Background: We determined changes in GluN2 subunit composition responsible for increased N-methyl-d-aspartate receptor (NMDAR) activity in hypothalamus in hypertension.
Results: Protein kinase CK2 mediates up-regulation of synaptic GluN2A, which increases NMDAR activity and neuronal excitability in hypertension.
Conclusion: Increased NMDAR activity in hypertension results from CK2-mediated GluN2A up-regulation.
Significance: Understanding GluN2 subunit changes in brain is important for developing new treatments for hypertension.
Keywords: Glutamate Receptors Ionotropic (AMPA, NMDA); Hypothalamus; Neurochemistry; Neurons; Synaptic Plasticity
Abstract
Increased glutamatergic input, particularly N-methyl-d-aspartate receptor (NMDAR) activity, in the paraventricular nucleus (PVN) of the hypothalamus is closely associated with high sympathetic outflow in essential hypertension. The molecular mechanisms underlying augmented NMDAR activity in hypertension are unclear. GluN2 subunit composition at the synaptic site critically determines NMDAR functional properties. Here, we found that evoked NMDAR-excitatory postsynaptic currents (EPSCs) of retrogradely labeled spinally projecting PVN neurons displayed a larger amplitude and shorter decay time in spontaneously hypertensive rats (SHRs) than in Wistar-Kyoto (WKY) rats. Blocking GluN2B caused a smaller decrease in NMDAR-EPSCs of PVN neurons in SHRs than in WKY rats. In contrast, GluN2A blockade resulted in a larger reduction in evoked NMDAR-EPSCs and puff NMDA-elicited currents of PVN neurons in SHRs than in WKY rats. Blocking presynaptic GluN2A, but not GluN2B, significantly reduced the frequency of miniature EPSCs and the firing activity of PVN neurons in SHRs. The mRNA and total protein levels of GluN2A and GluN2B in the PVN were greater in SHRs than in WKY rats. Furthermore, the GluN2B Ser1480 phosphorylation level and the synaptosomal GluN2A protein level in the PVN were significantly higher in SHRs than in WKY rats. Inhibition of protein kinase CK2 normalized the GluN2B Ser1480 phosphorylation level and the contribution of GluN2A to NMDAR-EPSCs and miniature EPSCs of PVN neurons in SHRs. Collectively, our findings suggest that CK2-mediated GluN2B phosphorylation contributes to increased synaptic GluN2A, which potentiates pre- and postsynaptic NMDAR activity and the excitability of PVN presympathetic neurons in hypertension.
Introduction
Essential (primary) hypertension is the most common form of hypertension accounting for an estimated 90–95% of all cases (1, 2). Without adequate treatment, hypertension can lead to stroke, coronary artery disease, or kidney failure. Although the mechanisms underlying the pathogenesis of essential hypertension are poorly understood, increased sympathetic outflow represents an important mechanism (3, 4). The hypothalamic paraventricular nucleus (PVN)2 is essential for the maintenance of hypertension via regulation of sympathetic outflow and arterial blood pressure (5, 6). Transplantation of embryonic hypothalamic tissues, especially the rostral hypothalamus containing the PVN, from spontaneously hypertensive rats (SHRs) into the brain of normotensive rats results in the development of hypertension in the latter (7, 8). The glutamatergic input and excitability of PVN neurons that project to the spinal cord sympathetic preganglionic neurons are increased in SHRs (5, 9). We have shown that pre- and postsynaptic N-methyl-d-aspartate receptors (NMDARs) of PVN presympathetic neurons are up-regulated and play a key role in the elevated sympathetic outflow in SHRs (5, 9, 10). However, the molecular mechanisms underlying increased NMDAR activity in the PVN in hypertension remain largely unknown.
NMDARs are critically involved in excitatory synaptic transmission and plasticity in various brain regions for a variety of diseases including hypertension (9, 11). Functional NMDARs are typically composed of two GluN1 subunits and two GluN2 (GluN2A–D) subunits (12). The identity of the GluN2 subunit determines many of the biophysical and pharmacological properties of downstream signaling, receptor trafficking, and synaptic targeting (13). Compared with the GluN2B subunit, the GluN2A subunit confers a higher affinity for glutamate, distinctly faster kinetics, greater channel open probability, and more prominent Ca2+-dependent desensitization (14–16). The number and subunit composition of synaptic NMDARs are not static but change dynamically during development and in response to neuronal activity or sensory experience (17–20). In the PVN, NMDARs are composed primarily of two GluN1 subunits and two GluN2A or GluN2B subunits (21). During postnatal development, a gradual switch from GluN2B to GluN2A at the synaptic site has been shown to occur in the PVN and many other brain regions (22). However, little is known about the composition of the synaptic GluN2 subunits in the PVN in essential hypertension.
In the present study, we determined the contribution of GluN2A and GluN2B subunits to increased pre- and postsynaptic NMDAR activity and the mechanism underlying increased GluN2A-mediated NMDAR activity of PVN presympathetic neurons in SHRs. Our findings indicate that GluN2A, but not GluN2B, mediates the increased presynaptic and postsynaptic NMDAR activity and the firing activity of PVN neurons in SHRs. Also, the protein kinase CK2 plays an essential role in the increased GluN2B Ser1480 phosphorylation level and GluN2A-mediated synaptic NMDAR activity of PVN neurons in SHRs. This new information is important for our understanding of the molecular mechanisms involved in synaptic plasticity of the hypothalamus and in the development of hypertension.
EXPERIMENTAL PROCEDURES
Animals
Male Wistar-Kyoto (WKY) rats and SHRs (13 weeks old; Harlan, Indianapolis, IN) were used in this study. SHRs are one of the most commonly used and best characterized animal models of essential hypertension. The surgical procedures and experimental protocols were approved by the Institutional Animal Care and Use Committee of The University of Texas M. D. Anderson Cancer Center and conformed to the National Institutes of Health guidelines on the ethical use of animals. Blood pressure was measured daily with a noninvasive tail-cuff system (IITC Life Science, Inc., Woodland Hills, CA) in a subset of animals for at least 1 week before the experiment. The systolic ABP was significantly higher in the 13-week-old SHRs (206.35 ± 4.12 mmHg, n = 20) than in the age-matched WKY rats (123.57 ± 3.58 mmHg, n = 20).
