Abstract
Histone methylation represents one of the most critical epigenetic events in DNA function regulation in eukaryotic organisms. Classic molecular biology and genetics tools provide significant knowledge about mechanisms and physiological roles of histone methyltransferases and demethylases in various cellular processes. In addition to this stream line, development and application of chemistry and chemistry-related techniques are increasingly involved in biological study, and provide information otherwise difficulty to obtain by standard molecular biology methods. Herein, we review recent achievements and progress in developing and applying chemical and biochemical approaches in the study of histone methylation, including chromatin immunoprecipitation (ChIP), chemical ligation, mass spectrometry (MS), biochemical assays, and inhibitor development. These technological advances allow histone methylation to be studied from genome-wide level to molecular and atomic levels. With ChIP technology, information can be obtained about precise mapping of histone methylation patterns at specific promoters, genes or other genomic regions. MS is particularly useful in detecting and analyzing methylation marks in histone and nonhistone protein substrates. Chemical approaches that permit site-specific incorporation of methyl groups into histone proteins greatly facilitate the investigation of the biological impacts of methylation at individual modification sites. Discovery and design of selective organic inhibitors of histone methyltransferases and demethylases provide chemical probes to interrogate methylation-mediated cellular pathways. Overall, these chemistry-related technological advances have greatly improved our understanding of the biological functions of histone methylation in normal physiology and diseased states, and also are of great potential to translate basic epigenetics research into diagnostic and therapeutic application in the clinic.
Keywords: histone methylation, demethylation, ChIP, mass spectrometry, chemical ligation, inhibitor
1. INTRODUCTION
The genomes of eukaryotic organisms are tightly packaged into chromatins, which form the structural basis of nuclear processes associated with genetic activity. The concept of epigenetics is defined to describe the changes in gene expression or function that do not entail alterations in the underlying DNA sequence.1-3 Over the past 15 years, significant efforts have been invested in understanding different aspects of epigenetic regulation in various cell and organism models. A myriad array of biological processes ranging from genomic imprinting, inactivation of X chromosome, to the establishment and maintenance of cell identity, are found to be tightly controlled by epigenetic mechanisms.4 Abnormal alterations of epigenetic landscapes, in conjunction with genetic changes, form the fundamental basis for the initiation and progression of many human diseases, especially cancer.5-7 Key discoveries in the cancer epigenetic field have dramatically changed our view of carcinogenesis. As early as 1983, Feinberg and colleagues observed a global reduction of DNA methylation content in colon cancer cells compared to the levels in normal colonic tissues.8 Since then increasing amounts of information have been explored about molecular epigenetic abnormalities in cancers. A number of tumor-specific genes are found to be regulated by DNA methylation and histone modifications on the chromatin template.9 For example, the global reduction of acetylated H4-K16 and trimethylated H4-K20 has been demonstrated to be a general feature of tumor cells that occur in the early stages of carcinogenesis and accumulate with tumor progression.10 Aberrant epigenetic silencing of tumor suppressor genes and aberrant epigenetic magnification of oncogenes are regarded as two key molecular mechanisms utilized by cancer cells to escape from cell checkpoint machinery.11 Compared to stable genetic alterations, epigenetic changes are relatively plastic and are likely reversible. Therefore, enzymes involved in the establishment and maintenance of epigenetic marks have been considered as a new class of drug targets, and small molecule inhibitors blocking the activities of these enzymes may lead to development of so-called epigenetic therapeutics.12,13
Nucleosome is the smallest structural unit of chromatin, in which 146 base pairs of DNA are wrapped around an octamer of core histones comprised of two copies of each of H2A, H2B, H3 and H4 proteins. The covalent modification of chromatin is manifested via two primary modes: DNA methylation and posttranslational modifications (PTMs) of histones. All of the four core histones contain a characteristic globular fold domain that engages in histone-histone and histone-DNA interaction to form the structured nucleosomal disc framework. On the other hand, the N-terminal sequences of the core histones are largely disordered in the crystal structures.14 These flexible tails protrude out of the nucleosome disc and are subject to various PTMs, including methylation, acetylation, and phosphorylation.15 Significant progress has been made in recent years in understanding the functional roles of these PTM marks in transcriptional regulation.16 It is important to point out that covalent modifications of histones, especially methylation, are now found to occur throughout all regions of histones, not merely the N-terminal tails. The mechanisms of these PTM marks in modulating chromatin remodeling and DNA function are being intensively studied. Importantly, widespread interplays between different histone PTM marks have been found or implicated, and histone modification patterns have been proposed to function as a set of regulatory “codes”, which are referred to as “histone codes”.17,18 These biochemical codes are “written” by specific chromatin modifying enzymes and then “read” by downstream effector proteins and protein complexes to signal a transcriptional “on-and-off” status of target genes.19,20 It remains a daunting task for biologists to decipher the epigenetic codes and code networks. Among different families of histone modifying enzymes, histone acetyltranfeases (HATs) are the best characterized to date.21-23 While histone acetylation is generally correlated with increased chromatin accessibility and transcriptional activity,24-26 the transcriptional impact of histone methylation depends on the specific contexts where the methylation mark is located. For example, methylation of histone H3 at lysine 4 is associated with actively transcribed genes, whereas H3 methylation at K9 is enriched at constitutively condensed chromatin and developmentally inactive global genes.27,28
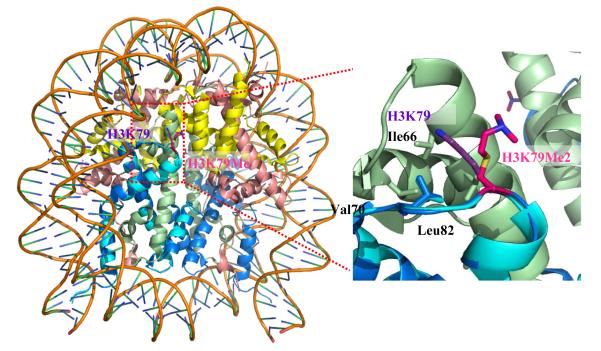
The field of histone methylation has been greatly invigorated by the development and application of various new methods and technologies. One recent example is that Luger and coworkers applied a unique chemical approach to install specific methylation analogs on recombinant histone H3 at the K79 site (i.e., H3Kc79me2).29 Crystal structures of the reconstituted nucleosomes were obtained to understand biochemical impacts of this important epigenetic mark (Figure 1). The unmodified H3K79 is in a position to make a weak contact with the L2 loop of H4. The added bulk from the two methyl groups on the ε-amine of H3Kc79me2 causes the side chain to assume alternative conformations, making them almost completely solvent accessible. This work offers interesting structural information about how H3K79 methylation alters the nucleosomal function and demonstrates the power of chemical approaches in epigenetics studies. In addition to offering biological insights into histone methylation, new experimental tools and approaches also present a great avenue that can potentially translate basic epigenetics biology into clinical applications.30 Herein, we discuss recent achievements and progress in developing and applying chemistry- and biochemistry-related approaches to the investigation of histone methylation, including chromatin immunoprecipitation (ChIP), mass spectrometry, site-specific labeling, biochemical methylation assays, and inhibitor development.
Figure 1.
Crystal structure of nucleosomes containing H3Kc79me2. The whole structure is shown on left and the zoomed area of H3K79 is shown on right. H2A is colored yellow, H2B colored pink, H3 without Kc79Me2 colored dark blue, H3 with Kc79Me2 colored light blue, and H4 colored green.
2. HISTONE METHYLTRANSFERASES AND DEMETHYLASES
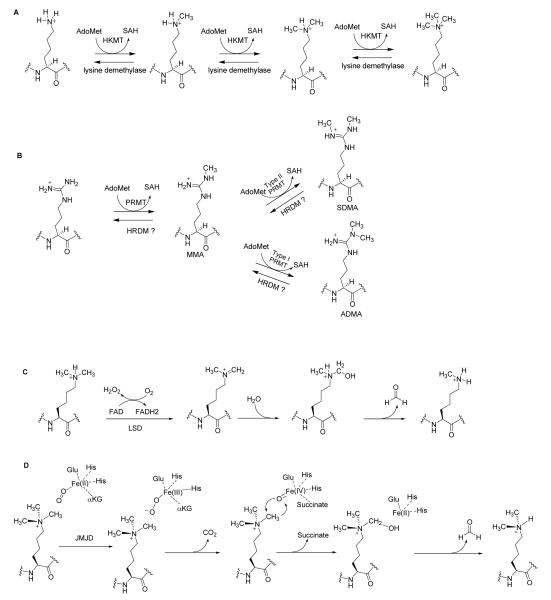
Histone methylation can be grouped into two major families, lysine methylation and arginine methylation, which are catalyzed by histone lysine methyltransferases (HKMTs) and protein arginine methyltransferases (PRMTs), respectively. Since 2004, a number of histone lysine demethylases has been discovered,21,31,32 revealing a dynamic nature of histone methylation regulation. Figure 2 illustrates the biochemical reactions of histone lysine methylation/demethylation and arginine methylation. The human proteins responsible for histone methylation and demethylation are summarized in Table 1.20,33-39
Figure 2.
Biochemical mechanisms of histone methylation and demethylation. (A) Lysine is methylated by HKMTs. (B) Type I and type II PRMTs methylate arginines and generate MMA, ADMA and SDMA. (C) LSD demethylates lysine via an amine oxidation reaction using FAD as a cofactor. (D) JMJD enzymes use αKG and Fe(II) as cofactors to demethylate the methylated lysines.
Table 1.
Protein members of human histone lysine methyltransferases (HKMTs) and protein arginine methyltrasferases (PRMTs) and lysine demethylases (KDMs).
