Abstract
Autocrine IFN-γ signaling is important for CD4 differentiation to T helper 1 effector cells, but it has been unclear whether it contributes to CD8 T cell differentiation. We show here that naïve murine CD8 T cells rapidly and transiently produce low levels of IFN-γ upon stimulation with Ag and B7-1, with production peaking at about 8 hours and declining by 24 hours. The autocrine IFN-γ signals for upregulation of expression of Tbet and GrzB, and induces weak cytolytic activity and effector IFN-γ production. IFN-α acts synergistically with IFN-γ to support development of strong effector functions, while IL-12 induces high T-bet expression and strong function in the absence of IFN-γ signaling. Thus, IFN-γ is not only an important CD8 T cell effector cytokine, it is an autocrine/paracrine factor whose contributions to differentiation vary depending on whether the response is supported by IL-12 or Type I IFN.
Introduction
Inflammatory cytokines have a fundamental role in activating T cell responses in that they can provide a third signal that determines whether the response of naïve T cells to Ag and costimulation results in tolerance induction, in their absence, versus full activation and memory development if they are present. For naïve CD8 T cells, IL-12 and Type I IFNs (IFN-α/β) appear to be the major signal 3 cytokines that support responses to pathogens, transplants, tumors and adjuvants (1–7). Although not as extensively studied, IL-1 may play a similar role in supporting CD4 T cell differentiation and survival (8–10). Consistent with these roles, administration of IL-12 or IFN-α can replace the need for adjuvant in supporting a CD8 T cell response to peptide Ag while IL-1 cannot (9, 11), and conversely, administration of IL-1 can replace the need for adjuvant in supporting a CD4 T cell response to peptide Ag while IL-12 cannot (8, 10). In both cases, responses depend upon direct cytokine signaling to the T cells.
IFN-γ is a major effector cytokine produced in large amounts by fully differentiated CD4 and CD8 T cells when they re-encounter Ag several days after their initial stimulation (12). It does not appear to have signal 3 activity with respect to determining whether the outcome of encounter with Ag leads to tolerance versus full activation, but low levels of IFN-γ produced within 24 hours of initial stimulation of CD4 T cells contributes to differentiation down the Th1 pathway (13–15). Whether IFN-γ signaling also contributes to Ag-driven differentiation of CD8 T cells has been less well-defined, and somewhat controversial. Naïve CD8 T cells responding to Listeria monocytogenes infection receive an IFN-γ signal within 12 hours of infection, as measured by IFN-γR-dependent phosphorylation of STAT1 (16), but the functional consequences of this signaling are unclear. Numerous studies have examined in vivo CD8 T cell responses in the absence of IFN-γ and have revealed varied effects depending on the model being examined, but in most cases it has not been determined whether these are due to involvement of a CD8 T cell intrinsic IFN-γ pathway. A few reports have provided evidence that IFN-γ signaling directly to the Ag-specific CD8 T cells contributes to the magnitude of primary and memory responses to viruses (17, 18), while others have indicated that T cell intrinsic effects are minimal in comparison to effects of IFN-γ signaling to other cell types of the host (19–21). Almost nothing is known at the mechanistic level as to how intrinsic IFN-γ signaling might influence CD8 T cell differentiation.
IFN-γ production by CD8 T cells is highest when the cells are re-stimulated with Ag at 48 to 72 hours after their initial stimulation, i.e. after differentiation to effector cells has occurred. However, some increase in IFN-γ mRNA is detected within 24 hours of in vitro stimulation with Ag and B7-1 (22), and naïve human CD8 T cells produce some IFN-γ within 24 hours of stimulation with anti-CD3 and anti-CD28 mAbs (23). Prompted by these suggestions that CD8 T cells may produce IFN-γ early in response to Ag, we have examined whether this is the case and whether the early IFN-γ might provide an autocrine signal to promote differentiation. As described here, naïve CD8 T cells produce low levels of IFN-γ within hours of stimulation with Ag and B7-1, and IFN-γ signaling can upregulate T-bet expression and support some limited development of weak but physiologically relevant effector functions, and can synergize with IFN-α in supporting development of strong function. In contrast to IFN-α, IL-12 supports development of strong effector functions in the absence of IFN-γ signaling.
Materials and Methods
Mice and reagents
TCR transgenic mice used included OT-I (24), pmel (25) and P14 (26) specific for H- 2Kb/OVA257–264, H-2Kb/gp10025-33 and H-2Db/GP33-41 respectively. B6.129S7-Ifngtm1Ts/J and B6.129S7-Ifngr1tm1Agt/J mice, deficient for IFN-γ (Ifng) and IFN-γR (Ifngr1) were obtained from The Jackson Laboratory (Bar Harbor, ME) and bred with OT-I mice. Mice deficient for the IL- 12βR1 and IFNAR1 receptors were obtained by crossing OT-I.IL-12βR1 −/− mice with OT-I.IFNAR1−/− mice and breeding to obtain OT-I.IL-12βR1−/−.IFNAR1−/− mice (7). Mice were maintained under specific pathogen-free conditions at the University of Minnesota, and these studies have been reviewed and approved by the Institutional Animal Care and Use Committee. C57BL/6NCr and C56BL/6.Ly5.2 mice were purchased from the National Cancer Institute. Peptide antigens OVA257-264 (SIINFEKL), and hgp10025-33 (KVPRNQDWL) were obtained from New England Peptide (Gardner, MA). Peptide antigen GP33-41 was a gift from Dr. David Masopust. All directly conjugated fluorescent Abs were purchased from eBioscience (San Diego, CA), Biolegend (San Diego, CA), or BD Biosciences (San Diego, CA).
Purification and in vitro stimulation of Naïve CD8 T cells
In some experiments, unseparated lymph node or spleen cells from TCR transgenic mice were used. In other experiments, lymph nodes were harvested from mice, pooled, and disrupted to obtain a single cell suspension. CD8+ CD44lo naïve cells were enriched by negative selection using magnetic MACS MicroBeads from Milteny Biotec (Auburn, CA). In brief, cells were coated with FITC-labeled Abs specific for CD4, B220, I-Ab, CD11c, and CD44. Anti-FITC MicroBeads were then added and the suspension passed over separation columns attached to a MACS magnet. Cells that did not bind were collected, and were >95% CD8+ and <0.5% CD44hi. Artificial APC (aAPC) were prepared by immobilizing DimerX H-2Kb:Ig fusion protein or H-2Db:Ig fusion protein (BD Biosciences) and murine recombinant B7-1/Fc chimeric protein (R&D Systems, Minneapolis, MN) on 5 micron diameter sulfated polystyrene latex microspheres, loaded with cognate peptide as previously described (9), and used at 2×105 aAPC/well. Alternatively, DimerX and B7-1/Fc were immobilized in flat-bottom microtiter wells and loaded with peptide Ag as previously described (27). 5×104 purified naïve CD8 T cells were stimulated by Ag in microtiter wells in 0.2 ml of RP-10 medium with 2.5 U/ml of recombinant IL-2 added. When added, murine rIL-12 (R&D Systems, Minneapolis, MN) was used at 2ng/ml and Universal Type I IFN (PBL Biomedical Laboratories, Piscataway, NJ) was used at 1000U/ml.