Retrograde Labeling of PVN Presympathetic Neurons
Spinally projecting PVN neurons were labeled retrogradely as we described previously (9, 10, 23). Briefly, rats were anesthetized by intraperitoneal injection of a mixture of ketamine (70 mg/kg) and xylazine (6 mg/kg). The spinal cord was exposed at the T2 and T4 levels, and 0.04-μm FluoSpheres (Molecular Probes) were pressure-ejected (Nanojector II; Drummond Scientific, Broomall, PA) bilaterally into the intermediolateral cell column (∼500 μm from the midline and ∼500 μm below the dorsolateral sulcus) via a glass pipette with three or four separate 50-nl injections. The rats were returned to their cages for 3–5 days to permit the transport of FluoSpheres into the PVN.
Brain Slice Preparation
Coronal hypothalamic slices (300 μm thick) containing the PVN were obtained from FluoSpheres-injected rats as we described previously (9, 23). Briefly, the rat was rapidly decapitated under anesthesia with isoflurane. The brain was quickly removed and sectioned with a vibrating microtome in an ice-cold artificial cerebral spinal fluid (aCSF) solution containing 126 mm NaCl, 3 mm KCl, 1.5 mm MgSO4, 2.4 mm CaCl2, 1.2 mm NaH2PO4, 10 mm glucose, and 26 mm NaHCO3 saturated with 95% O2 and 5% CO2. The slices were preincubated in the aCSF at 34 °C for at least 1 h before electrophysiological recordings. To verify the injection and diffusion sites of FluoSpheres, we sectioned the spinal cord at the injected level immediately after killing the rat and viewed the slice under a microscope. Data were collected for analysis only if the injection site was located within the intermediolateral cell column of the spinal cord.
Electrophysiological Recordings in Brain Slices
Labeled PVN neurons were visualized under an upright microscope (BX51WI; Olympus, Tokyo, Japan) with epifluorescence and infrared differential interference contrast optics and were recorded at 34 °C using borosilicate glass electrodes (resistance, 4–6 megohms). The pipette solution contained 110 mm Cs2SO4, 2.0 mm MgCl2, 0.5 mm CaCl2, 5.0 mm EGTA, 5.0 mm MgATP, 0.5 mm Na2GTP, and 10 mm HEPES with the pH adjusted to 7.3 by CsOH (280–300 mosmol). Signals were processed using an Axopatch 700B amplifier (Molecular Devices), filtered at 1–2 kHz, and digitized at 20 kHz.
NMDAR-mediated excitatory postsynaptic currents (EPSCs) were evoked by electrical stimulation (0.1 ms, 0.8 mA, 0.2 Hz) through a bipolar tungsten electrode connected to a stimulator. The tip of the stimulating electrode was placed on the ventral side ∼150 μm away from the neuron recorded. The neuron was held at 40 mV in the presence of the GABAA receptor antagonist bicuculline (10 μm) and the non-NMDAR antagonist 6-cyano-7-nitroquinoxaline-2,3-dione (20 μm) in the aCSF (10). The sodium channel blocker lidocaine N-ethyl bromide (10 mm) was added to the pipette solution to suppress the firing activity of recorded neurons.
In some experiments, NMDAR currents were also elicited by puff application of 100 μm NMDA directly to the recorded neuron at a holding potential of −60 mV (10). The tip of the puff pipette was placed ∼80 μm away from the recorded neuron. The NMDA was ejected onto the recorded neuron using a positive pressure system (4 psi, 15 ms; Toohey Company, Fairfield, NJ) in the presence of a low concentration of Mg2+ (0.1 mm), 10 μm glycine, and 1 μm tetrodotoxin in the aCSF. The miniature EPSCs (mEPSCs) of labeled PVN neurons were recorded at a holding potential of −60 mV in the presence of 10 μm bicuculline and 1 μm tetrodotoxin. The spontaneous firing activity of labeled PVN neurons was recorded using the current clamp mode without current injection (9, 10). The recording solutions were similar to those used for NMDAR-EPSC recordings except that Cs2SO4 in the pipette solution was replaced with potassium gluconate and QX-314 was not used. The spontaneous firing activity was recorded when it reached a steady state.
All drugs were freshly prepared in aCSF before the experiments and delivered via syringe pumps to reach their final concentrations. ZnCl2, 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (DRB), 4,5,6,7-tetrabromobenzotriazole. (TBB), glycine, and NMDA were purchased from Sigma-Aldrich. Ro25-6981, AP5, 6-cyano-7-nitroquinoxaline-2,3-dione, bicuculline, and tetrodotoxin were obtained from Ascent Scientific (Princeton, NJ). Lidocaine N-ethyl bromide was purchased from Alomone Labs (Jerusalem, Israel).
Western Immunoblotting
Rats were anesthetized with 2–3% isoflurane and decapitated. The coronal hypothalamic slices containing the PVN were sectioned, and the PVN tissue (∼ 0.5 mm in diameter) spanning from −1.08 bregma to −2.12 bregma was micropunched bilaterally on a cold plate under the microscope using the third ventricle as a reference (10). Total proteins were extracted with radioimmuneprecipitation assay buffer in the presence of the mixture protease inhibitors. Synaptosomes were isolated using a method reported previously (24). In brief, PVN tissues (each sample pooled from three rats) were homogenized in the ice-cold buffer (10 mm Tris-HCl, pH 7.4, 5 mm NaF, 1 mm Na3VO4, 1 mm EDTA, and 1 mm EGTA) containing 320 mm sucrose and the mixture protease inhibitors and were centrifuged for 10 min (1,000 × g at 4 °C) to remove nuclei and large debris. The supernatant was centrifuged at 10,000 × g to obtain the crude synaptosome fraction. The protein concentrations were determined using the Bradford protein assay. The total (20 μg) and synaptosome (60 μg) proteins were separated using 8% SDS-polyacrylamide gels and transferred to a polyvinylidene difluoride membrane (Millipore). The blot was probed with anti-GluN2A antibody (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA) or anti-GluN2B antibody (1:1,000; Santa Cruz Biotechnology) and then reprobed with anti-GAPDH antibody (1:2,000; Sigma-Aldrich) as loading controls. The ECL kit (GE Healthcare) was used to detect the protein band, and the band intensities were quantified by using ImageJ software. The specificity of the GluN2A and GluN2B antibodies has been shown previously (24). The amount of GluN2 subunits was quantified by normalizing the optical density of their protein band to that of GAPDH. The mean value of GluN2 subunits in WKY rats was considered to be 1.
