Abstract
AIM
To investigate the effect of Bak Foong Pills (BFP) on the expression of β-amyloid (Aβ) in rats retina with optic nerve transection, and its roles and possible mechanisms in protecting optic nerve damage.
METHODS
Seventy-two healthy, Sprague-Dawley, adult rats were randomly assigned to three groups: negative control group (control group), optic nerve transection group (model group) and BFP treatment group (BFP group, 100μg/mL) followed by establishing optic nerve transection model. The expression of Aβ was measured at 48 hours by Western-blotting. Moreover, the expressions of Bcl-2, Bax and Caspase-3 mRNA were evaluated at 48 hours by reverse transcriptase polymerase chain reaction (RT-PCR).
RESULTS
There were significant differences among the control, model and BFP groups in the expression of Aβ (all P<0.01). Aβ expression was significantly higher in the model and BFP groups than that in the control group (P<0.01), with a more significant reduction in the BFP group than that in the model group (P<0.01). Moreover, there were also significant differences among the three groups in the expressions of Bcl-2/Bax (Bcl-2: anti-apoptotic; Bax: proapoptotic) and Caspase-3 mRNA (proapoptotic) (all P<0.01). Bcl-2/Bax ratio was significantly lower and Caspase-3 mRNA expression was significantly higher in the model and BFP groups than those in the control group (P<0.01), with a significant growing of Bcl-2/Bax and reduction of Caspase-3 in the BFP group than those in the model group (P<0.01).
CONCLUSION
BFP can down-regulate Aβ expression in retina and may inhibit apoptosis and protect optic nerve by enhancing Bcl-2/Bax ratio and inhibiting Caspase-3 pathway.
Keywords: Bak Foong Pills, optic nerve damage, β-amyloid protein, Bcl-2/Bax, Caspase-3 mRNA
INTRODUCTION
Diabetic retinopathy, glaucoma, and age-related macular degeneration (AMD) are the most common causes of visual impairment and legal blindness in many countries[1]. One common denominator of these conditions is progressive loss of the neural cells of the eye, of which the mechanisms are very complicated. Apoptosis is widely considered as one of the most important reasons[2],[3]. β-amyloid (Aβ) protein is one of the most important apoptotic factors which is formed after sequential cleavage of the amyloid precursor protein (APP), a transmembrane glycoprotein of undetermined function. APP can be processed by α-, β- and γ-secretases; Aβ protein is generated by successive action of the β- and γ-secretases. Deposition of Aβ peptides in the brain and retina tissues results in neuroinflammation and neurovascular inflammation. Loss of the normal physiological functions of Aβ is also thought to contribute to neuronal dysfunction, including Alzheimer's disease (AD) and optic nerve diseases[4],[5]. In Alzheimer's disease, some experiment findings indicated that the significant increase in the amount of Caspase-3 (proapoptotic) and decrease in Bcl-2/Bax ratio (Bcl-2: anti-apoptotic; Bax: proapoptotic) is one of the most important apoptotic avenues induced by Aβ[6]. But there is still no demonstration of the similar nerve damage induced by Aβ in optic nerve diseases. We studied the expression of Aβ protein in optic nerve transection rats.
As a well-known gynaecological tonic in China, Bak Foong Pills (BFP, China registration #Z980035, also known as Bai Feng Wan) has long been recognized to have estrogen-like activities, such as its abilities to increase the expression of cystic fibrosis transmembrane-conductance regulator, reduce blood pressure, promote vasorelaxation, decrease serum triglyceride, and even its antiplatelet activity and stimulating effects on dopamine release of the brain. To explore the possible neuroprotective effect of BFP and its related molecular mechanism(s) in optic nerve damage, we examined the expression of some of the apoptotic factors such as Caspase-3, Bax, and Bcl-2 that we expected to get the impact from BFP.
