An update to a previous review of European treatment practices is presented based on discussions during an expert meeting that was convened to review novel agent data published or presented at medical meetings until the end of 2011 and to assess their impact on treatment strategies.
Keywords: Multiple myeloma, Novel agents, Thalidomide, Bortezomib, Lenalidomide
Abstract
The management of multiple myeloma has undergone profound changes over the recent past as a result of advances in our understanding of the disease biology as well as improvements in treatment and supportive care strategies. Notably, recent years have seen a surge in studies incorporating the novel agents thalidomide, bortezomib, and lenalidomide into treatment for different disease stages and across different patient groups. This article presents an update to a previous review of European treatment practices and is based on discussions during an expert meeting that was convened to review novel agent data published or presented at medical meetings until the end of 2011 and to assess their impact on treatment strategies.
Introduction
Multiple myeloma (MM) treatment practices have changed substantially in the recent past, most notably with the introduction of several novel agents—thalidomide, bortezomib, and lenalidomide—that are increasingly being incorporated into treatment strategies in everyday clinical practice. These highly active agents are providing tangible benefits for patients with a malignancy that remains incurable in the majority of cases. Importantly, improvements in survival outcomes have been noted, not only in young patients but also in the elderly patient population, for whom the prognosis used to be generally poor with conventional treatments [1–12].
This progress is being driven by intense research efforts into the biology of the disease and into the optimal use of novel agents in different treatment stages and across various age and risk groups, reflected in the large number of studies being reported as abstracts at hematology congresses and in numerous publications dedicated to MM. These trials are not only being conducted by national study groups but, in addition, there are numerous international initiatives and collaborations under the auspices of the European Myeloma Network (EMN), the International Myeloma Working Group (IMWG), and other groups, recognizing that through cooperation in large clinical trials it is possible to translate learnings from those trials more rapidly into clinical practice.
A European expert meeting was convened to discuss the impact of recent data on treatment strategies, and the results of those discussions are summarized in this article. During the discussions, emphasis was placed on providing an update to previously published treatment strategies [13, 14], with a particular focus on the management of frail elderly patients and patients with high-risk disease. The objective is to provide clear suggestions relevant for daily clinical practice based on the best available current evidence. Newer agents currently undergoing development were excluded from the discussions because access to these remains limited at the present time. For a discussion of these investigational agents, the reader is referred to a number of excellent review articles that have recently been published [15–19].
Frontline Treatment
Transplant Setting
For young patients, generally considered to be those aged <65 years, without significant comorbidity, high-dose therapy followed by autologous stem cell transplantation (ASCT) remains the standard of care [20, 21]. This treatment modality offers the chance for a prolonged disease- and treatment-free period, which is an important consideration for young patients in terms of quality of life (QoL), enabling patients to pursue normal activities of daily living.
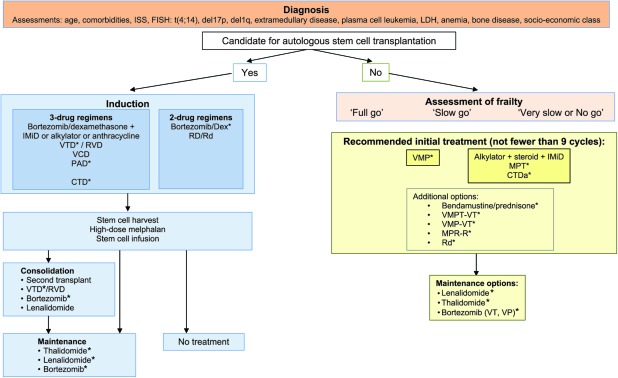
Prior to high-dose melphalan (HDM), patients typically receive induction therapy to reduce the tumor burden. Based on recent data, several effective induction regimens incorporating novel agents can be recommended, as outlined in Figure 1. Data from phase III randomized trials support the use of a combination consisting of bortezomib plus dexamethasone plus an immunomodulatory drug (IMiD) or alkylator or anthracycline (e.g. bortezomib, thalidomide, and dexamethasone [VTD], bortezomib, doxorubicin, and dexamethasone [PAD], bortezomib, cyclophosphamide, and dexamethasone [VCD], lenalidomide, bortezomib, and dexamethasone [RVD]), as well as the use of the combination of cyclophosphamide, thalidomide, and dexamethasone (CTD) as an oral alternative to vincristine, doxorubicin, and dexamethasone (VAD), which has as yet only been tested against cyclophosphamide plus VAD, but not compared with bortezomib- or lenalidomide-containing regimens. Of note, no phase III trial data are available regarding the VCD and RVD induction regimens; however, both are currently undergoing investigation and results from ongoing phase III trials are awaited. Efficacy data for the different combinations are summarized in Table 1. Although the use of three-drug regimens may be preferred because of the superior efficacy in terms of response rates, particularly good responses, for example very good partial response (VGPR) or better, as well as extended event-free (EFS), progression-free (PFS) and overall survival (OS), the use of two-drug regimens, such as bortezomib plus dexamethasone or lenalidomide plus dexamethasone, could be additional options. Of note, although bortezomib plus dexamethasone was found to be significantly superior to VAD in terms of complete response (CR)/near CR and VGPR rates, the PFS interval was not significantly longer [22]. The combination of lenalidomide plus dexamethasone as induction has not been examined in a randomized phase III trial. However, data regarding this induction regimen are available from the phase III Eastern Cooperative Oncology Group trial that compared lenalidomide plus high-dose dexamethasone (RD) with lenalidomide plus low-dose dexamethasone (Rd) (Table 1). In addition, Palumbo et al. [32] employed lenalidomide plus low-dose dexamethasone as induction therapy in a phase III trial designed to examine the role of transplantation by comparing a novel agent–containing regimen with tandem ASCT. Following four cycles of lenalidomide plus low-dose dexamethasone, patients were randomized to receive six cycles of melphalan, prednisone, and lenalidomide (MPR) or tandem ASCT followed by a second randomization to no maintenance versus maintenance with lenalidomide. With a median follow-up of 26 months, the 2-year PFS rate was significantly higher in patients randomized to the transplant arm, whereas the OS times were comparable in the two arms. Longer follow-up of the trial is awaited, and these data together with those from two further ongoing phase III trials—one a collaborative effort by the Dana-Farber Cancer Institute (DFCI) and the Intergroupe Francophone du Myélome (IFM) (IFM/DFCI 2009 trial) and the other an initiative within the EMN (EMN-02 trial)—will help to define the role of transplant in the novel agent era. Induction therapy is typically administered for a short period, three or four cycles, although some trials have also investigated a longer induction period of six cycles [25]. The choice of regimen is clearly influenced by the availability of the agents in the different countries. Of note, none of the novel agents is currently approved by the European Medicines Agency (EMA) for use in the frontline transplant setting, although in several countries their use prior to ASCT is reimbursed by local governments on the basis of favorable results from phase III trials. In the U.S., thalidomide in combination with dexamethasone is indicated for the treatment of patients with newly diagnosed MM [33]. Bortezomib has been approved by the U.S. Food and Drug Administration (FDA) for the treatment of MM without any limitation to treatment setting, whereas lenalidomide is currently not approved for frontline use but is indicated for the treatment of patients who have received at least one prior therapy in combination with dexamethasone [34].
Figure 1.
Multiple myeloma treatment tree outside clinical trials: frontline†.
*Data available from phase III randomized trial.
Abbreviations: CTDa, attenuated cyclophosphamide, thalidomide, and dexamethasone; EMA, European Medicines Agency; Dex, dexamethasone; IMiD, immunomodulatory drug; MPR-R, melphalan, prednisone, and lenalidomide plus lenalidomide maintenance; MPT, melphalan, prednisone, and thalidomide; RD, lenalidomide and high-dose dexamethasone; Rd, lenalidomide and low-dose dexamethasone; RVD, lenalidomide, bortezomib, and dexamethasone; VMP, bortezomib, melphalan, and prednisone; VMPT, VMP plus thalidomide; VP, bortezomib plus prednisone; VT, bortezomib plus thalidomide; VTD, bortezomib, thalidomide, and dexamethasone.
Table 1.
Summary of novel agent–based induction trials
aSignificant difference between arms.
bn = 40 in the Rd arm and n = 50 in the RD arm underwent ASCT.
cBest overall response to treatment.
Abbreviations: ASCT, autologous stem cell transplantation; CR, complete response; CTD, cyclophosphamide, thalidomide, and dexamethasone; CVAD, cyclophosphamide, vincristine, doxorubicin, and dexamethasone; HR, hazard ratio; NA, not available; nCR, near CR; OS, overall survival; PAD, bortezomib, doxorubicin, and dexamethasone; PFS, progression-free survival; RD, lenalidomide and high-dose dexamethasone; Rd, lenalidomide and low-dose dexamethasone; RVD, lenalidomide, bortezomib, and dexamethasone; TTP, time to progression; VAD, vincristine, doxorubicin, and dexamethasone; VCD, bortezomib, cyclophosphamide, and dexamethasone; VD, bortezomib and dexamethasone; VGPR, very good partial response; vtD, reduced dose bortezomib, reduced-dose thalidomide, and dexamethasone; VTD, bortezomib, thalidomide, and dexamethasone.
