Abstract
This cross-sectional clinical study compared inflammation, including expression of the chemokine interleukin (IL)-8 and intercellular cell adhesion molecule-1 (ICAM-1), in the stifle joints of 4 control dogs and 23 dogs with cranial cruciate ligament rupture (CCLR). The CCL, synovial membrane, meniscus, cartilage, and synovial fluid from the affected stifle joints of all the dogs were examined. Inflammatory cell counts were performed on the synovial fluid, and the tissues were processed for histologic study and immunohistochemical detection of IL-8 and ICAM-1. The synovial fluid from the stifle joints of the dogs with CCLR had an increased percentage of neutrophils (P = 0.054) and a decreased percentage of lymphocytes (P = 0.004) but not macrophages compared with the fluid from the control dogs. There was accumulation of inflammatory cells and increased expression of IL-8 and ICAM-1 in the vascular endothelium of the synovial membrane and the CCL of the dogs with CCLR. The increase in inflammatory cells in the stifle joints of dogs with CCLR may therefore be due to increased expression of IL-8 and ICAM-1 in the synovial membrane and the CCL after the injury. These data may help in understanding the mechanisms of inflammation associated with CCLR.
Résumé
Cette étude clinique par analyse transversale visait à comparer l’inflammation, incluant l’expression de l’interleukine (IL)-8 et de la molécule intercellulaire d’adhésion des cellules de type 1 (ICAM-1), dans les genoux de 4 chiens témoins et de 23 chiens avec rupture du ligament croisé cranial (RLCC). Le LCC, la membrane synoviale, les ménisques, le cartilage, et le liquide synovial provenant des articulations affectées de tous les chiens ont été examinés. Les dénombrements des cellules inflammatoires ont été effectués sur le liquide synovial, et les tissus ont été traités pour examen histologique et détection par immuno-histochimie d’IL-8 et d’ICAM-1. Comparativement au liquide synovial provenant des chiens témoins, le liquide synovial provenant de l’articulation du genou des chiens avec RLCC présentait une augmentation du pourcentage des neutrophiles (P = 0,054) et une diminution du pourcentage des lymphocytes (P = 0,004) mais pas des macrophages. Il y avait une augmentation des cellules inflammatoires et une augmentation de l’expression d’IL-8 et d’ICAM-1 dans l’endothélium vasculaire de la membrane synoviale et le LCC de chiens avec RLCC. L’augmentation des cellules inflammatoires dans les genoux de chiens avec RLCC pourrait ainsi être due à l’augmentation d’expression d’IL-8 et d’ICAM-1 dans la membrane synoviale et le LCC après la blessure. Ces données pourraient aider à comprendre le mécanisme de l’inflammation associée avec la RLCC.
(Traduit par Docteur Serge Messier)
Introduction
The canine cranial cruciate ligament (CCL) plays a large role in the craniocaudal stability of the stifle joint (1). The CCL is attached to the medial surface of the lateral femoral condyle and the craniomedial surface of the tibial plateau in all canine species. This ligament stabilizes the joint during the full range of motion (2,3). The CCL consists of twisted collagenous fascicles and fiber bundles that consist of fascicles, fibers, and fibrils. Because it has 2 attachment zones, the ligament can be divided into craniomedial and craniolateral functional components (1). Rupture of the CCL (CCLR), one of the most common orthopedic injuries in dogs, is the main cause of osteoarthritis of the stifle joint (4). Rupture causes instability of the joint, which results in cranial drawer instability, increased internal rotation, and hyperextension. Despite reconstruction of the ligament, there may be progressive stifle osteoarthritis secondary to the biomechanical alterations within the joint and cartilage damage (4).
Although CCLR may occur as a result of trauma, more often it is a consequence of degeneration of the ligament (5). Inflammation associated with joint trauma, sepsis, or immune-mediated disease is also considered to be an important cause of CCLR (6). The most common clinical picture of an affected stifle joint includes joint effusion, periarticular fibrosis, radiographic evidence of osteoarthritis and joint effusion, and cranial drawer instability. Radiographic assessment of the long-term results of all surgical repair techniques shows evidence of progressive osteoarthritis (7).
