Abstract
Artificial transcription factors (ATFs) are potent synthetic biology tools for modulating endogenous gene expression and precision genome editing. The ribbon–helix–helix (RHH) superfamily of transcription factors are widespread in bacteria and archaea. The principal DNA binding determinant in this family comprises a two-stranded antiparallel β-sheet (ribbons) in which a pair of eight-residue motifs insert into the major groove. Here, we demonstrate that ribbons of divergent RHH proteins are compact and portable elements that can be grafted into a common α-helical scaffold producing active ATFs. Hybrid proteins cooperatively recognize DNA sites possessing core tetramer boxes whose functional spacing is dictated by interactions between the α-helical backbones. These interactions also promote combinatorial binding of chimeras with different transplanted ribbons, but identical backbones, to synthetic sites bearing cognate boxes for each protein either in vitro or in vivo. The composite assembly of interacting hybrid proteins offers potential advantages associated with combinatorial approaches to DNA recognition compared with ATFs that involve binding of a single protein. Moreover, the new class of RHH ATFs may be utilized to re-engineer transcriptional circuits, or may be enhanced with affinity tags, fluorescent moieties or other elements for targeted genome marking and manipulation in bacteria and archaea.
INTRODUCTION
The activities of sequence-specific DNA binding proteins are vital to a multitude of cellular processes from transcriptional regulation to genome segregation, and from the movement of transposable elements to site-specific recombination (1). In particular, transcription factors that bind DNA are fundamental for controlling and tuning expression of single genes or multigene regulons in response to diverse environmental and metabolic cues. Transcription factors are equipped with a variety of binding domains that interact with DNA, most frequently with the major groove via α-helical structural elements (2).
There is now considerable interest in designing artificial transcription factors (ATFs) for rational rewiring of gene circuits in synthetic biology, for precision targeting and editing of novel genomic addresses, and for developing regulatable expression systems that control gene clusters introduced into heterologous hosts for metabolic engineering (3–5). Custom ATFs based on Cys2–His2–type zinc finger DNA binding proteins have emerged as specially promising candidates with far-reaching and diverse applications (6). Notably, zinc finger proteins fused to nuclease domains can be targeted to pre-defined genomic locations allowing for precise chromosome manipulation in a range of eucaryotes (7). Another class of ATF, transcription activator-like effectors, have been designed for specific binding and transcriptional regulation of endogenous genes (8,9). The rational design of miniature proteins that bind DNA in vitro also has met with significant, albeit limited, success (10,11). However, fabrication of miniature protein libraries that specifically recognize arrays of discrete DNA motifs has not yet been described.
Ribbon–helix–helix (RHH) transcription factors are found widely in bacteria and archaea. Although the primary sequences of RHH proteins are diverse, they possess a common homodimeric structure that comprises a pair of antiparallel β-strands (ribbons) framed within an α-helical scaffold (12) (Figure 1A). Typically, numerous RHH dimers assemble cooperatively on an array of regularly spaced DNA sites to exert transcriptional repression of a pertinent gene(s). Binding necessitates the assembly of at least two dimers on adjacent sites via protein–protein interactions mediated by the α-helical backbones. The ribbon is the principal binding determinant, inserting into the major groove of its site, the sequence of which is specific for each protein. The ribbon is a remarkably compact binding element as the paired β-strands each comprise only eight identical amino acids. Here, we demonstrate that ribbons with diverse sequences can be exchanged between different α-helical backbones to generate a new class of ATF that can be directed to synthetic DNA sites in vitro. Moreover, pairs of RHH ATFs that possess discrete ribbon motifs can be cooperatively targeted via their identical α-helical scaffolds to chimeric sites that possess core motifs for both proteins in vitro and in vivo. Members of this new type of ATF potentially may be deployed in innovative combinations to engineer novel regulatory circuits, or may be augmented with affinity tags, fluorescent moieties, or other modules that will permit targeted genome identification and manipulation in bacteria and archaea for which ATFs are scarce.
Figure 1.
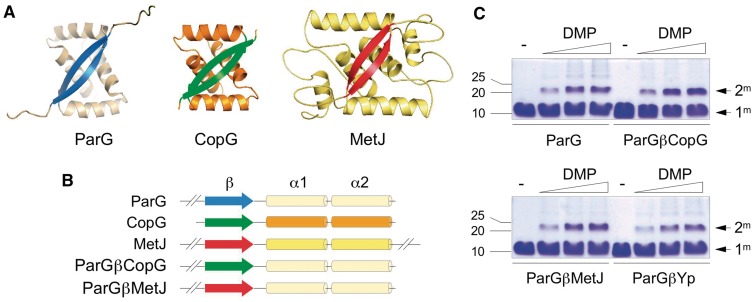
Organization of chimeric ParG:RHH proteins. (A) Tertiary structures of ParG (1P94.pdb), MetJ (1CMA.pdb) and CopG (2CPG.pdb) dimers. α-helices are shown in shades of orange and β-strands in blue (ParG), green (CopG) and red (MetJ). (B) Linear representations of ParG, CopG, MetJ and chimeras ParGβCopG and ParGβMetJ that comprise ribbons of CopG and MetJ, respectively, transplanted into the ParG α-helical scaffold. (C) Chemical cross-linking of ParG, ParGβCopG, ParGβMetJ and ParGβYp with dimethyl pimelimidate (0, 1, 5 and 10 mM, left to right) at 37°C for 60 min followed by SDS–PAGE. Monomeric (1m) and dimeric (2m) species, and positions of molecular weight markers (kDa) are shown.
MATERIALS AND METHODS
Bacterial strains and media
Escherichia coli DH5α was used for plasmid construction, and BL21(DE3) was used for protein overproduction. Strains were grown in Luria–Bertani medium with ampicillin (200 µg/ml) and/or spectinomycin (250 µg/ml) as appropriate for plasmid maintenance.
