterogenous in pediatric disorders [69].
Recommendations
Similar to prior recommendations in the context of adult neuroimaging treatment trials, investigators must specify a priori whether symptom profiles, behavioral performance, or neuroimaging measures are of primary interest. In the context of pediatric treatment trials, it is critical to consider developmental effects on the specified target of the intervention. Initial neuroimaging investigations of treatment trials should begin with studies using adult sample and then systematically downward extend to younger samples in order to methodically understand the complex interactions between development and treatment effects on brain, behavior, and symptoms.
Differences in Brain Morphometry
As in cross-sectional clinical fMRI studies, treatment fMRI studies are sensitive to potential differences in the size of various brain regions in clinical contexts [70]. Volume differences have been identified in key neuroanatomic regions in numerous neurodevelopmental disorders, including autism [71], Fragile X syndrome [72], and Williams Syndrome [73].
Recommendations
Normalization to standard stereotaxic space in fMRI treatment studies of neurodevelopmental disorders should be done with caution. When differences in brain morphology have been documented, regional mean signal change analyses performed in native subject space may be optimal [60, 74]. An additional consideration is that, although most fMRI studies test for regional differences in MR signal intensity, differences in brain morphometry may manifest in terms of the spatial extent, rather than magnitude, of brain activity [75]. Thus, effects of signal extent, as well as intensity, should be evaluated.
Analysis of Pediatric Neuroimaging Data
Although fMRI studies of neurodevelopmental disorders typically include pediatric samples [76, 77], and non-clinical fMRI studies have been carried out even with infants [78], best practices for the analysis of pediatric brain imaging data are still evolving [51]. Similar to cross-sectional pediatric functional brain imaging studies, researchers conducting pediatric treatment neuroimaging trials must decide whether to analyze pediatric brain imaging data using normalization to adult template brains, normalization to pediatric template brains, or in native space using either automated or expert manual segmentation.
Recommendations
Despite initial evidence that brain imaging data from children as young as 7 years old may be adequately normalized with adult brain templates [79, 80] the use of adult brain templates and atlases for normalization and segmentation pediatric brain imaging data may impose biases and limitations for estimates of both brain structure and brain function [81, 82]. Multiple pediatric brain templates and pediatric brain scan data repositories are available [81, 83–86]. These templates allow for automated analytic procedures with accurate spatial normalization for adults and children. Additionally, there is initial evidence that study-specific “internal” or “local” pediatric templates (i.e., derived by averaging brains of study subjects themselves) may provide reasonable estimates of brain structure relative to “external” pediatric templates [82]. In summary, although there is not yet clear consensus regarding whether to use adult template, pediatric template, or native space approaches, there is initial evidence that in the context of template approaches, pediatric templates, rather than adult reference data, should be used.
There are no studies addressing direct comparisons between results using expert manual segmentation of pediatric brain imaging data versus normalization to appropriate pediatric templates, and this more research is needed to provide firm guidance in this regard. It is clear that there is a great deal of individual variability in brain morphemetry, even in healthy adults, prompting some to suggest that normalization to standard stereotaxic space should be done with caution [74, 87], particularly in the context of pediatric samples [65]. It should be noted that currently available pediatric templates are derived from nonclinical samples, and thus the suitability of such templates for longitudinal studies involving samples with neurodevelopmental disorders in not yet known. Future research comparing expert hand tracing to age-appropriate pediatric samples derived from cohorts with different forms of psychiatric and neurodevelopmental disorders are needed before best practices can be determined. Future research is also needed to examine linkages between the differences in brain morphemetry across development and differences in the extent and magnitude of neural activations.
