Abstract
Terminalia chebula is a native plant from southern Asia to southwestern China that is used in traditional medicine for the treatment of malignant tumors and diabetes. This plant also has antibacterial and immunomodulatory properties. The present study assessed T. chebula extract-dependent protein expression changes in Jurkat cells. Matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry and Ingenuity Pathways Analysis (IPA) were performed to assess protein expression and networks, respectively. A comparative proteomic profile was determined in T. chebula extract (50 μg/mL)-treated and control cells; the expressions of β-tubulin, ring finger and CHY zinc finger domain containing 1, and insulin-like growth factor 1 receptor kinase were significantly down-regulated in T. chebula extract-treated Jurkat cells. Moreover, the molecular basis for the T. chebula extract-dependent protein expression changes in Jurkat cells was determined by IPA. Treatment with the T. chebula extract significantly inhibited nuclear factor-κB activity and affected the proteomic profile of Jurkat cells. The molecular network signatures and functional proteomics obtained in this study may facilitate the evaluation of potential antitumor therapeutic targets and elucidate the molecular mechanism of T. chebula extract-dependent effects in Jurkat cells.
Key Words • gallic acid, Ingenuity Pathways Analysis, Jurkat cells, nuclear factor-κB, Terminalia chebula extract
Introduction
Terminalia chebula is a medicinal plant native mainly to tropical regions.1 It has traditionally been used as an alternative medicine to treat cancers. T. chebula extract also exhibits a variety of biological functions, including anticancer, antidiabetic, and antibacterial activities.2,3 Recently, it was reported that chebulagic acid isolated from T. chebula extract inhibits cyclooxygenase and 5-lipoxygenase, key enzymes involved in inflammation and carcinogenesis.4
In this study, we used Jurkat cells, an immortalized T lymphocyte cell line. Jurkat cells have been used to determine the molecular mechanism of action of anticancer drugs and radiation.5 Our primary objective was to compare the protein expression profile of Jurkat cells treated with T. chebula extract with that of control cells. Proteomic techniques have emerged as a powerful tool to associate the broad-spectrum protein expression with specific cellular responses. In our study, a comparative protein expression signature was established for Jurkat cells treated with T. chebula extract alone or combined with tumor necrosis factor α (TNFα).
Recently, we reported the inhibitory effect of gallic acid from T. chebula extract on nuclear factor-κB (NF-κB) activity in Jurkat cells.6 NF-κB, a transcription factor that is considered a master regulator of inflammation, is a promising drug target because it regulates the expression of inflammatory cytokines, adhesion molecules, and other proteins in several cancers. Most NF-κB inhibitors act by compromising NF-κB interaction with target DNA. Many antioxidants inhibit NF-κB by reducing the phosphorylation of inhibitory IκB.7 We reported that T. chebula extract containing gallic acid can inhibit NF-κB activity and down-regulates interleukin-8 and monocyte chemotactic protein-1 transcription in Jurkat-NF-κB–β-lactamase (bla) cells.6 It is interesting that the molecular network characterized by Ingenuity Pathways Analysis (IPA) analysis showed that T. chebula extract treatment affected the protein degradation, amino acid metabolism, and behavior networks, whereas NF-κB and mitogen-activated protein kinase 1 (extracellular signal-regulated kinase/mitogen-activated protein kinase) linked to Akt, protein kinase C isoforms, insulin, and phosphoinositide 3-kinase, whose expressions were not up-regulated. This suggests that the T. chebula extract containing gallic acid is a direct, potent inhibitor of NF-κB and can reduce the NF-κB-dependent activity in Jurkat cells.
In this study, we used proteomic techniques to identify novel molecular targets of the gallic acid fractionated T. chebula extract in Jurkat cells. Jurkat cells are used for studies of various biological phenomena, such as apoptosis and cell engulfment.8,9 In particular, we detected differential expression of proteins, including β-tubulin, ring finger and CHY zinc finger domain containing 1 (RCHY1), and insulin-like growth factor 1 receptor kinase (IGF1R) in T. chebula extract-treated Jurkat cells. Overall, the effects suggest that the components of T. chebula extract including gallic acid may be a novel drug candidate that has possible research and clinical value.
Materials and Methods
Acquisition of T. chebula extract and reagents
Hot water (at 60°C for 4 h) T. chebula extract solution (10 mg/mL) was obtained from Sunten Pharmaceutical Co. (Taipei, Taiwan). Isolation and identification of gallic acid were described in our previous article.6 In brief, gallic acid was isolated from T. chebula extract by reversed-phase high-performance liquid chromatography (C18, YMC-ODS-A, 250×10 mm, i.d.) with a solvent system of 15% acetonitrile in water at a flow rate of 1.5 mL/min. The purity of gallic acid so obtained was greater than 98.0% by high-performance liquid chromatography. Mouse recombinant TNFα was acquired from Sigma-Aldrich (USA). Cell culture medium, RPMI 1640 medium, fetal bovine serum, and penicillin–streptomycin were purchased from Gibco Life Technologies (UK).
