Abstract
Dramatically improved survival associated with tyrosine kinase inhibitor (TKI) therapy has transformed the disease model for chronic myeloid leukemia (CML) to one of long-term management, but treatment success is challenged with poor medication adherence. Many risk factors associated with poor adherence can be ameliorated by close monitoring, dose modification, and supportive care. Controlling risk factors for poor adherence in combination with patient education that includes direct communication between the health care team and the patient are essential components for maximizing the benefits of TKI therapy.
Introduction
The last three decades have witnessed extraordinary advances in the treatment of chronic diseases, among which tyrosine kinase inhibitor (TKI) therapy for chronic myeloid leukemia (CML) is perhaps one of the most outstanding examples. Between 1975 and 1977, the 5-year relative survival rate of patients with CML was only 24% [1]. Allogeneic stem cell transplantation offered a potential cure for a small subset of patients; however, the risk of serious infection, graft-versus-host disease, relapse, and mortality presented substantial limitations [2,3]. The introduction of imatinib (Gleevec®, Novartis Pharmaceuticals Corporation, East Hanover, NJ) in 2001 replaced interferon-α as the standard of care for CML patients, with a result of remarkable improvement in patient survival. For example, the 8-year follow-up analysis of imatinib treatment in 553 CML patients participating in the International Randomized Study of Interferon versus STI571 demonstrated an overall survival (OS) rate of 85%; OS was 93% when only CML-related deaths and those before stem cell transplantation are considered [4]. With this prolonged survival and the recent approval of the more potent agents nilotinib (Tasigna®, Novartis Pharmaceuticals Corporation, East Hanover, NJ) and dasatinib (Sprycel®, Bristol-Myers Squibb Company, Princeton, NJ), which may be able to provide additional survival benefit, CML more closely resembles a chronic rather than a fatal disease. A new challenge has arisen, however, stemming directly from the success of TKI therapy. Poor medication adherence has come into focus as a major impediment to successful treatment of patients with CML: New evidence has shown that inadequate adherence to imatinib and suboptimal outcomes are inextricably linked [5-7].
Difficulty in maintaining high medication adherence is not limited to TKI therapy or patients with CML, however. Indeed, the problem is pervasive across chronic conditions. For example, a 2003 report from the World Health Organization found the average rate of long-term adherence for diseases such as asthma, diabetes, hypertension, and tuberculosis was only about 50% in developed countries, and assumed to be even lower in developing countries where health resources are fewer and access to care is unpredictable [8]. The report identified five key areas that affect adherence, including factors related to socioeconomics, the health care team or health system, therapy and interventions, the condition and its treatment, and factors intrinsic to patients themselves. Although some factors were relatively more important in one chronic illness than another, multiple factors often were involved, making poor adherence a multidimensional problem [8].
The impact of poor adherence across chronic conditions was demonstrated in a meta-analysis of 63 studies in cancer, diabetes, heart disease, hypercholesterolemia, hypertension, intestinal diseases, recurrent otitis media, sleep apnea, and transplant. The analysis found a significant difference in outcome between patients with high versus low adherence: high adherence reduced the risk of a poor outcome by 26% [9]. The degree to which patients adhere to medication also has larger societal ramifications. One large retrospective study found that high rates of medication adherence were associated with lower disease-related medical costs and significantly lower hospitalization rates for patients with diabetes, hypertension, hypercholesterolemia, and congestive heart failure [10]. Similar relationships were observed when adherence and health care costs were analyzed in patients with HIV [11] and osteoporosis [12].
Although it may be assumed that adherence is universally high in CML because the benefits of adhering to TKI treatment are clear, the literature suggests that long-term adherence will be problematic for some patients. Furthermore, adherence is not an “all or none” phenomenon but represents a range of behaviors, from taking all medications as prescribed, to an occasional skipped dose, to taking few or no doses at all [13]. It is essential for all clinicians who treat CML to be aware of the degree and impact of poor adherence to TKIs, the underlying risk factors, and strategies to ensure that patients continue TKI therapy to gain its full benefits.
