Abstract
Background
Evidence suggests that subgroups of patients with irritable bowel syndrome (IBS) are hyper-responsive to a variety of laboratory stress conditions.
Methods
This study compared sleep quality and night time plasma adrenocorticotropic hormone (ACTH) and serum cortisol levels in response to anticipation of public speaking between 43 women with IBS and 24 healthy control women. In addition, comparisons were made between subgroups within the IBS sample based on predominant stool patterns, 22 IBS-constipation and 21 IBS-diarrhea. Subjects slept three nights in a sleep laboratory, and on the third night serial blood samples were drawn every 20 minutes from 8 PM until awakening. Because subjects had different sleep onsets, each subject’s results were synchronized to the first onset of stage 2 sleep.
Key Results
Compared to the healthy control group, women with IBS had significantly worse sleep efficiency, and higher cortisol but not ACTH levels over the night. However, there were no IBS bowel pattern subgroup differences. Among IBS subjects, cortisol levels early in the night were higher than found in our previous study with a similar protocol but without the threat of public speaking. These results suggest that a social stressor, such as public speaking prior to bedtime, increases cortisol but not ACTH levels suggesting HPA dysregulation in women with IBS.
Conclusions & Inferences
This response to a social stressor contributes to our understanding of the relationship of stress to symptom expression in IBS.
Keywords: ACTH, cortisol, irritable bowel syndrome, sleep, social stress
Irritable bowel syndrome (IBS) is a functional gastrointestinal (GI) disorder that affects approximately 10–15% of the U.S. population. It is well established that women with IBS are more likely to seek health care services for their symptoms as compared to men [1]. While it is not clear whether stress initiates IBS, it has been shown that stress can trigger or exacerbate symptoms in approximately 75–80% of patients with IBS [2–4]. Patients with IBS report more chronic stress and that, at least for some patients, chronic stress is linked to the pathophysiology of IBS (for reviews see [2, 3, 5, 6]).
Several investigators have demonstrated alterations in the hypothalamic-pituitary-adrenal (HPA) axis as well as indicators of the autonomic nervous system (ANS) in patients (predominantly women) with IBS under baseline and acute laboratory and clinical stress conditions [7–12]. HPA dysregulation secondary to chronic stress may alter the response to acute stress exposure. This dysregulation may be due to down-regulation of the HPA axis subsequent to prolonged stress [7, 10]. However, in patients with IBS blunted [9], elevated [7], or unchanged [8] cortisol results are seen across studies. These inconsistent findings may be accounted for by differences in person characteristics such as prior history of or current psychological distress, cognitive appraisal of the stressor, presence of comorbid conditions, IBS symptom severity, history of early adverse events, age, poor sleep quality, as well as the type, intensity and duration of the acute stressor [13, 14]. In a prior study we noted IBS bowel pattern subgroup differences (IBS-constipation [IBS-C] greater than IBS-diarrhea [ IBS-D]) in cortisol during sleep [13]. Whether these subgroup differences exist in response to a social stressor was the impetus for the current study. In addition, we sought to determine whether objective sleep indices varied by group.
The type of laboratory stress used to elicit physiological responses has received considerable attention in the psychophysiological literature. Public speaking as well as anticipation of public speaking is considered a consistent acute social stressor for women [15]. Attentional biases to visceral, environmental as well as social stimuli exist within patients with IBS. However, it is unknown whether the increased sensitivity towards social threat is related to higher or lower cortisol levels. The purpose of the current study is to compare sleep quality as measured by polysomnography (PSG) and the patterns of adenocorticotropic hormone (ACTH) and cortisol secretion during the night in healthy control (HC) women and women with either IBS-C or IBS-D when faced with the prospect of public speaking in the morning. Based on prior work, we hypothesized that women with IBS compared to HC women would exhibit more time awake during the night, and higher ACTH and cortisol levels prior to and during sleep in anticipation of public speaking. In addition, we hypothesized that women with IBS-C would demonstrate higher levels of ACTH and cortisol relative to women with IBS-D.
MATERIALS AND METHODS
Subjects and setting
University institutional review board approval was obtained prior to recruitment and renewed annually. Women with IBS and HC women (ages 18–45) were recruited through community advertisements in the Pacific Northwest and screened by telephone for the eligibility. To be included in the IBS group subjects had to have a prior diagnosis of IBS for at least 6 months made by a primary health care provider (e.g., internist, gastroenterologist). Over the preceding 3 months they had to have abdominal discomfort or pain more than 25% of the time that was associated with two of three features: 1) relieved with defecation, 2) onset associated with a change in frequency of stool, or 3) onset associated with a change in form (appearance) of stool. Subjects were classified as IBS-C if they had greater than or equal to 25% of stools hard and lumpy and less than 25% loose (mushy) or watery. Criteria for IBS-D were ≥ to 25% of stools loose and watery and less than 25% hard or lumpy. The Rome III criteria were confirmed with the Rome III Diagnostic Questionnaire for Functional GI Disorders [16]. Women who met the criteria for IBS-mixed were excluded. Women in the HC group could not have other functional GI disorders or serious health problems. In both groups, women were excluded if they: 1) had a history of an organic GI disease, cardiac arrhythmia, renal, or gynecological pathology; 2) were currently taking medications, e.g., prokinetic drugs, laxatives (but not fiber supplements), anti-diarrheals or antispasmodics, for GI symptoms; 3) were currently taking other medications daily that would alter serotonin, catecholamines, or cortisol; 4) had a body mass index (BMI, kg/m2) > 35; 5) worked during the late evening and night; 6) had a known primary sleep disorder; 7) had severe co-morbid pain or psychiatric conditions; or 8) drank ≥ 300mg of caffeine-containing beverages in the afternoon-evening or drank three or more servings of alcohol containing beverages every day.
