Abstract
As a common feature of many neurological diseases and injury, the loss of axon pathways can have devastating effects on function. Here, we demonstrate a new strategy to restore damaged axon pathways using transplantable miniature constructs consisting of living neurons and axonal tracts internalized within hydrogel tubes. These hydrogel microconduits were developed through an iterative process to support neuronal survival and directed axon growth. The design included hollow agarose tubes providing a relatively stiff outer casing to direct constrained unidirectional outgrowth of axons through a central soft collagen matrix, with overall dimensions of 250 μm inner diameter ×500 μm outer diameter and extending up to several centimeters. The outer casing was also designed to provide structural support of neuronal/axonal cultures during transplantation of the construct. Using neuron culture conditions optimized for the microconduits, dissociated dorsal root ganglia neurons were seeded in the collagen at one end of the conduits. Over the following week, high-resolution confocal microscopy demonstrated that the neurons survived and the somata remained in a tight cluster at the original seeding site. In addition, robust outgrowth of axons from the neurons was found, with axon fascicles constrained in a longitudinal projection along the internal collagen canal and extending over 5 mm in length. Notably, this general geometry recapitulates the anatomy of axon tracts. As such, these constructs may be useful to repair damaged axon projections by providing a transplantable bridge of living axons. Moreover, the small size of the construct permits follow-on studies of minimally invasive transplantation into potentially sensitive regions of the nervous system.
Introduction
Disconnection of axon pathways is a common feature of many central nervous system (CNS) disorders, including neurodegenerative diseases and traumatic injury.1–5 However, functional regeneration rarely occurs in the CNS due to inhibition of axon growth and an absence of directed guidance (for review see Refs.6,7). Cell-based therapies targeting damaged axonal pathways have shown promise on several fronts, including release of neuroprotective factors, local release of neurotransmitters at distal targets of lost pathways, and to provide myelinating cells to facilitate remyelination of denuded axons.8–10 Nonetheless, these cell-replacement strategies are not designed to directly restore long-distance axon pathways.
To promote axon regeneration, strategies are being pursued that enhance the intrinsic ability of axons to regenerate11–13 and modify extrinsic factors to create an environment permissive for axonal outgrowth.14–17 To compliment these efforts, there has been substantial interest in developing transplantable “scaffolds” to directly facilitate axon regeneration. Typically, mechanisms of axonal pathfinding and guidance are investigated in vitro, with neurite outgrowth investigated across planar surfaces18–25 and more complex three-dimensional (3D) environments.26–30 Moreover, many scaffold-based strategies are being developed to promote and guide axonal regeneration following CNS injury in vivo.31–33 While directed axon growth has been demonstrated with these techniques, the number and length of axons growing along scaffolds has been limited. Further, scaffold-based approaches do not address the need to repair axon pathways where source neuronal somata are also lost, such as in nigro-striatal pathway degeneration in Parkinson's disease.
Recently, we have engineered transplantable nervous tissue constructs comprised of stretch grown axon fascicles spanning two populations of neurons to bridge extensive regions of nervous tissue damage.34–38 While promising, the large size of these constructs is not ideal for transplantation into discrete regions of the nervous system.
To address these technology gaps, we have combined cell-based therapy and scaffold approaches to create transplantable micron-scale tubular conduits containing unidirectional living axonal tracts in a preformed architecture (Fig. 1). Design criteria for microconduits included (1) a suitable microenvironment to support robust neural survival and axonal extension; (2) neuronal somata restricted to a single region with unidirectional axonal projections extending several millimeters, and (3) tubular constructs with a small diameter (≤500 μm) and sufficient rigidity to provide support and permit minimally invasive delivery in vivo. Comprised of an agarose exterior and a bioactive collagenous matrix interior, neuronal survival and unidirectional axonal outgrowth were optimized to permit axon extension over 5 mm in vitro. This design permits transplantation of the living microconduits into the CNS to repair lost axonal pathways.
FIG. 1.
Concept: three-dimensional (3D) microconduits with uniaxial axonal tracts. Our objective was to optimize 3D microconduits to promote neuronal survival at one end while facilitating unidirectional axonal extension through the conduit interior (A). Microscale tubular guidance channels were generated using an agarose exterior and a bioactive matrix interior (B). These agarose-collagen microconduits simultaneously provide comparatively rigid structural support (via agarose) and bioactive ligands (via collagen) to encourage neuronal survival, somata localization, and longitudinal neuritic extension in a 3D microenvironment. The size and geometry of these microconduits permit minimally invasive injection into neural tissue for targeted replacement of axonal tracts. Color images available online at www.liebertpub.com/tea
Materials and Methods
Three-dimensional microconduit fabrication
All components were from Invitrogen (Carlsbad, CA) or BD Biosciences (San Jose, CA) unless noted. Microconduits consisted of an agarose-collagen hydrogel molded into a cylindrical, pipe-like structure through which axon growth could occur (Fig. 1). The outer hydrogel structure was comprised of 1% agarose (Sigma-Aldrich, St. Louis, MO) in Dulbecco's phosphate-buffered saline (DPBS). The agarose solution was heated until liquid (maintained at 55°C) and drawn into microliter glass capillary tubes (Drummond Scientific, Broomall, PA) via capillary action to generate a cylinder with an outer diameter of 990 or 500 μm. Next, an inner column was created by positioning an acupuncture needle (diameter: 250 μm) (Seirin, Weymouth, MA) in the center of the liquid agarose-filled capillary tube. After cooling to room temperature, the capillary tubes were placed into a liquid collagenous solution (3.0 mg/mL, rat tail type I) and the central needle was retracted creating a negative pressure gradient that drew the collagen into a central column (i.e., clear of agarose) created by the evacuating needle. The cured microconduits, now consisting of an agarose tubular shell with a collagenous core, were gently pushed out of the capillary tubes and placed in DPBS where they were cut to 5–20 mm in length and sterilized under UV light (1 h). Microconduits were then either incubated at 37°C for a minimum of 30 min prior to neuronal addition or neurons were plated immediately prior to incubation (see cell plating protocol below).