Administration of a single dose of HDM of 200 mg/m2 is considered the standard conditioning regimen. Following the transplant procedure, the application of consolidation or maintenance therapy or both with the aim of decreasing the risk for relapse and extending PFS and OS times is an attractive approach. Whereas consolidation consists of the administration of a treatment for a limited period of time with the intention of inducing a deeper response following ASCT, maintenance therapy is administered for a prolonged period with the objective of maintaining the response achieved with the ASCT step. A number of options to consolidate a response are available, including the application of a second transplant and the administration of novel agents or novel agent combinations (Fig. 1), and available data with novel agents in this setting are summarized in Table 2A [31, 35–39]. These indicate that the use of consolidation improves the depth of response and might be considered as a reasonable treatment option following ASCT. VTD consolidation was examined in a phase III trial and found to be beneficial after high-dose therapy [37, 40]. However, further data, particularly phase III evidence, are needed to define the optimal strategy in this setting, that is, which agent, which regimen, and for how long.
Table 2.
Summary of novel agent data in the post-ASCT setting
aSignificant difference between arms.
Abbreviations: ASCT, autologous stem cell transplantation; CR, complete response; CTD, cyclophosphamide, thalidomide, and dexamethasone; EFS, event-free survival; HR, hazard ratio; IF, immunofixation-negative; IFN, interferon; NA, not available; nCR, near CR; OS, overall survival; PAD, bortezomib, doxorubicin, and dexamethasone; PCR, polymerase chain reaction; PD, progressive disease; PFS, progression-free survival; RVD, lenalidomide, bortezomib, and dexamethasone; sCR, stringent CR; TAD, thalidomide, doxorubicin, and dexamethasone; TD, thalidomide and dexamethasone; TT2, total therapy 2; TTP, time to progression; VGPR, very good partial response; VTD, bortezomib, thalidomide, and dexamethasone; vtD, reduced dose bortezomib, reduced-dose thalidomide, and dexamethasone.
Similarly, the application of maintenance treatment using one of the effective novel agents is an attractive concept, and a number of studies have investigated such an approach. Thalidomide has been extensively investigated in the post-ASCT setting and has a demonstrated PFS benefit in all trials (Table 2B) [27, 39, 41–50]; however, concerns exist about the shorter OS time following relapse as well as the toxicity associated with long-term treatment and the inability to overcome the poor prognosis related to high-risk cytogenetic abnormalities [51]. Lenalidomide may be a more optimal maintenance agent because of the absence of neurotoxicity, and convincing efficacy data in support of this treatment are available from trials conducted by the IFM and the Cancer and Leukemia Group B (CALGB), which examined lenalidomide versus placebo post-ASCT [39, 49] (Table 2B). In both trials, a highly significant longer PFS interval was observed for patients in the lenalidomide arm and, in addition, a survival benefit was evident in the CALGB trial at the last data update [49]. Unexpectedly, a higher incidence of secondary primary malignancies was noted in patients receiving lenalidomide maintenance therapy. Genotoxic chemotherapy appears to be a key cofactor, and hence more data on the safety of this therapy are needed before recommendations for routine practice can be made.
Bortezomib was also investigated in the maintenance setting in the phase III HOVON/GMMG (Stichting Hemato-Oncologie voor Volwassenen Nederland/German-Speaking Myeloma Multicenter Group) trial. That trial compared bortezomib as a backbone for the induction regimen (PAD) followed by HDM and bortezomib maintenance therapy with VAD followed by HDM and thalidomide maintenance treatment. The PFS and OS times were significantly longer in the bortezomib-containing arm, during both induction and maintenance, than in the control arm that employed thalidomide for maintenance only [27]. However, no PFS benefit was seen with bortezomib over thalidomide maintenance in a landmark analysis performed from the start of maintenance, and the design of the study does not allow for a dissection of the role of bortezomib maintenance therapy. Notably, a substudy restricted to German centers found that the adverse impact of del(17p13) on PFS and OS outcomes could be significantly reduced by bortezomib-based treatment [52]. The median PFS intervals were 26.2 months in the bortezomib-containing arm and 12 months in the control arm (p = .024), whereas the 3-year OS rates were 69% and 17%, respectively (p = .028). These results indicate that the long-term administration of bortezomib may be beneficial in patients carrying del(17p13).
Although recent trials have provided important results regarding the utility and benefit of consolidation and maintenance in the post-ASCT setting, further studies are needed to provide answers to the many open questions that remain, such as the duration of treatment, the impact on subsequent therapies, and who should receive post-ASCT treatment. In addition, the measurement of QoL data in the maintenance setting is of importance in order to thoroughly assess the benefit of long-term therapy. Only limited QoL data are currently available. Stewart et al. [48] could show that the use of thalidomide maintenance was associated with a significant negative impact on QoL across a range of domains examined, indicating that a careful weighing of the anticipated benefit versus possible toxicities and impact on QoL is needed when considering long-term therapy. It is important to note that lenalidomide has a toxicity profile that is distinct from that of thalidomide, in particular regarding nonhematological toxicities, and in addition to the substantial efficacy noted above, may therefore be superior as maintenance therapy. In addition, bortezomib was better tolerated when used as maintenance therapy than thalidomide in the HOVON/GMMG trial [27]. A recent IMWG publication provides a comprehensive review and analysis of the available data, as well as important considerations for clinical practice [53].
Nontransplant Setting
As in the transplant setting, novel agents also form an integral part of treatment strategies for patients not eligible for transplantation because of age or comorbidities. The following options are recommended based on data from randomized phase III trials (Table 3A) [4–9, 11, 54–56]: bortezomib plus melphalan plus prednisone (VMP) and the combination of an alkylator plus a steroid plus an IMiD, which includes the two regimens melphalan plus prednisone plus thalidomide (MPT) and attenuated CTD (CTDa) (Fig. 1). Both VMP and MPT are approved in this setting by the EMA and FDA. Bendamustine plus prednisone is another effective regimen that is also approved by the EMA. Additional options for the treatment of patients in the nontransplant setting include the following combinations: lenalidomide plus dexamethasone, VMPT-VT, VMP-VT, and MPR-R, and the available data for these are summarized in Table 3A.
Table 3.
Summary of data for novel agent regimens in the nontransplant setting
aSignificant difference between arms.
bResults according to induction arm: VMP or VTP, respectively; no significant difference in PFS or OS between VT or VP maintenance. PFS: VT, 32 mos; VP, 24 mos; p = .1. OS: HR, 1.2, 0.6–2.4.
cSignificant difference between MPR+R and MP arms.
d≥VGPR.
Abbreviations: CR, complete response; CTDa, attenuated cyclophosphamide, thalidomide, and dexamethasone; OS, overall survival; MP, melphalan and prednisone; MPR, melphalan, prednisone, and lenalidomide; MPR-R, MPR with lenalidomide maintenance; MPT, melphalan, prednisone, thalidomide; ORR, objective response rate; PFS, progression-free survival; PR, partial response; RD, lenalidomide and high-dose dexamethasone; Rd, lenalidomide and low-dose dexamethasone; T, thalidomide; TTF, time to treatment failure; VGPR, very good partial response; VMP, bortezomib, melphalan, and prednisone; VMPT, bortezomib, melphalan, prednisone, and thalidomide; VT, bortezomib and thalidomide; VTP, bortezomib, thalidomide, and prednisone.
In the nontransplant setting, it seems beneficial to give treatment for a prolonged period of time, with many investigators recommending the administration of no fewer than nine cycles, although formal proof for this notion has not been generated as yet.
The use of maintenance treatment in the nontransplant setting has been shown to lead to a longer PFS interval, and the following options have been investigated in phase III clinical trials: single-agent thalidomide, single-agent lenalidomide, and bortezomib in the combinations VT and VP (Table 3B) [7, 8, 54, 57, 58]. Despite the substantial benefit observed, that is, a median PFS time of up to 37 months [7], questions surrounding the use of maintenance therapy remain, such as the duration of treatment, optimal regimen, and impact on QoL. In addition, it is not clear if it is important to use different novel agent drug classes in induction and in maintenance or if the same agent should be used throughout when toxicity is not an issue.
Considerations in the Treatment of Frail Elderly Patients
The nontransplant population constitutes a highly heterogeneous patient group, encompassing fit elderly patients as well as those who are frail elderly, for whom treatment goals and strategies differ vastly. In particular, frail elderly patients present a significant challenge because of the physiological changes associated with aging, which result in compromised function, the presence of comorbidities, and a reduced ability to tolerate treatment and any associated toxicity, which has a significant impact on feasible treatment options. A summary of important factors to be considered in the treatment of elderly patients is as follows: lower functional capacity (performance status, activities of daily living [ADL score], cognitive function); comorbidities (renal, pulmonary, hepatic, cardiac, bone marrow); disability; frailty (weakness, poor endurance, weight loss, low physical activity, slow gait speed); a higher prevalence of unfavorable prognostic factors (β2-microglobulin ≥3.5 μg/mL, albumin <3.5 g/dL, hemoblobin <10 g/dL, International Staging System [ISS] stage III) [59]; polypharmacy; and a lower capacity to tolerate toxicity.
In discussion, a number of factors were identified that may be used to define the population of frail elderly patients. These include age, the presence and grade of comorbidities, compromised organ function (cardiac, pulmonary, hepatic, renal, bone marrow), overall function or ability (to pursue ADLs), and frailty.
A number of geriatric scales exist; however, there is limited published experience on their use in MM patients, and no consensus regarding a particular scale to be used was reached. The “timed up-and-go” assessment was considered to be the most straightforward method, highlighting the fact that the assessment of frailty is typically based on clinical judgment. There is a need for the validation of existing geriatric scales in MM patients.
