Abstract
Maintenance of ionic balance is essential for neuronal functioning. Hydrogen sulfide (H2S), a known toxic environmental gaseous pollutant, has been recently recognized as a gasotransmitter involved in numerous biological processes and is believed to play an important role in the neural activities under both physiological and pathological conditions. However, it is unclear if it plays any role in maintenance of ionic homeostasis in the brain under physiological/pathophysiological conditions. Here, we report by directly measuring Na+ activity using Na+ selective electrodes in mouse cortical slices that H2S donor sodium hydrosulfide (NaHS) increased Na+ influx in a concentration-dependent manner. This effect could be partially blocked by either Na+ channel blocker or N-methyl-D-aspartate receptor (NMDAR) blocker alone or almost completely abolished by coapplication of both blockers but not by non-NMDAR blocker. These data suggest that increased H2S in pathophysiological conditions, e.g., hypoxia/ischemia, potentially causes a disruption of ionic homeostasis by massive Na+ influx through Na+ channels and NMDARs, thus injuring neural functions. Activation of delta-opioid receptors (DOR), which reduces Na+ currents/influx in normoxia, had no effect on H2S-induced Na+ influx, suggesting that H2S-induced disruption of Na+ homeostasis is resistant to DOR regulation and may play a major role in neuronal injury in pathophysiological conditions, e.g., hypoxia/ischemia.
Keywords: hydrogen sulfide, Na+ homeostasis, ionotropic glutamate receptor, Na+ channel, delta-opioid receptor, cortex
Hydrogen sulfide (H2S) has been long known as a toxic environmental gaseous pollutant with the characteristic pungent odor of rotten eggs. Most of the environmental H2S comes from the industrial by products released into the atmosphere and from natural sources such as petroleum, sewage, volcanic gases, and sulfur springs. H2S is highly toxic and often fatal at higher concentrations, and a chronic exposure at lower concentrations or sublethal dose of H2S could be harmful as well (Integrated Risk Information System, 2003; Szabó, 2007). H2S toxicity in the central nervous system (CNS) can compromise neural functions (Abe and Kimura, 1996; Kilburn, 2003; Kombian et al., 1993; Partlo et al., 2001), alter metabolism (Elovaara et al., 1978; Hannah et al., 1989), exhaust mitochondrial cytochrome c oxidase and cause its deficiency (Meo et al., 2011), damage brain structures (Gaitonde and Sellar, 1987; Hooser et al., 2000; Solnyshkova, 2003; Solnyshkova et al., 2004), and lead to developmental abnormalities (Hannah and Roth, 1991) and neuronal death/loss (Brenneman et al., 2000; Cheung et al., 2007; Hooser et al., 2000). Therefore, it may cause an encephalopathic- and neurasthenic-like syndrome and some neurological and/or neuropsychological abnormalities (Gaitonde and Sellar, 1987; Hirsch, 2002; Kilburn, 2003; Meo et al., 2011).
Hydrogen sulfide's role as a neuromodulator in physiological functioning of the CNS was first recognized in 1996 (Abe and Kimura, 1996). H2S is now acknowledged as the third endogenous gaseous transmitter succeeding its two prior cousins—nitric oxide and carbon monoxide (Wang, 2002). H2S is produced endogenously in the body by the transsulfuration enzymes like cystathionine-γ-lyase, cystathionine β-synthase (CBS), and the recently discovered 3-mercaptopyruvate sulfurtransferase (Kamoun, 2004; Wang, 2002). In the brain, CBS is the main enzyme responsible for the endogenous production of H2S. The reported physiological levels in the human, rat, and bovine brain are relatively high, ranging from 50 to 160μM. As a gasotransmitter, H2S exerts multiple effects at physiological concentrations in the CNS, for example, it potentiates the activity of N-methyl-D-aspartate receptors (NMDARs) and enhances long-term potentiation (LTP) in the hippocampus associated with learning and memory (Abe and Kimura, 1996). H2S induces Ca2+ wave in neurons and glial cells, stimulates intracellular cyclic adenosine monophosphate (cAMP) production, regulates intracellular signaling activity (e.g., protein kinase A [PKA], mitogen-activated protein kinases), scavenges free radical species (such as hydrogen peroxide, nitric oxide, peroxynitrite, and hypochlorous acid), and increases intracellular glutathione (a major and effective antioxidant) levels (Tan et al., 2010). Abnormality of endogenous H2S production has been observed in several neurological diseases such as stroke (Qu et al., 2006; Ren et al., 2010), and Alzheimer's disease (AD) (Eto et al., 2002; Gong et al., 2011).
The role of H2S in the pathophysiological conditions in the brain remains incongruent. On the one hand, because of its ability to scavenge free radical species and increase the intracellular glutathione levels, H2S offers neuroprotection against oxidative stress (Tan et al., 2010). In sharp contrast, some studies have showed that H2S, even in physiological ranges, increases neuronal death (apoptosis and necrosis) and recruitment of death-inducing signal complexes associated with NMDAR-dependent pathways (Brenneman et al., 2000; Chen et al., 2011; Cheung et al., 2007; Hooser et al., 2000). As a matter of fact, NMDAR-dependent neuronal injury and functional changes are a well-known phenomenon associated with certain pathological conditions such as ischemia (Aarts et al., 2002; Lee et al., 1999) and AD (Bordji et al., 2011; Chohan and Iqbal, 2006; Doraiswamy, 2003; Farlow, 2004; Hu et al., 2012; Malinow, forthcoming; Parameshwaran et al., 2008). For example, NMDAR-mediated Ca2+ overload in ischemic stress can lead to severe neuronal injury/death (Lee et al., 1999), and amyloid beta– and tau–induced neurotoxicity and deleterious effects in synaptic transmission and plasticity that contribute to memory and cognitive deficits in AD have been shown to be mediated by NMDAR (Bordji et al., 2011; Chohan and Iqbal, 2006; Doraiswamy, 2003; Farlow, 2004; Hu et al., 2012; Malinow, forthcoming; Parameshwaran et al., 2008). Indeed, memantine, a noncompetitive NMDAR antagonist, has been approved in Europe for the treatment of moderately severe to severe AD and is an investigational drug in the United States (Doraiswamy, 2003; Farlow, 2004; Hu et al., 2012; Reisberg et al., 2003). Therefore, it is essential to clearly understand the role of H2S in brain pathophysiology, at various concentrations and under hypoxic or ischemic conditions.
Neuronal function is critically dependent on the maintenance of ionic homeostasis. A disruption of ionic homeostasis is considered to be a key initial step in neuronal injury/death that occurs in many pathological conditions (Chao and Xia, 2010). Na+ is the predominant ion in the extracellular space. Changes in the extracellular sodium concentration ([Na+]o) have profound effects on the cellular functions, e.g., neuronal excitability, intracellular Ca2+ homeostasis, pH stability, and glutamate uptake by altering the operating mode of Na+/Ca2+ exchange, Na+/H+ exchange, and Na+-glutamate cotransport (Calabresi et al., 1999; Kiedrowski, 2007; Rojas et al., 2007; Sheldon and Church, 2004). A large amount of Na+ influx and the subsequent Na+ overload can induce neuronal injury and death (Chao and Xia, 2010). It is unknown whether H2S has any effects on Na+ homeostasis in the brain. Studies have shown that H2S can modulate the neuronal activity through regulation of ionotropic glutamate receptors (Abe and Kimura, 1996; Cheung et al., 2007). A common feature of ionotropic glutamate receptor channels (including NMDAR channels and non-NMDAR channels) is Na+ permeability (Mayer and Westbrook, 1987). In addition, voltage-gated Na+ channels constitute the major route of Na+ influx into neurons for normal neuronal activities as well as under pathological conditions (e.g., ischemia) (Chao and Xia, 2010; Jarecki et al., 2010). Both ionotropic glutamate receptor channels and voltage-gated Na+ channels have been shown to mediate hypoxic/ischemic Na+ influx in the cortex (Chao et al., 2009; Chao and Xia, 2010; Kang et al., 2009; Chao, He, Yang, Bazzy-Asaad, Lazarus, Balboni, Kim, and Xia, unpublished data). However, it still remains to be determined if these channels are also involved in H2S regulation of Na+ homeostasis.
