Abstract
It is assumed that acute myocardial infarction affects renal function. To study the mechanism, we used mice following permanent ligation of their left coronary artery that results in extensive myocardial infarction. Soon after ligation, there was a marked rise in circulating pro-inflammatory cytokines and malondialdehyde (thiobarbituric acid-positive evidence of lipid peroxidation). Renal function had significantly declined by the third day in association with mild fibrosis, and swelling of glomeruli and tubules. There was a significant increase in the expression of the lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1), interelukin-1β, vascular cell adhesion molecule-1, and thiobarbituric acid-reactive substances in the kidney. Renal function showed some recovery by Day 21; however, there was progressive fibrosis of the kidneys. LOX-1 knockout mice had significantly diminished increases in systemic and renal pro-inflammatory cytokines, malondialdehyde, structural alterations, and decline in renal function than the wild-type mice following ligation of the left coronary artery. Cardiac function and survival rates were also significantly better in the LOX-1 knockout mice than in the wild-type mice. Hence, severe myocardial ischemia results in renal dysfunction and histological abnormalities suggestive of acute renal injury. Thus, LOX-1 is a key modulator among multiple mechanisms underlying renal dysfunction following extensive myocardial infarction.
Keywords: inflammation, LOX-1, myocardial infarction, renal function
Myocardial ischemia is the most common cause of mortality and morbidity in the developed countries and is rapidly becoming a common malady in the developing countries. Patients who survive acute myocardial infarction often develop cardiac functional impairment, which if sustained affects the kidneys. This has been addressed as cardiorenal syndrome. The simplistic view of cardiorenal syndrome is that a relatively normal kidney becomes dysfunctional because of sustained low cardiac output. This concept has been recently challenged, and a more articulated definition of cardiorenal syndrome has been presented.1 This includes a variety of acute or chronic conditions where the primary failing organ can be either the heart or the kidney.1 Thus, direct and indirect effects of each organ that is dysfunctional can initiate and perpetuate the combined disorder of the two organs through a complex combination of neurohormonal feedback mechanisms.1–3
There is a link between coronary heart disease and renal dysfunction, which may be a manifestation of endothelial/epithelial dysfunction leading to the syndrome of migration of oxidized lipids through the inter-endothelial junctions to the intima (atherogenesis) and to passage of plasma proteins (microalbuminuria) through the inter-epithelial junctions.4 It has been suggested that the abnormalities in renin–angiotensin system and oxidized low-density lipoproteins (ox-LDL) participate in the pathogenesis of this process through expression and activation of a lectin-like receptor LOX-1 in the heart and the kidney.4
Clinicians have suggested a renal functional defect during the acute phase of myocardial infarction, and attributed renal dysfunction to low cardiac output state and/or marked increase in venous pressure leading to kidney congestion.1,5 However, direct evidence for this phenomenon has not been presented. Nonetheless, it is well known that worsening renal function following myocardial infarction is a powerful and independent predictor of in-hospital and 1-year mortality.6,7
A plausible basis for this independent effect might be that an acute decline in renal function during myocardial infarction does not merely reflect severity of the illness, but involves complex cardiovascular pathobiology mediated through activation of inflammatory pathways.8,9 Acute myocardial infarction is associated with a systemic pro-inflammatory and pro-oxidant state. The activation of renin–angiotensin system contributes to this pro-inflammatory and pro-oxidant state in the injured myocardium. Renin–angiotensin system activation, especially when sustained, has been shown to induce fibroblast growth and cardiomyocyte hypertrophy via activation of angiotensin II type 1 receptor.
Our studies have shown that the pro-inflammatory and pro-oxidant state in the hearts of rats and mice subjected to acute myocardial ischemia is accompanied by a marked upregulation of LOX-1.10,11 LOX-1 is a pro-inflammatory molecule that is responsible for polymorphonuclear leukocyte (PMNL) accumulation in the ischemic and injured tissues.12,13 Expression and activation of LOX-1 also result in the release of oxidant species.14,15 The key evidence in favor of the pathogenic role of LOX-1 expression/activation came from studies wherein an antibody given before induction of myocardial ischemia reduced inflammatory and oxidant response and resultant injury to the heart.16 Hu et al.17 showed that mice with LOX-1 deletion (termed LOX-1 knockout or KO) have significantly less myocardial injury following coronary ligation than do wild-type (WT) mice subjected to the same degree of ischemia.
We hypothesized that the systemic inflammatory process present during and following acute myocardial infarction may affect renal function. We also posited that LOX-1 abrogation would limit renal dysfunction following myocardial infarction by inhibiting pro-inflammatory and prooxidative signaling.
RESULTS
Survival after total left coronary artery ligation
The survival rates in WT and LOX-1 KO mice that underwent sham left coronary artery (LCA) ligation for 3 weeks were comparable. On Day 3 post-LCA ligation, 79% of the LOX-1 KO mice survived (vs. 58% of WTmice, P < 0.05). LOX-1 KO mice had 25% improvement in survival over the WT mice over the 3-week period following LCA ligation. These data are similar to another report from our laboratory in a different group of mice.18
Heart weight after LCA ligation
The heart weight/tibia length ratio, an indicator of cardiac hypertrophy, was increased in both groups of mice subjected to LCA ligation. The increase in the heart weight/tibia length was less in the LOX-1 KO group (P = 0.001 vs. WT mice; Table 1).
Table 1.
