Abstract
Dehydroepiandrosterone (DHEA), 7-keto DHEA, and several comparison drugs (ethanol, chlordiazepoxide, rauwolscine, and RO15-4513) were administered to male rats responding under a multiple schedule of food and ethanol presentation to determine their selectively for decreasing ethanol-maintained responding. DHEA and 7-keto DHEA significantly decreased both ethanol- and food-maintained responding, compared to control, while also decreasing blood ethanol concentration (BEC). Acute ethanol administration also decreased responding for both food and ethanol; however, ethanol-maintained responding was more potently decreased than food-maintained responding. BEC remained relatively stable after increasing ethanol doses. Among the other drugs tested, RO15-4513 was the most selective for decreasing ethanol-maintained responding compared to food-maintained responding, and it decreased BECs as ethanol-maintained responding decreased. The largest dose of rauwolscine significantly decreased responding for food, while not affecting ethanol-maintained responding compared to control. Low to intermediate doses of rauwolscine produced small, non-significant increases in ethanol-maintained responding and BECs. Chlordiazepoxide produced significant decreases in food-maintained responding and the dose of ethanol presented, but only at the highest dose tested. Although DHEA and 7-keto DHEA did not decrease ethanol-maintained responding as selectively as ethanol or RO15-4513 under the multiple schedule, these neurosteroids may be valuable pharmacological tools in the development of new treatments for alcohol abuse and dependence.
Keywords: ethanol, dehydroepiandrosterone, rauwolscine, RO15-4513, operant responding, rat
Introduction
The suggestion that the neuroactive steroids could potentially serve as new pharmacotherapies for alcohol abuse closely followed the discovery of their capacity for mediating some of the effects of ethanol (Morrow et al., 1999; O’Dell et al., 2005). This notion is also consistent with data from this laboratory showing that the neurosteroid dehydroepiandrosterone (DHEA) can dose-dependently decrease voluntary ethanol intake in subjects with a long history of ethanol consumption (Gurkovskaya et al., 2009). Though the mechanism and selectivity of this effect are still unknown, ligands with the capacity for negatively modulating the GABAA receptor such as DHEA can reduce ethanol intake either directly by altering chloride flux through the GABAA receptor channel (June et al., 2001; June et al., 2003) or indirectly by altering the composition (i.e., subunits) of the GABAA receptor (Yu et al., 1996). This modification of GABAA receptor function could, in turn, alter the discriminative-stimulus or reinforcing effects of ethanol; however, neither DHEA nor its sulfated conjugate DHEA-S have been shown to markedly alter the discriminative stimulus effects of ethanol (Bienkowski and Kostowski, 1997; Gurkovskaya and Winsauer, 2009). Given these findings there still remains the possibility that DHEA may diminish the reinforcing effects of ethanol, or that it works by some other behavioral or pharmacological mechanism. For example, DHEA can decrease total caloric intake in both mice and leptin receptor-deficient Zucker rats (Porter and Svec, 1995; Svec and Porter, 1997), and this effect on appetitive behavior may decrease the effectiveness of ethanol as a reinforcer. The purpose of this study was to determine whether DHEA-induced decreases in ethanol intake are selective for ethanol or whether DHEA uniformly affects responding for all appetitive reinforcers (e.g., food). To eliminate the possibility that the interaction of DHEA with ethanol was the result of its metabolism to the sex hormones (i.e., testosterone and estradiol), 7-keto DHEA, which it is not metabolized to these hormones, was also tested (Davidson et al., 2000).
To establish food- and ethanol-maintained behavior, subjects were trained to respond under a multiple schedule. The use of schedule-controlled operant responding allowed for the precise measurement of food and ethanol consumption by only providing subjects access to discrete quantities of food and ethanol contingent upon specific response requirements. This has an advantage over many home-cage or voluntary drinking experiments in which measurements of ethanol and food intake can be corrupted by the loss of ethanol (e.g., due to spillage or by inversion of a bottle) or the loss of food (e.g., rodent chow) in the subject’s bedding. In addition, the effects of drugs on schedule-controlled behavior can be quantified easily using dependent measures such as response rate. Lastly, schedule-controlled responding limits the day-to-day variability of consummatory behavior when compared to subjects with unlimited access to food and water (Winsauer and Riley, 1988).
For comparison purposes, several other drugs that have been shown to affect either food or ethanol intake were also administered. Chlordiazepoxide, for example, is a positive allosteric modulator of the GABAA receptor complex that facilitates GABA binding by binding to the benzodiazepine receptor site (Serfozo and Cash 1992), and drugs with this mechanism of action have been shown to increase in food intake (Cooper and Moores, 1985; Sanger, 1984; Winsauer et al., 1994). Rauwolscine is an alpha type-2 receptor antagonist and drugs with this mechanism of action have been shown to decrease food intake (Callahan et al., 1984), but increase operant responding for ethanol reinforcement (Le et al., 2005; Marinelli et al., 2007). Finally, the negative GABAA receptor modulator RO15-4513 was administered because of its previously demonstrated capacity for decreasing ethanol intake in a variety of experimental procedures (Balakleevsky et al., 1990; McBride et al., 1988; Samson et al., 1987).
Methods
Subjects
Twenty-four male Long-Evans hooded rats (Harlan Laboratories, Indianapolis, IN) served as subjects. All subjects were 46-50 days of age and housed singly in polypropylene cages with hardwood chip bedding in a colony room maintained at 21±2°C with 50±10% relative humidity on a 14-h light/10-h dark cycle (lights on at 0600 h CST). Standard rodent chow (Rodent Diet 5001, PMI Inc., Brentwood, MO) was freely provided until postnatal day (PND) 79. Subjects earned 45-mg food pellets (TestDiets, a division of LabDiet, Richmond, IN) during the experimental sessions and, when necessary, were provided chow in the home cage after the test sessions in order to maintain them at 90% of their free-feeding weight. This was determined by averaging each subject’s weight over 10 days from PND 70 - 79. Subjects ranged in weight from 340 - 410 g. Water was freely available in their home cage. This study was carried out in accordance with the Institutional Animal Care and Use Committee of the Louisiana State University Health Sciences Center and the guidelines of the Committee on Care and Use of Laboratory Animal Resources, as adopted and promulgated by the U.S. National Institutes of Health.
Apparatus
Nine identical modular test chambers (Coulbourn Instruments, Allentown, PA, Model E10-10TC) configured specifically for rodents were used. Located on the front wall of each chamber were a houselight, speaker, a response lever (6.5 cm above the floor and centered), three colored stimuli above the lever, a pellet trough (2 cm above the floor and 5 cm to the left of the response lever), a concave liquid reservoir (2 cm above the floor and 5 cm to the right of the response lever) and a recessed drinking trough (centered 4 cm above the concave spout and 5 cm to the right of the response lever). The three stimuli above the lever were aligned horizontally and could be illuminated by three Sylvania 28ESB indicator lamps, one with a red plastic cap, one with a yellow cap, and one with a green cap. The response lever required a minimum force of 0.25 N for activation and responses produced an audible click of a feedback relay that was mounted behind the response panel. A liquid solenoid (Parker Hannifin Corp., New Britain, CT), also mounted behind each panel, dispensed liquid from a 50-ml centrifuge tube to the liquid reservoir in the chamber when activated. A separate feeder dispensed pellets to the pellet trough. Each chamber was enclosed within a sound-attenuating cubicle equipped with a fan for ventilation and white noise from the speaker was used to mask extraneous sounds. All test chambers were connected to a computer programmed in MED-PC for Windows, Version IV (MED Associates, Inc., St. Albans, VT).
Behavioral procedure
Subjects were trained to consume ethanol orally using a modified saccharin-fading procedure (Leonard et al., 2006; Samson, 1986). Prior to each daily training session, subjects were weighed and then placed in an illuminated operant chamber where each subject had access to a 50-ml plastic centrifuge tube, which contained a saccharin/ethanol solution. Each tube was fitted with a rubber stopper and metal sipper tube that was freely accessible to the subject from within the drinking trough. Rats were allowed 30 min of free access to the saccharin/ethanol solution. At the end of the 30-min session, the drinking tubes were removed and the subjects were weighed and then returned to their home cage. The volume of the solution consumed was determined by weighing the tubes before and after the session. The dose of ethanol was calculated as grams/kilogram (g/kg) body weight based on the volume of solution consumed and the mean body weight for each subject. All solutions were prepared fresh daily.
