Abstract
Purpose
We performed a comprehensive comparative study of the plan quality between volumetric modulated arc therapy (VMAT) and intensity-modulated radiation therapy (IMRT) for the treatment of prostate cancer.
Methods and Materials
Eleven patients with prostate cancer treated at our institution were randomly selected for this study. For each patient, a VMAT plan and a series of IMRT plans using an increasing number of beams (8, 12, 16, 20, and 24 beams) were examined. All plans were generated using our in-house-developed automatic inverse planning (AIP) algorithm. An existing 8-beam clinical IMRT plan, which was used to treat the patient, was used as the reference plan. For each patient, all AIP-generated plans were optimized to achieve the same level of planning target volume (PTV) coverage as the reference plan. Plan quality was evaluated by measuring mean dose to and dose-volume statistics of the organs-at-risk, especially the rectum, from each type of plan.
Results
For the same PTV coverage, the AIP-generated VMAT plans had significantly better plan quality in terms of rectum sparing than the 8-beam clinical and AIP-generated IMRT plans (p < 0.0001). However, the differences between the IMRT and VMAT plans in all the dosimetric indices decreased as the number of beams used in IMRT increased. IMRT plan quality was similar or superior to that of VMAT when the number of beams in IMRT was increased to a certain number, which ranged from 12 to 24 for the set of patients studied. The superior VMAT plan quality resulted in approximately 30% more monitor units than the 8-beam IMRT plans, but the delivery time was still less than 3 minutes.
Conclusions
Considering the superior plan quality as well as the delivery efficiency of VMAT compared with that of IMRT, VMAT may be the preferred modality for treating prostate cancer.
Keywords: Volumetric modulated arc therapy, Intensity-modulated radiation therapy, Treatment planning, Prostate cancer, OAR sparing
INTRODUCTION
Intensity-modulated radiation therapy (IMRT) is a commonly used method to treat prostate cancer. In IMRT, multiple beam angles are used and the intensity of each beam can be modulated by using multileaf collimators (MLCs), enabling the creation of complex yet highly conformal dose profiles. Volumetric modulated arc therapy (VMAT) has attracted increasing attention because of its greatly improved delivery efficiency over fixed-field IMRT. Unlike IMRT, which typically includes less than 10 fixed-field beam angles, VMAT includes a large number of beam directions from an arc trajectory and delivers doses dynamically during rotation of the gantry. From each direction, however, there is no beam modulation by the MLCs so the intensity from each beam direction is uniform in VMAT (1). While in some situations more than one arc is used in VMAT, the level of modulation from each beam direction is still much lower than that from each beam in fixed-field IMRT.
VMAT has been previously compared with IMRT for various types of cancer at different sites (1–23). Although it has been well established that VMAT results in improved delivery efficiency over IMRT (1–23), it is still unclear whether VMAT also generates a better plan quality than IMRT for prostate cancer treatment planning (3, 5, 10, 12, 13, 16, 17), as shown in table 1. All these studies employed 5–9 fixed gantry fields in IMRT and one or more full arcs in VMAT. As contradictory results from these studies suggest, there is still inconclusiveness about whether VMAT generates better plan quality than IMRT for prostate cancer radiation therapy. Besides, little detail was found in the published literatures on the time and effort spent in the inverse planning process or how the quality of the IMRT and VMAT plans are controlled, which strongly affects the planning outcomes and hence the plan quality comparison result.
Table.1.
Published studies comparing VMAT and IMRT treatment plans for prostate cancer.
| Author, year | Number of patients | IMRT/VMAT planning systems | Number of beams in IMRT | Number of arcs / range of angles | VMAT MUs / IMRT MUs | Plan quality: VMAT vs. IMRT |
|---|---|---|---|---|---|---|
| Zhang et al., 2010 | 11 | MSKCC/MSKCC* | 5 | 1/360° | 0.45 | Small difference; VMAT tended to result in slightly better normal tissue sparing |
| Rao et al., 2010 | 6 | Pinnacle/Pinnacle + inhouse | 7 | 1/356° | 0.86 | Similar |
| Boylan et al., 2010 | 5 | Pinnacle/Pinnacle + inhouse | 5 | 1/348° | 0.94 | VMAT shows slightly worse PTV coverage but better OAR sparing |
| Yoo et al., 2010 | 10 | Eclipse/Eclipse | 9 (primary) | 1/358° | 0.33 | For complex PTV: IMRT is better; for simple PTV: they are comparable |
| 2/358° x2 | 0.35 | |||||
| 7 (boost) | 1/358° | 0.57 | ||||
| 2/358° x2 | 0.62 | |||||
| Wolff et al., 2009 | 9 | Elekta/ERGO++ + AMOA | 7 | 1/360° | 0.68 | VMAT has better PTV coverage but also higher OAR dose |
| 1/360° + 2/100° | 0.71 | |||||
| Bedford et al., 2009 | 1 | Elekta/in-house | 5 | 1/350° | 1.07 | VMAT has small improvement in PTV coverage and OAR sparing, but higher volume of irradiated normal tissue |
| Palma et al., 2008 | 10 | Eclipse/Eclipse | 5 | 1/358° | 0.58 | VMAT has more favorable dose distribution and lower dose to rectum and femoral heads |
MSKCC = Memorial Sloan Kettering Cancer Center (MSKCC) treatment planning system
In the current study, we found that, when the plan qualities of both modalities were optimized to the best that we can achieve, VMAT generally had a superior plan quality compared to 8-beam IMRT with our institution’s standard 8-beam configuration (see Results section). However, intuitively, increased number of beam directions brings increased intensity modulations and therefore an improvement in IMRT plan quality. To our knowledge, the plan quality of VMAT has not been compared to that of IMRT when more than 9 fields are used. Furthermore, it has yet to be determined if, as the number of gantry angles used in IMRT increases, there exists a point beyond which having more modulation offsets having fewer beam directions, so that IMRT could generate a better quality plan than VMAT. Bortfeld explored this general question mathematically and found that the maximum required number of beams for IMRT is proportional to the radius of the tumor (11). It was claimed that using more beams beyond this limit will not improve the plan quality. However, since their derivations were based on a strongly simplified model, the result was much approximated and was not examined in real patients. Here, we explore this question and look for a real-world solution in the context of prostate cancer treatment planning.