REPRESENTATIVE RESULTS
Preliminary Case Study Findings
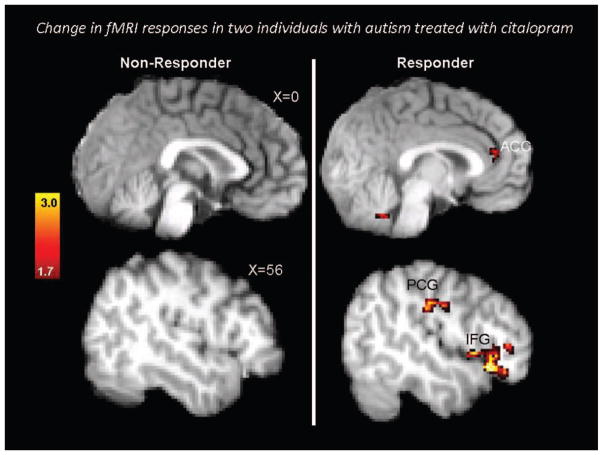
Figure 2 illustrates preliminary case study findings from two case studies randomized to the active study arm in a larger double-blind randomized controlled trial comparing citalopram treatment to placebo treatment in adults with diagnoses of Autistic Disorder or Asperger’s Disorder and moderate-to-high levels of repetitive behaviors [7]. Participants completed an fMRI oddball target detection task both before and after approximately 12 weeks of treatment with citalopram, a selective serotonin reuptake inhibitor. This task is sensitive to functional frontostriatal abnormalities in individuals with autism spectrum disorders that are correlated with the severity of repetitive behaviors [88, 89]. One participant (Case 1) showed no reductions in repetitive behaviors whereas the other (Case 2) showed marked reductions. Brain activation in relevant prefrontal regions, including the dorsal anterior cingulate cortex, increased in only the participant whose repetitive behavior symptoms improved. Although these are case study findings and thus must be interpreted with caution, these proof-of-principle findings suggest that functional brain imaging may be valuable for initial studies designed to elucidate potential mechanisms of action of targeted interventions. We highlight, however, the further research with adequate samples would be essential to derive any substantive findings from these case study reports.
FIGURE 2.
Single-subject fMRI activation change maps in native subject space depicting areas of statistically increased activation after citalopram treatment for repetitive behaviors in two individuals with autism spectrum disorders. The fMRI task was an oddball target detection paradigm designed to recruit brain areas subserving cognitive control. Case 1 (left) was a treatment nonresponder and Case 2 (right) was a treatment-responder. The figure illustrates increased activation in relevant brain areas in the treatment responder but not in the treatment nonresponder. Contrasts are thresholded at corrected Z > 1.7. Reprinted from Dichter et al (2010) [7].
Prediction of Treatment Response
Figure 3 illustrates results from an antidepressant treatment fMRI trial [5, 6]. Outpatients with unipolar major depressive disorder participated in a pretreatment fMRI scans that utilized a Wheel of Fortune decision-making and reward processing task [Ernst, 2004]. After the pre-treatment scans, patients received a 9-week course of behavioral activation treatment, a form of individual psychotherapy. The figure demonstrates an activation cluster during the reward selection phase of the task that predicted subsequent response to treatment. Future research will evaluate the prospective utility of this finding to predict treatment response in a new group of patients. More generally, this approach illustrates the potential utility of using pretreatment fMRI data to predict individualized treatment response.
FIGURE 3.
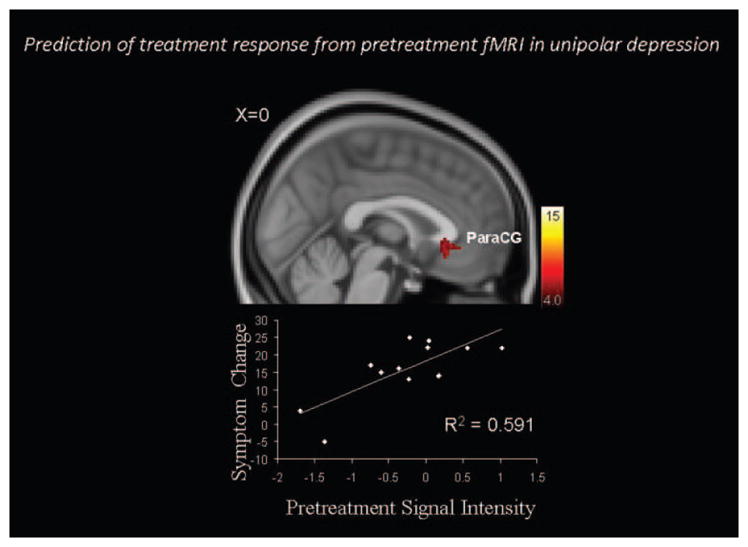
Relations between pre-treatment fMRI and response to psychotherapy treatment in unipolar depression. The top panel illustrates a cluster within the ventral paracingulate gyrus (ParaCG) that predicted change in Hamilton Rating Scale for Depression (HAM-D) [91] scores after psychotherapy in individuals with unipolar major depressive disorder. The fMRI task focused on decision making and reward processing. Contrasts are thresholded at corrected Z > 4.0. Adapted from Dichter et al (2009) [5].
