Abstract
OBJECTIVE:
There is controversy about appropriate methods to reduce sudden cardiac death (SCD) in young athletes, but there is limited evidence on costs or consequences of alternative strategies. The objective of this study was to evaluate the cost-effectiveness of adding electrocardiogram (ECG) screening to the currently standard practice of preparticipation history and physical examination (H&P) to reduce SCD.
METHODS:
Decision analysis modeling by using a societal perspective, with annual Markov cycles from age 14 until death. Three screening strategies were evaluated: (1) H&P, with cardiology referral if abnormal (current standard practice); (2) H&P, plus ECG after negative H&P, and cardiology referral if either is abnormal; and (3) ECG only, with cardiology referral if abnormal. Children identified with SCD-associated cardiac abnormalities were restricted from sports and received cardiac treatment. Main outcome measures were costs of screening and treatment, quality-adjusted life years (QALYs), and premature deaths averted.
RESULTS:
Relative to strategy 1, incremental cost-effectiveness is $68 800/QALY for strategy 2 and $37 700/QALY for strategy 3. Monte Carlo simulation revealed the chance of incremental cost-effectiveness compared with strategy 1 was 30% for strategy 2 and 66% for strategy 3 (assumed willingness to pay ≤$50 000/QALY). Compared with strategy 1, strategy 2 averted 131 additional SCDs at $900 000 per case, and strategy 3 averted 127 SCDs at $600 000 per case.
CONCLUSIONS:
Under a societal willingness to pay threshold of $50 000/QALY, adding ECGs to current preparticipation evaluations for athletes is not cost-effective, with costs driven largely by false-positive findings.
KEY WORDS: cost-effectiveness, ECG screening, sudden death
What’s Known on This Subject:
Sudden cardiac death in young athletes is an uncommon but devastating event. Addition of routine electrocardiogram (ECG) screening to standard preparticipation care may reduce the number of sudden deaths. Lack of data regarding effectiveness and costs has prevented widespread implementation.
What This Study Adds:
Adding ECG screening to current preparticipation evaluation is not cost-effective. Cost is driven primarily by the evaluation of the large number of false-positive findings. An ECG-only screening strategy is more cost-effective.
Sudden cardiac death (SCD) in an athlete is devastating to families and communities. The annual incidence of SCD in athletes is estimated from 1 to 6 per 100 000 persons.1–3 The most common conditions that increase SCD risk include hypertrophic cardiomyopathy (HCM), coronary artery anomalies of wrong sinus origin, arrhythmogenic right ventricular cardiomyopathy, and various arrhythmia syndromes.4–6 These diagnoses are typically undetected before SCD. Electrocardiograms (ECGs) may detect some cases during the asymptomatic phase, enabling interventions to reduce SCD risk.
Most clinical guidelines currently recommend that children considering competitive athletics be screened via careful history and physical examination (H&P) before sports participation, with cardiology referral and ECG in cases with abnormal cardiac examination, personal history of cardiac symptoms, or family history of SCD. However, some experts advocate adding ECGs to the current standard of preparticipation care.7–9 Critics of such a strategy cite lack of evidence of effectiveness and feasibility, implications for personnel requirements, cost considerations, and the negative impact of false-positive screening results.10–12 Rational policy-making regarding enhanced screening for athletes is limited by lack of evidence on its likely benefits and costs.
We conducted a modeled cost effectiveness analysis of 2 strategies that incorporate routine ECG screening as part of preparticipation evaluation of athletes, compared with the current standard. Previous economic evaluations of preparticipation ECG screening in athletes have had important methodological limitations5,13,14 or have not accounted for quality of life implications of the screening program as part of the primary outcome measure.15 We address these limitations here, with the objective of informing clinicians, policy-makers, and researchers.
Methods
A state-transition Markov model was constructed to evaluate the cost-effectiveness of 3 strategies to screen adolescent athletes in the United States for heart disease (HD) known to increase risk for SCD.16 The end points for the analysis are screening and treatment costs, quality-adjusted life years (QALYs), and premature deaths averted.
HD Screening Strategies
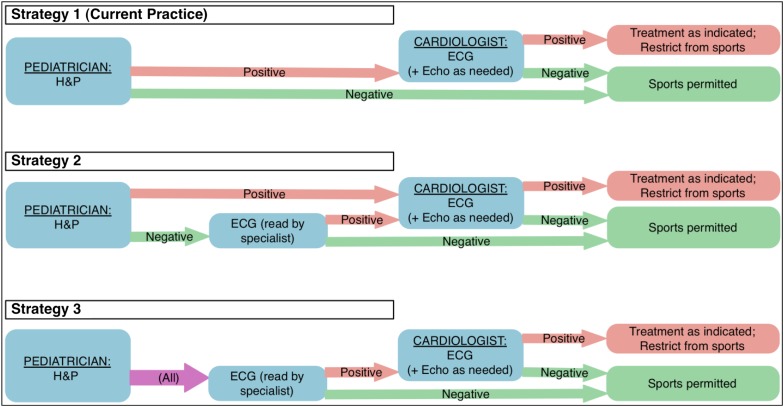
We compared 3 main screening strategies to identify SCD risk, shown in Fig 1: (1) H&P, with children with potential abnormalities being referred for pediatric cardiology evaluation. Strategy 1 is the current standard of care and was used as a reference to estimate the cost-effectiveness of 2 alternative strategies: (2) H&P, followed by ECG in children with negative H&P, with children with abnormalities on either test being referred for definitive cardiology evaluation; and (3) cardiac risk screening based on ECG only, with referral for cardiology evaluation for abnormal ECGs. To mirror the likely practice model in the community, and because H&P likely provides benefits beyond screening for cardiac risk, the model assumes that patients would still receive H&P under strategy 3 but that cardiology referral would be based solely on ECGs.
