Abstract
Overwhelming evidence implicates insulin-like growth factor (IGF) signaling in the growth and survival of many types of human cancer cells. Numerous inhibitors of the IGF1 receptor (IGF1R) have been developed, and they displayed remarkable antineoplastic activity in preclinical models and promising success in early phase clinical trials. However, while responses have been observed in numerous cancer types, they have occurred in a minority of patients, and serious toxicities have been observed. Identifying patients likely to benefit from anti-IGF1R therapy requires further characterizing the role of IGF1 signaling in various stages of tumorigenesis in order to identify critical downstream factors that may be used as predictors of response, or to serve as novel therapeutic targets. Recent microarray analyses have begun to unravel expression “signatures” specific for IGF1 that correlate with poor breast cancer prognosis and with response to anti-IGF1R inhibitors. In this review we briefly discuss the history of the IGF1 family in neoplasia, how it is targeted, results from clinical trials, and the quest for biomarkers that will predict response to IGF1R-targeted therapy.
Keywords: iomarkers, IGF1, targeted therapy, neoplasia, cancer, insulin, signaling, signatures
I. THE IGF FAMILY
The insulin-like growth factor (IGF) family is a crucial regulator of cell proliferation and survival, and IGF signaling is frequently altered in human cancer.1–4 The IGF family consists of two ligands (IGF1 and IGF2), two receptors (IGF1R and IGF2R), six insulin-like growth factor binding proteins (IGFBP1–6),5 and several IGFBP-related proteins (IGFBPrP). Insulin-receptor substrates 1 and 2 (IRS1 and IRS2) are key signaling intermediates, and well-documented downstream effectors are PI3K/AKT and MAPK/ ERK1.6–10 The consequence of signaling results in a temporal transcriptional response leading to a wide range of biological processes including cell proliferation and survival.
The vast majority of circulating levels of IGF1 are produced in the liver in response to growth hormone (GH).11,12 However, IGF signaling can also be amplified through autocrine and paracrine production by other organs or neoplastic cells.13,14 For example, stromal cells of the mammary connective tissue produce IGF1, leading to an increase in signaling.15,16 IGF ligands in circulation are bound by IGFBPs, 90% of which are bound to IGFBP3.17 IGFBPs regulate the local bioavailability of IGF1 and IGF2. The association of IGF with IGFBPs increases the stability of the ligand, allowing prolonged signaling.18 However, the role of IGFBPs in IGF signaling is complicated by the fact that they associate with the ligands with nearly equal affinity as the receptor, and thus compete with the receptor for binding, potentially decreasing receptor activation.5
Both IGF1 and IGF2 bind and activate IGF1R.4 IGF1R is a transmembrane receptor tyrosine kinase normally found as a heterotetramer19 composed of two extracellular ligand-binding alpha subunits and two transmembrane and intracellular tyrosine kinase catalytic beta subunits.20 Binding of the ligands results in autophosphorylation of the intracellular kinase domains and subsequent downstream signaling.4,20–22 IGF2R does not contain a kinase domain, and binding with IGF2 (IGF1 does not associate with IGF2R) does not result in downstream signaling. Generally, it is believed that IGF2R dampens IGF1R signaling by sequestering IGF2.23
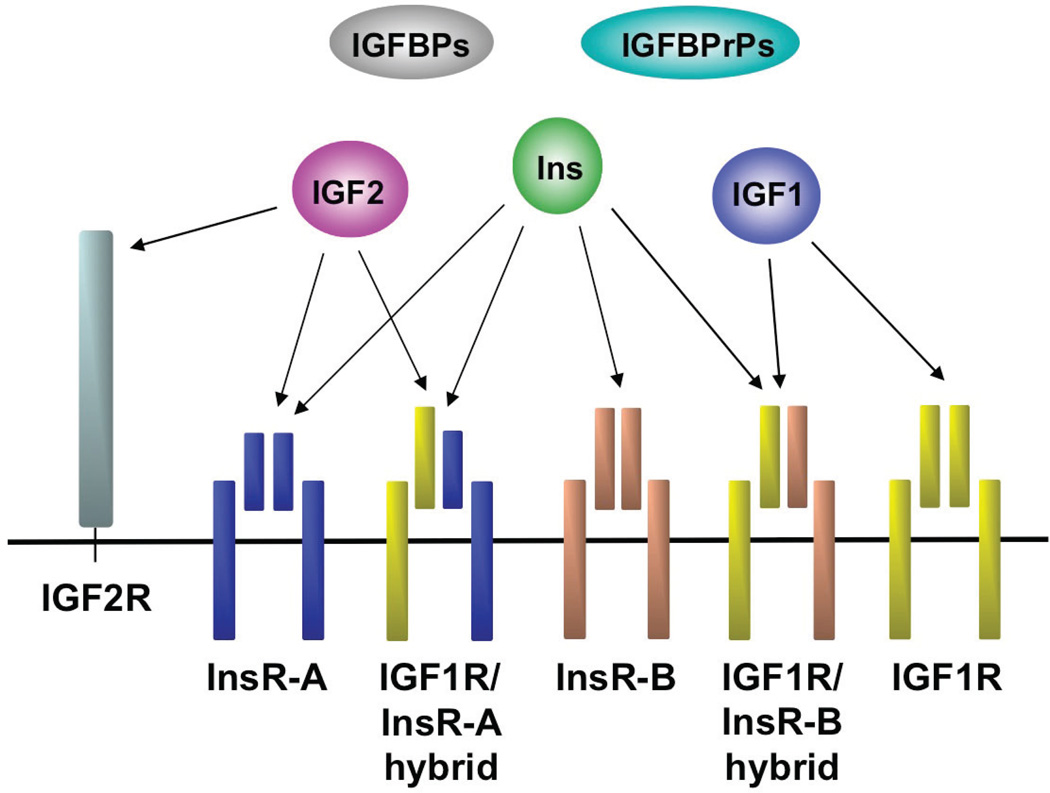
IGF1R signaling and regulation has several layers of complexity due to the close homology with the insulin receptor (InsR) (Fig. 1). IGFR1 and InsR share 84% similarity at the amino acid level and 100% in the ATP binding domain.4,24 There are two isoforms of the InsR that arise by alternative splicing: (1) InsR-A, which is normally expressed in fetal tissues, but is commonly expressed in human cancers as well, and (2) InsR-B, which is normally expressed in adult tissues. Both InsR isoforms bind IGF1, albeit with lower affinity than insulin, but InsR-A binds IGF2 with similar affinity to that of insulin.25 Furthermore, IGF1R binds insulin, but with much lower affinity than IGF1 or IGF2, resulting in activation of the receptor and downstream signaling.26,27 Additionally, IGF1R and InsR can form hybrid receptors consisting of one alpha and one beta subunit from each (Fig. 1). The hybrid receptors can bind insulin, IGF1, and IGF2 but are thought to preferentially support IGF signaling.28–30 Phosphorylation of IGF1R or hybrid receptor caused by IGF1 or IGF2 binding predominately leads to mitogenic signaling, as does InsR-A activation by IGF2.25,31,32 Conversely, phosphorylation of InsR-B by insulin or IGF1 results in metabolic signaling.33,34 Because both IGF1R and InsR activate similar downstream effectors, such as IRS1 and IRS2, it is unclear how they mediate distinct phenotypes, and it is uncertain whether blocking IGF1R activation will result in compensation through IGF signaling via the InsR, something that must be considered carefully when developing anti-IGF1R therapies.
FIGURE 1.
IGFR and InsR complexes. IGF1R and InsR exist as heterotetramers consisting of an external ligand-binding alpha subunit and an internal signaling beta subunit. In addition, InsR has a fetal (InsR-A) and an adult isoform (InsR-B) resulting from alternative splicing. IGF1R and InsR can form hybrid heterotetramers. IGF2R is homologous to the mannose-6-phosphate receptor. The ligands insulin (Ins), IGF1, and IGF2 exhibit selectivity in their receptor binding profiles, as indicated in the figure. IGF binding proteins (IGFBPs) and IGFBP-related proteins (IGFBPrPs) bind IGFs to regulate their bioavailability and activity.