Assay of cytotoxic activity and IFN-γ production
Cytolytic activity was determined in a standard 4 hr 51Cr-release assay using E.G7 cells as targets and EL-4 cells as controls. IFN-γ production was determined by intracellular staining of washed, fixed and permeabilized cells using an allophycocyanin-conjugated anti- IFN-γ mAb, followed by analysis by flow cytometry. In all experiments, 0.6 μl/ml of GolgiStop (BD Biosciences) was added for the last 4 hr of stimulation. For measuring effector IFN-γ of cells at the end of 3 days of culture, the cells were harvested, washed, resuspended in the presence of 1 μg/ml cognate peptide and incubated for 4 hr in the presence of GolgiStop prior to analysis.
Adoptive transfer, tumor growth and immunization
Adoptive transfer of either naïve or 3-day in vitro stimulated cells was done by i.v. injection of the indicated numbers of cells into the tail vein, and OT-I cells in the host mice were subsequently identified and quantified by flow cytometry based on CD8 and Thy1 expression as previously described (2, 11). For experiments examining effects of adoptive transfer of in vitro stimulated OT-I cells on tumor growth, mice were challenged by s.c. injection of B16.OVA tumor and the tumors allowed to grow until they were visible and palpable (8–10 days), and OT-I effector cells were then injected i.v. in the tail veins. Tumor growth was assessed by right angle measurements of the tumor mass, with results expressed as area (mm2).
Results
Naïve CD8 T cells produce IFNγ early in response to Ag and costimulation
When Ag, B7-1 and either IL-12 or IFN-α/β signals are provided in vitro, naïve CD8 T cells expand and differentiate over the course of 3 days to develop potent cytolytic activity and acquire the capacity to produce high levels of IFN-γ upon re-encounter with Ag (9, 28). A report of early production of low levels of IFN-γ by naïve human CD8 T cells (23) raised the possibility that this might also occur for murine cells, and if so, that autocrine IFN-γ signaling might contribute to differentiation. To examine this, spleen cells from three CD8 TCR transgenic mouse strains, OT-I (24), pmel (25) and P14 (26), were stained with anti-CD44 mAb and stimulated in vitro with cognate peptide for 4 or 8 hours, and IFN-γ production was then determined by intracellular staining. Despite being isolated from naïve mice, the CD8 T cell populations included cells having low, intermediate and high levels of CD44 (Fig. 1). Cells with the CD44hi memory phenotype probably arise from homeostatic expansion, and this is consistent with OT-I cells undergoing stronger homeostatic expansion than pmel cells (29) and having the highest proportion of CD44hi cells.
FIGURE 1. Naïve CD8 T cells produce IFN-γ early in response to Ag and B7-1-dependent costimulation.
Spleen cells from OT-I and pmel, and lymph node cells from P14 TCR transgenic mice (all 16 weeks of age) were stained with anti-CD44 mAb and stimulated with cognate peptide for 4 hr (middle row) or 8 hr (bottom row), permeabilized and stained with anti- IFN-γ mAb as described in Materials and Methods. (A) Top panel shows OT-I cells cultured for 4 hours in the absence of cognate peptide and bottom panel shows OT-I cells stimulated with Ag for 8 hours and stained with isotype control Ab. Isotype controls were done for all samples at both 4 and 8 hr, and in all cases showed fewer than 1.5 % IFNg + events in the CD44low/int gate. (B-D) Each column shows the CD44 expression profile (top row) for the indicated cell type, and dot plots for IFN-γ × CD44 for cells stimulated with Ag for 4 hr (middle row) and 8 hr (bottom row). For dot plots, percent of cells in each gated quadrant is shown, and the mean fluroscence intensity (MFI; geometric mean) for cells in some quadrants are shown. OT-I.IFN-γ−/− cells examined in parallel had fewer than 1.5% IFN-γ + events in the CD44 low/int gate at 4 or 8 hr (not shown). In an independent experiment examining cells stimulated for 8 hr, the percent IFN-γ+ CD44 low/int cells for OT-I, pmel, P14, and OT-I. IFN-γ−/− were 43.1%, 8.6%, 27.4% and 1.0% respectively.
For all 3 TCR transgenic cell types, a large fraction of the CD44hi population produced IFN-γ within 4 hr of stimulation, as expected for memory cells. In addition, however, significant fractions of the CD44low and CD44int populations also produced IFN-γ, albeit levels were lower based on mean fluorescent intensities (MFI)) and production appeared to be somewhat slower (Fig. 1). IFN-γ production by the CD44low and CD44int populations was quite comparable. While readily detectable, the levels of IFN-γ produced early by naïve cells were two to three logs lower than levels produced by effector cells after 3 days of differentiation and re-stimulation with Ag (see below).
In order to better define the stimulation requirements for early IFN-γ production, naïve CD44low OT-I cells were purified by negative selection (see Materials and Methods) and stimulated in vitro using aAPC having class I MHC-peptide complexes immobilized on the surface, with or without co-immobilized B7-1/Fc ligand. While IFN-γ production did not require B7-1-dependent costimulation, the fraction of CD44 low OT-I cells producing IFN-γ was increased 2- to 3-fold when B7-1 was present (Fig. 2A and B). The contribution from B7-1-dependent costimulation was not due to more IL-2 being produced, as addition of IL-2 to cultures stimulated with just Ag on aAPC did not increase IFN-γ production (Fig. 2B). The response of the purified naïve CD8 T cells to aAPC stimulation strongly argues that Ag and B7-1-dependent costimulation are the necessary and sufficient signals to activate this response, and that cytokines or other surface ligands on Ag presenting cells are not involved.
Figure 2. Early IFN-γ production by naïve cells is increased by B7-1-dependent costimulation but not IL-2, and is rapid and transient.
(A) Purified CD44low OT-I T cells were stimulated in vitro for 8 hr with Ag alone or Ag along with co-immobilized B7-1/Fc ligand (Ag/B7), and IFN- γ production was determined by IC staining. Results shown are mean and range for duplicate samples. Five independent experiments confirmed that Ag/B7 stimulation results in 2- to 3-fold greater numbers of IFN-γ+ cells than does Ag alone. (B) Purified CD44low OT-I (black line) and OT-I.IFNγ−/− (solid gray) T cells were stimulated with aAPC having just Ag, or Ag and B7-1 (Ag/B7) immobilized on the surface either without or with IL-2 added (2.5U/ml). IFN-γ production was determined by IC staining after 8 hours of stimulation. Percent IFN-γ+ OT-I cells is shown for the indicated gating. (C) Purified CD44 low OT-I cells were stimulated with Ag/B7 for varying times and IFN-γ production determined by IC staining. Shown are representative dot plots (CD44 × IFN-γ) of stimulated cells at 4 hr (top) and 8 hr (bottom). In all cases, samples stained with isotype control Ab had < 1% IFN-γ+ cells. (D) IFN-γ+ cells were determined as in (C), and are shown as the percent of total OT-I cells. Results are expressed as mean +/− SD at each time point for two independent experiments. Five independent experiments confirmed these results.
