Abstract
Hydrophilic matrix tablets are prone to mechanical stress while passing through the gastrointestinal tract, which may result in inappropriate drug-release characteristics. Intrinsic viscosity is a physical polymer property that can be directly compared across various types and grades of polymers and correlated with the mechanical susceptibility of swollen matrix tablets. Five tablet formulations containing different HPMC and HPC polymers were prepared and analyzed using an in vitro glass bead manipulation test. The dissolution rate results were modeled using the Korsmeyer–Peppas equation and a correlation was found between the fit constants k and n, goodness-of-fit measure parameters, and intrinsic viscosity. Moreover, the dissolution profiles were used to calculate the degree of mechanical susceptibility for each formulation, defined as the ratio of the average dissolution rate after manipulation and the initial dissolution rate before manipulation. It was confirmed that an increased intrinsic viscosity polymer value resulted in a decrease in mechanical susceptibility. Considering this, two simple rules were defined for designing robust matrix tablets with respect to mechanical stresses.
Key words: dissolution, HPC, HPMC, hydrophilic matrices, intrinsic viscosity, mechanical susceptibility
Introduction
Hydrophilic matrix tablets are one of the most common extended-release dosage forms. The mechanisms and modeling of drug release from hydrophilic matrices has been extensively studied and reviewed in the scientific literature (1–3), including in our previous work (4–6). In cases in which release from swollen tablets is predominately erosion-controlled, the tablet matrices are greatly influenced by hydrodynamic conditions, shear stress, and friction forces that occur in vivo or in vitro (7,8).
Mechanical stress applied to matrix tablets during gastrointestinal transit may lead to faster disruption of the gel layer and influence the plasma drug concentrations over time. This can change the therapeutic efficacy and safety and also result in non-bioequivalence of products in the case of generic drug development. Achieving product bioequivalence is especially critical when the formulations tested show different susceptibility to the mechanical stress applied because in vitro stress simulation tests are less predictive of in vivo behavior due to the complexity of the gastrointestinal tract (7,9).
In a fasting state, the stress on the tablet mainly depends on the migrating myoelectric complex, which cycles every 90 to 120 min and normally starts in the stomach. The movement of tablets during this phase is rapid and tablets are subject to increased mechanical stress, especially during gastric emptying (10,11). The destructive forces in the human stomach and small intestine were measured using a “Destructive force–Dependent Release System”, and the results obtained were 1.9 and 1.2 N, respectively (12). Theoretically, each matrix tablet with a swollen gel layer should be able to resist these mechanical forces in order to achieve the desired release profile.
Additionally, gastrointestinal motility is elevated during and after food intake. This causes even greater mechanical stress on matrix tablets. There is also evidence that food intake stimulates the transport of gastrointestinal content from the terminal small intestine into the colon, a mechanism that is known as gastroileal or gastro-ileocecal reflex, which can again exert elevated mechanical stress on the matrix tablet (10,13).
In the search for in vitro–in vivo correlation (IVIVC) or the in vitro–in vivo relationship (IVIVR), various gastrointestinal mechanical stress conditions can be simulated in vitro using a modified or novel dissolution apparatus. There are few examples, such as a rotation beaker apparatus (14), application of mechanical stress on swollen matrices with glass beads (15), or systems that simulate the pressure forces exerted by gut wall motility (16). These models were used to evaluate matrix tablet formulations and determine the robustness of the gel layer during dissolution testing by introducing the mechanical stress phase in the test.
The viscosity of the nascent gel layer formed on the matrix tablet has a major influence on both the water uptake kinetics and the erosion rate, and thereby determines the gel-layer robustness during periods of increased physical stress. The viscosity of the nascent gel layer can be adjusted by using different viscosity grades of the polymers and by varying the amount of the polymer in the tablet bulk (17).
The polymer viscosity grade is linked to the intrinsic viscosity of the polymer, which is in turn related to the polymer molecular weight, the degree and type of substitution, and the particular polymer–solvent system used. Several studies to date have described polymer properties that affect the release from matrix tablets (17,18). These studies predominantly evaluated the viscosity grades of the polymers tested, whereby viscosity was measured in water solution at a prescribed concentration. This polymer property is called apparent viscosity and needs to be experimentally determined separately for each polymer grade and concentration using a procedure that is time-consuming and subject to errors due to differences in methodology and experimental setup (own data). Moreover, various polymer types and grades employ different experimental procedures to characterize their apparent viscosity as part of their specification. As such, it is tedious and not straightforward to assess the equivalence of characteristics of different polymer types by simply comparing their apparent viscosities.
Intrinsic viscosity, however, is a physical polymer property independent from polymer concentration and can be directly compared across different types and grades of polymers, which makes it advantageous compared to apparent viscosity measurements. At low polymer concentrations, the intrinsic viscosity is related to the rate of increase of apparent viscosity as a function of polymer volume fraction. Therefore, polymers with higher intrinsic viscosity are more effective at increasing the apparent viscosity of the solution.
In our study, the intrinsic polymer viscosity was correlated specifically with the mechanical susceptibility (MS) of matrix tablets. The term “mechanical susceptibility” here refers to the decreased integrity of the swollen gel layer of matrix tablets after the application of mechanical stress. The more pronounced or higher the mechanical susceptibility of the gel layer, the faster the release of the incorporated drug substance after mechanical manipulation.
Our results show that an increased intrinsic viscosity value resulted in a decrease of mechanical susceptibility of the swollen gel layer. Considering this, two simple rules for designing robust matrix tablets with respect to the mechanical stresses was obtained. To establish the rules, formulations with different cellulose ether polymers—hydroxypropyl cellulose (HPC) and hydroxypropylmethyl cellulose (HPMC)—were prepared and their gel-layer robustness was evaluated using a specially designed dissolution testing procedure.
