Abstract
Stenotrophomonas maltophilia is increasingly being isolated from the respiratory tract of individuals with cystic fibrosis, and, because of its multidrug-resistant nature, the selection of suitable treatment regimens can be problematical. Etest methodology was used to facilitate MIC and antimicrobial combination testing on 80 isolates of S. maltophilia cultured from the respiratory tract of Scottish individuals with cystic fibrosis between 2001 and 2010. The overall rate of susceptibility for the 1,410 MIC tests was 23.1%, and resistance was 68.9%. The most active antimicrobials were minocycline, co-trimoxazole, and doxycycline, with 92.4%, 87.3%, and 58.8% of isolates being susceptible, respectively. Of the 517 combinations, 13.2% were synergistic, with the most synergistic being ticarcillin/clavulanate plus aztreonam (91.7% synergistic), ticarcillin/clavulanate plus colistin (40%), and ticarcillin/clavulanate plus levofloxacin (19.4%). Colistin plus tobramycin was the only antagonistic combination (0.2%). By the median susceptible breakpoint index, the most active combinations were minocycline plus co-trimoxazole (median index, 20), minocycline plus piperacillin-tazobactam (median, 20), and co-trimoxazole plus ceftazidime (median, 16.5). The increasing problem of multidrug resistance in organisms recovered from the respiratory tracts of individuals with cystic fibrosis is not going to go away. Current susceptibility testing methods do not address the slow-growing organisms associated with chronic infection, and interpretive standards are based on achievable blood levels of antimicrobials. Addressing these issues specifically for organisms recovered from the respiratory tracts of individuals with cystic fibrosis should lead to better therapeutic outcomes and improved wellbeing of individuals with cystic fibrosis.
INTRODUCTION
Cystic fibrosis (CF) is a life-shortening, autosomal recessive genetic condition caused by dysfunction of the cystic fibrosis transmembrane conductance regulator (CFTR) protein (21, 25, 31). Abnormal CFTR function in the lung results in thick, viscous secretions which are difficult to clear, and this in turn leads to chronic infection, decline in lung function, and eventually respiratory failure (25). Antimicrobial therapy has undoubtedly helped prolong the lives of individuals with CF, but a consequence of this is the colonization of the lungs with difficult-to-treat multidrug-resistant bacteria such as Pseudomonas aeruginosa, Burkholderia cepacia, and Stenotrophomonas maltophilia (4, 9). S. maltophilia is a ubiquitous organism commonly isolated from water, soil, and sewage and also from nosocomial environments. This organism can be the cause of respiratory, urinary, and bloodstream infections in hospitalized patients, especially in those who are immumocompromised or are in intensive care units and particularly in those patients who are catheterized or are receiving mechanical ventilation (22, 29). It has recently been shown to be a risk factor for pulmonary exacerbation in CF, but the role it plays in the decline of lung function is unclear (3, 11, 13, 30). Reports suggest that S. maltophilia acquisition in CF occurs in individuals who are older, have poorer lung function, and have advanced lung disease and that its detection does not independently affect short-term survival, that long-term chronic infection is unusual, and that accelerated deterioration in lung function is unlikely (12).
S. maltophilia infections are difficult to treat due high levels of intrinsic resistance and also acquired resistances possibly due to overuse of broad-spectrum-β-lactam antimicrobials (9).
In this work, we describe our experience of S. maltophilia isolates which have been referred to the Scottish Cystic Fibrosis Antibiotic Susceptibility Testing Service (CFASS) for MIC and antimicrobial combination testing (17). CFASS is based in the microbiology laboratory of Aberdeen Royal Infirmary and accepts Gram-negative non-lactose-fermenting bacterial isolates from all Scottish CF centers. The isolates which are identified at a local level by the referring centers are typically referred when the isolates are multidrug resistant or there are problems locally with identifying suitable treatment regimens. Etest methodology was used to facilitate the MIC and combination testing of the isolates, and the fractional inhibitory concentration index (FICI) and the susceptible breakpoint index (SBPI) were used to aid interpretation of the combination testing results (19).
MATERIALS AND METHODS
A total of 80 isolates of S. maltophilia were referred to CFASS for MIC and combination testing between May 2001 and August 2010. Confirmation of isolate identification was performed as previously described (19). MIC testing was performed on Mueller-Hinton agar (MHA) using Etest methodology according to the instructions of the manufacturers (AB Biodisk, Solna, Sweden, and bioMérieux, Basingstoke, United Kingdom) The antimicrobials tested were amikacin, gentamicin, netilmicin (testing discontinued 2006), tobramycin, ciprofloxacin, levofloxacin, aztreonam, ceftazidime, piperacillin (testing discontinued 2003), piperacillin-tazobactam, imipenem, meropenem, colistin, ticarcillin/clavulanate, ampicillin/sulbactam, chloramphenicol, minocycline, doxycycline (testing introduced October 2003), co-rimoxazole, and rifampin. For the purpose of this work, MICs falling between concentrations in the standard doubling-dilution scale were rounded up to the next doubling dilution (e.g., 0.38 = 0.5 mg/liter) and were interpreted as susceptible (S), intermediate (I), or resistant (R) according to the following criteria: levofloxacin, ceftazidime, ticarcillin/clavulanate, minocycline, chloramphenicol, and co-trimoxazole per Clinical and Laboratories Standards Institute (CLSI)-approved interpretive standards for S. maltophilia, ampicillin/sulbactam per CLSI interpretive standards for Acinetobacter spp., and rifampin per CLSI interpretive standards for Staphylococcus spp. The remainder were interpreted according to CLSI criteria for other nonenterobacteriaceae (5).