FIGURE 1.
Alternative strategies to screen for risk of SCD. Strategy 1 reflects current practice. Strategies 2 and 3 reflect alternative clinical decision rules for ECG and cardiologist referral.
Under each strategy, patients can be divided into 4 subgroups: those without HD who also screen negative (true-negatives); those with HD who are missed by the screening (false-negatives), who are treated like true-negatives; those with HD who are correctly identified and thus treated for their HD and restricted from athletic participation (true-positives); and those without HD who incorrectly screen positive (false-positives), who are treated and restricted unnecessarily.
HD Diagnosis and Treatment
We assumed that cardiology evaluations would always involve an ECG (ie, a second ECG under strategies 2 and 3), and 90% would also involve an echocardiogram. We assumed that the sensitivity and specificity of cardiology evaluation is high but not perfect; thus, some patients would receive a false-positive diagnosis after the initial cardiology evaluation. We assumed that cardiologists re-evaluate all patients with a positive diagnosis biannually for the first 3 years, leading to 20% of false-positives per year being correctly re-classified as true-negatives. After 3 years, diagnoses would be permanent, whether true or false.
We assumed that patients with HD would fall into 1 of 3 diagnostic categories: hypertrophic and other cardiomyopathies; arrhythmogenic syndromes (eg, long QT syndrome, catecholaminergic polymorphic ventricular tachycardia); and other (eg, myocarditis, congenital HD). Within each category, patients would be “low risk” or “high risk” for SCD, based on clinical characteristics at the time of diagnosis. Low-risk patients were treated with observation alone or with medication (β-blockers). All high-risk patients were treated with medication and had a fixed, yearly probability of undergoing implantable cardioverter-defibrillator (ICD) implantation. Low-risk patients had a small probability to transition to high risk. All patients diagnosed with HD were restricted from competitive athletics.
Markov Transition Model
We modeled HD screening at age 14 and treatment and outcomes up to a maximum attainable age of 100, divided in annual Markov cycles. The complete set of Markov states, and transitions between them, are shown in the online Supplement Fig 4. Transitions relate HD status, cardiology treatment (which includes restriction from sports participation), and mortality.
Table 1 presents our assumptions about model parameters and transition probabilities between Markov states. Parameter values were based on published research or on expert estimates within the research team when no relevant research was available. The model incorporated uncertainty in parameter values. Table 1 lists ranges for most parameters, which were assumed to follow a β distribution.
TABLE 1.
Model Parameters
| Parameter | Base Case | Range | Source |
|---|---|---|---|
| Unit costs | |||
| Initial patient visit (H&P) (CPT code 99203) | $92 | 67–117 | 30 |
| Cardiologist visit (CPT code 99244) | $185 | 150–220 | 30 |
| ECG (CPT code 93000) | $23 | 20–44 | 30 |
| Echocardiogram (CPT codes 93303+93320+93325) | $350 | 280–420 | 30 |
| Patient/parent time (per visit) | $20 | 17–41 | 30 |
| Annual cost of the β blockers | $380 | 300–460 | 31 |
| ICD (including surgery) | $27 741 | 25 741–33 741 | 32 |
| ICD battery replacement (including surgery), per replacement | $22 122 | 20 122–27 122 | 32 |
| Treatment volume | |||
| Fraction of cardiology visits that include echocardiogram, initial/screening | 0.90 | 0.85–0.95 | a |
| Visits for HD with SCD risk positives (cardiologist + ECG + 0.5 echo), per year | 2 | N/A | a |
| Days of β-blocker medication (if medicated), per year | 365 | N/A | a |
| Event probabilities, SCD and associated factors for nonathletes | |||
| Prevalence of HD with risk of SCD | 0.001 | 0.0005–0.0045 | 5 |
| Of death if no HD with SCD risk (by age and gender) | US life table | N/A | 33 |
| Of SCD if HD with low SCD risk, boy nonathlete (per year) | 0.01 | 0.008–0.012 | 17–20 |
| Of SCD if HD with low SCD risk, girl nonathlete (per year) | 0.004 | 0.003–0.005 | 17 |
| Ratio between SCD rates of high risk and low risk patients (multiplier) | 3 | 2.5–3.5 | 21,22 |
| Annual probability of a low SCD risk patient to develop high SCD risk HD | 0.005 | 0.003–0.007 | a |
| Age above which the SCD risk declines | 50 | 40–60 | a |
| Degree of SCD risk decline at age threshold (multiplier) | 0.5 | N/A | a |
| Event probabilities, sport and associated factors | |||
| Relative risk of SCD for boy athletes with HD with SCD risk (multiplier) | 2.5 | 1.4–3.4 | 17 |
| Relative risk of SCD for girl athletes with HD with SCD risk (multiplier) | 2.5 | 1.75–3.75 | 17 |
| Event probabilities, HCM and associated factors | |||
| Prevalence of HCM condition, among athletes with HD with SCD risk | 0.50 | 0.40–0.60 (A: related to B & C) | 34 |
| Fraction of athletes with HCM that are not treated (low risk) | 0.44 | 0.32–0.56 (X: related Y & Z) | 19 |
