Abstract
Cholesteryl ester transfer protein (CETP) facilitates the transfer of HDL cholesteryl ester to triglyceride-rich lipoproteins (TRL). This study aimed to determine the effects of CETP inhibition with torcetrapib on TRL composition and apoB-48 metabolism. Study subjects with low HDL cholesterol (<40 mg/dl), either untreated (n = 9) or receiving atorvastatin 20 mg daily (n = 9), received placebo for 4 weeks, followed by torcetrapib 120 mg once daily for the next 4 weeks. A subset of the subjects not treated with atorvastatin participated in a third phase (n = 6), in which they received torcetrapib 120 mg twice daily for an additional 4 weeks. At the end of each phase, all subjects received a primed-constant infusion of [5,5,5-2H3]L-leucine, while in the constantly fed state, to determine the kinetics of TRL apoB-48 and TRL composition. Relative to placebo, torcetrapib markedly reduced TRL CE levels in all groups (≥−69%; P < 0.005). ApoB-48 pool size (PS) and production rate (PR) decreased in the nonatorvastatin once daily (PS: −49%, P = 0.007; PR: −49%, P = 0.005) and twice daily (PS: −30%, P = 0.01; PR: −27%, P = 0.13) cohorts. In the atorvastatin cohort, apoB-48 PS and PR, which were already lowered by atorvastatin, did not change with torcetrapib. Our findings indicate that CETP inhibition reduced plasma apoB-48 concentrations by reducing apoB-48 production but did not have this effect in subjects already treated with atorvastatin.
Keywords: cholesteryl ester transfer protein, apolipoprotein, lipoprotein kinetics, postprandial metabolism, torcetrapib
Postprandial hypertriglyceridemia represents an independent risk factor for premature coronary heart disease (1–3). It is now established that the remnants of apoB-containing triglyceride-rich lipoproteins (TRL) may penetrate the arterial intima and be retained by the extracellular matrix, causing arterial cholesterol accumulation (4). In the postprandial state, in response to fat intake, plasma concentrations of triglycerides (TG) in chylomicrons, VLDL, and their remnants are elevated (5), thereby enhancing the probability of plaque formation and increasing the risk of atherosclerosis.
The apoB associated with TRL particles exists in two forms in plasma, apoB-100 and apoB-48, both of which are produced in humans by the same structural gene on chromosome 2 (6). ApoB-48, which results from tissue-specific mRNA editing introducing a premature stop codon at APOB codon 2153, is secreted by the intestine within chylomicrons in response to dietary fat. Although the synthesis of apoB-48 is constitutive, apoB-48 secretion requires association with TG via the intracellular action of microsomal triglyceride transfer protein, and the size and number of the assembled particles depend on the lipid content in the enterocytes (7–9). In plasma, the TG-enriched core is hydrolyzed by lipoprotein lipase, resulting in the delivery of free fatty acids to muscle and adipose tissue and the formation of chylomicron remnants. Unlike apoB-100, apoB-48 does not contain the LDL receptor-binding domain, and apoB-48-containing remnants are taken up by hepatic receptors that recognize apoE (10, 11).
The metabolism of TRL is linked to that of HDL through the activity of plasma enzymes and transport proteins. Cholesteryl ester transfer protein (CETP) plays a crucial role by mediating the bidirectional net exchange of cholesteryl ester (CE) in HDL for TG in the apoB-containing lipoproteins.
CETP activity is increased under postprandial conditions, and in dyslipidemia it can contribute significantly to an enhanced cholesterol burden of the atherogenic apoB-containing lipoproteins. In familial combined hyperlipidemia, Guerin and colleagues (12) have reported that the increased number of postprandial chylomicrons and large VLDL-1 acceptor particles, combined with the postprandial targeting of VLDL-1 as the preferred TRL acceptor of CE from HDL, results in enhanced CE transfer. Increased CETP activity, in turn, causes the accumulation of a population of CE-enriched atherogenic remnant particles containing apoB-48 and apoE (12). It is well known that plasma TG and HDL cholesterol levels are inversely related (13). One explanation for this potentially atherogenic relationship is that, in patients with elevated plasma TG levels, the CE content in HDL decreases as a consequence of enhanced CETP activity. Inhibition of CETP activity may, therefore, constitute a therapeutic approach that not only raises HDL cholesterol but also attenuates the atherogenic profile of apoB-48-containing lipoproteins (12, 14).
We have reported previously that partial inhibition of CETP activity with torcetrapib significantly increased plasma concentrations of HDL cholesterol, apoA-I, and large HDL particles and decreased LDL cholesterol, apoB, and small dense LDL concentrations in subjects with low baseline levels of HDL cholesterol (15–18). The HDL-raising effects mediated by torcetrapib in these subjects were associated with significant delays in the catabolism of HDL apoA-I, as well as significant increases in the clearance of VLDL apoB-100 and apoE. The aim of the present study was to determine the effects of torcetrapib-mediated inhibition of CETP on apoB-48 metabolism and TRL composition in the same subjects.
METHODS
Subjects
The subjects in this study were recruited at Tufts Medical Center (Boston, MA) and the University of Pennsylvania (Philadelphia, PA). Subjects were eligible if they met the following criteria: age 18 to 70 years, HDL cholesterol <40 mg/dl, TG <400 mg/dl, LDL cholesterol <160 mg/dl, and body mass index between 18 and 35 kg/m2. Subjects having a LDL cholesterol of <160 mg/dl while on a stable dose of atorvastatin 20 mg once daily were considered for the atorvastatin arm of the study, provided they met the other criteria. Exclusion criteria have been described previously in detail (15). The study protocol was approved by the human investigation review committee of each institution. Informed, written consent was obtained from each study subject.
Study design
This was a single-blinded, placebo-controlled, fixed sequence study designed to examine the effects of torcetrapib on plasma lipoproteins and lipoprotein metabolism in subjects with low HDL cholesterol. A total of 19 subjects were enrolled, with nine subjects studied while receiving atorvastatin treatment throughout the study. Both cohorts included one female subject, one subject with the 2/3 apoE genotype, and one subject with the 3/4 apoE genotype. All subjects were similar with respect to age and body mass index, but, as expected, the levels of LDL cholesterol and total apoB at randomization were lower in the atorvastatin cohort (Table 1). A detailed description of the study has been reported previously (15). Briefly, the study consisted of an introductory period lasting 2 to 4 weeks, during which the subjects were screened and, if necessary (subjects with a LDL cholesterol >160 mg/dl; n = 9), received atorvastatin 20 mg once daily throughout the study. All subjects were placed on placebo for 4 weeks, followed by torcetrapib 120 mg once daily for an additional 4 weeks. Six of the 10 subjects not taking atorvastatin also participated in a third phase, in which they were given torcetrapib 120 mg twice daily for 4 weeks. Only 9 of the 10 subjects not on atorvastatin were studied during the torcetrapib 120 mg once daily phase, due to unsatisfactory data (see below). There was no washout period between the phases.
TABLE 1.