| Class | Subfamily | Enzymes name, gene accession # | Substrates | Functions |
|---|---|---|---|---|
| HKMTs | SET1 | hMLL1 (KMT2A, NP_005924.2) hMLL2 (KMT2B, NP_003473.3) hMLL3 (KMT2C, NP_733751.2) hMLL4 (KMT2D, NP_055542.1) hMLL5 (KMT2E, NP_891847.1) |
H3K4 | Leukemogenic translocations |
| hSET1A(KMT2F, NP_004665.2) hSET1B (KMT2G, Q9UPS6.2) ASH1( KMT2H, NP_060959.2) |
Gene activation | |||
| SET2 | NSD1(KMT3B, NP_071900.2) NSD2(WHSC1, NP_001035889.1) NSD3(WHSC1L1, NP_075447.1) |
H3K36, K4, K20 |
Malformation syndrome and myelomas |
|
| hSETD2(KMT3A, NP_054878) | Angiogenesis | |||
| SMYD1-5 | H3K4 P53 | Muscle specific transcriptional modulator |
||
| SUV39 | hSUV39H1(KMT1A,NP_003164.1) hSUV39H2(KMT1B,NP_078946.1) |
H3K9 | Heterochromatic gene silencing |
|
| hG9a (KMT1C, NP_006700.3) | H3K9, WIZ, DNMT1, mAM |
Transcriptional repression of developmental genes |
||
| hGLP(KMT1D, NP_079033.4) | ||||
| hSEDB1 (KMT1E NP_036564.2) hSEDB2 (KMT1F NP_114121.1) |
H3K9 mAM |
Epigenetic regulation in development |
||
| EZH | EZH1 and 2 (KDM6A and B) | H3K27 H1K26 |
PRC2 mediated repression |
|
| PRDM | PRDM1 (BLIMP1,NP_001189.2) PRDM4 (PFM1, NP_036538.3) RIZ1 (KMT8, NP_036363.2) |
H3K9 | Gene repression, tumor suppression and carcinogenesis. |
|
| Other SET | PR-SET7/8 (KMT5A,NP_065115.3) SUV4-20H1 (KMT5B,NP_060105.3 ) SUV4-20H2 (KMT5C,NP_116090.2) SET7/9 (KMT7, NP_085151.1) |
P53, TAF, DNMT1, ERa ,H3K9 |
Modifying the interaction between target histone and non-histone proteins’ and DNA and other binding partners. |
|
| Non SET | hDOT1L (KMT4, NP_115871.1) | H3K79 | Telomeric silencing | |
| PRMTs |
type I PRMT |
hPRMT1 (AAF62893) | H4R3, NAB2P, NPL3P |
Interacting with the interferon-α receptor |
| hPRMT3 (AAC39837) | rpS2 | Ribosome regulators in ribosome assembly |
||
| hCARM1 (CARM1,NP_954592) | H3R2,R17, H3R26, CBP/P300 |
Promoting transcription | ||
| hPRMT6 (Q96LA8) | Tat HMGA1 DNA pol β |
Cell cycle progression and apoptosis |
||
| mPRMT8 (DAA01382) | Same as PRMT1 |
Same as PRMT1 | ||
|
type II PRMT |
hPRMT2 (AAH00727) | Catalytic inactive |
Not clear | |
| hPRMT5 (AAF04502) | H3R8, H4R3, MBD2 |
Transcription modulation | ||
| hPRMT7 (NP_061896) | Not clear | Chemotherapeutic toxicity |
||
| hPRMT9 (AAH64403) | Not clear | Not clear | ||
| KDMs | LSD | LSD1(KDM1A , NP_001009999.1) | H3K4, K9, P53 |
Transcriptional regulation |
| AOF1 (KDM1B ,NP_694587.3) | H3K4, K9, DNMT1 |
DNA methylation imprints |
||
| FBXL | FBXL11 (KDM2A, NP_036440.1) FBXL10 (KDM2B, NP_115979.3) |
H3K36me1 /2 |
Regulate transcriptional elongation |
|
| JMJD1 | JMJD1A (KDM3A, NP_060903.2) JMJD1B (KDM3B, NP_057688.2) JMJD1C (TRIP8, NP_116165.1 ) Hairless Protein(NP_005135) |
H3K9me1/ 2 |
Transcriptional co- repressors |
|
| JMJD2 | JMJD2A (KDM4A,NP_055478.2) JMJD2B (KDM4C, NP_055876.2) JMJD2C (KDM4B, NP_055830.1) JMJD2D (KDM4D, NP_060509.2) |
H3K9/K36 me2/3 |
Transcriptional elongation and repression |
|
| JMJD3 | UTX (KDM6A, NP_066963.2) UTY(NP_009056.3) JMJD3(KDM6B, NP_001073893.1) |
H3K27me2 /3 |
Transcriptional regulation for development |
|
| JARID | JARID1A (RBP2, NP_001036068.1) JARID1B (PLU-1, NP_006609.3) JARID1C(SMCX, NP_004178.2) JARID1D(SMCY, NP_001140177.1) |
H3K4me2/ 3 |
Transcriptional corepressors |
|
| JARID2(JMJ, NP_002043.2) | No activity | Cardiac development | ||
| PHF | PHF2(JHDM1E, NP_085150.1) PHF8(ZNF422, NP_055922.1) KIAA1718(JHDM1D, NP_085150.1 ) |
Neural differentiation | ||
| Other JMJD |
HSPBAP (NP_078886.2) | Hsp27 | Cell stress responses | |
| JMJD6 (NP_001074930.1) | Ribonucleo U2AF65 |
Regulation of RNA splicing |
||
| PLA2G4B (NP_001108105.1) FIH (NP_060372.2) JMJD4(NP_075383.1) JMJD5(NP_001138820.1) |
Not clear | Not clear |
A. Histone lysine methyltransferases
To date, more than 10 lysine methyltransferases have been identified in humans and many of them display unique substrate specificity (Table 1).40 With the exception of DOT1L, all HKMTs contain an evolutionally conserved SET (SU(VAR)3-9, enhancer-of-Zeste, Trihorax) domain responsible for the lysine methylation activity.41 It is noteworthy that the epsilon amino group of lysine residues can be methylated in the states of mono-, di-, and tri-methylation, and such differential methylation further enhances the functional diversity of lysine methylation. For example, dimethylation at H3K4 occurs at both inactive and active genes, whereas trimethylation is exclusive to active genes.42
SET1 proteins include MLL (myeloid/lymphoid or mixed-lineage leukemia protein) in mammal, TRX (trithorax) in drosophila, and SET1 in yeast. This family of proteins contains a SET domain and a following Post-SET region at the carboxyl terminus. SET1 proteins have the capacity to specifically methylate lysine 4 of histone H3 (H3K4), generating an epigenetic mark for active chromatin. More than 50 different MLL fusion partners have been identified.43 MLL rearrangements are found in more than 70% of infant leukemias, in approximately 10% in adults of therapy-related leukemias (AML), which are developed in patients previously treated with topoisomerase II inhibitors for other malignancies. Leukemogenic MLL translocations encode MLL fusion proteins that have lost H3K4 methyltransferase activity.43
The SET2 family includes three groups of highly related proteins: NSD (nuclear-receptor set domain) 1-3, the huntingtin-interactive protein SETD2/HYPB, and SMYD1-5 proteins. NSD proteins contain PWWP domain, HMG box, SET domain, and PHD-type zinc finger. NSD gene deletion and translocation cause malformation syndrome and myelomas. The methyltransferase activity of NSD depends on the nature of substrates and DNA may act as an allosteric effector for activity regulation.44 SETD2/HYPB protein belongs to a class of huntingtin-interacting proteins characterized by WW motifs. Except for the SET domain, SETD2 contains a novel transcriptional activation domain, which is associated with hyperphosphorylated RNA polymerase II. SETD2 is specific for H3K36 methylation and associated with active chromatin.45 A new study using knockout mice shows that SETD2 is required for embryonic vascular remodeling via its activity of H3K36 methylation.46 SMYD proteins contain a SET domain and a MYND-type zinc finger that directly binds to specific DNA sequences. The SMYD proteins might modulate transcription by two independent mechanisms: through chromatin structure modification via the H3K4 methylation and at the level of transcriptional elongation by recruitment of RNA polymerase II.47 SMYD proteins are shown to be skeletal/cardiac muscle specific transcriptional modulators in development.48
The human SUV39 proteins include SUV39H1/2, G9a, GLP and SETDB1/2. SUV39 proteins specifically methylate H3K9 by their SET domain and possess other domains that recognize epigenetic marks (e.g., SUV39H1/2 harbor chromodomain, G9a/GLP contain ankyrin repeats domain, and SETDB1/2 contain MBD domain). SUV39 proteins are related to heterochromatic gene silencing.49 Through formation of heteromeric complexes, G9a/GLP cooperatively exert H3K9 methyltransferase function in transcriptional repression of developmental genes.50 SETDB1 (SET domain bifurcated 1) is related to promoter H3K9 trimethylation and causes transcriptional repression.51 A new study on zebra fish provides unique evidence that SETDB2-mediated H3K9me3 is potentially involved in negative epigenetic regulation of dorsal organizer formation during early embryonic development.52
The EZH family contains two related members, which are homologous to the PcG protein Enhancer of Zeste (EZ).53 EZH proteins have no Post-SET domain, and their SET domain methylates H3K27. The PRDM family of proteins harbor a special SET domain at the N-terminus, which has 20–30% sequence identity to the SET domain and is considered as a subclass of SET domain named PR/SET.54 Some PRDM proteins mutate in the conserved motif (NHSCxPN) and do not have HKMT activities.55 PRDM proteins are often associated with gene repression, tumor suppression and carcinogenesis.56 A number of HKMTs have a SET domain that is not preceded by Post-SET domain and methylate histones or non-histone proteins (e.g. p53, DNMT1, etc.), which include SUV420H1, SUV420H2, SET7/9, and PR-SET7 (also called SET8/).57 Unlike other HKMTs, DOT1L does not contain SET domain. DOT1L is originally identified as a disrupter of telomeric silencing via its H3K79 methyltransferase activity.58
B. Histone arginine methyltransferases
Histone arginine methylation is catalyzed by PRMTs that are classified as type I (PRMT-1, -2, -3, -4, -6 and -8) and type II (PRMT-5, -7, -9) enzymes.59-62 Type I PRMTs catalyze the transfer of the methyl group from S-adenosyl-L-methionine (AdoMet, SAM) to the guanidino nitrogen atoms of arginine residues to produce ω-NG monomethylarginines (MMA) and ω-NG, NG-asymmetric dimethylarginines (ADMA). Type II PRMTs catalyze the formation of MMA and ω-NG,N’G-symmetric dimethylarginines (SDMA). Many PRMTs exhibit quite high substrate specificity and are correlated with different functions. Although not changing the charge state in substrates, the addition of methyl groups increases steric hindrance and removes amino hydrogens that might be involved in hydrogen bonding. Therefore, methylation could serve to modulate intra- or intermolecular interactions of the target proteins.37,63,64
Many PRMTs act as nuclear receptor coactivators, which implicates PRMTs as potential targets in the treatment of hormone-dependent tumors.62 PRMT1 is a component of the MLL complex, and required for leukemogenic transformation.65,66 PRMT1 interacts with the interferon-alpha receptor.67 Mice lacking a functional PRMT1 gene die at an early stage of embryogenesis.68 PRMT4 is better known as coactivator-associated methyltransferase 1 (CARM1), which methylates histone H3 at residues R2, R17, and R26 as well as CBP/p300.69 PRMT5 is the first enzyme found to synthesize SDMA in protein substrates.70 PRMT5 methylates histone H3R8, H4R3 and forms a complex with human SWI/SNF chromatin remodeling components BRG1 and BRM; this activity results in the repression of genes.71 PRMT6 methylates HIV-1 Tat and HMGA1 to affect their activities during cell cycle progression and apoptosis.72,73 PRMT7 is correlated with resistance of cellular toxicity to certain DNA-damaging chemotherapeutic agents.74 Inhibition of PRMT7 activity has great promise in treating cancer by sensitizing cancer cells to chemotherapeutics.62 PRMT8 is extremely similar to PRMT1, but PRMT8 is specifically expressed in brain tissue.75 The other PRMTs’ substrates and function are not quite clear yet. The enzymatic reaction of arginine methylation is shown in Figure 2B.
C. Histone lysine demethylases
For some time, histone methylations were considered like DNA methylation to be permanent marks. Since 2004, a large number of enzymes have been discovered with the ability to demethylate methylated histone lysine residues via amine oxidation, hydroxylation or deimination. The histone lysine demethylases (HKDM) discovered thus far are defined into two families: lysine-specific demethylase (LSD) proteins and Jumonji domain (JMJD) containing (JmjC) proteins. The JMJD family includes FBXL, JMJC1-3, JARID, PHF and other JMJD subfamily proteins. The enzymatic mechanisms of HKDMs are illustrated in Figure 2C and 2D.76 The identification of the amine oxidase LSD1 as a histone demethylase changed the perception of irreversible histone methylation. Through a flavin adenine dinucleotide (FAD)-dependent amine oxidase reaction, LSD removes one methyl group from histone substrate (Figure 2C).77 So far, only LSD1 and AOF1 are classified to belong to this family.78 LSD1 demethylates H3K4me2/me1, H3K9me2/me1 and nonhistone substrates, such as p53. LSD proteins are associated with different complexes, thereby participating in the regulation of transcriptional programs, heterochromatin spreading and stress-induced responses. Moreover, a recent paper showed that AOF1 demethylates H3K4 and is critical for establishing the DNA methylation imprints during oogenesis.79
The JMJD family of proteins belongs to the α-ketoglutarate (α-KG)-dependent oxygenase superfamily. A feature of these proteins is their ability to bind to Fe(II) ions and to hydroxylate protein substrates utilizing oxo-ferryl(IV) and αKG as cofactors (Figure 2D). FBXL proteins are the first JMJD histone demethylases identified.80 FBXL proteins contain F-box domain and JMJD domain which demethylates H3K36me1/2. The FBXL proteins are involved in regulating transcriptional elongation.80,81 The JMJD1 proteins have been implicated in the demethylation of H3K9me2 for many genes related to tumor suppression, androgen receptor targeting, and thyroid receptor responding.82 JMJD1 proteins act as transcriptional corepressors via removal of “activatory” methylation marks. JMJD2 proteins significantly decrease H3K9me3 and H3K9me2 levels, which are associated with transcriptional elongation and transcriptional repression.83 JMJD3 proteins are histone demethylases specific for H3K27me3/me2, which is essential for normal development.84 Thus, JMJD3 proteins play important roles in cell fate decision, counteracting pluripotency and transcriptional regulation of the INK4A-ARF locus. JARID1 proteins contain an ARID domain before the JMJD domain. JARID proteins can demethylate tri- and di-methylated histone H3 at Lys 4 (H3K4me3/2), which are often found at start sites and coding regions of transcribed genes. JARID1 works as transcriptional corepressors85 and a large number of point mutations have been found in X-linked mental retardation (XLMR).86 JARID2, also known as Jumonji (JMJ), is phylogeneticly related to the JARID1 family, but no enzymatic activity has been demonstrated. Gene knockout experiments indicate that JARID2 plays a role in cardiac development.87 PHF proteins contain a Cys4-His-Cys3 zinc finger-like PHD domain and may work on H3K9me2 and H3K27me2 repressing marks.88 Recent studies suggest that PHF proteins mediate transcriptional activation of genes involved in neural differentiation.89 In addition to the above mentioned JMJD proteins, there are some other JMJD proteins whose enzymatic activities are not clear yet. One of them is JMJD6, which had been previously defined as histone arginine demethylase,90 but its validity was recently questioned by another paper, which defined JMJD6 as a lysyl-hydroxylase.91
3. CHIP TECHNOLOGY FOR PROFILING OF HISTONE METHYLATIONS
Histone modifications play a critical role in influencing gene expression and genome function by establishing local and global chromatin environments and orchestrating DNA-based biological processes. In the last few years, remarkable progress has been seen in characterizing histone modifications on genome-wide scales.15 A major driving force has been the development and improvement of high-throughput sequencing by combining ChIP and DNA-microarray analysis (chip) techniques, which is abbreviated as “ChIP-on-chip”. In particular, the histone modification patterns in yeast genome have been extensively studied.92-95
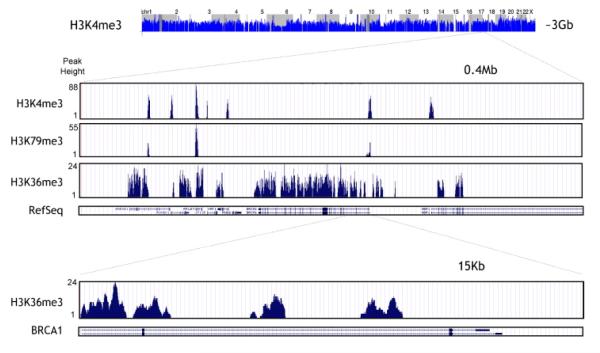
ChIP-based high-throughput screening has yielded mapping of the epigenome of human cancer cells.9 Figure 3 illustrates a zoomed window on the histone H3 methylation patterns in a cancer genome using the ChIP-on-chip approach.7 Such information on the distribution of epigenetic marks in normal and diseased states provides insights into the underlying molecular processes that drive pathogenesis. Herein we provide an overview of the ChIP technique, silico data analyses, and variations of ChIP, such as ChIP-on-chip and ChIP-sequencing (ChIP-seq).
