Background: LacdiNAc and sulfo-LacdiNAc modification of N-glycoproteins/peptides is well documented.
Results: O-Linked LacdiNAc and the novel phospho-LacdiNAc modification were detected and structurally characterized on core 2-glycans of six ECM-related proteins.
Conclusion: LacdiNAc and phospho-LacdiNAc are expressed on ECM proteins.
Significance: This novel modification opens new aspects in the posttranslational control of protein function.
Keywords: Carbohydrate Glycoprotein, Carbohydrate Structure, Extracellular Matrix Proteins, Glycosylation, Phosphorylation, LacdiNAc, ZP3, α-Dystroglycan, Mucin-type O-Linked Glycans, Phosphoglycans
Abstract
The terminal modification of glycans by β4 addition of N-acetylgalactosamine to N-acetylglucosamine with formation of the N,N-diacetyllactosediamine (LacdiNAc) moiety has been well documented for a number of N-linked glycoproteins and peptides, like neurohormones. Much less is known about O-glycoproteins in this regard because only human zona pellucida glycoprotein 3 (ZP3) and bovine proopiomelanocortin were reported to be LacdiNAc-modified. In searching for mammalian proteins modified with O-linked LacdiNAc we identified six positive species among nine endogenous and recombinant O-glycoproteins, which were extracellular matrix, or matrix-related proteins. These are ZP3 and the five novel LacdiNAc-positive species ECM1, AMACO, nidogen-1, α-dystroglycan, and neurofascin. The mass spectrometric analyses revealed a core 2-based tetrasaccharide as the common structural basis of O-linked LacdiNAc that could be further modified, similar to the type 2 LacNAc termini, with fucose, sialic acid, or sulfate. Here, we provide structural evidence for a novel type of mucin-type O-glycans that is strictly specific for LacdiNAc termini: sugar phosphorylation with formation of GalNAcβ1–4(phospho-)GlcNAc. The structural details of the phosphatase-labile compound were elucidated by MS2 analysis of tetralysine complexes and by MSn measurements of the permethylated glycan alditols. Phospho-LacdiNAc was detected in human HEK-293 as well as in mouse myoblast cells and in bovine brain tissue.
Introduction
The peripheral modification of N-linked glycans with LacdiNAc represents a unique protein-specific glycosylation found on a selected panel of glycoproteins and appears to play crucial roles in the regulation of circulatory half-life of hormones (1) and in cell recognition (2). The modification of N-linked glycans was detected in a series of mammalian glycoproteins including the glycoprotein luteinizing hormone (3), glycodelin (4), prolactin-like proteins (5), proopiomelanocortin (6), SorLA/LR11 (7), sialoadhesin (8), tenascin-R (9), and carbonic anhydrase VI (10). On glycodelin the LacdiNAc modification was claimed to have contraceptive functions.
The group of Jacques Baenziger could provide evidence that for the activity of β4-specific GalNAc transferases βGT3 and βGT4, a 19-meric peptide sequence within the target protein (LRRFIEQKITKRKKEKYWP) is necessary and sufficient (11). This determining cis-located peptide is characterized by a high content of basic amino acids and an α-helical structure. It is located at the C terminus of carbonic anhydrase VI and could induce the modification of a normally unmodified protein (transferrin) after recombinant translocation of the peptide.
As a peripheral modification of O-linked chains LacdiNAc4 modifications were found on murine zona pellucida glycoprotein (ZP3), which plays a role in initial sperm-egg binding (12) and on bovine proopiomelanocortin (13). There are only few reports on other LacdiNAc-modified O-glycoproteins. Among these, the oviductal mucins from Triturus alpestris (14) and cercarial glycoproteins from Schistosoma mansoni (15) have to be mentioned. Here, we provide evidence for the specific expression of LacdiNAc-terminating O-linked glycans on six extracellular matrix glycoproteins or membrane-tethered glycoproteins that fulfill functions by interacting with the extracellular matrix (ECM) network, like α-dystroglycan. None of these glycoproteins contains peptidic elements identical with or sequence homologous to those controlling the LacdiNAc modification of N-linked glycans.
The LacdiNAc dihexosamine has been shown previously to be the substrate for further modification by fucosylation, sialylation, or sulfation, which are, however, not confined to this terminal disaccharide and were found also on type 1 or type 2 lactosamines. Here, we describe for the first time the highly specific phosphorylation of the subterminal GlcNAc in LacdiNAc termini. This structural element (phosphorylated LacdiNAc) is unrelated to the previously reported phosphomannose-based dihexosamine claimed to form a functional part of the α-dystroglycan ligand which binds to laminin G domains (16).
EXPERIMENTAL PROCEDURES
Materials
All chemicals were purchased from Sigma-Aldrich and were of the grade “pro analysi.” Exceptions are marked in the text. Proteins were handled at +4 °C, long time storage was at −20 °C. The O-glycoprotein fetuin was bought from Sigma.
Cells and Cell Culture
Cell culture work was performed with media and plasticware from Biochrom (Berlin, Germany). Human embryonic kidney cell line EBNA-293 (Invitrogen) was cultivated at 37 °C and 5% CO2 in Dulbecco's minimal essential medium (DMEM), supplemented with 5% fetal calf serum (FCS), 100 units/ml penicillin, and 100 μg/ml streptomycin. Transfection was performed using the Superfect lipofection reagent (Qiagen). Transfected cells were cultivated in the presence of 5 μg/ml puromycin (Sigma). The supernatant was collected for purification of the recombinant proteins. For expression of proteins under serum-free conditions cells were washed with PBS and grown in the same medium without FCS for 3 days before collection of the supernatant.
C2F3 cells (kindly provided by Dr. Ursula Hartmann, Institute of Biochemistry II, University of Cologne) were cultivated at 37 °C and 7.5% CO2 in DMEM, supplemented with 20% FCS, 100 units/ml penicillin, and 100 μg/ml streptomycin. Transfection and protein expression were done as described for HEK-293 cells.
Preparation of Proteins from Bovine Brain Lysate
A piece of bovine brain was homogenized on ice in a glass tissue grinder in 9 μl of solubilization buffer (10 mm Tris-HCl, pH 7.2, 150 mm NaCl, 0,5% CHAPS, protease inhibitor mixture (Complete, Mini, EDTA-free Protease Inhibitor Mixture Tablets; Roche Applied Science), phosphatase inhibitor (PhosStop Mini Tablets; Roche Applied Science)) per mg of brain. After rotating for 30 min at 4 °C the crude lysate was sonicated on ice (5 times for 15 s) and cleared by dual centrifugation at 3,900 × g for 15 min. Proteins were precipitated by methanol/chloroform precipitation and delipidated by several methanol/chloroform (1:1 and 1:2, v/v) extractions.
Generation of Glycosylation Probes
Human ZP3-III and ZP3-IV were generated as truncated secretory fusion proteins lacking the cytosolic and transmembrane domains and C-terminal stretches of the ZP domain (p29-176 and p29-218). Using overhang primers with a noncomplementary 3′-extension with recognition sequences for the restriction enzymes BgIII and XbaI the PCR products were digested with endonucleases and cloned into the eukaryotic pCEP-PU vector V59 designed for the expression of C-terminally tagged (oligo-His and strepII) fusion proteins. The purification protocol is described elsewhere (17).
Human ECM1 (full-length construct) was generated similarly using overhang primers with recognition sequences for the restriction enzymes SpeI and NotI and cloned into the eukaryotic vector pCEP-PU designed for the expression of C-terminally His6-tagged fusion proteins.