II. THE ROLE OF IGF1 FAMILY MEMBERS IN ONCOGENIC SIGNALING AND CANCER
A. IGF1 levels
Alterations in the IGF signaling pathway have been described in multiple cancer types, including osteosarcomas, gynecological, gastrointestinal, prostate, lung, and breast, and several lines of evidence implicate IGF signaling in oncogenesis. This is not surprising given that IGF signaling results in cell proliferation, survival, and migration through the oncogenic PI3K/ AKT and Ras/MAPK/ERK1 cascades.10,35–39 In fact, elevated levels of IGF1 in serum are correlated with an increased risk of developing ductal carcinoma in situ and invasive breast cancer.40–43 Interestingly, antiestrogens that are effective in the treatment and prevention of breast cancer consistently lower IGF1 levels in serum.44 Increases in IGF signaling can be caused by increased production of IGF1 through endocrine, paracrine, or autocrine mechanisms, or it can reflect increased bioavailability due to the reduction of IGFBP3 expression. The tumor suppressor p53 is a key transcriptional regulator of the IGFBP3 gene. Mutations in p53, a hallmark in most cancers, can lead to a reduction of IGFBP3, and thus an increase in the concentration of free IGFs available to activate IGF1R.17 Accordingly, it was shown that an increased ratio of IGF1 to IGFBP3 is correlated with increased breast cancer risk.45 Finally, evidence from liver-specific IGF1-deficient (LID) mice confirms the importance of IGF levels in developing cancer. The LID mice, which have very low circulating IGF1 levels, develop colonic and mammary tumors less than their wild-type littermates and have lower cancer incidence. However, IGF1 administration to the LID mice results in the restoration of tumor growth and metastasis.46,47
B. IGF1R
IGF1R is often overexpressed and hyperactive in cancer cells, though rarely is it mutated.48,49 IGF1R is sufficient to transform several cell types, including immortalized mammary epithelial cells (MCF10A), and is necessary for transformation by a variety of known viral and cellular oncogenes including SV40 and c-Src, as assayed by focus-forming assays in NIH3T3 and mouse embryonic fibroblasts (MEFs).37,50–52 Activation of IGF1R is important for the expression of genes that regulate the cell cycle, survival, motility, attachment, and metastasis. Overexpression of a constitutively active or inducible IGF1R in the mouse mammary gland leads to rapid mammary tumorigenesis.35,36 High IGF1R activity can also make cancer cells resistant to chemotherapeutic agents and radiation through its capacity to inhibit apoptosis.53,54 In human tumors, high IGF1R expression and activation occurs in carcinomas that are resistant to chemotherapy, as well as HER2 and EGFR inhibitors, and is associated with lower patient survival.55–59 Conversely, inhibiting receptor signaling results in cancer cell apoptosis in vitro and prevents tumor formation in nude mice in vivo.60–62
C. IRSs
Several studies demonstrated the oncogenic potential of the IRSs and their importance in IGF1R-mediated transformation. Like IGF1R, overexpression of IRS1 and IRS2 leads to proliferation of mammary epithelial cells under serum-free conditions, colony formation in soft agar, and tumor growth in nude mice, while transgenic overexpression in mammary glands results in tumorigenesis and metastasis.10,38,39,63 Silencing IRS1 levels in mammary epithelial cells results in a 70% reduction of proliferation with concurrent induction of apoptosis. Interestingly, if IRS1 is activated directly, for example by v-src, then IGF1R is no longer a requirement for transformation.64–66 Furthermore, recently it was shown that IRS2 plays a pivotal role in tumor cell metabolism. It was demonstrated that IRS2 promotes invasion by sustaining aerobic glycolysis of mouse mammary tumor cells, by regulating the mammalian target of rapamycin (mTor)-dependent surface expression of glucose transporter 1 (Glut1).67 Further, IRS2 expression is positively regulated by hypoxia, which selects for tumor cells with increased metastatic potential, facilitating breast carcinoma cell survival and invasion especially under low oxygen conditions.68 Together these data stress the multifaceted importance of IRSs in cancer development and maintenance. Finally, it was demonstrated that an antibody to the IGF1R is ineffective in T47D-YA breast cancer cells unless IRS1 and IRS2 are expressed.69 This discovery further emphasizes our need to critically understand the role of the IRSs, other downstream effectors, and IRS-regulated target genes in order to properly develop effective IGF1R therapy.
III. TARGETING IGF1R
A. Overview
Given the importance of IGF signaling in neoplasia and metastasis, it is not surprising that considerable effort has been dedicated to inhibiting this pathway. Several therapeutic strategies have been developed, including humanized monoclonal anti-bodies directed against the extracellular portion of the receptor that prevent ligand binding, small-molecule inhibitors that act on the tyrosine kinase domain, siRNA and antisense approaches to reduce receptor expression, and the expression of dominant-negative truncated IGF1R proteins that interfere with receptor function.70–75 Of these strategies, the blocking antibodies and tyrosine kinase inhibitors are the most clinically relevant.66 Preclinical data in both in vitro and in vivo models of common human cancers demonstrate that targeting IGF1R results in impressive antineoplastic activity—preventing downstream signaling and decreasing tumor cell proliferation, as well as xenograft and tumor growth.4 Thus, preclinical data have validated IGF-IR as a therapeutic target, and over 30 monoclonal antibodies and tyrosine kinase inhibitors have been developed. Over a dozen of these are now undergoing clinical evaluation involving almost 30 combinations with other treatments in at least 16 tumor types (see references 76, 77).
B. Antibodies
Monoclonal antibodies are the major class of inhibitors receiving clinical attention. They inhibit IGF1R signaling by binding specifically to IGF1R, thereby preventing interaction with ligands, leading to internalization of the receptor.77 From a therapeutic standpoint the major advantage of the use of blocking antibodies, or so it is believed, is that the antibodies specifically inhibit IGF1R without altering InsR activity, reducing the concerns of deleterious metabolic side effects. Unfortunately, recent data demonstrate that this specificity is a double-edged sword because insulin may also play an important and independent role in tumorigenesis.33 Down-regulating the InsR in cancer cells and xenografts reduced cell proliferation, angiogenesis, lymphangiogenesis, and metastasis.78 Further, epidemiological studies have demonstrated that insulin therapy and insulin secretagogues correlate with an increased risk of cancer development.79,80 This is of particular interest because studies in cell lines demonstrated that pharmacologically inhibiting the IGF1R with monoclonal antibodies leads to a compensatory increase in InsR signaling.26,27,81,82 Therefore, it is possible that InsR signaling could compensate for IGF1R, leading to resistance to IGF1R-targeted therapy, and could theoretically lead to an increased cancer risk if IGF1R inhibition-induced hyperinsulinemia is not properly controlled.
C. Tyrosine Kinase Inhibitors
Small-molecule inhibitors of IGFIR generally function as ATP-competitive inhibitors by binding to the ATP-binding pocket of IGFIR, which prevents auto-phosphorylation of the intracellular kinase domain. Because of the high homology between IGF1R and InsR, most of these tyrosine kinase inhibitors bind with equal affinity to InsR and inhibit insulin signaling. Consequently, there are predictable metabolic side effects of targeting IGF1R with kinase inhibitors that must be treated. For example, an IGF1R TKI can block xenograft and mammary tumor growth, but when administered in vivo it causes hyperinsulinemia and hyperglycemia.35,83,84 Theoretically, because TKIs have a broader range of inhibition by targeting IGF1R, InsR, and IGF1R/ InsR hybrid receptors, they may be expected to be more effective antineoplastic agents than the antibodies.76 In a transgenic mouse model of a pancreatic B-cell neuroendocrine tumor, anti-IGF1R therapy with an inhibitory antibody increased expression of InsR, but did not reduce tumor growth. However, when InsR expression was suppressed and IGF1R was inhibited with the same antibody, tumor growth was impeded,33,82 giving credence to the notion that dual inhibitors are more effective.
