Introduction
Pre-emptive kidney transplant (PKT) – defined as transplant prior to dialysis - has numerous advantages as a treatment approach for patients with advanced renal disease. In the last 15 years, PKT has become more common and been performed at higher levels of estimated glomerular filtration rate (eGFR), particularly among recipients of live donor transplants, among whom timing of transplantation is easier to control. However, recent studies have raised important new concerns about unintended consequences of early versus late PKT. In this article, we review the convincing evidence that PKT offers diverse advantages for patients, discuss potential problems that might emerge from PKT at higher levels of renal function, examine the feasibility of a “just-in-time” PKT strategy for transplant centers, and discuss whether a new kidney allocation system could affect rates of PKT.
Plausible Benefits of Pre-Emptive Kidney Transplantation
When timed appropriately, PKT offers benefits that are intuitive to patients and providers. By avoiding hemodialysis, renal disease patients avoid the many health risks associated with dialysis – risks which are particularly elevated during the first 90 days of dialysis treatment. Multiple observational studies suggest that PKT recipients to enjoy better survival than kidney transplant recipients requiring dialysis.(1, 2) This mortality benefit is likely driven by lack of exposure to catheter-associated infections, the risks of vascular access placement, and cardiovascular complications (such as sudden cardiac death) that are more common when patients adapt to the rigors of dialysis. Also, accelerated atherosclerosis and vascular calcification attributed to dialysis(3, 4) may be avoided with PKT and therefore, long-term post-transplant cardiovascular complications may also be decreased by PKT.
Some of the improved outcomes associated with PKT may be attributed to candidate selection. PKT recipients differ in important ways from other recipients. Kasiske et al, using data from the United Network for Organ Sharing, reported that pre-emptive transplant is more likely among young, white, privately insured, employed and college-educated patients.(2) In a more recent study, Fissell et al. reported that better renal function at the time of listing for transplant is associated with a greater likelihood of PKT.(5) These findings strongly suggest that transplant candidates who succeed in achieving PKT are likely to have greater personal resources and physicians who advocate for their early progress through the complex system for kidney transplant evaluation.
When compared to transplantation after initiating dialysis, the advantages of PKT appear to extend to better post-transplant renal function, allograft survival, and overall costs of treatment. PKT recipients experience lower rates of delayed graft function (DGF) than kidney transplant recipients who have undergone dialysis.(2) For example, in the study by Kaskiske et al, 8.4 % of PKT recipients of deceased donor kidneys had DGF versus 25.6% of non-PKT recipients of deceased donor kidneys (p<0.001). Similarly, the rate of DGF was 2.6% among PKT recipients of live donor kidneys versus 6.1% among non-PKT recipients (p<0.001).(2) The lower rate of DGF associated with PKT may be explained by the benefits of residual renal function, which allows some recipients to avoid peri-operative complications of volume overload or hyperkalemia. In light of the residual renal function in PKT recipients, DGF in this group is probably best defined as failure of the serum creatinine to fall after kidney transplant, rather than the conventional definition of the need for post-transplant dialysis during the first post-transplant week.(6) However, residual renal function may not entirely explain the excellent early outcomes after PKT. PKT recipients also have lower rates of acute rejection, perhaps because dialysis has immune-modulatory effects that make recipients more likely to recognize the allograft as foreign despite immunosuppression. (7, 8)
Further, PKT recipients enjoy longer allograft life,(9) and this superior allograft survival is consistent among PKT recipients whether or not they experience DGF.(2) They may return to work sooner than recipients who have been on dialysis.(10) Although transplantation is initially a high-cost procedure, there is a break-even point after which the cumulative cost of the transplant, including maintenance care, becomes cheaper than maintenance dialysis. Because PKT avoids the high cost of chronic dialysis treatment, PKT appears to be cost-saving compared to kidney transplantation after dialysis.(11) These superior outcomes with PKT remain consistent after adjusting for demographic and clinical differences between PKT recipients and other recipients. Although most studies of PKT suffer from the potential for lead-time bias (because PKT patients undergo treatment earlier in their disease course), they suggest collectively that PKT offers important advantages with a plausible biological basis.
