Abstract
Recent years have seen a significant increase in understanding of the host genetic and genomic determinants of susceptibility to HIV-1 infection and disease progression, driven in large part by candidate gene studies, genome-wide association studies, genome-wide transcriptome analyses, and large-scale in vitro genome screens. These studies have identified common variants in some host loci that clearly influence disease progression, characterized the scale and dynamics of gene and protein expression changes in response to infection, and provided the first comprehensive catalogs of genes and pathways involved in viral replication. Experimental models of AIDS and studies in natural hosts of primate lentiviruses have complemented and in some cases extended these findings. As the relevant technology continues to progress, the expectation is that such studies will increase in depth (e.g., to include host whole exome and whole genome sequencing) and in breadth (in particular, by integrating multiple data types).
HIV-1 infection and disease progression are influenced by variants in genes required for optimal HIV-1 replication (e.g., transcription factors) and genes that actively thwart viral pathogenesis (e.g., restriction factors).
Host genetics has been of considerable interest to the field of HIV/AIDS since the identification of the role of CCR5Δ32 (Dean et al. 1996; Huang et al. 1996; Liu et al. 1996) in resistance to infection and of human leukocyte antigen (HLA) alleles in disease progression (Kaslow et al. 1996). Further observations confirm that there is a significant component of heredity in the susceptibility to HIV-1. Identical twins infected with the same viral strain progressed at a similar pace, whereas their fraternal twin had a different clinical course (Draenert et al. 2006). In vitro, the study of cells from large pedigrees—immortalized B lymphocytes from multigeneration families—allowed the identification of a host genetic contribution to >50% of the observed differences in cell susceptibility to transduction with a vesicular stomatitis virus (VSV)-pseudotyped HIV-1 vector (Loeuillet et al. 2008). In the larger context of infection by simian immunodeficiency viruses, host genetic variation influences transmission between species as well as replication and pathogenesis within individuals of the same species (Kirmaier et al. 2010). Host genetic variation includes fixed differences between species (divergence) and variation within populations (polymorphism). Genetic barriers to viral transmission and spread can arise owing to variation in genes required for optimal viral replication (e.g., receptors, transcription factors, chaperones, etc.) as well as variation in genes that actively thwart viral replication and pathogenesis (e.g., innate and adaptive immune effectors such as antibody and cytotoxic T lymphocyte (CTL) responses, and restriction factor loci such as TRIM5α, the APOBEC3 cluster, and tetherin). The influence of host selection on viral evolution manifests as changes in primary sequence (escape and reversion variants) in the viral genome structure, and over the long term, in the acquisition of accessory functions. In this article, we begin with an overview of the current understanding of genes and gene variants influencing susceptibility to HIV-1 in humans, and susceptibility to HIV/simian immunodeficiency virus (SIV) in nonhuman primate models of infection. This includes data from candidate gene studies, genome-wide association studies, and other genome-wide screens. We then analyze notable data on host genome pressure on the viral genome structure. Finally, we present a global view on the evolutionary genomics of susceptibility to HIV-1 and other retroviruses.
KEY ADVANCES
Human Genomics
Genome-Wide Association Studies
The HIV field was one of the first to embrace the opportunities offered by new technologies that allowed genome-wide association studies (GWASs). This approach, which assesses 500,000 to 1 million genetic variants (single nucleotide polymorphism, SNP) for each individual, had advantages and limitations (Telenti and Goldstein 2006). First, it allowed for the first time the non–a priori analysis, at genome-wide scale, of possible genetic determinants of a trait. This would allow discovery, unbiased validation of all previously reported variants, and scoring the contribution of the genetic variant in the context of other possible genome influences. However, the limitations of the approach include the need for sufficient power, requiring large numbers of subjects, and the identification of very precise study traits (Evangelou et al. 2011). In addition, only common variation (present in >5% in a given population) is investigated in GWASs. The need for large populations (power) is determined by the statistical requirements that impose a very strict threshold of significance (i.e., defined by P < 5 × 10−8 owing to correction for multiple comparisons) and by the limited contribution of any given genetic variant to the population phenotype. The study traits need to be defined by strict criteria to avoid heterogeneity in the phenotype—an important consideration because the field uses study outcomes, such as time to AIDS or death, which represent composite end points. Finally, the genotyping arrays used in GWASs target common variants that tag other variants; the actual causal variant or functional polymorphism is unlikely to be interrogated directly. Current arrays are generally adequate to capture common variation in Caucasians and Asians, although less effective in individuals of African ancestry.
Eight GWASs have been published during the period 2007–2010 (Table 1). The first study (Fellay et al. 2007) investigated the genomic determinants of viral set point after seroconversion—the relatively steady state of viral replication in the 3 years following a documented infection. As a secondary end point, the study evaluated disease progression, as defined by time to CD4+ T-cell count less than 350 cells/μL, or else initiation of treatment. This study identified three variants in chromosome 6: a variant in HCP5 that tagged the protective allele HLA-B*5707, a variant upstream of HLA-C associated with difference in HLA-C expression levels, and a variant in ZNRD1 that may be merely associated with other influences from the major histocompatibility complex (MHC) locus, or contribute directly to pathogenesis (Ballana et al. 2010). Extension of this work by the same group of researchers (Fellay et al. 2009) confirmed the various associations and ruled out major variants elsewhere in the genome with the exception of those in the CCR5-CCR2 locus (Fig. 1). The overall variance explained by the genome-wide significant hits was close to 20%—much larger than what has been observed in GWASs of other diseases or in studies of anthropomorphic diversity (height, weight) but still a small percentage of the total. Although the overwhelming message is one of confirmation of the critical importance of the HLA-B/HLA-C locus, some studies have reported additional candidate loci (Table 1).
Table 1.
Genome-wide association studies, 2007–2010
| Study/year | Trait | N | Population | Genome-wide significant hitsa | Comment |
|---|---|---|---|---|---|
| Fellay et al. 2007 | Viral load set point, disease progression | 486 (seroconverters) | Caucasian | rs2395029 (HCP5/HLA-B*57:01), rs9264942 (HLA-C), rs9261174 (ZNRD1) | |
| Dalmasso et al. 2008 | Plasma HIV-RNA levels and cellular HIV-DNA levels | 605 (seroconverters) | Caucasian | rs10484554, rs2523619, and rs2395029 (HLA-C, HLA-B locus) | |
| Limou et al. 2009 | HIV nonprogression | 275 (HIV-1+ nonprogressors), and 1352 (negative controls) | Caucasian | rs2395029 (HCP5/HLA-B*57:01) | Subset study (Limou et al. 2010) used to validate candidate rs2234358 (CXCR6) in three independent cohort studies (n = 1028) |
| Le Clerc et al. 2009 | Rapid disease progression | 85 (HIV-1+ rapid progressors), and 2049 (negative controls) | Caucasian | – | |
| Fellay et al. 2009 | Viral load set point, disease progression | 2554 (seroconverters and seroprevalent) | Caucasian | rs2395029, rs9264942, rs259919, rs9468692, rs9266409 (MHC locus), and rs333(CCR5Δ32) | Failed to validate previously reported candidate genes with the exception of CCR5/CCR2 variants |
| Herbeck et al. 2010 | Disease progression | 156 (rapid, moderate, and nonprogressors) | Caucasian | – | Among the 25 top-ranking variants, rs17762192 (PROX1) was validated in an independent replication cohort of 590 seroconverters |
| Pelak et al. 2010 | Viral set point | 515 (seroconverters) | African American | HLA-B*57:03 (rs2523608) | In contrast with the tagged HLA, rs2523608 does not reach genome-wide significance |
| International HIV Controllers Study 2010 | HIV nonprogression | 974 (HIV controllers), 2648 (HIV progressors) | Caucasian, African American, and Hispanics | 313 SNPs in the MHC locus captured by rs9264942, rs2395029, rs4418214, and rs3131018 as independent markers | Arg97, Cys67, Gly62, and Glu63, all in HLA-B; Ser77 in HLA-A; and Met304 in HLA-C collectively explain 20% of the observed variance in Caucasians |
aVaries between p < 10−7 and p < 10−8 depending on the study design.
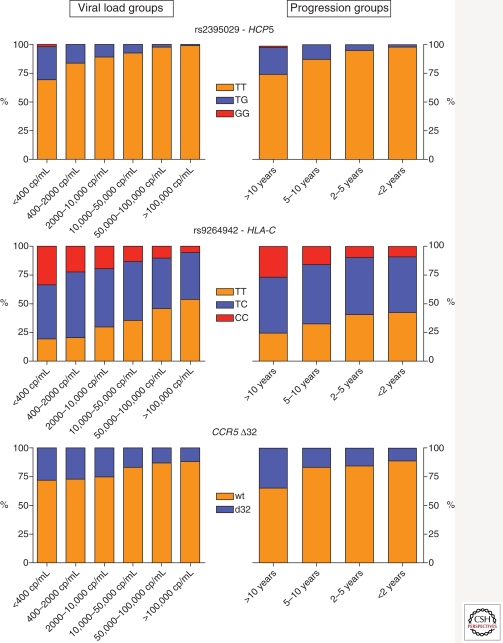
Figure 1.
Distribution of the protective alleles according to viral or clinical phenotypes. The bar graphs show the allelic distribution of three variants that have a genome-wide significant association with HIV-1 set point (left-hand-side graphs) and disease progression (right-hand-side graphs) in a population of 2362 HIV-infected individuals. The data on HCP5, a perfect tag of HLA*B57:01, illustrate the nature of the association between protective alleles and long-term nonprogression: although 31%–37% of elite controllers carry HLA*B57:01, only 6%–22% of HLA*B57:01 carriers are elite controllers. (Adapted, with permission, from Fellay et al. 2009; reprinted, with permission, from PLoS Genetics © 2009.)
