Abstract
Background: Pesticide–environment interactions are bidirectional. The environment alters pesticides by metabolism and photodegradation, and pesticides in turn change the environment through nontarget or secondary effects.
Objectives: Approximately 900 currently used commercial pesticides of widely diverse structures act by nearly a hundred mechanisms to control insects, weeds, and fungi, usually with minimal disruption of nature’s equilibrium. Here I consider some aspects of the discovery, development, and use of ecofriendly or green pesticides (i.e., pesticides that are safe, effective, and biodegradable with minimal adverse secondary effects on the environment). Emphasis is given to research in my laboratory.
Discussion: The need for understanding and improving pesticide–environment interactions began with production of the first major insecticide approximately 150 years ago: The arsenical poison Paris Green was green in color but definitely not ecofriendly. Development and use of other pesticides has led to a variety of problems. Topics considered here include the need for high purity [e.g., hexachlorocyclohexane and polychloroborane isomers and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T)], environmental degradation and the bioactivity of resulting photoproducts and metabolites, pesticide photochemistry (including the use of structural optimization, photostabilizers, and photosensitizers to achieve suitable persistence), the presence of multiple active ingredients in botanical insecticides, the need to consider compounds with common mechanisms of action, issues related to primary and secondary targets, and chemically induced or genetically modified changes in plant biochemistry. Many insecticides are bird, fish, and honeybee toxicants, whereas herbicides and fungicides pose fewer environmental problems.
Conclusion: Six factors have contributed to the greening of pesticide–environment interactions: advances in pesticide chemistry and toxicology, banning of many chlorinated hydrocarbons, the development of new biochemical targets, increased reliance on genetically modified crops that reduce the amount and variety of pesticides applied, emphasis on biodegradability and environmental protection, and integrated pest- and pesticide-management systems.
Keywords: ecochemistry, ecotoxicology, metabolism, pesticide, photochemistry
Commercially available pesticides currently include approximately 900 structurally diverse compounds (Tomlin 2009) that act by nearly a hundred mechanisms to control insects, weeds, and fungi (Casida 2009b). These pesticides meet the goals of green chemistry (i.e., they are safe, effective, and biodegradable with minimal environmental disruption) to varying degrees. In this commentary, I will review progress in the greening of pesticide–environment interactions, with emphasis on my own observations and research over six decades (Casida 2009a, 2010a, 2010b) concerning the chemistry and toxicology of pesticides in environmental systems [see Supplemental Material, Figure 1 (http://dx.doi.org/10.1289/ehp.1104405)].
Figure 1.
Paris Green, the first green pesticide, was green in color only (definitely not ecofriendly). Label from 1867 package. Reproduced with permission from Getty Images.
The meaning of a “green pesticide” has changed drastically in the last century and a half. Paris Green, the common name for cupric acetoarsenite, is an emerald-green powder containing 43% arsenic and was used from 1865 until the 1940s. It effectively controlled the Colorado potato beetle (Figure 1), chewing pests of cotton and many other crops, and mosquito larvae, with sustained U.S. use levels of about 4,000,000 lb/year (Shepard 1939). Other inorganic toxicants based on arsenic, copper, lead, mercury, sulfur, fluorine, and other compounds—supplemented with the botanicals nicotine, pyrethrum, and rotenone—were also part of the insecticide armamentarium. These compounds provided partial to adequate control for many major pests, but they were far from ideal for environmental safety.
Synthetic organic insecticides introduced in the 1940s and 1950s were far more effective than Paris Green and other early pesticides. DDT (dichlorodiphenyltrichloroethane), chlorinated benzene, chlorinated camphene, and the chlorinated cyclodienes were remarkably successful but introduced new problems of bird, fish, and honeybee toxicity and bioaccumulation through food webs (Stephenson and Soloman 2007; Ware and Whitacre 2004). Paul Müller was presented the Nobel Prize in Physiology and Medicine in 1948 for discovering DDT and its effectiveness in controlling insect-vectored human diseases (Dunlap 1981; Metcalf 1973; Müller 1959), but Rachel Carson was awarded the U.S. Presidential Medal of Freedom posthumously in 1980 for her book Silent Spring, which pleaded for banning this insecticide because of its effects on health and the environment (Carson 1962) [see Supplemental Material, Figure 2 (http://dx.doi.org/10.1289/ehp.1104405)]. DDT was highly restricted or banned in 1973 after 4–6 billion pounds had been used. Views on pesticide use and safety continue to differ between food and agricultural producers on one side and environmentalists and health officials on the other. Efforts to understand and cope with these problems were initiated by insect toxicologists who started or revitalized the fields of ecochemistry and ecotoxicology (Felsot 1985). A broad range of information is involved in estimating the environmental impact of specific pesticides (Kovach et al. 1992).
Figure 2.
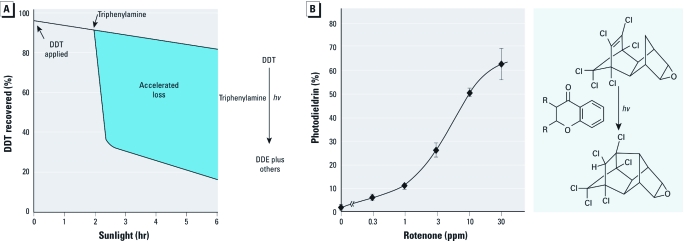
Photosensitizers accelerate insecticide residue loss on bean foliage exposed to sunlight as illustrated for (A) DDT (25 ppm) containing triphenylamine (50 ppm; time dependence) and (B) dieldrin (10 ppm) containing rotenone (concentration dependence, 1‑hr exposure; error bars are SDs; photosensitization was due to chromanone moiety). hv, light energy.
Ecochemistry
Impurities and adjuvants. In contrast to DDT, which is easy to prepare with high purity, insecticides obtained by chlorination of benzene (hexachlorocyclohexane) and camphene (toxaphene) are used as isomer mixtures with 12% active γ-hexachlorocyclohexane (lindane) (Brooks 1977) and 0.2–2% octachlorobornane (A-2), respectively [see Supplemental Material, Figure 3 (http://dx.doi.org/10.1289/ehp.1104405)] (Casida et al. 1974; Saleh et al. 1977; Turner et al. 1975, 1977). Much of the adverse chronic toxicology of technical hexachlorocyclohexane in mammals is probably due to the 5–14% β isomer, which is stored for prolonged periods in fat (Smith 1991). Technical toxaphene consists of several hundred hepta-, octa-, and nonachlorobornanes and related compounds, most but not all of which have been shown to be readily biodegradable based on studies of enzyme, organismal, and environmental fate of the commercial mixture and individual congeners (Maruya et al. 2005; Saleh et al. 1977; Vetter and Oehme 2000). Perhaps the most serious impurity problem was that of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), an impurity in the now-banned herbicide 2,4,5-T (2,4,5-trichlorophenoxyacetic acid; see Supplemental Material, Figure 4) with an oral LD50 (median lethal dose) that ranges from 0.6–2.1 μg/kg for guinea pigs to about 1,100–5,000 μg/kg for hamsters (Institute of Medicine 1994). Intraperitoneal administration of [3H]TCDD to mice revealed little or no metabolism, an exceptionally long persistence, and high localization in the hepatic endoplasmic reticulum (Vinopal and Casida 1973). Although these examples may be extreme, they highlight the need for high-purity pesticide products.
Figure 3.