The organ gravimetric parameters
| Parameters | WT ischemia (sham) |
LOX-1 KO ischemia (sham) |
WT ischemia (3 days) |
LOX-1 KO ischemia (3 days) |
WT ischemia (3 weeks) |
LOX-1 KO ischemia (3 weeks) |
|---|---|---|---|---|---|---|
| Body weight (g) | 25.75 ± 1.55 | 25.38 ± 1.82 | 22.31 ± 1.90 | 23.87 ± 1.24 | 26.29 ± 2.23 | 26.49 ± 3.02 |
| Heart weight (mg) | 123 ± 5.40 | 126 ± 6.10 | 157 ± 6.20* | 135 ± 7.03‡ | 179 ± 7.30* | 155 ± 7.72*,‡ |
| Heart weight per tibia length (mg/mm) | 7.00 ± 0.15 | 7.10 ± 0.20 | 9.13 ± 0.76* | 7.94 ± 0.71‡ | 10.91 ± 1.03* | 8.8 ± 0.68*,‡ |
| 1-Kidney weight (mg) | 167 ± 5.20 | 165 ± 4.55 | 232 ± 8.34* | 187 ± 9.47*,‡ | 227 ± 7.69* | 178 ± 7.32*,‡ |
| 1-Kidney weight per body weight (mg/g) | 7.10 ± 0.21 | 7.06 ± 0.19 | 9.33 ± 0.86* | 7.79 ± 0.53‡ | 8.76 ± 0.95* | 7.54 ± 0.38‡ |
Abbreviations: KO, knockout; LOX-1, low-density lipoprotein receptor-1; WT, wild type.
N=6 male mice in each group. Data are expressed as mean ± s.d.
P < 0.05 compared to corresponding sham ischemia group.
P < 0.05 compared to WT mice.
Left ventricular function after LCA ligation
There was a significant suppression of LV function as assessed by echocardiography on Day 3 as well as on Day 21 post-LCA ligation in the WT mice (P = 0.001. vs. sham ischemia WT mice; Figure 1). On Day 21, there was significant dilation of LV and thinning of LV anterior wall. Notably, LV function showed less deterioration in the LOX-1 KO mice subjected to LCA ligation on Day 3 as well as Day 21 (vs. WT mice) (Figure 1).
Figure 1. Representative M-mode echocardiography tracings and summary data.
Shown are the data on ejection fraction, left ventricular (LV) anterior wall systolic thickness, and LV internal diameter in end-diastole in the wild-type (WT) and low-density lipoprotein receptor-1 (LOX-1) knockout (KO) group at Days 3 and 21 (3 weeks) after total left coronary artery (LCA) ligation. Note that the changes were less pronounced in the LOX-1 KO mice subjected to the same duration of ischemia (P < 0.05 vs. WT). Data, shown as mean ± s.d., are based on measurements from six mice in each group. LVIDd, left ventricular internal diameter in diastole; LVIDs, left ventricular internal diameter in systole. *P < 0.05 vs. corresponding sham ischemia group; ‡P < 0.05 vs. WT group.
Arterial pressure after LCA ligation
Basal blood pressure was similar in the WT and LOX-1 KO mice and remained unchanged in all sham ischemia mice. Systolic blood pressure was lower in WTand LOX-1 KO mice during the 3-week period after LCA ligation than in corresponding groups of sham ischemia mice (P < 0.05). Although the systolic blood pressure was slightly lower in WT mice with LCA ligation compared with LOX-1 KO mice, the difference was not significant (Figure 2).
Figure 2. Renal function and blood pressure following left coronary artery (LCA) occlusion measured as glomerular filtration rate (GFR), serum blood urea nitrogen (BUN), and creatinine.
Note that the blood pressure was similar in wild-type (WT) and lipoprotein receptor-1 (LOX-1) knockout (KO) mice at all time points. Data, shown as mean ± s.d., are based on measurements in 8–10 animals in each group. *P < 0.05 vs. corresponding sham ischemia group; ‡P < 0.05 vs. WT group; n = 6.
Renal function after LCA ligation
As shown in Figure 2, serum creatinine and glomerular filtration rate (GFR) values were comparable and remained within the normal range in the WT and LOX-1 KO mice subjected to sham LCA occlusion. There was, however, a significant decline in GFR and rise in serum creatinine and blood urea nitrogen (BUN) on Day 3 in the WT mice with LCA ligation. In contrast, the decline in GFR and rise in serum creatinine were much less in the LOX-1 KO mice (P = 0.001).
On Day 21, there was a significant improvement in GFR and in serum creatinine and BUN in the WT mice with LCA ligation, although the values were still in the abnormal range. In contrast, serum BUN and creatinine and GFR values were in the normal range on Day 21 after LCA ligation in the LOX-1 KO mice (P < 0.05 vs. WT mice with LCA ligation).
