Abstract
BACKGROUND
Preoperative needle localization (NL) is the gold standard for lumpectomy of nonpalpable breast cancer. Hematoma ultrasound-guided (HUG) lumpectomy can offer several advantages. The purpose of this study was to compare the use of HUG with NL lumpectomy in a single surgical practice.
STUDY DESIGN
Patients with nonpalpable lesions who underwent NL or HUG lumpectomy from January 2007 to December 2009 by a single surgeon were identified from a breast surgery database. Ease of scheduling, volume excised, re-excision rates, operating room time, and health care charges were the main outcomes variables. Univariate and multivariate analyses were performed to compare the 2 groups.
RESULTS
Lumpectomy was performed in 110 patients, 55 underwent HUG and 55 underwent NL. Hematoma ultrasound-guided lumpectomy was associated with a nearly 3-fold increase in the odds ratio of additional tissue being submitted to pathology (p = 0.039), but neither the total amount of breast tissue removed, nor the need for second procedure were statistically different between the 2 groups. Duration of the surgical procedure did not vary between the 2 groups; however, the time from biopsy to surgery was shorter for HUG by an expected 9.7 days (p = 0.019), implying greater ease of scheduling. Mean charges averaged $250 less for HUG than for NL, but this difference was not statistically significant.
CONCLUSIONS
Hematoma ultrasound-guided is equivalent to NL with regard to volume of tissue excised, need for operative re-excision, and operating room time. Adoption of HUG in our practice allowed for more timely surgical care.
Early stage breast cancer can be treated appropriately by lumpectomy and radiation therapy, with axillary staging if the cancer is invasive. A greater emphasis on screening and early detection has resulted in an increase in the presentation of nonpalpable breast cancer. When lumpectomy is performed after percutaneous, image-guided biopsy of a nonpalpable lesion, some sort of procedure (usually preoperative needle localization [NL]) must be done to guide surgical excision. Several alternatives to NL, for example, radioactive seed implantation and MRI guidance, have been suggested.1,2 Thompson and colleagues demonstrated that intraoperative localization of the biopsy hematoma (hematoma ultrasound-guided [HUG] lumpectomy) could eliminate the need for NL3 without introducing the need for another invasive procedure. Hematoma ultrasound guidance was incorporated into our practice at the University of Iowa Hospitals and Clinics in 2007. We hypothesized that the use of this procedure could facilitate patient scheduling and decrease associated costs.
METHODS
Patient enrollment
Institutional Review Board approval was obtained for this single-institution, retrospective study from January 2007 to December 2009. During this period, one surgeon introduced and subsequently used the HUG procedure. Patients with nonpalpable lesions who underwent lumpectomy with NL or HUG localization performed by this surgeon were identified from a review of an electronic database. The number of days from diagnostic imaging, initial surgical consult, and initial biopsy to the surgical procedure, as well as the intraoperative volume of tissue excised for each participant were recorded. The number of patients requiring additional surgery due to inadequate margin clearance was documented, as were the health care charges. Mammography and ultrasound were used to detect calcifications and/or masses. The localization method was not randomized; lumpectomy via HUG localization or NL was performed based on surgeon preference after assessing the patient in clinic.