D. IGF1R Inhibitors in Clinical Trials
Comprehensive reviews summarizing results from clinical trials involving IGF1R inhibitors were recently published.76,77 Here we summarize, rather broadly, lessons learned from those trials. Phase I and phase II trials demonstrated that generally, anti-IGF1R therapy was well-tolerated with mostly mild adverse events,85–87 which include elevations of liver function tests, hyperglycemia, dehydration, asthenia, nausea, thrombocytopenia, and arthralgias.88 Early efficacy results were also very promising. A patient with chemo-refractory Ewing sarcoma had complete remission in a phase I trial in response to an anti-IGF1R blocking antibody, AMG 479.87 In a phase II trial conducted by the Sarcoma Alliance for Research through Collaboration, another anti-IGF1R antibody, R1507, showed a response rate of 14% when given to 125 patients with recurrent or refractory sarcomas,77,89 but acquired resistance was common with continued therapy. Phase Ia and phase II trials of combining figitumumab, another anti-IGF1R antibody, with cytotoxic chemotherapy for treatment of non-small-cell lung cancer (NSCLC) were also largely successful, with an increase of progressionfree survival in many patients.90 However, two large phase III trials of figitumumab in combination with carboplatin and paclitaxel for first line treatment of metastatic NSCLC and in combination with Tarceva as a second/third-line treatment for patients with previously treated advanced non-adenocarcinoma NSCLC, were stopped due to potential futility and the increased risk of severe toxicities.91 Figitumumab was given to patients in these trials irrespective of the expression of IGF1R in their tumors. Pre-clinical data clearly demonstrate that low IGF1R levels are a powerful negative predictor for lack of response with 95% specificity.92,93 Interestingly, survival hazard ratio estimates in the cancelled phase III trial favored treatment of patients with high baseline levels of IGF1 with figitumumab in combination with carboplatin and paclitaxel, and patients with low baseline levels of IGF1 with only carboplatin and paclitaxel.91 This demonstrates the need to further characterize IGFI signaling in order to understand how it leads to various stages of tumorigenesis and to identify downstream targets or other factors that could be used as predictors of response or targeted themselves.
IV. EFFECTS OF IGF1 SIGNALING ON TRANSCRIPTIONAL NETWORKS: IMPLICATIONS FOR TARGETING STRATEGIES
The expression of IGF1R is necessary for anti-tumor activity by targeted IGF1R therapy, but expression alone does not accurately predict whether anti-IGF1R therapy will be effective. Further, preclinical evidence demonstrated that an anti-IGFIR antibody was ineffective at treating breast cancer cells unless IRS1 and IRS2 were expressed,69 suggesting that the expression of ‘IRSs is also necessary for antitumor activity by IGF1R targeted therapy, but like IGF1R expression, it does not positively predict effectiveness. So it seems that the activation of the IGF1R pathway is a requirement for IGF1R therapy to be effective, and it is apparent that therapy can be effective in certain instances with tolerable side effects; but it is also apparent that other biomarkers must be identified so that patients may be properly divided into cohorts that will or will not respond to targeted IGF1R therapy. While the phosphorylation cascade that occurs following IGF1R activation is relatively well described, the effects of that cascade on gene expression are only recently beginning to be understood. The ability to define cancer subtypes and response to specific therapies using gene expression “signatures” has been demonstrated in multiple studies.94,95 Perhaps by defining an IGF1R signature and discovering the important components of that signature, we will be able to accurately predict antitumor response to IGF1R therapy.
Gene expression changes following IGF1 treatment have now been examined by microarray analyses in multiple mouse and human cell lines.15,96–102 The first study to examine IGF1-induced global gene expression was in mouse fibroblast NIH-3T3 cells overexpressing either IGF1R or InsR in an attempt to understand how insulin and IGF1 can signal through the same post-receptor signaling pathways, yet mediate distinct biological functions. They found that 30 of 2221 genes were significantly induced by IGF1 but not insulin, most of which are associated with mitogenesis and differentiation.97 Another study with similar goals examined gene expression following stimulation of artificial chimeric receptors (IGF1R and InsR) to ensure selective activation of each receptor in 3T3-L1 preadipocytes. They demonstrated that at 4 hours post-stimulation, 11 genes were differentially regulated between IGF1R and InsR, and that the differentially regulated genes are mostly involved in adhesion, transcription, transport, and proliferation.98 Of course, it is possible that genes that are regulated by both InsR and IGF1R are key to predict responsiveness to IGF1R therapy. Therefore, while these studies began to define target genes of IGF1 signaling in comparison to Ins signaling, there was still a need to decipher how activated IGF1R contributes to cancer progression.
One study attempted to answer this question by examining gene expression changes elicited by IGF1 in an immortalized breast epithelial cell line (184tert). They detected changes in 156 of 1920 examined genes, many of which, like the previous studies demonstrated, were associated with cancer progression, transcription, cell cycle, metabolism, and angiogenesis. They further used an in silico approach to examine the promoter regions of IGF1-regulated genes in order to elucidate the mechanism of regulation. They found that IGF1-induced genes commonly had CRE/AP1/AP2 coupled with SP1 and ETS transcription factor binding sites in their proximal promoter, whereas negatively regulated genes commonly had FKHR, Myc, and WT1 binding sites.99 It should be noted that they examined only approximately 300 base pairs upstream for the promoter analyses; therefore, common regulatory elements elsewhere would not have been observed. How IGF1 regulates the expression of its targets is still largely unknown.
In an additional study, expression profiling was performed on a breast cancer cell line, MCF7, stably overexpressing either IGF1 or IGF2. This revealed that 21 out of 22,500 transcripts examined were more than doubled by both IGF1 and IGF2, 9 by IGF1 alone, and 9 by IGF2 alone. IGF1 and IGF2 overexpression increased proliferation rates and the efficiency of tumor formation in mouse xenografts. Most of the genes up-regulated by IGF signaling in these cells were involved in amino acid and protein synthesis, suggesting that induction of cell proliferation and tumor formation by persistent IGF stimulation may be in part due to anabolic effects.102
In a more recent study, a set of more than 800 genes responsive to IGF1 signaling was identified that is present in a significant proportion of human breast cancers and indicates poor prognosis. In this study, temporal changes in gene expression were observed in MCF7 cells stimulated with IGF1, and the regulated gene sets were associated with cell proliferation, survival, metabolism, and DNA repair. These genes were enriched for transcriptional targets of the Ras/ERK1/2 and PI3K/AKT/mTOR pathways.96 Profiling of human breast tumors from several independent expression profile data sets103–105 found that the IGF1 signature correlated strongly with profiles of ER-negative breast tumors and ∼25% of ER-positive tumors that have low expression levels of estrogen receptor. Additionally the signature significantly correlated with adverse pathological (high grade, large tumors, nodal involvement) and clinical markers (ER− and PR−) and with poor prognosis in breast cancer.96 The IGF signature was reversed in cancer cell lines and xenografts when these were treated with three different anti-IGF1R therapies. Cell lines highly expressing the IGF1 signature and triple negative breast cancer (TNBC) tumorgrafts were highly sensitive to treatment with BMS-754807, a dual IGF1R/InsR TKI.106 Further, when BMS-754807 was given in combination with docetaxel, tumors were eradicated in TNBC tumorgrafts, and tumor reduction was associated with decreased proliferation, increased apoptosis, and mitotic catastrophe. This study provides preclinical data that suggest patient populations can be identified that will respond to IGF1R therapy by combining IGF1R expression and the IGF1 signature. However, the signature alone only weakly correlated with response, probably because the signature is similar to that elicited by other growth factor pathways.
Another group also defined an IGF signature in stromal fibroblasts. In that study, a drastic change in gene expression was observed. Over 370 genes had a greater than 1.5-fold induction following IGF1 stimulation, with a significant enrichment in proliferation-associated genes.15 Other genes were involved in angiogenesis, the p53 pathway, and integrin and Wnt signaling. Interestingly, unlike the previous study, they found that genes induced in MCF7 cells by IGF1 treatment were not significantly associated with the cell cycle or proliferation. However, the signature from the fibroblasts, like the signature from the epithelial cells in the previous study, when compared to microarrays from breast cancer biopsies of 295 patients, corresponds to increased risk of metastasis, lower survival rate, and loss of ER.15 In fact, their IGF1 signature is the inverse of the good-risk 70-gene signature. In this study they confirmed the validity of the IGF1 signature from primary breast and lung fibroblasts in four different human solid cancer publically available datasets and showed that it is predictive of outcome and can be used to divide patients into two groups with significantly different prognoses.
