Abstract
Background
As essential components of the innate immune system, dendritic cells (DCs) can interact directly with pathogens as well as participate in the adaptive immune response. In cells closely related to DCs such as macrophages and monocytes, prior exposure to minute amounts of endotoxin can lead to a refractory period where subsequent exposure to higher doses fails to induce an inflammatory response; little research has investigated this effect on DCs. This study tested if murine bone marrow-derived dendritic cells (BM-DCs) respond to endotoxin- and bacterial sonicate-induced tolerance by decreased inflammatory and increased anti-inflammatory response, and the role of IRAK-M, an intracellular negative regulator of TLR signaling, in this tolerance.
Results
Tolerized BM-DCs exhibited a significant drop in TNF-α and IL-12p70 production and increased IL-10 expression compared to untolerized cells. BM-DCs also showed the ability to develop heterotolerance, in which the LPS exposure alone was able to induce tolerance to Helicobacter pylori sonicate and TLR2 agonist Pam3Cys. Furthermore, the expression of IRAK-M was increased after restimulation of tolerized BM-DCs as determined qPCR and Western blot. IRAK-M exhibited a suppressive effect on surface expression of major histocompatibilty complex class II (MHC II) and CD80 in LPS-tolerized BM-DCs. IL-10 expression in bacterial sonicate-tolerized IRAK-M−/− BM-DCs was altered as compared to wild type BM-DCs, with tolerance-induced expression of IL-10 mitigated in tolerized IRAK-M−/− BM-DCs.
Conclusion
Along with endotoxin, bacterial sonicate is able to induce refractory tolerance in BM-DCs, and IRAK-M plays a role in modulating cell surface expression of MHC class II and CD80 and release of IL-10 during this tolerance.
Keywords: Dendritic cells, Immunomodulation, Immune reprogramming, Endotoxin tolerance, IRAK-M, Helicobacter pylori
1. Introduction
As essential components of the innate immune system, DCs can interact directly with pathogens as well as stimulate the adaptive immune response in their role as antigen presenting cells (APCs) [1]. In relation to T cell proliferation, DCs are able to skew T cell subsets based on cytokine expression [2]. DCs, as a subclass of leukocytes, are fairly heterogeneous and have complex physiological function, thus underscoring the need for a better understanding of their function.
Despite the knowledge gathered on their role in the adaptive immune response, there is comparatively little known about what leads to tolerization of DCs themselves. In monocytes and macrophages, which are closely related in function to dendritic cells, cellular tolerance has been shown to occur in septic patients with various TLR ligands, particularly the pyrogenic endotoxin (lipopolysaccharide, or LPS) derived from Gram-negative bacteria [3–7]. Monocytes, macrophages, and dendritic cells respond to bacterial components through what have been termed pattern recognition receptors (PRRs) [6,8–11]. Toll-like receptors (TLRs), necessary for the recognition of endotoxin and a wide array of pathogen-associated molecular patterns (PAMPs), are the major PPRs on APCs. These receptors are involved in cytokine response and co-stimulatory molecule expression during bacterial infection [6]. PAMPs bind to TLRs leading to cytokine activation through NF-κB and MAP kinase pathways, and these signaling pathways are essential to inflammation and eventual activation of the adaptive immune response [10]. Immune response and inflammation is a complex pathophysiological state, and endotoxin tolerance (ET) is a phenomenon in which re-exposure to endotoxin after an initial exposure to minute levels of endotoxin fails to induce fever and inflammation [12–18]. This form of hypo-responsiveness to otherwise stimulatory bacterial components suggests that intracellular TLR signaling that detects bacterial components is regulated within these cells and releases inflammatory signals. Further investigation into ET seems to suggest not just global immunosuppression of monocytes and macrophages during transient tolerance, but rather regulated increase and decrease of particular genes best characterized as “reprogramming” to better capture the altered, rather than globally decreased, function of the immune cells [6,9,19,20].
This ET phenomenon has been shown to occur in vivo and in vitro in human and animal models, with in vitro experiments mainly testing monocytes and macrophages [3,4,7,17,21–24]. This tolerant phenotype is correlated with the up-regulation of a number of cell receptors, enhanced phagocytosis, decreased antigen presentation, and a decrease in pro-inflammatory cytokine release [13,15,24–26]. ET has been identified as playing a role in diverse pathological settings involving different cell types [1,6,8,16,18,27,28]. However, the behavior of dendritic cells during this form of transient unresponsiveness, as well as how dendritic cells respond to aggregated bacterial components, has been comparatively unexplored.
A key immunomodulatory molecule that has been identified to be important during ET is interleukin-1 receptor-associated kinase-M (IRAK-M). In circulating monocytes of human septic patients that display refractory tolerance, IRAK-M is expressed in much higher levels and has been shown to be essential for ET in vitro in macrophages [5,7,23,29]. IRAK-M has been identified as a potent negative regulator of TLR signaling through MyD88, decreasing signaling through NF-κB and expression of pro-inflammatory cytokines (TNF-α, IL-1-β, IL-6, and IL-12B, among others) [29]. IRAK-M is up-regulated in macrophages and monocytes, and even “essential” for transient tolerance in these cells [3,29,30]. However, the role of IRAK-M has not been identified or characterized in transient tolerization of these cells to bacterial components. We show that endotoxin and bacterial sonicate are able to induce refractory tolerance in BM-DCs, and IRAK-M plays a role in modulating cell surface expression of MHC class II and CD80 and release of IL-10 during this tolerance. Unlike wild type cells that increase their IL-10 expression after developing tolerance, when IRAK-M−/− BM-DCs are tolerized they are unable to significantly elevate their IL-10 expression as compared to IRAK-M−/− BM-DCs that are untolerized.
2. Materials and methods
2.1. Mice
C57BL/6 mice were housed in the specific pathogen free animal maintenance facility at the University of Michigan Health System. IRAK-M−/− mice [29] were provided by Shizuo Akira (Osaka University) and bred in the breeding facility at the University of Michigan. The University of Michigan Animal Care and Use Committee approved all animal experiments. Mouse genotypes were confirmed by tail PCR.
2.2. Media and cytokines
For all experiments, bone marrow-derived DCs (BM-DCs) were cultured in complete medium consisting of RPMI-1640 (Sigma, Milwaukee, WI) with 9% heat-inactivated fetal calf serum (ISC Biosciences, Kaysville, UT), 2 mM added Glutamine (4 mM total), 100 U/ml Penicillin, and 100 μg/ml Streptomycin. The recombinant mouse cytokines GM-CSF (10 ng/ml) and IL-4 (10 ng/ml) (R&D Systems, Minneapolis, MN) were diluted in complete medium during culture for 6 days. After harvest of the cells at day 6, only mGM-CSF was included in the complete medium for the duration of the experiment through stimulation, rest, and re-stimulation periods.