A significant step forward in the understanding of the complexity of signals emerging from the MHC locus came from the International HIV Controllers Study (2010). This large consortium compared the genetic data of 974 subjects defined as viral controllers and 2648 progressors. After an initial step that confirmed the critical importance of the MHC region in the trait of nonprogression, the study proceeded to mapping putative causal variants within the HLA locus. This step is complex because this gene-rich region has high levels of genetic diversity and a complex pattern of linkage disequilibrium. The linkage disequilibrium reflects the measurement of nonrandom association between two or more alleles that occur together on a chromosome. The International HIV Controllers Study developed analytical tools that specified unique residues in the HLA-B groove as putative causal variants. These included Gly62, Glu63, Cys67, and Arg97, all in HLA-B. In addition, the study identified Ser77 in HLA-A and Met304 in HLA-C. With the exception of Met304 in the transmembrane domain of HLA-C, these residues are all located in the MHC class I peptide binding groove, underscoring that the conformational presentation of class I restricted epitopes to T cells play a key role in host control. Chimpanzees can be infected with HIV-1; however, most do not develop AIDS. The contemporary MHC class I repertoire of chimpanzees targets analogous conserved domains of HIV-1/SIVcpz to those targeted by human protective alleles HLA-B*57 and B*27, in particular, of the Gag protein (de Groot et al. 2010). The functional characteristics of the chimpanzee MCH-repertoire may be the result of a selective sweep caused by lentiviruses (de Groot et al. 2010). The consistent identification of HLA-B*57, HLA-B*27, and HLA-B*51 in host genome studies is attributed to fitness cost of mutating in the targeted viral epitopes and to the patterns of viral escape from CD8+ T-cell recognition. Differences in prevalence of the restricting HLA allele in different human groups consequently lead to HIV-1 adaptation to HLA at a population level (Kawashima et al. 2009).
Following the completion of the GWASs mentioned above, the field is planning a joint meta-analysis of all available data sets combining information presented in Table 1 and new GWASs. This approach can address issues of insufficient power and thus facilitate the identification of common variants that would be associated with smaller effects, or more rare variants included in the typing arrays. Follow-up of GWASs includes resequencing and functional analysis of the main hits and putative causal variants. This has been performed for HCP5 (Yoon et al. 2010), HLA-C (Thomas et al. 2009), and ZNRD1 (Ballana et al. 2010) hits. Of particular interest is the recent work that identifies the −35 SNP in HLA-C as a marker of variation at the binding of microRNA Hsa-miR-148a to its target site within the 3′ untranslated region of HLA-C (Kulkarni et al. 2011). This mechanism of posttranscriptional regulation results in relatively low surface expression of alleles that bind this microRNA and high expression of HLA-C alleles. The exact role of the individual HLA-B amino acids identified by the International HIV Controllers Study on epitope presentation, T-cell receptor binding, and CTL activity is the subject of intensive research in the field.
Vaccine Genomics
A particularly attractive use of GWASs is in the understanding of differences between individuals in the response to immunogens. Although vaccine genomics is in its infancy, some initial applications have been reported. A GWAS assessed determinants of HIV-specific T-cell responses to the MRKAd5 HIV-1 gag/pol/nef vaccine (Fellay et al. 2011) tested in 831 subjects of the Step HIV-1 vaccine trial, as measured by IFN-γ ELISpot assays. No genetic variant reached genome-wide significance, but polymorphisms located in the MHC showed the strongest association with response to the HIV-1 Gag protein. HLA-B alleles known to associate with differences in HIV-1 control were found to be responsible. The authors concluded that the host immunogenetic background needs to be considered in the analysis of immune responses to T-cell vaccines. Increasingly, vaccination studies will include host genetic analysis, informed consent, and DNA storage for later analysis.
Vacccine genomics can also address the selective pressure from vaccine-induced T-cell responses on HIV-1 infection in humans. Rolland et al. (2011) analyzed HIV-1 from 68 infected volunteers in the STEP trial to identify signatures distinguishing vaccine from placebo recipients. Gag amino acid 84, a site encompassed by several epitopes contained in the vaccine and restricted by HLA alleles common in the study cohort, was identified by this approach. Viral genome regions excluded from the vaccine components did not carry distinctive signatures of selective pressure from vaccine-induced T-cell responses on HIV-1 infection in humans.
Advanced Genome Analyses
Although the platforms and analytical tools for genome-wide genotyping are well established, it is clear that the GWAS approach will not capture some aspects of the host influence on the pathogenesis of HIV infection. Additional types of data include the transcriptome and proteome of the infected cell or individual. Further techniques use evolutionary and comparative genetic tools for the identification of host genes involved in genetic conflicts with lentiviral or retroviral pathogens, or large-scale functional genomics using loss-of-function (siRNA) and gain-of-function screens (Bushman et al. 2009; Telenti 2009).
Expression analyses have generated a collection of data from in vitro and in vivo studies on cell lines, whole blood, and cellular subsets. Many publications were based on earlier technologies interrogating small gene subsets, were limited by the number of samples, and rarely captured the dynamic nature of the transcriptome (Giri et al. 2006). Globally, this body of literature has described a number of features of the infectious process that massively modulates the antiviral defense systems (the interferon response, including the antiretroviral intrinsic cellular defense apparatus), as well as genes involved in the cell cycle and degradation/proteasome pathway (Rotger et al. 2010). Particularly relevant is the observation that elite controllers have CD4+ T-cell transcriptome profiles that are similar to those from individuals receiving effective treatment (Rotger et al. 2010). The transcriptome profile of a subset of elite controllers is indistinguishable from that of the uninfected individuals—at least in HLA-DR-CD4+ T-cell subsets that were the object of a recent study (Vigneault et al. 2011).
Increasingly, studies are successfully using expression data to single out genes for functional analyses. Notable examples include observations on SOCS1 (Rotger et al. 2011), BATF (Quigley et al. 2010), and CXCR6 (Paust et al. 2010) that originated in transcriptome analyses. The suppressor of cytokine signaling 1 (SOCS1) suppresses interferon signaling by direct binding to phosphorylated type I interferon and active JAK kinase and by orchestrating the events leading to proteasomal degradation of a number of target proteins. Although viruses such as HTLV-1 may use induction of SOCS1 to evade the antiviral effects of interferon signaling, it is differentially up-regulated in the nonpathogenic primate models (Bosinger et al. 2009; Jacquelin et al. 2009; Lederer et al. 2009) and in HIV-1 infected humans that tolerate high levels of viral replication and do not progress (Rotger et al. 2011). The basic leucine transcription factor, ATF-like (BATF), a transcription factor in the AP-1 family, was identified during the study of exhausted CD8+ T cells. PD-1 coordinately regulates a program of exhaustion genes in humans and mice, including up-regulation of BATF. Silencing BATF in T cells from individuals with chronic viremia rescues HIV-specific T-cell function (Quigley et al. 2010). The third example, CXCR6, emerged from the study of natural killer (NK) cells in mice (Paust et al. 2010). Hepatic NK cells, but not splenic or naive NK cells, develop specific memory of vaccines containing antigens from HIV-1 and other viruses (Paust et al. 2010). NK cell memory depends on CXCR6, a chemokine receptor on hepatic NK cells that is required for the persistence of NK memory.
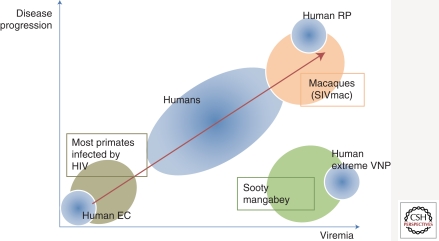
Additional efforts are also directed at comparative transcriptomics, which is the cross-species analysis of expression patterns of human and nonhuman primates during infection and disease (Rotger et al. 2011). HIV-infected subjects with rapid disease progression have gene expression patterns in CD4+ and CD8+ T cells similar to that in pathogenic SIV infection of rhesus macaque (Fig. 2). In contrast, humans that do not progress despite prolonged and extreme levels of viral replication share transcriptional features with the sooty mangabey model of natural infection, including a common profile of regulation of a set of genes that includes CASP1, CD38, LAG3, TNFSF13B, SOCS1, and EEF1D (Rotger et al. 2011).
Figure 2.
Schematic representation of the parallelism between human and nonhuman primate (NHP) models of HIV/SIV pathogenesis. EC, elite controllers; RP, rapid progressors; VNP, extreme viremic nonprogressors. (Adapted, with permission, from Guido Silvestri 2010.)
Next-generation sequencing offers an unprecedented opportunity to jointly analyze cellular and viral transcriptional activity. This approach served to show that at peak infection of SupT1 cells—a T-cell line—one transcript in 143 is of viral origin (0.7%), including a small component of antisense viral transcription (Lefebvre et al. 2011). Deep sequencing also showed very active transcription of repetitive elements and endogenous retroviruses: Approximately 0.4% of all cellular transcripts are of such origin whether the cell is infected or not (Lefebvre et al. 2011).
Proteomic studies analyzed 2000–3200 proteins and identified 15%–21% to be differentially expressed on infection (Chan et al 2007; Ringrose et al. 2008), including changes in the abundance of proteins with known interactions with HIV-1 viral proteins. The NCBI HIV-1 Human Protein Interaction Database (www.ncbi.nlm.nih.gov/RefSeq/HIVInteractions/) summarizes >3000 interactions with almost 1500 human genes (Fu et al. 2009). A complete library of viral-host protein coimmunoprecipitation relevant to the early innate immune responses to HIV is being built by the HINT consortium (HIV Networks Team, www.hint.org) and will be publicly available.
Genome-wide siRNA and shRNA screens have generated a comprehensive view of genes required for efficient viral replication (Brass et al. 2008; Konig et al. 2008; Zhou et al. 2008; Yeung et al. 2009). Individually, each published screen has identified a few hundred such candidate genes. However, there is a limited overlap across studies that has been attributed to differences in study design and to the type and source of the si/shRNA library (Bushman et al. 2009). The studies were also not specifically designed to identify restriction factors; no hits resulted in increasing levels of viral replication. Despite these limitations, meta-analysis of the data convincingly identified sets of genes and pathways that are common to two or more studies. A pattern emerged among the 34 shared genes that involve the nuclear pore machinery, the mediator complex, a number of key kinases, and components of the NF-κB complex. Studies under way address those various technical issues by the systematic comparison of multiple siRNA libraries, the development of experimental approaches that can identify restriction factors, the integration of these data with other sources of experimental data, notably with gene expression (Fellay et al. 2010), and by the use of primary cells.
One gain-of-function screen used a cDNA library representing 15,000 unique genes in an infectious HIV-1 system (Nguyen et al. 2007). A more recent overexpression screening approach aimed at characterizing the antiviral activity of more than 380 interferon-stimulated genes (ISGs) against a panel of viral pathogens (Schoggins et al. 2011). Broadly antiviral effectors included IRF1, C6orf150/MB21D1, HPSE, RIG-I/DDX58, MDA5/IFIH1, and IFITM3. In addition, anti-HIV activity was proposed for MX2, IFITM2, CD74, TNFRDF10A, IRF7, and UNC84B. Several ISGs, including ADAR, FAM46C, LY6E, and MCOLN2, enhanced the replication of certain viruses, highlighting the complexity in the type I interferon responses.