Considering the treatment of elderly patients, it is recommended that therapy be adjusted according to risk groups defined by age, comorbidity, disability, and frailty. As for other diseases, patients may be divided into specific groups, such as “full go,” “slow go,” and “very slow or no go,” and treatment should be adjusted accordingly by applying dose reductions and low-dose treatment [60]. There was consensus that the presence of frailty should not lead to a compromise in the choice of effective treatment as long as tolerability is acceptable and that the best available option should be administered to all patients.
A dose-reduction algorithm based on the presence of the risk factors contributing to frailty outlined above was recently published and is summarized in Table 4 [61]. Careful monitoring of patients and adherence to dose-reduction schema should ensure that patients are treated with the optimal regimen, thus maximizing beneficial outcomes.
Table 4.
Dose adjustment recommendations for the treatment of frail elderly patients
Adapted from Palumbo A, Anderson K. Multiple myeloma. N Engl J Med 2011;364:1046–1060, with permission.
There is substantial evidence from clinical trials demonstrating the utility of dose reduction of novel agents in the treatment of elderly patients. Hulin et al. [3] investigated thalidomide at 100 mg/day instead of 200 mg/day in combination with MP and could show that the treatment was effective with acceptable tolerability in patients aged >75 years. Notably, it was associated with both a significant PFS benefit and a significant OS benefit over treatment with MP. The combination of lenalidomide and low-dose dexamethasone presents another well-tolerated option for frail patients [56]. Two trials investigated a reduced frequency of administration of bortezomib, once weekly instead of twice weekly, at the usual dose of 1.3 mg/m2 in combination with MP [6, 7]. In both trials, the efficacy of once-weekly administration was found to be comparable with that of twice-weekly administration; however, tolerability was found to be markedly better. In particular, the incidence of peripheral neuropathy (PN) was substantially lower. Whereas in the Velcade® as Initial Standard Therapy in Multiple Myeloma (VISTA) trial, which investigated twice-weekly administration of bortezomib, the incidence of grade 3 or 4 sensory PN was 13%, this was lower, at 2% and 7%, in the Gruppo Italiano Malattie EMatologiche dell'Adulto (GIMEMA) and Programa para el Estudio y la Terapéutica de las Hemopatías Malignas y Grupo Español de Mieloma (PETHEMA/GEM) trials, respectively, investigating once-weekly administration of bortezomib [6, 7, 62]. In addition, once-weekly administration resulted in a substantially lower rate of treatment discontinuation, indicating that patients were able to remain on treatment for longer, thus maximizing the benefit of therapy.
Recently, another strategy to improve the tolerability of bortezomib was suggested. The s.c. administration of bortezomib was found to be similarly efficacious to i.v. administration (Table 5) but was substantially better tolerated, with significantly fewer all-grade, grade ≥2, and grade ≥3 PN events with s.c. administration than with i.v. administration [63]. It should be noted that these results were obtained in the relapsed setting. The s.c. administration of bortezomib was recently approved by the FDA [64] and is currently under evaluation by the EMA.
Table 5.
Summary of phase III trials of novel agent regimens in the relapse setting
aSignificant difference between arms.
bCR only.
cResponse after 8 cycles.
Abbreviations: CR, complete response; nCR, near CR; OS, overall survival; PR, partial response; TTP, time to progression.
Taken together, there has been substantial progress in treatment options for elderly patients, who should receive the most effective treatment possible considering individual patient factors, for example, frailty, tolerability, and QoL aspects. Careful assessment, close monitoring, and prompt dose adjustments are recommended to maximize treatment benefits in this population.
Relapsed Setting
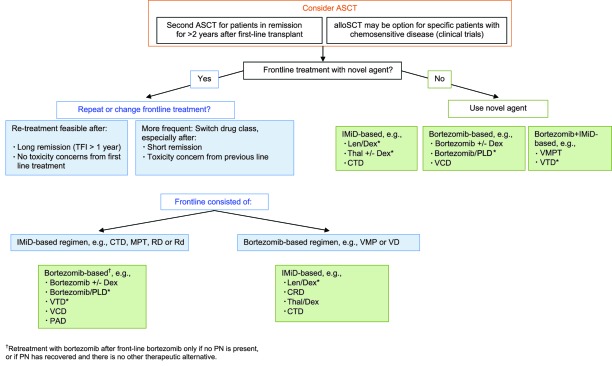
Figure 2 outlines decision points and possible treatment strategies at first relapse.
Figure 2.
Multiple myeloma treatment tree outside clinical trials: first relapse.
*Data available from phase III randomized trial.
Abbreviations: ASCT, allogeneic stem cell transplantation; autoSCT, autologous stem cell transplantation; CRD, cyclophosphamide, lenalidomide, and dexamethasone; Dex, dexamethasone; CTD, cyclophosphamide, thalidomide, and dexamethasone; IMiD, immunomodulatory drug; Len, lenalidomide; MPT, melphalan, prednisone, and thalidomide; PLD, pegylated liposomal doxorubicin; RD, lenalidomide and high-dose dexamethasone; Rd, lenalidomide and low-dose dexamethasone; TFI, treatment-free interval; Thal, thalidomide; VCD, bortezomib, cyclophosphamide, and dexamethasone; VD, bortezomib plus dexamethasone; VMP, bortezomib, melphalan, and prednisone; VMPT, VMP plus thalidomide; VTD, bortezomib, thalidomide, and dexamethasone.
The application of a second ASCT should be considered as a feasible option for patients in remission for >2 years following the first-line ASCT step. In addition, allogeneic transplantation (alloSCT) may be an option for specific patients, for example, those with high-risk factors. However, the use of alloSCT outside clinical trials is not recommended [65].
Thalidomide, bortezomib, and lenalidomide are key components in the treatment of relapsed disease as backbone agents for highly effective combination regimens, and available phase III data for these agents in the relapsed setting are summarized in Table 5. In addition, a range of other effective regimens that use additive or synergistic activity obtained through the combination of novel agents with each other and with anthracyclines, alkylating agents, and/or steroids are available. These combinations have been reviewed in a number of excellent articles and are therefore not discussed further here [66–76]. In addition, clinical trials of experimental agents also should be considered.
Retreatment Versus Switching Therapy
At relapse, the question of whether to repeat treatment with the agent or combination used upfront or change to another agent has to be considered, and this is influenced by the quality and duration of response to the first-line treatment as well as toxicity concerns. European experts concur that switching drug class is the more frequent approach, even after a long remission, to reduce the risk for the generation of resistant clones. Nevertheless, retreatment with components of the initial therapy presents a feasible option following a long treatment-free interval (>1 year) in the absence of tolerability concerns and is supported by evidence from studies [77]. In the MM009 and MM010 trials, treatment with lenalidomide plus dexamethasone was found to be effective regardless of prior thalidomide treatment [78], and a recent report suggested that treatment with thalidomide- or lenalidomide-based regimens following upfront use of these drugs is feasible [79]. Bortezomib retreatment was investigated in a number of trials, including the phase III VISTA trial and the phase II RETRIEVE (Retreatment after Initial Response to Velcade) study as well as in a number of other small prospective and retrospective studies, and results indicate that the repeated use of bortezomib in later lines results in responses in a substantial proportion of patients without concerns for cumulative toxicity or induction of resistance to the agent [5, 80–83]. These data indicate that, for patients who relapse following a durable response (i.e., longer than the median PFS time with the previous therapy), the same treatment can be repeated, whereas for patients relapsing following a short response (i.e., shorter than the median PFS time with the previous therapy), new regimens should be sequentially administered.
Factors Influencing Treatment Decisions
Aside from the quality of the first response, the choice of treatment at relapse is influenced by a host of patient- and disease-related factors, such as age, performance status, comorbidity, and toxicity associated with previous treatments. Treatment has to be adapted to the individual patient situation, but a number of general strategies can be proposed.
Renal Impairment
Bortezomib-based treatments are effective in patients with renal impairment, and the combination of bortezomib and high-dose dexamethasone may be considered as the treatment of choice, as recently recommended by the IMWG [84]. Reversal of renal insufficiency is observed in a substantial proportion of patients with bortezomib-based treatment. There is limited experience on the use of thalidomide in this setting. Nevertheless, careful administration appears feasible [84]. Lenalidomide-based treatment has been shown to be effective [85]; however, dose modification based on renal function is mandatory because of the renal clearance of the agent [84, 85].
Current or Recent Thromboembolic Event
A bortezomib-based treatment may be the preferred approach in patients with a recent thromboembolic event. However, lenalidomide-based treatment may also be feasible if prophylaxis guidelines are followed [86].
Treatment-Related PN
In the case of pre-existing PN, the use of a potentially neurotoxic agent should be approached with caution and with appropriate dose modification, route of administration adjustment, and schedule change as clinically indicated. Ideally, a non-neurotoxic regimen such as a lenalidomide-based combination should be chosen if available.
Based on the positive results of a phase III trial, s.c. administration of bortezomib presents an attractive option to reduce the incidence of PN. In addition, a number of excellent recent publications provided a review of the incidence, pathophysiology, and management of therapy-associated PN, and the reader is referred to these for further detail [87–90].