Figure 3.
High-resolution profiling of histone H3 methylation in a cancer genome. (permission from reference 7).
A. The basic principle of ChIP
ChIP is a powerful and widely applied technique to analyze the spatial and temporal association of specific proteins (e.g., transcription factors, nuclear receptors, etc.) with DNA and the dynamics of histone modifications including methylation. The ChIP technique enables precise mapping of temporal changes at specific promoters, genes or other genomic regions of interest at a unique resolution of up to the single nucleosome level (approximately 170 base pairs). In addition, by using ChIP it is possible to follow the distribution of histone methylation over the entire genome. Importantly, the application of ChIP allows one to gain unique insight into how genes are regulated in their native contexts.
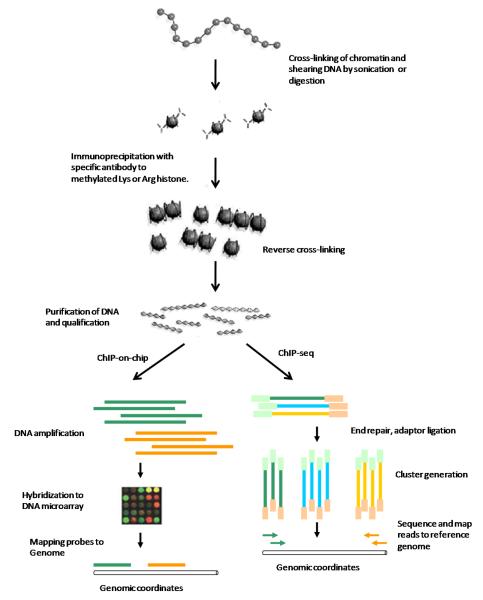
The basic principle of ChIP is based on the selective enrichment of a chromatin fraction containing a specific antigen (e.g. transcription factors, DNA binding proteins, modified histones, etc.) by an immunoprecipitation step. Specific antibodies that recognize the modified form of a protein are used to target its occupancy within DNA regions. There are two common types of ChIP protocols: Cross-linking ChIP (X-ChIP) which uses chromatin fixed with formaldehyde and fragmented by sonication and native chromatin ChIP (N-ChIP) which uses native chromatin prepared by nuclease digestion of cell nuclei.96 Figure 4 illustrates the basic steps of ChIP, ChIP-on-chip and ChIP-seq protocols. The enriched DNA fragments are amplified to obtain sufficient quantity by PCR (shown as long bars). The detection and analysis of immunoprecipitated DNA vary depending upon which questions are to be addressed, such as Southern blot, PCR, quantitative real-time PCR, hybridization on microarrays or deep sequencing (see below for details). Subsequent to the wet-lab steps, bioinformatics methods will be used to mine and analyze the enriched DNA regions.
Figure 4.
Schematic of chromatin immunoprecipation (ChIP), ChIP-on-Chip and ChIP-seq.
B. ChIP-on-chip
In this approach, the isolated DNA fragments are amplified to generate micrograms of fluorescently labeled DNA and then analyzed by hybridization to DNA microarrays. Depending on the contents of the microarrays, the ChIP-on-chip method can be divided into two groups: (a) Promoter tiling arrays. The probes are designed with a focus on specific genomic elements such as promoters. The advantage of promoter tiling arrays is low cost, but they are biased since the array design relies on known annotation. Thus, some relevant regions may not be covered.97 (b) Genome tiling arrays. The probe contents cover entire genomes. Thus, these arrays allow a global genome-wide analysis. However, experimental costs can be high because the entire genomic contents have to be distributed on several arrays.
Early studies with ChIP-on-chip focus on the histone modifications in S. cerevisiae 98,99 and D. melanogaster.100 Recently, ChIP-on-chip has been used to profile histone modifications in human genomes.101,102 The flow chart presented in Figure 4 shows how ChIP-on-chip can be used to study histone modifications. The enriched ChIP DNAs and control DNAs prepared from input chromatin are labeled with different color (e.g., green for the experiment and yellow for the control). The amplified DNAs are hybridized to a DNA microarray. In the last, the microarray probes are mapped to a reference genome to yield genomic coordinates.
C. ChIP-Seq
ChIP–Seq is a recently developed technique for analyzing immunoprecipitated DNA using the high-throughput technique developed by Solexa. To take the Illumina Genome Analyzer using Solexa technology as an example, the basic ChIP–Seq procedures are shown in Figure 4, comprised of the following four steps: (a) The ChIP DNA ends are repaired and ligated to a pair of adaptors, followed by limited PCR amplification. (b) The DNA molecules are bound to the surface of a flow cell that contains covalently bound oligonucleotides that recognize the adaptor sequences. (c) The Illumina Genome Analyzer automatically reads each individual DNA sequences during solid-phase PCR processing. (d) The resulting sequence reads are mapped to a reference genome to obtain genomic coordinates that correspond to the immunoprecipitated fragments.
ChIP-seq combines ChIP with deep sequencing methods which are normally provided by 454, Illumina or ABI. With these next-generation sequencing (NGS) technologies, production of several millions of sequence reads during each run becomes feasible. The early applications of ChIP–seq to profiling histone modifications were done in CD4+ T cells102 and mouse embryonic stem (ES) cells.103 Compared to ChIP–chip, ChIP-seq has the following advantages: (a) Being more quantitative. The sensitivity and scalability can be improved by increasing the number of sequencing runs to adjust the signal-to-noise ratio until saturation is reached. Thus, the modification levels at different genomic regions can be directly compared. (b) Lower costs. Compared to the whole genome tiling approaches, ChIP-seq requires minimal hands-on processing, fewer replicate experiments, and less input materials.104 (c) Low bias. Because each DNA is sequenced without hybridization, there are no issues associated with cross-hybridization. As the data obtained are sequence reads, ChIP-seq offers a rapid analysis pipeline (as long as a high-quality genome sequence is available for read mapping) and a great potential to detect mutations in binding-site sequences. However, the data amount of each single ChIP-seq run is in the terabyte range, which requires sophisticated methods for data handling and analysis (see the next section).
D. Analysis of genome-wide ChIP Data sets
To extract meaningful biological information from above genome-wide ChIP-on-chip and Chip-seq experiments, it requires advanced bioinformatics and biostatistics tools for data analyses. For a successful analysis, the common challenge is to find peaks of signals that correspond to particular regions of the genome and to determine at which threshold statistical significance exists. The ChIP data analysis includes two steps: 1) detection and definition of enriched regions, and 2) annotation and further analysis of enriched regions.
D1. Detection and definition of enriched regions
For ChIP-on-chip data sets, the first step is to identify probes and clusters that are significantly changed compared to background. Commonly, replicated probes with specific enrichment are compared to replicates of input DNA (or unspecific enrichment) controls. Regions are then defined by a sliding window or peak-finding algorithm. A brief introduction to the analysis of tiling microarray experiments has recently been published.105 Two of the most useful software packages are TileMap106 and the Tiling-array (MAT) algorithm.107 The output of this analytical step is the identification of chromosomal positions with a start and end position (so-called BED file format). BED file format provides a way to define data lines that are displayed in an annotation track. Data lines have three required fields and nine additional optional fields. Microarray data sets are commonly very large, and analytical precision is influenced by a number of variables. The background noise and appropriate normalization of the data are very important. Thus, the output BED file also includes information about data statistic normalization measure for significance and ratio.
For ChIP-seq, NGS platforms push the limits for the number of reads that can be produced in each run, a major bottle neck in data analysis will be the mapping of raw read sequences to a reference genome. The efficient large-scale alignment of nucleotide databases (ELAND) is a short-read mapping algorithm, which is available and effective in particular contexts. ELAND is a part of the Illumina Solexa sequencing analysis pipeline optimized to map very short reads, with the longest read possible being 32 bp in length, and ignores additional bases when the sequenced reads are longer. ELAND allows maximally two mismatches between the read and the genomic background sequence, which may not be sufficient for longer reads. If longer reads are desired, other alignment tools will need to be used. For example, the RMAP (http://rulai.cshl.edu/rmap) algorithm recently was published.108 It efficiently uses two different mapping criteria, both based on approximate matching of the read and the reference genome. The output format of ChIP-seq analysis is same as that of ChIP-on-chip, and also shows chromosomal positions that can be in a BED file format. The ChIP-seq can be directly compared to ChIP-on-chip data.
D2. Annotation and further analysis of enriched regions
Once sequence files are generated, the biological interpretation of these data will be visualized on a genome browser. The output files with the chromosomal positions of the enriched clusters (BED files) can be uploaded to current genome annotations. Public domain genome annotations, such as the genome browsers of UCSC (http://www.genome.ucsc.edu) and Ensembl (http://www.ensembl.org), can be used for data uploading.109 For regions that do not directly overlap with annotated genomic regions, the next upstream and downstream elements can be searched and a distance can be assigned on the plus and minus strand. To annotate the regions, a public domain named “Cis regulatory annotation system” (CEAS) package110 is available at http://ceas.cbi.pku.edu.cn. In addition, knowledge of overlapping or closest annotation (loci) opens new possibilities for subsequent pathway mining. Further data analysis will be project-specific and depends on the specific biological questions asked. Hodges et al.111 recently developed a method by using flexible, high-density microarrays to capture any desired fraction of the human genome. This methodology provides an adaptable route toward rapid and efficient resequencing of any sizeable, non-repeat portion of the human genome.
4. ANALYSIS OF HISTONE METHYLATION BY MASS SPECTROMETRY
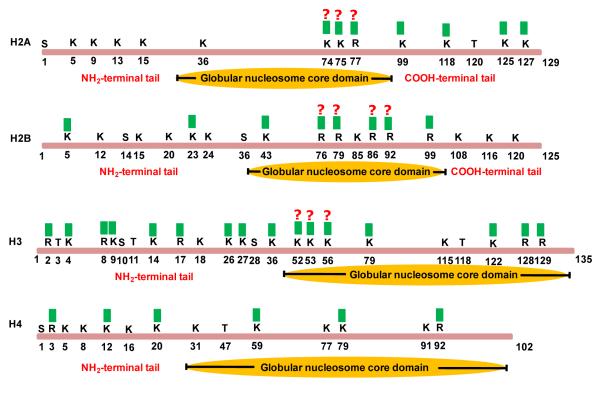
Mass spectrometry (MS) is increasingly used as a powerful analytical tool in histone methylation study.112-116 Thus far, a myriad of lysine and arginine residues in core histones are found to be methylated (Figure 5). As a matter of fact, the majority of these methylation sites were discovered by MS methods. The mass differences of methylated lysine or arginine residues from unmodified residues allow the unambiguous determination of these modifications on histones by MS analysis. The advantages of MS-based methods over traditional antibody-based technologies include complete and simultaneous characterization of multiple modifications (combinatorial codes) on a single histone protein without a priori knowledge of individual modifications.