Combination testing was usually performed using six pairs of antimicrobials which were selected based on their MICs, clinical information such as the individual's current antimicrobial therapy and allergies or intolerances, if known, and also at the clinician's specific request. Briefly, Mueller-Hinton agar plates (Oxoid, Basingstoke, United Kingdom) were inoculated per Etest instructions for MIC determinations. Two Etests, A and B, were applied to the inoculated agar, and the mixture was left at room temperature for 1 h to allow the antimicrobials to migrate into the agar. Etest A was then removed, and a fresh Etest B was placed on the imprint of Etest A, matching the antimicrobial gradient. Likewise, Etest B was replaced with a fresh Etest A. Incubation was carried out at 35°C in ambient air, and the MICs were read after 22 to 24 h.
FICI.
Indices derived from the combination MIC results were calculated using the MIC value read on the Etest strip, and the FICI was interpreted as follows:
where, in cases in which an MIC was found to be greater than the values in the antimicrobial range tested, the next doubling dilution above the highest value of the range tested was used to calculate the FICI (e.g., if an MIC of >256 mg/liter was found, then the FICI was calculated using 512 mg/liter) (1). The indices were interpreted as follows: an FICI of ≤0.5 = synergy, an FICI of >0.5 and ≤4.0 = no interaction, and an FICI of >4.0 = antagonism (23).
SBPI.
The SBPI was calculated as follows:
An SBPI of 2.0 indicates either that the MICs of antimicrobials A and B in combination are equivalent to their respective susceptible breakpoints or that the MIC of one of the antimicrobials in a combination is less than its susceptible breakpoint. It therefore follows that the greater the SBPI value, the more effective that combination is in vitro (19).
The combination results were reported in rank order of their SBPI results from the highest to the lowest SBPI. Any combination which was found to be antagonistic (FICI > 4.0) was not ranked, irrespective of the SBPI result, and was reported as being not recommended for therapy.
Questionnaire.
From April 2002, a questionnaire designed to assess the usefulness of CFASS was issued with each result's report, and clinicians were requested to return their yes/no responses to the questionnaire to CFASS.
Statistical methods.
Descriptive statistics were derived using Microsoft Office Excel 2003.
RESULTS
A total of 879 isolates from 218 individuals with CF were referred to CFASS for extended susceptibility testing between May 2001 and August 2010. Of these, 80 isolates (9.1% of the total) from 13 females and 20 males were identified at a local level by their 7 referring laboratories as being S. maltophilia. Of those 33 individuals, 15 have had only S. maltophilia isolates referred and 12 have had referrals of P. aeruginosa or Pseudomonas spp., whereas 5 have also had referrals of Sphingomonas spp., Chryseomonas spp., Alcaligenes spp., or Ralstonia pickettii, and 1 had a possible B. cepacia isolate referred. The age range for both males and females at the first S. maltophilia referral was 1 to 48 years (median, 19 years). Between 1 and 3 S. maltophilia (median, 1) isolates were received per single referral per person, and between 1 and 9 (median, 1) isolates were received per person during the course of the study. No S. maltophilia isolates were referred for testing during 2007.