MATERIALS AND METHODS
Materials
Seventy-two male Sprague-Dawley (SD) rats (Experimental Animal Center of the Second Xiangya Hospital of Central South University) (License No. SYXK (Xiang) 2004-0013), weighing 230-250g, were given free access to standard rat food and drinking water and cared for in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Then they were divided randomly into negative control group (control group), optic nerve transection group (model group) and Bak Foong Bills treatment group (BFP group), with 24 rats in each group. Optic nerve transection was induced in the model and BPF groups. Rats were anesthetized in a prone position via an intraperitoneal injection of 100g/L chloral hydrate (3.5mL/kg). The temporal eyelid incision was performed to expose the intraorbital optic nerve, which was transected approximately 1mm behind the globe by using microscissors and avoiding interfere with the blood supply (e.g. ophthalmic artery and ciliary vessels) and then sutured. The control animals were exposed to the same surgical procedures without optic nerve transection. BFP was provided by TongRenTang of China, dissolved in 9g/L of NaCl solution preparation of 6g/L mother liquor, sterilized by filter, and stored at -20°C. After the injury, 0.01mL BFP (100mg/L) was injected intravitreally into the eyes of the BFP group 1mm behind the limbus with a 10μL microsyringe. The needle was left in the vitreous cavity for a few seconds after injection to prevent reflux, and an equal volume of physiological saline was administered to the model and control groups. A total of 72 rats were included in this study. Some rats showed agitation for a short time following intravitreal injection and then recovered. No abnormal appearance or rejection occurred, and all rats survived. Rats were selected from each group at 48 hours post-surgery and the retina was removed, which were used for Western blotting and RT-PCR.
Methods
Detection of β-amyloid expression
Extracted protein was loaded on a 120g/L SDS-PAGE gel and transferred onto nitrocellulose membranes. Then proteins were then blocked and incubated with specific antibodies: β-actin (ProMab, USA), β-amyloid (Santa, USA). Membranes were subsequently washed, incubated with specific secondary horseradish peroxidase-conjugated antibodies, and revealed with the enhanced chemiluminescence kit (Life Science Prod Products, USA). The band intensity was analyzed by scanning densitometry.
Detection of Bcl-2, Bax and Caspase-3 mRNA expressions
Total RNA from retina was harvested using Trizol reagent according to the manufacturer's instructions. First-strand cDNA was synthesized by reverse transcriptase (Fermentas RevertAid first Strand cDNA Synthesis Kit) at 42°C using 1μg total RNA extract. RT-PCR was conducted in 0.2mL domed-cap pcr tubes (Axygen) with a total reaction volume of 25μL containing 1μL first-strand reaction product, 0.2nmoL/L specific upstream and downstream primers, and 12.5μL of 2×Taq polymerase buffer (Tiangeng, China). Amplification of cDNA fragments and analysis were carried out. Amplification of the housekeeping gene β-actin mRNA transcript, which served as a normalization standard was carried out. The primers and the conditions of RT-PCR were summarized in Table 1.
Table 1. Primers and conditions in RT-PCR.
| Gene | Primer | Product size | t/°C | |
| Bcl-2 | UpperLower | 5- actttgcagagatgtccagt -35- cggttcaggtactcagtcat -3 | 217bp | 58 |
| Bax | UpperLower | 5- tttgttacagggtttcatcc -35- gcagctccatattattgtcc -3 | 155bp | 53 |
| Caspase-3 | UpperLower | 5- aagactatccatggaagcaa -35- ctctctgaggttagctgcat-3 | 174bp | 55 |
| β-actin | UpperLower | 5-gagagggaaatcgtgcgtgac-35-catctgctggaaggtggaca-3 | 452bp | 61 |
Statistical Analysis
Statistical analysis was performed via SPSS 13.0 software and data were expressed as mean±SD. Results were analyzed using one-way analysis of variance and comparison among groups was performed using the LSD method. P<0.05 was considered statistically significant.
RESULTS
β-amyloid Expression
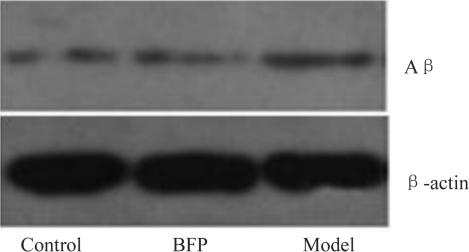
In the retina tissues of the control rats, the expression of Aβ was the weakest (Figure 1), while higher expressions were presented in the model and BFP groups. There was significant difference, compared with the control group (P<0.01). However, the level of Aβ reduced significantly in the BFP group than that in the model group (18.8%→10.6%, P<0.01).
Figure 1. Aβ expression of rat retina.