Rats initially received a 0.125% (w/v) saccharin sodium (Fischer Chemicals, Fair Lawn, NJ) solution that was gradually replaced with 32% ethanol (v/v) over subsequent training sessions (i.e., as the saccharin concentration was reduced from 0.125% to 0%, the ethanol concentration was increased from 1% to 32%). The solutions were presented in the following order: 0.125% saccharin/1% ethanol, 0.1% saccharin/2% ethanol, 0.05% saccharin/5% ethanol, 0.01% saccharin/8% ethanol, 0% saccharin/10% ethanol, 0% saccharin/18% ethanol, and 0% saccharin/32% ethanol. Each saccharin/ethanol solution was presented daily until either a stable level of intake was observed (± 20% of the mean for 3 consecutive days) or a maximum of 8 days had elapsed.
To move the subjects from voluntary consumption of ethanol to operant responding for ethanol, the green stimulus light above the lever was illuminated and every response on the lever dispensed 0.1 ml of 32% v/v ethanol to the concave liquid reservoir situated to the right of the lever and below the drinking trough. After rats reliably pressed the lever under this continuous reinforcement schedule (CRF), the number of responses necessary for reinforcement was increased until subjects were responding under a fixed-ratio (FR) 10 schedule (i.e., every ten lever presses dispensed 0.1 ml of 32% v/v ethanol). After five days of stable responding under this schedule (i.e., the volume of ethanol consumed did not vary by more than 30% from day to day), another component was added to create the multiple schedule. In this component, a red stimulus light above the lever was illuminated and rats responded under a continuous reinforcement (CRF) schedule in which a single lever press illuminated the pellet trough briefly (0.4 s) and dispensed a 45-mg food pellet. As training progressed under this multiple schedule, the number of responses required for food reinforcement was increased until rats were responding under a FR-20 schedule. Strict alternation of the two components occurred after 20 reinforcers or a specified period of time (i.e., five minutes for food components and 10 minutes for ethanol components), whichever occurred first. However, component alternations were restricted to the specified component duration during and after the testing of DHEA and 7-keto DHEA. The purpose of this manipulation was to limit the development of adventitiously or superstitiously reinforced responding (for an in-depth discussion of adventitious reinforcement, see Sidman, 1960). Thus, if 20 reinforcements occurred prior to the specified component duration, the discriminative stimuli over the lever were extinguished and responding had no programmed consequence until the component timed out. Each component was also followed by a one-minute change-over delay in which the stimuli above the lever were extinguished and responding on the lever had no programmed consequence; the house light remained illuminated. Sessions began with a different component each day and ended after 60 minutes. Given the aforementioned component durations, this meant that the subjects could obtain a maximum of 80 food reinforcers and 80 ethanol reinforcers per day. The administration of various drugs began after responding for food did not vary by more than 20% for 10 consecutive days.
Blood collection and blood ethanol concentration (BEC) determinations
To assess BEC, blood samples were collected by saphenous venepuncture immediately after the 60-minute behavioral session. Samples were collected after both vehicle and drug administration. Serum was isolated and stored at −80 °C until blood ethanol concentrations (mg/dl) were quantified in duplicate using the MicroStat GM7 Analyzer (Analox Instruments, Inc., Lunenburg, MA). The intra-assay coefficient of variation was 2.5%.
Drugs and drug administration
The acute effects of ethanol (0.32 – 1.8 g/kg), dehydroepiandrosterone (DHEA, 10 – 180 mg/kg), 7-keto dehydroepiandrosterone (7-keto DHEA, 10 – 56 mg/kg), chlordiazepoxide (1.8 – 32 mg/kg), rauwolscine (1.8 – 18 mg/kg) and RO15-4513 (1.8 – 10 mg/kg) were determined after responding under the multiple schedule had stabilized. In general, drugs were administered i.p. 15 min prior to the session and the injection volume was generally 0.1 ml/100 grams, with the exception of ethanol, which was injected as a 15% volume/volume (v/v) solution and required different volumes for each dose. Due to the limited solubility of DHEA and 7-keto DHEA, the injection volume also had to be increased for doses larger than 32 mg/kg. DHEA (Sigma-Aldrich Corp., St. Louis, MO) was dissolved in 45% 2-hydroxypropyl γ-cyclodextrin (Sigma-Aldrich Corp., St. Louis, MO) in saline. 7-keto DHEA (kindly provided by Dr. Henry Lardy, Department of Biochemistry and Institute for Enzyme Research, University of Wisconsin, Madison, WI) was dissolved in a vehicle consisting of 80% (v/v) propylene glycol, 8% polyethylene glycol, and 2% benzyl alcohol. Rauwolscine hydrochloride and chlordiazepoxide hydrochloride were both purchased from Sigma-Aldrich Corporation (St. Louis, MO) and dissolved in 0.9% sterile saline. RO15-4513 (ethyl 8-azido-6-dihydro-5-methyl-6-oxo-4H-imidazo[1,5-a]-[1,4]benzodiazepine-3-carboxylate, RBI, Natick, MA) was dissolved in 50% (v/v) propylene glycol, 11% polyethylene glycol, 2 % benzyl alcohol and 37% sterile water. Drug injections were generally administered on Tuesdays and Fridays, with vehicle or saline (control) injections administered on Thursdays. Baseline sessions (no injections) occurred on Mondays and Wednesdays. The highest dosages of all drugs were only administered once per week to avoid the development of acute tolerance and to limit any “carry over” effects. Doses of each drug were administered in a mixed order and at least 10 days of testing without drug occurred between drugs.
Statistical Analyses
A two-way analysis of variance (ANOVA) using SigmaStat (SYSTAT Software, Inc. Point Richmond,CA,USA) determined the effects of each drug on response rate for food- and ethanol-maintained responding. One-way ANOVA tests were used to isolate significant interactions and to analyze BECs and the dose of ethanol presented. Holm-Sidak post-hoc tests were used to compare drug sessions with control (saline or vehicle) sessions. The same post-hoc tests were used to conduct all pairwise comparisons when differences from control were not significant, but there was a main effect. Significance was accepted at α level ≤ 0.05 for all statistical tests.
In addition to the two-way ANOVA tests that were conducted, decreases in response rate for both food and ethanol were characterized by calculating the ED50 values for each drug. In the case of response rate, the ED50 value represented the estimated dose of drug that decreased response rates for each reinforcer to 50% of control. These values were determined by a linear regression analysis using two or more data points reflecting the downward slope of each curve. To visualize more easily the downward slopes of each curve, the raw data for each subject were first transformed to a percent of control. Finally, the curves for response rate were considered to be significantly different if the ED50 values for food-maintained responding fell outside of the 95% confidence intervals obtained for ethanol-maintained responding.
Results
Subjects were trained for an average of 35 days under the sucrose-fading procedure to stabilize intake for 32% v/v ethanol. The average dose presented to subjects when 32% ethanol was available was 1.1 g/kg (1.5 ml). Responding for ethanol under the operant procedure stabilized after an average of 23 days, while only 13 days were required to stabilize responding under the multiple schedule of ethanol and food presentation. Mean ethanol presentation increased from 1.1 g/kg (1.5 ml) to 2.5 g/kg (3.5 ml) after the component of food-maintained responding was added. Stable responding under the multiple schedule was also indicated by the pattern of responding under the FR schedule, which was characterized by a distinct “break and run” pattern of responding in which high, constant rates of responding were followed by brief periods with no responding prior to the initiation of each ratio.
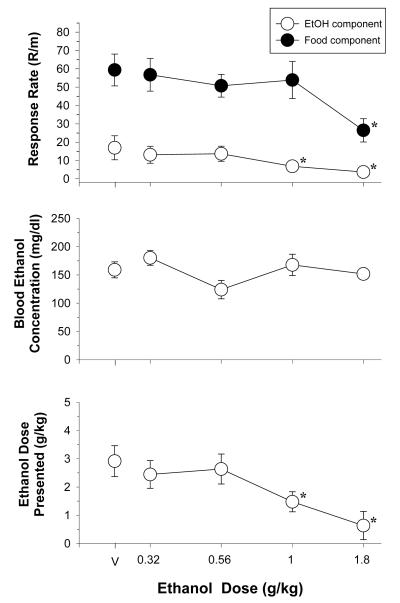
The effects of increasing doses of i.p.-administered ethanol on the response rate (response/min) for both food and ethanol presentation are plotted in the top panel of Figure 1. In general, the mean rate of responding in the ethanol component during these ethanol determinations was 16.94 ± 6.56 responses/minute, whereas the mean rate of responding in the food component was 59.39 ± 8.48 responses/minute. This difference in baseline rates of responding in each component also explains why the two-way ANOVA tests routinely found main effects for the type of reinforcer on response rates. For instance, a two-way ANOVA indicated there was a significant main effect of reinforcer [F(1,28)=31.450, p<0.001] and dose [F(4,28)= 10.96, p<0.001], as well as a significant reinforcer x dose interaction [F(4,28)=3.91, p<0.02] on response rate after ethanol administration. The reinforcer x dose interaction was also confirmed by one-way ANOVA tests, which confirmed the effects of dose [ethanol: F(4,28)=7.63, p<0.001, food: F(4,28)=7.85, p<0.001] and indicated that responding for ethanol was affected more potently than responding for food (i.e., 1 and 1.8 g/kg significantly reduced responding for ethanol when compared to control, whereas only 1.8 g/kg decreased responding for food). This difference in potency was also evident from the ED50 values, which indicated that the mean ED50 value for decreasing food-maintained responding was significantly larger than the ED50 value for ethanol-maintained responding (Table 1). In fact, for many of the subjects, the ED50 value for food-maintained responding could not be calculated because responding was not decreased by 50 percent.