Cell culture and treatments
Jurkat cells were obtained from the American Type Culture Collection (Manassas, VA, USA). Cell cultures were maintained at subconfluence in a 95% air/5% CO2 humidified atmosphere at 37°C in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum and 1% penicillin–streptomycin. Initial screening studies were performed to determine the concentration of T. chebula extract. Here, T. chebula extract (50 μg/mL) and TNFα (10 ng/mL), a 5-h treatment was used for all experiments.
Transfection and selection of cell line
Jurkat cells were transfected following the microporation procedure (iNCYTO, Korea) using the pLenti-bsd/NF-κB-bla CellSensor™ vector (catalog number K1128, Invitrogen). The total procedure was described previously.6
Protein sample preparation and two-dimensional gel electrophoresis
Protein sample preparation and two-dimensional gel electrophoresis (2DE) were performed on the whole-cell lysate protein fraction using our previously published methods.10 In brief, IPG dry strips (pH 4.0–7.0, 7 cm, Bio-Rad Laboratories, Hercules, CA, USA) were rehydrated for 12–16 h with 9.5 M urea containing 2% CHAPS and 40 mM dithiothreitol and loaded with 200 μg of the sample. Isoelectric focusing and stepwise electrophoresis were performed. Gel electrophoresis was performed in an Ettan DALTsix electrophoresis unit (GE Healthcare) at a constant 3 W per gel at 15°C until the blue dye exited the bottom of the gel. Next, the 2DE gels were stained with Coomassie Brilliant Blue dye. The resulting spots were imaged, and densitometry was performed using PDQuest software (Bio-Rad Laboratories). We performed three independent 2DE analyses and found similar gel images for individual experiment. The relative densitometry of each band was normalized to protein volume to determine the relative expression of each protein spot.
In-gel digestion
Protein spots were enzymatically digested in-gel in a manner similar to that previously described,11 using modified porcine trypsin (Promega, Madison, WI, USA). Gel pieces were washed with 50% acetonitrile to remove sodium dodecyl sulfate, salt and Coomassie stain. After that, gel pieces were dried to remove the solvent followed to rehydration with trypsin (8–10 ng/μL) and incubated for 8–10 h at 37°C. The proteolytic reaction was terminated by adding 5 μL of 0.5% trifluoroacetic acid. Tryptic peptides were recovered by extraction with 50% aqueous acetonitrile, and the aqueous-phase extractions of replicate gel pieces were combined. An aliquot of this solution was mixed with an equal volume of a saturated solution of α-cyano-4-hydroxycinnamic acid in 50% aqueous acetonitrile, and 1 μL of the mixture was spotted on the target plate.
Matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry and database analysis
Peptide analysis was performed using an Ultraflex matrix-assisted laser desorption/ionization (MALDI)–time-of-flight (TOF) mass spectrometer (Bruker Daltonics, Bremen, Germany). In brief, peptides were evaporated using an N2 laser (337 nm) in a delayed extraction approach and were accelerated with a 20-kV injection pulse for duration of flight analysis. Each spectrum represented the cumulative average of 300 laser shots. The ProFound search program was developed at Rockefeller University (http://prowl.rockefeller.edu/prowl-cgi/profound.exe) and was used for protein identification employing a Bayesian algorithm by peptide mass fingerprinting data. The presence of signal peptide is considered when such information is available in the corresponding National Center for Biotechnology Information GenPept format flatfile (www.ncbi.nlm.nih.gov/Entrez/batch.html). The following input parameters (mass tolerance±1; iodoacetamide modifications for amino acid residues) were applied. Spectra were internally calibrated using trypsin autodigestion ion peaks (m/z 842.510 and 2211.1046).
Western blotting assay
Western blotting was performed according to standard procedures.6 In brief, equal amounts of protein from whole-cell lysates (10 μg) were resolved by electrophoresis on 12% polyacrylamide gels. Proteins were then transferred to polyvinylidene difluoride membranes by electroblotting using the immersion method followed by blocking with 5% skimmed milk in 1% Tris-buffered saline containing Tween for 1 h and incubating overnight with primary antibodies at 4°C. After washing, the membranes were incubated for 1 h with the secondary antibodies. Blots were visualized with enhanced chemiluminescence (ECL™ Plus kit, GE Healthcare). The antibodies used were Rchy1/Pirh2 (sc-166293) and heat shock 70-kDa protein (HSP70) (sc-1060) from Santa Cruz Biotechnology Inc.
Canonical pathway analysis of datasets
IPA (Ingenuity® Systems [www.ingenuity.com], Mountain View, CA, USA) was conducted to determine the most significant canonical pathways in the datasets. Detailed methods have been described previously.12 In brief, genes from the dataset were considered for literature review. The significance of the association between the dataset and the canonical pathway was measured in two ways: (1) the ratio of the number of genes from the dataset that map to a given canonical pathway was divided by the total number of genes that map to the same canonical pathway; and (2) Fisher's exact test was used to calculate a P value to determine the probability that the association between the genes in the dataset and the canonical pathway could be explained by chance alone. After the datasets were uploaded, each gene identifier was mapped to its corresponding gene object in the IPA Knowledge Base, and these genes were overlaid onto a global molecular network. Gene networks were then algorithmically generated based on their connectivity.