Characteristics of subjects at randomization
| Torcetrapib Alone |
Atorvastatin + Torcetrapib |
||
| Variablea | 120 mg QD (n = 9) | 120 mg BID (n = 6)b | 120 mg QD (n = 9) |
| Age (yr) | 49 ± 4 | 48 ± 5 | 51 ± 3 |
| Sex (M/F) | 8/1 | 6/0 | 8/1 |
| Body-mass index | 28.1 ± 0.8 | 26.4 ± 0.6 | 28.9 ± 1.1 |
| Cholesterol, fasting (mg/dl) | |||
| Total | 201 ± 11 | 208 ± 14 | 152 ± 7c |
| LDL | 132 ± 9 | 137 ± 10 | 90 ± 8c |
| HDL | 33 ± 2 | 35 ± 2 | 32 ± 2 |
| Ratio total to HDL | 6.4 ± 0.5 | 6.2 ± 0.7 | 4.9 ± 0.4d |
| Triglycerides, fasting (mg/dl) | 160 ± 31 | 156 ± 41 | 145 ± 31 |
| Apolipoprotein, fasting (mg/dl) | |||
| Total B | 104 ± 4 | 103 ± 6 | 98 ± 7 |
| A-I | 117 ± 3 | 118 ± 4 | 114 ± 6 |
BID, twice daily; QD, once daily. Boldface indicates significance.
Data are presented as mean ± SEM. To convert the values for cholesterol to mmol/l, multiply by 0.02586. To convert the values for triglycerides to mmol/l, multiply by 0.01129. Significance for comparison between the torcetrapib alone cohorts and the atorvastatin+torcertpib cohort was tested by a two-independent sample t-test.
Five of the nine subjects in the torcetrapib 120 mg QD alone group and a sixth subject (who completed the placebo phase but had unsatisfactory enrichment data for the torcetrapib 120 mg QD phase) went on to receive 120 mg of torcetrapib twice daily for an additional 4 weeks. The characteristics of these six subjects did not differ significantly from the entire torcetrapib alone group.
P < 0.01 for comparison with the subjects who did not receive atorvastatin.
P = 0.05 for comparison with the torcetrapib alone 120 mg QD subjects.
At the end of each 4-week treatment period, the subjects underwent a primed-constant infusion of deuterated leucine, under constantly fed conditions, to determine the kinetics of apoB-48. As described previously (19), the subjects were fed hourly for 20 h with small identical meals (15% calories as protein, 49% as carbohydrate, 36% as fat; 180 mg cholesterol/1,000 kcal) starting 5 h before and continuing throughout the infusion. All plasma and TRL lipid and apolipoprotein levels have been shown in previous studies to be stable by the start of the infusion period and not to change significantly throughout the infusion period (19, 20). At 11 AM (0 h), [5,5,5-D3]L-leucine (C/D/N Isotopes, Pointe-Claire, Quebec) 10 μmol/kg body weight was injected intravenously as a bolus over 1 min and then by continuous infusion for 15 h at a rate of 10 μmol/kg body weight/h. Blood samples were collected into tubes containing EDTA (0.15%) just before the infusion (0 h) and at 30, 35, and 45 min and at 1, 1.5, 2, 3, 4, 6, 9, 12, 14, and 15 h during the infusion. The samples were placed on ice immediately. The TRL (d <1.006 g/ml) particles were isolated from fresh plasma by density ultracentrifugation (21), divided into aliquots for TRL apoB measurement or isolation, and stored at −80°C for no more than 3 years before analysis.
Apolipoprotein isotopic enrichment and kinetic analysis
As described previously (22), TRL proteins were separated by gradient SDS-PAGE and transferred to a Westran S polyvinylidene difluoride membrane (GE Life Sciences, Piscataway, NJ) using a Tris-glycine-methanol system. ApoB-48 bands were visualized by Coomassie blue R-250, excised from the membrane, and hydrolyzed with 12 N HCl at 110°C for 24 h. Amino acids were converted to n-propyl ester and heptafluorobutyramide derivatives and analyzed for isotopic enrichment by electron capture negative chemical ionization gas chromatography-mass spectrometry. A capillary column coated with DX-4 stationary phase was used to separate leucine from its isomer isoleucine. Selected ion monitoring at m/z 349 (derivatized leucine – HF)− and m/z 352 (derivatized D3-leucine – HF)− was used to determine the areas under the chromatographic peaks for each ion. Moles percent enrichment (D3-leucine/[D3-leucine + leucine]) for each sample was calculated from the area under the curve and converted to a tracer-tracee ratio (percent) to account for the isotopic enrichment of the D3-leucine tracer (23).
The SAAM II program (University of Washington, Seattle, WA) was used to determine the fractional catabolic rate (FCR) of apoB-48 using a previously described multicompartmental model (22, 24). The model assumed a constant enrichment of the precursor pool, as has been demonstrated in metabolic studies using a primed-constant infusion to administer labeled leucine (22, 24, 25). Determination of the FCR is independent of the level of precursor enrichment. ApoB-48 concentration was maintained constant over the 15 h infusion period. The production rate (PR) was computed as the product of the FCR and the pool size (PS), which equals the plasma concentration multiplied by plasma volume, estimated as 4.5% of body weight in kilograms.
One subject in the nonatorvastatin arm who also participated in the torcetrapib 120 mg twice daily phase had unsatisfactory enrichment data for the torcetrapib 120 mg once daily phase. The tracer data for the unsatisfactory phase were not modeled, and the data for this subject were included only in the torcetrapib 120 mg twice daily subset analysis.
Quantitation of apoB-48 and TRL lipids
Lipid and apolipoprotein levels were measured in fasting and nonfasting plasma samples as previously reported (15–18). Plasma levels of total cholesterol (TC) and TG were measured by automated enzymatic assays standardized through the Centers for Disease Control (Atlanta, GA) (26). The lipid composition of the nonfasting TRL fraction isolated from fresh plasma by density ultracentrifugation and stored at −80°C was measured using enzymatic reagents for total and free cholesterol and TG (Wako Chemicals, USA); CE concentration was calculated as the difference between total and free cholesterol. To determine the nonfasting concentration of apoB-48 in TRL (containing both chylomicrons and VLDL), the concentration of total apoB in TRL was measured with a noncompetitive ELISA using previously separated frozen TRL aliquots, in-house controls from pooled plasma, and immunoaffinity-purified polyclonal antibodies (Meridian Life Sciences, Saco, ME). The relative proportion of apoB within the TRL fraction that was apoB-48 and apoB-100 was assessed by densitometric scanning of 0.1% Coomassie blue R-250-stained SDS-PAGE gels and calculated to be the average of the 14 measurements obtained during the infusion (27).
Statistical analysis
The SAS System for Windows (release 9.2; SAS Institute) was used for statistical analysis. Significant differences in the means between placebo and torcetrapib phases within a given group were assessed by paired t-tests, whereas two-independent sample t-tests were used to detect differences between the nonatorvastatin and atorvastatin cohorts. A logarithmic transformation was applied to the data not normally distributed before formal analysis. The percent change relative to placebo was calculated on an individual basis and summarized descriptively by cohort. Spearman correlations were used in the correlation analyses. All data in the text, tables, and graphs are presented in the original scale of measurement as means ± SEM. P < 0.05 was considered significant.