Fig. 1.
Effects of peripheral ethanol administration in 8 rats responding under a multiple schedule of food (FR 20) and ethanol (FR 10) presentation. Unfilled circles represent response rate during the ethanol component, whereas filled circles represent response rate during the food component. The reinforcer in the ethanol component was 0.1 ml of 32% (v/v) ethanol, whereas the reinforcer in the food component was 45-mg food pellets. The dependent measures were response rate (response/min), blood ethanol concentration (mg/dl), and the ethanol dose presented (g/kg). The points and vertical lines above “V” indicate the mean and standard error of the mean (SEM) for sessions in which vehicle was administered (control). The mean rate of responding for food was 59.39 ± 8.48 responses/min, whereas the mean rate of responding for ethanol was 16.94 ± 6.56 responses/min. The points with vertical lines in the dose-effect data indicate the mean and SEM for sessions in which ethanol was administered. The points without vertical lines indicate instances in which the SEM is encompassed by the point. Asterisks indicate significant differences from control.
Table 1.
Individual and mean ED50 values in g/kg (ethanol) or mg/kg (DHEA, 7-keto DHEA, chlordiazepoxide, RO15-4513, and rauwolscine) for decreases in response rate in ethanol - and food - maintained responding.
| Ethanol | Food | Ethanol | Food | Ethanol | Food | |
|---|---|---|---|---|---|---|
| 899 | 0.67 | 1.71 | 27.61 | 50.70 | 35.73 | 35.81 |
| 1107 | 0.56 | --- | 41.30 | 37.33 | ||
| 1108 | 0.58 | 0.76 | 128.80 | 150.00 | 36.06 | 38.02 |
| 1109 | 0.71 | --- | --- | --- | 51.40 | --- |
| 1110 | 41.21 | 61.80 | 41.21 | 40.46 | ||
| 1111 | 0.81 | --- | 126.80 | 175.40 | --- | --- |
| 1112 | 0.92 | 1.42 | 65.16 | 81.28 | 43.65 | 43.15 |
| 1113 | 0.98 | --- | 119.90 | 131.80 | 36.81 | 37.33 |
| 1119 | 1.06 | --- | ||||
| 1212 | 30.13 | --- | ||||
| Mean | 0.79 | 1.30* | 84.92 | 108.50 | 39.54 | 38.68 |
| 95% CI | 0.63-0.94 | 36.85-132.98 | 34.20-44.88 |
| Subject | Chlordiazepoxide (mg/kg) | RO15-4513 (mg/kg) | ||
|---|---|---|---|---|
| Ethanol | Food | Ethanol | Food | |
| 1213 | 15.96 | 16.03 | 4.40 | 5.40 |
| 1214 | 23.33 | 16.22 | --- | --- |
| 1215 | 26.67 | 24.38 | 2.99 | 3.64 |
| 1216 | 16.98 | 16.41 | --- | 6.37 |
| 1217 | 25.47 | 24.89 | 4.26 | 6.64 |
| 1218 | 11.69 | 11.64 | 8.19 | --- |
| 1219 | --- | 31.12 | 5.19 | --- |
| 1220 | 20.80 | 24.21 | --- | --- |
| 1221 | 9.40 | 9.31 | --- | 3.78 |
| Mean | 18.79 | 19.36 | 5.01 | 5.17 |
| 95% CI | 13.49-24.09 | 2.59-7.43 | ||
| Subject | Rauwolscine (mg/kg) | |
|---|---|---|
| Ethanol | Food | |
| 1107 | --- | --- |
| 1108 | 12.19 | 6.04 |
| 1109 | 8.81 | 8.51 |
| 1110 | 15.49 | 11.94 |
| 1111 | --- | --- |
| 1112 | 16.63 | --- |
| 1212 | 15.45 | 13.21 |
| Mean | 13.71 | 9.93 |
| 95% CI | 9.73-17.69 | |
indicates a mean value for food-maintained responding that is outside of the confidence interval (C.I.) for ethanol-maintained responding
indicates instances in which the ED50 value could not be calculated due to the absence of substantial rate-decreasing effects, or the decreases in response rate were greater than 50% even at the lowest dose tested (as was true for RO15-4513).
For BEC, a one-way ANOVA indicated that there was a significant main effect of i.p. - administered ethanol [F(4,25)=3.75, p<0.02], but none of the doses of ethanol significantly differed from control (see Figure 1). Lastly, i.p. ethanol administration dose-dependently decreased the dose of ethanol presented as there was a significant main effect of dose [F(4,28)=19.48, p<0.001], and post-hoc tests indicated that both 1 and 1.8 g/kg significantly reduced the dose of ethanol presented.
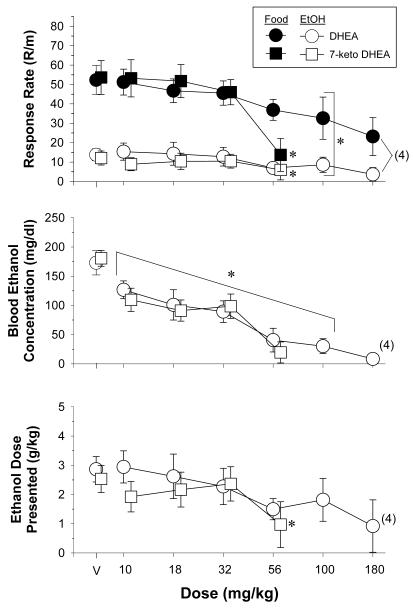
Figure 2 shows the effect of increasing doses of DHEA (n=7) and 7-keto DHEA (n=8) on response rates, BEC, and the dose of ethanol presented. As indicated by a two-way ANOVA, both the reinforcer [DHEA: F(1,28)= 54.38, p<0.001; 7-keto DHEA: F(1,28)= 21.48, p<0.002] and the doses of each drug [DHEA: F(5,28)=4.94, p<0.002; 7-keto DHEA: F(4,28)=10.07, p<0.001] had significant main effects on response rate. However, the reinforcer x dose interaction was only significant after 7-keto DHEA [7-keto DHEA: F(4,28)=7.98, p<0.001; DHEA F(5,28)=1.47, NS]. Essentially, this means that DHEA decreased the dose-effect curves for both ethanol and food in a parallel manner, whereas 7-keto DHEA had effects on response rate that depended on both the dose and the reinforcer. This was confirmed further by separate one-way ANOVA tests, which indicated that 56 mg/kg of 7-keto DHEA eliminated the significant difference between ethanol- and food-maintained responding by substantially decreasing food-maintained responding. The difference in the effects of DHEA and 7-keto DHEA on response rate were also evident in the individual ED50 values (see Table 1). For DHEA, the ED50 value for every subject was lower for ethanol-maintained responding than food-maintained responding; however, the mean for ED50 for food-maintained responding did not fall outside of the confidence interval established for ethanol-maintained responding. For 7-keto DHEA, on the other hand, both the individual and mean ED50 values were very similar for ethanol- and food-maintained responding because 56 mg/kg essentially eliminated responding for both reinforcers.
Fig. 2.
Effects of acute administration of DHEA (n=7) and 7-keto DHEA (n=9) in rats responding under a multiple schedule of food and ethanol presentation. Unfilled symbols represent response rate during the ethanol component, whereas filled symbols represent response rate during the food component. The dependent measures were response rate (response/min), blood ethanol concentration (mg/dl), and the ethanol dose presented (g/kg). The mean rate of responding for food was 46.42 ± 5.43 responses/min during testing with DHEA and 49.14 ± 8.48 responses/min during testing with 7-keto DHEA, whereas the mean rate of responding for ethanol was 13.95 ± 3.56 responses/min and 12.97 ± 3.97 responses/min, respectively. The numbers in parentheses indicate the number of subjects for that data point when it was less than 7 (DHEA) or 8 (7-keto DHEA) due to the elimination of responding in some subjects at the higher doses. Asterisks indicate significant differences from vehicle administration (control). The bracket in the top panel indicates that the main effect of DHEA dose was analyzed with the data for each reinforcer combined as the interaction was not significant. In the middle panel, all of the data points within the bracket were significantly different from vehicle (control).