Materials and methods
Materials
A model drug indapamide with pH independent solubility of less than 0.1 mg/ml measured in aqueous buffer media, pH 1 to 8 at 37°C, was purchased from Biocon, India. Excipients used were HPMC USP Type 2208, grades K100M, K15M, K4M (Dow Chemical Company, Midland, MI, USA), HPC HXF (Hercules, Aqualon, Wilmington, DE, USA) lactose monohydrate 200 mesh (Friesland Campina, Netherlands), polyvinylpyrolydone PVP K30 (BASF, Ludwigshafen, Germany), microcrystalline cellulose Avicel PH 102 (FMC BioPolymer, Drammen, Norway), colloidal silica dioxide Aerosil 200 (Degussa, Frankfurt, Germany) and magnesium stearate (Mallinckrodt Chemical Inc., St. Louis, MO, USA). Reagents for HPLC assay, acetonitrile, methanol, EDTA and glacial acetic acid were obtained from Merck, Germany.
Preparation of the Tablet Formulations Studied
Five tablet formulations containing 1.5 mg of sulfonamide model drug (indapamide) were prepared. The drug exhibits weakly acidic properties (pKa 8.8) and solubility less than 0.1 mg/ml in aqueous buffer solutions ranging from pH 1 to 8 at 37°C. Formulations containing various cellulose ether derivatives were prepared as follows (see Table I for compositions). A model drug, lactose monohydrate, and polyvinylpyrolydone were blended and granulated with purified water using a Collette Gral—PRO 25 (Machines Collette, Belgium) high shear granulator. The granules were dried under vacuum and sieved with Frewitt MG 636 (Key International, USA), with an oscillating sieve using a mesh size of 0.5 mm. The granulate loss on drying (LOD) was 2.6% tested 30 min at 80°C using loss on drying balance (Mettler-Toledo International Inc.). These granules were used for all five formulations investigated. For each formulation, the prescribed amount of granules, selected cellulose ether derivative, colloidal anhydrous silica, and microcrystalline cellulose were blended in a bin blender (Erweka, Heusenstamm, Germany). Finally, magnesium stearate was added to obtain the final mixture. The final mixture was compressed into tablet cores 8 mm in diameter using a tablet press (Ima Kilian LX 18, Cologne, Germany) with a compression force of 7 to 10 kN to obtain tablets with a hardness of 100 to 120 N and a target mass of 200 mg (Kraemer automatic tablet tester, Germany).
Table I.
Compositions of Tablet Formulations Studied (mg)
| Formulation | F1 | F2 | F3 | F4 | F5 |
|---|---|---|---|---|---|
| Granulate with model drug (indapamide) | 88.1 | 88.1 | 88.1 | 88.1 | 88.1 |
| HPMC USP Type 2208, K15M (DOW) | 70.0 | ||||
| HPMC USP Type 2208, K4M (DOW) | 70.0 | 100.0 | |||
| HPMC USP Type 2208, K100M (DOW) | 70.0 | ||||
| HPC HXF (Hercules, Aqualon) | 70.0 | ||||
| Microcrystalline cellulose | 40.0 | 40.0 | 10.0 | 40.0 | 40.0 |
| Colloidal anhydrous silica | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Magnesium stearate | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Target mass | 200.0 | 200.0 | 200.0 | 200.0 | 200.0 |
Intrinsic viscosity and apparent viscosity
Intrinsic viscosity [η] is a fundamental property of the solute and solvent combination. It is defined as the relative increase of viscosity of the solution ((η − η0)/η0) divided by the volume fraction of the solute (φ), taken at the limit of zero solute fraction (Eq. 1; 19,20).
 |
1 |
Intrinsic viscosity values for cellulose derivatives were calculated or obtained from the literature, as presented in Table II. Apparent viscosities were obtained from manufacturer specifications and confirmed by our own experimental data (Table II). For HPMC types, all manufacturer specifications were experimentally confirmed. In the case of HPC, the experimentally determined apparent viscosity was higher compared to values found in the literature due to a lower solution temperature during measurement (see Table II for details). Apparent viscosities were measured using a viscosimeter (LVF Brookfield, Middleboro, MA, USA) as described in the USP procedure for cellulose derivatives (23).
Table II.
Polymer Type and Amount (w/w%) in Tablet Formulations Studied, Intrinsic Viscosity, and Apparent Viscosity Data
| Formulation | F1 | F2 | F3 | F4 | F5 |
|---|---|---|---|---|---|
| Polymer | K15M | K4M | K4M | K100M | HPC HXF |
| Amount (w/w%) | 35% | 35% | 50% | 35% | 35% |
| Intrinsic viscosity (dL/g) | 8.98a | 7.37a | 7.37a | 11.01a | 10.20b |
| Apparent viscosity (mPas) | 11,250–21,000c (13,367 ± 2,173)e | 3,000–5,600c (3,003 ± 500)e | 3,000–5,600c (3,003 ± 500)e | 80,000–120,000c (88,000 ± 11,314)e | 14,000–18,000d (55,150 ± 7,000)e |
bObtained from literature (20)
cApparent viscosity, 2% in water at 20°C, mPas (product specification, DOW)
dApparent viscosity, 2% in water at 25°C, mPas, using cylinder type Brookfield LVF viscosimeter (22)
eApparent viscosity, 2% in water, at 20°C, mPas, using cylinder type Brookfield LVF viscosimeter (experimental data (n = 3), measured according to USP procedure for cellulose derivatives
In vitro Dissolution Test Simulating Transition through the Pylorus
Dissolution tests were performed using a dissolution tester (Erweka DT6, Heusenstamm, Germany) coupled with a Vankel automatic sampler (Vankel VK8000, USA). Standard vessels with baskets (USP Apparatus 1) were used at a stirring rate of 100 rpm and 900 ml of purified water as a dissolution medium. The water was degassed prior the test with a Caleva MD1000 degasser (UK) and the temperature was set to 37°C ± 0.5°C. For each time point, 1.7 ml of samples were automatically collected and filtered through 4.0 μm tip filters (Erweka, Heusenstamm, Germany) to 2.0 ml vials. The dissolution medium was not replaced.