Figure 5.
Distribution of methyl marks (labeled with green squares)) on the core histones.
Various MS techniques have been applied to histone methylation study. “Bottom-up” approaches analyze small pieces of peptides enzymatically digested from pre-separated or purified histones. The sequence information and the exact sites of methylation are usually determined by tandem mass spectrometry (MS/MS) analysis of these peptides.117-120 “Top-down” methods directly measure the masses of full length histones, and the methylation level and their relative stoichiometry can be obtained by MS profiling and protein fragmentation.121,122 Because a significant number of PTMs are located in the N-terminal region of the core histones, an alternative version of top-down method, “middle-town” approach has been applied to characterize large peptides that usually contain less than 50 N-terminal amino acid residues of histone tails. To quantitatively characterize the dynamic changes of histone methylation under different cellular or stimulus conditions, a variety of quantitative or semiquantitative MS methods has been developed, including label free approach, in vivo stable isotope labeling, and chemical derivatization.
A. MS methods for the detection and site mapping of histone methylations
A1. Bottom-up methods for histone methylation analysis
“Bottom-up” analysis is a widely used MS approach for PTM characterization. Histones extracted from cells are usually separated by high-performance liquid chromatography (HPLC) to reduce the complexicity of samples. Intact histone proteins are first enzymatically digested to a pool of peptides that are subsequently separated by HPLC before MS analysis. The resulting peptides can be analyzed directly by matrix-assisted laser desorption/ionization (MALDI) peptide finger printing or nanoelectrospray ionization (ESI) MS. The sequence of the peptides and the location of methylated amino acid residues can be determined by MS/MS by means of different types of MS fragmentation techniques such as collision-activated dissociation (CAD),123,124 electron capture dissociation (ECD),125 electron transfer dissociation (ETD),126,127 infrared multiphoton dissociation (IRMPD),128 and post-source decay (PSD)129. Numerous known and novel methylation sites have been detected in a variety of core and linker histone variants from mammals, yeasts and plants.130,131 132,133 A recent example includes a study of histone H3 and H4 variants of soybean and their PTMs using a MALDI-TOF/TOF mass spectrometer.134 Fragmentation analysis of selected peptides lead to the determination of mono-, di- and trimethylation at Lysine 4, Lysine 27 and Lysine 36 in histone H3 variants extracted from soybean leaves. In addition, the differences of the methylation patterns in these variants were detected.
Modification-specific signals, or marker ions, can be observed from the low mass range (0-170 m/z) in MS/MS spectra. These immonium ions and related fragments of modified amino acid residues produced during peptide fragmentation can be used for the identification and validation of histone methylations.120,135,136 For example, monomethylated and dimethylated lysine residues can be distinguished by the marker ions at 98 m/z and 112 m/z, respectively. Table 2 shows a list of histone modifications and their corresponding Δm values and marker ions. The detection and identification of methylated peptides can be validated by the observation of these characteristic marker ions or neutral loss, particularly in the cases that the MS/MS fragmentation patterns are insufficient to determine the nature of the modification.137,138
Table 2.
List of histone methylations.
| Modification | Δm (Da)a |
Immonium ions (m/z) | Neutral loss (Da)b |
References |
|---|---|---|---|---|
| Lysine monomethylation | 14.0156 | 84.081, 98.096, 143.118 |
138,293 | |
| Lysine dimethylation | 28.0312 | 84.081, 112.4 | 293 | |
| Lysine trimethylation | 42.0470 | 84.081, 143.154 | 59.073, 60.081 | 293 |
| Arginine monomethylation |
14.0156 | 32.049, 74.071, 57.055 |
31.042, 56.037, 73.064 |
294 |
| Arginine asymmetric dimethylation |
28.0312 | 46.065, 71.060, 88.087 |
45.058, 70.066, 87.080 |
294 |
| Arginine symmetric dimethylation |
28.0312 | 32.049, 71.060, 88.087 |
31.042, 70.066, 87.080 |
294 |
Δm is defined as the mass difference between modified and native amino acid residues.
Neutral loss is the loss of a specific neutral mass from precursor ions during tandem MS analysis.
In addition to the validation of sequencing data, the marker ions can also be utilized in the discovery of methylated peptides. In “immonium ion scanning” method, modified peptides can be specifically selected by monitoring their characteristic immonium ion(s) using tandem mass spectrometers, such as triple quadrupole instruments.139 Gouttas and coworkers investigated the application of “immonium ion scanning” strategy for the discovery of histone methylations.140 Their data reveal that the discovery rate of modified peptides can be improved up to 4-fold over control analyses where immonium ions are strong and unique. For instance, immonium ion scanning at m/z 98 dramatically increased the number of methyllysine-modified peptides that could be identified in an LC-MS/MS analysis of histone proteins.
A2. Top-Down strategy
High-resolution and high mass-accuracy mass spectrometers such as Fourier-transform ion cyclotron resonance mass spectrometry (FT-ICR-MS) in combining with protein fragmentation enable the direct profiling of intact histones and site mapping of their PTMs.141-146 In this top-down approach, purified or pre-fractionated histones are introduced into the mass spectrometer for the accurate measurement of precursor ion masses and the fragmentation of intact proteins can be achieved by tandem mass analysis via ECD or ETD techniques. The mass increase in the precursor ions and mass shift in the fragment ions containing methylations can be used to determine the presence of histone methylations.121,122,146 For example, Thomas and coworkers reported the top-down mass spectrometric characterization of human histone H3 using a Quadrupole-FT ICR MS instrument.122 They have observed a broad distribution of species differing by 14 Da and less than 3% unmodified protein for all three H3 variants. Through fragmentation of all species by ECD, they have founded about 5% methylation of lysine 4 and about 50% dimethylation of lysine 9.
A problem associated with top-down analysis is the partial oxidation of Met and Cys residues generated in vivo or during sample preparation and storage.147,148 These oxidations increase the sample complexicity and the difficulty in data interpretation for accurate profiling of intact proteins as well as site mapping of histone modifications. To circumvent this problem, a method that allows complete oxidization of partially oxidized histone proteins has been developed recently.149,150 Under mild performic acid treatment, methionine and cysteine can be completely oxidized while other residues remain unaffected. This leads to enhanced accuracy and sensitivity of top down analyses of combinatorially modified forms of histones.
A3. Middle-down approach
Due to the development of ECD and ETD tandem mass spectrometry techniques, an alternative version of top-down MS, middle-down mass spectrometry, has been recently popularized that allows the identification of protein modifications on single and large pieces of peptides (mass greater than ~3000 Da). This approach has proved to be particularly useful for the MS characterization of histone methylation and other modifications because histone tails containing the majority of modifications can be proteolytically generated and purified as a single piece of peptide for MS analysis.151-154 The N-terminal tails with less than 50 amino acid residues are usually produced from digestion by endoproteases such as Glu-C, Asp-N, Arg-C or trypsin after unmodified lysine residues are protected by derivatization. Subsequent MS/MS analysis of these tails is able to characterize the modifications on histones. An obvious advantage is that all histone modifications are included in a single peptide so that unambiguous distinction of the species with the same mass containing the same number of modification groups at different residues, or species with same nominal mass but containing different modifications can be achieved. Using middle-down approach, Garcia and colleagues have identified numbers of mono-, di-, or trimethyl modified lysine residues from 150 distinct histone histone H3.2 species.146 Taverna and co-workers investigated the long-distance combinatiorial linkage between methylation and acetylation on histone H3 N termini.20 MS analysis of the N-terminal H3 tails generated from Glu-C digestion revealed the correlations between K4 methylation and H3 acetylation and concurrent presentation of K4 and K27 methylation on one H3 species.
A4. Distinguish histone tri-methylation and acetylation
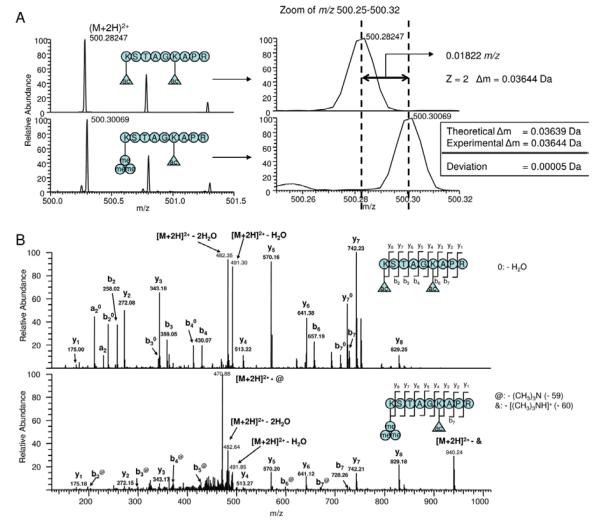
An analytical challenge in characterizing histone methylation by MS is to distinguish trimethylation from acetylation because the mass difference between trimethylation (42.0470 Da) and acetylation (42.0106 Da) is very small (0.0364 Da). One approach is to use an instrument with high-mass resolving power and high-mass accuracy, such as FT–ICR mass spectrometers.155-157 A mass accuracy within 2-5 ppm allows the differentiation of these two modifications in peptide mass fingerprinting or MS/MS analysis. For instance, the experimental mass of a yeast H3 peptide is consistent with trimethylation (1.1 ppm error) and not acetylation (44.1 ppm error). In addition, the modified residue K4me3 was revealed by two adjacent y-type ions (y-ions are a series of C-terminal fragments produced by random cleavage of the amide bond of a peptide during MS/MS analysis).
Trimethylation and acetylation can be also distinguished by the presence of diagnostic marker ions and neutral loss as above described.137,138 Fragmentation of trimethylated peptides usually generates a neutral loss of trimethyl amine at 59 Da whereas an acetylated peptide produces an ammonium ion at 126 Da during collision induced dissociation.135 A recent example reported by Trelle and coworkers reveals the distinction of H3K9 acetylation and H3K9 trimethylation in P. falciparum.138 The sequence of the peptide, K3MeSTAGKAcAPR, was determined by the complete y-ion series in its MS/MS spectrum. The presence of a trimethylated N-terminal lysine in the peptide is validated by the observation of an almost complete series of fragments corresponding to neutral loss of trimethylamine (59 Da) from b-ions (a series of N-terminal fragments produced in MS/MS) (Figure 6).138 Meanwhile, the acetylated peptide, KAcSTAGKAcAPR, is readily sequenced based on the almost complete series of y- and b-ions and no neutral loss is observed from the fragment ions of this peptide.
Figure 6.
Determination of trimethylated N-terminal lysine residues by mass spectrometry. (A) Mass spectra of the P. falciparum H3 peptides: (upper) KacSTAGKacAPR and (lower) Kme3STAGKacAPR. The theoretical Δm is calculated as the mass difference between a trimethylation and an acetylation (42.04695 - 42.010565 = 0.03693). (B) MS/MS spectra of these two peptides. (permission from reference 138)
Metabolic incorporation of heavy stable isotopes to the acetyl or methyl groups is an effective way to differentiate trimethylation and acetylation because they are no longer isobaric and can be easily distinguished by mass alone.158,159 By incubating cells in culture media containing methionine-methyl-D3, heavy methyl group can be incorporated into methylated residues and cause a mass shift of 3 Da or its multiples in modified peptides obtained from labeled cells. Therefore, a trimethylated peptide should have an isotopic partner that shifted 9 Da in mass while such mass change cannot be seen in an acetylated peptide.
Another approach for the determination of isobaric histone modifications by reversed-phase retention time and high mass accuracy was reported recently by Yang and colleagues.160 Their data showed that acetylation resulted in increased retention time for modified peptides in comparing with unmodified peptides, whereas trimethylation caused little change in retention time. Because reversed-phase liquid chromatography fractionation has been used in most experiments as a necessary step prior to MS analysis for peptide identification, this method provides a simple and effective means for unequivocally distinguishing between acetylated and trimethylated peptides.