| Fraction of athletes with HCM on medication (low risk) | 0.50 | 0.40–0.60 (Y: related to X & Z) | 19,35 |
| Fraction of athletes with HCM on medication (high risk) | 0.06 | 0.04–0.08 (Z: related to X & Y) | 35 |
| Probability of a high risk HCM patient to get an ICD per Markov cycle | 0.125 | 0.10–0.15 | 35 |
| SCD annual rate reduced by an implanted ICD for HCM patients (multiplier) | 0.05 (95% saved) | 0.03–0.10 | 21,36 |
| SCD annual rate reduced by medication for HCM patients (multiplier) | 0.95 (5% saved) | 0.90–1.00 | 36,37 |
| Event probabilities, arrhythmias and associated factors | |||
| Prevalence of arrhythmias, among athletes with HD with SCD risk | 0.25 | 0.20–0.30 (B: related to A & C) | 34 |
| Fraction of athletes with arrhythmias that are not treated (low risk) | 0.05 | 0.04–0.06 (related to next 2 parameters) | 38,39 |
| Fraction of athletes with arrhythmias on medication (low risk) | 0.80 | 0.76–0.84 | 38 |
| Fraction of athletes with arrhythmias on medication (high risk) | 0.15 | 0.12–0.18 | 38 |
| Probability of a high risk arrhythmias patient to get an ICD per Markov cycle | 0.125 | 0.10–0.15 | 38,39 |
| SCD annual rate reduced by an implanted arrhythmias for HCM patients (multiplier) | 0.05 (95% saved) | 0.03–0.10 | 40,41 |
| SCD annual rate reduced by medication for arrhythmias patients (multiplier) | 0.70 (30% saved) | 0.50–0.80 | 22 |
| Event probabilities, other HD (not related to HCM and arrhythmias) and associated factors | |||
| Prevalence of other HD, among athletes with HD with SCD risk | 0.25 | 0.10–0.40 (C: dependent on A & B – in practice, we fix A & B, then C = 1−A−B) | 34 |
| Fraction of athletes with other HD that are not treated (low risk) | 0.18 | 0.14–0.22 (related to next 2 parameters) | a |
| Fraction of athletes with other HD on medication (low risk) | 0.70 | 0.64–0.76 | a |
| Fraction of athletes with other HD on medication (high risk) | 0.12 | 0.10–0.14 | a |
| Probability of a high risk other HD patient to get an ICD, per Markov cycle | 0.125 | 0.10–0.15 | a |
| SCD annual rate reduced by an implanted other HD for HCM patients (multiplier) | 0.05 (95% saved) | 0.03–0.10 | a |
| SCD annual rate reduced by medication for other HD patients (multiplier) | 0.90 (10% saved) | 0.80–0.95 | a |
| Sensitivity and specificity for detecting HD with risk of SCD | |||
| Sensitivity (patient H&P) | 0.06 | 0.03–0.40 | 42,43 |
| Specificity (patient H&P) | 0.95 | 0.85–1.00 | 43 |
| Sensitivity (ECG) | 0.70 | 0.65–0.75 | 44 |
| Specificity (ECG) | 0.95 | 0.85–1.00 | 43,44 |
| Sensitivity (ECG + patient H&P; strategy 2) | 0.71 | 0.65–0.77 | a |
| Specificity (ECG + patient H&P; strategy 2) | 0.91 | 0.72–1.00 | a |
| Sensitivity of the cardiologist (ECG + echo as needed) | 0.90 | 0.80–1.00 | a,13 |
| Specificity of the cardiologist (ECG + echo as needed) | 0.98 | 0.92–1.00 | a,13 |
| Annual probability of reclassification of false-positives to true-negatives (for the first 3 y) | 0.20 | 0.10–0.30 | a |
| Annual probability of reclassification of true-positives to false-negatives (for the first 3 y) | 0.00 | 0.00–0.10 | a |
| Utilities and utility-related parameters | |||
| Utility state of a healthy person | 1.00 | N/A | a |
| Utility state of an otherwise healthy person with undiagnosed HD | 1.00 | N/A | a |
| Utility state of a patient with a positive screening until final decision of a cardiologist (average duration of this state: 3.5 d) | 0.90 | 0.85–0.95 | a |
| Utility state of a patient at the initial shock of a positive diagnosis | 0.90 | 0.85–0.95 | 21 |
| Utility state of a false-positive patient (FP diagnosis confirmed by a cardiologist) after the recovery of the initial shock (he/she is on medication) | 0.98 | 0.97–0.99 | a |
| Utility state of a person with HD with low risk of SCD (on medication) after the recovery of the initial shock (he/she is on medication) | 0.98 | 0.97–0.99 | a |
| Utility state of a person with HD with high risk of SCD (on medication) after the recovery of the initial shock (he/she is on medication) | 0.97 | 0.96–0.98 | a |
| Utility state of a person with HD left without treatment after the recovery of the initial shock | 0.98 | 0.97–0.99 | a |
| Utility state of a person with HD with high risk of SCD with implanted ICD (we have assumed 0.5 shocks per year; disutility = 0.01/ shock) | 0.885 | 0.845–0.925 | 45 |
| Disutility of battery replacement, per event | 0.01 | N/A | 21 |
| Average time between the prescreening and the visit to a cardiologist (d) | 3.5 | 2.5–4.5 | a |
| Duration of the recovery from the initial shock (y) | 0.5 | 0.10–1.00 | a |
| Other | |||
| Age of initiation of competitive sports | 14 | N/A | a |
| Maximum potential age | 100 | N/A | a |
| Initial fraction of cohort that is male | 0.59 | 0.55–0.63 | 23 |
| Interval between ICD battery replacements (y) | 7 | 5–9 | 21 |
| Maximum age for playing competitive sports (y) | 35 | 28–36 | a |
| Maximum age for possible transition from low SCD risk to high SCD risk | 50 | N/A | a |
| Discount rate | 0.03 | 0.00–0.05 | 24,46 |
CPT, Current Procedural Terminology; N/A, not applicable.