CD44 levels increase on stimulated cells beyond about 8 hours, so the time course for IFN-γ production by naïve CD44low cells in a mixed population cannot be determined. This can be determined using purified CD44low cells, however, since the purification yields a population that is predominantly CD44low, with only a small number of CD44int and almost no CD44hi cells (Fig. 2C). IFN-γ+ CD44low cells are readily detected within 4 hr (Fig. 2C top) and numbers increase by 8 hr (Fig. 2C bottom), when CD44 expression is beginning to increase on the stimulated cells. Beyond 8 hr, the response declines (Fig. 2D). Thus, IFN-γ production by the naïve CD44low cells is rapid and transient. In nine experiments examining Ag-stimulated IFN-γ production by purified CD44low OT-I cells obtained from mice ranging in age from 4 to 14 weeks, the percent of cells that were IFN-γ+ at 8 hrs was 23.8% +/− 5.8% (avg+/− SD), and there was no correlation between age of the mice and percent of IFN-γ producers.
A large fraction of CD44hi memory cells rapidly produce IFN-γ upon restimulation with Ag, and we therefore compared the Ag dose response for early IFN-γ production by the OT-I cells having low, intermediate and high levels of CD44. OT-I cells from LN of naïve mice have all three populations (Fig. 3A), and cells in each population produce IFN-γ within 8 hr of stimulation with aAPC (Ag and B7-1/Fc) (Fig. 3B and C). When IFN-γ production was examined at varied peptide Ag concentrations, the fraction of IFN-γ+ cells plateaued at about 25% for the CD44low/int cells and about 75% for the CD44hi cells (Fig. 3D). Similarly, the average amounts of IFN-γ produced as measured by mean fluorescence intensity plateaued at higher peptide concentrations, and levels were about two to three times higher for memory versus naïve cells (Fig. 3E). Thus, while both CD44 hi and CD44 low/int cells produce IFN-γ early after stimulation and the Ag dose responses are very similar (Fig. 3D and E), a smaller fraction of the CD44low/int population produces IFN-γ and they produce distinctly less than do CD44 hi cells.
FIGURE 3. Peptide Ag dose response for early IFN-γ production.

LN cells from OT-I mice (8 weeks old) were stained with anti-CD44 mAb and stimulated in vitro with aAPC prepared by immobilizing H-2Kb.DimerX and B7-1/Fc on the surface and pulsing with varying concentrations of peptide Ag prior to being washed and added to the cultures. After 8 hr of stimulation the cells were permeabilized and stained for IFN-γ. (A) CD44 expression on OT-I cells incubated in the absence of peptide Ag. (B) IFN- γ expression of OT-I cells incubated for 8 hr in the absence of Ag. (C) Representative dot plot of OT-I cells stimulated with peptide Ag (10−6 M) for 8 hr. (D) The percent of IFN-g+ cells of the total cells in the CD44low/int gate and in the CD44hi gate, using gates shown in C, at varying peptide concentrations. (E) The MFI (geometric mean) of the IFN-g+ cells in the CD44low/int and CD44hi gates. At the lowest peptide concentrations there were too few events to obtain meaningful MFI values.
Purified naïve cells from OT-I.Rag−/− CD8 T cells produced more early IFN-γ than did OT-I cells, ruling out a potential requirement for dual TCR expression (Fig. 4A-C). IL-12 and Type I IFN both stimulate development of strong effector IFN-γ production by cells stimulated with Ag and B7-1 for 3 days (28). To determine if endogenous levels of these cytokines in normal mice might be responsible for some in vivo priming of naïve cells for IFN-γ production, we examined early IFN-γ production by OT-I cells deficient for both the IL-12- and Type I IFN receptors (Fig. 4D-F). The receptor-deficient cells included CD44 low, CD44 int and CD44 hi populations (Fig. 4D). None of the cells produced IFN-g when cultured in the absence of Ag (Fig. 4E), but when Ag was present they exhibited the same pattern of early IFN-γ production as wt OT-I, with CD44hi cells making the highest levels and a fraction of the CD44low and CD44int cells making somewhat lower levels (Fig. 4F). The peptide dose response for the OT-I.IL-12βR−/−.IFNAR−/−cells was essential the same as for wild type OT-I (not shown). Thus, in vivo priming by IL-12 and/or Type I IFN is not required for naïve cells to have the capacity to produce early IFN-γ in response to Ag-dependent stimulation.
Figure 4. Naïve OT-I cells from Rag−/− mice and mice deficient for both IL-12 and Type I IFN receptors produce early IFN-γ.
(A-C) Purified CD44low OT-I.IFNγ−/− (A) OT-I (B), and OT-I.Rag−/− cells (C) were stimulated with aAPC having Ag and B7-I immobilized on the surface, and IFN-γ production determined by IC staining after 8 hours. Isotype control Ab staining is solid gray. Percent IFN-γ+ cells is shown for the indicated gating. Shown are representative histograms from triplicate samples. (D) CD8 T cells from OT-I cells deficient for IL-12 and Type I IFN receptors (OT-I.IL-12βR−/−.IFNAR−/−) were stimulated for 8 hr with aAPC having Ag and B7-I/Fc on the surface, stained with anti-CD44 mAb, and IFN-γ production determined by IC staining. The CD44 expression profile of cells at 8 hr is shown. (E) A representative dot plot (CD44 × IFN-γ) of cells stimulated with aAPC that were not pulsed with peptide Ag, i.e. a no Ag control. (F) A representative dot plot of cells stimulated with aAPC that had been pulsed with Ag. In an independent experiment, 13% of the CD44low/int cells produced IFN-γ. The peptide dose response for the OT-I.IL-12βR−/−.IFNAR−/− cells was essential the same as for wild type OT-I (not shown).
IFN-γ signals for development of weak effector functions, but is not required for IL-12-mediated development of effector functions
Optimal development of effector functions by CD8 T cells requires a third signal that can be provided by IL-12 or IFN-α/β (9, 28). However, some weak cytolytic activity develops in the absence IL-12 or IFN-α/β, and stimulation with just Ag and B7-1 upregulates weak and transient expression of genes for many effector proteins, including grzB and IFN-γ(22). In order to determine whether the IFN-γ produced early in response to stimulation through the TCR and CD28 might contribute to this limited differentiation of the cells, OT-I T cells that lacked the gene for either IFN-γ (OT-I. IFN-γ−/−) or the receptor for IFN-γ (OT-I. IFN-γR−/−) were examined.