B. MS strategies for the quantitative analysis of histone methylation
To fully understand the mechanism and functional role of histone methylation, it is important to accurately monitor how the levels of specific modifications change in response to different conditions, such as cell cycle changes, disease states or treatment with therapeutic agents. Early MS efforts focused on the confirmation of known modifications and the discovery of novel modification sites on histones. More recent studies have attempted to quantify the site occupancy of various histone modifications and compare the differently modified histone forms in samples from different cell states or treatments.161,162
B1. Label-free MS methods for the quantification of histone methylation
Top-down and middle-down are commonly used label-free methods for quantifying histone modifications. Recently Pesavento and colleagues applied top-down MS and hydrophilic-interaction liquid chromatography for the quantification of histone H4 combinatorial codes.150,163 By combining efficient separation of intact protein, high accuracy FT-MS measurement and ECD fragmentation, enhanced dynamic range (>104) has been achieved that allows the precise characterization and quantification of 42 forms uniquely modified by methylation and acetylation. The dynamic changes of some low abundant methylation forms including those with trimethylated Lys-20, monomethylated Arg-3, and the novel dimethylated Arg-3 (each <1% of all H4 forms) were also determined.163 In a middle-down approach, Phanstiel and co-workers reported the identification and quantification of 74 discrete combinatorial codes on the tail of histone H4 isoforms in differentiating human embryonic stem cells.151 The global isoform quantification was determined by calculating the percentage of the isoform peak areas of individual isoforms over those of all H4 isoforms whereas an MS/MS based strategy were used for the quantification of the isomers sharing identical normal mass but differing in the placement of modifications. In a recently reported middle-down approach on studying the H4 N-terminal tail modifications from four cell types,153 a 23-residue peptide covering the most heavily modified region of H4 was generated by Asp-N digestion and analyzed by MS analysis. Comparison of the MS spectra revealed distinct patterns of methylation for triacetylated H4 1-23 for each cell type and the relative abundance of these methylation forms were determined.
B2. Chemical derivatization with stable isotope labeling
A double derivatization method has been developed by Hunt’s group for the quantitative characterization of histone modifications.146,157,164 The first derivatization is applied to the free amino group in the N-terminus and endogenously unmodified or mono-methylated internal lysines so that large and reproducible tail peptides containing histone modifications can be generated by trypsin digestion that only cleaves histones at the C-terminal to arginine residues. This proteolytic process mimics Arg-C digestion but with high efficiency and specificity. For relative quantification, a secondary derivatization can be performed after trypsin digestion with an esterification reaction to modify carboxylic acid groups with a normal (D0-methanol) or stable isotope-labeled reagent (D4-methanol). In this way, equivalent peptides digested from two samples which contain identical modifications will appear as doublets separated by 3 Da (the mass difference between D3- and D0-methanol labeled peptides) in MS spectra. By comparing the abundance of each component of the doublet, the relative enrichment of specific modifications in the two samples can be determined.
To overcome the drawbacks involved in the secondary esterification reaction, such as moisture sensitivity and sample loss, Plazas-Mayorca and colleagues has reported an improved method that conduct the second derivatizatization with either d0- or d10-propionic anhydride on the newly formed free N-terminal amino groups.165 Therefore, differentially expressed histone methylation levels between samples can be directly detected by comparing the peak pairs separated by a +5 Da mass shift.
B3. Metabolic labeling MS methods for the characterization of dynamic changes of histone methylation
A metabolic labeling method, Stable Isotope Labeling by Amino acids in Cell culture (SILAC), which allows the incorporation of stable-isotope amino acids into proteins has been developed recently for quantitative proteomic analysis.166,167 Two populations of cells are grown in culture media that are identical except that one of them contains a ‘light’ and the other a ‘heavy’ form of a particular amino acid (e.g., 12C and 13C labeled L-arginine, respectively). Metabolic incorporation of stable-isotope amino acids results in. pairs of chemically identical peptides that can be detected by MS. The ratio of peak intensities in the mass spectrum for such peptide pairs accurately reflects the abundance ratio for the two proteins. Because of its high sequence coverage of identified proteins and high labeling efficiency and simplicity, this strategy has become one of the most popular methods for quantitative characterization of differentially expressed proteins and post-translational modifications.168,169
Various studies have applied the SILAC method for the analysis of histone modifications.170,133,171,172 For example, Bonenfant and colleagues analyzed the core histone modifications occurring through the cell cycle.173 A complex pattern of cycle-dependent methylation was observed: during G2/M, H3 Lys27 and Lys36 were decreased, whereas H4 Lys20 was increased. Their data reveal that mitosis is the period of the cell cycle during which many modifications exhibit dynamic changes.
Pulsed SILAC (pSILAC) is a variation of the SILAC method where the labeled amino acids are added to the growth medium for only a short period of time.174 This allows monitoring the differences in de novo protein production rather than raw concentration. In an application of pulsed SILAC on histone methylation, the modification profile of newly synthesized histones was tracked to monitor the correlation between histone methylation and cell cycle progression.171 The synchronized cells were pulse-labeled with an isotopically labeled arginine (15N) that is 4 Da heavier than the naturally occurring 14N isoform. Much slower lysine methylation kinetics in contrast to the highly dynamic lysine acetylation on the tails of newly synthesized histones was observed in this study.
Combining in vivo labeling with SILAC and top-down MS, Pesavento and coworkers analyzed the regulation of H4K20 methylation during the cell cycle.150 Almost all K20 methylations are observed to be progressive and targeted to newly synthesized H4. Their data suggest that methylation of H4K20 and acetylation of H4K16 are present on histone tail at the same time and are regulated independently, differing from the previous suggestions that K20 methylation and K16 acetylation are mutually antagonistic.
In another variation of SILAC, heavy-methyl SILAC method, the cells were cultured in media with 13CD3-methionine instead of heavy leucine, lysine or arginine as in regular SILAC approaches.159 The heavy methionine can be converted to 13CD3-adenosyl methionine, a sole biological methyl donor, and the methylated residues can be labeled with heavy methyl groups in newly modified histones. As in any approach that uses stable-isotope labeling, the identification and relative quantification of histone methylation can be achieved by measuring relative peak heights of methylated peptide pairs. The advantages of this method include increased confidence in methylation site mapping and quantification and distinction between trimethylation and acetylation. Using this method, Fodor and colleagues have demonstrated the functional role of a protein hydroxylase at pericentric heterochromatin in mammalian cells by monitoring lysine trimethyl states of H3K9me3.175 Zee and colleagues has reported the characterization of the steady-state kinetics of global methylation on a residue-specific basis using the combination of LC-MS and heavy methyl-SILAC labeling.172 Their work demonstrated progressively slower rates of the formation of mono-, di-, and trimethylated residues and different methylation rates associated with active genes and silent genes.
Together, recent successes have demonstrated the significant role of MS-based methods for the qualitative and quantitative characterization of histone methylation. Most of the strategies developed to analyze individual proteins are now applied to characterize the localization and dynamic changes of modified histones and variants in various species. Capability of MS approaches in complete and simultaneous analysis of the combination of methylation and other modifications on entire histone tails illustrate their tremendous potential in deciphering the histone combinatorial code.
5. SITE-SPECIFIC METHYLATION OF HISTONES FOR MECHANISTIC AND FUNCTIONAL ANALYSIS
All the four core histones, H2A, H2B, H3, and H4, share a similar structure, with a central “fold domain” and terminal tails at N and C ends.14 Close to one hundred PTMs have been discovered thus far, which are located in both the terminal tails and the fold domain.117,155,176-178 A great challenge in epigenetics research is to elucidate the biochemical effects of specific histone modifications on cell growth and differentiation. Histones isolated from mammalian cells possess complex and heterogeneous modification patterns,151,163,179 which makes it technically difficult to investigate the contribution from individual modification sites. For example, methyllysine residues are hypothesized to mediate interactions between nucleosomal histones and macromolecular complexes that regulate DNA transcription, replication, and repair.180 Investigating how histone modifications influence the activity of these DNA-regulating factors would be facilitated by a biochemical system that harbors the desired modification at selected specific residues. In practice, however, homogeneously modified histones are difficult to obtain from cell extracts. Current methods for introducing methylation into recombinant histones include enzymatic biosynthesis and semi-chemical synthesis. In a typical biosynthetic strategy, the modification is introduced by incubating a histone protein with a recombinant enzyme that catalyzes the formation of the PTM mark of interest. The enzymatic approach is often limited by the availability of active recombinant enzymes. Even with appropriate enzymes available, those biochemical reactions are difficult to drive to full completion; therefore the yield is usually far less than 100%. For example, Robinson et al181 recently used recombinant MOF to prepare histone H4 containing the acetyl-K16 mark and the yield of modification is about 30%. Also, in many circumstances, the specificity of enzyme is a big problem so that modifications at additional sites may also be introduced; therefore it is difficult to achieve chemo- and regio-selectivity. For histone methylation, no matter the methylation is on lysine or arginine residues, multiple methylated states can exist on a single amino acid residue, namely, three for lysine and two for arginine. Thus, the products of histone methylation are highly heterogeneous in terms of the degree of methylation. In this regard, chemical biology-based semi-synthetic approaches are particularly useful to facilitate creating homogeneous and chemically defined histone proteins which can be used directly for biochemical assays of the modification or to assemble reconstituted nucleosomal arrays.
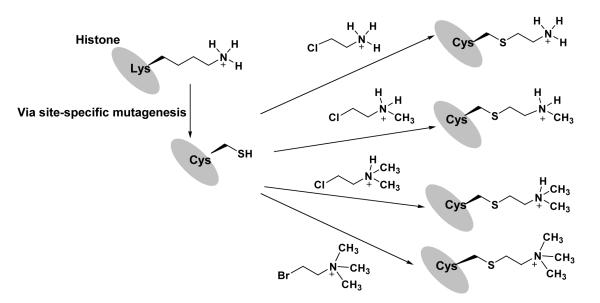
An interesting chemical strategy for the incorporation of methyl lysine marks into histones is recently developed by Shokat and colleagues.182,183 In this method, the lysine residue at a target site is first mutated to cysteine (e.g. H3-K79 to Cys) by site-directed mutagenesis. After protein expression and purification, the recombinant histone is then chemically labeled with a respective aminoethyl halide compound to form a methyl-lysine analog (MLA) group, i.e., N-methylated aminoethylcysteine, at the mutation site (Figure 7). By performing immunoanalysis of the modified histones, the authors concluded that MLA groups are effectively recognized by the antibodies that bind to the methylated lysine residues at mono-, di- and trimethylation states, suggesting that the MLA groups are structurally and functionally analogous to methylated lysines. With the same concept, Schultz and coworkers reported preparation of methyl lysine analogs by using phenylselenocysteine as a precursor, but the oxidation step may cause racemization at the α carbon of the amino acid.184 These chemical approaches allow for the rapid generation of large quantities of histone proteins in which the site and degree of methylation can be controlled throughout the entire sequence of each histone and provides a means to study lysine methylation in nucleosomes. In a recent example, chemical labeling was applied to make reconstituted nucleosomes containing H3-K79me analog marks for crystallography studies.29 However, a few technical caveats should be kept in mind when one considers using the chemical approaches for installation of specific methyllysine marks. First, to ensure chemo- and regio-specific labeling only at the methylation sites, the natural cysteines in a target protein need to be deleted or mutated. Fortunately, among the four core histones, only H3 contains a cysteine at site 110 and the C110A mutation does not seem to disrupt nucleosome function.183 Second, the chemical property difference of methyl-lysine analogs from their natural counterparts cannot be negligible in certain contexts; While these analogs are simple to employ, they are longer than the native amino acids by 0.3 Å,185 and the pKa of the ammonium protons decreases by 1.1 unit,186 which may lead to altered specificity or affinity in binding interactions.187 Third, the thioether linker is susceptible to oxidation (i.e., sulfoxide formation), thus reducing buffer condition needs to be used to avoid the thioether oxidation. Lastly, it is not compatible with the incorporation of different degrees of methylations at multiple sites on the same histone (e.g., H3-K4me2-K27me3). Despite these limitations, this simple lysine analog method provides a robust and affordable route to large quantities of homogeneously and site-specifically methylated histones to assess the biochemical mechanisms by which lysine methylation influences chromatin structure and function. In principle, this method can be modified to install other PTM analogs. For example, it is possible to introduce methyl-arginine analogs into histones in a similar manner, if the guanidino group is chemically stable enough when linked to cysteine thiol.
Figure 7.
A chemical approach to generating histones that contain site-specific methyl-lysine analogs.