DHEA [F(6,33)=15.08, p<0.001] and 7-keto DHEA [F(4,28)=18.11, p<0.001] also produced significant main effects on BEC, with post-hoc tests indicating that doses of 10-100 mg/kg of DHEA and 10-56 mg/kg of 7-keto DHEA significantly decreased BEC from control. The BEC data collected after 180 mg/kg of DHEA were not included in the repeated-measures analysis due to the fact that these data only represented 4 subjects; responding was eliminated for the other 3 subjects at 100 mg/kg. Unlike the effects of DHEA and 7-keto DHEA on BEC, the dose of ethanol presented (shown in the bottom panel) was only decreased significantly after 56 mg/kg of 7-keto DHEA [F(4,28)= 10.23, p<0.001] when compared to control. With regard to DHEA, the absence of an effect on the dose presented after 100 mg/kg was somewhat surprising given the significant decrease in BEC. However, there were two different within-session patterns of responding that may have contributed to the relative absence of mean effects on the ethanol dose presented compared to BEC (data not shown). The first pattern, which was the more typical, reflected the fact that responding for food occurred at higher mean rates than responding for ethanol, and that ethanol-maintained responding consistently waned over successive ethanol components. Furthermore, when DHEA was administered, responding for ethanol was dose-dependently decreased, whereas responding for food was only decreased after 100 mg/kg. These respective within-session patterns of responding contrasted with a second pattern, which was evident in at least 3 subjects. In this within-session pattern, responding for both food and ethanol occurred at high rates under control conditions and high doses of DHEA (56 and 100 mg/kg) produced relatively small decreases in responding for both food and ethanol. In other words, even though responding was decreased by DHEA, responding remained relatively high in both components and many components still alternated after 20 reinforcements rather than the designated time. More importantly, subjects with this pattern of responding were also found to have an excessive amount of liquid in the “drop” pans below the chamber floor following DHEA administration, indicating that the response rate for ethanol did not always accurately reflect the amount of ethanol consumed.
At this point in the study, the contingencies for component alternations were changed in an effort to bring the responding of all of the subjects under better stimulus control and to eliminate any adventitious responding in the ethanol components. More specifically, the contingency that allowed components to alternate after 20 reinforcers was removed and component alternations were limited to the respective component durations (i.e., 5 minutes for food components and 10 minutes for ethanol components). Although these data are not shown, instituting this manipulation revealed that some subjects were responding at very high rates in the ethanol component even in the absence of reinforcement; however, in the 8 weeks following the implementation of this manipulation, these subjects’ response rate in the ethanol component decreased and all of the ethanol presented was consumed. To verify the consumption of the ethanol, a small catch pan was placed underneath the concave liquid reservoir. Given that this restriction on component alternations brought responding in the ethanol component under better control by eliminating adventitious responding, the rest of the drugs (i.e., chlordiazepoxide, rauwolscine and RO15-4513) were tested with this restriction in place.
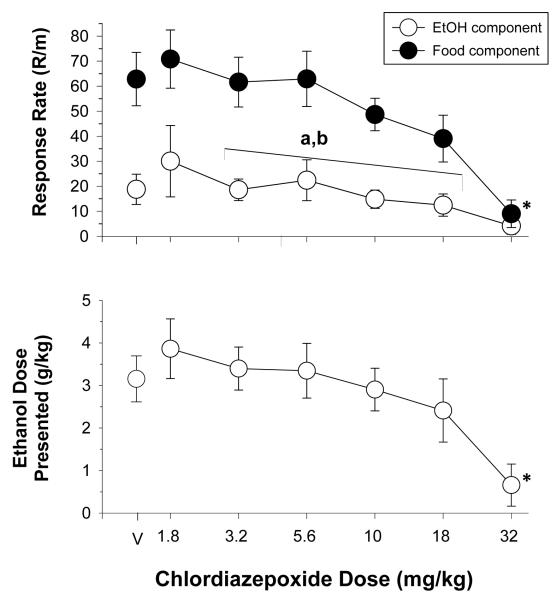
The data plotted in figure 3 show the effects of increasing doses of chlordiazepoxide on response rates, and the dose of ethanol presented for 9 rats. A two-way ANOVA indicated there were significant main effects of reinforcer [F(1,48)=45.78, p<0.001] and dose [F(6,48)= 8.070, p<0.001], as well as a significant reinforcer x dose interaction [F(6,48)=8.31, p<0.001]. The reinforcer x dose interaction was further examined by one-way ANOVA tests, which indicated that chlordiazepoxide significantly affected responding for food and ethanol [food: F(6,48)=11.87, p<0.001, ethanol: F(6,48)=2.5527, p<0.05], but differently in comparison to vehicle administration (control). More specifically, Holm-Sidak post hoc tests found that 32 mg/kg of chlordiazepoxide significantly decreased food-maintained, but not ethanol-maintained responding, when compared to control. However, the response rates for ethanol after 1.8 and 32 mg/kg were significantly different and this difference likely contributed to the significant interaction. In terms of the rate-decreasing effects of chlordiazepoxide, the ED50 values for ethanol- and food-maintained responding were very similar, as indicated by the individual ED50 values in Table 1. As shown in the lower panel of this figure, 32 mg/kg produced a significant decrease from control in the ethanol dose presented [F(6,48)=7.39, p<0.001].
Fig. 3.
Effects of acute administration of chlordiazepoxide in 9 rats responding under a multiple schedule of food and ethanol presentation. Unfilled circles represent response rate during the ethanol component, whereas filled circles represent response rate during the food component. The dependent measures were response rate (response/min) and dose of ethanol presented (g/kg). The mean rate of responding for food was 62.84 ± 10.68 responses/min, whereas the mean rate of responding for ethanol was 18.79 ± 6.05 responses/min. Letters indicate significant differences among doses (e.g., ‘a’ is significantly different from ‘b’, but not significantly different from ‘a,b’). Asterisks indicate significant differences from vehicle (control).
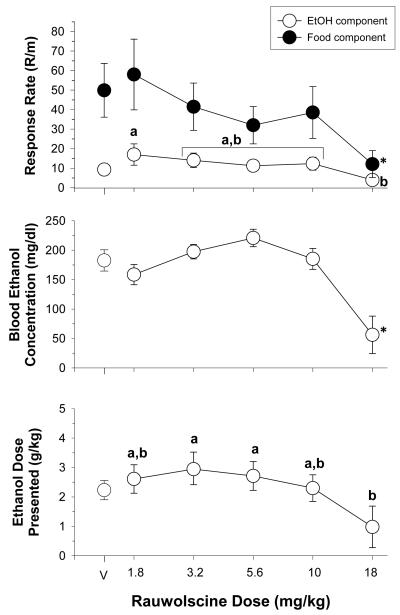
Figure 4 shows the effects of increasing doses of rauwolscine on response rates, blood ethanol concentration, and the dose of ethanol presented (g/kg) for 7 rats. With respect to response rate, a two-way ANOVA indicated there were significant main effecst of reinforcer [F(1,30)=10.30, p<0.02] and dose [F(5,30)= 3.83, p<0.01], as well as a significant reinforcer x dose interaction [F(5,30)=4.17, p<0.005]. In terms of the interaction, separate one-way ANOVA tests indicated that rauwolscine significantly affected responding for food [F(5,30)=4.02, p<0.01] and ethanol [F(5,30)=2.95, p<0.05], with 18 mg/kg producing a significant decrease in food-maintained responding when compared to control and a significant decrease in ethanol-maintained responding when compared to the lowest dose of rauwolscine (i.e., 1.8 m/kg). Unlike the other drugs tested, rauwolscine was the only drug that decreased food-maintained responding more potently than ethanol-maintained responding in every subject (Table 1). Rauwolscine dose also had a significant main effect on BEC [F(5,30)=11.34, p<0.001] and the dose of ethanol presented [F(5,30)=3.22, p<0.02], which post hoc tests indicated was largely due to the significant difference between 18 mg/kg and two of the intermediate doses (3.2 and 5.6 mg/kg).
Fig. 4.
Effects of acute administration of rauwolscine in 7 rats responding under a multiple schedule of food and ethanol presentation. Unfilled circles represent response rate during the ethanol component, whereas filled circles represent response rate during the food component. The dependent measures were response rate (response/min), blood ethanol concentration (mg/dl), and dose of ethanol presented (g/kg). The mean rate of responding for food was 49.90 ± 13.75 responses/min, whereas the mean rate of responding for ethanol was 9.30 ± 1.57 responses/min. Letters indicate significant differences among doses. For further detail, see legend for Fig. 3.