Gene synthesis and expression, and protein purification
RHH hybrids were generated by overlap extension PCR (13) using Phusion High-Fidelity DNA Polymerase (New England Biolabs) generating blunt-end amplicons. Briefly, hybrids were generated by splicing two amplicons synthesized using parG as template. The first amplicon (PCR-1) contained the ribbon coding sequence of interest at its 3′-end whereas the second amplicon (PCR-2) possessed the same ribbon sequence but at its 5′-end. PCR-3 was performed using equimolar concentrations of purified PCR-1 and PCR-2 as template without primers for the first 10 cycles, followed by an additional 26 cycles in which external primers were added. PCR-3 products containing the spliced recombinant sequence were cloned in pET22b(+) after digestion with NdeI and XhoI. Genes cloned in pET22b(+) also were amplified and subcloned between PfoI and NotI or NdeI and PacI sites in pCDFDuet-1. The gene for ParGYp was produced using a set of overlapping oligonucleotides that were 5′-phosphorylated, annealed, ligated in vitro and cloned in pET22b(+) between NdeI and XhoI sites. The sequences of all cloned inserts were verified. His-tagged proteins were purified as described previously (14).
Gel retardation assays
DNA fragments for gel retardation assays were generated by annealing complementary primers one of which was 5′-biotinylated, or were prepared by second strand synthesis of an unlabeled oligonucleotide using a biotinylated primer. Purification of the fragments and conditions for retardation assays were outlined in detail previously (15). Briefly, biotinylated DNA (2 nM) was incubated for 20 min at 25°C in binding buffer [10 mM Tris–HCl, pH 7.5, 50 mM NaCl, 5 mM MgCl2, 0.005–0.05 mg/ml poly(dI-dC)] with the ParG concentrations shown in figure legends. Reaction mixtures were electrophoresed on 7–10% polyacrylamide gels in 0.5 × Tris–borate-EDTA buffer, pH 8.3, for 50–70 min at 90 V at 22°C. DNA was transferred by capillary action or electroblotting to positively charged nylon membranes (Roche), and the transferred DNA fragments were immobilized by UV cross-linking. Biotin end-labeled DNA was detected using chemiluminescent nucleic acid detection reagents (Thermo Scientific Pierce) (14).
DNaseI footprinting and surface plasmon resonance
Preparation and purification of biotinylated PCR products, conditions for DNase I footprinting reactions, denaturing gel electrophoresis, and detection of biotinylated DNA followed procedures described in detail recently (15). Surface plasmon resonance was performed with a Biacore 3000 instrument (Biacore AB). A Streptavidin chip was primed with immobilization buffer (10 mM Tris–HCl, pH 7.5, 200 mM NaCl, 1 mM EDTA) and then washed with three consecutive 1-min injections (50 µl/min) of activation buffer (1 M NaCl, 50 mM NaOH). A 24-bp biotinylated double-stranded oligonucleotide bearing copGLparGR and a second fragment of identical length but unrelated sequence (5′-CCGACGAAGGAGGTGGCTGGTTGG-3′) were prepared at concentrations of 4 nM in immobilization buffer. The fragments were injected at 10 µl/min through separate flow cells until a value of ∼160 resonance units was detected. Running buffer [200 mM NaCl, 10 mM Tris–HCl pH 7.5, 1.25 ng/µl poly(dI-dC), 0.05% Tween-20, 1 mM DTT] was used both to prime the chip and to prepare protein dilutions at the desired working concentration (1.6 µM). Binding reactions were performed at 23°C in this buffer by injecting 200 µl protein into each flow cell at 50 µl/min. Binding curves were produced by subtracting the signal corresponding to the flow cell with the unrelated DNA sequence from the signal generated by the test sample. Data were analyzed using BIAevaluation software 3.1.
β-Galactosidase assays
124-bp fragments bearing POH1 or POH2 were cloned in the BamHI site upstream of a promoterless lac operon in pRS415 (16) producing transcriptional fusions in which the operon is expressed from the cloned promoter. Strain BL21 harboring the pRS415 derivative was cotransformed with pCDFDuet-1 plasmids encoding the test protein(s). β-galactosidase assays were performed with cells permeabilized with chloroform and SDS (17). Values shown are averages of at least three experiments performed in triplicate.
Kanamycin resistance assays
A promoterless aph gene derived from pET30b(+) was PCR amplified and inserted in place of the lac operon downstream of POH2 in pRS415. Strain DH5α bearing this plasmid and empty pCDFDuet-1 or pCDFDuet-1 encoding the test protein(s) was grown in ampicillin and spectinomycin for plasmid maintenance to OD600 ∼0.6. Cultures were reinoculated (2%) in fresh broth containing both antibiotics, as well as with and without kanamycin (1000 µg/ml). Growth was monitored further at OD600. The data shown are representative of experiments performed in triplicate.
RESULTS
Transplanting ribbon DNA recognition determinants into a common α-helical backbone
ParG is both a RHH centromere-binding protein and transcriptional repressor, and also modulates dynamics of the partner ParF segregation protein (14,15,18–23) (Figure 1). The antiparallel β-strands in ParG comprise symmetrical KRVNVNFD motifs (18), whereas the ribbon that contacts DNA in the CopG RHH protein has the sequence KRLTITLS (differences from ParG underlined) (24). The CopG ribbon was substituted in place of the native ribbon in ParG to produce hybrid protein ParGβCopG (Figure 1B and Supplementary Figure S1). This chimera cross-linked into a dimeric species as efficiently as ParG (14) indicating that the hybrid maintains tertiary structure (Figure 1C). The native ParG ribbon recognizes multiple degenerate 5′-ACTC-3′ boxes in both the centromere and OF operator located upstream of the parF–parG cassette, single dimers multimerizing cooperatively thereby coating the two sites. The 5′-ACTC-3′ boxes are separated invariably by 4-bp AT-rich spacers (15,23). Instead, CopG makes multiple contacts in its co-crystal structure with a 13-bp pseudosymmetric element that spans two 5′-TGCA-3′ motifs (24,25). The ParGβCopG hybrid did not bind a ParG site in which a pair of 5′-ACTC-3′ boxes were substituted with 5′-TGCA-3′ motifs for CopG. CopG makes additional base contacts outside of the 5′-TGCA-3′ motifs (24,25) and 5′-GCAC/T-3′ tetramers overlap these motifs in certain copG sites. Indeed, the co-crystal structure of CopG with DNA (PDB 1b01) reveals that the core base specific recognition contacts span a 5′-GCAC-3′ sequence element (24). The popG synthetic locus comprises a ParG site in which the 5′-ACTC-3′ boxes were replaced by 5′-GCAC/T-3′ boxes. This site was recognized very efficiently by ParGβCopG in gel retardation assays with an apparent Kd ∼1.2 µM (Figure 2A). Binding was specific and cooperative as ParGβCopG did not dock onto sites that possessed a single 5′-GCAC/T-3′ box, for example (see Figure 4B). Inserting T residues adjacent to both 5′-GCAC-3′ boxes in popG to restore the core 5′-TGCA-3′ motifs for CopG did not enhance binding by ParGβCopG (popG1 in Figure 2A; apparent Kd ∼1.2 µM). These results establish that ParGβCopG is a functional chimera with a DNA binding specificity which closely overlaps that of CopG from which the ribbon in this hybrid originates.