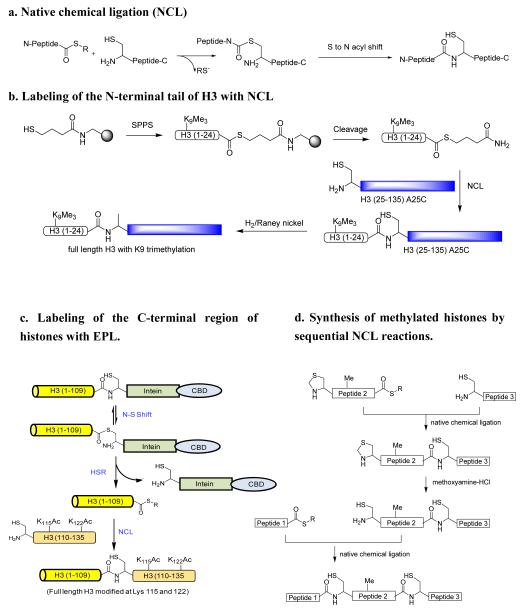
Native chemical ligation (NCL) represents another important technique for generating histones with specific PTM marks. NCL was first described by Kent’s group in 1994.188 This method involves the reaction between an unprotected peptide containing an amino-terminal cysteine and a second peptide with a carboxyl-terminal thioester in the typical pH range of 7—8.5. The first step is a transthioesterification reaction followed by a spontaneous intramolecular S to N acyl shift to generate an amide bond at the junction site (Figure 8a). It is noted that other cysteine residues that are present in the ligation peptides generally do not appreciably influence the efficiency of NCL.
Figure 8.
Native chemical ligation and its application in making site-specifically methylated histones.
Adding methylation marks to the amino-termini of histones using NCL
A significant number of lysine and arginine methylations occur on the flexible amino-terminal tails of the core histones. NCL has been demonstrated to conveniently make symisynthetic histones with specific methyl marks close to the N-terminus. In the work by He et al,189 a H3(1-24) peptide with a K9me3 and a C-terminal thioester was synthesized using solid phase peptide synthesis protocol (Figure 8b). The major part of histone H3, namely, H3(25-135)-A25C was recombinantly expressed in E. coli. The mutation of Ala 25 to Cys is needed for the subsequent ligation with the peptide thioester. In general, recombinant proteins expressed from E. coli have a methionine or acetyl modification on their N-terminal ends. This probably is the most challenging issue for ligating a peptide thioester to the N-terminus of a target protein. Fortuitously, the authors found by FT-ESI MS analysis that the alpha amino group of C25 was 90% free. It remains to determine whether this property holds true for all histone proteins that start with different amino acids on their N-termini. NCL was performed between H3(1-24)-K9me3 peptide thioester and H3(25-135)-A25C with the assistance of 2% thiophenol to form full length H3 protein containing K9me3. The cysteine residue at position 25 was converted to the natural residue Ala by desulfurization with hydrogen/Raney nickel. Clearly, this represents a successful example for installing methyl group to the lysine residues in the N-terminal regions of the core histones. In addition to its use in studying H3K9 methylation, the NCL strategy was utilized to generate several other homogeneously modified histones, including H4 with K16 acetylation,190 H4 with K5, 8 or 12 acetylations,189 and H3 with S10 phosphorylation.191 These examples illustrate that NCL is a robust tool to address the PTMs occurring on the N-terminal tails. Since methylation and many other PTMs on the N-terminal tails of the core histones have been shown to play critical roles in regulating the level of gene transcription, NCL will likely continue to be used to investigate the biochemical impact of PTMs on the N-termini of histones.
Adding methylation marks to the carboxyl end of the core histones using NCL
Following the pioneering work on NCL by Kent and coworkers in 1994, a significant breakthrough in the chemical ligation field is the discovery of intein-mediated generation of recombinant protein thioester and its merged utility with NCL. The protocols were first described in 1998 by Muir and Cole, et al,192 and independently by Xu and colleagues at New England Biolabs.193 In this method, a protein of interest is expressed in frame with an intein on the downstream. Autocatalysis of the intein results in the generation of a protein thioester bond between the target protein and the intein which is believed to occur via isomerization (N to S acyl shift). In the presence of appropriate thiol agents (e.g., thiophenol), a transthioesterification reaction occurs, which breaks the linkage between the target protein and the intein domain, and meanwhile forms a protein thioester that can then be reacted with an N-terminal cysteine-containing synthetic peptide via NCL to afford a semisynthetic protein carrying the desired, synthetically incorporated modifications. This technique is generally termed “expressed protein ligation (EPL)” or less frequently, “intein mediated ligation”. This technical advancement has significant values in providing experimental capability to interrogate protein function and structure and folding. Thus far, EPL has been widely utilized for making semi-synthetic proteins with different functional motifs such as fluorescent groups, PTM marks, unnatural amino acids, and isotopic probes (reviewed in 194,195).
EPL is particularly suited for making recombinant histone proteins with methyl marks close to their carboxyl termini (Figure 8c). MS analysis of endogeneous histones reveal that abundant methylation occurs at the carboxyl end of histones.155 Also, it was proposed that 30% of the CARM1-catalyzed methylation of H3 occurred at the C-terminus at one or more of the four clustered arginine residues (128/129/131/134).196 However, the biochemical and physiological functions of these methylations largely remain unknown. Use of EPL to make homogeneous nucleosomal histones with methyl groups close to the C-terminus will be of great value for understanding the biochemical impact of these PTMs in regulating chromatin structure and function. Recently, Manohar and coworkers used EPL to produce a semisynthetic histone H3 with acetyl marks at K115 and K122 (Figure 8c). Enzymatic and biophysical analyses of the reconstituted nucleosomes containing these two marks quite precisely characterized the biochemical consequence of the two acetyl marks at the histone DNA dyad interface; it reduces DNA-histone interaction, increases nucleosome mobility, and facilitates nucleosome repositioning.197 It might be seen in the near future that similar protocols of using EPL will be applied to studying histone methylations occurring in the carboxyl-terminal regions of histones.
Installing methylation marks in the middle regions of the core histones using NCL
In typical biochemical applications, NCL provides a convenient means for covalent linking of two unrelated peptides by forming a native peptide bond. Kent and colleagues recently further improved the technique so that it can be used for sequential ligation of multiple peptide segments. The key technical point is to protect the N-terminal cysteine residue of a peptide thioester with a removable protecting group such as acetamidomethyl (ACM)198 and thiazolidine-4-carboxo (Thz)199 (Figure 8d). In this protocol, the N-protected peptide thioester is first ligated on its carboxyl end with a cysteine-containing peptide via NCL. After the ligation, the N-terminal protecting group is removed by deprotecting reagents to generate a free cysteine residue on the N-terminus. The new free cysteine is subsequently used to react with peptide thioesters by another round of NCL reaction. Such a sequential NCL protocol can be itinerated and has been proven useful for the total synthesis of several small-size proteins, such as crambin,198 ubiquitin,199 and a HIV-1 protease.200 The four human core histones have sizes in the range of 102-135 AA, which could be synthetically accessed by using the sequential NCL reactions. It will be exciting to see whether histones harboring specific methyl marks can be synthesized with this methodology.
Limitations of NCL
As described above, NCL is particularly useful to prepare synthetic and semisynthetic histone and nonhistone proteins containing precisely designated PTM marks. Nonetheless, there are several technical limitations for using NCL to produce semi-synthesized histones with specific methylation patterns. NCL requires the synthesis of large quantities of modified peptide thioesters, which are most suitably made using Boc/trifluoroacetic acid (TFA) peptide chemistry. Although technically more amenable, the Fmoc/piperidine peptide synthetic protocol generally does not fit very well for the preparation of peptide thioesters. Also, NCL requires a cysteine at the ligation site. This is especially challenging for histone synthesis or semisynthesis because almost no cysteine residues are present in histones (the only one is H3 Cys110). Use of auxiliary groups201-203 or desulfurization after the ligation reaction 189,204,205 has been shown by several groups to expand NCL reactions beyond cysteine. In applying NCL for selective labeling of a recombinant protein, certain intrinsic properties of the protein target may be affected. For instance, use of thiol reagents could break disulfide bonds within proteins. If a protein exists as dimers or oligomers, the dimerization or oligomerization may be interfered because of the fusion of the target protein with intein. Even so, because of their facile chemistry and efficacy, NCL and EPL will continue to be an appealing chemical biology tool for polypeptide semisynthesis and engineering to facilitate protein functional study, including histone modifications.
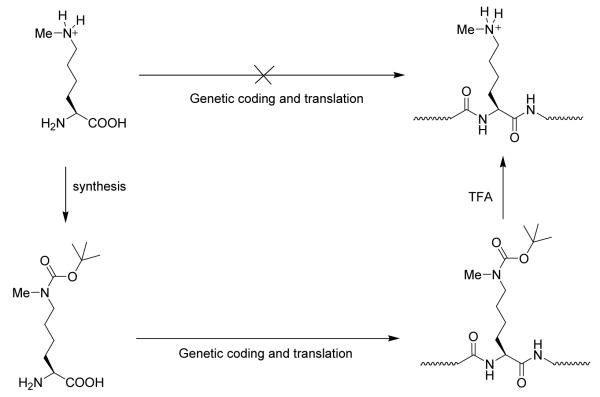
In addition to the aforementioned MLA and the NCL approaches, utilization of genetic methods for the incorporation of PTM marks into recombinant histones has recently been reported (Figure 9).187,206 This strategy evolves an orthogonal pyrrolysyl-tRNA synthetase/tRNACUA pair that specifically recognizes Nε-tert-butyloxycarbonyl-Nε-methyl-L-lysine and directs its incorporation into recombinant proteins such as histone H3. Nε-methyl-L-lysine was not used directly in the protein synthesis because the pyrrolysyl-tRNA synthetase did not accept methyl-lysine as a substrate. To load the methylation at H3K9 position, the codon for lysine 9 in the histone H3 gene was replaced with an amber codon. Following protein biosynthesis, the auxiliary Boc group was removed by TFA to reveal Nε-methyl-L-lysine. The authors nicely demonstrated that methylated H3 with this approach has all the expected biochemical activities for H3K9 methylation, e.g., recognition by heterochromatin protein 1 (HP1). This method is quite general, and can be potentially used for quantitative, site-specific incorporation of Nε-methyl-L-lysine in any recombinant proteins. However, it remains unclear at this time whether dimethylated and trimethylated lysines and methylated arginines can be installed with this genetic codon approach. Also, TFA treatment may destroy the three-dimensional structure of the recombinant protein product. Thus, a protein refolding step is likely required.
Figure 9.
A genetic codon approach to the site-selective incorporation of methyl lysines into recombinant histones.
6. DEVELOPMENT OF EPIGENETIC INHIBITORS FOR HISTONE METHYLATION
A. In silico screening for methyltransferase and demethylase inhibitors
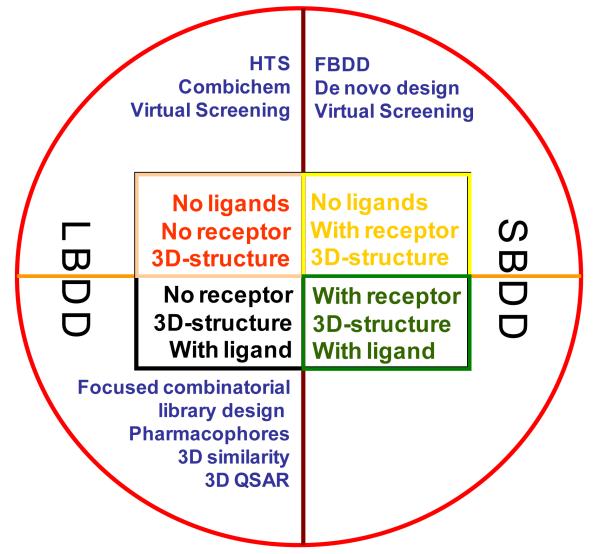
Up to now, the investigation of small molecule inhibitors against histone methyltransferases and demethylases is still in its infancy in contrast to that of histone deacetylase (HDAC) and DNA methyltransferase (DNMT) inhibitors. Given their powerfulness in speeding up hit identification in drug discovery processes, in silico screening approaches are of particular value in search for new methyltransferase and demethylase inhibitors. The in silico screening is defined as a computational technique used in drug discovery research based on the availability of small molecule (ligand) databases and crystal structures of enzymes (receptor). It can be classified as ligand-based drug design (LBDD) approach and receptor structure-based drug design (SBDD) approach207-210, as shown in Figure 10. By now, there are three LBDD approaches207, i.e., ligand-based quantitative structure activity relationship (i.e., QSAR, quantitative structure-activity relationship, sometimes referred to as QSPR: quantitative structure-property relationship), pharmacophore modeling, and similarity search, all of which are applicable to the drug discovery process. Based on the hypotheses that similar molecules have similar activities and on the principle of structure-activity correlation, QSAR is the process by which chemical structure is quantitatively correlated with a well defined process, such as biological activity or chemical descriptors. The pharmacophore modeling is used to define the essential features of one or more molecules with the same biological activity. Then a database of scaffold-diverse compounds can be searched for more compounds which share the same structural features. The similarity search approach offers three distinct access modes (i.e., exact match, partial match and best match) to identify potential hits from small molecule databases, thus being very useful in drug discovery.211 Due to its power to rapidly identify novel potential lead compounds, pharmacophore modeling is particularly crucial in the LBDD.
