Abstract
In this study, the effects of the wound‐covering materials, Acticoat® and Cutinova Hydro®, on wound healing have been studied in rabbit models with open and tissue‐lost wounds with full‐thickness flank excisions. Rabbits were used as subjects with three groups of four rabbits each, and trial periods of 7, 14 and 21{\uns}days. Four circular wounds, of 1.5 cm diameter were made two on the right (one of them control) and two on the left (one of them control) of the dorsal sides of the abdomen. Acticoat® and Cutinova Hydro® were applied on the wounds with suture for a period of 21 days and one each placed on the right and left sides as control with gauze. Biopsy specimens were taken from the animals at the end of the research period to check the length of the epithelium, epithelial thickness, size of wounds, wound granulation tissue formation and histopathological evaluation for clarity. The Acticoat® group showed better healing and scar formation compared to the Cutinova Hydro® group by macroscopic examination. Epithelial wound length and clarity in terms of statistical difference occurred on day 21 (P <0.05); while the length of the wound epithelium decreased patency, epithelial thickness on days~7, 14 and 21, showed no statistical differences (P >0.05). As a result, the Acticoat® wound dressing was determined as a more reliable for the early wound healing. This study has shown the short‐term clinical benefits of hydroactive, polyurethane dressings in the management of acute wounds. However, longer periods of wound healing procedure should be planned for reliable and safe results of wound dressing. It has also been concluded that microbiological analyses should be included for more robust and reliable comparisons.
Keywords: Acticoat®; Clinical‐pathological observation; Cutinova Hydro®; Wound healing
Introduction
The tissue‐lost wound is still an important issue in surgery in humans and animals. The biological processess of wound healing include cell regeneration, cell proliferation and collagen production. An understanding of the biology of wound healing is essential for a surgeon in order to minimise any adverse consequences. Therefore, new views of wound healing are being tried constantly.
The ideal wound dressing should maintain a moist environment, promote healing, enable gaseous exchange, protect from secondary infection, allow ongoing assessment, be comfortable, cause minimal pain to the patient, result in minimal scarring, be cost effective and enable removal without causing trauma 1, 2, 3, 4, 5.
Acticoat® is a relatively new form of silver antimicrobial barrier dressing that helps avoid the problems of earlier agents. It has rapid and sustained bactericidal activity against environmental organisms in partial‐ and full‐thickness wounds, which may reduce inflammation and promote healing 6, 7, 8. However, it has been reported to delay healing of skin graft donor site wounds compared to an alginate dressing (2). Acticoat® is useful as a burn wound dressing and can also be used on some fresh skin grafts as a post‐operative dressing and on other wounds 2, 7.
There are limited side effects to topical silver therapy; silver toxicity or argyrosis is uncommon and generally resolves with cessation of the therapy. The majority of the side effects of topical antimicrobial agents are associated with the comolecule sulphadiazine. Transient and self‐limiting leukopenia is found and the development of dermatitis and toxic epidermal necrolysis has also been reported (9).
Occlusive dressings (Cutinova Hydro®), which consist of a passive water vapour barrier, effectively create a moist wound healing environment. Interestingly, studies of occlusive dressings in the treatments of wounds at other sites have shown that this moist environment may offer benefits including increased reepithelialisation, dermal collagen and fibronectin synthesis and improved comfort and faster healing of donor site (10) or burn (11) or chronic wound (12) and reduced scarring (13) or chronic and acute wound incidence of infection (14) using hydrocolloid dressings. Moreover, it has been suggested that such a modulation of the wound healing response decreases scarring. Occlusive dressings have the ability to stimulate the release of platelet‐derived growth factor within the wounds. In this clinical setting, these dressings have no detrimental or indeed demonstrable effect on the bacterial microflora of the wound (15).
The goal of this study was to compare the healing effects of Acticoat® and Cutinova Hydro® on the epithelium and wound contraction with respect to protection, and healing and histopathological results.