After 1.5 h from commencing the test, tablets were transferred to plastic tubes containing 5 ml of medium and 9 g of glass beads with a density of approximately 2.5 g/ml and 1 cm in diameter. The tubes were vertically shaken for 10 min on a laboratory shaker (IKA, Staufen, Germany) at 300 strokes/min. After this manipulation, the tablets were transferred back to baskets and the dissolution test continued. Sampling times were 1.5, 2, 4, 6, 8, 12, 16, and 20 h. A similar dissolution test was previously used by Sako et al. to simulate situations in the gastrointestinal tract with elevated mechanical stress (15). For comparison, the dissolution test of formulation F4 was performed without the mechanical manipulation.
HPLC Assay
Samples collected from the in vitro dissolution test were analyzed for the amount of dissolved drug using a 2695D Waters HPLC system with UV detection at wavelength 254 nm. A chromatographic column Chromolith Speed ROD RP-18e (Merck, Germany) with dimensions 50 × 4.6 mm was thermostated at 36°C. The mobile phase was composed of acetonitrile/methanol/NaEDTA/glacial acetic acid in a ratio of 275:175:550:1 (volume ratio). A NaEDTA solution was prepared by dissolving 1 g of EDTA dihydrate in 5,000 ml of purified water. The mobile phase flow rate was 2.0 ml/min and 100 μl of sample solution was injected from the vials, which were maintained at 4°C. Retention time of the model drug was around 1 min; the run time of the analysis was 1.3 min.
Modeling and Evaluation of Mechanical Susceptibility
Dissolution testing data were manipulated and analyzed within the R software environment for statistical computing and graphics (24). The non-linear least squares method was used to fit the different dissolution profiles. The Korsmeyer–Peppas release rate constant k and the exponent n were calculated by fitting the dissolution curves to Eq. 2.
 |
2 |
Q(t) is the percentage of drug released at a given time point t. The exponent n was used as a criterion to evaluate the release-mechanism kinetics.
Moreover, the degree of MS was quantified by comparing the quantity of dissolved drug after the application of mechanical stress with the linear extrapolation curve defined by the initial slope of the dissolution curve (Eq. 3). The initial dissolution rate (ΔQ/Δt)initial was defined as the average dissolution rate at 1.5 h, immediately before the mechanical stress treatment.
 |
3 |
Results and discussion
The Influence of Mechanical Stress on In Vitro Drug Release from Various Cellulose Ether-Based Matrix Tablets
The results of the mechanical stress simulation test demonstrate that the dissolution profiles from various tablet formulations were very similar before mechanical manipulation. As evident from Fig. 1, the mechanical manipulation produced a “kink” in the dissolution curve at the 1.5 h time point. Afterwards, the drug release was the slowest for the formulation F5 containing HPC HXF, followed by F4 containing HPMC K100M and F1 with HPMC K15M (Fig. 1). These formulations were less affected by mechanical stress than F2 and F3 with HPMC K4M. The drug release from the F2 formulation was faster in comparison to F3 because the amount of the HPMC K4M as a matrix-forming agent was lower.
Fig. 1.
Mean dissolution profiles for tablet formulations tested (compositions from F1 to F5 are presented in Table I) under mechanical stress test conditions in water as a dissolution medium: a 20 h time scale with SD error bars (n = 3), b 3 h time scale, manipulation at 1.5 h is indicated
To gain more insight into drug-release mechanisms, the dissolution results obtained were used to calculate the Korsmeyer–Peppas constant k and the exponent n by fitting the data to Eq. 2 (Fig. 2). The exponent n can be used as a criterion to evaluate the release-mechanism kinetics and usually assumed values between 0.45 and 0.89 (for cylindrical shapes). When n is equal to 0.89, the release kinetic is of zero order, and hence, with values for n approaching 0.89, the dissolution profile of a drug becomes progressively more linear (1,2). The exponent n is also a descriptive measure of the drug-release mechanism. For values of n approaching 0.89, the drug release is more erosion-controlled, and for values of n approaching 0.45 the release is more diffusion-controlled (1,2).
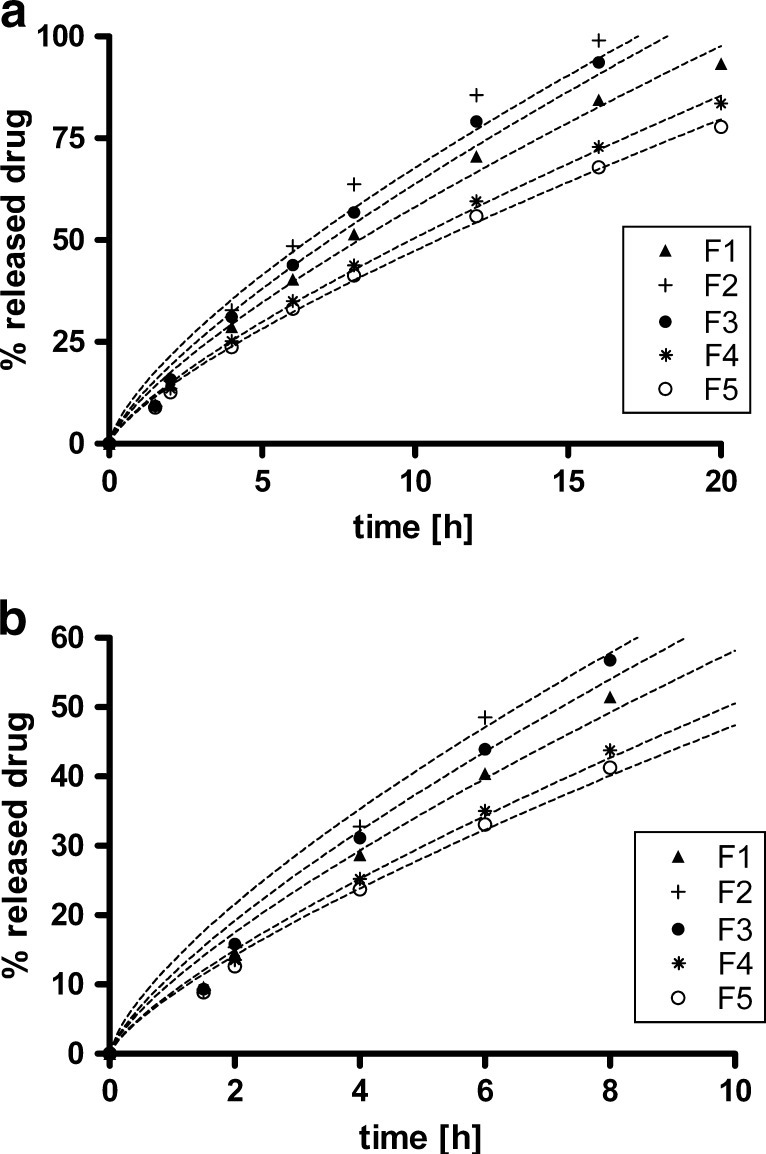
Fig. 2.