In this study, using an in-house-developed automatic inverse planning (AIP) algorithm, a comprehensive comparative treatment planning study of VMAT and IMRT was performed. Specifically, we compared the plan quality of VMAT to that of IMRT with 8, 12, 16, 20, and 24 fixed gantry fields to obtain a better understanding of the effect of the number of beam directions and the level of modulation on plan quality.
METHOD AND MATERIALS
Patients
Computed tomography data sets of 11 patients with prostate cancer who were treated with IMRT in our institution between 2009 and 2010 were randomly selected for this institutional review board approved study. The planning target volume (PTV) was defined as the prostate and the proximal seminal vesicles (n = 8), or the prostate and the entire seminal vesicle (n = 3) with a margin of 5 mm posterior and 7 mm in other directions. For all patients, the prescribed dose was 76 Gy delivered in 38 fractions. The rectum tolerance is 70 Gy covering less than 25% of the volume, the 90% isodose line falls wthin the half width of rectum, and the 50% isodose line falls within the full width of the rectum. For the bladder, 65 Gy and 40 Gy have to cover less than 25% and 50% of the volume, respectively. The femoral heads are limited to receive 50 Gy in less than 10% of the volume (24).
Automatic Inverse Planning (AIP) algorithm
To generate VMAT and IMRT plans for each patient, we used the AIP algorithm which was implemented in the Pinnacle3 v9.0 treatment planning system (Philips Nuclear Medicine, Fitchburg, WI) (25, 26). The AIP algorithm makes use of Pinnacle’s scripting language. It efficiently and automatically generates IMRT or VMAT plans by performing the following steps.
1. Planning structure generations
A set of planning structures is generated based on the physician-drawn PTV, OARs and normal tissue in order to facilitate the inverse planning. A brief description to each structure is listed in table 2. In addition to the basic structures, AIP creates two ring structures, FS-PTVRing and FS-Ring, which help to shape the isodose distribution and to reduce the appearance of hot spots in the corresponding areas.
Table.2.
Planning structures used in the AIP algorithm for IMRT and VMAT.
| FS-PTV | A copy of the physician-draw PTV structure |
| FS-PTVRing | 8 mm thick ring structure surrounding 2 mm expansion of the PTV |
| FS-Ring | 3 cm thick ring structure along the outer contour of the body |
| FS-NormalTissue | Entire body excluding the 10 mm expansion of the PTV |
| FS-BladderAvoid | The bladder structure avoiding 3 mm expansion of the PTV |
| FS-RectumAvoid | The rectum structure avoiding 3 mm expansion of the PTV |
| FS-FHAvoid | The femoral heads structure avoiding 3 mm expansion of the PTV |
2. Initial objective function setup
An initial set of objective functions, which applies to all prostate IMRT/VMAT patients, were determined based on our previous experience in prostate treatment planning and serves as the starting point of the optimization in AIP. The initial set of objective functions, as shown in table 3, gives tight dose constraints to the OARs and normal tissue but loose constraints to the PTV such that the initial optimization results in a plan with the best OAR sparing.
Table.3.