Figure 2.
Docking of ParGβCopG and ParGβMetJ onto hybrid sites. (A) The popG locus comprises a site for ParG binding in which 5′-ACTC-3′ boxes were replaced by 5′-GCAC-3′ core motifs recognized by ParGβCopG. The hybrid was tested in gel retardation assays with double-stranded oligonucleotides bearing popG, and with variant sites in which T residues were introduced 5′ of the tetramer motifs (popG1), left and right 5′-GCAC-3′ tetramers were inverted (popG2 and popG3, respectively), and AT contents of regions flanking the 5′-GCAC-3′ boxes were decreased (popG4). Open and filled arrows mark unbound DNA and nucleoprotein complexes, respectively. (B) Sequences derived from sites recognized by ParG and MetJ are represented by blue and red boxes, respectively. The pet1 site bears the sequence recognized by MetJ in the crystal structure; pet2 is similar to pet1 but carries a C-to-T mutation in the spacer between the two 5′-ACGT-3′ boxes; pet3 consists of a ParG site in which core 5′-ACTC-3′ motifs recognized by the protein are replaced with 5′-ACGT-3′ sequences for ParGβMetJ binding. The control is a 24-bp double-stranded oligonucleotide with a randomized sequence. Open and filled arrows mark unbound DNA and nucleoprotein complexes, respectively. (C) Testing ribbon sequence requirements for ParGβCopG binding. The ParG ribbon comprises KRVNVNFD that is altered to KRLTITLS in ParGβCopG which possesses the CopG ribbon (green). Amino acids one and two are common to parental and hybrid proteins. In ParGβCopG.1, residues 3–6 correspond to those in CopG, whereas residues 4 and 6 in ParGβCopG.2 are derived from CopG. Other residues originate from ParG. ParGβCopG.1 and ParGβCopG.2 were tested in gel retardation assays with the popG site. Proteins in panels A–C were used at 0, 0.5, 1.0 and 1.5 µM (left to right).
Figure 4.
Simultaneous binding of ParG and hybrid ParGβCopG to chimeric sites. (A) Sequences of binding sites for ParG, CopG and hybrid sites tested for binding by ParG and/or ParGβCopG. Sequences from sites recognized by ParG and CopG are highlighted in blue and green, respectively. Core recognition motifs for ParG and ParGβCopG—an inverted 5′-ACTC-3′ sequence and 5′-GCAC/T-3′, respectively—are boxed. (B) Gel retardation assays used proteins mixed in equimolar ratios to give final concentrations of 0, 0.5, 1.0 and 1.5 µM (left to right) that were analyzed for binding to the copGLparGR and parGLcopGR sites (left panels). In addition, sites harboring single base-pair deletions or insertions in the central AT-rich regions were tested (middle and right panels, respectively). Open and filled arrows mark unbound DNA and nucleoprotein complexes, respectively. (C) Surface plasmon resonance analysis of ParG and ParGβCopG binding individually and simultaneously to the copGLparGR site. (D) DNase I footprinting of ParG and ParGβCopG on copGLparGR. Proteins were mixed in equimolar ratios to give final concentrations of 0, 3, 9, 16 and 20 µM (left to right). The location of the copGLparGR site is highlighted. L, Maxam–Gilbert A + G sequencing ladder.
Two further characteristics of ParGβCopG and ParG binding were compared. First, ParG recognizes natural and synthetic sites possessing various arrangements of direct and inverted 5′-ACTC-3′ motifs (15,23). Similarly, as well as the popG site containing directly-oriented boxes, ParGβCopG bound substrates bearing inverted 5′-GCAC-3′ boxes (popG2 and popG3, apparent Kd ∼2.5 and 2.1 µM, respectively) (Figure 2A). Second, the 4-bp AT-rich spacers between 5′-ACTC-3′ boxes are read out indirectly by ParG (23). Artificial sites with more GC-rich spacers are bound poorly by the protein partly due to less effective interactions between dimers bound on neighboring boxes (15). Similarly, the popG4 site bearing flanking regions with reduced AT content was bound only weakly by ParGβCopG with an apparent Kd > 4 µM (Figure 2A). Thus, ParGβCopG retains the capacity of ParG dimers to bind to directly- and inversely-oriented core tetramer motifs and, like ParG, is sensitive to the AT-richness of the bases flanking these boxes.
The ribbon of MetJ, another archetypal RHH protein (26), was selected as a second example for designing hybrid proteins. MetJ binds a tandemly repeated 8 bp palindrome, or met-box, with the optimal sequence 5′-AGACGTCT-3′ (27). The MetJ ribbon that recognizes this box is KKITVSIP which differs at six positions (underlined) compared to the ParG β-strands. The ParG ribbon was replaced with the equivalent MetJ sequence producing ParGβMetJ (Figure 1B and Supplementary Figure S1). The protein formed dimers in solution (Figure 1C) and bound weakly to a pair of tandem met-boxes with apparent Kd > 4 µM (pet1 in Figure 2B). As outlined earlier, ParG binding to its native site requires a high AT content between core 5′-ACTC-3′ motifs (19). A single C-to-T transition between met-boxes in pet1 produced pet2 which was bound more avidly by ParGβMetJ (apparent Kd ∼1.6 µM). Alignment of MetJ dimers on met-boxes is centered on the 5′-ACGT-3′ sequence (27). Embedding a pair of 5′-ACGT-3′ boxes in a parG site (pet3) improved binding by ParGβMetJ further still (apparent Kd ∼1.2 µM) (Figure 2B) demonstrating that this core motif from the met-box is sufficient for docking of this hybrid protein.