Procedures
Initially, women gave informed consent to have their hematocrit level determined. >36% was considered in the normal range. If the hematocrit was low and the woman wanted to continue in the study, she was given a list of iron-rich food to enhance her diet; then, the hematocrit was rechecked a month later. Women who met the criteria returned to give informed consent, complete questionnaires, undergo a targeted physical assessment, review the daily diary, and tour the Sleep Laboratory. At home, they completed their diary each evening over one menstrual cycle, starting with their next menses. To standardize the menstrual cycle phase for testing, sleep testing was performed during the mid-luteal phase. Naturally menstruating women tested their urine for five days to determine the pre-ovulatory lutenizing hormone peak (ClearPlan Easy, Unipath Diagnostics Inc, Waltham, MA). For women on oral contraceptives the sleep laboratory visit was scheduled for the third week of their pill pack [17]. Prior to coming to laboratory, women were asked to refrain from drinking caffeine-containing beverages, taking acetaminophen, aspirin, or ibuprofen within 6 hours of their bedtime, or drinking alcohol during the 3-day laboratory assessment. Monetary compensation was provided.
Sleep Laboratory Protocol
Women slept in the sleep laboratory for 3 consecutive nights: adaptation, baseline, and the stress night when blood was drawn. The women arrived two-three hours before their usual bedtime, during which time electrodes were placed for the assessments. Subjects then relaxed in bed until the lights off. During the adaptation night apnea/hyponea and periodic leg movement disorder were assessed along with PSG. If a woman was positive for either disorder she was excluded from the study. On the second night (baseline), only PSG was assessed. On the stress night, women had an intravenous (IV) catheter inserted at 8pm; then, electrodes were placed for PSG. At the woman’s bedtime, the catheter was attached to a line that extended through a port to the monitoring room. A normal saline IV solution with 1,000 IU of heparin (15 ml/hour) was used. Blood samples for ACTH and cortisol were obtained every 20 minutes. During the evening women were reminded that in the morning they would be giving a 10-minute talk on their experiences in the study. The talk was given as the experimental stressor in a lecture room, research staff attended, and it was videotaped.
Measures
All subjects completed a demographic data sheet. Criteria for functional GI disorders was assessed with Functional GI Disorders-Rome III [16]. This questionnaire assesses the criteria for esophageal, gastroduodenal, bowel, functional abdominal pain, biliary, and anorectal disorders. Respondents were asked to describe their symptoms over the prior three months.
Recalled sleep quality
The Pittsburgh Sleep Quality Index (PSQI) and the daily diary were used. The PSQI assesses sleep quality and disturbances over the prior month. It is composed of 19 items that are scored to determine 7 component scores: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction [18]. Its reliability has been published elsewhere[18]. A global PSQI score > 5 yielded a diagnostic sensitivity of 89.6% and specificity of 86.5% (κ = .75, p < .001) in distinguishing good from poor sleepers [19].
Objective indicators of sleep
A standard PSG approach was used to measure electroencephalogram, electromyogram, and electrooculogram. Recordings were done using an Embla Recording Systems with Somnologica software 3.3.2 (Embla Systems, Thornton, CO). Sleep was scored using standard scoring criteria in 30 second epoch, with a combined computer and human scoring protocol. A computer sleep summary program calculated the sleep variables including sleep efficiency index (total sleep time/time in bed), sleep onset latency (minutes from lights out to the onset of stage 2 and rapid eye movement, REM), number of arousals measured by the return to stage 0 for at least 10 sec, and percent time spent awake (stage 0), in stage 2, in slow wave sleep stages 3 & 4 and in REM [20, 21] across time. Inter-rater agreement > 90% has been established for sleep scoring. Periodic leg movements were measured by placing a pair of silver/silver chloride electrodes on the anterior tibialis muscle of the leg contralateral to the subject’s preferred sleeping side [22]. Sleep apnea was assessed using ventilatory patterns as monitored by small bead thermistors detection of air flow from each nostril and the mouth [23].
Psychological Distress
The 53-item Brief Symptoms Index (BSI) was used. The subject was asked to consider the last 7 days then rate each symptom from 0 ‘not at all’ to 4 ‘extremely’ distressing. Internal consistency of the subscale scores are reported to range from α = .77 to .90 and test-retest reliability (one week interval) was .78 to .90 [24].