Glycosylation probes based on human α-dystroglycan (hDG6, hDG8, hDGdel3, hDGdel2) were generated as described previously (17). Endogenous α-dystroglycan was purified from human muscle as described (18).
The DNA of the mucin domain of neurofascin186, comprising amino acids 921–1020 and cloned into a pBluescript vector with the restriction sites NheI and XhoI, was ordered from MrGene (Regensburg, Germany) and transformed into the Escherichia coli strain DH5α (Invitrogen) according to standard protocols. Transformed bacteria were selected overnight on LB broth-agar plates (Invitrogen) containing 200 mg/ml ampicillin at 37 °C. Amplification occurred overnight in LB broth liquid medium (Invitrogen) with ampicillin in a 37 °C shaker. The DNA was purified by using a Plasmid Midi kit (Qiagen). Amplified cDNA was cloned into a modified version of the eukaryotic expression vector pCEP-PU (pCEP4) via the restriction sites (5′ → 3′) NheI, EcoRV, and BamHI/BglII. The vector has an N-terminal BM40 secretion signal, a CMV promoter, and a C-terminal strepII as well as a His8 tag. For selection of transfected cells the vector contains an ampicillin and a puromycin resistance gene. Expression and purification were done as described for the dystroglycan constructs.
A fusion protein containing the amino acids p2797–2939 of human MUC5-A/C was expressed and purified as described for neurofascin.
Expression and purification of MUC1-S and AMACO-P2 were described elsewhere (19, 20). Endogenous MUC1 was isolated from human skim milk (21). Recombinant mouse nidogen-1, expressed in HEK-293 cells, was a kind gift of Dr. Roswitha Nischt, Dermatology, University Clinic Cologne. Bovine fetuin was bought from Sigma-Aldrich.
Gel Electrophoresis and Western Blotting
SDS-gels were either stained with silver or blotted onto nitrocellulose membranes (Protan BA 83, Schleicher & Schuell) in a semidry transfer cell (Trans-Blot SD; Bio-Rad). The fusion proteins α-dystroglycan, neurofascin, MUC5-A/C, and ZP3-III were detected with anti-strepII mouse IgG (IBA, Göttingen, Germany). MUC1-S and endogenous MUC1 were detected with an anti-MUC1 ectodomain-specific antibody C595, AMACO, and ECM1 with anti-His antibody (Qiagen). LacdiNAc was detected with monoclonal antibody 273-3F2 (kindly provided by Dr. C. H. Hokke, Leiden University Medical Clinic, Leiden, The Netherlands) (22). As secondary antibody, a peroxidase-conjugated rabbit anti-mouse IgG (DAKO, Hamburg, Germany) was used. Protein-antibody conjugates were detected by enhanced chemiluminescence (Roche Applied Science).
β-Elimination of O-Glycans and Permethylation of Glycan Alditols
The glycan chains were released from the protein by reductive β-elimination. For this purpose the glycoproteins were incubated with 0.5 m NaBH4 in 50 mm NaOH for 18 h at 50 °C. The reaction was stopped by adding 1 μl of acetic acid. Salt was removed with a 50-μl Dowex 50W-X8 column (Bio-Rad) in a batch procedure. Excessive borate was codistilled as methylester in a stream of nitrogen by adding several 0.1-ml aliquots of 1% acetic acid in methanol.
Permethylation of the glycan chains was performed by a procedure based on the method of Yu et al. (23). Briefly, 30 μg of β-eliminated glycan alditols were dried and resuspended in 200 μl of NaOH/dimethyl sulfoxide suspension and kept at 4 °C until frozen. Then 100 μl of methyliodide was added, and the solution was kept under agitation for 4 h at 4 °C. The reaction was stopped with 200 μl of cold water on ice and neutralized with 1 μl of 30% acetic acid. The glycans were purified with a C18 Sep-Pak column (Waters) as described (23).
Linkage Analysis of Glycans
Partially methylated alditol acetates were prepared from permethylated samples according to Ref. 24 and analyzed by GC/MS on a 15-m RTX5-SILMS column (Restek, Bad Homburg, Germany) with an initial temperature of 60 °C. The temperature gradient was run from 60 to 100 °C with 40 °C/min followed by 10 °C/min up to 280 °C. Relative masses of ions formed in an electron beam at 70 eV were scanned between 100 and 450 Da.
Analysis of Glycopeptides
Glycopeptide analysis of the recombinant proteins was performed by LC-MS/MS of tryptic peptides on an ESI ion trap, the HCT ultra-ETDII PTMDiscovery-System (Bruker-Daltonics, Bremen, Germany) coupled with an online easy-nano-LC system (Proxeon, Odense, Denmark). The sample was separated on an analytical C18 column (75 μm × 10 cm) using gradient runs from 0 to 35% acetonitrile in 0.1% TFA for 30 min. Ions were scanned with 8100 atomic mass units/s in a range from m/z 300 to 2500 in MS mode and m/z 200 to 3000 in MS/MS mode. MS/MS spectra were generated by collision-induced dissociation (CID) fragmentation. Glycopeptide analysis of endogenous α-dystroglycan was performed on a hybrid linear ion trap-Fourier transform ion cyclotron resonance mass spectrometer (Thermo Electron, Bremen, Germany) and is described in detail elsewhere (18).
Global Cellular O-Glycome Analysis
Cells or tissue was lysed by sonication in reducing 4-fold concentrated SDS sample buffer with β-mercaptoethanol. Approximately, 15 μg was loaded on a discontinuous SDS-gel composed of a 3.5% stacking gel, followed by a <1-cm 7.5% polyacrylamide gel layer and an approximately 9-cm 35% polyacrylamide gel. After approximately 15 min, when the protein was concentrated in the intermediate 7.5% gel layer, the Coomassie-stained gel piece was excised, washed two times for 1 h with 1 ml of 50% acetonitrile, and dried by vacuum rotation before digestion with Pronase E (3–5 μg in 100 mm NH4HCO3 containing 1 mm CaCl2). (Glyco)peptides were eluted with 200 μl of H2O (two times), 200 μl of 50% acetonitrile, and 200 μl of 100% acetonitrile for 1 h each and dried in a SpeedVac before β-elimination.
Enzymatic Digestions
10-μg aliquots of protein were digested with 0.1 unit of trypsin (Promega) in 0.1 m NH4HCO3 for 16 h at 37 °C. Sulfatase (from Helix pomatia type H-1; Sigma-Aldrich) digestion (0.8 unit) was done with 5 μg of protein substrate dissolved in 200 mm sodium acetate, pH 5.0, at 37 °C overnight. Phosphatase digestion (1 unit, alkaline phosphatase from calf intestine; Sigma) was performed with 5 μg of protein substrate dissolved in 10% diethanolamine containing 5 mm MgCl2, pH 9.8, at 37 °C overnight.
ESI-MS/MS of Lysine-Glycan Ion Pairing
The analysis was performed according to a protocol by Zhang et al. (25). Briefly, trilysine was dissolved in methanol/water (1:1) containing 0.5% acetic acid. 5 μl of this solution was added to a dried oligosaccharide mixture, reductively released from 20 μg of glycoprotein. After mixing, the solution was applied to ESI-MS/MS analysis by direct infusion via a 20-μl sample loop. A flow of 20 μl/h of methanol/water (1:1) containing 0.5% acetic acid was applied. Ions were scanned with 8100 atomic mass units/s in a range from m/z 300 to 2000 in MS mode and m/z 300 to 1500 in MS/MS mode. MS/MS spectra were generated by CID fragmentation.
