Abstract
Motility, maturation and the acrosome reaction (AR) are fundamental functions of mammalian spermatozoa. While travelling through the female reproductive tract, spermatozoa must mature through a process named capacitation, so that they can reach the egg and undergo the AR, an exocytotic event necessary to fertilize the egg. Though Cl− is important for sperm capacitation and for the AR, not much is known about the molecular identity of the Cl− transporters involved in these processes. We implemented a modified perforated patch-clamp strategy to obtain whole cell recordings sealing on the head of mature human spermatozoa. Our whole cell recordings revealed the presence of a Ca2+-dependent Cl− current. The biophysical characteristics of this current and its sensitivity to niflumic acid (NFA) and 4,4′-diisothiocyano-2,2′-stilbene disulphonic acid (DIDIS) are consistent with those displayed by the Ca2+-dependent Cl− channel from the anoctamin family (TMEM16). Whole cell patch clamp recordings in the cytoplasmic droplet of human spermatozoa corroborated the presence of these currents, which were sensitive to NFA and to a small molecule TMEM16A inhibitor (TMEM16Ainh, an aminophenylthiazole). Importantly, the human sperm AR induced by a recombinant human glycoprotein from the zona pellucida, rhZP3, displayed a similar sensitivity to NFA, DIDS and TMEM16Ainh as the sperm Ca2+-dependent Cl− currents. Our findings indicate the presence of Ca2+-dependent Cl− currents in human spermatozoa, that TMEM16A may contribute to these currents and also that sperm Ca2+-dependent Cl− currents may participate in the rhZP3-induced AR.
Key points
Ion channels participate in crucial sperm functions such as motility, capacitation and the acrosome reaction.
Chloride, the main anion in physiological solutions, is deeply involved in sperm physiology.
We implemented a modified perforated patch-clamp strategy to obtain whole cell recordings sealing on the head of mature human spermatozoa to investigate their ion channels.
This work presents the first evidence for the presence of calcium-dependent chloride channels (CaCCs) in human spermatozoa; they could be constituted by TMEM16.
The CaCCs play an important role in the physiology of human spermatozoa and participate in the acrosome reaction.
Introduction
From their germinal niche till they reach and fertilize the egg, mammalian spermatozoa must travel a long and winding road. Upon ejaculation and during their transit through the female reproductive tract, spermatozoa acquire progressive motility and undergo molecular, biochemical and physiological changes referred to as capacitation that enable them to reach and fertilize the egg (Bailey, 2010). To accomplish fertilization, spermatozoa must carry out the acrosome reaction (AR) (reviewed in Darszon et al. 2011). This exocytotic reaction enables spermatozoa to penetrate the ZP matrix and fuse with the egg plasma membrane, generating a zygote. Though for many years it has been believed that the zona pellucida (ZP), a glycoproteinaceous matrix that surrounds the mammalian oocyte, is the physiological inducer of the AR, how and where this reaction occurs has been re-examined recently (Ganguly et al. 2010; Inoue et al. 2011; Jin et al. 2011). The human ZP matrix is composed of four glycoproteins designated as ZP1 to ZP4; ZP3 is believed to be the main AR inducer (Conner et al. 2005; Caballero-Campo et al. 2006; Litscher et al. 2009). The AR is a calcium-dependent process and it is inhibited by several ion channel blockers, evidencing their predominant role in this process (Espinosa et al. 1998; Mayorga et al. 2007). It is well established that motility, capacitation and the AR require diverse ions (Ca2+, HCO3−, Na+, K+ and Cl−) (Visconti et al. 1995; Salicioni et al. 2007; Darszon et al. 2011).
In mouse spermatozoa, the absence of external Cl− does not affect sperm viability, but capacitation-associated processes such as the increase in tyrosine phosphorylation, the increase in cAMP levels, hyperactivation, the ZP-induced AR and finally fertilization are abolished or significantly reduced (Wertheimer et al. 2008; Chen et al. 2009). Similar results have been found in human sperm (Yeung & Cooper, 2008). As in other cells, Cl− is the principal anion that among other important functions is implicated in sperm volume regulation and protection from osmotic stress (Furst et al. 2002; Yeung et al. 2005; Cooper & Yeung, 2007). Mammalian spermatozoa confront drastic osmotic changes along their journey to find the egg (Chen et al. 2010); for example, the acrosome swelling that occurs after binding to ZP leads to AR (Zanetti & Mayorga, 2009). Therefore, it is likely that Cl− plays a relevant role in sperm physiology. However, not much is known about the proteins that transport it across the membrane of this fundamental cell.
Many different cell types in which cell volume control and secretion are critical (i.e. epithelial cells in exocrine glands and trachea, airway, vascular smooth muscle cells, reproductive tract smooth muscle cells, oviduct and ductus epididymis cells, and mouse spermatids) express Ca2+-dependent Cl− channels (CaCCs), exhibiting similar biophysical, pharmacological and molecular features (Hartzell et al. 2005; Huang et al. 2009; Kunzelmann et al. 2011). Interestingly, niflumic acid (NFA) and 4,4′-diisothiocyanostilbene-2,2′-disulphonic acid (DIDS), two CaCC blockers, inhibit the ZP-induced mouse spermatozoa AR in a similar dose-dependent manner as that with which they block CaCCs, indicating their involvement in this exocytotic event (Espinosa et al. 1998).
The long journey of spermatozoa is accompanied by dynamic changes in the concentration of intracellular Ca2+ ([Ca2+]i) that trigger myriad signalling events which could include the modulation of CaCCs, as was demonstrated in other cells (Arreola et al. 1996). Though currents mediated by CaCCs, initially documented in Xenopus oocytes (Miledi, 1982), have now been recorded in many cells types, only recently has the transmembrane protein TMEM16A been identified as one of the major molecular counterparts of the CaCCs (Caputo et al. 2008; Schroeder et al. 2008; Yang et al. 2008). The development of small molecule inhibitors to investigate the contribution of TMEM16A to CaCC conductance in human tissues, airway, intestinal epithelial and salivary gland cells allows the pharmacological dissection of TMEM16A/CaCC function (Namkung et al. 2010).
In spite of the evidence suggesting a role for CaCCs in human sperm physiology, they have never been recorded directly in these cells. In this work, we used a modified perforated patch-clamp technique to obtain whole cell recordings sealing on the head of mature human spermatozoa. Our findings show that these sperm possess CaCCs with biophysical and pharmacological properties resembling those reported in other native cells (Hartzell et al. 2005) and in CaCC expression cloning experiments in amphibian and mammalian systems (Caputo et al. 2008; Schroeder et al. 2008; Yang et al. 2008). We corroborate this by showing that these currents can be recorded sealing in the cytoplasmic droplet, as has been done for CatSper, Hv and Slo3 (Kirichok et al. 2006; Lishko et al. 2010; Santi et al. 2010). Our results are also consistent with a significant contribution of TMEM16A like channels to the CaCC currents obtained by whole cell recordings from heads of human spermatozoa. In addition, our observations suggest that these channels are involved in the human sperm rhZP3-mediated AR.
Methods
Ethical approval
This study was approved by the Bioethics Committee at the Biotechnology Institute from the National Autonomous University of Mexico. All semen donors gave written informed consent.
Cell preparation, sperm capacitation and acrosome reaction assays
Ejaculates were obtained from healthy donors by masturbation after at least 48 h of sexual abstinence. Only semen samples that fulfilled the World Health Organization guidelines were selected for experiments (WHO, 2010). Highly motile sperm were recovered after a swim-up separation for 1 h in Ham's F-10 medium at 37°C in a humidified atmosphere of 5% CO2–95% air. For electrophysiological experiments the cells were stored in physiological salt solutions immediately after swim-up separation until used. For AR determinations the swim-up procedure was carried out supplementing the F-10 medium with 5 mg ml−1 of BSA and 2 mm CaCl2, pH 7.4. Cell concentration was then adjusted to 107 sperm ml−1 with the capacitating Ham's F-10 medium, and incubation was continued for 5 h. After capacitation, sperm were preincubated with CaCC inhibitors (NFA, DIDS or TMEM16Ainh, 15 min in all cases) and the AR was induced in capacitated cells incubated for 30 min with 10 ng μl−1 of recombinant human ZP3 (rhZP3), purified as reported previously (Jose et al. 2010). Staining with fluorescein isothiocyanate-labelled Pisum sativum agglutinin (FITC-PSA) was performed for evaluation of the AR. Sperm were washed with phosphate-buffered saline (PBS) and aliquots of the cell suspensions were smeared onto AR glass slides and then air dried. To permeabilize the plasma and outer acrosomal membranes, the slides were fixed with cold methanol for 30 s. Samples were incubated with FITC-PSA (25 μg ml−1 in PBS, pH 7.4) for 30 min at room temperature in a moisture chamber. The excess of FITC-PSA was washed out with distilled water, slides were dried again and finally the AR assays were evaluated with a fluorescence microscope (Zeiss Axioskop, excitation filter 450–490 nm, emission filter 520 nm). The AR patterns (bright fluorescence: acrosome intact; no fluorescence or only fluorescence of the equatorial segment: acrosome reacted) were evaluated in at least 200 cells per condition. Negative (no stimulation) and positive (A-23187 10 μm) controls were included in all experiments. For each experiment, AR indexes (ARIs) were calculated by subtracting the percentage of spontaneously reacted spermatozoa in the negative control (value of 1.8 ± 0.4%, n = 3), from the raw mean values for all experimental conditions, expressing the results as a percentage of the AR observed in the positive controls (value of 29.3 ± 0.6%, n = 3).