RESULTS
Effects of torcetrapib on nonfasting concentrations of apoB-48 and TRL lipids
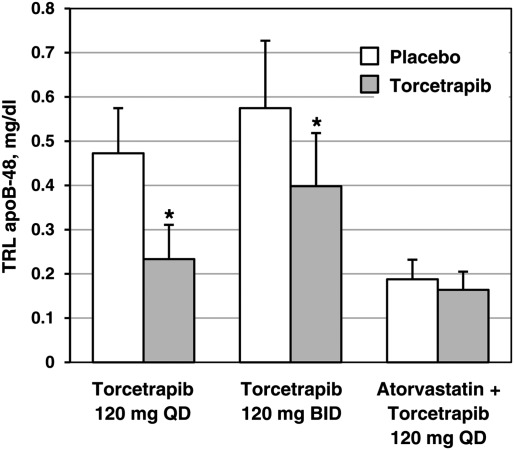
As reported previously (15), partial inhibition of CETP activity with torcetrapib significantly decreased fasting plasma concentrations of total apoB when the drug was given as monotherapy or in combination with atorvastatin. Torcetrapib also had notable effects on nonfasting concentrations of TRL apoB-48 (Fig. 1; see supplementary data for the effects on apoB-100, apoA-I, apoA-II, and apoE concentrations). Relative to placebo, TRL apoB-48 levels were decreased significantly, by 49 ± 9% (mean ± SEM; P = 0.007) in the nonatorvastatin 120 mg once daily group (from 0.47 ± 0.10 to 0.23 ± 0.08 mg/dl) and by 31 ± 6% (P = 0.009) in the nonatorvastatin 120 mg twice daily group (from 0.57 ± 0.15 to 0.40 ± 0.12 mg/dl). No significant decrease in TRL apoB-48 levels was observed in the atorvastatin cohort.
Fig. 1.
Effect of torcetrapib on the nonfasting plasma concentration of TRL apoB-48 in subjects with low HDL cholesterol. Relative to placebo, torcetrapib decreased apoB-48 levels by 49 ± 9% (from 0.47 ± 0.10 to 0.23 ± 0.08 mg/dl) in the torcetrapib 120 mg once daily group (n = 9), by 31 ± 6% (from 0.57 ± 0.15 to 0.40 ± 0.12 mg/dl) in the torcetrapib 120 mg twice daily group (n = 6), and by 2 ± 14% (from 0.19 ± 0.04 to 0.16 ± 0.04 mg/dl) in the atorvastatin group (n = 9). The effects on apoB-100, apoA-I, apoA-II, and apoE are shown in the supplementary data. Data are expressed as mean ± SEM. *P < 0.01 for comparison with the placebo phase. QD, once daily; BID, twice daily.
The effects of torcetrapib on nonfasting lipid concentrations are shown in Table 2. Relative to placebo, torcetrapib decreased plasma TG levels by 21 ± 8% (P = 0.04) in the once daily cohort, by 7 ± 8% (P not significant) in the twice daily cohort, and by 17 ± 9% (P = 0.06) in the atorvastatin cohort. Changes in TC levels were minimal in all three groups. Plasma TC and TG levels were, as expected, significantly higher in the nonatorvastatin cohort, compared with the levels measured in the atorvastatin cohort, during both the placebo and the torcetrapib phases.
TABLE 2.
Effects of torcetrapib on nonfasting plasma and TRL lipid levelsa
| Torcetrapib Alone |
Atorvastatin + Torcetrapib |
|||
| Parameter and Study Phaseb | 120 mg QD (n = 9) | 120 mg BID (n = 6) | 120 mg QD (n = 9) | P value, Statin vsNonstatinc |
| Plasma lipids | ||||
| Total cholesterol (mg/dl) | ||||
| Placebo | 195 ± 10 | 208 ± 12 | 142 ± 10 | 0.002 |
| Torcetrapib | 189 ± 10 | 204 ± 13 | 137 ± 7 | < 0.001 |
| Change (%) | −3 ± 3 | −2 ± 4 | −3 ± 3 | |
| P value | 0.35 | 0.70 | 0.32 | |
| Triglycerides (mg/dl) | ||||
| Placebo | 277 ± 34 | 278 ± 39 | 169 ± 20 | 0.02 |
| Torcetrapib | 208 ± 26 | 258 ± 50 | 140 ± 20 | 0.04 |
| Change (%) | −21 ± 8 | −7 ± 8 | −17 ± 9 | |
| P value | 0.04 | 0.38 | 0.06 | |
| TRL lipids | ||||
| Total cholesterol (mg/dl) | ||||
| Placebo | 32.5 ± 3.7 | 35.1 ± 5.7 | 17.3 ± 2.3 | 0.003 |
| Torcetrapib | 17.4 ± 2.1 | 20.4 ± 4.6 | 9.8 ± 1.6 | 0.01 |
| Change (%) | −42 ± 8 | −41 ± 7 | −41 ± 8 | |
| P value | < 0.001 | 0.004 | 0.002 | |
| Cholesteryl ester (mg/dl) | ||||
| Placebo | 26.8 ± 3.4 | 29.6 ± 5.4 | 12.7 ± 1.7 | 0.002 |
| Torcetrapib | 6.9 ± 1.2 | 7.0 ± 2.4 | 3.5 ± 0.4 | 0.02 |
| Change (%) | −73 ± 3 | −78 ± 5 | −69 ± 4 | |
| P value | < 0.001 | 0.002 | < 0.001 | |
| Triglycerides (mg/dl) | ||||
| Placebo | 202 ± 24 | 208 ± 32 | 123 ± 20 | 0.03 |
| Torcetrapib | 160 ± 23 | 200 ± 45 | 103 ± 20 | 0.04 |
| Change (%) | −17 ± 10 | −6 ± 9 | −16 ± 12 | |
| P value | 0.08 | 0.46 | 0.10 | |
| TG/CE ratio | ||||
| Placebo | 8.0 ± 0.6 | 7.7 ± 0.9 | 10.1 ± 0.9 | 0.07 |
| Torcetrapib | 26.8 ± 3.9 | 47.1 ± 12.1 | 29.1 ± 4.1 | 0.69 |
| Change (%) | 229 ± 31 | 550 ± 216 | 193 ± 35 | |
| P value | < 0.001 | 0.02 | 0.001 | |
BID, twice daily; CE, cholesteryl ester; TG, triglycerides; TRL, triglyceride-rich lipoprotein; QD, once daily. Bold face indicates significance.
Effects of torcetrapib on the composition of TRL, intermediate density lipoprotein (IDL), LDL, and HDL are shown in the supplementary data.
Data are presented as mean ± SEM. Significance for comparison of absolute values with placebo phase was determined using a paired t-test, with triglycerides being log-transformed before statistical analysis.
Significance for comparison between torcetrapib alone and atorvastatin + torcetrapib cohorts during placebo and torcetrapib 120 mg once daily phases was tested by a two-independent sample t-test.
Torcetrapib also reduced lipid concentrations within nonfasting TRL particles (Table 2; see supplementary data for effects on lipoprotein composition). Torcetrapib alone once daily decreased TRL TC by 42 ± 8% (P < 0.001) relative to placebo, TRL CE by 73 ± 3% (P < 0.001), and TRL TG by 17 ± 10% (P = 0.08). The corresponding decreases among subjects who received torcetrapib twice daily were −41 ± 7% (P = 0.004), −78 ± 5% (P = 0.002), and −6 ± 9% (P not significant), respectively. Torcetrapib given in combination with atorvastatin effected significant reductions in TRL TC (−41 ± 8%; P = 0.002) and CE (−69 ± 4%; P < 0.001) and a nonsignificant reduction in TRL TG (−16 ± 12%), relative to the levels measured during the placebo phase. The atorvastatin subjects also had significantly lower TRL TC, CE, and TG levels during the placebo (TC: P = 0.003; CE: P = 0.002; TG: P = 0.03) and the torcetrapib phases (TC: P = 0.01; CE: P = 0.02; TG: P = 0.04), compared with the nonatorvastatin once daily subjects, due to the known effects of atorvastatin.