Public Speaking Anxiety
The Personal Report of Communication Apprehension (PRCA-24) questionnaire is a 24-item scale that reflects anxiety in different communication settings: group discussion, meetings, interpersonal conversations, and public speaking [25, 26]. Each subscale has 6 items rated on a 5-point Likert scale (strongly agree to strongly disagree). It has been shown to be internally consistent α = .86 [27].
Anxiety, Depression, and Sleep Quality measured on each of the three sleep nights
Each evening in the sleep lab subjects filled out the anxiety and depression subscales of the 18-item short version of the BSI. Each morning they filled out the Morning Sleep Questionnaire (MSQ). A single question from this scale, “How would you rate the overall quality of your sleep?”, from 1 = “terrible” to 9 = “great”, was used.
Cortisol
Blood was collected in silicone coated tubes, allowed to coagulate, then centrifuged. The serum layer was removed and analyzed for cortisol measurement by chemiluminescence using the automated Immulite Analyzer (Diagnostic Products Co.). The intra-assay variation was 2.7% and the inter assay variation is 5.4%.
ACTH
For ACTH blood was collected in iced EDTA tubes. The plasma was separated from the cells by centrifuging in a refrigerated (−40°C) centrifuge, then aliquoted into 2 plastic tubes and frozen immediately at −70°C. ACTH was assayed by chemiluminescence using the automated Immulite Analyzer (Diagnostic Products Co.). Every tenth sample was done in duplicate. The intra-assay variation was 6.2% and the inter assay variation is 9.4%.
All assays were manually screened for data quality. The blood draw collection logs were used to evaluate and to exclude unusual values based on evidence of procedural issues [13]. Isolated missing or disqualified values interior to the within-subject hormone time series were replaced using running median smoothing and linear interpolation. In order to make the time course more comparable across subjects, the time base for each woman’s data sequence was synchronized to her first sustained passage into Stage 2 (or deeper) sleep. After applying a log transform, the within-subject hormone series were aggregated into hourly means, based on time blocks representing whole hours before and after the onset of sleep. Results are presented for the 8 hourly blocks from 2 hours before stage 2 sleep until 6 hours after stage two sleep.
Data Analysis
Demographic variables were compared between the IBS and HC groups using Student’s t-test statistics, Mann-Whitney U statistics, or Chi-square tests, depending on the distribution of the variables. The distributions of cortisol and ACTH were very skewed, and all analyses of these hormones were done on log-transformed data. Plots of geometric means across the nights in 3 groups (IBS-C, IBS-D and HC) were used to describe patterns of hormones. Differences between IBS and HC and between IBS-C and IBS-D in sleep quality variables were tested with ANCOVA analyses that controlled for age, BMI, and the value of the variable of interest on the previous night. At each hour during the night, hormone levels were analyzed with ANCOVA controlling for age, BMI, and median bedtime over the previous two weeks.
Our previous study [13] used essentially the same protocol as this study, but without the threat of public speaking. Therefore the effect of the public speaking threat, over and above the effect of sleeping in a sleep lab with IV blood draws all night long, was tested by comparing data from the two studies. ANCOVA was used to test for differences within HC and IBS groups between the two studies on hormone levels at each hour during the night.
RESULTS
Descriptive Characteristics
Forty-three women with IBS and 24 HC women slept in the laboratory on night 3 and contributed PSG data. Blood samples for cortisol and ACTH were collected on 42 IBS and 24 HC women, though blood collection was terminated early (less than 5 hours) due to technical problems with the blood draws for three of these women (two IBS, one HC).
There were no statistically significant differences in the demographic characteristics between the 43 women with IBS and the 24 HCs. The combined sample had mean ± sd age of 28 ± 6 years. Twenty-four percent of subjects were married or partnered, and 75% had a college degree. There were no significant differences in race (93% white for IBS versus 79% for HC, p = .10) and professional or manager job (29% versus 17%, p = .20).
Table 1 summarizes the retrospective measures of sleep quality, anxiety, depression, and public speaking anxiety, as well as daily ratings of sleep quality, anxiety and depression for 3 nights among the HC and IBS women. Retrospective repots of sleep quality, anxiety and depression, were somewhat worse in IBS than HC, though none of these differences were statistically significant. There were no IBS versus HC group or IBS bowel pattern subgroup differences in the public speaking anxiety scale scores. The IBS-C group did report somewhat higher levels of anxiety and depression as compared to the IBS-D group, though this was not significant (analyses not shown).
Table 1.