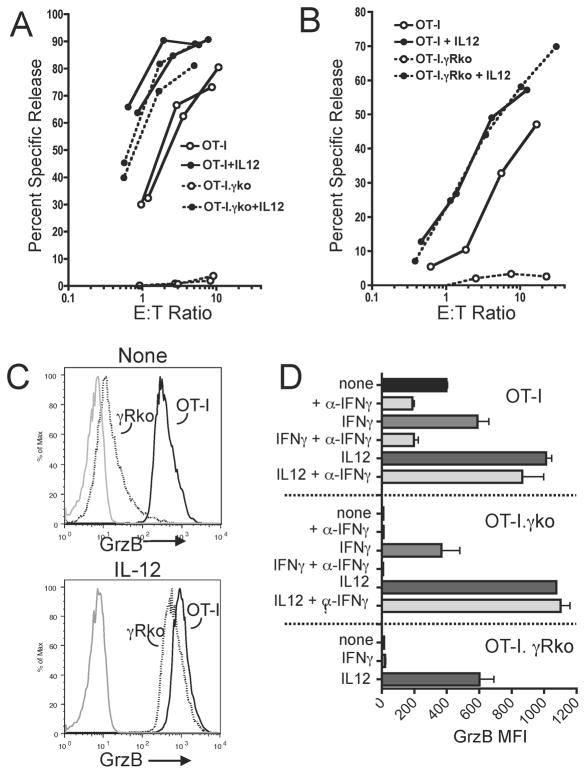
Stimulation of wt OT-I cells with Ag/B7 results in some Ag-specific cytolytic activity, but at a level that is about 80% to 90% less (based on comparison of E:T ratios required for a given level of lysis) than the activity of cells stimulated with Ag/B7 in the presence of IL-12 (Fig. 2A and B). This weak cytolytic activity is absent, however, in OT-I. IFN-γ−/− or OT-I. IFN-γR−/− cells stimulated with Ag/B7 (Fig. 2A and B). Thus, IFN-γ provides a signal necessary for development of weak cytolytic function in response to Ag/B7-dependent signals. However, it is not necessary for development of strong cytolytic activity when IL-12 is present; both OT-I. IFN-γ−/− and OT-I. IFN-γR−/− cells develop potent activity in response to Ag/B7 and IL-12 (Fig. 5A and B).
FIGURE 5. IFN-γ signals for development of weak effector functions, but is not required for IL-12-mediated development of effector functions.
Naïve OT-I, OT-I. IFN-γ−/− (OT-I.γ ko), and OT-I. IFN-γR−/− (OT-I.γ Rko) cells were stimulated for 3 days in vitro with Ag/B7 in the absence or presence of IL-12, washed and assayed. (A) OT-I (solid lines) and OT-I.γ ko (dashed lines) were stimulated for 3 days with Ag/B7 in the absence (open symbols) or presence of IL- 12 (filled symbols) and cytolytic activity determined in a 4 hr 51Cr-release assay. Separate lines are shown for cells from independent cultures of each cell type. (B) As in A, but examining OT-I.γ Rko cells (dotted lines). For nine independent experiments, cytolytic activity of OT-I cells in the absence of IL-12 was 12% +/− 9% (SD) of the activity of cells stimulated with IL-12, while no activity was detected for OT-I.γko or OT-I.γRko cells. (C) GrzB levels were determined by IC staining and representative histograms are shown. Solid lines are OT-I cells and dashed lines are OT-I. IFN-γRko cells stimulated with Ag/B7 in the absence (top) or presence (bottom) of IL- 12. Gray lines are isotype control Ab. (D) GrzB levels are shown as mean fluorescence intensity (MFI: geometric mean) of IC staining. Additions to the 3 day cultures with Ag/B7 are shown, and included neutralizing anti- IFN-γ Ab (α-IFNγ), IFN-γ and IL-12 in various combinations. Values are means and ranges for duplicate samples.
IFN-γ signaling effects on grzB expression paralleled the effects on development of cytolytic activity. Stimulation with Ag/B7 resulted in some upregulation of grzB expression in wt OT-I cells but upregulation was minimal in OT-I. IFN-γ−/− or OT-I. IFN-γR−/− cells (Fig. 5C and D). IL-12 increased GrzB expression in OT-I cells two- to three-fold, and comparably high levels were expressed in OT-I. IFN-γ−/− or OT-I. IFN-γR−/− cells when IL-12 was present. Thus, IFN-γ signaling supports upregulation of grzB but is not required for IL-12-dependent upregulation. This was further confirmed by examining effects of adding IFN-γ or neutralizing anti- IFN-γ mAb to cultures stimulated with Ag/B7 in the absence of IL-12 (Fig. 5D). Addition of IFN-γ to cultures of OT-I. IFN-γ−/− cells increased grzB expression to levels comparable to those in OT-I cells, and this increase was blocked when anti- IFN-γ mAb was also added. As expected, addition of IFN- γ to OT-I. IFN-γR−/− cells did not increase grzB levels. Addition of neutralizing anti- IFN-γ mAb partially inhibited Ag/B7-dependent upregulation in OT-I cells, while addition of IFN-γ increased expression (Fig. 5D). Anti- IFN-γ mAb eliminated the increase resulting from exogenous IFN-γ but some upregulation remained, which is likely due to autocrine signaling being less susceptible to blockade by ligand-neutralizing antibody (30). Consistent with grzB upregulation in response to IL-12 being independent of IFN-γ signaling, addition of anti- IFN-γ mAb to OT-I cells stimulated in the presence of IL-12 did not significantly reduce grzB expression.
Use of OT-I. IFN-γR−/− cells made it possible to also examine early IFN-γ signaling effects on development of the ability to produce IFN-γ as an effector cytokine. The receptor-deficient cells cannot receive an IFN-γ signal, but retain the ability to produce IFN- γ. Furthermore, the OT-I. IFN-γR−/− cells produce low levels of early (8 hr) in response to stimulation with Ag/B7 (Fig. 6, A-C). Over the 3-day course of differentiation, effector CD8 T cells can develop the ability to rapidly produce high levels of IFN-γ upon re-encountering Ag. To assess the signaling requirements for this response, cells were stimulated for 3 days, washed, incubated with peptide Ag for 4 hr in the presence of monensin to block secretion, and the level of IFN-γ production then determined by IC staining. Stimulating wt OT-I cells with just Ag/B7 results in some effector IFN-γ production by day 3, and this is substantially reduced by addition of anti- IFN-γ mAb during the 3 day culture period (Fig. 6D). Production of IFN-γ by OT-I cells is strongly increased if IL-12 is present during the culture period, and this is not blocked by the presence of anti- IFN-γ mAb during culture (Fig. 6E). In contrast to wild-type cells, OT-I. IFN-γR−/− cells do not make effector IFN-γ after stimulation for 3 days with just Ag/B7, although they do produce IFN-γ at 8 hr in response to this stimulus. The OT-I. IFN-γR−/− cells do produce a strong effector IFN-γ response, comparable to that of OT-I cells, when IL-12 is present during the 3 days of culture (Fig. 6G). Thus, as is the case for grzB expression and cytolytic function, early IFN-γ signaling promotes development of weak effector IFN-γ production capacity in cells responding to Ag/B7, but is not necessary for development of a strong response when IL-12 is present.
FIGURE 6. Early IFN-γ signals for development of weak late IFN- γ effector production, but is not required for IL-12-mediated development of strong IFN-γ effector function.
(A – C) OT-I, OT-I. IFN-γR−/−, and OT-I. IFN-γ−/− cells were stimulated for 8 hr with Ag/B7 and IFN-γ production determined by IC staining. (D – G) OT-I or OT-I. IFN-γR−/− cells were cultured for 3 days with Ag/B7 and either no addition (D and F) or with IL-12 added (E and G), and IFN-γ production then determined by IC staining after 4 hr re-stimulation with peptide Ag. Gray lines are isotype Ab controls. Thin lines in (D) and (E) are cells that had neutralizing anti- IFN-γAb present during the culture period. Representative histograms are shown.