Inflammation accompanies many diseases and is typically characterized by the expression of proinflammatory mediators, elaboration of adhesion molecules, and recruitment of inflammatory cells (8). There is evidence of inflammation in stifle joints with CCLR (5). Multiple cytokines, such as interleukin (IL)-1β, IL-6, IL-8, IL-10, IL-17, and tumor necrosis factor-alpha (TNF-α) are known to be involved in inflammatory joint diseases such as osteoarthritis (9). Among these, IL-8, which is produced by leukocytes, fibroblasts, endothelial cells, chondrocytes, and synoviocytes, is known to be a chemoattractant for neutrophils (10) and to induce the expression of adhesion molecules and neutrophil degranulation. The proinflammatory cytokines IL-1, IL-6, and TNF-α display many biologic functions in the early phase of joint and cartilage inflammation (5). Data show higher levels of cytokines such as IL-1β, IL-6, and TNF-α as well as markers of cartilage degradation in the synovial fluid (11). Synovial fluid from stifle joints with CCLR has more mononuclear cells but not granulocytes, which suggests a noninfectious cause (12). There is some evidence that mRNA expression of IL-8 is not altered in the joint with CCLR (2), and therefore tests for IL-8 mRNA as a specific marker of joint disease should not be used; there are also no data on the expression of IL-8 protein in CCLR. Inflammatory cell recruitment is mediated through coordinated expression of adhesion molecules such as selectins, integrins, and members of the immunoglobulin superfamily (13). The expression of intercellular adhesion molecule-1 (ICAM-1), a member of the immunoglobulin superfamily, is rapidly regulated after stimulation of endothelium, and this molecule is critical for the engagement of integrins expressed on neutrophils and monocytes (14). To our knowledge, there are no data on the immunohistologic expression of IL-8 and ICAM-1 in the synovial membrane and CCL of dogs with or without CCLR.
To better understand the mechanisms of inflammation associated with CCLR, we compared inflammation, including the presence of inflammatory cells and the expression of IL-8 and ICAM-1, in the stifle joints of dogs with CCLR.
Materials and methods
Experimental animals and protocols
The experimental protocols were approved by the University of Saskatchewan Campus Committee on Animal Care, and the experiments were conducted according to the Canadian Council on Animal Care guidelines (15). Before surgery the dog owners were asked to sign a consent form allowing their dogs to be part of this study. We included samples from 23 dogs out of the 25 dogs with CCLR and 4 control dogs without any restrictions on dog size or duration of their condition. The dogs’ mean age was 6.3 ± 2.6 (standard deviation) y and mean body weight 33.24 ± 13.08 kg. Samples were collected from 5 areas of the knee joint during surgery: the joint fluid (before skin incision), the synovial membrane (a sample 2 to 3 mm in diameter), the CCL, cartilage, and a portion of the meniscus if it was damaged and was to be resected. Joint condition was evaluated before and after surgery as to which limb was affected, the grade of lameness, joint effusion, capsular thickening, medial buttress, swelling on the medial side of the affected leg facing the other leg, crepitus, meniscal click, cranial drawer, and positive tibial compression. Records of the surgical procedures and the surgical findings were examined.
Collection and processing of joint fluid and tissues
Tissues were processed as described previously (16). Briefly, the joint fluid was collected in a tube containing ethylene diamine tetraacetic acid, smears were prepared immediately, and the rest of the fluid was stored at −80°C. The samples of synovial membrane and meniscus, as well as the samples of CCL, were fixed in 10% formalin for 24 h, washed 3 times with phosphate-buffered saline (PBS), and stored in PBS at 4°C. The tissues were dehydrated in ascending concentrations of ethanol and xylene and then embedded in paraffin. Sections 5 7mu;m thick were cut, placed on glass slides coated with poly-L-lysine solution (Sigma Diagnostics, St. Louis, Missouri, USA), and kept for 45 min at 55°C in an oven to increase tissue adherence. The tissue sections were stained with hematoxylin and eosin (H&E), whereas the synovial fluid smears were stained with Wright–Giemsa stain for differential leukocyte count. Histologic signs of inflammation were rated from “−” to “+++” according to severity: “−”, no signs of inflammation; “+”, few inflammatory cells and absence of a large area of inflammation; “++”, inflammatory cells and large areas of inflammation; and “+++”, intense inflammation in the entire tissue section.