Estimated by the authors due to lack of published evidence.
We estimated the prevalence of HD with SCD risk to be 0.1%.5 We estimated the annual rate of SCD for boy nonathletes with low-risk HD to be 1%,17–20 and the corresponding rate for girls to be 0.4%.17 High-risk disease elevated these risks by a factor of 3.21,22 Competitive athletics increased risk of SCD by a factor of 2.5 over baseline for boys and girls.17 We estimated that there are 1 million 14-year-old potential athletes each year and that 59% of these were boys.23
We assessed the costs of pharmacotherapy, associated medication management visits, and the value of patient/parent time associated with treatment. We assumed that initial ECGs in strategies 2 and 3 would be read by someone with appropriate training and that these ECGs would require a separate medical appointment.
Health state utilities for adolescents and young adults with HD are scarce in the literature. Values and associated ranges are listed in Table 1. We assumed that positive initial ECGs would reduce QALYs briefly due to stress, pending more definitive cardiology evaluation. We assumed that formal HD diagnosis reduced QALYs initially but that utility would recover on an exponential trajectory to near normal levels over time, except for patients who received an ICD implant.
Beyond Table 1, we made several additional assumptions that affect the decision model:
Prevalence of HD with SCD risk is equivalent between boys and girls.
The effective sensitivity and specificity of H&P and ECG are independent.
All people identified with HD comply with treatment recommendations and avoidance of competitive athletics.
Overall, 70% of high-risk patients will get an ICD within 10 years, approximately one-tenth of high-risk patients without ICDs each year.
After age 50, the annual rate of SCD due to HD diagnosed by screening decreases by half.
Analysis
We conducted analyses from the societal perspective. Costs and QALYs were discounted at 3% in the base case.24 Societal willingness to pay was assumed to lie below $50 000/QALY.25–28 Expected-value analysis and Monte Carlo simulations were performed. Costs and quality-adjusted life expectancies were calculated for each strategy and used to estimate incremental cost-effectiveness (ICE) relative to strategy 1. Monte Carlo simulation used 1000 trials, each with 1 000 000 patients, to assess the precision of cost-effectiveness estimates. In each trial, model parameters were each sampled randomly across their respective distributions.
One-way expected value sensitivity analyses were performed on all parameters. Analyses used TreeAge Pro Healthcare 2009 software.
Results
Base-Case Analysis
Table 2 presents the expected cost and effectiveness of strategies 1 to 3 and the expected ICE of strategies 2 and 3 relative to strategy 1. Relative to strategy 1, strategy 2 raises costs by $116.90 and adds 0.0017 QALYs per member of the screening cohort, corresponding to an ICE of $68 800/QALY, whereas strategy 3 raises costs by $75.05 and adds 0.0020 QALYs per capita for an ICE of $37 700/QALY. Strategy 3 dominates strategy 2, having both lower costs and better outcomes. Though strategy 2 captures slightly more true-positives, it also doubles the number of false-positives relative to strategy 3; the losses from the latter, due to stress and disutility of treatment, more than offset the gains from the former.
TABLE 2.
Resource Utilization and Number of SCDs (for 1-Million-Person Cohort) and Expected Costs per Capita, Effectiveness per Capita, and Incremental Cost-Effectiveness
| Strategy | Pediatric Cardiology Visits (‘000s) (95% CI) | ICD Implants (95% CI) | ICD Battery Replacements (95% CI) | SCDs (95% CI) | Cost per Capita | QALYs per Capita | Incremental, Relative to Strategy 1 | ||
|---|---|---|---|---|---|---|---|---|---|
| Cost | QALYs | Cost-Effectiveness ($/QALY) | |||||||
| 1 | 74 (13–200) | 9 (2–19) | 60 (15–130) | 433 (253–693) | $169.27 | 28.475237 | — | — | — |
| 2 | 203 (85–407) | 110 (63–175) | 725 (390–1225) | 302 (175–484) | $286.17 | 28.476938 | +$116.90 | +0.001701 | +$68 800 |
| 3 | 137 (67–261) | 107 (60–170) | 705 (380–1180) | 306 (176–495) | $244.32 | 28.477229 | +$75.05 | +0.001992 | +$37 700 |
Em dash denotes not applicable.
Monte Carlo Simulation
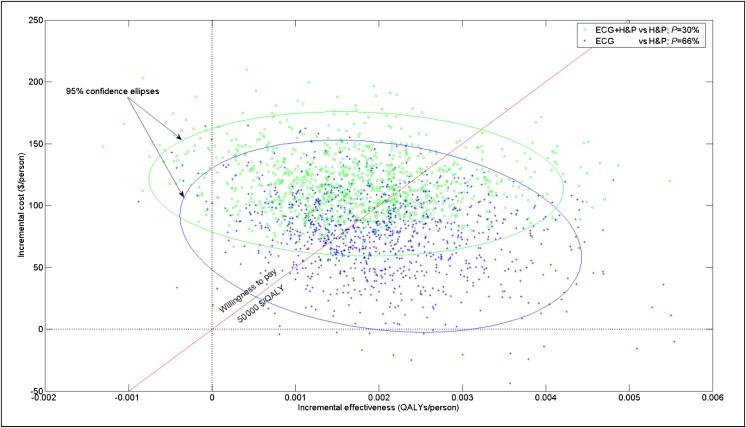
Figure 2 shows the incremental costs and effectiveness of strategies 2 and 3 relative to strategy 1. The diagonal line represents the target societal cost threshold of $50 000/QALY, with trials to the right of that line signifying lower (ie, more favorable) ICE. Based on that threshold, strategy 2 has a 30% probability of cost-effectiveness, relative to strategy 1, whereas strategy 3 has a 66% probability of cost-effectiveness. The relatively wide scatter for strategy 2 is due to the application of 2 screening procedures (H&P + ECG), each involving some uncertainty.