To determine if the weak function that develops in vitro in response to IFN-γ results in physiologically relevant in vivo function, cells were tested for their ability to mediate tumor growth control in an adoptive transfer model. OT-I and OT-I. IFN-γR−/− cells were stimulated for three days in vitro with Ag/B7in the absence or presence of IL-12, washed, and adoptively transferred into mice with progressing B16.OVA tumors growing subcutaneously. Mice had been inoculated 10 days earlier with B16.OVA, and all had visible, palpable tumors at the time of effector cell transfer. OT-I effector cells generated in the absence of IL-12 (OT-I/None) caused significant control of tumor growth (p<0.014 versus control), while OT-I. IFN-γR−/− effector cells (IFN-γRko/None) did not control tumor (Fig. 7). Thus, the weak function that develops in response to IFN-γ signaling can be sufficient to mediate significant in vivo control of Ag-bearing cells.
FIGURE 7. IFN-γ-dependent differentiation yields effector cells that can mediate tumor growth control.
OT-I and OT-I. IFN-γR−/− (OT-I.γ Rko)cells were stimulated in vitro with Ag/B7 for 3 days with no cytokine addition (None) or with IL-12 added. Cells were then washed and adoptively transferred into mice bearing subcutaneous B16.OVA tumors that had been growing for 10 days (7 mice/group), and tumor size was determined by two right-angle measurements on day 21 of tumor growth (i.e. 11 days after adoptive transfer). Results are shown as mean tumor area (mm2) +/− SD, and p values are by Student’s T test. Essentially the same results were obtained in a second independent experiment. Comparison of OT-I/IL-12 versus IFNγRko/IL-12 at 20 × 105 cell input yields a p value of 0.03, and comparision at 2 × 105 cell input yields a p value of 0.07.
Consistent with in vitro levels of effector function, OT-I and OT-I. IFN-γR−/− effector cells generated in the presence of IL-12 (OT-I/IL12 and IFN-γRko/IL12) mediated strong tumor growth control; tumor regression began within a few days of transfer and growth was controlled for more than 11 days following transfer (Fig 7). OT-I effector cells generated in the presence of IL-12 were greater than 10-times more effective than those generated in its absence. In addition, and consistent with in vitro development of strong effector function with IL-12 in the absence of IFN-γ signaling, OT-I. IFN-γR−/− cells generated in the presence of IL-12 mediated strong tumor control, and were only marginally if at all less effective than OT-I cells (Fig. 7).
IFN-γ can synergize with IFN α in supporting development of effector functions
IFN-α acts comparably to IL-12 as a third signal to support development of strong effector functions and long-term survival of naïve CD8 T cells responding to Ag and costimulation (7, 28). However, in contrast to IL-12 driven differentiation that is largely independent of IFN- γ signaling, responses to IFN-α exhibit a strong dependence on intact IFN-γ signaling. Cytolytic activity that develops in response to Ag/B7 and IFN-α is reduced greater than 90% in OT-I. IFN-γ−/− cells in comparison to OT-I cells (Fig. 8A), and this is also the case for OT-I. IFN-γR−/− cells (data not shown and Fig. 8C). Similarly, IFN-α supports development of strong effector IFN-γ production by OT-I cells and this is greatly reduced for OT-I. IFN-γR−/− cells, while both cell types produce comparable effector IFN-γ following stimulation in the presence of IL-12 (Fig. 8B).
FIGURE 8. IFN-γ and IFN-α synergize to induce development of strong effector functions.
(A) OT-I (solid lines) and OT-I. IFN-γ−/− (dashed lines) cells were stimulated for 3 days in vitro with Ag/B7 and no cytokine addition (None), or either IL-12 (1μg/ml) or IFN-α (1,000U/ml) added to the cultures. Cells were then harvested and cytolytic activity was determined in a 51Cr-release assay. (B) OT-I (black lines) and OT-I. IFN-γR−/− (gray lines) cells were stimulated as in A and harvested at day 3. Cells were re-stimulated for 4 hours with SIINFEKL peptide and IFN-γ production then determined by IC staining (B). (C) OT-I (solid lines) and OT-I. IFN-γR−/−(dashed lines) cells were stimulated for 3 days with Ag/B7 and varying amounts of IFN-α added to the cultures as indicated. On day 3 cells were harvested and cytolytic activity (top panel), GrzB expression (middle panel) and IFN-γ production (bottom panel) were determined. Cytolytic activity (top) is expressed as Lytic Units per 1×106 cells.
To rule out the possibility that the IFN-γ-dependence observed in these experiments might be due to sub-optimal levels of IFN-α, dose responses were examined for OT-I and OT-I. IFN-γR−/− cells. As shown in Fig. 8C, cytotoxicity (expressed as Lytic Units), upregulation of grzB expression and effector IFN-γ production were all highly dependent on intact IFN-γ signaling even at very high doses of IFN-α. Thus, signaling through both the Type I and Type II IFN receptors appears to be highly synergistic in driving effector differentiation of CD8 T cells responding to Ag and costimulation.
IFN-γ-dependent upregulation of T-box transcription factors
The T-box transcription factors Eomes and T-bet play central roles in the differentiation of CD8 T cells, influencing both effector and memory development (31–35). In previous studies examining Eomes mRNA, we had found that expression was high in naïve CD8 T cells and decreased in cells responding to just Ag/B7 over 3 days in vitro, but was maintained at a high level when either IL-12 or IFN-α was present (22). A somewhat different result was obtained when Eomes protein levels were determined by IC staining. Eomes was marginally detectable in naïve cells but was upregulated by stimulation with just Ag and B7-1 within 44 hrs, and at this time levels were essentially the same whether or not IL-12 was present (Fig. 9A). Levels were also the same for OT-I and OT-I. IFN-γ−/− cells. By 72 hours Eomes levels had decreased in cells stimulated with just Ag/B7 and remained somewhat higher if IL-12 was present, and here too levels were comparable for OT-I and OT-I. IFN-γ−/− cells. Thus, it appears that IFN-γ signaling is not involved in regulation of Eomes protein expression in cells responding to Ag and costimulation.
FIGURE 9. IFN-γ upregulates expression of T-bet but does not affect Eomes expression.

(A) OT-I and OT-I. IFN-γ−/− cells were stimulated with Ag/B7 without added cytokine (None) or with IL-12 added. At the indicated times cells were harvested and Eomes protein expression determined by IC staining. Expression in naïve cells (left panels) was also determined. Gray lines are isotype control Ab. (B) Cells were stimulated as above with no cytokine added (None), or with IFN-γ or IL-12 added. Cells were harvested after 72 hr and T-bet protein levels determined by IC staining. Gray line is isotype control Ab, and vertical dotted lines are to aid comparisons. (C) Cells were stimulated and T-bet expression levels determined as in B at the indicated times. Results are expressed as mean fluorescence intensity (MFI: geometric mean). (D) OT-I and OT-I. IFN-γ−/− cells were stimulated with Ag/B7 and varying concentrations of IFN-α. Cells were harvested at 72 hr and T-bet levels determined by IC staining. Results are expressed as MFI (geometric mean) of T-bet.