Using a different approach in an attempt to identify biomarkers for response to IGF1R targeted therapy, Huang and colleagues examined the gene signature of 28 sarcoma and neuroblastoma cell lines in response to BMS-536924, an IGF1R TKI. They identified genes differentially expressed between sensitive and resistant cell lines. Notably, IGF1, IGF2, and IGFIR were highly expressed in sensitive cell lines, while IGFBP3 and IGFBP6 were highly expressed in resistant lines.107
Finally, extending beyond gene expression signatures, one study has attempted to integrate genomic and transcriptomic features to predict response of colorectal cancer (CRC) to OSI-906, another small-molecule inhibitor of IGF1R/InsR.108 In contrast to using differentially expressed genes as a signature to predict sensitivity, the authors used the k-top scoring pair (k-TSP) algorithm and identified three gene pairs, from the expression profiles of four sensitive and four resistant CRC cell lines, as the final classifier: (PROM1>MTE1), (LY75>OXCT1), and (HSD17B2>CALD1).108 To improve the predictive power of the k-TSP classifier they integrated IGF1R copy number—they report a significant correlation between sensitivity to OSI-906 and an unbalanced IGF1R copy number gain based on ploidy—and KRAS mutational status. The integrated classifier was then used to correctly predict responsiveness to OSI-906 in 17 of 19 additional CRC cell lines and all six human CRC explants examined.108 Unfortunately, the classifier only correctly predicted one of two sensitive cell lines, so its use as a possible positive predictor of response needs further validation in a larger set of samples. However, the approach of integrating gene expression, copy number, and mutation data to predict sensitivity to IGF1R therapy is intriguing.
The culmination of these studies demonstrates the possibility of using gene expression as potential predictors of response to IGF1R therapy. Like all targeted therapies, inhibition of IGF1R is not and will not be effective in unselected populations, so it is essential to identify markers that will predict response to therapy, and there is still a great deal of work necessary to identify appropriate biomarkers.
V. CONCLUSIONS
It is unknown how IGF1R can signal through similar intermediates to those of other tyrosine kinases, like the highly homologous InsR, but stimulate different biological responses. Will inhibiting both IGF1R and InsR by RTK inhibitors be beneficial because of the role of insulin in tumorigenesis, or will the metabolic consequences of inhibiting InsR be too difficult to control? Distinct downstream targets could exist to elicit different biological responses between the closely related kinases, but what are these targets? Could they be used as biomarkers to predict response to IGF1R therapy? We along with others have recent microarray data that have begun to unravel expression signatures specific for IGF1R, which correspond to an increased risk of metastasis, lower survival rate, loss of estrogen receptor, and response to IGF1R inhibitor treatment. However, still relatively little is known about how IGF1 regulates this signature or what targets are important for the different biological responses to IGF1, such as proliferation, resistance to apoptosis, migration, and transformation. Inhibition of IGF1R can be an effective treatment, especially in combination with chemotherapy or radiation, but it can also be toxic and ineffective if given unselectively to patients. Defining critical downstream targets could lead to the development of novel therapeutic strategies, and may define what cancers will respond to anti-IGFIR therapy.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health P01CA30195 and P50CA58183 to C. Kent Osborne (Baylor College of Medicine) and R01CA94118 to Adrian V. Lee (University of Pittsburgh Cancer Institute). AVL is a recipient of an award from Susan G. Komen for the Cure.
ABBREVIATIONS
- IGF
insulin-like growth factor
- IGF1
insulin-like growth factor 1
- IGF2
insulin-like growth factor 2
- IGF1R
insulin-like growth factor 1 receptor
- IGF2R
insulin-like growth factor 2 receptor
- IGFBP1–6
insulin-like growth factor binding proteins 1–6
- IGFBPrP
insulin-like growth factor binding protein-related proteins
- IRS1-2
insulin-receptor substrate 1 and 2
- PI3K
phosphatidylinositol 3-kinase
- MAPK
mitogen-activated protein kinase
- ERK
extracellular signal-regulated kinase
- GH
growth hormone
- InsR
insulin receptor
- ATP
adenosine-5’-triphosphate
- LID
liver-specific IGF1-deficient
- MEFs
mouse embryonic fibroblasts
- EGFR
epidermal growth factor receptor
- SV40
simian virus 40
- mTor
mammalian target of rapamycin
- Glut1
glucose transporter 1
- siRNA
small interfering ribonucleic acid
- TKI
tyrosine-kinase inhibitor
- NSCLC
non-small-cell lung cancer
- WT1
Wilms tumor 1
- FKHR
forkhead homolog 1
- ER
estrogen receptor
- PR
progesterone receptor
- TNBC
triple negative breast cancer
REFERENCES
- 1.Baserga R, Peruzzi F, Reiss K. The IGF-1 receptor in cancer biology. Int J Cancer. 2003;107(6):873–877. doi: 10.1002/ijc.11487. [DOI] [PubMed] [Google Scholar]
- 2.Holly J. Insulin-like growth factor-1 and risk of breast cancer. Lancet. 1998;352(9137):1388. doi: 10.1016/S0140-6736(05)60781-7. [DOI] [PubMed] [Google Scholar]
- 3.Burroughs KD, Dunn SE, Barrett JC, Taylor JA. Insulin-like growth factor-I: a key regulator of human cancer risk? J Natl Cancer Inst. 1999;91(7):579–581. doi: 10.1093/jnci/91.7.579. [DOI] [PubMed] [Google Scholar]
- 4.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4(7):505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 5.Firth SM, Baxter RC. Cellular actions of the insulinlike growth factor binding proteins. Endocr Rev. 2002;23(6):824–854. doi: 10.1210/er.2001-0033. [DOI] [PubMed] [Google Scholar]
- 6.Myers MG, Jr., Sun XJ, Cheatham B, Jachna BR, Glasheen EM, Backer JM, White MF. IRS-1 is a common element in insulin and insulin-like growth factor-I signaling to the phosphatidylinositol 3’-kinase. Endocrinology. 1993;132(4):1421–1430. doi: 10.1210/endo.132.4.8384986. [DOI] [PubMed] [Google Scholar]
- 7.Ridderstrale M, Degerman E, Tornqvist H. Growth hormone stimulates the tyrosine phosphorylation of the insulin receptor substrate-1 and its association with phosphatidylinositol 3-kinase in primary adipocytes. J Biol Chem. 1995;270(8):3471–3474. doi: 10.1074/jbc.270.8.3471. [DOI] [PubMed] [Google Scholar]
- 8.Shepherd PR. Mechanisms regulating phosphoinositide 3-kinase signalling in insulin-sensitive tissues. Acta Physiol Scand. 2005;183(1):3–12. doi: 10.1111/j.1365-201X.2004.01382.x. [DOI] [PubMed] [Google Scholar]
- 9.Shepherd PR, Withers DJ, Siddle K. Phosphoinositide 3-kinase: the key switch mechanism in insulin signalling. Biochem J. 1998;333(Pt 3):471–490. doi: 10.1042/bj3330471. Epub 1998/07/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dearth RK, Cui X, Kim HJ, Kuiatse I, Lawrence NA, Zhang X, Divisova J, Britton OL, Mohsin S, Allred DC, Hadsell DL, Lee AV. Mammary tumorigenesis and metastasis caused by overexpression of insulin receptor substrate 1 (IRS-1) or IRS-2. Mol Cell Biol. 2006;26(24):9302–9314. doi: 10.1128/MCB.00260-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299(5611):1346–1351. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- 12.Thissen JP, Ketelslegers JM, Underwood LE. Nutritional regulation of the insulin-like growth factors. Endocr Rev. 1994;15(1):80–101. doi: 10.1210/edrv-15-1-80. [DOI] [PubMed] [Google Scholar]