Figure 10.
Approaches in LBDD and SBDD.
The SBDD approaches include docking in conjunction with score functions and de novo drug design. The docking of a library of small molecules into the structure of macromolecular targets and scoring of their potential complementarities to binding sites are widely used in hit identification and lead optimization.208 SBDD uses a known 3D structure of proteins to assist the development of new drug compounds. The 3D structure of protein targets is derived from X-ray crystallography or nuclear magnetic resonance (NMR) experiments or even by protein modeling. Based on these structures, researchers can precisely probe the interaction between a receptor and a potential drug compound. This ability of working at high resolution with both proteins and drug compounds makes SBDD one of the most powerful methods in drug design by pharmaceutical industry over the past twenty years.
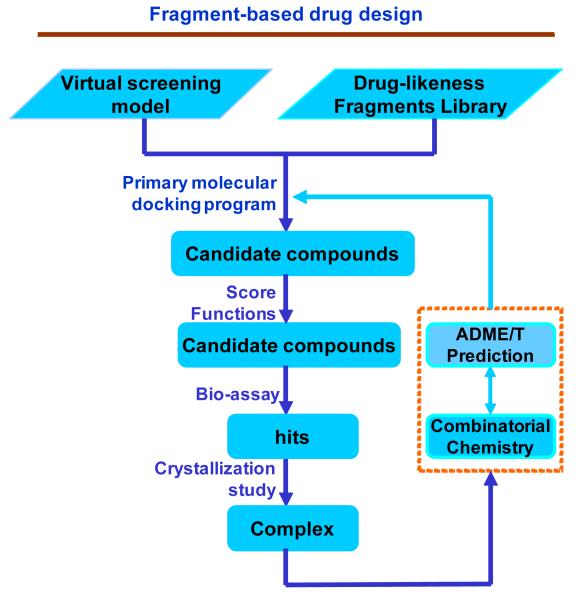
Recently, one promising in silico drug design approach named fragment-based drug design (FBDD) has emerged in the drug discovery process. In contrast to HTS, fragment-based lead discovery identifies low molecular weight chemical fragments (also known as scaffolds or templates, with molecular weights from 150 to 350 Da) from very small compound libraries. The strategy of FBDD is shown in Figure 11. These fragments are then linked and combined or optimized to generate lead compounds. This approach offers an alternative, complementary strategy to high throughput virtual screening (HTVS). It emphasizes efficiency and design, and lead compounds emanating from fragment-based drug discovery have a better chance of being successful. Recent data from Astex Technology indicate that, in a typical pharmaceutical company, 70% of initial hits ultimately fail, whereas 80% of fragment hits prove useful.212 Therefore, the use of the FBDD method to identify selective methyltransferase and demethylase inhibitors should be a promising and feasible approach.
Figure 11.
The fragment-based drug design workflow.
The purpose of in silico virtual screening approach is to come up with hits of novel chemical structures that bind to the macromolecular target of interest. In contrast to HTS, virtual screening approach is knowledge-based and utilizes a given 3-D structure of the target or a reference ligand with known binding conformation to identify more novel scaffolds with inhibitory activities. By now, despite that the application of virtual screening is still on its way, some successful cases have been reported in the identification of methyltransferases inhibitors.65,213,214 Based on the model of human PRMT1 complexed with SAH, Heinke and co-workers65 combined the pharmacophore search and the structure-based virtual screening approach to screen Chembridge database containing 328000 molecules. Among those candidate compounds top-ranked by the docking program and verified by pharmacophore model, nine compounds were identified as inhibitors against PRMT1 activity. Spannhoff and co-workers213 also modeled the human PRMT1 and Aspergillus nidulans PRMT1 (RmtA) 3-D structures using the rat PRMT3 X-ray structure as a template, and identified seven inhibitors of PRMTs by in vitro assay. Recently, by fragment-based drug design approach in conjunction with other LBDD and SBDD approaches, some potent inhibitors against PRMT1 have been identified and optimized.65,214
B. Biochemical assays for methyltransferase and demethylase inhibitor discovery
Having an effective assay is a key element for the successful investigation of enzymatic functions of histone methyltransferases and demethylases as well as for effective screening and characterization of methylation and demethylation inhibitors. Nowadays, HTS is an important method of the discovery of novel drugs and is relevant to the fields of biology and chemistry. HTS in conjunction with combinatorial libraries of small organic molecules can increase hits in the identification of target-specific ligands. HTS allows a researcher to quickly conduct millions of biochemical, genetic or pharmacological tests by using robotics, data processing and control programs, liquid handing devices, and sensitive detectors. By this approach, active compounds, antibodies or genes which regulate a particular biological pathway are rapidly identified as the starting points (lead compounds or hits) for further drug design. HTS has been successful in the discovery of histone deacetylase (HDAC)215,216 and DNMT1217 inhibitors. The first small molecule PRMT inhibitors reported by Bedford and co-workers were obtained via a random screening.218 Recently, chaetocin, a compound that reduces HKMTs activity against H3K9 dimethylation, was also identified by an HTS approach. Further, Kubicek and co-workers screened specific inhibitors of HKMTs using recombinant G9a as the target enzyme with a library containing 125000 small molecules.217 By this approach, a highly specific inhibitor named BIX-01294 (Figure 15) for G9a was identified.
Figure 15.
Inhibitors of HKMTs.
Radioisotope-labeled assays represent the gold standard approach for the in vitro measurement of HKMT and PRMT activities (Figure 12). In this isotopic approach, [3H] or [14C]-labeled AdoMet is used as a cofactor to generate the radioactive protein or peptide products. The amount of methylated products is quantified by loading the labeled histones onto P81 filter paper due to the cationic nature of histones. If the histone substrates contain affinity tags such as biotin, the products can be purified on affinity beads prior to scintillation counting. Alternatively, the reaction mixture can be separated on SDS-PAGE gels, and visualized and quantitated by autoradiography or phosphorimaging. In typical biochemical laboratories, the radioactive assay is implemented in test tube formats, but it can also be set up in the microplate format. Gowher et al 219 tested the methylation of a biotin-labeled H3 N-terminal peptide by Dim-5, a HKMT enzyme with specificity on H3K9. Following the methylation reaction, the products are bound to avidin-coated microplates by the strong biotin-avidin interaction. The plates are subsequently washed to remove the free [3H]-AdoMet and the bound radio-labeled peptide is released by the addition of HCl and the final radioactivity can be quantitated by scintillation counting. It remains to determine whether the microplate assay can be improved for HTS use. For instance, it needs to test whether the radioactive plates can be directly scanned on a Beta scanning instrument which is related to the amenability to HTS automation. In practice, whether and how the surface affects the methylation need to be addressed. Generally speaking, radioisotope-labeled assays suffer from several disadvantages: radioactive AdoMet is quite costly, exposure of laboratory personnel to radioactivity is a consideration, the disposal of radioactive waste, the nature of discontinuous nature (i.e., an endpoint assay), and the quantitation is technically challenging, in particular in gel-based measurements.220 Consequently, although the classic radioactive assay has been successfully used to measure methylase activities from various sources, appropriate homogeneous, non-radioactive, and spectrometric or fluorescent assays systems are highly needed for mechanistic dissection, functional evaluation, and inhibitor screening.
Figure 12.
General strategies for methyltransferase assays.
The design of nonradioactive methylation assays, however, is technically challenging because the substrates and products of PRMTs and HKMTs, i.e., SAM versus SAH, and peptide/protein versus methylated peptide/protein, exhibit little spectroscopic difference.221 In order to determine the enzymatic turnover of methyltranferaeses with spectrometric and fluorescent methods, several groups reported on carrying out the enzymatic methylation reactions in conjugation with additional enzymes that utilize SAH as a substrate and transform it into photoactively detectable products (Figure 12B). For instance, Hendricks et al222 described a coupled assay for salicylic acid carboxyl methyltransferase (SAMT). The SAMT reaction is carried out in the presence of recombinant AdoHcy nucleosidase (SAHN) which converts the SAH product to into adenine and S-ribosylhomocysteine. The latter compound is further cleaved by recombinant LuxS (S-ribosylhomocysteinase, EC 3.2. 1.148) to form homocysteine. Finally, homocysteine is quantitated with Ellman’s reagent. The Ellman’s reagent, 5,5′-dithiobis(2-nitrobenzoate) (DTNB) 223,224, is a standard reagent to detect thiol reagents such as homocysteine. The reaction product, 2-nitro-5-thiobenzoate (TNB), can be monitored by absorbance at 412 nm. Such a three-step coupled approach was also explored for the characterization of methylation by putrescine N-methyltransferase (PMT).225 In a related method, Hevel and colleagues used SAHN to convert SAH to adenine and S-ribosylhomocysteine.221 Instead of targeting S-ribosylhomocysteine, the authors added adenine deaminase to transform adenine to hypoxanthine, a spectrometrically active compound that can be measured by absorbance at 265 nm. The short absorption wavelength may be a limitation for the application of this assay.
In a fluorescent method, Wang et al226 performed catechol-O-methyltransferase-catalyzed methylation with the addition of AdoHcy hydrolase (SAHH) which converts AdoHcy to the thiol homocysteine. The homocysteine causes disulfide bond cleavage in a fluorescein-cystaminemethyl red reporter molecule and activates fluorescence emission of the fluorophore. In a similar manner, Collazo et al227 used SAHH-coupled assay to study histone H3 methylation by S. pombe CLR4, an H3 Lys-9-specific methyltransferase. In this case, the homocysteine is monitored through reaction with a sulfhydryl-sensitive fluorophore, ThioGlo 1, which fluoresces strongly upon conjugation of its maleimide moiety to a sulfhydryl group. These SAHH-coupled enzymatic methylation assays in conjugation with fluorescent signal readout seem simpler and more sensitive than the SAHN/LuxS-coupled spectrophotometric assays.
The major advantage of the coupled enzymatic assays is that they avoid using radioisotope-labeled materials and allow for the measurement of PRMT activity via absorption or fluorescent readouts. The limitations are that these methods are generally of lower sensitivity than radioactive methods and involve multiple additional components which necessitate experimental optimization prior to the methylation study. Further, when used for inhibitor screening, there is a chance that the coupling components may be inhibited, thus leading to false positive hits.
Antibody-based immunodetection methods are also broadly used for methylation and demethylation studies (Figure 12C). Like other PTMs such as protein phosphorylation and protein lysine acetylation, many primary antibodies have been developed and available for recognition of specific methylarginine and methyllysine marks in protein targets. After the methylation reaction with regular cold SAM, a primary antibody is added to capture the methylated substrates. Then, a secondary anti-IgG antibody is added to produce signal readouts. Commonly, this secondary antibody is labeled with horseradish peroxidase (HRP) so that a peroxidase substrate is added that results in the formation of a chemiluminescent product.218 In another way, the secondary antibody is labeled with lanthanide europium. After removing excess reagents by several rounds of washing, an enhancement chelating cocktail (e.g., the DELFIA inducer from Perkin Elmer) is added to release the europium label from the antibody and activate its photon emission. Finally, a time-resolved fluorescence (TRF) measurement (340/615 nm) is used for the quantitation of the activity of methyltransferases.213 The method of TRF detection has great advantage of reducing background fluorescence levels, thus increasing signal-to-noise ratios. Detection with europium-labeled secondary antibodies have been used in a number of cases, e.g., in vitro assay for the lysine methyltransferase G9a,217 cellular assay of histone acetylation,228 and screening of PRMT inhibitors.65,213 Antibody-based immunosorbent assays are valuable tools to identify methyl marks in protein substrates and to determine the relative changes in methylase activity. However, antibody-based immunosorbent methods have inherent shortcomings that need to bear in mind. The costliness of antibodies and lengthy procedure greatly limit its throughput. Often, the methylation patterns are complicated and availability of antibody is often an issue. Also, recognition of a methyl mark by a primary antibody is dependent on the specific contexts surrounding the methyl mark. Antibody-based approaches require a priori knowledge of a modification, can suffer from cross reactivity and epitope occlusion, and rarely detect more than a single PTM on a histone tail.116 Furthermore, antibody-based assays are difficult for accurate quantitation. In almost all cases, a control sample is used to achieve quantitation of target methylation in a relative manner, and information about the degree and yield of methylation reactions is difficult to obtain from an immunosorbent assay.