Primate Genetics
Nonhuman primates (NHP), whether naturally or experimentally infected with simian immunodeficiency viruses (SIVs), display phenotypic variation on multiple levels, including differences in relative susceptibility to infection, variability in both acute and long-term viral replication levels, differing rates of disease progression, and differences in degree of pathogenesis (see Klatt et al. 2011; Lifson and Haigwood 2011). NHP populations, including both wild populations and captive-bred colonies, comprise genetically variable, outbred individuals, and it is reasonable to assume that variation in virological phenotypes reflects, in part, host genetic variation. Phenotypic variation in SIV-infected NHPs provides a considerable but largely unexplored opportunity to examine the influence of host genetics on primate immunodeficiency virus replication and disease. In the case of rhesus macaques, the most commonly used NHP in AIDS research, the availability of whole genome sequence (WGS) data has facilitated discovery and cataloging of SNPs and copy-number variants that may prove useful for genetic and genomic analyses (Malhi et al. 2007; Lee et al. 2008). WGS data are also available for chimpanzees and a variety of other NHPs representing all main primate lineages (www.ensembl.org). Thus, comparative studies of the different SIVs and their respective primate hosts have the potential to identify and characterize genes that govern the transmission of viruses within and between populations.
Relevance to HIV infection and human disease exists on multiple fronts. For many gene products with well-established roles in HIV-1 infection or replication, there is evidence for an analogous or similar role for other SIV, and in such cases, the existence of animal models can confirm and even extend understanding of the biological relevance of such interactions (Fig. 2). Humans are frequently exposed to retroviruses of other, nonhuman primates (Wolfe et al. 2004), and SIV from chimpanzees and sooty mangabeys have made the jump several times (see Hahn et al. 2000; Apetrei et al. 2004; Sharp and Hahn 2011). Thus, in addition to reconstructing the natural history of the primate lentiviruses, comparative studies can reveal the role of genetics in determining which retroviruses jump from primates into humans, and allow us to ask whether adaptation to the human genetic landscape is a prerequisite for emergence of new viruses. As a practical matter, identification and molecular analysis of genes that interfere with cross-species transmission are also helping pave the way toward improved animal models of HIV infection and AIDS (Hatziioannou et al. 2006; Kamada et al. 2006; Ambrose et al. 2007; Igarashi et al. 2007; Hatziioannou et al. 2009). Finally, understanding the role of genetics in promoting the nonpathogenic outcome in natural hosts of SIV could some day suggest pharmacological strategies for uncoupling HIV infection from disease in humans.
Studies in Macaques (Macaca sp.)
In a striking parallel to the emergence of HIV-1 and HIV-2 in humans, the first SIV was isolated as an emerging pathogen in colonies of captive Asian macaques in the early 1980s (Daniel et al. 1985; Mansfield et al. 1995; Gardner 2003; Apetrei et al. 2004). The SIV-infected macaque has since served as the primary model for preclinical AIDS research, in part because there is still no practical, small animal model that faithfully recapitulates HIV infection and AIDS in all essential parameters. Among the practical difficulties encountered in the SIV/macaque model are animal-to-animal variability in susceptibility to infection, viral replication levels, and disease progression (not unlike the variation observed among HIV-infected humans). Although this variability can confound small-scale animal studies (for practical reasons, studies in NHPs are often underpowered), it also means that nonhuman primate models can be used to investigate the influence of host genetics on viral replication, emergence, and pathogenesis.
As a model system for understanding the influence of host genetic variation on virological outcomes, SIV-infected macaques have several advantages over analysis of human cohorts. Initial infection is established either by a cloned viral isolate of known sequence, or by a biological isolate that can be genetically defined by sequencing. Unlike naturally occurring infection in humans, most macaque cohorts consist of animals infected in parallel with identical or very similar viruses. Both the time and route of infection are known precisely. Because most or all animals in a study are under the care of a specific team of investigators following a set of standardized protocols, sample collection, evaluation, and care of animals in SIV cohorts are inherently more uniform. Furthermore, end points (such as definition of AIDS) are consistently applied. Finally, and most importantly, hypotheses can be tested or confirmed in either prospectively or retrospectively genotyped animals. Familial relationships are also often known for captive-bred animals, raising the possibility that approaches incorporating pedigree analysis might ultimately be applied to macaque cohorts. The primary disadvantage of macaque cohorts as subjects for genetic association studies is the relatively small size of any given study (typically <50 animals). Nonetheless, given the extensive use of the SIV/macaque model for the past 25 years and the widespread use of a limited number of closely related SIV strains, it should be feasible to assemble sufficiently large study cohorts by combining samples from multiple SIV studies. To date, GWASs have not been reported for SIV/macaque cohorts. However, studies focusing on specific genes/loci serve to illustrate the potential contributions of SIV cohorts to our understanding of host genetics and AIDS.
The MHC Locus in Primate Models
Perhaps the most significant contributions of SIV animal models to understanding the role of host genetics have been in elucidating the influence of MHC class I genes on lentiviral replication and disease progression, and the potential for vaccine-induced CTL responses. The first robust correlations between specific MHC genotypes and epitope-specific viral escape from CTL emerged from studies of SIV-infected rhesus macaques (Evans et al. 1999; Allen et al. 2000). For example, Evans et al. documented emergence of epitope-specific escape variants by tracking SIV sequences in MHC-defined macaques (Evans et al. 1999). Similarly, Allen et al. reported rapid emergence of CTL-escape variants in a Mamu-*A01-restricted Tat epitope during the first 8 wk of infection, corresponding to the primary onset of acute-phase virus-specific CTL responses (Allen et al. 2000). Such studies were corroborated by monoclonal-antibody-based depletion of CD8+ T cells in SIV-infected macaques, which led to transient increases in viral replication levels and provided confirmation that cellular immune responses have a major influence on viral replication (Schmitz et al. 1999). Later studies also revealed significant correlations between specific MHC alleles and control of SIV replication levels; most notably, MHC B-locus alleles Mamu-B*08 and B*17, which are associated with elite control of SIVmac infection in rhesus macaques (Yant et al. 2006; Loffredo et al. 2007a,b).
More recently, O’Connor and colleagues took advantage of a unique population of animals, the Mauritian cynomolgus macaques, to analyze the impact of MHC diversity on viral replication levels in vivo (O’Connor et al. 2010). Because Mauritian cynomolgus macaques display a limited number of MHC haplotypes, MHC-homozygosity is fairly common—a situation enabling direct comparison of viral replication in homozygous and heterozygous individuals, many with shared MHC haplotypes. In this study, MHC-homozygous animals infected with SIVmac239 had chronic-phase plasma viral RNA levels 80 times higher on average than MHC-heterozygous animals analyzed in parallel, suggesting that the increased breadth of potential virus-specific CD8+ T-lymphocyte responses in heterozygous individuals on average gave rise to significantly enhanced control of viral replication. These results provided strong experimental confirmation of prior studies describing heterozygous advantage in human HIV/AIDS cohorts (Carrington et al. 1999; Tang et al. 1999). As a practical matter, evidence of heterozygous advantage in these animals also lends credence to the hypothesis that an effective HIV-1 vaccine will be one that induces a broad range of virus-specific immune responses.
MHC class I molecules also interact with killer immunoglobulin-like receptors (KIR), influencing NK cell responses to HIV infection (Martin et al. 2002, 2007). Several candidate MHC-KIR interactions have been reported for rhesus macaques based on in vitro binding assays (Rosner et al. 2011). Colantonio and colleagues made the serendipitous discovery that a recombinant soluble MHC tetramer folded around certain SIV-derived peptides could bind to the surface of lymphocytes from a subset of uninfected animals with no prior exposure to SIV or SIV antigens (Colantonio et al. 2011). This observation led to the identification of a specific MHC-KIR interaction (Mamu-A1*00201/Mamu-KIR3DL05) and the first functional demonstration of ligand-mediated NK cell inhibition in primary macaque cells (Colantonio et al. 2011). Further investigation also revealed that the particular SIV peptide/epitope bound by the Mamu-A1*00201 tetramer influenced the ligand/KIR interaction. Although the impact of MHC-bound peptide on MHC-KIR interaction has been reported (Malnati et al. 1995; Peruzzi et al. 1996; Mandelboim et al. 1997; Rajagopalan et al. 1997; Zappacosta et al. 1997; Hansasuta et al. 2004; Thananchai et al. 2007), the analysis by Colantonio et al. pointed toward specific involvement of residues in the KIR molecule. By taking advantage of polymorphic variants of the rhesus macaque KIR3DL05 gene that differed in peptide selectivity, they pinpointed residues in the third loop of the KIR D1 domain that influence the peptide dependency of the MHC-KIR interaction (Colantonio et al. 2011). Structural models of MHC-KIR interaction place this loop in close proximity to the MHC-peptide surface (Boyington et al. 2000; Fan et al. 2001; Sharma et al. 2009). Importantly, genetic characterization of the rhesus macaque KIR locus and the development of reagents specific for macaques will permit incorporation of KIR genetics into animal models of AIDS and preclinical vaccine research.
Restriction Factors
The discovery of the host restriction factor TRIM5α illustrates the benefits of a comparative approach to the study of HIV and AIDS. The antiviral activity of TRIM5α was first identified by Stremlau and colleagues by screening a rhesus macaque cDNA library for genes that conferred resistance to HIV-1 infection of human cells (Stremlau et al. 2004). Similarly, an unusual Trim5 ortholog was identified as the source of a genetic block to HIV-1 infection in cells from South American owl monkeys (Aotus sp.) (Nisole et al. 2004; Sayah et al. 2004). It is now widely assumed that TRIM5 is a major modulator of cross-species transmission of retroviruses, and as a practical consequence, TRIM5 poses a significant genetic barrier to development of a NHP model of HIV-1 infection (Hatziioannou et al. 2006; Kamada et al. 2006; Ambrose et al. 2007; Igarashi et al. 2007; Hatziioannou et al. 2009).
Two independent association studies in SIV-infected rhesus macaques have shown the ability of TRIM5 to suppress lentiviral replication levels in vivo (Kirmaier et al. 2010; Lim et al. 2010a). It is noteworthy that both studies uncovered significant associations with relatively few animals (n < 100 animals in both cases). This differs from TRIM5 studies in human HIV/AIDS cohorts, where reported associations have been modest (Goldschmidt et al. 2006; Javanbakht et al. 2006; Speelmon et al. 2006). The Lim et al. study (Lim et al. 2010a), which focused on a cohort of SIVmac251-infected rhesus macaques, most closely resembles the situation in human cohorts. Just as HIV-1 is only weakly susceptible to human TRIM5, SIVmac strains are relatively resistant to rhesus macaque TRIM5. The enhanced ability to detect a significant effect in macaques was owed, in part, to the presence at high frequency of functionally distinct TRIM5 alleles in the rhesus macaque TRIM5 locus (Newman et al. 2006; Wilson et al. 2008). Lim et al. also revealed that complete viral resistance to TRIM5 is not required for pathogenesis—animals with restrictive alleles displayed lower but significant levels of SIVmac251 replication and developed AIDS—a fact that could not have been appreciated from tissue culture experiments alone.