FIGURE 2.
Incremental cost and effectiveness relative to current practice. The scatter plot shows the results of 1000 Monte Carlo simulation trials for strategies 2 (green circles) and 3 (blue pluses). The 2 ellipses correspond to the 95% CIs around the 2 respective sets of results. The diagonal line represents the target societal cost threshold of $50 000/QALY, with trials to the right of that line signifying lower (ie, more favorable) ICE. Listed “P” represents the fraction of Monte Carlo trials that lie on or to the right of the target threshold.
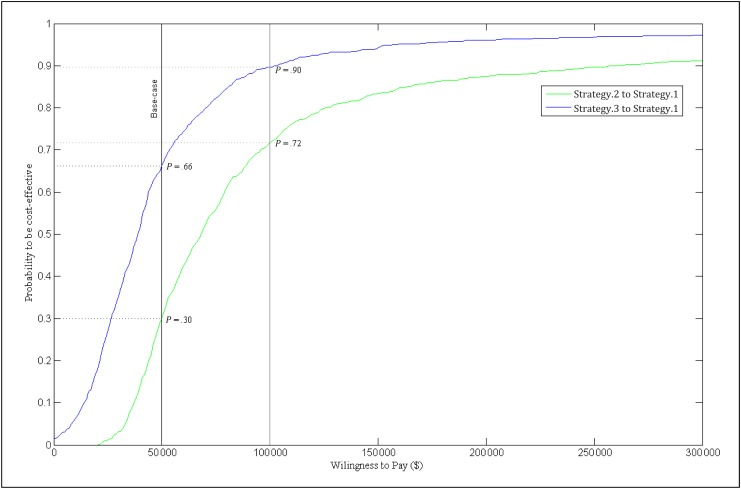
Figure 3 shows that if the target cost threshold is increased to $100 000/QALY, the probability of cost effectiveness increases to 72% for strategy 2 and 90% for strategy 3, relative to strategy 1.
FIGURE 3.
Societal acceptability curve. The societal acceptability curve plots the probability that strategies 2 and 3, respectively, are cost effective relative to strategy 1, for different societal willingness to pay thresholds, based on Monte Carlo simulation.
Table 2 shows the estimated number of SCDs per million-person cohort across the life course. Compared with strategy 1, strategy 2 is expected to avert 131 SCDs (95% confidence interval [CI]: 78–209) at a cost of $900 000 per averted SCD (95% CI: $0.6–$1.5 million), and strategy 3 averts 127 SCDs (95% CI: 77–198) at a cost of $600 000 per averted SCD (95% CI: $0.4–$1.0 million).
Table 2 also illustrates the resource utilization that flows from the 3 strategies.
Sensitivity Analysis
Sensitivity analysis demonstrates that ICE of strategies 2 and 3, relative to strategy 1, is sensitive to the specificity and sensitivity of the respective screening procedures (ie, H&P, ECG, and cardiology evaluation) and to SCD-related parameters (eg, HD prevalence, annual SCD rate for nonathletes, incremental SCD risk from sports, and age limit for competitive sports). For instance, the ICE for strategy 2 meets the target willingness to pay threshold of $50 000/QALY if HD prevalence rises to 1.45 cases per 1000 from the assumed base of 1 per 1000, whereas ICE for strategy 3 exceeds the target for HD prevalence below 0.75 cases per 1000 (Supplemental Fig 5). The model is also sensitive to the relative risk of SCD of athletes compared with nonathletes (Supplemental Fig 6). The base value of the relative risk is 2.5. The ICE of strategy 2 falls below the target threshold if the relative risk of SCD exceeds 3. In contrast, the ICE of strategy 3 exceeds the target if the relative risk of SCD falls below 2.1.
With respect to the specificity of screening methods, strategy 2 would meet the target cost threshold if ECG specificity rose from 0.95 to 0.98, whereas ICE of strategy 3 exceeds the target for ECG specificities of 0.93 or below (Supplemental Fig 7). The ICE of strategy 2 is insensitive to the specificity of H&P (Supplemental Fig 8). However, when specificity of H&P falls below 0.87, strategy 3 dominates strategy 1 (ie, better outcomes and lower cost); indeed, this is true generally when the specificity of ECG exceeds the specificity of H&P by 0.08 or more. In contrast, findings for strategies 2 and 3 are relatively insensitive to the sensitivity of ECG and of H&P (Supplemental Figs 9 and 10).
The ICE of strategy 2 relative to strategy 1 is very sensitive to the specificity of cardiologist assessment (Supplemental Fig 11). If this specificity falls by 0.05 or more from the base value of 0.98, strategy 2 becomes inferior to strategy 1, whereas raising it by 0.01 improves the ICE by a factor of 2. Given our assumption that ECG and H&P have equal specificity, strategy 3 is insensitive to the specificity of cardiologist assessment. Sensitivity of cardiologist assessment has a linear effect on the ICE of strategies 2 and 3 relative to strategy 1.
Findings are not sensitive to utilities or most costs. The exception is cost of ECGs, which has a linear effect on the ICE of strategies 2 and 3. The price of ICDs, while high per case, does not substantively affect the model because they are used so rarely.