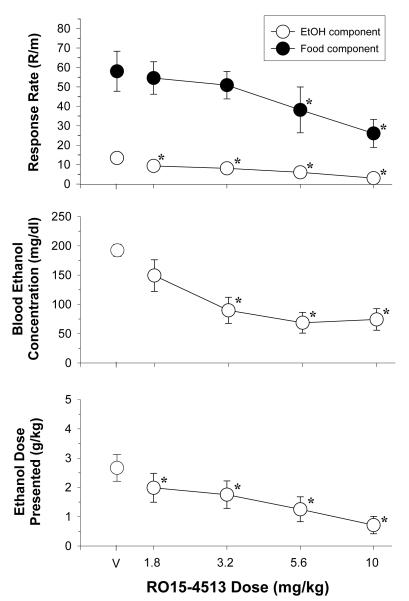
The data plotted in Figure 5 show the effects of increasing doses of RO15-4513 on response rates, blood ethanol concentration, and the dose of ethanol presented for 9 rats. A two-way ANOVA indicated there were significant effects of reinforcer [F(1,32)=37.45, p<0.001] and dose [F(4,32)= 6.65, p<0.001], as well as a significant reinforcer x dose interaction [F(4,28)=2.689, p<0.05]. The reinforcer x dose interaction was also confirmed by one-way ANOVA tests, as RO15-4513 significantly decreased responding for ethanol [F(4,32)=10.36, p<0.001] at all four doses tested, whereas it only decreased responding for food [F(4,32)=4.67, p<0.005] at two of the doses tested (i.e., 5.6 and 10 mg/kg). However, difference in potency was not reflected in the mean ED50 values (see Table 1), which were comparable for both ethanol- and food-maintained responding (i.e., 5.01 and 5.17 mg/kg, respectively). The similarity of the ED50 values may have resulted from the fact that the negative slope for the decrease in ethanol-maintained responding was very shallow and spanned all four doses of RO15-4513, whereas the negative slope for the decrease in food-maintained responding was quite steep and resulted from testing the two highest doses. The sensitivity of ethanol-maintained responding to RO15-4513 administration was also evident in the main effect of dose on BEC [F(4,32)=16.08, p<0.001] and on the ethanol dose presented [F(4,32)=9.43, p<0.001]. For example, when compared to control, 3.2-10 mg/kg significantly decreased BEC, whereas 1.8-10 mg/kg significantly decreased the ethanol dose presented.
Fig. 5.
Effects of acute administration of RO15-4513 in 9 rats responding under a multiple schedule of food and ethanol presentation. Unfilled circles represent response rate during the ethanol component, whereas filled circles represent response rate during the food component. The dependent measures were response rate (response/min), blood ethanol concentration (mg/dl), and dose of ethanol presented (g/kg). The mean rate of responding for food was 58.05 ± 10.32 responses/min, whereas the mean rate of responding for ethanol was 13.39 ± 3.02 responses/min. Asterisks indicate significant differences from control.
Discussion
In contrast to previous studies of DHEA in this laboratory that utilized a limited-access two-bottle choice test (Gurkovskaya et al., 2009; Leonard et al., 2006; Worrel et al., 2011), rats were trained under a multiple FR-20, FR-10 schedule maintained by both food and ethanol presentation to assess the effects of ethanol, DHEA, 7-keto DHEA, chlordiazepoxide, rauwolscine, and RO15-4513 on ethanol intake. Responding for ethanol under this multiple schedule produced BECs similar to, or higher than, many binge studies utilizing operant self-administration, home-cage two-bottle choice procedures, or non-contingent ethanol infusion (Ji et al., 2008; Prasad et al., 2001; Schulteis et al., 2008). Although operant responding for ethanol rarely produces high response rates, providing another reinforcer (e.g., food) has been shown to induce drinking behavior and produce BECs large enough to produce ataxia (Roehrs and Samson, 1981). In addition, the multiple schedule of food- and ethanol-reinforced responding was able to detect selective alterations in the intake of each type of reinforcer by drug administration irrespective of the differences in response rate. These findings would be consistent with a study by Ginsburg et al. (2005), which found that similar rates of responding were not necessary to detect the effects of a drug on responding maintained by different reinforcers. For example, these investigators equilibrated responding for both ethanol and food under a multiple schedule, and found that the selective serotonin reuptake inhibitor (SSRI) fluvoxamine can selectively decrease ethanol-maintained responding. This finding with fluvoxamine confirmed the results from other studies using different procedures and suggested that the selective effects of the SSRIs were not due to different baseline levels of responding. Thus, the selective decreases in ethanol-maintained responding should not be attributed solely to the lower response rates in the ethanol components compared to the food components.
Interestingly, i.p. administration of increasing doses of ethanol more potently decreased ethanol-maintained responding than food-maintained responding, but the decreases in ethanol-maintained responding did not significantly decrease BECs from control levels. This finding suggested the possibility that subjects were titrating their responding (i.e., consumption) to maintain a consistent concentration of ethanol in the blood. These data are reminiscent of other studies on ethanol intake in which acute administration of ethanol decreased ethanol preference, while producing stable BEC values (Leonard et al., 2006; Mormede et al., 2004; Samson et al., 2002). For example, Leonard et al. (2006) found that voluntary ethanol consumption by male rats decreased as the noncontingent doses of ethanol increased; however, the total ethanol dose was relatively stable after each ethanol dose. One argument against the notion that the rats were titrating their BEC is that the 1.8 g/kg dose decreased food-maintained responding even though BEC had not changed substantially; however, this was likely due to the large bolus injection of ethanol and the difference between cumulative and non-cumulative dosing.
Unlike acute ethanol administration, i.p. administration of DHEA and 7-keto DHEA produced significant decreases in BEC in the absence of significant, concomitant decreases in responding for ethanol or the ethanol dose presented. Given the previous research from this laboratory showing that DHEA and 7-keto DHEA can decrease ethanol intake (Gurkovskaya et al., 2009; Worrel et al., 2011), the effects of these drugs on BEC alone were puzzling. Moreover, even though the individual ED50 values indicated that ethanol-maintained responding was decreased more potently than food-maintained responding in every subject, the mean ED50 values were not significantly different because of the substantial variability across subjects. One possible explanation for the relative insensitivity of at least 3 of the 7 subjects to the dose of DHEA and 7-keto DHEA was that responding in the ethanol component was somehow superstitiously or adventitiously “chained” to responding in the food component (Boren, 1969); that is, subjects were responding as if the presentation of the stimuli for the food component were contingent upon responding during the ethanol component. Therefore, in these subjects, DHEA and 7-keto DHEA may only have been effective to the extent that these drugs can decrease food-maintained behavior or responding that is maintained by conditioned stimuli. In any case, restricting component alternations to the specified component duration substantially reduced the amount of unreinforced responding in the ethanol component and brought responding in this component under better control.
Among all of the drugs, RO15-4513 was the most selective drug for reducing ethanol-maintained responding compared to food-maintained responding, and this decrease in response rate resulted in a concomitant reduction in BECs and the ethanol dose presented. RO15-4513 has been hypothesized to reduce ethanol intake by opposing its effects at the GABAA receptor (McBride et al., 1988; Samson et al., 1987; Suzdak et al., 1986, 1988). For example, Suzdak et al. (1988) found that when 10 mg/kg of beta-carboline-3-carboxylate (β-CCE) was administered prior to RO15-4513, it completely antagonized the reduction in ethanol intoxication (e.g., ataxia, sedation, loss of muscle tone and righting reflex) produced by RO15-4513, demonstrating that the interactive effects of RO15-4513 with ethanol were mediated by its effects at the benzodiazepine receptor site on GABAA receptor complex. Interestingly, DHEA, like RO15-4513, has been shown to negatively modulate the GABAA receptor when administered alone (Imamura and Prasad,1998; Sieghart,1995), and this is thought to occur through a neurosteroid binding site(s) on this complex (Majewska et al., 1990; Hosie et al., 2006, 2007). Moreover, the capacity of both drugs to negatively modulate GABAA receptors likely plays a prominent role in attenuating the effects of ethanol, which acts as a positive modulator of GABAA receptors. The mechanism by which 7-keto DHEA interacts with ethanol has yet to be determined, although there is indirect evidence that 7-keto DHEA has similar effects to DHEA in reversing scopolamine-induced memory deficits in young mice (Shi et al., 2000). The proposed mechanism for this effect of DHEA, and presumably 7-keto DHEA, is by a reduction of GABAergic inhibition, which increases acetylcholine release and competes with scopolamine at muscarinic receptors (Brioni, 1993; Nabeshima et al., 1988a). As a more direct means of determining 7-keto DHEA’s mechanism of action, studies utilizing radiolabeled 7-keto DHEA, as well as whole-cell recording of chloride influx (e.g., patch clamp technique), will be necessary. In reference to the behavioral effects of DHEA and RO15-4513, both compounds have been shown to reverse the memory-impairing effects of ethanol (Castellano and Populin, 1990; Melchior and Ritzmann, 1996; Nabeshima et al., 1988b), and these effects have been thus far attributed to the capacity of these compounds to interfere with ethanol’s actions at the GABAA receptor.
