Abstract
Vascular calcification can occur in nearly all arterial beds and in both the medial and intimal layers. The initiating factors and clinical consequences depend on the underlying disease state and the location of the calcification. The best studied manifestation is coronary artery calcification, in part because of the obvious clinical consequences, but also because of CT based imaging modalities. In the general population, the presence of coronary artery calcification increases cardiovascular risk above that predicted by traditional Framingham risk factors, suggesting the presence of non-traditional risk factors. In patients with chronic kidney disease (CKD) coronary artery calcification is more prevalent and markedly more severe than in the general population. In these CKD patients, non-traditional risk factors such as oxidative stress, advanced glycation end-products and disordered mineral metabolism are also more prevalent and more severe and offer mechanistic insight into the pathogenesis of vascular calcification.
Keywords: Hypertension, diabetes, vascular calcification, Chronic kidney disease (CKD), Traditional risk factors, non-traditional risk factors, inflammation, mineral metabolism, dyslipidemia, calcium, phosphorus
Introduction
Calcium is the most common element in bone, and 99% of the total body calcium is in bone in the form of a calcium phosphate crystalline structure called hydroxyapatite. Calcium is also found outside of bone in a variety of tissues, broadly termed extra-skeletal calcification. In these sites, the calcium can be in multiple forms- including hydroxyapatite, magnesium whitlockite, and amorphous calcium phosphate. Calcification in arteries, or vascular calcification, can lead to cardiovascular morbidity and mortality [1]. While cellular necrosis may lead to calcium deposition within or around vascular smooth muscle cells (VSMC), over the last 15 years there has been an increased appreciation that vascular calcification is a cell-mediated process. Indeed, the majority of vascular calcification is thought to be purposely laid down by vascular smooth muscle cells that have de-differentiated to osteoblast/chondrocyte like cells.
Pathophysiology
Arterial calcification can occur in both intimal and medial layers. In the intima, atherosclerotic disease is characterized by fibrofatty plaque formation, and based on autopsy data and animal models. Calcification had been thought to occur late in the disease course. However, using intravascular ultrasound, the atherosclerosis can also be a circumferential lesion (without an obstructed lumen) with calcification earlier in the course of the disease [2]. The medial layer may also be affected, leading to thickening of the medial layer of larger elastic arteries resulting in arteriosclerosis of smaller elastic arteries classically described as Mönckeberg’s calcification, or medial calcinosis[3, 4]. This disease of small vessels is more common in patients with diabetes, renal failure and advanced aging and is associated with increased all-cause and cardiovascular mortality in diabetic patients without CKD[5], and in CKD patients with and without diabetes
The clinical manifestations of vascular calcification depend on the location within the arterial wall and the tissue perfused. Intimal, atherosclerotic calcification can lead to myocardial infarction from stenosis and acute thrombus, or ischemia in both coronary and peripheral arteries. Medial or circumferential calcification can lead to reduced compliance due to arterial stiffening, resulting in an impaired vasodilation during ischemia that, in theory could lead to arrhythmias and sudden death. With medial calcification (arteriosclerosis) of the aorta, there will be increased pulse wave velocity, elevated pulse pressure, and systolic hypertension. Lastly, calcification of the arterioles of the skin and other organs can lead to calciphylaxis and ischemic gut[7]. Arterial calcification can be detected by plain radiographs, CT methods including EBCT and multi-slice (spiral) CT, and ultrasound. Unfortunately, with the exception of intravascular ultrasound, none of these techniques can distinguish intimal from medial calcification. CT based methods are utilized for clinical research as they allow for quantitation. The higher the coronary artery calcification score, the greater the risk of cardiovascular mortality in both the general population[8] and in chronic kidney disease (CKD)[9].
Age is the strongest predictor of coronary artery disease[8] but multiple other clinical risk factors have been implicated in the pathogenesis of arterial calcification. Coronary artery calcification is also more prevalent and more severe among CKD patients than in the general population, and studies in CKD patients offer insight into the pathogenesis. In patients not yet on dialysis, over 50% have coronary artery calcification[10] whereas 70–90% of prevalent dialysis patients have significant coronary artery calcification[11, 12]. Histologic studies comparing dialysis patients to non-CKD patients who died of a coronary event showed that dialysis patients had more calcification in the atheromatous plaques, but not more plaque. Dialysis patients also had a thicker medial layer [13]. Studies evaluating distal segments of the coronary arteries found medial calcification adjacent to the internal elastic lamina in dialysis patients [14] and in patients with advanced CKD[15]. We found isolated medial calcification in the absence of intimal calcification in the inferior epigastric artery of patients undergoing a renal transplant[16]. Thus, calcification can occur both in intimal and medial arterial layers and in different vascular beds. In a study of 4544 patients, the presence of calcification in the thoracic aorta, carotids and iliac arteries were associated with all-cause mortality with hazard ratios of 2.1, 1.6, and 1.67, respectively, whereas coronary artery calcification was associated with a hazard ratio of 3.4 for cardiovascular mortality[17]. At the present time, it appears that there may be different initiating factors in different vascular beds and in the intima and media, but a common downstream process of de-differentiation to an osteoblast like phenotype.