Toxic photoproducts formed during insecticide photodecomposition on bean foliage illustrated by (A) heptachlor and (B) fipronil. The graph is adapted from Hainzl and Casida (1996). Potencies (micromolar IC50 values) are for the γ-aminobutyric acidAreceptor noncompetitive blocker site of mouse or rat brain membranes (Hainzl and Casida 1996; Lawrence and Casida 1984). hv, light energy.
Figure 4.
Pesticide-induced changes in plant biochemistry. (A) Secondary metabolites induced in six crops by acifluorfen with 48-hr sunlight exposure; pisatin in peas; glyceollins in soybeans; hemigossypol in cotton; phaseolin in beans; xanthotoxin in celery; and N-feruloyl-3-methoxytyramine in spinach. Error bars are SDs. Adapted from Kömives and Casida 1983. (B) Neonicotinoid insecticides induce salicylate-associated responses in plants. (C) Safener induces herbicide-detoxifying enzyme and cofactor in corn but not in weeds.
Solvents, emulsifiers, and a variety of other ingredients, most of which are classified as inert, are added to pesticides to maximize their effectiveness. Although these additives are carefully optimized, they sometimes create unanticipated problems. For example, a reaction between the organophosphorus (OP) insecticide dimethoate and a formulation solvent (2-methoxyethanol) greatly increases dimethoate’s mammalian toxicity without substantially affecting its insecticidal activity (Casida and Sanderson 1961), and a formulation ingredient (polyethoxylated tallowamine) for the active agent in the OP herbicide glyphosate induces apoptosis and necrosis in several human cell types (Benachour and Séralini 2009). These observations are relevant to both human health and environmental health.
Metabolism. Pesticide manufacturers must thoroughly characterize degradation by metabolism and photodecomposition to meet requirements for registration and establish residue tolerances, and this information is generally available in the public domain. Pesticides typically yield 10–100 metabolites and photoproducts with varied types and degrees of bioactivity, as illustrated by DDT [see Supplemental Material, Figure 5 (http://dx.doi.org/10.1289/ehp.1104405)]. For example, the dichloroethylene DDE (dichlorodiphenyldichloroethylene), a metabolite of DDT in insects and mammals, is a major persisting environmental pollutant (Smith 1991). Drosophila convert DDT to the noninsecticidal but miticidal dicofol (Tsukamoto 1959). DDA, the acetic acid derivative of DDT, is a urinary biomarker of human DDT exposure and also has herbicidal activity (Casida and Allen 1951). Finally, the dichloroethane reductive dechlorination product DDD was detected in Clear Lake, California, ecosystems 7 years after DDD use was discontinued. DDD in the lake appeared to originate from algal metabolism of DDT applied in surrounding agricultural areas (Miskus et al. 1965).
Metabolic activation and detoxification of sulfur-containing OP insecticides is even more complex. Metabolism of profenofos enantiomers yields a mixture of toxicants that includes direct-acting and cytochrome P450 (CYP450)-activated inhibitors of acetylcholinesterase (AChE) and other serine hydrolases (Glickman et al. 1984; Wing et al. 1983) [see Supplemental Material, Figure 6A (http://dx.doi.org/10.1289/ehp.1104405)], which may contribute to its lack of cross-resistance with many other OP insecticides. The OP acephate is bioactivated by deacetylation to methamidophos followed by S- or N-oxidation to the actual esterase inhibitor (see Supplemental Material, Figure 6B) (Mahajna and Casida 1998). However, the final bioactivated phosphorothiolate can subsequently block the amidase activation step and thus inhibit further bioactivation, which probably contributes to the relatively low mammalian toxicity of acephate (Mahajna et al. 1997).
Photochemistry. Pesticides must persist on crops long enough to ensure effectiveness but without causing food-residue problems. The appropriate duration of persistence is normally achieved by structural modifications that improve stability in light without compromising biodegradability. For example, the pyrethroid chrysanthemate and neonicotinoid nitromethylene compounds require photostabilization to be useful in agriculture (Chen and Casida 1969; Dureja et al. 1984; Holmstead et al. 1977; Kleier et al. 1985; Ruzo et al. 1982). The discovery by Farkas et al. (1959) that the insecticidal activity of a pyrethroid was retained when the chrysanthemate dimethylvinyl substituent was replaced with a dichlorovinyl moiety ultimately led to the independent development of the potent photostabilized but still biodegradable pyrethroids by Elliott and colleagues (Casida 2010b) [see Supplemental Material, Figure 7A (http://dx.doi.org/10.1289/ehp.1104405)]. Nithiazine, the primary lead compound for the neonicotinoids (i.e., the discovery from which other neonicotinoids were developed) (Soloway et al. 1979), had a photolabile nitromethylene substituent (Kleier et al. 1985). Its potency was greatly increased with the addition of a chloropyridinylmethyl substituent (prototype) and was subsequently photostabilized with the nitroimine equivalent imidacloprid, currently the most important of all insecticides (Kagabu 2003) (see Supplemental Material, Figure 7B).
Degradation of pesticide residues on leaf surfaces may be enhanced by adding photosensitizers such as triphenylamine to DDT (Figure 2A) or rotenone to dieldrin (Figure 2B), but this results in the formation of bioactive and persistent photoproducts (Ivie and Casida 1970, 1971; Lawrence and Casida 1984). Furthermore, photosensitizers such as rotenone decompose (Cheng et al. 1972) and may have to be reapplied. Pesticides can also serve as photostabilizers; for example, the herbicide trifluralin and other dinitroanilines are quite effective experimental additives when used in pyrethroids for photostabilization (Dureja et al 1984).
Environmentally generated pesticide photoproducts are relevant to efficacy and safety evaluations. Some insecticides are photoactivated to compounds with increased potency as toxicants or receptor inhibitors. For example, the poorly active Z-isomer of an oxime ether pyrethroid may be photoactivated to the highly effective E-isomer (Brown et al. 1983) [see Supplemental Material, Figure 8 (http://dx.doi.org/10.1289/ehp.1104405)]. The chlorinated cyclodienes generate several toxic photoproducts and metabolites, including photoheptachlor (generated directly from heptachlor) and photoheptachlor epoxide (generated from the CYP450 metabolite heptachlor epoxide) (Figure 3A) (Ivie et al. 1972; Lawrence and Casida 1984). Photochemical desulfinylation of the phenylpyrazole insecticide fipronil produces residues that have equal or greater potency but much greater persistence than the parent compound (Figure 3B) (Hainzl and Casida 1996; Hainzl et al. 1998), which must be considered in approved tolerances and uses for fipronil (Tomlin 2009).
Ecotoxicology
Botanical insecticides. For centuries botanicals have been a principal source of insecticides and insecticidal prototypes for structural optimization. The search for new sources continues, sometimes with surprising results. For example, Drosophila bioassays of parsnips unexpectedly revealed a new botanical insecticidal and synergistic natural product identified as myristicin (Lichtenstein and Casida 1963) that is related to dill apiole and parsley apiole, which were later recognized as acting the same way (de Almeida et al. 2009) [see Supplemental Material, Figure 9A (http://dx.doi.org/10.1289/ehp.1104405)]. Similarly, bioassays for house fly toxicity of extracts from 62 plants from central China used in medical practice led to the isolation and structural assignment of paeonol and jacaranone (Xu et al. 2003); another compound of very high potency was identified as terbufos, an extremely hazardous systemic OP insecticide (rat oral LD50 1.6 mg/kg) (Tomlin 2009) (see Supplemental Material, Figure 9B). Thus, botanical insecticides and herbal medicines can be contaminated with synthetic pesticides during production or harvest, thereby possibly confounding the potency of the natural products.