The effects of chlordiazepoxide on ethanol intake have been reported to be dependent on a variety of variables, such as the schedule of reinforcement, preference for ethanol, and the availability of alternative reinforcers (Barrett and Weinberg, 1975; Meert,1993; Samson and Grant, 1985). In the present study, only a high dose of chlordiazepoxide (32 mg/kg) significantly decreased food-maintained responding compared to control and ethanol-maintained responding compared to the lowest dose of chlordiazepoxide. Though chlordiazepoxide has previously been shown to produce hyperphagic effects in rats (Cooper and Moores, 1985; Sanger, 1984; Winsauer et al., 1994), food-maintained responding was not increased; however, this may have been difficult to show given that the FR-20 schedule of food presentation generated fairly high rates of responding, and benzodiazepines can produce rate-dependent effects. Rate dependence commonly refers to drugs that decrease high rates of responding and increase low rates of responding under certain operant schedules (Sanger and Blackman, 1976). Why chlordiazepoxide did not then increase ethanol-maintained responding is unclear. An interesting possibility is that the rate-dependent effects of chlordiazepoxide interfere with its capacity to decrease response rate in the ethanol component. Nonetheless, the interaction of chlordiazepoxide and ethanol in the present study was consistent with a study by Leonard et al. (2006) showing that another benzodiazepine, flunitrazepam, had marginal effects on ethanol intake.
Similar to chlordiazepoxide, the highest dose of rauwolscine tested significantly decreased food-maintained responding compared to control and significantly decreased ethanol-maintained responding compared to the lowest dose tested. The only difference between these drugs under this baseline was that low to intermediate doses of rauwolscine tended to produce small (non-significant, p=0.057) increases in ethanol-maintained responding. For example, the increase in ethanol-maintained responding after 5.6 mg/kg increased mean BEC for the group by almost 40 mg/dl. In previous studies, α2-receptor antagonists such as rauwolscine have been shown to increase drug-seeking behavior and ethanol intake (Cippitelli et al., 2010; Le et al., 2005; Marinelli et al., 2007), and these effects have frequently been attributed to their reported anxiogenic effects in both humans (Bremner et al., 1996b; Holmberg and Gershon, 1961) and non-humans (Bremner et al., 1996a; Eroglu and Guven, 1998; Lang and Gershon, 1963; Venault et al., 1993). Similar to the α2-receptor antagonists (e.g., rauwolscine and yohimbine), many of the negative GABAA receptor modulators (e.g., RO15-4513, DHEA-S, β-carbolines) have been shown to be anxiogenic, but these drugs do not uniformly increase drinking (Belzung et al., 1987, 1988; Frye and Lacey, 1999; Lister, 1987; Misslin et al., 1988). In this study, rauwolscine significantly decreased food-maintained responding at a dose of 18 mg/kg, which is in accordance with a study by Callahan et al. (1984) where rauwolscine produced hypophagic effects in both lean and genetically obese mice. The failure to find significant increases in drinking and BECs similar to other studies (Cippitelli et al., 2010; Le et al., 2005; Marinelli et al., 2007), however, may be due to the large volumes of ethanol that are already consumed during the session (i.e., a ceiling effect). To test this possibility, future studies will need to establish a behavioral baseline that maintains lower levels of ethanol consumption that can be increased more easily.
In summary, DHEA, 7-keto DHEA and RO15-4513 significantly decreased BECs at doses that did not affect food-maintained responding in rats. For RO15-4513, these decreases in BEC were concomitant with decreases in ethanol-maintained responding, whereas for DHEA and 7-keto DHEA the decreases in BEC were concomitant with decreases ethanol-maintained responding only in subjects that were not responding adventitiously or superstitiously in the ethanol component. The fact that neither DHEA nor RO15-4513 have been shown to alter the metabolism of ethanol (Buczek et al., 1997; Melchior and Ritzmann, 1992) also supports the similarity of the effects of DHEA and RO15-4513 in those subjects whose responding was controlled solely by ethanol presentation. One new aspect to the current findings with DHEA and 7-keto DHEA was that both drugs decreased BEC and ethanol-maintained behavior over a relatively compressed time period compared to previous studies from this laboratory. More specifically, in previous studies DHEA only decreased ethanol intake after multiple injections, which could not rule out genomic effects of DHEA as a potential mechanism of action (Gurkovskaya et al., 2009; Worrel et al., 2011). In the present study, these neurosteroids produced changes in drinking behavior within minutes, a timescale that would seem to preclude genomic effects (cf. Belelli and Lambert, 2005), which develop relatively slowly (over minutes to hours) and often persist long after the disappearance of the steroid from the brain. Although 7-keto DHEA was more potent than DHEA, and the effects were not identical, there is the possibility that the metabolism of DHEA to 7-keto DHEA may contribute to the effects of DHEA on ethanol-maintained responding. 7-keto DHEA would also have a distinct advantage over DHEA as a treatment for alcohol abuse and dependence in that it does not act as a precursor for the sex hormones (Lardy et al., 1998) and would be free of the adverse effects associated with increased production of testosterone and estradiol (Panjari and Davis, 2007). Furthermore, unlike the sulfated form of DHEA (DHEA-S) (Ticku and Kulkarni, 1988) and the negative modulator RO15-4513 (Karp et al., 2009), there is very little evidence that DHEA (Heuser et al., 1965) or 7-keto DHEA are proconvulsant unless administered in large concentrations directly into the brain. In contrast, RO15-4513 has been shown to be convulsant in rats at relatively high doses, and a study by Miczek and Weerts (1987) found that small doses of this compound (1 mg/kg) in primates produced overt seizure-related behaviors (e.g., tremors) including a fatal tonic-clonic seizure in one subject. Proconvulsant effects of this sort have also precluded its use in humans. Conversely, 7-keto DHEA does not produce toxicity in rats at doses as high as 2000 mg/kg and in monkeys at doses as high as 1000 mg/kg (Davidson et al., 2000). In addition, 7-keto DHEA has been shown to be well tolerated in humans at doses as high as 200 mg/kg and is available over the counter as well as in some nutritional supplements (cf. Arsenou et al., 2003). These characteristics of 7-keto DHEA suggest that it may have therapeutic advantages over DHEA and that both of these compounds may produce fewer of the adverse effects that are generally associated with other negative GABAA receptor modulators (Lardy et al., 1998).