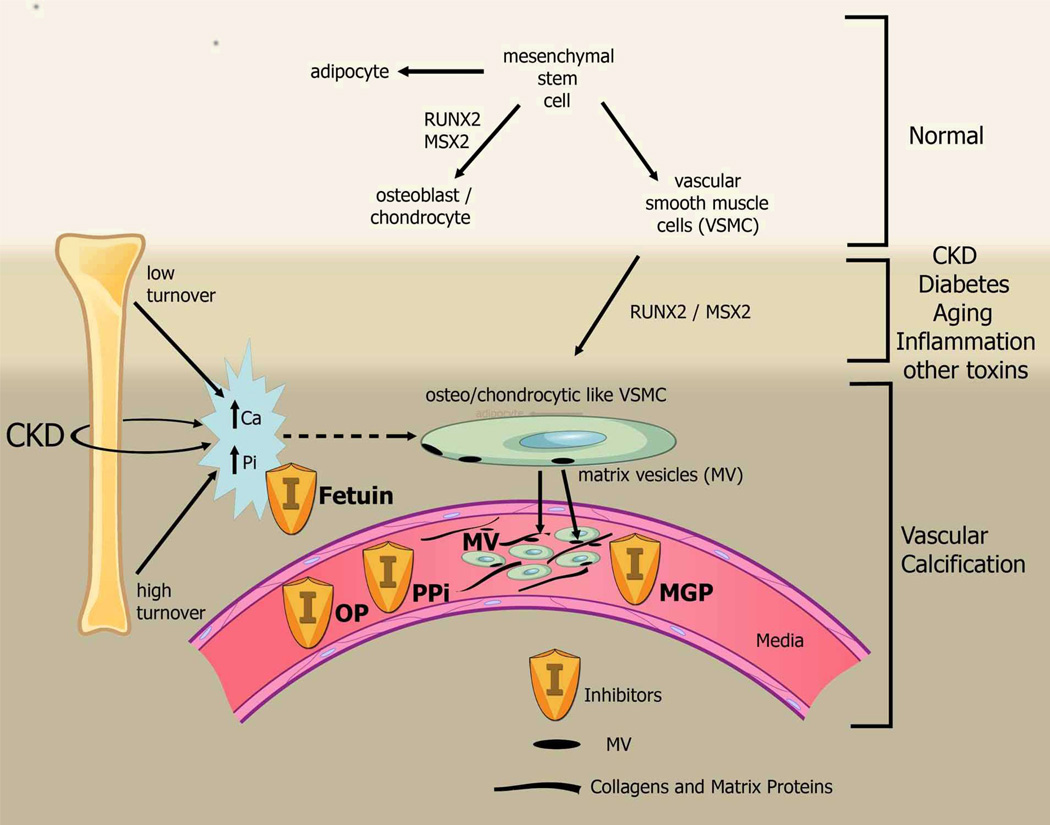
The mechanism of arterial calcification is complex, but multiple investigators agree that the first step appears to be de-differentiation or transformation of vascular smooth muscle cells (VSMC) to an osteoblast/chondrocytic phenotype (Figure 1). VSMC originate from a similar mesenchymal stem cell as osteoblasts, the latter occurring with upregulation of the transcription factor core binding factor alpha-1 (Cbfa-1) now called Runt-related transcription factor 2 (RUNX2) or msh homeobox 2 (MSX-2). These cells then do what a normal osteoblast does: secrete matrix proteins. We have shown that there is increased expression of RUNX2 by in situ hybridization in areas adjacent to calcification of the intima and the medial layer of inferior epigastric artery obtained from hemodialysis patients in association with ‘bone’ matrix proteins[16]. The signals that induce this transformation are multiple and will be described in detail below. Once the matrix is laid down, these cells then mineralize the matrix through the secretion of matrix vesicles [18], or through apoptosis[19]. Phosphorus and calcium increase the mineralizing potential of these matrix vesicles[18, 20]. The latter may explain why the disease is increased in CKD where disturbances of mineral metabolism are common and why there may be a relationship between bone mineralization and coronary calcification [21]. In both bone and arteries, there are inhibitors of calcification including matrix gla protein, pyrophosphate, and osteopontin and circulating inhibitors such as fetuin-A[22]. Thus, calcification is a balance between pro-mineralizing factors that stimulate VSMC de-differentiation and inhibitors of calcification. For the remainder of this review we will focus on the pro-mineralizing factors.
Figure 1.
Mesenchymal stem cells can differentiate to adipocytes, osteoblasts, chondrocytes, and vascular smooth muscle cells. The latter cells can also de-differentiate or transform to chondrocyte/osteoblast like cells by upregulation of transcription factors such as RUNX-2 and MSX2. These cells then lay down collagen and non-collagenous proteins in the intima or media AND incorporate calcium and phosphorus into matrix vesicles to initiate mineralization and further grow the mineral into hydroxyapatite. The overall positive calcium and phosphorus balance of most dialysis patients feeds both the cellular transformation and the generation of matrix vesicles. Ultimately, whether an artery calcifies or not, depends on the strength of the army of inhibitors standing by in the circulation and in the arteries. Reprinted with permission from[1].
Risk Factors
Vascular calcification can occur with either atherosclerosis or arteriosclerosis, both of which are increased with so-called “traditional” Framingham risk factors. Thus, many clinical series have tried to find associations with such risk factors with coronary artery or peripheral artery calcification. Everhart showed that risk factors for arterial calcification were impaired vibration, duration of diabetes, and high plasma glucose[23]. Patients with diabetes have increased coronary artery calcification, and the presence of diabetic nephropathy increases this risk[10, 24]. Lehto et al, in a study of 1059 patients with type II diabetes, demonstrated that medial arterial calcification of peripheral arteries was a strong independent predictor of total cardiovascular mortality, and also a significant predictor of future coronary heart disease events, stroke and amputation[5]. In contrast, dyslipidemia in the form of elevated LDL cholesterol does not seem to play a role in the coronary artery calcification in patients with CKD[25] whereas non-HDL cholesterol, but not LDL cholesterol, was associated with increased coronary artery calcification in asymptomatic adults without CKD[26]. Importantly, a recent meta-analysis demonstrated that statins improve atherosclerotic diseases, but have no effect on the reduction of coronary artery calcification [27]. Similarly, hypertension and smoking are not consistently identified risk factors for coronary artery calcification.
Clinically, the presence of calcification by EBCT increases the risk of mortality above that predicted by traditional “Framingham” risk factors. The presence of coronary calcification increases the area under the curve compared to traditional risk factors (0.84 vs 0.71, respectively, P <.001)[28]. In patients with CKD, there is a progressive increase in coronary artery calcification with loss of renal function, independent of traditional risk factors[29]. Multiple studies have demonstrated the added value of coronary artery calcification assessment on risk prediction including the multi-ethnic study of atherosclerosis (MESA)[30], Rotterdam[31], South Bay Heart Watch[32], and Heinz Nixdorf Recall[33]. The reader is referred to an excellent recent systematic review on this topic[34]. The remainder of this review focuses on animal and in vitro data examining some of the traditional and nontraditional risk factors associated with increased vascular calcification (Table 1).
Table 1.
Risk factors for vascular calcification
Traditional Risk Factors:
|
Non-traditional Risk Factors:
|
“Traditional” risk factors for vascular calcification
Hypertension
Hypertension is associated with vascular remodeling and arteriosclerosis. In clinical studies, hypertension is not a commonly cited risk factor for calcification, perhaps because the majority of subjects with calcification have hypertension as a clinical manifestation of the arteriosclerosis. The renin-angiotensin system is known to be a major pathogenic factor in VSMC apoptosis, growth and differentiation, and therefore it likely plays a role in calcification[35]. Armstrong et al fed rabbits an atherogenic diet with high dose vitamin D to induce calcification along the internal elastic lamina and the media layer. There was upregulation of BMP-2 and down regulation of alpha-smooth muscle actin suggesting a de-differentiation from a vascular smooth muscle cell phenotype to an osteoblast like phenotype. Furthermore, calcified arteries had upregulation of angiotensin 1 receptor and treatment with an angiotensin receptor blocker prevented the calcification[36]. In contrast, in 5/6th nephrectomized rats (a model of CKD), treatment with enalapril improved myocardial hypertrophy and progression of renal disease but had no effect on vascular calcification[37]. In a rat model of arterial calcification induced by intramuscular administration of vitamin D plus oral nicotine (VDN), increased calcium content of arteries was associated with increased levels of angiotensin II and adlosterone in the tissue; treatment with captopril or spironolactone reduced the calcification[38]. Thus, the renin-angiotensin and aldosterone pathway appears to play a role in arterial calcification. Whether this is due to the reduction of underlying remodeling (arteriosclerosis), or a direct inhibition of the osteogenic transformation will require additional studies.