MALDI-TOF-TOF MS of Methylated Glycans
MALDI MS was performed on a UltrafleXtreme instrument (Bruker Daltonics). The permethylated glycans (approximately 500 ng) contained in methanol were applied to the stainless steel target by mixing a 0.5-μl aliquot of sample with 1.0 μl of matrix (saturated solution of 2,5-dihydroxybenzoic acid in acetonitrile/0.1% TFA, 1:2). Analyses were performed by positive ion detection in the reflectron mode. Ionization of co-crystallized analytes was induced with a pulsed Smart-beam laser (accumulation of about 5,000 shots), and the ions were accelerated in a field of 20 kV and reflected at 23 kV.
ESI-MS/MS of Methylated Glycans
ESI-MSn of permethylated glycans, dissolved in 2% acetonitrile, 10% formic acid was performed as described for the glycopeptides by combined nano-LC using a gradient from 0 to 35% acetonitrile in 0.1% formic acid.
RESULTS
Extracellular Matrix Glycoproteins Are Modified with LacdiNAc-terminating O-Glycans
Endogenous ZP3 and proopiomelanocortin were until now the only characterized O-glycoproteins in mammals with proven LacdiNAc modifications on O-linked glycan chains (12, 13). Whereas proopiomelanocortin belongs to the group of pituitary hormones which are known to be modified with N-linked LacdiNAc, ZP3 belongs to the large group of ECM proteins. We asked whether other O-glycoproteins related to the group of ECM proteins are also modified specifically with LacdiNAc. A selection of mammalian O-glycoproteins was hence to be analyzed with respect to the O-glycosylation profiles. For this purpose the O-glycans were released from the proteins, permethylated, and analyzed by MALDI-MS. Analysis of the methylated O-glycan alditols from nine recombinant proteins (α-dystroglycan, ZP3, AMACO, ECM1, neurofascin, bone sialoprotein, MUC1, MUC5AC, and nidogen-1) and two endogenous proteins (α-dystroglycan and MUC1) demonstrated LacdiNAc expression in a truncation probe of ZP3 and in all other ECM-related glycoproteins (truncation or full-length probes of AMACO, ECM1, neurofascin, α-dystroglycan, nidogen-1, endogenous α-dystroglycan), but not in any of the other glycoproteins expressed in the same cell line (Table 1). The O-glycoprofiles of all samples were dominated by the highly abundant core 1- and core 2-based glycans (supplemental Figs. 1–8). In the LacdiNAc-positive samples at least one of four characteristic ion signals was registered in MALDI-MS. The masses m/z 1024, 1198, 1385, and 1559 correspond presumably to a LacdiNAc tetrasaccharide, its monofucosylated, its monosialylated, and its monofucosylated and -sialylated derivative (HN3, HN3F, HN3S, and HN3FS). The relative ratio of these specific ions varies strongly between 50% of all O-glycans on AMACO-P2 and <1% on neurofascin (supplemental Figs. 1–8). The core 2-based LacdiNAc structures were verified by MALDI-MS/MS analysis of postsource decay fragments. MS2 of the precursor ion at m/z 1024 (sodium adduct) revealed Y-type ions at m/z 284 and m/z 520 characteristic for a disubstituted HexNAc-ol. B-type ions at m/z 260 and m/z 505 (proton adduct) or 527 (sodium adduct) indicated a terminal HexNAc and a terminal HexNAc2, respectively (Fig. 1). A LacdiNAc modification of a core 1 O-glycan, which would be indicated by strong Z- or Y-fragments corresponding to a monosubstituted HexNAc-ol (m/z 298 or 316), an internal hexose (m/z 205), or a terminal trisaccharide consisting of HexNAc2Hex (B3α, m/z 731) was not detectable. This finding is in accordance with earlier results showing that O-glycoproteins from Chinese hamster ovary cells, which are lacking an active core 2-transferase, are not modified with LacdiNAc (26).
TABLE 1.
Mucin-type O-glycan composition of human and bovine O-glycoproteins
Purified recombinant probes were analyzed for their glycoprofiles by reductive β-elimination of O-linked glycans and analysis of the permethylated glycan alditols by MALDI-TOF-TOF MS.
| Protein | Core 1 | Core 2 | Core 2-LacdiNAc |
|---|---|---|---|
| ZP3 | + | + | + |
| α-Dystroglycan | + | + | + |
| Neurofascin | + | + | + |
| AMACO | + | + | + |
| ECM1 | + | + | + |
| Nidogen-1 | + | + | + |
| MUC1-S | + | + | − |
| MUC5-AC | + | + | − |
| Fetuin | + | + | − |
FIGURE 1.
MALDI-MS/MS of m/z 1024.5 from permethylated O-glycan alditols from recombinant neurofascin. These data are representative of the MS/MS data of m/z 1024 from all analyzed proteins. All fragment masses are sodium adducts if not indicated otherwise. The formation of the Y-ion m/z 284 indicates the presence of a disubstituted HexNAc-ol originating from a core 2 structure, whereas there is no signal corresponding to a linear core 1 tetrasaccharide with a monosubstituted HexNAc-ol (m/z 298 or 316).
The glycan analyses were supported by LC-MS/MS of tryptic peptides of the respective proteins. Tryptic peptides were analyzed by nano-LC coupled with ESI-ion trap MS/MS in the CID mode. On inspection of ion traces at m/z 204 and 407 (corresponding to the oxonium ions of terminal HexNAc and HexNAc2) we identified a series of peptides carrying LacdiNAc modified O-glycans, all presumably based on the core 2 structure according to the observed oxonium ions and the calculated monosaccharide compositions. Authenticity and biological significance of these findings on recombinant proteins are obvious when comparing the respective spectra of glycopeptides registered for recombinant and endogenous α-dystroglycan. O-Glycans with the monosaccharide composition H1N3 and carrying terminal HexNAc2 were identified on the same peptide (p471–479) (Fig. 2).
FIGURE 2.
ESI-MS/MS spectrum of the peptide LETASPPTR (molecular mass (M), 971.5 Da) modified with a core 2-based LacdiNAc tetrasaccharide (H1N3, M 1742.8 Da) from recombinant dystroglycan (hDG6), expressed in HEK-293 (1) (A) and endogenous α-dystroglycan purified from human muscle (B). Both spectra are based on the doubly charged precursor ion 871.92+, representing the peptide modified with Hex1HexNAc3 (771.3 Da). Both spectra show identical fragmentation patterns (with the exception of the fragment at m/z 1539.8) even though they were registered on different mass spectrometers. The fragment at m/z 1539.8 was not recorded in spectrum B due to a mass range restriction at m/z 1400.
A series of LacdiNAc-modified O-glycosylation sites was identified within the five recombinant and endogenous proteins by LC-MS/MS of the respective tryptic peptides. A number of peptides with LacdiNAc-O-glycan modification were found to carry additionally other mucin-type glycans or, in case of α-dystroglycan, O-mannosyl glycans. On the other hand, LacdiNAc was not found on all mucin-type O-glycosylation sites. (Fig. 3) The LacdiNAc glycopeptide species derived from ZP3-III were identified as p121–137, 138–145, and 152–156 (Table 2). The former two peptides include the three known O-glycosylation sites in ZP3, whereas the latter corresponds to an O-glycosylation site of an amino acid stretch linking the ZP3 sequence to the oligo-His tag. LacdiNAc-modified glycopeptide species derived from the human α-dystroglycan truncation construct hDG6 were identified as p1–17, 18–31, 87–106, 92–105, 111–115, 124–128, and 140–158 (Table 3). These peptides are located upstream of the mucin domain (p346–372) or within the mucin domain (p429–500) of endogenous human α-dystroglycan (Fig. 3). The corresponding data for the neurofascin, AMACO, and nidogen-1 truncation constructs are listed in Table 4. The MS/MS spectra revealed information about the glycans and the mass of the unmodified peptides. Due to intense fragmentation of the glycan moieties during CID-MS of glycopeptides peptide fragmentation is generally weak or not observed. However, as the recombinant proteins were purified (demonstrated by silver staining of SDS-gels; see Ref. 17) the observed LacdiNAc-modified peptides were undoubtedly derived from the respective protein. Examples for LacdiNAc-modified sites within authentic protein sequences are VSITR, LETASPPTR, APLAIAPPTETMAPPVR (α-dystroglycan), RTLETVR (AMACO), FTLSAR (neurofascin), and TTVFSEEK (ZP3) (refer to Fig. 3 for the rough localization of LacdiNAc-modified sites).