Discussion
A cost-effectiveness analysis of alternative strategies of incorporating ECG screening in the preparticipation evaluation of athletes indicates that adding ECG to routine H&P, and referring any patient to cardiology who screens positive on either assessment (strategy 2), has an ICE of $68 800 per QALY relative to current practice (strategy 1). This exceeds a target societal willingness to pay threshold of $50 000/QALY. Strategy 2 averts 130 SCDs per million-child cohort relative to current practice at a cost of $900 000 per averted SCD.
An alternate strategy of routine ECG screening, and cardiology referral based only on ECG results (strategy 3), has favorable ICE relative to current practice at $37 700 per QALY and averts 127 SCDs per cohort at a cost of $600 000 per life saved. The cost of H&P was included in strategy 3, even though clinical decisions are only informed by ECG. Although this induces a conservative bias, it is unlikely that the H&P can be removed from the preparticipation evaluation. This, in fact, is undesirable because the H&P may serve purposes beyond identifying SCD risk, such as identifying noncardiac risk factors for sudden death and other medical issues not directly related to sudden death risk.
This study aims to inform the debate over cardiovascular screening of adolescent athletes. In addition to the cost-effectiveness results, the model helps to inform several other aspects of this debate. First, the model provides an estimate of the total annual cost for adding a single ECG at age 14 to the current standard of care: ∼$116 million. Second, the model identifies important drivers of cost, particularly the rate of false-positives. Patients falsely diagnosed incur financial costs, and lower quality of life, associated with management of their “disease.” Our model assumes cardiology assessment is highly specific and also allows for reclassifying false-positive diagnoses to true-negative diagnoses. Nevertheless, with 200 000 annual referrals to a pediatric cardiologist under strategy 2, even a small false-positive rate has significant impact. False-positives are also driven by ECG specificity, especially if our base value of 0.95 turns out to be difficult to achieve in a mass screening program. Third, the model identifies resource and personnel needs that routine screening might require.
We note that some of the parameters to which our model is sensitive currently have limited empirical basis, particularly the specificity of ECG, the prevalence of relevant HD in this population, the rate of SCD, and the incremental risk of playing competitive sports. Research to improve the quality of data for such parameters could particularly advance the debate about cardiovascular screening.
Wheeler et al15 recently conducted an analogous study. Their findings were similar to those here regarding the estimated cost-effectiveness of H&P plus ECG (corresponding to our strategy 2) versus H&P alone (our strategy 1). However, these similar findings are potentially coincidental because the respective studies had many methodological differences. For instance, Wheeler et al15 assume that quality of life returns fully to “healthy” levels within 4 years of HD diagnosis, whereas we assume life-long decrements due to HD-associated treatment and sports restrictions. We permit some reclassification of false-positives after initial diagnosis, whereas Wheeler et al15 do not. We distinguish explicitly between different degrees of HD severity, which Wheeler et al15 do not, and we explicitly model the associated trajectories of treatment over the life course, whereas Wheeler et al15 use population averages. Wheeler et al15 do not explicitly enumerate the specificity of secondary testing (ie, cardiology evaluation), a parameter to which our model was particularly sensitive.
Wheeler et al15 use a base SCD risk ratio of ∼6 between people with HD who do and do not participate in sports; we use a value of 2.5. This highlights an important assumption underlying both models: that athletic activity increases the risk of SCD in sensitized populations. Although this assumption is supported by epidemiologic data from Italy,17 recent data from a Danish cohort suggests otherwise.29 We believe that a conservative estimate of relative risk is warranted. To illustrate the impact of this particular design difference, we re-estimated our model by using the higher SCD risk ratio. With this change, estimated ICE fell to $23 200/QALY for strategy 2 vs 1 (94% chance to fall under the $50 000/QALY threshold) and to $14 400/QALY for strategy 3 vs 1 (98% chance).
Finally, we note a difference in emphasis: Wheeler et al’s15 primary outcome was life years saved, whereas we focus on QALYs. In our view, quality of life issues are also central in assessing the net value of ECG screening, including the disutility associated with HD treatment and sports restrictions for both true-positives and (perhaps especially) false-positives. As Wheeler et al15 report in secondary analyses, and we find here, estimated cost effectiveness of enhanced screening is somewhat less favorable using the QALY metric.
This study has important limitations. First, certain assumptions represent an idealized world (ie, that patients will fully comply with activity restrictions). However, we wanted to evaluate the different screening strategies under best-case scenarios. Second, we classify diseases into 3 broad groups to model therapies. This is obviously a simplification; our model is more granular, though, than previous efforts, and sensitivity analyses suggest that costs and effectiveness of therapies are not prominent drivers of outcome. Third, certain costs were not included in the model, including costs for infrastructure, liability, and training ECG readers. Most critically, there is limited empirical evidence available regarding important model parameters. When data were available, they were derived primarily from cohort and observational studies, which are more susceptible to bias than randomized trials; in other cases (such as our assumption that ECG and H&P specificities are independent) we know of no available literature.
Conclusions
Under a societal willingness to pay threshold of $50 000/QALY, adding ECGs to current preparticipation evaluations for athletes is not cost-effective, whereas screening based only on ECGs has more favorable cost-effectiveness. Research to improve the quality of data available on those parameters that significantly influence cost-effectiveness would particularly advance the debate about cardiovascular screening.