Diabetes
Our laboratory examined the calcification and expression of ‘bone’ proteins in the inferior epigastric artery of patients undergoing a renal transplant. Patients with diabetes had increased calcification compared to non-diabetic patients and there was increased expression in the medial layer of bone matrix proteins in the arteries such as osteopontin, type I collagen and alkaline phosphatase[39]. In vitro, we have found that VSMC incubated with high glucose led to an increase in the expression of the osteoblast transcription factor RUNX2, BMP-2 and osteocalcin and enhanced calcification in bovine VSMC. The protein kinase C signaling pathway was involved in this high glucose-induced expression of RUNX2 and bone matrix proteins[39]. Another group found that when fed high fat diet, the Ldlr−/− diabetic mouse develops hyperglycemia, dyslipidemia and aortic calcification with concomitant upregulation of aortic BMP2 and Msx2 gene expression[40]. Increased glucose increased the BMP-2/Msx2-Wnt pathway, leading to an osteogenic phenotype in a subset of the myofibroblasts; inhibition of the BMP-2 pathway reduced arterial calcification[41]. Interestingly, the location of BMP2 and BMP4 differed in diabetic aortas in that BMP-4 was found in the endothelium and BMP2 throughout the vascular wall[41]. These results suggest that the increased vascular calcification in diabetes is at least partially due to the direct effects of hyperglycemia on transforming the VSMC to osteoblast like phenotype via multiple mechanisms.
Dyslipidemia
Although clinically the role of lipids in vascular calcification is unclear, during osteogenic differentiation, calcifying vascular cells (CVCs, a clone of VSMC that readily calcify) accumulate not only minerals but also lipids such as triglycerides[42]. In vitro, HDL inhibits the osteogenic differentiation pathway[42]. In CVCs, stearate, compared to other fatty acids, promoted mineralization whereas inhibition of acetyl-CoA carboxylase or acyl-CoA synthetase reduced mineralization[43]. In these same CVC, n-3 unsaturated fatty acids play a protective role through a p38-MAPK (mitogen-activated protein kinase) and PPARγ (peroxisome proliferator activated receptor gamma) dependent mechanism[44]. Finally, oxidized lipids such as oxysterols and oxidized phospholipids illicit procalcific effects in vascular cells as detailed below [45]. Thus, dyslipidemia, rather than elevated LDL cholesterol appears to be a major causative factor in vascular calcification.
Non- traditional risk factors
Inflammation
Inflammation is a known non-traditional risk factor for atherosclerosis and vascular disease in the normal population and in CKD and is associated with increased mortality[46, 47]. Both CRP [48] and inflammatory cytokines[49] are associated with increased coronary artery calcification in patients with CKD. Interestingly, osteogenesis is associated with local inflammation and macrophage infiltration in atherosclerosis in ApoE −/− mice as revealed by molecular imaging in vivo [50]. Tumor necrosis factor alpha can induce mineralization of calcifying vascular cells in vitro [51] and co-culture of these cells with monocyte/macrophages (the source of most cytokines) can accelerate mineralization [52]. In human VSMC, the phosphatidylinositol 3-kinase(PI3K)/Akt pathway may inhibit inflammation induced calcification, perhaps by mediating alkaline phosphatase which is a ‘marker’ of osteoblast phenotype but also a potent inhibitor of a naturally occurring inhibitor of calcification, pyrophosphate[53]. Cytokine stimulation of alkaline phosphatase from VSMCs probably plays an important role also in calcification associated with diabetes, since the TNF-α inhibitor infliximab was shown to reduce the osteogenic phenotype of VSMC and the extent of medial calcification in LDLR −/− diabetic mice, without reducing obesity, hypercholesterolemia and hyperglycemia[40].
Oxidative Stress
CKD is a state of increased oxidative stress due to impaired anti-oxidative mechanisms[54]. Elevations in asymmetric dimethylarginine, a naturally occurring inhibitor of NO synthase, are associated with increased intima-medial thickness in the carotid arteries, concentric left ventricular hypertrophy, and mortality in dialysis patients[55]. In a rat model of CKD, the antioxidant Tempol inhibited vascular calcification by reducing oxidative stress and inhibiting osteogenic transdifferentiation of vascular smooth muscle cells[56]. In the general population, there is growing evidence indicates that there is a correlation between oxidative stress and the development of vascular calcification[57–60]. Macrophages, endothelial cells, and smooth muscle cells produce reactive oxygen species such as hydrogen peroxide and superoxide anion in response to a number of stimuli. The free radical nitric oxide (NO) is generated from the endothelium from L -arginine by the enzyme NO synthase and leads to production of hydroxyl or peroxyl radicals. When VSMC are treated with β-glycerophosphate or uremic serum for 24 h, the production of H2O2 and early expression of NADPH oxidase sub-unit p22(phox) are increased. The elevated oxidative stress was associated with increased expression of RUNX2 and alkaline phosphatase and calcification of VSMC[57]. An important contributor to oxidative stress in atherosclerotic lesions is the formation of hydrogen peroxide from various sources in vascular cells[58]. A recent study by Byon et al[60] demonstrated that H2O2 induces a switch of VSMC from contractile to osteogenic phenotype associated with an increased expression of RUNX2 and calcification in VSMC. Furthermore, inhibition of H2O2-activated AKT signaling pathways blocked increased expression of RUNX2 and VSMC calcification[60]. A similar study has also demonstrated that advanced oxidation protein products (AOPPs) induce vascular calcification by promoting osteoblast differentiation of human vascular smooth muscle cells via the ERK signaling pathway[59]. In a rabbit model of atherogenesis fed high dose vitamin D, there was increased oxidative stress and aortic valve (AV) calcification/stenosis. The latter could be abrogated by the antioxidant lipoic acid[58]. Lipid oxidation products have direct effects on both bone-forming and bone-resorbing cells. Oxidized LDL directly inhibits differentiation of osteoblasts[45] while directly inducing differentiation of osteoclasts[61]. Oxidized lipids also regulate osteoclastogenic cytokines produced by osteoblasts[62]. Thus, oxidative stress may be causative in vascular calcification, and may also explain the relationship between increased coronary artery calcification and osteoporosis found in both CKD and the general population[63].
Advanced Glycation End-Products (AGEs)
Proteins can be modified indirectly by reactive carbonyl compounds formed by auto-oxidation of carbohydrates and lipids, leading to eventual formation of AGEs. AGEs have been found in arterial and cardiac tissue as well as atherosclerotic lesions in dialysis patients[64]. Circulating AGEs such as pentosidine are elevated in patients on dialysis[65]. AGE-modified elastin and calcification has been found to co-localize in the aortic media of dialysis patients and binding of mineral to elastin is thought to be an important factor in the pathogenesis of medial calcification [66]. In cultured VSMC, AGEs can accelerate calcification of microvascular pericytes[67]. AGEs induced the expression of RUNX2 mRNA and alkaline phosphatase activity and calcification[68]. The receptor for AGE (RAGE) is expressed in a variety of cells including VSMC[69] and these AGE mediated changes in VSMCs were partially attenuated by a neutralizing antibody to RAGE[70]. A study by Suga et al[71] demonstrated that activation of RAGE inhibited VSMC phenotypic gene expression and induces osteogenic differentiation of VSMC. This RAGE mediated effect was via Notch/Msx2 induction in VSMC. The results suggest that AGEs that accumulate in diabetes could elicit the osteoblastic differentiation of VSMCs, thereby contributing to vascular calcification via the RAGE pathway.
Abnormal mineral metabolism
Hyperphosphatemia
Abnormal mineral metabolism has been recognized as a nontraditional risk factor in the development of vascular calcification in CKD patients and is associated with increased mortality in both pre-dialysis and dialysis patients[1, 72]. Hyperphosphatemia is associated with the prevalence and progression of vascular calcification in dialysis patients[73]. Several studies have demonstrated that the use of non-calcium-based as compared with calcium-based phosphate binders attenuated vascular calcification and mortality in dialysis patients[9, 74]. In the general population, phosphorus levels in the upper quartile of the normal range are also associated with increased cardiovascular and all cause mortality[75].