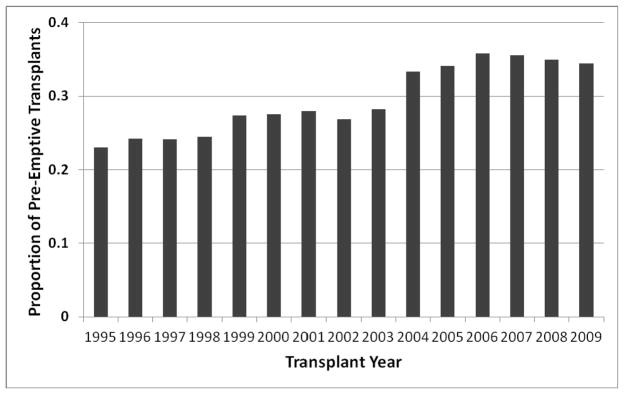
Given the excellent outcomes with PKT, this approach to kidney transplantation has become more common in the United States. The goal of expanding the practice of PKT has been termed the “Kidney First Initiative”.(12) PKT usually takes place in the context of live donor kidney transplantation, although it is occasionally feasible among deceased donor kidney recipients who are activated on the waiting list well before initiating dialysis or in those receiving a zero HLA antigen mismatched donor kidney. As shown in Figure 1, the percentage of adult live kidney donor recipients who were preemptive increased from 23% to 34% (p<0.001) from 1995 – 2009. During this period, however, the mean eGFR among pre-emptive kidney transplant recipients also increased substantially. In a national study using UNOS data, Grams et al reported that the mean recipient pre-transplant eGFR for PKT recipients rose from 9.2 ml/min/1.73 m2 in 1995 to 13.8 ml/min/1.73 m2 in 2009.(13)
Figure 1.
Proportion of pre-emptive kidney transplants among all live donor transplants in the US, by year*
* Based on data from the United Network for Organ Sharing. Table generated by Peter Reese.
Concerns about Timing: Does Renal Replacement Therapy at Higher Levels of Renal Function Provide Any Benefit to Patients?
Observational studies of kidney transplantation and a randomized trial of early versus late hemodialysis have led to important new concerns that earlier initiation of renal replacement therapy may provide little benefit or even cause harm to patients. These new data have called attention to the risks and costs of subjecting patients to PKT before it is needed.
Using detailed data from two Minnesota transplant centers, Akkina et al. contrasted outcomes for 839 kidney transplant recipients who were categorized by pre-transplant eGFR: <10.0 (Group 1), versus 10.0–14.9 (Group 2), and ≥15.0 ml/min/1.73 m2 (Group 3). Using Group 1 as a reference, there were no significant differences in all-cause graft failure for Group 2 (HR 0.99, p=0.94) or Group 3 (HR 1.35, p=0.16).(14) In the study of PKT recipients by Grams et al. cited earlier, the authors detected no differences in unadjusted patient or graft survival between recipients with pre-transplant eGFR<10, versus recipients with higher eGFRs. To address confounding associated with patient selection for PKT, the authors also performed a multivariable analysis using propensity-score adjustment and reported a trend toward higher mortality (HR 1.16, p=0.05) for PKT recipients with eGFR 15 – 20 versus recipients with eGFR<10 ml/min/1.73 m2.(13) It must be acknowledged that these studies were both observational and may be susceptible to residual confounding. For example, neither was able to adjust for important clinical considerations when initiating renal replacement therapy, such as the presence of uremia.