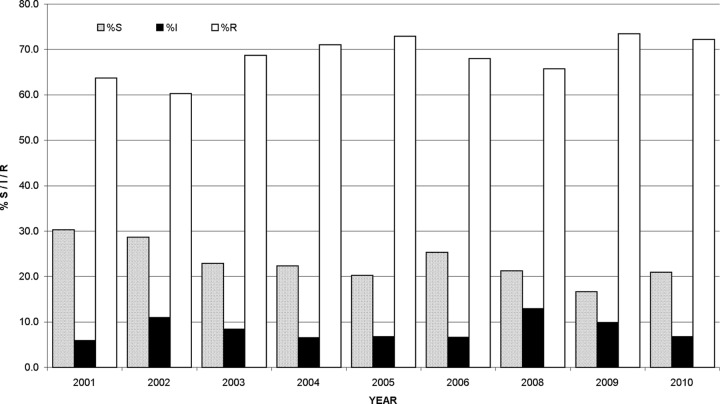
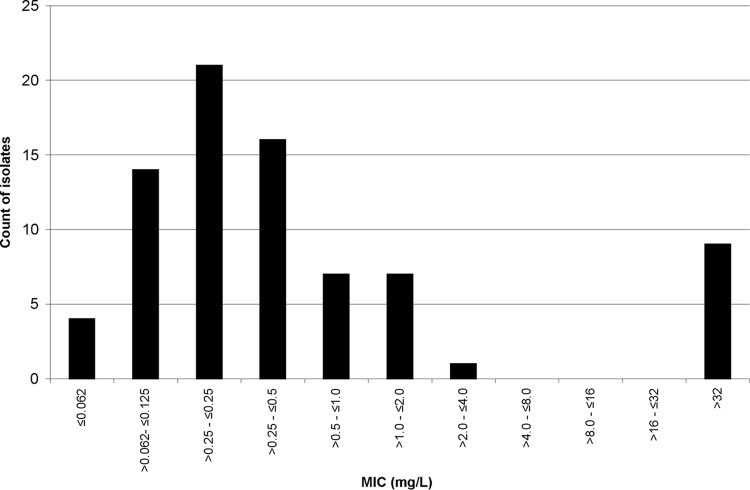
A total of 1,410 MIC determinations were performed on the 80 isolates of S. maltophilia. The results are presented in Table 1. Of those isolates, 23.1% were susceptible and 68.9% resistant to the antimicrobials tested. The results for the six antimicrobials determined with CLSI interpretive standards for S. maltophilia (n = 472) showed 48.7% of isolates to be susceptible and 38.4% resistant. Of the remaining 938 MICs with no CLSI-approved breakpoints, 10.2% were susceptible and 84.2% resistant. Figure 1 shows the annual percent S, I, and R results obtained. From 2001 to 2010, there were decreases in susceptibility of 9.3% and 15.3% for all antimicrobials tested and for the antimicrobials with CLSI interpretive guidelines for S. maltophilia, respectively (data not shown). Minocycline proved to be the most active single antimicrobial (92.4% S) followed by co-trimoxazole (87.3% S) and doxycycline (58.8% S). Gentamicin (13.3% S) was the most active aminoglycoside and ticarcillin/clavulanate (11.5% S) the most active β-lactam, and levofloxacin (45% S) was 5.1 times more active than ciprofloxacin. A bimodal distribution of the co-trimoxazole MICs was observed, but this was not the case for minocycline and doxycycline. The 10 co-trimoxazole-resistant isolates (Fig. 2) were referred from 8 patients. Of the 10 isolates, 6 had co-trimoxazole MICs > 32 mg/liter on first referral. MIC analysis of the first isolate referred from each individual (35 in total [2 individuals each had two isolates with distinct susceptibility patterns referred initially]) showed 26.2% and 54.5% of all antimicrobials and of the 6 antimicrobials with CLSI-approved breakpoints to be active, respectively. There was no difference in the order of the first 9 most active antimicrobials for all 80 isolates versus the order of the first-referred isolates from each individual (data not shown).
Table 1.
S. maltophilia MIC summary dataa
| Antimicrobial | No. of isolates tested | Category (no. of isolates) |
% in category |
MIC50 (mg/liter) | MIC90 (mg/liter) | Range (mg/liter) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S | I | R | S | I | R | Low | High | ||||
| MINb | 80 | 74 | 3 | 3 | 92.4 | 3.8 | 3.8 | 2 | 4 | 0.25 | 16 |
| SXTb | 79 | 69 | 0 | 10 | 87.3 | 0 | 12.7 | 0.50 | >32 | 0.012 | >32 |
| DOXc | 51 | 30 | 14 | 7 | 58.8 | 27.5 | 13.7 | 4 | 16 | 0.50 | 16 |
| CHLb | 78 | 37 | 22 | 19 | 47.4 | 28.2 | 24.4 | 16 | 64 | 2 | >256 |
| LVXb | 80 | 36 | 14 | 30 | 45.0 | 17.5 | 37.5 | 4 | >32 | 0.25 | >32 |
| COLc | 80 | 30 | 6 | 44 | 37.5 | 7.5 | 55.0 | 8 | >256 | 0.125 | >256 |
| GENc | 75 | 10 | 2 | 63 | 13.3 | 2.7 | 84.0 | >256 | >256 | 1 | >256 |
| TIMb | 78 | 9 | 19 | 50 | 11.5 | 24.4 | 64.1 | 256 | >256 | 1 | >256 |
| AMKc | 74 | 8 | 2 | 64 | 10.8 | 2.7 | 86.5 | >256 | >256 | 8 | >256 |
| CIPc | 80 | 7 | 14 | 59 | 8.8 | 17.5 | 73.7 | 16 | >32 | 0.50 | >32 |
| CAZb | 77 | 5 | 3 | 69 | 6.5 | 3.9 | 89.6 | >256 | >256 | 2 | >256 |
| ATMc | 70 | 4 | 0 | 66 | 5.7 | 0 | 94.3 | >256 | >256 | 2 | >256 |
| NETc | 42 | 2 | 0 | 40 | 4.8 | 0 | 95.2 | >256 | >256 | 2 | >256 |
| TOBc | 80 | 2 | 5 | 73 | 2.5 | 6.3 | 91.2 | 256 | >256 | 2 | >1024 |
| TZPc | 80 | 2 | 5 | 73 | 2.5 | 6.3 | 91.2 | >256 | >256 | 16 | >256 |
| SAMd | 64 | 1 | 0 | 63 | 1.6 | 0 | 98.4 | >256 | >256 | 4 | >256 |
| RIFe | 80 | 0 | 4 | 76 | 0 | 5.0 | 95.0 | 32 | >32 | 2 | >256 |
| PIPc | 12 | 0 | 0 | 12 | 0 | 0 | 100 | >256 | >256 | 256 | >256 |
| IPMc | 80 | 0 | 0 | 80 | 0 | 0 | 100 | >32 | >32 | >32 | >32 |
| MEMc | 70 | 0 | 0 | 70 | 0 | 0 | 100 | >32 | >32 | 32 | >32 |
S, susceptible; I, intermediate; R, resistant; MIN, minocycline; SXT, co-trimoxazole; DOX, doxycycline; CHL, chloramphenicol; LVX, levofloxacin; COL, colistin; GEN, gentamicin; TIM, ticarcillin/clavulanate; AMK, amikacin; CIP, ciprofloxacin; CAZ, ceftazidime; ATM, aztreonam; NET, netilmicin; TOB, tobramycin; TZP, piperacillin-tazobactam; SAM, ampicillin/sulbactam; RIF, rifampin; PIP, piperacillin; IPM, imipenem; MEM, meropenem.