Common mechanism of action. There are many examples of chemically diverse pesticides that act on a common primary molecular target. Consequently, it is important to sum the effects of pesticides that have a common mechanism of action when performing risk assessments [U.S. Environmental Protection Agency (EPA) 2011b] or evaluating environmental toxicology, such as effects on birds and fish exposed to multiple OPs and methylcarbamates (MCs) or on honeybees exposed to multiple neonicotinoids. In addition, common mechanisms of action are of great importance to pesticide management practices designed to avoid or forestall the selection of resistant strains by shifting from pesticides with one target site to pesticides that work through a different target, rather than enhancing cross-resistance by using pesticides that have a common target (Fungicide Resistance Action Committee 2010; Herbicide Resistance Action Committee 2010; Insecticide Resistance Action Committee 2011).
Secondary targets. Secondary targets for pesticides (i.e., molecular targets not related to their pesticidal activity) are best understood and perhaps of greatest concern for OPs and MCs (Casida and Quistad 2004, 2005). About 90 commercial insecticides that inhibit AChE as their primary target may act on other serine hydrolases as secondary targets. For example, OP-induced delayed neuropathy, first associated with tri-o-cresyl phosphate and then with the insecticide candidate mipafox and the insecticide leptophos, is now known to correlate with or result from inhibition of neuropathy target esterase (NTE) (Johnson and Glynn 2001), which has been identified as a lysophosphatidylcholine hydrolase (Quistad et al. 2003; Vose et al. 2007) [see Supplemental Material, Figure 10A (http://dx.doi.org/10.1289/ehp.1104405)]. In mice, the loss of NTE has linked OP exposure to hyperactivity (Winrow et al. 2003). Although hens are the standard model, other avians, sheep, water buffalo, and a variety of other mammals are all considered to be sensitive to effects on NTE (Ehrich and Jortner 2010; Wijeyesakere and Richardson 2010). OP-induced avian teratogenesis, first observed when pesticides were being injected into hen eggs (Roger et al. 1964), is attributable to inhibition of kynurenine formamidase activity and nicotinamide adenine dinucleotide biosynthesis (Seifert and Casida 1980) (see Supplemental Material, Figure 10B). Diazinon and carbaryl induce micromelia and abnormal feathering in hen eggs (Seifert and Casida 1980), but different skeletal defects have been noted for diazinon in bobwhite quail embryos (Meneely and Wyttenbach 1989). The cannabinoid syndrome from OPs involves inhibition of monoacylglycerol lipase and fatty acid amide hydrolase, elevated levels of the endocannabinoids (2-arachidonoyl glycerol and anandamide), and reduced amounts of arachidonic acid (Nomura and Casida 2011) (see Supplemental Material, Figure 10C), but the relevance to wildlife is unknown.
Changing plant biochemistry. Herbicides sometimes induce the synthesis of secondary plant substances in crops. At phytotoxic levels, protox inhibitors such as acifluorfen induce phenylalanine ammonia-lyase, which increases phytoalexins and stress metabolites in plants (e.g., pisatin in pea, glyceollin in soybean, and hemigossypol in cotton) (Kömives and Casida 1983) (Figure 4A). Similarly, application of inducers shortly before harvest might be used to elevate levels of desirable botanical products (Kömives and Casida 1982, 1983). Surprisingly, several neonicotinoid insecticides induce salicylate-associated responses in plants (Ford et al. 2010) (Figure 4B). For example, the chlorothiazolylcarboxylic acid metabolite of chlorothiazolyl neonicotinoids induces synthesis of salicylic acid in Arabidopsis. In contrast, imidacloprid is metabolized to a chlorohydroxypyridinylcarboxylic acid, which serves as a highly bioactive salicylic acid mimic (Ford et al. 2010).
Plant biochemistry is also intentionally altered to create herbicide-tolerant crops. This can be done on a temporary basis with a safener, or antidote, that enhances sulfate metabolism and elevates the cofactor and enzyme that detoxify the active form of the herbicide, for example, glutathione and glutathione S-transferase to detoxify thiocarbamate sulfoxides (bioactivated thiocarbamate herbicides) in corn but not in weeds (Adams et al. 1983; Casida 1978; Lay et al. 1975) (Figure 4C). In addition, 2-oxothiazolidine-4-carboxylic acid (a precursor of cysteine) may be effective in bioremediation to increase chloroacetanilide herbicide detoxification in poplar (Kömives et al. 2003). On a more long-term basis, this approach involves herbicide-tolerant, genetically modified crops (GMCs), for example, that overexpress a less-sensitive form of the 5-enolpyruvylshikimate 3-phosphate synthase target for glyphosate (Dill 2005). As an alternative, glufosinate-tolerant crops express N-acetyltransferase that detoxifies by forming N-acetylglufosinate. These GMCs may require only (or primarily) glyphosate or glufosinate for weed control, and thus fewer herbicides or smaller amounts of herbicides are applied (Phipps and Park 2002). However, selection of weed resistance to glyphosate threatens the continued effectiveness of GMCs, and GMC technology has not attained global public acceptance.
The Greening of Pesticide–Environment Interactions
Pesticides vary widely in environmental toxicity and impact. The most important and best available data on effects of pesticides on nontarget species are from acute and chronic exposure safety evaluations in mammals, with additional information on birds, fish, and honeybees (Table 1).
Table 1.