In the present study, we have tried to determine the role of H2S in Na+ homeostasis at different concentrations by directly measuring Na+ concentration in the cortical slices with Na+ selective electrodes. In addition, we have explored the possible involvement of ionotropic glutamate receptors and Na+ channels in the H2S effect in normoxic conditions. After exploring the pathophysiological role of H2S in the cortical tissues, we also checked the effect of activation of delta-opioid receptors (DOR) on H2S regulation of Na+ homeostasis because DOR has been well documented as a neuroprotector in the cortex (Chao and Xia, 2010). The results of this work will help us to better understand the mechanisms of hypoxic/ischemic injury in the cortex.
MATERIALS AND METHODS
Slice preparation.
The Animal Care and Use Committee of Yale University School of Medicine, accredited by the American Association for Accreditation for Laboratory Animal Care, approved all our experiments. Slices of the frontoparietal cortex were prepared following the protocol described in our previous studies (Chao et al., 2007a,b). Transverse cortical slices (400 μm) were cut from 24- to 32-day-old male C57BL/6 mice (Charles River Laboratories, Wilmington, MA) with a vibratome containing carbogen (95% O2, 5% CO2) saturated ice-cold standard artificial cerebrospinal fluid (ACSF) and then transferred to an incubation holder placed in a beaker containing 150 ml ACSF vigorously aerated with carbogen at ∼35°C. Standard ACSF consisted of (in mM) NaCl 125, KCl 3.1, NaHCO3 26, CaCl2 2.4, MgSO4 1.3, NaH2PO4 1.25, and dextrose 10 at pH 7.4. Slices were used for recording after an equilibration period of at least 90 min in carbogen saturated ACSF at ∼35°C. The recordings were made in the outer layer (corresponding to layer II and III) of the cortex.
A slice was transferred to the recording chamber (Model RC-22C; Warner Instrument Co., Hamden, CT) perfused with carbogen saturated ACSF (35.5 ± 0.5°C) at a flow rate of ∼3 ml/min. Slices were completely submerged 0.5–1 mm below the ACSF surface in the tissue chamber and kept under normoxic conditions for at least 15 min at ∼35.5°C before taking the experimental measurements.
Measurements of extracellular [Na+].
Extracellular Na+ concentrations ([Na+]o) were measured using Na+-sensitive microelectrodes. Na+-sensitive microelectrodes were prepared as described previously (Kang et al., 2009). Glass pipette–pulled electrodes were silanized by exposure to hexamethyldisilazane for Na+ electrode and subsequently baked at about 180°C for at least 2 h. The microelectrode tips were then broken back to ∼2 μm. The internal filling solution (150mM NaCl + 10mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) was injected into the electrode from the back. A cocktail mixture of internal filling fluid for Na+ electrodes (10% Na+ ionophore VI, 89.5% 2-nitrophenyl octyl ether, and 0.5% potassium tetraphenylborate), with height about 1 mm was sucked into the microelectrode tips. The reference electrode was an Ag/AgCl bridge electrode embedded in 2% agar in 3M KCl solution. Calibrations were carried out by detecting the responses generated in NaCl solutions (5, 10, 20, 50, 80, 100, 120, 150mM NaCl) in triplicate. For each concentration, the average voltage change in three separate tests was used as the final measurement for voltage change. In this range, the electrode response was near ideal, showing a logarithmic relationship to [Na+].
Electrical signals were monitored on an oscilloscope, recorded by a DC amplifier (Model IE-210, LPF 200; Warner Instrument Co.) and digitized by an Axon mini-digitizer acquisition system (Model miniDigi 1A; Axon Instruments, Union City, CA) at a sampling rate of 100 Hz. The following parameters were used for assessment of Na+ activity: (1) the maximal drop in [Na+]o induced by NaHS; (2) the time of occurrence of the maximal drop in [Na+]o induced by NaHS (Tmax), which refers to the time interval from the beginning of NaHS perfusion to the point of maximal [Na+]o drop; and (3) the recovery period that refers to the total time taken for Na+ levels to return to baseline during reperfusion with normal ACSF.
The slices were subjected to further experiments after recording a stable baseline for at least 5 min. All electrophysiological recordings were performed continuously for 40–60 min.
Drug administration.
Drugs were administered by switching from control superfusate to one containing drug(s) via a six-channel valve-controlled perfusion system (Model VC-6; Warner Instrument Co.). All drugs were perfused for 20 min before induction of [Na+]o drop and continued through the process. Under our experimental condition, H2S from NaHS could easily diffuse to the slice when NaHS reached the slice (theoretically no more than 20 s in maximum for total replacement of ACSF with NaHS-containing one in recording chamber). When NaHS arrives to the chamber, it quickly gets equilibrated with approximately one third of the H2S existing as the undissociated form (H2S), and the remaining two thirds existing as HS− at equilibrium with H2S (Abe and Kimura, 1996; Nagai et al., 2004). Because the perfusion was continuously made all the time, the H2S concentration was then maintained in a stable level in the whole duration of NaHS perfusion. Therefore, our experiment condition is well controlled and duplicable.
Chemicals.
Na+ ionophore VI (Fluka 71739), 2-nitrophenyl octyl ether (Fluka 73732), sodium tetraphenylborate (Fluka 72018), hexamethyldisilazane (Fluka 52619), and sodium hydrosulfide (NaHS) were purchased from Sigma Chemicals Co. (St Louis, MO). Tetrodotoxin citrate (TTX), (+)-MK 801 maleate, and CNQX disodium salt were purchased from Tocris Cookson Inc. (Ellisville, MI). UFP 512 (H-Dmt-Tic-NH-CH(CH2-COOH)-Bid), a specific and potent DOR agonist (Balboni et al., 2002), was synthesized by our research team.
As a H2S donor, NaHS was first prepared as a stock solution with the concentration being 1000 times higher than that of the final concentration used in the work and stored at 4°C in a sealed glass bottle immediately before recording, and the appropriate volume was added to ASCF immediately before slice perfusion with NaHS to obtain the expected concentration. As previously documented (Abe and Kimura, 1996; Nagai et al., 2004), with NaHS as a source of H2S, “in physiological saline, approximately one third of the H2S exists as the undissociated form (H2S), and the remaining two thirds exists as HS− at equilibrium with H2S. The use of NaHS enables us to define the concentrations of H2S in solution more accurately and reproducibly than bubbling H2S.”
The extracellular [Na+] was around 152mM in our slices, although most of our experiments were done with 150 and 300μM of NaHS with the maximal concentration being used at 1.2mM. Under such a condition, the influence of sodium ion from NaHS on the electrophysiological experiments is negligible (Abe and Kimura, 1996; Nagai et al., 2004).
NaHS did not change the pH of buffered ACSF (Abe and Kimura, 1996) in perfused slices under our electrophysiological conditions. Indeed, we randomly checked the pH of NaHS-containing ACSF in our experiments and found that even with 1200μM of NaHS, no pH changes of perfused ACSF occurred under our experimental condition.
Statistics.
All data are expressed as mean ± SEM, and the number of experiments (n) refers to the number of slices investigated. Except indicated in the text, one-way ANOVA followed by Newman Keuls test was used for multiple pairwise tests, and two-tailed unpaired Student's t-test was used for comparison of the two experimental groups. Observations were identified as significant if p < 0.05.
RESULTS
Concentration-Dependent Induction of Na+ Influx by H2S
To explore whether H2S regulates [Na+]o, we perfused the cortical slices with NaHS. As shown in Figures 1 and 2, NaHS at a concentration less than 100μM has very little effect on [Na+]o. Of the six slices perfused with 100μM NaHS, five did not show any appreciable change in [Na+]o, and the remaining one showed a slow and slight decrease in [Na+]o, as shown in Figure 2B. All these slices showed a large sudden drop in [Na+]o in response to oxygen-glucose deprivation (OGD), indicating that they have a reliable viability. With an increase in concentration (≥ 150μM), NaHS evoked a large drop in [Na+]o in almost all the slices investigated in a concentration-dependent fashion. With an increase in NaHS concentrations from 150 to 1200μM, there was shortening of the interval to maximal drop of [Na+]o and prolongation of the recovery time (Fig. 2). These results suggest that low concentrations of H2S have little effect on ionic homeostasis; whereas higher levels disrupt the ionic homeostasis in the cortex.