The combined data with ParGβCopG and ParGβMetJ illustrate that transplantation of diverse ribbons between distantly-related RHH proteins produces functional RHH hybrids. Second, the chimeras possess specific DNA binding activity and recognize 4-bp core motifs that are closely-related to motifs bound by the proteins from which the ribbons originate. Third, the chimeric sites with which the hybrid proteins interact retain features of sites bound by ParG—specifically 4-bp spacers that are AT-rich—that reflect interactions between the α-helical scaffolds of dimers bound at adjacent core sites.
Engineering a functional RHH hybrid necessitates splicing an entire ribbon sequence
Hydrophilic side-chains at positions 2, 4 and 6 within the eight amino acid β-strands of RHH factors make specific nucleotide base contacts during DNA binding (12). To ascertain whether replacing specifically these residues generated an active chimera, a hybrid was designed that contained residues 4 and 6 from CopG with other residues from ParG. The lysine and arginine residues at positions 1 and 2, respectively, are common to parental and hybrid proteins. A second variant possessed residues 3–6 from CopG and residues 7 and 8 from ParG. Neither the ParGβCopG.1 or ParGβCopG.2 variants bound the popG site in gel retardation assays (Figure 2C). ParG, like all DNA binding proteins, binds non-specifically at high concentrations in vitro (18). Similarly, ParGβCopG.1 and ParGβCopG.2 both bound non-specific DNA at concentrations similar to ParG, revealing that these hybrids retain some affinity for DNA, albeit of wider or unknown specificity (Supplementary Figure S2). Grafting specifically the hydrophilic amino acids from the CopG ribbon into ParG may produce hybrids that recognize different DNA sequences compared to the chimera possessing a complete β-strand exchange. However, transplanting an entire ribbon is more likely to produce RHH hybrids with predictable binding specificity than exchanging subsets of β-strand residues.
Database mining and fabrication of hybrids from uncharacterized RHH candidates
We previously have highlighted several ParG homologues from diverse bacterial species (18,21,28). In view of the continued expansion in genome sequencing, reiterative BLAST searching of the NCBI database was used here to identify further ParG homologues. Redundant entries with identical sequences were removed, thereby revealing > 250 unique sequence homologues. Although the tertiary structures of these proteins have not been elucidated, they are predicted RHH factors, like ParG. More than 90 of the homologues possess unique ribbon sequences that are distinct either from that found in prototypical ParG or in other homologues. The differences range from single amino acid variations to predicted ribbons in which all eight residues are dissimilar to those in ParG. Two homologues were selected for further analysis, ParGYp from Yersinia pseudotuberculosis and ParGAs from Aliivibrio salmonicida, which share moderate (46%) and low (18%) sequence identity, respectively, with archetypal ParG. The putative ribbons in ParGYp and ParGAs differ from ParG at four and seven positions, respectively (Figure 3A and Supplementary Figure S1).
Figure 3.
Design and binding of hybrids with ribbon motifs from putative RHH proteins. (A) Left: Sequences of the ParG β-strand and putative ribbons from homologues in Y. pseudotuberculosis and A. salmonicida (GenBank accession numbers CAQ76574 and CAQ81938, respectively). Differences from ParG are highlighted in magenta and black. These motifs were swapped into ParG to generate hybrids ParGβYp and ParGβAs. Right: Organization of 5′-ACTC-3′ boxes in the OF site bound by ParG (15), and 5′-GAC/TA-3′ and 5′-CATT/G-3′ motifs in the Y- and A-sites of Y. pseudotuberculosis and A. salmonicida, respectively. Arrow orientation indicates direct or inverted boxes. (B) Gel retardation assays with OF, the Y-site, ParG and ParGYp. (C) Gel retardation assays with OF, the Y-site, and ParGβYp. Assays in panels (B) and (C) used 0, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 1.0 and 1.5 µM (left to right) of the indicated proteins. (D) Gel retardation assays with OF, the A-site, and ParGβAs. Assays used 0, 0.1, 0.2, 0.3, 0.4 and 0.5 µM (left to right) ParGβAs. Open and filled arrows mark unbound DNA and nucleoprotein complexes, respectively, in panels A–D.
The degenerate 5′-ACTC-3′ motifs separated by 4-bp AT-rich spacers that comprise ParG binding sites (15,23) provide clues for in silico identification of equivalent sites for ParG homologues. Accordingly, examination of the regions upstream of parF homologues that precede genes for ParGYp and ParGAs pinpointed arrays of tetramer repeats separated by 4-bp AT-rich patches: the core motifs are 5′-GAC/T A-3′ and 5′-CATT/G-3′, respectively, forming the Y- and A-sites (Figure 3A). Purified ParGYp bound efficiently to a Y-site fragment, but did not interact with the OF site recognized by ParG. Conversely, ParG did not bind the Y-site demonstrating that the native proteins interact with the cognate sites, but not with non-cognate fragments (Figure 3B).
Hybrids were designed in which the putative ribbons of ParGYp and ParGAs were stitched into the ParG backbone, producing ParGβYp (Figure 1C) and ParGβAs (Supplementary Figure S1). The specificity of ParGβYp resembled that of ParGYp: the chimera bound the Y-site efficiently, did not bind a randomized DNA fragment (data not shown), and recognized the OF fragment only weakly (Figure 3C). The last may reflect indirect readout of the intervening AT-rich patches between the core motifs (23). Analogously, the ParGβAs hybrid assembled the A-site into a ladder of progressively more retarded complexes and ultimately into a major retarded species (Figure 3D). At low concentrations, ParGβAs bound the OF fragment only weakly (Figure 3D) which may reflect a broader binding specificity. In conclusion, the results establish that putative ribbon sequences from RHH factors whose tertiary structures are unelucidated can be substituted into the ParG α-helical scaffold producing functional ATFs whose specificity differs from that provided by the ParG ribbon. Importantly, this strategy also is a powerful approach to assign uncharacterized proteins as RHH factors.