Adherence in Patients with CML
The study of adherence to TKIs in patients with CML is relatively new, and all but one report to date have examined adherence only to imatinib. The Adherence Assessment with Glivec: Indicators and Outcomes (ADAGIO) study was a prospective, 90-day, observational study that used clinical interviews, self-reporting, and pill counts to assess adherence [6]. In this study, physicians estimated adherence among the 169 evaluable patients to be very high, believing that on average, 92.8% of patients were adherent to imatinib during the first month after diagnosis, and 87.4% after 12 months of treatment. Patients also reported high adherence rates, as indicated on a 100-point visual analog scale (VAS): the average VAS rating of adherence was 95.3 at enrollment and 95.7 at the 90-day follow-up. However, assessment by an adherence scale yielded quite different results from pill counting. Use of the Basel Assessment of Adherence Scale [14] indicated that approximately one-third of patients were nonadherent in the 30 days before and the 90 days during the study. Based on pill count, on average, 90.9% of the prescribed imatinib was taken; however, 71% of patients took less (with some patients taking as little as 29%), and 14.8% took more medication than prescribed (up to 202%). Only 14.2% of patients took their medication as prescribed [6].
A study from the Hammersmith Hospital in the United Kingdom examined adherence in 87 patients with chronic-phase CML who had been receiving imatinib 400 mg for a median of nearly 5 years [5]. Adherence was monitored for a median of 3 months using a microelectronic monitoring system, an electronic device fitted into the medication bottle cap that recorded each time the bottle was opened. The median adherence rate was 98%, but ranged from 24 to 104%. More than a quarter of the population (26%) was ≤90% adherent, and 14% of the population was ≤80% adherent [5].
Health care databases, which include electronic pharmacy claims and medical data, provide another means to assess adherence. A retrospective study by Darkow et al. analyzed treatment interruptions and the medication possession ratio (MPR) during 12 months of imatinib therapy in 267 participants in a managed care plan. A treatment interruption (failure to refill an imatinib prescription within 30 days of when the previous prescription ran out) was observed in 31% of patients, and the MPR (calculated as the total days when imatinib was available divided by 365 days × 100) was 77.7% [15]. Wu et al. also used commercial claims data and the MPR to determine imatinib adherence. In their study, 592 patients were categorized as having either a low (<85%) or high (≥85%) MPR. The mean MPR over 12 months was 79%; 40.9% of patients had a low MPR and 59% had a high MPR [16]. Furthermore, a retrospective cohort study used pharmacy data from an employee health information database to calculate the average 12-month MPR. Patients were considered to be adherent to imatinib if they maintained an average MPR of >85%. Among 430 patients, the mean MPR was 80%, with only 60% of patients reaching an MPR of >85% [17].
Information regarding nilotinib and dasatinib is limited. One recent study has examined adherence to these more potent TKIs in second-line settings [18]. Two large retrospective claims databases were combined; patients with CML who received one or more prescriptions of dasatinib (n = 452) or nilotinib (n = 69) were identified and followed for up to 6 months. Treatment adherence was measured by the proportion of days covered (PDC). Patients receiving nilotinib had better adherence compared with those receiving dasatinib, representing a difference of 17.1 days of coverage over 6 months. The mean (standard deviation) PDC with nilotinib was 0.79 (0.23) versus 0.69 (0.28) with dasatinib (P = 0.009) [18]. This finding was unexpected given that dasatinib has a once-daily regimen, whereas nilotinib is taken twice daily [18].
Although the studies described here used different methods to measure adherence, results were strikingly consistent: A substantial proportion of patients with CML have difficulty adhering to TKI treatment.
Impact of Poor Adherence on Treatment Response
Poor adherence to imatinib therapy can have profoundly negative consequences, including a suboptimal treatment response, imatinib resistance, and disease relapse [19]. In the ADAGIO study (N = 169), which used pill count as a surrogate measure of adherence, patients who were less adherent were more likely to have a suboptimal response than those who had good adherence [6]. Specifically, patients with a partial cytogenetic response had taken between 74 and 77% of the prescribed dose; patients with a complete cytogenetic response (CCyR) had taken between 90 and 93% of the prescribed dose [6].