Data processing was done using the KyPlot 2.0 program (KyensLab Inc, Tokyo, Japan). In all cases, differences between raw experimental and control data were tested by unpaired Student's t tests. Differences were considered significant when P < 0.05.
Whole-cell recording
We have applied the perforated-cell patch-clamp technique to record ionic currents in the whole cell mode in mature human spermatozoa plated on poly-lysine-coated coverslips. The patch pipette was applied at a 90 deg angle to the sperm cell head plasma membrane using a three-axis piezo nano-positioning with servo-control (PZ 62E, Physik Instrumente, Germany) as represented in the online supplemental material Fig. S1. We used a mixture of saponin/β-escin (100 μg ml−1/5 μm) in the pipette tip (∼1 μl) to induce controlled patch permeabilization, and were able to record stable whole-cell currents after patch perforation from dialysed spermatozoa. This strategy has been used to record ion currents from cardiac myocytes and neurons (Fan & Palade, 1998; Sarantopoulos et al. 2004). It consists in perforating cell attached patches to obtain whole cell records, using a mixture of detergents in the pipette tip. The detergents were loaded through the back of the pipette after filling it with solution. The success rate to perforate the patch and record stable whole cell ionic currents for an adequate amount of time was about 50%. To visually confirm electrical and diffusion access to all sperm compartments (head, middle and principal pieces; Fig. 1A), using the adapted perforated patch technique; we dialysed the cell with internal solution containing fluorescein (1 μm) and recorded the fluorescence in an inverted fluorescence microscope (Olympus IX71) with a CCD camera. Glass pipettes (1BBL, World Precision Instruments, Inc., Sarasota, FL, USA) were designed to have 5–7 MΩ pipette resistance, when filled with internal solution. The junction potential between internal and external solutions, measured with the offset correction of the patch amplifier, was usually about 4–6 mV. Membrane potential was always corrected for the liquid junction potential (∼4 mV). The chloride concentration was maintained at least at 5 mm to insure the proper operation of the Ag–AgCl electrodes. Changing the chloride concentration did have an effect on the liquid junction potential as we had to correct the pipette potential differentially for each concentration. The seal resistance was typically 3–5 GΩ. After sealing and achieving the whole cell configuration, capacitive transients were obtained and used to measure sperm capacitance and series resistance (Fig. S2) to monitor the suitability of the recording conditions with this method. Such transients are shown in Fig. S2A for the onset and offset. High temporal resolution (>20 kHz) recordings allowed estimates of clamp quality and time constants. Such a record is shown in Fig. S2B. A double exponential of the form I = Io+I1× (1 − exp(−t/τ1)) +I2× (1 − exp(−t/τ2)) was fitted to the capacitive transient decay. Typical values of the best fit parameters were −52, 38 and 14 pA for Io, I1 and I2, respectively, whereas the time constants were 23 and 625 μS for the fast and slow components, respectively. The amplitude of the fast component was about 2–3 times greater than that of the slow one, suggesting that the fast component may be related to clamp conditions in the head and surrounding regions through the neck, whereas the slow one may be related to the clamp conditions in the tail of the sperm. Hence, although the whole cell procedure reports currents from all over the sperm, the recording conditions are better and faster in the head and surrounding areas than in the tail. The area vs. time, charge (Q), under the capacitive transients, needed to estimate membrane capacitance (Cm), sperm area and series resistance is shown in Fig. S2. The average charge measured under these conditions was 24 ± 9 fC (n = 5) and the mean membrane capacitance of the whole sperm cell membrane was 1.3 ± 0.1 pF (n = 96). This measurement was quite similar to that reported by the capacitance compensation from the Axopatch 200B. The human sperm area determined by electron micrographs using a stereological analysis method is approximately 106 μm2 (Curry et al. 1996). The capacitance values found by us are consistent with this value, assuming the specific Cm to be 1 μF cm−2. For 1.3 pF average capacitance, the estimated sperm area under these assumptions is approximately 130 μm2. Recordings were started after at least 4 min dialysis to allow equilibration of the cytosolic content with the pipette solution.
Figure 1. Whole-cell patch-clamp recordings from mature human spermatozoa.
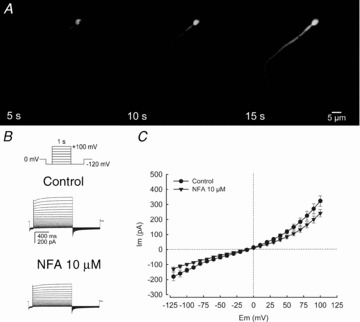
A, fluorescence image sequences of a spermatozoon after achieving the whole-cell configuration in the perforated-patch mode. Fluorescein (1 μm) diffused from the pipette throughout the interior of the spermatozoon in ∼15 s. The pipette fluorescence is not seen because the approach of the pipette is perpendicular to the sperm head (see supplemental Fig. S1). B, whole-cell currents elicited by voltage steps from a holding potential of 0 mV (inset: step protocol) in physiological salt solution conditions (see Methods) without and with NFA (10 μm). C, current–voltage relationships (I–V curve) of results as in B in the absence and presence of 10 μm NFA, showing inward and outward rectification at hyperpolarized and depolarized membrane potentials. Note that the macroscopic current family and I–V plot show a NFA-sensitive current component. For C, data represent means ± SEM with n = 4.
Whole-cell macroscopic currents were acquired at 10 kHz using an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA, USA) interfaced with a PC equipped with a DigiData 1300A (Molecular Devices) and filtered at 1–2 kHz (4-pole Bessel filter). Unless indicated otherwise in the figure, membrane potential was changed from −120 to 100 mV in 10 mV steps by delivering square pulses of 1 s duration every 5 s from a holding potential of 0 mV, followed by a 700 ms pulse to –120 mV. This protocol inactivates voltage dependent Ca2+ and K+ currents. The leak of the seal, calculated applying Ohm's law to current values measured at the middle of the capacitive transient, was very low under these circumstances (∼10–20 GΩ), suggesting that this technique did not alter the basic properties of the membrane.
During the course of this study Lishko et al. (2010) applied classical whole-cell patch clamp techniques for human sperm sealing at the cytoplasmic droplet. Using this strategy we were able to record CatSper currents stimulated by progesterone (Fig. 6A) and Ca2+-dependent Cl− currents inhibited by NFA and TMEM16Ainh (Fig. 6B and C).
Figure 6. Human spermatozoa patched at the cytoplasmic droplet display progesterone activated CatSper currents and CaCC currents inhibited by NFA and TMEM16Ainh.
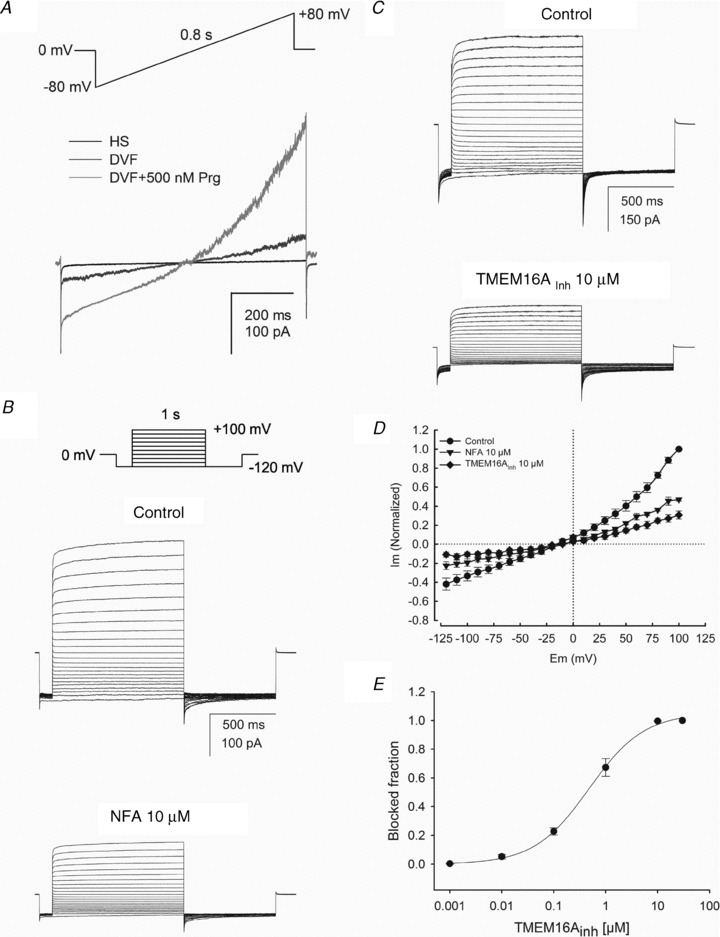
A, a representative monovalent whole-cell ICatSper recorded from a human spermatozoon in HS solution (baseline trace, black) and in divalent-free solution (DVF) in the absence (dark grey), and presence (grey) of 500 nm of progesterone (n = 4). B, whole-cell CaCC currents recorded from a human spermatozoon dialysed with pipette solution containing 250 nm free Ca2+ and exposed to a bath TEA-Cl standard solution. Currents were obtained at a holding potential of 0 mV with the indicated voltage step protocol (top panel) in the absence or presence of 10 μm NFA. Note that 10 μm NFA inhibited nearly 50% of the current indicating that it is a similar current to that observed in Fig. 4. C, family of CaCC currents recorded from another spermatozoon before and after exposure to 10 μm TMEM16Ainh. D, current–voltage relationship (I–V) of currents normalized with respect to maximal current in control conditions measurements at the end of each voltage pulse of recordings as in B and C. E, dose dependent blockade of CaCC currents by TMEM16Ainh. Current amplitudes were measured at +100 mV by averaging seven to nine original current traces and normalizing with respect to the maximal blocked fraction. For E and D data represent the mean ± SEM with n = 6.
