Abstract
Background
Angelman- and Rett-like syndromes share a range of clinical characteristics, including intellectual disability (ID) with or without regression, epilepsy, infantile encephalopathy, postnatal microcephaly, features of autism spectrum disorder, and variable other neurological symptoms. The phenotypic spectrum generally has been well studied in children; however, evolution of the phenotypic spectrum into adulthood has been documented less extensively. To obtain more insight into natural course and prognosis of these syndromes with respect to developmental, medical, and socio-behavioral outcomes, we studied the phenotypes of 9 adult patients who were recently diagnosed with 6 different Angelman- and Rett-like syndromes.
Methods
All these patients were ascertained during an ongoing cohort study involving a systematic clinical genetic diagnostic evaluation of over 250, mainly adult patients with ID of unknown etiology.
Results
We describe the evolution of the phenotype in adults with EHMT1, TCF4, MECP2, CDKL5, and SCN1A mutations and 22qter deletions and also provide an overview of previously published adult cases with similar diagnoses.
Conclusion
These data are highly valuable in adequate management and follow-up of patients with Angelman- and Rett-like syndromes and accurate counseling of their family members. Furthermore, they will contribute to recognition of these syndromes in previously undiagnosed adult patients.
Key Words: Adult phenotypes, Angelman- and Rett-like syndromes, CDKL5, Dravet syndrome, Kleefstra syndrome, Male MECP2, Phelan-McDermid syndrome, Pitt Hopkins syndrome
Introduction
Clinical characteristics of known syndromes including associated health and behavior issues and recognizable facial features are generally well studied and described in patients at childhood. Often little is known about evolution of the phenotype across life span. Information on health and social outcomes is necessary for an adequate management and follow-up of patients with specific intellectual disability (ID) syndromes and enables careful counseling of family members regarding prognosis, natural course of the disease, and life expectancy. Delineation of phenotypes at adult age will also contribute to recognition and diagnosis of ID syndromes in adults.
In the past decade improvements in diagnostic and research technologies have led to the identification of several novel chromosomal microdeletions and -duplications as well as single genes that are associated with known and novel ID syndromes, referred to as ‘Angelman- and Rett-like syndromes’ [Amir et al., 1999; Claes et al., 2001; Phelan et al., 2001; Kalscheuer et al., 2003; Wilson et al., 2003; Tao et al., 2004; Kleefstra et al., 2006; Zweier et al., 2007; Ariani et al., 2008; Papa et al., 2008; Zweier et al., 2010]. After the initial identification of the gene methyl CpG binding protein 2 (MECP2) as the cause of Rett syndrome [Amir et al., 1999], additional genes associated with a Rett-like/aspecific Rett syndrome phenotype have been subsequently identified. These include forkhead box G1 (FOXG1), cyclin-dependent kinase-like 5 (CDKL5), and myocyte enhancer factor 2C (MEF2C) [Kalscheuer et al., 2003; Tao et al., 2004; Ariani et al., 2008; Zweier et al., 2010]. Furthermore, in several patients with a clinical diagnosis of Angelman-like syndrome, mutations in MECP2 and CDKL5 have been identified [Watson et al., 2001; Moncla et al., 2002; Kleefstra et al., 2004; Russo et al., 2009]. In addition, several mutations in the gene transcription factor 4 (TCF4, Pitt Hopkins syndrome) have been identified in patients with Angelman-like phenotypes as well [Takano et al., 2010]. So, Angelman- and Rett-like syndromes have an overlapping phenotypic spectrum with heterogeneous underlying genetic defects which can mimic each other. The phenotypic spectrum comprises (severe) ID with or without regression, epilepsy, infantile encephalopathy, features of autism spectrum disorder, stereotypies and hand apraxia, postnatal microcephaly, autonomic dysfunction (including breathing anomalies, vasomotor disturbance, and gastro-intestinal symptoms), and other neurological symptoms, including ataxia and spasticity. Furthermore, various underlying genetic defects are thought to act in similar and/or interacting pathways. For example, MECP2 is thought to be involved in the epigenetic regulation of UBE3A (associated with Angelman syndrome) [Makedonski et al., 2005; Samaco et al., 2005]. Also, MECP2, CDKL5, and MEF2C were shown to interact directly which suggest involvement in a common pathway [Mari et al., 2005; Zweier et al., 2010].
During an ongoing cohort study among mainly adult patients with so far unexplained ID, we identified an associated genetic defect in 9 adult patients with Angelman- and Rett-like phenotypes. We found mutations in the genes TCF4, CDKL5, EHMT1 (euchromatic histone-lysine N-methyltransferase 1), MECP2, and SCN1A (sodium channel, voltage-gated, type I, alpha subunit), and small terminal deletions in chromosomal region 22q13.3, associated with Phelan-McDermid syndrome. Here we describe the evolution of their phenotypes up to different adult ages and give an overview of previously reported adult cases.
Patients and Methods
All patients were ascertained during an ongoing cohort study among patients with ID of unknown etiology. This study was approved by the local ethical committee. Patients were selected from a large population of approximately 4,000 people living in residential settings associated with 3 service providers for the care of people with ID in the eastern part of the Netherlands. Patients were selected on the base of an IQ level ≤70 in combination with the presence of one or more of the following features: micro- or macrocephaly, growth retardation or overgrowth, neurological features (e.g. ataxia, spasticity, neurodegenerative signs), congenital anomalies (i.e. congenital heart/renal/skin/genital defects), consanguinity and/or positive family history for ID, and/or dysmorphic features. More than 80% of the patients in the total cohort were adult patients. After obtaining written permission from the parents or legal representative for participation in this study, patients were invited to visit the department of human genetics at the Radboud University Nijmegen Medical Centre for an extensive multidisciplinary clinical evaluation. Specific DNA diagnostic tests were requested when indicated. In all patients, genome-wide SNP (single nucleotide polymorphism) array analysis was performed according to the standard Affymetrix GeneChip protocol (Affymetrix, Inc., Santa Clara, Calif., USA). In 8 patients the Affymetrix 250k array platform and in 1 patient (patient 7) the Affymetrix 2.7M array platform was used. Copy number estimates were determined using the updated version 2.0 of the CNAG (copy number analyzer for Affymetrix GeneChip mapping) software package (Affymetrix 250k SNP array) or the ChAS (chromosome analysis suite for Affymetrix GeneChip mapping) software package (Affymetrix 2.7M array). The normalized ratios were subsequently analyzed for genomic imbalances by a standard hidden Markov model. CNVs were mapped according to the UCSC genome browser build May 2004 (NCBI35/Hg17) for the Affymetrix 250k array platform and to build March 2006 (NCBI36/Hg18) for the Affymetrix 2.7M array platform.
All patients underwent screening metabolic tests in urine and serum as well, including quantification of lactate, amino acids, creatine biosynthesis, carnitines, organic acids, purines and pyrimidines, and transferrin subfractions.
Segregation Analysis
When possible, segregation of genetic/chromosomal variants was tested in the parents and/or other family members by SNP array, multiplex ligation-dependent probe amplification (MLPA), fluorescence in situ hybridization (FISH), or sequencing of the involved gene.
Results
Patient 1: Kleefstra Syndrome (EHMT1)
This female patient was ascertained at the age of 41 years. She was born at term after an uncomplicated pregnancy and delivery in breech position. Birth weight was >3 kg (>10th centile). At the age of 6 weeks she was admitted to the hospital because of persistent excessive regurgitation and weight loss, probably due to severe gastro-esophageal reflux. Her motor development was mildly delayed, with walking independently at the age of 18 months. Speech development was more severely delayed, and she was not able to speak single words until the age of 3 years. Contact making was diminished, and she had mood swings. An intravenous pyelogram at the age of 10 years because of recurrent urinary tract infections showed no abnormalities. Ophthalmological evaluation revealed substantial strabismus. At the age of 38 years her developmental age as assessed by the WISC-RN scale was 5.5 years with a discrepancy between verbal capacities (age equivalent 4.3 years) and performal capacities (age equivalent 6.6 years). She was able to walk, to talk in short simple sentences and needed some help in self care. Around the age of 38 years she presented with a remarkable change in her behavior pattern, including periods of apathy, decreased emotional responsiveness, staring, low motor activity, stupor, and sleep disturbance, characterized by frequent awakenings during the night and daytime sleepiness. Detailed behavioral characteristics have been published elsewhere [Verhoeven et al., 2011: patient 1]. Furthermore, impulsivity and aggressive outbursts were reported. She also showed focal myoclonic twitches of the right shoulder. Electro-encephalography was repeatedly normal. Brain MRI showed multifocal poorly defined abnormal white matter signal intensities, mainly localized deeply in the parietal lobe. In addition, bilaterally abnormal signal intensity in the globus pallidus was noticed. These abnormalities were not indicative for perinatal asphyxia. Cardiologic evaluation revealed hypertrophy of the left ventricle and paroxysmal atrial fibrillation. Upon physical examination at the age of 41 years, her height was 176 cm (75th centile), weight 103 kg (>98th centile), and head circumference 53.5 cm (10th centile). Facial dysmorphic features comprised a flat midface, slight upslant of the palpebral fissures, short philtrum, prognathism, and a full everted lower lip (fig. 1A, B). Chromosomal analysis by 250k SNP array analysis was normal, and screening metabolic tests revealed no abnormalities. Because of the clinical suspicion of Kleefstra syndrome without a deletion in chromosomal region 9q34.3, mutation analysis of EHMT1 was performed and revealed a pathogenic mutation in the splice site of intron 20 of the EHMT1 gene (c.3087+1G>T). Segregation analysis in the parents was not possible.
Fig. 1.
Clinical pictures. A, B Typical facial features of Kleefstra syndrome in patient 1. Note the midface hypoplasia, arched eyebrows and prognathism. C, D Typical facial appearance of Pitt Hopkins syndrome in patient 2. Note the coarse face with prognathism, broad mouth with down-turned corners and large nose. E, F Patient 3 with a mutation in MECP2 and his carrier sister (G, H). I, J Patient 4 with a mutation in CDKL5. Note her coarse face. K, L Patient 5 and 6 (M, N) with Dravet syndrome. O, P Patient 7 with Phelan-McDermid syndrome. Note the long face and full lips. Q–T Brother pair with Phelan-McDermid syndrome. Note the long face, prominent chin, and large ears in both brothers.