2.3. Generation of bone marrow-derived DCs
Murine femur and tibia bone marrow cells were suspended in PBS, depleted of RBCs, and cultured in complete medium with cytokines as described above. On day 3, 50% of the complete media was aspirated and replenished. On day 6, cells in culture were vigorously pipetted and harvested, and cells tightly adherent to the plate were discarded. DCs were enriched from these cells by gradient centrifugation (OptiPrep™, Sigma). DCs at the density interface were collected by gentle aspiration, washed, and cultured in complete medium with cytokines as described above. BM-DC purity was determined to be >70% as per their co-expression of CD11c and MHC class II by FACS analysis with anti-CD11c and anti-MHC class II fluorochromes (eBioscience, San Diego, CA).
2.4. Bacterial strains and culture conditions
Helicobacter pylori was grown on Campylobacter-selective agar (BD Diagnostics, Bedford, MA) and supplemented with 5% sterile horse blood, trimethoprim (5 μg/ml), vancomycin (10 μg/ml), and nystatin (10 μg/ml) for 2–4 days at 37 °C in a humidified microaerophilic chamber (BBL Gas System, with CampyPak Plus packs, BD Microbiology, Sparks, MD). In vitro experiments were performed using H. pylori strain SS1. To prepare the bacterial sonicate, bacteria were diluted in PBS (Invitrogen, Frederick, MD) to a concentration of 1 × 109/ml and subjected to repeated sonication in an ultrasonic bath. Protein levels were assayed using a BSA standard (Bio-Rad, Hercules, CA), and overall protein concentration was used as representative of proportional amounts of all bacterial components. LPS was purchased from Sigma and was derived from Escherichia coli strain O127:B8. LPS was diluted in PBS.
2.5. Tolerization and stimulation of DCs
DCs at 1 × 106 cells/ml were treated for 8 h with either 10 ng/ml LPS (unless otherwise indicated) or 2 μg/ml sonicate from E. coli or H. pylori produced as described above. After treatment, cells were washed 3 times with PBS (Invitrogen). Cells were then re-plated with complete media and allowed to rest overnight (16–18 h). A second stimulation was then administered of 100 ng/ml LPS or 10 μg/ml bacterial sonicate from E. coli or H. pylori (Supplementary Fig. 1). For co-culture with syngeneic splenocytes in a mixed-leukocyte reaction, spleens were harvested, crushed, filtered through 70 μm filters and red blood cells were lysed with ACK lysis solution. Splenocyte to BM-DC ratio was 10 to 1. For heterotolerance experiments, the second stimulation was either 10 μg/ml bacterial sonicate from H. pylori or 100 ng/ml Pam3Cys. Cells were collected at 3 h for mRNA analysis and 8 h for protein isolation. Supernatant was collected at 8 h for cytokine analysis. Trypan Blue viability testing showed no significant difference between cell viability after tolerization and re-stimulation in wild type or IRAK-M−/− cells (Supplementary Fig. 2). All experiments were repeated three times in duplicate.
2.6. mRNA isolation, cDNA synthesis, and qPCR
DCs were collected at three hours by media aspiration and cell scraping. Cells were promptly treated with TRIzol reagent as per instructions of manufacturer (Invitrogen). All mRNA samples used for analysis had A260/A280 ratios greater than 1.9. One μg of sample was used to synthesize cDNA using iScript cDNA synthesis kit as per instructions of manufacturer (Bio-Rad) and analyzed by qPCR using iQ SYBR Green Supermix as per instructions of manufacturer (Bio-Rad). GAPDH was used as an endogenous housekeeping reference gene. Murine primers (Invitrogen, Frederick, MD) were: GAPDH 5′ TCAAGAAGGT GGTGAAGCAGG3′, 5′TATTATGGGGGTCTGGGATGG3′; IL-10 5′CTT ACTGACTGGCATGAGGATCA3′, 5′AGCTGGTCCTTTGTTTGAAAGAAA3′; IRAK-M 5′TGAGCAACGGGACGCTTT3′, 5′GATTCGAACGTGCCAG GAA3′.
2.7. ELISA
At 8 h, cell-free supernatants were collected and analyzed for IFN-γ, TNF-α, IL-10, and IL-12p70. Cytokine measurements were performed by ELISA as per instructions of manufacturer (eBio-science/BD Biosciences, San Diego, CA/San Jose, CA).
2.8. Western blot
DCs were collected, washed, and lysed with lysis solution (Cell Signaling Technologies, Beverly, MA) containing protease and phosphatase inhibitors (Cell Signaling Technologies, Beverly, MA). Protein was then run on a SDS-PAGE (12%, continuous), transferred to PVDF (Bio-Rad), blotted with rabbit IgG anti-IRAK-M primary and goat IgG anti-rabbit secondary antibodies (Abcam, Cambridge, MA), and protein detection was carried out by chemiluminescence (Thermo Scientific Pierce). GAPDH was used as an endogenous housekeeping reference protein.
2.9. FACS analysis
DCs were washed twice with ice-cold PBS containing 0.5% bovine serum albumin (and sodium azide if cells were stored). After a 30-min incubation with 1 μg/100 μL Fc Block (BD Biosciences, San Jose, CA), the cells were incubated with MHC class II/CD11c FITC-conjugated and/or CD80 PE-conjugated antibodies or with isotype control antibodies. The cells were washed, fixed with cold 2% paraformaldehyde, and analyzed using a Coulter XL Flow Cytometer (Beckman Coulter, Miami, FL).
3. Results
3.1. Establishment of a model for bone marrow-derived dendritic cells tolerance with endotoxin, E. coli sonicate or H. pylori sonicate
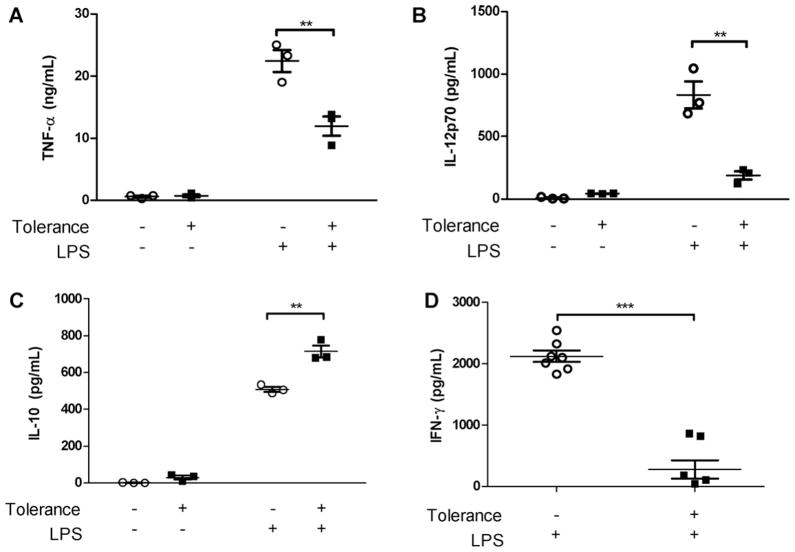
We used bone marrow-derived DCs (BM-DCs) to study further endotoxin tolerance in dendritic cells. When compared to controls not exposed to low-dose LPS or bacterial sonicate (H. pylori or E. coli), levels of the pro-inflammatory cytokines TNF-α and IL-12p70 were significantly decreased when cells were first treated with low dose LPS (Fig. 1A and B) or bacterial sonicate (Fig. 2A and B) for 8 h, allowed to rest overnight, and then subsequently stimulated with the same component at higher doses. The decreased cytokine production was not due to decreased cellular viability (Supplementary Fig. 2). In contrast to decreased TNF-α and IL-12p70, DCs in the same conditions show increased IL-10 expression upon low-dose LPS (Fig. 1C) or bacterial sonicate (Fig. 2C) exposure as compared to unexposed controls. When intracellular cytokines were examined, a similar pattern was seen in which the percentage of BM-DCs expressing intracellular TNF-α was decreased and the percentage expressing IL-10 was increased upon tolerization and re-stimulation with LPS, although only a small percent appear to express IL-10 (Supplementary Fig. 3A). Additionally, when high-dose LPS re-stimulated BM-DCs were co-cultured with syngeneic splenocytes in a mixed leukocyte reaction and the level of IFN-γ was measured, the re-stimulated BM-DCs were unable to elicit a strong IFN-γ response (Fig. 1D). Intracellular cytokine study revealed a comparable decrease in the percentage of CD4+ T cells expressing IFN-γ when cultured with tolerized and re-stimulated BM-DCs (Supplementary Fig. 3B).