Pulse protocols, and data capture and analysis were performed with pCLAMP software (Molecular Devices), SigmaPlot 9.0 (Systat Software Inc., San Jose, CA, USA) and Origin7.5 (OriginLab Corp., Northampton, MA, USA).
Solutions
Total whole-cell ionic currents were initially recorded using normal physiological salt solutions (HS) with the following composition; external solution (in mm): 135 NaCl, 5 KCl, 1.8 CaCl2, 1 MgSO4, 5 dextrose, 10 lactic acid, 1 sodium pyruvate, 10 Hepes, pH 7.35; and internal solution (in mm): 10 NaCl, 140 KCl, 1 MgSO4, 10 EGTA, 5 CaCl2, 5 ATP-Mg, 10 Hepes, pH 7.25. The standard external solution for recording Cl− currents had the following composition (in mm): 135 TEA-Cl, 0.5 CaCl2, 45 d-mannitol, 20 TES, pH 7.35 and 330 mosmol kg−1. The solutions with different Cl− concentrations are shown in Table 1. The tonicity of the internal solutions was 40 mosmol kg−1 more hypotonic than the external solutions, to get rid of swelling-activated Cl− channels possibly present in these cells (Yeung et al. 2005). The Ca2+-buffered pipette solutions containing free [Ca2+] near 0 (1 nm) and up to 1 μm were prepared as in Arreola et al. (1996). EGTA and N-(2-hydroxyethyl)ethylenedinitrilo-N,N′,N′-triacetic acid (HEDTA) were selected as high and low affinity Ca2+ buffers (Kd values of 95 nm and 3.5 μm, respectively), to prepare Ca2+ buffers in the nano- and micromolar range, respectively (Table 2). The pH of the pipette solutions was set to 7.25 with N-tris(hydroxymethyl)methyl-2-aminoethanesulphonic acid (TES). High concentrations of Ca2+ and pH buffers assured control of both Ca2+ and pH during the course of these experiments. To determine the free Ca2+ concentrations we used the Maxchelator (v.2.1.) software program (written by Chris Patton, Hopkins Marine Station, Stanford University). Monovalent cations were substituted by tetraethylammonium (TEA) in both the internal and external solutions to avoid contamination of the Cl− currents by K+ channels such as Slo3 or cationic currents through non-selective channels (Navarro et al. 2007; Martinez-Lopez et al. 2009; Santi et al. 2009). For recording monovalent CatSper currents, seals were formed in HS solution and pipettes were filled with (in mm): 135 caesium methanesulphonate (CsMeSO3), 5 CsCl, 10 EGTA, 5 Na-ATP, 0.5 Na-GTP, 10 Hepes, pH 7.3 adjusted with CsOH. Bath divalent-free solution (DVF) for recording monovalent CatSper currents contained the following (in mm): 140 CsMeSO3, 1 EDTA, 20 Hepes, pH 7.4 adjusted with CsOH. HS solution was used to record the baseline current which was then exchanged by DVF for measuring monovalent CatSper currents.
Table 1.
Composition of solutions with different Cl− concentrations (in mm)
| [Cl−]bath | TEA-Cl | CaCl2 | d-Mannitol | Dextrose | TES |
|---|---|---|---|---|---|
| 147 | 144 | 1.5 | 25 | — | 20 |
| 70 | 67 | 1.5 | 147 | 20 | 25 |
| 45 | 42 | 1.5 | 182 | 25 | 35 |
| 12 | 8 | 2 | 228 | 30 | 50 |
| [Cl−]pipette | Ca-gluconate | CaCl2 | EGTA-TEA | TEA-F | TES |
|---|---|---|---|---|---|
| 30 | 11.2 | 15 | 36.2 | 5 | 50 |
Table 2.
Composition of internal solutions with different Ca2+ concentrations (in mm)
| [Ca2+] | TEA-Cl | CaCl2 | Ca- gluconate | EGTA-TEA | HEDTA-TEA | TEA-F | TES | d-Mannitol |
|---|---|---|---|---|---|---|---|---|
| <10−6 | 47 | — | — | 20 | — | 5 | 50 | 74 |
| 25 × 10−6 | 37 | 5.2 | — | 25.2 | — | 5 | 50 | 94.5 |
| 100 × 10−6 | 5 | 21 | — | 41 | — | 5 | 50 | 10 |
| 250 × 10−6 | — | 23.5 | 2.7 | 36.2 | — | 5 | 50 | — |
| 10−3 | 29.9 | 8.6 | — | — | 38.6 | 5 | 50 | 18 |
Chemicals
Bovine serum albumin (BSA), CaCl2, A23187, Ham's F-10 medium, fluorescein isothiocyanate–Pisum sativum agglutinin (FITC-PSA), poly-lysine, niflumic acid, DIDS, saponin, β-escin, ionomycin, dimethyl sulfoxide (DMSO) and other inorganic salts were acquired from Sigma-Aldrich Chemical Co. (St Louis, MO, USA), and methanol was from J. T. Baker (Phillipsburg, NJ, USA). Stock solutions in DMSO were prepared for each compound and aliquots were stored at −20°C.
Statistical analysis
Numeric results are expressed as means ± standard error mean (SEM); n, mean number of individuals, tested and analysed with Student's t test. Two-tailed P values <0.05 were considered statistically significant.
Results
Whole-cell ionic currents
To investigate the mature human spermatozoa plasma membrane ionic currents, we applied the perforated-cell patch-clamp technique (see Methods) by attaching a patch pipette to the sperm head plasma membrane. Figure 1A shows the image of a spermatozoon after achieving the whole-cell configuration and dialysing with the fluorescent dye fluorescein (1 μm) through the patch pipette. Electrical access to all sperm compartments was corroborated by the fluorescence throughout the whole interior of the human spermatozoon. The average time necessary to accomplish cell dialysis after achieving a giga-ohm (GΩ) seal between the patch pipette and the plasma membrane of the sperm head was ∼15 s. Figure 1B shows whole-cell macroscopic currents obtained from the same spermatozoon using physiological salt solutions (HS). Under these recording conditions the macroscopic currents result from a mixture of cationic (K+, Ca2+ or Na+) and anionic (Cl−) conductances. As Cl− is essential for sperm physiology (pointed out in the Introduction) and its transport systems not well defined, we decided to explore the Cl− contribution to the sperm whole-cell macroscopic currents, obtained by this methodology. The application of NFA decreased the total current revealing the presence of a minor but significant and reproducible NFA-sensitive component possibly due to Cl− channels. The current–voltage relationship showed an inward and an outward rectifying component in the negative and positive membrane potential values; specifically, at negative (−120 mV) and positive (100 mV) voltages, 10 μm NFA reduced the conductance 40% and 17%, respectively (Fig. 1C).
Isolation and characterization of the Cl− conductance
Since NFA is best known as a blocker of Ca2+ regulated Cl− channels, we examined the Cl− dependence of the macroscopic current recorded in human spermatozoa using a modified bath solution containing different TEA-Cl concentrations (see Table 1 and Fig. 2A). To examine if the currents could include a voltage-activated Ca2+ channel (CaV) current, we applied a pre-pulse to –120 mV after holding the cells at 0 mV. Under these conditions we never observed such Ca2+ currents. When the bath solution contained high Cl− concentrations, robust currents were recorded and they decreased as the external Cl− concentration was reduced in the external medium. Lowering extracellular Cl− from 147 to 12 mm strongly reduced the outward currents; see records in Fig. 2A and I–V curve in Fig. 2B. Different extracellular Cl− concentrations, at a given intracellular Cl− concentration, elicited a shift in the reversal potential (Erev) (Fig. 2C). Between the extreme extracellular Cl− concentrations (147 and 12 mm), we found a shift of 49 ± 4 mV in the reversal potential (Erev). The expected shift for an ideal Cl− selective channel is 64 mV. Such a difference may indicate that the underlying channel has a small, but significant, permeability to gluconate, as it has been reported for several CaCCs (Frings et al. 2000; Kim et al. 2003). In addition, as there is external Ca2+ in the medium, CatSper (Lishko & Kirichok, 2010) and TRP-like channels could contribute to the currents.
Figure 2. The sperm macroscopic currents depend on the concentration of extracellular Cl−.
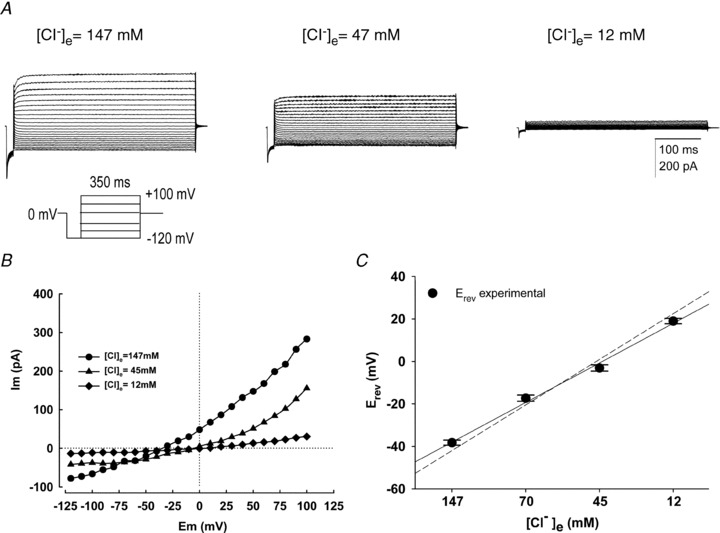
A, family of macroscopic currents obtained from a spermatozoon exposed to high and low extracellular Cl− concentrations ([Cl−]e) using TEA-Cl solutions (see Methods). Currents were elicited by voltage steps, indicted in the voltage step protocol, from a holding potential of 0 mV. Note that lowering of [Cl−]e from 147 to 12 mm strongly decreased outward currents. B, the I–V plot clearly shows the [Cl−]e dependence of the current and the reversal potential (Erev). Reducing [Cl−]e shifts Erev to the right, as expected for a Cl− selective channel. C, The relation between the measured Erev of the current and the calculated equilibrium potential for Cl− is very close. The dashed line is the fitting expected for an ideal Cl− channel. In all conditions [Ca2+]i = 250 nm. For C, data represent means ± SEM with n = 5.