The changes in TRL lipid concentrations in response to torcetrapib treatment translated into striking increases in the TG/CE ratio of TRL particles. Compared with the placebo phase, the TG/CE ratio during the torcetrapib phase increased by 229 ± 31% (P < 0.001) in the nonatorvastatin 120 mg once daily group, 550 ± 216% (P = 0.02) in the nonatorvastatin 120 mg twice daily group, and 193 ± 35% (P = 0.001) in the atorvastatin group.
Effects of torcetrapib on apoB-48 kinetic parameters
TRL apoB-48 PS and kinetic parameters at the end of the placebo and torcetrapib phases are presented in Table 3. In the nonatorvastatin cohort, relative to placebo, torcetrapib 120 mg once daily decreased TRL apoB-48 PS by 49 ± 9% (P = 0.007), whereas torcetrapib 120 mg twice daily reduced PS by 30 ± 6% (P = 0.01). Analysis of the kinetic data revealed that the reduction in apoB-48 PS in these subjects was primarily attributable to a torcetrapib-mediated reduction in production. TRL apoB-48 PR was decreased by 49 ± 8% (P = 0.005) with torcetrapib 120 mg once daily and by 27 ± 17% (P = 0.13) with torcetrapib 120 mg twice daily. TRL apoB-48 FCR was not altered significantly by either dose of torcetrapib. In the atorvastatin cohort, torcetrapib had no statistically significant effect on TRL apoB-48 PS, FCR, or PR. However, there was considerable interindividual variability in the response to torcetrapib in this cohort, and the data were reanalyzed, omitting one subject with the 3/4 apoE genotype who had marked increases in TRL apoB-48 PS (88%) and PR (39%). Although the percent decrease in PS and PR was greater (−14 ± 10% and −19 ± 12%, respectively) when this subject was omitted (data not shown), the torcetrapib-induced effect on TRL apoB-48 kinetic parameters, compared with placebo, remained nonsignificant.
TABLE 3.
Effects of torcetrapib on TRL apoB-48 kinetic parameters
| Torcetrapib Alone |
Atorvastatin + Torcetrapib |
|||
| Parameter and Study Phasea | 120 mg once daily (n = 9) | 120 mg twice daily (n = 6) | 120 mg once daily (n = 9) | P, Statin vs Nonstatinb |
| ApoB-48 PS (mg) | ||||
| Placebo | 18.5 ± 4.1 | 21.3 ± 5.8 | 7.4 ± 1.7 | 0.03 |
| Torcetrapib | 9.0 ± 2.9 | 14.8 ± 4.5 | 6.3 ± 1.6 | 0.64 |
| Change (%) | −49 ± 9 | −30 ± 6 | −3 ± 14 | |
| P value | 0.007 | 0.01 | 0.47 | |
| ApoB-48 FCR (pools/day) | ||||
| Placebo | 6.8 ± 0.8 | 7.0 ± 1.0 | 7.7 ± 1.0 | 0.55 |
| Torcetrapib | 7.1 ± 0.9 | 7.1 ± 1.6 | 7.1 ± 1.2 | 0.87 |
| Change (%) | 14 ± 17 | 4 ± 19 | −5 ± 12 | |
| P value | 0.83 | 0.82 | 0.37 | |
| ApoB-48 PR (mg/kg/day) | ||||
| Placebo | 1.42 ± 0.30 | 1.60 ± 0.36 | 0.57 ± 0.13 | 0.04 |
| Torcetrapib | 0.69 ± 0.21 | 1.07 ± 0.25 | 0.46 ± 0.09 | 0.59 |
| Change (%) | −49 ± 8 | −27 ± 17 | −12 ± 12 | |
| P value | 0.005 | 0.13 | 0.20 | |
FCR, fractional catabolic rate; PR, production rate; PS, pool size; TRL, triglyceride-rich lipoprotein. Boldface indicates significance.
Data are presented as mean ± SEM. Significance for comparison of absolute values with placebo phase was determined using a paired t-test on log-transformed data.
Significance for comparison between torcetrapib alone and atorvastatin+torcetrapib cohorts on placebo and torcetrapib 120 mg once daily was tested by a two-independent sample t-test.
As shown in Table 3, when the nonatorvastatin and atorvastatin cohorts were compared, the nonatorvastatin cohort on placebo had a mean TRL apoB-48 PS of 18.5 ± 4.1 mg, whereas the PS of the atorvastatin cohort was significantly smaller (7.4 ± 1.7 mg; P = 0.03). TRL apoB-48 PR in the atorvastatin cohort on placebo was also significantly lower than that of the nonatorvastatin cohort (P = 0.04). On torcetrapib, the differences between the two cohorts disappeared (PS: P = 0.64; PR: P = 0.59).
TRL composition and apoB-48 metabolism
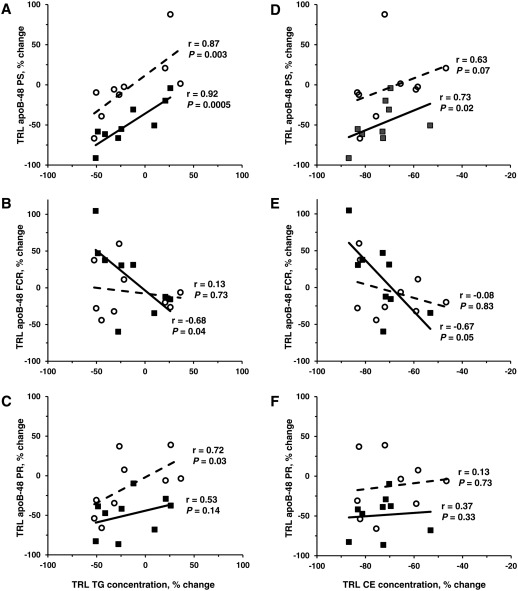
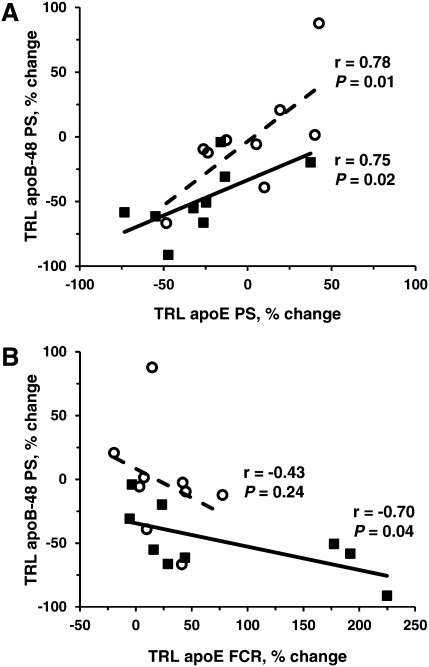
In the nonatorvastatin group at the end of the placebo phase, nonfasting plasma TG concentrations were associated with TRL apoB-48 PS (r = 0.67; P = 0.05), and apoB-48 FCR was associated with TRL apoE FCR (r = 0.68; P = 0.04). Correlations between the changes in TRL lipid concentrations and apoB-48 kinetic parameters after treatment with torcetrapib alone indicated, as shown in Fig. 2, that there was a significant relationship between the percent change in nonfasting TRL TG concentration and the percent change in apoB-48 PS (Fig. 2A: r = 0.92; P = 0.0005) and apoB-48 FCR (Fig. 2B: r = −0.68; P = 0.04), compared with placebo. Overall, subjects with the greatest reduction in TRL TG concentration in response to torcetrapib treatment had the greatest increase in apoB-48 FCR and, thus, had the greatest decrease in apoB-48 PS in response to torcetrapib treatment. Similar associations were observed between the percent change in nonfasting TRL CE concentration and the percent change in apoB-48 PS (Fig. 2D: r = 0.73; P = 0.02) and apoB-48 FCR (Fig. 2E: r = −0.67; P = 0.05). The associations between the percent change in TRL TG/CE ratio and the percent change in TRL apoB-48 parameters were not significant. In addition, as shown in Fig. 3, the percent change in apoB-48 PS in response to torcetrapib correlated positively with the percent change in TRL apoE PS (r = 0.75; P = 0.02) and inversely with the percent change in TRL apoE FCR (r = −0.70; P = 0.04). The association between apoB-48 FCR and TRL apoE FCR observed during the placebo phase did not remain after torcetrapib treatment.