Prolonged Treatment at Relapse
A further question in the relapsed setting concerns the duration of treatment and whether or not there is a role for maintenance therapy. Currently, no clear consensus regarding this matter exists. The combination of lenalidomide and dexamethasone at relapse is recommended until disease progression [91]. In addition, data from phase II studies demonstrated a benefit for prolonged treatment in the relapsed setting [92, 93]. Of note, patients with high-risk disease, for example, those with t(4,14), may benefit from continued therapy to delay progression as much as possible; however, a recent consensus statement by the IMWG concluded that the current level of evidence does not provide direction in deciding if patients in a specific risk group will benefit from maintenance therapy [94].
Start of Treatment at Relapse
Additional considerations in the relapsed setting concern the start of treatment. There is a need to distinguish between the presence of a biochemical relapse, characterized by a rise in M-component in the absence of symptoms, and a clinical relapse, when symptoms are present. In general, the indication to start treatment following progression is the presence of a clinical relapse. However, if the doubling time of the monoclonal protein is ≤2 months, treatment is indicated even in the absence of calcium elevation, renal insufficiency, anemia and bone abnormalities (CRAB criteria). A number of options exist to manage a biochemical relapse that can be guided by the rate of increase in paraprotein. On the one hand, close monitoring may be sufficient, whereas on the other hand, increasing the dose of the current treatment, the addition of another agent, interrupting treatment to allow the option of retreatment (for patients who return to a smoldering stage with upfront maintenance treatment), or switching to a different agent may be the appropriate choice of action.
MM is characterized by successive relapses interspaced with decreasing durations of remission. Treatment for these successive relapses requires a careful balance of efficacy and tolerability considerations, with QoL factors becoming increasingly crucial as the palliative component becomes the focus of disease management.
High-Risk MM
MM is a highly heterogeneous disease, both at the phenotypic and at the genotypic level. A number of patient- and disease-specific factors have been identified that signify high-risk disease. Patient-specific factors include age, the presence of comorbidities (e.g., renal failure, spinal cord compression), and aspects related to socioeconomic class, occupation, lifestyle, and family support. Disease-specific factors comprise genetic abnormalities detected by conventional cytogenetics, fluorescence in situ hybridization (FISH), or gene-expression profiling (GEP), the presence of extramedullary disease, renal failure resulting from MM, plasma cell leukemia, ISS stage, level of lactate dehydrogenase (LDH), a high proliferation rate or labeling index, the presence of anemia, and the phenotype of the tumor cell [95]. In addition, response to treatment, that is, the quality and duration of response, as well as the presence of early toxicity can denote high-risk disease.
Cytogenetic Abnormalities
The development and progression of MM are characterized by the occurrence of distinct cytogenetic abnormalities. For some of these, consensus exists regarding their prognostic impact, whereas for others data are controversial. The following categorization regarding the prognostic impact of the different abnormalities was agreed upon: (a) Abnormalities conferring a poor prognosis include any abnormality detected by conventional karyotyping or the presence of any of the following abnormalities detected by FISH: t(4,14), del17p, 1q gain. (b) Abnormalities not conferring a poor prognosis, that is, those that indicate standard-risk disease, include t(6,14), t(11,14), 5q amplification, hyperdiploidy, del13 without t(4, 14) and/or without del17p. (c) No consensus regarding the prognostic impact of the following abnormalities was reached: t(14,16), t(14,20), and del12p. Further data are needed to characterize these.
Assessment of Risk Factors
It is recommended that all patient- and disease-specific factors except labeling index form part of the examination in clinical trials, with the objective of obtaining important prognostic information and to inform future trial designs. Recommended parameters for assessment as part of standard examinations in routine practice are summarized as follows: age; the presence of comorbidities; ISS stage; the presence of t(4,14), del17p, or 1q gain using FISH; extramedullary disease; plasma cell leukemia; LDH level; the presence of anemia; bone survey; and socioeconomic class (occupation, lifestyle factors, family support).
Is There a Role for Risk-Adapted Treatment?
The approach of offering treatment targeted to specific patient- or disease-related factors is attractive based on positive experiences with other malignancies and is one of the hotly debated topics in MM treatment that has also been examined in a number of articles [17, 96–100].
A recent publication by the IMWG concluded that the current data are not sufficient to implement risk-adapted treatment approaches regarding cytogenetic abnormalities [94].
European experts recommend the assessment of risk factors to obtain important prognostic information but that these should not necessarily determine treatment decisions, with the exception of age as the only risk factor significantly impacting treatment decisions. It is recommended that all patients receive the most effective treatment regardless of risk status, meaning that highly effective regimens should not be reserved for high-risk disease because patients with standard-risk disease derive significant benefit from receiving highly effective treatment. For example, in the IFM 2005/01 trial, which compared bortezomib plus dexamethasone with VAD induction, patients with ISS stage 1 disease had a higher response rate than those with ISS stage 2 or ISS stage 3 disease [22]. Similarly, in the HOVON/GMMG-HD4 trial, which examined PAD versus VAD induction followed by bortezomib and thalidomide maintenance, respectively, patients with ISS stage 1 disease had longer PFS and OS times than those with ISS stage 2 or ISS stage 3 disease [27]. Notably, the total therapy approach pioneered by the Arkansas group was found to result in a 4-year CR rate of 89% in patients defined as low risk by GEP, corresponding to an estimated operational cure rate of 55% [101], unambiguously demonstrating the benefit of the application of highly effective combination regimens for patients with low-risk disease. On the other hand, it is recognized that this approach may lead to the overtreatment of some patients and to a limitation of treatment options in cases of relapse.
There are some data to suggest that bortezomib-containing regimens retain efficacy in the presence of some high-risk cytogenetic abnormalities [24, 52, 102–104]. For example, bortezomib plus dexamethasone, PAD, or VTD may be the preferred approach for induction treatment in the presence of t(4,14). In addition, the administration of bortezomib before and after ASCT in the HOVON/GMMG-HD4 trial (PAD induction, bortezomib maintenance) resulted in a significantly better outcome for patients with del17p than treatment in the control arm (VAD induction, thalidomide maintenance), suggesting that long-term administration of bortezomib may be beneficial for these patients [52]. The RVD combination is a preferred approach by some investigators [30, 100, 105]. For patients with del17p, an upfront alloSCT may be an option if the patient has a donor and is in a good general condition [106]. Taken together, although patients with high-risk disease have a better outcome with novel agent treatment than with conventional treatments (e.g., VAD), their survival times remain shorter than those seen for patients with standard-risk disease, and therefore novel approaches are still required for these patients.
Summary and Outlook
The management of MM has come a long way, and exciting developments are ongoing, not only in terms of new treatment options but also in our understanding of the biology of the malignancy, which is supported by the application of techniques such as GEP and single nucleotide polymorphism (SNP) arrays. The recent genome sequencing of myeloma samples is shedding light on important mutations and will contribute to a better understanding of the pathobiology of the malignancy as well as potential treatment targets [107].
The genotypic heterogeneity of MM is a challenge, and intense research efforts are ongoing to identify and characterize molecular factors that provide relevant prognostic information as well as to identify new targets for treatment [108, 109]. Using GEP, a number of signatures associated with a poor prognosis have been identified [110–117]. However, GEP is not only finding application in the molecular classification of patients but is also being investigated as a method to identify factors associated with toxicities seen with certain treatments [118–122], which may in the future enable the identification of patients likely to succumb to particular adverse events and may therefore provide a way of personalizing treatment. Moreover, molecular techniques may find application in guiding treatment decisions by predicting response to treatment [123].
Highly effective novel agents are inducing responses deeper than those previously obtained, and the use of techniques to measure responses both at the bone marrow level and outside the bone marrow will contribute to an accurate assessment of the efficacy of therapies in order to devise appropriate treatment strategies.
Patients resistant to novel agents present a particularly challenging group, as shown in a recent IMWG analysis in which patients who were refractory to novel agents had a median EFS duration of only 5 months and a median OS time of 9 months [124]. These patients require novel strategies, such as treatment with newer antimyeloma agents and sophisticated combinations [15]. A host of newer agents is currently undergoing investigation, including third-generation IMiDs (pomalidomide) and second-generation proteasome inhibitors (carfilzomib, marizomib), and also agents directed at novel targets that play an important role in myeloma disease biology, for example, monoclonal antibodies for CS1 (elotuzumab) or interleukin-6 (siltuximab [CNTO328]), histone deacetylase inhibitors (vorinostat, panobinostat), and mammalian target of rapamycin (mTor) inhibitors (temsirolimus), and ongoing clinical trials will define how these agents should be incorporated into existing treatment strategies.
The prognosis of MM patients has improved substantially, from a malignancy for which few treatment options were available and which was associated with a particularly bleak outlook to a disorder that some will argue should no longer be referred to as “incurable” [125, 126]. The substantial developments observed over the recent past are a result of the dedicated commitment of patients, clinicians, and nurses participating in national and international clinical trials. It is hoped that future concerted efforts will continue to drive such progress in the development of effective therapies to eradicate the tumor clone and the use of appropriate tools to assess the efficacy of treatment, with the ultimate aim of developing treatment strategies that offer QoL and survival prolongation, and that cure is no longer a dream [127].