Surgical procedure
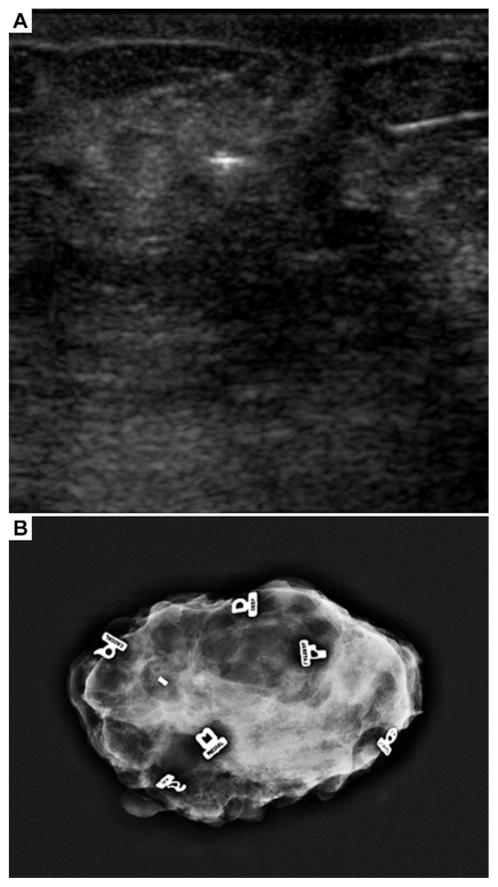
All patients who opted for lumpectomy (rather than mastectomy) for their treatment were considered for HUG. In addition, we did not attempt HUG in patients with >5 weeks from the time of core needle biopsy or in patients who had more than one area biopsied at the same setting in the same quadrant of the breast, to avoid the possibility of localizing the wrong site. Women who were candidates for HUG were then evaluated with surgeon-performed ultrasound in the clinic to image the biopsy cavity and/or clip (Fig. 1A). The location of the target was carefully noted with the patient and breast positioned as it would be for surgery and images were saved. If ultrasound visualization was successful in the clinic, the patient was scheduled for HUG lumpectomy and specimen x-ray. Visualization of the either the clip or the hematoma was considered successful. If visualization was not successful, the patient was scheduled for NL. Size of lesion and pattern of microcalcification identified on preoperative imaging did not play a role in assigning the patients to either group. At surgery, patients selected for HUG underwent ultrasound in the operating room and the site of the lesion was identified. The specimen was then excised in the usual fashion and oriented with margin markers (Fig. 1B). Specimen x-rays in 2 planes were obtained to confirm target acquisition and to assess margins. If any margin appeared close on the specimen x-ray, additional tissue was taken from that margin. All tissue was submitted for permanent sections. Performing intraoperative ultrasound with a sterile probe during the lumpectomy was initially done, as described previously, but was not believed to be necessary and the sterile probe was no longer used during the lumpectomy after our early experience. A single surgeon who was trained in breast ultrasound performed all ultrasound examinations and surgical procedures.
Figure 1.
(A) Preoperative breast ultrasound showing hematoma and biopsy clip. (B) X-ray of lumpectomy specimen showing biopsy clip and margin markers.
Statistical analysis
We used Student’s t-test for comparing numeric outcomes and contingency table analysis for categorical outcomes. If the distribution of numeric outcomes was skewed, non-parametric Wilcoxon rank sum test was performed. Statistics with both methods are reported. We performed chi-square test and Fisher’s exact test within contingency tables. We reported chi-square test results when the 2 test results were consistent and Fisher’s exact test when the results were not consistent. To best estimate the difference between the chosen procedures, multivariate regression analyses were used to adjust for potentially confounding factors. Log transformation of outcomes variables was performed where a log normal distribution described the variable better than a normal distribution. All statistical analyses were performed with STATA software, version 11.2 (Stata Corp) and p < 0.05 was considered significant.
RESULTS
Our study included a total of 110 female patients. Hematoma ultrasound-guided lumpectomy was performed in 55 patients and the remaining half underwent lumpectomy via NL. The demographic and other relevant clinical characteristics are summarized in Table 1. Both groups were comparable with respect to the physical characteristics of the patients and pathologic findings of the tumors. All patients had successful lumpectomy without any missed lesions. Ultrasound visualization of the biopsy site cavity and/or clip was more likely in the HUG group (p < 0.001). Several of the surgical characteristics of the lesions and operative details are summarized in Table 2. The tumor size in all 110 patients ranged from <0.1 cm to 1.9 cm and was not associated with any statistical difference between the 2 groups. The duration of the operative procedures performed in our study ranged from 0.6 to 3.6 hours; however, mean operative times (HUG 1.6 hours; NL 1.5 hours) were not statistically different between the 2 groups. Hematoma ultrasound-guided lumpectomy was associated with an increase in the proportion of patients who had intraoperative re-excision (HUG 84% vs NL 48%; p = 0.004). However, the total amount of breast tissue removed was not statistically different between the 2 groups (Table 2; Fig. 2). In addition, there was no statistical difference between the proportions of patients that required re-excision as a second operative procedure when comparing the 2 groups (Table 2; Fig. 3). All patients with a diagnosis of ductal carcinoma in situ in the HUG group had adequate margins, and the difference between HUG and NL re-excision for ductal carcinoma in situ was not statistically different.