Ca2+-dependent Cl− currents
To determine if the Cl− currents recorded in mature human spermatozoa were due to CaCCs, we varied the Ca2+ concentrations of the pipette internal solutions and applied voltage-step protocols to avoid other ionic currents as described in Fig. 3A. The macroscopic currents were negligible when [Ca2+]i was 1 nm and very small with 25 nm[Ca2+]i. Increasing the [Ca2+]i to 250 or 1000 nm induced the appearance of a robust current with an instantaneous component followed by a fractional slower component (Fig. 3A). This is clearly illustrated in the current–voltage (I–V) relationship at different [Ca2+]i concentrations (Fig. 3B) and especially at [Ca2+]i = 1000 nm where noticeable increments are recorded of the inward current at –120 mV and the outward-rectifying current at +100 mV. The currents at [Ca2+]i = 1000 nm are about 18- to 25-fold those at [Ca2+]i = 25 nm. Figure 3C shows steady-state average currents measured at −120 mV normalized against the current induced by 1000 nm of [Ca2+]i obtained from recordings in both the head, as well as by the now established protocol sealing at the cytoplasmic sperm droplet (Lishko et al. 2010, 2011). To provide a quantitative description of the Ca2+ dependence, we fitted the Hill equation to these data (continuous line) and obtained an apparent Kd of 236 nm and a Hill coefficient (nH) of 1.3. Figure 3D shows the relative current normalized against the current induced by 1000 nm of [Ca2+]i at +100 mV along with the fit of the Hill equation. The apparent Kd at +100 mV was 163 nm and nH of 1.9. The current at this potential was a very steep function of [Ca2+]i as indicated by the nH value. These results imply that the Ca2+ sensitivity of Cl− channels is a function of membrane potential as it has been reported for CaCCs in parotid acinar cells (Arreola et al. 1996). The Kd and nH values at negative and positive membrane potential are similar (nano-molar range) to those reported in rat parotid acinar cells (Kd = 300 nm and nH = 1.2 at –66 mV; Kd = 61 nm and nH = 2.7 at +74 mV) (Arreola et al. 1996). Consistently, they are also similar to those reported in heterologously expressed CaCCs (EC50 = 400 nm at 60 mV) (Yang et al. 2008).
Figure 3. Behaviour of the Cl− currents at different [Ca2+]i.
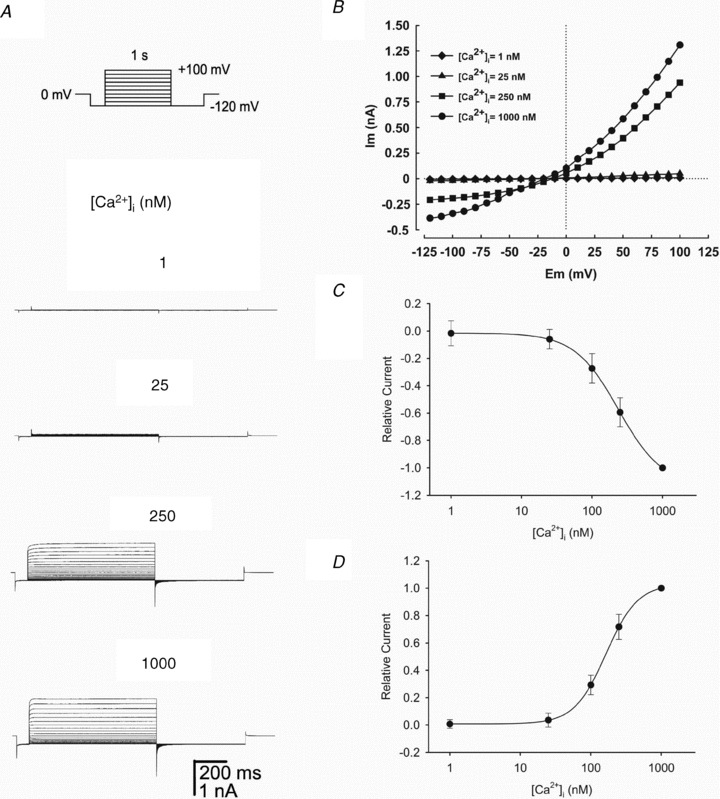
A, representative whole-cell currents recorded from one of four human spermatozoa dialysed with pipette solutions containing (from top to bottom) 1, 25, 250 and 1000 nm free Ca2+ with TEA-Cl standard solution in the bath. Currents were obtained from a holding potential of 0 mV with the voltage step protocol shown in the top panel. Note the strong [Ca2+]i dependence of the macroscopic current; voltage is not sufficient to activate the current in [Ca2+]i = 1 nm, and 25 nm activates only 4% of the current with respect to 1000 nm, the maximal concentration used. B, I–V plot of the currents obtained in A. C and D, illustrate concentration–response curves of the effect of different [Ca2+]i on the macroscopic inward and outward Cl− currents obtained from four independent experiments sealing on the sperm head and three on the cytoplasmic droplet (see Methods). Currents were normalized against the current obtained at 1000 nm[Ca2+]i recorded at –120 mV (C) and +100 mV (D). The continuous lines represent the data fitted to a Hill equation with the following parameters: Kd = 236 nm and nH = 1.3 at –120 mV and Kd = 163 nm and nH = 1.9 at +100 mV. Data represent means ± SEM with n = 7.
To further characterize the channels we recorded, we analysed the dependence of the gating parameters of CaCC activation with increasing [Ca2+]i. Figure S3A shows typical records of CaCC obtained with the same pulse protocol as described before and [Ca2+]i = 500 nm. The gating for activation of the outward currents was well described by a double exponential (see continuous line superimposed to the current trace). In Fig. S3B, we plotted the activation constants of the CaCC currents versus[Ca2+]i. These results show that elevating [Ca2+]i levels increased not only the currents amplitude, as described in Fig. 3, but also accelerated the activation kinetics.
Pharmacology of Ca2+-dependent Cl− currents
To examine the pharmacological properties of the putative CaCC currents recorded in mature human spermatozoa, two well known blockers of this channel were tested. We found that both NFA and DIDS potently affected the Ca2+-dependent Cl− currents in cells containing 250 nm of [Ca2+]i and under Cl− current recording conditions. As anticipated, the blockage became more pronounced as the NFA concentration increased (Fig. 4A). The blocking effect at the maximal NFA concentration (35 μm) tested was partially reversible upon washout. Figure 4B shows the I–V curves obtained after the application and washout of NFA. Best fit parameters of a Hill equation describing the blockage of CaCC versus NFA concentration yielded an IC50 = 8.8 ± 2 μm and an nH of 1.2. These results are similar to those reported from pharmacological studies in rabbit vascular smooth muscle cells (IC50 = 3.6 μm; Hogg et al. 1994), as well as in the heterologous expression of TMEM16A (IC50 = 29 μm; Schroeder et al. 2008). Figure 4C was constructed with the steady-state current values obtained at 100 mV.
Figure 4. Inhibition of the Ca2+-dependent Cl− currents by NFA.
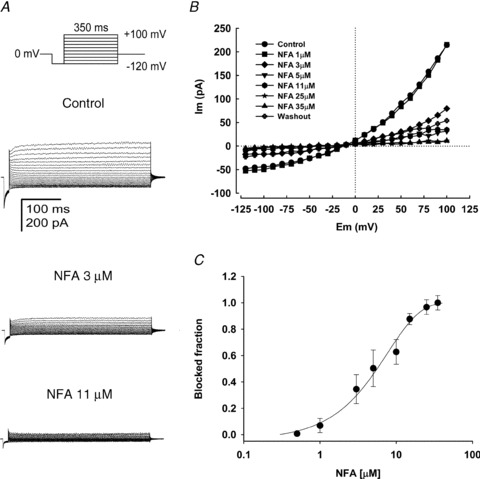
A, typical family of whole-cell currents recorded from human spermatozoa under three different conditions: control, 3 and 11 μm of NFA. Currents were obtained from holding potential of 0 mV with the indicated voltage step protocol (top panel) in the presence of external TEA-Cl standard solution and 250 nm free Ca2+ in the pipette solution. B, I–V plots of the results obtained within the indicated range of NFA concentrations (A displays only three conditions). The current blocked was close to 100% with 35 μm NFA and after 10 min of washout ∼25% of the control current was recovered. C, NFA dose-dependently blocks the Ca2+-dependent Cl− currents. The continuous line is the fit of the dose–response curve to the Hill equation which estimates an apparent IC50 = 8.8 ± 1.7 μm. For C, data represent means ± SEM with n = 5–7.