Supplementary Material
Glossary
- CI
confidence interval
- ECG
electrocardiogram
- H&P
history and physical examination
- HCM
hypertrophic cardiomyopathy
- HD
heart disease
- ICD
implantable cardioverter-defibrillator
- ICE
incremental cost-effectiveness
- QALY
quality-adjusted life year
- SCD
sudden cardiac death
Footnotes
All authors have made substantial contributions to the conception and design of the study, the acquisition, analysis, and interpretation of the data, and the drafting of the article. All authors have provided final approval of the article.
The views expressed in this article do not necessarily represent the views of the National Institute of Mental Health, the National Heart, Lung, and Blood Institute, the National Institutes of Health, the Department of Health and Human Services, or the US government.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: No external funding.
References
- 1.Maron BJ, Haas TS, Doerer JJ, Thompson PD, Hodges JS. Comparison of U.S. and Italian experiences with sudden cardiac deaths in young competitive athletes and implications for preparticipation screening strategies. Am J Cardiol. 2009;104(2):276–280 [DOI] [PubMed] [Google Scholar]
- 2.Atkins DL, Everson-Stewart S, Sears GK, et al. Resuscitation Outcomes Consortium Investigators . Epidemiology and outcomes from out-of-hospital cardiac arrest in children: the Resuscitation Outcomes Consortium Epistry-Cardiac Arrest. Circulation. 2009;119(11):1484–1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berger S, Utech L, Fran Hazinski M. Sudden death in children and adolescents. Pediatr Clin North Am. 2004;51(6):1653–1677, ix–x [DOI] [PubMed] [Google Scholar]
- 4.Maron BJ. Hypertrophic cardiomyopathy and other causes of sudden cardiac death in young competitive athletes, with considerations for preparticipation screening and criteria for disqualification. Cardiol Clin. 2007;25(3):399–414, vi [DOI] [PubMed] [Google Scholar]
- 5.Maron BJ, Thompson PD, Ackerman MJ, et al. American Heart Association Council on Nutrition, Physical Activity, and Metabolism . Recommendations and considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletes: 2007 update: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2007;115(12):1643–455 [DOI] [PubMed] [Google Scholar]
- 6.Ackerman MJ, Tester DJ, Driscoll DJ. Molecular autopsy of sudden unexplained death in the young. Am J Forensic Med Pathol. 2001;22(2):105–111 [DOI] [PubMed] [Google Scholar]
- 7.Myerburg RJ, Vetter VL. Electrocardiograms should be included in preparticipation screening of athletes. Circulation. 2007;116(22):2616–2626, discussion 2626 [DOI] [PubMed] [Google Scholar]
- 8.Corrado D, Pelliccia A, Bjørnstad HH, et al. Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Consensus Statement of the Study Group of Sport Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology . Cardiovascular pre-participation screening of young competitive athletes for prevention of sudden death: proposal for a common European protocol. Eur Heart J. 2005;26(5):516–524 [DOI] [PubMed] [Google Scholar]
- 9.Pelliccia A. The preparticipation cardiovascular screening of competitive athletes: is it time to change the customary clinical practice? Eur Heart J. 2007;28(22):2703–2705 [DOI] [PubMed] [Google Scholar]
- 10.Chaitman BR. An electrocardiogram should not be included in routine preparticipation screening of young athletes. Circulation. 2007;116(22):2610–2614, discussion 2615 [DOI] [PubMed] [Google Scholar]
- 11.Viskin S. Antagonist: routine screening of all athletes prior to participation in competitive sports should be mandatory to prevent sudden cardiac death. Heart Rhythm. 2007;4(4):525–528 [DOI] [PubMed] [Google Scholar]
- 12.Steinvil A, Chundadze T, Zeltser D, et al. Mandatory electrocardiographic screening of athletes to reduce their risk for sudden death proven fact or wishful thinking? J Am Coll Cardiol. 2011;57(11):1291–1296 [DOI] [PubMed] [Google Scholar]
- 13.Fuller CM. Cost effectiveness analysis of screening of high school athletes for risk of sudden cardiac death. Med Sci Sports Exerc. 2000;32(5):887–890 [DOI] [PubMed] [Google Scholar]
- 14.Drezner JA. Contemporary approaches to the identification of athletes at risk for sudden cardiac death. Curr Opin Cardiol. 2008;23(5):494–501 [DOI] [PubMed] [Google Scholar]
- 15.Wheeler MT, Heidenreich PA, Froelicher VF, Hlatky MA, Ashley EA. Cost-effectiveness of preparticipation screening for prevention of sudden cardiac death in young athletes. Ann Intern Med. 2010;152(5):276–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13(4):322–338 [DOI] [PubMed] [Google Scholar]
- 17.Corrado D, Basso C, Rizzoli G, Schiavon M, Thiene G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J Am Coll Cardiol. 2003;42(11):1959–1963 [DOI] [PubMed] [Google Scholar]
- 18.Berger S, Whitstone BN, Frisbee SJ, et al. Cost-effectiveness of Project ADAM: a project to prevent sudden cardiac death in high school students. Pediatr Cardiol. 2004;25(6):660–667 [DOI] [PubMed] [Google Scholar]
- 19.Maron BJ, Olivotto I, Spirito P, et al. Epidemiology of hypertrophic cardiomyopathy-related death: revisited in a large non-referral-based patient population. Circulation. 2000;102(8):858–864 [DOI] [PubMed] [Google Scholar]