In the Hammersmith Hospital study (N = 87), adherence was associated with achieving major molecular response (MMR; a 3-log reduction from baseline of BCR-ABL transcript levels or <0.1% on the international scale [IS]) and complete molecular response (CMR; an undetectable BCR-ABL transcript level after two consecutive polymerase chain reaction measurements, equivalent to <0.0032% on the IS) [5]. Highly adherent patients (those who took >90% of medication as prescribed) had a significantly higher 6-year probability than did less adherent patients (those who took ≤90% of medication as prescribed) of achieving MMR (95% vs. 28%, respectively; P < 0.001) and CMR (44% vs. 0%, respectively; P = 0.002). No MMRs were observed when adherence was ≤80%, and no CMRs were observed when adherence was ≤90%. Moreover, multivariate analysis identified adherence as one of two independent predictors for achieving MMR and the only independent predictor for achieving CMR. These data led the authors to conclude that good adherence was critical for achieving molecular responses in patients who had achieved CCyR with imatinib [5]. Although two patients harbored KD mutations, the authors acknowledge that there are insufficient data to relate adherence to mutations. This work was continued in a follow-up study in which the authors investigated the relationship between adherence to imatinib, as measured by microelectronic monitoring systems, and the probability of losing CCyR and of imatinib failure in the subsequent 2 years [7]. Patients with low adherence (≤85%; n = 23) had a higher probability than did adherent patients (>85% adherence; n = 64) of losing their CCyR at 2 years (26.8% vs. 1.5%, respectively; P = 0.0002) and a lower probability of remaining on imatinib (64.5% vs. 90.6%, respectively; P = 0.006). On multivariate analysis, the adherence rate and failure to achieve an MMR were the only independent predictors for loss of CCyR and discontinuation of imatinib therapy [7].
Recently, a third study, performed at the Adyar Cancer Institute, Chennai, India, has been reported in the literature [20]. The authors evaluated the records of 516 patients receiving imatinib for chronic-phase CML for adherence to therapy and patient outcomes. Nonadherence was defined as unwarranted treatment interruption for more than 1 week. For all patients, the estimated 5-year event-free survival (EFS) rate was 70.8% (95% confidence interval: 63.3–78.3), with a median follow-up of 39 months. Adherent patients demonstrated a 5-year EFS rate of 76.7%, but for nonadherent patients, the 5-year EFS rate was 59.8% (P = 0.011). These data provide a direct measure of the impact of adherence on survival.
Impact of Poor Adherence on Economic Outcomes
Poor adherence is also associated with greater overall health care utilization and medical costs in CML. In the retrospective analysis of US claims data reported by Darkow et al., MPR and costs were inversely related. After controlling for age, sex, number of concomitant medications, starting dose of imatinib, and cancer complexity in 267 identified CML patients, the MPR was inversely associated with health care costs, excluding the cost of imatinib (P < 0.001) and medical costs (P < 0.001). Every 10% point reduction in MPR was associated with a concomitant 14% increase in health care costs, excluding imatinib costs and a 15% increase in overall medical costs [15].
Similarly, the claims data analysis by Wu et al. found that in a study sample of 592 CML patients, a high MPR was associated with significantly lower disease-related and total health care costs, as well as lower resource utilization [16]. Patients with a low MPR (<85%) used more health care resources, including more frequent all-cause inpatient admissions (4.1 vs. 0.4; P < 0.001) and all-cause inpatient days (14.8 vs. 1.8; P < 0.001), than did patients with a high MPR (≥85%). Patients with a low MPR also had higher inpatient care costs, outpatient costs, and non-imatinib pharmacy costs (Fig. 1). Although patients with low MPRs had lower imatinib costs than did patients with high MPRs, the cost savings were offset by the increased costs of inpatient and outpatient care. The total cost of care for the low-MPR cohort was significantly higher than that for the high-MPR cohort (Fig. 1).
Figure 1.