Patient 2: Pitt Hopkins Syndrome (TCF4)
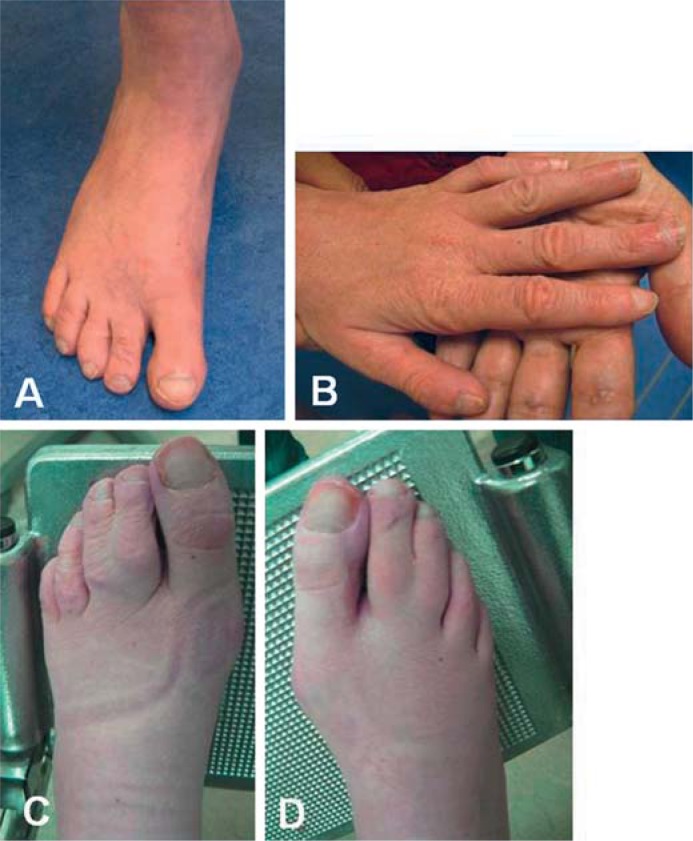
This male patient was ascertained at the age of 40 years. He was born at term after an uneventful pregnancy and delivery. Birth weight was 3,200 g (>20th centile). His psychomotor development was severely delayed. He was able to sit independently at the age of 4 years and able to walk at 8 years of age. He did not develop speech. His behavior was characterized by severe pica behavior, including the drift to ingest soft objectives such as socks. Several times he underwent a gastroscopy to remove socks from his stomach. To prevent further ingestion incidents, he was prescribed to wear a helmet with a face mask. In addition, he showed hyperactive and self-mutilation behaviors and had a high threshold of pain. During puberty he frequently had outbursts of crying, but later on he was generally in a happy mood. Apart from an atopic constitution presenting with allergy, asthma, and eczema, his general health was quite good. After a fall at the age of 36 years, in addition to a fresh compression fracture of the twelfth thoracic vertebra, multiple old fractures, including metacarpal, humeral, and femoral fractures, were detected and he was diagnosed with osteoporosis. Ophthalmological evaluation revealed myopia. Upon physical examination at the age of 40 years his height was 173 cm (>2nd centile), weight 56 kg (16th centile), and head circumference 53.5 cm (0.6th centile). He showed evident facial dysmorphism including remarkable prognathism, a broad nose with full tip, hyperplastic alae nasi, broad mouth with downturned corners, and widely spaced teeth (fig. 1C, D). The chest was asymmetric with a mild pectus excavatum, and he had a mild left convex scoliosis. His feet were flat, and both hands showed short fifth fingers and tapering of the fingers with concave nails (fig. 2A, B). On the right hand a single palmar crease was present. He preferred to sit on the ground with his legs sideward rotated to the left. He had difficulties to stand up from this position and showed a mildly increased muscular tone. Chromosomal analysis by 250k SNP array analysis was normal, and screening metabolic tests revealed no abnormalities. Because of some facial characteristics of Pitt Hopkins syndrome, mutation analysis of TCF4 was requested and revealed a known pathogenic mutation (c.1739G>A, p.Arg580Gln). Segregation analysis in the parents showed that the mutation had occurred de novo.
Fig. 2.
Clinical pictures. A, B Clubbing of toe- and fingernails in patient 2 with a mutation in TCF4. C, D 2–3 toe syndactyly in patient 7 who was diagnosed with Phelan-McDermid syndrome.
Patient 3: Familial MECP2 Mutation
This male patient was ascertained at the age of 27 years. Pregnancy and birth were uncomplicated. Birth weight was 3,750 g (50–75th centile). From the age of 24 months on his parents noticed a delay in his psychomotor development. He was able to walk independently between the age of 2 and 2.5 years and could speak a few single words. At the age of about 12 years he had developed tonic-clonic seizures. Seizures persisted on anti-epileptic drug treatment with a frequency of 1–2 times a day. Cerebral imaging by a CT scan at that time and later at adult age by MRI revealed no abnormalities. At adult age, his total IQ level as assessed by the WISC was 33. His behavior was characterized by a lack of initiative and he was very calm, introverted, and friendly. He had features of autism spectrum disorder and periodic tics. Since his early twenties, he showed a progressive decline in general functioning, including loss of speech (he was able to talk in 2–3 word sentences before), progressive neurological symptoms including walking difficulties with frequent falling, tremors of both arms, and apathy (showing significantly less initiative than before). He needed more help in self care as well. During the nights he frequently awakened and wandered around. After the start of anti-depressive drug treatment, he seemed to improve a little but did not regain his former level of functioning. On physical examination at the age of 27 years he had a height of 176 cm (16th centile), a weight of 51.5 kg (2nd centile), and a head circumference of 57 cm (25–50th centile). He had a mild brachycephaly and a long face with a pronounced chin. Other dysmorphic features included a full upturned tip of the nose and a broad mouth with full lips and everted lower lip (fig. 1E, F). He had narrow feet and hands with slender digits. His muscular tone was generally slightly increased and the reflexes were symmetrically brisk. There was a generalized muscular atrophy and a tremor of the arms.
The family history mentioned learning disabilities in the mother and his 3-year younger sister. The mother was admitted to a psychiatric hospital because of severe psychiatric problems. His sister had followed special education. At the age of 24 years she was employed as a packer at a distribution office and lived independently together with her husband. On physical examination she had a height of 167 cm (25th centile), a weight of 76 kg (90th centile), and a head circumference of 54.3 cm (20th centile). She had no evident dysmorphic features (fig. 1G, H).
Chromosomal analysis by 250k SNP array analysis and screening metabolic tests revealed no abnormalities. Because of the deterioration in functioning, neurological symptoms, and a family history suggestive for an X-linked inheritance pattern, mutation analysis of both ARX and MECP2 was performed. This revealed a pathogenic hemizygous late truncating mutation in MECP2 (c.1233_1243del, p.Ser411fs). Segregation analysis showed an identical heterozygous mutation in the sister. The mother was not available for testing.
Patient 4: Rett Syndrome Variant with Infantile Spasms (CDKL5)
This female patient was diagnosed at the age of 47 years. Pregnancy and birth were uncomplicated. She was doing well for the first 6 months, but then developed seizures and since then she had a delay in psychomotor development. Her motor development was only mildly delayed; however, she never learnt to speak. At adult age her ID was profound. The seizures persisted during her life, and despite treatment with multiple anti-epileptic drugs she suffered from frequent (atonic) seizures. In addition, she developed a mild spasticity. She had a friendly personality, and her behavior was characterized by hyperactivity, self-mutilation, and no sense of fear. At the age of 40 years a cerebral CT-scan, which was done because of a fall from a horse, showed signs of hypoplasia of the corpus callosum. The family history documented ID due to rhesusantagonism in 2 sons from 2 different brothers from the father. Upon physical examination at the age of 47 years she had a height of 162.5 cm (<16th centile), a weight of 45 kg (2nd centile), and a head circumference of 53 cm (<16th centile). She had prominent cheekbones, a mild prognathism, deep-set eyes, and blepharochalasis. The ears seemed to be slightly high positioned and she had a full upturned nose with hypertrophic alae nasi. The lips were full, and she missed several teeth due to caries (fig. 1I, J). She had thin limbs and small hands. Her feet showed slight clino- and syndactyly of the second and third toes. Chromosomal analysis by 250k SNP array analysis was normal, and screening metabolic tests revealed no abnormalities. Because of the severe ID combined with persistent therapy resistant seizures and the Rett syndrome-like phenotype, mutation analysis of MECP2, CDKL5, FOXG1, and SCN1A was performed, and a pathogenic splice site mutation in intron 7 of CDKL5 was found (c.464-1G>A). Segregation analysis in the parents was not performed.
Patients 5 and 6: Dravet Syndrome (SCN1A)
Patient 5
This male patient was diagnosed at the age of 34 years. He was born at term after an uncomplicated pregnancy and birth. Birth weight was 4,000 g (<90th centile). He was doing well for the first 5 months but then developed seizures after a vaccination. Since then he had regular absences but developed normally according to his parents. At the age of 2 years seizure frequency increased and his speech gradually declined. At 3 years of age he had a status epilepticus followed by a coma state of several days. After awakening he had a severely retarded psychomotor function and furthermore a right hemiplegia and was no longer able to sit or walk. Gradually the hemiplegia partially recovered and he learnt to walk again, though he did not regain the ability to speak. The epilepsy persisted throughout his life and he suffered from intractable seizures (for which he wore a helmet) despite treatment with multiple anti-epileptic drugs. At adult age he had a profound ID (age equivalent 10–18 months). His behavior was very friendly and characterized by little initiative, obsessive traits, and self-mutilation (pulling out his nails). He had a very high threshold of pain. He recurrently had upper and lower airway infections. Upon physical examination at the age of 34 years his height was 193 cm (90th centile), weight 84 kg (>50th centile), and head circumference 59 cm (80th centile). He had a slight micrognathia and several scars on his face due to recurrent epileptic falls. The ears were large and the lips full, but his appearance was not quite dysmorphic (fig. 1K, L). He was able to walk but he was somewhat hindered in his motor function by a mild right-sided hemiplegia. Chromosomal analysis by 250k SNP array analysis was normal, and screening metabolic tests revealed no abnormalities. Because of the severe ID and intractable seizures, mutation analysis of SCN1A was performed and revealed a pathogenic mutation in exon 26 (c.5304T>G, p.Ser1768Arg). Segregation analysis in the parents showed that the mutation had occurred de novo.