Table 1.
Demographic and Clinical Characteristics of the Patients
| Characteristic | HUG (n = 55) | NL (n = 55) | p Value |
|---|---|---|---|
| Age, y, mean ± SD | 58.1 ± 10.8 | 56.0 ± 12.1 | 0.341 |
| Age, y, n (%) | |||
| Younger than 50 | 13 (24) | 19 (35) | 0.208 |
| 50 or older | 42 (76) | 36 (65) | |
| Height, in, mean ± SD | 64.7 ± 2.8 | 64.8 ± 2.4 | 0.850 |
| Weight, lb, mean ± SD | 184.7 ± 49.2 | 186.0 ± 47.8 | 0.892 |
| BMI, mean ± SD | 30.9 ± 7.3 | 31.1 ± 7.4 | 0.903 |
| Diagnosis of biopsy, n (%) | |||
| Benign | 4 (7) | 9 (16) | 0.270 |
| In situ | 15 (27) | 22 (40) | |
| Invasive | 36 (66) | 24 (44) | |
| Observation on preoperative ultrasound | |||
| Biopsy cavity | 42 (76) | 6 (11) | <0.001 |
| Clip | 25 (45) | 3 (5) | <0.001 |
BMI, body mass index; HUG, hematoma ultrasound guided; NL, needle localization.
Table 2.
Surgical Characteristics of the Patients
| HUG | NL | p Value | HUG-NL* | |
|---|---|---|---|---|
| Imaging to operation, d | ||||
| Mean ± SD | 36.2 ± 25.9 | 60.9 ± 43.6 | <0.001 | −19.82 (p < 0.001) |
| Median | 32.0 | 42.0 | <0.001 | |
| Biopsy to operation, d | ||||
| Mean ± SD | 27.3 ± 38.1 | 51.2 ± 65.9 | 0.022 | −9.70 (p = 0.019) |
| Median | 20.0 | 26.5 | 0.007 | |
| First visit to operation, d | ||||
| Mean ± SD | 13.7 ± 7.8 | 34.7 ± 46.7 | 0.001 | −4.88 (p = 0.076) |
| Median | 13.0 | 13.0 | 0.120 | |
| ≥30 d | 1 (2%) | 16 (29%) | <0.001 | 0.05OR (p = 0.008) |
| Tumor size, cm | ||||
| Mean ± SD | 1.3 ± 0.7 | 1.5 ± 1.2 | 0.439 | 0.08 (p = 0.724) |
| Median | 1.2 | 1.3 | 0.916 | |
| Tissue volume excised, cm3 | ||||
| Mean ± SD | 354 ± 561 | 277 ± 213 | 0.346 | 3.20 (p = 0.927) |
| Median | 250 | 241 | 0.572 | |
| Operating room time, h | ||||
| Mean ± SD | 1.6 ± 0.7 | 1.5 ± 0.5 | 0.308 | 0.14 (p = 0.240) |
| Median | 1.6 | 1.4 | 0.453 | |
| Additional tissue to pathology, n (%) | ||||
| No | 11 (20) | 24 (44) | 0.008 | 2.87OR (p = 0.039) |
| Yes | 44 (80) | 31 (56) | ||
| Intraoperative re-excision, n (%) | ||||
| No | 9 (16) | 23 (42) | 0.004 | 3.07OR (p = 0.027) |
| Yes | 46 (84) | 32 (48) | ||
| Requiring additional operation, n (%) | ||||
| No | 46 (84) | 42 (76) | 0.340 | 0.54OR (p = 0.236) |
| Yes | 9 (16) | 13 (24) | ||
Controlling for age, body mass index, and tumor grade; outcomes variable log transformed for numeric variables.
HUG, hematoma ultrasound-guided; NL, needle localization; OR, odds ratio.