Materials and methods
Materials
Twelve rabbits were used as materials. The study was approved by the ethical committee of the university. The rabbits were kept 10 days in a suitable cage before starting the experiments. They were divided into three groups. Each group (periods of 7, 14 and 21 days) had four rabbits. All the rabbits were anaesthetised with 10 mg/kg, IM Xylazine (Rompun® Bayer) and 50 mg/kg IM Ketamine hydrochloride (Alfamine® EgeVet). The operation area was shaved, cleaned and disinfected on the right and the left sides of the spine and four 1·5 cm diameter circular tissues‐lost wounds made, two on the right (one of them control for Acticoat® with gauze) and two on left flank (one of them control for Cutinova Hydro® with gauze). The wounds on each side were made at a 3 cm distance from each other so as not to affect other groups. Acticoat® and Cutinova Hydro® were applied on only one wound each on right and left sides. Wound dressing with suture was performed on the rabbits for a period of 21 days. Cutinova Hydro® was changed every 3 days because of the loss of its absorbent characteristics. The Acticoat® was also changed every 3 days. Analgesic drugs flunixin and meglumin (1·1 mg/kg) S.C. were applied to all rabbits to relieve pain during post‐operative period.
Histopathological analysis
Biopsy samples were taken from 1 cm wide and 2 cm length sites in all the animals by using regular anaesthesia on days 7, 14 and 21. Tissues were fixed in 10% formalin and then embedded in paraffin wax blocks. Sections of 5 µm thickness were stained with hematoxylin–eosin and Van Gieson (VG) for these blocks. All sections were evaluated under the light microscope. The length of the wound epithelium, epithelial thickness and the size of the wounds were evaluated by measuring the opening with an ocular micrometer.
Statistical analysis
Analysis of variance and Duncan tests were performed using the SPSS 10.0 program. Results were expressed as mean ± SD and P < 0·05 was considered as the acceptable probability of a type 1 error (incorrectly rejecting the nypothesis of no difference between groups).
Results
Clinical findings
The rabbits were kept in suitable cages during research period. A rabbit with a sterile abscess was detected in the Acticoat® group on the seventh day. Sterile abscesses were also evident on the 14th day, in three out of the four samples in the Cutinova Hydro® group and the granulation tissue in the wound area of the abscess was not fully healed. The wound healing in groups according to the day of distrubition by clinical and macroscopic results are shown in Figure 1.
Figure 1.
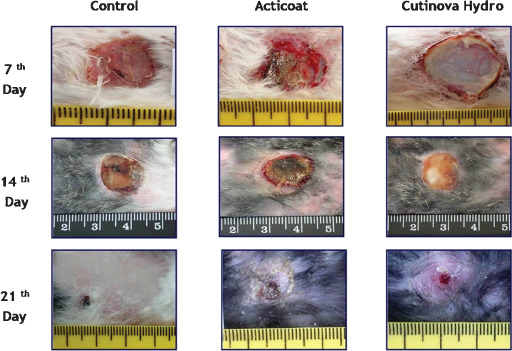
The wound healing in groups according to the day distribution by clinical and macroscopic results.
Histopathological findings
The distribution of histopathological findings for each group is given in Table 1.
Table 1.
Evaluation of histopathological findings in the groups *
| Groups | 7th Day | 14th Day | 21st Day | |
|---|---|---|---|---|
| The length of the epithelium | Control | 1294 ± 1355a,B | 2564 ± 593a,B | 6481 ± 2038a,A |
| Acticoat® | 913 ± 371a,B | 1731 ± 809a,B | 6556 ± 1888a,A | |
| Cutinova Hydro® | 870 ± 633a,B | 2569 ± 399a,B | 6188 ± 2625a,A | |
| Epithelial thickness | Control | 53 ± 37a,A | 106 ± 29a,A | 97 ± 32a,A |
| Acticoat® | 93 ± 53a,A | 101 ± 88a,A | 98 ± 16a,A | |
| Cutinova Hydro® | 54 ± 37a,A | 93 ± 43a,A | 88 ± 45a,A | |
| Size of wounds | Control | 10 688 ± 2904a,A | 7888 ± 3700a,A | 294 ± 587a,B |
| Acticoat® | 12 531 ± 3401a,A | 10 406 ± 4586a,A | 200 ± 400a,B | |
| Cutinova Hydro® | 11 656 ± 3902a,A | 9906 ± 1539a,A | 2656 ± 5312a,B |
*The same line for each parameter (A, B letters) and column (a, b letters) shows statistical differences. The length of the epithelium from the days (Acticoat®P < 0·01; Cutinova Hydro®P < 0·03; control P < 0·02), epithelial thickness (P > 0·05), size of wounds (Acticoat®P < 0·01, Cutinova Hydro®P < 0·022, the control P < 0·01).