CLSI-approved interpretive standards for S maltophilia.
CLSI-approved interpretive standards for nonenterobacteriaceae.
CLSI-approved interpretive standards for A. baumannii.
CLSI-approved interpretive standards for Staphylococcus species.
Fig 1.
Annual percentages of S, I, and R S. maltophilia isolates by MIC. No S. maltophilia isolates were submitted for testing during 2007.
Fig 2.
Co-trimoxazole MIC distribution for S. maltophilia.
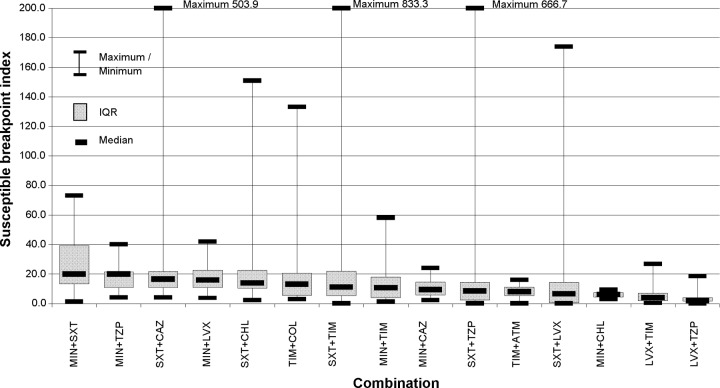
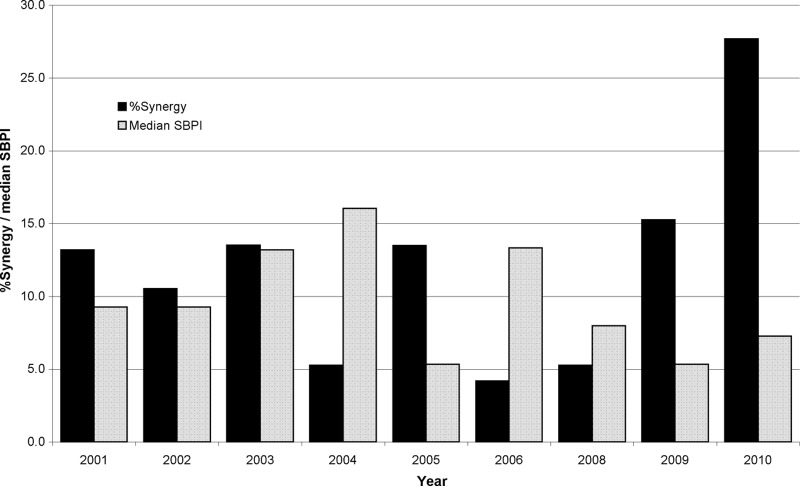
A total of 517 combination tests were performed on 60 pairs of antimicrobials. Of those combinations, 13.2% demonstrated synergy and 0.2% antagonism. Table 2 is a summary of the combination pairs tested, and Table 3 is a breakdown of the FICI and SBPI results according to the number of antimicrobials in the combination with CLSI-approved MIC breakpoints. A total of 15 combination pairs (362 combinations) were tested ≥10 times. Of these, the three most synergistic combinations were ticarcillin/clavulanate plus aztreonam (91.7%), ticarcillin/clavulanate plus colistin (40%), and ticarcillin/clavulanate plus levofloxacin (19.4%) but by median SBPI they ranked 11th, 6th, and 14th, respectively (Fig. 3). Minocycline plus co-trimoxazole and minocycline plus piperacillin-tazobactam with median SBPIs of 20 were ranked equal 1st, and co-trimoxazole plus ceftazidime (median SBPI, 16.5) was 3rd for those combinations tested ≥10 times when ranked by median SBPI (Fig. 3) but ranked 9th, 12th, and 7th by FICI, respectively. Antagonism was not found in those combinations tested ≥10 times. Synergy was found in 16 (10.3%) and antagonism in 1 (0.6%) of the 45 pairs which were tested <10 times (155 combinations). The increase in the number of synergistic combinations in 2009 and again in 2010 (Fig. 4) can be explained by the introduction of the testing of ticarcillin/clavulanate plus aztreonam in both years and also by testing of rifampin plus colistin in 2010. If these combinations were discounted, then the rates of synergy for 2009 and 2010 would be 10.7% (median SBPI, 4.8) and 13.2% (median SBPI, 7.3), respectively. To date, of the 5 combinations with rifampin plus colistin (median SBPI, 1.25), 4 have been synergistic. Interestingly, the 6 combinations with co-trimoxazole plus colistin have given a median SBPI of 34.5, with 2 combinations showing synergy.