To determine the GluN2B Ser1480 phosphorylation level in the PVN tissues, the specific phosphorylated GluN2B Ser1480 antibody (Pierce) was used for immunoblotting (25). During the initial protein extraction, PBS buffer was replaced with TBS buffer, and the phosphatase inhibitor was added.
Intracerebroventricular Injection
To determine whether CK2 is involved in the phosphorylation of GluN2B Ser1480 in the PVN, DRB (4 mm in 10 μl, three injections) or vehicle (0.5% dimethyl sulfoxide) was intracerebroventricularly injected 2 h before obtaining the PVN samples. A guide cannula was placed through a 2-mm burr hole drilled in the skull over the lateral ventricle in the coordinates of 1.5 mm lateral to the midline, 1.0 mm caudal to the bregma, and 3.0 mm ventral to the dura. Injections were performed by using a Hamilton syringe connected with an injection cannula, which was advanced 3.5 mm ventral from the surface of the dura. The tip of the injection cannula protruded 0.5 mm beyond the tip of the guide cannula. Each injection consisted of 10 μl of solution delivered over a period of 1 min.
PCR Analysis
Agarose gel electrophoresis was used to detect which GluN2 subunits are expressed in the PVN of WKY rats and SHRs. The total RNA from the PVN tissues was extracted by using TRIzol, and cDNA was synthesized by using SuperScript III (Invitrogen). PCR products were generated with LA TaqTM DNA polymerase (Takara), and GAPDH was used as an internal control. The sequence primers of individual GluN2 subunits that were used are shown in Table 1.
TABLE 1.
Primers used in PCR analysis
| Gene (accession no.) | Primersa | |
|---|---|---|
| GluN2A (Grin2a, NM_012573.3) | Fwd: | 5′-CGACCCCGGCAGCTTTGGAA-3′ |
| Rev: | 5′-GCGAGTGGGTCCGATTCTCTGC-3′ | |
| GluN2B (Grin2b, NM_012574.1) | Fwd: | 5′-ACGCCCATATGTTTGAGATGCCAGC-3′ |
| Rev: | 5′-ACCCGGTCAGGGTAGAGCGAC-3′ | |
| GluN2C (Grin2c, NM_012575.3) | Fwd: | 5′-CCC ATACCCGCCTGCCGTTC-3′ |
| Rev: | 5′-CTGTGCCTAGCCCCAGGGTCC-3′ | |
| GluN2D (Grin2d, NM_022797.1) | Fwd: | 5′-GGCTTAGACGGCGGCTGGTG-3′ |
| Rev: | 5′-GGGGCGGTGTGGGTGAGGTC-3′ | |
| GAPDH (NM_012828.2) | Fwd: | 5′-TGCCACTCAGAAGACTGTGG-3′ |
| Rev: | 5′-TTCAGCTCTGGGATGACCTT-3′ | |
a Fwd, forward; Rev, reverse.
We also used real-time PCR to determine the mRNA levels of GluN2A, GluN2B, and GluN2C subunits in the PVN in WKY rats and SHRs. The cDNA was quantified using SYBR Green PCR core reagents kit on the IQ5 system (Bio-Rad). The mRNA level of the GluN2 subunits was calculated by using the 2−ΔΔCt method and normalized by GAPDH (used as an internal control). The mean values in WKY rats were considered as 1.
Celiac Ganglionectomy and ABP Measurement with Radiotelemetry
Surgery was performed aseptically in SHRs anesthetized with 2% isofluorane, as we described previously (10). Briefly, the celiac ganglion area was exposed through a midline laparotomy. The celiac ganglion was identified near the superior mesenteric artery and celiac artery. For rats undergoing celiac ganglionectomy (CGx), the celiac plexus and all visible nerves were dissected and removed as completely as possible by stripping under a surgical microscope. In sham control rats, the celiac ganglion plexus was exposed but not disturbed.
To measure the ABP in SHRs, the Millar catheter of the telemetry system (Telemetry Research, Auckland, New Zealand) was inserted into the abdominal aorta below the bifurcation of the renal arteries (10). The rat was housed singly and injected with buprenorphine (0.3 mg/kg, subcutaneously) and penicillin for 3 days after surgery. The blood pressure signal was recorded every 2 days in conscious rats through the receiver and analyzed with the LabChart data acquisition system (AD Instruments, Bella Vista, Australia). Rats were used for brain slice recordings approximately 2 weeks after surgery.
Data Analysis
Data are presented as means ± S.E. The junction potential was corrected off-line according to the composition of the internal and external solutions used for recordings. The peak amplitude of NMDAR-EPSCs of PVN neurons was determined and analyzed using pClamp 10 software (Molecular Devices). The decay phase of NMDAR-EPSCs was fit with a double exponential function using the MiniAnalysis Program (Synaptosoft, Leonia, NJ), and decay kinetics are expressed as a weighted decay time constant (20). In all slice experiments, only one neuron was recorded for each slice, and at least four rats were used in each group. Student's t test was tested for comparisons of two data sets. For comparisons of more than two data sets, repeated measures ANOVA with Dunnett's test or two-way ANOVA with Bonferroni's post hoc test was performed. p < 0.05 was considered statistically significant.
RESULTS
Shortened Decay Time of NMDAR-EPSCs of PVN Neurons in SHRs
All whole cell patch clamp recordings were performed in retrogradely labeled PVN neurons in hypothalamic slices. The amplitude of NMDAR-EPSCs in labeled PVN neurons was significantly larger in 13-week-old SHRs than in age-matched WKY rats (Fig. 1, A and B). The decay kinetics of NMDAR-EPSCs depends largely on the GluN2 subunit composition (22). To measure the decay kinetics, we determined the weighted time constant (τw) by fitting the NMDAR-EPSCs with a double exponential function (20). The decay time of NMDAR-EPSCs of labeled PVN neurons was significantly less in SHRs than in WKY rats (Fig. 1, A and C).
FIGURE 1.
Changes in the decay kinetics of NMDAR-EPSCs of PVN neurons in SHRs. A, representative current traces showing original and normalized NMDAR-EPSCs of labeled PVN neurons from one SHR and one WKY rat. B and C, summary data showing the amplitude (B) and τw (C) of NMDAR-EPSCs of PVN neurons from SHRs and WKY rats (n = 7 neurons in each group). *, p < 0.05, compared with the WKY group. Error bars, S.E.