Patient 6
This patient was ascertained at the age of 49 years. Pregnancy and birth were uneventful. He developed seizures after vaccination at about 6 months of age. His psychomotor development was delayed. Motor milestones were only mildly delayed according to his brother. Speech development was more severely delayed, and he only learnt to speak a single word. At adult age he had a severe ID but had a good mobility. The epilepsy (mixed seizures) persisted throughout his life despite treatment with multiple anti-epileptic drugs. Seizures were provoked by psychological stress and constipation. He had a friendly personality and loved to get attention. Ophthalmological evaluation revealed a high hypermetropia and a substantial astigmatism. The family history of the mother was positive for epilepsy without developmental delay. At the age of 36 years conventional karyotyping and tests for Fragile X syndrome and Angelman syndrome were all normal. Upon physical examination at the age of 49 years his height was 175 cm (10th centile), weight 72.5 kg (84th centile), and head circumference 57.4 cm (<50th centile). He had minor facial dysmorphism including a high forehead, large lop ears, a prominent philtrum, and a full lower lip. His teeth had been removed because of caries (fig. 1M, N). On the feet he had a clinodactyly of the second toe and a cutaneous syndactyly of the second and third toes. His motor function was clumsy and he had mild spasticity. Chromosomal analysis by 250k SNP array analysis and screening metabolic tests revealed no abnormalities. Because of his severe ID and intractable seizures, mutation analysis of SCN1A was performed and revealed a frameshift mutation in exon 7 leading to a premature stop codon (c.3526delG, p.Glu1176fs). Parents were not available for segregation analysis.
Patients 7, 8, and 9: Phelan-McDermid Syndrome (22qter Deletion)
Patient 7
This male patient was ascertained at the age of 48 years. He was born as the fifth child of his parents. Pregnancy, delivery, and perinatal period were not remarkable. During his second year, his parents noticed a delay in his psychomotor development. He learnt to walk significantly later than his siblings, but his speech did not develop. At adult age his ID was severe. His behavior was not remarkable, and he had good social interactions. An abnormal breathing pattern, including intermittent irregular and rapid breathing was observed. At the age of 27 years he was diagnosed with hyperthyroidism, and treatment with Strumazol and Thyrax was started, but otherwise his general health was good. His vision was reported to be mildly impaired due to mild myopia and a slightly limited visual field of unknown cause. Since the age of 45 years his general functioning had declined remarkably after a hospital admission because of severe pneumonia complicated by respiratory insufficiency. He was no longer able to walk, had feeding problems due to swallowing difficulties, and became dependent on tube feeding. Contact making and social interaction diminished, and he also developed seizures. Cerebral imaging by both CT scan and MRI scan showed normal brain anatomy apart from mild enlargement of the cisterna magna and central atrophy. At the age of 49 years he died from pneumonia. The family history reported mild learning and behavior problems in an older sister and severe intellectual and physical disability in a son of a brother of the father.
Upon physical examination at the age of 48 years his height was on the 50th centile. Head circumference and weight were on the 80th centile. Facial appearance was coarse with bushy eyebrows, a short philtrum, a broad mouth with very full lips and widely placed dentition and he had large ears (fig. 1O, P). He had evident syndactyly of the second and third toes, with a nearly complete fusion on the right side (fig. 2C, D). He was wheelchair dependent and showed hypertonia with spastic posture of the hands and feet. Genetic tests included chromosomal analysis by 2.7M SNP array analysis and mutation analysis of TCF4 because of clinical similarities with Pitt Hopkins syndrome. 2.7M SNP array analysis identified a 1.8-Mb loss in chromosomal region 22q13.32q13.33 (47,782,571–49,543,031 Mb). Parents were not available for chromosomal analysis. Mutation analysis of TCF4 revealed no abnormalities.
Patients 8 and 9
This brother pair was ascertained at the age of 31 and 29 years, respectively. Both were born after an uneventful pregnancy and delivery and had normal birth parameters. During the neonatal period the older brother (patient 8) was treated with phototherapy because of hyperbilirubinemia. He was a very quiet baby. Since birth he had a mild spasticity of his right leg. He was treated with splints because of inverted position of his ankles. His psychomotor development was delayed. He was able to sit and walk independently at the age of 12 and 24 months, respectively. Speech development was delayed, but he learnt to speak simple sentences. Since childhood he had intermittently high levels of bilirubin and was diagnosed with Gilbert syndrome. At child age he underwent a unilateral orchidopexy because of torsio testis, and his phimosis was corrected. At adult age he had a moderate to severe ID. He was diagnosed with a bipolar mood disorder. During depressive episodes he suffered from sleeping disorders and refused to eat. In general, his behavior was friendly, but regularly he showed an aggressive behavior. Social interaction was good. Upon physical examination at the age of 31 years he had a high normal height (190 cm, 75th centile) and a normal weight (50th centile) and head circumference (20th centile). Hands and feet were large to normal. Facial characteristics included a long face with prominent chin and large ears (fig. 1Q, R). He had an increased lordosis of the lumbar spine which was suggestive for muscular hypotonia of the trunk. The feet were slightly inverted.
In contrast, the younger brother (patient 9) was a hyperactive baby with poor contact making. Since he was 2 months of age, his parents noticed that he developed abnormally. The motor development was mildly delayed with sitting independently at 13 months and walking independently at 20 months of age. Speech development was more severely delayed with the first single words at the age of 4 years. With age his social interaction improved, and he had good contact with his parents, brother, and care-takers; however, contact with housemates was limited. He showed mild features of autism spectrum disorder, including obsessive behaviors. His developmental level was somewhat lower than his brother's and corresponding with a developmental level of a 2–3-year-old child. His mood and behavior were changeable, including periods with hyperactivity and aggressive outbursts as well as periods with apathy. Now and then he had sleeping difficulties with awakening and wandering during the night. He was diagnosed with a bipolar mood disorder as well. At a young age he underwent an operation because of cryptorchidism and phimosis. His general health condition was good. Upon physical examination he had a normal height (185 cm, 50th centile) and weight (50–75th centile) and a small head circumference on the 2nd centile (54 cm). He had a brachycephaly. Just as his brother he had a prominent chin and large ears. In addition, he had a left-sided epicanthal fold, a subtle bifid point of the nose and horizontal ear lobe creases (fig. 1S, T). He had a similar posture like his brother, including an increased lordosis of the lumbar spine.
In both brothers brain MRI showed hypoplasia of the cerebellar vermis, an enlarged cisterna magna, and mild enlargement of the lateral ventricles.
Chromosomal analysis by 250k SNP array analysis identified an identical terminal loss of 2.12 Mb in chromosomal region 22q13.32q13.33 (47,35–49,47 Mb) in both brothers. Chromosomal analysis in blood lymphocytes from the parents both by SNP array analysis and FISH analysis, with probes localized on chromosomal regions 22q11 and 22q13, gave normal results. To further investigate the probability of the presence of the 22qter deletion in a mosaic pattern in one of the parents, we performed additional FISH experiments in buccal cells of the parents as well. Indications for a mosaic pattern in the parents were not found.
Evolution of Phenotypes in Present and Previous Cases
Kleefstra Syndrome. Adult patients with Kleefstra syndrome have rarely been reported before [Kleefstra et al. 2006, 2009; Verhoeven et al., 2010, 2011; Willemsen et al., this issue]. Table 1 summarizes the phenotypic features of the present and 12 previously reported adult patients diagnosed with Kleefstra syndrome. Developmental outcome varied from moderate to severe ID. Most patients have little speech ability, though mostly very primitive. Six out of 11 patients (in 2 patients a presence of regression was not reported) showed some kind of regression during adolescence or adulthood, which was in 5 patients reported to be associated with a concurrent striking behavioral change including periods of diminished responsiveness, hypoactivity, passivity, and catatonic phenomena. Seven out of 13 had (a history of) seizures, though in general not very severe. All adult patients had the typical facial features of Kleefstra syndrome. In the present patient the facial features were the major clue to the diagnosis in addition to the characteristic regressive behavioral pattern with onset at adult age. Microcephaly was present in 5 out of 13 patients, 5 out of 10 had a short adult height, and 5 out of 12 patients presented with obesity. In conclusion, most important diagnostic clues at adult age seem to be the typical facial features and behavior pattern which might be associated with regression of general functioning.
Table 1.
Clinical features in present and previously reported patients with (genetically confirmed) Kleefstra syndrome
| Present patient | Kleefstra et al. [2006] | Kleefstra et al. [2009] (2/6 described in more detail in Verhoeven et al. [2010]) | Willemsen et al. [this issue] (2/4 described in more detail in Verhoeven et al. [2011]) | Verhoeven et al. [2011] | Total | |
|---|---|---|---|---|---|---|
| Number of adults | 1 | 1 | 6 | 4 (excl. present patient 1) | 1 (other 2 adults are included in Willemsen et al. [this issue]) | 13 |
| Genetic defect | EHMT1 mutation: c.3087 +1 G >T | micro-deletion | 6/6 microdeletion | 3/4 microdeletion, 1/4 EHMT mutation | microdeletion | 11/13 microdeletion 2/13 EHMT1 mutation |
| Age, years | 41 | 36 | 20–59 | 18–32 | 19 | 18–59 |
| Gender | f | f | 4 m, 2 f | 3 f, 1 m | f | 5 m, 8 f |
| Developmental outcome | moderate ID, some speech | severe ID | 6/6 variable ID | moderate-severe ID | moderate ID | 13/13 moderate-severe ID |
| Motor symptoms | focal myclonic twitches shoulder, fixed flexure arms and hands | NR | 1/2 progressively immobile 1/2 frequent myoclonic twitches, slight hypertonia fixed flexure arms and hands in 2/2 | 1/4 rigid flexure of the arms and hands | slow, preservative and clumsy motor functioning at adult age | myoclonic twitches in 2 patients; immobility in 1 patient; fixed flexure extremities in 4 patients |
| Regression (of psy-chomotor function) | + | NR | 3/5 | 1/4 | + | 6/11 |
| Behavior/psychiatric | impulsivity and aggressive outbursts; around age 38 remarkable behavior change: periods of apathy, decreased emotional responsiveness, staring, low motor activity, stupor, and sleep disturbance | +, not further specified |
|
|
sleep disturbance; increase of behavior problems in adolescence with later on general inactivity and irritability, stereotypies | variable behavior problems in all; temporary (behavioral) regression/ increase of behavioral problems during adolescence; onset of typical behavior pattern at middle age including periods with passivity, decreased responsiveness and hypoactivity in a subset |
| Seizures | − | + | 4/6 | 2/4 | − | 7/13 |
| Recognizable facial features | + | + | + | + | + | present in all |
| Medical problems, age of onset (when documented) | hypertrophy of the left ventricle and paroxysmal atrial fibrillation, diagnosed around age 40 years | NR |
|
|
ventricular septum defect |
|
| Adult growth parameters | ||||||
| head circumference | normal | normal | 3/6 microcephaly | 1/3 microcephaly | 5/13 microcephaly | 5/13 microcephaly |
| height | normal | NR | 2/4 short stature | 2/4 short stature | 5/10 short stature | 5/10 short stature |
| weight | obesity | obesity | 1/5 obesity | 1/4 obesity | 5/12 obesity | 5/12 obesity |
| Other | brain MRI: poorly defined abnormal white matter signal intensities, manly localized deep in the parietal lobe, bilaterally abnormal signal intensity in the globus pallidus | brachydactyly |
|
|
− | 5/10 variable anomalies on brain MRI |
NR = Not reported; GOR = gastro-oesophageal reflux; VUR = vesicoureteral reflux.