Immunohistochemical studies
The immunohistochemical protocols have been described previously (16,17). Briefly, the sections were deparaffinized, rehydrated, and immersed in hydrogen peroxide (0.5% in methanol) to neutralize endogenous peroxidase. Sections exposed to pepsin (2 mg/mL of 0.01 N HCl) were blocked with bovine serum albumin (BSA) and incubated with primary antibodies (1:100 IL-8 and 1:50 ICAM-1 from mice; provided by Dr. C. Wayne Smith, Baylor College of Medicine, Houston, Texas, USA) and then secondary antibody [horseradish peroxidase (HRP)-conjugated polyclonal goat immunoglobulins against mouse antigen]. There were 3 types of control conditions: (a) primary and secondary antibodies were omitted, (b) only primary antibody was omitted, and (c) sections were stained with a von Willebrand antibody (1:200) and then incubated with secondary antibody (HRP-conjugated polyclonal goat immunoglobulins against rabbit antigen). All secondary antibodies were used at a 1:150 dilution and were procured from Dako Canada, Burlington, Ontario. After color development the sections were counterstained with methyl green.
Statistical analysis
The rank sum t-test (SigmaStat for Windows, version 3.11, Systat Software, San José, California, USA) was used to compare differences in differential leukocyte counts between the control dogs and those with CCLR. A P-value of less than 0.05 was considered significant. The data were expressed as medians and interquartile ranges.
Results
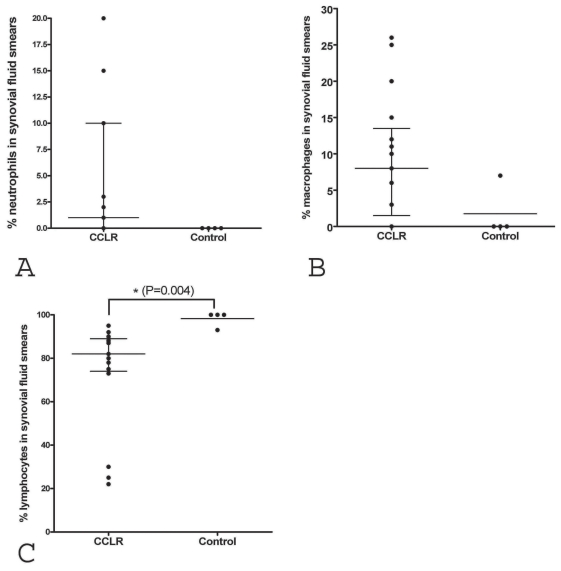
Compared with the control samples, the synovial fluid samples from the dogs with CCLR had an increased percentage of neutrophils (Figure 1A, P = 0.054), with a trend towards significance, no difference in percentage of macrophages (Figure 1B, P = 0.08), and a decreased percentage of lymphocytes (Figure 1C, P = 0.004).
Figure 1.
Differential leukocyte counts in smears of synovial fluid from dogs with cranial cruciate ligament rupture (CCLR) and control dogs. Compared with the control smears, the smears from dogs with CCLR showed a greater percentage of neutrophils (A, P = 0.054), a similar percentage of macrophages (B, P = 0.080), and a significantly lower percentage of lymphocytes (C, P = 0.004). Lines indicate median and interquartile range. Asterisk indicates a significant difference.
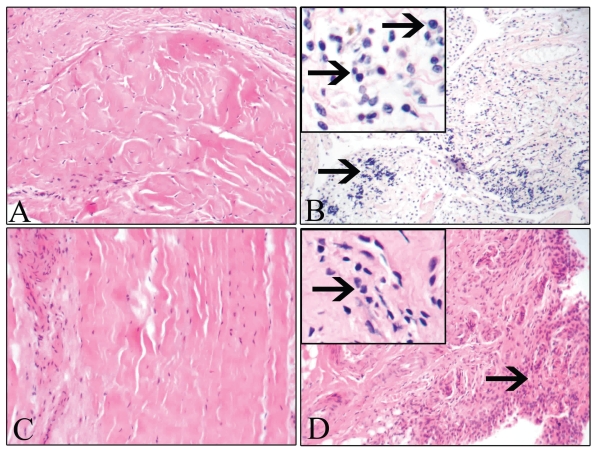
The histopathologic findings in the H&E-stained tissue sections from the stifle joints are summarized in Table I. Inflammation was observed in 1 of 8 cartilage samples, 15 of 20 CCL samples, 4 of 12 meniscus samples, and all 23 samples of synovial membrane from the dogs with CCLR. The synovium (Figure 2A) and the CCL (Figure 2C) from the control dogs did not show any accumulation of inflammatory cells. In contrast, the synovium (Figure 2B) from all the dogs with CCLR and the CCL (Figure 2D) from most of the dogs with CCLR contained macrophages and lymphocytes; a few neutrophils were detected in 3 of the 23 synovial tissue samples.