Mechanical stress simulation test: percent of dissolved drug versus time (h) for tablet formulations studied (compositions from F1 to F5 are presented in Table I) using water as a dissolution medium. Dashed lines represent curves after fitting the dissolution profiles to Eq. 2: a 20 h time scale, b same plot up to 10 h
For all formulations tested, in our case the exponent n value was approaching 0.89, hence the release mechanism was predominately erosion-controlled (Table III). In this case, the swollen polymer gel layer erodes from the matrix tablet surface due to the solubility of polymer in surrounding media and hydrodynamic stress. This mechanism is especially prominent in cases when solubility of the drug is low (note that the solubility of our drug was less than 0.1 mg/ml). After contact with the surrounding media, the matrix tablet swells and gel layer erodes from the surface, especially in the presence of other hydrodynamic forces; for example, gastrointestinal motility. The drug then dissolves and diffuses out from the eroded gel layer. In other words, the erosion of the hydrophilic polymer was faster compared to diffusion of the incorporated drug, and thus the release was more erosion-controlled (1,2).
Table III.
Korsmeyer–Peppas Release Rate Constants (k and n), Correlation Coefficient ( ), and Standardized Residual Error (
), and Standardized Residual Error ( ) for Tablet Formulations Studied
) for Tablet Formulations Studied
| Formulation | k | n |

|

|
|---|---|---|---|---|
| F1 | 10.3785 | 0.7482 | 0.99217 | 3.183 |
| F2 | 13.1492 | 0.7117 | 0.97153 | 7.013 |
| F3 | 11.3894 | 0.7481 | 0.98698 | 4.532 |
| F4 | 8.8124 | 0.7582 | 0.99742 | 1.586 |
| F5 | 8.4229 | 0.7499 | 0.99709 | 1.569 |
| F4 (no stress) | 7.1922 | 0.8156 | 0.99845 | 1.229 |
The best-fitted values k and n for each formulation were calculated using a non-linear least squares procedure. Correlation coefficients and standardized residual error of the fits were also calculated. The standardized residual error  is defined by
is defined by
 |
4 |
where εj is the fit error for time point j and m is the total number of data points. The standardized residual error is a convenient measure of fit prediction accuracy. The fit results are summarized in Table III.
In order to assess how much the “kinked” dissolution curves deviate from the Korsmeyer–Peppas model, we fitted the entire range instead of limiting the fit to the time interval where less than 60% of the drug is dissolved, as is usually done (25). This approach is validated by the good fit to data of formulation F4, where the dissolution test was performed without mechanical stress. In this case, the drug release was most linear and the fit was excellent throughout the entire range of time points (Table III). Using empirical dissolution models, such as the Weibull or Gompertz equations, we obtain better fits in the case of dissolution profiles for F1, F2, and F3. However, due to their empirical nature no mechanistic insight can be obtained from those results.
Correlation between Polymer Intrinsic Viscosity and Drug Release
The order of intrinsic viscosities of polymers investigated was (in increasing order): HPMC K4M < HPMC K15M < HPC HXF < HPMC K100M. The same polymer order was also determined for measured apparent viscosity. If viscosities are correlated with dissolution rates (Fig. 1, constant k in Table III), it is seen that lower intrinsic viscosities result in faster drug release. A similar pattern is also evident from apparent viscosities as given by our own measurements.