Pitt Hopkins Syndrome. The present patient is the oldest reported patient with Pitt Hopkins syndrome. As far as we know, 6 adult patients with genetically confirmed Pitt Hopkins syndrome have been reported in previous studies (table 2). All adults with Pitt Hopkins syndrome had a severely impaired developmental outcome. A minority suffered from seizures that presented at childhood. Late-onset seizures were not reported, suggesting that if seizures develop, they present at a young age. Medical problems were relatively mild, including mainly constipation, scoliosis, and aspecific ocular problems. Motor functioning was mildly impaired in some cases. Reported behavioral problems are diverse, but most patients have a happy disposition. Among this small group of adults with Pitt Hopkins syndrome, there are no indications for significant regression at adult age. The typical facial characteristics persist during adult age. In the present patient his facial gestalt was the clue to the diagnosis. In general, the combination of the typical facial characteristics, severe ID, the breathing pattern with periods of hyperventilation followed by apnoea, and the clubbing of finger- and toenails might suggest the diagnosis Pitt Hopkins syndrome in adults.
Table 2.
Clinical features in present and previously reported patients with (genetically confirmed) Pitt Hopkins syndrome
| Present patient | Zweier et al. [2007] | Zweier et al. [2008] | Rosenfeld et al. [2009] | Total | ||
|---|---|---|---|---|---|---|
| Number of adults | 1 | 2 | 3 | 1 | 7 | |
| Genetic defect | TCF4 mutation: c.1739G>A | TCF4 mutations:c.1153 C>T and IVS9–1G>C | TCF4 mutations: c.1699A>T, c.469C>T,c.1073G>T | 64-kb microdeletion 18q21.2 | 6 TCF4 mutations, 1 small microdeletion | |
| Age, years | 40 | 29 | 18–20 | 19 | 18–40 | |
| Developmental outcome | severe ID | severe ID | severe ID | severe ID | severe ID | |
| Motor symptoms | mild hypertonia | 1/2 ataxia | 1/3 ataxia | walks with assistance | 2/7 ataxia, 1/7 hypertonia | |
| 1/2 wide-based walking pattern | ||||||
| Regression (of psychomotor function) | − | NR | NR | NR | never reported | |
| Behavior/psychiatric | happy disposition, pica | 1/2 very anxious, self-mutilation | 2/3 happy disposition 1/3 violent outburst, stereotypies | happy disposition, bangs on objects, sleeping problems | mostly happy disposition, but diverse behavior problems do also occur | |
| Seizures | – | – | 1/3, since 9 months of age | – | 2/7, onset during childhood | |
| Recognizable facial features | + | + | 2/3 + | + | 7/7 | |
| 1/3 +/– | ||||||
| Medical problems, age of onset (when documented) | myopia, scoliosis, osteoporosis and atopic constitution, onset in childhood | 1/2 severe constipation, and strabismus in onset NR 1/2 lymphoma and dislocated hip, onset NR | 2/3 constipation, scoliosis 1/3 myopia, strabismus | strabismus, myopia | constipation, scoliosis, and aspecific ocular problems in several patients | |
| Adult growth parameters | ||||||
| head circumference | P0.6 | 2/2 <P3 | 2/3 <P3, 1/3 normal | P50 | 5/7 microcephaly | |
| height | >P2 | 2/2 <P3 | NR | P10–25 | 3/4 short stature | |
| weight | P16 | 2/2 <P3 | NR | P60 | 2/4 underweight | |
| Abnormal breathing patterna | – | 2/2 | 2/3 | +, breath holding | 5/7 | |
| Other | finger- and toenail clubbing | finger- and toenail clubbing in 1/2 | NR | NR | 2/3 nail clubbing |
NR = Not reported; P = percentile.a Comprising periods with hyperventilation followed by apnoea.
MECP Mutations in Males. MECP2 mutations are involved in Rett syndrome. Initially, it was thought that Rett syndrome occurs exclusively in females due to lethality of hemizygous mutations in males. Though, after the identification of the MECP2 gene in 1999, several MECP2 mutations have been detected in males as well. The phenotypic variability in males with MECP2 mutations is wide, and roughly 3 groups of phenotypes can be observed: (a) classical/atypical Rett syndrome occurring in males with a classical Rett syndrome mutation in mosaic pattern or in males with an XXY karyotype, (b) severe congenital encephalopathy, mostly associated with a mutation that causes Rett syndrome in females, and (c) a broad group with a wide phenotypic spectrum including variable levels of ID and variable occurrence of other neurological and/or psychiatric disorders [Moog et al., 2003, 2006; Villard, 2007]. The phenotypes caused by MECP2 duplications are not considered here. Mutations that cause the classical Rett syndrome phenotype in females are considered to be either prenatally lethal in males or leading to severe congenital encephalopathy and early death [Schüle et al., 2008]. The present patient can be assigned to the third group. Several males from X-linked ID families and single male patients with a phenotype fitting in the third group have been reported before [Meloni et al., 2000; Orrico et al., 2000; Couvert et al., 2001; Yntema et al., 2002]. Frameshift/truncating mutations, as observed in the present patient, infrequently have been reported in males before (2 previous reports) [Kleefstra et al., 2002; Moog et al., 2003]. Adult phenotypes of present and 23 previous cases are summarized in table 3. All patients (age range 21–56 years) have an impaired developmental outcome, though varying from mild to profound ID. From these data a significant phenotype-genotype correlation with respect to the degree of ID cannot be observed. In 2 of the families reported, identical mutations lead to a developmental level varying from mild to severe disability [Gendrot et al., 1999; Couvert et al., 2001; Gomot et al., 2003]. Almost all patients showed evident motor/neurological symptoms as tremors (46%), pyramidal signs (58%), and in 2 patients ataxic signs were observed. Interestingly, in one family tremors were reported in the carrier females as well [Couvert et al., 2001; Gomot et al., 2003]. Almost 30% of the males showed regression in functioning at adult age. One third of the patients had seizures, and with the exception of one, onset was reported at childhood age. A majority (64%) had microcephaly and short stature (67%). Four out of 24 developed kyphosis/scoliosis with age. Many were reported to have a friendly and cooperative behavior, though a few patients showed aggressive outbursts. Mood/depression and anxiety disorders were frequently observed. A subset of the patients had a passive, inactive personality, and some were shy/introverted in social interaction. In conclusion, clue features that might indicate the presence of a MECP2 mutation in adult males are variable ID in combination with neurological symptoms, including pyramidal signs and tremors, mood and anxiety disorders, and possibly regression at adult age. Facial features are not remarkable and do not contribute to the diagnosis.
Table 3.