Fig. 1.
Low-dose LPS induces tolerance of BM-DCs to high-dose LPS re-stimulation. BM-DCs exposed to low dose (10 ng/ml) LPS (tolerized group) or PBS (non-tolerized group), washed in PBS, rested overnight, was re-stimulated by higher dose LPS (100 ng/ml). (A and B) BM-DC in the tolerance group exhibit a significant ablation in secreted TNF-α and IL-12p70 compared to BM-DCs in the No-tolerance group. (C) BM-DCs in the tolerized group show a significant increase in secreted IL-10 upon second stimulation by higher dose LPS compared to DCs not exposed to low dose LPS. (D) High-dose LPS re-stimulated BM-DCs were co-cultured with syngeneic splenocytes for 72 h in a mixed leukocyte reaction and the level of IFN-γ was measured by ELISA. Tolerized BM-DCs show an inability to stimulate a type I helper T cell response (Th1) compared to BM-DCs in the non-tolerized group. All cytokine measurements determined by ELISA. 1st (low)/2nd (high) dose LPS = 10/100 ng/ml or PBS. Data represent mean ± SEM, n = 3 in duplicates (n = 7 for mixed leukocyte reaction). *p < 0.05, **p < 0.01, ***p < 0.001 compared to untolerized cells.
Fig. 2.
TNF-α, IL-12p70 and IL-10 expression in supernatant of DCs tolerized and stimulated with H. pylori SS1 sonicate or E. coli sonicate. (A and B) When tolerized with E. coli or H. pylori sonicate, BM-DCs exhibit a significant ablation in secreted TNF-α and IL-12p70 upon second stimulation by a higher dose of the same sonicate as compared to non-tolerized cells. Between stimulations cells are washed with PBS and rested overnight. Non-tolerized DCs display a clear TNF-α and IL-12p70 response to sonicate stimulation. (C) Sonicate tolerization leads to a significant increase in secreted IL-10 upon second stimulation by higher dose H. pylori sonicate or E. coli sonicate as compared to non-tolerized BM-DCs. All cytokine measurements determined by ELISA. 1st (low)/2nd (high) dose sonicate = 2/10 μg/ml or PBS. Data represent mean ± SEM, n = 3 in duplicates. *p < 0.05, **p < 0.01, ***p < 0.001 compared to untolerized cells.
This demonstrates the establishment of a model for dendritic cell LPS tolerization with respect to these cytokines, with an expected pattern of decreased inflammatory signaling (TNF-α and IL-12p70) and increased anti-inflammatory signaling (IL-10) by the BM-DCs themselves in addition to a weak IFN-γ production in a mixed-leukocyte reaction. Furthermore we show that BM-DCs can be tolerized with crude bacterial sonicate (H. pylori or E. coli) containing numerous TLR ligands and other PAMPs, indicating that multi-ligand stimulation does not prevent the development of a tolerogenic phenotype with respect to TNF-α, IL-12p70, and IL-10 (Fig. 2A–C).
3.2. BM-DCs exhibit heterotolerance when tolerized with LPS and subsequently stimulated with H. pylori sonicate or TLR2 agonist Pam3Cys
Instead of tolerization and re-exposure to the same stimulus, we tested heterotolerance of BM-DCs, defined as tolerance and re-stimulation performed by different stimuli. Specifically, cells were first treated with low dose E. coli LPS, allowed to rest overnight, and then stimulated with H. pylori sonicate. H. pylori sonicate was used due to its distinct immunomodulatory capabilities, and to provide contrasting H. pylori LPS stimulation to the initial tolerizing stimulus of E. coli LPS [31–35]. Despite exposure to different components, BM-DC tolerance was maintained when assayed for TNF-α and IL-10. This demonstrates that LPS alone, principally a TLR4 ligand, is sufficient to induce tolerance to bacterial stimulatory ligands which stimulate through various TLRs and PRRs (Fig. 3A). To test whether this effect would hold for re-stimulation with a TLR2 agonist, Pam3Cys was used to re-stimulate the cells after an initial tolerization with low dose LPS (Fig. 3B). The typical tolerance profile of decreased TNF-α and increased IL-10 was again observed, demonstrating heterotolerance with an initial TLR4 agonist followed by re-stimulation with a TLR2 agonist.
Fig. 3.
Heterotolerance in DCs exposed to low-dose LPS and subsequently exposed to H. pylori SS1 sonicate or TLR2 ligand. BM-DCs exposed to low-dose LPS (10 ng/ml), washed with PBS, rested overnight, and then exposed to H. pylori SS1 (10 μg/ml) sonicate or TLR2 ligand (Pam3Cys, EMC microcollections, Tubingen, Germany, 100 ng/ml). BM-DCs from the tolerized group exhibit a similar cytokine expression as homotolerized cells (exposed to a stimulus at a low dose and re-stimulated with the same stimulus at a higher dose) or PBS. Heterotolerized cells show ablated TNF-β expression and/or IL-12 and increased IL-10 expression when subjected first to low dose LPS then H. pylori sonicate or Pam3Cys compared to no-tolerance group BM-DCs. All cytokine measurements determined by ELISA. Data represent mean ± SEM, n = 3 (for H. pylori group) or 4 (for Pam3Cys group) in duplicates. *p < 0.05, **p < 0.01, ***p < 0.001 compared to untolerized cells.