Initial inverse planning objectives that are loaded into the Pinnacle treatment planning system for prostate cancer.
| ROI | Type | Constrain | Target cGy | Volume (%) | Weight | a |
|---|---|---|---|---|---|---|
| FS-PTV | Uniform Dose | N | 7900 | 0.01 | ||
| FS-PTV | Max Dose | N | 7900 | 0.01 | ||
| FS-PTV | Min Dose | N | 7900 | 0.01 | ||
| FS-PTVRing | Max Dose | N | 7000 | 0.005 | ||
| FS-Ring | Max DVH | N | 4000 | 0 | 0.002 | |
| FS-NormalTissue | Max DVH | N | 4000 | 0 | 0.01 | |
| FS-BladderAvoid | Max EUD | N | 1600 | 0.01 | 2 | |
| FS-RectumAvoid | Max EUD | N | 1600 | 0.01 | 5 | |
| FS-FHAvoid | Max EUD | N | 3500 | 0.01 | 50 |
3. Objective function parameter optimization (OFPO)
The flowchart of the OFPO process is shown in Fig.1. Three to six rounds of optimizations are applied for each patient, depending on the PTV coverage resulted from each round of optimization. Once the PTV D95 exceeds a predefined threshold (Dthreshold), OFPO terminates. In each round of optimization after the initial one, the weights of the PTV objectives are increased and optimization will be continued. Hence, a plan with improved PTV coverage and therefore reduced OAR sparing will be resulted. Beyond 3 rounds of optimizations, a copy of each resulted plan will be saved and, finally, 1 to 4 plans will be resulted, each with a different PTV/OAR compromise. An example is shown in Fig.2. The physician can choose the most preferable plan from them.
Fig.1.

Flowchart of the OFPA algorithm for prostate IMRT and VMAT, where i is the index of the optimization cycle, which has an upper limit of 6. Dthreshold and fweight are arbitrarily chosen to be 76.4 Gy and 5, respectively, based on experience.
Fig.2.

Dose-volume histograms of three plans resulted from one AIP execution with different trade-offs between PTV coverage and rectum sparing.
Note that the AIP program is not a treatment planning system but rather an inverse planning technique built in conjunction with the Pinnacle3 treatment planning system. The AIP program utilizes Pinnacle3’s built-in functions, which include its dose calculation and optimization plug-ins. In this study, all AIP-generated VMAT plans were optimized using the SmartArc module in Pinnacle3, which uses an optimization algorithm described by Bzdusek et al. (27). All AIP-generated IMRT plans in this study were optimized with the direct machine parameter optimization (DMPO) module (28), which directly optimizes MLC leaf positions and segment weights so that there is no need for fluence conversion and the plan quality will not degrade during delivery. When the same beam configuration is used, the AIP algorithm has been shown to consistently generate plans that are superior or comparable to those developed manually by experienced dosimetrists at our institution (25, 26).
Planning study design
For each patient, the clinically utilized IMRT plan, which was generated by an experienced dosimetrist at our institution prior to this study, was used as the reference plan. These plans utilized an 8-beam configuration, standard for prostate cancer treatment in our institution.
In this study, we generated a VMAT plan using the AIP algorithm for each patient. The AIP-generated VMAT plans used two 360° arcs (one rotating clockwise and the other rotating counter-clockwise), which produces better plan quality than using a single 360° arc. A comparison between using 1 and 2 arcs can be found in the Discussions section. A total of 91 control points were created through each arc using 4° spacing, which is Pinnacle’s default value and exhibited good dose calculation accuracy (29). We have found that a denser spacing of 2° is not necessary because it brings little improvement in plan quality but much prolonged optimization time. All VMAT plans were generated with variable dose rate as well as variable gantry rotation speed.
Also, we generated a series of IMRT plans using 8, 12, 16, 20, and 24 beam angles with the AIP algorithm for each patient. The 8-beam configuration used our institution’s standard template. For the 12-, 16-, 20-, and 24-beam IMRT configurations, beam angles were more densely selected near the tangent direction along the intersection between the rectum and the PTV and sparsely selected at other directions. Such a beam angle distribution produces a sharp dose fall-off at the rectum. Selected beam angles varied among patients, but most of the beams angles were the same. For the 24-beam IMRT plans, however, uniformly distributed beam angles were found to yield a better plan quality than beam angles selected using the approach mentioned above. Therefore, we used a uniform beam angle distribution for the 24-beam IMRT plans. In our study, an AIP-generated plan with a higher number of beams was not simply calculated from the same set of objectives as the other plans nor was it built upon the plan with a lower number of beams. Instead, the AIP algorithm was executed independently for each beam configuration, which resulted in a different set of objective parameters. Of the generated IMRT plans with different PTV/OAR compromises after each AIP execution, we selected the IMRT plan that had the same level of PTV coverage as the clinical IMRT plan to directly compare OAR sparing among the plans.
More details on the inverse planning parameter settings for IMRT and VMAT are listed in table 4. The maximum number of segments for IMRT and the maximum delivery time in seconds for VMAT were both set to 100 because further increasing the limits does not help to improve the plan quality. All IMRT plans in this study used the step-and-shoot technique and all plans deliver 6-MV photons.
Table.4.