Graphical representation of networks/pathways
Genes or gene products are represented as nodes, and the biological relationship between two nodes is represented as an edge (line). All edges are supported by the reference literature, from the canonical information stored in the IPA Knowledge Base. The intensity of the node color indicates the degree of up-regulation (red) or down-regulation (green).
Results
The protein expression profiles of Jurkat-NF-κB–bla cells treated with the T. chebula extract and the combination of T. chebula extract and TNFα were analyzed. In our previous study, we reported that T. chebula extract (50 μg/mL) inhibited NF-κB activity and strongly suppressed inhibitory IκBα phosphorylation in Jurkat-NF-κB–bla cells.6 Cells treated with T. chebula extract (50 μg/mL) for 5 h and incubated with T. chebula extract exhibited 100% viability in comparison with untreated cells (data not shown). Therefore, this extract had a minimal effect on Jurkat-NF-κB–bla cell viability, although effects on cell viability were observed at higher doses (100–200 μg/mL) (data not shown). Proteins that showed changes more than twofold from three independent experiments were selected and analyzed subsequently by MALDI-TOF mass spectrometry (MS) using trypsin. We compared the protein expression pattern of Jurkat-NF-κB–bla cells with or without extract treatment. Based on the ProFound database results, we identified protein expression that was up-regulated and down-regulated in Jurkat-NF-κB–bla cells.
Effects of T. chebula extract treatment
The effects of T. chebula extract treatment on protein expression were analyzed. The expressions of many proteins were up-regulated in T. chebula extract-treated Jurkat-NF-κB–bla cells, such as cell growth regulator with EF-hand domain 1, ankyrin repeat and MYND domain containing 2, KIAA0518, DOCK2, 14-3-3 proteins, and tropomyosin 4. β-Tubulin, RCHY1, IGF1R, and human Dutp pyrophosphatase complex with Dudp, TBC1 domain family, member 13 were among the proteins with reduced expression (Table 1 and Supplementary Fig. S1A; Supplementary Data are available online at www.liebertonline.com/jmf), and the decreases were significant for β-tubulin, RCHY1, and IGF1R. β-Tubulin is necessary for cellular component movement, microtubule-based movement, spindle assembly, and protein polymerization. Therefore, T. chebula extract treatment may negatively affect Jurkat-NF-κB–bla cells.
Table 1.
Effects of T. chebula Extract Treatment on Protein Expression in Jurkat-Nuclear Factor-κB-β-Lactamase Cells
| Protein | Spot number | NC | TC | Ratio | Est'd Z | Accession number |
|---|---|---|---|---|---|---|
| DOCK2 protein | 605 | 1 | 5193 | 5193.00 | 0.34 | gi54261667 |
| Bip protein | 2407 | 1 | 3219 | 3219.00 | 2.03 | gi6470150 |
| Transmembrane and coiled-coil domains 4 | 1307 | 1 | 2853 | 2853.00 | 0.96 | gi31565770 |
| Tropomyosin 4 isoform 2 | 2212 | 1 | 2584 | 2584.00 | 2.43 | gi4507651 |
| β 4 spectrin isoform σ2 | 3606 | 1 | 2195 | 2195.00 | 0.58 | gi11602887 |
| Lactamase, β2 | 7113 | 1 | 2145 | 2145.00 | 0.22 | gi7705793 |
| Cell growth regulator with EF-hand domain 1 | 111 | 1 | 2061 | 2061.00 | 2.43 | gi21961322 |
| 14-3-3n | 1106 | 1 | 1408 | 1408.00 | 1.33 | gi437363 |
| Chain A, cryogenic crystal structure of human myeloperoxidase isoform C | 1105 | 1 | 1406 | 1406.00 | 0.94 | gi7766941 |
| NLRC5 protein | 302 | 1 | 1313 | 1313.00 | 0.79 | gi30048125 |
| Chain A, Cryogenic crystal structure of human co-chaperone P23 | 1107 | 1 | 1225 | 1225.00 | 1.34 | gi9257073 |
| Transmembrane protein 74 | 1108 | 1 | 1056 | 1056.00 | 0.99 | gi23308545 |
| Ankyrin repeat and MYND domain containing 2 | 205 | 1 | 930 | 930.00 | 2.43 | gi28461129 |
| Laminin B1 | 2108 | 1 | 703 | 703.00 | 0.39 | gi186915 |
| KIAA0518 protein | 407 | 1 | 560 | 560.00 | 1.17 | gi3043560 |
| Sarcomeric tropomyosin κ | 1104 | 702 | 1 | 0.00 | 2.43 | gi48660012 |
| LOH12CR1 | 6108 | 1515 | 1 | 0.00 | 0.28 | gi15072483 |
| Chain A, human Dutp pyrophosphatase complex with Dudp | 8108 | 1620 | 1 | 0.00 | 2.43 | gi34810576 |
| Chain A, structure of insulin-like growth factor 1 receptor kinase | 7101 | 1627 | 1 | 0.00 | 0.17 | gi762941 |
| Ring finger and CHY zinc finger domain containing 1 | 6202 | 1840 | 1 | 0.00 | 1.28 | gi28703891 |
| v-crk sarcoma virus CT10 oncogene homolog (avian)-like | 7302 | 2060 | 1 | 0.00 | 2.3 | gi4885153 |
| HDCMC28P | 7401 | 2189 | 1 | 0.00 | 0.22 | gi7643778 |
| TBC1 domain family, member13 | 4106 | 2215 | 1 | 0.00 | 2.43 | gi55960036 |
| Tubulin, β | 1511 | 7678 | 1 | 0.00 | 1.42 | gi55961564 |
Est'd Z, estimated Z score as a measure of confidence for Profound-based identification; NC, negative (untreated control); TC, T. chebula extract (50 μg/mL)-treated.