Figure 2.

Comparison of the volume of total tissue removed.
Figure 3.

Re-excision rates between hematoma ultrasound-guided and needle localization.
The difference in the mean time from diagnostic biopsy to lumpectomy was 23.9 days and was statistically different comparing the 2 approaches (p = 0.022). Controlling for clinical characteristics with multivariate analysis, the time from diagnostic biopsy to surgery was 9.7 days shorter for HUG compared with NL (p = 0.019). When compared with NL patients, HUG patients had a decrease of 24.7 days from diagnostic imaging to lumpectomy and a reduction of 21 days from initial surgical consult to lumpectomy (p < 0.001 and p = 0.001, respectively). In addition, there was a significant decrease in the number of patients, as the time from first surgical visit to operation was >30 days (HUG vs NL: 1 vs 16; p < 0.001), and these differences remained significant on multivariate analysis (p < 0.008). This finding implied a greater ease of scheduling for patients undergoing HUG lumpectomy. A preliminary institutional analysis of related charges associated with each group revealed that HUG patients incurred a mean decrease in hospital charges of $67 and a mean decrease in professional charges of $184, with a total decrease for combined charges of $250. This amount increased to >$665 when controlling for covariates. However, because of large variation in charges, the differences failed to reach statistical significance.
We also noted a learning curve effect. In the first year of adoption of HUG, the surgeon had chosen one third of the patients to undergo HUG vs NL. That number increased to two thirds in the third year (p = 0.002). Although there is no statistically significant difference in operating room time between the 2 groups, initially HUG patients averaged 2.1 hours of operating room time. This decreased to 1.4 hours (which is less than operating room time for NL patients) in the third year (p = 0.002).
DISCUSSION
Increased awareness, widespread screening, and improved imaging methods have led to earlier detection of breast cancer, with a corresponding increase in the number of nonpalpable abnormalities.4,5 In most cases, the diagnosis of both invasive carcinoma and ductal carcinoma in situ are confirmed via image-guided core needle biopsy. During core breast biopsy, a clip is placed in the area to be sampled. The biopsy procedure usually creates a small cavity, which turns into a small hematoma. When subsequent excision (lumpectomy) is warranted, the gold standard for localizing nonpalpable lesions is preoperative NL. Needle location involves the insertion of a needle into the location of the previously placed biopsy clip for surgical guidance. Incidental clip migration6,7 and misplacement8 are undesirable complications. The migration,9 kinking, and/or fracture of the needle10 are some reported complications of needle localization. In addition, the fact that the correct placement of the needle is dependent on the proper position of the clip amplifies the error rate of NL. Vasovagal episodes and patient discomfort have been reported.11 Additionally, there can be logistical difficulties with NL because coordination between surgeon and radiologist is necessary for optimal outcomes.
Consequently, several alternatives to NL have been developed,2,12,13 of which HUG is one. We identified HUG as the simplest available option. After every biopsy, a hematoma remains within the biopsy site. Thompson and colleagues hypothesized that the hematoma could be used as a physiological marker that can be detected via ultrasound at the time of resection,3 and introduced the term hematoma ultrasound-guided to describe the procedure. They have subsequently reported their 10-year successful experience with this method,14 showing HUG lumpectomy to be safe and accurate in their practice. By eliminating the need for needle localization, it simplifies scheduling and improves patient comfort. Few subsequent reports have evaluated the adoption of this technique. We introduced this procedure to our practice in January 2007. During our 3-year experience, a total of 136 patients underwent lumpectomies performed by a single surgeon and, of those, 110 patients had nonpalpable lesions, with 55 eligible for HUG. When NL is used, close coordination with the radiologist is crucial. The goal is to excise the tumor without removing a large amount of additional tissue and, as such, the importance of a precise NL is paramount. In the radiological suite, it is easier to insert the wire from the superior or lateral position with respect to the chest wall, so the trajectory of the wire might not be optimal for incision planning. The patient is in a different position during the NL (seated, leaning forward) than in the operating room (supine), which can considerably change the position of the localizing wire with respect to the tumor. The classic NL complication of a broken or dislodged wire is not an issue with HUG lumpectomy and, if the lesion is missed (which did not occur in our series), intraoperative ultrasound can be used to determine the location of the tumor.