Mechanisms of Treatment Effects
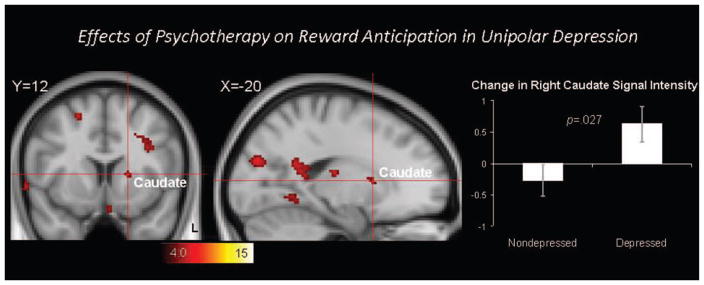
Figure 4 illustrates the effects of psychotherapy on brain responses during reward anticipation in the same group of outpatients with unipolar depression [5]. Depressed outpatients repeated the same reward-processing task after completing the course of psychotherapy. To model the effects of repeated scans, a group of matched nondepressed control participants were scanned on two occasions separated by the same length of time. The clusters in the figure, including the right caudate nucleus, indicate brain regions with significantly different change in activation in the depressed group relative to the non-depressed group. This approach illustrates not only the potential to use functional brain imaging to probe the effects of an intervention of brain function, but one approach to controlling for repeated scan sessions when examining treatment effects.
FIGURE 4.
The effects of psychotherapy on reward anticipation in unipolar depression were examined by comparing the change in brain activation in depression patients scanned before and after treatment with the change in brain activation observed in controls scanned twice. The red clusters reflect significant Group (depressed, non-depressed) X Time (Time 1, Time 2) interaction effects, and include the right caudate cluster highlighted by the cross hair. The bar graph illustrates that the depressed group showed significantly increased activation in this right caudate cluster after psychotherapy. Contrasts are thresholded at corrected Z > 4.0. Error bars represent standard errors of the mean. Adapted from Dichter et al (2009) [5].
SUMMARY
The emergence of fMRI as a powerful cognitive neuroscience tool to study brain processes has facilitated clinical research into the neural underpinnings of psychiatric disorders. A relatively novel application of fMRI is to study the effects of behavioral and psychopharmacologic treatments for psychiatric disorders. The ultimate promise of this approach is to understand the potential mechanisms of action of effective interventions, to stimulate the development of novel compounds by understanding the neurobiological effects of treatment agents, to better understand treatment response heterogeneity, and to prospectively predict treatment response via pretreatment fMRI scans. Advances in pediatric fMRI allow for inclusion of young children in fMRI protocols [64], enabling the use of fMRI to investigate treatment effects in pediatric samples with neurodevelopmental disorders.
Despite the knowledge to be gained by using fMRI in clinical trial contexts, this approach holds a number of methodological challenges. Although optimal designs are in many cases context-specific, general recommendations include evaluation of fMRI test–retest stability, standardization of fMRI task selection, selection of optimal comparison groups and study designs, a priori identification of primary outcome measures, matching or controlling for differences in fMRI task performance, side effects and dosing, the use of quantitative fMRI metrics, covariation of physiologic signals, adequate control of subject motion, fMRI acclimation, and the use of pediatric templates when templates are used. The adoption of best practices will increase the pace of fMRI intervention studies, thereby facilitating the understanding of treatment effects and the development of novel interventions.
Acknowledgments
Preparation of this manuscript was supported by K23 MH081285 to G. Dichter.
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Footnotes
We thank an anonymous reviewer for raising this point.