The agarose-collagen architecture of the microconduit was assessed by premixing collagen and agarose with different fluorophores and imaging performed using confocal microscopy. Specifically, agarose was premixed with Alexa Fluor 546 (excitation: 556 nm/emission: 573 nm) and collagen solution with Lucifer Yellow (LY; ex: 425 nm/em: 528 nm).
Neuronal cell culture
All procedures involving animals were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania and followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23; revised 1996). Dorsal root ganglia (DRG) were isolated from embryonic day 15 Sprague-Dawley rats (Charles River, Wilmington, MA). Briefly, timed-pregnant rats were euthanized using carbon dioxide. The uterus was removed by Caesarian section, and each fetus was removed from the amniotic sac and placed in cold Leibovitz-15 medium. The spinal cords were removed and individual DRG were isolated using fine forceps. To dissociate the tissue, DRG explants were exposed to prewarmed trypsin (0.25%)+EDTA (1 mM) and were placed at 37°C for 1 h. Following the addition of neurobasal medium+5% fetal bovine serum (FBS), the tissue was triturated and then centrifuged at 1000 rpm for 5 min. The supernatant was aspirated, and the cells were resuspended at 5×106 cells/mL in neurobasal medium+2% B-27+500 μM L-glutamine+1% penicillin/streptomycin+1% FBS+2 mg/mL glucose (Sigma-Aldrich)+10 ng/mL 2.5S nerve growth factor+10 mM FdU (Sigma-Aldrich) and 10 mM uridine (Sigma-Aldrich). Using a micropipette, ∼2–10 μL of cell solution was precisely delivered to one end of the microconduits. Also using a micropipette, a gentle negative pressure gradient was applied to the other end in some cases. The cultures were placed in a humidified tissue culture incubator (37°C and 5% CO2) for 3 h to allow cells to attach, after which, media was added to the culture vessel. The culture media was changed every 2–3 days in vitro (DIV) by replacement with fresh prewarmed media. All outcome measures were assessed at 6–7 DIV.
Cell viability assay, immunocytochemistry, and confocal microscopy
Living and dead/dying cells were labeled using calcein AM and ethidium homodimer-1 (EthD-1), respectively. Microconduits were rinsed in buffer and incubated with 2 μM calcein AM and 4 μM EthD-1 at 37°C for 30 min and rinsed in PBS. Calcein AM crosses the plasma membrane of all cells and the AM portion of the molecule is cleaved inside metabolically active cells, leaving the calcein to fluoresce bright green (ex: 494 nm/em: 517 nm). EthD-1 only enters cells with a compromised plasma membrane and then irreversibly binds DNA causing the nuclei to fluoresce red (ex: 528 nm/em: 617 nm).
For immunocytochemistry, microconduits were fixed in 3.7% formaldehyde for 30 min, rinsed in PBS, and permeabilized using 0.3% Triton X100 plus 4% goat or horse serum for 20 min. Primary antibodies were added (in PBS+4% serum) at 37°C for 4 h. The primary antibodies were the following neuronal markers: (1) MAP-2 (SMI-52R, 1:1000, Covance, Princeton, NJ), a microtubule-associated protein primarily in neuronal somata and dendrites, (2) tau (A0024, 1:400; Dako, Carpinteria, CA), a microtubule-associated protein primarily expressed in axons, (3) β-tubulin III (T8578, 1:500, Sigma-Aldrich), a microtubule element primarily expressed in neurons, and (4) GFAP (ab53554, 1:50; Abcam, Cambridge, MA), an intermediate filament-associated protein expressed in glial cells. After rinsing, Alexa 488 goat anti-mouse IgG, Alexa 546 goat anti-rabbit, Alexa 594 donkey anti-goat, and/or Alexa 488 donkey anti-mouse IgG secondary antibodies (1:500 in PBS+4% serum) were added at 18–24°C for 2 h.
Microconduits were fluorescently imaged using a laser scanning confocal microscope (BioRad Radiance 2000-MP on an Eclipse TE300; Nikon, Melville, NY or an LSM 710 on an Axio Observer Z1; Zeiss, Oberkochen, Germany). For each microconduit, multiple confocal z-stacks were digitally captured and analyzed. All confocal reconstructions were from full thickness z-stacks (≥250 μm when imaging cells within the inner diameter; ≥500–1000 μm when imaging externalized clusters of neuronal somata).