Table I.
Semiquantitative subjective grading of inflammation in stifle joint tissues of dogs with cranial cruciate ligament (CCL) rupture and control dogs
| Dog (case number) | Cartilage | CCL | Meniscus | Synovium | History of lameness |
|---|---|---|---|---|---|
| 1 (102865) | − | ++ | − | +++ | |
| 2 (112314) | +++ | +++ | ++ | ||
| 3 (120993) | − | − | − | ++a | 3 wk for right hind leg |
| 4 (122151) | − | +++ | 6 mo of abnormal gait, 2 wk non-weight-bearing | ||
| 5 (122345) | − | ++ | − | +++ | 5 mo for right hind leg, 5 wk (acute) for left hind leg |
| 6 (123330) | − | − | ++a | ||
| 7 (123970) | +++ | − | +++ | 3 wk for right hind leg (partial rupture), 2 wk for left hind leg (complete rupture + meniscus damage) | |
| 8 (126598) | + | ++a | 2 wk but not normal (lame after exercise) for 1 to 2 y before partial rupture diagnosed | ||
| 9 (127129) | − | +++ | ++ | 2 mo | |
| 10 (128791) | +++ | ++ | |||
| 11 (131689) | − | + | ++ | ||
| 12 (131792) | + | ||||
| 13 (132119) | +++ | ||||
| 14 (132235) | − | +++ | 3 mo; left hind leg needed second surgery 10 d after first surgery | ||
| 15 (117803) | − | ||||
| 16 (138509) | ++ | − | + | 3 mo | |
| 17 (138436) | +++ | +++ | |||
| 18 (119059) | − | +++ | 6 mo | ||
| 19 (138644) | +++ | − | ++ | 5 to 6 wk | |
| 20 (138638) | ++ | − | ++ | 6 mo bilaterally, then 1 wk acutely for left hind leg and 8 mo acutely for right hind leg | |
| 21 (129866) | ++ | ++ | ++ | Left leg 8 to 9 mo intermittently, then 1 mo constantly (complete rupture); right leg partial rupture for perhaps 2 y | |
| 22 (139237) | ++ | At least 9 mo | |||
| 23 (139460) | + | + | ++ | 4 to 5 mo | |
| 24 (139546) | − | ++ | 1 mo; acute onset | ||
| 25 (138083) | +++ | + | +++ | 6 wk; acute onset |
Presence of neutrophils. In all other tissue sections the inflammatory cells were macrophages and lymphocytes.
Figure 2.
No inflammation was observed in the synovial membrane (A) and CCL (C) from the control dogs, whereas there were accumulations of inflammatory cells (arrows) in the synovium (B) and the CCL (D) of dogs with CCLR. Hematoxylin and eosin; original magnifications ×400; Inset ×1000.
