Ceriops tagal commonly occurs along the Gujarat coast of India. It has evolved a high degree of salinity tolerance and optimal growth occurs at 12.6 ppt. This is related to an adaptive regulation of hydration and ionic content.
Abstract
Background and aims
Mangroves of Western Gujarat (India) are subject to die-back. Salinity intolerance is one possible cause, especially in young plants. We therefore quantified the extent to which young plants of one widely occurring mangrove species (Ceriops tagal) tolerate high salt in terms of establishment, growth, water status, proline content and mineral accumulation.
Methodology
In a greenhouse study, juvenile plants were established from mature propagules over 40 days in soil containing added NaCl, raising soil water salinity to 0.2, 2.5, 5.1, 7.7, 10.3, 12.6, 15.4, 17.9, 20.5 and 23.0 ppt (w/v). Growth and physiological characteristics were monitored over the subsequent 6 months.
Principal results
Despite a negative relationship between the percentage of young plant establishment and salt concentration (50 % loss at 22.3 ppt), the remaining plants proved highly tolerant. Growth, in dry weight, was significantly promoted by low salinity, which is optimal at 12.6 ppt. Water content, leaf expansion and dry matter accumulation in tissues followed a similar optimum curve with leaf area being doubled at 12.6 ppt NaCl. Salinity >12.6 and <23 ppt inhibited plant growth, but never to below control levels. Root:shoot dry weight ratios were slightly reduced by salinity (maximum 19 %), but the water potential of roots, leaves and stems became more negative as salinity increases while proline increases in all tissues. The concentration of Na increased, whereas concentrations of K, Ca, N and P decreased and that of Mg remained stable.
Conclusions
Ceriops tagal has a remarkably high degree of salinity tolerance, and shows an optimal growth when soil water salinity is 12.6 ppt. Salinity tolerance is linked to an adaptive regulation of hydration and ionic content. The cause of localized die-back along the coastal region of Gujarat is thus unlikely to be a primary outcome of salinity stress although amendments with Ca and K, and perhaps proline, may help protect against extreme salinity.
Introduction
Mangroves are woody plants that inhabit intertidal zones with high salinity (Shan et al., 2008; Parida and Jha, 2010) and can tolerate a wide range of salinities under natural conditions (Suarez et al., 1998). In nature, the growth and physiological tolerance mechanisms of mangroves vary due to a complex physical structure and differences in flooding regime, tidal inundation, rapid influx of extra nutrients and soil type (Clough, 1984; Naidoo, 1987). Mangroves display a range of adaptive responses to their specific habitat, including salt exclusion by root ultrafiltration (Scholander, 1968), salt secretion (elimination of substances not metabolically changed) via glands (Roth, 1992), ion accumulation in leaf cells (Popp, 1994), leaf succulence (Roth, 1992) and accumulation of organic acids as osmotica to counter toxic effects of salinity (Popp, 1984). Like other halophytes, mangroves decrease their water and osmotic potentials to maintain leaf turgor at high salinity (Naidoo, 1987; Khan et al., 2000a,b). The level of salinity required for optimal growth varies from 10 to 50 % seawater (Downton, 1982; Clough, 1984; Naidoo, 1987; Lin and Sternberg, 1992, 1995; Ball and Pidsley, 1995; Patel NT et al., 2010), and a decline in growth occurs with a further increase in salinity. Lowered water potentials and an associated accumulation of inorganic ions are common patterns observed in mangrove plants in extreme saline habitats (Ball and Farquhar, 1984; Naidoo, 1987; Patel NT et al., 2010) and are seen as a mechanism for maintaining turgor.
Mangroves are common along the coasts of Western Gujarat (India), but the stands are repeatedly decimated and fragmented (Patel, 2009). Little information exists about the salt tolerance of mangroves of the coastal region of Gujarat, and this information is crucial for the success of mangrove restoration efforts in that region. Ceriops tagal is one of the most common species in the coastal region of Gujarat. Its range extends from the semi-arid (near-arid) region of Saurashtra to the saline desert of Kutch, adjacent to the border of Pakistan. Ball (1988) reported that C. tagal is a salt-tolerant mangrove with the competitive ability to grow in highly saline and poorly inundated locations. Aziz and Khan (2001) and Khan and Aziz (2001) reported that C. tagal from Pakistan showed an optimal growth at 50 % seawater salinity. It is assumed that mangroves growing along the coasts of Western Gujarat have tolerance to arid conditions and high salinities (Patel, 2009). Thus, the present study investigated the tolerance of C. tagal of Gujarat at increasing salinity levels, by measuring plant establishment from propagules, juvenile plant growth, and water and mineral status. Such studies have been lacking up to now for mangroves of arid regions.
Materials and methods
Study area
The study was performed in a greenhouse of the botanical garden of Saurashtra University at Rajkot (22°18′N latitude, 70°56′E longitude), Gujarat, India. For propagule establishment and growth of young plants, the top 15 cm of black-cotton soil (vertisol), which is predominant in the Saurashtra region of Gujarat, was collected from an agricultural field near Saurashtra University. This soil is a clayey loam, containing 19.6 % sand, 20.3 % silt and 60.1 % clay by weight of dry soil. The available soil water between wilting coefficient and field capacity ranged from 18.3 to 35.0 %. The total organic carbon content was 1.3 % and pH was 7.2. The salinity of the soil was 0.2 ppt (w/v of soil water). Nitrogen, phosphorus, potassium, calcium and sodium concentrations were 0.15, 0.05, 0.03, 0.05 and 0.002 % by weight of dry soil, respectively. This soil is fertile and used for intensive agriculture. Physical and chemical properties of the soil have been reported earlier (Pandya et al., 2004).
Salinization of soil
Surface soil was collected, air dried and passed through a 2-mm mesh. Twelve samples of soil, each of 100 kg, were separately spread, about 50 mm thick, over polyethylene sheets. Sodium chloride (NaCl) amounting to 0, 280, 690, 1410, 1900, 2400, 2900, 3300, 3800, 4200, 4700 and 5170 g was then allocated to each sample and thoroughly mixed to give interstitial soil water salinities of 0.2, 2.5, 5.1, 7.7, 10.3, 12.6, 15.4, 17.9, 20.5, 23.0, 25.6 and 28.2 ppt, measured by electrical conductivity in a soil suspension prepared in distilled water with a 1:2 soil:water ratio following Patel AD et al. (2010). The suspension was shaken and allowed to stand overnight. Thereafter, the electrical conductivity of the supernatant solution was determined with a conductivity meter (Systronics, Model No. 304, India). The salinity of untreated control soil was 0.2 ppt. Seawater salinity at Jamnagar coast in Saurashtra varies from 28.8 to 30.7 ppt during the rainy (monsoon) season, which favours the establishment of propagules. Thus, soil salinity in the present investigation was not imposed above 28.2 ppt.
Plant material
Propagules (turions) of C. tagal develop on branches of the plants. Mature propagules were collected on 14 December 2007 from the Jamnagar coast (22°27′N latitude, 70°07′E longitude) of the Saurashtra region of Western Gujarat with the help of Gujarat Forest Department. Mature propagules were yellowish green, approx. 6.5 g (fresh weight), 17–20 cm long and 0.8–1.0 cm wide. Young propagules are brighter green, and were avoided because of poor germination.