The randomized, controlled Initiating Dialysis Early and Late (IDEAL) Study provides complementary evidence that initiating renal replacement therapy at higher levels of eGFR may not benefit patients but does come at a higher cost to the healthcare system. The IDEAL study randomized participants to a strategy of dialysis initiation at eGFR of 10.0 – 14.0 10 ml/min/1.73 m2 (early initiation) versus 5.0 to 7.0 10 ml/min/1.73 m2 (late initiation). eGFRs were estimated using the Cockcroft-Gault equation. The primary outcome was death from any cause. Although most patients in the late initiation group actually initiated dialysis at a higher-than-planned eGFR (mean 9.8 ml/min/1.73 m2) due to symptoms, the late initiation group did start dialysis approximately 6 months later than the early initiation group. (15) No difference in mortality (p=0.75) or major adverse events was observed between groups. A follow-up study reported that medical costs were higher in the early initiation group, but quality of life was similar for individuals randomized to early versus late initiation of dialysis.(16)
In light of these findings about the lack of benefit associated with renal replacement therapy at higher eGFRs, it is appropriate to consider the specific risks associated with PKT. Kidney transplant surgery is associated with higher mortality than dialysis in the early post-transplant period,(17) due to peri-operative risks and intense immunosuppression that promotes both infections and morbidities such as diabetes. Ideally, a patient would not be exposed to these risks until their renal disease had advanced to the point where uremia or other issues such as volume status were no longer feasibly managed with medical therapy. PKT using a living donor also exposes the donor to the small but real risks of nephrectomy, which encompasses operative complications as well as the burdens of missing work and lost productivity.(18, 19)
Achieving Just-in-Time Live Donor Kidney Transplant: Harder than It Sounds
The optimal treatment approach for PKT would be “just-in-time” transplantation, at the lowest level of renal function when the patient requires renal replacement therapy. However, kidney transplantation – particularly when a live donor is involved – involves a complex series of considerations and operational challenges that may make the “just-in-time” strategy difficult to achieve.
The over-riding challenge for transplant centers in achieving “just-in-time” PKT is orchestrating the testing and schedules of live kidney donors and recipients. For example, it is common to see potential recipients for transplant evaluation when their GFR is close to 20 ml/min/1.73 m2. Depending upon their rate of GFR loss, the interval from evaluation to PKT may be more than 12 months. Potential living kidney donors commonly come forward and are evaluated around the time of the initial recipient evaluation. Donor enthusiasm and willingness to complete testing are often highest at this initial evaluation. The donor may also have specific temporal requirements (e.g. due to work or childcare schedules). Some donors also travel long distances to the recipient’s center. These donor considerations could plausibly lead to scheduling a transplant earlier than is necessary for the recipient.
Another common challenge in optimizing PKT timing is participation in a kidney paired donor exchange. Although a small percentage of all live donor transplants currently involve donor exchange, that percentage is rising quickly.(20) The coordination of multiple simultaneous transplants is likely to involve compromises between optimal timing for any one participant in the exchange versus the real risks that a delay will prevent an exchange or larger chain from taking place at all.(21) Clinician teams will be motivated by the recognition that for most patients with advanced renal disease, undergoing successful kidney transplantation is a higher priority than achieving “just-in-time” transplantation.
Nonetheless, with close communication between donor and recipient teams, centers have an opportunity to reverse the trend of PKT at ever-higher levels of renal function that have been reported. In spite of the complicated operational challenges, transplant staff representing both the donors and the recipients should recognize their duties to move forward with kidney transplant only when the recipient has strong indications for renal replacement therapy that involve more than just a low eGFR, while respecting donor autonomy.(22)
Future Directions: Pre-Emptive Transplant under Proposed New Allocation Systems for Kidney Allografts
With the possible implementation of a new national deceased donor kidney allocation system, the Organ Procurement and Transplant Network (OPTN) Kidney Committee has given careful consideration to the effects of allocation policy on both living donor kidney transplants in general and PKT rates. The proposed new system would involve “longevity matching” – allocation of the “best quality” 20% of kidney allografts to the 20% of adult patients on the waiting list with the longest estimated post-transplant survival (EPTS) time.(23) Simulations of the effects of this new allocation system suggest that there will be little effect on PKT rates.