Goldstein and colleagues first reported evidence for variation in inherent susceptibility of T lymphocytes from naïve, uninfected rhesus macaques to infection with SIV strain SIVsmE543-3 (Goldstein et al. 2000). They further showed that susceptibility of an animal’s cells to infection in tissue culture correlated with in vivo susceptibility to SIVsmE543-3 infection. These results argued for the existence of an intrinsic cellular block to infection unrelated to virus-specific adaptive immunity. More recently, Kirmaier et al. found that this inherent resistance to SIVsmE543-3 was due to rhesus macaque TRIM5, and that variation in susceptibility of animals to SIVsmE543 infection correlated with allelic variation in the rhesus macaque TRIM5 gene (Kirmaier et al. 2010). Compared to SIVmac251, which is well adapted to rhesus macaques as a host, the impact of rhesus TRIM5 polymorphism on SIVsmE543 was far more dramatic (2–3 log differences in viral loads). The greater susceptibility of SIVsmE543-3 to multiple alleles of rhesus TRIM5 likely reflects its derivation by brief passage of a sooty mangabey virus (SIVsm) through only two rhesus macaques (Hirsch et al. 1997).
Restriction of HIV-1 by the most common alleles of rhesus macaque TRIM5 also poses a barrier to development of simian-tropic strains of HIV-1 (Ambrose et al. 2007). However, additional genetic barriers remain, including those imposed by the APOBEC3 and BST2/Tetherin loci. Although intraspecies surveys of NHP BST2/Tetherin and APOBEC3 loci have been limited, some reports suggest that these genes may also be polymorphic in rhesus macaques (Weiler et al. 2006; Jia et al. 2009; McNatt et al. 2009). Whether allelic variants of tetherin and APOBEC3 have an impact on viral infection or pathogenesis in the macaque model remains to be seen (a weak correlation between APOBEC3G variation and SIVmac replication levels in vivo has been reported [Weiler et al. 2006]). In contrast to rhesus macaques, pig-tailed macaques (species Macaca nemestrina) uniformly carry a single allele of TRIM5 (TRIM5CypA) that does not restrict HIV-1 infection in tissue culture assays (Liao et al. 2007), raising the possibility that this species may provide a more permissive host for developing an experimental model of HIV-1 infection (see Igarashi et al. 2007; Hatziioannou et al. 2009; Lifson and Haigwood 2011).
Although SIV-infected macaque cohorts have not been routinely subjected to gene association or GWASs, the impact of host genes and genetic variation on SIV replication and disease is disclosed by adaptive countermeasures acquired by viruses during replication in vivo. Kirmaier et al. identified specific amino acid alterations in the SIVsmE543 CA protein that emerged in vivo in several animals bearing suppressive TRIM5 genotypes. Such changes also appeared during the emergence of SIV in rhesus macaque colonies in the 1970s (Kirmaier et al. 2010). Among its many functions, the SIVmac Nef protein clearly prevents viral inhibition by rhesus macaque tetherin in tissue culture assays (Jia et al. 2009; Zhang et al. 2009). Compelling evidence in support of a similar role in vivo came from retrospective analysis of animals infected with a nef-deleted variant of SIVmac239. Replication of the SIVmac239 nef mutants was initially attenuated in vivo, and strains that eventually grew out in these animals had acquired novel anti-tetherin activity through adaptive changes in the viral Env protein (Serra-Moreno et al. 2011). Taken together, the emergence of adaptations to overcome CTL and restriction factors in animals with defined genotypes provides evidence that expression of these host genes (e.g., MHC, TRIM5, and tetherin) has biological relevance, by inhibiting viral replication in vivo, consistent with their observed mechanisms of action in the laboratory.
Chemokine Receptors and Chemokines
Mutations analogous to the Δ32 base-pair deletion in the CCR5 coding sequence in humans have also been found in two closely related nonhuman primates that serve as natural hosts of SIV infection (Chen et al. 1998; Palacios et al. 1998; Riddick et al. 2010). At least two distinct mutations are found in the CCR5 gene of sooty mangabeys (Cercocebus atys), the natural hosts of SIVsm (Riddick et al. 2010). Both are deletion mutations, encompassing 2 and 24 nucleotides of coding sequence, respectively. In one colony of captive sooty mangabeys, the frequencies of the Δ2 and Δ24 alleles were reported to be 26% and 3%, respectively (Riddick et al. 2010). The Δ24 variant is also present at high frequency in red-capped mangabeys (Cercocebus torquatus), and the naturally occurring SIV that is endemic to these animals (SIVrcm) can use a different molecule (CCR2b) as a coreceptor for viral entry (Chen et al. 1998).
Only as recently as 2004, researchers came to recognize that copy-number variation (CNV) is a major source of human genetic diversity (Iafrate et al. 2004; Sebat et al. 2004). CNV, which can encompass expansions and contractions of large segments of chromosomal DNA (and the genes contained therein), can result in phenotypic diversity. Nonhuman primates with relevance to AIDS and AIDS research, including chimpanzees and Indian origin rhesus macaques, also display significant levels of genome-wide CNV (Perry et al. 2006; Lee et al. 2008). In 2005, Gonzalez et al. reported an inverse correlation between copy number of CCL3L1, which encodes a ligand for CCR5, and susceptibility to HIV-1 infection (Gonzalez et al. 2005). Interestingly, a similar link between CCL3L1 copy number and disease progression in SIVmac-infected macaques has also been described (Degenhardt et al. 2009). The correlations have intuitive appeal, as ligands of CCR5 have been shown experimentally to inhibit HIV-1 replication (Cocchi et al. 1995; Menten et al. 1999, 2002; Nibbs et al. 1999; Xin et al. 1999). However, the link between CCL3L1 copy number and HIV-1 infection has been called into question by subsequent, independent studies, which have failed to reproduce the correlation (Bhattacharya et al. 2009; Urban et al. 2009). Likewise, after correcting for known influence of the MHC class I and TRIM5 loci on SIV infection, Lim et al. did not find a significant correlation between CCL3L1 copy number and replication of SIVmac in rhesus macaques (Lim et al. 2010b). At present, the original claims remain controversial and additional work may be needed to resolve the discrepancies; the fact that CCL3L1 is copy number variable in rhesus macaques raises the possibility that some issues may be addressable experimentally.
Joint Viral-Host Genome Analysis
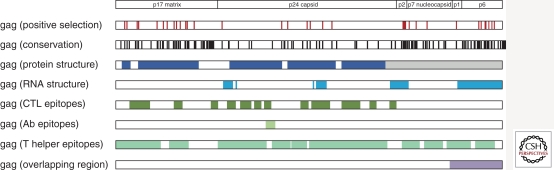
A particularly attractive application of genomics is in the analysis of the reciprocal genetic signals resulting from the interaction between the host and the pathogen. The HIV-1 genome is conducive to such analyses because the expected plasticity and mutability would effectively reflect the genetic signals of escape. However, there are constraints to viral escape, which reflect RNA and protein structural requirements (Watts et al. 2009) that may translate into loss of fitness. Although the HIV-1 genome is considered to be highly variable, 77% of amino acid positions are conserved, whereas 10% of the genome is under positive selection. This class of sites defines critical residues in host-pathogen interaction, whether resulting from CTL or other host-selective pressures. Although half of the sites under positive selection in the HIV-1 genome are mapped to CTL epitopes, there is considerable interest in identifying the nature of the pressures that are driving evolution in nonepitope sites. These may reflect pressure from KIR or host restriction factors. Figure 3 depicts the superposed signals and influences that may account for conservation and variation in the viral genome.
Figure 3.
Multilayer representation of HIV-1 clade B Gag. The various information layers align the sites under positive selective pressure (red), conservation scores (<90% conserved, black), the structured domains at the protein (dark blue), and viral RNA level (light blue) (Watts et al. 2009), the position of CTL (dark green), antibody (light green), and T helper epitopes (turquoise) compiled in the Los Alamos HIV database, and the Gag region overlapping with the viral protease (purple).
Studies of monozygotic twins infected with the same viral strain (Draenert et al. 2006) showed the power of the host genome to control and drive viral diversity. The initial CD8+ T-cell response targeted 17 epitopes, 15 of which were identical in each twin. Three years after infection, 14 of 15 initial responses were still detectable, whereas of four responses that declined in both twins, three showed mutations at the same residue. The antibody responses cross-neutralized the other twin’s virus, with similar changes in the pattern of evolution in the envelope gene. These results indicate a considerable concordance of cellular and humoral immune responses and HIV evolution in the same genetic environment (Draenert et al. 2006). To discover a larger number of host HLA-viral genome mutual associations at the population level, Moore et al. (2002) and Bhattacharya et al. (2007) searched for signatures of selection driven by specific HLA alleles across the viral genome. The challenge here is to expand the analysis to identify all possible driving forces, which may include mechanisms in addition to CTL pressure. Eventually, there is a need to conduct a full discovery effort that considers both the viral and the corresponding host at the genome level.
NEW RESEARCH AREAS
Next-Generation Sequencing
The field of host genomics is facing a change in paradigm (Fellay et al. 2010). GWASs served to understand the role of common variants in HIV-1 disease. However, the extent of variance explained—around 20% of viral load or disease progression—indicates that other factors are yet to be discovered. New technology in genome sequencing now allows the study of rare variation, whether through the capture and sequencing of the whole exome (the 2% of the genome that is protein coding), or increasingly through the deep sequencing of the whole genome or transcriptome (RNA-seq). These sequencing and resequencing tools associated with effective decoding bioinformatic support are also becoming affordable. A number of projects are under way in the HIV field that use next-generation sequencing for the study of extremes of disease (elite controllers vs. rapid progressors), and of unique populations that are resistant to infection (noninfected exposed hemophiliacs). Next-generation sequencing also offers an unprecedented opportunity to jointly analyze cellular and viral transcriptional activity (Lefebvre et al. 2011).
Evolutionary Genomics
The close association of retroviruses with the human, primate, and mammalian genome supports the notion that there has been significant coevolution, which results in signals of selection in both genomes. The availability of complete genomes for dozens of mammals and, as of 2010, nine primates representing all main primate and prosimian groups permits screening for signals of genetic conflict genome-wide. The analyses can extend from the comparative assessment of the coding regions of single individuals, to the analyses of whole genome signatures and the inclusion of multiple individuals. When applied to human diversity, genome evolutionary studies mark regions that likely reflect the impact of pathogens and population bottlenecks. These genes and genetic regions can inform next-generation sequencing projects (King et al. 2010), as analysis of the latter will need to be supported by priors—functional or other—because they test a very large sampling space. That is, genome sequencing generates very large numbers of variants that cannot be prioritized based solely on statistics. A new range of tools are now available for the identification of evolutionary signals during human population diversification (Grossman et al. 2010).