Table 2.
S. maltophilia antimicrobial combination testing results—percent synergy by fractional inhibitory concentration indexa
| Second antimicrobial | Value(s) for first antimicrobial or combination |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMK | GEN | TOB | CIP | LVX | ATM | CAZ | TZP | MEM | COL | TIM | CHL | MIN | DOX | SXT | RIF | |
| AMK | 10.8 | 1 | 2 | 5 | 1 | |||||||||||
| GEN | 13.3 | 3 | 1 | 9 | 3 | 1 | 4 | |||||||||
| TOB | 2.5 | 2 | 5 | 6 | 1 | 1 | 1 | 1 | 1 | |||||||
| CIP | 0 | 8.8 | 2 | 6 | 6 | 5 | 1 | 6 | 4 | |||||||
| LVX | 0 | 0 | 0 | 45.0 | 8 | 19 | 9 | 36 | 5 | 35 | 12 | |||||
| ATM | 0 | 1 | 5.7 | 12 | 3 | 1 | 4 | |||||||||
| CAZ | 1 | 5 | 3 | 0 | 3 | 6.5 | 7 | 8 | 15 | 22 | 1 | |||||
| TZP | 1 | 4 (10.5) | 2.5 | 4 | 1 | 11 | 23 | |||||||||
| MEM | 0.0 | 1 | ||||||||||||||
| COL | 1 ANT | 3 | 1 | 1 | 0 | 0 | 37.5 | 10 | 1 | 7 | 6 | 5 | ||||
| TIM | 0 | 2 | 0 | 1 | 7 (19.4) | 11 (91.7) | 4 (40) | 11.5 | 3 | 43 | 41 | |||||
| CHL | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 47.4 | 10 | 20 | |||||
| MIN | 1 | 0 | 1 (2.9) | 1 | 1 (6.7) | 0 (0) | 0 | 1 (2.3) | 0 (0) | 92.4 | 53 | 1 | ||||
| DOX | 0 | 58.8 | 1 | |||||||||||||
| SXT | 0 | 0 | 0 (0) | 0 | 1 (4.5) | 1 (4.3) | 2 | 6 (14.6) | 0 (0) | 2 (3.8) | 0 | 87.3 | 1 | |||
| RIF | 0 | 0 | 4 | 0 | 0 | 0.0 | ||||||||||
Highlighted figures, percentages of susceptible isolates at drug MIC; figures to right of highlights, numbers of combinations tested; figures to left of highlights, numbers of synergistic combinations (percent synergy where combination tested ≥10 times); 1 ANT, 1 combination antagonistic; AMK, amikacin; GEN, gentamicin; TOB, tobramycin; CIP, ciprofloxacin; LVX, levofloxacin; ATM, aztreonam; CAZ, ceftazidime; TZP, piperacillin-tazobactam; MEM, meropenem; COL, colistin; TIM, ticarcillin/clavulanate; CHL, chloramphenicol; MIN, minocycline; DOX, doxycycline; SXT, co-trimoxazole; RIF, rifampin.
Table 3.
Combination testing results according to the number of CLSI-approved antimicrobials for S. maltophilia in the combination
| Parameter | Value(s) for antimicrobials according to the indicated no. of CLSI-approved breakpoints in the combinationa |
||
|---|---|---|---|
| 2 | 1 | 0 | |
| Total no. of combinations | 311 | 171 | 35 |
| No. of pairs tested | 14 | 35 | 11 |
| No. (%) synergistic | 23 (7.4) | 36 (21.1) | 9 (25.7) |
| No. (%) antagonistic | 0 (0) | 0 (0) | 1 (2.9) |
| Median SBPI | 10.7 | 5.8 | 2.1 |
The antimicrobials used were levofloxacin, ceftazidime, ticarcillin/clavulanate, chloramphenicol, minocycline, and co-trimoxazole. SBPI, susceptible breakpoint index.
Fig 3.
Interquartile ranges (IQRs) of S. maltophilia susceptibility breakpoint indices for combinations tested ≥10 times. MIN, minocycline; SXT, co-trimoxazole; TZP, piperacillin-tazobactam; CAZ, ceftazidime; LVX, levofloxacin; CHL, chloramphenicol; TIM, ticarcillin/clavulanate; COL, colistin; ATM, aztreonam.
Fig 4.
Percent synergistic combinations and median susceptible breakpoint indices for drugs against S. maltophilia.
Of the 56 questionnaires issued (covering 63 isolates), 32 were returned to CFASS. The questionnaire and responses are set out in Table 4.
Table 4.