Inverse planning parameters for VMAT and IMRT.
| minimum segment area (cm2) | 2 |
| minimum segment Mus | 1 |
| minimum number of leaf pairs | 2 |
| minimum leaf end separation (cm) | 1.5 |
| maximum number of iterations | 25 |
| convolution dose iteration | 5 |
| maximum number of segments (IMRT) | 100 |
| maximum delivery time (second) (VMAT) | 100 |
| dose engine | CC Convolution |
Plan evaluation
PTV coverage was evaluated using the conformality index (CI) and the heterogeneity index (HI), which were calculated as follows (30):
where is the target volume covered by the prescribed dose, TV is the target volume, and is the volume enclosed by the prescribed isodose surface and
where D1and D95 are, respectively, the dose encompassing 1% and 95% of the target volume.
The IMRT and VMAT plans were evaluated using dose-volume histograms (DVHs). To quantitatively measure the OAR sparing of each plan, we calculated the mean OAR volume within the 30-, 40-, 50-, 60-, and 70-Gy isodose lines and the average mean dose to the rectum and the bladder. The total MUs per fraction were also compared to assess the delivery time for each plan. Statistical analysis was performed using the two-sided paired t-test. A p-value <=0.05 was defined as statistically significant.
RESULTS
All of the AIP-generated IMRT and VMAT plans were reviewed by a radiation oncologist (A. K. Lee, MD) and were considered as acceptable for patient treatment. All the AIP-generated plans in this study achieved a similar level of PTV coverage as the 8-beam clinical IMRT plans previously generated by experienced dosimetrists. The average CI and HI values for the 11 patients for each category of plans are similar, as shown in Fig.3.
Fig.3.

Average PTV conformality index and heterogeneity index values for the 11 patients for each type of plan.
We summarized the number of patients whose IMRT plan in each category achieved better rectum sparing than their VMAT plan. As shown in Fig.4, no patients received better rectum sparing from their 8-beam clinical or AIP-generated IMRT plans than from their VMAT plans. For one patient, the 12-beam IMRT plan achieved better rectum sparing (14% lower mean rectal dose, 15% less volume receiving 0–30Gy dose and only 1% larger volume receiving 40–70Gy dose) than the VMAT plan. The number of patients keeps increasing with the number of beams in the IMRT plans. All patients received better rectum sparing from their 24-beam IMRT plan than from their VMAT plan.
Fig.4.

Number of patients who received better rectum sparing from each type of IMRT plan than from their VMAT plan.
Fig.5 and Fig.6 depict quantitative dose–volume measures in the rectum and bladder from the IMRT and VMAT plans that had a similar level of PTV coverage. In general, the 8-beam AIP-generated IMRT plans had similar rectum and bladder sparing to the 8-beam clinical IMRT plans but inferior to that of the AIP-generated VMAT plans. As the number of beams used in IMRT increased, the level of rectum sparing achieved by these plans improved, eventually reaching a level similar to that of the VMAT plans. Table 5 shows the p values for the differences between doses delivered by the VMAT plans and those delivered by the various IMRT plans. For all the dosimetric indices in the rectum, the VMAT plans had a highly significant advantage (p < 0.0001) over the 8-beam clinical and AIP-generated IMRT plans. However, as more beams were included in the IMRT plans, the dosimetric advantage of the VMAT plans became less significant because the IMRT plan qualities were improved. For all the dosimetric indices in the bladder, the VMAT plans and the AIP-generated 8-beam IMRT plans were, on average, better or at least no worse than the 8-beam clinical IMRT plans. Although the trend for each individual patient was not as clear as in the rectum, dose levels in the bladder were well within the standard dose constraints (24) in all plans generated in this study.
Fig.5.

Dose statistics in the rectum for the 11 patients from the 8-beam clinical IMRT plan (a), 8-(b), 12- (c), 16- (d), 20- (e) and 24-beam (f) AIP-generated IMRT plans, and the AIP-generated VMAT plan (g).
Fig.6.

Dose statistics in the bladder for the 11 patients from the 8-beam clinical IMRT plan (a), 8- (b), 12- (c), 16- (d), 20- (e) and 24-beam (f) AIP-generated IMRT plans, and the AIP-generated VMAT plan (g).
Table.5.
p-values for the comparison on the dosimetric indices of VMAT with IMRT using different numbers of beams. Statistically significant values (p < 0.05) are highlighted.