Increased Ratio of GluN2A- to GluN2B-mediated NMDAR Currents of PVN Neurons in SHRs
GluN2A and GluN2B are the predominant GluN2 subunits in the PVN of the hypothalamus (21). Because the GluN2A subunit shows faster decay kinetics than does the GluN2B subunit (22), it is possible that the shortened decay time of NMDAR-EPSCs results from an increased ratio of GluN2A to GluN2B subunits at the synaptic site in the PVN of SHRs. We used the GluN2A-selective blocker Zn2+ (300 nm) (26, 27) and the GluN2B-selective antagonist Ro25-6981 (0.6 μm) (28, 29) to determine the contribution of GluN2A and GluN2B to NMDAR-EPSCs of labeled PVN neurons in WKY rats and SHRs. Because Ro25-6981 is highly specific for GluN2B, we first applied Ro25-6981 for 6 min and then applied both Ro25-6981 and Zn2+ for another 6 min. In labeled PVN neurons from WKY rats, bath application of Ro25-6981 caused a large reduction in the amplitude of NMDAR-EPSCs (52.55 ± 4.16%), and subsequent application of Zn2+ decreased the amplitude by only another 19.97 ± 3.45% (n = 7; Fig. 2, A–C). However, in labeled PVN neurons from SHRs, application of Ro25-6981 only decreased 29.99 ± 5.24% of the amplitude of NMDAR-EPSCs, and subsequent application of Zn2+ caused a large reduction in the amplitude (49.34 ± 3.65%, n = 7; Fig. 2, A–C). The remaining EPSCs of PVN neurons in SHRs were abolished by 50 μm AP5, which specifically blocks NMDARs (Fig. 2A). The τw of remaining NMDA currents after treatment with Ro25-6981 and Zn2+ did not differ significantly between WKY rats and SHRs (80.18 ± 9.67 versus 75.05 ± 6.39, p > 0.05). Thus, our data suggest that NMDAR currents of labeled PVN neurons are largely mediated by the Zn2+-sensitive GluN2A subunit in SHRs. In contrast, the major portion of NMDAR currents is mediated by the Ro25-6981-sensitive GluN2B subunit in WKY rats (Fig. 2C).
FIGURE 2.
Changes in GluN2A- and GluN2B-mediated NMDAR-EPSCs of PVN neurons in SHRs. A, representative current traces showing NMDAR-EPSCs inhibited by 0.6 μm Ro25-6981 and 300 nm Zn2+ in labeled PVN neurons from one SHR and one WKY rat. The remaining NMDAR-EPSCs in the SHRs were completely blocked by a high concentration (50 μm) of AP5. B and C, summary data showing inhibition of NMDAR-EPSCs of labeled PVN neurons by Ro25-6981 and Zn2+ in SHRs and WKY rats (n = 7 in each group). D, original current traces showing NMDAR-EPSCs blocked by 5 μm AP5 in labeled PVN neurons from one SHR and one WKY rat. E and F, summary data showing inhibition of NMDAR-EPSCs by 5 μm AP5 in labeled PVN neurons in SHRs (n = 9) and WKY rats (n = 7). *, p < 0.05, compared with the WKY group. #, p < 0.05, compared with the respective base-line control value. Error bars, S.E.
To further ensure the contribution of GluN2A to the NMDAR activity of PVN presympathetic neurons in SHRs, we used a lower concentration of AP5, which preferentially blocks the GluN2A subunit at 5 μm (30, 31). Application of 5 μm AP5 decreased the amplitude of NMDAR-EPSCs of labeled PVN neurons by 29.03 ± 1.58% (n = 7) in WKY rats but by 47.17 ± 1.93% (n = 9) in SHRs (Fig. 2, D–F). Furthermore, bath application of 5 μm AP5 produced a larger decrease in the amplitude of currents elicited by puff application of 100 μm NMDA to labeled PVN neurons in SHRs than in WKY rats (n = 7 in each group; Fig. 3).
FIGURE 3.
GluN2A-mediated postsynaptic NMDAR currents of PVN neurons are increased in SHRs. A, representative traces show the effect of 5 μm AP5 on NMDAR currents produced by puff application of 100 μm NMDA to labeled PVN neurons from one WKY rat and one SHR. B and C, group data show inhibition of puff NMDA-induced NMDAR currents by 5 μm AP5 in labeled PVN neurons from SHRs and WKY rats (n = 7 in each group). *, p < 0.05, compared with the WKY group. #, p < 0.05, compared with the respective base-line control value. Error bars, S.E.
Presynaptic GluN2A, but Not GluN2B, Contributes to Increased Synaptic Glutamate Release to PVN Neurons in SHRs
We have shown that presynaptic NMDAR activity regulates the glutamate release to PVN presympathetic neurons in SHRs, but not in WKY rats (10). To determine whether up-regulation of presynaptic NMDARs in SHRs is also mediated by the GluN2A subunit, we blocked postsynaptic NMDARs by adding the specific NMDA channel blocker MK-801 (1 mm) into the recording pipette solution (32). Bath application of 300 nm Zn2+ or 5 μm AP5 to block GluN2A significantly reduced the frequency of mEPSCs of labeled PVN neurons in SHRs (n = 7 in each group; Fig. 4, A and B). However, blocking GluN2B with 0.6 μm Ro25-6981 failed to affect the frequency of mEPSCs in these neurons significantly (Fig. 4C).
FIGURE 4.
Presynaptic GluN2A-containing NMDARs are increased in PVN neurons in SHRs. A, original traces and summary data show the effect of 300 nm Zn2+ on the frequency and amplitude of mEPSCs of labeled PVN neuron in SHRs (n = 7). B, raw traces and group data show the effect of 5 μm AP5 on the frequency and amplitude of mEPSCs of labeled PVN neuron in SHRs (n = 7). C, original traces and summary data show the effect of 0.6 μm Ro25-6981 on the frequency and amplitude of mEPSCs of labeled PVN neuron in SHRs (n = 7). *, p < 0.05, compared with the base-line control value. Error bars, S.E.