Paleovirology and Protein Reconstruction
The infection of the germline can lead to viral genes becoming inherited as host alleles. A broad range of retroviral and nonretroviral virus groups are now recognized as part of modern genomes (Johnson 2010; Katzourakis et al. 2010). The long-lasting association of retroviral genomes with the mammal genome, as well as exogenous retro/lentiviral infections, could influence contemporary susceptibility to HIV-1 in humans. Paleovirology is a novel field of research that uses genome data to reconstruct extinct viruses and the ancestral state of restriction factors (Emerman et al. 2010). Specific examples include the reconstruction of the core protein of PtERV, a 4-million-year-old endogenous retrovirus identified in the chimpanzee and gorilla genomes (Kaiser et al 2007). The resurrection of this virus could show that the human variant of TRIM5α actively prevented infection by this virus. A second example of reconstruction, this time of 20-million-year-old TRIM5α, suggested that restriction of HIV-1 has decreased during evolution leading to humans (Goldschmidt et al. 2008). Soll et al. investigated two chimpanzee endogenous retrovirus-1 and -2 (CERV1 and CERV2) relatives of modern murine leukemia viruses (MLVs) that are present in the genomes of Old World primates, but absent from the human genome (Soll et al. 2010). Using CERV2 Env-pseudotyped MLV vectors, Soll et al. identified copper transport protein 1 (CTR1) as a receptor that was presumably used by CERV2 during its ancient exogenous replication in primates. CTR1-inactivating mutations may represent an evolutionary barrier to the acquisition of CERV2 resistance in primates. In addition, the reconstruction of the first examples of endogenous lentiviruses, PSIV in lemurs (Gilbert et al. 2009) and RELIK in rabbits (Katzourakis et al. 2007; Keckesova et al. 2009), opens the door to the study of the activity of restriction factors on these ancient elements (Rahm et al. 2011).
Data Integration, Network, and Systems Biology
There is an urgent need to design experiments that will allow the incorporation of multiple types of genomic information with function. An excellent example from immunology is the identification of regulatory networks that control the transcriptional response of mouse primary dendritic cells to toll-like receptor agonists (Amit et al. 2009). This network model identified 24 core regulators and 76 fine tuners that explain how pathogen-sensing pathways achieve specificity. This study established a broadly applicable approach to dissecting the regulatory networks controlling transcriptional responses in mammalian cells. A second paradigmatic use of systems biology is the study of heterogeneity in cell populations during viral infection (Snijder et al. 2009). Much of the variation in viral infection, endocytosis, and membrane lipid composition is determined by the adaptation of cells to their population context. Perturbation screens, combined with quantitative modeling of single cells, revealed the molecular networks that underlie the heterogeneous patterns in cell populations that likely mediate collective behavior. Although developing system and network approaches in in vitro models is reductionist, the next challenge is to set the conditions for HIV-1 systems biology in vivo that can create series of perturbations, implement the iterative acquisition of high throughput data, and model the observed variation.
CONCLUSIONS
The field of host genomics is at a crossroads thanks to the availability of new technology and sequences of human and primate genomes. Some steps have been completed, such as clarifying the role of common variants in HIV viral load and disease progression, and the description of transcription in infected individuals. The next studies will be technology driven, but will also be designed to capture and integrate multiple types of data. These include the study of the genetics of resistance to infection and disease mediated by rare human variants, the use of deep RNA sequencing to improve the quantification of transcriptome changes in specific cell populations, and the capture of the dynamic processes in gene expression. At the postgenome level, there is a need for completing functional analysis of human variants or of candidate regulatory genes identified through the novel approaches. The integration of data and system biology will generate new lists of candidates for such functional studies.
Primate genetics is also progressing, led by the identification of important variants that stratify the susceptibility to infection in the macaque model. Live-attenuated SIV mutants and chimeric viruses (SIV-HIV hybrids, or SHIVs) first revealed the presence of dominant-acting genetic barriers to lentiviral replication in nonhuman primates, some of which have been identified. However, despite more than 25 years of work, cohorts of SIV-infected macaques have yet to be analyzed by large-scale gene association or GWASs.
Understanding of the host genome should inform the study of the viral genome, and vice versa. The plasticity of the viral genome provides several kinds of information: from the structural requirements for viral function, to the faithful reflection of host pressures exerted during cross-species transmission, adaptation to a new host, and spread in a genetically diverse population. Research priorities include the mapping of all sites in the viral genome that are the likely result of host-selective pressure, followed by the identification of the host factors that exert those pressures, and their characterization. A general framework needs to be designed that will address whether the role of the respective host factors is to act as cross-species and interindividual barriers of transmission, to exert control of viral replication in the infected individual, or to limit pathogenicity. Progress in the HIV-1 field will benefit from improved understanding of the evolution of the innate immunity against retroviruses, and this should clarify the genetic conflicts between retrovirus and the host.
ACKNOWLEDGMENTS
Work in the laboratory of A.T. is supported by the Swiss National Science Foundation. Work in the laboratory of W.E.J. is supported by the National Institutes of Health. We thank Jacques Fellay for comments and for Figure 1, and Joke Snoeck for data on Figure 3.
Footnotes
Editors: Frederic D. Bushman, Gary J. Nabel, and Ronald Swanstrom
Additional Perspectives on HIV available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Allen TM, O’Connor DH, Jing P, Dzuris JL, Mothe BR, Vogel TU, Dunphy E, Liebl ME, Emerson C, Wilson N, et al. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407: 386–390 [DOI] [PubMed] [Google Scholar]
- Ambrose Z, KewalRamani VN, Bieniasz PD, Hatziioannou T 2007. HIV/AIDS: In search of an animal model. Trends Biotechnol 25: 333–337 [DOI] [PubMed] [Google Scholar]
- Amit I, Garber M, Chevrier N, Leite AP, Donner Y, Eisenhaure T, Guttman M, Grenier JK, Li W, Zuk O, et al. 2009. Unbiased reconstruction of a mammalian transcriptional network mediating pathogen responses. Science 326: 257–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apetrei C, Robertson DL, Marx PA 2004. The history of SIVS and AIDS: Epidemiology, phylogeny and biology of isolates from naturally SIV infected non-human primates (NHP) in Africa. Front Biosci 9: 225–254 [DOI] [PubMed] [Google Scholar]
- Ballana E, Senserrich J, Pauls E, Faner R, Mercader JM, Uyttebroeck F, Palou E, Mena MP, Grau E, Clotet B, et al. 2010. ZNRD1 (zinc ribbon domain-containing 1) is a host cellular factor that influences HIV-1 replication and disease progression. Clin Infect Dis 50: 1022–1032 [DOI] [PubMed] [Google Scholar]
- Bhattacharya T, Daniels M, Heckerman D, Foley B, Frahm N, Kadie C, Carlson J, Yusim K, McMahon B, Gaschen B, et al. 2007. Founder effects in the assessment of HIV polymorphisms and HLA allele associations. Science 315: 1583–1586 [DOI] [PubMed] [Google Scholar]
- Bhattacharya T, Stanton J, Kim EY, Kunstman KJ, Phair JP, Jacobson LP, Wolinsky SM 2009. CCL3L1 and HIV/AIDS susceptibility. Nat Med 15: 1112–1115 [DOI] [PubMed] [Google Scholar]
- Bosinger SE, Li Q, Gordon SN, Klatt NR, Duan L, Xu L, Francella N, Sidahmed A, Smith AJ, Cramer EM, et al. 2009. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest 119: 3556–3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyington JC, Motyka SA, Schuck P, Brooks AG, Sun PD 2000. Crystal structure of an NK cell immunoglobulin-like receptor in complex with its class I MHC ligand. Nature 405: 537–543 [DOI] [PubMed] [Google Scholar]
- Brass AL, Dykxhoorn DM, Benita Y, Yan N, Engelman A, Xavier RJ, Lieberman J, Elledge SJ 2008. Identification of host proteins required for HIV infection through a functional genomic screen. Science 319: 921–926 [DOI] [PubMed] [Google Scholar]
- Bushman FD, Malani N, Fernandes J, D’Orso I, Cagney G, Diamond TL, Zhou H, Hazuda DJ, Espeseth AS, Konig R, et al. 2009. Host cell factors in HIV replication: Meta-analysis of genome-wide studies. PLoS Pathog 5: e1000437 10.1371/journal.ppat.1000437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington M, Nelson GW, Martin MP, Kissner T, Vlahov D, Goedert JJ, Kaslow R, Buchbinder S, Hoots K, O’Brien SJ 1999. HLA and HIV-1: Heterozygote advantage and B*35-Cw*04 disadvantage. Science 283: 1748–1752 [DOI] [PubMed] [Google Scholar]
- Chan EY, Qian WJ, Diamond DL, Liu T, Gritsenko MA, Monroe ME, Camp DG, Smith RD, Katze MG 2007. Quantitative analysis of human immunodeficiency virus type 1-infected CD4+ cell proteome: Dysregulated cell cycle progression and nuclear transport coincide with robust virus production. J Virol 81: 7571–7583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Kwon D, Jin Z, Monard S, Telfer P, Jones MS, Lu CY, Aguilar RF, Ho DD, Marx PA 1998. Natural infection of a homozygous Δ24 CCR5 red-capped mangabey with an R2b-tropic simian immunodeficiency virus. J Exp Med 188: 2057–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P 1995. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science 270: 1811–1815 [DOI] [PubMed] [Google Scholar]