FIGURE 3.
Schematic domain topology of LacdiNAc-modified glycoproteins. Only those O-glycosylation sites that were either published previously or identified in the course of this study are shown. The recombinantly expressed and analyzed sequence stretch of the respective protein is marked by a box. Squares, mucin-type O-glycans; L, LacdiNAc-modified O-glycans.
TABLE 2.
Tryptic glycopeptides of ZP3 fusion proteins ZP3-III and ZP3-IV detected by LC-ESI-MS
Glycopeptides in the mixture of tryptic ZP3 peptides were identified by inspection of mass-specific ion traces at m/z 407 and 487 in the LC-MS chromatograms.
| Detected ions with m/z 407 immonium ion | Calculated core peptide mass | Peptide identity | Glycan composition |
|---|---|---|---|
| 673.42+ | 571.4 | p152–156 (SLVPR) | H1N3 |
| 712.12+ | 571.4 | p152–156 | H1N3 + P |
| 1031.53+ | 571.4 | p152–156 | H5N7F2 |
| 856.32+ | 940.5 | p138–145 (TTVFSEEK) | H1N3 |
| 896.72+ | 940.5 | p138–145 | H1N3 + P |
| 936.53+ | 940.5 | p138–145 | H4N6 |
| 1031.53+ | 940.5 | p138–145 | H4N6S1 |
| 984.53+ | 940.5 | p138–145 | H4N6F1 |
| 774.54+ | 940.5 | p138–145 | H4N6F2 |
| 1011.83+ | 940.5 | p138–145 | H4N6F1 + P |
| 1018.93+ | 940.5 | p138–145 | H5N5S1 |
| 970.53+ | 940.5 | p138–145 | H5N5F1 |
| 1066.53+ | 940.5 | p138–145 | H5N5F1S1 |
| 921.73+ | 1914.0 | p121–137 (QGNVSSQAILPTWLPFR) | H1N3 + P |
| 971.23+ | 1914.0 | p121–137 | H1N3 F1 + P |
| 763.34+ | 1914.0 | p121–137 | H2N4 |
| 1066.53+ | 1914.0 | p121–137 | H2N4 F1 |
| 1082.53+ | 2330.2 | p118–137 (YPRQGNVSSQAILPTWLPFR) | H1N3 F1 |
TABLE 3.
Tryptic glycopeptides of α-dystroglycan fusion proteins detected by LC-ESI-IT
Glycopeptides in the mixture of tryptic α-dystroglycan peptides were identified by inspection of mass-specific ion traces at m/z 407 and 487 in the LC-MS chromatograms.
| Detected ions with m/z 407 immonium ion | Calculated core peptide mass | Peptide identity | Glycan composition |
|---|---|---|---|
| 670.32+ | 571.4 | SLVPR | H1N3 |
| 710.22+ | 571.4 | SLVPR | H1N3 +P |
| 743.42+ | 571.4 | SLVPR | H1N3F1 |
| 674.62+ | 575.4 | VSITR | H1N3 |
| 818.52+ | 575.4 | VSITR | H1N3S1 |
| 714.32+ | 575.4 | VSITR | H1N3 +P |
| 858.42+ | 575.4 | VSITR | H1N3S1 +P |
| 936.52+ | 575.4 | VSITR | H3N4 |
| 628.03+ | 626.3 | PRTPR | H1N5 +P |
| 868.53+ | 818.4 | TTTSGVPR | H3N6 +P |
| 717.13+ | 971.5 | LETASPPTR | H3N2S |
| 872.42+ | 971.5 | LETASPPTR | H1N3 |
| 912.52+ | 971.5 | LETASPPTR | H1N3 +P |
| 703.83+ | 971.5 | LETASPPTR | H2N4 |
| 923.54+ | 1087.6 | LIRTTTSGVPR | H3N9S1 |
| 684.73+ | 1279.7 | DPVPGKPTVTIR | H1N3 |
| 671.23+ | 1279.7 | DPVPGKPTVTIR | H2N2 |
| 953.13+ | 1436.7 | PATPSTDSTTTTTR | H2N5 +P |
| 863.14+ | 1453.9 | PVPRVTTKVSITR | H3N6S1 |
| 901.03+ | 1527.8 | VSITRLETASPPTR | H3N3 +P |
| 751.33+ | 1536.9 | DPVPGKPTVTIRTR | H1N2F1 |
| 770.03+ | 1536.9 | DPVPGKPTVTIRTR | H1N3 |
| 796.23+ | 1536.9 | DPVPGKPTVTIRTR | H1N3 +P |
| 873.53+ | 1556.8 | TTTSGVPRGGEPNQR | H1N3F2 |
| 842.33+ | 1592.8 | PATPSTDSTTTTTRR | H2N3 |
| 797.33+ | 1662.9 | GAIIQTPTLGPIQPTR | H2N2 |
| 920.73+ | 1662.9 | GAIIQTPTLGPIQPTR | H3N3 |
| 821.93+ | 1731.9 | APLAIPPTETMAPPVR | H2N2 |
| 835.93+ | 1731.9 | APLAIPPTETMAPPVR | H1N3 |
| 883.73+ | 1731.9 | APLAIPPTETMAPPVR | H1N3F1 |
| 932.73+ | 1731.9 | APLAIPPTETMAPPVR | H1N3S1 |
| 862.03+ | 1731.9 | APLAIPPTETMAPPVR | H1N3 +P |
| 959.53+ | 1731.9 | APLAIPPTETMAPPVR | H1N3S1 +P |
| 984.03+ | 1731.9 | APLAIPPTETMAPPVR | H2N4 +P |
| 997.63+ | 1731.9 | APLAIPPTETMAPPVR | H1N5 +P |
| 1146.43+ | 1731.9 | APLAIPPTETMAPPVR | H2N6 +2P |
| 829.33+ | 1918.9 | PATPSTDSTTTTTRRPTK | H1N2 |
| 839.63+ | 1949.0 | VSTPKPATPSTDSTTTTTR | H1N2 |
| 959.93+ | 2024.1 | TTTSGVPRGGEPNQRPELK | H1N3 +P |
| 1081.13+ | 2024.1 | TTTSGVPRGGEPNQRPELK | H2N4 +P |
| 957.34+ | 2040.1 | LETASPPTRIRTTTSGVPR | H6N4 |
| 1044.24+ | 2105.1 | VSTPKPATPSTDSTTTTTRR | H6N5 +P |
TABLE 4.