Plant establishment
For each level of soil salinity, 20 polyethylene bags (20.5 cm wide and 41 cm long) were each filled with 5 kg of soil. About 2.5 L of tap water were added to the soil in each bag until the water level was 2 cm above the soil surface. Bags were kept in an uncontrolled greenhouse under natural temperature and light. Ten propagules were planted (propagules were inserted up to one-third of their length into the soil) in each bag on 15 December 2007. Tap water was added daily to compensate for evapotranspiration loss. Plant establishment was recorded daily over 40 days, establishment being defined as the date when the first pair of leaves unfurled. A linear model was fitted to the cumulative proportion of established plants and the increasing soil salinity using the expression:
where  denotes the proportion of cumulative established plants, X is soil salinity and β0 and β1 are coefficients of linear regression. The salt concentration at which plant establishment was reduced to 50 % (SE50) was estimated using the model.
denotes the proportion of cumulative established plants, X is soil salinity and β0 and β1 are coefficients of linear regression. The salt concentration at which plant establishment was reduced to 50 % (SE50) was estimated using the model.
Plant growth
The two plants that established first were left in each bag, the others being removed as they appeared. At high salinities, young plants opened their first pair of leaves between 9 and 30 d later than controls. The full opening of the first pair of leaves defined the establishment of young plants. No further experiments were conducted on plants grown in soils at the two highest salinities (25.6 and 28.2 ppt) because (i) mortality of established plants did not occur at all salinity levels and (ii) Khan and Aziz (2001) and Aziz and Khan (2001) found that plant dry weight of C. tagal was maximum in 50 % seawater and higher salinity (100 % seawater) was without effect compared to controls. At the two highest salinities, established plants were therefore expected to grow similarly to control plants. Following 40 days for establishment, the smaller of the two plants was removed, leaving the more vigorous of the pair for further study. Thus, 20 single replicate plants were factorialized with 10 grades of soil salinity (0.2, 2.5, 5.1, 7.7, 10.3, 12.6, 15.4, 17.9, 20.5 and 23.0 ppt). The 200 growth bags were arranged in 20 completely randomized blocks. Plants were watered daily to maintain the water level above the soil surface and the experiment was terminated after 6 months. The mean daily maximum temperature of the greenhouse decreased from 29.5 ± 0.6 °C in December 2007 to 28.1 ± 0.4 °C in February 2008, and increased thereafter to 40.7 ± 0.4 °C in May 2008. The mean maximum temperature then decreased to 37.6 ± 0.3 °C until final harvesting on 15 June 2008. Plants were then washed with tap water to remove soil particles and morphological characteristics of each plant recorded, including shoot height, root length (as length of longest root) and leaf area (using outline tracings on graph paper). Fresh and dry weights of leaves, stems and roots were then determined. Water content (%) was calculated from fresh and dry weights. Data recorded for morphological characteristics, dry weight and water content were analysed by multivariate analysis of variance (MANOVA), using SYSTAT version 12 (Badashah and Nath, 2007) to assess the effect of salinity levels on plant growth variables considered simultaneously. A second-order polynomial model was fitted to the data for the examined parameters and increasing soil salinity using the expression:
where Y is the examined parameters, X is soil salinity, α is the Y-intercept, and β1 and β2 are constants.
Determination of water potential and proline content
Ten additional plants grown in the soil at each level of salinity were used for water potential and proline determinations 15 days before the termination of the growth experiment. Water potential of leaves, stems and roots was measured using a Dewpoint Potential Meter WP4 (Decagon Devices, Inc., USA) following Patel AD et al. (2010). All the measurements were taken between 7.30 and 10.00 a.m. Proline concentrations were estimated following Bates et al. (1973), using an extract of 0.5 g of fresh plant material in aqueous sulfosalicylic acid. The extracted proline was reacted with ninhydrin to form a chromophore read at 520 nm absorbance. Data were analysed by one-way ANOVA and linear regression.
Mineral analyses of plant materials
Mineral analyses were performed on leaves, stems and root tissues. Plant parts were pooled into one composite sample per salinity level separately. Plant samples were ground using a mortar and pestle, and three subsamples were analysed. Total nitrogen was determined by the Kjeldahl method and phosphorus content was estimated by the chlorostannous molybdophosphoric blue colour method in sulphuric acid (Piper, 1944). Concentrations of Ca, Mg, Na and K were determined by Shimadzu double-beam atomic absorption spectrophotometer AA-6800 (Shimadzu Corporation, Kyoto, Japan) after tri-acid (HNO3:H2SO4:HClO4 at a ratio of 10:1:4) digestion. Mineral data (mg g−1 dry weight) were analysed by one-way ANOVA and linear regression.
Results
Effect of salinity on plant establishment
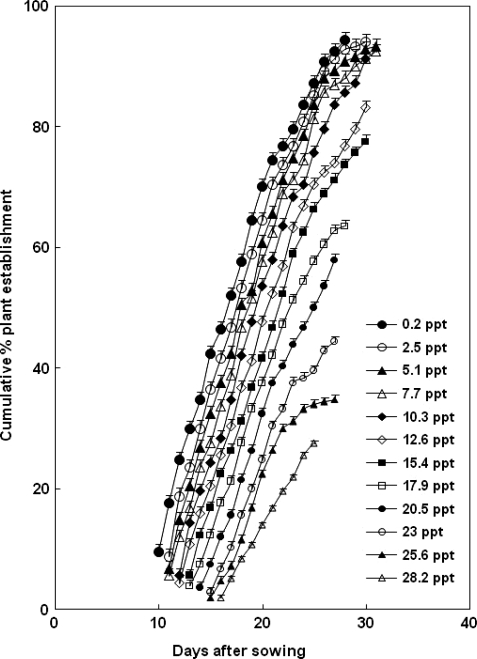
Under non-saline control conditions (0.2 ppt salinity), the first pair of leaves started to open 9 days after planting and 94.4 % of the propagules were established (i.e. showed signs of leaf opening) over 28 days (Fig. 1). Under saline treatments, the appearance of the first pair of unfurled leaves on propagules did not occur until 1–6 days later, with higher salinities causing longer delays. Leaves continued to unfurl for up to 31 days at 5.1 and 7.7 ppt salinity, and for at least 25 days at 28.2 ppt. Total propagule establishment decreased from 94.0 % at 2.5 ppt to 27.6 % at 28.2 ppt. There was a significant reduction in the percentage of plant establishment with increasing soil salinity according to the following expression: Y = 107.270 − 2.561X (Radj2 = 0.907, P < 0.001), where Y is arcsine (degrees) of proportion of cumulative plant establishment and X is salt concentration.
Fig. 1.
Cumulative establishment of propagules of C. tagal over 40 days after planting in response to increasing soil salinity. Error bars represent the SE, n = 200. Units of salinity are those measured in the soil water. The salinity of soil not receiving NaCl addition was 0.2 ppt. Percentage establishment indicates the percentage of 10 propagules (planted in each bag) that produced open leaves.