Comparisons of healthy control (HC) women and women with irritable bowel syndrome (IBS) on measures of sleep quality, anxiety, depression and public speaking fear
| HC (N=24) Mean (SD) |
IBS (N=43) Mean (SD) |
p-value | |
|---|---|---|---|
| Retrospective Measures at Baseline | |||
| Sleep quality: PSQI | 3.5 (1.9) | 4.3 (1.9) | .108 |
| Anxiety: BSI-53 | 0.28 (0.38) | 0.52 (0.54) | .067 |
| Depression: BSI-53 | 0.28 (0.54) | 0.51 (0.68) | .166 |
| Public Speaking subscale of PRCA | 17.2 (5.9) | 17.6 (4.9) | .781 |
| Nightly Measures in the Sleep Lab (daily self report) | |||
| Sleep quality: Night 1 | 5.1 (1.6) | 4.9 (1.2) | .661 |
| Sleep quality: Night 2 | 6.5 (1.3) | 6.2 (1.4) | .257 |
| Sleep quality: Night 3 | 5.2 (2.0) | 4.3 (1.9) | .074 |
| Anxiety: Night 1 | 0.22 (0.32) | 0.34 (0.34) | .149 |
| Anxiety: Night 2 | 0.15 (0.26) | 0.27 (0.36) | .147 |
| Anxiety: Night 3 | 0.14 (0.21) | 0.27 (0.32) | .079 |
| Depression: Night 1 | 0.17 (0.41) | 0.33 (0.43) | .149 |
| Depression: Night 2 | 0.12 (0.25) | 0.29 (0.53) | .160 |
| Depression: Night 3 | 0.15 (0.30) | 0.22 (0.40) | .425 |
PSQI = Pittsburgh Sleep Quality Index; BSI = Brief Symptom Inventory; PRCA = Personal Report of Communication Apprehension.
Table 2 shows means and standard deviations of the sleep measures from PSG on night 3. Several of these measures show significantly worse sleep quality in the IBS group than in the HC group. In general the differences are more significant when the analysis controlled for the value of the measure on night 2 (baseline). PSG sleep quality differences between IBS versus HC remained significant after controlling for anxiety on night 3 (Table 2).
Table 2.
Night 3 sleep polysomnographic indices (Mean [SD)] of healthy controls (HC), patients with IBS and separate IBS-constipation (IBS-C) and IBS-diarrhea (IBS-D)
| HC (N=24) |
IBS (N=43) |
P1 | P2 | P3 | IBS-C (N=22) |
IBS-D (N=21) |
P4 | |
| Total time in bed (min) |
482(33) | 476(37) | NS | NS | NS | 476(29) | 476(44) | NS |
| Total time asleep (min) |
393(44) | 361(58) | .010 | .008 | .025 | 351(63) | 372(52) | NS |
| Sleep efficiency index % |
81.5(8.3) | 76.2(13.3) | .037 | .009 | .023 | 74.0(13.7) | 78.5(12.7) | NS |
| Sleep latency lights out to S2 |
23.1(23.8) | 26.0(27.7) | NS | NS | NS | 28.8(32.5) | 23.1(22.0) | NS |
| Sleep latency S1 to REM |
80.2(50.4) | 101.0(65.4) | NS | .165 | .163 | 92.7(61.2) | 109.7(70.0) | NS |
| Sleep latency S1 to SWS |
15.4(13.7) | 15.9(10.1) | NS | NS | NS | 18.3(11.0) | 13.4(8.6) | NS |
| Sleep Fragmentation index |
7.6(2.8) | 7.7(3.0) | NS | NS | NS | 8.2(3.7) | 7.2(2.1) | NS |
| % time awake | 14.1(7.6) | 18.5(10.2) | .02 | .007 | .015 | 19.8(10.6) | 17.2(9.8) | NS |
| % time in S2 | 37.2(9.4) | 36.9(8.8) | NS | .024 | .028 | 37.9(10.1) | 35.8(7.3) | NS |
| % time in SWS | 21.3(9.3) | 20.6(7.9) | NS | NS | NS | 18.3(7.3) | 23.1(7.9) | NS |
| % time in REM | 21.2(5.2) | 17.5(5.6) | .003 | .004 | .015 | 16.5(5.9) | 18.6(5.2) | NS |
P1 = p-value for testing IBS versus HC, controlling for age and BMI.
P2 = p-value for testing IBS versus HC, controlling for age, BMI, and Night 2 value of the measure.
P3 = p-value for testing IBS versus HC, controlling for age, BMI, and Night 2 value of the measure, and anxiety that evening.
P4 = p-value for testing IBS-C versus IBS-D, controlling for age, BMI, and Night 2 value of the measure.
NS = p > .20
There were 8 IBS subjects with relatively poor sleep on night 3, of whom 5 (62%) reported high anxiety on that night. Of the 34 IBS subjects with relatively good sleep only 5 (15%) reported high anxiety, and only 2 (8%) of the 24 HC subjects (p = .002) reported high anxiety. Seven of the 8 IBS subjects with poor sleep are IBS-C (88%), whereas 15 of 35 IBS subjects with relatively good sleep are IBS-C (43%) (p = .046).
The geometric mean plasma ACTH levels are shown in Figure 1 and Table 3. There were no statistically significant differences between IBS and HC groups. There were no IBS-C versus IBS-D differences in ACTH levels.
Figure 1.
Plasma adrenocorticotropic hormone (ACTH) levels prior to and during sleep in IBS (N=42) versus HC (N=24) subjects. Vertical intervals show geometric means and 95% confidence interval. Note that the values on the ordinate are shown in linear measurement units, but are plotted on an axis with logarithmic spacing. IBS = Irritable Bowel Syndrome; HC = Healthy Controls.