- 13.Ellis MJ, Jenkins S, Hanfelt J, Redington ME, Taylor M, Leek R, Siddle K, Harris A. Insulin-like growth factors in human breast cancer. Breast Cancer Res Treat. 1998;52(1–3):175–184. doi: 10.1023/a:1006127621512. [DOI] [PubMed] [Google Scholar]
- 14.Marshman E, Streuli CH. Insulin-like growth factors and insulin-like growth factor binding proteins in mammary gland function. Breast Cancer Res. 2002;4(6):231–239. doi: 10.1186/bcr535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rajski M, Zanetti-Dallenbach R, Vogel B, Her-rmann R, Rochlitz C, Buess M. IGF-I induced genes in stromal fibroblasts predict the clinical outcome of breast and lung cancer patients. BMC Med. 2010;8:1. doi: 10.1186/1741-7015-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yee D, Paik S, Lebovic GS, Marcus RR, Favoni RE, Cullen KJ, Lippman ME, Rosen N. Analysis of insulin-like growth factor I gene expression in malignancy: evidence for a paracrine role in human breast cancer. Mol Endocrinol. 1989;3(3):509–517. doi: 10.1210/mend-3-3-509. [DOI] [PubMed] [Google Scholar]
- 17.Furstenberger G, Senn HJ. Insulin-like growth factors and cancer. Lancet Oncol. 2002;3(5):298–302. doi: 10.1016/s1470-2045(02)00731-3. [DOI] [PubMed] [Google Scholar]
- 18.Biddinger SB, Ludwig DS. The insulin-like growth factor axis: a potential link between glycemic index and cancer. Am J Clin Nutr. 2005;82(2):277–278. doi: 10.1093/ajcn.82.2.277. [DOI] [PubMed] [Google Scholar]
- 19.Ullrich A, Gray A, Tam AW, Yang-Feng T, Tsubokawa M, Collins C, Henzel W, Le Bon T, Kathuria S, Chen E, Jacobs S, Francke U, Ramachandran J, Fujita-Yamaguchi Y. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986;5(10):2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deutsch E, Tao Y, Pinzi V, Bourhis J. Mechanisms of disease: signaling of the insulin-like growth factor I receptor pathway-therapeutic perspectives in cancer. Nature Clin Pract Oncol. 2007;4(10):591–602. doi: 10.1038/ncponc0934. [DOI] [PubMed] [Google Scholar]
- 21.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7(2):85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 22.Tollefsen SE, Stoszek RM, Thompson K. Interaction of the alpha beta dimers of the insulin-like growth factor I receptor is required for receptor autophosphorylation. Biochemistry. 1991;30(1):48–54. doi: 10.1021/bi00215a008. [DOI] [PubMed] [Google Scholar]
- 23.Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75(1):73–82. [PubMed] [Google Scholar]
- 24.Chitnis MM, Yuen JS, Protheroe AS, Pollak M, Macaulay VM. The type 1 insulin-like growth factor receptor pathway. Clin Cancer Res. 2008;14(20):6364–6370. doi: 10.1158/1078-0432.CCR-07-4879. [DOI] [PubMed] [Google Scholar]
- 25.Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, Goldfine ID, Belfiore A, Vigneri R. Insulin receptor isoform A, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol. 1999;19(5):3278–3288. doi: 10.1128/mcb.19.5.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dinchuk JE, Cao C, Huang F, Reeves KA, Wang J, Myers F, Cantor GH, Zhou X, Attar RM, Gottardis M, Carboni JM. Insulin receptor (IR) pathway hyperactivity in IGF-IR null cells and suppression of downstream growth signaling using the dual IGF-IR/IR inhibitor, BMS-754807. Endocrinology. 2010;151(9):4123–4132. doi: 10.1210/en.2010-0032. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Pelzer AM, Kiang DT, Yee D. Downregulation of type I insulin-like growth factor receptor increases sensitivity of breast cancer cells to insulin. Cancer Res. 2007;67(1):391–397. doi: 10.1158/0008-5472.CAN-06-1712. [DOI] [PubMed] [Google Scholar]
- 28.Slaaby R, Schaffer L, Lautrup-Larsen I, Andersen AS, Shaw AC, Mathiasen IS, Brandt J. Hybrid receptors formed by insulin receptor (IR) and insulin-like growth factor I receptor (IGF-IR) have low insulin and high IGF-1 affinity irrespective of the IR splice variant. J Biol Chem. 2006;281(36):25869–25874. doi: 10.1074/jbc.M605189200. [DOI] [PubMed] [Google Scholar]
- 29.Pandini G, Vigneri R, Costantino A, Frasca F, Ippolito A, Fujita-Yamaguchi Y, Siddle K, Goldfine ID, Belfiore A. Insulin and insulin-like growth factor-I (IGF-I) receptor overexpression in breast cancers leads to insulin/IGF-I hybrid receptor overexpression: evidence for a second mechanism of IGF-I signaling. Clin Cancer Res. 1999;5(7):1935–1944. [PubMed] [Google Scholar]
- 30.Pandini G, Frasca F, Mineo R, Sciacca L, Vigneri R, Belfiore A. Insulin/insulin-like growth factor I hybrid receptors have different biological characteristics depending on the insulin receptor isoform involved. J Biol Chem. 2002;277(42):39684–39695. doi: 10.1074/jbc.M202766200. [DOI] [PubMed] [Google Scholar]
- 31.Zhao R, DeCoteau JF, Geyer CR, Gao M, Cui H, Casson AG. Loss of imprinting of the insulin-like growth factor II (IGF2) gene in esophageal normal and adenocarcinoma tissues. Carcinogenesis. 2009;30(12):2117–2722. doi: 10.1093/carcin/bgp254. [DOI] [PubMed] [Google Scholar]
- 32.Murphy SK, Huang Z, Wen Y, Spillman MA, Whitaker RS, Simel LR, Nichols TD, Marks JR, Berchuck A. Frequent IGF2/H19 domain epigenetic alterations and elevated IGF2 expression in epithelial ovarian cancer. Mol Cancer Res. 2006;4(4):283–292. doi: 10.1158/1541-7786.MCR-05-0138. [DOI] [PubMed] [Google Scholar]
- 33.Gallagher EJ, LeRoith D. Minireview: IGF, insulin, and cancer. Endocrinology. 2011;152(7):2546–2551. doi: 10.1210/en.2011-0231. [DOI] [PubMed] [Google Scholar]
- 34.Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/ insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30(6):586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- 35.Carboni JM, Lee AV, Hadsell DL, Rowley BR, Lee FY, Bol DK, Camuso AE, Gottardis M, Greer AF, Ho CP, Hurlburt W, Li A, Saulnier M, Velaparthi U, Wang C, Wen ML, Westhouse RA, Wittman M, Zimmermann K, Rupnow BA, Wong TW. Tumor development by transgenic expression of a constitutively active insulin-like growth factor I receptor. Cancer Res. 2005;65(9):3781–3787. doi: 10.1158/0008-5472.CAN-04-4602. [DOI] [PubMed] [Google Scholar]
- 36.Jones RA, Campbell CI, Gunther EJ, Chodosh LA, Petrik JJ, Khokha R, Morehead RA. Transgenic overexpression of IGF-IR disrupts mammary ductal morphogenesis and induces tumor formation. Oncogene. 2007;26(11):1636–1644. doi: 10.1038/sj.onc.1209955. [DOI] [PubMed] [Google Scholar]
- 37.Kim HJ, Litzenburger BC, Cui X, Delgado DA, Grabiner BC, Lin X, Lewis MT, Gottardis MM, Wong TW, Attar RM, Carboni JM, Lee AV. Constitutively active type I insulin-like growth factor receptor causes transformation and xenograft growth of immortalized mammary epithelial cells and is accompanied by an epithelial-to-mesenchymal transition mediated by NF-kappaB and snail. Mol Cell Biol. 2007;27(8):3165–3125. doi: 10.1128/MCB.01315-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanaka S, Ito T, Wands JR. Neoplastic transformation induced by insulin receptor substrate-1 overexpression requires an interaction with both Grb2 and Syp signaling molecules. J Biol Chem. 1996;271(24):14610–14616. doi: 10.1074/jbc.271.24.14610. [DOI] [PubMed] [Google Scholar]