Clinician responses to CFASS feedback questionnaires (n = 32)a
| Question | No. (%) of requested responses |
No. (%) of other responses |
||
|---|---|---|---|---|
| Yes | No | N/A | NR | |
| Has the current CFASS report helped in the management of the current infective exacerbation of CF? | 15 (46.9) | 17 (53.1) | 0 (0) | 0 (0) |
| Will this report help in the management of subsequent infective exacerbations of CF in this patient? | 28 (87.5) | 3 (9.4) | 0 (0) | 1 (3.1) |
| Has this report helped in the initial choice of antibiotic treatment? | 9 (28.1) | 23 (71.9) | 0 (0) | 0 (0) |
| Has this report led to a change in antibiotic treatment? | 12 (37.5) | 19 (59.4) | 0 (0) | 1 (3.1) |
| Has this report confirmed the existing choice of treatment where clinical progress was satisfactory? | 11 (34.4) | 16 (50.0) | 3 (9.4) | 2 (6.2) |
| Has this report confirmed the existing choice of treatment where there was clinical doubt? | 10 (31.2) | 15 (46.9) | 3 (9.4) | 4 (12.5) |
n, number of responses; N/A, not applicable; NR, no response.
DISCUSSION
It is widely reported in the literature that S. maltophilia is increasingly being isolated from the airways of individuals with CF, and the cause and effect are still being debated (3, 4, 11, 13). One thing agreed, however, is that the multidrug-resistant nature of S. maltophilia poses problems for the clinician in the selection of appropriate antimicrobial therapy. Aggressive antimicrobial therapy has been suggested as a possible cause for the increase in S. maltophilia isolation (9). In contrast to the findings of one cohort study looking at S. maltophilia acquisition factors, CFASS had more referrals from males than females (20 versus 13) (12). Whether this is a true reflection of S. maltophilia in the Scottish CF population is uncertain, as we can comment only on what is referred. The high rate of resistance found in this study serves to underline the resistant nature of S. maltophilia present in the Scottish CF population.
Co-trimoxazole, ticarcillin/clavulanate, minocycline, doxycycline, levofloxacin, and chloramphenicol have all been reported as being the most active single agent against S. maltophilia isolated from individuals with and without CF (6, 9, 11, 18, 22, 27, 29). Co-trimoxazole is often the drug of first choice for therapy, although increasing in vitro resistance is being reported (2, 9, 14). Of the patients in our study, 24.2% had co-trimoxazole-resistant S. maltophilia, and, like Valdezate et al., we also found a bimodal distribution of MICs (29). We can draw no firm conclusions regarding trends in susceptibility patterns of individual antimicrobials due to the low numbers of isolates tested on an annual basis (data not shown) and do not know if the referred S. maltophilia were from individuals with transient infection or individuals who were colonized with this organism.
The selection of empirical therapy for the treatment of multidrug-resistant organisms which infect the respiratory tract of individuals with CF is not an easy task. CFASS takes into account the MICs of the antimicrobials, current antimicrobial therapy, and antimicrobial allergy or intolerance (if known) when selecting combinations for testing and uses the recently described SBPI to aid ranking of the combination results in the order of their in vitro effectiveness (19).
Of the 517 combinations tested, 13.2% were synergistic and only 1 was antagonistic. Combinations of ticarcillin/clavulanate plus aztreonam have been reported by other workers as having synergistic activity against S. maltophilia (4, 11, 14) and have proved to be our most synergistic combination to date. The resistance of S. maltophilia to β-lactam antimicrobials is due to the expression of two ß-lactamases, L1 and L2. The metallo-β-lactam L1 is inhibited by aztreonam but not by clavulanic acid, and the serine ß-lactamase L2 is inhibited by clavulanic acid, thus inactivating the bacterium's β-lactam resistance mechanisms (14). However, by median SBPI the combination of ticarcillin/clavulanate plus aztreonam ranked only 11th in our league table of combinations tested ≥10 times. Giamarellos-Bourboulis et al. reported synergy with combinations of rifampin plus colistin and co-trimoxazole plus colistin, which we also found. Colistin targets the bacterial cell membrane, resulting in increased permeability of the cell envelope which facilitates the uptake of the partner antimicrobial into the bacterium (10). To date, rifampin plus colistin (tested 5 times) has been showing good synergy, although its median SBPI of 1.25 is disappointingly low. Of the 6 combinations of co-trimoxazole plus colistin which we have tested, 2 have been synergistic, and the median SBPI of 34.5 exceeds that of minocycline plus co-trimoxazole, which tops our league table of combinations tested ≥10 times according to median SBPI indices.
The data presented in Table 3 demonstrate that the most synergy was found in the combinations which did not have antimicrobials with CLSI-approved breakpoints for S. maltophilia. In contrast, higher median SBPIs were achieved in those combinations where both antimicrobials had CLSI-approved breakpoints. A possible explanation for this may be that the antimicrobials which have CLSI-approved breakpoints for S. maltophilia were more active by our MIC testing than those without. Perhaps the SBPI partially explains nonsynergistic combinations achieving successful therapeutic outcomes and also the lack of correlation between in vitro synergy and clinical efficacy, as it reflects antimicrobial breakpoints, unlike the FICI (6, 28).