FIG. 1.
Extracellular Na+ response to NaHS at different concentrations. The bars indicate the response rate of the examined cortical slices. Note that NaHS at concentration less than 100μM has very little effect on extracellular Na+ activity in all slices. With the increase in its concentration (≥ 150μM), NaHS evokes a large drop in [Na+]o (a direct index of Na+ influx) in a concentration-dependent fashion in almost all the slices that were investigated. Chi-square test showed a statistically significant difference in comparison with control when the concentration of NaHS increased to 150 (p = 0.0013), 300 (p < 0.0001), 600 (p = 0.0005), and 1200μM (p = 0.0001).
FIG. 2.
NaHS-evoked Na+ influx in the cortical slices. Trace recordings of (A) control, (B–F) 100, 150, 300, 600, 1200μM of NaHS respectively. (G–I) Statistical results of each recording parameter. ***p < 0.001 as compared with the control; ###p < 0.001 as compared with 100μM of NaHS; &p < 0.05, &&p < 0.01, &&&p < 0.001 as compared with 150μM of NaHS; +p < 0.05, +++p < 0.001 as compared with 300μM of NaHS. Note that NaHS, at the concentrations ≥ 150μM, evoked a large concentration-dependent fall in [Na+]o in almost all the slices investigated with a significantly shortened interval to maximal fall in [Na+]o and prolonged the recovery time from Na+ drop.
Effect of Blocking Na+ Channels on H2S-Evoked Na+ Influx
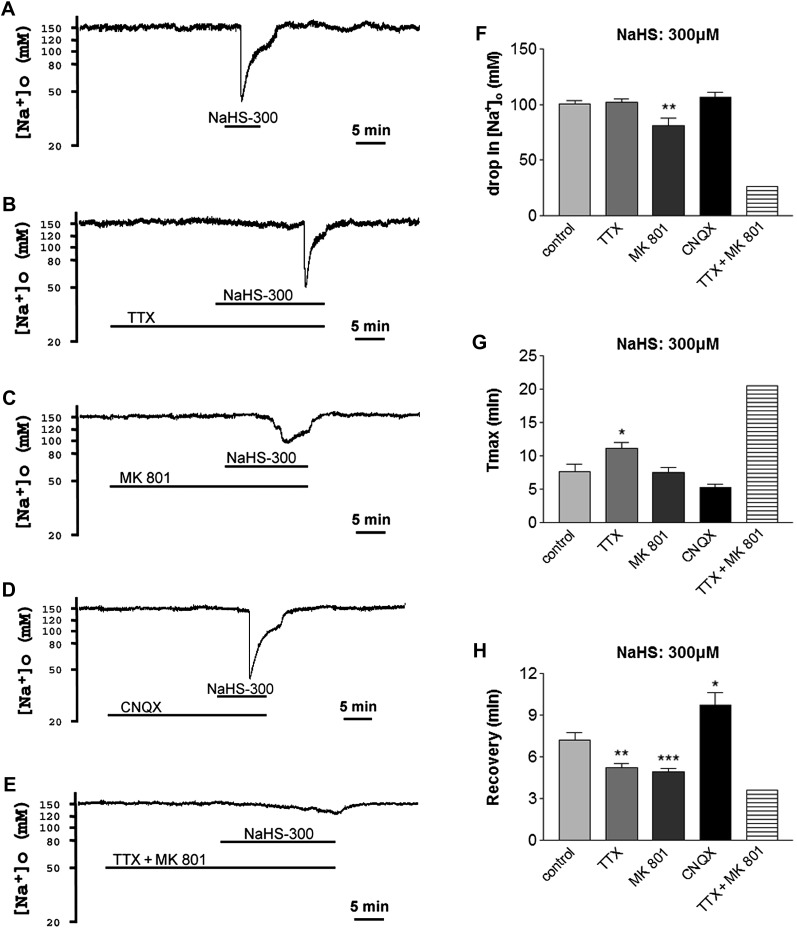
Voltage-gated Na+ channels comprise the major route of Na+ influx into neurons under both normal and pathophysiological conditions (e.g., ischemia) (Chao and Xia, 2010). These channels are well-known mediators of hypoxic/ischemic Na+ influx in the cortex (Chao et al., 2009; Kang et al., 2009; Chao, He, Yang, Bazzy-Asaad, Lazarus, Balboni, Kim, and Xia, unpublished data). To investigate their possible involvement in NaHS-induced Na+ influx, we applied TTX, a Na+ channel blocker, to the cortical slices and examined its effect on NaHS-induced disruption of Na+ homeostasis.
In eight of nine cortical slices, NaHS at 150μM evoked a sudden drop of 92.1 ± 8.4mM in [Na+]o that appeared after 13.1 ± 1.7 min of NaHS perfusion and took 4.7 ± 0.6 min to recover from peak drop to baseline after reperfusion with normal ACSF. In the presence of TTX (1μM), all the seven slices that were investigated (100%) did not have any response to NaHS (150μM) (p = 0.0004, chi-square test), i.e., NaHS (150μM) did not induce any appreciable change in [Na+]o (Fig. 3A). OGD test showed that these slices had good viability with an obvious sudden drop in [Na+]o in response to OGD in all these seven slices. With an increase in concentration to 300μM, NaHS evoked a sudden drop of 100.5 ± 3.0mM in [Na+]o that appeared after 7.7 ± 1.1 min of perfusion with NaHS and took 7.2 ± 0.6 min for recovery from peak drop to baseline after reperfusion with normal ACSF in 12 cortical slices investigated. In the presence of TTX (1μM), 21% (3 of 14) slices showed no response to NaHS (300μM) (p = 0.088, chi-square test), and the OGD test in these slices had reliable viability with an obvious sudden drop in [Na+]o in response to OGD. In the remaining slices (11 of 14), TTX (1μM) significantly postponed the occurrence of peak Na+ drop and accelerated the recovery from peak drop (n = 11), whereas there was no difference in the drop of [Na+]o between TTX (1μM) group (n = 11) and control (n = 12) group (Figs. 3B and 4).
FIG. 3.
Differential effects of TTX, MK 801, CNQX, and UFP 512 on extracellular Na+ response to 150 (A) and 300 (B) μM NaHS. The bars indicate the response rate of the cortical slices investigated. Na+ channel blocker TTX (1μM) completely blocked extracellular Na+ response to 150μM of NaHS in all the seven slices investigated (100%) (p = 0.0004, chi-square test). In the presence of NMDAR antagonist MK 801 (10μM) and non-NMDAR antagonist CNQX (10μM), respectively, 67 and 73% slices no longer showed responses to NaHS (150μM) (p = 0.011 and 0.006, respectively, chi-square test). Activation of DOR with UFP 512 (5μM) did not increase the number of slices that showed a lack of response to 150μM of NaHS (p = 0.881, chi-square test). In contrast, in the presence of TTX (1μM) and MK 801 (10μM), respectively, there were 21 and 18% of the slices showing lack of response to 300μM of NaHS (p = 0.088 and 0.124, respectively, chi-square test). In the presence of CNQX (10μM) or UFP 512 (5μM), all slices still responded to 300μM NaHS, as seen in NaHS alone. However, NaHS (300μM)-evoked drop in [Na+]o was completely blocked by coperfusion of TTX (1μM) and MK 801 (10μM) in eight of nine slices investigated (89%) (p < 0.0001, chi-square test).
FIG. 4.
Different roles of Na+ channels, NMDAR, and non-NMDAR in NaHS-evoked Na+ influx. Trace recordings of (A) control, (B) TTX (1μM), (C) MK 801 (10μM), (D) CNQX (10μM), and (E)TTX + MK 801. (F–H) Statistical results of each recording parameter. *p < 0.05, **p < 0.01, ***p < 0.001 as compared with the control. Note that 300μM NaHS-evoked Na+ influx could be partially attenuated by Na+ channel blocker TTX or NMDAR antagonist MK 801 but could not be decreased by non-NMDAR antagonist CNQX.