- 39.Ito T, Sasaki Y, Wands JR. Overexpression of human insulin receptor substrate 1 induces cellular transformation with activation of mitogen-activated protein kinases. Mol Cell Biol. 1996;16(3):943–951. doi: 10.1128/mcb.16.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279(5350):563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 41.Giovannucci E. Insulin-like growth factor-I and binding protein-3 and risk of cancer. Horm Res. 1999;513(Suppl):34–41. doi: 10.1159/000053160. [DOI] [PubMed] [Google Scholar]
- 42.Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363(9418):1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 43.Rinaldi S, Cleveland R, Norat T, Biessy C, Rohrmann S, Linseisen J, Boeing H, Pischon T, Panico S, Agnoli C, Palli D, Tumino R, Vineis P, Peeters PH, van Gils CH, Bueno-de-Mesquita BH, Vrieling A, Allen NE, Rodam A, Bingham S, Khaw KT, Manjer J, Borgquist S, Dumeaux V, Torhild Gram I, Lund E, Trichopoulou A, Makrygiannis G, Benetou V, Molina E, Danate Suarez I, Barricarte Gurrea A, Gonzalez CA, Tormo MJ, Altzibar JM, Olsen A, Tjonneland A, Gronbaek H, Overvad K, Clavel-Chapelon F, Boutron-Ruault MC, Morois S, Slimani N, Boffetta P, Jenab M, Riboli E, Kaaks R. Serum levels of IGF-I, IGFBP-3 and colorectal cancer risk: results from the EPIC cohort, plus a meta-analysis of prospective studies. Int J Cancer. 2010;126(7):1702–1715. doi: 10.1002/ijc.24927. [DOI] [PubMed] [Google Scholar]
- 44.Pollak M. IGF-I physiology and breast cancer. Recent Results Cancer Res. 1998;152:63–70. doi: 10.1007/978-3-642-45769-2_6. [DOI] [PubMed] [Google Scholar]
- 45.Li BD, Khosravi MJ, Berkel HJ, Diamandi A, Dayton MA, Smith M, Yu H. Free insulin-like growth factor-I and breast cancer risk. Int J Cancer. 2001;91(5):736–739. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1111>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 46.Wu Y, Yakar S, Zhao L, Hennighausen L, LeRoith D. Circulating insulin-like growth factor-I levels regulate colon cancer growth and metastasis. Cancer Res. 2002;62(4):1030–1035. [PubMed] [Google Scholar]
- 47.Wu Y, Cui K, Miyoshi K, Hennighausen L, Green JE, Setser J, LeRoith D, Yakar S. Reduced circulating insulin-like growth factor I levels delay the onset of chemically and genetically induced mammary tumors. Cancer Res. 2003;63(15):4384–4388. [PubMed] [Google Scholar]
- 48.Papa V, Gliozzo B, Clark GM, McGuire WL, Moore D, Fujita-Yamaguchi Y, Vigneri R, Goldfine ID, Pezzino V. Insulin-like growth factor-I receptors are overexpressed and predict a low risk in human breast cancer. Cancer Res. 1993;53(16):3736–3740. [PubMed] [Google Scholar]
- 49.Resnik JL, Reichart DB, Huey K, Webster NJ, Seely BL. Elevated insulin-like growth factor I receptor autophosphorylation and kinase activity in human breast cancer. Cancer Res. 1998;58(6):1159–1164. [PubMed] [Google Scholar]
- 50.Sell C, Dumenil G, Deveaud C, Miura M, Coppola D, DeAngelis T, Rubin R, Efstratiadis A, Baserga R. Effect of a null mutation of the insulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol Cell Biol. 1994;14(6):3604–3612. doi: 10.1128/mcb.14.6.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaleko M, Rutter WJ, Miller AD. Overexpression of the human insulinlike growth factor I receptor promotes ligand-dependent neoplastic transformation. Mol Cell Biol. 1990;10(2):464–473. doi: 10.1128/mcb.10.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sell C, Rubini M, Rubin R, Liu JP, Efstratiadis A, Baserga R. Simian virus 40 large tumor antigen is unable to transform mouse embryonic fibroblasts lacking type 1 insulin-like growth factor receptor. Proc Natl Acad Sci U S A. 1993;90(23):11217–11221. doi: 10.1073/pnas.90.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dunn SE, Ehrlich M, Sharp NJ, Reiss K, Solomon G, Hawkins R, Baserga R, Barrett JC. A dominant negative mutant of the insulin-like growth factor-I receptor inhibits the adhesion, invasion, and metastasis of breast cancer. Cancer Res. 1998;58(15):3353–3361. [PubMed] [Google Scholar]
- 54.Dunn SE, Hardman RA, Kari FW, Barrett JC. Insulin-like growth factor 1 (IGF-1) alters drug sensitivity of HBL100 human breast cancer cells by inhibition of apoptosis induced by diverse anticancer drugs. Cancer Res. 1997;57(13):2687–2693. [PubMed] [Google Scholar]
- 55.Bender LM, Nahta R. Her2 cross talk and therapeutic resistance in breast cancer. Front Biosci. 2008;13:3906–3912. doi: 10.2741/2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chakraborty AK, Liang K, DiGiovanna MP. Co-targeting insulin-like growth factor I receptor and HER2: dramatic effects of HER2 inhibitors on nonoverexpressing breast cancer. Cancer Res. 2008;68(5):1538–1545. doi: 10.1158/0008-5472.CAN-07-5935. [DOI] [PubMed] [Google Scholar]
- 57.Lu D, Zhang H, Koo H, Tonra J, Balderes P, Prewett M, Corcoran E, Mangalampalli V, Bassi R, Anselma D, Patel D, Kang X, Ludwig DL, Hicklin DJ, Bohlen P, Witte L, Zhu Z. A fully human recombinant IgG-like bispecific antibody to both the epidermal growth factor receptor and the insulin-like growth factor receptor for enhanced antitumor activity. J Biol Chem. 2005;280(20):19665–19672. doi: 10.1074/jbc.M500815200. [DOI] [PubMed] [Google Scholar]
- 58.Lu Y, Zi X, Zhao Y, Mascarenhas D, Pollak M. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin) J Natl Cancer Inst. 2001;93(24):1852–1857. doi: 10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- 59.Ueda S, Hatsuse K, Tsuda H, Ogata S, Kawarabayashi N, Takigawa T, Einama T, Morita D, Fukatsu K, Sugiura Y, Matsubara O, Mochizuki H. Potential crosstalk between insulin-like growth factor receptor type 1 and epidermal growth factor receptor in progression and metastasis of pancreatic cancer. Mod Pathol. 2006;19(6):788–796. doi: 10.1038/modpathol.3800582. [DOI] [PubMed] [Google Scholar]
- 60.Min Y, Adachi Y, Yamamoto H, Imsumran A, Arimura Y, Endo T, Hinoda Y, Lee CT, Nadaf S, Carbone DP, Imai K. Insulin-like growth factor I receptor blockade enhances chemotherapy and radiation responses and inhibits tumour growth in human gastric cancer xenografts. Gut. 2005;54(5):591–600. doi: 10.1136/gut.2004.048926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wen B, Deutsch E, Marangoni E, Frascona V, Maggiorella L, Abdulkarim B, Chavaudra N, Bourhis J. Tyrphostin AG 1024 modulates radiosensitivity in human breast cancer cells. Br J Cancer. 2001;85(12):2017–2021. doi: 10.1054/bjoc.2001.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perer ES, Madan AK, Shurin A, Zakris E, Romeguera K, Pang Y, Beech DJ. Insulin-like growth factor I receptor antagonism augments response to chemoradiation therapy in colon cancer cells. J Surg Res. 2000;94(1):1–5. doi: 10.1006/jsre.2000.5923. [DOI] [PubMed] [Google Scholar]