Ecotoxicology of some major pesticides.a
| Year intro | LD50 (mg/kg)b | LC50 (ppm) | LD50c | t½ (days) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pesticide type | Mammal | Bird | Fish | Honeybee | Soil | |||||||
| Insecticides | ||||||||||||
| Paris greend | 1867 | 22 | Toxic | High | ||||||||
| DDT | 1944 | 113 to > 1,000 | Moderate | 0.004–0.009 | 5 | 90–10,000 | ||||||
| Lindane | 1945 | 59–270 | 120–130 | 0.02–0.06 | 0.01 | |||||||
| Toxaphened | 1947 | 40–112 | 80–250 | < 0.05 | 22–80 | |||||||
| Endosulfan | 1955 | 70–110 | 205–1,000 | 0.002 | Low | 150–240 | ||||||
| Carbaryl | 1957 | 264–710 | 1,000–3,000 | 1.3–10 | 0.18 | 7–28 | ||||||
| Chlorpyrifos | 1965 | 135–2,000 | 32–490 | 0.002–0.54 | 0.36 | 7–56 | ||||||
| Deltamethrin | 1974 | 87 to > 10,000 | > 2,250 | 0.00091–0.0014 | 0.023 | 8–28 | ||||||
| Diflubenzuron | 1975 | > 4,640 | > 5,000 | > 65 | > 100 | 3.2 | ||||||
| Methoprene | 1975 | > 10,000 | 0.37 | > 1,000 | 10 | |||||||
| Abamectin | 1985 | 10–221 | 85 to > 2,000 | 0.003–0.01 | Toxic | Rapid | ||||||
| Imidacloprid | 1991 | 450 | 31–152 | 211–237 | High | 0.17 | ||||||
| Fipronil | 1993 | 95–97 | 11 to > 2,000 | 0.085–0.43 | High | |||||||
| Tebufenozide | 1994 | > 5,000 | > 2,150 | 3–5.7 | > 234 | 7–66 | ||||||
| Spinosad | 1997 | 3,783 to > 5,000 | > 2,000 | 3.5–30 | 0.0029 | 9–17 | ||||||
| Flonicamid | 2000 | 884–1,768 | > 2,000 | > 100 | > 60 | 1.1 | ||||||
| Tolfenpyrad | 2002 | 107–386 | 0.0029 | |||||||||
| Chlorantraniliprole | 2006 | > 5,000 | > 2,250 | > 14 | > 104 | < 60–365 | ||||||
| Spirotetramat | 2006 | > 2,000 | > 2,000 | 2.2–2.5 | 107 | < 1 | ||||||
| Pyrifluquinazon | 2009 | 300–2,000 | 1,360 | 4.4 | ||||||||
| Herbicides | ||||||||||||
| 2,4-D | 1942 | 138–764 | 472 to > 1,000 | > 100 | 104 | < 7 | ||||||
| Atrazine | 1957 | > 1,332–3,992 | 940–4,273 | 4.3–76 | > 97 | 16–117 | ||||||
| Trifluralin | 1961 | 5,545–6,293 | > 2,000 | 0.088 | > 100 | 25–201 | ||||||
| Paraquat | 1962 | 22–157 | 75–175 | 26–135 | 15 | < 7 | ||||||
| Alachlor | 1969 | 930–1,350 | 1,536 | 2.1–5.3 | > 94 | 8–17 | ||||||
| Glyphosate | 1974 | 3,530 to > 10,000 | > 3,851 | 97 to > 1,000 | 100 | 27–146 | ||||||
| Chlorsulfuron | 1982 | 5,545–6,293 | > 5,000 | > 50 to > 980 | > 100 | 28-42 | ||||||
| Glufosinate | 1981 | 200–2,000 | 710 to > 1,000 | > 100 | 7–20 | |||||||
| Mesotrione | 2001 | > 5,000 | > 2,000 | > 120 | > 11 | 3–7 | ||||||
| Fungicides | ||||||||||||
| Maneb | 1950 | > 5,000 | 1.8 | Nontoxic | 25 | |||||||
| Captan | 1952 | 9,000 | 2,000 to > 5,000 | 0.034–0.3 | 91 | 1 | ||||||
| Benomyl | 1970 | > 5,000 | 0.27–4.2 | > 50 | 0.8 | |||||||
| Triadimefon | 1976 | 250–1,000 | > 2,000 | 4–10. | 6–18 | |||||||
| Metalaxyl | 1979 | 633–788 | 923–1,466 | > 100 | 269 | 29 | ||||||
| Azoxystrobin | 1996 | > 5,000 | > 2,000 | 0.47–1.6 | > 25 | 70 | ||||||
| Abbreviations: intro, introduced; LC50, median lethal concentration; t½, half-life. aData from Tomlin (2009) except as indicated. bAcute oral LD50 values are for the range of species described in the cited study. cLD50 data are presented as µg/bee by oral exposure except for benomyl, chlorsulfuron, and spinosad, for which data represent contact exposure. Toxicity levels are given as nontoxic, low, moderate, toxic, and high. dDatafor Paris Green and toxaphene from Negherbon (1959). | ||||||||||||
Mammals. Most acute pesticide toxicity problems in mammals are caused by OPs (analogs of chlorpyrifos) and MCs (analogs of carbaryl). Although several insecticides have oral LD50 values < 100 mg/kg, the general trend in the last 15 years has been to develop only compounds with reduced mammalian toxicity. Herbicides (other than paraquat) and fungicides are generally less toxic to mammals than are insecticides. The acute oral LD50 values tabulated for laboratory mammals are an indicator, but not always reliable predictor, of acute or chronic toxicity to nontarget mammals.
Birds. Acute toxicity trends are generally similar for mammals and birds (Table 1). Chronic toxicity also plays a large role in environmental avian responses. The canary was the classical sentinel of toxic gas in coal mines, a role played by the robin and peregrine falcon with environmental DDT exposure; the insecticides underwent food chain accumulation and biomagnification leading to thinning of egg shells and population declines of birds (Carson 1962). Tolerances for carbofuran were revoked (U.S. EPA 2011a) and use of diazinon on golf courses was cancelled (U.S. EPA 2004), in part because of bird mortality. Other insecticides known to cause bird mortality events include the OPs monocrotophos, dicrotophos, methamidophos, and parathion, and the MC aldicarb (Friend and Franson 1999). Strychnine and 4-aminopyridine used as avicides are not only highly toxic to target birds but also pose secondary hazards to predatory and scavenger animals.
Fish. Most neurotoxic insecticides have high to ultrahigh fish toxicity, but this is also the case for some nonneuroactive herbicides (e.g., trifluralin) and fungicides (azoxystrobin, benomyl, and captan) (Table 1). Endosulfan, the last of the major chlorinated cyclodienes, was the cause of one of the worst ecological disasters in history (Greve and Wit 1971) when about 70 lb spilled into the Rhine river, killing millions of fish through much of Germany and into the Netherlands [see Supplemental Material, Figure 11A (http://dx.doi.org/10.1289/ehp.1104405)]. Despite its ultrahigh fish toxicity, endosulfan continues to be used for pest management in some countries. The γ-aminobutyric acid–gated chloride channel is the molecular target of several very potent fish toxins, specifically, endosulfan, lindane, toxaphene, and fipronil (Ratra et al. 2001). Toxicity to fish is also a major limiting factor in the use of pyrethroids, such as fenvalerate, particularly when agriculture and aquaculture are in proximity or intermixed (Haya 1989); however, this risk is minimized by proper application methods and the very low field rates required for pest control (Coats 2008). The search for pyrethroids with reduced fish toxicity led to the discovery of the nonester fenvalerate analogs etofenprox and silafluofen, which resulted in expanded use and improved environmental safety in rice production (Tomlin 2009) (see Supplemental Material, Figure 12).
Another pesticide spill may have been California’s worst inland environmental disaster. A tank car of metam sodium, a soil fungicide, tipped over into the Sacramento River, where it degraded into methyl isothiocyanate (the primary active product) and hydrogen sulfide (Carlock and Dotson 2010; Rubin 2004). Further breakdown probably involved methyldithiocarbamate sulfenic acid as an intermediate (Kim et al. 1994; Lam et al. 1993) [see Supplemental Material, Figure 11B (http://dx.doi.org/10.1289/ehp.1104405)]. Although most of these compounds are water reactive and biodegradable, it took many months for organisms in the exposed area to recover (Carlock and Dotson 2010; Gherman 1997).
Fish kill with a pesticide is sometimes intentional. For example, the biodegradable and photolabile rotenone in the form of derris resin (Cheng et al. 1972; Fukami et al. 1967; Schuler and Casida 2001) was used to remove invasive northern pike and other rough fish (i.e., less desirable fish) before reintroducing trout into Lake Davis in California (California Department of Fish and Game 2004, 2008). Lake Davis was treated with derris in 1997 and again 10 years later in an attempt to suppress or eradicate the rough fish. At one time rotenone was also a candidate anticancer agent (Fang and Casida 1998; Gerhäuser et al. 1995) and a model for Parkinson’s disease (Caboni et al. 2004). The primary target of rotenone is reduced nicotinamide adenine dinucleotide oxidase (Horgan et al. 1968; Schuler and Casida 2001), but rotenone also inhibits induced ornithine decarboxylase activity, which serves as an anticancer model (Fang and Casida 1998; Gerhäuser et al. 1995) [see Supplemental Material, Figure 11C (http://dx.doi.org/10.1289/ehp.1104405)]. From the derris added to Lake Davis, 40 components were identified and their inhibitory activity for NADH oxidase correlated with that for the anticancer model (Fang and Casida 1998).