- Colantonio AD, Bimber BN, Neidermyer WJ, Reeves RK, Alter G, Altfeld M, Johnson RP, Carrington M, O'Connor DH, Evans DT 2011. KIR polymorphisms modulate peptide-dependent binding to an MHC class I ligand with a Bw6 motif. PLoS Pathog 7: e1001316 10.1371/journal.ppat.1001316 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalmasso C, Carpentier W, Meyer L, Rouzioux C, Goujard C, Chaix ML, Lambotte O, Avettand-Fenoel V, Le CS, de Senneville LD, et al. 2008. Distinct genetic loci control plasma HIV-RNA and cellular HIV-DNA levels in HIV-1 infection: The ANRS genome wide association 01 study. PLoS One 3: e3907 10.1371/journal.pone.0003907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel MD, Letvin NL, King NW, Kannagi M, Sehgal PK, Hunt RD, Kanki PJ, Essex M, Desrosiers RC 1985. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science 228: 1201–1204 [DOI] [PubMed] [Google Scholar]
- Dean M, Carrington M, Winkler C, Huttley GA, Smith MW, Allikmets R, Goedert JJ, Buchbinder SP, Vittinghoff E, Gomperts E, et al. 1996. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia growth and development study, multicenter AIDS cohort study, multicenter hemophilia cohort study, San Francisco City cohort, ALIVE study. Science 273: 1856–1862 [DOI] [PubMed] [Google Scholar]
- Degenhardt JD, de Candia P, Chabot A, Schwartz S, Henderson L, Ling B, Hunter M, Jiang Z, Palermo RE, Katze M, et al. 2009. Copy number variation of CCL3-like genes affects rate of progression to simian-AIDS in Rhesus Macaques (Macaca mulatta). PLoS Genet 5: e1000346 10.1371/journal.pgen.1000346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot NG, Heijmans CM, Zoet YM, de Ru AH, Verreck FA, van Veelen PA, Drijfhout JW, Doxiadis GG, Remarque EJ, Doxiadis II, et al. 2010. AIDS-protective HLA-B*27/B*57 and chimpanzee MHC class I molecules target analogous conserved areas of HIV-1/SIVcpz. Proc Natl Acad Sci 107: 15175–15180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draenert R, Allen TM, Liu Y, Wrin T, Chappey C, Verrill CL, Sirera G, Eldridge RL, Lahaie MP, Ruiz L, et al. 2006. Constraints on HIV-1 evolution and immunodominance revealed in monozygotic adult twins infected with the same virus. J Exp Med 203: 529–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerman M, Malik HS 2010. Paleovirology—Modern consequences of ancient viruses. PLoS Biol 8: e1000301 10.1371/journal.pbio.1000301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelou E, Fellay J, Colombo S, Martinez-Picado J, Obel N, Goldstein DB, Telenti A, Ioannidis JP 2011. Impact of phenotype definition on genome-wide association signals: Empirical evaluation in HIV-1 infection. Am J Epidemiol 173: 1336–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DT, O’Connor DH, Jing P, Dzuris JL, Sidney J, da Silva J, Allen TM, Horton H, Venham JE, Rudersdorf RA, et al. 1999. Virus-specific cytotoxic T-lymphocyte responses select for amino-acid variation in simian immunodeficiency virus Env and Nef. Nat Med 5: 1270–1276 [DOI] [PubMed] [Google Scholar]
- Fan QR, Long EO, Wiley DC 2001. Crystal structure of the human natural killer cell inhibitory receptor KIR2DL1-HLA-Cw4 complex. Nat Immunol 2: 452–460 [DOI] [PubMed] [Google Scholar]
- Fellay J, Shianna KV, Ge D, Colombo S, Ledergerber B, Weale M, Zhang K, Gumbs C, Castagna A, Cossarizza A, et al. 2007. A whole-genome association study of major determinants for host control of HIV-1. Science 317: 944–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellay J, Ge D, Shianna KV, Colombo S, Ledergerber B, Cirulli ET, Urban TJ, Zhang K, Gumbs CE, Smith JP, et al. 2009. Common genetic variation and the control of HIV-1 in humans. PLoS Genet 5: e1000791 10.1371/journal.pgen.1000791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellay J, Shianna KV, Telenti A, Goldstein DB 2010. Host genetics and HIV-1: The final phase? PLoS Pathog 6: e1001033 10.1371/journal. ppat.1001033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellay J, Frahm N, Shianna KV, Cirulli ET, Casimiro DR, Robertson MN, Haynes BF, Geraghty DE, McElrath MJ, Goldstein DB 2011. Host genetic determinants of T cell responses to the MRKAd5 HIV-1 gag/pol/nef vaccine in the step trial. J Infect Dis 203: 773–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W, Sanders-Beer BE, Katz KS, Maglott DR, Pruitt KD, Ptak RG 2009. Human immunodeficiency virus type 1, human protein interaction database at NCBI. Nucleic Acids Res 37: D417–D422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner MB 2003. Simian AIDS: An historical perspective. J Med Primatol 32: 180–186 [DOI] [PubMed] [Google Scholar]
- Gilbert C, Maxfield DG, Goodman SM, Feschotte C 2009. Parallel germline infiltration of a lentivirus in two Malagasy lemurs. PLoS Genet 5: e1000425 10.1371/journal.pgen.1000425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri MS, Nebozhyn M, Showe L, Montaner LJ 2006. Microarray data on gene modulation by HIV-1 in immune cells: 2000–2006. J Leukoc Biol 80: 1031–1043 [DOI] [PubMed] [Google Scholar]
- Goldschmidt V, Bleiber G, May M, Martinez R, Ortiz M, Telenti A 2006. Role of common human TRIM5α variants in HIV-1 disease progression. Retrovirology 3: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt V, Ciuffi A, Ortiz M, Brawand D, Munoz M, Kaessmann H, Telenti A 2008. Antiretroviral activity of ancestral TRIM5α. J Virol 82: 2089–2096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S, Brown CR, Dehghani H, Lifson JD, Hirsch VM 2000. Intrinsic susceptibility of rhesus macaque peripheral CD4+ T cells to simian immunodeficiency virus in vitro is predictive of in vivo viral replication. J Virol 74: 9388–9395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez E, Kulkarni H, Bolivar H, Mangano A, Sanchez R, Catano G, Nibbs RJ, Freedman BI, Quinones MP, Bamshad MJ, et al. 2005. The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility. Science 307: 1434–1440 [DOI] [PubMed] [Google Scholar]
- Grossman SR, Shylakhter I, Karlsson EK, Byrne EH, Morales S, Frieden G, Hostetter E, Angelino E, Garber M, Zuk O, et al. 2010. A composite of multiple signals distinguishes causal variants in regions of positive selection. Science 327: 883–886 [DOI] [PubMed] [Google Scholar]
- Hahn BH, Shaw GM, De Cock KM, Sharp PM 2000. AIDS as a zoonosis: Scientific and public health implications. Science 287: 607–614 [DOI] [PubMed] [Google Scholar]
- Hansasuta P, Dong T, Thananchai H, Weekes M, Willberg C, Aldemir H, Rowland-Jones S, Braud VM 2004. Recognition of HLA-A3 and HLA-A11 by KIR3DL2 is peptide-specific. Eur J Immunol 34: 1673–1679 [DOI] [PubMed] [Google Scholar]
- Hatziioannou T, Princiotta M, Piatak M Jr, Yuan F, Zhang F, Lifson JD, Bieniasz PD 2006. Generation of simian-tropic HIV-1 by restriction factor evasion. Science 314: 95. [DOI] [PubMed] [Google Scholar]
- Hatziioannou T, Ambrose Z, Chung NP, Piatak M Jr, Yuan F, Trubey CM, Coalter V, Kiser R, Schneider D, Smedley J, et al. 2009. A macaque model of HIV-1 infection. Proc Natl Acad Sci 106: 4425–4429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbeck JT, Gottlieb GS, Winkler CA, Nelson GW, An P, Maust BS, Wong KG, Troyer JL, Goedert JJ, Kessing BD, et al. 2010. Multistage genomewide association study identifies a locus at 1q41 associated with rate of HIV-1 disease progression to clinical AIDS. J Infect Dis 201: 618–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch V, Adger-Johnson D, Campbell B, Goldstein S, Brown C, Elkins WR, Montefiori DC 1997. A molecularly cloned, pathogenic, neutralization-resistant simian immunodeficiency virus, SIVsmE543–3. J Virol 71: 1608–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Paxton WA, Wolinsky SM, Neumann AU, Zhang L, He T, Kang S, Ceradini D, Jin Z, Yazdanbakhsh K, et al. 1996. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med 2: 1240–1243 [DOI] [PubMed] [Google Scholar]
- Iafrate AJ, Feuk L, Rivera MN, Listewnik ML, Donahoe PK, Qi Y, Scherer SW, Lee C 2004. Detection of large-scale variation in the human genome. Nat Genet 36: 949–951 [DOI] [PubMed] [Google Scholar]
- Igarashi T, Iyengar R, Byrum RA, Buckler-White A, Dewar RL, Buckler CE, Lane HC, Kamada K, Adachi A, Martin MA 2007. Human immunodeficiency virus type 1 derivative with 7% simian immunodeficiency virus genetic content is able to establish infections in pig-tailed macaques. J Virol 81: 11549–11552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International HIV Controllers Study 2010. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 330: 1551–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquelin B, Mayau V, Targat B, Liovat AS, Kunkel D, Petitjean G, Dillies MA, Roques P, Butor C, Silvestri G, et al. 2009. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest 119: 3544–3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javanbakht H, An P, Gold B, Petersen DC, O’Huigin C, Nelson GW, O’Brien SJ, Kirk GD, Detels R, Buchbinder S, et al. 2006. Effects of human TRIM5α polymorphisms on antiretroviral function and susceptibility to human immunodeficiency virus infection. Virology 354: 15–27 [DOI] [PubMed] [Google Scholar]
- Jia B, Serra-Moreno R, Neidermyer W, Rahmberg A, Mackey J, Fofana IB, Johnson WE, Westmoreland S, Evans DT 2009. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog 5: e1000429 10.1371/journal.ppat.1000429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson WE 2010. Endless forms most viral. PLoS Genet 6: e1001210 10.1371/journal.pgen.1001210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser SM, Malik H, Emerman M 2007. Restriction of an extinct retrovirus by the human TRIM5 α antiviral protein. Science 316: 1756–1758 [DOI] [PubMed] [Google Scholar]
- Kamada K, Igarashi T, Martin MA, Khamsri B, Hatcho K, Yamashita T, Fujita M, Uchiyama T, Adachi A 2006. Generation of HIV-1 derivatives that productively infect macaque monkey lymphoid cells. Proc Natl Acad Sci 103: 16959–16964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaslow RA, Carrington M, Apple R, Park L, Munoz A, Saah AJ, Goedert JJ, Winkler C, O'Brien SJ, Rinaldo C, et al. 1996. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. NatMed 2: 405–411 [DOI] [PubMed] [Google Scholar]