Hematoma ultrasound-guided lumpectomy does require that the operating surgeon be trained in the use of ultrasound, but breast ultrasound is now considered part of the breast surgeon’s practice, and there are multiple training programs. Similarly, access to an ultrasound machine is required, but this should also be a normal part of the breast surgeon’s clinic and operating room environment. When HUG is used, the need and importance for interdepartmental coordination and communication is drastically reduced (if not virtually eliminated), as the localization performed by radiology is no longer a part of the procedure. As a result, the accuracy and precision of excision is improved because the potential for errors due to wire placement and wire-related complications is abolished and better patient comfort is established.
However, HUG does have some downsides. A potential drawback associated with HUG is the reabsorption of the hematoma, which, on average, occurs within 14 days.15 Arentz and colleagues noted that most hematomas are completely absorbed by 5 weeks.14 Once the hematoma is reabsorbed, only the clip remains, and the hematoma can no longer be used for guidance. However, in some cases, the tumor itself can be visualized by ultrasound, allowing ultrasound-guided lumpectomy. A second concern is the potential that the hematoma and cancer are not located in the same place. Although this is a theoretical concern, the site of the hematoma and cancer were the same in all patients in this series. In our study, none of the patients had palpable hematomas.
There will always be patients in whom HUG is difficult or not advisable. We do not use the procedure in women who have undergone neoadjuvant chemotherapy because the hematoma will be absent. We do not attempt the procedure in women who have had multiple targets biopsied in the same breast because of concern that we will localize the wrong hematoma. Hematoma ultrasound-guided lumpectomy is also not advocated for diagnostic biopsies, as we find this modality to be best suited for cases where a non-palpable lesion has been biopsied and requires excision. Some cases are best managed by using bracketing wires to localize the lesion, but in our study, we considered the use of HUG only if patients were candidates for localization using a single wire. In addition, we carefully select patients in the clinic by performing an ultrasound to confirm that we can image the hematoma and/or clip. Our experience has warranted HUG to be applicable only when the lesion is nonpalpable and when visualization of the hematoma and/or clip (not the lesion) is confirmed preoperatively via ultrasound. Most of the patients in our HUG group had a mass-like lesion or cluster microcalcifications without a mass identified on diagnostic imaging, none of them had scattered microcalcifications. This would suggest that HUG is associated with the pattern of distribution of the lesion; however, using HUG, we only tried to identify the biopsy cavity and/or the clip, not the lesion itself. In addition, we did not find the type of lesion to impact our results. Although intuitively one might postulate that it would be more difficult to visualize the hematoma or clip in obese women, we did not observe a difference in body mass index in our series between those who underwent HUG and those who underwent NL (Table 1). The HUG procedure is also not performed in women who have had a fine-needle aspiration biopsy, as there would hardly be a hematoma at the biopsy site that could be detected via ultrasound.
Concerning the issue of hematoma reabsorption, our data suggest that HUG procedures on average are performed closer to the time of biopsy (when the hematoma should be most evident) than NL procedures. This implies that HUG procedures are easier to schedule than NL procedures. The scheduling difference might reflect the fact that NL procedures must be scheduled with 2 departments, but HUG needs only to be scheduled with 1. Our radiologists have a limited number of NL placements they are able to perform on a daily basis and this is often a limiting factor in scheduling. NL patients were not scheduled with any less urgency than HUG patients, even given the potential concern of the hematoma reabsorbing. There is also the potential of optimizing cancer care by shortening the time from diagnosis to definitive cancer surgery; however, several weeks is not likely to be biologically significant.