Acknowledgments
Advisory Board meeting supported by Janssen Pharmaceutical Companies of Johnson & Johnson in EMEA. Unrestricted educational grant provided by Janssen Pharmaceutical Companies of Johnson & Johnson in EMEA to assist with editorial support. The authors take full responsibility for the content of the paper but thank Pia Sondergeld (Ammonitesystems) supported by Janssen for medical writing assistance.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Heinz Ludwig, Hervé Avet-Loiseau, Joan Bladé, Mario Boccadoro, Jamie D. Cavenagh, Michele Cavo, Faith Davies, Javier de La Rubia, Sosana Delimpasi, Meletios Dimopoulos, Johannes Drach, Hermann Einsele, Thierry Facon, Hartmut Goldschmidt, Urs Hess, Ulf-Henrik Mellqvist, Philippe Moreau, Jesûs San-Miguel, Pia Sondergeld, Pieter Sonneveld, Miklos Udvary, Antonio Palumbo
Collection and/or assembly of data: Heinz Ludwig, Hervé Avet-Loiseau, Joan Bladé, Mario Boccadoro, Jamie D. Cavenagh, Michele Cavo, Faith Davies, Javier de La Rubia, Sosana Delimpasi, Meletios Dimopoulos, Johannes Drach, Hermann Einsele, Thierry Facon, Hartmut Goldschmidt, Urs Hess, Ulf-Henrik Mellqvist, Philippe Moreau, Jesûs San-Miguel, Pia Sondergeld, Pieter Sonneveld, Miklos Udvary, Antonio Palumbo
Data analysis and interpretation: Heinz Ludwig, Hervé Avet-Loiseau, Joan Bladé, Mario Boccadoro, Jamie D. Cavenagh, Michele Cavo, Faith Davies, Javier de La Rubia, Sosana Delimpasi, Meletios Dimopoulos, Johannes Drach, Hermann Einsele, Thierry Facon, Hartmut Goldschmidt, Urs Hess, Ulf-Henrik Mellqvist, Philippe Moreau, Jesûs San-Miguel, Pia Sondergeld, Pieter Sonneveld, Miklos Udvary, Antonio Palumbo
Manuscript writing: Heinz Ludwig
Final approval of manuscript: Heinz Ludwig, Hervé Avet-Loiseau, Joan Bladé, Mario Boccadoro, Jamie D. Cavenagh, Michele Cavo, Faith Davies, Javier de La Rubia, Sosana Delimpasi, Meletios Dimopoulos, Johannes Drach, Hermann Einsele, Thierry Facon, Hartmut Goldschmidt, Urs Hess, Ulf-Henrik Mellqvist, Philippe Moreau, Jesûs San-Miguel, Pia Sondergeld, Pieter Sonneveld, Miklos Udvary, Antonio Palumbo
References
- 1.Facon T, Mary JY, Hulin C, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99–06): A randomised trial. Lancet. 2007;370:1209–1218. doi: 10.1016/S0140-6736(07)61537-2. [DOI] [PubMed] [Google Scholar]
- 2.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hulin C, Facon T, Rodon P, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. J Clin Oncol. 2009;27:3664–3670. doi: 10.1200/JCO.2008.21.0948. [DOI] [PubMed] [Google Scholar]
- 4.San Miguel JF, Schlag R, Khuageva NK, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 5.San Miguel JF, Schlag R, Khuageva NK, et al. Continued overall survival benefit after 5 years' follow-up with bortezomib-melphalan-prednisone (VMP) versus melphalan-prednisone (MP) in patients with previously untreated multiple myeloma, and no increased risk of second primary malignancies: Final results of the phase 3 VISTA trial [abstract 476] Blood. 2011;118:476. [Google Scholar]
- 6.Mateos MV, Oriol A, Martínez-López J, et al. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: A randomised trial. Lancet Oncol. 2010;11:934–941. doi: 10.1016/S1470-2045(10)70187-X. [DOI] [PubMed] [Google Scholar]
- 7.Palumbo A, Bringhen S, Rossi D, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: A randomized controlled trial. J Clin Oncol. 2010;28:5101–5109. doi: 10.1200/JCO.2010.29.8216. [DOI] [PubMed] [Google Scholar]
- 8.Palumbo A, Delforge M, Catalano J, et al. A phase 3 study evaluating the efficacy and safety of lenalidomide combined with melphalan and prednisone in patients ≥ 65 years with newly diagnosed multiple myeloma (NDMM): Continuous use of lenalidomide vs fixed-duration regimens [abstract 622] Blood. 2010;116:273. [Google Scholar]
- 9.Palumbo A, Adam Z, Kropff M, et al. A phase 3 study evaluating the efficacy and safety of lenalidomide (Len) combined with melphalan and prednisone followed by continuous lenalidomide maintenance (MPR-R) in patients (pts) ≥ 65 years (yrs) with newly diagnosed multiple myeloma (NDMM): Updated results for pts aged 65–75 yrs enrolled in MM-015 [abstract 475] Blood. 2011;118:475. [Google Scholar]
- 10.Wijermans P, Schaafsma M, Termorshuizen F, et al. Phase III study of the value of thalidomide added to melphalan plus prednisone in elderly patients with newly diagnosed multiple myeloma: The HOVON 49 study. J Clin Oncol. 2010;28:3160–3166. doi: 10.1200/JCO.2009.26.1610. [DOI] [PubMed] [Google Scholar]
- 11.Fayers PM, Palumbo A, Hulin C, et al. Thalidomide for previously untreated elderly patients with multiple myeloma: Meta-analysis of 1685 individual patient data from 6 randomized clinical trials. Blood. 2011;118:1239–1247. doi: 10.1182/blood-2011-03-341669. [DOI] [PubMed] [Google Scholar]
- 12.Pulte D, Gondos A, Brenner H. Improvement in survival of older adults with multiple myeloma: Results of an updated period analysis of SEER data. The Oncologist. 2011;16:1600–1603. doi: 10.1634/theoncologist.2011-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ludwig H, Beksac M, Bladé J, et al. Current multiple myeloma treatment strategies with novel agents: A European perspective. The Oncologist. 2010;15:6–25. doi: 10.1634/theoncologist.2009-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ludwig H, Beksac M, Bladé J, et al. Multiple myeloma treatment strategies with novel agents in 2011: A European perspective. The Oncologist. 2011;16:388–403. doi: 10.1634/theoncologist.2010-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dimopoulos MA, San-Miguel JF, Anderson KC. Emerging therapies for the treatment of relapsed or refractory multiple myeloma. Eur J Haematol. 2011;86:1–15. doi: 10.1111/j.1600-0609.2010.01542.x. [DOI] [PubMed] [Google Scholar]
- 16.Lonial S, Mitsiades CS, Richardson PG. Treatment options for relapsed and refractory multiple myeloma. Clin Cancer Res. 2011;17:1264–1277. doi: 10.1158/1078-0432.CCR-10-1805. [DOI] [PubMed] [Google Scholar]
- 17.Mitsiades CS, Davies FE, Laubach JP, et al. Future directions of next-generation novel therapies, combination approaches, and the development of personalized medicine in myeloma. J Clin Oncol. 2011;29:1916–1923. doi: 10.1200/JCO.2010.34.0760. [DOI] [PubMed] [Google Scholar]
- 18.Offidani M, Corvatta L, Morabito F, et al. How to treat patients with relapsed/refractory multiple myeloma: Evidence-based information and opinions. Expert Opin Investig Drugs. 2011;20:779–793. doi: 10.1517/13543784.2011.575060. [DOI] [PubMed] [Google Scholar]
- 19.Richardson PG, Lonial S, Jakubowiak AJ, et al. Monoclonal antibodies in the treatment of multiple myeloma. Br J Haematol. 2011 Jul 21; doi: 10.1111/j.1365-2141.2011.08790.x. [Epub ahead of print]. doi: 10.1111/j.1365–2141.2011.08790.x. [DOI] [PubMed] [Google Scholar]
- 20.Cavo M, Rajkumar SV, Palumbo A, et al. International Myeloma Working Group consensus approach to the treatment of multiple myeloma patients who are candidates for autologous stem cell transplantation. Blood. 2011;117:6063–6073. doi: 10.1182/blood-2011-02-297325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moreau P, Avet-Loiseau H, Harousseau JL, et al. Current trends in autologous stem-cell transplantation for myeloma in the era of novel therapies. J Clin Oncol. 2011;29:1898–1906. doi: 10.1200/JCO.2010.32.5878. [DOI] [PubMed] [Google Scholar]
- 22.Harousseau JL, Attal M, Avet-Loiseau H, et al. Bortezomib plus dexamethasone is superior to vincristine plus doxorubicin plus dexamethasone as induction treatment prior to autologous stem-cell transplantation in newly diagnosed multiple myeloma: Results of the IFM 2005–01 phase III trial. J Clin Oncol. 2010;28:4621–4629. doi: 10.1200/JCO.2009.27.9158. [DOI] [PubMed] [Google Scholar]
- 23.Rajkumar SV, Jacobus S, Callander NS, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: An open-label randomised controlled trial. Lancet Oncol. 2010;11:29–37. doi: 10.1016/S1470-2045(09)70284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cavo M, Tacchetti P, Patriarca F, et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction treatment before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: A randomised phase 3 study. Lancet. 2010;376:2075–2085. doi: 10.1016/S0140-6736(10)61424-9. [DOI] [PubMed] [Google Scholar]
- 25.Rosinol L, Cibeira MT, Mateos MV, et al. Results of pre- and post-autologous stem cell transplantation (ASCT) with three induction regimens in multiple myeloma (MM): Superiority of VTD (bortezomib/thalidomide/dexamethasone) over TD and VBMCP/VBAD plus bortezomib (VBMCP/VBAD/V) [abstract P-138] Haematologica. 2011;96(suppl 1):S69. [Google Scholar]
- 26.Moreau P, Avet-Loiseau H, Facon T, et al. Bortezomib plus dexamethasone versus reduced-dose bortezomib, thalidomide plus dexamethasone as induction treatment before autologous stem cell transplantation in newly diagnosed multiple myeloma. Blood. 2011;118:5752–5758. doi: 10.1182/blood-2011-05-355081. [DOI] [PubMed] [Google Scholar]
- 27.Sonneveld P, Schmidt-Wolf I, van der Holt B, et al. HOVON-65/GMMG-HD4 randomized phase III trial comparing bortezomib, doxorubicin, dexamethasone (PAD) vs VAD followed by high-dose melphalan (HDM) and maintenance with bortezomib or thalidomide in patients with newly diagnosed Multiple Myeloma (MM) [abstract 40] Blood. 2010;116:23. [Google Scholar]
- 28.Morgan GJ, Davies FE, Gregory WM, et al. Cyclophosphamide, thalidomide, and dexamethasone as induction therapy for newly diagnosed multiple myeloma patients destined for autologous stem-cell transplantation: MRC Myeloma IX randomized trial results. Haematologica. 2012;97:442–450. doi: 10.3324/haematol.2011.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Einsele H, Liebisch P, Langer C, et al. Velcade, intravenous cyclophosphamide and dexamethasone (VCD) induction for previously untreated multiple myeloma (German DSMM XIa Trial) [abstract 131] Blood. 2009;114:59. [Google Scholar]
- 30.Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010;116:679–686. doi: 10.1182/blood-2010-02-268862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roussel M, Robillard N, Moreau P, et al. Bortezomib, lenalidomide, and dexamethasone (VRD) consolidation and lenalidomide maintenance in frontline multiple myeloma patients: Updated results of the IFM 2008 phase II VRD intensive program [abstract 1872] Blood. 2011;118:1872. [Google Scholar]
- 32.Palumbo A, Cavallo F, Hardan I, et al. Melphalan/prednisone/lenalidomide (MPR) versus high-dose melphalan and autologous transplantation (MEL200) in newly diagnosed multiple myeloma (MM) patients <65 years: Results of a randomized phase III study [abstract 3069] Blood. 2011;118:3069. [Google Scholar]
- 33.U.S. Food and Drug Administration. THALOMID. [accessed November 6, 2011]. Available at http://www.fda.gov.