DIDS added to the bath solution had a similar blocking effect to NFA on the currents. Figure 5A shows typical recordings of a family of currents evoked by voltage steps from −120 to +100 mV under control conditions and after adding DIDS (20 μm). The current decreased more evidently in the positive range of the membrane potential. At 100 mV DIDS (20 μm) blocked about ∼52% of the control current. I–V plots of the controls and 20 μm DIDS are shown in Fig. 5B. The DIDS dose–response curve is shown in Fig. 5C. The normalized blockage fraction of the outward currents was plotted against the DIDS concentration in a semi log scale. The best fit of a Hill equation is the continuous line between the data points. Best fit parameters were IC50 = 16 ± 1.4 μm and nH = 1.4. These results are similar to those reported from pharmacological studies in rat vascular smooth muscle cells (IC50 = 16.5 μm; Baron et al. 1991), as well as in the heterologous expression of TMEM16A (IC50 = 24 μm; Schroeder et al. 2008). This pharmacological profile further suggests that the outward rectifying currents recorded from the head of mature human spermatozoa in our preparation are Ca2+-dependent chloride channels.
Figure 5. Inhibition of the Ca2+-dependent Cl− currents by DIDS.
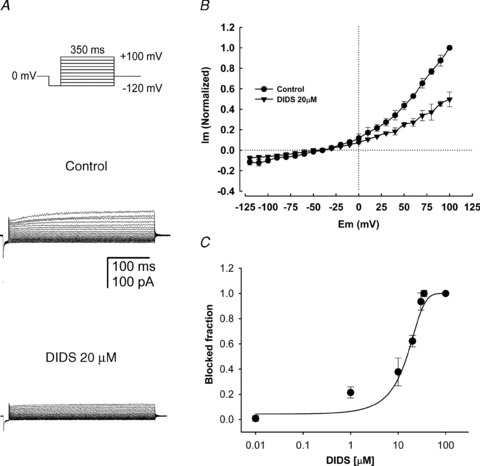
A, control whole-cell currents recorded from a human spermatozoon and blockage caused by 20 μm DIDS. Currents were obtained from a holding potential of 0 mV with the indicated voltage-step protocol (top panel) and recorded in the presence of external TEA-Cl standard solution and 250 nm free Ca2+ in the pipette solution. DIDS (20 μm) blocked the macroscopic currents by ∼55%. B, I–V relationships from five different spermatozoa without (control) or with 20 μm DIDS, which significantly inhibits the current (∼60% at 100 mV). C, DIDS dose-dependently blocks the Ca2+-dependent Cl− currents. The continuous line is the fit of the dose–response data to the Hill equation which estimates an apparent IC50 = 16 ± 1.4 μm. For B and C, data represent means ± SEM with n = 5–7.
Whole-cell patch clamp recordings in the cytoplasmic droplet without detergent reveal Ca2+-dependent Cl− currents blocked by TMEM16Ainh, a small molecule TMEM16A Cl− channel inhibitor
The use of detergent to obtain perforated whole cell patch clamp recordings in human spermatozoa could alter the observed Ca2+-dependent Cl− currents. To rule out this possibility, similar experiments were carried sealing in the cytoplasmic droplet and achieving the whole-cell configuration as described by Kirichok et al. (2006) and Lishko et al. (2010). Monovalent ICatSper currents stimulated by progesterone were recorded as a positive control using this methodology (Fig. 6A). This later experiment revealed NFA sensitive Ca2+-dependent Cl− currents displaying similar characteristics (Fig. 6B) to those described in the human sperm perforated patch recordings with similar reversal potential (approximately –15 mV) and outward rectification to depolarization voltages. The shape of currents generated by the voltage steps, the tail current after repolarizing to –120 mV and the clear activation by 250 nm Ca2+ are the same as those obtained using the perforated patch. Figure 6B shows a typical family of CaCC currents with [Ca2+]i = 250 nm in control conditions and after application of NFA 10 μm, using the same voltage step protocol and solutions described earlier. NFA inhibited nearly 50% of CaCC currents, as shown previously in Fig. 4.
To investigate the contribution of TMEM16A to the human sperm CaCC currents, we used TMEM16Ainh, a small molecule (aminophenylthiazole) TMEM16A inhibitor (generously provided by Dr Verkman, University of California). Figure 6C shows CaCC currents obtained before and after application of 10 μm TMEM16Ainh. A similar percentage of inhibition of the CaCC currents at +100 mV was observed with 10 μm NFA (∼50%) and 10 μm TMEM16Ainh (∼60%) (Fig. 6B and C). The I–V plots of the blockage of the CaCC currents by 10 μm NFA and 10 μm TMEM16Ainh are illustrated in Fig. 6D. The normalized blockage fraction of the outward currents (100 mV) was plotted against the TMEM16Ainh concentration in a semi log scale. The best fit to a Hill equation is the continuous line between the data points. Best fit parameters were IC50 = 482 nm and nH = 0.9 (Fig. 6E). These findings indicate that a major component of the CaCC currents is blocked by TMEM16Ainh (approximately 60%) and appears to be carried through TMEM16A channels.
Acrosome reaction assays
Our previous work in mouse spermatozoa suggested the participation of Cl− channels in the induction of AR (Espinosa et al. 1998). Considering this we tested if the CaCC blockers could inhibit the rhZP3-induced AR in the human spermatozoa. The results in Fig. 7 show that in the absence of rhZP3 the control (no addition) and the addition of DMSO (drugs vehicle) displayed a low acrosome reaction index (ARI) (∼1%). This index relates the AR induced by A23187, the maximum considered as ∼100%, to that induced by rhZP3 (8–10%). Application of rhZP3 elevated the ARI to ∼30%. Figure 7A and B illustrate the NFA and DIDS dose-dependent inhibition of the acrosome reaction. The estimated IC50 for these compounds (3 and 1 μm, respectively) are similar to those determined for the CaCCs. Figure 7C shows that the application of 10 and 20 μm TMEM16Ainh diminished the ARI to about 5–10% (AR was ∼6-fold less than the control with rhZP3). These results indicate that CaCCs, and possibly TMEM16A channels, have an essential role in the human sperm AR as anticipated from our results from mice spermatozoa.
Figure 7. The rhZP3-induced AR in human spermatozoa is inhibited by CaCC/ TMEM16Ainh.
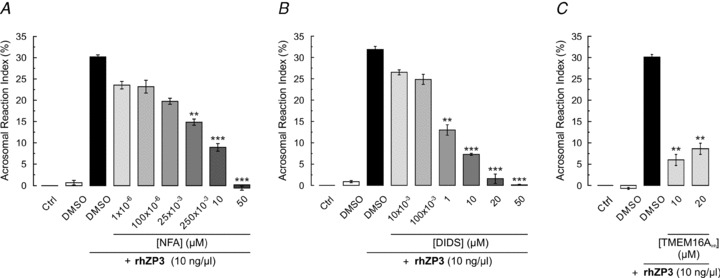
Motile human spermatozoa were obtained by the swim-up technique and capacitated during 5 h. Sperm populations were preincubated for 15 min with different concentrations of NFA, DIDS and TMEM16Ainh. The AR was induced with rhZP3 (10 ng μl−1). Cells were fixed with cold methanol and the acrosomal status was evaluated after staining sperm with FITC-PSA. Acrosomal reaction was expressed as an index (ARI, see Methods) and was used to estimate the percentage of AR inhibition (see Methods for details). NFA (A) and DIDS (B), two inhibitors of CaCCs, inhibited 90% of the AR. TMEM16Ainh (C) blocked approximately 80%, indicating that TMEM16A channels may have an important contribution in the AR. For all data, error bars represent ± SEM with n = 4–6. Statistical comparisons according to Student's unpaired t test indicate: *P < 0.05; **P < 0.01; ***P < 0.001 versus spermatozoa incubated with 0.1% DMSO + 10 ng μl−1 of rhZP3.
Discussion
In the present study, we demonstrated that human mature spermatozoa possess a typical Ca2+-dependent Cl− current. We implemented a modified perforated patch-clamp strategy to obtain whole cell recordings on the head of mature human spermatozoa by including detergents in the patch pipette (see Methods). This strategy represents an alternative to sealing on the cytoplasmic droplet, which has already opened new avenues for electrophysiological research in mouse and human spermatozoa (Lishko et al. 2010, 2011). Our new approach offers, in principle, an alternative to whole cell recordings in species where the sperm cytoplasmic droplet is lost at or after ejaculation (e.g. bull, mouse, domestic cat) (Axner & Linde Forsberg, 2007; Cooper, 2011), or in those cases in which mature spermatozoa do not possess a droplet at all (e.g. sea urchin, starfish, and fish) (Pudney, 1995; Arakaki et al. 1998; Smith & Ryan, 2010). Previous studies of single channel activity in cell-attached patches from the head of human spermatozoa revealed the presence of a Cl−-permeable channel in the equatorial region. However, its full characterization was precluded by the presence of different channel types closely associated in the same patch and the fact that channels were recorded in cell attached conditions without knowing the membrane potential and without controlling the intracellular ionic conditions (Jimenez-Gonzalez et al. 2007).