Reepithelialisation began on the seventh day for each of the three groups and early wound granulation tissue began to fill the work area, but no difference was observed between the groups in terms of reepithelialisation and connective tissue formation (Figure 2). The thickness of the epithelium forming at the edges of the wound reepithelialisation for the Acticoat® group was observed to be higher than that in the Cutinova Hydro® and control groups. Abscess formation was found for one animal in Acticoat® group but there were no abscess in control and Cutinova Hydro® groups on seventh day. Re‐epithelialisation and granulation tissue formation in the control group and Acticoat® group were seen on the 14th day, the wound area was completely filled with granulation tissue in all samples; in the remaining sections under the newly formed epithelium were seen to be enriched collagen and fibrositis in granulation tissue (Figure 3).
Figure 2.

Histopathological changes on the 7th day. Reepithelialisation area (arrow) (scanning tissue section), collagen (c), fibroblast (f). Haematoxylin–eosin (H&E ×20 magnification), vG (×400 magnification).
Figure 3.

Histopathological changes on the 14th day. Reepithelialisation area (arrow) (scanning tissue section), collagen (c), fibroblast (f). Haematoxylin–eosin (H&E ×20 magnification), vG (×400 magnification).
A rabbit with abscess was detected in Acticoat® group on seventh day. It was also evident on the 14th day. Also in three of the four samples in the Cutinova Hydro® group, the granulation tissue in the wound area of the abscess forming in the rabbits was not fully healed. Formation of edges of the wound epithelium and the length of the reepithelialisation were observed in Acticoat® group when compared to Cutinova Hydro® group (Table 1). The thickness of the edges of the wound epithelium in the control group was found to be higher than that in the other groups. VG dyeing showed more intense collagen maturation in Acticoat® group and the control groups, whereas fibroblasts were pointed out more in Cutinova Hydro® group (Figure 3). One animal out of all groups did not have complete epithelialisation on 21st day, but formation and epithelial thickness of reepithelialisation was determined to be better in Acticoat® group than others. While collagen‐rich granulation tissue was observed in the control and Acticoat® groups by VG dyeing, fibroblasts were observed to be more intense and less collagen‐rich in the Cutinova Hydro® group (Figure 4).
Figure 4.

Histopathological changes on 21st day. Reepithelialisation area (arrow) (scanning tissue section), collagen (c), fibroblast (f). Haematoxylin–eosin (H&E ×20 magnification), vG (×400 magnification).
In addition, samples taken from Acticoat® group showed foreign bodies estimated to be the ruins of silver and giant cells on 14th and 21st days. Results were evaluated according to days, and the length of the epithelium increased steadily in all the groups, 21st day showed a statistical difference, which forms the size of the wound in parallel and 7, 14 and 21 day results showed a decrease and this situation is considered to be statistically significant; the epithelial thickness at 7, 14 and 21 days, and the absence of statistical difference was detected (Table 1).
Discussion
The reaction of the organism to the process of wound healing is to provide wound closure as soon as possible. In this process, it is the physician's duty to help the subject, to protect the wound against external influences and micro‐organisms, to facilitate epithelialisation and granulation tissue formation. For this purpose, a wide variety of wound dressing materials have been tried from past to present. In this study, the effect of Cutinova Hydro® to facilitate reepithelialisation with keeping the wound area moist and effect of Acticoat® to prevent infections with antimicrobial effect were investigated on the healing of wounds in rabbits with open and the tissue‐lost wounds.
Hydrocolloids is detained in the moist area around the wound surface and reepithelialisation was expended by keeping the environment by creating a barrier between the wounds and prevent infection 4, 15. Cutinova Hydro® increases growth hormone concentration of protein in wound epithelium and occurrence of acute facial wounds accelerates (16) and an average rate of moist wound healing dressings that provided an increase of 3–4 days have also been reported (17); the present study in terms of reepithelialisation of Cutinova Hydro® is behind 7 days in comparison with other two groups; the 14th day epithelium showed a significant increase in day length and was considered the best. The 21st day results in all three groups were similar for reepithelialisation.
Natural and experimental studies in animals and in human clinical reepithelialisation showed rapid formation for the early periods. In humans, the studies reported in the absence of difference compared to control in terms of wound infection 4, 15 in the treatment of a patient with vasculitic ulcers as a barrier to bacteria emphasised the importance of Cutinova Hydro®(16). The use of hydrocolloids on the infected wounds increases infection. Therefore, for severe necrotic and infected wounds this dressing should not be used; it should be used for light and medium‐grade serous wounds (18). In the study, 14th day of Cutinova Hydro® group, three out of four samples show that Cutinova Hydro® was not considered successful in protecting against early wounds. But the 21st day results in the three groups (except for one animal) showed healing of wounds; the one animal that was not healed could be because of wounds that might be infected during dressing or application before or after with Cutinova Hydro® on 14th day. Histopathological examination shows that 7th and 14th days are of less collagen density than that of fibroblast. Granulation tissue was not completed because of infection in Cutinova Hydro® group.