Renal morphology in mice with LCA ligation
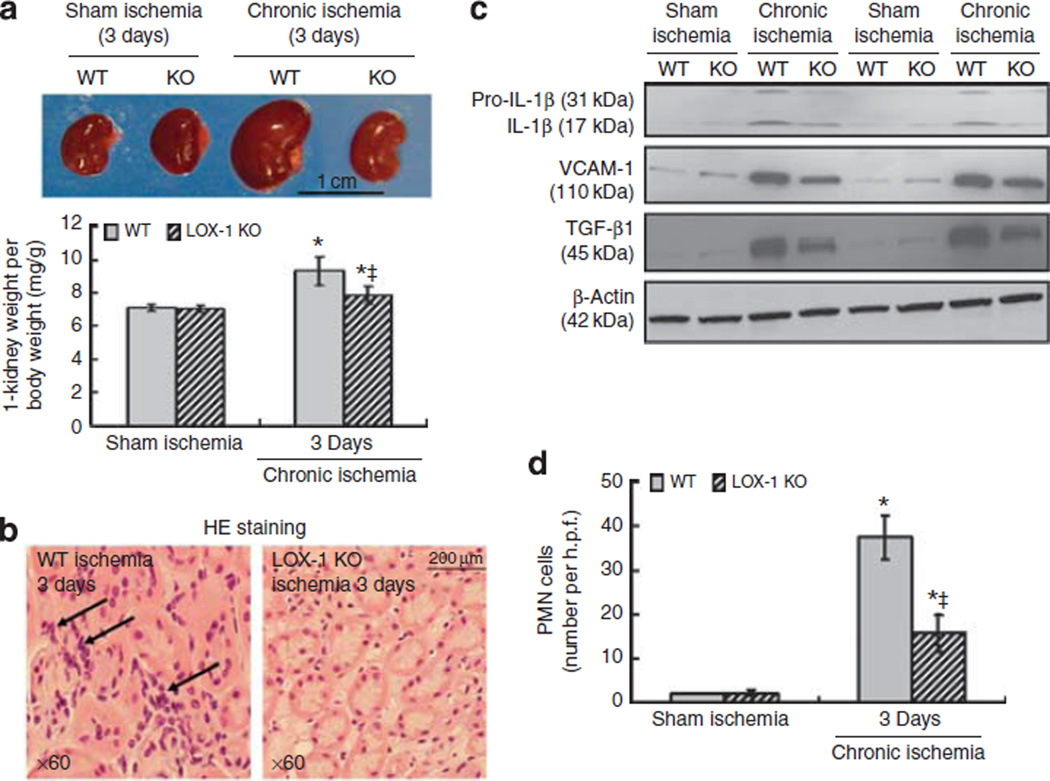
The kidney weight was markedly increased on Day 3 in both groups of mice subjected to LCA ligation (P < 0.05 vs. kidney weight in respective groups of mice subjected to sham ischemia), but the increase was less in the LOX-1 KO mice (P<0.05 vs. WT mice; Figure 3a). Kidney weight on Day 21 post-LCA ligation was less than on Day 3, although still more than in the corresponding groups of mice subjected to sham ischemia. These data are summarized in Table 1.
Figure 3. Kidney weight and inflammation following left coronary artery (LCA) ligation.
(a) Representative pictures of kidneys from the wild-type (WT) and lipoprotein receptor-1 (LOX-1) knockout (KO) groups. The lower panel shows summary data, shown as mean ± s.d., on kidney weight per body weight (mg/g, n = 6). *P < 0.05 compared to corresponding sham ischemia; ‡P < 0.05 compared to WT groups subjected to total LCA ligation. (b) Renal histology shows polymorphonuclear leukocytes (PMNLs) in the peritubular regions (original magnification × 600). In contrast to WT mice, the number of PMNLs was much less in the LOX-1 KO mice. (c) Representative protein expression of interleukin-1β (IL-1β), vascular cell adhesion molecule-1 (VCAM-1), and tumor growth factor-β1 (TGF-β1) in the mice kidneys. (d) Quantification of accumulation of PMNLs. H.p.f.: high power field. At least 100 glomeruli and tubules were examined in each slice, and at least four slices were examined in each kidney and the data averaged. *P < 0.05 compared to corresponding sham ischemia; ‡P < 0.05 compared to WT groups subjected to total LCA ligation.
There were striking changes in the morphology of kidneys from WT and LOX-1 mice subjected to LCA ligation. On Day 3 post-LCA ligation, there was marked swelling of the glomeruli and tubules, and an extensive presence of casts in the tubules in the WT mice (Figure 3b). There was a small amount of fibrosis throughout the medulla in the periglomerular and perivascular regions (Figure 4). Further, there was accumulation of PMNLs throughout the kidney on Day 3 post-LCA occlusion in the WT mice, and the accumulation of PMNLs was much less in the LOX-1 KO mice. Representative examples are shown in Figure 3b, and the summary of data is presented in Figure 3d. Expression of pro-inflammatory molecules, pro-interelukin-1β (IL-1β), vascular cell adhesion molecule-1 (VCAM-1), and tumor growth factor-β1 (TGF-β1), was increased markedly on Day 3 after LCA ligation in the kidneys of WT mice (P < 0.05; Figure 3c and Supplementary Figure S3 online); the LOX-1 KO mice had a much smaller increase in pro-IL-1β, VCAM-1, and TGF-β1 expression (P < 0.05 vs. WT mice).
Figure 4. Accelerated fibrosis in the kidney after prolonged myocardial ischemia.
(a) Representative sections of kidney stained with Masson’s trichrome (original magnification × 200) after left coronary artery (LCA) ligation. (b) Kidney section using Masson’s trichrome staining (original magnification × 600) revealed less tubulointerstitial fibrosis and glomerulosclerosis in the lipoprotein receptor-1 (LOX-1) knockout (KO) mice at Day 3 after LCA occlusion. (c) Summary data, shown as mean ± s.d., on the area of collagen deposition in the kidneys of WT and LOX-1 KO mice. At least four slices were examined in each kidney and summary data were obtained from six mice in each group. *P < 0.05 compared to corresponding sham ischemia group; ‡P < 0.05 compared to WT chronic ischemia. (d) Representative western blots of fibrosis-related proteins (collagen IV-α2 and procollagen-1) and β-actin expression.