Results
Verification of 3D microconduit architecture
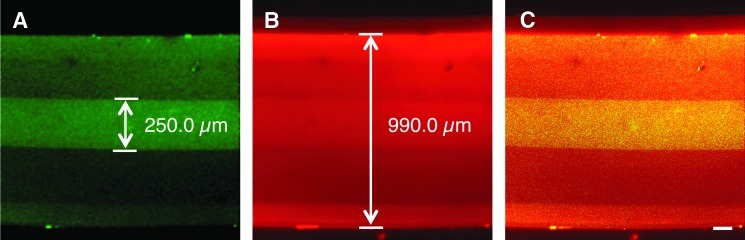
The desired architecture of the microconduits, designed to consist of an agarose tubular shell and a collagenous inner column, was verified using confocal microscopy with different fluorophores separately mixed with the agarose and collagen. Specifically, collagen was premixed with LY and the agarose was premixed with Alexa-546. Confocal microscopy revealed a hyper-intense LY signal in the center of the conduits with Alexa-546 labeling the entire construct (Fig. 2). The relevant dimensions of the microconduit including outer and inner diameters matched expected values. In particular, the collagenous core measured 250 μm, matching the diameter of the needle used to make this inner column (Fig. 2).
FIG. 2.
Verification of agarose-collagen architecture in 3D microconduits. Confocal reconstruction of a representative fluorescently labeled microconduit with collagenous core. To image the location of collagen within the agarose tubes, collagen was mixed with Lucifer Yellow (LY; green) prior to being drawn into agarose conduits that were premixed with Alexa-546 (red). Confocal microscopy revealed collagen in the center of the conduits based on hyper-intense LY signal (A). Although the Alexa-546 diffused into the collagen core during the imaging period, the borders of the agarose tubes were sufficiently denoted by the Alexa-546 signal (B) with overlay (C). The microconduit outer diameter (Alexa-546+) measured 990 μm and the collagenous core (LY+) measured 250 μm. Scale bar: 100 μm. Color images available online at www.liebertpub.com/tea
Neuronal penetration and survival in 3D microconduits
Neurons from DRG were plated at one end of the microconduits and maintained for 1 week in culture. In initial studies, neurons were added to the end of the microconduits immediately following the addition of collagen to the central core. A cell viability assay revealed surviving neuronal clusters at the extreme of the microconduit with a paucity of dead cells (Fig. 3). Interestingly, using this methodology, the neuronal somata did not maintain position at the microconduit extreme, but rather appeared to migrate into the inner diameter along the agarose wall. Neuritic outgrowth was apparent connecting individual somata; however, this growth was multidirectional reflecting the neuronal somatic distribution. Overall, these observations revealed that the microconduits provided a permissive environment for neuronal survival and neuritic extension. However, this microconduit iteration did not satisfy our design criteria of enabling neuronal survival at one end while facilitating unidirectional axonal extension through the conduit interior.
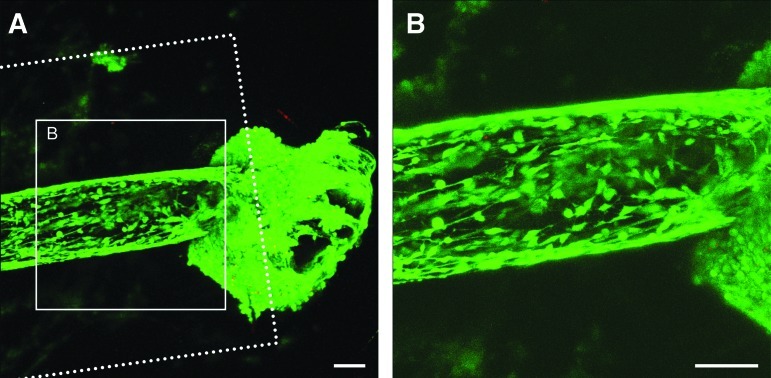
FIG. 3.
Neuronal growth and penetration within 3D microconduits. Confocal reconstructions of neurons adjacent to and within microconduits stained to denote live cells (green) and the nuclei of dead cells (red) at 6 days in vitro (DIV). Initial studies utilized larger diameter microconduits: 990 μm OD (denoted by dashed lines) with a 250 μm ID (denoted by cellular distribution). Live neurons were observed lining the interior of the microconduits (A). Robust neuronal survival was observed within the microconduits. These neurons appeared to migrate from a large cluster of cells located immediately external to the longitudinal end of the microconduit (B). Scale bars: 100 μm. Color images available online at www.liebertpub.com/tea
Neurite penetration into 3D microconduits
To achieve our design objectives, we modified the collagenous matrix and the cell density to improve neuronal position and axonal outgrowth. In particular, we preincubated the microconduits following collagen addition, which separated in time the matrix gelation from cell addition, which previously happened contemporaneously. This resulted in the majority of neuronal somata being restricted to the 3D microconduit exterior and a lower cell density within the inner diameter. Neuronal survival was very high, with modest cell death both within and outside the microconduits. Moreover, long neurites projected through the interior of the microconduits, with neurite penetration into the construct over 5 mm in some cases. This iteration resulted in robust neuronal survival at the construct extreme, a low cell density within conduits, and enhanced neuritic extension through the microconduits.