Total dry weight (biomass) of plants
The MANOVA showed a low value of Wilk's lambda (e.g. 0.08 with an associated d.f. of 90, 1237), while a high value for each of Pillai Trace (e.g. 1.47 with an associated d.f. of 90, 1701) and Hotelling–Lawley Trace (e.g. 6.24 with an associated d.f. of 90, 1613) demonstrated a statistically significant (P < 0.001) effect of salinity on the examined growth variables when considered simultaneously. Salinity significantly (P < 0.001) promoted the total dry weight (shoots + roots; Fig. 2). Total dry weight increased up to 12.6 ppt salinity, but declined with further increases in salinity although dry weights did not fall below control values even at the highest salt concentration (23.0 ppt). The optimum dry weights were at moderate salinities (5.1–12.6 ppt). There was a quadratic relationship between the salt concentration in soil and the dry weight of the whole plant (Y = 2551.30 + 117.25X − 4.83X2, r2 = 0.291, P < 0.001).
Fig. 2.
Effect of increasing soil salinity on total dry weight of C. tagal plants after 6 months growth from propagules. A fitted quadratic polynomial curve (Y = 2243.60 + 354.02X − 30.89X2, r2 = 0.858, P < 0.001) is shown. The data points show treatment means ± SE. n = 20. The large bar represents LSD at P < 0.05.
Stem and root elongation, and leaf expansion
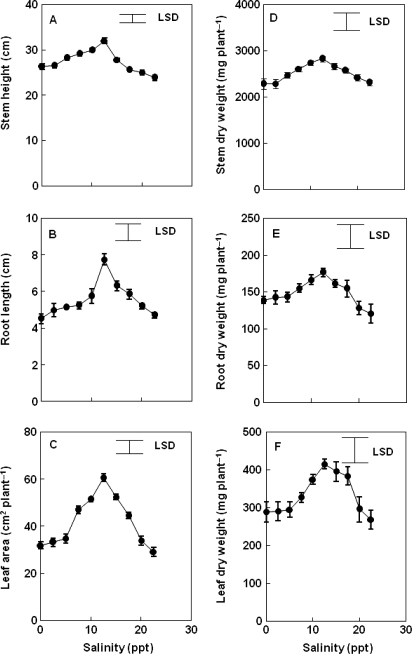
Salinity significantly stimulated (P < 0.001) stem and root elongation, although final lengths declined at concentrations over 12.6 ppt to levels similar to those of controls (Fig. 3A and B). Actual stem heights were considerably greater than root lengths under all treatments. There was a quadratic relationship between shoot height (r2 = 0.412, P < 0.001) or root length (r2 = 0.227, P < 0.001) and salt concentration. A positive relationship was obtained between stem height (r2 = 0.176, P < 0.001) or root length (r2 = 0.107, P < 0.001) and total dry weight of plants. Leaf area was doubled by increasing salinity to 12.6 ppt (P < 0.001) but was decreased by higher salinities, although never to below that of control plants (Fig. 3C). A quadratic relationship was obtained between leaf area and salt concentration in soil (r2 = 0.537, P < 0.001). There was a positive relationship between leaf area and total dry weight of plants (r2 = 0.227, P < 0.001).
Fig. 3.
Effect of increasing soil salinity on size of C. tagal plants after 6 months growth from propagules. (A) Stem height, (B) length of longest root, (C) leaf area, (D) stem dry weight, (E) root dry weight and (F) leaf dry weight. The large bar represents LSD at P ≤ 0.05. Bars on symbols represent the SE, n = 20. Some error bars are smaller than the symbols.
Dry weight of tissues
The dry weight of leaves, stems, shoots (leaves + stems) and roots was significantly promoted (P < 0.001) by salinity up to 12.6 ppt, but, at higher salinity, declined to values similar to those of controls (Figs 3D–F and 4A). There was a quadratic relationship between the salt concentration and dry weight (r2 = 0.146, 0.229, 0.278, 0.141, P < 0.001) for leaves, stems, shoots and roots, respectively. A positive relationship was obtained for dry weight of leaves (r2 = 0.340, P < 0.001), stems (r2 = 0.921, P < 0.001) and roots (r2 = 0.104, P < 0.001) with total dry weight of plants. The root:shoot dry weight ratio was 0.06 for plants grown in control soil (Fig. 4B) and did not change with increasing soil salinity.
Fig. 4.
Effect of increasing soil salinity on (A) dry weight of shoot (leaf + stem) and (B) root:shoot dry weight ratios of C. tagal plants after 6 months growth from propagules. The large bar represents the LSD at P ≤ 0.05. Effect of salinity on root:shoot dry weight ratio was not statistically significant. Bars on symbols represent the SE, n = 20. Some error bars are smaller than the symbols.
Water content and water potential of tissues
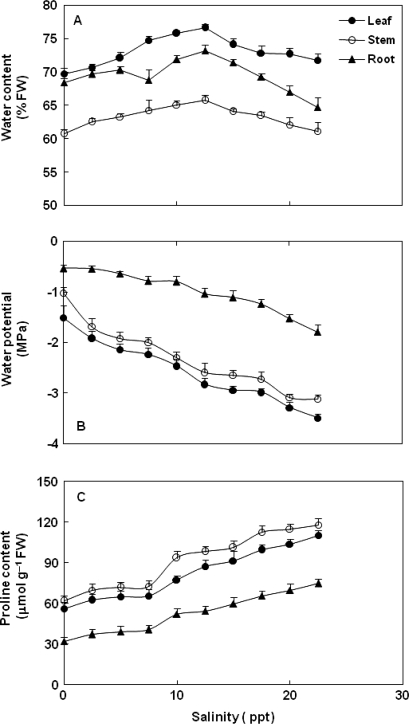
Water content (%) increased significantly (P < 0.001) in leaves, stems and roots up to 12.6 ppt salinity, but declined with further increases in salinity while always remaining above the control values (Fig. 5A). A quadratic relationship was obtained between soil salinity and water content of leaves (r2 = 0.219, P < 0.001), stems (r2 = 0.211, P < 0.001) and roots (r2 = 0.128, P < 0.001). Tissues differed significantly (P < 0.001) in their water content. Moreover, the maximum water content was in leaves and the minimum in stems. Water potentials became more negative (P < 0.001) as soil salinity increased (Fig. 5B), but never dropped below ∼3.4 MPa. There was a negative relationship between soil salinity and the water potential of leaves (r2 = 0.891, P < 0.001), stems (r2 = 0.869, P < 0.001) and roots (r2 = 0.824, P < 0.001). Tissues differed (P < 0.001) in their water potential, with leaves the most negative and roots the least.
Fig. 5.
Effect of increasing soil salinity on (A) water content, (B) water potential and (C) proline content of C. tagal plants after 6 months growth from propagules. Water content was determined at final harvest. Water potential and proline concentrations were measured 15 days before harvest. Error bars represent the SE, n = 20 for water content and n = 5 for water potential and proline content.