Tryptic glycopeptides of neurofascin, AMACO, and nidogen-1 fusion proteins detected by LC-ESI-MS
Glycopeptides in the mixture of tryptic glycoprotein peptides were identified by inspection of mass-specific ion traces at m/z 407 and 487 in the LC-MS chromatograms.
| Detected ions with m/z 407 immonium ion | Calculated core peptide mass | Peptide identity | Glycan composition |
|---|---|---|---|
| 714.02+ | 694.4 | FTLSAR (neurofascin) | H2N2 |
| 805.92+ | 694.4 | FTLSAR | H1N3F1 |
| 773.32+ | 694.4 | FTLSAR | H1N3 + P |
| 582.43+ | 1013.5 | YRFTLSAR | H2N2 |
| 677.43+ | 1013.5 | YRFTLSAR | H2N2S1 |
| 937.43+ | 1964.0 | IHESAPDEQSLEGSLVPR | H1N3 + P |
| 637.45+ | 1964.0 | IHESAPDEQSLEGSLVPR | H2N4 + P |
| 628.95+ | 1964.0 | IHESAPDEQSLEGSLVPR | H3N3 + P |
| 766.33+ | 874.4 | RTLETVR(AMACO) | H1N4F1S1 |
| 646.53+ | 874.4 | RTLETVR | H1N3S1 |
| 672.93+ | 874.4 | RTLETVR | H1N3S1 + P |
| 803.23+ | 1488.8 | ILSPGYEATERPR(nidogen-1) | H1N3F1 |
| 849.83+ | 1488.8 | ILSPGYEATERPR | H1N3S1 |
Supporting evidence for LacdiNAc expression in the recombinant glycosylation probes was provided by Western blot analyses using a highly specific monoclonal antibody to the LacdiNAc disaccharide GalNAcβ1–4GlcNAcβ1- (22). Specificity of antibody binding was verified by performance of inhibition assays with 50 mm GalNAc. Binding of the antibody to the LacdiNAc-positive proteins supported previous MS analyses All other applied O-glycoproteins, like MUC1, MUC5AC (data not shown), or fetuin were negative (Fig. 4).
FIGURE 4.

Western blot analysis of recombinant and native O-glycoproteins with a specific anti-LacdiNAc-antibody. 5 μg of each protein was loaded onto the SDS-gel. Due to differences in protein molecular mass and in the degree of O-glycosylation, the staining intensities of individual proteins cannot be regarded as quantitatively comparable. First lane, hDG8; second lane, ECM1; third lane, AMACO-P2; fourth lane, nidogen-1; fifth lane, ZP3-IV; sixth lane, MUC1-S; seventh lane, bovine fetuin. The asterisk marks artificial staining of keratin.
In summary, significant amounts of LacdiNAc-modified core 2-based O-glycans were found on ECM proteins (ZP3, AMACO, ECM1, nidogen-1) or membrane-tethered glycoproteins with functional relation to the ECM (α-dystroglycan, neurofascin) (supplemental Figs. 1–8). All LacdiNAc-positive O-glycoproteins were initially expressed in HEK-293 cells. To demonstrate expression of LacdiNAc also in other cell lines and mammalian species we expressed an α-dystroglycan probe (hDG6) in mouse myoblasts (C2F3). This protein was also modified with LacdiNAc O-glycans (data not shown). LacdiNAc was additionally found on endogenous human α-dystroglycan (Fig. 2). The LacdiNAc modification on the panel of glycoproteins under study was clearly protein-specific because some proteins expressed in HEK-293 cells were LacdiNAc-negative (endogenous MUC1 from skim milk, bovine fetuin, and recombinant MUC1-S and MUC5AC). LacdiNAc expression on ECM proteins is dependent on the expression of an N-acetyl-β-glucosaminyl-glycoprotein β-4-N-acetylgalactosaminyltransferase and on the ability to form core 2 structures (26). Both requirements are given in HEK-293 cells and obviously also in other cell lines and tissues used in this study.
Novel LacdiNAc-specific Glycan Phosphorylation on ECM Proteins
Careful inspection of LC-MS/MS spectra revealed for some of the LacdiNAc-positive glycopeptides additional oxonium ions at m/z 487 (i.e. for recombinant α-dystroglycan shown in Fig. 5). The mass increment of 80 Da, which adds to the LacdiNAc-specific oxonium ion at m/z 407, indicates a phosphorylation or sulfation of the terminal di-N-acetylhexosamine moiety. The spectra revealed ion series with mass differences indicating the sequential losses of terminal HexNAc and subterminal P-HexNAc (Fig. 5). The potentially phosphorylated glycopeptide species derived from ZP3-III were identified as p121–137, 138–145, and 152–156. Peptides p121–137 (calculated core peptide mass 1914.0) and p138–145 (calculated core peptide mass 940.5) were both substituted with either Hex1HexNAc3P1 or fucosylated derivatives thereof (Table 2).
FIGURE 5.
ESI-MS/MS spectrum of the N-terminal tryptic peptide (m/z 1733) from the deletion construct of human α-dystroglycan, hDGdel2 (1). Fragmentation pattern and oxonium ions at m/z 407 (HexNAc2 + H+) and m/z 487 (HexNAc2p + H+) indicate a modification with the phosphorylated LacdiNAc tetrasaccharide (H1N3P). M, molecular mass.
Potentially phosphorylated glycopeptide species derived from the human α-dystroglycan truncation construct hDG6 were identified as p1–17, 18–31, 87–106, 92–105, 111–115, 124–128, and 140–158 (Table 3). Phosphorylation could therefore be detected on almost all LacdiNAc-modified glycopeptides. The corresponding data for the neurofascin, AMACO, and nidogen-1 truncation constructs are listed in Table 4. Inspection of ion traces at m/z 407 and 487 of MS/MS spectra registered for the human MUC1 and MUC5AC truncation constructs did not give any indication for the presence of potentially phosphorylated LacdiNAc moieties. The 80-Da modification was found on almost all LacdiNAc-modified O-glycopeptides in minor amounts. Only some LacdiNAc-modified peptides could not be detected as phosphorylated species, possibly for quantitative reasons. Comparing ion-specific traces registered in the LC-chromatograms of tryptic peptides the relative ratio of a phosphoglycopeptide versus its nonphosphorylated counterpart appears to make up only a minor portion (Fig. 6 and supplemental Fig. 9). However, such estimations are prone to a high degree of uncertainty due to strongly varying ionization efficiencies in mass spectrometry and correspondingly fluctuating response factors of phosphorylated versus nonphosphorylated peptides. Analysis of permethylated O-glycan alditols revealed an even smaller relative amount. This may be due to the instability of phosphoester linkages under high pH condition as applied during β-elimination to release the glycan chains from the protein backbone. A reliable quantitation on the level of methylated glycan alditols was therefore not possible.
FIGURE 6.
Selected ion traces of the LC-chromatogram of tryptic peptides from recombinant α-dystroglycan (hDGdel2) that were either untreated or treated with sulfatase or phosphatase prior to analysis by ESI-MS. The ion trace of the tryptic peptide (SLVPR + HexNAc + H+, m/z 774.4 in blue) is shown compared with the phosphoglycan-specific ion trace (m/z 487, HexNAc2p + H+ in red). Glycan composition and peptide identity were confirmed for each chromatographic peak by MS/MS (data not shown).
Structural Characterization of Endogenous Phospho-LacdiNAc
Modifications with a sulfate group (HSO4−, 97,0705 g/mol) or with a phosphate group (H2PO4−, 96,9871 g/mol) cannot be distinguished unambiguously by mass spectrometry due to the very small incremental mass difference. To get more detailed information on the glycosidic structure and the identity of the inorganic substituent, three independent strategies were followed: enzymatic removal of the phosphate, MS/MS fragment analysis of glycan ion complexes with tetralysine, and MSn of methylated glycan alditols in an ESI-ion trap.