3.3. IRAK-M transcript and protein expression are up-regulated in BM-DCs during LPS tolerance
BM-DCs tolerized with LPS were assayed for the presence of IRAK-M transcript and protein in order to determine if enhanced expression of this negative regulator of TLR signaling occurred during endotoxin tolerance. IRAK-M was shown to be up-regulated in macrophages and monocytes when these cells were tolerized, and has even been shown to be “essential” to tolerance [6]. Our results indicate significantly increased expression of IRAK-M transcript in tolerized BM-DCs as determined by qPCR (Fig. 4A), as well as an increase in IRAK-M protein expression as determined by Western blot (Fig. 4B). Given the similar increase in IRAK-M during tolerance in macrophages and monocytes, these results suggest the possibility of IRAK-M having an analogous role in tolerance of dendritic cells.
Fig. 4.
IRAK-M mRNA and protein expression in endotoxin-tolerized wild type BM-DCs (A) qPCR 3 h after 2nd stimulation. BM-DCs were exposed for 8 h to low-dose LPS (10 ng/ml), washed with PBS, rested overnight, and re-stimulated with high-dose LPS (100 ng/ml). Cells were lysed and mRNA purified and copied to produce cDNA for qPCR analysis. After exposure to low-dose LPS there is a significantly increased production of IRAK-M transcript upon second stimulation by a higher dose of LPS. Data normalized to housekeeping gene GAPDH. (B) Western blot 8 h after re-stimulation with high-dose LPS. BM-DCs exposed to low dose LPS, washed with PBS, rested overnight, and re-stimulated with high dose LPS show increased intracellular IRAK-M expression compared to cells not exposed to low dose LPS. 1st (low)/2nd (high) dose LPS = 10/100 ng/ml or PBS. Data represent mean ± SEM, n = 3 in duplicates. *p < 0.05, **p < 0.01.
3.4. IRAK-M inhibits LPS-tolerized BM-DC surface expression of MHC class II and CD80
Major histocompatibility complex class II (MHC II) and co-stimulatory molecule CD80 are utilized by DCs for antigen presentation and priming the T cells against presented antigens, respectively. BM-DCs were tolerized with LPS and then re-stimulated with PBS or LPS and analyzed by flow cytometry to determine the percentage of the BM-DC population expressing MHC II and CD80. Tolerized IRAK-M−/− BM-DCs had higher expression of MHC II and CD80 compared to tolerized wild type BM-DCs, and this effect was even seen for MHC II in IRAK-M−/−cells without tolerization (Fig. 5). Tolerization status does not affect the expression of surface markers on wild type cells. With LPS re-stimulation, the expression of MHC II and CD80 was up-regulated in IRAK-M−/− BM-DCs but not wild type BM-DCs (Fig. 5). There was no change in CD86 or CD40 expression (data not shown). These findings in cells without IRAK-M expression indicate that IRAK-M has a suppressive role in MHC II and CD80 expression, and maintains MHC II and CD80 levels stable during the tolerization process.
Fig. 5.
IRAK-M inhibits surface expression of MHC class II and CD80 in LPS-tolerized BM-DCs. BM-DCs were tolerized with LPS and then re-stimulated with PBS or LPS. The expression of major histocompatibility complex class II (MHC II) and co-stimulatory molecule CD80 were measured by FACS. Parts (A) and (B) are single-stained cells (for either MHC II or CD80), with an arbitrary intensity threshold to determine positive or negative expression, while part (C) is a representative plot of double-stained cells (for both MHC II and CD80) gated for double-positive wild type BM-DCs. (A) On initial stimulation and without further stimulation after tolerance, the expression of MHC II is significantly higher in IRAK-M−/− LPS-tolerized BM-DCs as compared to wild type LPS-tolerized BM-DCs. After LPS re-stimulation, further increase in MHC II was observed in IRAK-M−/− LPS-tolerized BM-DCs but not in wild type LPS-tolerized BM-DCs. (B) The pattern of expression of CD80 in IRAK-M−/− and wild type LPS-tolerized BM-DCs was found to be similar to that of MHC II, however only during tolerance are significant increases found in IRAK-M−/− cells as compared to wild type. (C) Representative flow plot demonstrates increase of MHC II and CD80 double-positive cells in IRAK-M−/− BM-DCs compared to wild type with gating held constant. Largest population of MHC II and CD80 double-positive cells is seen in tolerized IRAK-M−/− BM-DCs that have been re-stimulated with LPS. 1st (low)/2nd (high) dose LPS = 10/100 ng/ml or PBS. Data represent mean ± SEM, n = 3 in duplicates. *p < 0.05, **p < 0.01, ***p < 0.001.
3.5. IRAK-M−/− BM-DCs display altered IL-10 expression during bacterial sonicate tolerance
The decreased expression of IL-12p70 and TNF-α in IRAK-M−/− BM-DCs during LPS tolerance was unaffected as compared to tolerized wild type BM-DCs, and even enhanced with respect to TNF-α (Fig. 6A and B). IL-10 expression was likewise unaffected in IRAK-M−/− versus wild type BM-DCs, maintaining its tendency to increase during tolerance (Fig. 6C). IRAK-M does not appear to play a suppressive role in the expression of these cytokines in LPS-specific tolerance, with only a small (yet statistically significant) effect on TNF-α. We then tested the development of tolerance to E. coli and H. pylori sonicate in IRAK-M−/− BM-DCs. As in IRAK-M−/− cells tolerized with LPS, bacterial sonicate-tolerized IRAK-M−/− BM-DCs maintained decreased expression of IL-12p70 and TNF-α during this tolerance (Fig. 7A, B, D, and E).
Fig. 6.

TNF-α, IL-12p70 and IL-10 expression in supernatant of wild type vs IRAK-M−/− DCs tolerized with LPS. (A and B) Tolerized BM-DCs exhibit a significant ablation in secreted TNF-α and IL-12p70 upon second stimulation by higher dose LPS in both IRAK-M−/− or wild type mice. (C) IL-10 expression in BM-DCs was increased in both tolerized IRAK-M−/− BM-DCs and wild type BM-DCs compared to untolerized cells of the same type. Fold change indicates the change in cytokine expression when cells are tolerized and compared to non-tolerized cells of the same type, either wild type or IRAK-M−/−. An asterisk (*) after the IRAK-M−/− fold change indicates the change as being significantly different as compared to wild type cells. All cytokine measurements determined by ELISA. 1st (low)/2nd (high) dose LPS = 10/100 ng/ml or PBS. Data represent mean ± SEM, n = 3 in duplicates. *p < 0.05, **p < 0.01, ***p < 0.001 compared to untolerized cells.
Fig. 7.