When intracellular staining was used to examine levels of T-bet protein at day 3, expression was found to be upregulated in wt OT-I in response to Ag/B7. Addition of IFN-γ to the cultures had no effect, but stronger upregulation occurred when IL-12 was added (Fig. 9B). This is consistent with previous results showing a similar pattern of regulation of T-bet mRNA expression in response to stimulation with two or three signals (22). The level of T-bet expression in OT-I. IFN-γ−/− and OT-I. IFN-γR−/− cells stimulated with Ag/B7 was greatly reduced in comparison to levels in OT-I cells, but the strong upregulation that occurs in the presence of IL-12 was comparable for OT-I and the deficient cells (Fig. 9B). Addition of IFN-γ to cultures of OT-I. IFN-γ−/− increased T-bet expression to the same level as occurs in OT-I cells, but as expected had no effect on levels in OT-I. IFN-γR−/− cells. Examination of the time course for T-bet expression over the 3 days while differentiation is occurring further confirmed that T-bet expression is low at all times in OT-I. IFN-γ−/− cells in comparison to OT-I, but comparable if IL-12 was present (Fig. 9C). Thus, IFN-γ-dependent induction of effector functions likely involves, at least in part, stimulation of increased expression of T-bet.
T-bet expression was also examined in OT-I and OT-I. IFN-γ−/− cells stimulated over a broad range of IFN-α concentrations (Fig. 9D). As expected, OT-I cells upregulated T-bet in the absence of IFN-α, and expression increased with increasing concentrations of IFN-α. Minimal upregulation occurred for OT-I. IFN-γ−/− cells in the absence of IFN-α, but expression increased with increasing concentrations of IFN-α. The effects of IFN-γ and IFN-α signaling on T-bet levels appeared to be simply additive, and not synergistic as was seen for development of function (Fig. 8).
Discussion
The results described here demonstrate that naïve CD8 T cells rapidly and transiently produce low levels of IFN-γ that provides a signal to support limited differentiation and development of weak effector functions, and this is likely mediated in part by upregulation of T-bet expression. Cytolytic activity and effector IFN-γ production are about ten-fold less than that of effector cells that differentiate in response to IL-12 or Type I IFN, but the cells can nevertheless have substantial in vivo function as demonstrated by their ability to control tumor growth. Unlike IL- 12 or Type I IFN, autocrine IFN-γ does not appear to program cells to survive long-term in vivo. When OT-I cells are stimulated in vitro as described here and are adoptively transferred into mice the cells do not survive long-term unless IL-12 was present during the in vitro stimulation (7), i.e. the autocrine IFN-γ signal does not program the cells to survive. Thus, it appears that IFN-γ signaling, along with Ag and costimulation, supports development of a short-lived population of suboptimal effector cells. These are not the equivalent of the ‘short-lived effector cells’ (SLEC) defined by high KLRG1 expression that arise in response to infections (36, 37), since fewer than 5% of the cells express KLRG1 either in vitro or following transfer into normal mice (data not shown). IL-12 or Type I IFN can provide a third signal that supports development of optimal effector functions and memory, and differentiation in response to these cytokines displays quite distinct dependencies on IFN-γ signaling. Responses driven by IL-12 are largely independent of IFN-γ, while IFN-α and IFN-γ act synergistically to promote optimal development of functions.
Haring et. al. (16) have shown that naïve CD8 T cells responding to Listeria monocytogenes infection receive an IFN-γ signal within 12 hours of infection, as measured by IFN- γR-dependent phosphorylation of STAT1. Beyond that time, as Ag-driven expansion is occurring, the IFN-γR2 chain is down-modulated and the cells become non-responsive to IFN-γ. Using the in vitro stimulation conditions described here, oligonucleotide microarray analysis showed that mRNA expression levels for IFN-γR1 and IFN-γR2 declined more than 8 to 10-fold within 24 hr of stimulation with Ag and B7-1, and neither IL-12 nor IFN-α affected the levels (22). These observations suggest that the IFN-γ signals that drive differentiation are delivered early in the response, during the same period when naïve cells are producing IFN-γ. By 24 hours after initial encounter with Ag, IFN-γ production is declining (Fig. 2C) and the ability of the cells to receive an IFN-γ signal is attenuated (16). By 48 to 72 hours the cells have acquired the ability to produce high levels of effector IFN-γ when they re-encounter Ag (Fig. 6 and data not shown), but during this time they remain refractory to IFN-γ signaling, and only regain partial responsiveness during the contraction phase of the response (16).
There are successive stages of IFN-γ production by CD8 T cells, with naïve cells poised to produce low levels of IFN-γ within hours of TCR and CD28 signaling, and signals from the IFN- γ then acting in a feed-forward manner to prime cells to make moderate levels of IFN- γ at later times when they re-encounter Ag (Fig. 6). IL-12 or IFN-α signals support a third stage characterized by very high levels of IFN-γ production in response to Ag (Fig. 6, E and G). T-bet and Eomes contribute to effector IFN-γ production (30, 31, 33–35, 38) and early IFN-γ signaling upregulates T-bet expression (Fig. 9B). However, regulation of the levels of IFN-γ production do not appear to simply be determined by T-bet and Eomes levels since IFN-γ production is highest on day 3 when levels of both transcription factors have substantially declined (Fig. 9). The differing stages of IFN-γ production in CD8 T cells are likely to result in part from differences in chromatin modification states of the IFN-γ gene, as is the case for CD4 T cells (12). IL-12 has been shown to cause long-range hyperacetylation in the promoter and exon regions of the IFN-γ gene in CD8 T cells stimulated with anti-CD3 mAb (39).
Early signaling via autocrine IFN-γ plays an important role in CD4 T cell differentiation that in some respects resemble the role demonstrated here for CD8 T cells, but in other respects is quite distinct. Naïve CD4 T cells produce low levels of IFN-γ within 24 hours of stimulation with Ag, and this early IFN-γ acts in an autocrine manner to induce Stat1-dependent upregulation of T-bet that is important for driving Th1 differentiation (13, 14). T-bet in turn induces IL-12R expression, and IL-12 can then signal in a Stat4-dependent manner to further increase expression of T-bet and IFN-γ(13, 15). A recent study examining the kinetics of these processes has suggested a model in which differentiation of Th1 cells is mediated by positive feedback loops of TCR- IFN-γ-Stat1-T-bet and IL-12-Stat4-T-bet signaling that act sequentially in an interlinked manner (15). The sequential nature of the pathway is enforced, at least in part, by TCR signals repressing IL-12R expression so that IL-12R is only upregulated upon termination of TCR signaling. Thus, in both CD4 and CD8 T cells stimulation with Ag results in rapid production of low levels of IFN-γ that upregulate T-bet expression in an autocrine manner. For CD8 T cells this is sufficient to result in some limited effector functions, including late effector IFN-γ production. Unlike CD4 T cells, IFN-γ signaling to upregulate IL-12R on CD8 T cells is not necessary, since IL-12 can stimulate strong effector functions and long-term survival in its absence. It also appears unlikely that the IFN-γ signaling contribution to IFN-α-dependent responses results from upregulation of the Type I IFN receptor, since the IFN-α dose-response profiles for T-bet upregulation are the same in the absence or presence of IFN-γ signaling (Fig. 9D). IFN-γ can mediate cross-regulation of numerous signaling pathways, including ‘priming’ of macrophages for enhanced type I IFN responses (40, 41).