Acknowledgements
This work was supported in part by a grant from the National Institute on Alcohol Abuse and Alcoholism AA019848 (M.W.H.).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arsenou ES, Fousteris MA, Koutsourea AI, Nikolaropoulos SS. 7-keto-delta(5)-steroids: key-molecules owning particular biological and chemical interest. Mini Rev Med Chem. 2003;3:557–567. doi: 10.2174/1389557033487953. [DOI] [PubMed] [Google Scholar]
- Balakleevsky A, Colombo G, Fadda F, Gessa GL. Ro 19-4603, a benzodiazepine receptor inverse agonist, attenuates voluntary ethanol consumption in rats selectively bred for high ethanol preference. Alcohol Alcohol. 1990;25:449–452. [PubMed] [Google Scholar]
- Barrett JE, Weinberg ES. Effects of chlordiazepoxide on schedule-induced water and alcohol consumption in the squirrel monkey. Psychopharmacologia. 1975;40:319–328. doi: 10.1007/BF00421470. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Belzung C, Misslin R, Vogel E, Dodd RH, Chapouthier G. Anxiogenic effects of methyl-beta-carboline-3-carboxylate in a light/dark choice situation. Pharmacol Biochem Behav. 1987;28:29–33. doi: 10.1016/0091-3057(87)90006-2. [DOI] [PubMed] [Google Scholar]
- Belzung C, Misslin R, Vogel E. Does RO 15-4513 reverse the anxiolytic effects of ethanol by its intrinsic properties? Pharmacol Biochem Behav. 1988;30:867–870. doi: 10.1016/0091-3057(88)90112-8. [DOI] [PubMed] [Google Scholar]
- Bienkowski P, Kostowski W. Discriminative stimulus properties of ethanol in the rat: effects of neurosteroids and picrotoxin. Brain Res. 1997;753:348–352. doi: 10.1016/s0006-8993(97)00165-0. [DOI] [PubMed] [Google Scholar]
- Boren JJ. Some variables affecting the superstitious chaining of responses. J Exp Anal Behav. 1969;12:959–969. doi: 10.1901/jeab.1969.12-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse. 1996a;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse. 1996b;23:39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Brioni JD. Role of GABA during the multiple consolidation of memory. Drug Dev Res. 1993;28:3–27. [Google Scholar]
- Buczek Y, Tomkins DM, Le AD, Sellers EM. Opposite effects of Ro 15-4513 on acquisition and maintenance of ethanol drinking behavior in male wistar rats. Alcohol Clin Exp Res. 1997;21:1667–1675. [PubMed] [Google Scholar]
- Callahan MF, Beales M, Oltmans GA. Yohimbine and rauwolscine reduce food intake of genetically obese (obob) and lean mice. Pharmacol Biochem Behav. 1984;20:591–599. doi: 10.1016/0091-3057(84)90309-5. [DOI] [PubMed] [Google Scholar]
- Castellano C, Populin R. Effect of ethanol on memory consolidation in mice: antagonism by the imidazobenzodiazepine Ro 15-4513 and decrement by familiarization with the environment. Behav Brain Res. 1990;40:67–72. doi: 10.1016/0166-4328(90)90044-f. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Damadzic R, Hansson AC, Singley E, Sommer WH, Eskay R, Thorsell A, Heilig M. Neuropeptide Y (NPY) suppresses yohimbine-induced reinstatement of alcohol seeking. Psychopharmacology (Berl) 2010;208:417–426. doi: 10.1007/s00213-009-1741-y. [DOI] [PubMed] [Google Scholar]
- Cooper SJ, Moores WR. Chlordiazepoxide-induced hyperphagia in non-food-deprived rats: effects of Ro15-1788, CGS 8216 and ZK 93 426. Eur J Pharmacol. 1985;112:39–45. doi: 10.1016/0014-2999(85)90236-5. [DOI] [PubMed] [Google Scholar]
- Davidson M, Marwah A, Sawchuk RJ, Maki K, Marwah P, Weeks C, Lardy H. Safety and pharmacokinetic study with escalating doses of 3-acetyl-7-oxo-dehydroepiandrosterone in healthy male volunteers. Clin Invest Med. 2000;23:300–310. [PubMed] [Google Scholar]
- Erogl L, Guven O. The effects of moclobemide on the yohimbine-induced anxiogenic action in the elevated plus-maze. Pharmacol Res. 1998;37:137–143. doi: 10.1006/phrs.1997.0275. [DOI] [PubMed] [Google Scholar]
- Frye CA, Lacey EH. The neurosteroids DHEA and DHEAS may influence cognitive performance by altering affective state. Physiol Behav. 1999;66:85–92. doi: 10.1016/s0031-9384(98)00256-x. [DOI] [PubMed] [Google Scholar]
- Ginsburg BC, Koek W, Javors MA, Lamb RJ. Effects of fluvoxamine on a multiple schedule of ethanol- and food-maintained behavior in two rat strains. Psychopharmacology (Berl) 2005;180:249–257. doi: 10.1007/s00213-005-2156-z. [DOI] [PubMed] [Google Scholar]
- Gurkovskaya OV, Winsauer PJ. Discriminative stimulus effects of ethanol, pregnanolone, and dehydroepiandrosterone (DHEA) in rats administered ethanol or saline as adolescents. Pharmacol Biochem Behav. 2009;93:82–90. doi: 10.1016/j.pbb.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurkovskaya OV, Leonard ST, Lewis PB, Winsauer PJ. Effects of pregnanolone and dehydroepiandrosterone on ethanol intake in rats administered ethanol or saline during adolescence. Alcohol Clin Exp Res. 2009;33:1252–1264. doi: 10.1111/j.1530-0277.2009.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser G, Ling GM, Buchwald NA. Sedation or seizures as dose-dependent effects of steroids. Arch Neurol. 1965;13:195–203. doi: 10.1001/archneur.1965.00470020085012. [DOI] [PubMed] [Google Scholar]
- Holmberg G, Gershon S. Autonomic and psychic effects of yohimbine hydrochloride. Psychopharmacologia. 1961;2:93–106. doi: 10.1007/BF00592678. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, Smart TG. Neurosteroid binding sites on GABA(A) receptors. Pharmacol Ther. 2007;116:7–19. doi: 10.1016/j.pharmthera.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Imamura M, Prasad C. Modulation of GABA-gated chloride ion influx in the brain by dehydroepiandrosterone and its metabolites. Biochem Biophys Res Commun. 1998;243:771–775. doi: 10.1006/bbrc.1998.8177. [DOI] [PubMed] [Google Scholar]
- Ji D, Gilpin NW, Richardson HN, Rivier CL, Koob GF. Effects of naltrexone, duloxetine, and a corticotropin-releasing factor type 1 receptor antagonist on binge-like alcohol drinking in rats. Behav Pharmacol. 2008;19:1–12. doi: 10.1097/FBP.0b013e3282f3cf70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June HL, Harvey SC, Foster KL, McKay PF, Cummings R, Garcia M, Mason D, Grey C, McCane S, Williams LS, Johnson TB, He X, Rock S, Cook JM. GABA(A) receptors containing (alpha)5 subunits in the CA1 and CA3 hippocampal fields regulate ethanol-motivated behaviors: an extended ethanol reward circuitry. J Neurosci. 2001;21:2166–2177. doi: 10.1523/JNEUROSCI.21-06-02166.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June HL, Foster KL, McKay PF, Seyoum R, Woods JE, Harvey SC, Eiler WJ, Grey C, Carroll MR, McCane S, Jones CM, Yin W, Mason D, Cummings R, Garcia M, Ma C, Sarma PV, Cook JM, Skolnick P. The reinforcing properties of alcohol are mediated by GABA(A1) receptors in the ventral pallidum. Neuropsychopharmacology. 2003;28:2124–2137. doi: 10.1038/sj.npp.1300239. [DOI] [PubMed] [Google Scholar]
- Karp G, Bentov Y, Masalha R, Ifergane G. Onset of late posttraumatic seizure after dehydroepiandrosterone treatment. Fertil Steril. 2009;91:931–932. doi: 10.1016/j.fertnstert.2008.08.115. [DOI] [PubMed] [Google Scholar]
- Lang WJ, Gershon S. Effects of psychoactive drugs on yohimbine induced responses in conscious dogs. A proposed screening procedure for anti-anxiety agents. Arch Int Pharmacodyn Ther. 1963;142:457–472. [PubMed] [Google Scholar]
- Lardy H, Kneer N, Wei Y, Partridge B, Marwah P. Ergosteroids. II: Biologically active metabolites and synthetic derivatives of dehydroepiandrosterone. Steroids. 1998;63:158–165. doi: 10.1016/s0039-128x(97)00159-1. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl) 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Leonard ST, Gerak LR, Gurkovskaya O, Moerschbaecher JM, Winsauer PJ. Effects of gamma-hydroxybutyric acid and flunitrazepam on ethanol intake in male rats. Pharmacol Biochem Behav. 2006;85:780–786. doi: 10.1016/j.pbb.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Lister RG. Interactions of Ro 15-4513 with diazepam, sodium pentobarbital and ethanol in a holeboard test. Pharmacol Biochem Behav. 1987;28:75–79. doi: 10.1016/0091-3057(87)90015-3. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Demirgoren S, Spivak CE, London ED. The neurosteroid dehydroepiandrosterone sulfate is an allosteric antagonist of the GABAA receptor. Brain Res. 1990;526:143–146. doi: 10.1016/0006-8993(90)90261-9. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Funk D, Juzytsch W, Harding S, Rice KC, Shaham Y, Le AD. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2007;195:345–355. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Lumeng L, Li TK. Effects of Ro 15-4513, fluoxetine and desipramine on the intake of ethanol, water and food by the alcohol-preferring (P) and - nonpreferring (NP) lines of rats. Pharmacol Biochem Behav. 1988;30:1045–1050. doi: 10.1016/0091-3057(88)90137-2. [DOI] [PubMed] [Google Scholar]