On Day 21 post-LCA ligation, the swelling of glomeruli and tubules was less as was the presence of casts in the tubules as compared with Day 3 post-LCA ligation. However, fibrosis was more extensive (Figure 4a–c), and it was present in the periglomerular and perivascular regions, and also in-between tubules. Changes in glomeruli, tubules, and the extent of fibrosis were qualitatively similar in the WT and LOX-1 KO mice, but quantitatively less marked in the LOX-1 KO mice (P < 0.05).
Markers of collagen formation in the kidney
Molecular markers of collagen formation (collagen IV-α2 and procollagen-I) were increased in the renal tissue of WT mice on Day 3 post-LCA ligation, and the increase in these markers persisted on Day 21. Increase in the markers of collagen formation was much less in the LOX-1 KO mice despite the same site of LCA ligation (Figure 4d).
Assessment of systemic inflammatory state
Pro-inflammatory cytokines, IL-1β, IL-6, interferon-γ, and tumor necrosis factor-α (TNF-α), as well as immunomodulatory cytokines, IL-10, were measured during the first 3 days post-LCA ligation. Serum levels of IL-1β, IL-6, and TNF-α were markedly elevated in the WT mice with peak at 24 h, followed by a steady decline to normal level by 72 h. The increase in pro-inflammatory and immunomodulatory cytokines was less in the LOX-1 KO mice (P = 0.001 vs. WT mice; Figure 5).
Figure 5. Serum concentration of pro-inflammatory cytokines during the first 3 days after left coronary artery (LCA) ligation.
Summary data, shown as mean ± s.d., from six mice in each group. ‡P < 0.05 vs. WT group. GM-CSF, granulocyte–macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; KO, knockout; TNF, tumor necrosis factor; WT, wild type.
Assessment of pro-oxidant state and collagen markers in the kidney
On Day 3 post-LCA ligation, there was a significant increase in the serum malondialdehyde and the kidney thiobarbituric acid-reactive substances in the WT mice; again, the increase in the serum malondialdehyde and the kidney thiobarbituric acid-reactive substances was less pronounced in the LOX-1 KO mice (P < 0.05). The pro-oxidant state declined modestly in both groups on Day 21 (Figure 6a and b).
Figure 6. Oxidative stress and activation of lipoprotein receptor-1 (LOX-1) and nuclear factor-κB (NF-κB) pathway in the kidney.
(a) Serum malondialdehyde (MDA) levels (n = 12–15 mice in each group). (b) Levels of thiobarbituric acid-reactive substances (TBARS, n = 6) in the kidney. (c) Representative western blots of LOX-1, IκBα, and its phosphorylation. (d) Quantification of western blot data (n = 4). A.u. indicates arbitrary units. Data are shown as mean ± s.d. *P < 0.05 compared to corresponding sham ischemia; ‡P < 0.05 compared to WT chronic ischemia. KO, knockout; WT, wild-type.
LOX-1 was expressed in the kidneys only modestly at baseline (sham ischemia group), and its expression increased markedly following LCA ligation in the WT mice. As expected, there was no LOX-1 in the LOX-1 KO mice either before or after LCA ligation (Figure 6c and d).
Increase in LOX-1 in the WT mice was associated with enhanced phosphorylation of IκBα (P < 0.05 vs. sham ischemia group). The activation of IκBα protein was less pronounced in the LOX-1 KO mice kidneys (P = 0.001 vs. WT mice) (Figure 6c and d).
DISCUSSION
This study presents novel data on the development of renal dysfunction in mice that had been subjected to total and permanent LCA ligation. On Day 3 post-LCA ligation, there was marked glomerular and tubular swelling and extensive deposits of cast in the tubular lumen, and minimal, but definite, evidence of fibrosis and activation of markers of collagen formation. Further, there was a significant decrease in GFR and a marked rise in serum BUN and creatinine, indicating renal dysfunction. These observations on renal dysfunction and altered morphology are reminiscent of acute renal injury seen in extensive tissue ischemia, trauma, or infection. On Day 21 after LCA ligation, renal function had improved compared to Day 3. The observations of some improvement in renal function at 3 weeks post-myocardial infarction are consistent with the natural history of acute renal injury. However, there was evidence of ongoing fibrosis in the kidneys at this time point when the LV was dilated, and cardiac remodeling had set in.
The acute deterioration in renal function identified soon after LCA ligation was associated with a rise in serum markers of inflammation (IL-1β, IL-6, TNF-α, and interferon-γ), suggesting a state of systemic inflammation. There was also evidence of inflammation in the kidney as the expression of several pro-inflammatory molecules, pro-IL-1β, IL-1β, and VCAM-1, was increased and there was evidence of PMNL infiltration in the renal tissues. Although the generalized inflammatory state following myocardial infarction has been known for quite some time,10 the evidence for inflammatory state in the kidney has not been presented thus far.
We suggest that the alterations in renal function and morphology, including fibrosis, are a direct result of a systemic inflammatory state following significant myocardial ischemia. In keeping with our postulate, Fujiu et al.19 have recently reported the association of inflammation and fibrosis in the kidney.