Simultaneous binding of native and chimeric RHH factors to hybrid DNA sites
RHH proteins typically do not bind a single core DNA motif, but identical RHH dimers instead assemble cooperatively on adjacent motifs. For ParG, cooperativity is promoted by interactions between the α-helical backbones across AT-rich spacers of appropriate length that traverse the core motifs. The backbone sequences differ between disparate RHH proteins implying that dimer–dimer interactions are not viable between these proteins. By contrast, chimeras based on ParG possess diverse ribbons but the α-helical scaffold sequences are identical (Figure 1B and Supplementary Figure S1), potentially permitting cooperative docking of RHH proteins with different binding specificities on artificial DNA sites bearing the corresponding motifs. A synthetic site was designed that included a core 5′-ACTC-3′ box from the OF operator bound by ParG (15) fused to a segment derived from a CopG binding site that includes the 5′-GCAC/T-3′ motif bound by ParGβCopG (copGLparGR in Figure 4A). Neither ParG or ParGβCopG alone bound this site in gel retardation or surface plasmon resonance assays verifying that a fragment bearing different subsites is not recognized by the proteins individually (Figure 4B and C). However, when ParG and ParGβCopG were incubated in equimolar ratios with copGLparGR a single retarded species was observed in gel retardation and a strong combined response was evident in surface plasmon resonance. Moreover, DNase I footprinting demonstrated that ParG and ParGβCopG protected a cloned copGLparGR locus specifically (Figure 4D). By contrast with copGLparGR, the parGLcopGR fragment comprises the left and right segments, respectively, of ParG and CopG sites (Figure 4A). This fragment was bound more weakly by ParG and ParGβCopG than the copGLparGR site which may reflect the dispositions of flanking AT bases in the two sites.
The copGLparGR and parGLcopGR sites were designed so that core motifs for ParG and ParGβCopG binding were separated by 4-bp spacers found invariably in natural ParG binding sites. This spacing accommodates cooperative interactions between the proteins’ common α-helical backbones. Altering the interbox spacing in copGLparGR or parGLcopGR by deletion or insertion of a single base pair reduced simultaneous binding by the proteins, emphasizing that the spacer length between core motifs is crucial for optimal binding of ParG-based dimers that possess distinct ribbon sequences (Figure 4B). In summary, the combined data illustrate that RHH proteins with identical α-helical backbones, but heterologous ribbon sequences, can be guided concurrently to a chimeric site designed with subsites for both proteins.
Transcriptional repression by RHH hybrids in vivo
RHH proteins are transcriptional repressors that control expression of single genes or more complex regulons (12). The potential of RHH hybrids to act as transcriptional repressors in vivo was tested by replacing the variant 5′-ACTC-3′ motifs recognized by ParG in the OF operator with 5′-GCAC-3′ boxes for ParGβCopG (Figure 5A). The resulting hybrid promoter-operator (POH1) was inserted as a transcriptional fusion upstream of the lac operon in vector pRS415 (16) driving expression of > 2000 U of β-galactosidase activity in E. coli. Provision of ParGβCopG in trans from a compatible vector reduced expression ∼10-fold (Figure 5B) whereas native ParG repressed expression only ∼2-fold. Thus, the engineered ParGβCopG protein is a functional transcriptional repressor that downregulates a synthetic promoter-operator in vivo.
Figure 5.
Chimera ParGβCopG is a functional transcriptional repressor in vivo. (A) The regulatory region (POF) for parF-parG expression comprises a characteristic Gram-negative promoter in which an array of eight 5′-ACTC-3′ boxes (blue) recognized by ParG is situated downstream of the putative −10 motif (15). These tetramer boxes were replaced by 5′-GCAC-3′ motifs (green) derived from a copG site (Figure 1) in the synthetic POH1 promoter-operator, and by alternating 5′-GCAC-3′ and 5′-ACGT-3′ boxes (red) in POH2—the latter are recognized by ParGβMetJ. (B) Synthetic promoter-operators were cloned as transcriptional fusions to a lacZ gene in pRS415. Intrinsic promoter activities when empty pCDFDuet-1 was present in trans and repression of these activities by ParGβCopG and/or ParGβMetJ produced from pCDFDuet-1 were tested. (C) Resensitization of E. coli to kanamycin. Native regulatory signals controlling expression of aph were supplanted by the POH1 promoter-operator. ParGβCopG provided in trans from a compatible pCDFDuet-1 based vector repressed expression from POH1 and ablated kanamycin resistance (filled diamonds). ParGβMetJ alone (filled squares) and ParGβCopG and ParGβMetJ simultaneously (open triangles) repressed aph expression to intermediate levels. Open circles show growth of a strain bearing the POH1-aph fusion and empty pCDFDuet-1. The inset shows that strains carrying the POH1-aph fusion and either pCDFDuet-1 (open circles) or pCDFDuet-1 expressing ParGβCopG (filled diamonds) grew similarly. (D) Interaction between positively charged lysine at β-strand position one and negatively charged glutamate approaching from a nearby α-helix may assist in stabilizing the ribbon in ParG and functional hybrids. A similar interaction will occur at the other ribbon extremity.
As RHH proteins with identical backbones, but disparate ribbons, can interact in vitro as described earlier, the potential of hybrid pairs to act coordinately on an artificial promoter-operator in vivo was examined. The POH2 sequence includes the OF regulatory region except that alternating 5′-ACTC-3′ motifs were substituted with 5′-GCAC-3′ and 5′-ACGT-3′ boxes for ParGβCopG and ParGβMetJ, respectively (Figure 5A). Unregulated POH2 expressed ∼1500 U of β-galactosidase activity when placed upstream of the lac operon (Figure 5B). ParGβMetJ alone repressed expression from POH2 modestly, whereas ParGβCopG reduced β-galactosidase values ∼10-fold. Interestingly, concomitant production of ParGβMetJ and ParGβCopG repressed expression from POH2 to an intermediate level compared to repression by the separate proteins (Figure 5B). This may result from partial occupation of the hybrid operator by the proteins, may reflect formation of heterodimeric hybrids or differences in protein half-lives may elicit the intermediate repression pattern. Similar repression profiles were noted with other combinations of RHH protein, including wild-type ParG and ParGβCopG on a hybrid operator that possessed binding sites for both proteins (M. Zampini and F. Hayes, unpublished data). Pairs of Arc RHH variants with surface patches of excess positive or negative charge have been described. Some of these variants folded preferentially as heterodimers (29). Analogous charge swaps at the ParG dimer-dimer interface may assist in enforcing tight combinatorial control of ParG chimeras in vivo.