Blocking GluN2A-mediated NMDAR Activity Reduces Firing Activity of PVN Neurons in SHRs
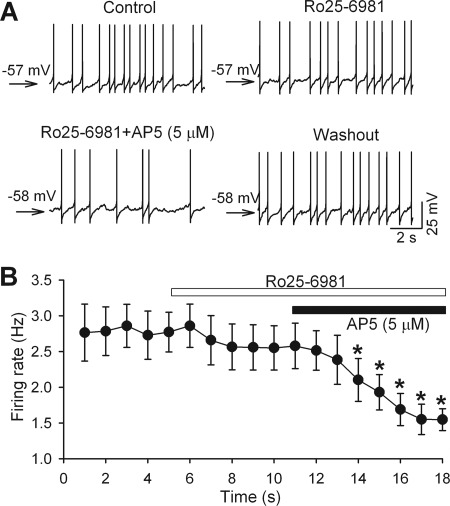
We have shown that blocking either presynaptic or postsynaptic NMDARs reduces the firing activity of PVN presympathetic neurons in SHRs, but not in WKY rats (9, 10). We next determined the relative contribution of GluN2A and GluN2B subunits to the increased firing activity of PVN neurons in SHRs. Bath application of 0.6 μm Ro25-6981 failed to alter the firing activity of labeled PVN neurons significantly. In contrast, subsequent application of 5 μm AP5 significantly reduced the firing activity of these PVN neurons (n = 7; Fig. 5).
FIGURE 5.
GluN2A-NMDARs contribute to increased firing activity of PVN neurons in SHRs. A, original recordings showing the differential effects of 0.6 μm Ro25-6981 and 5 μm AP5 on the firing activity of one labeled PVN neuron in a SHR. B, summary data showing the effects of Ro25-6981 and AP5 on the firing activity of labeled PVN neurons in SHRs (n = 7). *, p < 0.05, compared with the base-line control value. Error bars, S.E.
CGx Has No Effect on GluN2A- and GluN2B-mediated NMDAR Activity of PVN Neurons in SHRs
To determine further whether an increased ratio of GluN2A- to GluN2B-mediated NMDAR currents in the PVN is the cause or result of hypertension in SHRs, we examined the effect of lowering blood pressure with CGx on GluN2A- and GluN2B-mediated NMDAR-EPSCs of PVN neurons in SHRs. CGx, but not sham surgery, significantly lowered the mean ABP in SHRs for at least 2 weeks after surgery (Fig. 6A). Blocking GluN2B with 0.6 μm Ro25-6981 decreased only 24.19 ± 3.67% of the amplitude of NMDAR-EPSCs of labeled PVN neurons of SHRs subjected to CGx. By comparison, blocking GluN2A with 5 μm AP5 caused a large reduction in the amplitude of NMDAR-EPSCs (57.53 ± 4.47%, n = 7) in these rats (Fig. 6, B–D). The proportion of NMDAR-EPSCs inhibited by blockade of GluN2A or GluN2B in SHRs subjected to CGx was similar to that seen in sham-operated SHRs (n = 7; Fig. 6, B–D).
FIGURE 6.
GluN2A- and GluN2B-mediated NMDAR-EPSCs in PVN neurons in SHRs subjected to CGx. A, original recordings and group data show the effect of CGx on the ABP in SHRs (Sham, n = 6; CGx, n = 8). B–D, representative examples and summary data show the effects of 0.6 μm Ro25-6981 and 5 μm AP5 on NMDAR-EPSCs of labeled PVN neurons in SHRs subjected to CGx and sham surgery (n = 7 in each group). *, p < 0.05, compared with the sham group. #, p < 0.05, compared with the respective base-line control value. Error bars, S.E.
mRNA Level of GluN2A and GluN2B in PVN Is Increased in SHRs
In both WKY rats and SHRs, only the mRNA of GluN2A, GluN2B, and GluN2C, but not GluN2D, was detectable in the PVN tissue with agarose gel electrophoresis (Fig. 7A). Real-time PCR analysis showed that the mRNA level of GluN2A and GluN2B, but not GluN2C, in the PVN was significantly greater in SHRs than in WKY rats (Fig. 7A).
FIGURE 7.
Changes in the mRNA and protein levels of GluN2A and GluN2B subunits in the PVN in SHRs. A, representative agarose gel image shows the presence of the mRNA of GluN2A-C, but not GluN2D, subunits in the PVN (top), and real-time PCR data show changes in the mRNA levels of GluN2A-C in the PVN in SHRs and WKY rats (n = 6 in each group; bottom). B, representative gel images (20 μg of protein/lane) and group data show differences in the total protein levels of GluN2A and GluN2B subunits in PVN tissues in SHRs and WKY rats (n = 6 in each group). C, original gel images (60 μg of protein/lane) and group data show differences in the synaptosomal protein levels of GluN2A and GluN2B subunits in the PVN in SHRs and WKY rats (n = 6 in each group). D, original gel images (40 μg of protein/lane) and group data show differences in the level of phosphorylated GluN2B Ser1480 in the PVN in SHRs and WKY rats (n = 6 in each group). Molecular mass is indicated on the right side of the gel image. GAPDH was probed as a protein loading control. *, p < 0.05, compared with the WKY group. Error bars, S.E.
Synaptic GluN2A Protein and Phosphorylated GluN2B Ser1480 Levels in PVN Are Increased in SHRs
Next, we used Western immunoblotting to determine the protein levels of GluN2A and GluN2B in the whole tissue lysate and the synaptosomal fraction in the PVN of SHRs and WKY rats. The total GluN2A and GluN2B protein levels were significantly greater in SHRs than in WKY rats (Fig. 7B). Furthermore, the protein level of the GluN2A subunit in the synaptosomal fraction was significantly higher in the SHRs than in the WKY rats (Fig. 7C). However, the GluN2B protein level in the synaptosomes did not differ significantly between SHRs and WKY rats (Fig. 7C). In addition, the phosphorylated GluN2B Ser1480 protein level in the PVN was significantly higher in SHRs than in WKY rats (Fig. 7D).
Protein Kinase CK2 Mediates Increased GluN2A-mediated NMDAR Activity of PVN Neurons in SHRs
The protein kinase CK2 is involved in the developmental switch from GluN2B- to GluN2A-containing NMDARs in the cerebral cortex (25). We have shown that the CK2 activity is increased in the PVN and contributes to the elevated NMDAR activity and sympathetic vasomotor tone in SHRs (10). Therefore, we determined whether CK2 plays a role in the increased GluN2A-mediated NMDAR activity of PVN neurons in SHRs. We assessed GluN2A- and GluN2B-mediated NMDAR-EPSCs of labeled PVN neurons using 5 μm AP5 and 0.6 μm Ro25-6981, respectively, in the brain slices of SHRs pretreated with the specific CK2 inhibitor DRB (100 μm, 2–3 h), TBB (2 μm, 2–3 h) (10, 33), or vehicle. Compared with the vehicle only, treatment with DRB or TBB (10, 33) significantly reduced the amplitude of NMDAR-EPSCs of PVN neurons (Fig. 8, A and B). In DRB- and TBB-treated brain slices, blocking GluN2B with Ro25-6981 produced a large reduction in the amplitude of NMDAR-EPSCs of labeled PVN neurons (47.51 ± 3.76%, n = 7). In these neurons, blocking GluN2A with 5 μm AP5 decreased only 31.58 ± 2.32% of the amplitude (Fig. 8, A–C). Acute treatment with DRB (30 min) had no effects on NMDAR-EPSCs in SHRs. Hence, CK2 inhibition in SHRs normalized the proportion of GluN2A- and GluN2B-mediated NMDAR currents to the level observed in WKY rats.