Neuronal polarity and axonal presence in 3D microconduits
A key consideration in satisfying our design criteria was the establishment of distinct neuronal somatic and axonal regions within the 3D microconduits. Therefore, we optimized the cell delivery methodology to provide a microliter scale volume directly within the inner diameter of the microconduits. Following this delivery, we sought to verify the presence of neuronal somata at one end with axonal tracts projecting through the interior of the microconduits. Accordingly, we utilized immunocytochemistry with antibodies for MAP-2 and tau to label neuronal somata/dendrites and axons, respectively, in conjunction with high-resolution confocal microscopy. This analysis revealed a dense cluster of neuronal somata located at one end of the 3D microconduits (Fig. 4). These neurons would typically coalesce into a 3D ganglion with tear-drop morphology within the microconduits. In addition, axonal projections were observed extending longitudinally across the inner diameter of the microconduits (Fig. 4). Interestingly, the structure and location of these axons changed profoundly based on distance from the neuronal ganglion. For instance, projecting from a ganglion, a large tightly coalesced bundle of axons (fascicle) was sometimes observed, suggesting axons departed in the center (collagenous) portion of the conduits. Typically within 1–2 mm, the axons from a dense fascicle had branched to run longitudinally at the agarose-collagen border. This growth of axons along the interior walls of the agarose microconduits—at the agarose-collagen border—was readily observed by 3D confocal microscopy (Fig. 5). As growth occurred longitudinally, increased axonal branching with the formation of axonal spines in the leading segment was observed (Fig. 5). This analysis revealed that we had satisfied our design criteria, thereby creating 3D microconduits encapsulating healthy neuronal somata largely restricted to a dense ganglion at the microconduit extreme, whereas the deep interior was composed almost exclusively of long axons projecting several millimeters.
FIG. 4.
Neuronal polarity and axonal presence in 3D microconduits. Confocal reconstructions of neuronal constructs stained via immunocytochemistry to denote neuronal somata/dendrites (MAP-2; green) and axons (tau; red) at 6 DIV. A dense cluster of neuronal somata was located at one end with axonal projections extending longitudinally across the microconduit (A–C) (scale bar: 250 μm). Neuronal somata were restricted to a dense ganglion at the microconduit extreme, whereas the interior was composed exclusively of long axons projecting several millimeters (D, E) (scale bar: 50 μm). Color images available online at www.liebertpub.com/tea
FIG. 5.
Axonal distribution, branching, and structure in 3D microconduits. Higher magnification confocal reconstructions from demonstrative regions in figure 4. A large tightly coalesced bundle of axons (fascicle) projecting from a ganglion, suggesting axons departed ganglia in the center (collagenous) portion of the conduits (A). As growth occurred longitudinally, increased axonal branching (B) with the formation of axonal spines (C) in the leading segment was observed. Scale bar (A–C) 20 μm. Axonal spines (D, E) denoted by white arrows (scale bar: 10 μm). Confocal reconstructions from specific levels of the z-stacks were useful to determine the spatial location of extended axons. Schematic of imaging direction, sub-fields from the full-thickness z-stack, with hypothetical representative axons shown as green circles in cross section (F). This analysis methodology revealed that, following axonal branching, extension occurred at the agarose-collagen border, potentially exploiting the duel benefits of agarose stiffness and collagen presence (G–I). Scale bar (G–I) 20 μm. Color images available online at www.liebertpub.com/tea
Neuronal versus glial penetration into the microconduits
To eliminate the possibility that the projections into the microconduit were a result of glial rather than axonal outgrowth, staining was used to differentiate between glial and neuronal processes. Accordingly, we utilized immunocytochemistry with antibodies for β-tubulin III and GFAP to label neuronal somata/neurites and glial somata/processes, respectively, in conjunction with high-resolution confocal microscopy. This analysis revealed that in addition to axons, glial processes were present within the microconduit interior (Fig. 6). In particular, glial processes penetrated ∼1 mm into the microconduits. High-magnification confocal reconstructions demonstrated that while neuronal and glial processes exhibited colocalized outgrowth initially, purely axonal projections extended deep into the microconduits. There was no observed glial presence at the end of the axonal projections (Fig. 6). Similar results were obtained when labeling for tau or neurofilament with GFAP (data not shown).
FIG. 6.
Neuronal versus glial penetration into the microconduits. Confocal reconstructions of neuronal constructs stained via immunocytochemistry to denote neuronal somata/axons (β-tubulin III; green), cell nuclei (Hoechst; blue), and glial somata/processes (GFAP; red) at 7 DIV. Axons extended unidirectionally into the microconduit interior (A), while both neuronal and glial somata remained in a dense cluster at the end of the construct (B). Glial processes extended ∼1 mm into the microconduit interior, and were primarily colocalized with axons over this span (C, D). Scale bar (A–D) 100 μm. Higher magnification confocal reconstructions from demonstrative regions in (D) show that although glial processes were present in the initial region of the microconduit, purely axonal projections extended deep into the microconduit interior (E–G). Scale bars (E, G) 50 μm. Scale bar (F) 40 μm. Higher magnification confocal reconstructions from regions in (E) and (G) show that while there was no glial presence at the leading extremities of the axonal projections (H), glial and axonal processes initially grew in tandem, often intertwining (I). Scale bar (H, I) 40 μm. Color images available online at www.liebertpub.com/tea
Discussion
Here, microtissue engineered conduits were created composed of tubular hydrogel constructs containing living axonal tracts extending for several millimeters in vitro. This is the first demonstration of robust survival and long-distance unidirectional axonal extension achieved using relatively low cell density neuronal cultures (limited to hundreds to thousands of neurons) within a 3D self-contained microenvironment. As such, microconduit encased axons are potentially transplantable in vivo with the aim of reconnecting neuronal populations lost through disease or injury.