Effects of T. chebula extract treatment in combination with TNFα
To analyze the T. chebula extract effect in the presence of an inducer, we treated the cells with a combination of T. chebula extract and TNFα. The effects of T. chebula extract treatment in combination with TNFα are listed in Table 2. Indolethylamine N-methyltransferase, apolipoprotein B-100 precursor (APOB), C14orf21, ring finger protein 20, and R32184 expressions were significantly up-regulated. In addition, APOB expression was increased in treated cells. APO-B100 is a low-density lipoprotein that is synthesized exclusively in the liver. It modulates the inflammatory IκB kinase–NF-κB signaling cascade and the secretion of apolipoprotein B-100-containing lipoproteins.13 Proteins of the R32184/WDR18 family are involved in a variety of cellular processes, including cell cycle progression, signal transduction, apoptosis, and gene regulation. Among the proteins with decreased expression after treatment with T. chebula extract and TNFα, changes in expression of HSP70 8 isoform 1 (HSPA8), putative DNA binding protein, and polyribonucleotide nucleotidyltransferase 1 were significant (Supplementary Fig. S1B).
Table 2.
Effects of T. chebula Extract Treatment in Combination with Tumor Necrosis Factor α on Protein Expression in Jurkat-Nuclear Factor-κB-β-Lactamase Cells
| Protein | Spot number | NC | TC + TNFα | Ratio | Est'd Z | Accession number |
|---|---|---|---|---|---|---|
| R32184_1 | 8113 | 1 | 6439 | 6439.00 | 2.43 | gi3025445 |
| Indolethylamine N-methyltransferase | 3107 | 1 | 1952 | 1952.00 | 2.43 | gi6580817 |
| Ring finger protein 20 | 6612 | 1 | 1143 | 1143.00 | 0.56 | gi55665535 |
| Apolipoprotein B100 precursor | 5619 | 1 | 865 | 865.00 | 1.23 | gi178792 |
| C14orf21 protein | 5512 | 1 | 817 | 817.00 | 0.44 | gi19263717 |
| Laminin B2 | 306 | 990 | 8012 | 8.09 | 1.05 | gi34238 |
| Hypothetical protein | 4403 | 1454 | 5051 | 3.47 | 1.24 | gi57999530 |
| Kinesin light chain 1P | 8107 | 1209 | 3936 | 3.26 | 1.4 | gi30409772 |
| p37 AUF1 | 8106 | 2900 | 9373 | 3.23 | 0.98 | gi433344 |
| Pre-B-cell leukemia homeobox 3 isoform 1 | 1306 | 734 | 1757 | 2.39 | 2.43 | gi5453852 |
| KIAA1125 protein | 6601 | 1444 | 1044 | 0.72 | 1.17 | gi6329749 |
| Mitogen-activated protein kinase kinase 5 isoform B | 2217 | 17,904 | 1770 | 0.10 | 2.43 | gi4506101 |
| Phosphoribosylaminoimidazole carboxylase, phosphoribosylaminoimidazole succinocarboxamide synthetase isoform 2 | 6211 | 542 | 1 | 0.00 | 1.35 | gi5453539 |
| FLJ10305 | 7309 | 600 | 1 | 0.00 | 0.58 | gi48146803 |
| Truncated tenascin XB | 6208 | 709 | 1 | 0.00 | 2.43 | gi6448761 |
| Heat shock 70-kDa protein 8 isoform 1 | 6411 | 724 | 1 | 0.00 | 1.36 | gi5729877 |
| Solute carrier family 24 (sodium/potassium/calcium exchanger), member 1 | 6606 | 735 | 1 | 0.00 | 1.17 | gi4759128 |
| EH domain containing 4 | 6409 | 976 | 1 | 0.00 | 0.16 | gi7212811 |
| DDX60 protein | 2206 | 1284 | 1 | 0.00 | 2.43 | gi50959706 |
| COG4 protein | 2410 | 1553 | 1 | 0.00 | 0.66 | gi39645057 |
| PNPT1 protein | 1206 | 1576 | 1 | 0.00 | 2.43 | gi34785309 |
| Putative DNA binding protein | 3311 | 2137 | 1 | 0.00 | 0.66 | gi15706422 |
| Immunoglobulin heavy chain | 6109 | 2608 | 1 | 0.00 | 0.23 | gi4096428 |
| Chain A, binary complex of human type-I IMP dehydrogenase with 6-Cl-Imp | 7206 | 9278 | 1 | 0.00 | 1.32 | gi33357127 |
6-Cl-IMP, 6-chloropurine riboside 5′-monophosphate; COG4, component of oligomeric Golgi complex 4; PNPT1, polyribonucleotide nucleotidyltransferase 1.