Effects of Blocking Ionotropic Glutamate Receptor Channels on H2S-Evoked Na+ Influx in the Cortical Slices
Na+ permeability is a common feature of ionotropic glutamate receptors (Mayer and Westbrook, 1987). These receptors are also mediators of hypoxic/ischemic Na+ influx in the cortex (Chao and Xia, 2010). To investigate their possible involvement in NaHS-induced Na+ influx, we applied MK 801, an NMDAR blocker, and CNQX, a non-NMDAR blocker, respectively, to the cortical slices and examined their effects on NaHS-induced disruption of Na+ homeostasis.
In the presence of MK 801 (10μM) and CNQX (10μM), respectively, 67% (8 of 12) and 73% (8 of 11) slices no longer showed responses to 150μM NaHS (p = 0.011 and 0.006, respectively, chi-square test) (Fig. 3A). In the remaining four slices, MK 801 (10μM) decreased the drop in [Na+]o from 92.1 ± 8.4mM to 59.5 ± 20.1mM. Given to the limited number of slices that showed a response to NaHS (150μM) with a huge variation, there is no statistically significant difference between MK 801 group and control with respect to the drop in [Na+]o, Tmax, and recovery. Similarly, the remaining three slices in CNQX group also showed no significant change in extracellular Na+ activity in response to NaHS (150μM) when compared with the control group.
In 300μM NaHS perfused slices with perfusion of MK 801 (10μM), 18% slices showed no response to NaHS (300μM) (p = 0.124, chi-square test), and the OGD test in these slices had reliable viability with an obvious sudden drop in [Na+]o in response to OGD. In contrast, in the presence of CNQX (10μM), there was no increase in the number of the slices that showed a lack of response to NaHS (300μM), all the nine slices investigated showed a greater response of [Na+]o to NaHS (300μM) (Fig. 3B). To further explore the effect of MK 801, CNQX on NaHS-induced changes in [Na+]o, we analyzed [Na+]o changes of slices that showed response to 300μM NaHS. As shown in Figure 4, MK 801 (10μM) significantly attenuated NaHS (300μM)-evoked drop in [Na+]o and accelerated the recovery from peak drop (n = 14) but had little effect on the occurrence of peak Na+ drop (Tmax). Unlike MK 801 (10μM) that totally blocked NaHS (300μM)-evoked changes in [Na+]o in some slices, CNQX (10μM) could not completely prevent NaHS (300μM)-evoked changes in [Na+]o in nine slices investigated (Fig. 3B). For all these slices, CNQX (10μM) had no effect on NaHS (300μM)-evoked drop in [Na+]o (Fig. 4). The presence of CNQX (10μM) delayed the recovery from peak Na+ drop, contrary to the acceleration noted with either TTX (1μM) or MK 801 (10μM) (Fig. 4).
Because MK 801 (10μM) significantly reduced NaHS (300μM)-evoked drop in [Na+]o but had little effect on the occurrence of peak Na+ drop (Tmax) (n = 14), whereas TTX (1μM) significantly delayed the occurrence of peak Na+ drop with little effect on the drop of [Na+]o (n = 11), we coapplied TTX (1μM) and MK 801 (10μM) to the cortical slices and examined their combined effect on NaHS (300μM)-evoked disruption of extracellular Na+ homeostasis. We observed a complete blockade of NaHS (300μM)-evoked drop in [Na+]o following a coperfusion of TTX (1μM) and MK 801 (10μM) in eight of nine slices investigated (89%) (p < 0.0001, chi-square test) (Fig. 3B). The only slice that showed a slight response to NaHS (300μM) is shown in Figure 4. In the presence of both TTX (1μM) and MK 801 (10μM), 20 min of NaHS (300μM) perfusion induced [Na+]o only a minor decrease from the baseline (around 152mM) to 126mM with a rapid recovery from Na+ drop when normal ACSF was reintroduced in this slice. This observation suggested that TTX (1μM) and MK 801 (10μM) additively blocked almost all the effects of NaHS (300μM) on Na+ homeostasis.
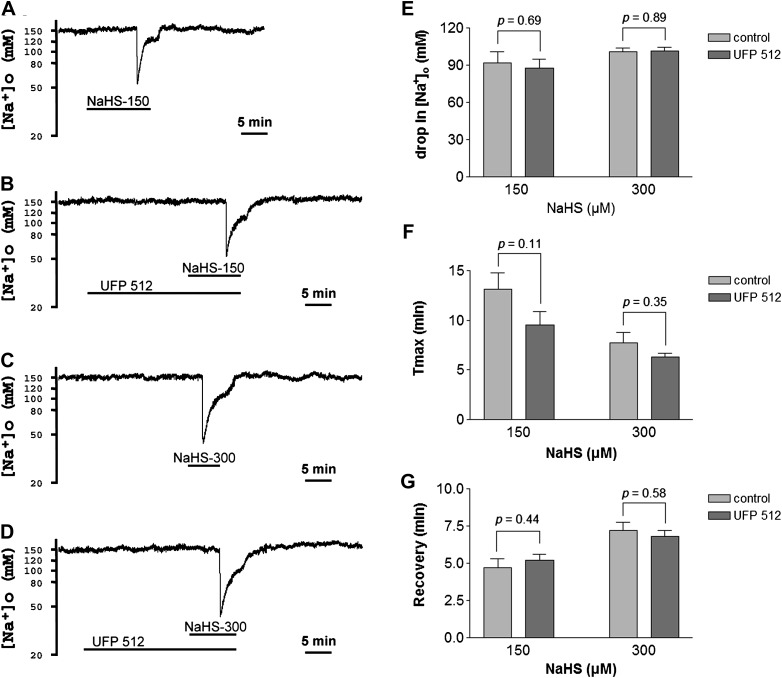
Effect of DOR Activation on H2S-Evoked Na+ Influx
Because our previous work has shown that DOR activation reduces Na+ currents and attenuates anoxia/ischemia-induced Na+ influx in the cortex (Kang et al., 2009; Chao, He, Yang, Bazzy-Asaad, Lazarus, Balboni, Kim, and Xia, unpublished data), we further investigated if DOR activation can attenuate H2S-evoked Na+ influx. We perfused the cortical slices with UFP 512, a specific and potent DOR agonist (Balboni et al., 2002; Chao et al., 2007a). We chose 5μM UFP 512 for the perfusion because this concentration is more effective in suppressing anoxia/ischemia-induced Na+ influx in the cortex (Chao, He, Yang, Bazzy-Asaad, Lazarus, Balboni, Kim, and Xia, unpublished data). As shown in Figures 3 and 5, neither 150μM nor 300μM of NaHS-induced disruption of extracellular Na+ homeostasis could be attenuated by 5μM of UFP 512. The perfusion of UFP 512 (5μM) did not increase the number of slices showing a lack of response to NaHS (150 or 300μM) (p = 0.881 for 150μM NaHS, chi-square test). Furthermore, UFP 512 neither decreased the peak drop in [Na+]o, delayed the occurrence of peak drop, nor accelerated the recovery from [Na+]o drop evoked by NaHS at the concentrations of 150–300μM.
FIG. 5.
Effect of UFP 512 on NaHS-evoked Na+ influx in the cortex. Trace recordings of (A) 150μM of NaHS (NaHS-150), (B) NaHS-150 + UFP 512 (5μM), (C) 300μM of NaHS (NaHS-300), and (D) NaHS-300 + UFP 512 (5μM). (E–G) Statistical results of each recording parameter. Note that activation of DOR with UFP 512 did not affect the NaHS-evoked Na+ influx in the cortex.
DISCUSSION
The major findings in the present study are: (1) NaHS, a donor of H2S, in lower concentrations (< 150μM) did not, whereas in higher concentrations did disrupt the Na+ homeostasis under normoxic condition in a concentration-dependent manner; (2) NaHS (150 or 300μM)-evoked Na+ influx could be partially blocked by either Na+ channel blocker TTX or NMDAR blocker MK 801 but could not be blocked by non-NMDAR blocker CNQX (NaHS in 300μM); (3) TTX and MK 801 additively attenuated almost all of NaHS-induced Na+ influx; and (4) activation of the DOR with UFP 512, which significantly attenuated hypoxic/ischemic Na+ influx, has little effect on NaHS-evoked Na+ influx.