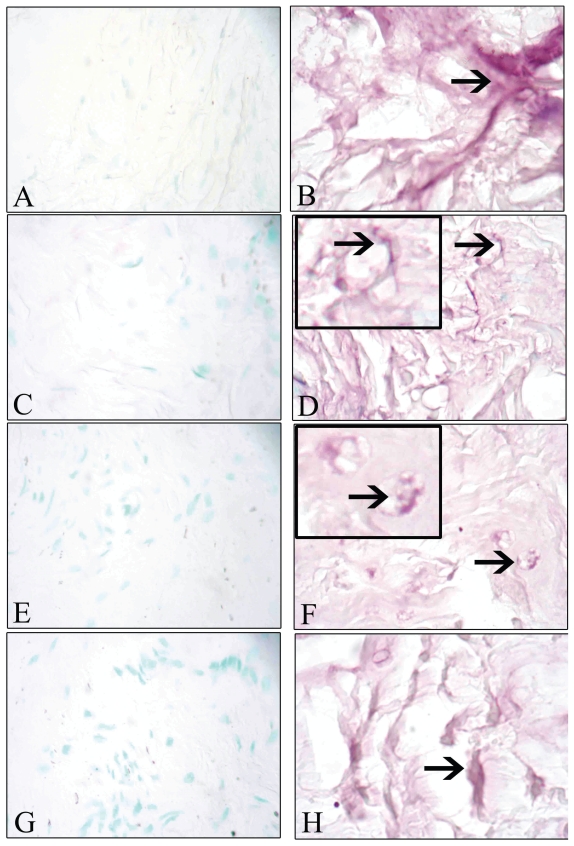
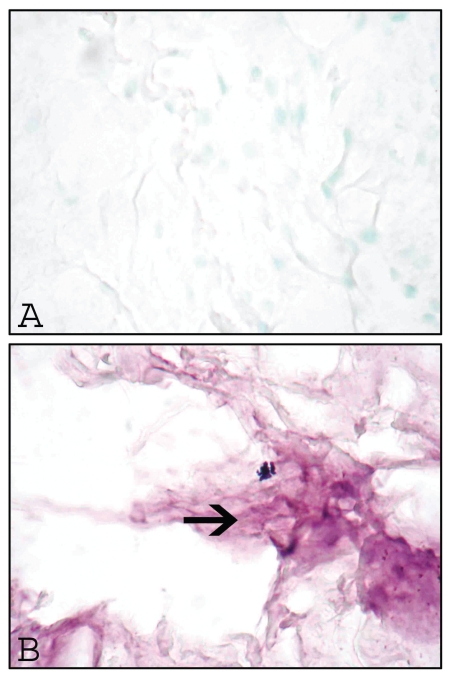
No staining for IL-8 was observed in sections of the synovial membrane (Figure 3A) and the CCL (Figure 3E) from the control dogs. In contrast, IL-8 staining was present in the synovial membrane of 21 out of 23 (Figure 3B) and the CCL of 19 out of 23 (Figure 3F) dogs with CCLR; it was observed mainly in vascular endothelial cells. Synovial membrane (Figure 3C) and CCL (Figure 3G) from the control dogs did not show staining for ICAM-1, whereas synovial membrane (Figure 3D) and CCL (Figure 3H) from the 21 dogs out of 23 dogs with CCLR showed staining for ICAM-1, again mostly in vascular endothelial cells. Omission of the primary antibody resulted in no staining (Figure 4A), whereas replacement with a von Willebrand antibody resulted in staining of the synovial vascular endothelial cells (Figure 4B).
Figure 3.
No staining for interleukin (IL)-8 or intercellular cell adhesion molecule-1 (ICAM-1) was detected in the synovial membrane (A and C, respectively) or the CCL (E and G, respectively) of the control dogs, whereas staining (arrows) for IL-8 and ICAM-1 was seen in the synovium (B and D, respectively) and the CCL (F and H, respectively) of dogs with CCLR, mainly in vascular endothelial cells. Original magnifications ×400.
Figure 4.
Omission of the primary antibody resulted in no staining (A), whereas use of a von Willebrand antibody instead of the primary antibody resulted in staining of the vascular endothelial cells of the synovium (B, arrow). Original magnifications ×400.
Discussion
The pathogenesis of inflammation accompanying CCLR remains poorly understood. Most previous studies focused on the analysis of synovial fluid (2,11,12,18), which is important and adds to our understanding of the pathogenesis of CCLR by providing information on the identity of infiltrating cells. However, there is also a need to investigate tissue expression of chemokines and adhesion molecules to understand the migration of inflammatory cells into the joint.
We found an increase in the percentage of neutrophils (P = 0.054) in the synovial fluid of dogs with CCLR compared with control dogs. Neutrophil migration occurs with inflammation in various tissues, and neutrophil quantification is used to detect inflammation (19,20). It is generally accepted that CCLR may not involve infectious agents or their byproducts, such as lipopolysaccharides and lipotechoic acids, which stimulate inflammation via the Toll-like receptors (5,7). Therefore, the accumulation of inflammatory cells may be due to trauma associated with the CCLR or secondary inflammation associated with the chronic instability and degenerative changes after CCLR.