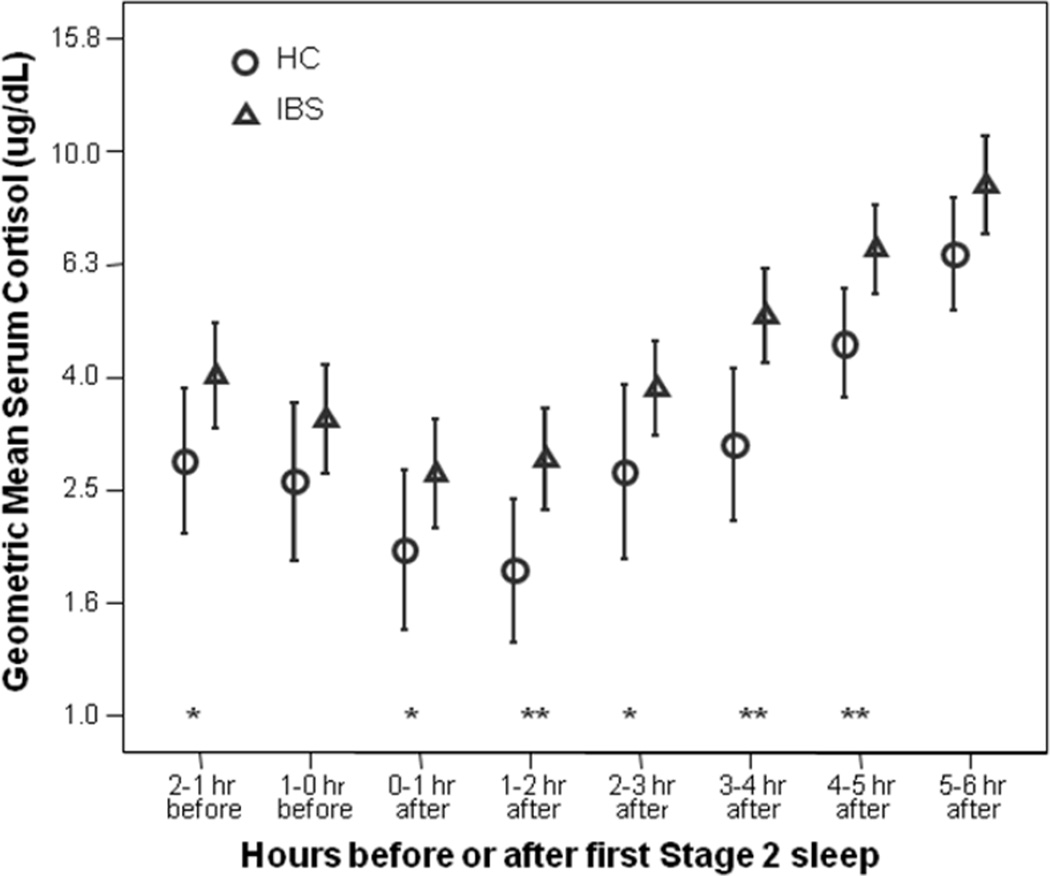
The geometric mean serum cortisol profiles are plotted in Figure 2. The omnibus main effects for group (p = .001) and the time (p < .001) were statistically significant, but the interaction effect for group and time was not significant (p = .161). The IBS group maintained cortisol values that were 46% higher than the HC women. There were no significant IBS-C versus IBS-D differences in cortisol levels.
Figure 2.
Serum cortisol levels prior to and during sleep in IBS (N=42) versus HC (N=24) subjects. Vertical intervals show geometric means and 95% confidence intervals. Note that the values on the ordinate are shown in linear measurement units, but are plotted on an axis with logarithmic spacing. IBS = Irritable Bowel Syndrome; HC = Healthy Controls; * = p-value < 0.05, ** = p-value < .01, from ANCOVA controlling for age, body mass index, and median bedtime over prior two weeks.
As seen in Figure 3, a comparison of this study to the previous study (without public speaking threat) shows similar cortisol levels across the night among HC women; however among IBS women, cortisol levels were much higher before sleep and for two hours after stage 2 sleep in the current study than in the previous study. At these times, there is a statistically significant interaction between study and diagnosis group (IBS vs HC). Objective sleep quality, as measured by sleep efficiency index, did not differ significantly between the two studies among either HC or IBS women. The cortisol results did not change substantially if sleep efficiency index on nights 2 and 3 was controlled for.
Figure 3.
Serum cortisol levels prior to and during sleep comparing results from two studies, stratified into HC (N=31 Study A, N=24 Study B) and IBS (N=30 Study A, N=42 Study B). Vertical intervals show geometric means and 95% confidence intervals. Note that the values on the ordinate are shown in linear measurement units, but are plotted on an axis with logarithmic spacing. Study A = prior study, with no threat of public speaking; Study B = current study, with threat of public speaking but otherwise similar to study A; IBS = Irritable Bowel Syndrome; HC = Healthy Controls; * = p-value < 0.05, ** = p-value < .01, from ANCOVA controlling for age and body mass index.