Silver nanocystalline‐containing (Acticoat®) silver preparations due to the antibacterial effect of wound healing have enhanced the formation of granulation tissue by preventing infections (19). Metalloproteinase levels and decreased number of inflammatory cells were seen in swine models in Acticoat®. By increasing apoptosis of inflammatory cells, wound healing on the 7th day has been reported to enhance growth of granulation tissue, and contamination is rarely seen (20). In the study, the length of the epithelium on the 7th day has proven to be better when compared with Cutinova Hydro®. Demling and DeSanti (6) early in wet‐surface wounds, according to the standard antibiotic solutions, expressed reepithelialisation to have 40% increase in Acticoat® group. The 14th day rate of reepithelialisation was far behind other groups, in this situation as reported by Innes et al. (2001), in the drying zone, due to Acticoat® and reepithelialisation was interpreted as caused by the delay. But 21st day Acticoat® group is the best group in terms of reepithelialisation. In addition, on 14th day three animals had an infection in the Cutinova Hydro® group. Acticoat® group takes same day of infection only the formation of an animal infection, the idea that to be antimicrobial effective of Acticoat®(19) has supported.
This observation is consistent with earlier clinical observations which suggested that initiation of fibroplasia in the silver‐treated wounds preceded that in the control wounds which has also been supported (20). It has been shown that fibroblasts were noted in the wound bed of the silver‐treated wounds by 24 hours; this suggests that fibroplasia began approximately at 48–72 hours. In addition, the extent of inflammation at the wound site was also decreased in the silver‐treated wounds (20). A topical pure silver delivery system (Acticoat®) provides the opportunity to more scientifically study the effect of silver on reepithelialisation in burn patients. The silver is delivered to the wound surface in a moist healing environment, which is recognised to be the optimum environment for all phases of healing. The effect of silver ions delivered to a wound in a moist environment versus the use of a standard topical antibiotic solution on the rate of reepithelialisation across meshed skin graft placed on an excised burn wound is studied. The importance of moisture for reepithelialisation is well recognised. The objective was to determine, after early excision, whether silver ions increased the rate of reepithelialisation, unrelated to its antimicrobial activity effect. Topical silver has been reported to increase wound healing unrelated to its antibacterial properties (6). Acticoat® has a useful role as a burn wound dressing and can be continued to use on burn wounds as well as on some fresh skin grafts as a post‐operative dressing and on other wounds 2, 7.
On checking the condition and improvement in the macroscopic study (Figure 1), the wounds look much better in Acticoat® group when compared with Cutinova Hydro® group, and the scar formation is significantly evident in Acticoat® group. As suggested by some authors 17, 21, 22, occlusive dressings reduce wound pain and scar formation. Silver policrystal of size 10–15 nm prompts wound healing and reduced scar formation has also been reported (23).
The Acticoat® wound dressing was determined superior considering that they have the ability to protect and promote healing during early period of wound healing. This study has also shown the short‐term clinical benefits of hydroactive, polyurethane dressings in the management of acute wounds. But, longer periods of wound healing procedure should be planned for reliable and safe results of wound dressing. It has also been concluded that microbiological analyses should include more robust and reliable comparisons.