References
- 1.March JS, Silva SG, Compton S, Shapiro M, Califf R, Krishnan R. The case for practical clinical trials in psychiatry. Am J Psychiat. 2005;162(5):836–46. doi: 10.1176/appi.ajp.162.5.836. [DOI] [PubMed] [Google Scholar]
- 2.Summerfelt WT, Meltzer HY. Efficacy vs. effectiveness in psychiatric research. Psychiatr Serv. 1998;49(6):834–5. doi: 10.1176/ps.49.6.834. [DOI] [PubMed] [Google Scholar]
- 3.Webb S. Drugmakers dance with autism. Nat Biotechnol. 2010;28(8):772–4. doi: 10.1038/nbt0810-772. [DOI] [PubMed] [Google Scholar]
- 4.Dichter G, Sikich Greeter S, Alderman C, Rittenberg A, Holtzclaw T, et al. fMRI of citalopram treatment in autism. Presented at the 10th Annual International Meeting for Autism Research (IMFAR); 2010 May. [Google Scholar]
- 5.Dichter GS, Felder JN, Petty C, Bizzell J, Ernst M, Smoski MJ. The effects of psychotherapy on neural responses to rewards in major depression. Biol Psychiat. 2009;66(9):886–97. doi: 10.1016/j.biopsych.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dichter GS, Felder JN, Smoski MJ. The effects of brief behavioral activation therapy for depression on cognitive control in affective contexts: an fMRI investigation. J Affect Disorders. 2010 doi: 10.1016/j.jad.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dichter GS, Felder J, Sikich L, Mahorney S, Bodfish J. fMRI activation change tracks medication-induced reductions in repetitive behaviors in autism: two case studies. Neurocase. 2010;16(4):307–16. doi: 10.1080/13554790903559671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siegle GJ, Carter CS, Thase ME. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. Am J Psychiat. 2006;163(4):735–8. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- 9.Tomarken AJ. A psychometric perspective on psychophysiological measures. Psychol Assess. 1995;7(3):387–95. [Google Scholar]
- 10.Carter CS, Barch DM, Buchanan RW, Bullmore E, Krystal JH, Cohen J, et al. Identifying cognitive mechanisms targeted for treatment development in schizophrenia: an overview of the first meeting of the cognitive neuroscience treatment research to improve cognition in schizophrenia initiative. Biol Psychiat. 2008;64(1):4–10. doi: 10.1016/j.biopsych.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caceres A, Hall DL, Zelaya FO, Williams SC, Mehta MA. Measuring fMRI reliability with the intra-class correlation coefficient. Neuroimage. 2009;45(3):758–68. doi: 10.1016/j.neuroimage.2008.12.035. [DOI] [PubMed] [Google Scholar]
- 12.Raemaekers M, Vink M, Zandbelt B, van Wezel RJ, Kahn RS, Ramsey NF. Test-retest reliability of fMRI activation during prosaccades and antisaccades. Neuroimage. 2007;36(3):532–42. doi: 10.1016/j.neuroimage.2007.03.061. [DOI] [PubMed] [Google Scholar]
- 13.Zandbelt BB, Gladwin TE, Raemaekers M, van Buuren M, Neggers SF, Kahn RS, et al. Within-subject variation in BOLD-fMRI signal changes across repeated measurements: quantification and implications for sample size. Neuroimage. 2008;42(1):196–206. doi: 10.1016/j.neuroimage.2008.04.183. [DOI] [PubMed] [Google Scholar]
- 14.Specht K, Willmes K, Shah NJ, Jancke L. Assessment of reliability in functional imaging studies. J Magn Reson Im. 2003;17(4):463–71. doi: 10.1002/jmri.10277. [DOI] [PubMed] [Google Scholar]
- 15.Aron AR, Gluck MA, Poldrack RA. Long-term test-retest reliability of functional MRI in a classification learning task. Neuroimage. 2006;29(3):1000–6. doi: 10.1016/j.neuroimage.2005.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friedman L, Stern H, Brown GG, Mathalon DH, Turner J, Glover GH, et al. Test-retest and between-site reliability in a multicenter fMRI study. Hum Brain Mapp. 2008;29(8):958–72. doi: 10.1002/hbm.20440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manoach DS, Halpern EF, Kramer TS, Chang Y, Goff DC, Rauch SL, et al. Test-retest reliability of a functional MRI working memory paradigm in normal and schizophrenic subjects. Am J Psychiat. 2001;158(6):955–8. doi: 10.1176/appi.ajp.158.6.955. [DOI] [PubMed] [Google Scholar]