Beneficial insects. Honeybees are generally no more sensitive than other insects to insecticides (Hardstone and Scott 2010). However, honeybee losses pose a major problem for agriculture. Pesticides with an LD50 < 1 μg/bee include some insecticidal chlorinated hydrocarbons (e.g., lindane), OPs and MCs (carbaryl and chlorpyrifos), pyrethroids (deltamethrin), neonicotinoids (imidacloprid), and microbials (spinosad), but not any of the herbicides and fungicides listed in Table 1. Currently, pesticide levels are high in North American apiaries (Mullin et al. 2010). It is possible to design analogs with low toxicity for honeybees. For example, parathion is highly toxic to bees, whereas its diisopropyl analog is much less harmful (Camp et al. 1969). Many potential uses of imidacloprid and clothianidin are restricted or banned in France, Germany, and Italy because of high bee toxicity, but other neonicotinoids, such as the cyanoimines thiacloprid and acetamiprid, are less toxic to bees (Iwasa et al. 2004).
Insect pests may be adequately controlled by natural predators and parasites until these enemies are removed by insecticide exposure. Integrated pest management programs were therefore developed to optimize biocontrol agents and minimize insecticide effects on biological control (Huffaker and Messenger 1976). Favored chemicals are those with high selectivity for pests versus predators and parasites, including natural and synthetic insecticides, insect growth regulators, and pheromones.
Other organisms. Other organisms may be adversely affected by pesticides. For example, earthworms are very sensitive to benomyl fungicide (Karnak and Hamelink 1982; van Gestel 1992), and frogs as tadpoles are sensitive to the lethal effects of endosulfan (Jones et al. 2009). The toxicity and symptomology of pyrethroids in frogs are similar to those in mammals (Cole and Casida 1983). A large number and great variety of pesticides are reported to have reproductive and endocrine-disrupting effects in mammals and wildlife (Colborn et al. 1993). For example, atrazine at environmentally relevant doses has been reported to induce endocrine disruption and demasculinization in frogs (Hayes et al. 2002), although this controversial finding has not been repeated by other laboratories and is not considered to be relevant in safety evaluation (U.S. EPA 2007).
Conclusion
There has clearly been a greening of pesticide–environment interactions involving improved pest specificity, less nontarget toxicity, lower persistence, and reduced use rates. These successes were sometimes accompanied by unexpected problems, unanticipated hazards, and even major environmental accidents, most of which were solved or placed in risk perspective by fundamental investigations, including studies from my laboratory. Safety has been substantially increased by integrating information related to pharmacokinetic and pharmacodynamic behaviors and operational factors (targeting and use rates), and as our knowledge continues to improve, we can look forward to even greener pesticide–environment interactions.
Supplemental Material
Acknowledgments
This paper was presented in part at the First International Conference on Agri-Environmental Chemistry and Toxicology organized by T. Kömives, T. Nemeth, and J.E.C. for the Hungarian Academy of Sciences and held 20–22 September 2011 in Budapest, Hungary.
I thank A. Anton and A. Szekacs for excellent arrangements and the conference co-participants for outstanding contributions. I received valuable advice and assistance from S. Kodani and F. Collazo of the Environmental Chemistry and Toxicology Laboratory at Berkeley.
Footnotes
The author declares he has no actual or potential competing financial interests.
References
- Adams CA, Blee E, Casida JE. Dichloroacetamide herbicide antidotes enhance sulfate metabolism in corn roots. Pestic Biochem Physiol. 1983;19:350–360. [Google Scholar]
- Benachour N, Séralini GE. Glyphosate formulations induce apoptosis and necrosis in human umbilical, embryonic and placental cells. Chem Res Toxicol. 2009;22:97–105. doi: 10.1021/tx800218n. [DOI] [PubMed] [Google Scholar]
- Brooks GT. Washington, DC: American Chemical Society, 1–20; 1977. Chlorinated insecticides: retrospect and prospect. In: Pesticide Chemistry in the 20th Century (Plimmer JR, Kearney PC, Kohn GK, Menn JJ, Ries S, eds). ACS Symposium Series, Vol 37. [Google Scholar]
- Brown MA, Gammon DW, Casida JE. Oxime ether pyrethroids and hydroxylamine ether propyrethroids: photochemistry, biological activity, and metabolism. J Agric Food Chem. 1983;31:1091–1096. [Google Scholar]
- Caboni P, Sherer TB, Zhang N, Taylor G, Na HM, Greenamyre JT, et al. Rotenone, deguelin, their metabolites, and the rat model of Parkinson’s disease. Chem Res Toxicol. 2004;17:1540–1548. doi: 10.1021/tx049867r. [DOI] [PubMed] [Google Scholar]
- California Department of Fish and Game. Lake Davis Northern Pike Eradication Options. 2004. Available: http://www.dfg.ca.gov/lakedavis/history/options.pdf [accessed 17 February 2012]
- California Department of Fish and Game. The Lake Davis Update. 2008. Available: http://www.dfg.ca.gov/lakedavis/newsletters/2008_april.pdf [accessed 17 February 2012]
- Camp HB, Fukuto TR, Metcalf RL. Selective toxicity of isopropyl parathion. Effect of structure on toxicity and anticholinesterase activity. J Agric Food Chem. 1969;17:243–248. [Google Scholar]
- Carlock LL, Dotson TA. In: Hayes’ Handbook of Pesticide Toxicology, Vol 2 (Krieger RI, ed). 3rd ed. Boston:Academic Press, 2293–2306; 2010. Metam-sodium. [Google Scholar]
- Carson R. Boston: Houghton Mifflin; 1962. Silent Spring. [Google Scholar]
- Casida JE. In: Chemistry and Action of Herbicide Antidotes (Pallos FM, Casida JE, eds). New York:Academic Press, 161–164; 1978. Herbicide antidotes: progress and prospects. [Google Scholar]
- Casida JE. In: Wolf Prize in Agriculture (Chet I, ed). Singapore:World Scientific Publishing, 383–431; 2009a. Autobiographical sketch and selected publications. [Google Scholar]
- Casida JE. Pest toxicology: the primary mechanisms of pesticide action. Chem Res Toxicol. 2009b;22:609–619. doi: 10.1021/tx8004949. [DOI] [PubMed] [Google Scholar]
- Casida JE. Curious about pesticide action. J Agric Food Chem. 2010a;59:2762–2769. doi: 10.1021/jf102111s. [DOI] [PubMed] [Google Scholar]
- Casida JE. Michael Elliott’s billion dollar crystals and other discoveries in insecticide chemistry. Pest Manag Sci. 2010b;66:1163–1170. doi: 10.1002/ps.1982. [DOI] [PubMed] [Google Scholar]