Under physiological conditions, Na+ concentrations, like other cations (K+ and Ca2+), are under a dynamic balance that often fluctuate transiently with neuronal activity within a small range but are brought back to their previous levels via multiple intrinsic mechanisms (Chao and Xia, 2010). A large alteration in extracellular/intracellular Na+ concentrations may trigger an event that leads to neuronal injury and death. For example, large Na+ influx induces cellular injury manifested by acute functional and morphological changes, e.g., loss of electrophysiological response to stimulus, cell swelling, bleb formation, and membrane injury (Calabresi et al., 1999; Friedman and Haddad, 1994; Hasbani et al., 1998; Itoh et al., 1998; Petrat et al., 2006; Shi et al., 2005). Removal of extracellular Na+ or blockade of Na+ entry, therefore, prevents hypoxic/ischemic neuronal damage and death (Banasiak et al., 2004; Breder et al., 2000; Friedman and Haddad, 1994; Probert et al., 1997; Raley-Susman et al., 2001). In the present study, we found that NaHS in lower concentrations (< 150μM) did not evoke an appreciable change in the extracellular Na+ homeostasis; however, at higher concentrations (150–1200μM), NaHS evoked a concentration-dependent large decrease in the [Na+]o under normoxic condition. This observation suggests that H2S in relatively lower physiological levels has little effect on Na+ homeostasis, but it can disrupt Na+ homeostasis at levels beyond physiological. Because Na+ overload due to large Na+ influx induces neuronal injury and death, high-concentration H2S-induced disruption of Na+ homeostasis may be harmful in the cortex. In fact, exposure to H2S, even in lower concentrations, has been shown to impair the functions of the CNS (Brenneman et al., 2000; Hannah and Roth, 1991; Kombian et al., 1993). In higher concentrations, H2S has been reported to suppress synaptic transmission (Abe and Kimura, 1996; Kombian et al., 1993) and cause structural damage (Solnyshkova et al., 2004) and neuronal death (Cheung et al., 2007). Cheung et al. (2007) reported that NaHS at < 200μM induces apoptosis, whereas at concentrations > 200μM, it induces necrosis in the primary cultured mouse cortical neurons. The necrotic neuronal death, cytoplasmic edema, and the vacuolated appearance in the cerebral cortex were also observed in H2S-exposed animals (Hooser et al., 2000; Solnyshkova et al., 2004). These features were also observed in the cells with intracellular Na+ overload (Friedman and Haddad, 1994; Hasbani et al., 1998; Itoh et al., 1998; Petrat et al., 2006; Shi et al., 2005). Therefore, high-concentration H2S induced large increase in Na+ influx that could be responsible for H2S-induced necrotic death, cytoplasmic edema, and the vacuolated appearance in the cerebral cortex. In addition, H2S-evoked disruption of Na+ homeostasis in the cortex may also be related to the phenomenon of H2S inhalation-induced unconsciousness/unresponsiveness seen in both humans as well as the animals. H2S inhalation has been reported to result in unconsciousness/unresponsiveness in some victim patients as well as livestock (Hirsch, 2002; Hooser et al., 2000; Integrated Risk Information System, 2003; Kilburn, 2003). As has been reported, a large Na+ influx causes widespread depolarization of the brain, disappearance of ongoing electrical activity, and interruption/blockade of synaptic transmission (Calabresi et al., 1999; Raley-Susman et al., 2001) and consequently makes the brain unresponsive, hence, unconscious in certain conditions such as stroke, epileptic seizures, and poisoning. The present observation of H2S-evoked Na+ influx could also explain, at least partially, the effect of H2S inhalation through this phenomenon.
Our data suggest that ionotropic glutamate receptor channels act as a direct mediator of H2S-evoked Na+ influx because the ionotropic glutamate receptor blockers attenuate the H2S-evoked Na+ influx in the cortex, and both NMDA and non-NMDAR channels are permeable to Na+ (Mayer and Westbrook, 1987). However, they also mediate H2S-evoked Na+ influx via an indirect mechanism. Besides the permeability to Na+, these two receptor channels are also Ca2+ permeable (Mayer and Westbrook, 1987). It has been indicated that H2S can induce the rise in intracellular Ca2+ through Ca2+ channels and thus induced Ca2+ releases from internal Ca2+ stores in neurons as well as glial cells (Tan et al., 2010). Increased [Ca2+]i can induce Na+ entry by activation of plasma Na+/Ca2+ exchangers to extrude excessive Ca2+ (Blaustein and Lederer, 1999). Therefore, ionotropic glutamate receptors also participate indirectly in the Na+ entry via Na+/Ca2+ exchangers in the cortex. We found NMDAR blocker MK 801 attenuates Na+ influx evoked by both 150 and 300μM NaHS. In contrast, non-NMDAR blocker CNQX could only attenuate the H2S-induced Na+ influx when H2S was in lower concentrations but had little effect on Na+ influx at higher concentrations of H2S. This observation was similar to that of H2S-induced cortical neuronal death made by Cheung et al. (2007) who showed that MK 801 and CNQX could selectively block neuronal death induced by H2S in lower concentrations. In high concentrations of H2S, however, only MK 801, but not CNQX, selectively blocked H2S-induced neuronal death (Cheung et al., 2007). Our results indicate that MK 801 (10μM) significantly diminishes NaHS (300μM)-evoked drop in [Na+]o but does not affect the occurrence of peak Na+ drop (vs. TTX [1μM] that significantly delayed this interval but did not affect the level of peak drop in [Na+]o). Altogether, these observations suggest that NMDAR channels play a more dominant role than non-NMDAR channels in H2S-induced Na+ entry.
The mechanisms underlying H2S-evoked Na+ influx through ionotropic glutamate receptor channels need further elucidation. One possibility is that H2S interacts with disulfides bonds or free thiols (Mustafa et al., 2009) in the ionotropic glutamate receptors in a way of redox modulation to modify the gating properties of the receptor channels and therefore increases Na+ permeability and Na+ influx. This is supported by the notion that disulfide bonds play a role in the modulation of NMDAR function, and H2S might be able to modify two cysteine residues (Cys744 and Cys798) of NR1 subunit of NMDAR to activate NMDAR (Aizenman et al., 1989; Sullivan et al., 1994). However, this may not be the case under conditions, for example, in NaHS potentiation of the induction of LTP in the hippocampus (Abe and Kimura, 1996). Alternatively, H2S-induced activation of cAMP/PKA pathway (Kimura, 2000) may be responsible for NMDAR activation and the subsequently Na+ influx because it has been shown previously that an increased production of cAMP and PKA activation by H2S in the neurons and glial cells phosphorylate NMDAR subunits at specific sites so as to enhance NMDA currents (Kimura, 2000; Leonard and Hell, 1997).