Comparative network analysis of drug effects using IPA software
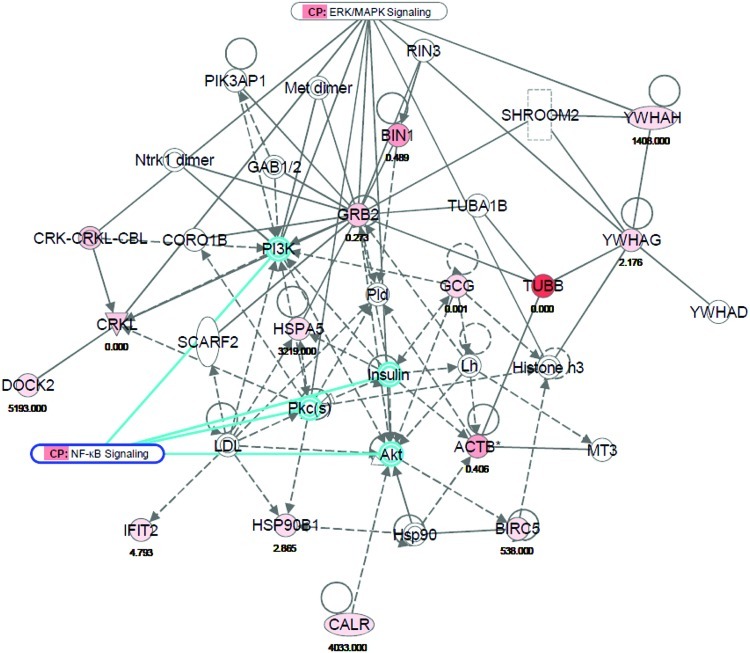
IPA analysis was used to identify gene networks, canonical pathways, and diseases (biomarkers). This network analysis can provide primary information about physical connectivity and functional relationships between proteins. Comparative analysis of untreated Jurkat-NF-κB–bla cells with cells treated with the T. chebula extract and TNFα combination is shown in Table 3. The top-scoring gene networks identified in the negative control and T. chebula extract-treated cells where interactive genes involved in protein degradation, amino acid metabolism, and behavior (Fig. 1). For the negative control and T. chebula/TNFα combined treatment, the top-scoring proteins are involved in molecular networks of immunological disease, inflammatory disease, and carbohydrate metabolism (Supplementary Fig. S2). β-Tubulin expression was higher in the negative control-treated cells than in T. chebula-treated cells. We focused on the NF-κB and extracellular signal-regulated kinase/mitogen-activated protein kinase signaling molecules in a network where the expression of Akt, protein kinase C isoforms, insulin, and phosphoinositide 3-kinase was not increased.
Table 3.
Interactive Molecules of Networks That Had the Top Scores in Jurkat-Nuclear Factor-κB–β-Lactamase Cells Treated with T. chebula Extract and Tumor Necrosis Factor α, Alone and in Combination
| Analysis | Molecules in network | Score | Focus molecules | Top functions |
|---|---|---|---|---|
| NC versus TNFα+TC | APOB, ATR (includes EG:545), BCL6, C4ORF14, CBR3, COG4, CUL4B, EPO, FIG4, GAB1, GRWD1, HNF4A, HSPA8, MAP2K5, MAP3K, MAP3K2, NFKB1, ONECUT1, PACSIN3, PPP1CA, RFWD2 (includes EG:64326), RNF20, SCFD1, SLC24A1, SON, SQSTM1, STARD7, SYNE1, TNS1, TUBB6, VAC14, VCL, WDR18, WDR51B, ZMYND8 | 41 | 17 | Immunological disease, inflammatory disease, carbohydrate metabolism |
| NC versus TC | ACTB, Akt, BIN1, BIRC5, CALR, CORO1B, CRK-CRKL-CBL, CRKL, DOCK2, GAB1/2, GCG, GRB2, Histone h3, Hsp90, HSP90B1, HSPA5, IFIT2, insulin, LDL, Lh, Met dimer, MT3, Ntrk1 dimer, PI3K, PIK3AP1, Pkc(s), Pld, RIN3, SCARF2, SHROOM2, TUBA1B, TUBB, YWHAD, YWHAG, YWHAH | 32 | 14 | Protein degradation, amino acid metabolism, behavior |
APOB, apolipoprotein B-100 precursor; ATR, ataxia telangiectasia and Rad3 related; BCL6, B-cell CLL/lymphoma 6; BIN1, bridging integrator 1; CBR3, carbonyl reductase 3; COG4, component of oligomeric golgi complex 4; CRKL, v-crk sarcoma virus CT10 oncogene homolog; DOCK2, dedicator of cytokinesis 2; EPO, erythropoietin; GAB1, GRB2-associated binding protein 1; GRB2, growth factor receptor-bound protein 2; HNF4A, hepatocyte nuclear factor 4A; HSPA8, heat shock 70-kDa protein 8 isoform 1; Hsp90/HSP90, heat shock 90-kDa protein; LDL, low-density lipoprotein; MAPK, mitogen-activated protein kinase; NFKB1, nuclear factor-κB 1; PI3K, phosphoinositide 3-kinase; TUBB, β-tubulin; WDR18, WD repeat domain 18; ZMYND8, zinc finger, MYND-type containing 8.