Bcl-2, Bax and Caspase-3 mRNA Expressions
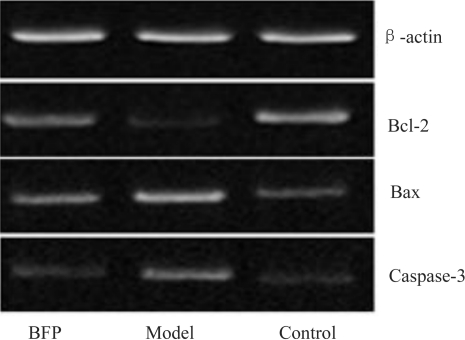
In the retina tissues of the control rats, the expression of Bcl-2 was the highest (Figure 2), while much less expression was presented in the model group and lower expression was presented in the BFP group. There were significant differences, compared with the control group (both P<0.01). However, after BFP intravitreal injection, the level of Bcl-2 had an obvious increase in the BFP group compared with the model group (P<0.01), but was still slightly lower than that in the control group.
Figure 2. Bcl-2, Bax and Caspase-3 expressions in rat retina (RT-PCR).
In the retina tissues of control rats, the expressions of Bax and Caspase-3 were the lowest. And higher expressions were noted in the model groups with statistically significant differences, compared with the control group (all P<0.01). However, after BFP intravitreal injection, the levels of Bax and Caspase-3 obviously decreased in the BFP groups, also there were significant difference, compared with the model group (P<0.01). Moreover, the ratio of Bcl-2/Bax decreased significantly in the model group and reduced in the BFP group compared with the control group (P<0.01). But in the the BFP group the ratio of Bcl-2/Bax increased evidently, also there were significant difference, compared with the model group (P<0.01, Table 2).
Table 2. Bcl-2, Bax and Caspase-3 mRNA in rat retinas.
| Groups | BFP | Model | Control |
| Bcl-2 | 49.77 | 36.43 | 18.04 |
| Bax | 21.51 | 58.38 | 26.42 |
| Caspase-3 | 71.06 | 30.26 | 17.05 |
DISCUSSION
Optic nerve damage may be caused by glaucoma, diabetes, trauma, degeneration and so on. One common feature of these conditions is progressive loss of the neural cells of the eye, for example: photoreceptors, interneurons, and especially retinal ganglion cells. Retinal ganglion cells are usually not able to regenerate their axons after optic nerve injury or in degenerative disorders, resulting in lifelong visual loss[3]. Recent years have seen enormous progress in the study of apoptosis mechanism and treatment options to stop the progression of age-related macular degeneration from a neovascular state to fibrosis, to slow down the worsening of glaucoma by reducing intraocular pressure, and to prevent the deterioration of diabetic retinopathy by optimizing glycemic control and treating retinal neovascularization early[7]-[9]. However, irreversible visual loss still occurs in a significant proportion of cases. Research is aimed at developing novel anti-apoptotic factors and mechanism and novel treatments using neuroprotective and regenerative strategies. Since optic nerve is one part of central nerve system, the view “a sick eye in a sick body” is more and more accepted by ophthalmologists. Therefore, those nerve apoptosis factors are more and more concerned, which play important roles in central nerve diseases, such as Alzheimer's disease and Parkinson's disease[10],[11]. β-amyloid protein is a well-known key element in central nerve degeneration diseases. In our study we found that in the optic nerve transection model rats, the expression of Aβ was significantly higher than the control group, which suggested that Aβ played an important role in the optic nerve damage via apoptosis, as demonstrated in central nerve degeneration diseases.