Using the perforated patch-clamp strategy we recorded robust whole-cell Cl− currents, activated by depolarization and Ca2+ (Kd = 163 nm), and blocked by NFA (IC50 = 8.8 μm) and DIDS (IC50 = 16 μm) in mature human spermatozoa (Figs. 2–5). Indirect evidence for other Cl− channels such as CFTR and the GABAA receptor has been reported in mouse and human spermatozoa (Meizel, 1997; Ritta et al. 1998; Gong et al. 2001; Hernandez-Gonzalez et al. 2007). However, the biophysical and pharmacological characteristics of the Cl− currents described in the present work, plus their [Ca2+]i dependence, indicate that they are mediated by CaCCs (Suzuki et al. 2006). The [Cl−]e, [Ca2+]i and voltage-dependent patterns displayed by the human spermatozoa Cl− currents reported in this paper are quite characteristic of CaCCs and in particular of the TMEM16A channels heterologously expressed. As in the heterologous expressed TMEM16A channels, these currents also show a high [Ca2+]i-dependent time constant of activation (Fig. S3). Besides the biophysical properties mentioned above, the pharmacological profile of the spermatozoa Ca2+-dependent Cl− currents closely follows that shown by native CaCCs and heterologously expressed TMEM16A channels (IC50 of 16–250 μm for DIDS and 2–44 μm for NFA) (Hartzell et al. 2005; Caputo et al. 2008; Schroeder et al. 2008; Yang et al. 2008). In general the IC50 values for drugs and the calcium dependence obtained for CaCCs in native cells are lower than those calculated in heterologous expression systems. These observations may indicate subtle but important differences between CaCC isoforms, lipid environments or the presence of specific auxiliary subunits capable of modifying the properties of the channels. It will be important to investigate these possibilities in the future.
We have corroborated that the Cl− currents recorded with the perforated patch are reproduced in cytoplasmic droplet recordings. Furthermore, we show that these later currents are inhibited by the small molecule TMEM16A inhibitor, which is consistent with the proposal that TMEM16A channels may significantly contribute to the CaCC currents in human mature spermatozoa (Fig. 6B–E).
Two families of proteins, the bestrophins and the anoctamins (e.g. bestrophin 1 and TMEM16A, respectively), recapitulate the properties of CaCCs, but TMEM16A shows most of the biophysical and pharmacological properties that have been attributed to CaCC in various tissues. Additionally, bestrophin 1 knockout mice show normal fertility and thriving (Kunzelmann et al. 2009). Though our results do not rule out a partial contribution of bestrophins or other TMEM16 family members as part of the CaCC currents we recorded in the head and the cytoplasmic droplet of human spermatozoa, their overall characteristics resemble mostly those described for TMEM16A CaCC (Almaca et al. 2009).
TMEM16A is expressed in different tissues such as smooth muscle cells of reproductive tracts like the oviduct and ductus epididymis (Huang et al. 2009). Its high expression in these reproductive tissues is consistent with our findings and raises the possibility that this channel could be implicated in physiological processes such as capacitation and the AR of the human spermatozoa, as we suggest here (Fig. 7). The TMEM16 family is composed of 10 members (A through K), also known as anoctamins 1 through 10. Although only TMEM16A and TMEM16B have been shown to express functional CaCCs (Schroeder et al. 2008), other members of the family may also form Cl− channels (Galietta, 2009). The sequence identity between the TMEM16 family members is relatively high, in particular in the transmembrane regions (about 50–60%). Furthermore, it is not clear yet whether the Cl− channel formed by TMEM16A is a monomer or a hetero-oligomer formed by other proteins and/or other members of the TMEM16 family (Galietta, 2009). From the reports currently known and our results, we suggest that the major component of the TMEM16 family present in the human spermatozoa head, is TMEM16A. We attempted to identify this protein using a TMEM16A antibody which in total protein extracts detected a faint band of the reported molecular mass (117 kDa). This protein was enriched by immunoprecipitation yielding a single band of the correct molecular mass. As this antibody did not reliably work to immunolocalize the channel in sperm and we did not have the peptide to compete, or access to the null TMEM16A mouse to establish the specificity of the antibody, we consider these results preliminary.
It has been shown that NFA and DIDS block mice spermatozoa Cl− channels. NFA was reported to inhibit a Ca2+ induced hyperpolarization which is partially driven by Cl− and the AR induced by solubilized ZP (Espinosa & Darszon, 1995; Espinosa et al. 1998). With this in mind, the effect of these compounds plus TMEM16Ainh was tested on the rhZP3-induced AR in human spermatozoa. Consistent with our previous results in mice, both NFA and DIDS inhibited the rhZP3-induced AR in a dose-dependent manner with IC50 values of 3 and 1 μM, respectively, which resemble those found for the blocking of the CaCC sperm currents (Fig. 7). In addition, the application of saturating concentrations of the TMEM16Ainh (10 and 20 μm) inhibited nearly 80% of the AR, indicating TMEM16A channels may participate in the human spermatozoa AR.
In recent times a remarkable pharmacological overlap has been revealed between Ca2+-dependent Cl− channels and the large conductance, Ca2+-gated K+ channels (BKCa or KCa1.1) (Greenwood & Leblanc, 2007; Sones et al. 2009). A wide range of structurally disparate agents considered to be Cl− channel blockers, such as NFA, anthracene-9-carboxylate and ethacrynic acid, enhance KCa1.1 currents (Ottolia & Toro, 1994; Greenwood & Large, 1995; Toma et al. 1996). As evidence suggests the possible presence of BKCa in mammalian sperm (Wu et al. 1998; Rossato et al. 2001), the combined effects of NFA could enhance its inhibitory potency on the AR. This reaction involves prominent sperm head membrane rearrangements triggered by [Ca2+]i changes (∼25–1000 nm) leading to acrosome swelling and a regulatory volume decrease which may involve CaCCs. By blocking CaCCs, NFA, DIDS and TMEM16Ainh could alter cell volume regulation, which might influence the distance between the outer acrosomal membrane and the plasma membrane, which is critical for acrosome exocytosis (Rossato et al. 1996; Zanetti & Mayorga, 2009).
Recently, Sones et al. (2010) showed that cholesterol depletion increases the amplitude of Ca2+-dependent Cl− currents from murine portal vein myocytes. The cholesterol removing agent methyl-β-cyclodextrin (M-βCD) affected the distribution of KCa1.1 and TMEM16A in lipid rafts. Cholesterol removal from the sperm plasma membrane during capacitation results in its reorganization, which is essential for the induction of the AR (Osheroff et al. 1999; Visconti et al. 1999; Bou Khalil et al. 2006; Girouard et al. 2008). It is possible that these mechanisms could modulate the AR by regulating Ca2+-dependent Cl− channels together with other sperm ion channels such as K+, Ca2+ and TRP channels (De Blas et al. 2009; Jose et al. 2010; Santi et al. 2010).
In summary, our data demonstrate the presence of robust CaCC currents in human spermatozoa. Their pharmacological and biophysical characteristics are consistent with TMEM16A channels contributing importantly to them. Our findings also indicate that CaCC currents, and possibly TMEM16A, actively participate in the AR triggered by rhZP3 in human spermatozoa.
Future studies are necessary to determine the precise localization and the physiological mechanisms that control the activity of the TMEM16A channels and how they modulate the AR.
Acknowledgments
We thank Orlando Trujillo Domínguez and Yoloxochitl Sanchez Guevara for their technical assistance, Dr Alan S. Verkman for the generous donation of the TMEM16A inhibitor used in this study, Dr Enrique Balderas for composing supplementary Figure 1 and Dr Froylán Gomez-Lagunas for valuable suggestions. This work was supported by NIH Grants R01 HD038082-07A1 (to P.V.), CONACyT-Mexico, (49113 to A.D., 47011 and 99333 to C.T.), DGAPA/UNAM (IN211809 and IN225406 to A.D., IN204109 and IN202212-3 to C.T. and IN217409 and IN204112–3 to C.B.). A.D. and G.F. are also grateful for international cooperation aid from CSIC (UdelaR, Uruguay) and from the SSRREE (México). We have no conflict of interest or disclosures.
Glossary
Abbreviations
- AR
acrosome reaction
- CaCC
calcium-dependent chloride channel
- cAMP
adenosine 3′,5′-cyclic monophosphate
- DIDS
4,4′-diisothiocyano-2,2′-stilbene disulphonic acid
- NFA
niflumic acid
- rhZP3
recombinant human glycoprotein from the zona pellucida number 3
- TMEM16A
transmembrane protein 16A, also called anoctamin-1
- TMEM16Ainh
a small molecule TMEM16A inhibitor
- ZP
zona pellucida
Author contributions
The conception and design of the electrophysiological experiments was done by G.O., G.F. and A.D. The acrosome reaction assays were performed by O.J., C.L.T. and A.D. The collection, analysis and interpretation of electrophysiological data were performed by G.O., and G.F. The drafting of the article and critical revision for important intellectual content were done by G.O., G.F., A.D., O.J., C.L.T. and C.B. All authors provided final approval of the version to be published. All experiments were carried out at the Universidad Nacional Autónoma de México.