FIGURE 8.
CK2 inhibition normalizes GluN2A-mediated pre- and postsynaptic NMDAR activity and the phosphorylated GluN2B level in the PVN in SHRs. A–C, representative traces and group data show the effects of 0.6 μm Ro25-6981 and 5 μm AP5 on electrically evoked NMDAR-EPSCs of labeled PVN neurons in brain slices of SHRs treated with 100 μm DRB, 2 μm TBB, or vehicle (n = 7 in each group). D, summary data show the effect of 5 μm AP5 on the frequency of mEPSCs of labeled PVN neurons in brain slices of SHRs treated with 100 μm DRB, 2 μm TBB, or vehicle (n = 7 in each group). E, representative gel images (40 μg of protein/lane) and group data show the effect of DRB on the level of phosphorylated GluN2B Ser1480 in the PVN in SHRs (n = 6 in each group). Molecular mass is indicated on the right side of the gel image. *, p < 0.05, compared with the vehicle group. #, p < 0.05, compared with the respective base-line control value. Error bars, S.E.
Compared with treatment with vehicle only, treatment with DRB or TBB also significantly decreased the base-line frequency of mEPSCs of labeled PVN neurons in SHRs (n = 7, Fig. 8D). Furthermore, bath application of 5 μm AP5 failed to significantly alter the frequency of mEPSCs in these neurons in DRB- or TBB-treated brain slices from SHRs (Fig. 8D).
In additional experiments, we determined whether CK2 plays a role in increased phosphorylation of GluN2B Ser1480 in the PVN in SHRs. Compared with treatment with vehicle only, treatment with DRB significantly reduced the phosphorylated GluN2B Ser1480 protein level in the PVN in SHRs (Fig. 8E).
DISCUSSION
Although the etiology of essential hypertension is poorly understood, there is considerable evidence showing that essential hypertension is initiated primarily from the central nervous system and is later reinforced by nonneural factors. Forebrain structures, especially the hypothalamic PVN, are involved in the regulation of the sympathetic nervous system (34) and play an important role in the pathogenesis of hypertension (8, 35). We have shown that the activity of both pre- and postsynaptic NMDARs is potentiated (9, 10) and is critically involved in the hyperactivity of PVN presympathetic neurons and increased sympathetic vasomotor tone in SHRs (5). However, the underlying mechanisms of increased NMDAR activity of PVN presympathetic neurons in hypertension are not fully understood.
GluN2 subunit composition determines NMDAR functional properties such as affinity for glutamate, sensitivity to Mg2+, channel conductance and open probability, and deactivation time (12, 16, 36). Because the GluN2A subunit has higher glutamate affinity and greater open probability than does the GluN2B subunit, an increase in the GluN2A/GluN2B ratio can result in potentiated NMDAR activity (14–16). We found that blocking GluN2A with Zn2+ or a low concentration of AP5 caused a larger reduction in NMDAR-EPSCs of PVN neurons in SHRs than in WKY rats; blocking GluN2B with Ro25-6981 had a similar but smaller effect. In addition, GluN2A-mediated NMDAR currents of PVN neurons induced by puff NMDA application were similarly increased in SHRs compared with WKY rats. An increase in the synaptic GluN2A subunit in the PVN is further supported by our finding that the decay time of NMDAR-EPSCs of PVN neurons was significantly shorter in SHRs than in WKY rats, which is consistent with the studies showing that GluN2A can accelerate the deactivation kinetics of synaptic NMDARs (14–16). Therefore, our data indicate that GluN2A is the predominant subunit that mediates increased postsynaptic NMDAR activity of PVN neurons in this animal model of hypertension.
We found it interesting that when postsynaptic NMDARs were blocked with intracellular dialysis of MK-801, blockade of GluN2A, but not GluN2B, significantly reduced the frequency of mEPSCs of PVN neurons in SHRs. Hence, an increase in synaptic GluN2A levels also accounts for the augmented presynaptic NMDAR activity in the PVN in these rats. Furthermore, we found that blocking GluN2A-containing NMDARs with a low concentration of AP5 significantly reduced the firing activity of PVN neurons in SHRs in anesthetic-free brain slices, whereas blocking GluN2B with Ro25-6981 had no significant effect. Our data thus suggest that up-regulation of synaptic GluN2A contributes to the increased NMDAR activity and excitability of PVN presympathetic neurons in hypertension. Because lowering blood pressure with CGx in SHRs did not significantly affect the increased activity of NMDARs or the ratio of GluN2A- to GluN2B-mediated NMDAR-EPSCs, the increased synaptic GluN2A activity may be the cause of hypertension rather than a secondary adaptive response to high blood pressure in SHRs. We observed that blocking GluN2A and GluN2B with Zn2+ and Ro25-6981, respectively, did not abolish NMDAR-EPSCs of PVN neurons in SHRs. The small NMDAR-EPSCs remaining after blocking of the GluN2A and GluN2B subunits may be mediated by triheteromeric GluN1/2A/2B subunits (37). Although GluN2C was detectable in the PVN, its expression level did not differ between WKY rats and SHRs. Also, the decay time of GluN1/GluN2C currents is very slow, which cannot explain the kinetics of the remaining NMDAR currents.
Our study revealed that the mRNA level and the total protein levels of both GluN2A and GluN2B subunits in the PVN were significantly greater in SHRs than in WKY rats. However, an increase in the GluN2A protein level in the synaptosomes was not associated with a significant change in the GluN2B level in the PVN in SHRs. Thus, an increase in the ratio of GluN2A- to GluN2B-mediated NMDAR-EPSCs of PVN neurons in SHR is not simply due to a switch of these two subunits at the synaptic site. It has been shown that synaptic GluN2A, but not GluN2B, protein levels are increased in the visual cortex during development or after visual stimulation (20, 38, 39). Our findings suggest that an increase in GluN2A-mediated NMDAR activity of PVN presympathetic neurons in hypertension likely results from GluN2A up-regulation and its increased trafficking to synaptic sites.