Voltage-gated Na+ channels constitute the major route for Na+ influx into the neurons in normal neuronal activities as well as certain pathophysiological conditions (e.g., ischemia) (Chao and Xia, 2010; Jarecki et al., 2010). H2S is known to target various ion channels such as KATP channels, Ca2+ channels, BK channels, and Cl− channels (Tang et al., 2010). It is not known whether H2S targets voltage-gated Na+ channels to regulate Na+ influx in the cortex. We found that TTX (1μM) completely abolished Na+ influx induced by NaHS at lower concentrations in the cortex in all the slices we investigated and significantly delayed the occurrence of peak Na+ drop and accelerated the recovery from peak drop but had little effect on the drop of [Na+]o evoked by higher concentration of NaHS (300μM). There are at least two ways to interpret these results. The straightforward explanation is that TTX-sensitive voltage-gated Na+ channels are one of the pathways of H2S-induced Na+ influx; therefore, H2S-induced Na+ influx can be blocked by TTX, just like that in 150μM of NaHS-perfusing slices. Another possibility is that TTX-sensitive voltage-gated Na+ channels are indirectly involved in H2S-evoked Na+ influx. Perfusion of TTX can lower the excitability and block the synaptic neurotransmission and therefore postpone the occurrence of peak Na+ drop, as it does in 300μM of NaHS-perfusing slices. Though a recently published study showed that NaHS increases peak sodium currents in myocytes obtained from the circular smooth muscle layer of human jejunum and rightly shifts the voltage dependence of Na+ current inactivation and activation in HEK293 cells heterologously expressing myocyte-type Nav1.5 (Strege et al., 2011), evidence has shown that in the brain, NaHS-induced inward currents of neurons and suppression of synaptic transmission are insensitive to TTX (Abe and Kimura, 1996; Kombian et al., 1993), making it unlikely that voltage-dependent Na+ channels may be involved in the brain. In our study, unlike NMDAR blocker that significantly attenuated NaHS (300μM)-evoked fall in [Na+]o without affecting the occurrence of peak Na+ drop, TTX greatly deferred the onset of NaHS (300μM)-evoked peak Na+ influx, while did not affect the peak drop in [Na+]o. Therefore, it is very likely that TTX-sensitive voltage-gated Na+ channels are indirectly involved in H2S-evoked Na+ influx in the cortex. Coperfusion of TTX (1μM) and MK 801 (10μM) almost completely eliminated the NaHS (300μM)-evoked Na+ influx, suggesting Na+ channels and NMDAR channels as synergistically acting mediators of H2S-evoked Na+ influx in the brain.
As to the resultant CNS lesions, H2S shares some common features with anoxia/ischemia (Hooser et al., 2000). In fact, earlier reports indicated that H2S can inhibit cytochrome oxidase in mitochondria through depleting cellular energy (Smith and Gosselin, 1966; Stene et al., 1976), and H2S toxic effects deriving from cellular anoxia are comparable to those of cyanide anion (Brierley et al., 1977). As it has been known, anoxia/ischemia induces a massive Na+ influx and subsequently triggers neuronal injury and death in the brain (Chao and Xia, 2010), which is similar to the observations of the present study with high-concentration NaHS perfusion in the brain slices. Interestingly, recently published reports on a rat stroke model have shown that H2S levels as well as the expression of H2S synthesizing enzymes in the lesioned cortex and hippocampus are significantly high within 24 h of cerebral ischemia, and a pretreatment with high-concentration NaHS further induces an increase in infarct volume and neuronal injury (Qu et al., 2006; Ren et al., 2010), and a latest report also indicated a significant increase in H2S production in the brain during hypoxic stress (Kwiatkoski et al., 2012). Therefore, H2S disruption of Na+ homeostasis is a likely mechanism of hypoxic/ischemic neuronal injury and death.
The features of H2S neurotoxicity (Abe and Kimura, 1996; Cheung et al., 2007; Hooser et al., 2000; Kombian et al., 1993; Solnyshkova et al., 2004) are similar, to a certain degree, to that in hypoxic/ischemic conditions (Chao and Xia, 2010; Krnjević, 2008). We previously found that DOR neuroprotection is related to its ability to attenuate anoxic/ischemic Na+ influx via regulation of Na+ channels in the cortex (Chao et al., 2009; Kang et al., 2009; Chao, He, Yang, Bazzy-Asaad, Lazarus, Balboni, Kim, and Xia, unpublished data). In the present study, we could not show an attenuation of H2S-evoked Na+ influx after DOR activation under similar experimental conditions. DOR attenuation of anoxic/ischemic disruption of ionic homeostasis including Na+ influx and K+ leakage is largely dependent on the activation of protein kinase C (PKC) and in part on the inhibition of Ca2+ entry (Chao et al., 2007a,b; Pamenter and Buck, 2008). In contrast, the response of neurons to H2S is predominantly cAMP-PKA–dependent (Abe and Kimura, 1996; Kimura, 2000). H2S possesses the ability to increase intracellular Ca2+ levels in neurons as well as glial cells via Ca2+ entry and internal Ca2+ mobilization, which testifies the role of H2S as a gasotransmitter to facilitate Ca2+-mediated signaling between neurons and glial cells (Tan et al., 2010). However, H2S-induced neuronal injury and death seem independent of Ca2+ influx or internal Ca2+ mobilization (Cheung et al., 2007). Even though DOR activation can reduce NMDAR-mediated Ca2+ influx under anoxic condition (Pamenter and Buck, 2008), our data suggest that DOR activation cannot attenuate H2S-evoked Na+ influx through NMDAR owing to the difference in target and signaling molecules of DOR involved in hypoxia from those of H2S exposure. On the other hand, our present observations regarding DOR effects on H2S-evoked Na+ influx reaffirm our previous reports that DOR activation specifically attenuates hypoxic/ischemic (vs. H2S evoked) disruption of ionic homeostasis (such as Na+ influx and K+ leakage) via PKC-dependent and PKA-independent pathway as well as in part by inhibition of Ca2+ entry in the cortex (Chao et al., 2007a,b; Kang et al., 2009; Chao, He, Yang, Bazzy-Asaad, Lazarus, Balboni, Kim, and Xia, unpublished data).
In conclusion, our data suggest that H2S in physiological concentrations exerts a minor effect on ionic homeostasis as a gasotransmitter, whereas at superphysiological levels it can be harmful and neurotoxic as it can disrupt the ionic homeostasis by markedly increasing Na+ influx through its action on ionotropic glutamate receptor channels, which cannot be attenuated by DOR activation that is neuroprotective against hypoxic/ischemic disruption of ionic homeostasis and insults.
FUNDING
National Institutes of Health (AT-004422) and Vivian L . Smith Neurological Foundation.
References
- Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang Y-T, Salter MW. Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science. 2002;298:846–850. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenman E, Lipton SA, Loring RH. Selective modulation of NMDA responses by reduction and oxidation. Neuron. 1989;2:1257–1263. doi: 10.1016/0896-6273(89)90310-3. [DOI] [PubMed] [Google Scholar]
- Balboni G, Salvadori S, Guerrini R, Negri L, Giannini E, Jinsmaa Y, Bryant SD, Lazarus LH. Potent δ-opioid receptor agonists containing the Dmt-Tic pharmacophore. J. Med. Chem. 2002;45:5556–5563. doi: 10.1021/jm020336e. [DOI] [PubMed] [Google Scholar]