Proline content of tissues
Proline concentrations (on a fresh weight basis) increased (P < 0.001) in leaves, stems and root tissues linearly with soil salinity (Fig. 5C). Stems contained slightly more proline than leaves, with roots containing about half the concentration of leaves. There was a positive relationship between salt and proline concentrations of leaves (r2 = 0.871, P < 0.001), stems (r2 = 0.863, P < 0.001) or roots (r2 = 0.850, P < 0.001). A significant inverse relationship was obtained between water potential (more negative values) and the proline content of leaves (r2 = 0.865, P < 0.001), stems (r2 = 0.811, P < 0.001) and roots (r2 = 0.748, P < 0.001).
Mineral accumulation
The concentration of Na on a dry weight basis was greater than that of K, P, Ca and Mg in all tissues of plants under both control and saline conditions (Table 1). Sodium increased significantly (P < 0.001) in leaves, stems and roots with increased soil salinity, giving a statistically significant positive relationship (P < 0.01). K in leaves, stems and roots decreased (P < 0.01) with increasing salinity, giving a negative relationship between tissue K and salt in the soil (P < 0.01). The Na:K ratio increased (P < 0.001) with salinity in all three tissues. There was a positive relationship between the Na:K ratio in tissues and salt concentration (P < 0.01).
Table 1.
Effect of soil salinity on nutrient concentration of tissues (leaf, stem and root) of C. tagal as indicated by mean ± SEM and estimated linear regression coefficients
| Tissue | Salinity (ppt) | N (mg g−1 d.m.) | P (mg g−1 d.m.) | K (mg g−1 d.m.) | Na (mg g−1 d.m.) | Ca (mg g−1 d.m.) | Mg (mg g−1 d.m.) | Na:K ratio | Ca:Na ratio |
|---|---|---|---|---|---|---|---|---|---|
| Leaf | 0.2 | 25.0 ± 1.5 | 2.3 ± 0.1 | 6.9 ± 0.3 | 5.9 ± 0.9 | 5.1 ± 0.3 | 1.5 ± 0.0 | 0.9 ± 0.1 | 0.9 ± 0.0 |
| 2.5 | 24.0 ± 1.3 | 2.2 ± 0.1 | 6.6 ± 0.1 | 6.0 ± 0.8 | 5.1 ± 0.1 | 1.5 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | |
| 5.1 | 23.0 ± 1.2 | 2.2 ± 0.1 | 6.5 ± 0.3 | 9.0 ± 0.6 | 4.9 ± 0.4 | 1.4 ± 0.0 | 1.4 ± 0.1 | 0.5 ± 0.0 | |
| 7.7 | 22.0 ± 1.7 | 2.2 ± 0.1 | 6.5 ± 0.3 | 10.2 ± 0.8 | 4.7 ± 0.1 | 1.4 ± 0.0 | 1.6 ± 0.0 | 0.5 ± 0.0 | |
| 10.3 | 22.0 ± 1.2 | 2.2 ± 0.2 | 6.3 ± 0.2 | 10.9 ± 0.9 | 4.6 ± 0.2 | 1.4 ± 0.0 | 1.7 ± 0.1 | 0.4 ± 0.0 | |
| 12.6 | 20.0 ± 1.7 | 2.1 ± 0.2 | 6.1 ± 0.3 | 11.3 ± 1.1 | 4.5 ± 0.1 | 1.4 ± 0.0 | 1.8 ± 0.0 | 0.4 ± 0.0 | |
| 15.4 | 20.0 ± 1.2 | 1.8 ± 0.1 | 6.0 ± 0.1 | 11.6 ± 0.6 | 4.3 ± 0.2 | 1.3 ± 0.1 | 1.9 ± 0.1 | 0.4 ± 0.0 | |
| 17.9 | 19.0 ± 1.2 | 1.7 ± 0.2 | 5.8 ± 0.2 | 11.6 ± 0.9 | 4.1 ± 0.2 | 1.3 ± 0.0 | 2.0 ± 0.2 | 0.4 ± 0.0 | |
| 20.5 | 18.0 ± 1.0 | 1.5 ± 0.2 | 5.6 ± 0.2 | 12.1 ± 0.8 | 4.1 ± 0.2 | 1.3 ± 0.1 | 2.2 ± 0.1 | 0.3 ± 0.0 | |
| 23 | 18.0 ± 0.7 | 1.5 ± 0.1 | 5.6 ± 0.1 | 12.5 ± 0.3 | 4.0 ± 0.1 | 1.3 ± 0.0 | 2.3 ± 0.1 | 0.3 ± 0.0 | |
| α | 24.712 | 2.38 | 6.847 | 6.838 | 5.136 | NS | 0.971 | 0.763 | |
| β | −0.202 | −0.023 | −0.037 | 0.284 | −0.034 | NS | 0.039 | −0.023 | |
| r2 | 0.601 | 0.609 | 0.614 | 0.77 | 0.624 | NS | 0.852 | 0.721 | |
| F-value | 3.569a | 4.958a | 3.697a | 27.393b | 3.965a | NS | 24.109b | 42.803b | |
| LSD0.05 | 4 | 0.4 | 0.7 | 1.4 | 0.6 | NS | 0.3 | 0.1 | |
| Stem | 0.2 | 23.0 ± 1.0 | 2.0 ± 0.1 | 4.4 ± 0.1 | 9.9 ± 0.4 | 4.7 ± 0.1 | 1.3 ± 0.0 | 2.3 ± 0.1 | 0.5 ± 0.0 |
| 2.5 | 22.0 ± 1.3 | 2.0 ± 0.3 | 4.4 ± 0.1 | 10.5 ± 0.2 | 4.7 ± 0.1 | 1.3 ± 0.0 | 2.4 ± 0.2 | 0.4 ± 0.0 | |
| 5.1 | 21.0 ± 1.0 | 2.0 ± 0.2 | 4.3 ± 0.0 | 10.9 ± 0.2 | 4.5 ± 0.2 | 1.3 ± 0.0 | 2.5 ± 0.1 | 0.4 ± 0.0 | |
| 7.7 | 21.0 ± 0.9 | 1.9 ± 0.2 | 4.3 ± 0.1 | 11.1 ± 0.1 | 4.4 ± 0.1 | 1.3 ± 0.0 | 2.6 ± 0.2 | 0.4 ± 0.0 | |
| 10.3 | 20.0 ± 1.2 | 1.9 ± 0.1 | 4.3 ± 0.1 | 11.5 ± 0.3 | 4.2 ± 0.1 | 1.3 ± 0.0 | 2.7 ± 0.2 | 0.4 ± 0.0 | |
| 12.6 | 20.0 ± 0.6 | 1.9 ± 0.1 | 4.3 ± 0.0 | 11.7 ± 0.2 | 4.2 ± 0.1 | 1.3 ± 0.1 | 2.7 ± 0.1 | 0.4 ± 0.0 | |
| 15.4 | 19.0 ± 1.1 | 1.7 ± 0.1 | 4.2 ± 0.1 | 11.8 ± 0.2 | 4.1 ± 0.3 | 1.2 ± 0.1 | 2.8 ± 0.1 | 0.3 ± 0.0 | |
| 17.9 | 18.0 ± 1.1 | 1.4 ± 0.2 | 4.1 ± 0.1 | 12.1 ± 0.1 | 4.0 ± 0.1 | 1.2 ± 0.1 | 2.9 ± 0.1 | 0.3 ± 0.0 | |
| 20.5 | 18.0 ± 0.9 | 1.3 ± 0.1 | 4.0 ± 0.1 | 12.4 ± 0.3 | 3.9 ± 0.1 | 1.2 ± 0.1 | 3.1 ± 0.1 | 0.3 ± 0.0 | |
