By delivering three previously determined cardiac-inducing factors—Gata4, Mef2c, and Tbx5 (GMT)—into the heart proper by retroviral infection, Srivastava and colleagues recently demonstrated the reprogramming of resident fibroblasts to functional cardiomyocytes (CMs) with end points of improved cardiac function and reduced scarring.1 As reported in Nature, the conversion of scar-forming cells to new heart muscle represents a major advance in the development of therapeutic approaches to regenerating damaged heart tissue.
When Takahashi and Yamanaka derived induced pluripotent stem cells (iPSCs) by transducing mouse embryonic fibroblasts with four transcription factors in 2006,2 they revolutionized our thinking on the plasticity of differentiated cell types. These seminal findings have since been extended to the reprogramming of a variety of adult cells from a variety of species, including humans (reviewed in ref. 3).
iPSCs now offer the possibility of deriving patient-specific lines, followed by directed differentiation in a dish, to model the cellular basis of disease (for example, long QT syndrome4,5). They have also been widely applied to chemical screens that either evaluate the toxicology and efficacy of existing drugs or identify novel therapeutic compounds. That said, iPSCs remain largely a developmental model with regard to differentiation, such that functional maturation of derivatives is, at present, sufficiently compromised to preclude their direct use for transplantation in regenerative medicine per se.
To address this shortcoming, investigators have sought to bypass the stem cell stage in reprogramming and move directly to induce specialized cell types from the fibroblast. This was first demonstrated by Wernig and colleagues, who revealed that a combination of three factors—Ascl1, Brn2, and Myt1l—could convert dermal fibroblasts into functional neurons,6 closely followed by Srivastava and colleagues, who reprogrammed committed fibroblasts (cardiac and dermal) to cardiomyocytes.7 Starting with an initial cocktail of 14 candidate cardiac-inducing molecules, fine-tuned down to three transcription factors (GMT), the latter team was able to increase the efficiency of direct cardiomyocyte transduction from 1.7% to 20%. This level of efficiency is significantly higher than reprogramming to iPSCs and was explained at the time as arising through the bypassing of precardiac developmental stages such as mesoderm or cardiac progenitors. Although induced cardiomyocytes (iCMs) displayed expression of cardiac proteins and electrical properties that “matured” a week later in culture, they still essentially exhibited a more neonatal-like phenotype. Consequently, the ability to establish iCMs in vivo became a significant priority not only as a proof of principle for those contemplating therapeutic regeneration of lost cardiac muscle but also to assess whether, in the heart proper, the environment would be more conducive to enhanced structural and functional maturation.
It is against this backdrop that the study by Srivastava and colleagues1 is significant, in demonstrating the direct in situ reprogramming of cardiac fibroblasts into iCMs. Myocardial infarction (MI) was induced in mice that then received retroviral delivery of genes encoding the GMT factors into the myocardium at the border zone of injury, along with a red dye (dsRed) that marks actively dividing cells. Successful targeting of fibroblasts and their derivatives was demonstrated via profiling of the signature markers Thy1 and vimentin, alongside lineage tracing using periostin-Cre driver and R26R-LacZ reporter strains. Four weeks after MI, β-galactosidase-positive (β-gal+) cells (arising from the fibroblast lineage) stained positively for α-actinin, a marker of cardiomyocyte differentiation, and exhibited the sarcomeric structure and rod-shaped morphology characteristic of mature cardiac muscle. Other nonmyocyte cells, such as endothelial or circulating blood precursors, were ruled out as having been reprogrammed by alternate (Tie2+) lineage tracing. Cell fusion was excluded on the basis of pulse-labeling experiments, which indicated that preexisting yellow fluorescent protein–positive (YFP+) cardiomyocytes (in tamoxifen-treated αMHC-merCreMer;R26R-YFP hearts) were refreshed post-MI by YFP–/dsRed+ cardiomyocytes derived from the virally infected cells. CMs were then isolated into cultures from infarcted/infected hearts at the same stage as for the in vivo experiments. In vitro analyses confirmed that only GMT/dsRed-infected cells were β-gal+ (compared with dsRed-alone controls) and, as such, derived from the periostin-positive fibroblast lineage. Most of the iCMs in vitro resembled CMs that were β-gal– from the same preparation, although they presented with a range of maturity as evidenced by electron micrographs of shortened sarcomeres and more diffuse Z-bands relative to endogenous CMs.