In vitro, phosphate increased the calcification of VSMC in dose-dependent manner[76]. High phosphate induced the loss of VSMC markers, such as smooth muscle (SM) alpha-actin and SM22α and increased the expression of the osteochondrogenic markers Runx2, osterix, osteopontin, and alkaline phosphatase[77, 78]. We have demonstrated that bovine VSMC incubated with calcification media (10 mM β-glycerolphosphate as a phosphate donor) generated cellular matrix vesicles that have high annexin II and VI content and the ability to mineralize extracellular matrix compared to that from bovine VSMC incubated without phosphate[18]. The matrix vesicles serve as nucleation sites for calcification, similar to the vesicles that bud from osteoblasts and hypertrophic chondrocytes in normal bone formation. Phosphate transport to cells is primarily mediated by sodium-dependent phosphate (NaPi) co-transporters[79] and treatment with phosphonoformic acid (PFA, a competitive inhibitor of NaPi transport) inhibits phosphate uptake and VSMC osteochondrogenic differentiation[76]. The type III NaPi co-transporters, PiT-1, is highly expressed in VSMC[80] and the knockdown PiT-1 with siRNA suppressed phosphate-induced calcification and blocked induction of the osteogenic markers Runx2/Cbfa1 and osteopontin[80]. However, our group has shown that bovine VSMCs incubated with pooled uremic sera from dialysis patients had increased calcification, above that induced by phosphorus but only when phosphorus is available[81]. The addition of PFA (inhibitor of NaPi transport) or levamisole (inhibitor of alkaline phosphatase) only partially inhibited uremic serum-induced osteopontin upregulation. The cyclic adenosine monophosphate (cAMP)/protein kinase A signaling pathway was involved in uremic serum-induced upregulation of RUNX2 and alkaline phosphatase[82]. High phosphate may also regulate matrix mineralization through elastin degradation. A soluble elastin-derived peptide can induce mineralization of human VSMCs in the presence of high phosphorus concentration[83]. Treatment of rat VSMC with elastin peptide induced the expression of elastin–laminin receptors along with increased expression of osteoblastic transcription factor RUNX2 and alkaline phosphatase[84]. TGF-β which is known to upregulate RUNX2[85], had synergistic effect on VSMC phenotypic change. In a rat aortic ring model, treatment with high phosphate and warfarin increased matrix metalloproteinase 9 (MMP-9) activity followed by transforming growth factor-β (TGF-β) signaling and aortic calcification[86]. We have recently demonstrated that MMP-2 and MMP-9 expression and activity are increased with progressive CKD, and blockade of MMP activity can inhibit arterial calcification[87]. This matrix degradation or alteration may be an initial step in calcification.
Hypercalcemia
There is an association with elevated serum calcium and the development of vascular calcification in the CKD population [72]. In addition, the use of calcium containing phosphate binders which induce positive calcium balance is associated with increased arterial calcification in the majority of studies[88]. In vitro, calcium alone can increase human VSMCs calcification[89]. Furthermore, calcium and phosphorus had synergistic roles in inducing mineralization of VSMC[90]. In an aorta ring culture model, elevated calcium was more potent than phosphorus to induce VSMC calcification for a given concentration of calcium and phosphorus, called the Ca × P product[91] [92]. Calcium also stimulates VSMC matrix vesicle release [90]. We have demonstrated that calcified VSMC derived cellular MV are enriched with annexin II and VI but with little fetuin-A[18]. Furthermore, blockade of annexin calcium channel activity with K201or the L-type calcium channel blocker verapamil significantly inhibit MV activity and the calcification of VSMC [20]. Shanahan and colleagues also demonstrated that blockade of intracellular calcium increase can inhibit MV calcification[93]. It is clear that abnormal mineral metabolism contributes to the development of vascular calcification by multiple mechanisms.
FGF23
The hormone fibroblast growth factor 23 (FGF23) is predominately expressed in osteocytes and is involved in mineral homeostasis by inducing hyperphosphaturia, inhibiting calcitriol synthesis and inhibiting PTH secretion[94]. In the kidney it exerts its biological functions by binding to the FGF receptor in the presence of the cofactor Klotho[95]. Several studies have demonstrated that FGF23 is associated with coronary artery and aortic vascular calcification in CKD and dialysis patients[96, 97]. Targeted deletion of FGF23 or Klotho in mice resulted in hyperphosphatemia and vascular calcification[95, 98]. In moderately uremic mice fed high-phosphate diets, elevated serum FGF-23 and osteopontin levels, but not serum phosphorus levels, were associated with extensive arterial-medial calcifications[99]. A recent study by Takei et al has demonstrated that the expression of stanniocalcin (STC) 2, a calcium/phosphate-regulating hormone, is increased and co-localized in calcified lesions of FGF23 or Klotho null mice[100]. Although the mechanism by which FGF23 affects vascular calcification is not clear at present, these data suggest that another mechanism by which phosphate affects vascular calcification may be through phosphorus-mediated elevation of FGF-23 levels.
In conclusion
CKD represents a model of accelerated vascular calcification and provides insight into the pathogenesis of arterial calcification and the importance of non-traditional risk factors.
Contributor Information
Neal X. Chen, Division of Nephrology, Department of Medicine, Indiana University School of Medicine, 950 West Walnut, Street, R2-Room 219, Indianapolis, Indiana 46202, USA. xuechen@iupui.edu, Phone: 317-274-3724, Fax: 317-274-8575.
Sharon M. Moe, Division of Nephrology, Department of Medicine, Indiana University School of Medicine, 950 West Walnut Street, R2-Room E202C, Indianapolis, Indiana 46202, USA. Roudebush Veterans Affairs Medical Center, 1481 West 10th Street, Indianapolis, Indiana 46202. smoe@iupui.edu, Phone: 317-278-2868, Fax: 317-274-8575.