Previous studies from our laboratory showed overexpression of LOX-1 in the myocardium following a brief period of ischemia.10,20 Acute ischemia is associated with activation of renin–angiotensin system and generation of large amounts of angiotensin II and expression of its type 1 receptor.18 Activation of angiotensin type 1 receptor activates NADPH oxidases in various tissues and circulating cells.21,22 The NADPH oxidase system is perhaps the most potent reactive oxygen species-generating system in vascular tissues. Reactive oxygen species are known to be potent pro-inflammatory and tissue injurious molecules.23 Other recent studies from our laboratory18 show that total and permanent LCA ligation similar to the one used in this study results in a sustained activation of LOX-1-NADPH oxidase-inflammation cascade in the myocardium. These observations suggest an important relationship between oxidant stress and inflammation. It is of note that reactive oxygen species per se can induce LOX-1 expression.24–26 There is also an important role for redox-sensitive transcription factors, such as nuclear factor-κB (NF-κB), in reactive oxygen species-mediated inflammation.25 Activation of NF-κB promotes the expression of pro-inflammatory cytokines, such as TNF-α and IL-6.
LOX-1 itself can serve as a pro-inflammatory adhesion molecule,27 and its expression and activation can lead to reactive oxygen species generation.27,28 In this study, we found that the levels of oxidation and inflammatory markers in the serum and the kidney were elevated in the WT mice after LCA occlusion, and the rise in levels of these markers was much less marked in the LOX-1 KO mice.
It is intriguing that LOX-1 expression increased in the kidneys of WT mice subjected to LCA ligation without direct injury to the kidney. The blood pressure fell soon after LCA ligation as a result of myocardial dysfunction. It is possible that the pattern of injury identified in this study is a reflection of ‘hemodynamic shock’ to the kidneys. However, the decline in blood pressure was only modest and similar in both WT and LOX-1 KO mice, suggesting that the decline in blood pressure is not the sole etiology of deterioration of renal function and extensive pathological alterations in the renal tissues. The pattern of renal dysfunction and injury improved over the 3 weeks following LCA ligation, indicated by an improvement in renal function and morphology. However, there was progressive fibrosis in renal tissues in the WT mice and deterioration in LV function at this time point.
It is likely that the interaction between oxidant stress, angiotensin II, and LOX-1 is responsible for growth and activation of fibroblasts and generation of collagen. Chen et al.29–31 showed that collagen formation by fibroblasts can be reduced by treatment with angiotensin II type 1 receptor blockers, peroxisome proliferator-activated receptor-γ ligands, and LOX-1 antibody. In the kidneys of mice with chronic LCA ligation, there was increased expression of TGF-β1, a potent inducer of fibrosis. Hu et al.32 showed a cross-talk between TGF-β1 and LOX-1 with a critical interactive role of reactive oxygen species, mitogen-activated protein kinases, and NF-κB in this cross-talk. In keeping with the hypothesis of a cross-talk between TGF-β1, reactive oxygen species, and LOX-1, there was much more TGF-β1 expression in the renal tissues of WT mice compared with LOX-1 KO mice, both subjected to total and permanent LCA ligation.
This study shows that following LCA ligation and extensive myocardial necrosis, there was intense collagen deposition in the kidney, presumably as a result of inflammation and oxidant stress resulting in fibroblast growth and activation. There was a significant alteration in the expression of NF-κB regulator IκBα. The alterations in renal morphology and function were much less in the kidneys of LOX-1 KO mice Day 3 as well as on Day 21 post-LCA occlusion, indicating a prominent role for LOX-1 in collagen synthesis and its deposition in the kidney after prolonged and extensive myocardial ischemia.
Kelly et al.33 identified LOX-1 expression in the proximal tubules in 32-week-old obese rats with uncontrolled diabetes and dyslipidemia. Dominguez et al.34 from the same laboratory showed that inhibition of LOX-1 with a binding antibody can effectively reverse critical pathogenic elements of nephropathy in the obese ZS rats with diabetes and dyslipidemia. Their study showed that blockade of LOX-1 limits renal lipotoxicity, inflammation, and peritubular fibrosis. In keeping with these findings, Hu et al.26 observed extensive areas of fibrosis in the kidney of WT mice subjected to angiotensin II infusion for 4 weeks; importantly, renal fibrosis and dysfunction was reduced by LOX-1 abrogation despite angiotensin II infusion. These investigators also indicated that the state of oxidant stress and inflammation is the basis of fibroblast growth and collagen accumulation in the kidney and deterioration of renal function.
In this study, we have shown a pathogenic role of LOX-1 in the determination of LV dysfunction in keeping with another study in a separate group of animals.18 LOX-1 abrogation attenuated systemic inflammation, reactive oxygen species generation, and IκBα activation in the kidney. Importantly, the LOX-1 KO mice showed less PMNL accumulation, glomerular/interstitial damage, and fibrosis in the kidney despite similar degree and duration of myocardial ischemia. It is possible that preserved renal function and morphology in these mice relates to improved cardiac function, but the limitation of inflammation and oxidant stress by LOX-1 deletion perhaps played a more significant role.
Important questions that arise from this study and will need to be addressed in future studies are: (1) Are there are other mediators of renal injury during myocardial infarction besides systemic inflammatory cytokine release and a ‘shock-like state’? In this context, expression of endothelin-1 in the kidney may be of interest as this cytokine is a potent vasoconstrictor and can reduce GFR, is NF-κB-dependent, and oxidant-inducible; (2) What is the fate and long-term functional consequences of renal fibrosis? (3) What is the appropriate therapy of acute renal injury that accompanies extensive myocardial injury?