We noted that the increase in synaptosomal GluN2A levels was much larger than that of the total GluN2A level in the PVN in SHRs. Also, the increase in the total GluN2B protein level was not associated with a parallel increase in its synaptic protein level in hypertensive rats. We thus explored the mechanism that might be involved in facilitating the trafficking of GluN2A, but not GluN2B, to the synaptic site in the PVN in SHRs. CK2 can phosphorylate Ser1480 of the GluN2B subunit to limit GluN2B synaptic insertion but promote GluN2A trafficking to the synaptic site (25, 40). Interestingly, we found that the phosphorylated GluN2B Ser1480 level in the PVN was significantly increased in SHRs. Furthermore, inhibiting CK2 significantly reduced the phosphorylated GluN2B level, suggesting that the increase in the synaptic GluN2A level may result from increased phosphorylation of GluN2B by CK2 in the PVN in SHRs. We also found that inhibiting CK2 normalized the ratio of GluN2A- to GluN2B-mediated NMDAR activity and diminished the effect of GluN2A inhibition on the frequency of mEPSCs of PVN neurons in SHRs. Therefore, our findings suggest that increased CK2 activity augments the NMDAR function by increasing the GluN2A level at pre- and postsynaptic sites in the PVN in SHRs. Increased CK2 activity may also minimize an increase in the synaptic GluN2B level even though the total GluN2B protein level is increased in the PVN in SHRs. Nevertheless, the precise mechanisms underlying the influence of CK2 on GluN2A and GluN2B subunits in the PVN are not clear. It is possible that increased CK2 activity in hypertension may promote GluN2A trafficking indirectly by phosphorylating other CK2 substrates such as PSD-95 (41) and protein phosphatase 2A (42). Because the CK2 activity does not change in all brain regions, such as the brainstem and prefrontal cortex, in SHRs (10), it is unlikely that increased GluN2A-mediated NMDAR activity is a general phenomenon in the central nervous system in hypertension.
To conclude, our study provides novel evidence that GluN2A-mediated NMDAR activity is profoundly increased at both pre- and postsynaptic sites in the hypothalamus in hypertension. The increase in synaptic GluN2A-NMDAR activity is mediated by CK2 and is a critical contributor to the hyperexcitability of PVN presympathetic neurons in hypertension. This new information greatly improves our understanding of the molecular mechanisms and synaptic plasticity involved in the pathogenesis of essential hypertension. On the basis of our findings, CK2 and GluN2A may represent new potential therapeutic targets for treatment of neurogenic hypertension.
This study was supported, in whole or in part by National Institutes of Health Grant R01 HL077400. This work was also supported by a postdoctoral fellowship from the American Heart Association South Central Affiliate (to Z.-Y. Y.) and the N. G. and Helen T. Hawkins Endowment (to H.-L. P.).
- PVN
- paraventricular nucleus
- ABP
- arterial blood pressure
- aCSF
- artificial cerebrospinal fluid
- CGx
- celiac ganglionectomy
- CK
- casein kinase
- DRB
- 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole
- EPSC
- excitatory postsynaptic current
- mEPSC
- miniature excitatory postsynaptic current
- NMDAR
- N-methyl-d-aspartate receptor
- SHR
- spontaneously hypertensive rat
- τw
- weighted time constant
- TBB
- 4,5,6,7-tetrabromobenzotriazole
- WKY
- Wistar-Kyoto.
REFERENCES
- 1. Carretero O. A., Oparil S. (2000) Essential hypertension: part I, definition and etiology. Circulation 101, 329–335 [DOI] [PubMed] [Google Scholar]
- 2. Oparil S., Zaman M. A., Calhoun D. A. (2003) Pathogenesis of hypertension. Ann. Intern. Med. 139, 761–776 [DOI] [PubMed] [Google Scholar]
- 3. Judy W. V., Watanabe A. M., Henry D. P., Besch H. R., Jr., Murphy W. R., Hockel G. M. (1976) Sympathetic nerve activity: role in regulation of blood pressure in the spontaneously hypertensive rat. Circ. Res. 38, 21–29 [DOI] [PubMed] [Google Scholar]
- 4. Anderson E. A., Sinkey C. A., Lawton W. J., Mark A. L. (1989) Elevated sympathetic nerve activity in borderline hypertensive humans: evidence from direct intraneural recordings. Hypertension 14, 177–183 [DOI] [PubMed] [Google Scholar]
- 5. Li D. P., Pan H. L. (2007) Glutamatergic inputs in the hypothalamic paraventricular nucleus maintain sympathetic vasomotor tone in hypertension. Hypertension 49, 916–925 [DOI] [PubMed] [Google Scholar]
- 6. Allen A. M. (2002) Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension 39, 275–280 [DOI] [PubMed] [Google Scholar]
- 7. Eilam R., Malach R., Segal M. (1994) Selective elimination of hypothalamic neurons by grafted hypertension-inducing neural tissue. J. Neurosci. 14, 4891–4902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Eilam R., Malach R., Bergmann F., Segal M. (1991) Hypertension induced by hypothalamic transplantation from genetically hypertensive to normotensive rats. J. Neurosci. 11, 401–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li D. P., Yang Q., Pan H. M., Pan H. L. (2008) Pre- and postsynaptic plasticity underlying augmented glutamatergic inputs to hypothalamic presympathetic neurons in spontaneously hypertensive rats. J. Physiol. 586, 1637–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ye Z. Y., Li D. P., Li L., Pan H. L. (2011) Protein kinase CK2 increases glutamatergic input in the hypothalamus and sympathetic vasomotor tone in hypertension. J. Neurosci. 31, 8271–8279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lau C. G., Zukin R. S. (2007) NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat. Rev. Neurosci. 8, 413–426 [DOI] [PubMed] [Google Scholar]
- 12. Furukawa H., Singh S. K., Mancusso R., Gouaux E. (2005) Subunit arrangement and function in NMDA receptors. Nature 438, 185–192 [DOI] [PubMed] [Google Scholar]
- 13. Cull-Candy S. G., Leszkiewicz D. N. (2004) Role of distinct NMDA receptor subtypes at central synapses. Sci. STKE 2004, re16. [DOI] [PubMed] [Google Scholar]