- 34.U.S. Food and Drug Administration. VELCADE. [accessed November 6, 2011]. Available at http://www.fda.gov.
- 35.Mellqvist UH, Gimsing P, Hjertner O, et al. Improved progression free survival with bortezomib consolidation after high dose melphalan; results of a randomized phase III trial [abstract O-11] Haematologica. 2011;96:S31. [Google Scholar]
- 36.Ladetto M, Ferrero S, Drandi D, et al. Long-term results of the GIMEMA VTD consolidation trial in autografted multiple myeloma patients (VEL-03–096): Impact of minimal residual disease detection by real time quantitative PCR on late recurrences and overall survival [abstract 827] Blood. 2011;118:827. [Google Scholar]
- 37.Cavo M, Pantani L, Patriarca F, et al. Superior complete response rate (CR) and progression-free survival (PFS) with bortezomib-thalidomide-dexamethasone (VTD) versus thalidomide-dexamethasone (TD) as consolidation therapy after autologous stem-cell transplantation (ASCT) in multiple myeloma (MM) [abstract 1871] Blood. 2011;118:1879. [Google Scholar]
- 38.Roussel M, Dörr G, Vaillant W, et al. Consolidation with bortezomib, thalidomide and dexamethasone after high dose therapy is feasible , safe and effective in de novo multiple myeloma patients who already received new drugs containing-induction regimen [abstract 3041] Blood. 2010;116:3041. [Google Scholar]
- 39.Attal M, Olivier P, Cances Lauwers V, et al. Maintenance treatment with lenalidomide after transplantation for myeloma: Analysis of secondary malignancies within the IFM 2005–02 trial. Haematologica. 2011;96:S23. [Google Scholar]
- 40.Terragna C, Durante S, Zamagni E, et al. Molecular remission after bortezomib-thalidomide-dexamethasone (VTD) compared with thalidomide-dexamethasone (TD) as consolidation therapy following double autologous transplantation (ASCT) for multiple myeloma (MM): Results of a qualitative and quantitative analysis [abstract P-224] Haematologica. 2011;96(suppl 1):S96. [Google Scholar]
- 41.Spencer A, Prince HM, Roberts AW, et al. Consolidation therapy with low-dose thalidomide and prednisolone prolongs the survival of multiple myeloma patients undergoing a single autologous stem-cell transplantation procedure. J Clin Oncol. 2009;27:1788–1793. doi: 10.1200/JCO.2008.18.8573. [DOI] [PubMed] [Google Scholar]
- 42.Attal M, Harousseau JL, Leyvraz S, et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108:3289–3294. doi: 10.1182/blood-2006-05-022962. [DOI] [PubMed] [Google Scholar]
- 43.Barlogie B, Attal M, Crowley J, et al. Long-term follow-up of autotransplantation trials for multiple myeloma: Update of protocols conducted by the Intergroupe Francophone du Myelome, Southwest Oncology Group, and University of Arkansas for Medical Sciences. J Clin Oncol. 2010;28:1209–1214. doi: 10.1200/JCO.2009.25.6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barlogie B, Tricot G, Anaissie E, et al. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. N Engl J Med. 2006;354:1021–1030. doi: 10.1056/NEJMoa053583. [DOI] [PubMed] [Google Scholar]
- 45.Barlogie B, Pineda-Roman M, van Rhee F, et al. Thalidomide arm of Total Therapy 2 improves complete remission duration and survival in myeloma patients with metaphase cytogenetic abnormalities. Blood. 2008;112:3115–3121. doi: 10.1182/blood-2008-03-145235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lokhorst HM, van der Holt B, Zweegman S, et al. A randomized phase 3 study on the effect of thalidomide combined with Adriamycin, dexamethasone, and high-dose melphalan, followed by thalidomide maintenance in patients with multiple myeloma. Blood. 2010;115:1113–1120. doi: 10.1182/blood-2009-05-222539. [DOI] [PubMed] [Google Scholar]
- 47.Morgan GJ, Gregory WM, Davies FE, et al. The role of maintenance thalidomide therapy in multiple myeloma: MRC Myeloma IX results and meta-analysis. Blood. 2012;119:7–15. doi: 10.1182/blood-2011-06-357038. [DOI] [PubMed] [Google Scholar]
- 48.Stewart AK, Trudel S, Bahlis NJ, et al. A randomized phase iii trial of thalidomide and prednisone as maintenance therapy following autologous stem cell transplantation (ASCT) in patients with multiple myeloma (MM): The NCIC CTG MY.10 trial [abstract 39] Blood. 2010;116:23. [Google Scholar]
- 49.McCarthy P, Owzar K, Anderson K, et al. Phase III Intergroup study of lenalidomide versus placebo maintenance therapy following single autologous stem cell transplant (ASCT) for multiple myeloma (MM): CALGB ECOG BMT-CTN 100104. Haematologica. 2011;96:S23. [Google Scholar]
- 50.Rosiñol L, Cibeira MT, Mateos MV, et al. A phase III PETHEMA/GEM randomized trial of postransplant (ASCT) maintenance in multiple myeloma: Superiority of bortezomib/thalidomide compared with thalidomide and alfa-2b interferon [abstract 3962] Blood. 2011;118:3962. [Google Scholar]
- 51.Cavo M, Pantani L, Tacchetti P, et al. Thalidomide maintenance in multiple myeloma: Certainties and controversies. J Clin Oncol. 2009;27:e186–e187. doi: 10.1200/JCO.2009.24.0150. [DOI] [PubMed] [Google Scholar]
- 52.Neben K, Lokhorst HM, Jauch A, et al. Administration of bortezomib before and after autologous stem cell transplantation improves outcome in multiple myeloma patients with deletion 17p. Blood. 2012;119:940–948. doi: 10.1182/blood-2011-09-379164. [DOI] [PubMed] [Google Scholar]
- 53.Ludwig H, Durie BG, McCarthy P, et al. IMWG consensus on maintenance therapy in multiple myeloma. Blood. 2012;119:3003–3015. doi: 10.1182/blood-2011-11-374249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morgan GJ, Davies FE, Gregory WM, et al. Cyclophosphamide, thalidomide, and dexamethasone (CTD) as initial therapy for patients with multiple myeloma unsuitable for autologous transplantation. Blood. 2011;118:1231–1238. doi: 10.1182/blood-2011-02-338665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pönisch W, Mitrou PS, Merkle K, et al. Treatment of bendamustine and prednisone in patients with newly diagnosed multiple myeloma results in superior complete response rate, prolonged time to treatment failure and improved quality of life compared to treatment with melphalan and prednisone—a randomized phase III study of the East German Study Group of Hematology and Oncology (OSHO) J Cancer Res Clin Oncol. 2006;132:205–212. doi: 10.1007/s00432-005-0074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vesole DH, Jacobus S, Rajkumar SV, et al. Lenalidomide plus low-dose dexamethasone (Ld): Superior one and two year survival regardless of age compared to lenalidomide plus high-dose dexamethasone (LD) [abstract 308] Blood. 2010;116:308. [Google Scholar]
- 57.Palumbo A, Bringhen S, Liberati AM, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: Updated results of a randomized, controlled trial. Blood. 2008;112:3107–3114. doi: 10.1182/blood-2008-04-149427. [DOI] [PubMed] [Google Scholar]
- 58.Mateos MV, Oriol A, Teruel AI, et al. Maintenance therapy with bortezomib plus thalidomide (VT) or bortezomib plus prednisone (VP) in elderly myeloma patients included in the GEM2005MAS65 Spanish randomized trial [abstract 477] Blood. 2011;118:477. doi: 10.1182/blood-2012-05-427815. [DOI] [PubMed] [Google Scholar]
- 59.Ludwig H, Bolejack V, Crowley J, et al. Survival and years of life lost in different age cohorts of patients with multiple myeloma. J Clin Oncol. 2010;28:1599–1605. doi: 10.1200/JCO.2009.25.2114. [DOI] [PubMed] [Google Scholar]
- 60.Palumbo A, Bringhen S, Ludwig H, et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: A report of the European Myeloma Network (EMN) Blood. 2011;118:4519–4529. doi: 10.1182/blood-2011-06-358812. [DOI] [PubMed] [Google Scholar]
- 61.Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 62.Bringhen S, Larocca A, Rossi D, et al. Efficacy and safety of once-weekly bortezomib in multiple myeloma patients. Blood. 2010;116:4745–4753. doi: 10.1182/blood-2010-07-294983. [DOI] [PubMed] [Google Scholar]
- 63.Moreau P, Pylypenko H, Grosicki S, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: A randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12:431–440. doi: 10.1016/S1470-2045(11)70081-X. [DOI] [PubMed] [Google Scholar]
- 64.U.S. Food and Drug Administration. Label approved on 01/23/2012 for VELCADE, NDA no. 021602. [accessed March 11, 2012]. Available at http://www.fda.gov.