- 18.Wei X, Yoo SS, Dickey CC, Zou KH, Guttmann CR, Panych LP. Functional MRI of auditory verbal working memory: long-term reproducibility analysis. Neuroimage. 2004;21(3):1000–8. doi: 10.1016/j.neuroimage.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 19.Wagner K, Frings L, Quiske A, Unterrainer J, Schwarzwald R, Spreer J, et al. The reliability of fMRI activations in the medial temporal lobes in a verbal episodic memory task. Neuroimage. 2005;28(1):122–31. doi: 10.1016/j.neuroimage.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 20.McGonigle DJ, Howseman AM, Athwal BS, Friston KJ, Frackowiak RS, Holmes AP. Variability in fMRI: an examination of intersession differences. Neuroimage. 2000;11(6 Pt 1):708–34. doi: 10.1006/nimg.2000.0562. [DOI] [PubMed] [Google Scholar]
- 21.Barch DM, Mathalon DH. Using brain imaging measures in studies of procognitive pharmacologic agents in schizophrenia: psychometric and quality assurance considerations. Biol Psychiat. 2011;70(1):13–8. doi: 10.1016/j.biopsych.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poldrack RA. Region of interest analysis for fMRI. Soc Cogn Affect Neurosci. 2007;2(1):67–70. doi: 10.1093/scan/nsm006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luna B, Velanova K, Geier CF. Methodological approaches in developmental neuroimaging studies. Hum Brain Mapp. 2010;31(6):863–71. doi: 10.1002/hbm.21073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casey BJ, Cohen JD, Jezzard P, Turner R, Noll DC, Trainor RJ, et al. Activation of prefrontal cortex in children during a non-spatial working memory task with functional MRI. Neuroimage. 1995;2(3):221–9. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- 25.Barch DM, Carter CS, Arnsten A, Buchanan RW, Cohen JD, Geyer M, et al. Selecting paradigms from cognitive neuroscience for translation into use in clinical trials: proceedings of the third CNTRICS meeting. Schizophr Bull. 2009;35(1):109–14. doi: 10.1093/schbul/sbn163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sikich L. Psychopharmacologic treatment studies in Autism. In: Schopler E, Yirmiya N, Shulman C, Marcus LM, editors. The research basis for autism intervention. New York: Kluver Academic/Plenum Publishers; 2001. [Google Scholar]
- 27.Braunholtz DA, Edwards SJ, Lilford RJ. Are randomized clinical trials good for us (in the short term)? Evidence for a “trial effect”. J Clin Epidemiol. 2001;54(3):217–24. doi: 10.1016/s0895-4356(00)00305-x. [DOI] [PubMed] [Google Scholar]
- 28.Diederich NJ, Goetz CG. The placebo treatments in neurosciences: new insights from clinical and neuroimaging studies. Neurology. 2008;71(9):677–84. doi: 10.1212/01.wnl.0000324635.49971.3d. [DOI] [PubMed] [Google Scholar]
- 29.Enck P, Benedetti F, Schedlowski M. New insights into the placebo and nocebo responses. Neuron. 2008;59(2):195–206. doi: 10.1016/j.neuron.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 30.Ernst E. Placebo: new insights into an old enigma. Drug Discov Today. 2007;12(9–10):413–8. doi: 10.1016/j.drudis.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Oken BS. The placebo treatments in neurosciences: new insights from clinical and neuroimaging studies. Neurology. 2009;72(23):2053. doi: 10.1212/01.wnl.0000349697.25117.41. author reply 2053–54. [DOI] [PubMed] [Google Scholar]
- 32.Holcomb HH, Cascella NG, Thaker GK, Medoff DR, Dannals RF, Tamminga CA. Functional sites of neuroleptic drug action in the human brain: PET/FDG studies with and without haloperidol. Am J Psychiat. 1996;153(1):41–9. doi: 10.1176/ajp.153.1.41. [DOI] [PubMed] [Google Scholar]
- 33.DeRubeis RJ, Siegle GJ, Hollon SD. Cognitive therapy versus medication for depression: treatment outcomes and neural mechanisms. Nat Rev Neurosci. 2008;9(10):788–96. doi: 10.1038/nrn2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehto SM, Tolmunen T, Joensuu M, Saarinen PI, Valkonen-Korhonen M, Vanninen R, et al. Changes in midbrain serotonin transporter availability in atypically depressed subjects after one year of psychotherapy. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(1):229–37. doi: 10.1016/j.pnpbp.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 35.Haut KM, Lim KO, MacDonald A., 3rd Prefrontal cortical changes following cognitive training in patients with chronic schizophrenia: effects of practice, generalization, and specificity. Neuropsychopharmacology. 2010;35(9):1850–9. doi: 10.1038/npp.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kennedy SH, Konarski JZ, Segal ZV, Lau MA, Bieling PJ, McIntyre RS, et al. Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. Am J Psychiat. 2007;164(5):778–88. doi: 10.1176/ajp.2007.164.5.778. [DOI] [PubMed] [Google Scholar]