Fig. 2.
The relationship between the torcetrapib-mediated percent change in nonfasting TRL TG concentration and the percent change in TRL apoB-48 PS (A), FCR (B), and PR (C) and between the percent change in nonfasting TRL CE concentration and the percent change in TRL apoB-48 PS (D), FCR (E), and PR (F), compared with placebo. In the nonatorvastatin cohort, the percent change in TRL TG concentration correlated positively with the percent change in apoB-48 PS and inversely with the percent change in apoB-48 FCR, whereas in the atorvastatin cohort, the percent change in TRL TG concentration was associated with the percent change in apoB-48 PS and PR. The percent change in TRL CE concentration correlated positively with the percent change in apoB-48 PS and inversely with the percent change in apoB-48 FCR in the nonatorvastatin cohort, but only with the percent change in apoB-48 PS in the atorvastatin cohort. Closed square and bold line indicate the nonatorvastatin group (n = 9); open circle and dotted line indicate the atorvastatin group (n = 9).
Fig. 3.
The relationship between the percent change in nonfasting TRL apoB-48 PS and in TRL apoE PS (A) and between the percent change in TRL apoB-48 PS and in TRL apoE FCR (B) compared with placebo. The relationship between the percent change in TRL apoB-48 PS and in TRL apoE PS was significant in both the nonatorvastatin and the atorvastatin groups, but only the nonatorvastatin group showed a significant relationship between the percent change in TRL apoB-48 PS and the percent change in TRL apoE FCR. Closed square and bold line indicate the nonatorvastatin group (n = 9); open circle and dotted line indicate the atorvastatin group (n = 9).
In the atorvastatin subjects after torcetrapib treatment, there was a significant relationship between the percent change in nonfasting TRL TG concentration and the percent change in apoB-48 PS (Fig. 2A: r = 0.87; P = 0.003), compared with placebo, and there was a trend toward significance between the percent change in nonfasting TRL CE levels and the percent change in apoB-48 PS (Fig. 2D: r = 0.63; P = 0.07). No associations between the percent change in TRL lipids and the percent change in apoB-48 FCR were observed. The percent change in TRL TG concentration and the percent change in apoB-48 PR, compared with placebo, were associated (Fig. 2C: r = 0.72; P = 0.03); however, when the subject with the 3/4 apoE genotype was omitted from the analysis, the association did not remain (r = 0.54; P = 0.16). The percent change in TRL apoB-48 PS correlated with the percent change in TRL apoE PS (Fig. 3A: r = 0.78; P = 0.01) but not with the percent change in TRL apoE FCR (Fig. 3B: r = −0.43; P = 0.24).
DISCUSSION
Interest in CETP inhibition as an effective strategy to reduce coronary heart disease (CHD) risk remains despite the termination of torcetrapib development due to excess all-cause mortality in CHD patients (28). There is clear evidence that torcetrapib has off-target, compound-specific effects independent of CETP inhibition (28–30) that are not associated with other CETP inhibitors, such as anacetrapib (31) and dalcetrapib (32), currently being tested in large clinical trials. With this in mind, mechanistic studies conducted with torcetrapib may provide important insight into the effect of CETP inhibition on lipoprotein metabolism.
We have reported previously that torcetrapib-mediated inhibition of CETP markedly reduced plasma concentrations of TRL particles, small dense LDL, and apoB in subjects with low HDL cholesterol (15, 17). These beneficial effects were associated with significant increases in the catabolism of VLDL apoB-100 and apoE (17, 18). In the present study, torcetrapib markedly reduced TRL CE levels, increasing greatly the TG/CE ratio in TRL particles, when the drug was given as monotherapy or in combination with atorvastatin. In contrast, concentrations of TRL apoB-48 were lowered significantly only in the subjects treated with torcetrapib alone. Analysis of the apoB-48 kinetic data revealed that the decrease in apoB-48 concentration in the nonatorvastatin cohort was due to a significant reduction in apoB-48 PR, with no change in the FCR.
Our finding of dramatic reductions in TRL CE concentrations is consistent with the concept that the inhibition of CETP activity by torcetrapib markedly decreases the amount of CE transferred from other lipoproteins to TRL. Although a significant fraction of TRL CE in the fasting and the fed state is most likely in the apoB-100-containing TRL particles (33), effective inhibition of CETP activity presumably reduces the CE content of apoB-48-containing TRL particles as well, lowering net delivery of TRL CE to the liver. It can be hypothesized that, as a result, biliary cholesterol excretion would probably decrease, less endogenous cholesterol would be available to the intestine for absorption, and lipidation of newly synthesized apoB-48 would be reduced. Plasma sterol markers of cholesterol absorption, which could substantiate this possible mechanism by which apoB-48 production would decrease, were not assessed in this study. However, it has been shown recently in hamsters, an animal model expressing CETP, that torcetrapib had no significant effect on cholesterol absorption or synthesis biomarkers (34). This observation concurs with our previous report that, in subjects with low HDL cholesterol, torcetrapib treatment up-regulated hepatic clearance of apoB-100-containing lipoproteins (17, 18) and did not substantially alter plasma lathosterol levels (cholesterol synthesis biomarker) or fecal neutral sterol and bile acid content (16, see supplementary data), suggesting that the rate of reverse cholesterol transport is not compromised. Taken together, these data suggest that apoB-48 secretion may not be affected by torcetrapib through this pathway.
There is evidence that CETP inhibition may influence enterocyte intracellular lipid metabolism and, hence, apoB-48 production. Niesor et al. (34) demonstrated that after oral dosing of radiolabeled cholesterol, dalcetrapib-treated hamsters had significant increases in plasma HDL cholesterol, accompanied by increases in cholesterol absorption biomarkers, with no change in nonHDL cholesterol. From the data, they hypothesized a preferential increase in the lipidation of nascent HDL by intestinal ATP-binding cassette (ABC) A1. It has also been reported that torcetrapib analogs altered intestinal lipid and lipoprotein processing in rats, reducing the number of chylomicron particles and the proportion of lipids carried by chylomicrons in lymph while increasing the proportion of lipids transported by HDL in lymph (35). Whether or not torcetrapib-mediated inhibition of CETP in humans affects the preferential partitioning of intestinal cholesterol to HDL remains to be determined. Regardless of the precise mechanism, decreased availability of lipids for chylomicron particle formation would result in enhanced intracellular degradation of apoB-48 and, thus, less apoB-48 secretion.