- 65.Lokhorst H, Einsele H, Vesole D, et al. International Myeloma Working Group consensus statement regarding the current status of allogeneic stem-cell transplantation for multiple myeloma. J Clin Oncol. 2010;28:4521–4530. doi: 10.1200/JCO.2010.29.7929. [DOI] [PubMed] [Google Scholar]
- 66.Kropff M, Giongco-Baylon H, Hillengass J, et al. Thalidomide versus dexamethasone for the treatment of relapsed and/or refractory multiple myeloma: results from OPTIMUM, a randomized trial. Haematologica. 2011 Dec 1; doi: 10.3324/haematol.2011.044271. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dimopoulos MA, Chen C, Spencer A, et al. Long-term follow-up on overall survival from the MM-009 and MM-010 phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia. 2009;23:2147–2152. doi: 10.1038/leu.2009.147. [DOI] [PubMed] [Google Scholar]
- 68.Richardson PG, Sonneveld P, Schuster M, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: Final time-to-event results of the APEX trial. Blood. 2007;110:3557–3560. doi: 10.1182/blood-2006-08-036947. [DOI] [PubMed] [Google Scholar]
- 69.Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: Combination therapy improves time to progression. J Clin Oncol. 2007;25:3892–3901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- 70.Garderet L, Iacobelli S, Moreau P, et al. Bortezomib (VELCADE)-thalidomide-dexamethasone (VTD) is superior to thalidomide-dexamethasone (TD) in patients with multiple myeloma (MM) progressing or relapsing after autologous transplantation [abstract P-150] Haematologica. 2011;96(suppl 1):S72. [Google Scholar]
- 71.Kastritis E, Palumbo A, Dimopoulos MA. Treatment of relapsed/refractory multiple myeloma. Semin Hematol. 2009;46:143–157. doi: 10.1053/j.seminhematol.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 72.Laubach JP, Mahindra A, Mitsiades CS, et al. The use of novel agents in the treatment of relapsed and refractory multiple myeloma. Leukemia. 2009;23:2222–2232. doi: 10.1038/leu.2009.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richardson PG, Laubach J, Mitsiades C, et al. Tailoring treatment for multiple myeloma patients with relapsed and refractory disease. Oncology (Williston Park) 2010;24(suppl 2):22–29. [PubMed] [Google Scholar]
- 74.Lonial S. Relapsed multiple myeloma. Hematology Am Soc Hematol Educ Program. 2010;2010:303–309. doi: 10.1182/asheducation-2010.1.303. [DOI] [PubMed] [Google Scholar]
- 75.Moehler T, Goldschmidt H. Therapy of relapsed and refractory multiple myeloma. Recent Results Cancer Res. 2011;183:239–271. doi: 10.1007/978-3-540-85772-3_11. [DOI] [PubMed] [Google Scholar]
- 76.van de Donk NW, Lokhorst HM, Dimopoulos M, et al. Treatment of relapsed and refractory multiple myeloma in the era of novel agents. Cancer Treat Rev. 2011;37:266–283. doi: 10.1016/j.ctrv.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 77.Shah N, Lonial S. Evidence-based mini-review: Treatment options for patients with relapsed/refractory myeloma previously treated with novel agents and high-dose chemotherapy and autologous stem-cell transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:310–313. doi: 10.1182/asheducation-2010.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang M, Dimopoulos MA, Chen C, et al. Lenalidomide plus dexamethasone is more effective than dexamethasone alone in patients with relapsed or refractory multiple myeloma regardless of prior thalidomide exposure. Blood. 2008;112:4445–4451. doi: 10.1182/blood-2008-02-141614. [DOI] [PubMed] [Google Scholar]
- 79.Madan S, Lacy MQ, Dispenzieri A, et al. Efficacy of retreatment with immunomodulatory drugs (IMiDs) in patients receiving IMiDs for initial therapy of newly diagnosed multiple myeloma. Blood. 2011;118:1763–1765. doi: 10.1182/blood-2011-04-350009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sood R, Carloss H, Kerr R, et al. Retreatment with bortezomib alone or in combination for patients with multiple myeloma following an initial response to bortezomib. Am J Hematol. 2009;84:657–660. doi: 10.1002/ajh.21517. [DOI] [PubMed] [Google Scholar]
- 81.Hrusovsky I, Emmerich B, von Rohr A, et al. Bortezomib retreatment in relapsed multiple myeloma - results from a retrospective multicentre survey in Germany and Switzerland. Oncology. 2010;79:247–254. doi: 10.1159/000322866. [DOI] [PubMed] [Google Scholar]
- 82.Petrucci T, Blau I, Corradini P, et al. Efficacy and safety of retreatment with bortezomib in patients with multiple myeloma: Interim results from RETRIEVE, a prospective international phase 2 study [abstract 377] Haematologica. 2010;95(suppl 2):152. [Google Scholar]
- 83.Taverna C, Voegeli J, Trojan A, et al. Bortezomib retreatment in patients with relapsed multiple myeloma in Switzerland [abstract P-193] Haematologica. 2011;96(suppl 1):S86. [Google Scholar]
- 84.Dimopoulos MA, Terpos E, Chanan-Khan A, et al. Renal impairment in patients with multiple myeloma: A consensus statement on behalf of the International Myeloma Working Group. J Clin Oncol. 2010;28:4976–4984. doi: 10.1200/JCO.2010.30.8791. [DOI] [PubMed] [Google Scholar]
- 85.Ludwig H, Spicka I, Thaler J, et al. Lenalidomide-dexamethasone (LD) as treatment of acute cast nephropathy-induced renal failure (ARF) in multiple myeloma (MM). A phase II study [abstract 306] Haematologica. 2011;96(suppl 2):127. [Google Scholar]
- 86.Palumbo A, Rajkumar SV, Dimopoulos MA, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22:414–423. doi: 10.1038/sj.leu.2405062. [DOI] [PubMed] [Google Scholar]
- 87.Delforge M, Bladé J, Dimopoulos MA, et al. Treatment-related peripheral neuropathy in multiple myeloma: The challenge continues. Lancet Oncol. 2010;11:1086–1095. doi: 10.1016/S1470-2045(10)70068-1. [DOI] [PubMed] [Google Scholar]
- 88.Mohty B, El-Cheikh J, Yakoub-Agha I, et al. Peripheral neuropathy and new treatments for multiple myeloma: Background and practical recommendations. Haematologica. 2010;95:311–319. doi: 10.3324/haematol.2009.012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sonneveld P, Jongen JL. Dealing with neuropathy in plasma-cell dyscrasias. Hematology Am Soc Hematol Educ Program. 2010;2010:423–430. doi: 10.1182/asheducation-2010.1.423. [DOI] [PubMed] [Google Scholar]
- 90.Richardson PG, Delforge M, Beksac M, et al. Management of treatment-emergent peripheral neuropathy in multiple myeloma. Leukemia. 2012;26:595–608. doi: 10.1038/leu.2011.346. [DOI] [PubMed] [Google Scholar]
- 91.San-Miguel JF, Dimopoulos MA, Stadtmauer EA, et al. Effects of lenalidomide and dexamethasone treatment duration on survival in patients with relapsed or refractory multiple myeloma treated with lenalidomide and dexamethasone. Clin Lymphoma Myeloma Leuk. 2011;11:38–43. doi: 10.3816/CLML.2010.n.120. [DOI] [PubMed] [Google Scholar]
- 92.Benevolo G, Larocca A, Gentile M, et al. The efficacy and safety of bortezomib and dexamethasone as a maintenance therapy in patients with advanced multiple myeloma who are responsive to salvage bortezomib-containing regimens. Cancer. 2011;117:1884–1890. doi: 10.1002/cncr.25743. [DOI] [PubMed] [Google Scholar]
- 93.Offidani M, Corvatta L, Polloni C, et al. Thalidomide, dexamethasone, Doxil and Velcade (ThaDD-V) followed by consolidation/maintenance therapy in patients with relapsed-refractory multiple myeloma. Ann Hematol. 2011;90:1449–1456. doi: 10.1007/s00277-011-1217-0. [DOI] [PubMed] [Google Scholar]
- 94.Munshi NC, Anderson KC, Bergsagel PL, et al. Consensus recommendations for risk stratification in multiple myeloma: Report of the International Myeloma Workshop Consensus Panel 2. Blood. 2011;117:4696–4700. doi: 10.1182/blood-2010-10-300970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gkotzamanidou M, Kastritis E, Gavriatopoulou MR, et al. Increased serum lactate dehydrogenase should be included among the variables that define very-high-risk multiple myeloma. Clin Lymphoma Myeloma Leuk. 2011;11:409–413. doi: 10.1016/j.clml.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 96.Kumar SK, Mikhael JR, Buadi FK, et al. Management of newly diagnosed symptomatic multiple myeloma: Updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines. Mayo Clin Proc. 2009;84:1095–1110. doi: 10.4065/mcp.2009.0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ailawadhi S, Masood A, Sher T, et al. Treatment options for multiple myeloma patients with high-risk disease. Med Oncol. 2010 Apr 14; [Epub ahead of print] [Google Scholar]