- 37.Brody AL, Saxena S, Stoessel P, Gillies LA, Fairbanks LA, Alborzian S, et al. Regional brain metabolic changes in patients with major depression treated with either paroxetine or interpersonal therapy: preliminary findings. Arch Gen Psychiatry. 2001;58(7):631–40. doi: 10.1001/archpsyc.58.7.631. [DOI] [PubMed] [Google Scholar]
- 38.Zubieta JK, Stohler CS. Neurobiological mechanisms of placebo responses. Ann NY Acad Sci. 2009;1156:198–210. doi: 10.1111/j.1749-6632.2009.04424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown GG, Perthen JE, Liu TT, Buxton RB. A primer on functional magnetic resonance imaging. Neuropsychol Rev. 2007;17(2):107–25. doi: 10.1007/s11065-007-9028-8. [DOI] [PubMed] [Google Scholar]
- 40.Brown GG, Eyler Zorrilla LT, Georgy B, Kindermann SS, Wong EC, Buxton RB. BOLD and perfusion response to finger-thumb apposition after acetazolamide administration: differential relationship to global perfusion. J Cereb Blood Flow Metab. 2003;23(7):829–37. doi: 10.1097/01.WCB.0000071887.63724.B2. [DOI] [PubMed] [Google Scholar]
- 41.Buxton RB, Wong EC, Frank LR. Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magn Reson Med. 1998;39(6):855–64. doi: 10.1002/mrm.1910390602. [DOI] [PubMed] [Google Scholar]
- 42.van Zijl PC, Eleff SM, Ulatowski JA, Oja JM, Ulug AM, Traystman RJ, et al. Quantitative assessment of blood flow, blood volume and blood oxygenation effects in functional magnetic resonance imaging. Nat Med. 1998;4(2):159–67. doi: 10.1038/nm0298-159. [DOI] [PubMed] [Google Scholar]
- 43.Hoge RD, Atkinson J, Gill B, Crelier GR, Marrett S, Pike GB. Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: the deoxyhemoglobin dilution model. Magn Reson Med. 1999;42(5):849–63. doi: 10.1002/(sici)1522-2594(199911)42:5<849::aid-mrm4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 44.Hyder F, Kida I, Behar KL, Kennan RP, Maciejewski PK, Rothman DL. Quantitative functional imaging of the brain: towards mapping neuronal activity by BOLD fMRI. NMR Biomed. 2001;14(7–8):413–31. doi: 10.1002/nbm.733. [DOI] [PubMed] [Google Scholar]
- 45.Liu TT, Wong EC, Frank LR, Buxton RB. Analysis and design of perfusion-based event-related fMRI experiments. Neuroimage. 2002;16(1):269–82. doi: 10.1006/nimg.2001.1038. [DOI] [PubMed] [Google Scholar]
- 46.Restom K, Perthen JE, Liu TT. Calibrated fMRI in the medial temporal lobe during a memory-encoding task. Neuroimage. 2008;40(4):1495–502. doi: 10.1016/j.neuroimage.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tomarken AJ. Focus chapter: methodological issues in psychophysiological research. In: Kendall PC, Butcher JN, Holmbeck GN, editors. Handbook of research methods in clinical psychology. 2. New York: John Wiley & Sons, Inc; 1999. [Google Scholar]
- 48.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiat. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 49.Bent S, Hendren RL. Improving the prediction of response to therapy in autism. Neurotherapeutics. 2010;7(3):232–40. doi: 10.1016/j.nurt.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raemaekers M, du Plessis S, Ramsey NF, Weusten JM, Vink M. Test-retest variability underlying fMRI measurements. Neuroimage. 2011 doi: 10.1016/j.neuroimage.2011.11.061. [DOI] [PubMed] [Google Scholar]
- 51.Bookheimer SY. Methodological issues in pediatric neuroimaging. Ment Retard Dev Disabil Res Rev. 2000;6(3):161–5. doi: 10.1002/1098-2779(2000)6:3<161::AID-MRDD2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 52.Gaab N, Gabrieli JD, Deutsch GK, Tallal P, Temple E. Neural correlates of rapid auditory processing are disrupted in children with developmental dyslexia and ameliorated with training: an fMRI study. Restor Neurol Neurosci. 2007;25(3–4):295–310. [PubMed] [Google Scholar]