FIG. 1.
Molecular network of protein degradation, amino acid metabolism, and behavior for NC-treated cells in comparison with TC extract-treated cells. The direct (solid line) and indirect (broken line) interaction of both up-regulated (red) or down-regulated (green) genes associated with nuclear factor-κB (NF-κB) signaling were identified in Jurkat-NF-κB–bla cells. This dataset was analyzed using Ingenuity Pathway Analysis. The node color is indicative of gene expression, and the color intensity is proportional to the gene expression fold change. ERK, extracellular signal-regulated kinase. Color images available online at www.liebertonline.com/jmf
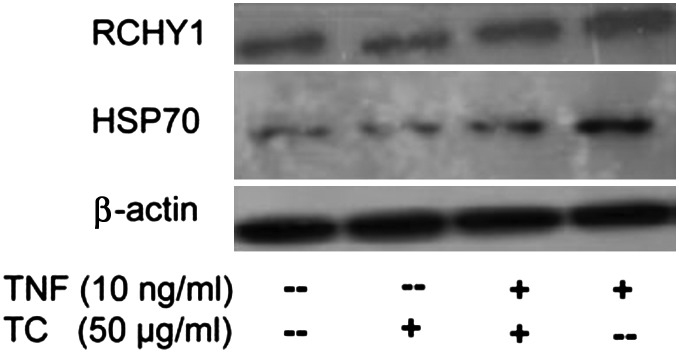
T. chebula extract component gallic acid inhibits RCHY1/Pirh2 and HSP70 in Jurkat cells
To validate the protein expression, we applied the gallic acid fraction of the T. chebula extract where the reduced expression of RCHY1/Pirh2 and HSP70 was found in Jurkat cells. The gallic acid fractionated T. chebula extract and combined treatment (5 h) with TNFα reduced the RCHY1/Pirh2 and HSP70 band compared to untreated control and only TNFα treatment, respectively (Fig. 2).
FIG. 2.
TC extract inhibited the expression of ring finger and CHY zinc finger domain containing 1 (RCHY1) and heat shock 70-kDa protein (HSP70). Cells were incubated for 5 h. The gallic acid fractionated TC extract (50 μg/mL) and combined treatment with TC extract and TNFα inhibits RCHY1/Pirh2 and HSP70 expression markedly than the untreated control and only TNFα. β-Actin expression was detected as a control. Data shown are representative of three independent experiments.
Discussion
Total protein of the cells was extracted after treatment. T. chebula extract inhibited NF-κB activity in Jurkat cells in a dose-dependent manner. After 2DE and MALDI-TOF-MS, proteins were identified in drug-treated Jurkat-NF-κB–bla cells. The protein expression in cells treated with the T. chebula extract and TNFα in combination was compared with the negative control. For the T. chebula extract treatment, we focused on proteins whose expressions were down-regulated to determine novel protein drug targets in lymphoma cells. Among the proteins with down-regulated expression, the expression of β-tubulin, RCHY1, and IGF1R was significant (Table 1). β-Tubulin is necessary for cellular component movement and cell motility. Suppression of β-tubulin inhibits Jurkat cell proliferation and survival. We determined the down-regulation of RCHY1, which is a ubiquitin-protein E3 ligase and promotes p53 degradation. The RCHY1 gene encodes a RING-H2 domain-containing protein with intrinsic ubiquitin-protein ligase activity. It was reported that RCHY1 (Pirh2) physically interacts with p53 and promotes ubiquitination of p53, independent of Mdm2.14 Furthermore, RCHY1 represses p53 activity, including p53-dependent transactivation and growth inhibition. RCHY1 is overexpressed in both human and murine lung cancers as determined by Pirh2 mRNA and protein expression in lung neoplastic tissues and is uninvolved with adjacent lung tissue. Increased RCHY1 expression may degrade wild-type p53 and reduce its tumor suppression function in tumor cells.15 Regarding these findings, RCHY1 may be a potential target of the T. chebula extract. The expression of IGF1R was down-regulated in T. chebula extract-treated cells. IGF1R triggers a cascade of intracellular signaling via tyrosine autophosphorylation and promotes cell survival and proliferation.16 Previous studies have implicated IGF1R in several cancers, including breast and prostate cancer.17 In some scenarios, its anti-apoptotic properties allow cancerous cells to resist the cytotoxicity of chemotherapeutic drug treatment and radiotherapy. IGFIR expression is increased in the majority of primary and metastatic prostate cancers.18 Therefore, T. chebula extract-dependent reduction of IGF1R expression may represent a beneficial anticancer activity.