In summary, we have presented a provocative observation of renal enlargement, extensive injury, and dysfunction following extensive myocardial infarction in mice. Since LOX-1 deletion reduced the markers of renal injury and dysfunction despite similar site of LCA occlusion, it is tempting to postulate that the state of generalized inflammation and oxidant stress following myocardial infarction affects the kidney that undergoes morphological and functional changes.
MATERIALS AND METHODS
Animal Protocol
C57BL/6 mice (also referred to as wild-type mice) were obtained from Jackson Laboratories (Bar Harbor, ME). The homozygous LOX-1 KO mice were developed and backcrossed eight times with C57BL/6 strain to replace the genetic background as described previously.35 C57BL/6 and homozygous LOX-1 KO (on C57BL/6 background) mice were bred by brother–sister mating and housed in a breeding colony. All experimental procedures were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee.
Genotyping
LOX-1 genotypes were verified by polymerase chain reaction analysis of genomic DNA extracted from the tail.
Details of the methodology have been published recently.18
Left coronary artery ligation and determination of left ventricular function
Animals were anesthetized, intubated, and mechanically ventilated. Body temperature was maintained between 37.0 °C and 37.5 °C with a heating pad. After equilibration period of 5 min, under aseptic conditions, left thoracotomy was performed in the 4th intercostal space and the pericardium opened. A 8-0 silk suture was passed around the proximal LCA and the LCA totally occluded permanently. This resulted in extensive infarct and LV dysfunction. Another group of animals underwent the same procedure, but no LCA ligation (sham ischemia). Additional details of the protocol are provided elsewhere.18 LV ejection fraction, wall thickness, and chamber dimensions were measured by echocardiography on Days 3 and 21 after LCA ligation, as described recently.18
Determination of blood pressure
Systolic blood pressure was measured through a tail-cuff apparatus (AD Instruments, Bella Vista, NSW, Australia) in conscious mice 3 days before and then on days 0, 3, and 21 after LCA ligation. Systolic blood pressure values were derived from an average of two cycles for 20 measurements per animal at each time point. Three preliminary training sessions were performed during 1 week before starting the experiment to ensure accurate measurements. In preliminary studies, the accuracy of tail cuff was established by comparing data obtained with biotelemetry system (Data Sciences, St Paul, MN).
Measurement of renal function
GFR in mild anesthetized mice was measured by fluorescein isothiocyanate-inulin clearance, as described previously,36 and expressed as microliters of fluorescein isothiocyanate-inulin cleared per minute. GFR values were corrected for kidney weight. Serum creatinine and BUN were determined using the diagnostic kit from International Bio-Analytical Industries (Boca Raton, FL).
Morphological and histological assessment
Hearts and kidneys were harvested and weighted. Heart weight is shown as the ratio of tibia length. Kidney weight is shown as the ratio of kidney weight to body weight (mg/g). Multiple renal longitudinal sections were stained with hematoxylin–eosin and Masson’s trichrome. Sections from the entire kidney were analyzed by an individual blinded to the study groups. PMNLs were counted in kidney sections stained with hematoxylin–eosin at original magnification × 600. Interstitial fibrosis was assessed in sections stained with Mason’s trichrome, and the area of interstitial fibrosis was quantified. At least four slices were examined in each kidney with six mice in each group.
Determination of oxidative stress
Lipid peroxidation was determined in the renal cortical tissue as thiobarbituric acid-reactive substances,37 and expressed as micromoles per gram wet weight. Malondialdehyde levels were expressed as mmol/l.38
Assessment of systemic inflammatory state by multiplex system
To assess the release of chemokines and cytokines in systemic circulation, the serum was obtained at 24, 48, and 72 h post-surgery. Chemokines and cytokines were measured by the Bio-Plex Protein Array System and a mouse cytokine 8-plex panel (Bio-Rad Laboratories, Hercules, CA), according to the manufacturer’s instructions. Concentrations of each cytokine were determined using Bio-Plex Manager software (Bio-Rad). The panel includes the following cytokines: IL-1β, IL-2, IL-4, IL-6, IL-10, granulocyte–macrophage colony-stimulating factor, interferon-γ, and TNF-α. The assay range was from 0.2 to 5000 pg/ml.
Western blot analysis
Inflammation-related molecules, LOX-1, IL-1β, and VCAM-1, were measured in the renal tissues by western blotting. Several collagen-related signals (collagen IV-α2 and procollagen-1) were also measured. IκBα and its phosphorylated form, as well as β-actin, were measured by western blotting. Primary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), Cell Signaling Technology (Danvers, MA) or Abcam (Cambridge, MA). Anti-LOX-1 antibody was obtained from Dr T Sawamura. The densities of protein bands were quantified by Quantity One Image Analyzer (Bio-Rad), and the band was normalized to that of β-actin.
Statistical analysis
Data are presented as mean ± s.d. Mice survival was analyzed by Kaplan–Meier analysis, and between-groups differences were tested by the log-rank test. Between-groups means were compared by one-way analysis of variance, followed by Student–Newman–Keuls test. A P < 0.05 was considered significant.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported in part by a grant 30801082 (to JL) from the National. Nature Science Foundation of China and Merit Review Award from the Department of Veterans Affairs (to JLM).
Footnotes
DISCLOSURE
All the authors declared no competing interests.
SUPPLEMENTARY MATERIAL
Figure S3. Summary data on the expression of IL-1β, VCAM-1, and TGF-β1 proteins (n = 4).