- 53.Small SL, Flores DK, Noll DC. Different neural circuits subserve reading before and after therapy for acquired dyslexia. Brain Lang. 1998;62(2):298–308. doi: 10.1006/brln.1998.1951. [DOI] [PubMed] [Google Scholar]
- 54.Richards TL, Berninger VW. Abnormal fMRI connectivity in children with dyslexia during a phoneme task: before but not after treatment 1. J Neurolinguist. 2008;21(4):294–304. doi: 10.1016/j.jneuroling.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aylward EH, Richards TL, Berninger VW, Nagy WE, Field KM, Grimme AC, et al. Instructional treatment associated with changes in brain activation in children with dyslexia. Neurology. 2003;61(2):212–9. doi: 10.1212/01.wnl.0000068363.05974.64. [DOI] [PubMed] [Google Scholar]
- 56.Church JA, Petersen SE, Schlaggar BL. The “Task B problem” and other considerations in developmental functional neuroimaging. Hum Brain Mapp. 2010;31(6):852–62. doi: 10.1002/hbm.21036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.American Psychiatric Association, American Psychiatric Association Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 58.Yoo SS, Choi BG, Juh R, Pae CU, Lee CU. Head motion analysis during cognitive fMRI examination: application in patients with schizophrenia. Neurosci Res. 2005;53(1):84–90. doi: 10.1016/j.neures.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 59.Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci U S A. 1992;89(12):5675–9. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hunt RH, Thomas KM. Magnetic resonance imaging methods in developmental science: a primer. Dev Psychopathol. 2008;20(4):1029–51. doi: 10.1017/S0954579408000497. [DOI] [PubMed] [Google Scholar]
- 61.Hajnal JV, Myers R, Oatridge A, Schwieso JE, Young IR, Bydder GM. Artifacts due to stimulus correlated motion in functional imaging of the brain. Magnet Reson Med. 1994;31:283–91. doi: 10.1002/mrm.1910310307. [DOI] [PubMed] [Google Scholar]
- 62.Jezzard P. Physiological noise: strategies for correction. In: Moonen C, Bandettini PA, editors. Medical radiology: functional MRI. New York: Springer-Verlag; 1999. pp. 173–81. [Google Scholar]
- 63.Voyvodic JT. Real-time fMRI paradigm control, physiology, and behavior combined with near real-time statistical analysis. Neuroimage. 1999;10(2):91–106. doi: 10.1006/nimg.1999.0457. [DOI] [PubMed] [Google Scholar]
- 64.Raschle NM, Lee M, Buechler R, Christodoulou JA, Chang M, Vakil M, et al. Making MR imaging child’s play – pediatric neuroimaging protocol, guidelines and procedure. J Vis Exp. 2009;(29) doi: 10.3791/1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kotsoni E, Byrd D, Casey BJ. Special considerations for functional magnetic resonance imaging of pediatric populations. J Magn Reson Im. 2006;23(6):877–86. doi: 10.1002/jmri.20578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klonoff EA, Janata JW, Kaufman B. The use of systematic desensitization to overcome resistance to magnetic resonance imaging (MRI) scanning. J Behav Ther Exp Psychiatry. 1986;17(3):189–92. doi: 10.1016/0005-7916(86)90026-1. [DOI] [PubMed] [Google Scholar]
- 67.Slifer KJ, Koontz KL, Cataldo MF. Operant-contingency-based preparation of children for functional magnetic resonance imaging. J Appl Behav Anal. 2002;35(2):191–4. doi: 10.1901/jaba.2002.35-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thomas KM, Casey BJ. Functional MRI in pediatrics. In: Moonen CTW, Bandettini PA, editors. Functional MRI. New York: Springer; 1999. pp. 513–23. [Google Scholar]
- 69.Casey BJ, Thomas KM, Welsh TF, Badgaiyan RD, Eccard CH, Jennings JR, et al. Dissociation of response conflict, attentional selection, and expectancy with functional magnetic resonance imaging. Proc Natl Acad Sci U S A. 2000;97(15):8728–33. doi: 10.1073/pnas.97.15.8728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Castellanos FX, Giedd JN, Hamburger SD, Marsh WL, Rapoport JL. Brain morphometry in Tourette’s syndrome: the influence of comorbid attention-deficit/hyperactivity disorder. Neurology. 1996;47(6):1581–3. doi: 10.1212/wnl.47.6.1581. [DOI] [PubMed] [Google Scholar]