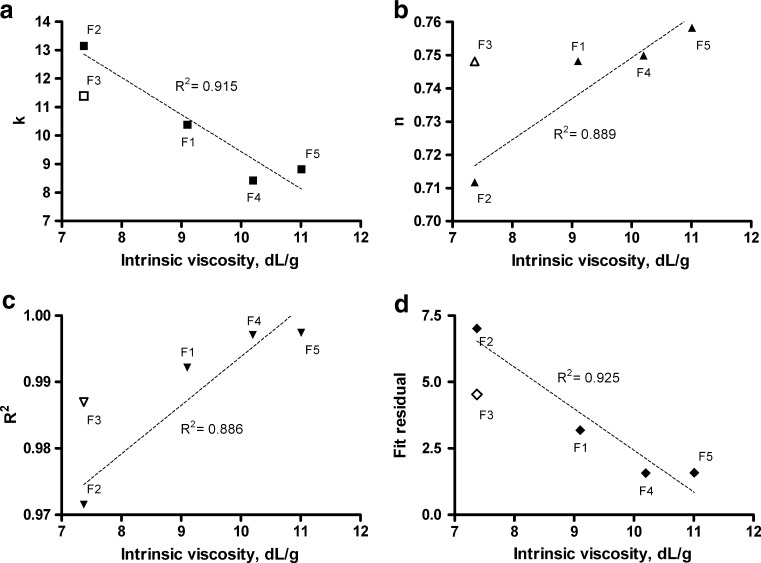
In addition, we used the correlation between fitted Korsmeyer–Peppas constants and intrinsic viscosity to evaluate the influence of intrinsic viscosity on the release mechanism (Fig. 3). The data suggested that the best-fitted Korsmeyer–Peppas constants (k and n) and resulting goodness-of-fit measures ( and
and  ) are correlated to the polymer intrinsic viscosity. The plots in Fig. 3 suggest that higher intrinsic viscosity is related to more linear drug-release profiles (higher values of exponent n) and in overall slower drug release (lower values of k). More importantly, both goodness-of-fit measures,
) are correlated to the polymer intrinsic viscosity. The plots in Fig. 3 suggest that higher intrinsic viscosity is related to more linear drug-release profiles (higher values of exponent n) and in overall slower drug release (lower values of k). More importantly, both goodness-of-fit measures,  and
and  , improve with higher intrinsic viscosity, indicating decreasing deviation from the Korsmeyer–Peppas model. This suggests the hypothesis that formulations utilizing polymers of higher intrinsic viscosity are more resilient to mechanical stress and are therefore less prone to the effects of mechanical stress. The concentration of polymer is important as well, as can be observed by comparing formulations F2 and F3 on Fig. 3.
, improve with higher intrinsic viscosity, indicating decreasing deviation from the Korsmeyer–Peppas model. This suggests the hypothesis that formulations utilizing polymers of higher intrinsic viscosity are more resilient to mechanical stress and are therefore less prone to the effects of mechanical stress. The concentration of polymer is important as well, as can be observed by comparing formulations F2 and F3 on Fig. 3.
Fig. 3.
Relationship of Korsmeyer–Peppas fit constants (k and n) and goodness-of-fit measures ( and
and  ) with respect to the polymer intrinsic viscosity. Open points were not included in the linear trend but are plotted on the same figure to show the effect of increased polymer concentration (F3 versus F2)
) with respect to the polymer intrinsic viscosity. Open points were not included in the linear trend but are plotted on the same figure to show the effect of increased polymer concentration (F3 versus F2)
In addition, the linear trends of the best-fitted Korsmeyer–Peppas constants and goodness-of-fit measures were plotted as a function of polymer intrinsic viscosity. These roughly linear trends reduce in significance if plotted against measured apparent viscosity, as seen from the comparison in Table IV.
Table IV.
Coefficients of determination for linear trends of Korsmeyer–Peppas constants (k and n) and goodness-of-fit measures ( and
and  ) against intrinsic viscosity and measured apparent viscosity. Higher values in the case of intrinsic viscosity imply superior correlation with that parameter
) against intrinsic viscosity and measured apparent viscosity. Higher values in the case of intrinsic viscosity imply superior correlation with that parameter
| Values | Coefficient of determination | |
|---|---|---|
| Intrinsic viscosity | Apparent viscosity | |
| k | 0.915 | 0.675 |
| n | 0.889 | 0.569 |

|
0.886 | 0.681 |

|
0.925 | 0.548 |
The results above suggest that the effect of mechanical stress on tablets is largely dependent on the rheological properties of the polymer matrix at various degrees of hydration, ranging from the partially hydrated core to the continuously eroding gel layer. It has previously been reported that the kinetics of the relevant processes of matrix hydration and polymer erosion both scale with inverse powers of the average molecular weight of the matrix polymer (19). As given by the Mark–Houwink–Sakurada relationship, the average molecular weight is related to the polymer intrinsic viscosity by the following relationship,
 |
5 |
where K and α are independent of molecular weight but dependent on the polymer–solvent system being studied. The values of α range from 0.5 for a poor solvent up to 0.85 for a good solvent. In the case of HPMC in water, the best-fitting value is estimated to be 0.821. Furthermore, the rate of polymer erosion at constant hydrodynamic stress can be expressed as (19)
 |
6 |
Here b and β are again certain time-varying constants solely dependent on the nature of the polymer and the exact hydrodynamic conditions. Combining Eqs. 5 and 6, one obtains
 |
7 |
Because the formulations studied are shown to be erosion-controlled, our results suggest that the above reasoning is applicable to periods of increased mechanical stress. Specifically, assuming that the constants  and
and  are comparable between different HPC and HPMC polymer grades at increased levels of mechanical stress, we hypothesize that polymers with higher intrinsic viscosity are more effective at limiting the rate of erosion, thus explaining the observed correlation between best-fitting Korsmeyer–Peppas constants and goodness-of-fit measures.
are comparable between different HPC and HPMC polymer grades at increased levels of mechanical stress, we hypothesize that polymers with higher intrinsic viscosity are more effective at limiting the rate of erosion, thus explaining the observed correlation between best-fitting Korsmeyer–Peppas constants and goodness-of-fit measures.