- Banasiak KJ, Burenkova O, Haddad GG. Activation of voltage-sensitive sodium channels during oxygen deprivation leads to apoptotic neuronal death. Neuroscience. 2004;126:31–44. doi: 10.1016/S0306-4522(03)00425-1. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Lederer WJ. Sodium/calcium exchange: Its physiological implications. Physiol. Rev. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- Bordji K, Becerril-Ortega J, Buisson A. Synapses, NMDA receptor activity and neuronal Aβ production in Alzheimer's disease. Rev. Neurosci. 2011;22:285–294. doi: 10.1515/RNS.2011.029. [DOI] [PubMed] [Google Scholar]
- Breder J, Sabelhaus CF, Opitz T, Reymann KG, Schrőder UH. Inhibition of different pathways influencing Na+ homeostasis protects organotypic hippocampal slice cultures from hypoxic/hypoglycemic injury. Neuropharmacology. 2000;39:1779–1787. doi: 10.1016/s0028-3908(00)00027-7. [DOI] [PubMed] [Google Scholar]
- Brenneman KA, James RA, Gross EA, Dorman D. Olfactory neuron loss in adult male CD rats following subchronic inhalation exposure to hydrogen sulfide. Toxicol. Pathol. 2000;28:326–333. doi: 10.1177/019262330002800213. [DOI] [PubMed] [Google Scholar]
- Brierley JB, Prior PF, Calverley J, Brown AW. Cyanide intoxication in Macaca mulatta . J. Neurol. Sci. 1977;31:133–157. doi: 10.1016/0022-510x(77)90011-9. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Marfia GA, Centonze D, Pisani A, Bernardi G. Sodium influx plays a major role in the membrane depolarization induced by oxygen and glucose deprivation in rat striatal spiny neurons. Stroke. 1999;30:171–179. doi: 10.1161/01.str.30.1.171. [DOI] [PubMed] [Google Scholar]
- Chao D, Balboni G, Lazarus LH, Salvadori S, Xia Y. Na+ mechanism of δ-opioid receptor induced protection from anoxic K+ leakage in the cortex. Cell. Mol. Life Sci. 2009;66:1105–1115. doi: 10.1007/s00018-009-8759-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao D, Bazzy-Asaad A, Balboni G, Xia Y. δ-, but not μ-, opioid receptor stabilizes K+ homeostasis by reducing Ca2+ influx in the cortex during acute hypoxia. J. Cell. Physiol. 2007a;212:60–67. doi: 10.1002/jcp.21000. [DOI] [PubMed] [Google Scholar]
- Chao D, Donnelly DF, Feng Y, Bazzy-Asaad A, Xia Y. Cortical δ-opioid receptors potentiate K+ homeostasis during anoxia and oxygen-glucose deprivation. J. Cereb. Blood Flow Metab. 2007b;27:356–368. doi: 10.1038/sj.jcbfm.9600352. [DOI] [PubMed] [Google Scholar]
- Chao D, Xia Y. Ionic storm in hypoxic/ischemic stress: Can opioid receptors subside it? Prog. Neurobiol. 2010;90:439–470. doi: 10.1016/j.pneurobio.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MJ, Peng ZF, Manikandan J, Melendez AJ, Tan GS, Chung CM, Li QT, Tan TM, Deng LW, Whiteman M, et al. Gene profiling reveals hydrogen sulphide recruits death signaling via the N-methyl-D-aspartate receptor identifying commonalities with excitotoxicity. J. Cell. Physiol. 2011;226:1308–1322. doi: 10.1002/jcp.22459. [DOI] [PubMed] [Google Scholar]
- Cheung NS, Peng ZF, Chen MJ, Moore PK, Whiteman M. Hydrogen sulfide induced neuronal death occurs via glutamate receptor and is associated with calpain activation and lysosomal rupture in mouse primary cortical neurons. Neuropharmacology. 2007;53:505–514. doi: 10.1016/j.neuropharm.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Chohan MO, Iqbal K. From tau to toxicity: Emerging roles of NMDA receptor in Alzheimer's disease. J. Alzheimer's Dis. 2006;10:81–87. doi: 10.3233/jad-2006-10112. [DOI] [PubMed] [Google Scholar]
- Doraiswamy PM. Alzheimer's disease and the glutamate NMDA receptor. Psychopharmacol. Bull. 2003;37:41–49. [PubMed] [Google Scholar]
- Elovaara E, Tossavainen A, Savolainen H. Effect of subclinical hydrogen sulfide intoxication on mouse brain protein metabolism. Exp. Neurol. 1978;62:93–98. doi: 10.1016/0014-4886(78)90043-2. [DOI] [PubMed] [Google Scholar]
- Eto K, Asada T, Arima K, Makifuchi T, Kimura H. Brain hydrogen sulfide is severely decreased in Alzheimer's disease. Biochem. Biophys. Res. Commun. 2002;293:1485–1488. doi: 10.1016/S0006-291X(02)00422-9. [DOI] [PubMed] [Google Scholar]
- Farlow MR. NMDA receptor antagonists. A new therapeutic approach for Alzheimer's disease. Geriatrics. 2004;59:22–27. [PubMed] [Google Scholar]
- Friedman JE, Haddad GG. Anoxia induces an increase in intracellular sodium in rat central neurons in vitro. Brain Res. 1994;663:329–334. doi: 10.1016/0006-8993(94)91281-5. [DOI] [PubMed] [Google Scholar]
- Gaitonde UB, Sellar RJ. Long term exposure to hydrogen sulphide producing subacute encephalopathy in a child. Br. Med. J. 1987;294:614. doi: 10.1136/bmj.294.6572.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong QH, Shi XR, Hong ZY, Pan LL, Liu XH, Zhu YZ. A new hope for neurodegeneration: Possible role of hydrogen sulfide. J. Alzheimer's Dis. 2011;24(Suppl. 2):173–182. doi: 10.3233/JAD-2011-110128. [DOI] [PubMed] [Google Scholar]
- Hannah RS, Hayden LJ, Roth SH. Hydrogen sulfide exposure alters the amino acid content in developing rat CNS. Neurosci. Lett. 1989;99:323–327. doi: 10.1016/0304-3940(89)90467-9. [DOI] [PubMed] [Google Scholar]
- Hannah RS, Roth SH. Chronic exposure to low concentrations of hydrogen sulfide produces abnormal growth in developing cerebellar Purkinje cells. Neurosci. Lett. 1991;122:225–228. doi: 10.1016/0304-3940(91)90864-p. [DOI] [PubMed] [Google Scholar]
- Hasbani MJ, Hyrc KL, Faddis BT, Romano C, Goldberg MP. Distinct role for sodium, chloride, and calcium in excitotoxic dendritic injury and recovery. Exp. Neurol. 1998;154:241–258. doi: 10.1006/exnr.1998.6929. [DOI] [PubMed] [Google Scholar]
- Hirsch AR. Hydrogen sulfide exposure without loss of consciousness: Chronic effects in four cases. Toxicol. Ind. Health. 2002;18:51–61. doi: 10.1191/0748233702th131oa. [DOI] [PubMed] [Google Scholar]
- Hooser SB, van Alstine W, Kiupel M, Sojka J. Acute pit gas (hydrogen sulfide) poisoning in confinement cattle. J. Vet. Diagn. Investig. 2000;12:272–275. doi: 10.1177/104063870001200315. [DOI] [PubMed] [Google Scholar]
- Hu NW, Ondrejcak T, Rowan MJ. Glutamate receptors in preclinical research on Alzheimer's disease: Update on recent advances. Pharmacol. Biochem. Behav. 2012;100:855–862. doi: 10.1016/j.pbb.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Integrated Risk Information System. Toxicological Review of Hydrogen Sulfide. 2003. U.S. Environmental Protection Agency, Washington, DC. EPA/635/R-03/005. Available at: www.epa.gov/iris. [Google Scholar]
- Itoh T, Itoh A, Horiuchi K, Pleasure D. AMPA receptor-mediated excitotoxicity in human NT2-N neurons results from loss of intracellular Ca2+ homeostasis following marked elevation of intracellular Na+ . J. Neurochem. 1998;71:112–124. doi: 10.1046/j.1471-4159.1998.71010112.x. [DOI] [PubMed] [Google Scholar]
- Jarecki BW, Piekarz AD, Jackson JO, Cummins TR. Human voltage-gated sodium channel mutations that cause inherited neuronal and muscle channelopathies increase resurgent sodium currents. J. Clin. Investig. 2010;120:369–378. doi: 10.1172/JCI40801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoun P. Endogenous production of hydrogen sulfide in mammals. Amino Acids. 2004;26:243–254. doi: 10.1007/s00726-004-0072-x. [DOI] [PubMed] [Google Scholar]
- Kang X, Chao D, Gu Q, Ding G, Wang Y, Balboni G, Lazarus LH, Xia Y. δ-Opioid receptors protect from anoxic disruption of Na+ homeostasis via Na+ channel regulation. Cell. Mol. Life Sci. 2009;66:3505–3516. doi: 10.1007/s00018-009-0136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiedrowski L. Critical role of sodium in cytosolic [Ca2+] elevations in cultured hippocampal CA1 neurons during anoxic depolarization. J. Neurochem. 2007;100:915–923. doi: 10.1111/j.1471-4159.2006.04308.x. [DOI] [PubMed] [Google Scholar]