DISCUSSION
The results of the current study show that during the night prior to a public speaking task, IBS subjects had less time asleep, worse sleep efficiency, decreased stage 2 and decreased REM sleep as compared to HCs. Night-time serum cortisol, but not plasma ACTH, levels were increased in women with IBS relative to a HCs. Compared to the previous study which did not have a public speaking threat, women with IBS showed elevated cortisol levels just before and just after falling asleep. This finding is consistent with the conjecture that women with IBS are especially reactive to the public speaking threat.
Self-reported sleep quality was reduced and evening anxiety was somewhat higher in the IBS group than HC, but not significantly so. There were no differences in self-reported apprehension about public speaking. There were no IBS-C versus IBS-D subgroup differences found in any of the study measures.
A number of studies have examined self report of sleep quality in patients with IBS [31, 32]. As a group, patients with IBS report poor sleep, including difficulty getting to sleep and staying sleep and feeling unfreshed in the morning [33]. However, laboratory PSG studies provide conflicting data [33, 34]. In an earlier study, we failed to find IBS versus control differences in PSG variables in women studied in a sleep laboratory for 3 nights despite higher levels of subjectively poor sleep [13]. In the current study, the introduction of the anticipation of public speaking stressor elicited several sleep stage differences between IBS and HC women. These group differences became even more significant when the sleep stage data from the second night (or baseline) were controlled for in the analysis. This suggests that IBS versus HC sleep stage differences are associated with stressor exposure or stressor anticipation rather than an underlying PSG alteration.
Both HC and IBS women exhibited the expected pattern of night-time ACTH release, that is, lower levels during the early part of the night with rising levels later in the night. The lack of difference between HC and IBS is consistent with the night time ACTH pattern observed in the Chang et al [7] study. In a carefully controlled study of 24-hour measures of cortisol and ACTH levels these investigators found little difference in ACTH levels in the first few hours after lights out and the hour before that. However, during the day and around the time of morning awakening plasma ACTH levels were significantly lower compared to a HC comparison group, suggesting HPA dysfunction. It can be speculated that the public speaking threat counteracted the tendency for ACTH to otherwise be lower early in the early morning hours in women with IBS.
In contrast to ACTH levels, serum cortisol levels were higher in women with IBS than HC. These findings are consistent with Chang et al [7] who found marginally higher plasma cortisol levels between 02:00am and 06:00am in women with IBS [7]. Combined with Chang’s finding of lower ACTH levels during the day and early morning hours our findings support the hypothesis that HPA dysfunction is present in women with IBS. These findings differ from our results found in a separate sample of IBS and control women studied in a sleep laboratory using a similar IV insertion but no public speaking threat [13]. In that study we found no IBS versus HC group differences in cortisol levels at any time point during the night [13]. This suggests that anticipation of public speaking on top of the IV placement separated the IBS from the HC group. It is interesting to note that while women with IBS did not report greater fear of public speaking than HC, they did report more anxiety (not quite statistically significant) prior to bedtime.
It can be conjectured that the anticipation of the public speaking task resulted in increased adrenal sensitivity to ACTH beyond that associated with needle placement and the inconvenience and discomfort of sleeping with an IV line. Given that this increased sensitivity was present 2–3 hours before bedtime supports the hypothesis that at least a subset of women with IBS exhibit increased adrenal sensitivity to ACTH that persists during sleep. Veldhuis [29] reported that variations in the pulsatility of the ACTH-cortisol response is influenced by gender (endogenous sex steroids), age, and BMI. In the current study controlling for BMI, IBS versus control group differences persisted. As such, increased sensitivity to ACTH could be related to other factors including variations in intraadrenal circadian pacemakers, systemic and local cytokines, or other peripheral neuropeptides [29]. However, we did not control for childhood trauma. In a small study of healthy women, Heim and colleagues [30] found increased pituitary–adrenal responses to emotional stressors in women who reported a history of sexual or physical abuse during childhood. Videlock and colleagues [14] found increases in salivary cortisol in response to sigmoidoscopy in those who reported adverse childhood events (e.g., divorce, loss of a parent, or abuse). However, in a prior study we found no differences in first void urine cortisol levels among women with IBS with a history of abuse, women with IBS without a history of abuse and a HC group with no history of abuse [28]. These contradictory findings suggest that differences in ACTH-cortisol response may appear only when the individual is challenged with a real or anticipated stressor.
The current study did not find statistically significant differences between IBS subgroups on third night sleep PSG variables, ACTH or cortisol. In our earlier study (without a public speaking stressor) [13] when IBS subgroups based on predominant bowel pattern were compared differences in cortisol emerged (ACTH levels were not measured). Women with IBS-C had elevated cortisol levels throughout much of the sleep period, whereas women with IBS-D were lower. The primary limitation of this study is the self selection of subjects. Those IBS patients who were willing to undergo three nights in a sleep laboratory with an intravenous line during the third night may be a select subset of the population of IBS patients. Subjects were recruited with community advertisements and were screened for conditions and medications that might influence sleep and/or cortisol measures. Thus, the subjects may not be typical of women with IBS who present clinically, especially to consultants in tertiary GI clinics. It should be noted that although the levels of cortisol found in IBS subjects were higher relative to controls the levels are within a normal range.