Evaluation of Mechanical Susceptibility
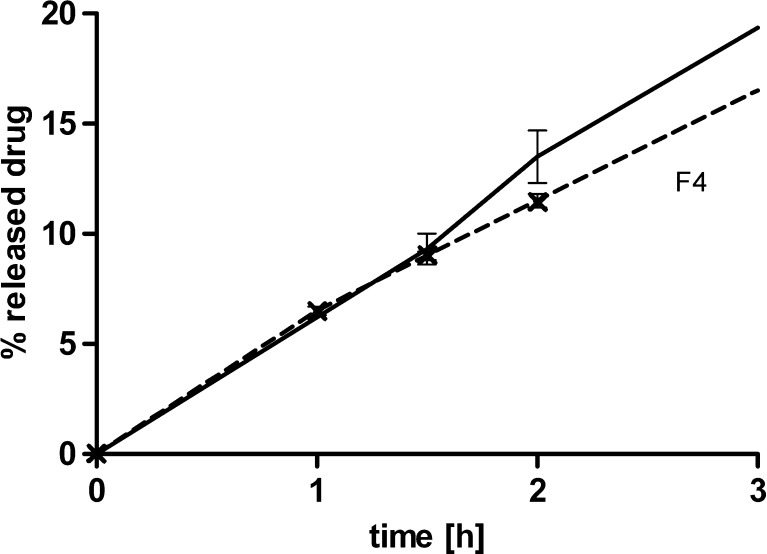
The effect of mechanical stress on matrix tablets was made more apparent by comparing the quantity of dissolved drug after the application of mechanical stress to the zero offset linear extrapolation curve defined by the initial slope of the dissolution curve, as described mathematically by MS in Eq. 3. Equivalently, MS can be regarded as the ratio of the average dissolution rate after manipulation and the initial dissolution rate before manipulation. The motivation for this definition was that the effect of mechanical manipulation at an early time point, where approximately 10% of the drug was dissolved, will mainly manifest itself as a linear translation of the results upwards from the reference dissolution curve one would obtain with a test without manipulation. This was confirmed by comparing dissolution results with and without mechanical manipulation (Fig. 4).
Fig. 4.
Percent of released drug versus time for Formulation F4 tested with mechanical manipulation (straight line) and without manipulation (dashed line). Note that the linear assumption of release until 1.5 h is justified by the dissolution result at 1 h of sampling time point
As defined, MS is just one of the possible measures to quantify the effect of mechanical stress. However, for time points where the amount of dissolved drug is low (below ∼50%), a simple interpretation is available. A MS value of 1 indicates that there is no observable mechanical susceptibility; the dissolution curve is linear. MS values >1 signify that the drug release is faster after mechanical manipulation. More precisely, a MS value of 1.1 indicates in relative terms that the amount of drug released is 10% higher at that particular time point as a consequence of the mechanical stress application compared to drug released from a non-manipulated tablet. Values below 1 imply that the dissolution curve is significantly departing from the zero-order kinetics and that the mechanical susceptibility is no longer relevant at these late time points.
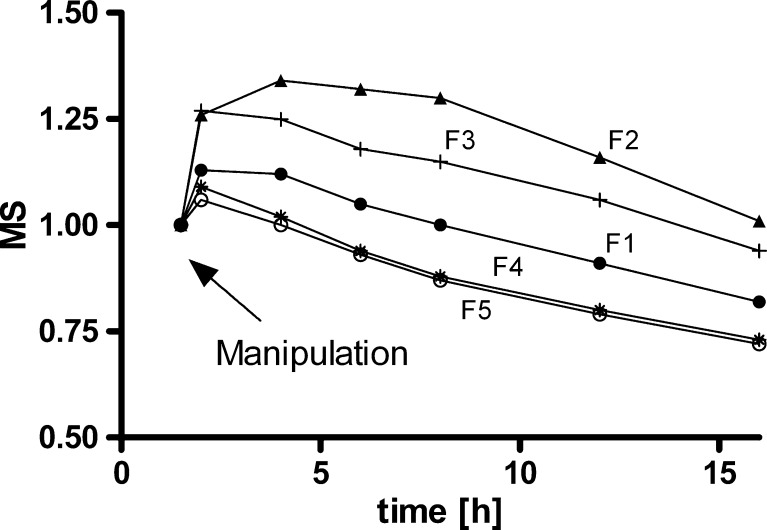
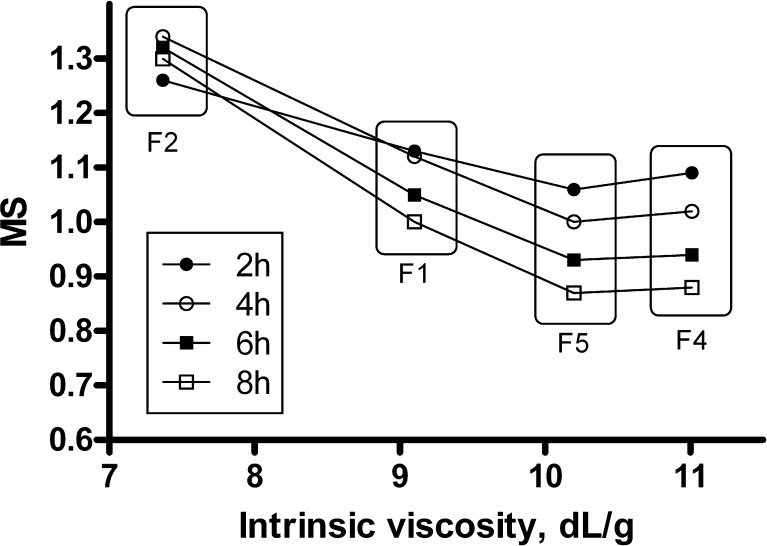
In Fig. 5, the time dependence of calculated MS after the application of mechanical stress is shown separately for each formulation studied. For composition F1, containing HPMC K15M, the maximum MS value of 1.13 was smaller than that of 1.34 in the case of F2, which contains HPMC of lower viscosity K4M (Table V, Fig. 5). Similarly the composition F3 showed a maximum MS of 1.27, which demonstrated that the increased amount of low-viscosity HPMC from 35 to 50% was not enough to reduce the magnitude of the mechanical susceptibility (Table V, Fig. 5). For composition F4, with HPMC K100M, the MS was low, and the maximum increase in drug release just after application of stress was 9% (a MS value of 1.09). Similarly, the mechanical susceptibility of HPC HXF sample F5 was also low, with MS only 6% (Table V, Fig. 5).
Fig. 5.
The degree of mechanical susceptibility (MS), after mechanical stress application for formulations tested. Ratios greater than 1 indicated that tablets were mechanically susceptible
Table V.