References
Papers of particular interest, published recently, have been highlighted as:
* Of importance
** Of major importance
- 1.Moe SM, Chen NX. Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2008;19(2):213–216. doi: 10.1681/ASN.2007080854. [DOI] [PubMed] [Google Scholar]
- 2.Ibanez B, Badimon JJ, Garcia MJ. Diagnosis of atherosclerosis by imaging. Am J Med. 2009;122(1 Suppl):S15–S25. doi: 10.1016/j.amjmed.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Moe SM, Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res. 2004;95(6):560–567. doi: 10.1161/01.RES.0000141775.67189.98. [DOI] [PubMed] [Google Scholar]
- 4.Proudfoot D, Shanahan CM, Weissberg PL. Vascular calcification: new insights into an old problem [editorial; comment] Journal of Pathology. 1998;185(1):1–3. doi: 10.1002/(SICI)1096-9896(199805)185:1<1::AID-PATH89>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 5.Lehto S, Niskanen L, Suhonen M, Ronnemaa T, Laakso M. Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol. 1996;16(8):978–983. doi: 10.1161/01.atv.16.8.978. [DOI] [PubMed] [Google Scholar]
- 6.London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18(9):1731–1740. doi: 10.1093/ndt/gfg414. [DOI] [PubMed] [Google Scholar]
- 7.Moe SM, Chen NX. Calciphylaxis and vascular calcification: a continuum of extra-skeletal osteogenesis. Pediatr Nephrol. 2003;18:969–975. doi: 10.1007/s00467-003-1276-0. [DOI] [PubMed] [Google Scholar]
- 8.Raggi P, Gongora MC, Gopal A, Callister TQ, Budoff M, Shaw LJ. Coronary artery calcium to predict all-cause mortality in elderly men and women. J Am Coll Cardiol. 2008;52(1):17–23. doi: 10.1016/j.jacc.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int. 2007 doi: 10.1038/sj.ki.5002059. [DOI] [PubMed] [Google Scholar]
- 10.Mehrotra R, Budoff M, Hokanson JE, Ipp E, Takasu J, Adler S. Progression of coronary artery calcification in diabetics with and without chronic kidney disease. Kidney Int. 2005;68(3):1258–1266. doi: 10.1111/j.1523-1755.2005.00522.x. [DOI] [PubMed] [Google Scholar]
- 11.Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, Raggi P. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005;68(4):1815–1824. doi: 10.1111/j.1523-1755.2005.00600.x. [DOI] [PubMed] [Google Scholar]
- 12.Moe SM, O'Neill KD, Reslerova M, Fineberg N, Persohn S, Meyer CA. Natural history of vascular calcification in dialysis and transplant patients. Nephrol Dial Transplant. 2004;19(9):2387–2393. doi: 10.1093/ndt/gfh303. [DOI] [PubMed] [Google Scholar]
- 13.Schwarz U, Buzello M, Ritz E, Stein G, Raabe G, Wiest G, Mall G, Amann K. Morphology of coronary atherosclerotic lesions in patients with end-stage renal failure. Nephrology, Dialysis, Transplantation. 2000;15(2):218–223. doi: 10.1093/ndt/15.2.218. [DOI] [PubMed] [Google Scholar]
- 14.Gross ML, Meyer HP, Ziebart H, Rieger P, Wenzel U, Amann K, Berger I, Adamczak M, Schirmacher P, Ritz E. Calcification of coronary intima and media: immunohistochemistry, backscatter imaging, and x-ray analysis in renal and nonrenal patients. Clin J Am Soc Nephrol. 2007;2(1):121–134. doi: 10.2215/CJN.01760506. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura S, Ishibashi-Ueda H, Niizuma S, Yoshihara F, Horio T, Kawano Y. Coronary calcification in patients with chronic kidney disease and coronary artery disease. Clin J Am Soc Nephrol. 2009;4(12):1892–1900. doi: 10.2215/CJN.04320709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moe SM, O'Neill KD, Duan D, Ahmed S, Chen NX, Leapman SB, Fineberg N, Kopecky K. Medial artery calcification in ESRD patients is associated with deposition of bone matrix proteins. Kidney Int. 2002;61(2):638–647. doi: 10.1046/j.1523-1755.2002.00170.x. [DOI] [PubMed] [Google Scholar]
- 17.Allison MA, Hsi S, Wassel CL, Morgan C, Ix JH, Wright CM, Criqui MH. Calcified atherosclerosis in different vascular beds and the risk of mortality. Arterioscler Thromb Vasc Biol. 2012;32(1):140–146. doi: 10.1161/ATVBAHA.111.235234. [DOI] [PubMed] [Google Scholar]
- 18.Chen NX, O'Neill KD, Chen X, Moe SM. Annexin-mediated matrix vesicle calcification in vascular smooth muscle cells. J Bone Miner Res. 2008;23(11):1798–1805. doi: 10.1359/JBMR.080604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shroff RC, McNair R, Figg N, Skepper JN, Schurgers L, Gupta A, Hiorns M, Donald AE, Deanfield J, Rees L, Shanahan CM. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation. 2008;118(17):1748–1757. doi: 10.1161/CIRCULATIONAHA.108.783738. [DOI] [PubMed] [Google Scholar]
- 20. Chen NX, Kircelli F, O'Neill KD, Chen X, Moe SM. Verapamil inhibits calcification and matrix vesicle activity of bovine vascular smooth muscle cells. Kidney Int. 2010;77(5):436–442. doi: 10.1038/ki.2009.481. This study provides an evidence of important of extracellular calcium in matrix vesicle activity and calcification.
- 21.Barreto DV, Barreto Fde C, Carvalho AB, Cuppari L, Draibe SA, Dalboni MA, Moyses RM, Neves KR, Jorgetti V, Miname M, Santos RD, Canziani ME. Association of changes in bone remodeling and coronary calcification in hemodialysis patients: a prospective study. Am J Kidney Dis. 2008;52(6):1139–1150. doi: 10.1053/j.ajkd.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 22.Ketteler M, Biggar PH. Review article: Getting the balance right: assessing causes and extent of vascular calcification in chronic kidney disease. Nephrology (Carlton) 2009;14(4):389–394. doi: 10.1111/j.1440-1797.2009.01149.x. [DOI] [PubMed] [Google Scholar]
- 23.Everhart JE, Pettitt DJ, Knowler WC, Rose FA, Bennett PH. Medial arterial calcification and its association with mortality and complications of diabetes. Diabetologia. 1988;31(1):16–23. doi: 10.1007/BF00279127. [DOI] [PubMed] [Google Scholar]
- 24.Mehrotra R, Budoff M, Christenson P, Ipp E, Takasu J, Gupta A, Norris K, Adler S. Determinants of coronary artery calcification in diabetics with and without nephropathy. Kidney Int. 2004;66(5):2022–2031. doi: 10.1111/j.1523-1755.2004.00974.x. [DOI] [PubMed] [Google Scholar]
- 25.Chertow GM, Raggi P, Chasan-Taber S, Bommer J, Holzer H, Burke SK. Determinants of progressive vascular calcification in haemodialysis patients. Nephrol Dial Transplant. 2004 doi: 10.1093/ndt/gfh125. [DOI] [PubMed] [Google Scholar]
- 26.Orakzai SH, Nasir K, Blaha M, Blumenthal RS, Raggi P. Non-HDL cholesterol is strongly associated with coronary artery calcification in asymptomatic individuals. Atherosclerosis. 2009;202(1):289–295. doi: 10.1016/j.atherosclerosis.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Henein MY, Owen A. Statins moderate coronary stenoses but not coronary calcification: results from meta-analyses. Int J Cardiol. 2011;153(1):31–35. doi: 10.1016/j.ijcard.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 28.Raggi P, Cooil B, Callister TQ. Use of electron beam tomography data to develop models for prediction of hard coronary events. Am Heart J. 2001;141(3):375–382. doi: 10.1067/mhj.2001.113220. [DOI] [PubMed] [Google Scholar]
- 29.Budoff MJ, Rader DJ, Reilly MP, Mohler ER, 3rd, Lash J, Yang W, Rosen L, Glenn M, Teal V, Feldman HI. Relationship of estimated GFR and coronary artery calcification in the CRIC (Chronic Renal Insufficiency Cohort) Study. Am J Kidney Dis. 2011;58(4):519–526. doi: 10.1053/j.ajkd.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Detrano R. The ethnic-specific nature of mechanisms for coronary heart disease. J Am Coll Cardiol. 2003;41(1):45–46. doi: 10.1016/s0735-1097(02)02619-0. [DOI] [PubMed] [Google Scholar]
- 31.Elias-Smale SE, Proenca RV, Koller MT, Kavousi M, van Rooij FJ, Hunink MG, Steyerberg EW, Hofman A, Oudkerk M, Witteman JC. Coronary calcium score improves classification of coronary heart disease risk in the elderly: the Rotterdam study. J Am Coll Cardiol. 2010;56(17):1407–1414. doi: 10.1016/j.jacc.2010.06.029. [DOI] [PubMed] [Google Scholar]
- 32.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. Jama. 2004;291(2):210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 33.Kalsch H, Lehmann N, Mohlenkamp S, Neumann T, Slomiany U, Schmermund A, Stang A, Moebus S, Bauer M, Mann K, Jockel KH, Erbel R. Association of coronary artery calcium and congestive heart failure in the general population: Results of the Heinz Nixdorf Recall study. Clin Res Cardiol. 2010;99(3):175–182. doi: 10.1007/s00392-009-0104-3. [DOI] [PubMed] [Google Scholar]
- 34. Peters SA, Bakker M, den Ruijter HM, Bots ML. Added value of CAC in risk stratification for cardiovascular events: a systematic review. Eur J Clin Invest. 2012;42(1):110–116. doi: 10.1111/j.1365-2362.2011.02555.x. This systemic review provides important informations regarding the risk factors for coronary artery calcification
- 35. Savoia C, Burger D, Nishigaki N, Montezano A, Touyz RM. Angiotensin II and the vascular phenotype in hypertension. Expert Rev Mol Med. 2011;13:e11. doi: 10.1017/S1462399411001815. This reviw describes the roles of renin-angiotensin systems in VSMC growth, apoptosis, differentiation and calcifiation.