| 23 | 17.0 ± 1.0 | 1.3 ± 0.1 | 3.9 ± 0.1 | 12.6 ± 0.1 | 3.9 ± 0.1 | 1.2 ± 0.1 | 3.2 ± 0.1 | 0.3 ± 0.0 | |
| α | 22.717 | 2.124 | 4.447 | 10.209 | 4.685 | NS | 2.279 | 0.455 | |
| β | −0.156 | −0.022 | −0.013 | 0.107 | −0.024 | NS | 0.025 | −0.007 | |
| r2 | 0.6 | 0.546 | 0.572 | 0.921 | 0.612 | NS | 0.865 | 0.844 | |
| F-value | 3.524a | 3.887a | 3.822a | 43.740b | 3.795a | NS | 20.926b | 16.877b | |
| LSD0.05 | 3.1 | 0.4 | 0.3 | 1.1 | 0.5 | NS | 0.4 | 0 | |
| Root | 0.2 | 21.0 ± 1.2 | 1.3 ± 0.1 | 3.1 ± 0.2 | 11.4 ± 0.3 | 4.2 ± 0.1 | 1.2 ± 0.0 | 3.3 ± 0.4 | 0.4 ± 0.0 |
| 2.5 | 20.0 ± 0.6 | 1.3 ± 0.1 | 3.0 ± 0.1 | 11.5 ± 0.1 | 4.2 ± 0.2 | 1.2 ± 0.1 | 3.4 ± 0.0 | 0.4 ± 0.0 | |
| 5.1 | 19.0 ± 1.0 | 1.2 ± 0.2 | 2.9 ± 0.1 | 12.3 ± 0.3 | 4.1 ± 0.1 | 1.2 ± 0.1 | 3.6 ± 0.2 | 0.3 ± 0.0 | |
| 7.7 | 19.0 ± 1.2 | 1.2 ± 0.1 | 2.9 ± 0.1 | 12.4 ± 0.3 | 3.9 ± 0.1 | 1.1 ± 0.1 | 3.8 ± 0.2 | 0.3 ± 0.0 | |
| 10.3 | 18.0 ± 0.6 | 1.1 ± 0.1 | 2.7 ± 0.2 | 13.5 ± 0.3 | 3.9 ± 0.3 | 1.1 ± 0.0 | 4.2 ± 0.4 | 0.3 ± 0.0 | |
| 12.6 | 18.0 ± 0.9 | 1.1 ± 0.1 | 2.6 ± 0.1 | 14.1 ± 0.2 | 3.8 ± 0.1 | 1.0 ± 0.1 | 4.4 ± 0.1 | 0.3 ± 0.0 | |
| 15.4 | 17.0 ± 0.8 | 1.0 ± 0.0 | 2.6 ± 0.1 | 14.6 ± 0.2 | 3.7 ± 0.1 | 1.0 ± 0.1 | 4.8 ± 0.3 | 0.3 ± 0.0 | |
| 17.9 | 17.0 ± 0.6 | 0.9 ± 0.1 | 2.4 ± 0.1 | 14.9 ± 0.3 | 3.6 ± 0.2 | 1.0 ± 0.1 | 5.4 ± 0.4 | 0.2 ± 0.0 | |
| 20.5 | 16.0 ± 1.0 | 0.9 ± 0.1 | 2.3 ± 0.1 | 15.1 ± 0.2 | 3.4 ± 0.1 | 1.0 ± 0.1 | 6.0 ± 0.4 | 0.2 ± 0.0 | |
| 23 | 16.0 ± 0.8 | 0.8 ± 0.0 | 2.3 ± 0.2 | 15.3 ± 0.1 | 3.4 ± 0.2 | 1.0 ± 0.1 | 6.1 ± 0.3 | 0.2 ± 0.0 | |
| α | 20.534 | 1.335 | 3.134 | 11.382 | 4.272 | NS | 2.992 | 0.369 | |
| β | −0.135 | −0.015 | −0.025 | 0.188 | −0.025 | NS | 0.084 | −0.007 | |
| r2 | 0.589 | 0.605 | 0.642 | 0.949 | 0.6 | NS | 0.856 | 0.882 | |
| F-value | 3.500a | 3.630a | 4.614a | 120.856b | 3.758a | NS | 14.149b | 23.545b | |
| LSD0.05 | 2.7 | 0.3 | 0.4 | 1.1 | 0.5 | NS | 0.9 | 0 |
Relationships are significant at P < 0.01.
NS, non-significant.
aF-values are significant at P < 0.01.
bF-values are significant at P < 0.001.
Concentrations of N, P and Ca decreased significantly (P < 0.01) in leaves, stems and roots in response to increasing salt concentration in soil. A negative relationship was obtained between N, P and Ca concentration in tissues and salt concentration (P < 0.01). The Ca:Na ratio in tissues decreased (P < 0.001) with increasing salinity of soil. A negative relationship (P < 0.01) was obtained between the Ca:Na ratio in tissues and salt concentration of soil. The concentration of Mg in tissues did not change with increasing salt concentration.
Discussion
It is well established that salinity can reduce and/or delay germination of halophyte seeds (Khan and Ungar, 1984; Ungar, 1996; Katembe et al., 1998; Li et al., 2002; Patel NT et al., 2010). Similarly, with C. tagal propagules, salinity over the range 2.5–28.2 ppt delayed propagule establishment and reduced the final percentage of plant establishment to below the control value of 94.4 %. The establishment percentage of C. tagal was reduced to 50 % (SE50) at a salinity of 22.3 ppt, whereas a 50 % reduction in seed germination (SG50) for Cassia montana, a halophyte tree in the coastal region of Saurashtra, was already obtained at 6.0 dS m−1 (3.8 ppt; Patel and Pandey, 2007). This confirms the high tolerance of the mangrove to salinity. Ceriops tagal is characterized by reduced but still considerable establishment of propagules under saline conditions. The capacity of C. tagal to invade upper estuarine habitats may thus be dependent on the tolerance shown to salt at this very early developmental stage (Harradine, 1982; Krauss et al., 1998). Adaptation of viviparous propagules to saline environments starts when they are still attached to the mother tree, by continuously absorbing salt from the tree or by a desalinating process (Joshi et al., 1972; Zheng et al., 1999). The tolerance of C. tagal also extended to young plants. At all tested salinity levels (0.2–28.2 ppt), no mortality was recorded once the first leaves appeared. Ye et al. (2005) reported that for Hong Kong Avicennia marina seedlings, the establishment percentage was 100 % at salinities ranging from 0 to 35 ppt.