Because waiting time points will commence when a candidate’s eGFR falls below 20 ml/min/1.73 m2 and because waiting time will remain a predominant factor in a candidate’s priority for receiving a kidney, the number of PKTs with deceased donor kidneys should remain stable. The effect of the new allocation system on living donor kidney transplantation also merits consideration. In a recent perspective piece, Hippen et al. expressed concern that the new kidney allocation system will shorten waiting times for young patients (those likely to have the longest EPTS). Since young patients are far more likely to have potential live donors, live donor transplant rates overall may decline – a serious unintended consequence of the proposed OPTN policy.(24) However, given that the expected number of candidates in the “top 20%” category is far larger than the number of longevity matched kidneys available, the OPTN Kidney Committee believes that waiting times will remain long. Faced with a long waiting time, young patients with access to the best deceased donor kidneys in the new allocation system will still have a strong incentive to pursue live kidney transplantation instead. Thus, rates of PKT involving live donor kidneys should also not change substantially in the context of the new allocation system. Of course, definitive data on the diverse consequences of a new kidney allocation system will not be available until the system is implemented.
Summary
As PKT has been identified as the optimal treatment for many ESRD patients, the transplant community now faces the challenging task of refining the delivery of this complex care. For eligible patients, PKT facilitates the avoidance of dialysis and its associated morbidities. There is also a significant potential savings in costs with PKT to the health care system. However, recent lessons from both transplantation and dialysis have raised concerns about the optimal timing of PKT. PKT at higher levels of eGFR may not benefit recipients and has the potential to expose live donors to harm earlier than necessary. Ongoing dissemination of information about the benefits and optimal timing of PKT to the transplant community and referring providers will be necessary to make progress toward the goal of “just-in-time” kidney transplantation.
Key Clinical Points.
Compared to recipients transplanted after dialysis, pre-emptive kidney transplant recipients enjoy longer recipient and allograft survival.
Pre-emptive kidney transplant recipients experience fewer complications such as delayed graft function.
Concerns have been raised that pre-emptive kidney transplantation is increasingly being performed at higher levels of pre-transplant eGFR, without evident benefit to recipients.
“Just-in-time” kidney transplantation would be optimal for patients with advanced kidney disease, but requires careful communication between recipient and live donor teams at the transplant center.
Acknowledgments
Funding sources: Dr. Reese is supported by NIH grant K23 - DK078688-01.
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meier-Kriesche HU, Port FK, Ojo AO, et al. Effect of waiting time on renal transplant outcome. Kidney Int. 2000;58:1311–1317. doi: 10.1046/j.1523-1755.2000.00287.x. [DOI] [PubMed] [Google Scholar]
- 2.Kasiske BL, Snyder JJ, Matas AJ, Ellison MD, Gill JS, Kausz AT. Preemptive kidney transplantation: the advantage and the advantaged. J Am Soc Nephrol. 2002;13:1358–1364. doi: 10.1097/01.asn.0000013295.11876.c9. [DOI] [PubMed] [Google Scholar]
- 3.Cheung AK, Sarnak MJ, Yan G, et al. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int. 2000;58:353–362. doi: 10.1046/j.1523-1755.2000.00173.x. [DOI] [PubMed] [Google Scholar]
- 4.Raggi P, Boulay A, Chasan-Taber S, et al. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002;39:695–701. doi: 10.1016/s0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 5.Fissell RB, Srinivas T, Fatica R, et al. Preemptive renal transplant candidate survival, access to care, and renal function at listing. Nephrol Dial Transplant. 2012 doi: 10.1093/ndt/gfs012. [DOI] [PubMed] [Google Scholar]