- Kilburn KH. Effects of hydrogen sulfide on neurobehavioral function. South. Med. J. 2003;96:639–646. doi: 10.1097/01.SMJ.0000072361.86796.56. [DOI] [PubMed] [Google Scholar]
- Kimura H. Hydrogen sulfide induces cyclic AMP and modulates the NMDA receptor. Biochem. Biophys. Res. Commun. 2000;276:129–133. doi: 10.1006/bbrc.1999.1915. [DOI] [PubMed] [Google Scholar]
- Kombian SB, Reiffenstein RJ, Colmers WF. The actions of hydrogen sulfide on dorsal raphe serotonergic neurons in vitro. J. Neurophysiol. 1993;70:81–96. doi: 10.1152/jn.1993.70.1.81. [DOI] [PubMed] [Google Scholar]
- Krnjević K. Electrophysiology of cerebral ischemia. Neuropharmacology. 2008;55:319–333. doi: 10.1016/j.neuropharm.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Kwiatkoski M, Soriano RN, Francescato HDC, Batalhao ME, Coimbra TM, Carnio EC, Branco LGS. Hydrogen sulfide as a cryogenic mediator of hypoxia-induced anapyrexia. Neuroscience. 2012;201:146–156. doi: 10.1016/j.neuroscience.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Lee JM, Zipfel GJ, Choi DW. The changing landscape of ischemic brain injury mechanism. Nature. 1999;399:A7–A14. doi: 10.1038/399a007. [DOI] [PubMed] [Google Scholar]
- Leonard AS, Hell JW. Cyclic AMP-dependent protein kinase and protein kinase C phosphorylate N-methyl-D aspartate receptors at different sites. J. Biol. Chem. 1997;272:12107–12115. doi: 10.1074/jbc.272.18.12107. [DOI] [PubMed] [Google Scholar]
- Malinow R. New developments on the role of NMDA receptors in Alzheimer's disease. Curr. Opin. Neurobiol. Forthcoming doi: 10.1016/j.conb.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog. Neurobiol. 1987;28:197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Meo ID, Fagiolari G, Prelle A, Viscorni C, Zeviani M, Tiranti V. Chronic exposure to sulfide causes accelerated degradation of cytochrome c oxidase in ethymalonic encephalopathy. Antioxid. Redox Signal. 2011;15:353–362. doi: 10.1089/ars.2010.3520. [DOI] [PubMed] [Google Scholar]
- Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, Snyder SH. H2S signals through protein S-sulfhydration. Sci. Signal. 2009;2:ra72. doi: 10.1126/scisignal.2000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Tsugane M, Oka J, Kimura H. Hydrogen sulfide induces calcium waves in astrocytes. FASEB J. 2004;18:557–559. doi: 10.1096/fj.03-1052fje. [DOI] [PubMed] [Google Scholar]
- Pamenter ME, Buck LT. δ-Opioid receptor antagonism induces NMDA receptor-dependent excitotoxicity in anoxic turtle cortex. J. Exp. Biol. 2008;211:3512–3517. doi: 10.1242/jeb.021949. [DOI] [PubMed] [Google Scholar]
- Parameshwaran K, Dhanasekaran M, Suppiramaniam V. Amyloid beta peptides and glutamatergic synaptic dysregulation. Exp. Neurol. 2008;210:7–13. doi: 10.1016/j.expneurol.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Partlo LA, Sainsbury RS, Roth SH. Effects of repeated hydrogen sulphide (H2S) exposure on learning and memory in the adult rat. Neurotoxicology. 2001;22:177–189. doi: 10.1016/s0161-813x(01)00016-x. [DOI] [PubMed] [Google Scholar]
- Petrat F, Li T, Dehne N, de Groot H, Rauen U. Sodium as the major mediator of NO-induced cell death in cultured hepatocytes. Life Sci. 2006;79:1606–1615. doi: 10.1016/j.lfs.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Probert AW, Borosky S, Marcoux FW, Taylor P. Sodium channel modulators prevent oxygen and glucose deprivation injury and glutamate release in rat neocortical cultures. Neuropharmacology. 1997;36:1031–1038. doi: 10.1016/s0028-3908(97)00072-5. [DOI] [PubMed] [Google Scholar]
- Qu K, Chen CPLH, Halliwell B, Moore PK, Wong PTH. Hydrogen sulfide is a mediator of cerebral ischemia damage. Stroke. 2006;37:889–893. doi: 10.1161/01.STR.0000204184.34946.41. [DOI] [PubMed] [Google Scholar]
- Raley-Susman KM, Kass IS, Cottrell JE, Newman RB, Chambers G, Wang J. Sodium influx blockade and hypoxic damage to CA1 pyramidal neurons in rat hippocampal slices. J. Neurophysiol. 2001;86:2715–2726. doi: 10.1152/jn.2001.86.6.2715. [DOI] [PubMed] [Google Scholar]
- Reisberg B, Doody R, Stöffler A, Schmitt F, Ferris S, Möbius HJ. Memantine in moderate-to-severe Alzheimer's disease. N. Engl. J. Med. 2003;348:1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- Ren C, Du A, Li D, Sui J, Mayhan WG, Zhao H. Dynamic change of hydrogen sulfide during global cerebral ischemia-reperfusion and its effect in rats. Brain Res. 2010;1345:197–205. doi: 10.1016/j.brainres.2010.05.017. [DOI] [PubMed] [Google Scholar]
- Rojas H, Colina C, Ramos M, Benaim G, Jaffe EH, Caputo C, DiPolo R. Na+ entry via glutamate transporter activates the reverse Na+/Ca2+ exchange and triggers Ca2+ i-induced Ca2+ release in rat cerebellar type-1 astrocytes. J. Neurochem. 2007;100:1188–1202. doi: 10.1111/j.1471-4159.2006.04303.x. [DOI] [PubMed] [Google Scholar]
- Sheldon C, Church J. Reduced contribution from Na+/H+ exchange to acid extrusion during anoxia in adult rat hippocampal CA1 neurons. J. Neurochem. 2004;88:594–603. doi: 10.1046/j.1471-4159.2003.02169.x. [DOI] [PubMed] [Google Scholar]
- Shi X, Gillespie PG, Nuttall AL. Na+ influx triggers bleb formation on inner hair cells. Am. J. Physiol. Cell Physiol. 2005;288:C1332–C1341. doi: 10.1152/ajpcell.00522.2004. [DOI] [PubMed] [Google Scholar]
- Smith RP, Gosselin RE. On the mechanism of sulfide inactivation by methemoglobin. Toxicol. Appl. Pharmacol. 1966;8:159–172. doi: 10.1016/0041-008x(66)90112-8. [DOI] [PubMed] [Google Scholar]
- Solnyshkova TG. Demyelination of nerve fibers in the central nervous system caused by chronic exposure to natural hydrogen sulfide-containing gas. Bull. Exp. Biol. Med. 2003;136:328–332. doi: 10.1023/b:bebm.0000010943.77392.5c. [DOI] [PubMed] [Google Scholar]
- Solnyshkova TG, Shakhlamov VA, Volodina EP. Cerebral cortex ultrastructure during exposure to hydrogen sulfide gas. Neurosci. Behav. Physiol. 2004;34:343–345. doi: 10.1023/b:neab.0000018744.97789.c4. [DOI] [PubMed] [Google Scholar]
- Stene RJ, Slosberg B, Beachman BE. Hydrogen sulfide intoxication. Ann. Intern. Med. 1976;85:756–758. doi: 10.7326/0003-4819-85-6-756. [DOI] [PubMed] [Google Scholar]
- Strege PR, Bernard CE, Kraichely RE, Mazzone A, Sha L, Beyder A, Gibbons SJ, Linden DR, Kendrick ML, Sarr MG, et al. Hydrogen sulfide is a partially redox-independent activator of the human jejunum Na+ channel, Nav1.5. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;300:G1105–G1114. doi: 10.1152/ajpgi.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JM, Traynelis SF, Chen HS, Escobar W, Heinemann SF, Lipton SA. Identification of two cysteine residues that are required for redox modulation of the NMDA subtype of glutamate receptor. Neuron. 1994;13:929–936. doi: 10.1016/0896-6273(94)90258-5. [DOI] [PubMed] [Google Scholar]
- Szabó C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- Tan BH, Wong PTH, Bian JS. Hydrogen sulfide: A novel signaling molecule in the central nervous system. Neurochem. Int. 2010;56:3–10. doi: 10.1016/j.neuint.2009.08.008. [DOI] [PubMed] [Google Scholar]
- Tang G, Wu L, Wang R. Interaction of hydrogen sulfide with ion channels. Clin. Exp. Pharmacol. Physiol. 2010;37:753–763. doi: 10.1111/j.1440-1681.2010.05351.x. [DOI] [PubMed] [Google Scholar]
- Wang R. Two's company, three's a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]