Clinical features of present and previously reported male patients with ‘mild’ MECP2 mutations that do not cause classical Rett syndrome in female patients or severe neonatal encephalopathy/prenatal death in males
| Present patient | Gendrot et al. [1999]; Couvert et al. [2001]; Gomot et al. [2003] (MRX16) | Gomot et al. [2003]; Yntema et al. [2002](T44) | Couvert et al. [2001]; Gomot et al. [2003](T36) | Orrico et al.[2000] (X307, MR48, MR50, X308) | Lindsay et al. [1996]; Klauck et al. [2002] | Meloni et al. [2000]; Claes et al. [1997] | Total | |
|---|---|---|---|---|---|---|---|---|
| Number of adults | 1 | 7 | 3 | 4 | 4 | 4 | 1 | 24 |
| Mutation/ domain | frameshift: c.1233_1243del (p.Ser411fs)/C-terminus | missense: c.410A>G (p.Glu137Gly)/ MBD | in frame deletion c.1161_1400del/C-terminus | missense: c.499C>T (p.Arg167Trp)/Co-repressor interacting region | missense: c.493C>T (p. Ala-140Val)/MBD | missense:c.493 C>T (p.Ala140Val)/BD | nonsense:c.1216 C>T (p.Glu406X)/C-terminus | 19/24 missense 1/24 nonsense, 1/24 frameshift, 3/24 in frame deletion |
| 5/24 in C-terminus 4/24 in co-repressor interacting region | ||||||||
| Age, years | 27 | 21–56 | 24–55 | 25–50 | 27–40 | 25–41 | 39 | 21–56 |
| Developmental outcome | severe ID | 6/7 mild/moderate ID | 1/3 mild ID | 4/4 mild ID | 4/4 severe ID | 4/4 moderate ID | severe ID | 24/24 variable ID |
| 1/7 profound ID | ||||||||
| 1/3 moderate ID | ||||||||
| 1/3 severe ID | ||||||||
| Motor symptoms | mild spasticity, hypertonia, brisk reflexes, generalized muscular atrophy; frequent falling; resting tremor | 5/6 hyperreflexia (1 NR) | 1/3 hypertonia | 3/4 resting tremor 3/4 hyperreflexia 1/4 ataxia | 4/4 resting tremor | 3/4 resting tremor 3/4 hyperreflexia 2/4 hypertonia | spasticity, hyperreflexia, choreoathetotic movemements, ataxic gait | 11/24 tremor 14/24 pyramidal signs 2/24 ataxia |
| Regression (of psycho-motor function) | +, global decline since early twenties | 4/6 language regression | − | 1/4 deterioration of articulation | – | – | +, around age 30 motor decline/ progressive spasticity | 7/24 |
| Behavior/ psychiatric | passivity, lack of initiative, cooperative, calm, shy and friendly; depression, sleep disturbance | 4/7 introvert/ timid/shy | 1/3 irritability, depression, emotional disturbance | 3/4 depression | slow, friendly | 3/4 pleasant | NR | 6/23 shy/timid |
| 3/7 anxiety | 1/3 poor contact and violence | 1/4 aggression | 2/4 aggression | 10/23 mood/anxiety disorder | ||||
| 2/7 passivity/slowness | 1/3 shy, quiet, anxious, and attention problems | 1/4 anxiety | 4/23 aggression 4/23 passivity/hypoactivity | |||||
| Seizures | +, grand mal, onset age 12 | 1/7, single seizure | 1/3 | 2/4 | NR | 1/4 | +, grand mal onset age 27 | 7/20 |
| Recognizable facial features | – | – | – | NR | NR | – | – | not present |
| Medical problems, age of onset (when documented) | – | 1/7 severe myopia 3/7 kyphosis/ scoliosis | NR | NR | NR | 1/4 congestive heart failure, onset age 39 1/4 kyphoscoliosis | bilateral cataract age 30, episodes of unexplained diarrhea; death at age 39 from pneumonia | variable, 4/24 kyphosis/ scoliosis |
| Adult growth parameters | ||||||||
| head circumference | P25–P50 | 3/7 <P3 | 1/3 normal, 2/3 NR | 2/4 <P3 | 4/4 normal | 4/4 normal | normal | 5/22 <P3 |
| height | P16 | 6/7 <P3 | 2/3 normal, 1/3 NR | 4/4 <P3 | NR | 2/4 short stature | NR | 12/18 short stature |
| weight | P2 | >P98 in 2/5 | NR | 3/4 obesity | NR | 1/4 obesity | NR | 6/14 obesity |
| Other | – | – | – | – | – | macro-orchidism, pyramidal signs | siallorhoe | no consistent other features |
| Family history | learning disabilities in mother and sister, mother has severe psychiatric problems | patients are from the same family, carrier females all normal intelligent | 4 affected males in one family, no information about carrier females | progressive tremor in 2 carrier females, normal intelligence in carrier females | mother and carrier sister have mild ID | mother low intelligence | borderline intelligence in 1 of 2 carrier females; severe ID in nephew | all familial cases; in 4/6 families carrier females have mild ID/learning difficulties (NR in 1 family) |
MRX/T/X/MR numbers in the heading refer to the family identification numbers in the original papers.
MBD = Methyl-binding domain; P = percentile.
Rett Syndrome Variant with Infantile Spasms (CDKL5 Mutations). The present patient is the oldest patient with a mutation in CDKL5 reported. Table 4 summarizes the clinical features of the present and 9 other adult female patients [Tao et al., 2004; Weaving et al., 2004; Archer et al., 2006; Bahi-Buisson et al., 2008a, b; Erez et al., 2009]. The developmental outcome is very poor. Remarkably, in one patient (twin sister of a severely affected patient) the developmental level was reported to be only mildly impaired [Weaving et al., 2004]. Six out of 10 patients were not able to speak. All patients showed motor symptoms, including mostly pyramidal signs, but also cerebellar symptoms were reported. Secondary locomotor tract deformities including scoliosis and/or contractures were present in 6 out of 8 patients. Five out of 9 patients had signs of autonomic dysregulation (intestinal symptoms, breathing anomalies, and vasomotor disturbance). Regression was reported in 4 out of 10 patients. Seizure outcome was very poor. Nine patients suffered from persistent intractable seizures into adulthood. Behavior characteristics that were frequently present included (hand)stereotypies and poor social interaction. Sleeping problems were documented in 4 patients. Small hands and/or feet were also more often reported (4/7). In conclusion, the consistent clinical features at adult age included a very poor developmental and seizure outcome in combination with other neurological signs including pyramidal tract and cerebellar and autonomic signs. Major health problems were secondary to neurological problems. Behavior symptoms included Rett-like behaviors such as (hand) stereotypies.
Table 4.
Clinical features in present and previously reported patients with CDKL5 mutations
| Present patient | Weaving et al. [2004] | Weaving et al. [2004] | Tao et al. [2004] | Archer et al. [2006] | Bahi-Buisson et al. [2008a, b] | Erez et al. [2009] | Total | |
|---|---|---|---|---|---|---|---|---|
| Mutation | c.464–1G>A | c.183delT | IVS13–1G>A | 525A>T (R175S) | c.del2362_2366 | C1892.1893dupTA/ c.229delGAAG | Del exon 1a–3 | |
| Number of adults | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 10 |
| Age, years | 47 | 19 (twins) | 28 | 41 (twins) | 18 | 19, 26 | 20 | 18–47 |
| Sex | f | f | f | f | f | f | f | 10 f |
| Developmental outcome | severe ID, no speech | severe ID, no speech in twin 1, mild ID in twin 2 | severe ID, no speech | severe ID in both, no speech | severe ID, speech in phrases | severe ID, first patient able to speak 3–word sentences, second patient no speech | severe ID, no speech | 9/10 severe ID 1/10 mild ID (speech is rare) |
| Motor signs | spastic tetra–plegia, unable to walk without support, ataxia | wheelchair bound, generalized spasticity, hyperreflexia (twin 1) | unsteady gait | one is wheelchair bound, twin sister is able to walk since age 8 with support; in both mild ataxia | motor dyspraxia, limited hand skills, hypotonia, but walks and swims | unable to walk, limited hand skills | wheelchair bound, hyporeflexia | 10/10, comprising pyramidal and sometimes cerebellar components |
| Regression (of psycho–motor function) | +, decline motor functioning | twin 1: + normal development until 10 months, then loss of skills | +, between ages 2 and 5 years loss of skills | not significant | – | – | +, between ages 4 and 7 years | 4/10 |
| Behavior/ psychiatric | self–mutilation, stereotypic movements (hand biting), hyperactivity, no sense of fear | twin 1: poor eye contact, hand stereotypies | hand stereotypies, dystonia, sleep disturbances | hand stereotypies, mood swings, little eye contact and interaction with environment in both | hand stereotypies, autism, bruxism, mood lability, sleep disturbance | hand stereotypies, autistic features, sleep disturbances (both); bruxism (2) | NR | 8/9 hand stereo typies 6/9 poor social interaction/autism 4/9 sleep disturbance |
| Seizures | ||||||||
|
6 months | 1/2: 9 weeks | 6 weeks | 10 weeks | 2 months | 6–12 weeks | first 2 weeks of life, | |
|
atonic, intractable; persistent seizures despite multiple medications | 1/2 infantile spasms, later mixeda seizure disorder 1/2: no seizures | severe infantile spasms, later mixed intractable seizures | infantile spasms until 6 months in both, later absences (in one of the twins) | West syndrome, subsequent severe mixed seizure disorder | no infantile spasms 1/2 refractory seizures 1/2 seizure arrest at 2 weeks | +, mixed, intractable and therapy resistent | 9/10 9/9 early onset 8/9 persistent severe seizure disorder into adulthood |
| Facial features | coarseness | NR | no dysmorphism | NR | NR | NR | coarseness, bilateral exotropia | 2/3 coarseness (possibly due to anti-epileptic medication) |
| Medical problems | Pes equino-varus (related to spasticity), gastrostomy tube | thoracolumbal scoliosis | scoliosis | progressive dorso-lumbal scoliois in and flexion contractures in both | NR | gastrostomy tube | 6/8 locomotor tract problems secondary to spasticity 2 patients with gastrostomy because of feeding difficulties | |
| Adult growth parameters | ||||||||
|
<P16 | 1/2 normal | NR | 2/2 P5–P15 | normal | 2/2 microcephaly | normal | 2/9 microcephaly |
|
<P16 | 1/2 <P3 1/2 P3–P10 | NR | NR | normal | NR | normal | 1/8 short stature |
|
P2 | 1/2 <P3 1/2 P10–P25 | NR | NR | NR | NR | normal | 2/6 cachexia |
| Autonomic features | constipation | 1/2: breathing abnormalities, peripheral vasomotor disturbance; severe constipation | + | episodes of hyperventilation, abdominal distention in both | 1/2 | NR | 5/9 | |
| Other | small hands, corpus callosum agenesis | small hands and feet; male brother also affected, died at age 16 of respiratory failure | small feet, hirsutism | NR | NR | NR | acne, hirsutism | 4/7 small hands and/or feet |
P = Percentile.
Mixed: tonic-clonic, myoclonic, absences.
Dravet Syndrome. In contrast to the other reported syndromes in the present study, follow-up of Dravet syndrome patients into adulthood has more often been documented before [Jansen et al., 2006; Akiyama et al., 2010]. The phenotype of 37 adult patients, including the present 2 patients, is summarized in table 5. Age ranged from 18–49 years. The majority of patients had a poor developmental outcome with severe ID in more than 80%. Seventy-five percent had motor symptoms (including pyramidal, cerebellar, and extrapyramidal symptoms), varying from clumsiness to being bedridden. Regression was uncommon. In 86% of all patients, intractable seizures with a history of early onset in the first year of life persisted into adulthood. Behavior characteristics were not systematically reported, though the present 2 patients both had a friendly and cooperative personality. Medical problems were not systematically reported. Poor developmental and seizure outcome in combination with motor symptoms but lack of other specific features might be indicative of Dravet syndrome in adult male and female patients.
Table 5.