- 20.Colan SD, Lipshultz SE, Lowe AM, et al. Epidemiology and cause-specific outcome of hypertrophic cardiomyopathy in children: findings from the Pediatric Cardiomyopathy Registry. Circulation. 2007;115(6):773–781 [DOI] [PubMed] [Google Scholar]
- 21.You JJ, Woo A, Ko DT, Cameron DA, Mihailovic A, Krahn M. Life expectancy gains and cost-effectiveness of implantable cardioverter/defibrillators for the primary prevention of sudden cardiac death in patients with hypertrophic cardiomyopathy. Am Heart J. 2007;154(5):899–907 [DOI] [PubMed] [Google Scholar]
- 22.Hobbs JB, Peterson DR, Moss AJ, et al. Risk of aborted cardiac arrest or sudden cardiac death during adolescence in the long-QT syndrome. JAMA. 2006;296(10):1249–1254 [DOI] [PubMed] [Google Scholar]
- 23.Associations NFoSHS. NFHS participation survey 2007–2008. Available at: www.nfhs.org/core/contentmanager/uploads2007-08%20participation%20survey.pdf. Accessed December 10, 2010
- 24.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276(15):1253–1258 [PubMed] [Google Scholar]
- 25.Filion KB, Roy AM, Baboushkin T, Rinfret S, Eisenberg MJ. Cost-effectiveness of drug-eluting stents including the economic impact of late stent thrombosis. Am J Cardiol. 2009;103(3):338–344 [DOI] [PubMed] [Google Scholar]
- 26.Greenberg D, Bakhai A, Cohen DJ. Can we afford to eliminate restenosis? Can we afford not to? J Am Coll Cardiol. 2004;43(4):513–518 [DOI] [PubMed] [Google Scholar]
- 27.Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making. 2000;20(3):332–342 [DOI] [PubMed] [Google Scholar]
- 28.Tengs TO, Adams ME, Pliskin JS, et al. Five-hundred life-saving interventions and their cost-effectiveness. Risk Anal. 1995;15(3):369–390 [DOI] [PubMed] [Google Scholar]
- 29.Holst AG, Winkel BG, Theilade J, et al. Incidence and etiology of sports-related sudden cardiac death in Denmark—implications for preparticipation screening. Heart Rhythm. 2010;7(10):1365–1371 [DOI] [PubMed] [Google Scholar]
- 30.Centers for Medicare and Medicaid Services. Medicare physician fee schedule. 2009. Available at: www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. Accessed March 15, 2009
- 31.Healthcare T. Red Book 2008. New York, NY: Thomson Reuters; 2008 [Google Scholar]
- 32.Medtronic. Reimbursement guide for ICD implants, March 2010. Available at: www.medtronic.com/wcm/groups/mdtcom_sg/@mdt/@crdm/documents/documents/2010-reimb-guide-icd.pdf. Accessed October 15, 2010
- 33.Arias E. United States life tables, 2004. Natl Vital Stat Rep. 2007;56(9):1–39 [PubMed] [Google Scholar]
- 34.Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980-2006. Circulation. 2009;119(8):1085–1092 [DOI] [PubMed] [Google Scholar]
- 35.Maron BJ, Casey SA, Poliac LC, Gohman TE, Almquist AK, Aeppli DM. Clinical course of hypertrophic cardiomyopathy in a regional United States cohort. JAMA. 1999;281(7):650–655 [DOI] [PubMed] [Google Scholar]
- 36.Maron BJ, Spirito P, Shen WK, et al. Implantable cardioverter-defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. JAMA. 2007;298(4):405–412 [DOI] [PubMed] [Google Scholar]
- 37.Melacini P, Maron BJ, Bobbo F, et al. Evidence that pharmacological strategies lack efficacy for the prevention of sudden death in hypertrophic cardiomyopathy. Heart. 2007;93(6):708–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Etheridge SP, Sanatani S, Cohen MI, Albaro CA, Saarel EV, Bradley DJ. Long QT syndrome in children in the era of implantable defibrillators. J Am Coll Cardiol. 2007;50(14):1335–1340 [DOI] [PubMed] [Google Scholar]
- 39.Goldenberg I, Moss AJ, Bradley J, et al. Long-QT syndrome after age 40. Circulation. 2008;117(17):2192–2201 [DOI] [PubMed] [Google Scholar]
- 40.Goldenberg I, Moss AJ, Maron BJ, Dick AW, Zareba W. Cost-effectiveness of implanted defibrillators in young people with inherited cardiac arrhythmias. Ann Noninvasive Electrocardiol. 2005;10(suppl 4):67–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zareba W, Moss AJ, Daubert JP, Hall WJ, Robinson JL, Andrews M. Implantable cardioverter defibrillator in high-risk long QT syndrome patients. J Cardiovasc Electrophysiol. 2003;14(4):337–341 [DOI] [PubMed] [Google Scholar]
- 42.Maron BJ, Shirani J, Poliac LC, Mathenge R, Roberts WC, Mueller FO. Sudden death in young competitive athletes. Clinical, demographic, and pathological profiles. JAMA. 1996;276(3):199–204 [PubMed] [Google Scholar]
- 43.Baggish AL, Hutter AM, Jr, Wang F, et al. Cardiovascular screening in college athletes with and without electrocardiography: A cross-sectional study. Ann Intern Med. 2010;152(5):269–275 [DOI] [PubMed] [Google Scholar]
- 44.Fuller CM, McNulty CM, Spring DA, et al. Prospective screening of 5,615 high school athletes for risk of sudden cardiac death. Med Sci Sports Exerc. 1997;29(9):1131–1138 [DOI] [PubMed] [Google Scholar]
- 45.Sears SF, Hazelton AG, St Amant J, et al. Quality of life in pediatric patients with implantable cardioverter defibrillators. Am J Cardiol. 2011;107(7):1023–1027 [DOI] [PubMed] [Google Scholar]
- 46.Gold MR, Siegel JE, Russell LB, Weinstein MC, eds. Cost-Effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.