In addition, DOCK2 expression was up-regulated in T. chebula extract-treated Jurkat-NF-κB–bla cells. The DOCK2 gene encodes a hematopoietic cell-specific CDM family protein that is indispensable for Rac-dependent lymphocyte chemotaxis.19 The expression of proteins associated with the KIAA0518 protein complex and proteins encoded by the MGA/MAX gene was also increased. MLL1, ASH2L, HCFC1/HCF1 WDR5, and RBBP5 are components of the KIAA0518 protein complex that methylates lysine-4 of histone H3 and activates gene transcription.20 The protein expression of two key components of this complex were up-regulated by T. chebula extract treatment.
In cells treated with a combination of the T. chebula extract and TNFα, the expression of HSPA8 was most significantly down-regulated. Previous studies have shown that HSP70 is present on the surface of many cancer cells, monocytes, and umbilical vein endothelial cell lines.21–23 Flow cytometric analysis confirmed the presence of HSP70 and HSPA8 on the surface of leukemic cancer cell lines.24 The T. chebula extract-dependent suppression of HSPA8 expression in TNFα-treated Jurkat cells may represent a novel therapeutic approach to treat lymphoma. We also validated a decreased HSP70 expression in Jurkat cells treated with T. chebula extract and with combined treatment with T. chebula extract and TNFα (Fig. 2).
IPA analysis of protein expression in negative control cells and cells treated with the T. chebula extract determined that the proteins with the top scores are involved in protein degradation, amino acid metabolism, and behavior (Fig. 1). The NF-κB and extracellular signal-regulated kinase/mitogen-activated protein kinase signaling pathways in this network were linked to Akt, protein kinase C isoforms, insulin, and phosphoinositide 3-kinase, whose expressions were not up-regulated. This suggests that treatment with the T. chebula extract can also inhibit NF-κB as we reported in our previous study.6 A previous study reported that a T. chebula extract compound, chebulagic acid, also inhibits NF-κB activity.25
Overall, proteomic MALDI-TOF-MS and IPA analysis uncovered key proteins and signaling networks that may be responsible for the inhibitory effects of T. chebula extract treatment in Jurkat cells. Our results suggest that the T. chebula extract treatment mainly suppresses the expression of β-tubulin, RCHY1, IGF1R, and HSP70, which may negatively affect lymphoma cells. IPA analysis identified interacting proteins that were differentially expressed in T. chebula extract-treated Jurkat cells and suppressed NF-κB signaling. Moreover, our study suggests that the T. chebula extract component gallic acid can inhibit RCHY1 and HSP70 in Jurkat cells, potentiating the anticancer effect of T. chebula extract. Future studies are necessary to identify the potential drug targets of other active compounds from T. chebula extract.
Supplementary Material
Acknowledgments
The authors thank Dr. Hyi-Seung Lee and Md. Abdul Mojid Mondol of the Korean Ocean Research and Development Institute for technical assistance and co-operation. This study was supported by Science and Technology Research Grant number HY-2010-N from Hanyang University.
Author Disclosure Statement
M.S.K. and S.L.L. are employees of Macro/care Co. The remaining authors have declared that no competing interests exist.
References
- 1.Fabry W. Okemo PO. Ansorg R. Antibacterial activity of East African medicinal plants. J Ethnopharmacol. 1998;60:79–84. doi: 10.1016/s0378-8741(97)00128-1. [DOI] [PubMed] [Google Scholar]
- 2.Sabu MC. Kuttan R. Anti-diabetic activity of medicinal plants and its relationship with their antioxidant property. J Ethnopharmacol. 2002;81:155–160. doi: 10.1016/s0378-8741(02)00034-x. [DOI] [PubMed] [Google Scholar]
- 3.Saleem A. Husheem M. Harkonen P. Pihlaja K. Inhibition of cancer cell growth by crude extract and the phenolics of Terminalia chebula Retz. fruit. J Ethnopharmacol. 2002;81:327–336. doi: 10.1016/s0378-8741(02)00099-5. [DOI] [PubMed] [Google Scholar]
- 4.Reddy DB. Reddy TC. Jyotsna G, et al. Chebulagic acid, a COX-LOX dual inhibitor isolated from the fruits of Terminalia chebula Retz., induces apoptosis in COLO-205 cell line. J Ethnopharmacol. 2009;124:506–512. doi: 10.1016/j.jep.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Abraham RT. Weiss A. Jurkat T cells and development of the T-cell receptor signalling paradigm. Nat Rev Immunol. 2004;4:301–308. doi: 10.1038/nri1330. [DOI] [PubMed] [Google Scholar]
- 6.Das ND. Jung KH. Park JH, et al. Terminalia chebula extract acts as a potential NF-kappaB inhibitor in human lymphoblastic T cells. Phytother Res. 2011;25:927–934. doi: 10.1002/ptr.3398. [DOI] [PubMed] [Google Scholar]