| AIP-VMAT vs. | rectum | Bladder | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| mean | V30 | V40 | V50 | V60 | V70 | mean | V30 | V40 | V50 | V60 | V70 | |
| 8-beam clinical IMRT | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.084 | 0.029 | 0.019 | 0.013 | 0.012 | 0.013 |
| 8-beam AIP-IMRT | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.153 | 0.037 | 0.020 | 0.011 | 0.007 | 0.019 |
| 12-beam AIP-IMRT | 0.022 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | 0.002 | 0.807 | 0.148 | 0.054 | 0.012 | 0.002 | 0.003 |
| 16-beam AIP-IMRT | 0.709 | 0.019 | 0.033 | 0.089 | 0.265 | 0.863 | 0.441 | 0.683 | 0.509 | 0.340 | 0.164 | 0.238 |
| 20-beam AIP-IMRT | 0.948 | 0.007 | 0.018 | 0.049 | 0.137 | 0.747 | 0.616 | 0.374 | 0.147 | 0.154 | 0.146 | 0.294 |
| 24-beam AIP-IMRT | 0.156 | 0.130 | 0.156 | 0.196 | 0.326 | 0.521 | 0.107 | 0.489 | 0.533 | 0.419 | 0.589 | 0.625 |
Fig.7 shows the DVHs of a typical patient. The PTV received similar coverage from each type of plan. The rectum received a much lower dose from the VMAT plan than from the 8-beam IMRT plans. However, as the number of beams in IMRT increases, the rectum dose from the IMRT plan decreases. In the 20-beam AIP-generated IMRT plan, the rectum DVH was only slightly higher than that of the VMAT plan in the mid-dose region, but it was superior to that of the VMAT plan in both the low- and high-dose regions. The 24-beam AIP-generated IMRT plan was almost identical to the 20-beam AIP-generated IMRT plan.
Fig.7.
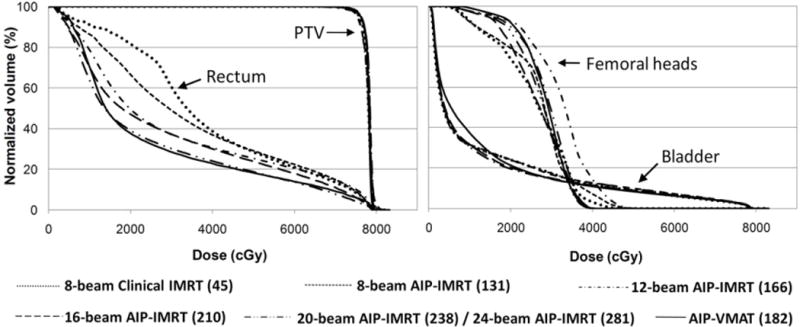
Dose-volume histograms of the PTV, rectum, and bladder from IMRT plans with different numbers of beams and the AIP-generated VMAT plan for a typical patient. The numbers in parentheses in the legend give the total number of control points for each plan.
For the patient presented in Fig.7, note the change in the total number of control points of each plan. In the case of the 16-beam AIP-generated IMRT plan, the total number of control points was 210 (9–19 control points from each beam direction), which was 15% higher than that of the VMAT plan (182), but the level of rectum sparing from this IMRT plan did not reach the same level as that from the VMAT plan. In the case of the 20-beam AIP-generated IMRT plan, the total number of control points was 238 (7–19 control points from each beam direction), which compensated for the missing beam angles and resulted in the same level of rectum sparing as the VMAT plan.
Fig.8 shows the isodose distributions from the different types of plans for a typical patient whose IMRT plan required 24 beams to be comparable to the VMAT plan in terms of rectum sparing. Although the 24-beam IMRT and the VMAT plans delivered a higher dose to the femoral heads than the 8-beam clinical IMRT plan, they are both well within the standard dose constraints for femoral heads (V50 less than 10% (24)).
Fig.8.

Dose distributions represented by isodose lines from the 8-beam clinical and AIP-generated IMRT plans, the 24-beam AIP-generated IMRT plan, and the AIP-generated VMAT plan.
The comparison of the total MUs used in each plan showed that the average total MU usage increased significantly as the number of beams used in IMRT increased. The AIP-generated VMAT plans used about 30% more total MUs than the 8-beam clinical and AIP-generated IMRT plans, but only about 4% more total MUs than the 24-beam AIP-generated IMRT plans (Fig.9). The delivery time for a typical VMAT plan was 2.6 minutes; the delivery times for 8-, 12-, 16-, 20-, and 24-beam IMRT plans were respectively 4.7, 7, 9.3, 11.7, and 14 minutes, which included the beam-on time and loading time of each beam.
Fig.9.

Average total MUs of the 11 patients.
DISCUSSION
In this study, we compared the quality of VMAT plans to that of a series of IMRT plans using increasing numbers of beams. We showed that the AIP-generated VMAT plans resulted in significantly better rectum sparing than the IMRT plans using the standard 8-beam configuration currently being utilized at our institution. When more gantry angles were added to the IMRT plans, we found that the VMAT plan quality was still consistently better until the number of beams in IMRT reached 12–16 beams. At this point, which varied among the patients examined, the IMRT plan quality became similar to or slightly better than that of VMAT. Beyond this point, the plan quality of IMRT does not improve noticeably further even if more beams are used. This indicates that, for prostate cancer, the plan quality of VMAT is a limit to which the plan quality of IMRT converges as increasing numbers of beams are used.