- 36. Armstrong ZB, Boughner DR, Drangova M, Rogers KA. Angiotensin II type 1 receptor blocker inhibits arterial calcification in a pre-clinical model. Cardiovasc Res. 2011;90(1):165–170. doi: 10.1093/cvr/cvq391. This article provides the evidence of angiotensin receptor in the development of arterial calcification.
- 37.Tokumoto M, Mizobuchi M, Finch JL, Nakamura H, Martin DR, Slatopolsky E. Blockage of the renin-angiotensin system attenuates mortality but not vascular calcification in uremic rats: sevelamer carbonate prevents vascular calcification. Am J Nephrol. 2009;29(6):582–591. doi: 10.1159/000192844. [DOI] [PubMed] [Google Scholar]
- 38.Wu SY, Yu YR, Cai Y, Jia LX, Wang X, Xiao CS, Tang CS, Qi YF. Endogenous aldosterone is involved in vascular calcification in rat. Exp Biol Med (Maywood) 2012;237(1):31–37. doi: 10.1258/ebm.2011.011175. [DOI] [PubMed] [Google Scholar]
- 39.Chen NX, Duan D, O'Neill K D, Moe SM. High glucose increases the expression of Cbfa1 and BMP-2 and enhances the calcification of vascular smooth muscle cells. Nephrol Dial Transplant. 2006;21(12):3435–3442. doi: 10.1093/ndt/gfl429. [DOI] [PubMed] [Google Scholar]
- 40.Al-Aly Z, Shao JS, Lai CF, Huang E, Cai J, Behrmann A, Cheng SL, Towler DA. Aortic Msx2-Wnt calcification cascade is regulated by TNF-alpha-dependent signals in diabetic Ldlr−/− mice. Arterioscler Thromb Vasc Biol. 2007;27(12):2589–2596. doi: 10.1161/ATVBAHA.107.153668. [DOI] [PubMed] [Google Scholar]
- 41.Bostrom KI, Jumabay M, Matveyenko A, Nicholas SB, Yao Y. Activation of vascular bone morphogenetic protein signaling in diabetes mellitus. Circ Res. 2011;108(4):446–457. doi: 10.1161/CIRCRESAHA.110.236596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parhami F, Basseri B, Hwang J, Tintut Y, Demer LL. High-density lipoprotein regulates calcification of vascular cells. Circ Res. 2002;91(7):570–576. doi: 10.1161/01.res.0000036607.05037.da. [DOI] [PubMed] [Google Scholar]
- 43. Ting TC, Miyazaki-Anzai S, Masuda M, Levi M, Demer LL, Tintut Y, Miyazaki M. Increased lipogenesis and stearate accelerate vascular calcification in calcifying vascular cells. J Biol Chem. 2011;286(27):23938–23949. doi: 10.1074/jbc.M111.237065. This article provides evidence for lipid metabolism and lipidgenesis as a risk factor for vascular calcification.
- 44.Abedin M, Lim J, Tang TB, Park D, Demer LL, Tintut Y. N-3 fatty acids inhibit vascular calcification via the p38-mitogen-activated protein kinase and peroxisome proliferator-activated receptor-gamma pathways. Circ Res. 2006;98(6):727–729. doi: 10.1161/01.RES.0000216009.68958.e6. [DOI] [PubMed] [Google Scholar]
- 45.Parhami F, Morrow AD, Balucan J, Leitinger N, Watson AD, Tintut Y, Berliner JA, Demer LL. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arteriosclerosis, Thrombosis & Vascular Biology. 1997;17(4):680–687. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- 46.Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107(3):363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 47.Kimmel PL, Phillips TM, Simmens SJ, Peterson RA, Weihs KL, Alleyne S, Cruz I, Yanovski JA, Veis JH. Immunologic function and survival in hemodialysis patients. Kidney Int. 1998;54(1):236–244. doi: 10.1046/j.1523-1755.1998.00981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oh J, Wunsch R, Turzer M, Bahner M, Raggi P, Querfeld U, Mehls O, Schaefer F. Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation. 2002;106(1):100–105. doi: 10.1161/01.cir.0000020222.63035.c0. [DOI] [PubMed] [Google Scholar]
- 49.Stompor T, Krasniak A, Sulowicz W, Dembinska-Kiec A, Janda K, Wojcik K, Tabor B, Kowalczyk-Michalek ME, Zdzienicka A, Janusz-Grzybowska E. Changes in common carotid artery intima-media thickness over 1 year in patients on peritoneal dialysis. Nephrol Dial Transplant. 2005;20(2):404–412. doi: 10.1093/ndt/gfh597. [DOI] [PubMed] [Google Scholar]
- 50.Aikawa E, Nahrendorf M, Figueiredo JL, Swirski FK, Shtatland T, Kohler RH, Jaffer FA, Aikawa M, Weissleder R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation. 2007;116(24):2841–2850. doi: 10.1161/CIRCULATIONAHA.107.732867. [DOI] [PubMed] [Google Scholar]
- 51.Tintut Y, Patel J, Parhami F, Demer LL. Tumor necrosis factor-alpha promotes in vitro calcification of vascular cells via the cAMP pathway. Circulation. 2000;102(21):2636–2642. doi: 10.1161/01.cir.102.21.2636. [DOI] [PubMed] [Google Scholar]
- 52.Tintut Y, Patel J, Territo M, Saini T, Parhami F, Demer LL. Monocyte/macrophage regulation of vascular calcification in vitro. Circulation. 2002;105(5):650–655. doi: 10.1161/hc0502.102969. [DOI] [PubMed] [Google Scholar]
- 53.Okazaki H, Shioi A, Hirowatari K, Koyama H, Fukumoto S, Ishimura E, Nishizawa Y. Phosphatidylinositol 3-kinase/Akt pathway regulates inflammatory mediators-induced calcification of human vascular smooth muscle cells. Osaka City Med J. 2009;55(2):71–80. [PubMed] [Google Scholar]
- 54.Amore A, Coppo R. Immunological basis of inflammation in dialysis. Nephrol Dial Transplant. 2002;17(Suppl 8):16–24. doi: 10.1093/ndt/17.suppl_8.16. [DOI] [PubMed] [Google Scholar]
- 55.Zoccali C, Mallamaci F, Tripepi G. Novel cardiovascular risk factors in end-stage renal disease. J Am Soc Nephrol. 2004;15(Suppl 1):S77–S80. doi: 10.1097/01.asn.0000093240.84097.fe. [DOI] [PubMed] [Google Scholar]
- 56. Yamada S, Taniguchi M, Tokumoto M, Toyonaga J, Fujisaki K, Suehiro T, Noguchi H, Iida M, Tsuruya K, Kitazono T. The antioxidant tempol ameliorates arterial medial calcification in uremic rats: Important role of oxidative stress in the pathogenesis of vascular calcification in chronic kidney disease. J Bone Miner Res. 2011 doi: 10.1002/jbmr.539. This study demosntrated that oxidative stress induced by uremia may play a role in the development of vascular calcification in CKD.