Supplementary material is linked to the online version of the paper at http://www.nature.com/ki
REFERENCES
- 1.Ronco C, Haapio M, House AA, et al. Cardiorenal syndrome. J Am Coll Cardiol. 2008;52:1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 2.Shlipak MG, Massie BM. The clinical challenge of cardiorenal syndrome. Circulation. 2004;110:1514–1517. doi: 10.1161/01.CIR.0000143547.55093.17. [DOI] [PubMed] [Google Scholar]
- 3.Martínez-Santos P, Vilacosta I. Cardiorenal syndrome: an unsolved clinical problem. Int J Nephrol. 2011:913029. doi: 10.4061/2011/913029. (E-pub ahead of print 29 May 2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gobal F, Deshmukh A, Shah S, et al. Triad of metabolic syndrome, chronic kidney disease, and coronary heart disease with a focus on microalbuminuria death by overeating. J Am Coll Cardiol. 2011;57:2303–2308. doi: 10.1016/j.jacc.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 5.van Kimmenade RR, Pinto Y, Januzzi JL., Jr When renal and cardiac insufficiencies intersect: is there a role for natriuretic peptide testing in the ‘cardio-renal syndrome’? Eur Heart J. 2007;28:2960–2961. doi: 10.1093/eurheartj/ehl399. [DOI] [PubMed] [Google Scholar]
- 6.Jose P, Skali H, Anavekar N, et al. Increase in creatinine and cardiovascular risk in patients with systolic dysfunction after myocardial infarction. J Am Soc Nephrol. 2006;17:2886–2891. doi: 10.1681/ASN.2006010063. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg A, Hammerman H, Petcherski S, et al. Inhospital and 1-year mortality of patients who develop worsening renal function following acute ST-elevation myocardial infarction. Am Heart J. 2005;150:330–337. doi: 10.1016/j.ahj.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 8.Berl T, Henrich W. Kidney-heart interactions: epidemiology, pathogenesis, and treatment. Clin J Am Soc Nephrol. 2006;1:8–18. doi: 10.2215/CJN.00730805. [DOI] [PubMed] [Google Scholar]
- 9.Tokuyama H, Kelly DJ, Zhang Y, et al. Macrophage infiltration and cellular proliferation in the non-ischemic kidney and heart following prolonged unilateral renal ischemia. Nephron Physiol. 2007;106:54–62. doi: 10.1159/000103910. [DOI] [PubMed] [Google Scholar]
- 10.Li D, Williams V, Liu L, et al. LOX-1 inhibition in myocardial ischemia–reperfusion injury: modulation of MMP-1 and inflammation. Am J Physiol Heart Circ Physiol. 2002;283:H1795–H1801. doi: 10.1152/ajpheart.00382.2002. [DOI] [PubMed] [Google Scholar]
- 11.Li D, Williams V, Liu L, et al. Expression of lectin-like oxidized low-density lipoprotein receptors during ischemia–reperfusion and its role in determination of apoptosis and left ventricular dysfunction. J Am Coll Cardiol. 2003;41:1048–1055. doi: 10.1016/s0735-1097(02)02966-2. [DOI] [PubMed] [Google Scholar]
- 12.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hasslacher J, Bijuklic K, Bertocchi C, et al. Levosimendan inhibits release of reactive oxygen species in polymorphonuclear leukocytes in vitro and in patients with acute heart failure and septic shock: a prospective observational study. Crit Care. 2011;15:R166. doi: 10.1186/cc10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li D, Saldeen T, Romeo F, et al. Oxidized LDL upregulates angiotensin II type 1 receptor expression in cultured human coronary artery endothelial cells: the potential role of transcription factor NF-kappaB. Circulation. 2000;102:1970–1976. doi: 10.1161/01.cir.102.16.1970. [DOI] [PubMed] [Google Scholar]
- 15.Cominacini L, Pasini AF, Garbin U, et al. Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-kappaB through an increased production of intracellular reactive oxygen species. J Biol Chem. 2000;275:12633–12638. doi: 10.1074/jbc.275.17.12633. [DOI] [PubMed] [Google Scholar]
- 16.Ishino S, Mukai T, Kuge Y, et al. Targeting of lectin like oxidized low-density lipoprotein receptor 1 (LOX-1) with 99mTc-labeled anti-LOX-1 antibody: potential agent for imaging of vulnerable plaque. J Nucl Med. 2008;49:1677–1685. doi: 10.2967/jnumed.107.049536. [DOI] [PubMed] [Google Scholar]
- 17.Hu C, Dandapat A, Chen J, et al. LOX-1 deletion alters signals of myocardial remodeling immediately after ischemia–reperfusion. Cardiovasc Res. 2007;76:292–302. doi: 10.1016/j.cardiores.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Lu J, Wang X, Wang W, et al. LOX-1 abrogation reduces cardiac hypertrophy and collagen accumulation following chronic ischemia in the mouse. Gene Ther. 2012;19:522–531. doi: 10.1038/gt.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujiu K, Manabe I, Nagai R. Renal collecting duct epithelial cells regulate inflammation in tubulointerstitial damage in mice. J Clin Invest. 2011;121:3425–3441. doi: 10.1172/JCI57582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu C, Chen J, Dandapat A, et al. LOX-1 abrogation reduces myocardial ischemia–reperfusion injury in mice. J Mol Cell Cardiol. 