The results described here predict that the importance of an IFN-γ signal to a CD8 response will vary depending on whether the response is supported by IL-12 or IFN-α/β, and this has been observed. The response to LCMV infection is highly dependent on IFN-α/β signaling directly to the CD8 T cells (1, 5, 6), and Whitton and co-workers showed that P14 TCR transgenic CD8 T cells that lacked the IFN-γ receptor were also strongly impaired in their response to LCMV (17, 18). In contrast, OT-I cells lacking the IFN-γ receptor made normal responses to immunization with peptide Ag and LPS (20), which induces IL-12 production. Direct IFN-γ signaling to CD8 T cells in vivo may also have effects on proliferation and/or survival that are not apparent in the experiments described here. In addition to synergizing with IFN-α/β, the weak effector functions that develop in response to IFN-γ alone could potentially have a role in helping to initiate a response by mediating killing of cells bearing foreign Ag even in the absence of overt stimulation of APC and in the absence of inflammatory cytokines. This limited cytolysis could then make Ag available to APC for presentation to CD4 helper T cells that, if response occurs, could then provide help to the CD8 T cells to yield full differentiation and optimal effector functions, as well as survival signals leading to development of memory. If help is not generated, the short-lived nature of the effectors that arise in response to IFN-γ signaling would insure that a pathological response to inappropriate Ag does not develop. IFN-γ produced early by CD8 T cells responding to Ag presented on a DC may also promote IL-12 production by activated DC to further support the response (42).
The low level of IFN-γ produced early by naïve CD8 T cells appears sufficient to provide optimal signaling, at least in vitro. Supplementing cultures with exogenous IFN-γ does not significantly increase responses beyond those reached in response to just the endogenously produced cytokine. It appears likely that IFN-γ signaling to the naïve cells occurs in an autocrine manner, and this is consistent with the inability to fully block the endogenous IFN-γ-mediated effects by addition of neutralizing Ab, while the same Ab can block IFN-γ-effects mediated by exogenous addition of IFN-γ to OT-I. IFN-γ−/− cells. The source of IFN-γ to support responses in vivo may vary, and include both autocrine and paracrine sources. Stimulated dendritic cells (DC) can produce IFN-γ, but CD4−8− DC produce much more than do the CD4-8+ DC that play the major role in Ag cross-presentation to CD8 T cells (43). Antigen-specific CD8 T cells responding to infections or tumors represent very heterogeneous populations with respect to surface marker expression and functions, including levels of grzB expression and ability to produce effector IFN-γ, and the basis for this heterogeneity is not well understood. It is likely influenced by many parameters, including TCR affinities for Ag and the extent and duration of Ag exposure. In addition, though, it seems likely that much of the heterogeneity derives from the cells having differing exposure to cytokines, including IFN-γ, IFN-α/β and IL-12, as they are responding to Ag.
In the classical ‘two-signal’ model for T cell activation, signals from the TCR and a costimulatory receptor, usually CD28, are required to activate the cells and avoid tolerance. A requirement for a third signal for naïve CD8 T cells was revealed with the finding that activation through just TCR and CD28 receptors stimulated proliferation of the cells, but was not sufficient to support optimal development of effector functions and memory, and the cells were rendered tolerant. Signaling via IL-12 or IFN-α/β could support full activation, and responses still required costimulation through the CD28 receptor. Thus, the cytokines do not provide an alternate form of costimulation, but rather a distinct third signal. In support of this ‘three-signal’ model, either IL-12, Type I IFNs or both have been shown to support in vivo responses to pathogens, transplants, tumors and adjuvants (2–5, 7, 44). The results described in this report show that IFN-γ alone does not provide a third signal, in that it does not stimulate optimal development of function nor does it provide a survival signal to allow an expanded memory population to develop. However, it does appear to be necessary for IFN-α/β, but not for IL-12, to have full signal 3 activity. Thus, the signal 3 cytokines that support CD8 T cell responses are IL-12 and IFN-γ:IFN-α/β.
Acknowledgments
We would like to thank Drs. Stephen Jameson and Brian Fife for helpful comments and suggestions, and critical reading of the manuscript.
Footnotes
This work was supported by grants AI35296 and AI34824 from the National Institutes of Health.
References
- 1.Aichele P, Unsoeld H, Koshella M, Schweier O, Kalinke U, Vucikuja S. Cutting Edge: CD8 T cells specific for lymphocytic choriomeningitis virus require Type I IFN receptor for clonal expansion. J Immunol. 2006;176:4525–4529. doi: 10.4049/jimmunol.176.8.4525. [DOI] [PubMed] [Google Scholar]
- 2.Curtsinger J, Gerner MY, Lins DC, Mescher MF. Signal 3 availability limits the CD8 T cell response to a solid tumor. J Immunol. 2007;178:6752–6760. doi: 10.4049/jimmunol.178.11.6752. [DOI] [PubMed] [Google Scholar]
- 3.Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Curr Op Immunol. 2010;22:1–8. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Filatenkov AA, Jacovetty EL, Fischer UB, Curtsinger JM, Mescher MF, Ingulli E. CD4 T Cell-Dependent Conditioning of Dendritic Cells to Produce IL-12 Results in CD8-Mediated Graft Rejection and Avoidance of Tolerance. J Immunol. 2005;174:6909–6917. doi: 10.4049/jimmunol.174.11.6909. [DOI] [PubMed] [Google Scholar]
- 5.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–650. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson LJ, Kolumam GA, Thomas S, Murali-Krishna K. Innate inflammatory signals induced by various pathogens differentially dictate the IFN-I dependence of CD8 T cells for clonal expansion and memory formation. J Immunol. 2006;177:1746–1754. doi: 10.4049/jimmunol.177.3.1746. [DOI] [PubMed] [Google Scholar]
- 7.Xiao Z, Casey KA, Jameson SC, Curtsinger JM, Mescher MF. Programming for CD8 T cell memory development requires IL-12 or Type I IFN. J Immunol. 2009;182:2786–2794. doi: 10.4049/jimmunol.0803484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ben-Sasson SZ, Hu-Li J, Quiel J, Cauchetaux S, Ratner M, Shapira I, Dinarello CA, Paul WE. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci U S A. 2009;106:7119–7124. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF. Inflammatory cytokines provide third signals for activation of naïve CD4+ and CD8+ T cells. J Immunol. 1999;162:3256–3262. [PubMed] [Google Scholar]
- 10.Pape KA, Khoruts A, Mondino A, Jenkins MK. Inflammatory cytokines enhance the in vivo clonal expansion and differentiation of antigen-activated CD4+ T cells. J Immunol. 1997;159:591–598. [PubMed] [Google Scholar]
- 11.Schmidt CS, Mescher MF. Adjuvant effect of IL-12: conversion of peptide antigen administration from tolerizing to immunizing for CD8+ T cells in vivo. J Immunol. 1999;163:2561–2567. [PubMed] [Google Scholar]