- Casida JE, Allen TC. A laboratory method for evaluating the phytotoxicity or phytostimulation of insecticides. Science. 1951;113:553–555. doi: 10.1126/science.113.2941.553. [DOI] [PubMed] [Google Scholar]
- Casida JE, Holmstead RL, Khalifa S, Knox JR, Ohsawa T, Palmer RJ, et al. Toxaphene insecticide: a complex biodegradable mixture. Science. 1974;183:520–521. doi: 10.1126/science.183.4124.520. [DOI] [PubMed] [Google Scholar]
- Casida JE, Quistad GB. Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem Res Toxicol. 2004;17:983–998. doi: 10.1021/tx0499259. [DOI] [PubMed] [Google Scholar]
- Casida JE, Quistad GB. Serine hydrolase targets of organophosphorus toxicants. Chem Biol Interact. 2005;157-158:277–283. doi: 10.1016/j.cbi.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Casida JE, Sanderson DM. Toxic hazard from formulating the insecticide dimethoate in methyl ‘Cellosolve’. Nature. 1961;189:507–508. doi: 10.1038/189507a0. [Letter] [DOI] [PubMed] [Google Scholar]
- Chen Y-L, Casida JE. Photodecomposition of pyrethrin I, allethrin, phthalthrin, and dimethrin. Modifications in the acid moiety. J Agric Food Chem. 1969;17:208–215. [Google Scholar]
- Cheng H-M, Yamamoto I, Casida JE. Rotenone photodecomposition. J Agric Food Chem. 1972;20:850–856. [Google Scholar]
- Coats JR. In: The Toxicology of Fishes (Di Giulio RT, Hinton DE, eds). Boca Raton, FL:CRC Press, 805–817; 2008. Toxicology of synthetic pyrethroid insecticides in fish: a case study. [Google Scholar]
- Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993;101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole LM, Casida JE. Pyrethroid toxicology in the frog. Pestic Biochem Physiol. 1983;20:217–224. [Google Scholar]
- de Almeida RRP, Souto RNP, Bastos CN, da Silva MHL, Maia JGS. Chemical variation in Piper aduneum and biological preparation of its dillapiole-rich essential oil. Chem Biodivers. 2009;6:1427–1434. doi: 10.1002/cbdv.200800212. [DOI] [PubMed] [Google Scholar]
- Dill CM. Glyphosate-resistant crops: history, status and future. Pest Manag Sci. 2005;61:219–224. doi: 10.1002/ps.1008. [DOI] [PubMed] [Google Scholar]
- Dunlap TR. Princeton, NJ: Princeton University Press; 1981. DDT: Scientists, Citizens, and Public Policy. [Google Scholar]
- Dureja P, Casida JE, Ruzo LO. Dinitroanilines as photostabilizers for pyrethroids. J Agric Food Chem. 1984;32:246–250. [Google Scholar]
- Ehrich M, Jortner BS. In: Handbook of Pesticide Toxicology (Krieger R, ed). Amsterdam:Elsevier, 1479–1504; 2010. Organophosphorus-induced delayed neuropathy. [Google Scholar]
- Fang N, Casida JE. Anticancer action of cubé insecticide: correlation for rotenoid constituents between inhibition of NADH:ubiquinone oxidoreductase and induced ornithine decarboxylase activities. Proc Natl Acad Sci USA. 1998;95:3380–3384. doi: 10.1073/pnas.95.7.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas J, Kourim P, Sorm F. Relation between chemical structure and insecticidal activity in pyrethroid compounds. I. An analogue of chrysanthemic acid containing chlorine in the side chain. Collect Czechoslov Chem Commun. 1959;24:2230–2236. [Google Scholar]
- Felsot AS. Early contributions of insect toxicology to the evolution of environmental toxicology. Illinois Nat Hist Surv Bull. 1985;33:199–218. [Google Scholar]
- Friend M, Franson JC, eds. Field Manual of Wildlife Diseases: General Field Procedures and Diseases of Birds. U.S. Department of the Interior, U.S. Geological Survey. 1999. Available: http://www.nwhc.usgs.gov/publications/field_manual/field_manual_of_wildlife_diseases.pdf [accessed 17 February 2012].
- Ford KA, Casida JE, Chandran D, Gulevich AG, Okrent RA, Durkin KA, et al. Neonicotinoid insecticides induce salicylate-associated plant defense responses. Proc Natl Acad Sci USA. 2010;107:17527–17532. doi: 10.1073/pnas.1013020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami J-I, Yamamoto I, Casida JE. Metabolism of rotenone in vitro by tissue homogenates from mammals and insects. Science. 1967;155:713–716. doi: 10.1126/science.155.3763.713. [DOI] [PubMed] [Google Scholar]
- Fungicide Resistance Action Committee. FRAC Code List: Fungicides Sorted by Mode of Action. 2010. Available: http://www.frac.info/frac/publication/anhang/FRAC_Code_List_2010 [accessed 17 February 2012]
- Gerhäuser C, Mar W, Lee SK, Suh N, Luo Y, Kosmeder J, et al. Rotenoids mediate potent cancer chemopreventative activity through transcriptional regulation of ornithine decarboxylase. Nat Med. 1995;1:260–266. doi: 10.1038/nm0395-260. [DOI] [PubMed] [Google Scholar]
- Gherman E. Reactions of a Kindly Nature. A Toxic Nightmare: The Dunsmuir Metam Sodium Spill Revisited. Sonoma County Free Press. 1997. Available: http://www.sonomacountyfreepress.com/reaction/a_toxic_nightmare.html [accessed 17 February 2012]
- Glickman AH, Wing KD, Casida JE. Profenofos insecticide bioactivation in relation to antidote action and the stereospecificity of acetylcholinesterase inhibition, reactivation, and aging. Toxicol Appl Pharmacol. 1984;73:16–22. doi: 10.1016/0041-008x(84)90047-4. [DOI] [PubMed] [Google Scholar]
- Greve PA, Wit SL. Endosulfan in the Rhine River. J Water Pollut Control Fed. 1971;43:2338–2348. [PubMed] [Google Scholar]
- Hainzl D, Casida JE. Fipronil insecticide: novel photochemical desulfinylation with retention of neurotoxicity. Proc Natl Acad Sci USA. 1996;93:12764–12767. doi: 10.1073/pnas.93.23.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainzl D, Cole LM, Casida JE. Mechanisms for selective toxicity of fipronil insecticide and its sulfone metabolite and desulfinyl photoproduct. Chem Res Toxicol. 1998;11:1529–1535. doi: 10.1021/tx980157t. [DOI] [PubMed] [Google Scholar]
- Hardstone MC, Scott JG. Apis mellifera more sensitive to insecticides than other insects? Pest Manag Sci. 2010;66:1171–1180. doi: 10.1002/ps.2001. [DOI] [PubMed] [Google Scholar]
- Haya K. Toxicity of pyrethroid insecticides to fish. Environ Toxicol Chem. 1989;81:381–391. [Google Scholar]
- Hayes TB, Collins A, Lee M, Mendoza M, Noriega N, Stuart AA, et al. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proc Natl Acad Sci. 2002;99:5476–5480. doi: 10.1073/pnas.082121499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbicide Resistance Action Committee. The World of Herbicides According to HRAC Classification on Mode of Action 2010. 2010. Available: http://www.hracglobal.com/Portals/5/moaposter.pdf [accessed 16 February 2012]
- Holmstead RL, Casida JE, Ruzo LO. Photochemical reactions of pyrethroid insecticides. Am Chem Soc Symp Ser. 1977;42:137–146. [Google Scholar]