TNF-α, IL-12p70 and IL-10 in supernatant of wild type compared to IRAK-M−/− DCs tolerized with H. pylori sonicate or E. coli sonicate. (A and B, D and E) BM-DCs tolerized with E. coli or H. pylori sonicate exhibit a significant decrease in secreted TNF-α and IL-12p70 upon second stimulation by higher dose E. coli or H. pylori sonicate compared to untolerized cells from both IRAK-M−/− or wild type mice. (C and F) IL-10 demonstrates mitigated increase during tolerance by the lack of IRAK-M. Tolerized wild type BM-DCs show an expected IL-10 increase upon re-stimulation, however tolerized IRAK-M−/− BM-DCs failed to significantly increase IL-10 expression as compared to untolerized IRAK-M−/− BM-DCs. Fold change indicates the change in cytokine expression when cells are tolerized and compared to non-tolerized cells of the same type, either wild type or IRAK-M−/−. An asterisk (*) after the IRAK-M−/− fold change indicates the change as being significantly different as compared to wild type cells. All cytokine measurements determined by ELISA. 1st (low)/2nd (high) dose sonicate = 2/10 μg/ml or PBS. Data represent mean ± SEM, n = 3 in duplicates. *p < 0.05, **p < 0.01, ***p < 0.001 compared to untolerized cells of the same genotype.
Additionally, IRAK-M appears to play a role in IL-10 expression during bacterial sonicate tolerance. In contrast to bacterial sonicate tolerized wild type BM-DCs, no significant induction of IL-10 production was observed in sonicate tolerized IRAK-M−/− BM-DCs compared to non-tolerized (Fig. 7C, F). Unlike wild type BM-DCs which display increased IL-10 after tolerance and re-stimulation, when IRAK-M−/− BM-DCs are tolerized they do not significantly elevate their IL-10 expression as compared to IRAK-M−/− BM-DCs that are untolerized (indicated with a “NS” on Fig. 7C and F), although a trend toward difference is seen. This cannot be explained by a difference in viability of wild type as compared to IRAK-M−/− BM-DCs (Supplementary Fig. 2). Fold change comparisons are also provided to demonstrate a quantitative difference in the change in TNF-α and IL-10 expression in tolerized compared to non-tolerized cells, with an asterisk (*) indicating significant difference in that change between IRAK-M−/− and wild type cells.
When intracellular expression of TNF-α and IL-10 was examined, and an analogous pattern was seen in which the percent of cells expressing TNF-α was decreased in tolerized IRAK-M−/− BM-DCs compared to non-tolerized cells. An increase in the percent expressing IL-10 was observed only in LPS tolerization, whereas the percent of E. coli- and H. pylori-tolerized IRAK-M−/− BM-DCs expressing intracellular IL-10 decreased slightly (Supplementary Fig. 3C). Overall, this indicates that IRAK-M does not play a significant role in maintaining a tolerogenic phenotype in LPS tolerance, but does appear important in bacterial sonicate tolerance by promoting the expression of IL-10.
4. Discussion
Recent studies in dendritic cell tolerance have demonstrated their behavior in response to individual ligands [11,36–38]. This study aimed to develop a model of endotoxin tolerance within murine bone marrow-derived dendritic cells (BM-DCs), observe tolerance with crude bacterial components, as well as investigate the role of IRAK-M in DC tolerance. IRAK-M was identified as essential to endotoxin tolerance in macrophages and monocytes. Overall, the role of dendritic cells in adaptive tolerance has been investigated, but much investigation remains surrounding how DCs themselves might be tolerized [39,40].
We established a tolerance model to imitate what would, in vivo, be primary and secondary exposures [6]. This was developed in order to determine dendritic cell behavior when exposed to multiple stimulatory ligands, or tolerance in which the tolerizing stimulus and secondary stimulus are distinct from each other (heterotolerance) [41]. Multiple ligand stimulation is more representative of in vivo conditions since varied bacterial components typically stimulate dendritic cells [31,42,43]. E. coli was taken as an archetypal model of Gram (−) bacterial exposure, and H. pylori was chosen due to its varied immunomodulatory capabilities, ability to chronically colonize the gastric epithelium and interact with DCs, and complex pathological manifestations [31,32,44–48].
Monocyte-derived cells can be tolerized and display a modulated cytokine response, with significantly decreased pro-inflammatory cytokines and increased anti-inflammatory cytokines [1,3–6,11,17,19,23,27,37,38,49–59]. Furthermore, these cells display a regular pattern of decreased antigen presentation, enhanced phagocytosis, and expression of negative regulators, particularly IRAK-M [13,15,24–26]. This type of tolerance has been implicated in a wide array of acute and chronic diseases [6]. In addition to LPS from the two bacteria used in this study (H. pylori and E. coli), the sonicate would also include various cell components which can lead to inflammatory signaling via TLR and other receptors [10].
The current study has demonstrated that BM-DCs possess the ability to become tolerized, and that this tolerance can occur with aggregated microbial components. Tolerized BM-DCs showed significantly decreased expression of the inflammatory cytokines TNF-α and IL-12p70. Conversely, IL-10 expression was increased when BM-DCs were tolerized with LPS or bacterial sonicate. IL-10 notably has a strong influence on the development of regulatory T cells [60]. Additionally, we showed that tolerized BM-DCs were not able to stimulate a robust IFN-γ when co-cultured with spleen-derived T cells as is seen with non-tolerized BM-DCs, indicating tolerized BM-DCs might be less able to stimulate an inflammatory Th1 response. Results from intracellular staining demonstrate that a smaller percentage of CD4+ cells express IFN-γ when cultured with tolerized BM-DCs. However, conclusions about Th1 priming capacity cannot be made without further experiments.
We further tested heterotolerance in BM-DCs to determine if the modulated cytokine expression could be maintained when the cells are tolerized and stimulated with distinct stimuli. In our study the initial tolerizing stimulus was LPS, recognized by TLR4, followed by secondary exposure to H. pylori sonicate, which does not contain LPS that signals through TLR4 [34]. We also performed heterotolerance with Pam3Cys, which signals through TLR2, as the secondary stimulus. These heterotolerized cells maintained the expected decrease in TNF-α expression and increase in IL-10 expression.
Proceeding from these cytokines studies, we investigated the role of IRAK-M in the tolerance of BM-DCs. IRAK-M was studied since it is expressed in high amounts in tolerized monocytes and macrophages, and essential to the development of tolerance within these cells [3–5,7,23,27–29,61]. IRAK-M interacts with IRAK-1 and IRAK-4 to prevent dissociation from the TLR4/MyD88 adapter complex and leading to NF-κB activation [10,29]. The current study shows BM-DCs tolerized with LPS exhibit significantly increased expression of IRAK-M at both the mRNA and protein levels.
Investigation beyond correlative participation of IRAK-M in tolerance showed that the absence of IRAK-M increases the expression of MHC II and CD80 in LPS tolerance, as well as decreasing IL-10 expression and modulating TNF-α expression during bacterial sonicate tolerance. IRAK-M appeared to have no role in suppressing the expression of IL-12p70 in either LPS or bacterial sonicate tolerance. These results clarify the specific role of IRAK-M in tolerized BM-DCs; IRAK-M suppresses MHC II and CD80 upon induction of LPS tolerance and prevents significantly increased IL-10 release in cells tolerized with bacterial sonicate and re-stimulated (although a trend toward difference is seen). IRAK-M, through its effects on IL-10, may also exert effects on the skewing of T cells toward a regulatory phenotype.