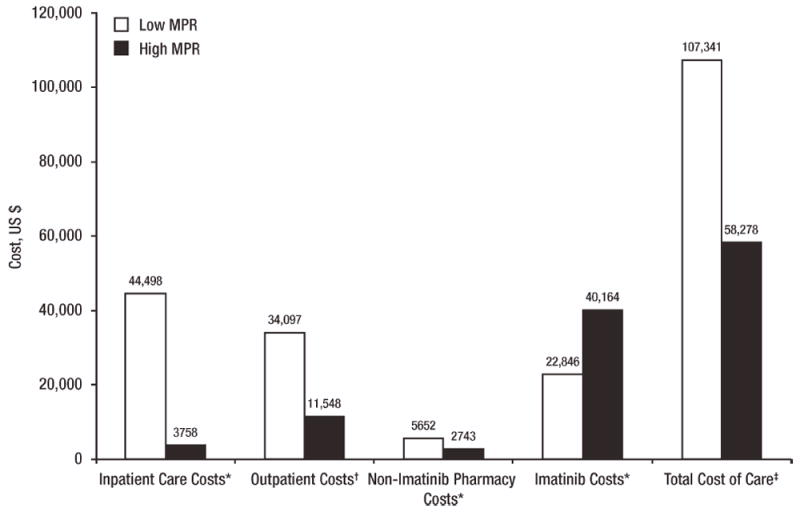
Association between adherence and costs (2005 US dollars) [16]. MPR, medication possession ratio. Note: Costs are over the first year after initiation of imatinib. Low MPR: <85%; high MPR: ≥85%. *P < 0.001. †P = 0.001. ‡P = 0.021.
Predictive Factors of Nonadherence in CML
Studies have identified a number of variables that affect adherence to TKIs. Predictors of poor adherence from two of the most recent studies found a range of factors, many of which can be addressed by close patient monitoring and proactive management (Table I). Modifiable risk factors identified by St. Charles et al. in 430 evaluable CML patients included a starting imatinib dose of >400 mg, a longer time lag between CML diagnosis and imatinib prescription fill, a greater number of concomitant prescriptions, and a higher percentage of copayment [17].
TABLE I.
| Authors | Study design | No. of patients | Adherence measure | Patient-related predictors of poor adherence |
|---|---|---|---|---|
| St. Charles et al. [17] | Retrospective cohort study using an employee-based database containing pharmacy and medical data | 430 | MPR over 12 months | • Younger agea |
| • Shorter exposure to imatinib | ||||
| • Index imatinib dose >400 mg | ||||
| • Longer time lag between CML diagnosis to imatinib prescription fill | ||||
| • More concomitant prescriptions | ||||
| • Higher copayment (>$5/day) | ||||
| Marin et al. [5] | Prospective study that monitored adherence during long-term imatinib treatment | 87 | MMS over 3 months | • Younger ageb |
| • AEs (asthenia, nausea, muscle cramps, and bone or joint pains) | ||||
| • Patients who took imatinib independently of meals | ||||
| • Unexplained fivefold increases in BCR-ABL1 transcript levels | ||||
| Noens et al. [6] | Prospective, observational, multicenter, noninterventional study | 169 | Clinical interviews, self-reporting, and pill counts over a 90-day period | Associated with increased nonadherencec |
| • Older age | ||||
| • Longer time since diagnosis of CML | ||||
| • Living alone | ||||
| • Male sex | ||||
| • Longer time on imatinib | ||||
| • Imatinib dose ≥600 mg/day | ||||
| • Higher degrees of chronic care received | ||||
| • Higher self-reported functional status/quality of life | ||||
| Associated with better adherencec | ||||
| • Knowledge of disease and treatment | ||||
| • More medications taken per day | ||||
| • Secondary school graduate or higher | ||||
| • Long-term medication behavior self-efficacy |
AE, adverse event; CML, chronic myeloid leukemia; MMS, microelectronic monitoring system; MPR, medication possession ratio.
Inclusion criteria restricted age to 18–65 years; mean age: 50 years.
Median age at enrollment: 50.7 years (range: 25.5–89.0 years).
These are not independent factors and should be interpreted as part of a canonical model of multiple complementary variables. They are presented in descending order of canonical loading (correlation of the individual variables and their respective variables).
The study from the Hammersmith Hospital (N = 87) found that common adverse events (AEs: asthenia, nausea, muscle cramps, and bone or joint pains) and taking imatinib independently of meals (a contributor to gastrointestinal tract upset) were associated with significantly lower adherence rates [5]. These data are consistent with a recent study in which patients with CML were asked to report current symptoms, how these symptoms interfered with daily activities, and how they were managed to maintain acceptable quality of life. A total of 44 symptoms were reported on a symptom inventory; fatigue, pain, and nausea were most frequent. Notably, such symptoms interfered with adherence, leading some patients to stop or consider stopping treatment or to decrease the dose or frequency of treatment [21].