Enzymatic Approach
To identify the inorganic substituent we digested the tryptic peptides derived from hDGDel3 with either phosphatase or sulfatase to perform differential analyses by LC-ESI-MS/MS. By comparing the extracted ion trace at m/z 487 (HexNAc2 + 80 + H+) registered for the enzyme-treated and untreated sample, we could demonstrate that the phosphatase treatment resulted in nearly quantitative removal of the respective ion signals (Fig. 6 and supplemental Fig. 9) whereas the treatment with sulfatase resulted in no obvious changes in signal intensities. The activity of the two hydrolases toward sulfated or phosphorylated glycans was tested with standard compounds (chondroitin disaccharide 4S, GlcNAc 6-phosphate), and the reaction products were analyzed by MALDI- and GC-MS (data not shown).
MS2 of Tetralysine Ion Pair Complexes
We further tried to discriminate between sulfate and phosphate substitution by analysis of the distinct fragmentation of ion pairs formed between tetralysine and the modified glycans. Due to differences in the chemical bond stabilities it is possible to discriminate between sulfate and phosphate modifications by different MS2 fragmentation patterns of the ion-pairing complexes (25).
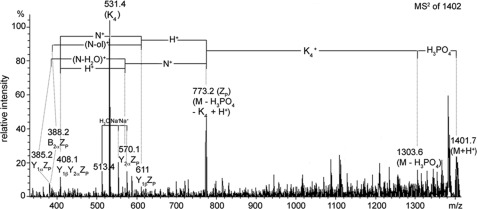
We performed MS2 of the precursor ion at m/z 1401.8, a complex of tetralysine and the (sulfate- or phosphate-modified) core 2-tetrasaccharide alditol GalNAc1–4GlcNAc1-6(Gal1–3)GalNAc-ol derived from hDG8 (HN3P-K4). Ligand dissociation into the free tetralysine (m/z 530.4) and the free glycan chain, typical for a phosphoglycan, was observed. A complex containing glycan-linked sulfate would be more stable, resulting in a dissociation between the sugar moiety and the sulfate and recognizable in a fragment corresponding to M-80-K4. Another hint to a phospho modification is the elimination of phosphoric acid (−98.0 Da) and a Y1αΖP fragment (m/z 385.2) which arises from splitting of the dehydrated N-acetylhexosamine (m/z 185.1) (Fig. 7). These data clearly indicate a modification of the subterminal GlcNAc with a phosphate and allow the tentative linkage assignment to 3-GlcNAc because the only alternative, a 6-linked phosphate, would not result in the elimination of phosphoric acid (25). No complex formation of tetralysine and a sulfated glycan (expected fragment signal at m/z 792.4, K4-S) was observed.
FIGURE 7.
MS2 spectrum of the tetralysine-phosphoglycan complex K4/H1N3-P (molecular mass, 1402 Da) (K4, tetralysine). Elimination of phosphate as H3PO4 (98 Da) and ligand dissociation into free tetralysine (5304 Da) and the glycan chain indicate a modification with phosphate. Sulfate substitution would be indicated by desulfation of glycan without ligand dissociation from tetralysine. The fragment spectrum reveals a phospho modification of the subterminal HexNAc.
MSn Analyses
In the first approach the oligosaccharides were liberated from the protein core by reductive β-elimination followed by methylation under conditions that retain sugar-linked phosphate groups. Because phosphate groups become mono- or dimethylated during this process (incremental mass increase of 94 or 108 Da) whereas sulfate groups stay unmethylated (incremental mass increase of 80 Da) this approach reveals further evidence for a discrimination of sulfate and phosphate groups (23). ESI-MS1 survey spectra registered with direct infusion indicated the presence of phosphorylated species. We observed a low intensity signal at m/z 1104 corresponding to the sodium adduct of the tetrasaccharide (H1N3), modified with a monomethylated phosphate. The masses m/z 1072 (Na+) and m/z 1050 (H+) belong to the same compound after elimination of methanol (supplemental Fig. 10).
MS2 of the phosphorylated, methanol-eliminated LacdiNAc glycan at m/z 1050 showed intense B1α (m/z 260), B2α (m/z 553), and Y1α (m/z 498) fragments of a terminal N-acetylhexosamine, of a subterminal phosphorylated di-N-acetylhexosamine, and of the residual core 2 structure. The B1α fragment indicates an unsubstituted terminal HexNAc, which confirms a phosphorylation of the subterminal N-acetylhexosamine (Fig. 8). Several minor fragments, including cross-ring fragmentations, support the proposed structure. A fragment of m/z 360.2 can be explained by an 1,3XGlcNAcZα2Zβ1 fragmentation of a 3,4-substituted subterminal HexNAc, pointing to a 3-phosphoester linkage (Fig. 8A) and supporting the linkage information obtained by tetralysine complex fragmentation (see above). Not even minor fragments support the alternative HexNAc substitution, a 6-phosphoesther linkage. Irrespective of this, the 3-phosphoester linkage is still a tentative assignment, and further experiments are necessary for an unequivocal confirmation of the linkage. MS2 spectra of the fragment at m/z 1104 (no methanol elimination) showed exactly the same fragmentation pattern due to elimination of methanol during the fragmentation process.
FIGURE 8.
ESI-MSn spectra of permethylated O-glycan alditols from recombinant α-dystroglycan (hDG8). A, MS2 spectrum of the permethylated phosphoglycan H1N3p + H+ (m/z 1050) as a methanol elimination product. The structures of the glycan alditol at m/z 1050.5 and of the fragment at m/z 360.2 are included. The fragment at m/z 360.2 can be interpreted as indicating a 3-phosphoester of the subterminal GlcNAc. B, MS3 spectrum of the B2α-fragment (N2P) at m/z 553.5 revealing a terminal HexNAc (no methanol elimination) and the elimination of methaphosphate (79 Da). All fragments are proton adducts.
MSn analysis further supported the evidence of a phosphorylated GlcNAc. MS3 of the B2α fragment led to elimination of 79 Da, a mass difference that can only be explained by elimination of metaphosphoric acid (PO3) (Fig. 8B). Methanol was therefore obviously eliminated from the phosphate group and not from the sugar moiety or by a cyclization in between.
Phospho-LacdiNAc Is Expressed in Mouse Myoblasts and Bovine Brain Tissue
LacdiNAc and phospho-LacdiNAc were initially detected on proteins, which were expressed recombinantly in HEK-293 cells. To demonstrate expression of the phosphoglycan in other cell types we expressed the fusion protein hDG6 in the mouse myoblast cell line C2F3. ESI-MS analysis of the tryptic digest revealed phosphorylated LacdiNAc glycans on the same peptides as derived from dystroglycan expressed in HEK cells (data not shown).
Further evidence for phosphorylated LacdiNAc expression on endogenous glycoproteins was provided by O-glycan analyses of bovine brain tissue. ESI-MS of the permethylated O-glycome revealed low amounts of the phosphoglycan among a majority of other mucin-type O-glycans (Fig. 9).
FIGURE 9.
ESI-MS/MS of precursor ion at m/z 1072. 6 from permethylated bovine brain O-glycans corresponds to the sodium adduct of phosphorylated tetrasaccharide HN3P (M-32).
Formation of Phospho-LacdiNAc Is Independent of Variations in Cell Culture Conditions
To evaluate whether phospho-LacdiNAc formation is induced artificially under certain conditions of cell growth, we expressed a recombinant probe (hDG8) in HEK-293 cells (i) at subconfluence in the presence of FCS, (ii) in the presence of FCS, but at confluence with high cell densities, and (iii) without FCS at confluence. Secretory fusion proteins generated under all conditions were found to be modified with phosphorylated LacdiNAc. A small reduction of approximately 10% was observed for expression in the absence of FCS, when analyzed by LC-ESI-MS/MS (supplemental Fig. 11).