Repression of a second reporter gene by RHH hybrids was examined by placing POH2 upstream of a promoterless kanamycin resistance determinant. Escherichia coli bearing the transcriptional fusion was resistant to >1000 µg/ml of the antibiotic compared to a strain without the fusion that was sensitive to <50 µg/ml kanamycin. ParGβCopG provided in trans repressed expression from POH2, dramatically inhibiting growth in the presence of the antibiotic (Figure 5C). Production of ParGβCopG in the absence of kanamycin did not block growth (Figure 5C, inset). As noted with the POH2-lac fusion, ParGβMetJ alone or with ParGβCopG repressed expression of the POH2-aph fusion to intermediate levels. Overall, the data establish that RHH hybrids can be targeted to synthetic operator sites in vivo. Moreover, combinations of chimeric RHH proteins and operators potentially can be employed to achieve gradations in gene expression.
DISCUSSION
The most successful strides in designing ATFs have been made by engineering extant proteins. Random or site-directed mutagenesis of DNA recognition elements can generate functional derivatives with altered binding properties, although developing variants with new specificities by this route is challenging (30,31). Fusing multiple DNA binding domains with distinct recognition patterns in tandem to generate multidomain proteins that bind extended DNA sites has been fruitful in the case of artificial zinc finger proteins (6,7). Swapping integral DNA recognition determinants between protein scaffolds has proven more taxing: active hybrids have been produced when similar α-helical DNA binding elements have been transplanted between closely related proteins, but exchange between more disparate proteins often generates disrupted or non-functional variants (32,33). By contrast, we have demonstrated here that the ribbon of the RHH superfamily is a compact and highly portable element that can be grafted in its entirety into a universal α-helical scaffold to produce functional chimeras. Among eight residues that comprise the ribbon, the designed hybrids contained four to seven amino acids that differ from those in the supplanted ParG ribbon (Supplementary Figure S1). The spliced antiparallel β-strand structures apparently can be stably accommodated within the common α-helical backbone of ParG and make appropriate DNA major groove contacts. Thus, the interactions that underpin the RHH fold in native proteins are maintained in the hybrids. To our knowledge, the only comparable study with RHH proteins involved swapping residues between ribbons of Arc and Mnt repressors with simultaneous exchange of additional residues outside of the ribbons. These multiple changes were accompanied by alterations in binding specificity (34,35).
Non-specific binding is characteristic of all sequence specific DNA binding proteins when tested at elevated concentrations in vitro. Not all RHH hybrids that we designed were active: chimeras bearing the β-strand motifs from the Arc, ArtA and ParD (36–38) proteins swapped into the ParG backbone did not bind DNA, even non-specifically at high protein concentrations (Supplementary Figures S1 and S3). The tertiary structures of these inactive variants may be disrupted. Proper packing of the ribbon within the α-helical scaffold will influence the stability and functionality of the chimeric proteins. Thus, packing incompatibilities could be associated with the failure of the Arc ribbon to be spliced on the ParG backbone, as it contains phenylalanine as one of the ribbon–helix interface residues (36). Interestingly, each of the ribbons that produced a functional chimera when transplanted into ParG possesses a positively-charged lysine residue at position one of the β-strand, like ParG itself (Figures 2 and 3). By contrast, the Arc, ArtA and ParD ribbons have neutral residues at this position (Supplementary Figure S1). Similarly, the ω and CcdA RHH repressors have non-charged residues at the first positions in their β-strands (39,40) and these ribbons also produced inactive chimeras when grafted onto the ParG backbone (Supplementary Figures S1 and S3). However, when the first positions in the β-strands of these hybrids were altered to lysine, the resulting proteins bound non-specific DNA avidly in the case of the modified ω ribbon and showed a preference for RYRY core motifs in the case of the CcdA ribbon (unpublished data). Further analysis is required to determine the binding specificities of these hybrids and whether they match those associated with the parental ribbons. The interaction of lysine residues at the N-termini of the β-strands with negatively-charged glutamate residues that approach from nearby α-helices may assist in clamping the ribbon into a stable configuration in ParG and in active hybrids (Figure 5D). Determination of the tertiary structures of selected hybrids, both functional and non-functional, will clarify this hypothesis.
ParG recognizes degenerate 5′-ACTC-3′ boxes that are separated consistently by AT-rich spacers. Cooperative dimer–dimer interactions mediated by ParG α-helical scaffolds across the AT-rich regions dictate that cores invariably possess centre-to-centre distances of 4-bp resulting in regular positioning of ParG dimers along the DNA (15,23). Similarly, other RHH proteins generally bind non-palindromic motifs, although often these are extended sequences > 4-bp whose spacing varies from protein to protein (12). Combining several RHH hybrids with different specificities to form composite ATFs and the use of longer binding sites will allow the recognition of extended, more specific sequences in whole bacterial genomes. Moreover, each functional hybrid described here required only substitution of tetramer boxes within a ParG site for efficient binding while adhering to the centre-to-centre distance between these motifs observed with ParG. As illustrated by the ParGYp and ParGAs examples (Figure 3), a very powerful strategy ensues for identification of tetrameric core motifs recognized by hybrid RHH repressors, as well as for pinpointing putative proteins as bona fide RHH factors. This approach also may clarify whether it is feasible to decipher a code that relates ribbon sequences to the DNA sites that they recognize. Elucidation of this code may be simplified using ribbons spliced into a universal α-helical scaffold, as outlined here, so that any contribution of the scaffold to binding is normalized.