We noticed expression of IL-8 on vascular endothelium in both the synovial membrane and the CCL of most of the dogs with ligament rupture. The migration of inflammatory cells is facilitated by the expression of chemokines such as IL-8 and adhesion molecules such as ICAM-1 (19,21–25). Inflammatory cells migrate into affected tissues through a multistep process that includes rolling, adhesion, and transmigration across the capillaries and venules. Rolling and slowing of neutrophils is mediated through chemokines such as IL-8 that are expressed on the vascular endothelium and engagement of adhesive proteins called selectins (22). The IL-8 expressed in the vascular endothelium of synovial membrane and the CCL may be important or even critical in attracting neutrophils into the joint.
The chemokine-induced slowing of neutrophils in the capillaries and venules is followed by their tighter engagement by ICAM-1 expressed on vascular endothelium and β2 integrins expressed on neutrophils, which results in arrest of neutrophils and activation of cell signaling in both endothelium and neutrophils (14,26,27). Canine neutrophils express integrins such as β2 and β3, which participate in their recruitment (28,29). Although there is some information on the expression of ICAM-1 on the vascular endothelium of myocardium and synovial membrane in dogs (30,31), no data are available for synovial membrane. and CCL in the dogs with CCLR. The expression of ICAM-1 on the vascular endothelium of the synovial membrane and the CCL is an integral step in the tight adhesion of neutrophils to the vascular endothelium. These first data for this process in the dog suggest a role for ICAM-1 in the recruitment of inflammatory cells in CCLR. The expression of IL-8 and ICAM-1 associated with accumulation of lymphocytes and neutrophils in synovial fluid and inflammatory cells in the synovial membrane and the CCL appears to be a direct consequence of CCLR or the physical instability induced by rupture. Although the expression of chemokines and adhesion molecules is mostly induced by microbes and their products such as lipopolysaccharides, there is evidence that trauma itself can induce the expression of these substances (32–34). Therefore, we believe that IL-8 and ICAM-1 expression leading to accumulation of inflammatory cells in synovial fluid is due to trauma to the tissues.
We recognize some of the limitations of this study. First, there is a need to analyze mRNA expression of IL-8 and ICAM-1 and to phenotype by means of flow cytometry the inflammatory cells in the synovial fluid. Second, long-term follow-up of the expression of inflammatory markers in the synovial fluid and tissue components of the joint is needed. Third, we believe that quantitative analysis of the inflammatory markers in the joint tissues by means of enzyme-linked immunosorbent assay would strengthen the immunohistologic evidence.
In conclusion, we have provided possibly the first data on the immunohistologic expression of IL-8 and ICAM-1 in the synovial membrane and the CCL of dogs with CCLR. These data may assist in a better understanding of the pathogenesis of CCLR.
Acknowledgments
The work was supported through a grant from the Companion Animal Health Fund of the Western College of Veterinary Medicine, University of Saskatchewan, Saskatoon, Saskatchewan, to Dr. Baljit Singh and Dr. Cindy Shmon. Mustafa El-Hadi was the recipient of an Undergraduate Summer Research Award from the Natural Sciences and Engineering Research Council of Canada, and Andrea Aebisher was a Merck–Mérial Veterinary Scholar. Dr. Charavaryamath was supported through a Graduate Merit Scholarship from the University of Saskatchewan, a Founding Chairs Graduate Fellowship from the Institute of Agricultural, Rural and Environmental Health, University of Saskatchewan, and a scholarship from the Canadian Institutes of Health Research Strategic Training Program in Public Health and the Agricultural Rural Ecosystem and Partner Institutes, including the Institute of Cancer Research, the Institute of Circulatory and Respiratory Health, the Institute of Infection and Immunity, the Institute of Population and Public Health, and the University of Saskatchewan. The authors declare no conflict of interest.