DISCUSSION
We describe a novel modification of mucin-type O-linked glycans on ECM proteins: phosphorylated LacdiNAc. The structure, a core 2-based tetrasaccharide with a terminal GalNAcβ1–4(phospho-GlcNAcβ1–6) moiety, is specifically confined to the LacdiNAc unit and not found on the structurally similar LacNAc type 2 disaccharide. Whereas other modifications of LacdiNAc, like fucosylation, sialylation, or sulfation, are shared among these two related structures, phosphorylation appears to be a unique feature of LacdiNAc. The phospho modification was detected at substoichiometric levels on the LacdiNAc glycan which may reflect a highly regulated expression.
The LacdiNAc dihexosamine has been reported on a variety of N-glycoproteins and hormonal N-glycopeptides, but so far only on proopiomelanocortin and ZP3 as a terminal modification of O-linked glycans. Proopiomelanocortin belongs to the group of pituitary hormones which are known to be modified with LacdiNAc. ZP3 is an ECM protein of the zona pellucida that is involved in the fertilization process. In this study we were able to detect LacdiNAc in a variety of other O-glycoproteins, which have in common that they either belong to the functional group of secretory/shed ECM proteins (ZP3, AMACO, ECM1, nidogen-1) or to membrane-bound glycoproteins that interact with ECM proteins (α-dystroglycan, neurofascin). This finding may be related to functional implications of the glycan. The relative amounts of LacdiNAc glycans within the core 2 O-glycan pool varied strongly between 1 and 50%, which may reflect different substrate qualities of the protein species or variation in the preparation of recombinant probes. All O-glycoproteins expressing LacdiNAc were also positive for its phosphorylated derivative P-LacdiNAc, however at a substoichiometric level that escaped reliable quantitation. Neither LacdiNAc nor P-LacdiNAc is confined to human HEK-293 cell expression, because they can be found also on O-linked glycans from mouse cell lines (myoblast cells) and on endogenous human proteins (α-dystroglycan). P-LacdiNAc expression is also not restricted to recombinant proteins as we could detect it in a global O-glycome analysis of bovine brain tissue.
Glycan phosphorylation in mammals is commonly known as a targeting signal for proteins destined for transport to the lysosome. The modification described here has, however, no obvious structural or functional relationship to mannose 6-phosphate. The P-LacdiNAc-modified proteins play roles in the ECM context. The only structurally related, mammalian O-glycan phosphorylation described so far was detected on O-mannose-based glycans in human α-dystroglycan (16). The previously described structure has a terminal dihexosamine unit in common with the structure reported here; however, the terminal GalNAc is linked β1–3 to the subterminal GlcNAc, which is distinct from the LacdiNAc moiety, and the phosphate group was reported to be substituted at the core mannose. Unexpectedly, the various truncation and deletion constructs of hDG expressed in the same cellular model as used in the previous study (HEK-293 cells) did not express detectable amounts of O-mannose-based phosphoglycans. Neither on the glycopeptide (ESI-MS/MS) or glycoprotein levels (top-down sequencing by in-source decay MALDI-MS) nor reductively eliminated glycans (prior to or after metal chelate affinity chromatography) revealed any indication of the presence of phosphorylated mannose-based glycans (data not shown).
The modification with P-LacdiNAc of ZP3 may be of particular biological relevance, because earlier studies have shown that this glycoprotein serves as the primary sperm receptor and that the binding of spermatozoa to ZP3 is regulated primarily by glycan side chains. The structure of the O-linked chains, however, that were claimed to be involved in the fertilization process, were quite different, including terminal disaccharides Galα1–3Gal, Galβ1–4GlcNAc, and GalNAcβ1–4GlcNAc (4, 27–29). It has been controversially discussed whether sulfation may be important in human sperm-ZP3 interactions similar to those involving sulfate on high affinity ligands for selectins (27, 28). In the same manner also phosphorylated glycans, in particular P-LacdiNAc, could play a role in the regulation of sperm-egg binding or in the fertilization process in general. A follow-up study will have to elucidate these potential functions of the novel determinant.
Acknowledgments
We thank Dr. Neil Smyth (University of Southampton, UK), Dr. Raimund Wagener (University of Cologne), and Dr. Roswita Nischt (University Clinic Cologne), for the recombinant fusion proteins ECM1, AMACO-P2, and nidogen-1, respectively; Prof. Lothar Elling (University of Aachen) for a recombinant mutant form of β4-galactosyltransferase; and Prof. Cornelis Hokke (University of Leiden) for the anti-LacdiNAc antibody. We thank Dr. Stefan Müller (Central Bioanalytics, Center for Molecular Medicine Cologne) for technical support during the performance of MSn measurements on a HCT ETDII PTM Discovery.
This work was supported by the Deutsche Forschungsgemeinschaft Grants HA 2092/21-1 (to F.-G. H.) and BR 3979/1-1 (to I. B.) and by Swedish Research Council Grant 8266 (to A. G.).

This article contains supplemental Figs. 1–11.
- LacdiNAc
- (N,N-diacetyllactosediamine)
- ZP3
- zona pellucida glycoprotein 3
- CID
- collision-induced dissociation
- ECM
- extracellular matrix
- ESI
- electrospray ionization
- MS/MS
- tandem MS.
REFERENCES
- 1. Manzella S. M., Hooper L. V., Baenziger J. U. (1996) Oligosaccharides containing β-1,4-linked N-acetylgalactosamine, a paradigm for protein-specific glycosylation. J. Biol. Chem. 271, 12117–12120 [DOI] [PubMed] [Google Scholar]
- 2. Lowe J. B., Marth J. D. (2003) A genetic approach to mammalian glycan function. Annu. Rev. Biochem. 72, 643–691 [DOI] [PubMed] [Google Scholar]
- 3. Green E. D., van Halbeek H., Boime I., Baenziger J. U. (1985) Structural elucidation of the disulfated oligosaccharide from bovine lutropin. J. Biol. Chem. 260, 15623–15630 [PubMed] [Google Scholar]
- 4. Dell A., Morris H. R., Easton R. L., Panico M., Patankar M., Oehniger S., Koistinen R., Koistinen H., Seppala M., Clark G. F. (1995) Structural analysis of the oligosaccharides derived from glycodelin, a human glycoprotein with potent immunosuppressive and contraceptive activities. J. Biol. Chem. 270, 24116–24126 [DOI] [PubMed] [Google Scholar]
- 5. Manzella S. M., Dharmesh S. M., Cohick C. B., Soares M. J., Baenziger J. U. (1997) Developmental regulation of a pregnancy-specific oligosaccharide structure, NeuAcα2,6GalNAcβ1,4GlcNAc, on select members of the rat placental prolactin family. J. Biol. Chem. 272, 4775–4782 [DOI] [PubMed] [Google Scholar]