2008;44:76–83. doi: 10.1016/j.yjmcc.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Anilkumar N, Weber R, Zhang M, et al. Nox4 and nox2 NADPH oxidases mediate distinct cellular redox signaling response to agonist stimulation. Arterioscler Thromb Vasc Biol. 2008;28:1347–1354. doi: 10.1161/ATVBAHA.108.164277. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed A, Fujisawa T, Nui XL, et al. Angiopoietin-2 confers atheroprotection in apoE−/− mice by inhibiting LDL oxidation via nitric oxide. Circ Res. 2009;104:1333–1336. doi: 10.1161/CIRCRESAHA.109.196154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cominacini L, Pasini AF, Garbin U, et al. The platelet–endothelium interaction mediated by lectin-like oxidized low-density lipoprotein receptor-1 reduces the intracellular concentration of nitric oxide in endothelial cells. J Am Coll Cardiol. 2003;41:499–507. doi: 10.1016/s0735-1097(02)02811-5. [DOI] [PubMed] [Google Scholar]
- 24.Lu J, Mitra S, Wang X, et al. Oxidative stress and lectin-like ox-LDL-receptor LOX-1 in atherogenesis and tumorigenesis. Antioxid Redox Signal. 2011;15:2301–2333. doi: 10.1089/ars.2010.3792. [DOI] [PubMed] [Google Scholar]
- 25.Mehta JL. Oxidized or native low-density lipoprotein cholesterol: which is more important in atherogenesis? J Am Coll Cardiol. 2006;48:980–982. doi: 10.1016/j.jacc.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Hu C, Kang BY, Megyesi J, et al. Deletion of LOX-1 attenuates renal injury following angiotensin II infusion. Kidney Int. 2009;76:521–527. doi: 10.1038/ki.2009.234. [DOI] [PubMed] [Google Scholar]
- 27.Honjo M, Nakamura K, Yamashiro K, et al. Lectin-like oxidized LDL receptor-1 is a cell-adhesion molecule involved in endotoxin-induced inflammation. Proc Natl Acad Sci USA. 2003;100:1274–1279. doi: 10.1073/pnas.0337528100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doerries C, Grote K, Hilfiker-Kleiner D, et al. Critical role of the NAD(P)H oxidase subunit p47phox for left ventricular remodeling/dysfunction and survival after myocardial infarction. Circ Res. 2007;100:894–903. doi: 10.1161/01.RES.0000261657.76299.ff. [DOI] [PubMed] [Google Scholar]
- 29.Chen K, Chen J, Li D, et al. Angiotensin II regulation of collagen type I expression in cardiac fibroblasts: modulation by PPAR-gamma ligand pioglitazone. Hypertension. 2004;44:655–665. 661. doi: 10.1161/01.HYP.0000144400.49062.6b. [DOI] [PubMed] [Google Scholar]
- 30.Chen K, Chen J, Liu Y, et al. Adhesion molecule expression in fibroblasts: alteration in fibroblast biology after transfection with LOX-1 plasmids. Hypertension. 2005;46:622–627. doi: 10.1161/01.HYP.0000179045.95915.b0. [DOI] [PubMed] [Google Scholar]
- 31.Chen K, Li D, Zhang X, et al. Anoxia–reoxygenation stimulates collagen type-I and MMP-1 expression in cardiac fibroblasts: modulation by the PPAR-gamma ligand pioglitazone. J Cardiovasc Pharmacol. 2004;44:682–687. doi: 10.1097/00005344-200412000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Hu C, Dandapat A, Sun L, et al. Regulation of TGFbeta1-mediated collagen formation by LOX-1: studies based on forced overexpression of TGFbeta1 in wild-type and lox-1 knock-out mouse cardiac fibroblasts. J Biol Chem. 2008;283:10226–10231. doi: 10.1074/jbc.M708820200. [DOI] [PubMed] [Google Scholar]
- 33.Kelly KJ, Burford JL, Dominguez JH. Postischemic inflammatory syndrome: a critical mechanism of progression in diabetic nephropathy. Am J Physiol Renal Physiol. 2009;297:F923–F931. doi: 10.1152/ajprenal.00205.2009. [DOI] [PubMed] [Google Scholar]
- 34.Dominguez JH, Mehta JL, Li D, et al. Anti-LOX-1 therapy in rats with diabetes and dyslipidemia: ablation of renal vascular and epithelial manifestations. Am J Physiol Renal Physiol. 2008;294:F110–F119. doi: 10.1152/ajprenal.00013.2007. [DOI] [PubMed] [Google Scholar]
- 35.Mehta JL, Sanada N, Hu CP, et al. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ Res. 2007;100:1634–1642. doi: 10.1161/CIRCRESAHA.107.149724. [DOI] [PubMed] [Google Scholar]
- 36.Qi Z, Whitt I, Mehta A, et al. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol. 2004;286:F590–F596. doi: 10.1152/ajprenal.00324.2003. [DOI] [PubMed] [Google Scholar]
- 37.Jia Z, Aoyagi T, Yang T. mPGES-1 protects against DOCA-salt hypertension via inhibition of oxidative stress or stimulation of NO/cGMP. Hypertension. 2010;55:539–546. doi: 10.1161/HYPERTENSIONAHA.109.144840. [DOI] [PubMed] [Google Scholar]
- 38.Hu C, Dandapat A, Sun L, et al. Modulation of angiotensin II-mediated hypertension and cardiac remodeling by lectin-like oxidized low-density lipoprotein receptor-1 deletion. Hypertension. 2008;52:556–562. doi: 10.1161/HYPERTENSIONAHA.108.115287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.