During the placebo phase, subjects in the atorvastatin arm of this study had apoB-48 concentrations that were markedly lower than the levels observed in the nonatorvastatin cohort. Studies using oral fat load tests have reported lower apoB-48 and TG concentrations during atorvastatin treatment, compared with placebo, in subjects with hypertriglyceridemia (36), hypercholesterolemia (37), familial dysbetalipoproteinemia (38, 39), and CHD (40, 41). The reductions have been associated with enhanced clearance of apoB-48 (22) and chylomicron-like emulsions (42) in subjects with dyslipidemia. In subjects with diabetes, Hogue et al. (43) found that atorvastatin reduced TRL apoB-48 concentrations primarily by decreasing its production. In the current study of subjects with low HDL, apoB-48 FCR on placebo was similar in the atorvastatin and the nonatorvastatin cohorts, but the rate of production on placebo was significantly lower in the atorvastatin cohort. The atorvastatin subjects on placebo also had substantially lower serum concentrations of lathosterol (reduced cholesterol synthesis) and, in turn, decreased fecal sterol content (16, see supplementary data), parameters associated with chylomicron production. Moreover, atorvastatin therapy in hyperlipidemic men has been shown to stimulate intestinal mRNA expression of the LDL receptor and Niemann-Pick C1Like 1 and to decrease mRNA levels of ABCG5 and ABCG8, without changing significantly the expression of apoB-48 (44). There is, however, a great deal of individual variability in these studies, and the precise effect that statin therapy has on apoB-48 metabolism may depend on the underlying lipoprotein disorder. In addition, the present study was not designed to examine the effect of atorvastatin on apoB-48 metabolism, but only that of torcetrapib on this parameter, in subjects either untreated or on atorvastatin at baseline.
Overall, the data indicate that, in contrast to torcetrapib monotherapy, torcetrapib added to atorvastatin had no significant effect on plasma apoB-48 levels. Our results agree with those of Guerin and colleagues (45), who have reported that torcetrapib 60 mg once daily in combination with atorvastatin 10 mg once daily had only a minor effect on chylomicron levels. In our view, the one significant effect of adding torcetrapib to atorvastatin therapy was the marked reduction in TRL CE concentration.
In the nonatorvastatin subjects, the percent increase in apoB-48 FCR in response to torcetrapib was a strong predictor of the torcetrapib-mediated percent decrease in TRL TG and TRL CE levels. Torcetrapib treatment, however, did not enhance the overall catabolism of apoB-48 in any of the cohorts. This outcome, which holds even when the statistical analysis controlled for differences in apoE genotype (data not shown), underscores the concept that the effects of torcetrapib on the metabolism of TRL apoB-48-containing particles differ mechanistically from those on TRL apoB-100-containing particles (17), despite the presence of apoE, a ligand for hepatic catabolism of both classes of TRL. It is known that TRL apoE is a major determinant of TRL apoB-100 and TRL apoB-48 clearance because these parameters are markedly decreased in human familial apoE deficiency (46). In the present study, the procedure used to isolate TRL from whole plasma did not separate apoB-48-containing particles from apoB-100-containing particles; hence, the torcetrapib-mediated effects on the lipid and apoE content of TRL are influenced by alterations in the metabolism not only of apoB-48-containing TRL but also of apoB-100-containing TRL. Based on the associations we did observe, we could speculate that apoE-mediated removal of apoB-48-containing lipoproteins is related to the changes in apoE FCR effected by CETP inhibition. Subjects in the nonatorvastatin group with the greatest reduction in apoB-48 PS in response to torcetrapib had the greatest increase in TRL apoE FCR, indicating that the mechanism of chylomicron clearance is consistent with known results. However, enhanced apoE-mediated clearance is not the main mechanism by which torcetrapib lowers apoB-48 PS. As discussed above, torcetrapib decreases apoB-48 concentration primarily by decreasing the production rate of apoB-48.
Several limitations are noteworthy. The small number of subjects in each cohort underscores the marked variability in torcetrapib-induced response, especially in the atorvastatin group. The feeding protocol introduces frequent doses of fat into the intestine, which may result in the formation of smaller, less triglyceride-rich apoB-48-containing lipoproteins; however, previous use of the same feeding protocol has demonstrated that both atorvastatin and extended-release niacin treatment enhanced the fractional catabolism of TRL apoB-100 and TRL apoB-48, with no significant change in production (22, 25). Measurements of plasma markers of cholesterol absorption, like cholestanol, campesterol, and β-sitosterol, could corroborate the unexpected decrease in apoB-48 production. Further studies to distinguish the effects of CETP inhibition on the metabolism of chylomicron-specific lipids and apolipoproteins and to define the molecular mechanism underlying the observed decrease in apoB-48 production are required.
In conclusion, our results provide new insights into the mechanisms by which CETP inhibition attenuates the atherogenic profile of apoB-48-containing lipoproteins in subjects with low HDL cholesterol. These data indicate that torcetrapib reduces apoB-48 levels by decreasing production of apoB-48. Adding torcetrapib treatment to atorvastatin therapy had no further benefit on apoB-48 concentrations already lowered by atorvastatin. This alteration in apoB-48 metabolism may be another mechanism by which CETP inhibition contributes to the reduction of CHD risk in humans.
Supplementary Material
Acknowledgments
The authors thank the nursing and dietary staff of each clinical research center, as well as the laboratory staff at each site, for excellent technical assistance. The authors are also grateful to Julian B. Marsh and Stefania Lamon-Fava for their contributions to the discussion.
Footnotes
Abbreviations:
- CE
- cholesteryl ester
- CETP
- cholesteryl ester transfer protein
- CHD
- coronary heart disease
- FCR
- fractional catabolic rate
- PR
- production rate
- PS
- pool size
- TC
- total cholesterol
- TG
- triglycerides
- TRL
- triglyceride-rich lipoprotein
This work was supported by the Department of Clinical Research, Medicinal Products Research and Development, Pfizer, Inc., Groton, CT. Additional support was provided by US Department of Agriculture Research Service Contract 53-3K-06 (E.J.S.), by Project Grant P50 HL083813-01 from the National Institutes of Health (E.J.S.), by Public Health Services Research Grant M01-RR00040 from the National Institutes of Health (D.J.R.), and by Grant UL-1RR024134 from the National Center for Research Resources (D.J.R.). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the US Department of Agriculture or the National Institutes of Health. Support for the clinical studies was provided by the General Clinical Research Center of Tufts Medical Center and the General Clinical Research Center of the University of Pennsylvania. P.H.R.B. is a fellow of the National Health and Medical Research Council of Australia.