Strategies to Improve Adherence
Strategies to improve adherence must take patients’ reasons for missing doses into consideration and address them directly. In-depth interviews were conducted with 21 of the 87 CML patients who participated in the Hammer-smith Hospital adherence trial to explore reasons why doses of imatinib were missed [22]. In agreement with the adherence literature at large, the results showed that patients’ reasons could be categorized as either being unintentional or intentional, with some overlap [22]. Unintentional nonadherence is when patients are hindered from taking their medication as prescribed by reasons beyond their control; intentional nonadherence is when patients consciously decide to miss doses [8]. In interviews with CML patients, the most common reason for unintentional nonadherence was forgetting doses, and the most common reason for deciding to miss doses was to avoid experiencing AEs. Surprisingly, many patients did not think missing “the odd dose” mattered and based this belief on communication with health care professionals [22]. These results suggest that to improve TKI treatment adherence in patients with CML, it is important not only to elucidate methods that will help patients remember to take doses but also to address AEs appropriately and promptly and to explain the importance of treatment adherence. Preliminary data from an online survey of 405 physicians who treat CML patients in Brazil, France, Italy, Spain, and Russia suggested that individual patient counseling on adherence and involvement of the patient in established adherence protocols positively affects adherence [23]. It is also important to involve the patients when deciding on what strategy may be most helpful for each individual [24].
Health care professionals play an important role in encouraging patient adherence to TKI therapy. They can advise patients on adherence aids, such as pillboxes and alarms, which patients who experience unintentional nonadherence may find beneficial. In addition, they can provide guidance to address patients’ concerns regarding AEs and make sure patients understand how to optimally manage their illness and treatment. Improved patient–physician communication should be promoted to support adherence, especially in regard to involving patients in treatment decisions [24].
Side effects associated with poor adherence can be managed by prompt attention and supportive care. National Comprehensive Cancer Network® Clinical Practice Guidelines in Oncology® recommend examining adherence in patients who failed to achieve a hematologic or cytogenetic response with TKI therapy [25]. Even Grades 1 and 2 treatment-related AEs should be regularly monitored, because their persistence can adversely impact adherence. Furthermore, low-grade AEs can become chronic, and supportive care should be optimized early to prevent AEs from becoming more severe. Based on clinical experience, an algorithm has been provided to address patients who present with resistance by failing to achieve treatment milestones for TKI therapy (Fig. 2). Although specific to side effects, this algorithm can be modified based on the particular risk factor or factors that are identified in individual patients. If side effects continue despite supportive care, a change to another TKI should be considered. Notably, patient education with every step is essential.
Figure 2.
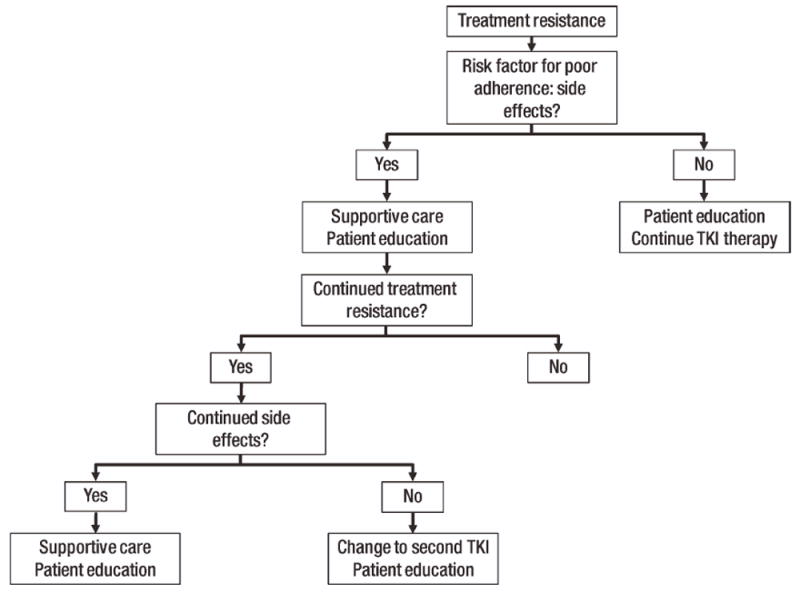
Algorithm for managing modifiable risk factors for poor adherence to tyrosine kinase inhibitor (TKI) therapy: side effects. Treatment resistance is defined as failing to achieve treatment milestones. Note: This algorithm can be modified based on the particular risk factor or factors that are identified in individual patients.