- 63.D’Ambrosio C, Keller SR, Morrione A, Lienhard GE, Baserga R, Surmacz E. Transforming potential of the insulin receptor substrate 1. Cell Growth Differ. 1995;6(5):557–562. Epub 1995/05/01. [PubMed] [Google Scholar]
- 64.Cesarone G, Garofalo C, Abrams MT, Igoucheva O, Alexeev V, Yoon K, Surmacz E, Wickstrom E. RNAi-mediated silencing of insulin receptor sub- strate 1 (IRS-1) enhances tamoxifen-induced cell death in MCF-7 breast cancer cells. J Cell Biochem. 2006;98(2):440–450. doi: 10.1002/jcb.20817. [DOI] [PubMed] [Google Scholar]
- 65.Sun H, Tu X, Baserga R. A mechanism for cell size regulation by the insulin and insulin-like growth factor-I receptors. Cancer Res. 2006;66(23):11106–11109. doi: 10.1158/0008-5472.CAN-06-2641. [DOI] [PubMed] [Google Scholar]
- 66.Baserga R. Customizing the targeting of IGF-1 receptor. Future Oncol. 2009;5(1):43–50. doi: 10.2217/14796694.5.1.43. [DOI] [PubMed] [Google Scholar]
- 67.Pankratz SL, Tan EY, Fine Y, Mercurio AM, Shaw LM. Insulin receptor substrate-2 regulates aerobic glycolysis in mouse mammary tumor cells via glucose transporter 1. J Biol Chem. 2009;284(4):2031–2037. doi: 10.1074/jbc.M804776200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mardilovich K, Shaw LM. Hypoxia regulates insulin receptor substrate-2 expression to promote breast carcinoma cell survival and invasion. Cancer Res. 2009;69(23):8894–8901. doi: 10.1158/0008-5472.CAN-09-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Byron SA, Horwitz KB, Richer JK, Lange CA, Zhang X, Yee D. Insulin receptor substrates mediate distinct biological responses to insulin-like growth factor receptor activation in breast cancer cells. Br J Cancer. 2006;95(9):1220–1228. doi: 10.1038/sj.bjc.6603354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arteaga CL, Kitten LJ, Coronado EB, Jacobs S, Kull FC, Jr., Allred DC, Osborne CK. Blockade of the type I somatomedin receptor inhibits growth of human breast cancer cells in athymic mice. J Clin Invest. 1989;84(5):1418–1423. doi: 10.1172/JCI114315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Surmacz E. Growth factor receptors as therapeutic targets: strategies to inhibit the insulin-like growth factor I receptor. Oncogene. 2003;22(42):6589–6597. doi: 10.1038/sj.onc.1206772. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y, Sun Y. Insulin-like growth factor receptor-1 as an anti-cancer target: blocking transformation and inducing apoptosis. Curr Cancer Drug Targets. 2002;2(3):191–207. doi: 10.2174/1568009023333863. [DOI] [PubMed] [Google Scholar]
- 73.Bohula EA, Salisbury AJ, Sohail M, Playford MP, Riedemann J, Southern EM, Macaulay VM. The efficacy of small interfering RNAs targeted to the type 1 insulin-like growth factor receptor (IGF1R) is influenced by secondary structure in the IGF1R transcript. J Biol Chem. 2003;278(18):15991–15997. doi: 10.1074/jbc.M300714200. [DOI] [PubMed] [Google Scholar]
- 74.Sachdev D, Hartell JS, Lee AV, Zhang X, Yee D. A dominant negative type I insulin-like growth factor receptor inhibits metastasis of human cancer cells. J Biol Chem. 2004;279(6):5017–5024. doi: 10.1074/jbc.M305403200. [DOI] [PubMed] [Google Scholar]
- 75.Garcia-Echeverria C, Pearson MA, Marti A, Meyer T, Mestan J, Zimmermann J, Gao J, Brueggen J, Capraro HG, Cozens R, Evans DB, Fabbro D, Furet P, Porta DG, Libetanz J, Martiny-Baron G, Ruetz S, Hofmann F. In vivo antitumor activity of NVP-AEW541—a novel, potent, and selective inhibitor of the IGF-IR kinase. Cancer Cell. 2004;5(3):231–239. doi: 10.1016/s1535-6108(04)00051-0. [DOI] [PubMed] [Google Scholar]
- 76.Gualberto A, Pollak M. Emerging role of insulin-like growth factor receptor inhibitors in oncology: early clinical trial results and future directions. Oncogene. 2009;28(34):3009–3021. doi: 10.1038/onc.2009.172. [DOI] [PubMed] [Google Scholar]
- 77.Golan T, Javle M. Targeting the insulin growth factor pathway in gastrointestinal cancers. Oncology (Williston Park) 2011;25(6):518–526. 29. [PubMed] [Google Scholar]
- 78.Zhang H, Fagan DH, Zeng X, Freeman KT, Sachdev D, Yee D. Inhibition of cancer cell proliferation and metastasis by insulin receptor downregulation. Oncogene. 2010;29(17):2517–2527. doi: 10.1038/onc.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hemkens LG, Grouven U, Bender R, Gunster C, Gutschmidt S, Selke GW, Sawicki PT. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia. 2009;52(9):1732–1744. doi: 10.1007/s00125-009-1418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52(9):1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 81.Buck E, Gokhale PC, Koujak S, Brown E, Eyzaguirre A, Tao N, Rosenfeld-Franklin M, Lerner L, Chiu MI, Wild R, Epstein D, Pachter JA, Miglarese MR. Compensatory insulin receptor (IR) activation on inhibition of insulin-like growth factor-1 receptor (IGF-1R): rationale for cotargeting IGF-1R and IR in cancer. Mol Cancer Ther. 2010;9(10):2652–2664. doi: 10.1158/1535-7163.MCT-10-0318. [DOI] [PubMed] [Google Scholar]
- 82.Ulanet DB, Ludwig DL, Kahn CR, Hanahan D. Insulin receptor functionally enhances multistage tumor progression and conveys intrinsic resistance to IGF-1R targeted therapy. Proc Natl Acad Sci U S A. 2010;107(24):10791–10798. doi: 10.1073/pnas.0914076107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Novosyadlyy R, Lann DE, Vijayakumar A, Rowzee A, Lazzarino DA, Fierz Y, Carboni JM, Gottardis MM, Pennisi PA, Molinolo AA, Kurshan N, Mejia W, Santopietro S, Yakar S, Wood TL, LeRoith D. Insulin-mediated acceleration of breast cancer development and progression in a nonobese model of type 2 diabetes. Cancer Res. 2010;70(2):741–751. doi: 10.1158/0008-5472.CAN-09-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wittman M, Carboni J, Attar R, Balasubramanian B, Balimane P, Brassil P, Beaulieu F, Chang C, Clarke W, Dell J, Eummer J, Frennesson D, Gottardis M, Greer A, Hansel S, Hurlburt W, Jacobson B, Krishnananthan S, Lee FY, Li A, Lin TA, Liu P, Ouellet C, Sang X, Saulnier MG, Stoffan K, Sun Y, Velaparthi U, Wong H, Yang Z, Zimmermann K, Zoeckler M, Vyas D. Discovery of a (1H-benzoimidazol-2-yl)-1H-pyridin-2-one (BMS-536924) inhibitor of insulin-like growth factor I receptor kinase with in vivo antitumor activity. J Med Chem. 2005;48(18):5639–5643. doi: 10.1021/jm050392q. [DOI] [PubMed] [Google Scholar]
- 85.Haluska P, Shaw HM, Batzel GN, Yin D, Molina JR, Molife LR, Yap TA, Roberts MA, Sharma A, Gualberto A, Adjei AA, de Bono JS. Phase I dose escalation study of the anti insulin-like growth factor-I receptor monoclonal antibody CP-751871 in patients with refractory solid tumors. Clin Cancer Res. 2007;13(19):5834–5840. doi: 10.1158/1078-0432.CCR-07-1118. [DOI] [PubMed] [Google Scholar]
- 86.Lacy MQ, Alsina M, Fonseca R, Paccagnella ML, Melvin CL, Yin D, Sharma A, Enriquez Sarano M, Pollak M, Jagannath S, Richardson P, Gualberto A. Phase I, pharmacokinetic and pharmacodynamic study of the anti-insulinlike growth factor type 1 Receptor monoclonal antibody CP-751871 in patients with multiple myeloma. J Clin Oncol. 2008;26(19):3196–3203. doi: 10.1200/JCO.2007.15.9319. [DOI] [PubMed] [Google Scholar]