From another perspective, our results demonstrate that the difference in the plan quality of VMAT and IMRT is due to the difference in the number of beam angles and the level of modulation from each angle used in the two modalities. Our results show that having a large number of beam angles but few modulations (control points) from each angle is superior (in terms of plan quality) to having many modulations from each angle but a small number of beam angles. However, a large number of modulations from many beam angles in IMRT may compensate for the insufficient number of beams and produce a plan quality similar to that of VMAT, when the number of beams in IMRT is sufficiently large.
Because our in-house AIP algorithm was developed to generate treatment plans with optimal plan quality, all the AIP-generated plans in this study have the best quality that we can achieve, which enabled us to perform a fair comparison of the two modalities. We applied a quality control (QC) method recently published by Moore et al. (31) in evaluating the quality of plans involved in this study. It was found that the rectum dose measured from the AIP-generated plans is consistently close to the “best organ sparing” predicted by the QC model presented in the paper. The relative model excess, which gives the normalized difference between measured and predicted dose, obtained from our AIP-generated 8-field IMRT plans ranges from −0.2 to 0.22Gy, which is within the expected range for plans after applying the QC procedure (-0.8 to 0.22Gy). The clinically treated IMRT plans have also resulted in a similar range. This result shows that both the clinical and the AIP-generated IMRT plans have achieved similar level of rectum sparing as the well-quality-controlled plans. For IMRT plans with a larger number of beams, the algorithm optimizes the plan in the same way as it does for 8-field IMRT so that we expect it to generate the same high level of plan quality for these beam configurations.
The AIP-generated VMAT plans generated in this study resulted in considerably higher MU usage than the 8-beam IMRT plans, which is inconsistent with the results reported by other groups, who have found that VMAT plans usually reduce MU usage compared to IMRT plans (3, 10, 12, 13, 16, 17). For studies conducted in the Varian planning system (3, 16), the significant reduction in MU usage of VMAT probably comes from the difference in the optimization algorithms used for VMAT and IMRT. Comparing to studies conducted in Pinnacle (12, 13), the discrepancy may be due to the fact that, in the AIP algorithm, we focused exclusively on improving the plan quality, especially by reducing the rectum dose, so that achievable optimal plans were generated but not plans with higher delivery efficiency or lower MU usage. We believe that the higher MU usage resulting from our plans is a consequence of their highly conformal dose distributions and their superior OAR sparing. To confirm this, we manually designed a VMAT plan for one patient using a set of objectives that has loose dose constraints for the rectum and bladder compared to those used in the AIP algorithm. This manual VMAT plan resulted in dramatically reduced MU usage (363 MU) compared to that of the corresponding AIP-generated VMAT plan (1038 MU), but it also resulted in inferior OAR sparing (Fig.10). As far as delivery time is concerned, the 1038-MU plan took only 10% longer to deliver than the 363-MU plan. Admittedly, higher MU has its drawbacks such as the potential increase in total body dose because of scattering and leakage from MLCs (32). Therefore, in future improvement of the AIP algorithm, efforts will be spent on reducing the MU usage while maintaining the plan quality.
Fig.10.

DVH curves from two VMAT plans with different MUs.
All the VMAT plans generated in this study used two 360° arcs instead of one 360° arc because we found that dual-arc is superior to single-arc in terms of the compromise between plan quality and delivery efficiency. Fig.11 shows the typical DVH plots of a single-arc and a dual-arc VMAT plan. Dual-arc produced better rectum and bladder sparing than single-arc. In fact, dual-arc VMAT plans typically used less than 15% more total MUs than single-arc VMAT plans. Therefore, in this study we used dual-arc VMAT plans for the comparison with IMRT.
Fig.11.

Dose-volume histograms of the PTV, rectum, and bladder from the single- and dual-arc VMAT plans for a typical patient.
The effect of the number of beams on plan quality has been previously studied by Pirzkall et al., using a completely different approach from ours, who found that less than 9 beams may result in increased dose in regions far away from the target. In their work, all IMRT plans were generated “using the same dose-volume constraints” (33). In contrast, every plan in our study was generated completely independently from other plans, as described in the Methods section. Specifically, the objective function parameters of every plan were optimized for each beam angle configuration, ensuring a high plan quality of each case.
This work also suggests the effectiveness of the AIP algorithm for prostate cancer treatment planning. For the few manually-designed clinical VMAT plans that were available in our institution, the AIP-generated VMAT plans produced at least a comparable plan quality. The AIP algorithm also consistently produced IMRT plans that were comparable, if not superior, to the dosimetrists’ manual IMRT plans with the same beam angle configurations for all patients that were examined.
One might argue that IMRT with a large number of beams (> 8) is not clinically practical considering its lower delivery efficiency. In this study, we chose to use IMRT with a larger number of fields to obtain a better understanding of the capability of IMRT and the differences between IMRT and VMAT plan quality. Furthermore, novel technologies that enable the more efficient delivery of fixed-field IMRT, such as the one used in the TrueBeam system (Varian Medical Systems, Palo Alto, California), and continuing improvements in IMRT delivery techniques will make it possible to deliver IMRT plans with a large number of beams more efficiently in the near future, so we must continue to expand our understanding of this modality.