Clinical features of present and previously reported patients with genetically confirmed Dravet syndrome
| Present patient 5 | Present patient 6 | Jansen et al. [2006] | Akiyama et al. [2010] | Total | |
|---|---|---|---|---|---|
| Number of patients | 1 | 1 | 10 | 25 | 37 |
| Mutation | c.5304T>G (p.Ser1768Arg) | c.3526delG (p.Glu1176fs) | NR | 14/25 missense | missense> frameshift> nonsense |
| 5/25 nonsense | |||||
| 6/25 frameshift | |||||
| Age, years | 34 | 49 | mean 26 (range 18–47) | 18–43 | 18–49 |
| Gender | m | m | NR | 9 m, 16 f | 11 m, 16 f, 10 NR |
| Developmental outcome | severe ID, no speech | severe ID, very little speech | 1/10 normal | 24/25 severe ID | 31/37 severe ID |
| 4/10 moderate ID |
|
4/37 moderate ID | |||
| 5/10 severe ID |
|
1/37 mild ID | |||
|
1/37 no ID | ||||
|
|||||
|
|||||
| Motor symptoms | mild right-sided hemiplegia, after epileptic state, able to walk | clumsiness, spasticity | 5/10 pyramidal | 2/25 bedridden | 30/37 motor signs, comprising pyramidal, cerebellar and extrapyramidal signs |
| 3/10 cerebellar | 11/25 clumsy | ||||
| 4/10 extrapyramidal | 7/25 ataxia | ||||
| 2/10 no motor signs | 5/25 no motor signs | ||||
| Regression | +, onset at age 2 years | – | uncommon | 5/25 | 6/27 |
| Behavior/ psychiatric | very friendly, little initiative, obsessive traits, self-mutilation, very high threshold of pain | friendly personality, loves to get attention | NR | NR | 2/2 cooperative and friendly personality |
| Seizures | |||||
|
5 months | 6 months | 3–8 months | <first year of life | 37/37 onset within first year of life |
|
absences, tonic-clonic | mixed | at onset: 1/10 myclonic, 4/10 generalized tonic-clonic, 5/10 febrile; later: mixed seizures in 8/10 (tonic-clonic, absences, mycoclonic, partial) | generalized at adult age, only in 6 mixed seizures | variable types of seizures |
|
once epileptic state, intractable seizures despite multiple anti-epileptic drugs | intractable seizures despite multiple anti-epileptic drugs | intractable seizures in 10/10 | intractable in 20/25; in 5 seizures suppressed for 1–3 years | 32/37 intractable seizures persisting throughout adulthood |
| Recognizable facial features | – | – | NR | NR | not present |
| Medical problems | upper and lower airway infections | high hypermetropia and a substantial astigmatism | NR | 1 patient described as case report: pneumonia | 2/3 airway infection (not systematically reported) |
| Growth parameters | normal | normal | NR | NR | 12/12 normal |
NR = Not reported.
Phelan-McDermid Syndrome. Clinical features of the present patients and 15 previously reported adult patients are summarized in table 6. The age of the patients ranged from 19–49 years, and the present patient 7 was the oldest one reported. Developmental outcome varied from mild to severe ID. Most patients did have some speech ability. We observed a tendency to deterioration in motor functioning above the age of 40 years, including presence of pyramidal signs. The present patient 7 showed a severe decline in motor functioning as well as in cognitive and health performance. About half of the patients did at least have one seizure during lifetime, but severe seizure disorders were not reported. The majority of the adult patients had variable behavior problems, including autistic behavioral features, though in some patients these seemed to improve with age. Other features that were more frequently reported included a long face and large ears, large and/or slender hands/feet, and hypotonia with/without lumbar lordosis. In 4 patients, including the present patients 8 and 9, results of cerebral imaging by MRI were reported. Interestingly, in 3 of these patients similar anomalies, comprising cerebellar vermis hypoplasia, enlargement of the cisterna magna, and dilatation of ventricles, were observed.
Table 6.
Clinical features of present and previously reported patients with Phelan-McDermid syndrome
| Present patient 7 | Present patients 8, 9 | Flint et al. [1995] | Slavotinek et al. [1997] | Anderlid et al. [2002] | Tabolacci et al. [2005] | Dhar et al. [2010] | Misceo etal. [2011] | Luciani etal. [2003] | Manning etal. [2004] | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of adults | 1 | 2 | 1 | 1 | 1 | 1 (from brother pair) | 2 | 1 | 7 | 1 | 18 |
| Genetic defect | 1.8–Mb deletion | 2.12–Mb deletion | 130–kb terminal deletion | submicro scopic deletion | 100–kb terminal 22ql3.3 deletion | 3.5–Mb deletion | 5.65–Mb and 5.79–Mb deletions | intragenic SHANK3 deletion due to translocation Xq21.33and 22ql3.33 | 6withr(22), 1 with t(22) | 46,XX, der(22) t(14;22) (q32.31;ql3.33) | 9/18 22ql3.3 1 intragenic SHANK3 deletion |
| 6/18 ring chromosome 22 2/18 balanced translocation | |||||||||||
| Age, years | 48 | 31,29 | 22 | 26 | 33 | 24 | 19, 31 | 20 | 20–36 | 46 | 19–49 |
| Gender | m | m | f | f | f | m | m,f | f | 3m,4f | f | 8 m, 10 f |
| Developmental outcome | severe ID, no speech | moderate–severe ID | severe ID, no speech | learning difficulties | mild ID | severe ID, limited speech | significant speech delay | moderate ID, simple speech, difficult to understand | 7/7, in 2/7 severe (all r(22)) | + | 18/18, variable, most have some speech |
| Motor signs | hypertonia, progressive spasticity and wheelchair bound after age 45; swallowing difficulties after age 45 | 1/2 mild spasticity right leg 2/2 truncal hypotonia | fully mobile | NR | ataxic gait | hypotonia | NR | oral: poor articulation | 5/7 hypotonia | hyperreflexia and clonus, ataxia, decreased ambulation | tendency to deterioration in motor functioning above age 40, with onset of pyramidal signs |
| Regression | +, general decline in functioning | no | NR | NR | +, at adult age, decline in speech, motor function, daily living functions, incontinence | NR | NR | NR | NR | +? limited early history | 2–3/18 (at age>35 years) |
| Behavior/ psychiatric | not remarkable | bipolar mood disorder, sleep disturbance and high threshold of pain in both; mild features of autism spectrum disorder, improving with age (pat. 9) | NR | NR | autistic features: lack in contact, stereotypic movements | quiet and loving, but stubborn; peculiar catatonic–like episodes, high threshold of pain | +, not further specified | hand stereotypies, sometimes irritated and aggressive; more social and less ritualistic than in childhood | 7/7 very severe | NR | 14/15 variable, tendency to improvement of social interactions with age? |
| Seizures | +, onset at age 45 years | no | NR | NR | + | +, febrile, incidental | 1: no, 2: yes | NR | 2/7 | +, focal and petit mal | 7/15 |
| Facial dysmorphic features | coarse, bushy eyebrows, short philtrum, broad mouth, full lips, widely placed dentition, large ears | 1/2 long face 2/2 prominent chin, large ears | micrognathia, simple ears, prominent nose, gap between incisors | wide broad forehead, bulbous nose tip, prominent columella, high palate | long face, high nasal bridge, broad nose | asymmetry of the face, midface hypoplasia, square forehead, prominent supraorbital ridges, bulbous tip of the nose, short philtrum. large ears, prominent lower lip, high arched palate, gap between incisors | NR | periorbital puffiness, broad nasal bridge, slightly low–set and outwardly rotated ears, high and narrov palate, narrow bitemporal diameter, microretrognathia, low hairline neck | 5/7 dysplastic ears | narrowed bitemporal diameter, epicanthal folds, periorbital fullness, flat midface | long face, large ears; midface hypoplasia, periorbital fullness are more often reported |
| Medical problems, age of onset (when documented) | hyperthyroidism since age 27 years, died at the age of 49 years from pneumonia | Gilbert syndrome (pat. 8) since childhood | – | mitral valve prolapse, diagnosed at age 18 years | – | right convex scoliosis, diagnosed at age 22 years; constipation, since first years of life | recurrent upper respiratory tract infections in 1/2 | – | NR | NR | variable |
| Growth parameters | |||||||||||
|
P50 | P75 (pat. 8); | NR | P75–90 | slender build | P25 | 1: accelerated, | P75 | 6/7 normal | NR | normal-increased in |
| P50 (pat. 9) | 2: normal | 1/6 short stature | majority | ||||||||
|
P80 | P50 (pat. 8); | NR | NR | NR | >P97 | NR | P90 | NR | NR | normal-increased in majority |
| P50–75 (pat. 9) | |||||||||||
|
P80 | P20 (pat. 8); | NR | >P97 | NR | >P98 | NR | PIO | NR | NR | normal-increased in majority |
|
P2 (pat. 9) | ||||||||||
| Other | intermittent irregular and rapid breathing; 2/3 syndactyly of the toes | 1/2 endorotation ankles, normal-large hands and feet 2/2 genital anomalies 2/2 on brain MRI hypoplasia of the cerebellar vermis, enlarged cisterna magna, mild dilatation lateral ventricles | 2/3 long, thin fingers, 2/3 syndactyly of the toes, club feet deformity | long thin fingers, nasal speech, unusual hair texture, recurrent miscarriages | long fingers and toes, normal brain MRI | large hands and feet, persistence of fetal finger pads. 2/3 syndactyly of the toes brain MRI: cortical atrophy, pachygyria, cerebellar vermis hypoplasia, enlarged cisterna and ventricles | NR | prominent lumbar lordosis; slender hands with mild webbing between fingers, webbing between 2/3 toes | NR | NR | lumbar lordosis, probably related to truncal hypotonia 2/3 syndactyly toes, large and/or slender hands; 3/4 similar anomalies on brain MRI |
NR = Not reported; P = percentile.
Discussion
The adult phenotype of most of the presented syndromes has not been systematically reviewed and described in previous reports, except for SCN1A mutations involved in Dravet syndrome [Jansen et al., 2006; Akiyama et al., 2010].