REFERENCES
- 1.Zilversmit D. B. 1995. Atherogenic nature of triglycerides, postprandial lipidemia, and triglyceride-rich-remnant lipoproteins. Clin. Chem. 41: 153–158 [PubMed] [Google Scholar]
- 2.Karpe F. 1999. Postprandial lipoprotein metabolism and atherosclerosis. J. Intern. Med. 246: 341–355 [DOI] [PubMed] [Google Scholar]
- 3.Patsch J. R., Miesenböck G., Hopferwieser T., Mühlberger V., Knapp E., Dunn J. K., Gotto A. M., Jr, Patsch W. 1992. Relation of triglyceride metabolism and coronary artery disease. Studies in the postprandial state. Arterioscler. Thromb. 12: 1336–1345 [DOI] [PubMed] [Google Scholar]
- 4.Proctor S. D., Vine D. F., Mamo J. C. L. 2002. Arterial retention of apolipoprotein B48- and B100-containing lipoproteins in atherogenesis. Curr. Opin. Lipidol. 13: 461–470 [DOI] [PubMed] [Google Scholar]
- 5.Otokozawa S., Ai M., Van Himbergen T., Diffenderfer M. R., Asztalos B. F., Tanaka A., Lamon-Fava S., Schaefer E. J. 2009. Fasting and postprandial apolipoprotein B-48 levels in healthy, obese, and hyperlipidemic subjects. Metabolism. 58: 1536–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Young S. G. 1990. Recent progress in understanding apolipoprotein B. Circulation. 82: 1574–1594 [DOI] [PubMed] [Google Scholar]
- 7.Hussain M. M. 2000. A proposed model for the assembly of chylomicrons. Atherosclerosis. 148: 1–15 [DOI] [PubMed] [Google Scholar]
- 8.Davidson N. O., Shelness G. S. 2000. Apolipoprotein B: mRNA edition, lipoprotein assembly, and presecretory degradation. Annu. Rev. Nutr. 20: 169–193 [DOI] [PubMed] [Google Scholar]
- 9.Silva K. D. R. R., Kelly C. N. M., Jones A. E., Smith R. D., Wootton S. A., Miller G. J., Williams C. M. 2003. Chylomicron particle size and number, factor VII activation and dietary monounsaturated fatty acids. Atherosclerosis. 166: 73–84 [DOI] [PubMed] [Google Scholar]
- 10.Mahley R. W., Rall S. C., Jr 2000. Apolipoprotein E: far more than a lipid transport protein. Annu. Rev. Genomics Hum. Genet. 1: 507–537 [DOI] [PubMed] [Google Scholar]
- 11.Kowal R. C., Herz J., Goldstein J. L., Esser V., Brown M. S. 1989. Low density lipoprotein receptor-related protein mediates uptake of cholesteryl esters derived from apoprotein E-enriched lipoproteins. Proc. Natl. Acad. Sci. USA. 86: 5810–5814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerin M., Egger P., Soudant C., Le Goff W., van Tol A., Dupuis R., Chapman M. J. 2002. Cholesteryl ester flux from HDL to VLDL-1 is preferentially enhanced in type IIB hyperlipidemia in the postprandial state. J. Lipid Res. 43: 1652–1660 [DOI] [PubMed] [Google Scholar]
- 13.Schaefer E. J., Levy R. I., Anderson D. W., Danner R. N., Brewer H. B., Jr, Blackwelder W. C. 1978. Plasma-triglycerides in regulation of H.D.L.-cholesterol levels. Lancet. 312: 391–393 [DOI] [PubMed] [Google Scholar]
- 14.Lamarche B., Uffelman K. D., Carpentier A., Cohn J. S., Steiner G., Barrett P. H., Lewis G. F. 1999. Triglyceride enrichment of HDL enhances in vivo metabolic clearance of HDL apoA-I in healthy men. J. Clin. Invest. 103: 1191–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brousseau M. E., Schaefer E. J., Wolfe M. L., Bloedeon L. T., Digenio A. G., Clark R. W., Mancuso J. P., Rader D. J. 2004. Effects of an inhibitor of cholesteryl ester transfer protein on HDL cholesterol. N. Engl. J. Med. 350: 1505–1515 [DOI] [PubMed] [Google Scholar]
- 16.Brousseau M. E., Diffenderfer M. R., Millar J. S., Nartsupha C., Asztalos B. F., Welty F. K., Wolfe M. L., Rudling M., Björkhem I., Angelin B., et al. 2005. Effects of cholesteryl ester transfer protein inhibition on high-density lipoprotein subspecies, apolipoprotein A-I metabolism, and fecal sterol excretion. Arterioscler. Thromb. Vasc. Biol. 25: 1057–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Millar J. S., Brousseau M. E., Diffenderfer M. R., Barrett P. H. R., Welty F. K., Faruqi A., Wolfe M. L., Nartsupha C., Digenio A. G., Mancuso J. P., et al. 2006. Effects of the cholesteryl ester transfer protein inhibitor torcetrapib on apolipoprotein B100 metabolism in humans. Arterioscler. Thromb. Vasc. Biol. 26: 1350–1356 [DOI] [PubMed] [Google Scholar]
- 18.Millar J. S., Brousseau M. E., Diffenderfer M. R., Barrett P. H. R., Welty F. K., Cohn J. S., Wilson A., Wolfe M. L., Nartsupha C., Schaefer P. M., et al. 2008. Effects of the cholesteryl ester transfer protein inhibitor torcetrapib on VLDL apolipoprotein E metabolism. J. Lipid Res. 49: 543–549 [DOI] [PubMed] [Google Scholar]
- 19.Cohn J. S., Wagner D. A., Cohn S. D., Millar J. S., Schaefer E. J. 1990. Measurement of very low density and low density lipoprotein apolipoprotein (apo) B-100 and high density lipoprotein apoA-I production in human subjects using deuterated leucine. Effect of fasting and feeding. J. Clin. Invest. 85: 804–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lichtenstein A. H., Hachey D. L., Millar J. S., Jenner J. L., Booth L., Ordovas J., Schaefer E. J. 1992. Measurement of human apolipoprotein B-48 and B-100 kinetics in triglyceride-rich lipoproteins using [5,5,5-2H3]leucine. J. Lipid Res. 33: 907–914 [PubMed] [Google Scholar]
- 21.Havel R. J., Eder H., Bragdon J. 1955. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J. Clin. Invest. 34: 1345–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamon-Fava S., Diffenderfer M. R., Barrett P. H. R., Buchsbaum A., Matthan N. R., Lichtenstein A. H., Dolnikowski G. G., Horvath K., Asztalos B. F., Zago V., et al. 2007. Effects of different doses of atorvastatin on human apolipoprotein B-100, B-48, and A-I metabolism. J. Lipid Res. 48: 1746–1753 [DOI] [PubMed] [Google Scholar]
- 23.Cobelli C., Toffolo G., Bier D., Nosadini R. 1987. Models to interpret kinetic data in stable isotope tracer studies. Am. J. Physiol. 253: E551–E564 [DOI] [PubMed] [Google Scholar]
- 24.Welty F. K., Lichtenstein A. H., Barrett P. H. R., Dolnikowski G. G., Schaefer E. J. 1999. Human apolipoprotein (apo) B-48 and apoB-100 kinetics with stable isotopes. Arterioscler. Thromb. Vasc. Biol. 19: 2966–2974 [DOI] [PubMed] [Google Scholar]
- 25.Lamon-Fava S., Diffenderfer M. R., Barrett P. H. R., Buchsbaum A., Nyaku M., Horvath K. V., Asztalos B. F., Otokozawa S., Ai M., Matthan N. R., et al. 2008. Extended-release niacin alters the metabolism of plasma apolipoprotein (apo) A-I and apoB-containing lipoproteins. Arterioscler. Thromb. Vasc. Biol. 28: 1672–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNamara J. R., Schaefer E. J. 1987. Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clin. Chim. Acta. 166: 1–8 [DOI] [PubMed] [Google Scholar]