In a preliminary experiment with LPS tolerized and re-stimulated cells, there is evidence for an increase in Creb1 mRNA expression (Supplementary Fig. 4). Crib1 is a transcription factor that regulates diverse cellular responses including promoting IL-10 production and inhibiting NF-κB activation, and this may be one component that links IRAK-M expression with suppressed IL-10 in tolerized cells [62]. IRAK-4 is a target of IRAK-M in post-translational protein interaction, thus possibly explaining the lack of influence on IRAK-4 mRNA [29].
Although not entirely clear, the contrasting effects of IRAK-M in this BM-DC model as compared to its effects in macrophages demonstrated in other studies is perhaps due to their functional differences (i.e., greater involvement in antigen-specific adaptive responses by DCs, as opposed to greater involvement of macrophages in innate inflammation) [40,63]. Thus a negative regulator of inflammation like IRAK-M would be more important to maintaining the inflammatory response under control in macrophages innately, perhaps explaining the larger intracellular role that IRAK-M seems to play in modulating the inflammatory cytokine response in macrophages.
5. Conclusions
We show that along with endotoxin (LPS), bacterial sonicate is able to induce refractory tolerance in BM-DCs. This type of refractory tolerance has been implicated in complex pathologies ranging from sepsis to cystic fibrosis [1,6,8,16,18,27,28], with many clinical implications in elucidating the mechanism of its development. We have demonstrated that IRAK-M plays a role in suppressing cell surface expression of MHC class II, the principal antigen-presenting molecule, and CD80, a T cell co-stimulatory molecule, as well as modulating TNF-α expression and increasing IL-10 expression during this tolerance. IRAK-M, an upstream inhibitor of NF-κB signaling, had been shown to be essential to endotoxin tolerance in macrophages, yet remained largely uncharacterized in dendritic cells [3–5,7,23,27–29,61]. Our results suggest that an inflammatory stimulus containing ligands of various TLRs and other PRRs such as in bacterial sonicate, and not LPS alone, is associated with IRAK-M involvement in the reprogramming of BM-DC IL-10 expression during tolerance.
Supplementary Material
Acknowledgments
Acknowledgements and funding
Grant support: This study was supported by a grant from the Undergraduate Research Opportunity Program at the University of Michigan and grants from the National Institutes of Health (Grant nos. K08 DK081678-01; R01 DK087708-01; R03 DK081678-01). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- BM-DC (DC)
bone marrow-derived dendritic cell (dendritic cell)
- LPS
lipopolysaccharide (endotoxin)
- TNF-α
tumor necrosis factor-alpha
- TGF-β
transforming growth factor beta
- IL
interleukin
- IRAK
interleukin 1 receptor-associate kinase
- NF-κB
nuclear factor kappa B
- TLR
Toll-like receptor
- qPCR
quantitative polymerase chain reaction
- APC
antigen presenting cell
- PRR
pattern recognition receptor
- PAMP
pathogen associate molecular pattern
- MAP kinase
mitogen-activated protein kinase
- ET
endotoxin tolerance
- MyD88
myeloid differentiation factor 88
- TRIF
TIR-domain-containing adapter-inducing interferon-β
- IκB
inhibitor of nuclear factor kappa B kinase
- GM-CSF
granulocyte/macrophage-colony stimulating factor
- PBS
phosphate buffered saline
- GAPDH
glyceraldehyde-3 phosphate dehydrogenase
- ELISA
enzyme-linked immunosorbent assay
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.imlet.2012.03.006.
Footnotes
Conflicts of interest
There is no conflict of interest declared for any author.
Authors’ contributions
Tyler Cole designed the study and worked with Jay Luther, Jiajie Zhang, Chun-Chia Chen, and Min Zhang for data acquisition. Tyler Cole teamed with Chun-Chia Chen, Min Zhang, Ted Standiford and John Y. Kao for data analysis and interpretation. Technical and material support were provided by Min Zhang, Ted Standiford, John Y. Kao and Michael Newstead. John Y. Kao played a pivotal role as the principal investigator and revised the manuscript for intellectual content (for which he teamed with Ted Standiford), assisted in statistical analysis, and obtained funding. For study concept, design and supervision, he partnered with Tyler Cole.
Contributor Information
Tyler S. Cole, Email: tscole@umich.edu.
Min Zhang, Email: minzhang@umich.edu.
Theodore J. Standiford, Email: tstandif@umich.edu.
Michael Newstead, Email: newstead@umich.edu.
Jay Luther, Email: jayluthe@med.umich.edu.
Jiajie Zhang, Email: jiajiez@umich.edu.
Chun-Chia Chen, Email: chunchia@med.umich.edu.
John Y. Kao, Email: jykao@med.umich.edu.
References
- 1.Cherayil BJ. How not to get bugged by bugs: mechanisms of cellular tolerance to microorganisms. Curr Opin Gastroenterol. 2003;19:572–7. doi: 10.1097/00001574-200311000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Palucka K, Banchereau J. Dendritic cells: a link between innate and adaptive immunity. J Clin Immunol. 1999;19:12–25. doi: 10.1023/a:1020558317162. [DOI] [PubMed] [Google Scholar]
- 3.Liu ZJ, Yan LN, Li XH, Xu FL, Chen XF, You HB, et al. Up-regulation of IRAK-M is essential for endotoxin tolerance induced by a low dose of lipopolysaccharide in Kupffer cells. J Surg Res. 2008;150:34–9. doi: 10.1016/j.jss.2007.12.759. [DOI] [PubMed] [Google Scholar]
- 4.del Fresno C, Garcia-Rio F, Gomez-Pina V, Soares-Schanoski A, Fernandez-Ruiz I, Jurado T, et al. Potent phagocytic activity with impaired antigen presentation identifying lipopolysaccharide-tolerant human monocytes: demonstration in isolated monocytes from cystic fibrosis patients. J Immunol. 2009;182:6494–507. doi: 10.4049/jimmunol.0803350. [DOI] [PubMed] [Google Scholar]
- 5.Deng JC, Cheng G, Newstead MW, Zeng X, Kobayashi K, Flavell RA, et al. Sepsis-induced suppression of lung innate immunity is mediated by IRAK-M. J Clin Invest. 2006;116:2532–42. doi: 10.1172/JCI28054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475–87. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Escoll P, del Fresno C, Garcia L, Valles G, Lendinez MJ, Arnalich F, et al. Rapid up-regulation of IRAK-M expression following a second endotoxin challenge in human monocytes and in monocytes isolated from septic patients. Biochem Biophys Res Commun. 2003;311:465–72. doi: 10.1016/j.bbrc.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 8.Lemaire LC, van der Poll T, van Lanschot JJ, Endert E, Buurman WA, van Deventer SJ, et al. Minimally invasive surgery induces endotoxin-tolerance in the absence of detectable endotoxemia. J Clin Immunol. 1998;18:414–20. doi: 10.1023/a:1023282706945. [DOI] [PubMed] [Google Scholar]