BFP has long been recognized to have estrogen-like activities, such as its ability to increase the expression of cystic fibrosis transmembrane-conductance regulator, reduce blood pressure, improve vasorelaxation, decrease serum triglyceride, and even have its antiplatelet activity and stimulating effects on dopamine release of the brain. Although BFP contains phytoestrogens such as ginseng that may act similarly to estrogen, which has been shown to prevent neuronal degeneration caused by increased oxidative stress and apoptosis, other components of BFP may act through different mechanisms, the details of which remain to be elucidated. Liu et al[12] has reported an ability of BFP to reverse MPTP-induced reduction in tyrosine hydroxylase expression, suggesting that it has neuroprotective effects, as well as the underlying mechanism(s), different from that of estrogen. It has been reported that BFP has neuroprotective effects to hippocampal neuron injury induced by β-amyloid. We found that although higher expression of β-amyloid was identified in the BFP group compared with the normal control group, the level of Aβ reduced more significantly in BFP treatment group than that in the optic nerve transection group. The results suggest that BFP had neuroprotective effects in optic nerve damage. Moreover, the results of RT-PCR showed that the ratio of Bcl-2/Bax decreased more significantly and the level of caspase-3 increased evidently in model group compared with control group, which may suggest that β-amyloid induce optic nerve apoptosis by down-regulating the ratio of Bcl-2/Bax and up-regulating the gene expression of caspase-3. RT-PCR results also demonstrated that the ratio of Bcl-2/Bax increased more significantly and the level of caspase-3 decreased more evidently in the BFP group than those in the model group. Therefore, we can conclude that BFP could block the excessive apoptosis of optic nerve neuron by enhancing the ratio of Bcl-2/Bax and reducing the gene expression of caspase-3, aiming at the neuroprotective effects. Apoptosis of optic nerve can be inhibited by BFP by protecting from many kinds of optic nerve diseases. However, more extensive and deeper research is still needed to develop novel treatments using neuroprotective and regenerative strategies.
REFERENCES
- 1.Bunce C, Wormald R. Leading causes of certification for blindness and partial sight in England & Wales. BMC Public Health. 2006;6:58. doi: 10.1186/1471-2458-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dratviman-Storobinsky O, Hasanreisoglu M, Offen D, Barhum Y, Weinberger D, Goldenberg-Cohen N. Progressive damage along the optic nerve following induction of crush injury or rodent anterior ischemic optic neuropathy in transgenic mice. Mol Vis. 2008;14:2171–2179. [PMC free article] [PubMed] [Google Scholar]
- 3.Parrilla-Reverter G, Agudo M, Sobrado-Calvo P, Salinas-Navarro M, Villegas-Perez MP, Vidal-Sanz M. Effects of different neurotrophic factors on the survival of retinal ganglion cells after a complete intraorbital nerve crush injury: a quantitative in vivo study. Exp Eye Res. 2009;89(1):32–41. doi: 10.1016/j.exer.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Perl DP. Neuropathology of Alzheimer's disease. Mt Sinai J Med. 2010;77(1):32–42. doi: 10.1002/msj.20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jellinger KA. Recent advances in our understanding of neurodegeneration. J Neural Transm. 2009;116(9):1111–1162. doi: 10.1007/s00702-009-0240-y. [DOI] [PubMed] [Google Scholar]
- 6.Craft JM, Watterson DM, Van Eldik LJ. Human amyloid beta-induced neuroinflammation is an early event in neurodegeneration. Glia. 2006;53(5):484–490. doi: 10.1002/glia.20306. [DOI] [PubMed] [Google Scholar]
- 7.Chakravarthy U, Evans J, Rosenfeld PJ. Age related macular degeneration. BMJ. 2010;340:c981. doi: 10.1136/bmj.c981. [DOI] [PubMed] [Google Scholar]
- 8.Maier PC, Funk J, Schwarzer G, Antes G, Falck-Ytter YT. Treatment of ocular hypertension and open angle glaucoma: meta-analysis of randomized controlled trials. BMJ. 2005;331:134. doi: 10.1136/bmj.38506.594977.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parravano M, Menchini F, Virgili G. Antiangiogenic therapy with anti-vascular endothelial growth factor modalities for diabetic macular oedema. Cochrane Database Syst Rev. 2009:CD007419. doi: 10.1002/14651858.CD007419.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Perl DP. Neuropathology of Alzheimer's disease. Mt Sinai J Med. 2010;77(1):32–42. doi: 10.1002/msj.20157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jellinger KA. Recent advances in our understanding of neurodegeneration. J Neural Transm. 2009;116(9):1111–1162. doi: 10.1007/s00702-009-0240-y. [DOI] [PubMed] [Google Scholar]
- 12.Liu B, Xie JX, Rowlands DK, Gou YL, Leung CC, Chung YW, Chan HC. Neuroprotective Effects of Bak Foong pill in 1-methyl-4-phenyl-1, 2, 3, 6-tetrahyrdropyridine (MPTP)-induced Parkinson's disease model mice. Biol Pharm Bull. 2004;27(8):1245–1250. doi: 10.1248/bpb.27.1245. [DOI] [PubMed] [Google Scholar]