References
- 1. Arıcan M, Ozturk A. A clinical study using collagenase enzyme in the treatment of open or infected wounds. Eurasian J Vet Sci 1999;15:153–8. [Google Scholar]
- 2. Innes ME, Umraw N, Fish JS, Gomez M, Cartotto RC. The use of silver coated dressings on donor site wounds: a prospective, controlled matched pair study. Burns 2001;27:621–7. [DOI] [PubMed] [Google Scholar]
- 3. Ogurtan Z, Hatipoglu F, Ceylan C. The effect of Alkanna tinctoria Tausch on burn wound healing in rabbits. Dtsch Tierarztl Wochenschr 2002;109:481–5. [PubMed] [Google Scholar]
- 4. Wynne R, Holsworth L, Flavell O. Effect of three wound dressings on infection, healing comfort, and cost in patients with sternotomy wounds. Chest 2004;125:43–9. [DOI] [PubMed] [Google Scholar]
- 5. Yavru N, Koc Y, Arıcan M, Doğruer Y. The effect of granulated sugar application on healing in the treatment of infected wounds and abscesses. Eurasian J Vet Sci 1992;8:64–8. [Google Scholar]
- 6. Demling RH, DeSanti MDL. The rate of re‐epithelialization across meshed skin grafts is increased with exposure to silver. Burns 2002;28:264–6. [DOI] [PubMed] [Google Scholar]
- 7. Dunn K, Jones VE. The role of Acticoat with nanocrystalline silver in the management of burns. Burns 2004;30Suppl:1S–9S. [DOI] [PubMed] [Google Scholar]
- 8. Holder IA, Durkee P, Supp AP, Boyce ST. Assessment of a silver‐coated barrier dressing for potential use with skin grafts on excised burns. Burns 2003;29:445–8. [DOI] [PubMed] [Google Scholar]
- 9. Fraser JF, Bodman J, Sturgess R, Faoagali J, Kimble RM. An in vitro study of the anti‐microbial efficacy of a 1% silver sulphadiazine and 0.2% chlorhexidine digluconate cream 1% silver sulphadiazine cream and a silver coated dressing. Burns 2003;30:1–7. [DOI] [PubMed] [Google Scholar]
- 10. Wiechula R. The use of moist wound‐healing dressings in the management of split‐thickness skin graft donor sites: a systematic review. Int J Nurs Pract 2003;9:S9–S17. [DOI] [PubMed] [Google Scholar]
- 11. Wyatt D, McGowan DN, Najarian MP. Comparison of a hydrocolloid dressing and silver sulfadiazine cream in the outpatient management of second‐degree burns. J Trauma 1990;30:857–65. [DOI] [PubMed] [Google Scholar]
- 12. Kerstein MD, Gemmen E, van Rijswijk L, Lyder CH, Phillips T, Xakellis G, Golden K, Harrington C. Cost and cost effectiveness of venous and pressure ulcer protocols of care. Dis Manag Health Outcomes 2001;9:651–63. [Google Scholar]
- 13. Hein NT, Prawer SE, Katz HI. Facilitated wound healing using transparent film dressing following Mohs micrographic surgery. Arch Dermatol 1988;124:903–6. [PubMed] [Google Scholar]
- 14. Hutchinson JJ, Lawrence JC. Wound infection under occlusive dressings. J Hosp Infect 1991;17:83–94. [DOI] [PubMed] [Google Scholar]
- 15. Thomas DW, Hill CM, Lewis MAO, Stephens P, Walker R, Weth AVD. Randomized clinical trial of the effect of semi‐occlusive dressings on the microflora and clinical outcome of acute facial wounds. Wound Rep Reg 2000;8:258–63. [DOI] [PubMed] [Google Scholar]
- 16. Paggi B, Campton GA, Orsted H, Teot L, Ockenfels HM. Growth factors and interactive dressing in wound repair. EWMA J 2002;2: 17–23. [Google Scholar]
- 17. Gunes UY, Eser I. Nemli yara iyilesmesi ve oklusif pansumanların nemli yara iyilesmesindeki önemi. CU Hemsirelik Yuksekokulu Dergisi 2006;10:57–65. [Google Scholar]
- 18. Seyhan T, Ertas NM. New dressing materials and their usage in plastic surgery clinics. Mediforum 2006;1–3:1–5. [Google Scholar]
- 19. Thomas S. Surgical Materials Testing Lab. SMTL Dressings DataCard: “Acticoat 7”. http://www.dressings.org/Dressings/acticoat‐7.html (accessed on 30 January 2004).
- 20. Wright JB, Lam K, Buret AG, Olson ME, Burrell RE. Early healing events in a porcine model of contaminated wounds: effects of nanocrystalline silver on matrix metalloproteinases, cell apoptosis and healing. Wound Rep Reg 2002;10:141–51. [DOI] [PubMed] [Google Scholar]
- 21. Baranoski S. Wound dressings: a myriad of challenging decisions. Home Healthc Nurse 2005;23:307–17. [DOI] [PubMed] [Google Scholar]
- 22. McIsaac C. Managing wound care outcomes. Ostomy Wound Manage 2005;51:54–6. [PubMed] [Google Scholar]
- 23. Burrell RE, Nadworny P. Nanocrystalline silver: novel structure and activity. In: Proceedings of the 3rd International Conference on the Development of BME in Vietnam, Vol. 27; 2010; Ho Chi Minh City, Vietnam, 2010:6–9.