- 14. Monyer H., Sprengel R., Schoepfer R., Herb A., Higuchi M., Lomeli H., Burnashev N., Sakmann B., Seeburg P. H. (1992) Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science 256, 1217–1221 [DOI] [PubMed] [Google Scholar]
- 15. Williams K., Russell S. L., Shen Y. M., Molinoff P. B. (1993) Developmental switch in the expression of NMDA receptors occurs in vivo and in vitro. Neuron 10, 267–278 [DOI] [PubMed] [Google Scholar]
- 16. Vicini S., Wang J. F., Li J. H., Zhu W. J., Wang Y. H., Luo J. H., Wolfe B. B., Grayson D. R. (1998) Functional and pharmacological differences between recombinant N-methyl-d-aspartate receptors. J. Neurophysiol. 79, 555–566 [DOI] [PubMed] [Google Scholar]
- 17. Barria A., Malinow R. (2002) Subunit-specific NMDA receptor trafficking to synapses. Neuron 35, 345–353 [DOI] [PubMed] [Google Scholar]
- 18. Bellone C., Nicoll R. A. (2007) Rapid bidirectional switching of synaptic NMDA receptors. Neuron 55, 779–785 [DOI] [PubMed] [Google Scholar]
- 19. Carmignoto G., Vicini S. (1992) Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science 258, 1007–1011 [DOI] [PubMed] [Google Scholar]
- 20. Matta J. A., Ashby M. C., Sanz-Clemente A., Roche K. W., Isaac J. T. (2011) mGluR5 and NMDA receptors drive the experience- and activity-dependent NMDA receptor NR2B to NR2A subunit switch. Neuron 70, 339–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Herman J. P., Eyigor O., Ziegler D. R., Jennes L. (2000) Expression of ionotropic glutamate receptor subunit mRNAs in the hypothalamic paraventricular nucleus of the rat. J. Comp. Neurol. 422, 352–362 [PubMed] [Google Scholar]
- 22. Monyer H., Burnashev N., Laurie D. J., Sakmann B., Seeburg P. H. (1994) Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron 12, 529–540 [DOI] [PubMed] [Google Scholar]
- 23. Li D. P., Chen S. R., Pan H. L. (2003) Angiotensin II stimulates spinally projecting paraventricular neurons through presynaptic disinhibition. J. Neurosci. 23, 5041–5049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dunah A. W., Sirianni A. C., Fienberg A. A., Bastia E., Schwarzschild M. A., Standaert D. G. (2004) Dopamine D1-dependent trafficking of striatal N-methyl-d-aspartate glutamate receptors requires Fyn protein tyrosine kinase but not DARPP-32. Mol. Pharmacol. 65, 121–129 [DOI] [PubMed] [Google Scholar]
- 25. Sanz-Clemente A., Matta J. A., Isaac J. T., Roche K. W. (2010) Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors. Neuron 67, 984–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Erreger K., Traynelis S. F. (2008) Zinc inhibition of rat NR1/NR2A N-methyl-d-aspartate receptors. J. Physiol. 586, 763–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paoletti P., Ascher P., Neyton J. (1997) High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J. Neurosci. 17, 5711–5725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fischer G., Mutel V., Trube G., Malherbe P., Kew J. N., Mohacsi E., Heitz M. P., Kemp J. A. (1997) Ro 25–6981, a highly potent and selective blocker of N-methyl-d-aspartate receptors containing the NR2B subunit: characterization in vitro. J. Pharmacol. Exp. Ther. 283, 1285–1292 [PubMed] [Google Scholar]
- 29. Chamberlain S. E., Yang J., Jones R. S. (2008) The role of NMDA receptor subtypes in short-term plasticity in the rat entorhinal cortex. Neural Plast. 2008, 872456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li R., Huang F. S., Abbas A. K., Wigström H. (2007) Role of NMDA receptor subtypes in different forms of NMDA-dependent synaptic plasticity. BMC Neurosci. 8, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kimura R., Matsuki N. (2008) Protein kinase CK2 modulates synaptic plasticity by modification of synaptic NMDA receptors in the hippocampus. J. Physiol. 586, 3195–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhou H. Y., Chen S. R., Chen H., Pan H. L. (2010) Opioid-induced long-term potentiation in the spinal cord is a presynaptic event. J. Neurosci. 30, 4460–4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lieberman D. N., Mody I. (1999) Casein kinase-II regulates NMDA channel function in hippocampal neurons. Nat. Neurosci. 2, 125–132 [DOI] [PubMed] [Google Scholar]
- 34. Swanson L. W., Sawchenko P. E. (1983) Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu. Rev. Neurosci. 6, 269–324 [DOI] [PubMed] [Google Scholar]
- 35. Ciriello J., Kline R. L., Zhang T. X., Caverson M. M. (1984) Lesions of the paraventricular nucleus alter the development of spontaneous hypertension in the rat. Brain Res. 310, 355–359 [DOI] [PubMed] [Google Scholar]
- 36. Groc L., Heine M., Cousins S. L., Stephenson F. A., Lounis B., Cognet L., Choquet D. (2006) NMDA receptor surface mobility depends on NR2A-2B subunits. Proc. Natl. Acad. Sci. U.S.A. 103, 18769–18774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Neyton J., Paoletti P. (2006) Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J. Neurosci. 26, 1331–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu X. B., Murray K. D., Jones E. G. (2004) Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development. J. Neurosci. 24, 8885–8895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Quinlan E. M., Philpot B. D., Huganir R. L., Bear M. F. (1999) Rapid, experience-dependent expression of synaptic NMDA receptors in visual cortex in vivo. Nat. Neurosci. 2, 352–357 [DOI] [PubMed] [Google Scholar]
- 40. Chung H. J., Huang Y. H., Lau L. F., Huganir R. L. (2004) Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. J. Neurosci. 24, 10248–10259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Soto D., Pancetti F., Marengo J. J., Sandoval M., Sandoval R., Orrego F., Wyneken U. (2004) Protein kinase CK2 in postsynaptic densities: phosphorylation of PSD-95/SAP90 and NMDA receptor regulation. Biochem. Biophys. Res. Commun. 322, 542–550 [DOI] [PubMed] [Google Scholar]
- 42. Hériché J. K., Lebrin F., Rabilloud T., Leroy D., Chambaz E. M., Goldberg Y. (1997) Regulation of protein phosphatase 2A by direct interaction with casein kinase 2α. Science 276, 952–955 [DOI] [PubMed] [Google Scholar]