Using a protocol in which patients are carefully identified as candidates for HUG lumpectomy (single lesion, short time from biopsy), screened with surgeon-performed ultrasound in the clinic (to confirm adequate visualization of the cavity and/or clip), and then localized with surgeon-performed ultrasound in the operating room, careful specimen orientation and specimen radiography, we have been able to duplicate the success of NL with easier scheduling and probably less discomfort to the patient. When first described, Thompson and colleagues3 covered the ultrasound transducer with sterile plastic sheath and used it to localize the lesion and to plan the incision. The transducer was also used inside the incision to further help with dissection as well as ex vivo assessment of margin adequacy. In our experience with HUG, once the patient was in the final position for surgery, we used the transducer to delineate the site of incision, to note the depth from the skin and the distance to the fascia, and to make the incision directly over this marked site. We chose not to involve the probe in the sterile field after some cases failed to demonstrate any added benefit. We prefer to use specimen x-ray (rather than specimen ultrasound), as many of our patients have residual microcalcifications that would not be easily visible on ultrasound. This does require ordering the specimen x-ray, which could potentially introduce some delay; however, our operative time was not different between the 2 groups (Table 2). Additional shave margins were selectively taken in HUG and NL groups if specimen x-ray showed micro-calcifications, or if the clip or the hematoma was close to one or more margins (the clip was always in the primary specimen). We did not assess sonographic visibility of the primary lesion in cases of small solid tumors; clearly, the presence of such a target, although rare, would facilitate ultrasound-guided excision. One might hypothesize that the larger the tumor size the more likely the tumor, rather than the smaller hematoma, would be detected on ultrasound (both preoperative and intraoperative) and, therefore, HUG would be used for the larger invasive tumors. Our analysis indicated that tumor size was not statistically significant between the 2 groups (p = 0.724) and therefore did not affect the use of HUG.
When introducing a new technique, there are always concerns about inferior treatment. The goals of lumpectomy are the removing the tumor mass with a margin of normal surrounding tissue and achieving cosmetic outcomes that are acceptable to the patient. When it comes to cosmesis, the precise amount of normal breast tissue that should be resected around a malignant lesion has been debated and varies among practices. At our institution, we have adopted guidelines that support a 2-mm margin. Our data demonstrate that HUG had no difference in volume of tissue removed or need for repeat surgery, and no missed lesions (in either group).
It should be noted that this study is not without limitations. The use of a single surgeon’s preference to allocate the 2 groups does create the potential for selection bias. All patients who met basic criteria were evaluated for HUG and the increasing adoption over time supports a learning curve effect. However, the major objective of the study was to examine the feasibility of introducing a new technique into the surgical practice for patients undergoing breast-conservation surgery. In addition, it is unlikely that HUG will ever be applicable to all patients and, therefore, selective use of the technique likely reflects its practical application in surgical practice.
CONCLUSIONS
Our study confirms that HUG is a suitable alternative to NL in patients who have lesions, hematoma, biopsy cavity, or clip that can be visualized via ultrasound. In this regard, ultrasound-guided lumpectomy might be a more accurate description of the HUG procedure. Hematoma ultrasound-guided lumpectomy not only provides enhanced patient comfort and experience, it also allows physicians to be more efficient and effective without compromising patient care or cosmetic result. Additional investigations will be needed to determine whether HUG can be performed at a lower cost than NL. The caliber of treatment offered by HUG is at least as safe and as effective as NL. This is at the very least enough to warrant additional comparative analysis between HUG and the current gold standard. Hematoma ultrasound-guided lumpectomy proved to be a useful addition to our practice, and we were able to incorporate it without difficulties.
Acknowledgments
Supported by the National Institutes of Health grant T32CA148062 (principal investigator: R J Weigel) and by a generous gift from the Kristen Olewine Milke Breast Cancer Research Fund.
Footnotes
Disclosure Information: Nothing to disclose.
Abstract presented at the 64th Annual Cancer Symposium of the Society of Surgical Oncology, San Antonio, TX, March 2011.