Importantly, gene expression profiles for iCMs more closely resembled those for endogenous CMs, relative to cardiac fibroblasts, and revealed downregulation of fibroblast-specific genes such as periostin and Fsp1. In the heart proper, iCMs were structurally integrated by virtue of adherens (N-cadherin+) and gap junction (Cx43+) formation, although only 4% had a Cx43 localization pattern indistinguishable from endogenous CMs. To determine their functionality, appropriate intercellular handling of calcium [Ca2+], as the prelude to synchronous contraction, was demonstrated, and single-cell patch clamping of iCMs isolated from periostin-Cre;R26R-EYFP mice revealed contractile responses to electrical stimulation, intracellular Ca2+ release, and cell-shortening parameters akin to the responses of adult ventricular myocytes. The effect of the iCMs after MI, as determined by magnetic resonance imaging, was improved cardiac function up to 12 weeks after injury. This was accompanied by reduced indicators of cardiac stress and significantly reduced scar area, which was attributable in part to the presence of reprogrammed iCMs as observed within the scar of GMT-infected mice.
Finally, to enhance the GMT effect, the authors targeted an increase in the Thy1+ fibroblast cells, through the addition of thymosin β4 (Tβ4), a highly pleiotropic actin-monomer binding protein that has been shown to activate epicardial cells, resulting in an expansion of derivative fibroblasts.8,9 Intramyocardial injection of Tβ4 before retroviral infection increased the percentage of Thy1+ and vimentin-positive fibroblasts. The number of cells subsequently infected with GMT was doubled following Tβ4 treatment, and the number of β-gal+ iCMs residing in the infarct/border zone increased from 35% to 51%. The downstream consequence of combined Tβ4 plus GMT was a synergistic improvement in cardiac function at 8 weeks post-MI.
This study is a highly significant addition to the paradigm of regenerating adult heart tissue from endogenous cells. Although bypassing some of the issues that complicate the alternative approach of cell transplantation (e.g., choice of type of cell and an allogeneic vs. autologous source, in vitro expansion, and cell survival following engraftment), there remains the issue of optimal mode and site of delivery. The use of retroviruses is problematic, not only because of a potentially risky off-target profile through genome integration but also because—extrapolating to humans—retroviruses raise an innate immune response and are neutralized by complement. Viral vectors will need to be replaced, therefore, by alternate reprogramming vehicles such as synthetic modified RNA10 or small molecules11—the latter requiring exhaustive screens to assess molecular effects that are at least comparable to GMT. The improvement in efficiency of reprogramming could be extended beyond expanding the potential target fibroblast population to address why it is that not all fibroblasts within the infarct border zone appear reprogrammable or why they adopt a range of “partial” iCM phenotypes in terms of maturation. Related to this, subsequent attempts by other groups to repeat the induction of dermal (tail tip) fibroblasts to cardiomyocytes have proven largely unsuccessful in vitro, whereas cardiac fibroblast reprogramming appears robust, as demonstrated in both of the studies by Srivastava and colleagues. It will, therefore, be of interest to investigate what might be special about cardiac fibroblasts, relative to their dermal counterparts. Developmental origin may play a significant role. Despite the assertion by Srivastava et al. that cardiac fibroblasts are “embryologically distinct from cardiomyocytes in their origin,”1 they do in fact arise from a progenitor population within the proepicardial organ that can give rise to cardiomyocytes.12,13 This mutual origin, if supported by a common progenitor, hints at the possibility of retention of some form of memory (epigenetic?) of lineage multipotency.
Other interesting questions remain. For example, what is the role of injury signaling in the reprogramming process in vivo, and what are the efficiency and maturation status of reprogrammed iCMs in the intact (uninjured) heart? It might be that important inflammatory cues or release of injury-induced signals, such as the plethora of trophic molecules from activated epicardium post-MI,8 play a key role in reprogramming or the maturation of iCMs—or both (Figure 1). What about regional reprogramming in the injured heart? In the present study, iCMs adopt an adult ventricular electrophysiological phenotype, which may reflect the ventricular origin of the fibroblasts. Do reprogrammed atrial fibroblasts produce atrial-like iCMs, and how might pacemaker CMs of the conduction system be induced?