Evaluation of Mechanical Susceptibility (MS) Calculated from Eq. 3, after the Application of Mechanical Stress
| Formulation | F1 | F2 | F3 | F4 | F5 | |
|---|---|---|---|---|---|---|
| Degree of mechanical susceptibility, MS | ||||||
| Dissolution time point (h) | 1.5 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2 | 1.13 | 1.26 | 1.27 | 1.09 | 1.06 | |
| 4 | 1.12 | 1.34 | 1.25 | 1.02 | 1.00 | |
| 6 | 1.05 | 1.32 | 1.18 | 0.94 | 0.93 | |
| 8 | 1.00 | 1.30 | 1.15 | 0.88 | 0.87 | |
| 12a | 0.91 | 1.16 | 1.06 | 0.80 | 0.79 | |
| 16a | 0.82 | 1.01 | 0.94 | 0.73 | 0.72 | |
aAt these time points the dissolution curves already deviate significantly from zero-order kinetics
Limiting Mechanical Susceptibility: a Simple Rule
Applying mechanical stress after 1.5 h evidently caused some abrasion of the matrix gel layer, resulting in faster drug release. Relating in vitro data with known in vivo physiology of the gastrointestinal tract, one can assume that formulations F1, F4, and F5 would hardly be affected by stress conditions in the gastrointestinal tract (transition through the pylorus, the presence of food in the stomach, and motility of the gastrointestinal tract after consumption of a meal). For these formulations, the MS was below the 15% increase of the initial dissolution rate.
The main question that remains is how to select polymers for matrix tablets that are not affected by mechanical stress expressed through gastrointestinal transit. We tried to postulate a simple rule based on intrinsic polymer properties like intrinsic viscosity. As seen from Fig. 6, the mechanical susceptibility decreases with increasing intrinsic viscosity, and then appears to reach a plateau for intrinsic viscosities >10 dL/g. Comparing compositions F2 and F3, one can see that increasing the amount of polymer lowers the MS (Fig. 7), but not as much as by using polymers of higher intrinsic viscosity.
Fig. 6.
Mechanical susceptibility (MS) immediately after mechanical stress application for tested formulations F1 to F5 as a function of polymer intrinsic viscosity
Fig. 7.
Comparison of mechanical susceptibility (MS) immediately after mechanical stress application for tested formulations F2 and F3 with various amounts of HPMC K4M polymer
Based on Figs. 6 and 7, the following two simple formulation rules for selecting polymers for a matrix tablet, where the goal of the formulation is to limit mechanical susceptibility, can be postulated (Table VI). The higher the intrinsic viscosity of the polymer, the lower the MS limit that can be achieved. To assure robust matrices, polymers with higher intrinsic viscosities should be used. If only polymer with low intrinsic viscosity is available or should be incorporated into the formulation, a greater proportion of it should be used to achieve the desired limit on mechanical susceptibility.
Table VI.
Proposed Polymer Amounts and Approximate Intrinsic Viscosities Relating to Mechanical Susceptibility (MS) Limits
| MS limit | Polymer amount (w/w) | Intrinsic viscosity (approximate; dL/g) |
|---|---|---|
| <1.10 | 35% | >10.0 |
| <1.15 | 35% | >9.0 |
| <1.25 | 35% | >8.5 |
| <1.25 | 50% | >7.5 |
Without additional measurement points and testing additional polymer types and grades, it is difficult to search for a more detailed functional relationship between mechanical susceptibility and intrinsic viscosity. It is clear that additional factors, such as polymer and excipient particle size, polymer swelling index, and dissolution medium, will all influence the release of the drug from the matrix. However, because we used matrices with a relatively low proportion of matrix-forming agents, where the erosion process is much more expressed compared to tablets with high polymer proportions, we strongly believe that our rules regarding mechanical susceptibility can be extended to tablets with higher polymer amounts. Nonetheless, we should keep in mind that increasing the amount of polymer with low intrinsic viscosity is not as effective as replacing it with polymer of higher intrinsic viscosity.
This rudimentary study shows that there appears to be a minimum threshold value to intrinsic viscosity that assures a controlled, non-accelerated, drug-dissolution profile even in the presence of increased stress conditions in the gastrointestinal tract. Intrinsic viscosity is thus shown to be an important formulation property related to the safety and efficacy of the product under various conditions of product administration.
Conclusions
This study demonstrated that polymer physical parameter intrinsic viscosity can be used instead of apparent viscosity to predetermine the robustness of matrix tablets. A simple and effective experimental procedure was used to quantify the mechanical susceptibility of swollen matrix tablets. Based on these results, two simple rules were postulated that can be used to guide the development of matrix tablet formulations that ensure matrices with low susceptibility to mechanical stress. The first is that one has to use polymer with high intrinsic viscosity to formulate tablets with low mechanical susceptibility. The second rule is that increasing the amount of polymer with low intrinsic viscosity is not as effective as replacing it with polymer of higher intrinsic viscosity. The rules obtained can save time, enabling quick decisions and results in the competitive environment of the pharmaceutical industry.