In the CNS, targeted reconnection of neurons via axon regeneration is a formidable challenge due to the long distances the axons would have to extend to reach suitable targets in an environment that is highly nonpermissive for axon growth. Moreover, few cell-based strategies for CNS repair have addressed recapitulating the lost neuroanatomy of axon tracts spanning populations of neurons, particularly in remote regions of the nervous system. The present microconduit strategy provides engineered neural tissue in a preformed 3D architecture that can be tailored to a size to span specific lost axonal pathways. Further, these microconduits provide a new investigative platform for the evaluation of 3D axonal path finding in vitro.
The microconduits were designed to comprise a population of neuronal somata at one end, with long unidirectional axon-only tracts projecting longitudinally through the interior column. Moreover, the physical parameters were intended to provide sufficient structural support while being of suitable size to potentially permit minimally invasive in vivo delivery. For these design criteria, we used a stepwise iterative process to modify matrix properties (e.g., collagen gelation prior to or concurrent with cell addition) and cell factors (density and delivery methodology) to optimize the structural and geometric properties of the constructs. The resultant agarose-collagen microconduits simultaneously provide relatively rigid structural support (via agarose) and bioactive ligands (via collagen) to encourage neuronal survival, somatic localization, and longitudinal neuritic extension in a 3D microenvironment. Moreover, the final size of the microconduits (500 μm outer diameter) is suitable for surgical implantation using a 20-gauge needle.
Following the introduction of cells to the conduits, in some cases axonal outgrowth initially projected from the ganglion at one end as a single dense fascicle centrally within the collagenous matrix. However, as growth progressed through the length of the conduit, this fascicle separated into individual axons, typically within 1–2 mm. In addition, axons relocated from the central channel to grow along the inner agarose-collagen border, potentially to exploit both the stiffness of the agarose and the presence of ligands within the collagen. Alternatively, preferential growth along the agarose-collagen border may occur due to intrinsic programming that promotes multidirectional outgrowth, increasing the likelihood that axons locate synaptic partners. Such intrinsic outgrowth would be physically constrained by the agarose barrier, forcing growth to occur along its surface. Notably, leading axonal segments several millimeters from the neuronal somata had a high density of axonal spines, suggesting that axons may be actively seeking synaptic partners. In addition, the observation that the axon fascicles could extend several millimeters through the conduit demonstrates the feasibility of long-distance directed axonal outgrowth through a 3D matrix.
To confirm that the observed outgrowth in the microconduits was axonal, immunocytochemistry was performed to exclude the possibility that the extending processes were glial. For this analysis, we labeled the microconduits with antibodies for β-tubulin III and GFAP, as the satellite cells in DRG are multipotential glial precursors and neither MAP-2 nor tau is exclusively a neuronal marker.39–41 This revealed that despite our mitotic inhibitor treatment, a notable glial component remained after 1 week in culture. Interestingly, over approximately the first millimeter there appeared to be preferential axonal growth along glial processes. This phenomenon may recapitulate a developmental process and has been previously described in aligned astrocytes in vitro.42 However, this analysis confirmed that the deepest projections into the microconduits interior were exclusively axonal.
This approach using 3D microconduits may be complimentary to the many other biomaterial-based strategies being developed to promote axonal regeneration.31–33,43–46 Similar in concept to tubular guidance conduits being developed to facilitate repair following spinal cord injury31–33 and peripheral nerve injury,43,44 the microconduits are of a size that would permit use in brain. In addition, while nano- and/or microfibers have shown promise by eliciting longitudinal axonal outgrowth in vitro,43,47 these strategies may have limited ability to directly elicit targeted axonal growth over long distances in the complex microenvironment of the brain. Moreover, the nature of the materials and fabrication techniques lends itself to the generation of constructs that could be tailored to the desired geometry of transplant required.
The microconduit approach may appear similar in concept to our previous strategy of creating living axonal tracts engineered using axonal “stretch-growth,”36,38,48 however, there are important differences. Stretch grown axonal constructs are typically bidirectional with axons spanning two populations of neurons. The axonal stretch-growth process produces relatively large planar tissue, which is rolled around the longitudinal axis prior to transplantation.37 In contrast, the microconduit encased axonal constructs are unidirectional and designed to allow minimally invasive (due to smaller size) implantation in remote regions of the nervous system that would not tolerate substantial manipulation, such as the brainstem and thalamus. In addition, the microconduit design constrains the direction of axon outgrowth. Nonetheless, following transplantation the general repair strategy between these two technologies is similar by providing living axons that can bridge damaged regions of the nervous system. Survival and integration of the microconduit constructs following transplantation appears feasible since the stretch-grown constructs have been successfully used for repair of both peripheral nerve34 and spinal cord injury35 in rodents.
In addition to potentially reconnecting neuronal populations directly, microconduits may also guide host axonal regeneration by exploiting axon-mediated axonal outgrowth. Specifically, microconduits could provide a living scaffold along which directed host axonal regeneration occurs to permit targeted nerve tract reestablishment. While axon-mediated axonal outgrowth has been studied in the context of developmental neurobiology, the mechanism and potential role of this mode of axonal growth in regeneration is unknown. As such, microconduit constructs may provide a useful investigative platform to examine axon-mediated axonal outgrowth as a regenerative strategy in a 3D environment.