In all probability, in the time required for the local laboratory to isolate the causative organisms from the respiratory secretions of an infective exacerbation, for the submission of the organisms to CFASS for further investigation, and for CFASS to report back to the clinician, the individual will have undergone a course of antimicrobial therapy. This is reflected in the responses to the questionnaire, where 53.1% of respondents stated that the CFASS report had not helped in the management of the current infective exacerbation and 87.5% stated that the report would be helpful in the management of subsequent infective exacerbations.
Undoubtedly, antimicrobial therapy has helped prolong the lives of people with CF, but at what cost? We now have the dilemma of multidrug-resistant organisms colonizing the lungs and causing infective episodes coupled with more unusual organisms, including S. maltophilia, being implicated as causative infecting agents (15). Methods such the checkerboard, Etest, time-kill, and multiple-combination bactericidal testing are used to assess the in vitro activity of antimicrobial combinations. Each method has its own advantages and disadvantages; comparison of in vitro results from different methods is difficult, and very few clinical studies have been undertaken to assess therapeutic outcomes guided by the in vitro results generated by these methods (24, 26).
There is a real need to readdress the whole area of the microbiology of the CF lung, starting at grassroots level. Molecular methods have made inroads into the identification and reclassification of bacteria (8, 15). As far as we are aware, there are no specific guidelines formulated for the susceptibility testing of organisms which have been recovered from the respiratory secretions of people with CF, especially for those organisms associated with chronic infection. Laboratories and clinicians have to rely on published antimicrobial breakpoints formulated for the treatment of rapidly growing organisms from acute infection for the interpretation of susceptibility testing results. These breakpoints are based on achievable blood levels of antimicrobials and not levels achievable in the lung, as is the case with aerosolized antimicrobials, where local levels greatly exceed those achievable by systemic administration (7). The converse also applies, where achievable levels in the lung can be less than blood levels (20).
It is unfortunate that we are unable to comment on any treatment outcomes based on the recommendations of CFASS, but, that said, feedback from the users of CFASS indicates that they find the extended susceptibility testing results helpful in guiding therapy (16).
ACKNOWLEDGMENTS
CFASS is funded for adult patient isolate testing by the National Services Division of the Common Services Agency of the Scottish Executive. Astra Zeneca funded some of the testing of pediatric patient isolates.
We thank the laboratories and clinicians who use CFASS for their support.
I.M.G. is a consultant to and/or speaker board member of Astra Zeneca, Merck Sharp and Dohme, Novartis, and Pfizer.
Footnotes
Published ahead of print 14 May 2012
REFERENCES
- 1. Bonapace CR, White RL, Friedrich LV, Bosso JA. 2000. Evaluation of antibiotic synergy against Acinetobacter baumannii: a comparison with Etest, time kill and checkerboard methods. Diagn. Microbiol. Infect. Dis. 38:43–50 [DOI] [PubMed] [Google Scholar]
- 2. Bonfiglio G, et al. 2000. Levofloxacin in vitro activity and time-kill evaluation of Stenotrophomonas maltophilia clinical isolates. J. Antimicrob. Chemother. 45:115–117 [DOI] [PubMed] [Google Scholar]
- 3. Chandra S, et al. 2008. Stenotrophomonas maltophilia in cystic fibrosis. Chest 134(Suppl.):S57002 http://meeting.chestpubs.org/cgi/content/abstract/134/4/s57002 [Google Scholar]
- 4. Chernish RN, Aaron SD. 2003. Approach to resistant gram-negative bacterial pulmonary infections in patients with cystic fibrosis. Curr. Opin. Pulm. Med. 9:509–515 [DOI] [PubMed] [Google Scholar]
- 5. Clinical and Laboratories Standards Institute 2010. Performance standards for antimicrobial susceptibility testing: 20th informational supplement M100-S20. CLSI, Wayne, PA [Google Scholar]
- 6. Denton M, Kerr KG. 1998. Microbiological and clinical aspects of infection associated with Stenotrophomonas maltophilia. Clin. Microbiol. Rev. 11:57–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dudley MN, Loutit J, Griffith DC. 2008. Aerosol antibiotics: considerations in pharmacological and clinical evaluation. Curr. Opin. Biotech. 19:637–643 [DOI] [PubMed] [Google Scholar]