We described the phenotype of 9 adults with 6 different Angelman- and Rett-like syndromes and reviewed previously reported adult cases in the literature. As in childhood patients, adult patients with the reported Angelman- and Rett-like syndromes showed an overlapping phenotypic spectrum as well. Despite the relatively low numbers of patients in each group that have been reported so far, a tendency to specific phenotypic differences at adult age with respect to evolution of the phenotype and outcome could be observed, though conclusions are hampered by the small numbers (table 7). Patients with Kleefstra syndrome, males with MECP2 mutations, and females with CDKL5 mutations showed a further decline in functioning/regression at adult age, whereas patients with Pitt Hopkins syndrome and Dravet syndrome seemed to have a relatively stable performance. The prognosis with regard to the outcome of epilepsy showed also significant differences, with the poorest outcome in Dravet syndrome patients and patients with CDKL5 mutations. Motor symptoms were clearly present in some syndromes, including male Rett syndrome, Kleefstra syndrome (catatonic phenomena), and in patients with CDKL5 mutations, but absent or minor in other syndromes. Distinctive behavior patterns could be observed as well, for example adult onset of periods with passivity, decreased responsiveness and hypoactivity in Kleefstra syndrome, and Rett syndrome like behaviors in patients with CDKL5 mutations. Major medical problems, except for severe epilepsy, were infrequently reported in adult patients with the presented Angelman- and Rett-like syndromes. Observed medical problems were mostly secondary to motor complications, for example secondary scoliosis, contractures, feeding difficulties, and airway infections due to spasticity, which were predominantly observed in patients with mutations in CDKL5, MECP2, and SCN1A.
Table 7.
Distinguishing features in adult patients with different Angelman- and Rett-like syndromes
| Kleefstra syndrome | Pitt Hopkins syndrome | MECP2 mutations in males | CDKL5 mutations | Dravet syndrome | Phelan-McDermid syndrome | |
|---|---|---|---|---|---|---|
| Facial appearance | recognizable | recognizable | non-recognizable | non-recognizable | non-recognizable | subtle, including long face, large ears |
| Epilepsy | +/–, mild | +/–, mild | +/–, mild | +, intractable | +, intractable | +/–, mild |
| Motor symptoms | +/–, catatonic phenomena | +/– mild | +, pyramidal, tremors, cerebellar | +, pyramidal, cerebellar | +, pyramidal, cerebellar | – |
| Degree of ID | moderate-severe | severe | mild-profound | mostly severe | severe | mild-severe |
| Regression | + (6/11), at adult age | – | + (1/3) at adult age | + (4/10) | – | +/– |
| Typical behavior/ psychiatric features | adult age onset periods with passivity, decreased responsiveness and hypoactivity; sleep disturbance | mostly happy disposition, but diverse behavior problems including pica and self-mutilation | mood/depression and anxiety disorders; shyness and passivity | (hand) stereotypies and poor social interaction | friendly and coop erative in general | autistic features, mood disorders |
| Signs of autonomic dysfunction | – | abnormal breathing pattern, constipation | – | breathing anomalies, cold and purple feet/vasomotor disturbance, severe constipation | – | +/– abnormal breathing pattern in present patient 7 |
| Growth parameters | microcephaly, short stature, and obesity in about 50%; obesity | microcephaly and short stature in majority | microcephaly and short stature in majority | normal-decreased | normal | normal-increased |
| Medical problems and/or other distinctive features | no consistently occurring medical problems | typical clubbing of finger- and toenails | spinal column deformity | spinal column deformity, scolio–sis; small hands and/or feet; feeding problems/gastrostomy | airway infections more often reported | long and/or slender fingers/toes, 2/3 syndactyly toes |
| Life expectancy (oldest reported patient, years) | 59 | 40 | 56 | 47 | 49 | 49 (died at this age) |
Patients with Kleefstra syndrome and Pitt Hopkins syndrome retain the recognizable facial features at adult age, and thus, in these patients their facial appearance is an important clue to the diagnosis.
In conclusion, the combination of different key clinical features at adult age might be helpful to distinguish specific Angelman- and Rett-like syndromes from each other. These key features comprise the degree of ID, seizure characteristics, motor symptoms, occurrence of regression and characteristic facial appearance (Pitt Hopkins syndrome and Kleefstra syndrome). Knowledge about and insight in syndrome specific adult clinical characteristics facilitates adequate management and follow-up of patients with Angelman- and Rett-like syndromes and enables careful counseling of family members regarding prognosis, natural course of the disease, and life expectancy.
Acknowledgements
We thank the participating patients and their families and the participating specialist physicians for people with intellectual disability. In addition, we thank Prof. W.M.A. Verhoeven associated with the Vincent van Gogh Institute for Psychiatry, Centre of Excellence for Neuropsychiatry, Venray, The Netherlands and the Erasmus University Medical Centre, Department of Psychiatry, Rotterdam, the Netherlands for his valuable contribution to the discussion on the adult phenotypes. This work was supported by grants from the Consortium ‘Stronger on your own feet’ (to T.K., J.H.M.R., H.M.J.S.-L.V. and M.H.W), GENCODYS, an EU FP7 large-scale integrating project grant (Grant agreement no. 241995), and The Netherlands Organization for Health Research and Development (ZonMw Fellowship grant no. 907-00–365 to T.K.).
References
- Akiyama M, Kobayashi K, Yoshinaga H, Ohtsuka Y. A long-term follow-up study of Dravet syndrome up to adulthood. Epilepsia. 2010;51:1043–1052. doi: 10.1111/j.1528-1167.2009.02466.x. [DOI] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Anderlid BM, Schoumans J, Annerén G, Tapia-Paez I, Dumanski J, et al. FISH-mapping of a 100-kb terminal 22q13 deletion. Hum Genet. 2002;110:439–443. doi: 10.1007/s00439-002-0713-7. [DOI] [PubMed] [Google Scholar]
- Archer HL, Evans J, Edwards S, Colley J, Newbury-Ecob R, et al. CDKL5 mutations cause infantile spasms, early onset seizures, and severe mental retardation in female patients. J Med Genet. 2006;43:729–734. doi: 10.1136/jmg.2006.041467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariani F, Hayek G, Rondinella D, Artuso R, Mencarelli MA, et al. FOXG1 is responsible for the congenital variant of Rett syndrome. Am J Hum Genet. 2008;83:89–93. doi: 10.1016/j.ajhg.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahi-Buisson N, Kaminska A, Boddaert N, Rio M, Afenjar A, et al. The three stages of epilepsy in patients with CDKL5 mutations. Epilepsia. 2008a;49:1027–1037. doi: 10.1111/j.1528-1167.2007.01520.x. [DOI] [PubMed] [Google Scholar]
- Bahi-Buisson N, Nectoux J, Rosas-Vargas H, Milh M, Boddaert N, et al. Key clinical features to identify girls with CDKL5 mutations. Brain. 2008b;131:2647–2661. doi: 10.1093/brain/awn197. [DOI] [PubMed] [Google Scholar]
- Claes S, Devriendt K, D'Adamo P, Meireleire J, Raeymaekers P, et al. X-linked severe mental retardation and a progressive neurological disorder in a Belgian family: clinical and genetic studies. Clin Genet. 1997;52:155–161. doi: 10.1111/j.1399-0004.1997.tb02536.x. [DOI] [PubMed] [Google Scholar]
- Claes L, Del-Favero J, Ceulemans B, Lagae L, Van Broeckhoven C, et al. De novo mutations in the sodium-channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am J Hum Genet. 2001;68:1327–1332. doi: 10.1086/320609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvert P, Bienvenu T, Aquaviva C, Poirier K, Moraine C, et al. MECP2 is highly mutated in X-linked mental retardation. Hum Mol Genet. 2001;10:941–946. doi: 10.1093/hmg/10.9.941. [DOI] [PubMed] [Google Scholar]
- Dhar SU, del Gaudio D, German JR, Peters SU, Ou Z, et al. 22q13.3 deletion syndrome: clinical and molecular analysis using array CGH. Am J Med Genet A. 2010;152A:573–581. doi: 10.1002/ajmg.a.33253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez A, Patel AJ, Wang X, Xia Z, Bhatt SS, et al. Alu-specific microhomology-mediated deletions in CDKL5 in females with early-onset seizure disorder. Neurogenetics. 2009;10:363–369. doi: 10.1007/s10048-009-0195-z. [DOI] [PubMed] [Google Scholar]
- Flint J, Wilkie AO, Buckle VJ, Winter RM, Holland AJ, et al. The detection of subtelomeric chromosomal rearrangements in idiopathic mental retardation. Nat Genet. 1995;9:132–140. doi: 10.1038/ng0295-132. [DOI] [PubMed] [Google Scholar]
- Gendrot C, Ronce N, Raynaud M, Ayrault AD, Dourlens J, et al. X-linked nonspecific mental retardation (MRX16) mapping to distal Xq28: linkage study and neuropsychological data in a large family. Am J Med Genet. 1999;83:411–418. [PubMed] [Google Scholar]
- Gomot M, Gendrot C, Verloes A, Raynaud M, David A, et al. MECP2 gene mutations in non-syndromic X-linked mental retardation: phenotype-genotype correlation. Am J Med Genet A. 2003;123A:129–139. doi: 10.1002/ajmg.a.20247. [DOI] [PubMed] [Google Scholar]
- Jansen FE, Sadleir LG, Harkin LA, Vadlamudi L, McMahon JM, et al. Severe myoclonic epilepsy of infancy (Dravet syndrome): recognition and diagnosis in adults. Neurology. 2006;67:2224–2226. doi: 10.1212/01.wnl.0000249312.73155.7d. [DOI] [PubMed] [Google Scholar]