- 57.Sutra T, Morena M, Bargnoux AS, Caporiccio B, Canaud B, Cristol JP. Superoxide production: a procalcifying cell signalling event in osteoblastic differentiation of vascular smooth muscle cells exposed to calcification media. Free Radic Res. 2008;42(9):789–797. doi: 10.1080/10715760802400766. [DOI] [PubMed] [Google Scholar]
- 58.Liberman M, Bassi E, Martinatti MK, Lario FC, Wosniak J, Jr, Pomerantzeff PM, Laurindo FR. Oxidant generation predominates around calcifying foci and enhances progression of aortic valve calcification. Arterioscler Thromb Vasc Biol. 2008;28(3):463–470. doi: 10.1161/ATVBAHA.107.156745. [DOI] [PubMed] [Google Scholar]
- 59.You H, Yang H, Zhu Q, Li M, Xue J, Gu Y, Lin S, Ding F. Advanced oxidation protein products induce vascular calcification by promoting osteoblastic trans-differentiation of smooth muscle cells via oxidative stress and ERK pathway. Ren Fail. 2009;31(4):313–319. doi: 10.1080/08860220902875182. [DOI] [PubMed] [Google Scholar]
- 60.Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, McDonald JM, Chen Y. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283(22):15319–15327. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tintut Y, Parhami F, Tsingotjidou A, Tetradis S, Territo M, Demer LL. 8-Isoprostaglandin E2 enhances receptor-activated NFkappa B ligand (RANKL)-dependent osteoclastic potential of marrow hematopoietic precursors via the cAMP pathway. J Biol Chem. 2002;277(16):14221–14226. doi: 10.1074/jbc.M111551200. [DOI] [PubMed] [Google Scholar]
- 62.Tseng W, Lu J, Bishop GA, Watson AD, Sage AP, Demer L, Tintut Y. Regulation of interleukin-6 expression in osteoblasts by oxidized phospholipids. J Lipid Res. 2010;51(5):1010–1016. doi: 10.1194/jlr.M001099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Demer LL. Vascular calcification and osteoporosis: inflammatory responses to oxidized lipids. Int J Epidemiol. 2002;31(4):737–741. doi: 10.1093/ije/31.4.737. [DOI] [PubMed] [Google Scholar]
- 64.Niwa T, Katsuzaki T, Miyazaki S, Miyazaki T, Ishizaki Y, Hayase F, Tatemichi N, Takei Y. Immunohistochemical detection of imidazolone, a novel advanced glycation end product, in kidneys and aortas of diabetic patients. J Clin Invest. 1997;99(6):1272–1280. doi: 10.1172/JCI119285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miyata T, Sprague SM. Advanced glycation of beta 2-microglobulin in the pathogenesis of bone lesions in dialysis-associated amyloidosis. Nephrology, Dialysis, Transplantation. 1996;11(Suppl 3):86–90. doi: 10.1093/ndt/11.supp3.86. [DOI] [PubMed] [Google Scholar]
- 66.Sakata N, Noma A, Yamamoto Y, Okamoto K, Meng J, Takebayashi S, Nagai R, Horiuchi S. Modification of elastin by pentosidine is associated with the calcification of aortic media in patients with end-stage renal disease. Nephrol Dial Transplant. 2003;18(8):1601–1609. doi: 10.1093/ndt/gfg200. [DOI] [PubMed] [Google Scholar]
- 67.Yamagishi S, Fujimori H, Yonekura H, Tanaka N, Yamamoto H. Advanced glycation endproducts accelerate calcification in microvascular pericytes. Biochem Biophys Res Commun. 1999;258(2):353–357. doi: 10.1006/bbrc.1999.0625. [DOI] [PubMed] [Google Scholar]
- 68.Tanikawa T, Okada Y, Tanikawa R, Tanaka Y. Advanced glycation end products induce calcification of vascular smooth muscle cells through RAGE/p38 MAPK. J Vasc Res. 2009;46(6):572–580. doi: 10.1159/000226225. [DOI] [PubMed] [Google Scholar]
- 69.Naka Y, Bucciarelli LG, Wendt T, Lee LK, Rong LL, Ramasamy R, Yan SF, Schmidt AM. RAGE axis: Animal models and novel insights into the vascular complications of diabetes. Arterioscler Thromb Vasc Biol. 2004;24(8):1342–1349. doi: 10.1161/01.ATV.0000133191.71196.90. [DOI] [PubMed] [Google Scholar]
- 70.Ren X, Shao H, Wei Q, Sun Z, Liu N. Advanced glycation end-products enhance calcification in vascular smooth muscle cells. J Int Med Res. 2009;37(3):847–854. doi: 10.1177/147323000903700329. [DOI] [PubMed] [Google Scholar]
- 71.Suga T, Iso T, Shimizu T, Tanaka T, Yamagishi S, Takeuchi M, Imaizumi T, Kurabayashi M. Activation of receptor for advanced glycation end products induces osteogenic differentiation of vascular smooth muscle cells. J Atheroscler Thromb. 2011;18(8):670–683. doi: 10.5551/jat.7120. [DOI] [PubMed] [Google Scholar]
- 72.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: a national study. American Journal of Kidney Diseases. 1998;31(4):607–617. doi: 10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 73.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342(20):1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 74.Chertow GM, Burke SK, Raggi P. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62(1):245–252. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 75.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005;112(17):2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. [DOI] [PubMed] [Google Scholar]
- 76.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87(7):E10–E17. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 77.Jono S, Peinado C, Giachelli CM. Phosphorylation of osteopontin is required for inhibition of vascular smooth muscle cell calcification. J Biol Chem. 2000;275(26):20197–20203. doi: 10.1074/jbc.M909174199. [DOI] [PubMed] [Google Scholar]
- 78.Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM. Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res. 2001;89(12):1147–1154. doi: 10.1161/hh2401.101070. [DOI] [PubMed] [Google Scholar]
- 79.Werner A, Dehmelt L, Nalbant P. Na+-dependent phosphate cotransporters: the NaPi protein families. Journal of Experimental Biology. 1998;201(Pt 23):3135–3142. doi: 10.1242/jeb.201.23.3135. [DOI] [PubMed] [Google Scholar]