Supplementary material
Supplemental Figure 1
Supplemental Figure 2
Supplemental Figure 3
References
- Almaca J, Tian Y, Aldehni F, Ousingsawat J, Kongsuphol P, Rock JR, Harfe BD, Schreiber R, Kunzelmann K. TMEM16 proteins produce volume-regulated chloride currents that are reduced in mice lacking TMEM16A. J Biol Chem. 2009;284:28571–28578. doi: 10.1074/jbc.M109.010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakaki Y, Uehara T, Fagoonee I. Comparative studies of the genus Echinometra from Okinawa and Mauritius. Zoolog Sci. 1998;15:159–168. doi: 10.2108/zsj.15.159. [DOI] [PubMed] [Google Scholar]
- Arreola J, Melvin JE, Begenisich T. Activation of calcium-dependent chloride channels in rat parotid acinar cells. J Gen Physiol. 1996;108:35–47. doi: 10.1085/jgp.108.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axner E, Linde Forsberg C. Sperm morphology in the domestic cat, and its relation with fertility: a retrospective study. Reprod Domest Anim. 2007;42:282–291. doi: 10.1111/j.1439-0531.2007.00780.x. [DOI] [PubMed] [Google Scholar]
- Bailey JL. Factors regulating sperm capacitation. Syst Biol Reprod Med. 2010;56:334–348. doi: 10.3109/19396368.2010.512377. [DOI] [PubMed] [Google Scholar]
- Baron A, Pacaud P, Loirand G, Mironneau C, Mironneau J. Pharmacological block of Ca2+-activated Cl− current in rat vascular smooth muscle cells in short-term primary culture. Pflugers Arch. 1991;419:553–558. doi: 10.1007/BF00370294. [DOI] [PubMed] [Google Scholar]
- Bou Khalil M, Chakrabandhu K, Xu H, Weerachatyanukul W, Buhr M, Berger T, Carmona E, Vuong N, Kumarathasan P, Wong PT, Carrier D, Tanphaichitr N. Sperm capacitation induces an increase in lipid rafts having zona pellucida binding ability and containing sulfogalactosylglycerolipid. Dev Biol. 2006;290:220–235. doi: 10.1016/j.ydbio.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Caballero-Campo P, Chirinos M, Fan XJ, Gonzalez-Gonzalez ME, Galicia-Chavarria M, Larrea F, Gerton GL. Biological effects of recombinant human zona pellucida proteins on sperm function. Biol Reprod. 2006;74:760–768. doi: 10.1095/biolreprod.105.047522. [DOI] [PubMed] [Google Scholar]
- Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, Pfeffer U, Ravazzolo R, Zegarra-Moran O, Galietta LJ. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- Chen Q, Peng H, Lei L, Zhang Y, Kuang H, Cao Y, Shi QX, Ma T, Duan E. Aquaporin3 is a sperm water channel essential for postcopulatory sperm osmoadaptation and migration. Cell Res. 2010;21:922–933. doi: 10.1038/cr.2010.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WY, Xu WM, Chen ZH, Ni Y, Yuan YY, Zhou SC, Zhou WW, Tsang LL, Chung YW, Hoglund P, Chan HC, Shi QX. Cl− is required for HCO3− entry necessary for sperm capacitation in guinea pig: involvement of a Cl−/HCO3− exchanger (SLC26A3) and CFTR. Biol Reprod. 2009;80:115–123. doi: 10.1095/biolreprod.108.068528. [DOI] [PubMed] [Google Scholar]
- Conner SJ, Lefievre L, Hughes DC, Barratt CL. Cracking the egg: increased complexity in the zona pellucida. Hum Reprod. 2005;20:1148–1152. doi: 10.1093/humrep/deh835. [DOI] [PubMed] [Google Scholar]
- Cooper TG. The epididymis, cytoplasmic droplets and male fertility. Asian J Androl. 2011;13:130–138. doi: 10.1038/aja.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper TG, Yeung CH. Involvement of potassium and chloride channels and other transporters in volume regulation by spermatozoa. Curr Pharm Des. 2007;13:3222–3230. doi: 10.2174/138161207782341240. [DOI] [PubMed] [Google Scholar]
- Curry MR, Millar JD, Tamuli SM, Watson PF. Surface area and volume measurements for ram and human spermatozoa. Biol Reprod. 1996;55:1325–1332. doi: 10.1095/biolreprod55.6.1325. [DOI] [PubMed] [Google Scholar]
- Darszon A, Nishigaki T, Beltran C, Trevino CL. Calcium channels in the development, maturation, and function of spermatozoa. Physiol Rev. 2011;91:1305–1355. doi: 10.1152/physrev.00028.2010. [DOI] [PubMed] [Google Scholar]
- De Blas GA, Darszon A, Ocampo AY, Serrano CJ, Castellano LE, Hernandez-Gonzalez EO, Chirinos M, Larrea F, Beltran C, Trevino CL. TRPM8, a versatile channel in human sperm. PloS One. 2009;4:e6095. doi: 10.1371/journal.pone.0006095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa F, Darszon A. Mouse sperm membrane potential: changes induced by Ca2+ FEBS Lett. 1995;372:119–125. doi: 10.1016/0014-5793(95)00962-9. [DOI] [PubMed] [Google Scholar]
- Espinosa F, de la Vega-Beltran JL, Lopez-Gonzalez I, Delgado R, Labarca P, Darszon A. Mouse sperm patch-clamp recordings reveal single Cl− channels sensitive to niflumic acid, a blocker of the sperm acrosome reaction. FEBS Lett. 1998;426:47–51. doi: 10.1016/s0014-5793(98)00305-6. [DOI] [PubMed] [Google Scholar]
- Fan JS, Palade P. Perforated patch recording with β-escin. Pflugers Arch. 1998;436:1021–1023. doi: 10.1007/pl00008086. [DOI] [PubMed] [Google Scholar]
- Frings S, Reuter D, Kleene SJ. Neuronal Ca2+-activated Cl− channels – homing in on an elusive channel species. Prog Neurobiol. 2000;60:247–289. doi: 10.1016/s0301-0082(99)00027-1. [DOI] [PubMed] [Google Scholar]
- Furst J, Gschwentner M, Ritter M, Botta G, Jakab M, Mayer M, Garavaglia L, Bazzini C, Rodighiero S, Meyer G, Eichmuller S, Woll E, Paulmichl M. Molecular and functional aspects of anionic channels activated during regulatory volume decrease in mammalian cells. Pflugers Arch. 2002;444:1–25. doi: 10.1007/s00424-002-0805-1. [DOI] [PubMed] [Google Scholar]
- Galietta LJ. The TMEM16 protein family: a new class of chloride channels? Biophys J. 2009;97:3047–3053. doi: 10.1016/j.bpj.2009.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly A, Bukovsky A, Sharma RK, Bansal P, Bhandari B, Gupta SK. In humans, zona pellucida glycoprotein-1 binds to spermatozoa and induces acrosomal exocytosis. Hum Reprod. 2010;25:1643–1656. doi: 10.1093/humrep/deq105. [DOI] [PubMed] [Google Scholar]
- Girouard J, Frenette G, Sullivan R. Seminal plasma proteins regulate the association of lipids and proteins within detergent-resistant membrane domains of bovine spermatozoa. Biol Reprod. 2008;78:921–931. doi: 10.1095/biolreprod.107.066514. [DOI] [PubMed] [Google Scholar]
- Gong XD, Li JC, Cheung KH, Leung GP, Chew SB, Wong PY. Expression of the cystic fibrosis transmembrane conductance regulator in rat spermatids: implication for the site of action of antispermatogenic agents. Mol Hum Reprod. 2001;7:705–713. doi: 10.1093/molehr/7.8.705. [DOI] [PubMed] [Google Scholar]
- Greenwood IA, Large WA. Comparison of the effects of fenamates on Ca-activated chloride and potassium currents in rabbit portal vein smooth muscle cells. Br J Pharmacol. 1995;116:2939–2948. doi: 10.1111/j.1476-5381.1995.tb15948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood IA, Leblanc N. Overlapping pharmacology of Ca2+-activated Cl− and K+ channels. Trends Pharmacol Sci. 2007;28:1–5. doi: 10.1016/j.tips.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Hartzell C, Putzier I, Arreola J. Calcium-activated chloride channels. Annu Rev Physiol. 2005;67:719–758. doi: 10.1146/annurev.physiol.67.032003.154341. [DOI] [PubMed] [Google Scholar]
- Hernandez-Gonzalez EO, Trevino CL, Castellano LE, de la Vega-Beltran JL, Ocampo AY, Wertheimer E, Visconti PE, Darszon A. Involvement of cystic fibrosis transmembrane conductance regulator in mouse sperm capacitation. J Biol Chem. 2007;282:24397–24406. doi: 10.1074/jbc.M701603200. [DOI] [PubMed] [Google Scholar]
- Hogg RC, Wang Q, Large WA. Action of niflumic acid on evoked and spontaneous calcium-activated chloride and potassium currents in smooth muscle cells from rabbit portal vein. Br J Pharmacol. 1994;112:977–984. doi: 10.1111/j.1476-5381.1994.tb13177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Rock JR, Harfe BD, Cheng T, Huang X, Jan YN, Jan LY. Studies on expression and function of the TMEM16A calcium-activated chloride channel. Proc Natl Acad Sci U S A. 2009;106:21413–21418. doi: 10.1073/pnas.0911935106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue N, Satouh Y, Ikawa M, Okabe M, Yanagimachi R. Acrosome-reacted mouse spermatozoa recovered from the perivitelline space can fertilize other eggs. Proc Natl Acad Sci U S A. 2011;108:20008–20011. doi: 10.1073/pnas.1116965108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Gonzalez MC, Gu Y, Kirkman-Brown J, Barratt CL, Publicover S. Patch-clamp ‘mapping’ of ion channel activity in human sperm reveals regionalisation and co-localisation into mixed clusters. J Cell Physiol. 2007;213:801–808. doi: 10.1002/jcp.21153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, Baba SA, Chiba K, Hirohashi N. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc Natl Acad Sci U S A. 2011;108:4892–4896. doi: 10.1073/pnas.1018202108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose O, Hernandez-Hernandez O, Chirinos M, Gonzalez-Gonzalez ME, Larrea F, Almanza A, Felix R, Darszon A, Trevino CL. Recombinant human ZP3-induced sperm acrosome reaction: evidence for the involvement of T- and L-type voltage-gated calcium channels. Biochem Biophys Res Commun. 2010;395:530–534. doi: 10.1016/j.bbrc.2010.04.059. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Shin SY, Lee JE, Kim JH, Uhm DY. Ca2+-activated Cl− channel currents in rat ventral prostate epithelial cells. Prostate. 2003;55:118–127. doi: 10.1002/pros.10214. [DOI] [PubMed] [Google Scholar]