CONCLUSION
We have demonstrated that the VMAT technique combined with our in-house AIP algorithm generates significantly superior plans compared to the 8-beam clinical IMRT plans used for prostate cancer treatment at our institution. For IMRT plan quality to be improved such that it is comparable to that of an optimized VMAT plan, a sufficiently large number of beams has to be used. However, this would come at the expense of even longer dose delivery, increased treatment times (leading to increased intra-fractional motion) and higher economic cost. Considering the superior delivery efficiency of VMAT and the fact that the optimized VMAT plan quality in terms of both DVH and conformality of dose distribution well exceeds that of clinical IMRT plans, VMAT may be the preferred modality for treating prostate cancer.
Acknowledgments
The University of Texas MD Anderson Cancer Center is supported by grant CA16672.
Footnotes
CONFLICTS OF INTEREST NOTIFICATION
There are no actual or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys. 2008;35:310–317. doi: 10.1118/1.2818738. [DOI] [PubMed] [Google Scholar]
- 2.Cozzi L, Dinshaw KA, Shrivastava SK, et al. A treatment planning study comparing volumetric arc modulation with RapidArc and fixed field IMRT for cervix uteri radiotherapy. Radiotherapy and Oncology. 2008;89:180–191. doi: 10.1016/j.radonc.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Palma D, Vollans E, James K, et al. Volumetric Modulated Arc Therapy for Delivery of Prostate Radiotherapy: Comparison with Intensity-Modulated Radiotherapy and Three-Dimensional Conformal Radiotherapy. Int J Radiat Oncol Biol Phys. 2008;72:996–1001. doi: 10.1016/j.ijrobp.2008.02.047. [DOI] [PubMed] [Google Scholar]
- 4.Popescu CC, Olivotto IA, Beckham WA, et al. Volumetric Modulated Arc Therapy Improves Dosimetry and Reduces Treatment Time Compared to Conventional Intensity-Modulated Radiotherapy for Locoregional Radiotherapy of Left-Sided Breast Cancer and Internal Mammary Nodes. Int J Radiat Oncol Biol Phys. 2008;76:287–295. doi: 10.1016/j.ijrobp.2009.05.038. [DOI] [PubMed] [Google Scholar]
- 5.Bedford JL. Treatment planning for volumetric modulated arc therapy. Med Phys. 2009;36:5128–5138. doi: 10.1118/1.3240488. [DOI] [PubMed] [Google Scholar]
- 6.Clivio A, Fogliata A, Franzetti-Pellanda A, et al. Volumetric-modulated arc radiotherapy for carcinomas of the anal canal: A treatment planning comparison with fixed field IMRT. Radiotherapy and Oncology. 2009;92:118–124. doi: 10.1016/j.radonc.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 7.Stieler F, Wolff D, Lohr F, et al. A fast radiotherapy paradigm for anal cancer with volumetric modulated arc therapy (VMAT) Radiation Oncology. 2009;4:11. doi: 10.1186/1748-717X-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanetti E, Clivio A, Nicolini G, et al. Volumetric modulated arc radiotherapy for carcinomas of the oropharynx, hypo-pharynx and larynx: A treatment planning comparison with fixed field IMRT. Radiotherapy and Oncology. 2009;92:111–117. doi: 10.1016/j.radonc.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 9.Verbakel W, Cuijpers JP, Hoffmans D, et al. Volumetric Intensity-Modulated Arc Therapy Vs. Conventional IMRT in Head-and-Neck Cancer: A Comparative Planning and Dosimetric Study. Int J Radiat Oncol Biol Phys. 2009;74:252–259. doi: 10.1016/j.ijrobp.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 10.Wolff D, Stieler F, Welzel G, et al. Volumetric modulated arc therapy (VMAT) vs. serial tomotherapy, step-and-shoot IMRT and 3D-conformal RT for treatment of prostate cancer. Radiotherapy and Oncology. 2009;93:226–233. doi: 10.1016/j.radonc.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Bortfeld T. The number of beams in IMRT-theoretical investigations and implications for single-arc IMRT. Phys Med Biol. 2010;55:83–97. doi: 10.1088/0031-9155/55/1/006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boylan CJ, Golby C, Rowbottom CG. A VMAT planning solution for prostate patients using a commercial treatment planning system. Phys Med Biol. 2010;55:N395–N404. doi: 10.1088/0031-9155/55/14/N01. [DOI] [PubMed] [Google Scholar]
- 13.Rao M, Yang WS, Chen F, et al. Comparison of Elekta VMAT with helical tomotherapy and fixed field IMRT: Plan quality, delivery efficiency and accuracy. Med Phys. 2010;37:1350–1359. doi: 10.1118/1.3326965. [DOI] [PubMed] [Google Scholar]