- Horgan DJ, Singer TP, Casida JE. Studies on the respiratory chain-linked reduced nicotinamide adenine dinucleotide dehydrogenase. XIII. Binding sites of rotenone, piericidin A, and amytal in the respiratory chain. J Biol Chem. 1968;243:834–843. [PubMed] [Google Scholar]
- Huffaker CB, Messenger PS, eds. New York:Academic Press, 1–788. 1976. Theory and Practice of Biological Control. [Google Scholar]
- Insecticide Resistance Action Committee. IRAC MoA Classification Scheme. Version 7.1. 2011. Available: http://www.irac-online.org/wp-content/uploads/2009/09/MoA_Classification.pdf [accessed 16 February 2012]
- Institute of Medicine. Washington, DC: National Academies Press; 1994. Veterans and Agent Orange: Health Effects of Herbicides Used in Vietnam. Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides. [PubMed] [Google Scholar]
- Ivie GW, Casida JE. Enhancement of photoalteration of cyclodiene insecticide chemical residues by rotenone. Science. 1970;167:1620–1622. doi: 10.1126/science.167.3925.1620. [DOI] [PubMed] [Google Scholar]
- Ivie GW, Casida JE. Photosensitizers for the accelerated degradation of chlorinated cyclodienes and other insecticide chemicals exposed to sunlight on bean leaves. J Agric Food Chem. 1971;19:410–416. [Google Scholar]
- Ivie GW, Knox JR, Khalifa S, Yamamoto I, Casida JE. Novel photoproducts of heptachlor epoxide, trans-chlordane, and trans-nonachlor. Bull Environ Contam Toxicol. 1972;7:376–382. doi: 10.1007/BF01684465. [DOI] [PubMed] [Google Scholar]
- Iwasa T, Motoyama N, Ambrose JT, Roe RM. Mechanism for the differential toxicity of neonicotinoid insecticides in the honey bee Apis mellifera. Crop Prot. 2004;23:371–378. [Google Scholar]
- Johnson MK, Glynn P. In: Handbook of Pesticide Toxicology, Vol 2 (Krieger R, ed). San Diego, CA:Academic Press, 953–965; 2001. Neuropathy target esterase. [Google Scholar]
- Jones DK, Hammond JJ, Relyea RA. Very highly toxic effects of endosulfan across nine species of tadpoles: lag effects and family-level sensitivity. Environ Toxicol Chem. 2009;28:1939–1945. doi: 10.1897/09-033.1. [DOI] [PubMed] [Google Scholar]
- Kagabu S. Weinheim, Germany: Wiley-VCH, 193–212; 2003. Molecular design of neonicotinoids: past, present and future. In: Chemistry of Crop Protection (Voss A, Ramos G, eds) [Google Scholar]
- Karnak RE, Hamelink JL. A standardized method of determining the acute toxicity of chemicals to earthworms. Ecotoxicol Environ Saf. 1982;6:216–222. [Google Scholar]
- Kim J-H, Lam W-W, Quistad GB, Casida JE. Sulfoxidation of the soil fumigants metam, methyl isothiocyanate, and dazomet. J Agric Food Chem. 1994;42:2019–2024. [Google Scholar]
- Kleier D, Holden I, Casida JE, Ruzo LO. Novel photoreactions of an insecticidal nitromethylene heterocycle. J Agric Food Chem. 1985;33:998–1000. [Google Scholar]
- Kömives T, Casida JE. Diphenyl ether herbicides: effects of acifluorfen on phenylpropanoid biosynthesis and phenylalanine ammonia-lyase activity in spinach. Pestic Biochem Physiol. 1982;18:191–196. [Google Scholar]
- Kömives T, Casida JE. Acifluorfen increases the leaf content of phytoalexins and stress metabolites in several crops. J Agric Food Chem. 1983;31:751–755. [Google Scholar]
- Kömives T, Gullner G, Rennenberg H, Casida JE. Ability of poplar (Populus spp.) to detoxify chloroacetanilide herbicides. Water Air Soil Pollut Focus. 2003;3:277–283. [Google Scholar]
- Kovach J, Petzoldt C, Degnil J, Tette J. A method to measure the environmental impact of pesticides. New York’s Food Life Sci Bull. 1992. pp. 1–8. Available: http://dspace.library.cornell.edu/bitstream/1813/5203/1/FLS-139.pdf [accessed 15 February 2012]
- Lam W-W, Kim J-H, Sparks SE, Quistad GB, Casida JE. Metabolism in rats and mice of the soil fumigants metham, methyl isothiocyanate, and dazomet. J Agric Food Chem. 1993;41:1497–1502. [Google Scholar]
- Lawrence LJ, Casida JE. Interactions of lindane, toxaphene and cyclodienes with brain-specific t-butylbicyclophosphorothionate receptor. Life Sci. 1984;35:171–178. doi: 10.1016/0024-3205(84)90136-x. [DOI] [PubMed] [Google Scholar]
- Lay M-M, Hubbell JP, Casida JE. Dichloroacetamide antidotes for thiocarbamate herbicides: mode of action. Science. 1975;189:287–289. doi: 10.1126/science.1145201. [DOI] [PubMed] [Google Scholar]
- Lichtenstein EP, Casida JE. Myristicin, an insecticide and synergist occurring naturally in the edible parts of parsnips. J Agric Food Chem. 1963;11:410–415. [Google Scholar]
- Mahajna M, Casida JE. Oxidative bioactivation of methamidophos insecticide: synthesis of N-hydroxymethamidophos (a candidate metabolite) and its proposed alternative reactions involving N → O rearrangement or fragmentation through a metaphosphate analogue. Chem Res Toxicol. 1998;11:26–34. doi: 10.1021/tx9701135. [DOI] [PubMed] [Google Scholar]
- Mahajna M, Quistad GB, Casida JE. Acephate insecticide toxicity: safety conferred by inhibition of the bioactivating carboxyamidase by the metabolite methamidophos. Chem Res Toxicol. 1997;10:64–69. doi: 10.1021/tx9601420. [DOI] [PubMed] [Google Scholar]
- Maruya KA, Smalling KL, Mora MA. Residues of toxaphene in insectivorous birds (Petrochelidon spp.) from the Rio Grande, Texas. Arch Environ Contam Toxicol. 2005;48:567–574. doi: 10.1007/s00244-004-0142-9. [DOI] [PubMed] [Google Scholar]
- Meneely GA, Wyttenbach CR. Effects of the organophosphate insecticides diazinon and parathion on bobwhite quail embryos: skeletal defects and acetylcholinesterase activity. J Exp Zool. 1989;252:60–70. doi: 10.1002/jez.1402520109. [DOI] [PubMed] [Google Scholar]
- Metcalf RL. A century of DDT. J Agric Food Chem. 1973;21:511–519. doi: 10.1021/jf60188a040. [DOI] [PubMed] [Google Scholar]
- Miskus RP, Blair DP, Casida JE. Conversion of DDT to DDD by bovine rumen fluid, lake water, and reduced porphyrins. J Agric Food Chem. 1965;13:481–483. [Google Scholar]
- Müller P. Basel: Berkhäuser Verlag; 1959. The Insecticide Dichlorodiphenyltrichloroethane and Its Significance. Vol II. [Google Scholar]
- Mullin CA, Frazier M, Frazier JL, Ashcraft S, Simonds R, vanEngelsdorp D, et al. 2010High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS ONE 5e9754; doi: 10.1371/journal.pone.0009754[Online 19 March 2010] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negherbon WO. Philadelphia, PA: WB Saunders; 1959. Handbook of Toxicology. Vol III. Insecticides, A Compendium. [Google Scholar]