- Kirichok Y, Navarro B, Clapham DE. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature. 2006;439:737–740. doi: 10.1038/nature04417. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K, Kongsuphol P, Aldehni F, Tian Y, Ousingsawat J, Warth R, Schreiber R. Bestrophin and TMEM16-Ca2+ activated Cl− channels with different functions. Cell Calcium. 2009;46:233–241. doi: 10.1016/j.ceca.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K, Kongsuphol P, Chootip K, Toledo C, Martins JR, Almaca J, Tian Y, Witzgall R, Ousingsawat J, Schreiber R. Role of the Ca2+-activated Cl− channels bestrophin and anoctamin in epithelial cells. Biol Chem. 2011;392:125–134. doi: 10.1515/BC.2011.010. [DOI] [PubMed] [Google Scholar]
- Lishko PV, Botchkina IL, Fedorenko A, Kirichok Y. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell. 2010;140:327–337. doi: 10.1016/j.cell.2009.12.053. [DOI] [PubMed] [Google Scholar]
- Lishko PV, Botchkina IL, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature. 2011;471:387–391. doi: 10.1038/nature09767. [DOI] [PubMed] [Google Scholar]
- Lishko PV, Kirichok Y. The role of Hv1 and CatSper channels in sperm activation. J Physiol. 2010;588:4667–4672. doi: 10.1113/jphysiol.2010.194142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litscher ES, Williams Z, Wassarman PM. Zona pellucida glycoprotein ZP3 and fertilization in mammals. Mol Reprod Dev. 2009;76:933–941. doi: 10.1002/mrd.21046. [DOI] [PubMed] [Google Scholar]
- Martinez-Lopez P, Santi CM, Trevino CL, Ocampo-Gutierrez AY, Acevedo JJ, Alisio A, Salkoff LB, Darszon A. Mouse sperm K+ currents stimulated by pH and cAMP possibly coded by Slo3 channels. Biochem Biophys Res Commun. 2009;381:204–209. doi: 10.1016/j.bbrc.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayorga LS, Tomes CN, Belmonte SA. Acrosomal exocytosis, a special type of regulated secretion. IUBMB Life. 2007;59:286–292. doi: 10.1080/15216540701222872. [DOI] [PubMed] [Google Scholar]
- Meizel S. Amino acid neurotransmitter receptor/chloride channels of mammalian sperm and the acrosome reaction. Biol Reprod. 1997;56:569–574. doi: 10.1095/biolreprod56.3.569. [DOI] [PubMed] [Google Scholar]
- Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci. 1982;215:491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- Namkung W, Phuan PW, Verkman AS. TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. J Biol Chem. 2010;286:2365–2374. doi: 10.1074/jbc.M110.175109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro B, Kirichok Y, Clapham DE. KSper, a pH-sensitive K+ current that controls sperm membrane potential. Proc Natl Acad Sci U S A. 2007;104:7688–7692. doi: 10.1073/pnas.0702018104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osheroff JE, Visconti PE, Valenzuela JP, Travis AJ, Alvarez J, Kopf GS. Regulation of human sperm capacitation by a cholesterol efflux-stimulated signal transduction pathway leading to protein kinase A-mediated up-regulation of protein tyrosine phosphorylation. Mol Hum Reprod. 1999;5:1017–1026. doi: 10.1093/molehr/5.11.1017. [DOI] [PubMed] [Google Scholar]
- Ottolia M, Toro L. Potentiation of large conductance KCa channels by niflumic, flufenamic, and mefenamic acids. Biophys J. 1994;67:2272–2279. doi: 10.1016/S0006-3495(94)80712-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudney J. Spermatogenesis in nonmammalian vertebrates. Microsc Res Tech. 1995;32:459–497. doi: 10.1002/jemt.1070320602. [DOI] [PubMed] [Google Scholar]
- Ritta MN, Calamera JC, Bas DE. Occurrence of GABA and GABA receptors in human spermatozoa. Mol Hum Reprod. 1998;4:769–773. doi: 10.1093/molehr/4.8.769. [DOI] [PubMed] [Google Scholar]
- Rossato M, Di Virgilio F, Rizzuto R, Galeazzi C, Foresta C. Intracellular calcium store depletion and acrosome reaction in human spermatozoa: role of calcium and plasma membrane potential. Mol Hum Reprod. 2001;7:119–128. doi: 10.1093/molehr/7.2.119. [DOI] [PubMed] [Google Scholar]
- Rossato M, Di Virgilio F, Foresta C. Involvement of osmosensitive calcium influx in human sperm activation. Mol Hum Reprod. 1996;2:903–909. doi: 10.1093/molehr/2.12.903. [DOI] [PubMed] [Google Scholar]
- Salicioni AM, Platt MD, Wertheimer EV, Arcelay E, Allaire A, Sosnik J, Visconti PE. Signalling pathways involved in sperm capacitation. Soc Reprod Fertil Suppl. 2007;65:245–259. [PubMed] [Google Scholar]
- Santi CM, Butler A, Kuhn J, Wei A, Salkoff L. Bovine and mouse SLO3 K+ channels: evolutionary divergence points to an RCK1 region of critical function. J Biol Chem. 2009;284:21589–21598. doi: 10.1074/jbc.M109.015040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi CM, Martinez-Lopez P, de la Vega-Beltran JL, Butler A, Alisio A, Darszon A, Salkoff L. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett. 2010;584:1041–1046. doi: 10.1016/j.febslet.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarantopoulos C, McCallum JB, Kwok WM, Hogan Q. β-Escin diminishes voltage-gated calcium current rundown in perforated patch-clamp recordings from rat primary afferent neurons. J Neurosci Methods. 2004;139:61–68. doi: 10.1016/j.jneumeth.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, Ryan MJ. Evolution of sperm quality but not quantity in the internally fertilized fish Xiphophorus nigrensis. J Evol Biol. 2010;23:1759–1771. doi: 10.1111/j.1420-9101.2010.02041.x. [DOI] [PubMed] [Google Scholar]
- Sones WR, Davis AJ, Leblanc N, Greenwood IA. Cholesterol depletion alters amplitude and pharmacology of vascular calcium-activated chloride channels. Cardiovasc Res. 2010;87:476–484. doi: 10.1093/cvr/cvq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sones WR, Leblanc N, Greenwood IA. Inhibition of vascular calcium-gated chloride currents by blockers of KCa1.1, but not by modulators of KCa2.1 or KCa2.3 channels. Br J Pharmacol. 2009;158:521–531. doi: 10.1111/j.1476-5381.2009.00332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Morita T, Iwamoto T. Diversity of Cl− channels. Cell Mol Life Sci. 2006;63:12–24. doi: 10.1007/s00018-005-5336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma C, Greenwood IA, Helliwell RM, Large WA. Activation of potassium currents by inhibitors of calcium-activated chloride conductance in rabbit portal vein smooth muscle cells. Br J Pharmacol. 1996;118:513–520. doi: 10.1111/j.1476-5381.1996.tb15432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti PE, Bailey JL, Moore GD, Pan D, Olds-Clarke P, Kopf GS. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development. 1995;121:1129–1137. doi: 10.1242/dev.121.4.1129. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Galantino-Homer H, Ning X, Moore GD, Valenzuela JP, Jorgez CJ, Alvarez JG, Kopf GS. Cholesterol efflux-mediated signal transduction in mammalian sperm. β-Cyclodextrins initiate transmembrane signaling leading to an increase in protein tyrosine phosphorylation and capacitation. J Biol Chem. 1999;274:3235–3242. doi: 10.1074/jbc.274.5.3235. [DOI] [PubMed] [Google Scholar]
- Wertheimer EV, Salicioni AM, Liu W, Trevino CL, Chavez J, Hernandez-Gonzalez EO, Darszon A, Visconti PE. Chloride Is essential for capacitation and for the capacitation-associated increase in tyrosine phosphorylation. J Biol Chem. 2008;283:35539–35550. doi: 10.1074/jbc.M804586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. WHO Laboratory Manual for the Examination and Processing of Human Semen. Geneva: WHO; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WL, So SC, Sun YP, Chung YW, Grima J, Wong PY, Yan YC, Chan HC. Functional expression of P2U receptors in rat spermatogenic cells: dual modulation of a Ca2+-activated K+ channel. Biochem Biophys Res Commun. 1998;248:728–732. doi: 10.1006/bbrc.1998.9051. [DOI] [PubMed] [Google Scholar]
- Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, Park SP, Lee J, Lee B, Kim BM, Raouf R, Shin YK, Oh U. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Barfield JP, Cooper TG. Chloride channels in physiological volume regulation of human spermatozoa. Biol Reprod. 2005;73:1057–1063. doi: 10.1095/biolreprod.105.044123. [DOI] [PubMed] [Google Scholar]
- Yeung CH, Cooper TG. Potassium channels involved in human sperm volume regulation–quantitative studies at the protein and mRNA levels. Mol Reprod Dev. 2008;75:659–668. doi: 10.1002/mrd.20812. [DOI] [PubMed] [Google Scholar]
- Zanetti N, Mayorga LS. Acrosomal swelling and membrane docking are required for hybrid vesicle formation during the human sperm acrosome reaction. Biol Reprod. 2009;81:396–405. doi: 10.1095/biolreprod.109.076166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