Author Contributions
Study conception and design: Larrieux, Liao, Scott-Conner, Weigel
Acquisition of data: Larrieux, Cupp
Analysis and interpretation of data: Larrieux, Cupp, Liao, Scott-Conner, Weigel
Drafting of manuscript: Larrieux, Scott-Conner, Weigel
Critical revision: Larrieux, Cupp, Liao, Scott-Conner, Weigel
References
- 1.Jakub JW, Gray RJ, Degnim AC, et al. Current status of radioactive seed for localization of non palpable breast lesions. Am J Surg. 2010;199:522–528. doi: 10.1016/j.amjsurg.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Tomikawa M, Hong J, Shiotani S, et al. Real-time 3-dimensional virtual reality navigation system with open MRI for breast-conserving surgery. J Am Coll Surg. 2010;210:927–933. doi: 10.1016/j.jamcollsurg.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 3.Thompson M, Henry-Tillman R, Margulies A, et al. Hematoma-directed ultrasound-guided (HUG) breast lumpectomy. Ann Surg Oncol. 2007;14:148–156. doi: 10.1245/s10434-006-9076-y. [DOI] [PubMed] [Google Scholar]
- 4.Gordon PB. Ultrasound for breast cancer screening and staging. Radiolog Clin N Am. 2002;40:431–441. doi: 10.1016/s0033-8389(01)00014-8. [DOI] [PubMed] [Google Scholar]
- 5.Nothacker M, Duda V, Hahn M, et al. Early detection of breast cancer: benefits and risks of supplemental breast ultrasound in asymptomatic women with mammographically dense breast tissue. A systematic review. BMC Cancer. 2009;9:335. doi: 10.1186/1471-2407-9-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kass R, Kumar G, Klimberg VS, et al. Clip migration in stereotactic biopsy. Am J Surg. 2002;184:325–331. doi: 10.1016/s0002-9610(02)00952-2. [DOI] [PubMed] [Google Scholar]
- 7.Bernaerts A, De Schepper A, Jr, Van Dam P, Pouillon M. Clip migration after vacuum-assisted stereotactic breast biopsy: a pitfall in preoperative wire localization. JBR-BTR. 2007;90:172–175. [PubMed] [Google Scholar]
- 8.Birdwell RL, Jackman RJ. Clip or marker migration 5–10 weeks after stereotactic 11-gauge vacuum-assisted breast biopsy: report of two cases. Radiology. 2003;229:541–544. doi: 10.1148/radiol.2292021594. [DOI] [PubMed] [Google Scholar]
- 9.Seifi A, Axelrod H, Nascimento T, et al. Migration of guidewire after surgical breast biopsy: an unusual case report. Cardiovasc Intervent Radiol. 2009;32:1087–1090. doi: 10.1007/s00270-009-9620-9. [DOI] [PubMed] [Google Scholar]
- 10.Montrey JS, Levy JA, Brenner RJ. Wire fragments after needle localization. AJR Am J Roentgenol. 1996;167:1267–1269. doi: 10.2214/ajr.167.5.8911193. [DOI] [PubMed] [Google Scholar]
- 11.Nakamura Y, Urashima M, Matsuura A, et al. Stereotactic directional vacuum-assisted breast biopsy using lateral approach. Breast Cancer. 2010;17:286–289. doi: 10.1007/s12282-009-0162-4. [DOI] [PubMed] [Google Scholar]
- 12.Mullen DJ, Eisen RN, Newman RD, et al. The use of carbon marking after stereotactic large-core-needle breast biopsy. Radiology. 2001;218:255–260. doi: 10.1148/radiology.218.1.r01ja33255. [DOI] [PubMed] [Google Scholar]
- 13.Van Esser S, Hobbelink M, Van der Ploeg IM, et al. Radio guided occult lesion localization (ROLL) for non-palpable invasive breast cancer. J Surg Oncol. 2008;98:526–529. doi: 10.1002/jso.21143. [DOI] [PubMed] [Google Scholar]
- 14.Arentz C, Baxter K, Boneti C, et al. Ten-year experience with hematoma-directed ultrasound-guided (HUG) breast lumpectomy. Ann Surg Oncol. 2010;17:378–383. doi: 10.1245/s10434-010-1230-x. [DOI] [PubMed] [Google Scholar]
- 15.Liberman L, Hann LE, Dershaw DD, et al. Mammographic findings after stereotactic 14-gauge vacuum biopsy. Radiology. 1997;203:343–347. doi: 10.1148/radiology.203.2.9114086. [DOI] [PubMed] [Google Scholar]