- 14.Scorsetti M, Bignardi M, Clivio A, et al. Volumetric Modulation Arc Radiotherapy Compared With Static Gantry Intensity-Modulated Radiotherapy for Malignant Pleural Mesothelioma Tumor: A Feasibility Study. Int J Radiat Oncol Biol Phys. 2010;77:942–949. doi: 10.1016/j.ijrobp.2009.09.053. [DOI] [PubMed] [Google Scholar]
- 15.Shaffer R, Nichol AM, Vollans E, et al. A Comparison of Volumetric Modulated Arc Therapy and Conventional Intensity-Modulated Radiotherapy for Frontal and Temporal High-Grade Gliomas. Int J Radiat Oncol Biol Phys. 2010;76:1177–1184. doi: 10.1016/j.ijrobp.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Yoo S, Wu QJ, Lee WR, et al. Radiotherapy Treatment Plans With RapidArc for Prostate Cancer Involving Seminal Vesicles and Lymph Nodes. Int J Radiat Oncol Biol Phys. 2010;76:935–942. doi: 10.1016/j.ijrobp.2009.07.1677. [DOI] [PubMed] [Google Scholar]
- 17.Zhang PP, Happersett L, Hunt M, et al. Volumetric Modulated Arc Therapy: Planning and Evaluation for Prostate Cancer Cases. Int J Radiat Oncol Biol Phys. 2010;76:1456–1462. doi: 10.1016/j.ijrobp.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 18.Wu QJ, Yoo S, Kirkpatrick JP, et al. Volumetric arc intensity-modulated therapy for spine body radiotherapy: comparison with static intensity-modulated treatment. Int J Radiat Oncol Biol Phys. 2009;75:1596–1604. doi: 10.1016/j.ijrobp.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Bertelsen A, Hansen CR, Johansen J, et al. Single arc volumetric modulated arc therapy of head and neck cancer. Radiotherapy and Oncology. 2010;95:142–148. doi: 10.1016/j.radonc.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Guckenberger M, Richter A, Krieger T, et al. Is a single arc sufficient in volumetric-modulated arc therapy (VMAT) for complex-shaped target volumes? Radiotherapy and Oncology. 2009;93:259–265. doi: 10.1016/j.radonc.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Matuszak MM, Yan D, Grills I, et al. Clinical Applications of Volumetric Modulated Arc Therapy. Int J Radiat Oncol Biol Phys. 2010;77:608–616. doi: 10.1016/j.ijrobp.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 22.Zacarias AS, Brown MF, Mills MD. Volumetric modulated arc therapy (VMAT) treatment planning for superficial tumors. Med Dosim. 2010;35:226–229. doi: 10.1016/j.meddos.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Popple RA, Fiveash JB, Brezovich IA, et al. RapidArc Radiation Therapy: first year experience at the University of Alabama at Birmingham. Int J Radiat Oncol Biol Phys. 2010;77:932–941. doi: 10.1016/j.ijrobp.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Chao KSC. Practical essential of intensity modulated radiation therapy. 2. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 25.Zhang X, Li X, Quan EM, et al. A methodology for automatic intensity-modulated radiation treatment planning for lung cancer. Phys Med Biol. 2011;56:3873–3893. doi: 10.1088/0031-9155/56/13/009. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Pan X, Li Y, et al. Automated treatment planning for radiation therapy. 2010 Pending for US patent.
- 27.Bzdusek K, Friberger H, Eriksson K, et al. Development and evaluation of an efficient approach to volumetric arc therapy planning. Med Phys. 2009;36:2328–2339. doi: 10.1118/1.3132234. [DOI] [PubMed] [Google Scholar]
- 28.Hardemark B, A L, H R, et al. RaySearch White Paper – Direct machine parameter optimization with RayMachine in Pinnacle. 2003. [Google Scholar]
- 29.Feygelman V, Zhang G, Stevens C. Initial dosimetric evaluation of SmartArc – a novel VMAT treatment planning module implemented in a multi-vendor delivery chain. Journal of applied clinical medical physics / American College of Medical Physics. 2010;11:99–116. doi: 10.1120/jacmp.v11i1.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feuvret L, Noel G, Mazeron JJ, et al. Conformity index: A review. Int J Radiat Oncol Biol Phys. 2006;64:333–342. doi: 10.1016/j.ijrobp.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 31.Moore KL, Brame RS, Low DA, et al. Experience-based quality control of clinical intensity-modulated radiotherapy planning. Int J Radiat Oncol Biol Phys. 2011:1–7. doi: 10.1016/j.ijrobp.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 32.Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys. 2006;65:1–7. doi: 10.1016/j.ijrobp.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 33.Pirzkall A, Carol MP, Pickett B, et al. The effect of beam energy and number of fields on photon-based IMRT for deep-seated targets. Int J Radiat Oncol Biol Phys. 2002;53:434–442. doi: 10.1016/s0360-3016(02)02750-5. [DOI] [PubMed] [Google Scholar]