- 87.Tolcher AW, Sarantopoulos J, Patnaik A, Papadopoulos K, Lin CC, Rodon J, Murphy B, Roth B, McCaffery I, Gorski KS, Kaiser B, Zhu M, Deng H, Friberg G, Puzanov I. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J Clin Oncol. 2009;27(34):5800–5807. doi: 10.1200/JCO.2009.23.6745. [DOI] [PubMed] [Google Scholar]
- 88.Maki RG. Small is beautiful: insulin-like growth factors and their role in growth, development, and cancer. J Clin Oncol. 2010;28(33):4985–4995. doi: 10.1200/JCO.2009.27.5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pappo AS, Patel S, Crowley J, Reinke DK, Staddon AP, Kuenkele K, Chawla SP, Benjamin RS, Helman LJ, Bake LH. Activity of R1507, a monoclonal antibody to the insulin-like growth factor-1 receptor (IGF1R), in patients (pts) with recurrent or refractory Ewing’s sarcoma family of tumors (ESFT): results of a phase II SARC study. 2010 ASCO Annual Meeting, J Clin Oncol. 2010;28(suppl):15s. abstr 10000. [Google Scholar]
- 90.Karp DD, Paz-Ares LG, Novello S, Haluska P, Garland L, Cardenal F, Blakely LJ, Eisenberg PD, Langer CJ, Blumenschein G, Johnson FM, Green S, Gualberto A. Phase II study of the antiinsulin-like growth factor type 1 receptor antibody CP-751871 in combination with paclitaxel and carboplatin in previously untreated, locally advanced, or metastatic non-small-cell lung cancer. J Clin Oncol. 2009;27(15):2516–2522. doi: 10.1200/JCO.2008.19.9331. [DOI] [PubMed] [Google Scholar]
- 91.Jassem J, Langer CJ, Karp DD, Mok T, Benner RJ, Green SJ, Park K, Novello S, Strausz J, Gualberto A. Randomized, open label, phase III trial of figitumumab in combination with paclitaxel and carboplatin versus paclitaxel and carboplatin in patients with non-small cell lung cancer (NSCLC) 2010 ASCO Annual Meeting, J Clin Oncol. 2010;28(suppl):15s. abstr 7500. [Google Scholar]
- 92.Zha J, O’Brien C, Savage H, Huw LY, Zhong F, Berry L, Lewis Phillips GD, Luis E, Cavet G, Hu X, Amler LC, Lackner MR. Molecular predictors of response to a humanized anti-insulin-like growth factor-I receptor monoclonal antibody in breast and colorectal cancer. Mol Cancer Ther. 2009;8(8):2110–2121. doi: 10.1158/1535-7163.MCT-09-0381. [DOI] [PubMed] [Google Scholar]
- 93.Lee AV, Yee D. Targeting IGF-1R: at a crossroad. Oncology (Williston Park) 2011;25(6):535–536. [PMC free article] [PubMed] [Google Scholar]
- 94.Bild AH, Yao G, Chang JT, Wang Q, Potti A, Chasse D, Joshi MB, Harpole D, Lancaster JM, Berchuck A, Olson A, Marks JR, Dressman HK, West M, Nevins JR. Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature. 2006;439(7074):353–357. doi: 10.1038/nature04296. [DOI] [PubMed] [Google Scholar]
- 95.Ramaswamy S, Golub TR. DNA microarrays in clinical oncology. J Clin Oncol. 2002;20(7):1932–1941. doi: 10.1200/JCO.2002.20.7.1932. [DOI] [PubMed] [Google Scholar]
- 96.Creighton CJ, Casa A, Lazard Z, Huang S, Tsimelzon A, Hilsenbeck SG, Osborne CK, Lee AV. Insulinlike growth factor-I activates gene transcription programs strongly associated with poor breast cancer prognosis. J Clin Oncol. 2008;26(25):4078–4085. doi: 10.1200/JCO.2007.13.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dupont J, Khan J, Qu BH, Metzler P, Helman L, LeRoith D. Insulin and IGF-1 induce different patterns of gene expression in mouse fibroblast NIH-3T3 cells: identification by cDNA microarray analysis. Endocrinology. 2001;142(11):4969–4975. doi: 10.1210/endo.142.11.8476. [DOI] [PubMed] [Google Scholar]
- 98.Mulligan C, Rochford J, Denyer G, Stephens R, Yeo G, Freeman T, Siddle K, O’Rahilly S. Microarray analysis of insulin and insulin-like growth factor-1 (IGF-1) receptor signaling reveals the selective up-regulation of the mitogen heparin-binding EGF-like growth factor by IGF-1. J Biol Chem. 2002;277(45):42480–42487. doi: 10.1074/jbc.M206206200. [DOI] [PubMed] [Google Scholar]
- 99.Oh JS, Kucab JE, Bushel PR, Martin K, Bennett L, Collins J, DiAugustine RP, Barrett JC, Afshari CA, Dunn SE. Insulin-like growth factor-1 inscribes a gene expression profile for angiogenic factors and cancer progression in breast epithelial cells. Neoplasia. 2002;4(3):204–217. doi: 10.1038/sj.neo.7900229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Palsgaard J, Brown AE, Jensen M, Borup R, Walker M, De Meyts P. Insulin-like growth factor I (IGF-I) is a more potent regulator of gene expression than insulin in primary human myoblasts and myotubes. Growth Horm IGF Res. 2009;19(2):168–178. doi: 10.1016/j.ghir.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 101.Yoshida E, Atkinson TG, Chakravarthy B. Neuroprotective gene expression profiles in ischemic cortical cultures preconditioned with IGF-1 or bFGF. Brain Res Mol Brain Res. 2004;131(1–2):33–50. doi: 10.1016/j.molbrainres.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 102.Pacher M, Seewald MJ, Mikula M, Oehler S, Mogg M, Vinatzer U, Eger A, Schweifer N, Varecka R, Sommergruber W, Mikulits W, Schreiber M. Impact of constitutive IGF1/IGF2 stimulation on the transcriptional program of human breast cancer cells. Carcinogenesis. 2007;28(1):49–59. doi: 10.1093/carcin/bgl091. [DOI] [PubMed] [Google Scholar]
- 103.van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, van der Velde T, Bartelink H, Rodenhuis S, Rutgers ET, Friend SH, Bernards R. A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med. 2002;347(25):1999–2009. doi: 10.1056/NEJMoa021967. [DOI] [PubMed] [Google Scholar]
- 104.Wang Y, Klijn JG, Zhang Y, Sieuwerts AM, Look MP, Yang F, Talantov D, Timmermans M, Meijer-van Gelder ME, Yu J, Jatkoe T, Berns EM, Atkins D, Foekens JA. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet. 2005;365(9460):671–679. doi: 10.1016/S0140-6736(05)17947-1. [DOI] [PubMed] [Google Scholar]
- 105.Miller LD, Smeds J, George J, Vega VB, Vergara L, Ploner A, Pawitan Y, Hall P, Klaar S, Liu ET, Bergh J. An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc Natl Acad Sci U S A. 2005;102(38):13550–13555. doi: 10.1073/pnas.0506230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Litzenburger BC, Creighton CJ, Tsimelzon A, Chan BT, Hilsenbeck SG, Wang T, Carboni JM, Gottardis MM, Huang F, Chang JC, Lewis MT, Rimawi MF, Lee AV. High IGF-IR activity in triple-negative breast cancer cell lines and tumorgrafts correlates with sensitivity to anti-IGF-IR therapy. Clin Cancer Res. 2011;17(8):2314–2327. doi: 10.1158/1078-0432.CCR-10-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang F, Greer A, Hurlburt W, Han X, Hafezi R, Wittenberg GM, Reeves K, Chen J, Robinson D, Li A, Lee FY, Gottardis MM, Clark E, Helman L, Attar RM, Dongre A, Carboni JM. The mechanisms of differential sensitivity to an insulin-like growth factor-1 receptor inhibitor (BMS-536924) and rationale for combining with EGFR/HER2 inhibitors. Cancer Res. 2009;69(1):161–170. doi: 10.1158/0008-5472.CAN-08-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pitts TM, Tan AC, Kulikowski GN, Tentler JJ, Brown AM, Flanigan SA, Leong S, Coldren CD, Hirsch FR, Varella-Garcia M, Korch C, Eckhardt SG. Development of an integrated genomic classifier for a novel agent in colorectal cancer: approach to individualized therapy in early development. Clin Cancer Res. 2010;16(12):3193–3204. doi: 10.1158/1078-0432.CCR-09-3191. [DOI] [PMC free article] [PubMed] [Google Scholar]