- Katzourakis A, Gifford RJ 2010. Endogenous viral elements in animal genomes. PLoS Genet 6: e1001191 10.1371/journal.pgen.1001191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzourakis A, Tristem M, Pybus OG, Gifford RJ 2007. Discovery and analysis of the first endogenous lentivirus. Proc Natl Acad Sci 104: 6261–6265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima Y, Pfafferott K, Frater J, Matthews P, Payne R, Addo M, Gatanaga H, Fujiwara M, Hachiya A, Koizumi H, et al. 2009. Adaptation of HIV-1 to human leukocyte antigen class I. Nature 458: 641–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keckesova Z, Ylinen LM, Towers GJ, Gifford RJ, Katzourakis A 2009. Identification of a RELIK orthologue in the European hare (Lepus europaeus) reveals a minimum age of 12 million years for the lagomorph lentiviruses. Virology 384: 7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CR, Rathouz PJ, Nicolae DL 2010. An evolutionary framework for association testing in resequencing studies. PLoS Genet 6: e1001202 10.1371/journal.pgen.1001202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirmaier A, Wu F, Newman RM, Hall LR, Morgan JS, O’Connor S, Marx PA, Meythaler M, Goldstein S, Buckler-White A, et al. 2010. TRIM5 suppresses cross-species transmission of a primate immunodeficiency virus and selects for emergence of resistant variants in the new species. PLoS Biol 8: e1000462 10.1371/journal.pbio.1000462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Klatt NR, Silvestri G, Hirsch V 2011. Nonpathogenic simian immunodeficiency virus infections. Cold Spring Harb Perspect Med 10.1101/cshperspect.a007153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig R, Zhou Y, Elleder D, Diamond TL, Bonamy GM, Irelan JT, Chiang CY, Tu BP, De Jesus PD, Lilley CE, et al. 2008. Global analysis of host-pathogen interactions that regulate early-stage HIV-1 replication. Cell 135: 49–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni SS, Savan R, Qi Y, Gao X, Yuki Y, Bass SE, Martin MP, Hunt P, Deeks S, Telenti A, et al. 2011. Differential microRNA regulation of HLA-C expression and it association with HIV control. Nature 472: 495–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Clerc S, Limou S, Coulonges C, Carpentier W, Dina C, Taing L, Delaneau O, Labib T, Sladek R, Deveau C, et al. 2009. Genomewide association study of a rapid progression cohort identifies new susceptibility alleles for AIDS (ANRS Genomewide Association Study 03). J Infect Dis 200: 1194–1201 [DOI] [PubMed] [Google Scholar]
- Lederer S, Favre D, Walters KA, Proll S, Kanwar B, Kasakow Z, Baskin CR, Palermo R, McCune JM, Katze MG 2009. Transcriptional profiling in pathogenic and non-pathogenic SIV infections reveals significant distinctions in kinetics and tissue compartmentalization. PLoS Pathog 5: e1000296 10.1371/journal.ppat.1000296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AS, Gutierrez-Arcelus M, Perry GH, Vallender EJ, Johnson WE, Miller GM, Korbel JO, Lee C 2008. Analysis of copy number variation in the rhesus macaque genome identifies candidate loci for evolutionary and human disease studies. Hum Mol Genet 17: 1127–1136 [DOI] [PubMed] [Google Scholar]
- Lefebvre G, Desfarges S, Uyttebroeck F, Muñoz M, Beerenwinkel N, Rougemont J, Telenti A, Ciuffi A 2011. Analysis of HIV-1 expression level and sense of transcription by high-throughput sequencing of the infected cell. J Virol 85: 6205–6211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao CH, Kuang YQ, Liu HL, Zheng YT, Su B 2007. A novel fusion gene, TRIM5-Cyclophilin A in the pig-tailed macaque determines its susceptibility to HIV-1 infection. AIDS 21 (Suppl 8): S19–S26 [DOI] [PubMed] [Google Scholar]
- *.Lifson JD, Haigwood NL 2011. Lessons in nonhuman primate models for AIDS vaccine research: From minefields to milestones. Cold Spring Harb Perspect Med 10.1101/cshperspect.a007310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SY, Rogers T, Chan T, Whitney JB, Kim J, Sodroski J, Letvin NL 2010a. TRIM5α modulates v control in Rhesus Monkeys. PLoS Pathog 6: e1000738 10.1371/journal.ppat.1000738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SY, Chan T, Gelman RS, Whitney JB, O’Brien KL, Barouch DH, Goldstein DB, Haynes BF, Letvin NL 2010b. Contributions of Mamu-A*01 status and TRIM5 allele expression, but not CCL3L copy number variation, to the control of SIVmac251 replication in Indian-origin rhesus monkeys. PLoS Genet 6: e1000997 10.1371/journal.pgen.1000997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limou S, Le Clerc S, Coulonges C, Carpentier W, Dina C, Delaneau O, Labib T, Taing L, Sladek R, Deveau C, et al. 2009. Genomewide association study of an AIDS-nonprogression cohort emphasizes the role played by HLA genes (ANRS Genomewide Association Study 02). J Infect Dis 199: 419–426 [DOI] [PubMed] [Google Scholar]
- Limou S, Coulonges C, Herbeck JT, van Manen D, An P, Le Clerc S, Delaneau O, Diop G, Taing L, Montes M, et al. 2010. Multiple-cohort genetic association study reveals CXCR6 as a new chemokine receptor involved in long-term nonprogression to AIDS. J Infect Dis 202: 908–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R, MacDonald ME, Stuhlmann H, Koup RA, Landau NR 1996. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 86: 367–377 [DOI] [PubMed] [Google Scholar]
- Loeuillet C, Deutsch S, Ciuffi A, Robyr D, Taffe P, Munoz M, Beckmann JS, Antonarakis SE, Telenti A 2008. In vitro whole-genome analysis identifies a susceptibility locus for HIV-1. PLoS Biol 6: e32 10.1371/journal.pbio.0060032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffredo JT, Friedrich TC, Leon EJ, Stephany JJ, Rodrigues DS, Spencer SP, Bean AT, Beal DR, Burwitz BJ, Rudersdorf RA, et al. 2007a. CD8+ T cells from SIV elite controller macaques recognize Mamu-B*08-bound epitopes and select for widespread viral variation. PLoS One 2: e1152 10.1371/journal.pone.0001152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffredo JT, Maxwell J, Qi Y, Glidden CE, Borchardt GJ, Soma T, Bean AT, Beal DR, Wilson NA, Rehrauer WM, et al. 2007b. Mamu-B*08-positive macaques control simian immunodeficiency virus replication. J Virol 81: 8827–8832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi RS, Sickler B, Lin D, Satkoski J, Tito RY, George D, Kanthaswamy S, Smith DG 2007. MamuSNP: A resource for Rhesus Macaque (Macaca mulatta) genomics. PLoS ONE 2: e438 10.1371/journal.pone.0000438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malnati MS, Peruzzi M, Parker KC, Biddison WE, Ciccone E, Moretta A, Long EO 1995. Peptide specificity in the recognition of MHC class I by natural killer cell clones. Science 267: 1016–1018 [DOI] [PubMed] [Google Scholar]
- Mandelboim O, Wilson SB, Vales-Gomez M, Reyburn HT, Strominger JL 1997. Self and viral peptides can initiate lysis by autologous natural killer cells. Proc Natl Acad Sci 94: 4604–4609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield KG, Lerch NW, Gardner MB, Lackner AA 1995. Origins of simian immunodeficiency virus infection in macaques at the New England Regional Primate Research Center. J Med Primatol 24: 116–122 [DOI] [PubMed] [Google Scholar]
- Martin MP, Gao X, Lee JH, Nelson GW, Detels R, Goedert JJ, Buchbinder S, Hoots K, Vlahov D, Trowsdale J, et al. 2002. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet 31: 429–434 [DOI] [PubMed] [Google Scholar]
- Martin MP, Qi Y, Gao X, Yamada E, Martin JN, Pereyra F, Colombo S, Brown EE, Shupert WL, Phair J, et al. 2007. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat Genet 39: 733–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNatt MW, Zang T, Hatziioannou T, Bartlett M, Fofana IB, Johnson WE, Neil SJ, Bieniasz PD 2009. Species-specific activity of HIV-1 Vpu and positive selection of tetherin transmembrane domain variants. PLoS Pathog 5: e1000300 10.1371/journal.ppat.1000300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menten P, Struyf S, Schutyser E, Wuyts A, De Clercq E, Schols D, Proost P, Van Damme J 1999. The LD78β isoform of MIP-1α is the most potent CCR5 agonist and HIV-1-inhibiting chemokine. J Clin Invest 104: R1–R5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menten P, Wuyts A, Van Damme J 2002. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev 13: 455–481 [DOI] [PubMed] [Google Scholar]
- Moore CB, John M, James IR, Christiansen FT, Witt CS, Mallal SA 2002. Evidence of HIV-1 adaptation to HLA-restricted immune responses at a population level. Science 296: 1439–1443 [DOI] [PubMed] [Google Scholar]
- Newman RM, Hall L, Connole M, Chen GL, Sato S, Yuste E, Diehl W, Hunter E, Kaur A, Miller GM, et al. 2006. Balancing selection and the evolution of functional polymorphism in Old World monkey TRIM5α. Proc Natl Acad Sci 103: 19134–19139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen DG, Yin H, Zhou Y, Wolff KC, Kuhen KL, Caldwell JS 2007. Identification of novel therapeutic targets for HIV infection through functional genomic cDNA screening. Virology 362: 16–25 [DOI] [PubMed] [Google Scholar]
- Nibbs RJ, Yang J, Landau NR, Mao JH, Graham GJ 1999. LD78β, a non-allelic variant of human MIP-1α (LD78α), has enhanced receptor interactions and potent HIV suppressive activity. J Biol Chem 274: 17478–17483 [DOI] [PubMed] [Google Scholar]
- Nisole S, Lynch C, Stoye JP, Yap MW 2004. A Trim5-cyclophilin A fusion protein found in owl monkey kidney cells can restrict HIV-1. Proc Natl Acad Sci 101: 13324–13328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor SL, Lhost JJ, Becker EA, Detmer AM, Johnson RC, Macnair CE, Wiseman RW, Karl JA, Greene JM, Burwitz BJ, et al. 2010. MHC heterozygote advantage in simian immunodeficiency virus-infected Mauritian cynomolgus macaques. Sci Trans Med 2: 22ra18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios E, Digilio L, McClure HM, Chen Z, Marx PA, Goldsmith MA, Grant RM 1998. Parallel evolution of CCR5-null phenotypes in humans and in a natural host of simian immunodeficiency virus. Curr Biol 8: 943–946 [DOI] [PubMed] [Google Scholar]
- Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, Szczepanik M, Telenti A, Askenase PW, Compans RW, et al. 2010. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol 11: 1127–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelak K, Goldstein DB, Walley NM, Fellay J, Ge D, Shianna KV, Gumbs C, Gao X, Maia JM, Cronin KD, et al. 2010. Host determinants of HIV-1 control in African Americans. J Infect Dis 201: 1141–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry GH, Tchinda J, McGrath SD, Zhang J, Picker SR, Caceres AM, Iafrate AJ, Tyler-Smith C, Scherer SW, Eichler EE, et al. 2006. Hotspots for copy number variation in chimpanzees and humans. Proc Natl Acad Sci 103: 8006–8011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peruzzi M, Parker KC, Long EO, Malnati MS 1996. Peptide sequence requirements for the recognition of HLA-B*2705 by specific natural killer cells. J Immunol 157: 3350–3356 [PubMed] [Google Scholar]
- Quigley M, Pereyra F, Nilsson B, Porichis F, Fonseca C, Eichbaum Q, Julg B, Jesneck JL, Brosnahan K, Imam S, et al. 2010. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD-1 inhibits T cell function by upregulating BATF. Nat Med 16: 1147–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahm N, Yap M, Snoeck J, Zoete V, Munoz M, Radespiel U, Zimmermann E, Michielin O, Stoye JP, Ciuffi A, et al. 2011. Unique spectrum of activity of prosimian TRIM5α against exogenous and endogenous retroviruses. J Virol 85: 4173–4183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Long EO 1997. The direct binding of a p58 killer cell inhibitory receptor to human histocompatibility leukocyte antigen (HLA)-Cw4 exhibits peptide selectivity. J Exp Med 185: 1523–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddick NE, Hermann EA, Loftin LM, Elliott ST, Wey WC, Cervasi B, Taaffe J, Engram JC, Li B, Else JG, et al. 2010. A novel CCR5 mutation common in sooty mangabeys reveals SIVsmm infection of CCR5-null natural hosts and efficient alternative coreceptor use in vivo. PLoS Pathog 6: e1001064 10.1371/journal.ppat.1001064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringrose JH, Jeeninga RE, Berkhout B, Speijer D 2008. Proteomic studies reveal coordinated changes in T-cell expression patterns upon infection with human immunodeficiency virus type 1. J Virol 82: 4320–4330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland M, Tovanabutra S, deCamp AC, Frahm N, Gilbert PB, Sanders-Buell E, Heath L, Magaret CA, Bose M, Bradfield A, et al. 2011. Genetic impact of vaccination on breakthrough HIV-1 sequences from the STEP trial. Nat Med 17: 366–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner C, Kruse PH, Hermes M, Otto N, Walter L 2011. Rhesus macaque inhibitory and activating KIR3D interact with Mamu-A-encoded ligands. J Immunol 186: 2156–2163 [DOI] [PubMed] [Google Scholar]
- Rotger M, Dang KK, Fellay J, Heinzen EL, Feng S, Descombes P, Shianna KV, Ge D, Gunthard HF, Goldstein DB, et al. 2010. Genome-wide mRNA expression correlates of viral control in CD4+ T-cells from HIV-1-infected individuals. PLoS Pathog 6: e1000781 10.1371/journal.ppat.1000781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotger M, Dalmau J, Rauch A, McLaren P, Bosinger SE, Marttinez R, Sandler NG, Roque A, Liebner J, Battegay M, et al. 2011. Comparative transcriptome analysis of extreme phenotypes of human HIV-1 infection and sooty mangabey and rhesus macaque models of SIV infection. J Clin Invest 121: 2391–2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayah DM, Luban J 2004. Selection for loss of Ref1 activity in human cells releases human immunodeficiency virus type 1 from cyclophilin A dependence during infection. J Virol 78: 12066–12070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, et al. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283: 857–860 [DOI] [PubMed] [Google Scholar]
- Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, Rice CM 2011. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472: 481–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebat J, Lakshmi B, Troge J, Alexander J, Young J, Lundin P, Maner S, Massa H, Walker M, Chi M, et al. 2004. Large-scale copy number polymorphism in the human genome. Science 305: 525–528 [DOI] [PubMed] [Google Scholar]
- Serra-Moreno R, Jia B, Breed M, Alvarez X, Evans DT 2011. Compensatory changes in the cytoplasmic tail of gp41 confer resistance to tetherin/BST-2 in a pathogenic nef-deleted SIV. Cell Host Microbe 9: 46–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D, Bastard K, Guethlein LA, Norman PJ, Yawata N, Yawata M, Pando M, Thananchai H, Dong T, Rowland-Jones S, et al. 2009. Dimorphic motifs in D0 and D1+D2 domains of killer cell Ig-like receptor 3DL1 combine to form receptors with high, moderate, and no avidity for the complex of a peptide derived from HIV and HLA-A*2402. J Immunol 183: 4569–4582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Sharp PM, Hahn BH 2011. Origins of HIV and the AIDs pandemic. Cold Spring Harb Perspect Med 10.1101/cshperspect.a006841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestri G 2010. Pathogenic vs. nonpathogenic retrovirus infections. Presented at the Conference on Retroviruses and Opportunistic Infections. San Francisco, February 16–19, 2010 [Google Scholar]
- Snijder B, Sacher R, Ramo P, Damm EM, Liberali P, Pelkmans L 2009. Population context determines cell-to-cell variability in endocytosis and virus infection. Nature 461: 520–523 [DOI] [PubMed] [Google Scholar]
- Soll SJ, Neil SJ, Bieniasz PD 2010. Identification of a receptor for an extinct virus. Proc Natl Acad Sci 107: 19496–19501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speelmon EC, Livingston-Rosanoff D, Li SS, Vu Q, Bui J, Geraghty DE, Zhao LP, McElrath MJ 2006. Genetic association of the antiviral restriction factor TRIM5α with human immunodeficiency virus type 1 infection. J Virol 80: 2463–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J 2004. The cytoplasmic body component TRIM5α restricts HIV-1 infection in Old World monkeys. Nature 427: 848–853 [DOI] [PubMed] [Google Scholar]
- Tang J, Costello C, Keet IP, Rivers C, Leblanc S, Karita E, Allen S, Kaslow RA 1999. HLA class I homozygosity accelerates disease progression in human immunodeficiency virus type 1 infection. AIDS Res Hum Retroviruses 15: 317–324 [DOI] [PubMed] [Google Scholar]
- Telenti A 2009. HIV-1 host interactions—Integration of large scale datasets. F1000 Biol Rep 1: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telenti A, Goldstein DB 2006. Genomics meets HIV. Nat Rev Microbiol 4: 9–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thananchai H, Gillespie G, Martin MP, Bashirova A, Yawata N, Yawata M, Easterbrook P, McVicar DW, Maenaka K, Parham P, et al. 2007. Cutting edge: Allele-specific and peptide-dependent interactions between KIR3DL1 and HLA-A and HLA-B. J Immunol 178: 33–37 [DOI] [PubMed] [Google Scholar]
- Thomas R, Apps R, Qi Y, Gao X, Male V, O’hUigin C, O’Connor G, Ge D, Fellay J, Martin JN, et al. 2009. HLA-C cell surface expression and control of HIV/AIDS correlate with a variant upstream of HLA-C. Nat Genet 41: 1290–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban TJ, Weintrob AC, Fellay J, Colombo S, Shianna KV, Gumbs C, Rotger M, Pelak K, Dang KK, Detels R, et al. 2009. CCL3L1 and HIV/AIDS susceptibility. Nat Med 15: 1110–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneault F, Woods M, Buzon MJ, Li C, Pereyra F, Crosby SD, Rychert J, Church G, Martinez-Picado J, Rosenberg ES, et al. 2011. Transcriptional profiling of CD4 T cells identifiesd subgroups of HIV-1 elite controllers. J Virol 85: 3015–3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts JM, Dang KK, Gorelick RJ, Leonard CW, Bess JW Jr, Swanstrom R, Burch CL, Weeks KM 2009. Architecture and secondary structure of an entire HIV-1 RNA genome. Nature 460: 711–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler A, May GE, Qi Y, Wilson N, Watkins DI 2006. Polymorphisms in eight host genes associated with control of HIV replication do not mediate elite control of viral replication in SIV-infected Indian rhesus macaques. Immunogenetics 58: 1003–1009 [DOI] [PubMed] [Google Scholar]
- Wilson SJ, Webb BL, Maplanka C, Newman RM, Verschoor EJ, Heeney JL, Towers GJ 2008. Rhesus macaque TRIM5 alleles have divergent antiretroviral specificities. J Virol 82: 7243–7247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe ND, Switzer WM, Carr JK, Bhullar VB, Shanmugam V, Tamoufe U, Prosser AT, Torimiro JN, Wright A, Mpoudi-Ngole E, et al. 2004. Naturally acquired simian retrovirus infections in central African hunters. Lancet 363: 932–937 [DOI] [PubMed] [Google Scholar]
- Xin X, Shioda T, Kato A, Liu H, Sakai Y, Nagai Y 1999. Enhanced anti-HIV-1 activity of CC-chemokine LD78β, a non-allelic variant of MIP-1α/LD78α. FEBS Lett 457: 219–222 [DOI] [PubMed] [Google Scholar]
- Yant LJ, Friedrich TC, Johnson RC, May GE, Maness NJ, Enz AM, Lifson JD, O’Connor DH, Carrington M, Watkins DI 2006. The high-frequency major histocompatibility complex class I allele Mamu-B*17 is associated with control of simian immunodeficiency virus SIVmac239 replication. J Virol 80: 5074–5077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung ML, Houzet L, Yedavalli VS, Jeang KT 2009. A genome-wide short hairpin RNA screening of jurkat T-cells for human proteins contributing to productive HIV-1 replication. J Biol Chem 284: 19463–19473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon W, Ma BJ, Fellay J, Huang W, Xia SM, Zhang R, Shianna KV, Liao HX, Haynes BF, Goldstein DB 2010. A polymorphism in the HCP5 gene associated with HLA-B*5701 does not restrict HIV-1 in vitro. AIDS 24: 155–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappacosta F, Borrego F, Brooks AG, Parker KC, Coligan JE 1997. Peptides isolated from HLA-Cw*0304 confer different degrees of protection from natural killer cell-mediated lysis. Proc Natl Acad Sci 94: 6313–6318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wilson SJ, Landford WC, Virgen B, Gregory D, Johnson MC, Munch J, Kirchhoff F, Bieniasz PD, Hatziioannou T 2009. Nef proteins from simian immunodeficiency viruses are tetherin antagonists. Cell Host Microbe 6: 54–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Xu M, Huang Q, Gates AT, Zhang XD, Castle JC, Stec E, Ferrer M, Strulovici B, Hazuda DJ, et al. 2008. Genome-scale RNAi screen for host factors required for HIV replication. Cell Host Microbe 4: 495–504 [DOI] [PubMed] [Google Scholar]