Microconduit constructs may also serve as an in vitro test bed for the investigation of factors influencing neuronal survival and axonal outgrowth/directionality. Here, microconduits may be engineered to simultaneously vary and/or provide gradients in mechanical properties, internal core ligand presence/density/stiffness, and potentially provide chemotaxic signals. Typically, the effects of these factors on neurite outgrowth have been examined using planar surfaces (e.g., mechanical properties,20–22 ligand type/density,23–25 and chemotaxic gradients18,19). However, axons in the brain must traverse a complex 3D environment to reach appropriate targets. Accordingly, factors influencing axonal extension in 3D matrices have been investigated to determine the relative contributions of matrix mechanical properties, haptotaxic, and chemotaxic cues in a microenvironment more representative of the in vivo situation.26–30 Additionally, the importance of geometric guidance cues including surface curvature is increasingly being recognized.43,47,49,50 To date, the ability to systematically vary these parameters in a true 3D environment has been elusive. As such, 3D microconduits may provide a new approach to understanding both individual and synergistic influences on axonal extension and directionality.
Conclusions
We have developed a microtissue engineering technique to generate long, living axonal tracts encased in microconduit hydrogels for repair of the nervous system. The microconduits were designed with a relatively stiff hydrogel outer casing to direct constrained unidirectional outgrowth of axons through a central soft proteinaceous canal. The stiff outer casing additionally provides structural support to protect the engineered microtissue during transportation and transplantation of the construct. Moreover, the small size of the construct will permit minimally invasive implantation into sensitive regions of the nervous system. Overall, this neural microtissue engineering strategy represents the first approach capable of simultaneously providing neuronal replacement and re-creating long-distance axonal connections, with the added benefit of miniature dimensions enabling minimally invasive delivery.
Acknowledgments
We would like to thank Kevin D. Browne, Kevin Hou, and William Okech for assistance in these studies. Financial support was provided by the Department of Defense (grant No. SC090019) and the National Institutes of Health (grant Nos. NS048949, NS038104, NS056202, and NRSA NS043126).
Disclosure Statement
No competing financial interests exist.
References
- 1.Tallantyre E.C. Bo L. Al-Rawashdeh O. Owens T. Polman C.H. Lowe J.S. Evangelou N. Clinico-pathological evidence that axonal loss underlies disability in progressive multiple sclerosis. Mult Scler. 2010;16:406. doi: 10.1177/1352458510364992. [DOI] [PubMed] [Google Scholar]
- 2.Belal A. Ylikoski J. Pathology as it relates to ear surgery II. Labyrinthectomy. J Laryngol Otol. 1983;97:1. doi: 10.1017/s0022215100093737. [DOI] [PubMed] [Google Scholar]
- 3.Levin P.S. Newman S.A. Quigley H.A. Miller N.R. A clinicopathologic study of optic neuropathies associated with intracranial mass lesions with quantification of remaining axons. Am J Ophthalmol. 1983;95:295. doi: 10.1016/s0002-9394(14)78297-2. [DOI] [PubMed] [Google Scholar]
- 4.Cheng H.C. Ulane C.M. Burke R.E. Clinical progression in Parkinson disease and the neurobiology of axons. Ann Neurol. 2010;67:715. doi: 10.1002/ana.21995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marshall V.G. Bradley W.G., Jr. Marshall C.E. Bhoopat T. Rhodes R.H. Deep white matter infarction: correlation of MR imaging and histopathologic findings. Radiology. 1988;167:517. doi: 10.1148/radiology.167.2.3357964. [DOI] [PubMed] [Google Scholar]
- 6.Curinga G. Smith G.M. Molecular/genetic manipulation of extrinsic axon guidance factors for CNS repair and regeneration. Exp Neurol. 2008;209:333. doi: 10.1016/j.expneurol.2007.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huebner E.A. Strittmatter S.M. Axon regeneration in the peripheral and central nervous systems. Results Probl Cell Differ. 2009;48:339. doi: 10.1007/400_2009_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings B.J. Uchida N. Tamaki S.J. Salazar D.L. Hooshmand M. Summers R. Gage F.H. Anderson A.J. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A. 2005;102:14069. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim H.J. Stem cell potential in Parkinson's disease and molecular factors for the generation of dopamine neurons. Biochim Biophys Acta. 2011;1812:1. doi: 10.1016/j.bbadis.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Orlacchio A. Bernardi G. Martino S. Stem cells: an overview of the current status of therapies for central and peripheral nervous system diseases. Curr Med Chem. 2010;17:595. doi: 10.2174/092986710790416272. [DOI] [PubMed] [Google Scholar]