Growth of young C. tagal plants was stimulated by low salinity and was optimal at 12.6 ppt. Similar results have also been reported for other halophytes (Naidoo and Raghunanan, 1990; Ayala and O'Leary, 1995; Khan et al., 2000a; Patel and Pandey, 2007; Patel NT et al., 2010). Soil salinity at 12.6 ppt approximately equals 41 % rainy season seawater of the Jamnagar coast. Similarly, optimum growth of seedlings was obtained at 50 % seawater for A. marina from Sunderban (Karim and Karim, 1993), for A. marina, C. tagal and Rhizophora mucronata from Pakistan (Khan and Aziz, 2001), and for Sonneratia alba from Australia (Ball and Pidsley, 1995). Other studies have reported lower optimal ∼25 % seawater for A. marina and Rhizophora stylosa (Downton, 1982; Clough, 1984; Naidoo, 1987; Burchett et al., 1989). This suggests that C. tagal growing along the semi-arid and arid coasts of Gujarat has considerable salinity tolerance. As a result, plants of C. tagal along the Gujarat coasts in India have approximately a similar salinity tolerance to those of the Karachi coast in Pakistan. Under natural conditions in Gujarat, propagules of C. tagal establish during the rainy season when the salinity level of seawater is somewhat diluted.
High soil salinity affects plant growth due to low water potential, ion toxicities, nutrient deficiencies or a combination of these (Khan et al., 2000a). Patel and Pandey (2007) reported that seedlings of C. montana grow optimally at 7.9 dS m−1 (5.1 ppt) salinity. Evidently, C. tagal can be grouped among such highly salt-tolerant plants. The water content of its tissues increased until 12.6 ppt and then declined with increasing salinity while never dehydrating to below control levels. A similar result was reported for Suaeda fruticosa (Khan et al., 2000a) and Aegiceras corniculatum (Patel and Pandey, 2009). Halophytes are known to adjust their tissue water potentials to below those of the soil water (Ungar, 1991). Ceriops tagal showed a progressive decrease in the water potential of leaves and stems with an increase in salinity, indicating that it adopts an osmoconforming strategy, as previously described for this species by Khan and Aziz (2001).
Ceriops tagal has the ability to exclude salts via root ultrafiltration (Hagemeyer, 1997). In the present experiment, a high Na concentration was maintained in tissues with an increase in salinity. It was presumably achieved by compartmentalizing Na+ into vacuoles, as has been found in mangroves and other halophytes (Flowers et al., 1977; Li et al., 2008). High internal salt concentrations benefit mangrove plants by lowering the internal water potential, thereby driving water uptake from soil with high salinity and low soil water potential. Yeo (1983) has suggested that Na+ and Cl− accumulated in leaf tissue provide osmotic adjustment and turgor to maintain growth. This would explain the remarkable ability of C. tagal to expand its leaves more vigorously and improve its tissue hydration at modest salinity levels, and avoid dehydrating to below control levels when higher salinities were imposed.
In the present study, the decrease of K+ concentration with increasing soil salinity suggests that Na+ inhibited the K+ uptake. The Na:K ratio increased in leaves and stems with an increase in salinity, indicating an increase in transportation of Na+ from root to shoot. Tattini et al. (1995) reported that the Na:K ratio increases in salt-tolerant species with increasing salinity in the external medium, because mass transport of sodium takes place from root to shoot via the transpiration stream.
Because of its zwitterionic, high-hydrophilic non-toxic character, proline can act as a ‘compatible solute’ and its accumulation is a common response to salinity and related stresses (Stewart and Lee, 1974; Storey et al., 1977), allowing it to function at high concentrations to effect osmotic adjustment, stabilization of macromolecules and regulate cellular redox status (Storey et al., 1977; Yamada et al., 2005). The increase of proline concentrations in C. tagal with increasing Na concentration indicates that higher proline accumulation may help alleviate NaCl stress in C. tagal, although the increase may be too small to influence osmotic balance significantly. Proline accumulation was greater in shoots than in roots. This confirms the observation of Munns (2002) that organic solutes are often lower in roots than shoots. Popp and Albert (1995) reported that C. tagal accumulates cyclitols along with Na+ and Cl− to maintain osmotic balance.
In general, salinity reduces N accumulation in plants (Feigin, 1985), and we observed a steady reduction in above- and below-ground tissues as salt application increases, with the greatest reduction (28 %) being in leaves. The effect may be ascribed to chloride toxicity (Torres and Bingham, 1973). Similarly, decreases in P concentration were also seen, but both the decline in N and P occurred at low and medium salinities where growth was stimulated, indicating a strong regulation that avoided debilitatingly large declines at modest levels of salinity.
Salinity also decreased Ca2+, suggesting that Na+ reduced internal concentrations in roots and shoots that might have slowed the growth as optimal levels of salt were exceeded. Uptake of Ca2+ may decrease because of ion interactions, precipitation and increase in ionic strength (Janzen and Chang, 1987). It is evidenced that in salt-stressed roots of cotton, Na+ displaced membrane-associated Ca, which was believed to be primarily at the plasma membrane (Cramer et al., 1985). In addition, NaCl salinity displaced membrane-associated Ca on protoplasts of corn (Lynch et al., 1987) and plasma membrane vesicles of melon (Yermiyahu et al., 1994). One consequence of the displacement of membrane-associated Ca2+ by Na+ is the immediate increase in K+ efflux across the plasma membrane of salt-stressed cotton roots (Cramer et al., 1985). This effect may be related to the rapid depolarization of the membrane potential upon salinization (Cramer, 1997). In the present study, the increased efflux of K+ might be one of the reasons for the significant decrease in K concentration in tissues of C. tagal in response to NaCl salinity. Shabala et al. (2003) reported that in addition to significant Na+ uptake into the root epidermis of barley, the onset of salt stress caused rapid and prolonged efflux of H+, K+ and NH4+. But not all key nutrient ions were depressed by salinity. Magnesium concentrations stood out as being very stable and this may be a key feature supporting faster growth at low and moderate salinities, and protecting C. tagal from Mg2+ decreasing to below control levels at high salinities. As is well known, Mg2+ has central roles in chlorophyll structure, as an enzyme cofactor and in exporting photosynthate (Marschner and Cakmak, 1989). The mechanism behind this magnesium homeostasis is unknown.
Conclusions and forward look
High percentages of propagules established successfully under salinity stress, 50 % establishment being achieved at 22.3 ppt NaCl. Subsequent stem and root elongation, and especially leaf expansion, were increased substantially at low and moderate salinities (≤12.6 ppt) while greater salinity failed to slow growth to below control values. Dry matter accumulation in tissues of plants behaved similarly. These findings demonstrate that C. tagal is a highly salt-tolerant mangrove at this key early stage in its life cycle. The tolerance is linked to a proportionate regulation of tissue water potentials, which were seen to decrease with an increase in salinity, thereby helping to maintain normal tissue hydration (the osmoconforming strategy). An increased accumulation of proline may also have contributed to this stabilization. A significant increase in Na and a decrease in K, N, P and Ca in tissues may inhibit the growth of the plants in the more extreme saline habitats.