- 12.Schoenborn JR, Wilson CB. Regulation of interferon-gamma during innate and adaptive immune responses. Adv Immunol. 2007;96:41–101. doi: 10.1016/S0065-2776(07)96002-2. [DOI] [PubMed] [Google Scholar]
- 13.Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naïve CD4+ T cells. Nat Immunol. 2002;3:506–508. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 14.Lighvani AA, Frucht DM, Jankovic D, Yamane H, Aliberti J, Hissong BD, Nguyen BV, Gadina M, Sher A, Paul WE, O’Shea JJ. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci U S A. 2001;98:15137–15142. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schultz EG, Mariani L, Radbruch A, Hofer T. Sequential polarization and imprinting of Type I helper lymphocytes by interferon-g and interleukin-12. Immunity. 2009;30:673–683. doi: 10.1016/j.immuni.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Haring JS, Corbin GA, Harty JT. Dynamic regulation of IFN-gamma signaling in antigen-specific CD8+ T cells responding to infection. J Immunol. 2005;174:6791–6802. doi: 10.4049/jimmunol.174.11.6791. [DOI] [PubMed] [Google Scholar]
- 17.Whitmire JK, Eam B, Benning N, Whitton JL. Direct interferon-gamma signaling dramatically enhances CD4+ and CD8+ T cell memory. J Immunol. 2007;179:1190–1197. doi: 10.4049/jimmunol.179.2.1190. [DOI] [PubMed] [Google Scholar]
- 18.Whitmire JK, Tan JT, Whitton JL. IFN-gamma acts directly on CD8+ T cells to increase their abundance during virus infection. J Exp Med. 2005;201:1053–1059. doi: 10.1084/jem.20041463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puliaev R, Nguyen P, Finkelman FD, Via CS. Differential requirement for IFN-gamma in CTL maturation in acute murine graft-versus-host disease. J Immunol. 2004;173:910–919. doi: 10.4049/jimmunol.173.2.910. [DOI] [PubMed] [Google Scholar]
- 20.Sercan O, Stoycheva D, Hammerling GJ, Arnold B, Schuler T. IFN-gamma receptor signaling regulates memory CD8+ T cell differentiation. J Immunol. 2010;2010:2855–2862. doi: 10.4049/jimmunol.0902708. [DOI] [PubMed] [Google Scholar]
- 21.Tewari K, Nakayama Y, Suresh M. Role of direct effects of IFN-gamma on T cells in the regulation of CD8 T cell homeostasis. J Immunol. 2007;179:2115–2125. doi: 10.4049/jimmunol.179.4.2115. [DOI] [PubMed] [Google Scholar]
- 22.Agarwal PA, Raghavan A, Nandiwada SL, Curtsinger JM, Bohjanen PR, Mueller DL, Mescher MF. Gene regulation and chromatin remodeling by IL-12 and Type I interferon in programming for CD8 T cell effector function and memory. J Immunol. 2009;183:1695–1704. doi: 10.4049/jimmunol.0900592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mailliard RB, Egawa S, Cai Q, Kalinska A, Bykovskaya SN, Lotze MT, Kapsenberg ML, Storkus WJ, Kalinski P. Complementary dendritic cell-activating function of CD8+ and CD4+ T cells: helper role of CD8+ T cells in the development of T helper type 1 responses. J Exp Med. 2002;195:473–483. doi: 10.1084/jem.20011662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hogquist K, Jameson S, Heath W, Howard J, Bevan M, Carbone F. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 25.Palmer DC, Balasubramaniam S, Hanada K-i, Wrzesinski C, Yu Z, Farid S, Theoret MR, Hwang LN, Klebanoff CA, Gattinoni L, Goldstein AL, Yang JC, Restifo NP. Vaccine-Stimulated, Adoptively Transferred CD8+ T Cells Traffic Indiscriminately and Ubiquitously while Mediating Specific Tumor Destruction. J Immunol. 2004;173:7209–7216. doi: 10.4049/jimmunol.173.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pircher H, Burki K, Lange R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 27.Curtsinger JM, Johnson CM, Mescher MF. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J Immunol. 2003;171:5165–5171. doi: 10.4049/jimmunol.171.10.5165. [DOI] [PubMed] [Google Scholar]
- 28.Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Cutting Edge: Type I IFNs Provide a Third Signal to CD8 T Cells to Stimulate Clonal Expansion and Differentiation. J Immunol. 2005;174:4465–4469. doi: 10.4049/jimmunol.174.8.4465. [DOI] [PubMed] [Google Scholar]
- 29.Johnson LDS, Jameson SC. Self-specific CD8+ T cells maintain a semi-naïve state following lymphopenia-induced proliferation. J Immunol. 2010;184:5604–5611. doi: 10.4049/jimmunol.1000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lauffenburger DA, Oehrtman GT, Walker L, Wiley HS. Real-time quantitative measurement of autocrine ligand binding indicates that autocrine loops are spatially localized. Proc Natl Acad Sci USA. 1998;95:15368–15373. doi: 10.1073/pnas.95.26.15368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Intlekofer AM, Takemoto N, Kao C, Banerjee A, Schambach F, Northrop JK, Shen H, Wherry EJ, Reiner SL. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J Exp Med. 2007;204:2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Intlekofer AM, Takemoto N, Wherry EJ, Longworth AA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 33.Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, Reiner SL. Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan BM, Juedes A, Szabo SJ, von Herrath M, Glimcher LH. Antigen-driven effector CD8 T cell function regulated by T-bet. Proc Natl Acad Sci U S A. 2003;100:15818–15823. doi: 10.1073/pnas.2636938100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szabo SJ, Sullivan BM, Stemmann C, Satoskar AR, Sleckman BP, Glimcher LH. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science. 2002;295:338–342. doi: 10.1126/science.1065543. [DOI] [PubMed] [Google Scholar]
- 36.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL- 12. J Immunol. 2007;179:2074–2081. doi: 10.4049/jimmunol.179.4.2074. [DOI] [PubMed] [Google Scholar]
- 38.Glimcher LH, Townsend MJ, Sullivan BM, Lord GM. Recent developments in the transcriptional regulation of cytolytic effector cells. Nat Rev Immunol. 2004;4:900–911. doi: 10.1038/nri1490. [DOI] [PubMed] [Google Scholar]
- 39.Zhou W, Chang S, Aune TM. Long-range histone acetylation of the Ifng gene is an essential feature of T cell differentiation. Proc Natl Acad Sci USA. 2004;101:2440–2445. doi: 10.1073/pnas.0306002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu X, Herrero C, Li WP, Antoniv TT, Falck-Pederson E, Koch AE, Woods JM, Haines GK, Ivashkiv LB. Sensitization of IFN-gamma Jak-STAT signaling during macrophage activation. Nat Immunol. 2002;3:859–866. doi: 10.1038/ni828. [DOI] [PubMed] [Google Scholar]
- 41.Hu X, Ivashkiv LB. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity. 2009;31:539–550. doi: 10.1016/j.immuni.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leibundgut-Landmann S, Osorio F, Brown GD, Reis e Sousa C. Stimulation of dendritic cells via the dectin-1/Syk pathway allows priming of cytotoxic T-cell responses. Blood. 2008;112:4971–4980. doi: 10.1182/blood-2008-05-158469. [DOI] [PubMed] [Google Scholar]
- 43.Hochrein H, Shortman K, Vremec D, Scott B, Hertzog P, O’Keeffe M. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J Immunol. 2001;166:5448–5455. doi: 10.4049/jimmunol.166.9.5448. [DOI] [PubMed] [Google Scholar]
- 44.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]