- Nomura DK, Casida JE. Activity-based protein profiling of organophosphorus and thiocarbamate pesticides reveals multiple serine hydrolase targets in mouse brain. J Agric Food Chem. 2011;59:4860–4867. doi: 10.1021/jf101747r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipps RH, Park JR. Environmental benefits of genetically modified crops: global and European perspectives on their ability to reduce pesticide use. J Anim Feed Sci. 2002;11:1–18. [Google Scholar]
- Quistad GB, Barlow C, Winrow CJ, Sparks SE, Casida JE. Evidence that mouse brain neuropathy target esterase is a lysophospholipase. Proc Natl Acad Sci USA. 2003;100:7983–7987. doi: 10.1073/pnas.1232473100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratra GS, Kamita SG, Casida JE. Role of human GABAA receptor β3 subunit in insecticide toxicity. Toxicol Appl Pharmacol. 2001;172:233–240. doi: 10.1006/taap.2001.9154. [DOI] [PubMed] [Google Scholar]
- Roger J-C, Chambers H, Casida JE. Nicotinic acid analogs: effects on response of chick embryos and hens to organophosphate toxicants. Science. 1964;144:539–540. doi: 10.1126/science.144.3618.539. [DOI] [PubMed] [Google Scholar]
- Rubin AL. Sacramento, CA: California Environmental Protection Agency, Medical Toxicology Branch, Department of Pesticide Regulation; 2004. Metam Sodium (Sodium N-Methyldithiocarbamate). Risk Characterization Document. [Google Scholar]
- Ruzo LO, Smith IH, Casida JE. Pyrethroid photochemistry: photooxidation reactions of the chrysanthemates phenothrin and tetramethrin. J Agric Food Chem. 1982;30:110–115. [Google Scholar]
- Saleh MA, Turner WV, Casida JE. Polychlorobornane components of toxaphene insecticide: structure-toxicity relations and metabolic reductive dechlorination. Science. 1977;198:1256–1258. doi: 10.1126/science.929197. [DOI] [PubMed] [Google Scholar]
- Schuler F, Casida JE. The insecticide target in the PSST subunit of complex I. Pest Manag Sci. 2001;57:932–940. doi: 10.1002/ps.364. [DOI] [PubMed] [Google Scholar]
- Seifert J, Casida JE. Mechanisms of teratogenesis induced by organophosphorus and methylcarbamate insecticides. Prog Pestic Biochem. 1980;1:219–246. [Google Scholar]
- Shepard HH. Minneapolis, MN: Burgess Publishing Company; 1939. The Chemistry and Toxicology of Insecticides. [Google Scholar]
- Smith AG. In: Handbook of Pesticide Toxicology, Vol 2, Classes of Pesticides (Hayes WG Jr, Laws ER Jr, eds). New York:Academic Press, 731–915; 1991. Chlorinated hydrocarbon insecticides. [Google Scholar]
- Soloway SB, Henry AC, Kollmeyer WD, Padgett WM, Powell JE, Roman SA, et al. In: Advances in Pesticide Science, Vol 2 (Geissbühler H, ed). New York:Pergamon, 206–217; 1979. Nitromethylene heterocycles as insecticides. [Google Scholar]
- Stephenson GR, Soloman KR. Guelph, Ontario, Canada:Canadian Network of Toxicology Centres Press. 2007. Pesticides and the Environment. [Google Scholar]
- Tomlin CDS. Alton, UK: British Crop Production Council; 2009. The Pesticide Manual: A World Compendium. 15th ed. [Google Scholar]
- Tsukamoto M. Metabolic fate of DDT in Drosophila melanogaster. I. Identification of a non-DDE metabolite. Botyu-Kagaku. 1959;24:141–151. [Google Scholar]
- Turner WV, Engel JL, Casida JE. Toxaphene components and related compounds: preparation and toxicity of some hepta-, octa-, and nonachlorobornanes, hexa- and heptachlorobornenes, and a hexachlorobornadiene. J Agric Food Chem. 1977;25:1394–1401. doi: 10.1021/jf60214a050. [DOI] [PubMed] [Google Scholar]
- Turner WV, Khalifa S, Casida JE. Toxaphene toxicant A. Mixture of 2,2,5-endo,6-exo,8,8,9,10-octachlorobornane and 2,2,5-endo,6-exo,8,9,9,10-octachlorobornane. J Agric Food Chem. 1975;23:991–994. doi: 10.1021/jf60201a050. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency) Washington, DC: U.S. Environmental Protection Agency; 2004. Interim Reregistration Eligibility Decision (IRED); Diazinon. EPA 738-R-04-006. [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency) White Paper on the Potential for Atrazine to Affect Amphibian Gonadal Development. Washington, DC:U.S.EPA. 2007. Available: http://www.epa.gov/scipoly/sap/meetings/2007/october/2007_amphibian_white_paper.pdf [accessed 17 February 2012]
- U.S. EPA (U.S. Environmental Protection Agency) Carbofuran Cancellation Process. 2011a. Available: http://www.epa.gov/opp00001/reregistration/carbofuran/carbofuran_noic.htm [accessed 17 February 2012]
- U.S. EPA (U.S. Environmental Protection Agency) Common Mechanism Groups; Cumulative Exposure and Risk Assessment. 2011b. Available: http://www.epa.gov/oppsrrd1/cumulative/common_mech_groups.htm [accessed 17 February 2012]
- van Gestel CA. Validation of earthworm toxicity tests by comparison with field studies: a review of benomyl, carbendazim, carbofuran, and carbaryl. Ecotoxicol Environ Saf. 1992;23:221–236. doi: 10.1016/0147-6513(92)90060-g. [DOI] [PubMed] [Google Scholar]
- Vetter W, Oehme M. In: The Handbook of Environmental Chemistry. New Types of Persistent Halogenated Compounds (Paasivirta J, ed). Heidelberg:Springer-Verlag, 237–287; 2000. Toxaphene. Analysis and environmental fate of congeners. [Google Scholar]
- Vinopal JH, Casida JE. Metabolic stability of 2,3,7,8-tetrachlorodibenzo-p-dioxin in mammalian liver microsomal systems and in living mice. Arch Environ Contam Toxicol. 1973;1:122–132. doi: 10.1007/BF01986002. [DOI] [PubMed] [Google Scholar]
- Vose SC, Holland NT, Eskenazi B, Casida JE. Lysophosphatidylcholine hydrolases of human erythrocytes, lymphocytes and brain: sensitive targets of conserved specificity for organophosphorus delayed neurotoxicants. Toxicol Appl Pharmacol. 2007;224:98–104. doi: 10.1016/j.taap.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware GW, Whitacre DM. Willoughby, OH: MeisterPro Information Resources; 2004. The Pesticide Book. 6th ed. [Google Scholar]
- Wijeyesakere SJ, Richardson RJ. In: Hayes’ Handbook of Pesticide Toxicology, Vol 2 (Krieger R, ed). 3rd ed. Amsterdam:Elsevier, 1435–1455; 2010. Neuropathy target esterase. [Google Scholar]
- Wing KD, Glickman AH, Casida JE. Oxidative bioactivation of S-alkyl phosphorothiolate pesticides: stereospecificity of profenofos insecticide activation. Science. 1983;219:63–65. doi: 10.1126/science.6849116. [DOI] [PubMed] [Google Scholar]
- Winrow CJ, Hemming ML, Allen DM, Quistad GB, Casida JE, Barlow C. Loss of neuropathy target esterase in mice links organophosphate exposure to hyperactivity. Nat Genet. 2003;33:477–485. doi: 10.1038/ng1131. [DOI] [PubMed] [Google Scholar]
- Xu H, Zhang N, Casida JE. Insecticides in Chinese medicinal plants: survey leading to jacaranone, a neurotoxicant and glutathione-reactive quinol. J Agric Food Chem. 2003;51:2544–2547. doi: 10.1021/jf021164x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.