Conclusions
Over the past decade, the introduction of TKI therapy for treatment of CML patients has resulted in increased survival and improved quality of life. Poor long-term adherence to TKI therapy, however, has arisen as a critical new challenge in the management of this disease. Poor medication adherence to imatinib negatively impacts treatment efficacy and leads to higher morbidity and mortality by contributing to resistance, suboptimal cytogenetic and molecular responses, and higher risk of disease progression [5,7,19]. Further studies are needed to learn the impact of imatinib pill dosage on adherence (e.g., four 100 mg tablets vs. the 400 mg tablet), if adherence differs among patients under the care of community versus academic oncologists, and how the frequency of visits and milestone follow-up testing impacts adherence. Additional studies also are needed to investigate adherence to dasatinib and nilotinib, to determine if the impact of poor adherence observed with imatinib can be generalized across all available TKI therapies.
Although patients and physicians believe adherence to imatinib to be high, both groups overestimate the actual degree of adherence [6]. Data suggest that at least one-third of patients are poorly adherent to imatinib treatment regimens [5,6,15,17]. Understanding the underlying intentional and unintentional causes of nonadherence, investigating and addressing modifiable risk factors, and educating patients on the need to take medication as prescribed will do much to help patients achieve maximum benefits from their treatment.
Acknowledgments
The authors thank Mariana Ovnic, David Keleti, and Patricia Segarini of Percolation Communications LLC for their medical editorial assistance.
Contract grant sponsors: Novartis Pharmaceuticals Corporation (E.J., H.K., L.E., A.M.C, D.M.), Bristol-Myers Squibb Company, Pfizer Inc. (H.K., L.E., D.M.).
Footnotes
Conflict of interest: Nothing to report
References
- 1.American Cancer Society. Cancer Facts & Figures 2010. Atlanta, GA: American Cancer Society; 2010. [October 11, 2011]. Available at: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-026238.pdf. [Google Scholar]
- 2.Robin M, Guardiola P, Devergie A, et al. A 10-year median follow-up study after allogeneic stem cell transplantation for chronic myeloid leukemia in chronic phase from HLA-identical sibling donors. Leukemia. 2005;19:1613–1620. doi: 10.1038/sj.leu.2403821. [DOI] [PubMed] [Google Scholar]
- 3.Tomblyn M, Chiller T, Einsele H, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: A global perspective. Biol Blood Marrow Transplant. 2009;15:1143–1238. doi: 10.1016/j.bbmt.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deininger M, O’Brien SG, Guilhot F, et al. International randomized study of interferon vs STI571 (IRIS) 8-year follow up: Sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. Blood (ASH Annual Meeting Abstracts) 2009;114:1126. [Google Scholar]
- 5.Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28:2381–2388. doi: 10.1200/JCO.2009.26.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noens L, van Lierde MA, De Bock R, et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: The ADAGIO study. Blood. 2009;113:5401–5411. doi: 10.1182/blood-2008-12-196543. [DOI] [PubMed] [Google Scholar]
- 7.Ibrahim AR, Eliasson L, Apperley JF, et al. Poor adherence is the main reason for loss of CCyR and imatinib failure for chronic myeloid leukemia patients on long-term therapy. Blood. 2011;117:3733–3736. doi: 10.1182/blood-2010-10-309807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabaté E, editor. Adherence to Long-Term Therapies: Evidence for Action. Geneva, Switzerland: World Health Organization; 2003. [October 11, 2011]. Available at: http://whqlibdoc.who.int/publications/2003/9241545992.pdf. [Google Scholar]
- 9.DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: A meta-analysis. Med Care. 2002;40:794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS. Impact of medication adherence on hospitalization risk and healthcare cost. Med Care. 2005;43:521–530. doi: 10.1097/01.mlr.0000163641.86870.af. [DOI] [PubMed] [Google Scholar]