ParG multimerizes cooperatively on native DNA sites via backbone interactions between dimers bound at neighboring core motifs (15,23). Athough the RHH hybrids described here possess different ribbons, they are constructed with identical ParG backbones. These common α-helical scaffolds can be used to target pairs of distinct hybrids to synthetic sites that are designed with core motifs for both ribbons in vitro (Figure 4). An intriguing picture also emerged with analogous experiments in vivo. A single hybrid efficiently repressed an artificial promoter-operator in which core motifs for ParG were replaced by motifs for the hybrid (Figure 5B). Thus, the artificial RHH proteins are functional transcriptional repressors that retain the capacity to interfere with the action of RNA polymerase (25,41). Moreover, a promoter-operator designed with alternating core motifs for two different RHH hybrids was repressed strongly by one hybrid, weakly by the second hybrid, and to an intermediate level by both chimeras simutaneously (Figure 5B and C). These results are specially tantalizing as they establish that combinatorial use of RHH hybrids potentially can be used to modulate differential expression of target genes. Achieving gradations of expression is useful, for example, when analyzing toxic genes or when over- and under-expression of a gene of interest is sought to examine effects on cellular pathways. Combinatorial use of RHH ATFs as part of a molecular toolbox also has promise, for example, in targeting specific genomic loci with fluorescent fusion proteins to track movement of these sites during procaryotic chromosome replication and segregation. The hybrids also could be deployed as molecular roadblocks at specific loci to inhibit bacteriophage propagation, may be supplemented with peptide tags or developed into a novel two-hybrid system to study protein–protein interactions in vivo. The composite assembly of interacting RHH ATFs offers advantages associated with combinatorial approaches to DNA recognition compared with existing classes of ATFs that involve binding of a single protein (42). Moreover, the ParG C-terminus accommodates both short peptide affinity tags and > 200 amino acid domains without disrupting DNA binding or dimerization (14), indicating the suitability of the RHH fold for adding effector modules to innovate novel ATFs in procaryotes.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online: Supplementary Figures 1–3.
FUNDING
Biotechnology and Biological Sciences Research Council (to F.H.). Funding for open access charge: The University of Manchester.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Daniela Barillà and Maria Schumacher for incisive reading of the manuscript.
REFERENCES
- 1.Stormo GD, Zhao Y. Determining the specificity of protein-DNA interactions. Nat. Rev. Genet. 2010;11:751–760. doi: 10.1038/nrg2845. [DOI] [PubMed] [Google Scholar]
- 2.Garvie CW, Wolberger C. Recognition of specific DNA sequences. Mol. Cell. 2001;8:937–946. doi: 10.1016/s1097-2765(01)00392-6. [DOI] [PubMed] [Google Scholar]
- 3.Ansari AZ, Mapp AK. Modular design of artificial transcription factors. Curr. Opin. Chem. Biol. 2002;6:765–772. doi: 10.1016/s1367-5931(02)00377-0. [DOI] [PubMed] [Google Scholar]
- 4.Collins CH, Yokobayashi Y, Umeno D, Arnold FH. Engineering proteins that bind, move, make and break DNA. Curr. Opin. Biotechnol. 2003;14:371–378. doi: 10.1016/s0958-1669(03)00091-0. [DOI] [PubMed] [Google Scholar]
- 5.Khalil AS, Collins JJ. Synthetic biology: applications come of age. Nat. Rev. Genet. 2010;11:367–379. doi: 10.1038/nrg2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beerli RR, Barbas CF., III Engineering polydactyl zinc-finger transcription factors. Nat. Biotechnol. 2002;20:135–141. doi: 10.1038/nbt0202-135. [DOI] [PubMed] [Google Scholar]
- 7.Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. Genome editing with engineered zinc finger nucleases. Nat. Rev. Genet. 2010;11:636–646. doi: 10.1038/nrg2842. [DOI] [PubMed] [Google Scholar]
- 8.Miller JC, Tan S, Qiao G, Barlow KA, Wang J, Xia DF, Meng X, Paschon DE, Leung E, Hinkley SJ, et al. A TALE nuclease architecture for efficient genome editing. Nat. Biotechnol. 2011;29:143–148. doi: 10.1038/nbt.1755. [DOI] [PubMed] [Google Scholar]
- 9.Zhang F, Cong L, Lodato S, Kosuri S, Church GM, Arlotta P. Efficient construction of sequence-specific TAL effectors for modulating mammalian transcription. Nat. Biotechnol. 2011;29:149–153. doi: 10.1038/nbt.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chin JW, Schepartz A. Concerted evolution of structure and function in a miniature protein. J. Am. Chem. Soc. 2001;123:2929–2930. doi: 10.1021/ja0056668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart AL, Park JH, Waters ML. Redesign of a WW domain peptide for selective recognition of single-stranded DNA. Biochemistry. 2011;50:2575–2784. doi: 10.1021/bi101116a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schreiter ER, Drennan CL. Ribbon-helix-helix transcription factors: variations on a theme. Nat. Rev. Microbiol. 2007;5:710–720. doi: 10.1038/nrmicro1717. [DOI] [PubMed] [Google Scholar]
- 13.Higuchi R, Krummel B, Saiki RK. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988;16:7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barillà D, Hayes F. Architecture of the ParF-ParG protein complex involved in procaryotic DNA segregation. Mol. Microbiol. 2003;49:487–499. doi: 10.1046/j.1365-2958.2003.03564.x. [DOI] [PubMed] [Google Scholar]
- 15.Zampini M, Derome A, Bailey SES, Barillà D, Hayes F. Recruitment of the ParG segregation protein to different affinity DNA sites. J. Bacteriol. 2009;191:3832–3841. doi: 10.1128/JB.01630-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simons RW, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 17.Miller JH. A Short Course in Bacterial Genetics: A Laboratory Manual for Escherichia coli and Related Bacteria. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 1992. pp. 72–74. [Google Scholar]
- 18.Golovanov AP, Barillà D, Golovanova M, Hayes F, Lian LY. ParG, a protein required for active partition of bacterial plasmids, has a dimeric ribbon-helix-helix structure. Mol. Microbiol. 2003;50:1141–1153. doi: 10.1046/j.1365-2958.2003.03750.x. [DOI] [PubMed] [Google Scholar]