- 98.Rajkumar SV. Multiple myeloma: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol. 2011;86:57–65. doi: 10.1002/ajh.21913. [DOI] [PubMed] [Google Scholar]
- 99.Rajkumar SV, Gahrton G, Bergsagel PL. Approach to the treatment of multiple myeloma: A clash of philosophies. Blood. 2011;118:3205–3211. doi: 10.1182/blood-2011-06-297853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Richardson PG, Laubach J, Mitsiades CS, et al. Managing multiple myeloma: The emerging role of novel therapies and adapting combination treatment for higher risk settings. Br J Haematol. 2011;154:755–762. doi: 10.1111/j.1365-2141.2011.08791.x. [DOI] [PubMed] [Google Scholar]
- 101.Nair B, van Rhee F, Shaughnessy JD, Jr, et al. Superior results of Total Therapy 3 (2003–33) in gene expression profiling-defined low-risk multiple myeloma confirmed in subsequent trial 2006–66 with VRD maintenance. Blood. 2010;115:4168–4173. doi: 10.1182/blood-2009-11-255620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Avet-Loiseau H, Leleu X, Roussel M, et al. Bortezomib plus dexamethasone induction improves outcome of patients with t(4;14) myeloma but not outcome of patients with del(17p) J Clin Oncol. 2010;28:4630–4634. doi: 10.1200/JCO.2010.28.3945. [DOI] [PubMed] [Google Scholar]
- 103.Avet-Loiseau H, Magrangeas F, Moreau P, et al. Molecular heterogeneity of multiple myeloma: Pathogenesis, prognosis, and therapeutic implications. J Clin Oncol. 2011;29:1893–1897. doi: 10.1200/JCO.2010.32.8435. [DOI] [PubMed] [Google Scholar]
- 104.Cavo M, Bringhen S, Terragna C, et al. Bortezomib-based induction treatments improve outcomes of newly diagnosed multiple myeloma patients with high-risk cytogenetic abnormalities [abstract 781] Blood. 2010;116:342. [Google Scholar]
- 105.Rajkumar SV. Treatment of multiple myeloma. Nat Rev Clin Oncol. 2011;8:479–491. doi: 10.1038/nrclinonc.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Knop S, Liebisch P, Hebart H, et al. Up front allogeneic stem cell transplant is superior to tandem high-dose melphalan in cytogenetically defined ultra high-risk myeloma [abstract V677] Onkologie. 2011;34(suppl 6):199. [Google Scholar]
- 107.Chapman MA, Lawrence MS, Keats JJ, et al. Initial genome sequencing and analysis of multiple myeloma. Nature. 2011;471:467–472. doi: 10.1038/nature09837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Corthals SL, Sun SM, Kuiper R, et al. MicroRNA signatures characterize multiple myeloma patients. Leukemia. 2011;25:1784–1789. doi: 10.1038/leu.2011.147. [DOI] [PubMed] [Google Scholar]
- 109.van Duin M, Broyl A, de Knegt Y, et al. Cancer testis antigens in newly diagnosed and relapse multiple myeloma: Prognostic markers and potential targets for immunotherapy. Haematologica. 2011;96:1662–1669. doi: 10.3324/haematol.2010.037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Shaughnessy JD, Jr, Zhan F, Burington BE, et al. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood. 2007;109:2276–2284. doi: 10.1182/blood-2006-07-038430. [DOI] [PubMed] [Google Scholar]
- 111.Decaux O, Lodé L, Magrangeas F, et al. Prediction of survival in multiple myeloma based on gene expression profiles reveals cell cycle and chromosomal instability signatures in high-risk patients and hyperdiploid signatures in low-risk patients: A study of the Intergroupe Francophone du Myélome. J Clin Oncol. 2008;26:4798–4805. doi: 10.1200/JCO.2007.13.8545. [DOI] [PubMed] [Google Scholar]
- 112.Broyl A, Hose D, Lokhorst H, et al. Gene expression profiling for molecular classification of multiple myeloma in newly diagnosed patients. Blood. 2010;116:2543–2553. doi: 10.1182/blood-2009-12-261032. [DOI] [PubMed] [Google Scholar]
- 113.Dickens NJ, Walker BA, Leone PE, et al. Homozygous deletion mapping in myeloma samples identifies genes and an expression signature relevant to pathogenesis and outcome. Clin Cancer Res. 2010;16:1856–1864. doi: 10.1158/1078-0432.CCR-09-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kumar SK, Uno H, Jacobus SJ, et al. Impact of gene expression profiling-based risk stratification in patients with myeloma receiving initial therapy with lenalidomide and dexamethasone. Blood. 2011;118:4359–4362. doi: 10.1182/blood-2011-03-342089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Moreaux J, Klein B, Bataille R, et al. A high-risk signature for patients with multiple myeloma established from the molecular classification of human myeloma cell lines. Haematologica. 2011;96:574–582. doi: 10.3324/haematol.2010.033456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Walker BA, Leone PE, Chiecchio L, et al. A compendium of myeloma-associated chromosomal copy number abnormalities and their prognostic value. Blood. 2010;116:e56–e65. doi: 10.1182/blood-2010-04-279596. [DOI] [PubMed] [Google Scholar]
- 117.Sawyer JR. The prognostic significance of cytogenetics and molecular profiling in multiple myeloma. Cancer Genet. 2011;204:3–12. doi: 10.1016/j.cancergencyto.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 118.Johnson DC, Corthals S, Ramos C, et al. Genetic associations with thalidomide mediated venous thrombotic events in myeloma identified using targeted genotyping. Blood. 2008;112:4924–4934. doi: 10.1182/blood-2008-02-140434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Broyl A, Corthals SL, Jongen JL, et al. Mechanisms of peripheral neuropathy associated with bortezomib and vincristine in patients with newly diagnosed multiple myeloma: A prospective analysis of data from the HOVON-65/GMMG-HD4 trial. Lancet Oncol. 2010;11:1057–1065. doi: 10.1016/S1470-2045(10)70206-0. [DOI] [PubMed] [Google Scholar]
- 120.Corthals SL, Kuiper R, Johnson DC, et al. Genetic factors underlying the risk of bortezomib induced peripheral neuropathy in multiple myeloma patients. Haematologica. 2011;96:1728–1732. doi: 10.3324/haematol.2011.041434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Favis R, Sun Y, van de Velde H, et al. Genetic variation associated with bortezomib-induced peripheral neuropathy. Pharmacogenet Genomics. 2011;21:121–129. doi: 10.1097/FPC.0b013e3283436b45. [DOI] [PubMed] [Google Scholar]
- 122.Johnson DC, Corthals SL, Walker BA, et al. Genetic factors underlying the risk of thalidomide-related neuropathy in patients with multiple myeloma. J Clin Oncol. 2011;29:797–804. doi: 10.1200/JCO.2010.28.0792. [DOI] [PubMed] [Google Scholar]
- 123.Rajpal R, Dowling P, Meiller J, et al. A novel panel of protein biomarkers for predicting response to thalidomide-based therapy in newly diagnosed multiple myeloma patients. Proteomics. 2011;11:1391–1402. doi: 10.1002/pmic.201000471. [DOI] [PubMed] [Google Scholar]
- 124.Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: A multicenter International Myeloma Working Group study. Leukemia. 2012;26:149–157. doi: 10.1038/leu.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fassas A, Shaughnessy J, Barlogie B. Cure of myeloma: Hype or reality? Bone Marrow Transplant. 2005;35:215–224. doi: 10.1038/sj.bmt.1704757. [DOI] [PubMed] [Google Scholar]
- 126.Barlogie B, Shaughnessy JD., Jr Genomics and cure in multiple myeloma [abstract] Haematologica. 2011;96(suppl 1):S10. [Google Scholar]
- 127.San-Miguel JF, Mateos MV. Can multiple myeloma become a curable disease? Haematologica. 2011;96:1246–1248. doi: 10.3324/haematol.2011.051169. [DOI] [PMC free article] [PubMed] [Google Scholar]