In summary we found that when public speaking is anticipated, there were distinguishing differences in the nocturnal profiles for serum cortisol between the IBS women as a whole and the HC women, but no differences occurred among the IBS subgroups defined by bowel pattern predominance.
ACKNOWLEDGMENTS AND DISCLOSURES
This study was supported by NINR, NIH NR01094 and P30 NR04001. We want to thank Mr. Ernest Tolentino and Ms. Joyce Tsuji for their assistance with the laboratory assays, Mr. James Rothermel for the polysomnography analyses and especially, the women who gave so generously of their time to participate in this study.
MH, MJ, KC, and RB designed, initiated and supervised the study. RB and KC performed the statistical analyses. AP recruited subjects and managed the sleep laboratory protocol. All authors participated in the writing of the manuscript as well as providing constructive criticism and approving the final submission.
Abbreviations
- ACTH
Adenocorticotropic Hormone
- ANCOVA
Analysis of Covariance
- ANS
Autonomic Nervous System
- BMI
Body Mass Index, (weight(kg)/height (m)2)
- GI
Gastrointestinal
- HC
Healthy Control
- HPA
Hypothalamic-Pituitary-Adrenal
- IBS
Irritable Bowel Syndrome
- IBS-C
IBS-Constipation
- IBS-D
IBS-Diarrhea
- IV
Intravenous
- PSQI
Pittsburgh Sleep Quality index
- PSG
Polysomnography
- REM
Rapid Eye Movement
- SD
Standard Deviation
- SE
Standard Error
Footnotes
COMPETING INTERESTS
The authors have no competing interests.
REFERENCES
- 1.Chang L, Toner BB, Fukudo S, et al. Gender, age, society, culture, and the patient's perspective in the functional gastrointestinal disorders. Gastroenterology. 2006;130:1435–1446. doi: 10.1053/j.gastro.2005.09.071. [DOI] [PubMed] [Google Scholar]
- 2.Chang L. The role of stress on physiologic responses and clinical symptoms in irritable bowel syndrome. Gastroenterology. 2011;140:761–765. doi: 10.1053/j.gastro.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larauche M, Mulak A, Tache Y. Stress and visceral pain: From animal models to clinical therapies. Exp Neurol. 2011 May; doi: 10.1016/j.expneurol.2011.04.020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levy RL, Cain KC, Jarrett M, Heitkemper MM. The relationship between daily life stress and gastrointestinal symptoms in women with irritable bowel syndrome. J Behav Med. 1997;20:177–193. doi: 10.1023/a:1025582728271. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka Y, Kanazawa M, Fukudo S, Drossman DA. Biopsychosocial model of irritable bowel syndrome. J Neurogastroenterol Motil. 2011;17:131–139. doi: 10.5056/jnm.2011.17.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vicario M, Alonso C, Guilarte M, et al. Chronic psychosocial stress induces reversible mitochondrial damage and corticotropin-releasing factor receptor type-1 upregulation in the rat intestine and IBS-like gut dysfunction. Psychoneuroendocrinology. 2011 Jun; doi: 10.1016/j.psyneuen.2011.05.005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Chang L, Sundaresh S, Elliott J, et al. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol Motil. 2009;21:149–159. doi: 10.1111/j.1365-2982.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elsenbruch S, Lucas A, Holtmann G, et al. Public speaking stress-induced neuroendocrine responses and circulating immune cell redistribution in irritable bowel syndrome. Am J Gastroenterol. 2006;101:2300–2307. doi: 10.1111/j.1572-0241.2006.00837.x. [DOI] [PubMed] [Google Scholar]
- 9.FitzGerald LZ, Kehoe P, Sinha K. Hypothalamic--pituitary-- adrenal axis dysregulation in women with irritable bowel syndrome in response to acute physical stress. West J Nurs Res. 2009;31:818–836. doi: 10.1177/0193945909339320. [DOI] [PubMed] [Google Scholar]
- 10.Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29:83–98. doi: 10.1016/s0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- 11.Mazur M, Furgala A, Jablonski K, et al. Dysfunction of the autonomic nervous system activity is responsible for gastric myoelectric disturbances in the irritable bowel syndrome patients. J Physiol Pharmacol. 2007;58(Suppl 3):131–139. [PubMed] [Google Scholar]