- 7.Bharti AC. Donato N. Singh S. Aggarwal BB. Curcumin (diferuloylmethane) down-regulates the constitutive activation of nuclear factor-kappa B and IkappaBalpha kinase in human multiple myeloma cells, leading to suppression of proliferation and induction of apoptosis. Blood. 2003;101:1053–1062. doi: 10.1182/blood-2002-05-1320. [DOI] [PubMed] [Google Scholar]
- 8.Arur S. Uche UE. Rezaul K, et al. Annexin I is an endogenous ligand that mediates apoptotic cell engulfment. Dev Cell. 2003;4:587–598. doi: 10.1016/s1534-5807(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 9.Wang P. Song JH. Song DK. Zhang J. Hao C. Role of death receptor and mitochondrial pathways in conventional chemotherapy drug induction of apoptosis. Cell Signal. 2006;18:1528–1535. doi: 10.1016/j.cellsig.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Das ND. Park JH. Jung KH, et al. Sodium arsenite dependent protein expression analysis on human embryonic carcinoma (NCCIT) cell line. Toxicol Lett. 2011;207:149–158. doi: 10.1016/j.toxlet.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Shevchenko A. Wilm M. Vorm O. Mann M. Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 12.Li CJ. Li RW. Wang YH. Elsasser TH. Pathway analysis identifies perturbation of genetic networks induced by butyrate in a bovine kidney epithelial cell line. Funct Integr Genomics. 2007;7:193–205. doi: 10.1007/s10142-006-0043-2. [DOI] [PubMed] [Google Scholar]
- 13.Tsai J. Zhang R. Qiu W. Su Q. Naples M. Adeli K. Inflammatory NF-kappaB activation promotes hepatic apolipoprotein B100 secretion: evidence for a link between hepatic inflammation and lipoprotein production. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1287–G1298. doi: 10.1152/ajpgi.90540.2008. [DOI] [PubMed] [Google Scholar]
- 14.Leng RP. Lin Y. Ma W, et al. Pirh2, a p53-induced ubiquitin-protein ligase, promotes p53 degradation. Cell. 2003;112:779–791. doi: 10.1016/s0092-8674(03)00193-4. [DOI] [PubMed] [Google Scholar]
- 15.Duan W. Gao L. Wu X. Zhang Y. Otterson GA. Villalona-Calero MA. Differential response between the p53 ubiquitin-protein ligases Pirh2 and MdM2 following DNA damage in human cancer cells. Exp Cell Res. 2006;312:3370–3378. doi: 10.1016/j.yexcr.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 16.LeRoith D. Werner H. Beitner-Johnson D. Roberts CT., Jr Molecular and cellular aspects of the insulin-like growth factor I receptor. Endocr Rev. 1995;16:143–163. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]
- 17.Jones HE. Goddard L. Gee JM, et al. Insulin-like growth factor-I receptor signalling and acquired resistance to gefitinib (ZD1839; Iressa) in human breast and prostate cancer cells. Endocr Relat Cancer. 2004;11:793–814. doi: 10.1677/erc.1.00799. [DOI] [PubMed] [Google Scholar]
- 18.Hellawell GO. Turner GD. Davies DR. Poulsom R. Brewster SF. Macaulay VM. Expression of the type 1 insulin-like growth factor receptor is up-regulated in primary prostate cancer and commonly persists in metastatic disease. Cancer Res. 2002;62:2942–2950. [PubMed] [Google Scholar]
- 19.Fukui Y. Hashimoto O. Sanui T, et al. Haematopoietic cell-specific CDM family protein DOCK2 is essential for lymphocyte migration. Nature. 2001;412:826–831. doi: 10.1038/35090591. [DOI] [PubMed] [Google Scholar]
- 20.Wysocka J. Swigut T. Milne TA, et al. WDR5 associates with histone H3 methylated at K4 and is essential for H3 K4 methylation and vertebrate development. Cell. 2005;121:859–872. doi: 10.1016/j.cell.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 21.Shin BK. Wang H. Yim AM, et al. Global profiling of the cell surface proteome of cancer cells uncovers an abundance of proteins with chaperone function. J Biol Chem. 2003;278:7607–7616. doi: 10.1074/jbc.M210455200. [DOI] [PubMed] [Google Scholar]
- 22.Asea A. Kraeft SK. Kurt-Jones EA, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 23.Triantafilou K. Triantafilou M. Dedrick RL. A CD14-independent LPS receptor cluster. Nat Immunol. 2001;2:338–345. doi: 10.1038/86342. [DOI] [PubMed] [Google Scholar]
- 24.Hantschel M. Pfister K. Jordan A, et al. Hsp70 plasma membrane expression on primary tumor biopsy material, bone marrow of leukemic patients. Cell Stress Chaperones. 2000;5:438–42. doi: 10.1379/1466-1268(2000)005<0438:hpmeop>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy DB. Reddanna P. Chebulagic acid (CA) attenuates LPS-induced inflammation by suppressing NF-kappaB and MAPK activation in RAW 264.7 macrophages. Biochem Biophys Res Commun. 2009;381:112–117. doi: 10.1016/j.bbrc.2009.02.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.