- 71.Sears LL, Vest C, Mohamed S, Bailey J, Ranson BJ, Piven J. An MRI study of the basal ganglia in autism. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23(4):613–24. doi: 10.1016/s0278-5846(99)00020-2. [DOI] [PubMed] [Google Scholar]
- 72.Cohen JD, Nichols T, Brignone L, Hall SS, Reiss AL. Insular volume reduction in fragile X syndrome. Int J Dev Neurosci. 2011 doi: 10.1016/j.ijdevneu.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martens MA, Reutens DC, Wilson SJ. Auditory cortical volumes and musical ability in Williams syndrome. Neuropsychologia. 2010;48(9):2602–9. doi: 10.1016/j.neuropsychologia.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 74.Devlin JT, Poldrack RA. In praise of tedious anatomy. Neuroimage. 2007;37(4):1033–41. doi: 10.1016/j.neuroimage.2006.09.055. discussion 1050–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, et al. A shift from diffuse to focal cortical activity with development. Dev Sci. 2006;9(1):1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- 76.Passarotti AM, Sweeney JA, Pavuluri MN. Emotion processing influences working memory circuits in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2010;49(10):1064–80. doi: 10.1016/j.jaac.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Di Martino A, Kelly C, Grzadzinski R, Zuo XN, Mennes M, Mairena MA, et al. Aberrant striatal functional connectivity in children with autism. Biol Psychiat. 2010 doi: 10.1016/j.biopsych.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298(5600):2013–5. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- 79.Burgund ED, Kang HC, Kelly JE, Buckner RL, Snyder AZ, Petersen SE, et al. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage. 2002;17(1):184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- 80.Kang HC, Burgund ED, Lugar HM, Petersen SE, Schlaggar BL. Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage. 2003;19(1):16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- 81.Sanchez CE, Richards JE, Almli CR. Neurodevelopmental MRI brain templates for children from 2 weeks to 4 years of age. Dev Psychobiol. 2012;54(1):77–91. doi: 10.1002/dev.20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoon U, Fonov VS, Perusse D, Evans AC. The effect of template choice on morphometric analysis of pediatric brain data. Neuroimage. 2009;45(3):769–77. doi: 10.1016/j.neuroimage.2008.12.046. [DOI] [PubMed] [Google Scholar]
- 83.Waber DP, De Moor C, Forbes PW, Almli CR, Botteron KN, Leonard G, et al. The NIH MRI study of normal brain development: performance of a population based sample of healthy children aged 6 to 18 years on a neuropsychological battery. J Int Neuropsychol Soc. 2007;13(5):729–46. doi: 10.1017/S1355617707070841. [DOI] [PubMed] [Google Scholar]
- 84.Almli CR, Rivkin MJ, McKinstry RC. The NIH MRI study of normal brain development (Objective-2): newborns, infants, toddlers, and preschoolers. Neuroimage. 2007;35(1):308–25. doi: 10.1016/j.neuroimage.2006.08.058. [DOI] [PubMed] [Google Scholar]
- 85.Evans AC. The NIH MRI study of normal brain development. Neuroimage. 2006;30(1):184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 86.Leppert IR, Almli CR, McKinstry RC, Mulkern RV, Pierpaoli C, Rivkin MJ, et al. T(2) relaxometry of normal pediatric brain development. J Magn Reson Im. 2009;29(2):25–67. doi: 10.1002/jmri.21646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wilke M, Schmithorst VJ, Holland SK. Assessment of spatial normalization of whole-brain magnetic resonance images in children. Hum Brain Mapp. 2002;17(1):48–60. doi: 10.1002/hbm.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dichter GS, Felder JN, Bodfish JW. Autism is characterized by dorsal anterior cingulate hyperactivation during social target detection. Soc Cogn Affect Neurosci. 2009;4(3):215–26. doi: 10.1093/scan/nsp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shafritz KM, Dichter GS, Baranek GT, Belger A. The neural circuitry mediating shifts in behavioral response and cognitive set in autism. Biol Psychiat. 2008;63(10):974–80. doi: 10.1016/j.biopsych.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44(1):162–7. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 91.Hamilton MA. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]