References
- 1.de Rooster H, de Bruin T, van Bree H. Morphologic and functional features of the canine cruciate ligaments. Vet Surg. 2006;35:769–780. doi: 10.1111/j.1532-950X.2006.00221.x. [DOI] [PubMed] [Google Scholar]
- 2.de Bruin T, de Rooster H, van Bree H, Cox E. Interleukin-8 mRNA expression in synovial fluid of canine stifle joints with osteoarthritis. Vet Immunol Immunopathol. 2005;108:387–397. doi: 10.1016/j.vetimm.2005.06.013. Epub 2005 Aug 15. [DOI] [PubMed] [Google Scholar]
- 3.Slocum B, Slocum TD. Tibial plateau leveling osteotomy for repair of cranial cruciate ligament rupture in the canine. Vet Clin North Am Small Anim Pract. 1993;23:777–795. doi: 10.1016/s0195-5616(93)50082-7. [DOI] [PubMed] [Google Scholar]
- 4.Vasseur PB, Berry CR. Progression of stifle osteoarthrosis following reconstruction of the cranial cruciate ligament in 21 dogs. JAm Anim Hosp Assoc. 1992;28:129–136. [Google Scholar]
- 5.Doom M, de Bruin T, de Rooster H, van Bree H, Cox E. Immunopathological mechanisms in dogs with rupture of the cranial cruciate ligament. Vet Immunol Immunopathol. 2008;125:143–161. doi: 10.1016/j.vetimm.2008.05.023. Epub 2008 Jun 3. [DOI] [PubMed] [Google Scholar]
- 6.Comerford EJ, Smith K, Hayashi K. Update on the aetiopatho-genesis of canine cranial cruciate ligament disease. Vet Comp Orthop Traumatol. 2011;24:91–98. doi: 10.3415/VCOT-10-04-0055. [DOI] [PubMed] [Google Scholar]
- 7.Harasen G. Canine cranial cruciate ligament rupture in profile. Can Vet J. 2003;44:845–846. [PMC free article] [PubMed] [Google Scholar]
- 8.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 9.Maccoux LJ, Salway F, Day PJR, Clements DN. Expression profiling of select cytokines in canine osteoarthritis tissues. Vet Immunol Immunopathol. 2007;118:59–67. doi: 10.1016/j.vetimm.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Yu CL, Sun KH, Shei SC, et al. Interleukin 8 modulates interleukin-1 beta, interleukin-6 and tumor necrosis factor-alpha release from normal human mononuclear cells. Immunopharmacology. 1994;27:207–214. doi: 10.1016/0162-3109(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 11.Hay CW, Chu Q, Budsberg SC, Clayton MK, Johnson KA. Synovial fluid interleukin 6, tumor necrosis factor, and nitric oxide values in dogs with osteoarthritis secondary to cranial cruciate ligament rupture. Am J Vet Res. 1997;58:1027–1032. [PubMed] [Google Scholar]
- 12.Johnson KA, Hay CW, Chu Q, Roe SC, Caterson B. Cartilage-derived biomarkers of osteoarthritis in synovial fluid of dogs with naturally acquired rupture of the cranial cruciate ligament. Am J Vet Res. 2002;63:775–781. doi: 10.2460/ajvr.2002.63.775. [DOI] [PubMed] [Google Scholar]
- 13.Wang Q, Doerschuk CM. The signaling pathways induced by neutrophil–endothelial cell adhesion. Antioxid Redox Signal. 2002;4:39–47. doi: 10.1089/152308602753625843. [DOI] [PubMed] [Google Scholar]
- 14.Mayadas TN, Cullere X. Neutrophil β2 integrins: Moderators of life or death decisions. Trends Immunol. 2005;26:388–395. doi: 10.1016/j.it.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Olfert ED, Cross BM, McWilliam AA, editors. Guide to the Care and Use of Experimental Animals. 2nd ed. Vol. 1. Ottawa, Ontario: Canadian Council on Animal Care; 1993. [Last accessed November 7, 2011]. Available from www.ccac.ca/en/CCAC_Programs/Guidelines_Policies/GUIDES/ENGLISH/toc_v1.htm. [Google Scholar]
- 16.Wassef A, Janardhan K, Pearce JW, Singh B. Toll-like receptor 4 in normal and inflamed lungs and other organs of pig, dog and cattle. Histol Histopathol. 2004;19:1201–1208. doi: 10.14670/HH-19.1201. [DOI] [PubMed] [Google Scholar]