- 11.Munakata J, Benner JS, Becker S, et al. Clinical and economic outcomes of nonadherence to highly active antiretroviral therapy in patients with human immunodeficiency virus. Med Care. 2006;44:893–899. doi: 10.1097/01.mlr.0000233679.20898.e9. [DOI] [PubMed] [Google Scholar]
- 12.Hiligsmann M, Rabenda V, Gathon HJ, et al. Potential clinical and economic impact of nonadherence with osteoporosis medications. Calcif Tissue Int. 2010;86:202–210. doi: 10.1007/s00223-009-9329-4. [DOI] [PubMed] [Google Scholar]
- 13.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 14.Cleemput I, Dobbels F. Measuring patient-reported outcomes in solid organ transplant recipients: An overview of instruments developed to date. Pharmacoeconomics. 2007;25:269–286. doi: 10.2165/00019053-200725040-00002. [DOI] [PubMed] [Google Scholar]
- 15.Darkow T, Henk HJ, Thomas SK, et al. Treatment interruptions and non-adherence with imatinib and associated healthcare costs: A retrospective analysis among managed care patients with chronic myelogenous leukaemia. Pharmacoeconomics. 2007;25:481–496. doi: 10.2165/00019053-200725060-00004. [DOI] [PubMed] [Google Scholar]
- 16.Wu EQ, Johnson S, Beaulieu N, et al. Healthcare resource utilization and costs associated with non-adherence to imatinib treatment in chronic myeloid leukemia patients. Curr Med Res Opin. 2010;26:61–69. doi: 10.1185/03007990903396469. [DOI] [PubMed] [Google Scholar]
- 17.St Charles M, Bollu VK, Hornyak E, et al. Predictors of treatment non-adherence in patients treated with imatinib mesylate for chronic myeloid leukemia. Blood (ASH Annual Meeting Abstracts) 2009;114:2209. [Google Scholar]
- 18.Wu EQ, Guerin A, Yu AP, et al. Retrospective real-world comparison of medical visits, costs, and adherence between nilotinib and dasatinib in chronic myeloid leukemia. Curr Med Res Opin. 2010;26:2861–2869. doi: 10.1185/03007995.2010.533648. [DOI] [PubMed] [Google Scholar]
- 19.O’Dwyer M, Atallah E. Practical considerations for the management of patients in the tyrosine kinase inhibitor era. Semin Hematol. 2009;46(2 Suppl 3):S16–S21. doi: 10.1053/j.seminhematol.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Ganesan P, Sagar TG, Dubashi B, et al. Nonadherence to imatinib adversely affects event free survival in chronic phase chronic myeloid leukemia. Am J Hematol. 2011;86:471–474. doi: 10.1002/ajh.22019. [DOI] [PubMed] [Google Scholar]
- 21.Williams LA, Ault P, Cleeland CS, et al. Symptom burden in chronic myeloid leukemia (CML) J Clin Oncol (ASCO Annual Meeting Proceedings) 2010;28(Suppl 1):6133. [Google Scholar]
- 22.Eliasson L, Clifford S, Barber N, Marin D. Exploring chronic myeloid leukemia patients’ reasons for not adhering to the oral anticancer drug imatinib as prescribed. Leuk Res. 2011;35:626–630. doi: 10.1016/j.leukres.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 23.Guilhot F, Coombs J, Zernovak O, et al. A global retrospective and physician-based analysis of adherence to tyrosine kinase inhibitor (TKI) therapies for chronic myeloid leukemia (CML) Blood (ASH Annual Meeting Abstracts) 2010;116:1514. [Google Scholar]
- 24.Nunes V, Neilson J, O’Flynn N, et al. Clinical guidelines and evidence review for medicines adherence: Involving patients in decisions about prescribed medicines and supporting adherence. London: National Collaborating Centre for Primary Care and Royal College of General Practitioners; 2009. [October 11, 2011]. Available at: http://www.nice.org.uk/nicemedia/live/11766/42971/42971.pdf. [PubMed] [Google Scholar]
- 25.NCCN. National Comprehensive Cancer Network: Clinical Practice Guidelines in Oncology Chronic Myelogenous Leukemia Version 2. 2011. [October 11, 2011]. Available at: http://www.nccn.org/professionals/physician_gls/PDF/cml.pdf. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Chronic Myelogenous Leukemia V.2.2012 © 2011 National Comprehensive Cancer Network, Inc All rights reserved The NCCN Guidelines® may not be reproduced in any form for any purpose without the express written permission of the NCCN To view the most recent and complete version of the NCCN Guidelines, go online to NCCN.org NATIONAL COMPREHENSIVE CANCER NETWORK®, NCCN®, NCCN GUIDELINES®, and all other NCCN Content are trademarks owned by the National Comprehensive Cancer Network, Inc.