- 6. Skelton T. P., Kumar S., Smith P. L., Beranek M. C., Baenziger J. U. (1992) Proopiomelanocortin synthesized by corticotrophs bears asparagine-linked oligosaccharides terminating with SO4-4GalNAcβ1,4GlcNAcβ1,2Manα. J. Biol. Chem. 267, 12998–13006 [PubMed] [Google Scholar]
- 7. Fiete D., Mi Y., Oats E. L., Beranek M. C., Baenziger J. U. (2007) N-Linked oligosaccharides on the low density lipoprotein receptor homolog SorLA/LR11 are modified with terminal GalNAc-4-SO4 in kidney and brain. J. Biol. Chem. 282, 1873–1881 [DOI] [PubMed] [Google Scholar]
- 8. Martínez-Pomares L., Crocker P. R., Da Silva R., Holmes N., Colominas C., Rudd P., Dwek R., Gordon S. (1999) Cell-specific glycoforms of sialoadhesin and CD45 are counter-receptors for the cysteine-rich domain of the mannose receptor. J. Biol. Chem. 274, 35211–35218 [DOI] [PubMed] [Google Scholar]
- 9. Woodworth A., Pesheva P., Fiete D., Baenziger J. U. (2004) Neuronal-specific synthesis and glycosylation of tenascin-R. J. Biol. Chem. 11, 10413–10421 [DOI] [PubMed] [Google Scholar]
- 10. Hooper L. V., Beranek M. C., Manzella S. M., Baenziger J. U. (1995) Differential expression of GalNAc-4-sulfotransferase and GalNAc-transferase results in distinct glycoforms of carbonic anhydrase VI in parotid and submaxillary glands. J. Biol. Chem. 270, 5985–5993 [DOI] [PubMed] [Google Scholar]
- 11. Miller E., Fiete D., Blake N. M., Beranek M., Oates E. L., Mi Y., Roseman D. S., Baenziger J. U. (2008) A necessary and sufficient determinant for protein-selective glycosylation in vivo. J. Biol. Chem. 283, 1985–1991 [DOI] [PubMed] [Google Scholar]
- 12. Dell A., Chalabi S., Easton R. L., Haslam S. M., Sutton-Smith M., Patankar M. S., Lattanzio F., Panico M., Morris H. R., Clark G. F. (2003) Murine and human zona pellucida 3 derived from mouse eggs express identical O-glycans. Proc. Natl. Acad. Sci. U.S.A. 100, 15631–15636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Siciliano R. A., Morris H. R., Bennett H. P., Dell A. (1994) O-Glycosylation mimics N-glycosylation in the 16-kDa fragment of bovine proopiomelanocortin: the major O-glycan attached to Thr-45 carries SO4-4GalNAcβ1–4GlcNAcβ-1-, which is the archetypal nonreducing epitope in the N-glycans of pituitary glycohormones. J. Biol. Chem. 269, 910–920 [PubMed] [Google Scholar]
- 14. Florea D., Maes E., Guérardel Y., Page A., Zanetta J. P., Cogalniceanu D., Strecker G. (2006) Structure elucidation of NeuAc, NeuGc, and Kdn-containing O-glycans released from Triturus alpestris oviductal mucins: characterization of the poly-LacdiNAc sequence, HSO3(4)(GalNAcβ1–4GlcNAcβ1–3)1–3GalNAcβ1–4(GlcNAcβ1–3)0–1GlcNAcβ1-6GalNAc-ol. Glycoconj. J. 23, 377–399 [DOI] [PubMed] [Google Scholar]
- 15. Huang H. H., Tsai P. L., Khoo K. H. (2001) Selective expression of different fucosylated epitopes on two distinct sets of Schistosoma mansoni cercarial O-glycans: identification of a novel core type and Lewis X structure. Glycobiology 11, 395–406 [DOI] [PubMed] [Google Scholar]
- 16. Yoshida-Moriguchi T., Yu L., Stalnaker S. H., Davis S., Kunz S., Madson M., Oldstone M. B., Schachter H., Wells L., Campbell K. P. (2010) O-Mannosyl phosphorylation of α-dystroglycan is required for laminin binding. Science 327, 88–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Breloy I., Schwientek T., Gries B., Razawi H., Macht M., Albers C., Hanisch F. G. (2008) Initiation of mammalian O-mannosylation in vivo is independent of a consensus sequence and controlled by peptide regions within and upstream of the α-dystroglycan mucin domain. J. Biol. Chem. 283, 18832–18840 [DOI] [PubMed] [Google Scholar]
- 18. Nilsson J., Nilsson J., Larson G., Grahn A. (2010) Characterization of site-specific O-glycan structures within the mucin-like domain of α-dystroglycan from human skeletal muscle. Glycobiology 20, 1160–1169 [DOI] [PubMed] [Google Scholar]
- 19. Müller S., Hanisch F. G. (2002) Recombinant MUC1 probe authentically reflects cell-specific O-glycosylation profiles of endogenous breast cancer mucin: high density and prevalent core 2-based glycosylation. J. Biol. Chem. 277, 26103–26112 [DOI] [PubMed] [Google Scholar]
- 20. Gebauer J. M., Müller S., Hanisch F. G., Paulsson M., Wagener R. (2008) O-Glucosylation and O-fucosylation occur together in close proximity on the first epidermal growth factor repeat of AMACO (VWA2 protein). J. Biol. Chem. 283, 17846–17854 [DOI] [PubMed] [Google Scholar]
- 21. Hanisch F. G., Uhlenbruck G., Peter-Katalinic J., Egge H., Dabrowski J., Dabrowski U. (1989) Structures of neutral O-linked polylactosaminoglycans on human skim milk mucins: a novel type of linearly extended poly-N-acetyllactosamine backbones with Galβ(1–4)GlcNAcβ(1–6) repeating units. J. Biol. Chem. 264, 872–883 [PubMed] [Google Scholar]
- 22. van Remoortere A., Hokke C. H., van Dam G. J., van Die I., Deelder A. M., van den Eijnden D. H. (2000) Various stages of schistosoma express Lewis(x), LacdiNAc, GalNAcβ1–4 (Fucα1–3)GlcNAc and GalNAcβ1–4(Fucα1–2Fucα1–3)GlcNAc carbohydrate epitopes: detection with monoclonal antibodies that are characterized by enzymatically synthesized neoglycoproteins. Glycobiology 10, 601–609 [DOI] [PubMed] [Google Scholar]
- 23. Yu S. Y., Wu S. W., Hsiao H. H., Khoo K. H. (2009) Enabling techniques and strategic workflow for sulfoglycomics based on mass spectrometry mapping and sequencing of permethylated sulfated glycans. Glycobiology 19, 1136–1149 [DOI] [PubMed] [Google Scholar]
- 24. Albersheim P., Nevins D. J., English P. D., Karr A. (1967) A method for the analysis of sugars in plant cell-wall polysaccharides by gas-liquid chromatography. Carbohydr. Res. 5, 340–345 [Google Scholar]
- 25. Zhang Y., Jiang H., Go E. P., Desaire H. (2006) Distinguishing phosphorylation and sulfation in carbohydrates and glycoproteins using ion-pairing and mass spectrometry. J. Am. Soc. Mass Spectrom. 17, 1282–1288 [DOI] [PubMed] [Google Scholar]
- 26. Do K. Y., Do S. I., Cummings R. D. (1997) Differential expression of LacdiNAc sequences (GalNAcβ1–4GlcNAc-R) in glycoproteins synthesized by Chinese hamster ovary and human 293 cells. Glycobiology 7, 183–194 [DOI] [PubMed] [Google Scholar]
- 27. Benoff S. (1997) Carbohydrates and fertilization: an overview. Mol. Hum. Reprod. 3, 599–637 [DOI] [PubMed] [Google Scholar]
- 28. Chapman N. R., Barratt C. L. (1996) The role of carbohydrate in sperm-ZP3 adhesion. Mol. Hum. Reprod. 2, 767–774 [DOI] [PubMed] [Google Scholar]
- 29. Morris H. R., Dell A., Easton R. L., Panico M., Koistinen H., Koistinen R., Oehninger S., Patankar M. S., Seppala M., Clark G. F. (1996) Gender-specific glycosylation of human glycodelin affects its contraceptive activity. J. Biol. Chem. 271, 32159–32167 [DOI] [PubMed] [Google Scholar]