- 11.Jain A. Brady-Kalnay S.M. Bellamkonda R.V. Modulation of Rho GTPase activity alleviates chondroitin sulfate proteoglycan-dependent inhibition of neurite extension. J Neurosci Res. 2004;77:299. doi: 10.1002/jnr.20161. [DOI] [PubMed] [Google Scholar]
- 12.Liu K. Lu Y. Lee J.K. Samara R. Willenberg R. Sears-Kraxberger I. Tedeschi A. Park K.K. Jin D. Cai B. Xu B. Connolly L. Steward O. Zheng B. He Z. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13:1075. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yip P.K. Wong L.F. Sears T.A. Yanez-Munoz R.J. McMahon S.B. Cortical overexpression of neuronal calcium sensor-1 induces functional plasticity in spinal cord following unilateral pyramidal tract injury in rat. PLoS Biol. 2010;8:e1000399. doi: 10.1371/journal.pbio.1000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradbury E.J. Moon L.D. Popat R.J. King V.R. Bennett G.S. Patel P.N. Fawcett J.W. McMahon S.B. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 15.Stichel C.C. Hermanns S. Luhmann H.J. Lausberg F. Niermann H. D'Urso D. Servos G. Hartwig H.G. Muller H.W. Inhibition of collagen IV deposition promotes regeneration of injured CNS axons. Eur J Neurosci. 1999;11:632. doi: 10.1046/j.1460-9568.1999.00466.x. [DOI] [PubMed] [Google Scholar]
- 16.Mingorance A. Sole M. Muneton V. Martinez A. Nieto-Sampedro M. Soriano E. del Rio J.A. Regeneration of lesioned entorhino-hippocampal axons in vitro by combined degradation of inhibitory proteoglycans and blockade of Nogo-66/NgR signaling. FASEB J. 2006;20:491. doi: 10.1096/fj.05-5121fje. [DOI] [PubMed] [Google Scholar]
- 17.Tang X.Q. Heron P. Mashburn C. Smith G.M. Targeting sensory axon regeneration in adult spinal cord. J Neurosci. 2007;27:6068. doi: 10.1523/JNEUROSCI.1442-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao X. Shoichet M.S. Investigating the synergistic effect of combined neurotrophic factor concentration gradients to guide axonal growth. Neuroscience. 2003;122:381. doi: 10.1016/j.neuroscience.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 19.Cao X. Shoichet M.S. Defining the concentration gradient of nerve growth factor for guided neurite outgrowth. Neuroscience. 2001;103:831. doi: 10.1016/s0306-4522(01)00029-x. [DOI] [PubMed] [Google Scholar]
- 20.Leach J.B. Brown X.Q. Jacot J.G. Dimilla P.A. Wong J.Y. Neurite outgrowth and branching of PC12 cells on very soft substrates sharply decreases below a threshold of substrate rigidity. J Neural Eng. 2007;4:26. doi: 10.1088/1741-2560/4/2/003. [DOI] [PubMed] [Google Scholar]
- 21.Flanagan L.A. Ju Y.E. Marg B. Osterfield M. Janmey P.A. Neurite branching on deformable substrates. Neuroreport. 2002;13:2411. doi: 10.1097/01.wnr.0000048003.96487.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang F.X. Yurke B. Firestein B.L. Langrana N.A. Neurite outgrowth on a DNA crosslinked hydrogel with tunable stiffnesses. Ann Biomed Eng. 2008;36:1565. doi: 10.1007/s10439-008-9530-z. [DOI] [PubMed] [Google Scholar]
- 23.Adams D.N. Kao E.Y. Hypolite C.L. Distefano M.D. Hu W.S. Letourneau P.C. Growth cones turn and migrate up an immobilized gradient of the laminin IKVAV peptide. J Neurobiol. 2005;62:134. doi: 10.1002/neu.20075. [DOI] [PubMed] [Google Scholar]
- 24.Tong Y.W. Shoichet M.S. Enhancing the neuronal interaction on fluoropolymer surfaces with mixed peptides or spacer group linkers. Biomaterials. 2001;22:1029. doi: 10.1016/s0142-9612(00)00338-0. [DOI] [PubMed] [Google Scholar]
- 25.Li G.N. Liu J. Hoffman-Kim D. Multi-molecular gradients of permissive and inhibitory cues direct neurite outgrowth. Ann Biomed Eng. 2008;36:889. doi: 10.1007/s10439-008-9486-z. [DOI] [PubMed] [Google Scholar]
- 26.Blewitt M.J. Willits R.K. The effect of soluble peptide sequences on neurite extension on 2D collagen substrates and within 3D collagen gels. Ann Biomed Eng. 2007;35:2159. doi: 10.1007/s10439-007-9389-4. [DOI] [PubMed] [Google Scholar]
- 27.Cullen D.K. Lessing M.C. LaPlaca M.C. Collagen-dependent neurite outgrowth and response to dynamic deformation in three-dimensional neuronal cultures. Ann Biomed Eng. 2007;35:835. doi: 10.1007/s10439-007-9292-z. [DOI] [PubMed] [Google Scholar]
- 28.Dodla M.C. Bellamkonda R.V. Anisotropic scaffolds facilitate enhanced neurite extension in vitro. J Biomed Mater Res A. 2006;78:213. doi: 10.1002/jbm.a.30747. [DOI] [PubMed] [Google Scholar]
- 29.Ju Y.E. Janmey P.A. McCormick M.E. Sawyer E.S. Flanagan L.A. Enhanced neurite growth from mammalian neurons in three-dimensional salmon fibrin gels. Biomaterials. 2007;28:2097. doi: 10.1016/j.biomaterials.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willits R.K. Skornia S.L. Effect of collagen gel stiffness on neurite extension. J Biomater Sci Polym Ed. 2004;15:1521. doi: 10.1163/1568562042459698. [DOI] [PubMed] [Google Scholar]
- 31.Moore M.J. Friedman J.A. Lewellyn E.B. Mantila S.M. Krych A.J. Ameenuddin S. Knight A.M. Lu L. Currier B.L. Spinner R.J. Marsh R.W. Windebank A.J. Yaszemski M.J. Multiple-channel scaffolds to promote spinal cord axon regeneration. Biomaterials. 2006;27:419. doi: 10.1016/j.biomaterials.2005.07.045. [DOI] [PubMed] [Google Scholar]