- 9.Foster SL, Medzhitov R. Gene-specific control of the TLR-induced inflammatory response. Clin Immunol. 2009;130:7–15. doi: 10.1016/j.clim.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–45. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- 11.Chamorro S, Garcia-Vallejo JJ, Unger WW, Fernandes RJ, Bruijns SC, Laban S, et al. TLR triggering on tolerogenic dendritic cells results in TLR2 up-regulation and a reduced proinflammatory immune program. J Immunol. 2009;183:2984–94. doi: 10.4049/jimmunol.0801155. [DOI] [PubMed] [Google Scholar]
- 12.Andreasen AS, Krabbe KS, Krogh-Madsen R, Taudorf S, Pedersen BK, Moller K. Human endotoxemia as a model of systemic inflammation. Curr Med Chem. 2008;15:1697–705. doi: 10.2174/092986708784872393. [DOI] [PubMed] [Google Scholar]
- 13.Munoz C, Misset B, Fitting C, Bleriot JP, Carlet J, Cavaillon JM. Dissociation between plasma and monocyte-associated cytokines during sepsis. Eur J Immunol. 1991;21:2177–84. doi: 10.1002/eji.1830210928. [DOI] [PubMed] [Google Scholar]
- 14.Munoz C, Carlet J, Fitting C, Misset B, Bleriot JP, Cavaillon JM. Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Invest. 1991;88:1747–54. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolk K, Docke WD, von Baehr V, Volk HD, Sabat R. Impaired antigen presentation by human monocytes during endotoxin tolerance. Blood. 2000;96:218–23. [PubMed] [Google Scholar]
- 16.Draisma A, Pickkers P, Bouw MP, van der Hoeven JG. Development of endotoxin tolerance in humans in vivo. Crit Care Med. 2009;37:1261–7. doi: 10.1097/CCM.0b013e31819c3c67. [DOI] [PubMed] [Google Scholar]
- 17.Cavaillon JM, Adrie C, Fitting C, Adib-Conquy M. Endotoxin tolerance: is there a clinical relevance. J Endotoxin Res. 2003;9:101–7. doi: 10.1179/096805103125001487. [DOI] [PubMed] [Google Scholar]
- 18.Cavaillon JM. The nonspecific nature of endotoxin tolerance. Trends Microbiol. 1995;3:320–4. doi: 10.1016/s0966-842x(00)88963-5. [DOI] [PubMed] [Google Scholar]
- 19.Chan C, Li L, McCall CE, Yoza BK. Endotoxin tolerance disrupts chromatin remodeling and NF-kappaB transactivation at the IL-1beta promoter. J Immunol. 2005;175:461–8. doi: 10.4049/jimmunol.175.1.461. [DOI] [PubMed] [Google Scholar]
- 20.Foster SL, Hargreaves DC, Medzhitov R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature. 2007;447:972–8. doi: 10.1038/nature05836. [DOI] [PubMed] [Google Scholar]
- 21.Rayhane N, Fitting C, Lortholary O, Dromer F, Cavaillon JM. Administration of endotoxin associated with lipopolysaccharide tolerance protects mice against fungal infection. Infect Immun. 2000;68:3748–53. doi: 10.1128/iai.68.6.3748-3753.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zacharioudaki V, Androulidaki A, Arranz A, Vrentzos G, Margioris AN, Tsatsanis C. Adiponectin promotes endotoxin tolerance in macrophages by inducing IRAK-M expression. J Immunol. 2009;182:6444–51. doi: 10.4049/jimmunol.0803694. [DOI] [PubMed] [Google Scholar]
- 23.Hassan F, Islam S, Tumurkhuu G, Dagvadorj J, Naiki Y, Komatsu T, et al. Involvement of interleukin-1 receptor-associated kinase (IRAK)-M in toll-like receptor (TLR) 7-mediated tolerance in RAW 264. 7 macrophage-like cells. Cell Immunol. 2009;256:99–103. doi: 10.1016/j.cellimm.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Nolan A, Kobayashi H, Naveed B, Kelly A, Hoshino Y, Hoshino S, et al. Differential role for CD80 and CD86 in the regulation of the innate immune response in murine polymicrobial sepsis. PLoS One. 2009;4:e6600. doi: 10.1371/journal.pone.0006600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolk K, Kunz S, Crompton NE, Volk HD, Sabat R. Multiple mechanisms of reduced major histocompatibility complex class II expression in endotoxin tolerance. J Biol Chem. 2003;278:18030–6. doi: 10.1074/jbc.M207714200. [DOI] [PubMed] [Google Scholar]
- 26.Medvedev AE, Kopydlowski KM, Vogel SN. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and toll-like receptor 2 and 4 gene expression. J Immunol. 2000;164:5564–74. doi: 10.4049/jimmunol.164.11.5564. [DOI] [PubMed] [Google Scholar]
- 27.del Fresno C, Soler-Rangel L, Soares-Schanoski A, Gomez-Pina V, Gonzalez-Leon MC, Gomez-Garcia L, et al. Inflammatory responses associated with acute coronary syndrome up-regulate IRAK-M and induce endotoxin tolerance in circulating monocytes. J Endotoxin Res. 2007;13:39–52. doi: 10.1177/0968051907078623. [DOI] [PubMed] [Google Scholar]
- 28.van’t Veer C, van den Pangaart PS, van Zoelen MA, de Kruif M, Birjmohun RS, Stroes ES, et al. Induction of IRAK-M is associated with lipopolysaccharide tolerance in a human endotoxemia model. J Immunol. 2007;179:7110–20. doi: 10.4049/jimmunol.179.10.7110. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi K, Hernandez LD, Galan JE, Janeway CA, Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell. 2002;110:191–202. doi: 10.1016/s0092-8674(02)00827-9. [DOI] [PubMed] [Google Scholar]
- 30.Liew FY, Xu D, Brint EK, O’Neill LA. Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol. 2005;5:446–58. doi: 10.1038/nri1630. [DOI] [PubMed] [Google Scholar]