References
- 1.Liu P, Ju T, Qiu Y. Diffusion-controlled drug delivery systems. In: Li X, Jasti BR, editors. Design of controlled release drug delivery systems. United States: McGraw-Hill; 2006. pp. 107–137. [Google Scholar]
- 2.Wang Z, Shmeis RA. Dissolution controlled drug delivery systems. In: Li X, Jasti BR, editors. Design of controlled release drug delivery systems. United States: McGraw-Hill; 2006. pp. 139–172. [Google Scholar]
- 3.Siepmann J, Peppas NA. Hydrophilic matrices for controlled drug delivery: an improved mathematical model to predict the resulting drug release kinetics (the “Sequential Layer” model) Pharm Res. 2000;17(10):1290–1298. doi: 10.1023/A:1026455822595. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner S, Kristl J, Peppas N. Network structure of cellulose ethers used in pharmaceutical applications during swelling and at equilibrium. Pharm Res. 2002;19:1084–1090. doi: 10.1023/A:1019891105250. [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner S, Lahajnar G, Sepe A, Kristl J. Investigation of the state and dynamics of water in hydrogels of cellulose ethers by 1H NMR spectroscopy. AAPS Pharm Sci Tech. 2002;3(4):E36. doi: 10.1208/pt030436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baumgartner S, Lahajnar G, Sepe A, Kristl J. Quantitative evaluation of polymer concentration profile during swelling of hydrophilic matrix tablets using 1H NMR and MRI methods. Eur J Pharm Biopharm. 2005;59:299–306. doi: 10.1016/j.ejpb.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 7.McConnell EL, Fadda HM, Basit AW. Gut instincts: explorations in intestinal physiology and drug delivery. Int J Pharm. 2008;364:213–226. doi: 10.1016/j.ijpharm.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Abrahamsson B, Alpsten M, Bakec B, Larsson A, Sjoegren J. In vitro and in vivo erosion of two different hydrophilic gel matrix tablets. Eur J Pharm Biopharm. 1998;46:69–75. doi: 10.1016/S0939-6411(98)00002-2. [DOI] [PubMed] [Google Scholar]
- 9.Mudie DM, Amidon GL, Amidon GE. Physiological parameters for oral delivery and in vitro testing. Mol Pharm. 2010;7(5):1388–1405. doi: 10.1021/mp100149j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weitschies W, Blume H, Mönnikes H. Magnetic marker monitoring: high resolution real-time tracking of oral solid dosage forms in the gastrointestinal tract. Eur J Pharm Biopharm. 2010;74:93–101. doi: 10.1016/j.ejpb.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Goodman K, Hodges LA, Band J, Stevens HNE, Weitschies W, Wilson CG. Assessing gastrointestinal motility and disintegration profiles of magnetic tablets by a novel magnetic imaging device and gamma scintigraphy. Eur J Pharm Biopharm. 2010;74:84–92. doi: 10.1016/j.ejpb.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Kamba M, Seta Y, Kusai A, Nishimura K. Comparison of the mechanical destructive force in the small intestine of dog and human. Int J Pharm. 2002;237:139–149. doi: 10.1016/S0378-5173(02)00043-1. [DOI] [PubMed] [Google Scholar]
- 13.Abrahamsson B, Albery T, Eriksson A, Gustafsson I, Sjoberg M. Food effects on tablet disintegration. Eur J Pharm Sci. 2004;22:165–172. doi: 10.1016/j.ejps.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Abrahamsson B, Pal A, Sjoberg AM, Carlsson M, Laurell E, Brasseur JG. A novel in vitro and numerical analysis of shear-induced drug release from extended-release tablets in the fed stomach. Pharm Res. 2005;22:1215–1226. doi: 10.1007/s11095-005-5272-x. [DOI] [PubMed] [Google Scholar]
- 15.Sako K, Sawada T, Nakashima H, Yokohama S, Sonobe T. Influence of water soluble fillers in hydroxypropylmethylcellulose matrices on in vitro and in vivo drug release. J Con Rel. 2002;81:165–172. doi: 10.1016/S0168-3659(02)00067-6. [DOI] [PubMed] [Google Scholar]
- 16.Garbacz G, Wedemeyer R, Nagel S, Giessmann T, Mönnike H, Wilson CG, Siegmund W, Weitschies W. Irregular absorption profiles observed from diclofenac extended release tablets can be predicted using a dissolution test apparatus that mimics in vivo physical stresses. Eur J Pharm Biopharm. 2008;70:421–428. doi: 10.1016/j.ejpb.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 17.Campos-Aldrete ME, Villafuerte-Robles L. Influence of the viscosity grade and the particle size of HPMC on metronidazole release from matrix tablet. Eur J Pharm Biopharm. 1997;43:173–178. doi: 10.1016/S0939-6411(96)00004-5. [DOI] [Google Scholar]
- 18.Patel VF, Patel NM. Statistical evaluation of influence of viscosity and content of polymer on dipyridamole release from floating matrix tablets: a technical note. AAPS Pharm Sci Tech. 2007;8(3):E69. doi: 10.1208/pt0803069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell SA, Balwinski KM. A framework to investigate drug release variability arising from hypromellose viscosity specifications in controlled release matrix tablets. J Pharm Sci. 2008;97:2277–2285. doi: 10.1002/jps.21145. [DOI] [PubMed] [Google Scholar]
- 20.Fettaka M, Issaadi R, Moulai-Mostefa N, Dez I, Le Cerf D, Picton L. Thermo sensitive behavior of cellulose derivatives in dilute aqueous solutions: From macroscopic to mesoscopic scale. J Colloid Interface Sci. 2011;357(2):372–378. doi: 10.1016/j.jcis.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 21.Hiemenz PC, Lodge TP. Polymer Chemistry, Second Edition. Florida: CRC, Taylor & Francis Group: Boca Raton. 2007; p. 338–339.
- 22.Feller RM, Wilt M. Evaluation of Cellulose Ethers for Conservation. The Getty Conservation Institute. 1990. http://www.getty.edu/conservation/publications_resources/pdf_publications/ethers.pdf. Accessed 7 Oct 2011.
- 23.USP-NF Online. US Pharmacopoeia, Rockville. http://www.uspnf.com. Accessed 7 Oct 2011.
- 24.R Development Core Team. R Foundation for Statistical Computing, Vienna, 2010. http://www.r-project.org. Accessed 16 Dec 2011.
- 25.Korsmeyer RW, Gurny R, Doelker E, Buri P, Peppas NA. Mechanisms of solute release from porous hydrophilic polymers. Int J Pharm. 1983;15:25–35. doi: 10.1016/0378-5173(83)90064-9. [DOI] [PubMed] [Google Scholar]