- 32.Silva N.A. Salgado A.J. Sousa R.A. Oliveira J.T. Pedro A.J. Leite-Almeida H. Cerqueira R. Almeida A. Mastronardi F. Mano J.F. Neves N.M. Sousa N. Reis R.L. Development and characterization of a novel hybrid tissue engineering-based scaffold for spinal cord injury repair. Tissue Eng Part A. 2010;16:45. doi: 10.1089/ten.TEA.2008.0559. [DOI] [PubMed] [Google Scholar]
- 33.Tsai E.C. Dalton P.D. Shoichet M.S. Tator C.H. Synthetic hydrogel guidance channels facilitate regeneration of adult rat brainstem motor axons after complete spinal cord transection. J Neurotrauma. 2004;21:789. doi: 10.1089/0897715041269687. [DOI] [PubMed] [Google Scholar]
- 34.Huang J.H. Cullen D.K. Browne K.D. Groff R. Zhang J. Pfister B.J. Zager E.L. Smith D.H. Long-term survival and integration of transplanted engineered nervous tissue constructs promotes peripheral nerve regeneration. Tissue Eng Part A. 2009;15:1677. doi: 10.1089/ten.tea.2008.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iwata A. Browne K.D. Pfister B.J. Gruner J.A. Smith D.H. Long-term survival and outgrowth of mechanically engineered nervous tissue constructs implanted into spinal cord lesions. Tissue Eng. 2006;12:101. doi: 10.1089/ten.2006.12.101. [DOI] [PubMed] [Google Scholar]
- 36.Pfister B.J. Iwata A. Meaney D.F. Smith D.H. Extreme stretch growth of integrated axons. J Neurosci. 2004;24:7978. doi: 10.1523/JNEUROSCI.1974-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfister B.J. Iwata A. Taylor A.G. Wolf J.A. Meaney D.F. Smith D.H. Development of transplantable nervous tissue constructs comprised of stretch-grown axons. J Neurosci Methods. 2006;153:95. doi: 10.1016/j.jneumeth.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 38.Smith D.H. Wolf J.A. Meaney D.F. A new strategy to produce sustained growth of central nervous system axons: continuous mechanical tension. Tissue Eng. 2001;7:131. doi: 10.1089/107632701300062714. [DOI] [PubMed] [Google Scholar]
- 39.Fex Svenningsen A. Colman D.R. Pedraza L. Satellite cells of dorsal root ganglia are multipotential glial precursors. Neuron Glia Biol. 2004;1:85. doi: 10.1017/S1740925X04000110. [DOI] [PubMed] [Google Scholar]
- 40.Geisert E.E., Jr. Johnson H.G. Binder L.I. Expression of microtubule-associated protein 2 by reactive astrocytes. Proc Natl Acad Sci U S A. 1990;87:3967. doi: 10.1073/pnas.87.10.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X. Chen Y. Wang C. Huang L.Y. Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci U S A. 2007;104:9864. doi: 10.1073/pnas.0611048104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.East E. de Oliveira D.B. Golding J.P. Phillips J.B. Alignment of astrocytes increases neuronal growth in three-dimensional collagen gels and Is maintained following plastic compression to form a spinal cord repair conduit. Tissue Eng Part A. 2010;16:3173. doi: 10.1089/ten.tea.2010.0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wen X. Tresco P.A. Effect of filament diameter and extracellular matrix molecule precoating on neurite outgrowth and Schwann cell behavior on multifilament entubulation bridging device in vitro. J Biomed Mater Res A. 2006;76:626. doi: 10.1002/jbm.a.30520. [DOI] [PubMed] [Google Scholar]
- 44.Wen X. Tresco P.A. Fabrication and characterization of permeable degradable poly(DL-lactide-co-glycolide) (PLGA) hollow fiber phase inversion membranes for use as nerve tract guidance channels. Biomaterials. 2006;27:3800. doi: 10.1016/j.biomaterials.2006.02.036. [DOI] [PubMed] [Google Scholar]
- 45.Cai J. Peng X. Nelson K.D. Eberhart R. Smith G.M. Permeable guidance channels containing microfilament scaffolds enhance axon growth and maturation. J Biomed Mater Res A. 2005;75:374. doi: 10.1002/jbm.a.30432. [DOI] [PubMed] [Google Scholar]
- 46.Kim Y.T. Haftel V.K. Kumar S. Bellamkonda R.V. The role of aligned polymer fiber-based constructs in the bridging of long peripheral nerve gaps. Biomaterials. 2008;29:3117. doi: 10.1016/j.biomaterials.2008.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cullen D.K. Patel A.R. Doorish J.F. Smith D.H. Pfister B.J. Developing a tissue-engineered neural-electrical relay using encapsulated neuronal constructs on conducting polymer fibers. J Neural Eng. 2008;5:374. doi: 10.1088/1741-2560/5/4/002. [DOI] [PubMed] [Google Scholar]
- 48.Smith D.H. Stretch growth of integrated axon tracts: extremes and exploitations. Prog Neurobiol. 2009;89:231. doi: 10.1016/j.pneurobio.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smeal R.M. Rabbitt R. Biran R. Tresco P.A. Substrate curvature influences the direction of nerve outgrowth. Ann Biomed Eng. 2005;33:376. doi: 10.1007/s10439-005-1740-z. [DOI] [PubMed] [Google Scholar]
- 50.Smeal R.M. Tresco P.A. The influence of substrate curvature on neurite outgrowth is cell type dependent. Exp Neurol. 2008;213:281. doi: 10.1016/j.expneurol.2008.05.026. [DOI] [PubMed] [Google Scholar]