- 31.Kao JY, Zhang M, Miller MJ, Mills JC, Wang B, Liu M, et al. Helicobacter pylori immune escape is mediated by dendritic cell-induced Treg Skewing and Th17 suppression in mice. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luther J, Dave M, Higgins PD, Kao JY. Association between Helicobacter pylori infection and inflammatory bowel disease: a meta-analysis and systematic review of the literature. Inflamm Bowel Dis. 2009 doi: 10.1002/ibd.21116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rad R, Ballhorn W, Voland P, Eisenacher K, Mages J, Rad L, et al. Extracellular and intracellular pattern recognition receptors cooperate in the recognition of Helicobacter pylori. Gastroenterology. 2009;136:2247–57. doi: 10.1053/j.gastro.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 34.Yokota S, Ohnishi T, Muroi M, Tanamoto K, Fujii N, Amano K. Highly-purified Helicobacter pylori LPS preparations induce weak inflammatory reactions and utilize Toll-like receptor 2 complex but not Toll-like receptor 4 complex. FEMS Immunol Med Microbiol. 2007;51:140–8. doi: 10.1111/j.1574-695X.2007.00288.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhang M, Berndt BE, Eaton KA, Rathinavelu S, Pierzchala A, Kao JY. Helicobacter pylori-pulsed dendritic cells induce H. pylori -specific immunity in mice. Helicobacter. 2008;13:200–8. doi: 10.1111/j.1523-5378.2008.00606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geisel J, Kahl F, Muller M, Wagner H, Kirschning CJ, Autenrieth IB, et al. IL-6 and maturation govern TLR2 and TLR4 induced TLR agonist tolerance and cross-tolerance in dendritic cells. J Immunol. 2007;179:5811–8. doi: 10.4049/jimmunol.179.9.5811. [DOI] [PubMed] [Google Scholar]
- 37.Karp CL, Wysocka M, Ma X, Marovich M, Factor RE, Nutman T, et al. Potent suppression of IL-12 production from monocytes and dendritic cells during endotoxin tolerance. Eur J Immunol. 1998;28:3128–36. doi: 10.1002/(SICI)1521-4141(199810)28:10<3128::AID-IMMU3128>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 38.Albrecht V, Hofer TP, Foxwell B, Frankenberger M, Ziegler-Heitbrock L. Tolerance induced via TLR2 and TLR4 in human dendritic cells: role of IRAK-1. BMC Immunol. 2008;9:69. doi: 10.1186/1471-2172-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 40.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 41.Yanagawa Y, Onoe K. Enhanced IL-10 production by TLR4- and TLR2-primed dendritic cells upon TLR restimulation. J Immunol. 2007;178:6173–80. doi: 10.4049/jimmunol.178.10.6173. [DOI] [PubMed] [Google Scholar]
- 42.Romagnani S. Regulation of the T cell response. Clin Exp Allergy. 2006;36:1357–66. doi: 10.1111/j.1365-2222.2006.02606.x. [DOI] [PubMed] [Google Scholar]
- 43.Kao JY, Rathinavelu S, Eaton KA, Bai L, Zavros Y, Takami M, et al. Helicobacter pylori-secreted factors inhibit dendritic cell IL-12 secretion: a mechanism of ineffective host defense. Am J Physiol Gastrointest Liver Physiol. 2006;291:G73–81. doi: 10.1152/ajpgi.00139.2005. [DOI] [PubMed] [Google Scholar]
- 44.Gatta L, Vakil N, Leandro G, Di Mario F, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and meta-analysis of randomized controlled trials in adults and children. Am J Gastroenterol. 2009 doi: 10.1038/ajg.2009.555. [DOI] [PubMed] [Google Scholar]
- 45.Souza RC, Lima JH. Helicobacter pylori and gastroesophageal reflux disease: a review of this intriguing relationship. Dis Esophagus. 2009;22:256–63. doi: 10.1111/j.1442-2050.2008.00911.x. [DOI] [PubMed] [Google Scholar]
- 46.Wang C, Yuan Y, Hunt RH. Helicobacter pylori infection and Barrett’s esophagus: a systematic review and meta-analysis. Am J Gastroenterol. 2009;104:492–500. doi: 10.1038/ajg.2008.37. (quiz 491, 501) [DOI] [PubMed] [Google Scholar]
- 47.Lahner E, Annibale B, Delle Fave G. Systematic review: Helicobacter pylori infection and impaired drug absorption. Aliment Pharmacol Ther. 2009;29:379–86. doi: 10.1111/j.1365-2036.2008.03906.x. [DOI] [PubMed] [Google Scholar]
- 48.Zhang YY, Xia HH, Zhuang ZH, Zhong J. Review article: ‘true’ re-infection of Helicobacter pylori after successful eradication – worldwide annual rates, risk factors and clinical implications. Aliment Pharmacol Ther. 2009;29:145–60. doi: 10.1111/j.1365-2036.2008.03873.x. [DOI] [PubMed] [Google Scholar]
- 49.Fransen JH, Hilbrands LB, Ruben J, Stoffels M, Adema GJ, van der Vlag J, et al. Mouse dendritic cells matured by ingestion of apoptotic blebs induce T cells to produce interleukin-17. Arthritis Rheum. 2009;60:2304–13. doi: 10.1002/art.24719. [DOI] [PubMed] [Google Scholar]
- 50.Biswas SK, Tergaonkar V. Myeloid differentiation factor 88-independent Toll-like receptor pathway: sustaining inflammation or promoting tolerance. Int J Biochem Cell Biol. 2007;39:1582–92. doi: 10.1016/j.biocel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 51.Fitting C, Dhawan S, Cavaillon JM. Compartmentalization of tolerance to endotoxin. J Infect Dis. 2004;189:1295–303. doi: 10.1086/382657. [DOI] [PubMed] [Google Scholar]
- 52.Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat Med. 2009;15:496–7. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fan H, Cook JA. Molecular mechanisms of endotoxin tolerance. J Endotoxin Res. 2004;10:71–84. doi: 10.1179/096805104225003997. [DOI] [PubMed] [Google Scholar]
- 54.Wysocka M, Robertson S, Riemann H, Caamano J, Hunter C, Mackiewicz A, et al. IL-12 suppression during experimental endotoxin tolerance: dendritic cell loss and macrophage hyporesponsiveness. J Immunol. 2001;166:7504–13. doi: 10.4049/jimmunol.166.12.7504. [DOI] [PubMed] [Google Scholar]
- 55.Mages J, Dietrich H, Lang R. A genome-wide analysis of LPS tolerance in macrophages. Immunobiology. 2007;212:723–37. doi: 10.1016/j.imbio.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 56.Kwan WH, Boix C, Gougelet N, Fridman WH, Mueller CG. LPS induces rapid IL-10 release by M-CSF-conditioned tolerogenic dendritic cell precursors. J Leukoc Biol. 2007;82:133–41. doi: 10.1189/jlb.0406267. [DOI] [PubMed] [Google Scholar]
- 57.Kobayashi KS, Flavell RA. Shielding the double-edged sword: negative regulation of the innate immune system. J Leukoc Biol. 2004;75:428–33. doi: 10.1189/jlb.0703321. [DOI] [PubMed] [Google Scholar]
- 58.De Nardo D, Nguyen T, Hamilton JA, Scholz GM. Down-regulation of IRAK-4 is a component of LPS- and CpG DNA-induced tolerance in macrophages. Cell Signal. 2009;21:246–52. doi: 10.1016/j.cellsig.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 59.Chang J, Kunkel SL, Chang CH. Negative regulation of MyD88-dependent signaling by IL-10 in dendritic cells. Proc Natl Acad Sci U S A. 2009;106:18327–32. doi: 10.1073/pnas.0905815106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 61.Mandrekar P, Bala S, Catalano D, Kodys K, Szabo G. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. J Immunol. 2009;183:1320–7. doi: 10.4049/jimmunol.0803206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wen AY, Sakamoto KM, Miller LS. The role of the transcription factor CREB in immune function. J Immunol. 2010;185:6413–9. doi: 10.4049/jimmunol.1001829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.