- 17.Singh B, Rawlings N, Kaur A. Expression of integrin alphavbeta3 in pig, dog and cattle. Histol Histopathol. 2001;16:1037–1046. doi: 10.14670/HH-16.1037. [DOI] [PubMed] [Google Scholar]
- 18.Pelletier JP, Jovanovic D, Fernandes JC, et al. Reduced progression of experimental osteoarthritis in vivo by selective inhibition of inducible nitric oxide synthase. Arthritis Rheum. 1998;41:1275–1286. doi: 10.1002/1529-0131(199807)41:7<1275::AID-ART19>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 19.Gillitzer R, Goebeler M. Chemokines in cutaneous wound healing. J Leukoc Biol. 2001;69:513–521. [PubMed] [Google Scholar]
- 20.Schramm R, Thorlacius H. Neutrophil recruitment in mast cell-dependent inflammation: Inhibitory mechanisms of glucocorticoids. Inflamm Res. 2004;53:644–652. doi: 10.1007/s00011-004-1307-8. [DOI] [PubMed] [Google Scholar]
- 21.Owen CA, Campbell EJ. The cell biology of leukocyte-mediated proteolysis. J Leukoc Biol. 1999;65:137–150. doi: 10.1002/jlb.65.2.137. [DOI] [PubMed] [Google Scholar]
- 22.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 23.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood. 2005;105:4191–4199. doi: 10.1182/blood-2004-12-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller WA. Leukocyte–endothelial cell interactions in the inflammatory response. Lab Invest. 2002;82:521–533. doi: 10.1038/labinvest.3780446. [DOI] [PubMed] [Google Scholar]
- 25.Kawabata K, Hagio T, Matsuoka S. The role of neutrophil elastase in acute lung injury. Eur J Pharmacol. 2002;451:1–10. doi: 10.1016/s0014-2999(02)02182-9. [DOI] [PubMed] [Google Scholar]
- 26.Ley K. Integration of inflammatory signals by rolling neutrophils. Immunol Rev. 2002;186:8–18. doi: 10.1034/j.1600-065x.2002.18602.x. [DOI] [PubMed] [Google Scholar]
- 27.Lindbom L, Werr J. Integrin-dependent neutrophil migration in extravascular tissue. Semin Immunol. 2002;14:115–121. doi: 10.1006/smim.2001.0348. [DOI] [PubMed] [Google Scholar]
- 28.Tarnow I, Kristensen AT, Krogh AK, et al. Effects of physiologic agonists on canine whole blood flow cytometry assays of leukocyte–platelet aggregation and platelet activation. Vet Immunol Immunopathol. 2008;123:345–352. doi: 10.1016/j.vetimm.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 29.Lu H, Ballantyne C, Smith CW. LFA-1 (CD11a/CD18) triggers hydrogen peroxide production by canine neutrophils. J Leukoc Biol. 2000;68:73–80. [PubMed] [Google Scholar]
- 30.Cutolo M, Sulli A, Barone A, Seriolo B, Accardo S. Macrophages, synovial tissue and rheumatoid arthritis. Clin Exp Rheumatol. 1993;11:331–339. [PubMed] [Google Scholar]
- 31.Sun B, Fan H, Honda T, et al. Activation of NF kappa B and expression of ICAM-1 in ischemic-reperfused canine myocardium. J Mol Cell Cardiol. 2001;33:109–119. doi: 10.1006/jmcc.2000.1280. [DOI] [PubMed] [Google Scholar]
- 32.Van Griensven M, Krettek C, Pape HC. Immune reactions after trauma. Eur J Trauma. 2003;29:181–192. [Google Scholar]
- 33.Feuerstein GZ, Wang X, Barone FC. Inflammatory gene expression in cerebral ischemia and trauma. Potential new therapeutic targets. Ann N Y Acad Sci. 1997;825:179–193. doi: 10.1111/j.1749-6632.1997.tb48428.x. [DOI] [PubMed] [Google Scholar]
- 34.Morganti-Kossmann MC, Rancan M, Stahel PF, Kossmann T. Inflammatory response in acute traumatic brain injury: A double-edged sword. Curr Opin Crit Care. 2002;8:101–105. doi: 10.1097/00075198-200204000-00002. [DOI] [PubMed] [Google Scholar]