- 8. Elborn JS. 2008. Identification and management of unusual pathogens in cystic fibrosis. J. R. Soc. Med. 101(Suppl.):S2–S5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gales AC, et al. 2001. Emerging importance of multidrug-resistant Acinetobacter species and Stenotrophomonas maltophilia as pathogens in seriously ill patients: geographic patterns, epidemiological features, and trends in the SENTRY Antimicrobial Surveillance Program (1997–1999). Clin. Infect. Dis. 32(Suppl. 2):104–113 [DOI] [PubMed] [Google Scholar]
- 10. Giamarellos-Bourboulis EJ, Karnesis L, Giamarellou H. 2002. Synergy of colistin with rifampicin and trimethoprim/sulfamethoxazole on multidrug-resistant Stenotrophomonas maltophilia. Diagn. Microbiol. Infect. Dis. 44:259–263 [DOI] [PubMed] [Google Scholar]
- 11. Goss CH, Burns JL. 2007. Exacerbations in cystic fibrosis. 1: Epidemiology and pathogenesis. Thorax 62:360–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Goss CH, Otto K, Aitken ML, Rubenfeld GD. 2002. Detecting Stenotrophomonas maltophilia does not reduce survival of patients with cystic fibrosis. Am. J. Respir. Crit. Care Med. 166:356–361 [DOI] [PubMed] [Google Scholar]
- 13. Graff GR, Burns JL. 2002. Factors affecting the incidence of Stenotrophomonas maltophilia isolation in cystic fibrosis. Chest 121:1754–1760 [DOI] [PubMed] [Google Scholar]
- 14. Krueger TS, Clark EA, Nix DE. 2001. In vitro susceptibility of Stenotrophomonas maltophilia to various antimicrobial combinations. Diagn. Microbiol. Infect. Dis. 41:71–78 [DOI] [PubMed] [Google Scholar]
- 15. Lipuma JJ. 2010. The changing microbial epidemiology in cystic fibrosis. Clin. Microbiol. Rev. 23:299–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. MacKenzie FM, Griffiths C, Dempsey O, Devereaux G, Gould IM. 2005. Combination antibiotic susceptibility testing in cystic fibrosis. Lancet 366:1693. [DOI] [PubMed] [Google Scholar]
- 17. MacKenzie FM, et al. 2004. Antibiograms of resistant Gram-negative bacteria from Scottish CF patients. J. Cyst. Fibros. 3:151–157 [DOI] [PubMed] [Google Scholar]
- 18. Marchac V, Equi A, Le Bihan-Benjamin C, Hodson M, Bush A. 2004. Case-control study of Stenotrophomonas maltophilia acquisition in cystic fibrosis patients. Eur. Respir. J. 23:98–102 [DOI] [PubMed] [Google Scholar]
- 19. Milne KEN, Gould IM. 2010. Combination testing of multidrug-resistant cystic fibrosis isolates of Pseudomonas aeruginosa: use of a new parameter, the susceptible breakpoint index. J. Antimicrob. Chemother. 65:82–90 [DOI] [PubMed] [Google Scholar]
- 20. Moriarty TF, McElnay JC, Elborn JS, Tunney MM. 2007. Sputum antibiotic concentrations: implications for treatment of cystic fibrosis lung infection. Paediatr. Pulmonol. 42:1008–1017 [DOI] [PubMed] [Google Scholar]
- 21. Moskowitz SM, et al. 2008. Clinical practice and genetic counseling for cystic fibrosis and CFTR-related disorders. Genet. Med. 10:851–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nicodemo AC, Araujo MRE, Ruiz AS, Gales AC. 2004. In vitro susceptibility of Stenotrophomonas maltophilia isolates: comparison of disc diffusion, Etest and agar dilution methods. J. Antimicrob. Chemother. 53:604–608 [DOI] [PubMed] [Google Scholar]
- 23. Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 52:1 doi:10.1093/jac/dkg301 [DOI] [PubMed] [Google Scholar]
- 24. Pankey GA, Ashcraft DS. 2010. In vitro synergistic/additive activity of levofloxacin with meropenem against Stenotrophomonas maltophilia. Diagn. Microbiol. Infect. Dis. 67:297–300 [DOI] [PubMed] [Google Scholar]
- 25. Rowe SM, Clancy JP. 2006. Advances in cystic fibrosis therapies. Curr. Opin. Pediatr. 18:604–613 [DOI] [PubMed] [Google Scholar]
- 26. Saiman L. 2007. Clinical utility of synergy testing for multidrug-resistant Pseudomonas aeruginosa isolated from patients with cystic fibrosis: ‘the motion for’. Paediatr. Respir. Rev. 8:249–255 [DOI] [PubMed] [Google Scholar]
- 27. San Gabriel P, et al. 2004. Antimicrobial susceptibility and synergy studies of Stenotrophomonas maltophilia isolates from patients with cystic fibrosis. Antimicrob. Agents Chemother. 48:168–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Song W, Woo HJ, Kim JS, Lee KM. 2003. In vitro activity of β-lactams in combination with other antimicrobial agents against resistant strains of Pseudomonas aeruginosa. Int. J. Antimicrob. Agents 21:8–12 [DOI] [PubMed] [Google Scholar]
- 29. Valdezate S, Vindel A, Loza E, Baquero F, Canton R. 2001. Antimicrobial susceptibilities of unique Stenotrophomonas maltophilia clinical strains. Antimicrob. Agents Chemother. 45:1581–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Waters V, et al. 2012. Chronic Stenotrophomonas maltophilia infection and exacerbation outcomes in cystic fibrosis. J. Cyst. Fibros. 11:8–13 [DOI] [PubMed] [Google Scholar]
- 31. Zemanick ET, et al. 2010. Measuring and improving respiratory outcomes in cystic fibrosis lung disease: opportunities and challenges to therapy. J. Cyst. Fibros. 9:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]