Future work could usefully examine the ability of exogenous proline, and Ca and K to ameliorate the injurious effects of high concentrations of NaCl, although the high tolerance to low and moderate salinities that we demonstrated here strongly suggests that mangrove die-back on the Gujarat coast may have other causes which now merit further investigation.
Sources of funding
This study was supported with Departmental Special Assistance using funds provided by the Government of India University Grants Commission, New Delhi.
Contributions by authors
N.T.P. and A.N.P. contributed to a similar extent to the experimental work and to writing the paper. A.G. performed the statistical analyses.
Conflict of interest statement
None declared.
References
- Ayala F, O'Leary JW. Growth and physiology of Salicornia bigelovii Torr. at suboptimal salinity. International Journal of Plant Science. 1995;156:197–205. [Google Scholar]
- Aziz I, Khan MA. Experimental assessment of salinity tolerance of Ceriops tagal seedlings and saplings from the Indus delta, Pakistan. Aquatic Botany. 2001;70:259–268. [Google Scholar]
- Badashah SN, Nath RV., Multivariate analysis of variance. SYSTAT 12 manual – Statistics-III. San Jose, CA, USA: SYSTAT Software Inc. Technology; 2007. pp. 223–260. [Google Scholar]
- Ball MC. Ecophysiology of mangroves. Trees. 1988;2:129–142. [Google Scholar]
- Ball MC, Farquhar GD. Photosynthetic and stomatal responses of two mangrove species, Aegiceras corniculatum and Avicennia marina to long term salinity and humidity conditions. Plant Physiology. 1984;74:1–6. doi: 10.1104/pp.74.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball MC, Pidsley SM. Growth responses to salinity in relation to distribution of two mangrove species, Sonneratia alba and S. lanceolata. Functional Ecology. 1995;9:77–85. [Google Scholar]
- Bates LS, Waldren RP, Teare FD. Rapid determination of free proline from water stress studies. Plant and Soil. 1973;39:205–207. [Google Scholar]
- Burchett MD, Clarke CJ, Field CD, Pulkownik A. Growth and respiration in two mangrove species at a range of salinities. Physiologia Plantarum. 1989;75:299–303. [Google Scholar]
- Clough BF. Growth and salt balance of the mangroves Avicennia marina (Forssk.) Vierh. and Rhizophora stylosa Griff. in relation to salinity. Australian Journal of Plant Physiology. 1984;11:419–430. [Google Scholar]
- Cramer GR. Uptake and role of ions in salt tolerance. In: Jaiwal PK, Singh RP, Gulati A, editors. Strategies for improving salt tolerance in higher plants. New Delhi: Oxford & IBH Publishing Co. Pvt. Ltd; 1997. pp. 55–86. [Google Scholar]
- Cramer GR, Lauchli A, Polito VS. Displacement of Ca2+ by Na+ from the plasmalemma of root cells. A primary response to salt stress? Plant Physiology. 1985;79:207–211. doi: 10.1104/pp.79.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downton WJS. Growth and osmotic relations of the mangrove Avicennia marina, as influenced by salinity. Australian Journal of Plant Physiology. 1982;9:519–528. [Google Scholar]
- Feigin A. Fertilization management of crops irrigated with saline water. Plant and Soil. 1985;89:285–299. [Google Scholar]
- Flowers TJ, Troke PF, Yeo AR. The mechanism of salt tolerance in halophytes. Annual Review of Plant Physiology. 1977;28:89–121. [Google Scholar]
- Hagemeyer J. Salt. In: Prasad MHV, editor. Plant ecophysiology. Toronto: John Wiley and Sons, Inc; 1997. pp. 173–206. [Google Scholar]
- Harradine AR. Effect of salinity on germination and growth of Pennisetum macrourum in southern Tasmania. Journal of Applied Ecology. 1982;19:273–282. [Google Scholar]
- Janzen HH, Chang C. Cation nutrition of barley as influenced by soil solution composition in a saline soil. Canadian Journal of Soil Science. 1987;67:619–629. [Google Scholar]
- Joshi GV, Pimplaskar M, Bhosale LJ. Physiological studies in germination of mangroves. Botanica Marina. 1972;45:91–95. [Google Scholar]
- Karim J, Karim A. Effect of salinity on the growth of some mangrove plants in Bangladesh. In: Lieth H, Al Masoom A, editors. Towards the rational use of high salinity tolerant plants. Dordrecht: Kluwer; 1993. pp. 187–192. [Google Scholar]
- Katembe WJ, Ungar IA, Mitchell JP. Effect of salinity on germination and seedlings growth of two Atriplex species (Chenopodiaceae) Annals of Botany. 1998;82:167–175. [Google Scholar]
- Khan MA, Aziz I. Salinity tolerance in some mangrove species from Pakistan. Wetlands Ecology and Management. 2001;9:219–223. [Google Scholar]
- Khan MA, Ungar IA. The effect of salinity and temperature on the germination of polymorphic seeds and growth of Atriplex triangularis Willd. American Journal of Botany. 1984;71:481–489. [Google Scholar]
- Khan MA, Ungar IA, Showalter AM. The effect of salinity on the growth, water status, and ion content of a leaf succulent perennial halophyte, Suaeda fruiticosa (L.). Forssk. Journal of Arid Environments. 2000a;45:73–84. [Google Scholar]
- Khan MA, Ungar IA, Showalter AM. Effects of salinity on growth, water relations and ion accumulation of the subtropical perennial halophyte, Atriplex griffithii Moq. var. stocksii Boiss. Annals of Botany. 2000b;85:225–232. [Google Scholar]
- Krauss KW, Chambers JL, Allen JA. Salinity effects and differential germination of several half-sib families of baldcypress from different seed sources. New Forest. 1998;15:53–68. [Google Scholar]
- Li H, Kefu Z, Xiufeng W. The inhibition of salinity on the germination of halophyte seeds. Journal of Shandong Agricultural University (Natural Science) 2002;33:170–173. [Google Scholar]
- Li N, Chen S, Zhou X, Li C, Shao J, Wang R, Fritz E, Hüttermann A, Polle A. Effect of NaCl on photosynthesis, salt accumulation and ion compartmentation in two mangrove species, Kandelia candel and Bruguiera gymnorhiza. Aquatic Botany. 2008;88:303–310. [Google Scholar]
- Lin G, Sternberg LSL. Effects of growth form, salinity, nutrient and sulfide on photosynthesis, carbon isotope discrimination and growth of red mangrove (Rhizophora mangle L.) Australian Journal of Plant Physiology. 1992;19:509–517. [Google Scholar]
- Lin G, Sternberg L. Variation in propagule mass and its effect on carbon assimilation and seedling growth of red mangrove (Rhizophora mangle L.). in Florida, USA. Journal of Tropical Ecology. 1995;11:109–119. [Google Scholar]