Figure 1.
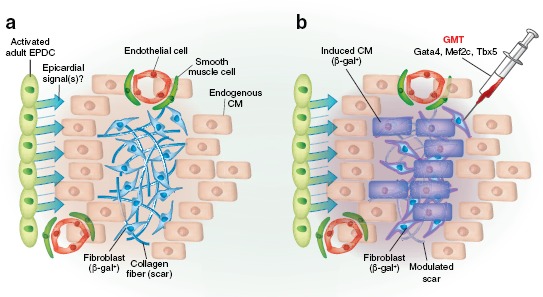
Scar to muscle conversion after myocardial infarction in a periostin-LacZ reporter mouse. (a) Activated β-gal+ (blue) fibroblasts lay down collagen fibers and establish a scar to patch the lost muscle tissue. Retroviral infection by three cardiac-inducing factors—Gata4, Mef2c, and Tbx5 (GMT)—reprograms a proportion of the fibroblasts to form induced cardiomyocytes (iCMs), which not only replaces lost endogenous CMs but also modulates the scar region for repopulation. (b) β-gal+ iCMs are structurally and functionally similar to endogenous CMs, and their maturation is probably a product of the local environment, including signals from activated epicardial cells (EPDCs).
Arguably, one of the most fascinating observations in this study was the reprogramming of fibroblasts residing in the scar to iCMs (Figure 1). It was unclear whether these targeted fibroblasts were actively fibrotic and had adopted a myofibroblast phenotype, but nevertheless their reprogramming to CMs in this region was highly significant. Fibrosis and scarring constitute the default wound-healing process in adult mammalian hearts and not only establish a noncontractile region within the ventricle that can ultimately result in pathological remodeling (progression to heart failure) but also create a hostile environment in terms of regenerative cell repopulation; converting scar to new muscle, therefore, effectively kills two (nonregenerative) birds with one stone.
Whether GMT reprogramming of fibroblasts to iCMs extends beyond rodents into large animals for preclinical studies, and humans for patient trials, is the ultimate question. Certainly as a therapeutic, in situ reprogramming has clear advantages over cell transplantation (which has been subjected extensively to trials; reviewed in ref. 14), not the least of which is the lower cost and shorter delivery time to patients.
Acknowledgments
P.R.R. is supported by a British Heart Foundation Chair in Regenerative Medicine.
REFERENCES
- Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L.et al. (2012In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes Naturee-pub ahead of print 18 April 2012. [DOI] [PMC free article] [PubMed]
- Takahashi K., and, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Hanna JH, Saha K., and, Jaenisch R. Pluripotency and cellular reprogramming: facts, hypotheses, unresolved issues. Cell. 2010;143:508–525. doi: 10.1016/j.cell.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott-Flugel L.et al. (2010Patient-specific induced pluripotent stem-cell models for long-QT syndrome N Engl J Med 3631397–1409. [DOI] [PubMed] [Google Scholar]
- Itzhaki I, Maizels L, Huber I, Zwi-Dantsis L, Caspi O, Winterstern A.et al. (2011Modelling the long QT syndrome with induced pluripotent stem cells Nature 471225–229. [DOI] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature. 2010;463:1035–1041. doi: 10.1038/nature08797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieda M, Fu JD, gado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG.et al. (2010Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors Cell 142375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Honor LB, He H, Ma Q, Oh JH, Butterfield C.et al. (2011Adult mouse epicardium modulates myocardial injury by secreting paracrine factors J Clin Invest 1211894–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart N, Bollini S, Dube KN, Vieira JM, Zhou B, Davidson S.et al. (2011De novo cardiomyocytes from within the activated adult heart after injury Nature 474640–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F.et al. (2010Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA Cell Stem Cell 7618–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira JM., and, Riley PR.2012Chemical genetics and its potential in cardiac stem cell therapy Br J Pharmacole-pub ahead of print 2 March 2012. [DOI] [PMC free article] [PubMed]
- Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K.et al. (2008A myocardial lineage derives from Tbx18 epicardial cells Nature 454104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J.et al. (2008Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart Nature 454109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, Watt S.et al. (2012Stem cell treatment for acute myocardial infarction Cochrane Database Syst Rev 2CD006536. [DOI] [PubMed] [Google Scholar]