- 12.Posserud I, Agerforz P, Ekman R, Bjornsson ES, Abrahamsson H, Simren M. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut. 2004;53:1102–1108. doi: 10.1136/gut.2003.017962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burr RL, Jarrett ME, Cain KC, Jun SE, Heitkemper MM. Catecholamine and cortisol levels during sleep in women with irritable bowel syndrome. Neurogastroenterol Motil. 2009;21:e1148–e1197. doi: 10.1111/j.1365-2982.2009.01351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Videlock EJ, Adeyemo M, Licudine A, et al. Childhood trauma is associated with hypothalamic-pituitary-adrenal axis responsiveness in irritable bowel syndrome. Gastroenterology. 2009;137:1954–1962. doi: 10.1053/j.gastro.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwerdtfeger A, Rosenkaimer AK. Depressive symptoms and attenuated physiological reactivity to laboratory stressors. Biol Psychol. 2011;87:430–438. doi: 10.1016/j.biopsycho.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Drossman DA, Corazziari E, Delvaux M, et al. Rome III: The functional gastrointestinal disorders. 3rd ed. McLean, VA: Degnon Associates; 2006. [Google Scholar]
- 17.Shechter A, Varin F, Boivin DB. Circadian variation of sleep during the follicular and luteal phases of the menstrual cycle. Sleep. 2010;33:647–656. doi: 10.1093/sleep/33.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 19.Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53:737–740. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- 20.Armitage R, Hoffmann R, Fitch T, Morel C, Bonato R. A comparison of period amplitude and power spectral analysis of sleep EEG in normal adults and depressed outpatients. Psychiatry Res. 1995;56:245–256. doi: 10.1016/0165-1781(95)02615-4. [DOI] [PubMed] [Google Scholar]
- 21.Drewes AM, Nielsen KD, Taagholt SJ, Bjerregard K, Svendsen L, Gade J. Sleep intensity in fibromyalgia: focus on the microstructure of the sleep process. Br J Rheumatol. 1995;34:629–635. doi: 10.1093/rheumatology/34.7.629. [DOI] [PubMed] [Google Scholar]
- 22.Stiasny K, Oertel WH, Trenkwalder C. Clinical symptomatology and treatment of restless legs syndrome and periodic limb movement disorder. Sleep Med Rev. 2002;6:253–265. doi: 10.1053/smrv.2001.0193. [DOI] [PubMed] [Google Scholar]
- 23.American Academy of Sleep Medicine Task Force. Sleep. Vol. 22. The Report of an American Academy of Sleep Medicine Task Force; 1999. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research; pp. 667–689. [PubMed] [Google Scholar]
- 24.Derogatis L. SCL-90R: Administration, Scoring and Procedures Manual. NCS Pearson, Inc; 1994. [Google Scholar]
- 25.McCroskey J. An introduction to rhetorical communication. Prentice Hall, Inc; 1993. [Google Scholar]
- 26.McCroskey J, Beatty M. Communication and personality. In: McCroskey J, Daly J, Martin M, Beatty M, editors. Communication apprehension. Cresskill, NJ: Hampton Press; 1998. pp. 215–231. [Google Scholar]
- 27.Levine T, McCroskey J. Measuring trait communication apprehension: A test of rival measurement models of the PRCA-24. Communication Monographs. 1996;57:62–62. [Google Scholar]
- 28.Heitkemper M, Jarrett M, Cain K, et al. Increased urine catecholamines and cortisol in women with irritable bowel syndrome. Am J Gastroenterol. 1996;91:906–913. [PubMed] [Google Scholar]
- 29.Veldhuis JD, Iranmanesh A, Roelfsema F, et al. Tripartite control of dynamic ACTH-cortisol dose responsiveness by age, body mass index, and gender in 111 healthy adults. J Clin Endocrinol Metab. 2011;96:2874–2881. doi: 10.1210/jc.2011-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heim C, Newport DJ, Heit S, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- 31.Chen CL, Liu TT, Yi CH, Orr WC. Evidence for altered anorectal function in irritable bowel syndrome patients with sleep disturbance. Digestion. 2011;84:247–251. doi: 10.1159/000330847. [DOI] [PubMed] [Google Scholar]
- 32.Kanaly T, Shaheen NJ, Vaughn BV. Gastrointestinal physiology and digestive disorders in sleep. Curr Opin Pulm Med. 2009 doi: 10.1097/MCP.0b013e3283318539. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Rotem AY, Sperber AD, Krugliak P, Freidman B, Tal A, Tarasiuk A. Polysomnographic and actigraphic evidence of sleep fragmentation in patients with irritable bowel syndrome. Sleep. 2003;26:747–752. doi: 10.1093/sleep/26.6.747. [DOI] [PubMed] [Google Scholar]
- 34.Elsenbruch S, Thompson JJ, Hamish MJ, Exton MS, Orr WC. Behavioral and physiological sleep characteristics in women with irritable bowel syndrome. Am J Gastroenterol. 2002;97:2306–2314. doi: 10.1111/j.1572-0241.2002.05984.x. [DOI] [PubMed] [Google Scholar]
- 35.Chang L, Mayer EA, Johnson T, FitzGerald LZ, Naliboff B. Differences in somatic perception in female patients with irritable bowel syndrome with and without fibromyalgia. Pain. 2000;84:297–307. doi: 10.1016/s0304-3959(99)00215-8. [DOI] [PubMed] [Google Scholar]