- 19.Carmelo E, Barillà D, Golovanov AP, Lian LY, Derome A, Hayes F. The unstructured N-terminal tail of ParG modulates assembly of a quaternary nucleoprotein complex in transcription repression. J. Biol. Chem. 2005;280:28683–28691. doi: 10.1074/jbc.M501173200. [DOI] [PubMed] [Google Scholar]
- 20.Barillà D, Rosenberg MF, Nobbmann U, Hayes F. Bacterial DNA segregation dynamics mediated by the polymerizing protein ParF. EMBO J. 2005;24:1453–1464. doi: 10.1038/sj.emboj.7600619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barillà D, Carmelo E, Hayes F. The tail of the ParG DNA segregation protein remodels ParF polymers and enhances ATP hydrolysis via an arginine finger-like motif. Proc. Natl Acad. Sci. USA. 2007;104:1811–1816. doi: 10.1073/pnas.0607216104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machón C, Fothergill TJG, Barillà D, Hayes F. Promiscuous stimulation of ParF protein polymerization by heterogeneous centromere binding factors. J. Mol. Biol. 2007;374:1–8. doi: 10.1016/j.jmb.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 23.Wu M, Zampini M, Bussiek M, Hoischen C, Diekmann S, Hayes F. Segrosome assembly at the pliable parH centromere. Nucleic Acids Res. 2011;39:5082–5097. doi: 10.1093/nar/gkr115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomis-Rüth FX, Solá M, Acebo P, Párraga A, Guasch A, Eritja R, González A, Espinosa M, del Solar G, Coll M. The structure of plasmid-encoded transcriptional repressor CopG unliganded and bound to its operator. EMBO J. 1998;17:7404–7415. doi: 10.1093/emboj/17.24.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernández-Arriaga AM, Rubio-Lepe TS, Espinosa M, del Solar G. Repressor CopG prevents access of RNA polymerase to promoter and actively dissociates open complexes. Nucleic Acids Res. 2009;37:4799–4811. doi: 10.1093/nar/gkp503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Augustus AM, Reardon PN, Heller WT, Spicer LD. Structural basis for the differential regulation of DNA by the methionine repressor MetJ. J. Biol. Chem. 2006;281:34269–34276. doi: 10.1074/jbc.M605763200. [DOI] [PubMed] [Google Scholar]
- 27.He YY, Stockley PG, Gold L. In vitro evolution of the DNA binding sites of Escherichia coli methionine repressor, MetJ. J. Mol. Biol. 1996;255:55–66. doi: 10.1006/jmbi.1996.0006. [DOI] [PubMed] [Google Scholar]
- 28.Hayes F. The partition system of multidrug resistance plasmid TP228 includes a novel protein that epitomizes an evolutionarily-distinct subgroup of the ParA superfamily. Mol. Microbiol. 2000;37:528–541. doi: 10.1046/j.1365-2958.2000.02030.x. [DOI] [PubMed] [Google Scholar]
- 29.Nohaile MJ, Hendsch ZS, Tidor B, Sauer RT. Altering dimerization specificity by changes in surface electrostatics. Proc. Natl Acad. Sci. USA. 2001;98:3109–3114. doi: 10.1073/pnas.051624498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salinas RK, Folkers GE, Bonvin AMJJ, Das D, Boelen R, Kaptein R. Altered specificity in DNA binding by the lac repressor: a mutant lac headpiece that mimics the gal repressor. Chembiochem. 2005;6:1628–1637. doi: 10.1002/cbic.200500049. [DOI] [PubMed] [Google Scholar]
- 31.Desai TA, Rodionov DA, Gelfand MS, Alm EJ, Rao CV. Engineering transcription factors with novel DNA-binding specificity using comparative genomics. Nucleic Acids Res. 2009;37:2493–2503. doi: 10.1093/nar/gkp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wharton RP, Brown EL, Ptashne M. Substituting an α-helix switches the sequence-specific DNA interactions of a repressor. Cell. 1984;38:361–369. doi: 10.1016/0092-8674(84)90491-4. [DOI] [PubMed] [Google Scholar]
- 33.Kolkhof P, Teichmann D, Kisters-Woike B, von Wilcken-Bergmann B, Müller-Hill B. Lac repressor with the helix-turn-helix motif of λ cro binds to lac operator. EMBO J. 1992;11:3031–3038. doi: 10.1002/j.1460-2075.1992.tb05373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knight KL, Sauer RT. DNA binding specificity of the Arc and Mnt repressors is determined by a short region of N-terminal residues. Proc. Natl Acad. Sci. USA. 1989;86:797–801. doi: 10.1073/pnas.86.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raumann BE, Knight KL, Sauer RT. Dramatic changes in DNA-binding specificity caused by single residue substitutions in an Arc/Mnt hybrid repressor. Nat. Struct. Biol. 1995;2:1115–1122. doi: 10.1038/nsb1295-1115. [DOI] [PubMed] [Google Scholar]
- 36.Raumann BE, Rould MA, Pabo CO, Sauer RT. DNA recognition by β-sheets in the Arc repressor-operator crystal structure. Nature. 1994;367:754–757. doi: 10.1038/367754a0. [DOI] [PubMed] [Google Scholar]
- 37.Ni L, Jensen SO, Ky Tonthat N, Berg T, Kwong SM, Guan FH, Brown MH, Skurray RA, Firth N, Schumacher MA. The Staphylococcus aureus pSK41 plasmid-encoded ArtA protein is a master regulator of plasmid transmission genes and contains a RHH motif used in alternate DNA-binding modes. Nucleic Acids Res. 2009;37:6970–6983. doi: 10.1093/nar/gkp756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oberer M, Zangger K, Gruber K, Keller W. The solution structure of ParD, the antidote of the ParDE toxin antitoxin module, provides the structural basis for DNA and toxin binding. Protein Sci. 2007;16:1676–1688. doi: 10.1110/ps.062680707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murayama K, Orth P, de la Hoz AB, Alonso JC, Saenger W. Crystal structure of ω transcriptional repressor encoded by Streptococcus pyogenes plasmid pSM19035 at 1.5 Å resolution. J. Mol. Biol. 2001;314:789–796. doi: 10.1006/jmbi.2001.5157. [DOI] [PubMed] [Google Scholar]
- 40.Madl T, Van Melderen L, Mine N, Respondek M, Oberer M, Keller W, Khatai L, Zangger K. Structural basis for nucleic acid and toxin recognition of the bacterial antitoxin CcdA. J. Mol. Biol. 2006;364:170–185. doi: 10.1016/j.jmb.2006.08.082. [DOI] [PubMed] [Google Scholar]
- 41.Smith TL, Sauer RT. Role of operator subsites in Arc repression. J. Mol. Biol. 1996;264:233–242. doi: 10.1006/jmbi.1996.0637. [DOI] [PubMed] [Google Scholar]
- 42.Moretti R, Ansari AZ. Expanding the specificity of DNA targeting by harnessing cooperative assembly. Biochimie. 2008;90:1015–1025. doi: 10.1016/j.biochi.2008.02.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.