- 6.Yarlagadda SG, Coca SG, Garg AX, et al. Marked variation in the definition and diagnosis of delayed graft function: a systematic review. Nephrol Dial Transplant. 2008;23:2995–3003. doi: 10.1093/ndt/gfn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaul H, Girndt M, Sester U, Sester M, Kohler H. Initiation of hemodialysis treatment leads to improvement of T-cell activation in patients with end-stage renal disease. Am J Kidney Dis. 2000;35:611–616. doi: 10.1016/s0272-6386(00)70006-0. [DOI] [PubMed] [Google Scholar]
- 8.Mange KC, Joffe MM, Feldman HI. Dialysis prior to living donor kidney transplantation and rates of acute rejection. Nephrol Dial Transplant. 2003;18:172–177. doi: 10.1093/ndt/18.1.172. [DOI] [PubMed] [Google Scholar]
- 9.Mange KC, Joffe MM, Feldman HI. Effect of the use or nonuse of long-term dialysis on the subsequent survival of renal transplants from living donors. N Engl J Med. 2001;344:726–731. doi: 10.1056/NEJM200103083441004. [DOI] [PubMed] [Google Scholar]
- 10.Katz SM, Kerman RH, Golden D, et al. Preemptive transplantation--an analysis of benefits and hazards in 85 cases. Transplantation. 1991;51:351–355. [PubMed] [Google Scholar]
- 11.Haller M, Gutjahr G, Kramar R, Harnoncourt F, Oberbauer R. Cost-effectiveness analysis of renal replacement therapy in Austria. Nephrol Dial Transplant. 2011;26:2988–2995. doi: 10.1093/ndt/gfq780. [DOI] [PubMed] [Google Scholar]
- 12.Abecassis M, Bartlett ST, Collins AJ, et al. Kidney transplantation as primary therapy for end-stage renal disease: a National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. Clin J Am Soc Nephrol. 2008;3:471–480. doi: 10.2215/CJN.05021107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grams ME, Massie AB, Coresh J, Segev DL. Trends in the timing of pre-emptive kidney transplantation. J Am Soc Nephrol. 2011;22:1615–1620. doi: 10.1681/ASN.2011010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akkina SK, Connaire JJ, Snyder JJ, Matas AJ, Kasiske BL. Earlier is not necessarily better in preemptive kidney transplantation. Am J Transplant. 2008;8:2071–2076. doi: 10.1111/j.1600-6143.2008.02381.x. [DOI] [PubMed] [Google Scholar]
- 15.Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363:609–619. doi: 10.1056/NEJMoa1000552. [DOI] [PubMed] [Google Scholar]
- 16.Harris A, Cooper BA, Li JJ, et al. Cost-effectiveness of initiating dialysis early: a randomized controlled trial. Am J Kidney Dis. 2011;57:707–715. doi: 10.1053/j.ajkd.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725–1730. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 18.Segev DL, Muzaale AD, Caffo BS, et al. Perioperative mortality and long-term survival following live kidney donation. JAMA. 2010;303:959–966. doi: 10.1001/jama.2010.237. [DOI] [PubMed] [Google Scholar]
- 19.Nanidis TG, Antcliffe D, Kokkinos C, et al. Laparoscopic versus open live donor nephrectomy in renal transplantation: a meta-analysis. Ann Surg. 2008;247:58–70. doi: 10.1097/SLA.0b013e318153fd13. [DOI] [PubMed] [Google Scholar]
- 20.Axelrod DA, McCullough KP, Brewer ED, Becker BN, Segev DL, Rao PS. Kidney and pancreas transplantation in the United States, 1999–2008: the changing face of living donation. Am J Transplant. 2010;10:987–1002. doi: 10.1111/j.1600-6143.2010.03022.x. [DOI] [PubMed] [Google Scholar]
- 21.Akkina SK, Muster H, Steffens E, Kim SJ, Kasiske BL, Israni AK. Donor exchange programs in kidney transplantation: rationale and operational details from the north central donor exchange cooperative. Am J Kidney Dis. 2011;57:152–158. doi: 10.1053/j.ajkd.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beauchamp TLCJ. Principles of Biomedical Ethics. New York, NY: Oxford University Press; 2001. [Google Scholar]
- 23.Organ Procurement and Transplantation Network. OPTN/UNOS Kidney Transplantation Committee Meeting; February 6, 2012; Chicago, IL. [Accessed: April 16, 2012.]. URL: http://optn.transplant.hrsa.gov/CommitteeReports/interim_main_KidneyTransplantationCommittee_3_7_2012_11_36.pdf. [Google Scholar]
- 24.Hippen BE, Thistlethwaite JR, Jr, Ross LF. Risk, prognosis, and unintended consequences in kidney allocation. N Engl J Med. 2011;364:1285–1287. doi: 10.1056/NEJMp1102583. [DOI] [PubMed] [Google Scholar]