- Meert TF. Effects of various serotonergic agents on alcohol intake and alcohol preference in Wistar rats selected at two different levels of alcohol preference. Alcohol Alcohol. 1993;28:157–170. [PubMed] [Google Scholar]
- Melchior CL, Ritzmann RF. Dehydroepiandrosterone enhances the hypnotic and hypothermic effects of ethanol and pentobarbital. Pharmacol Biochem Behav. 1992;43:223–227. doi: 10.1016/0091-3057(92)90661-x. [DOI] [PubMed] [Google Scholar]
- Melchior CL, Ritzmann RF. Neurosteroids block the memory-impairing effects of ethanol in mice. Pharmacol Biochem Behav. 1996;53:51–56. doi: 10.1016/0091-3057(95)00197-2. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Weerts EM. Seizures in drug-treated animals. Science. 1987;235:1127a. doi: 10.1126/science.235.4793.1127a. [DOI] [PubMed] [Google Scholar]
- Misslin R, Belzung C, Vogel E. Interaction of RO 15-4513 and ethanol on the behaviour of mice: antagonistic or additive effects? Psychopharmacology (Berl) 1988;94:392–396. doi: 10.1007/BF00174695. [DOI] [PubMed] [Google Scholar]
- Mormede P, Colas A, Jones BC. High ethanol preferring rats fail to show dependence following short- or long-term ethanol exposure. Alcohol Alcohol. 2004;39:183–189. doi: 10.1093/alcalc/agh037. [DOI] [PubMed] [Google Scholar]
- Morrow AL, Janis GC, VanDoren MJ, Matthews DB, Samson HH, Janak PH, Grant KA. Neurosteroids mediate pharmacological effects of ethanol: a new mechanism of ethanol action? Alcohol Clin Exp Res. 1999;23:1933–1940. doi: 10.1111/j.1530-0277.1999.tb04094.x. [DOI] [PubMed] [Google Scholar]
- Nabeshima T, Noda Y, Itoh K, Kameyama T. Role of cholinergic and GABAergic neuronal systems in cycloheximide-induced amnesia in mice. Pharmacol Biochem Behav. 1988a;31:405–409. doi: 10.1016/0091-3057(88)90366-8. [DOI] [PubMed] [Google Scholar]
- Nabeshima T, Tohyama K, Kameyama T. Reversal of alcohol-induced amnesia by the benzodiazepine inverse agonist Ro 15-4513. Eur J Pharmacol. 1988b;155:211–217. doi: 10.1016/0014-2999(88)90506-7. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Purdy RH, Covey DF, Richardson HF, Roberto M, Koob GF. Epipregnanolone and a novel synthetic neuroactive steroid reduce alcohol self-administration in rats. Pharmacol Biochem Behav. 2005;81:543–550. doi: 10.1016/j.pbb.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Panjari M, Davis SR. DHEA therapy for women: effect on sexual function and wellbeing. Hum Reprod Update. 2007;13:239–248. doi: 10.1093/humupd/dml055. [DOI] [PubMed] [Google Scholar]
- Porter JR, Svec F. DHEA diminishes fat food intake in lean and obese Zucker rats. Ann NY Acad Sci. 1995;774:329–331. doi: 10.1111/j.1749-6632.1995.tb17400.x-i1. [DOI] [PubMed] [Google Scholar]
- Prasad RM, Doubinskaia I, Singh DK, Campbell G, Mace D, Fletcher A, Dendle P, Yurek DM, Scheff SW, Kraemer PJ. Effects of binge ethanol administration on the behavioral outcome of rats after lateral fluid percussion brain injury. J Neurotrauma. 2001;18:1019–1029. doi: 10.1089/08977150152693719. [DOI] [PubMed] [Google Scholar]
- Roehrs TA, Samson HH. Ethanol reinforced behavior assessed with a concurrent schedule. Pharmacol Biochem Behav. 1981;15:539–544. doi: 10.1016/0091-3057(81)90204-5. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Samson HH, Grant KA. Chlordiazepoxide effects on ethanol self-administration: dependence on concurrent conditions. J Exp Anal Behav. 1985;43:353–364. doi: 10.1901/jeab.1985.43-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH, Tolliver GA, Pfeffer AO, Sadeghi KG, Mills FG. Oral ethanol reinforcement in the rat - effect of the partial inverse benzodiazepine agonist Ro15-4513. Pharmacol Biochem Behav. 1987;27:517–519. doi: 10.1016/0091-3057(87)90357-1. [DOI] [PubMed] [Google Scholar]
- Samson HH, Chappell A, Legg B. Effects of self-administered alcohol or sucrose preloads on subsequent consumption in the rat. J Stud Alcohol. 2002;63:107–113. [PubMed] [Google Scholar]
- Sanger DJ. Chlordiazepoxide-induced hyperphagia in rats: lack of effect of GABA agonists and antagonists. Psychopharmacology (Berl) 1984;84:388–392. doi: 10.1007/BF00555218. [DOI] [PubMed] [Google Scholar]
- Sanger DJ, Blackman DE. Rate-dependent effects of drugs: a review of the literature. Pharmacol Biochem Behav. 1976;4:73–83. doi: 10.1016/0091-3057(76)90178-7. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Archer C, Tapert SF, Frank LR. Intermittent binge alcohol exposure during the periadolescent period induces spatial working memory deficits in young adult rats. Alcohol. 2008;42:459–467. doi: 10.1016/j.alcohol.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serfozo P, Cash DJ. Effect of a benzodiazepine (chlordiazepoxide) on a GABAA receptor from rat brain. Requirement of only one bound GABA molecule for channel opening. FEBS Lett. 1992;310:55–59. doi: 10.1016/0014-5793(92)81145-c. [DOI] [PubMed] [Google Scholar]
- Shi J, Schulze S, Lardy HA. The effect of 7-oxo-DHEA acetate on memory in young and old C57BL/6 mice. Steroids. 2000;65:124–129. doi: 10.1016/s0039-128x(99)00094-x. [DOI] [PubMed] [Google Scholar]
- Sidman M. Control techniques. In: Sidman M, editor. Tactics in scientific research. Authors Cooperative, Inc.; Boston: 1960. p. 341363. [Google Scholar]
- Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- Suzdak PD, Glowa JR, Crawley JN, Schwartz RD, Skolnick P, Paul SM. A selective imidazobenzodiazepine antagonist of ethanol in the rat. Science. 1986;234:1243–1247. doi: 10.1126/science.3022383. [DOI] [PubMed] [Google Scholar]
- Suzdak PD, Paul SM, Crawley JN. Effects of Ro15-4513 and other benzodiazepine receptor inverse agonists on alcohol-induced intoxication in the rat. J Pharmacol Exp Ther. 1988;245:880–886. [PubMed] [Google Scholar]
- Svec F, Porter J. The effect of dehydroepiandrosterone (DHEA) on Zucker rat food selection and hypothalamic neurotransmitters. Psychoneuroendocrinology. 1997;22(Suppl 1):S57–S62. doi: 10.1016/s0306-4530(97)00004-8. [DOI] [PubMed] [Google Scholar]
- Ticku MK, Kulkarni SK. Molecular interactions of ethanol with GABAergic system and potential of RO15-4513 as an ethanol antagonist. Pharmacol Biochem Behav. 1988;30:501–510. doi: 10.1016/0091-3057(88)90487-x. [DOI] [PubMed] [Google Scholar]
- Venault P, Jacquot F, Save E, Sara S, Chapouthier G. Anxiogenic-like effects of yohimbine and idazoxan in two behavioral situations in mice. Life Sci. 1993;52:639–645. doi: 10.1016/0024-3205(93)90455-c. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, Riley AL. Cholecystokinin potentiates the rate-decreasing effects of morphine on schedule-controlled behavior in rats. Pharmacol Biochem Behav. 1988;30:569–575. doi: 10.1016/0091-3057(88)90067-6. [DOI] [PubMed] [Google Scholar]
- Winsauer PJ, Verrees JF, O’Halloran KP, Bixler MA, Mele PC. Effects of chlordiazepoxide, 8-OH-DPAT and ondansetron on radiation-induced decreases in food intake in rats. J Pharmacol Exp Ther. 1994;270:142–149. [PubMed] [Google Scholar]
- Worrel ME, Gurkovskaya OV, Leonard ST, Lewis PB, Winsauer PJ. Effects of 7-keto dehydroepiandrosterone on voluntary ethanol intake in male rats. Alcohol. 2011;45:349–354. doi: 10.1016/j.alcohol.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Follesa P, Ticku MK. Down-regulation of the GABA receptor subunits mRNA levels in mammalian cultured cortical neurons following chronic neurosteroid treatment. Brain Res Mol Brain Res. 1996;41:163–168. doi: 10.1016/0169-328x(96)00087-3. [DOI] [PubMed] [Google Scholar]