- 27.Zilversmit D. B., Shea T. M. 1989. Quantitation of apoB-48 and apoB-100 by gel scanning or radio-iodination. J. Lipid Res. 30: 1639–1646 [PubMed] [Google Scholar]
- 28.Barter P. J., Caulfield M., Eriksson M., Grundy S. M., Kastelein J. J. P., Komajda M., Lopez-Sendon J., Mosca L., Tardif J-C., Waters D. D., Shear C. L., Revkin J. H., Buhr K. A., Fisher M. R., Tall A. R., Brewer B. for the ILLUMINATE Investigators 2007. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357: 2109–2122 [DOI] [PubMed] [Google Scholar]
- 29.Nicholls S. J., Tuzcu E. M., Brennan D. M., Tardif J-C., Nissen S. E. 2008. Cholesteryl ester transfer protein inhibition, high-density lipoprotein raising, and progression of coronary atherosclerosis: insights from ILLUSTRATE (Investigation of Lipid Level Management Using Coronary Ultrasound to Assess Reduction of Atherosclerosis by CETP Inhibition and HDL Elevation). Circulation. 118: 2506–2514 [DOI] [PubMed] [Google Scholar]
- 30.Forrest M. J., Bloomfield D., Briscoe R. J., Brown P. N., Cumiskey A-M., Ehrhart J., Hershey J. C., Keller W. J., Ma X., McPherson H. E., et al. 2008. Torcetrapib-induced blood pressure elevation is independent of CETP inhibition and is accompanied by increased circulating levels of aldosterone. Br. J. Pharmacol. 154: 1465–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krishna R., Anderson M. S., Bergman A. J., Jin B., Fallon M., Cote J., Rosko K., Chavez-Eng C., Lutz R., Bloomfield D. M., et al. 2007. Effect of the cholesteryl ester transfer protein inhibitor, anacetrapib, on lipoproteins in patients with dyslipidaemia and on 24-h ambulatory blood pressure in healthy individuals: two double-blind, randomized placebo-controlled phase I studies. Lancet. 370: 1907–1914 [DOI] [PubMed] [Google Scholar]
- 32.Stein E. A., Stroes E. S. G., Steiner G., Buckley B. M., Capponi A. M., Burgess T., Niesor E. J., Kallend D., Kastelein J. J. P. 2009. Safety and tolerability of dalcetrapib. Am. J. Cardiol. 104: 82–91 [DOI] [PubMed] [Google Scholar]
- 33.Cohn J. S., Johnson E. J., Millar J. S., Cohn S. D., Milne R. W., Marcel Y. L., Russell R. M., Schaefer E. J. 1993. Contribution of apoB-48 and apoB-100 triglyceride-rich lipoproteins (TRL) to postprandial increase in the plasma concentration of TRL triglycerides and retinyl esters. J. Lipid Res. 34: 2033–2040 [PubMed] [Google Scholar]
- 34.Niesor E. J., Chaput E., Staempfli A., Blum D., Derks M., Kallend D. 2011. Effect of dalcetrapib, a CETP modulator, on non-cholesterol sterol markers of cholesterol homeostasis in healthy subjects. Atherosclerosis. 219: 761–767 [DOI] [PubMed] [Google Scholar]
- 35.Trevaskis N. L., Shanker R. M., Charman W. N., Porter C. H. J. 2010. The mechanism of lymphatic access of two cholesteryl ester transfer protein inhibitors (CP524,515 and CP532,623) and evaluation of their impact on lymph lipoprotein profiles. Pharm. Res. 27: 1949–1964 [DOI] [PubMed] [Google Scholar]
- 36.Parhofer K. G., Laubach E., Barrett P. H. R. 2003. Effect of atorvastatin on postprandial lipoprotein metabolism in hypertriglyceridemic patients. J. Lipid Res. 44: 1192–1198 [DOI] [PubMed] [Google Scholar]
- 37.Otokozawa S., Ai M., Van Himbergen T., Asztalos B. F., Tanaka A., Stein E. A., Jones P. H., Schaefer E. J. 2009. Effects of intensive atorvastatin and rosuvastatin treatment on apolipoprotein B-48 and remnant lipoprotein cholesterol levels. Atherosclerosis. 205: 197–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ishigami M., Yamashita S., Sakai N., Hirano K., Hiraoka H., Nakamura T., Matsuzawa Y. 2003. Atorvastatin markedly improves type III hyperlipoproteinemia in association with reduction of both exogenous and endogenous apolipoprotein B-containing lipoproteins. Atherosclerosis. 168: 359–366 [DOI] [PubMed] [Google Scholar]
- 39.Guerin M., Egger P., Le Goff W., Soudant C., Dupuis R., Chapman M. J. 2002. Atorvastatin reduces postprandial accumulation and cholesteryl ester transfer protein-mediated remodeling of triglyceride-rich lipoprotein subspecies in type IIB hyperlipidemia. J. Clin. Endocrinol. Metab. 87: 4991–5000 [DOI] [PubMed] [Google Scholar]
- 40.Dane-Stewart C. A., Watts G. F., Pal S., Chan D., Thompson P., Hung J., Mamo J. C. L. 2003. Effect of atorvastatin on apolipoprotein B48 metabolism and low-density lipoprotein receptor activity in normolipidemic patients with coronary artery disease. Metabolism. 52: 1279–1286 [DOI] [PubMed] [Google Scholar]
- 41.Schaefer E. J., McNamara J. R., Tayler T., Daly J. A., Gleason J. A., Seman L. J., Ferrari A., Rubenstein J. J. 2002. Effects of atorvastatin on fasting and postprandial lipoprotein subclasses in coronary heart disease patients versus control subjects. Am. J. Cardiol. 90: 689–696 [DOI] [PubMed] [Google Scholar]
- 42.Sposito A. C., Santos R. D., Amâncio R. F., Ramiers J. A. F., Chapman M. J., Maranhão R. C. 2003. Atorvastatin enhances the plasma clearance of chylomicron-like emulsions in subjects with atherogenic dyslipidemia: relevance to the in vivo metabolism of triglyceride-rich lipoproteins. Atherosclerosis. 166: 311–321 [DOI] [PubMed] [Google Scholar]
- 43.Hogue J-C., Lamarche B., Deshaies Y., Tremblay A. J., Bergeron J., Gagné C., Couture P. 2008. Differential effect of fenofibrate and atorvastatin on in vivo kinetics of apolipoproteins B-100 and B-48 in subjects with type 2 diabetes mellitus with marked hypertriglyceridemia. Metabolism. 57: 246–254 [DOI] [PubMed] [Google Scholar]
- 44.Tremblay A. J., Lamarche B., Lemelin V., Hoos L., Benjannet S., Seidah N. G., Davis H. R., Jr, Couture P. 2011. Atorvastatin increases intestinal expression of NPC1L1 in hyperlipidemic men. J. Lipid Res. 52: 558–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guerin M., Le Goff W., Duchene E., Julia Z., Nguyen T., Thuren T., Shear C. L., Chapman M. J. 2008. Inhibition of CETP by torcetrapib attenuates the atherogenicity of postprandial TG-rich lipoproteins in type IIB hyperlipidemia. Arterioscler. Thromb. Vasc. Biol. 28: 148–154 [DOI] [PubMed] [Google Scholar]
- 46.Schaefer E. J., Gregg R. E., Ghiselli G., Forte T. M., Ordovas J. M., Zech L. A., Brewer H. B., Jr 1986. Familial apolipoprotein E deficiency. J. Clin. Invest. 78: 1206–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.