- 80.Li X, Yang HY, Giachelli CM. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circ Res. 2006;98(7):905–912. doi: 10.1161/01.RES.0000216409.20863.e7. [DOI] [PubMed] [Google Scholar]
- 81.Chen NX, O'Neill KD, Duan D, Moe SM. Phosphorus and uremic serum up-regulate osteopontin expression in vascular smooth muscle cells. Kidney Int. 2002;62(5):1724–1731. doi: 10.1046/j.1523-1755.2002.00625.x. [DOI] [PubMed] [Google Scholar]
- 82.Chen NX, Duan D, O'Neill K D, Wolisi GO, Koczman JJ, Laclair R, Moe SM. The mechanisms of uremic serum-induced expression of bone matrix proteins in bovine vascular smooth muscle cells. Kidney Int. 2006 doi: 10.1038/sj.ki.5001663. [DOI] [PubMed] [Google Scholar]
- 83.Hosaka N, Mizobuchi M, Ogata H, Kumata C, Kondo F, Koiwa F, Kinugasa E, Akizawa T. Elastin degradation accelerates phosphate-induced mineralization of vascular smooth muscle cells. Calcif Tissue Int. 2009;85(6):523–529. doi: 10.1007/s00223-009-9297-8. [DOI] [PubMed] [Google Scholar]
- 84.Simionescu A, Philips K, Vyavahare N. Elastin-derived peptides and TGF-beta1 induce osteogenic responses in smooth muscle cells. Biochem Biophys Res Commun. 2005;334(2):524–532. doi: 10.1016/j.bbrc.2005.06.119. [DOI] [PubMed] [Google Scholar]
- 85.Lee KS, Kim HJ, Li QL, Chi XZ, Ueta C, Komori T, Wozney JM, Kim EG, Choi JY, Ryoo HM, Bae SC. Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol Cell Biol. 2000;20(23):8783–8792. doi: 10.1128/mcb.20.23.8783-8792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bouvet C, Moreau S, Blanchette J, de Blois D, Moreau P. Sequential activation of matrix metalloproteinase 9 and transforming growth factor beta in arterial elastocalcinosis. Arterioscler Thromb Vasc Biol. 2008;28(5):856–862. doi: 10.1161/ATVBAHA.107.153056. [DOI] [PubMed] [Google Scholar]
- 87. Chen NX, O’Neill Kalisha D., Chen Kiattisunthorn Xianming, Kraiwporn, Gattone Vincent H., Moe Sharon M. Activation of Arterial Matrix Metalloproteinases Leads to Vascular Calcification in Chronic Kidney Disease. American Journal of Nephrology. 2011;Vol. 34(No. 3) doi: 10.1159/000330175. This study suggests that the matrix degradation plays an important role in vascular calcification in CKD.
- 88.Moe SM, Chertow GM. The Case against Calcium-Based Phosphate Binders. Clin J Am Soc Nephrol. 2006;1(4):697–703. doi: 10.2215/CJN.00560206. [DOI] [PubMed] [Google Scholar]
- 89.Yang H, Curinga G, Giachelli CM. Elevated extracellular calcium levels induce smooth muscle cell matrix mineralization in vitro. Kidney Int. 2004;66(6):2293–2299. doi: 10.1111/j.1523-1755.2004.66015.x. [DOI] [PubMed] [Google Scholar]
- 90.Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol. 2004;15(11):2857–2867. doi: 10.1097/01.ASN.0000141960.01035.28. [DOI] [PubMed] [Google Scholar]
- 91.Lomashvili KA, Cobbs S, Hennigar RA, Hardcastle KI, O'Neill WC. Phosphate-induced vascular calcification: role of pyrophosphate and osteopontin. J Am Soc Nephrol. 2004;15(6):1392–1401. doi: 10.1097/01.asn.0000128955.83129.9c. [DOI] [PubMed] [Google Scholar]
- 92. Shroff RC, McNair R, Skepper JN, Figg N, Schurgers LJ, Deanfield J, Rees L, Shanahan CM. Chronic mineral dysregulation promotes vascular smooth muscle cell adaptation and extracellular matrix calcification. J Am Soc Nephrol. 2010;21(1):103–112. doi: 10.1681/ASN.2009060640. This study demonstrates an important role of calcium in VSMC calcification.
- 93.Kapustin AN, Davies JD, Reynolds JL, McNair R, Jones GT, Sidibe A, Schurgers LJ, Skepper JN, Proudfoot D, Mayr M, Shanahan CM. Calcium regulates key components of vascular smooth muscle cell-derived matrix vesicles to enhance mineralization. Circ Res. 2011;109(1):e1–e12. doi: 10.1161/CIRCRESAHA.110.238808. [DOI] [PubMed] [Google Scholar]
- 94.Wolf M. Fibroblast growth factor 23 and the future of phosphorus management. Curr Opin Nephrol Hypertens. 2009;18(6):463–468. doi: 10.1097/MNH.0b013e328331a8c8. [DOI] [PubMed] [Google Scholar]
- 95.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390(6655):45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 96.Balci M, Kirkpantur A, Gulbay M, Gurbuz OA. Plasma fibroblast growth factor-23 levels are independently associated with carotid artery atherosclerosis in maintenance hemodialysis patients. Hemodial Int. 2010;14(4):425–432. doi: 10.1111/j.1542-4758.2010.00480.x. [DOI] [PubMed] [Google Scholar]
- 97. Desjardins L, Liabeuf S, Renard C, Lenglet A, Lemke HD, Choukroun G, Drueke TB, Massy ZA. FGF23 is independently associated with vascular calcification but not bone mineral density in patients at various CKD stages. Osteoporos Int. 2011 doi: 10.1007/s00198-011-1838-0. This study demonstrates an important role of FGF23 in vascular calcification in CKD.
- 98.Stubbs J, Liu S, Quarles LD. Role of fibroblast growth factor 23 in phosphate homeostasis and pathogenesis of disordered mineral metabolism in chronic kidney disease. Semin Dial. 2007;20(4):302–308. doi: 10.1111/j.1525-139X.2007.00308.x. [DOI] [PubMed] [Google Scholar]
- 99.El-Abbadi MM, Pai AS, Leaf EM, Yang HY, Bartley BA, Quan KK, Ingalls CM, Liao HW, Giachelli CM. Phosphate feeding induces arterial medial calcification in uremic mice: role of serum phosphorus, fibroblast growth factor-23, and osteopontin. Kidney Int. 2009;75(12):1297–1307. doi: 10.1038/ki.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Takei Y, Yamamoto H, Sato T, Otani A, Kozai M, Masuda M, Taketani Y, Muto-Sato K, Lanske B, Takeda E. Stanniocalcin 2 is associated with ectopic calcification in alpha-klotho mutant mice and inhibits hyperphosphatemia-induced calcification in aortic vascular smooth muscle cells. Bone. 2012 doi: 10.1016/j.bone.2012.01.006. [DOI] [PubMed] [Google Scholar]