- Kalscheuer VM, Tao J, Donnelly A, Hollway G, Schwinger E, et al. Disruption of the serine/threonine kinase 9 gene causes severe X-linked infantile spasms and mental retardation. Am J Hum Genet. 2003;72:1401–1411. doi: 10.1086/375538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauck SM, Lindsay S, Beyer KS, Splitt M, Burn J, et al. A mutation hot spot for nonspecific X-linked mental retardation in the MECP2 gene causes the PPM-X syndrome. Am J Hum Genet. 2002;70:1034–1037. doi: 10.1086/339553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleefstra T, Yntema HG, Oudakker AR, Romein T, Sistermans E, et al. De novo MECP2 frameshift mutation in a boy with moderate mental retardation, obesity and gynaecomastia. Clin Genet. 2002;61:359–362. doi: 10.1034/j.1399-0004.2002.610507.x. [DOI] [PubMed] [Google Scholar]
- Kleefstra T, Yntema HG, Nillesen WM, Oudakker AR, Mullaart RA, et al. MECP2 analysis in mentally retarded patients: implications for routine DNA diagnostics. Eur J Hum Genet. 2004;12:24–28. doi: 10.1038/sj.ejhg.5201080. [DOI] [PubMed] [Google Scholar]
- Kleefstra T, Brunner HG, Amiel J, Oudakker AR, Nillesen WM, et al. Loss-of-function mutations in euchromatin histone methyl transferase 1 (EHMT1) cause the 9q34 subtelomeric deletion syndrome. Am J Hum Genet. 2006;79:370–377. doi: 10.1086/505693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleefstra T, van Zelst-Stams WA, Nillesen WM, Cormier-Daire V, Houge G, et al. Further clinical and molecular delineation of the 9q subtelomeric deletion syndrome supports a major contribution of EHMT1 haploinsufficiency to the core phenotype. J Med Genet. 2009;46:598–606. doi: 10.1136/jmg.2008.062950. [DOI] [PubMed] [Google Scholar]
- Lindsay S, Splitt M, Edney S, Berney TP, Knight SJ, et al. PPM-X: a new X-linked mental retardation syndrome with psychosis, pyramidal signs, and macroorchidism maps to Xq28. Am J Hum Genet. 1996;58:1120–1126. [PMC free article] [PubMed] [Google Scholar]
- Luciani JJ, de Mas P, Depetris D, Mignon-Ravix C, Bottani A, et al. Telomeric 22q13 deletions resulting from rings, simple deletions, and translocations: cytogenetic, molecular, and clinical analyses of 32 new observations. J Med Genet. 2003;40:690–696. doi: 10.1136/jmg.40.9.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makedonski K, Abuhatzira L, Kaufman Y, Razin A, Shemer R. MeCP2 deficiency in Rett syndrome causes epigenetic aberrations at the PWS/AS imprinting center that affects UBE3A expression. Hum Molec Genet. 2005;14:1049–1058. doi: 10.1093/hmg/ddi097. [DOI] [PubMed] [Google Scholar]
- Manning MA, Cassidy SB, Clericuzio C, Cherry AM, Schwartz S, et al. Terminal 22q deletion syndrome: a newly recognized cause of speech and language disability in the autism spectrum. Pediatrics. 2004;114:451–457. doi: 10.1542/peds.114.2.451. [DOI] [PubMed] [Google Scholar]
- Mari F, Azimonti S, Bertani I, Bolognese F, Colombo E, et al. CDKL5 belongs to the same molecular pathway of MeCP2 and it is responsible for the early-onset seizure variant of Rett syndrome. Hum Mol Genet. 2005;14:1935–1946. doi: 10.1093/hmg/ddi198. [DOI] [PubMed] [Google Scholar]
- Meloni I, Bruttini M, Longo I, Mari F, Rizzolio F, et al. A mutation in the Rett syndrome gene, MECP2, causes X-linked mental retardation and progressive spasticity in males. Am J Hum Genet. 2000;67:982–985. doi: 10.1086/303078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misceo D, Rødningen OK, Barøy T, Sorte H, Mellembakken JR, et al. A translocation between Xq21.33 and 22q13.33 causes an intragenic SHANK3 deletion in a woman with Phelan-McDermid syndrome and hypergonadotropic hypogonadism. Am J Med Genet A. 2011;155:403–408. doi: 10.1002/ajmg.a.33798. [DOI] [PubMed] [Google Scholar]
- Moncla A, Kpebe A, Missirian C, Mancini J, Villard L. Polymorphisms in the C-terminal domain of MECP2 in mentally handicapped boys: implications for genetic counselling. Eur J Hum Genet. 2002;10:86–89. doi: 10.1038/sj.ejhg.5200761. [DOI] [PubMed] [Google Scholar]
- Moog U, Smeets EE, van Roozendaal KE, Schoenmakers S, Herbergs J, et al. Neurodevelopmental disorders in males related to the gene causing Rett syndrome in females (MECP2) Eur J Paediatr Neurol. 2003;7:5–12. doi: 10.1016/s1090-3798(02)00134-4. [DOI] [PubMed] [Google Scholar]
- Moog U, Van Roozendaal K, Smeets E, Tserpelis D, Devriendt K, et al. MECP2 mutations are an infrequent cause of mental retardation associated with neurological problems in male patients. Brain Dev. 2006;28:305–310. doi: 10.1016/j.braindev.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Orrico A, Lam C, Galli L, Dotti MT, Hayek G, et al. MECP2 mutation in male patients with non-specific X-linked mental retardation. FEBS Lett. 2000;481:285–288. doi: 10.1016/s0014-5793(00)01994-3. [DOI] [PubMed] [Google Scholar]
- Papa FT, Mencarelli MA, Caselli R, Katzaki E, Sampieri K, et al. A 3 Mb deletion in 14q12 causes severe mental retardation, mild facial dysmorphisms and Rett-like features. Am J Med Genet A. 2008;146A:1994–1998. doi: 10.1002/ajmg.a.32413. [DOI] [PubMed] [Google Scholar]
- Phelan MC, Rogers RC, Saul RA, Stapleton GA, Sweet K, et al. 22q13 deletion syndrome. Am J Med Genet. 2001;101:91–99. doi: 10.1002/1096-8628(20010615)101:2<91::aid-ajmg1340>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Rosenfeld JA, Leppig K, Ballif BC, Thiese H, Erdie-Lalena C, et al. Genotype-phenotype analysis of TCF4 mutations causing Pitt-Hopkins syndrome shows increased seizure activity with missense mutations. Genet Med. 2009;11:797–805. doi: 10.1097/GIM.0b013e3181bd38a9. [DOI] [PubMed] [Google Scholar]
- Russo S, Marchi M, Cogliati F, Bonati MT, Pintaudi M, et al. Novel mutations in the CDKL5 gene, predicted effects and associated phenotypes. Neurogenetics. 2009;10:241–250. doi: 10.1007/s10048-009-0177-1. [DOI] [PubMed] [Google Scholar]
- Samaco RC, Hogart A, LaSalle JM. Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Hum Molec Genet. 2005;14:483–492. doi: 10.1093/hmg/ddi045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüle B, Armstrong DD, Vogel H, Oviedo A, Francke U. Severe congenital encephalopathy caused by MECP2 null mutations in males: central hypoxia and reduced neuronal dendritic structure. Clin Genet. 2008;74:116–126. doi: 10.1111/j.1399-0004.2008.01005.x. [DOI] [PubMed] [Google Scholar]
- Slavotinek A, Maher E, Gregory P, Rowlandson P, Huson SM. The phenotypic effects of chromosome rearrangement involving bands 7q21.3 and 22q13.3. J Med Genet. 1997;34:857–861. doi: 10.1136/jmg.34.10.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabolacci E, Zollino M, Lecce R, Sangiorgi E, Gurrieri F, et al. Two brothers with 22q13 deletion syndrome and features suggestive of the Clark-Baraitser syndrome. Clin Dysmorphol. 2005;143:127–132. [PubMed] [Google Scholar]
- Takano K, Lyons M, Moyes C, Jones J, Schwartz CE. Two percent of patients suspected of having Angelman syndrome have TCF4 mutations. Clin Genet. 2010;78:282–288. doi: 10.1111/j.1399-0004.2010.01380.x. [DOI] [PubMed] [Google Scholar]
- Tao J, Van Esch H, Hagedorn-Greiwe M, Hoffmann K, Moser B, et al. Mutations in the X-linked cyclin-dependent kinase-like 5 (CDKL5/STK9) gene are associated with severe neurodevelopmental retardation. Am J Hum Genet. 2004;75:1149–1154. doi: 10.1086/426460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven WM, Kleefstra T, Egger JI. Behavioral phenotype in the 9q subtelomeric deletion syndrome: a report about two adult patients. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:536–541. doi: 10.1002/ajmg.b.31015. [DOI] [PubMed] [Google Scholar]
- Verhoeven WM, Egger JI, Vermeulen K, van de Warrenburg BP, Kleefstra T. Kleefstra syndrome in three adult patients: further delineation of the behavioral and neurological phenotype shows aspects of a neurodegenerative course. Am J Med Genet A. 2011;155A:2409–2415. doi: 10.1002/ajmg.a.34186. [DOI] [PubMed] [Google Scholar]
- Villard L. MECP2 mutations in males. J Med Genet. 2007;44:417–423. doi: 10.1136/jmg.2007.049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P, Black G, Ramsden S, Barrow M, Super M, et al. Angelman syndrome phenotype associated with mutations in MECP2, a gene encoding a methyl CpG binding protein. J Med Genet. 2001;38:224–228. doi: 10.1136/jmg.38.4.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaving LS, Christodoulou J, Williamson SL, Friend KL, McKenzie OL, et al. Mutations of CDKL5 cause a severe neurodevelopmental disorder with infantile spasms and mental retardation. Am J Hum Genet. 2004;75:1079–1093. doi: 10.1086/426462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HL, Wong AC, Shaw SR, Tse WY, Stapleton GA, et al. Molecular characterisation of the 22q13 deletion syndrome supports the role of haploinsufficiency of SHANK3/ PROSAP2 in the major neurological symptoms. J Med Genet. 2003;40:575–584. doi: 10.1136/jmg.40.8.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yntema HG, Oudakker AR, Kleefstra T, Hamel BC, van Bokhoven H, et al. In-frame deletion in MECP2 causes mild nonspecific mental retardation. Am J Med Genet. 2002;107:81–83. doi: 10.1002/ajmg.10085. [DOI] [PubMed] [Google Scholar]
- Zweier C, Peippo MM, Hoyer J, Sousa S, Bottani A, et al. Haploinsufficiency of TCF4 causes syndromal mental retardation with intermittent hyperventilation (Pitt-Hopkins syndrome) Am J Hum Genet. 2007;80:994–1001. doi: 10.1086/515583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier C, Sticht H, Bijlsma EK, Clayton-Smith J, Boonen SE, et al. Further delineation of Pitt-Hopkins syndrome: phenotypic and genotypic description of 16 novel patients. J Med Genet. 2008;45:738–744. doi: 10.1136/jmg.2008.060129. [DOI] [PubMed] [Google Scholar]
- Zweier M, Gregor A, Zweier C, Engels H, Sticht H, et al. Mutations in MEF2C from the 5q14.3q15 microdeletion syndrome region are a frequent cause of severe mental retardation and diminish MECP2 and CDKL5 expression. Hum Mutat. 2010;31:722–733. doi: 10.1002/humu.21253. [DOI] [PubMed] [Google Scholar]