- Lynch J, Cramer GR, Lauchli A. Salinity reduces membrane-associated calcium in corn root protoplasts. Plant Physiology. 1987;83:390–394. doi: 10.1104/pp.83.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H, Cakmak I. High light intensity enhances chlorosis and necrosis in leaves of zinc, potassium and magnesium deficient bean Phaseolus vulgaris plants. Journal of Plant Physiology. 1989;134:308–315. [Google Scholar]
- Munns R. Comparative physiology of salt and water stress. Plant, Cell and Environment. 2002;25:239–250. doi: 10.1046/j.0016-8025.2001.00808.x. [DOI] [PubMed] [Google Scholar]
- Naidoo G. Effects of salinity and nitrogen on growth and plant water relations in the mangrove Avicennia marina (Forsk.) Vierh. New Phytologist. 1987;107:317–326. doi: 10.1111/j.1469-8137.1987.tb00183.x. [DOI] [PubMed] [Google Scholar]
- Naidoo GR, Raghunanan R. Salt tolerance in the succulent halophyte, Sarcocornia natalensis. Journal of Experimental Botany. 1990;41:497–502. [Google Scholar]
- Pandya DH, Mer RK, Prajith PK, Pandey AN. Effect of salt stress and manganese supply on growth of barley seedlings. Journal of Plant Nutrition. 2004;27:1361–1379. [Google Scholar]
- Parida AK, Jha B. Salt tolerance mechanisms in mangroves: a review. Trees–Structure and Function. 2010;24:199–217. [Google Scholar]
- Patel AD, Pandey AN. Effect of soil salinity on growth, water status and nutrient accumulation in seedlings of Cassia montana (Fabaceae) Journal of Arid Environments. 2007;70:174–182. [Google Scholar]
- Patel AD, Jadeja HR, Pandey AN. Effect of salinisation of soil on growth, water status and nutrient accumulation in seedlings of Acacia auriculiformis (Fabaceae) Journal of Plant Nutrition. 2010;33:914–932. [Google Scholar]
- Patel NT. Effect of salinisation of soil on growth and nutrient accumulation in seedlings of certain plant species on Gujarat coasts. Rajkot, India: University of Saurashtra; 2009. PhD Thesis. [Google Scholar]
- Patel NT, Pandey AN. Salinity tolerance of Aegiceras corniculatum (L.) Blanco from Gujarat coasts of India. Anales de Biología. 2009;31:93–104. [Google Scholar]
- Patel NT, Yadav DR, Ghosh D, Pandey AN. Salinity tolerance of Rhizophora mucronata Lam. from Gujarat coasts of India. Botanica Marina. 2010;53:213–222. [Google Scholar]
- Piper CS. Soil and plant analysis. New York: Wiley Interscience; 1944. [Google Scholar]
- Popp M. Chemical composition of Australian mangroves. I. Inorganic ions and organic acids. Zeitschrift für Pflanzenphysiologie. 1984;113:395–409. [Google Scholar]
- Popp M. Salt resistance in herbaceous halophytes and mangroves. In: Behnke HD, Luttge U, Esser K, editors. Progress in botany. Berlin: Springer; 1994. pp. 416–429. [Google Scholar]
- Popp M, Albert R. The role of organic solutes in salinity adaptations of mangroves and herbaceous halophytes. In: Khan MA, Ungar IA, editors. Biology of salt tolerant plants. Karachi: Department of Botany, University of Karachi; 1995. pp. 416–429. [Google Scholar]
- Roth LC. Hurricane and mangrove vegetation: effects of Huricane Joan, October 1988, on the vegetation of Isla del Venado- Bluefields, Nicaragua. Biotropica. 1992;24:375–384. [Google Scholar]
- Scholander PF. How mangroves desalinate seawater. Plant Physiology. 1968;21:251–261. [Google Scholar]
- Shabala S, Shabala L, Volkenburgh EV. Effect of calcium on root development and root ion fluxes in salinised barley seedlings. Functional Plant Biology. 2003;30:507–514. doi: 10.1071/FP03016. [DOI] [PubMed] [Google Scholar]
- Shan L, RenChao Z, SuiSui D, SuHua S. Adaptation to salinity in mangroves: implication on the evolution of salt tolerance. Chinese Science Bulletin. 2008;53:1708–1715. [Google Scholar]
- Stewart GR, Lee JA. The role of proline accumulation in halophytes. Planta. 1974;120:279–289. doi: 10.1007/BF00390296. [DOI] [PubMed] [Google Scholar]
- Storey R, Ahmed N, Wyn Jones RG. Taxonomic and ecological aspects of the distribution of glycinebetaine and related compounds in plants. Oecologia. 1977;27:319–332. doi: 10.1007/BF00345565. [DOI] [PubMed] [Google Scholar]
- Suarez N, Sobrado MA, Medina E. Salinity effects on the leaf water relations components and ion accumulation patterns in Avicennia germinans L. seedlings. Oecologia. 1998;114:299–304. doi: 10.1007/s004420050451. [DOI] [PubMed] [Google Scholar]
- Tattini M, Gucci R, Coradeschi MA, Ponzio C, Everard JD. Growth, gas exchange and ion content in Olea europeaea plants during salinity stress and subsequent relief. Physiologia Plantarum. 1995;95:203–210. [Google Scholar]
- Torres BC, Bingham FT. Salt tolerance of Mexican wheat. I. Effect of NO3 and NaCl on mineral nutrition, growth and grain production of four wheats. Soil Science Society of America Proceedings. 1973;37:711–715. [Google Scholar]
- Ungar IA. Ecophysiology of vascular halophytes. Boca Raton, FL: CRC Press; 1991. [Google Scholar]
- Ungar IA. Effect of salinity on seed germination, growth and ion accumulation of Atriplex patula (Chenopodiaceae) American Journal of Botany. 1996;83:604–607. [Google Scholar]
- Yamada M, Morishita H, Urano K, Shiozaki N, Yamaguchi-Shinozaki K, Shinozaki K, Yoshiba Y. Effects of free proline accumulation in petunias under drought stress. Journal of Experimental Botany. 2005;56:1975–1981. doi: 10.1093/jxb/eri195. [DOI] [PubMed] [Google Scholar]
- Yeo AR. Salinity resistance: physiologies and prices. Physiologia Plantarum. 1983;58:214–222. [Google Scholar]
- Yermiyahu U, Nir S, Ben-Hayyim G, Kafkafi U. Quantitative competition of calcium with sodium or magnesium for sorption sites on plasma membrane vesicles of melon (Cucumis melo L.) root cells. Journal of Membrane Biology. 1994;138:55–63. doi: 10.1007/BF00211069. [DOI] [PubMed] [Google Scholar]
- Ye Y, Tam NF, Lu C, Wong Y. Effects of salinity on germination, seedling growth and physiology of three salt secreting mangrove species. Aquatic Botany. 2005;83:193–205. [Google Scholar]
- Zheng WJ, Wang WQ, Lin P. Dynamics of element contents during the development of hypocotyls and leaves of certain mangrove species. Journal of Experimental Marine Biology and Ecology. 1999;233:247–257. [Google Scholar]