We recently illustrated a single-step strategy for detecting PRMT1-catalyzed methylation by fluorescence measurement (Figure 12D).229 In this approach, a fluorescein-labeled peptide molecule (e.g., histone H4 peptide and glycine- and arginine-rich peptide) was synthesized and used as a PRMT1 substrate. Interestingly, the fluorescence intensity of the fluorophore changed during the time course of substrate methylation and the intensity was sensitive to the presence of PRMT1 inhibitors such as sinefungin. Also a dramatic shift in the anisotropy of the fluorescently labeled peptide substrate was exhibited upon its association with PRMT1. This strategy is particularly useful for studying interactions between PRMTs and their substrates and can be applied to investigate inhibitors and ligands that target the substrate binding pocket of the enzymes. The advantage of this approach in PRMT inhibitor study includes merits of “mix-and-measure” assay, simplicity of data analysis, and sensitivity to the progression of both substrate binding and methylation. The major shortcoming of this assay is that a high amount of proteins is needed to produce the wanted changes in fluorescence readouts, which may limit its practical use in HTS. Nevertheless, this is a unique approach for both inhibitory and mechanistic probing.
Histone lysine methylation has been shown to be a biochemically reversible process. Although not validated, it is quite possible that demethylases may exist in cells for arginine demethylation as well. To set up a biochemical demethylase assay, methylated histones need to be prepared as substrates. For small peptide substrates, solid phase peptide synthesis is the best way to make the sequence containing desired methylated residues. For histone protein or nucleosome substrates, methylation can be introduced by incubating them with AdoMet and appropriate recombinant histone methyltransferases. For the demethylation on lysine residues, no matter the demethylation is catalyzed by LSD or JMJD type demethylases, the reaction generates unmethylated protein product and small molecule byproduct formaldehyde, the latter of which can be used to quantify the enzymatic turnover of demethylation. Typically, the demethylase activity is determined by measuring the amounts of generated formaldehyde by the Nash-Hantzsch reaction.80,230 In this protocol, formaldehyde is reacted with acetylacetone (2,4-pentanedione) in the presence of ammonia to produce 3,5-diacetyl-1,4-dihydropyridine (DDL). DDL is a photosensitive molecule that can be measured either by photometric absorbance at 412 nm 230 or by fluorescence at 510 nm.231 The colorimetric detection sensitivity of Nash reagents is considered poor. Other derivatization reagents can be used as alternatives for the determination of formaldehyde, e.g., Purpald (4-amino-3-hydrazino-5-mercapto-1,2,4-triazole) and N-methylbenzothiazolinone-2-hydrazone (MBTH),232, tryptophan-sulfuric acid-iron reagents,233 and 2,4-dinitrophenylhydrazine.234 In addition to colorimetric and fluorescent detection of the formaldehyde product, a radioactive approach may also be used. In this case, radioactive methylated histone substrates containing 3H or 14C-labeled methyl groups are needed in the demethylase reaction to produce a radioactive formaldehyde product. After the demethylation reaction is over, radioactive formaldehyde is purified by the removal of all proteins by trichloroacetic acid (TCA) precipitation. Formaldehyde in the TCA supernatant is then converted to DDL by Nash reagents. Next, DDL is extracted into organic phase with 1-pentanol and subjected to liquid scintillation counting. It is worthwhile to note that, for all these approaches, the generated formaldehyde or DDL can be separated by passing a HPLC column and then subject to quantitative analysis. The radioactive protocol offers a better assay sensitivity.231
Another approach to measure the amounts of released formaldehyde makes use of formaldehyde dehydrogenase (FDH), which oxidizes formaldehyde to formic acid, meanwhile reducing NAD+ to NADH. The amounts of NADH generated can be measured by absorbance at wavelength of 340 nm 235 or by fluorescence emission at 465 nm.236-238 In addition to the measurement of formaldehyde, demethylated histone peptide products can be directly measured by MALDI-MS,237,238 thus providing an optional approach for counterscreen or secondary assays of primary hits.
In the LSD1-catalyzed demethylation, hydrogen peroxide (H2O2) is produced as a side product and its production is stoichiometric with each demethylation cycle. Therefore, a peroxidase-coupled assay can be performed to quantify the amounts of released H2O2.78,239 It is worthwhile to point out that the reaction of LSD-catalyzed demethylation (Methyl-histone + H2O + O2 → histone + HCHO + H2O2) is formally similar to the oxidation reaction catalyzed by amine oxidases which can be formulated as: RCH2NR1R2 + H2O + O2 → RCHO + NHR1R2 + H2O2. The peroxidase-linked spectrophotometric approach is a continuous assay which is suitable for measuring kinetic time courses of the oxidation reaction.
In a typical peroxidase-coupled assay, the reaction mixture consists of demethylation ingredients (LSD1, methylated histone, FAD), 4-aminoantipyrine, vanillic acid, and HRP. In this course, the released H2O2 will promote the condensation reaction between 4-aminoantipyrine and vanillic acid, which is catalyzed by the peroxidase, to generate a stoichiometric amount of quinoneimine dye (Figure 13). The formation of this product is monitored at wavelength of 498 nm.240 This assay is robust, continuous, rapid, and suited for automation, thus convenient for implementation in microtiter plate formats.241 Except vanillic acid as the chromogen, other compounds of choice include 3,5-dichloro-2-hydroxybenzenesulfonic acid,78 2,2′-azino-bis(3-ethylbenthiazoline-6-sulfonic acid) (ABTS),242 and 2,4-dichloro-phenol.240 Different chromogens will affect slightly the maximum wavelength of the spectrophotometric absorption. Also, some chromogens may inhibit the amine oxidase of study and should be avoided.240,241
Figure 13.
Coupled demethylation assays analyzing the production of hydrogen peroxide.
Alternatively, hydrogen peroxide can be measured by the use of luminol as a chemiluminescent substrate (Figure 13).243,244 This detection method is the same as the widely used HRP/luminol protocol in Western blotting experiments. In the reaction, luminol is oxidized by peroxide with the catalysis of HRP and results in generation of an excited state product called 3-aminophthalate*. This product decays to a ground energy state by releasing photons of light, i.e., chemiluminescence. Several commercial demethylase assay kits are currently available and are all based on the chemical principles described above.
C. Inhibitors of HKMTs
Histone methyltransferases and demethylases have received considerable attention of investigation because of their critical function in regulating many epigenetic processes such as gene expression, heterochromatin formation, and X-chromosome inactivation.13,245,246 Despite that various epigenetic targets have been explored with drug discovery approaches, so far only HDAC and DNMT inhibitors are FDA approved for the treatment of human cancers or are currently investigated in clinical tests.247 The identification of inhibitors against histone methylation and demethylation is still in an early stage of development. AdoMet analogs represent the first set of methyltransferase inhibitors. These compounds include methylthioadenosine (MTA), SAH,248 sinefungin249, adenosine dialdehyde, and cycloleucine (Figure 14).250-252 However, due to structural similarity to AdoMet, they target all the methyltransferases, such as DNMTs, HKMTs, PRMTs and unrelated N- and O-methyltransferases.253 Therefore, these compounds are non-specific methyltransferase inhibitors.
Figure 14.
AdoMet analogs as methyltransferase inhibitors.
A few small molecule compounds such as chaetocin,254 BIX-01294,217 BIX-01338,217 UNC0224,255 DZNep256 (Figure 15) have been identified with anti-HKMT activity by random or virtual screening approaches. The first inhibitor of HKMTs is a natural fungal substance named chaetocin. It is initially isolated from the fermentation broth of Chaetomium minutum and belongs to the class of 3-6 epidithio-deketopiperazines (ETPs). Chaetocin is a weak inhibitor of certain H3K9-specific HKMTs such as mouse G9a and Neurospora crassa DIM5 with micromolar IC50 values (2.5 and 3 μM, respectively). It shows strong inhibition of SU(VAR)3-9 and human ortholog of dSU(VAR)3-9 with IC50 of 600 nM and 800 nM, and acts competitively versus AdoMet. The activity of chaetocin against hSU(VAR)3-9 in cells and its cytotoxic effects were observed.254
BIX-01294 inhibits dimethylation of lysine 9 on histone H3 (H3K9me2) at low micromolar level and is an uncompetitive inhibitor against AdoMet. BIX-01294 acts as an inhibitor against G9a in vitro (IC50 = 2.7 μM) and does not affect the activity of SUV39H1 or PRMT1. BIX-01294 was also observed to specifically reduce the methylation level of H3K9 while other lysine methylation sites like H3K27 or H4K20 were not changed. However, BIX-01338, another small molecule identified from the same study of BIX-01294, was found to be a nonspecific inhibitor which can affect the methylation activity of almost all tested enzymes. In addition, BIX-01338 plays as a dual inhibitor against arginine and lysine methyltransferase.217 Based on BIX-01294, Jin and co-workers255 performed molecule design, synthesis and SAR analysis, and identified UNC0224 that potently and selectively targeted G9a with an IC50 of 15 nM.
Recently, 3-deazaneplanocin A (DZNep, Figure 15) was reported to selectively inhibit H3K27me3 and H4K20me3.257 However, Miranda and co-workers report that DZNep acts as a non-specific inhibitor against histone methylation and is not as selective for H3K27me3 and H4K20me3 as previously reported.256 DZNep is structurally analogous to sinefungin and adenosine dialdehyde (Figure 14).
D. Inhibitors of PRMTs
As aforementioned, PRMT members are classified into type I (e.g., PRMT1, 4, and 6) and type II (PRMT5, 7, and 9) subfamilies.62 Type I PRMTs catalyze the formation of MMA and ADMA. Type II PRMTs catalyze the formation of MMA and SDMA. The first reported small molecule PRMT inhibitors were identified from a random screening of 9000 library compounds by Bedford and coworkers in 2004.218 Among those inhibitors, AMI-1 and AMI-6 (Figure 16) specifically target PRMTs but not HKMTs in vitro. AMI-5, also named eosin, was identified in the same study and shown to inhibit both PRMTs and HKMTs. In 2007, by in silico studies, Ragno and coworkers used AMI-5 as the lead compound to design a series of new inhibitors against PRMTs and then performed SAR study for the lead optimization.258 The lead structure was optimized to curcumin-like scaffolds with bromo- or dibromophenol substructures, which are known to be a scaffold of HAT inhibitors.259 Indeed, by in vitro assays, anti-HAT and anti-sirtuin activities were observed for several compounds. Among them, the compound 4b is an inhibitor against hPRMT1 with an IC50 value of 14μM (Figure 16).260
Figure 16.
Inhibitors of PRMTs.
In 2007, Jung and coworkers applied target-based in silico virtual screening approach and identified seven small molecules for PRMT1 inhibition with an IC50 below 55 μM.213 Among them, two inhibitors, stilbamidine and allantodapsone, were found to inhibit PRMT1 activity in vitro and H4R3 methylation in HepG2 cells (Figure 16).213 Interestingly, further selectivity study showed that allantodapsone also had in vitro inhibitory activity against SET7/9. Furthermore, by screening small fragment molecules with molecular weights less than 200 with a FBDD approach, an α-methylthiolycolic amide was identified against PRMT1.65 Due to its poor drug-likeness (the drug-likeness is referred to the Lipinski’s Rule of Five261) and lack of chemical stability, the authors performed similarity search and found a compound named RM65 (Figure 16) to inhibit both the aspergillus and human enzymes with same strength (55.4 μM).262,263 Furthermore, treatment of HepG2 cells with RM65 resulted in a robust hypomethylation on histone H4R3 and did not show inhibitory activity against SET7/9.214 Recently, Heinke et al65 conducted a virtual screening of the small molecule collection of Chembridge database on the basis of a PRMT1–allantodapsone interaction model. From that study, several new lead inhibitors (e.g., compound 6 and 9 in Figure 16) were identified against PRMT1 with submicromolar potency. In the PRMT family, CARM1 has attracted particular interest because it is involved in hormone-responsive carcinogenesis.264-268 Inhibitors that selectively block CARM1 activities in the cell may be of therapeutic merits. Based on a random HTS hit, Purandare and coworkers performed synthetic optimization and obtained a set of pyrazole amide inhibitors for CARM1 (compound 7b), the best of which showed an IC50 of 80 nM.269 However, whether these pyrazole inhibitors are effective in tumor suppression has not yet been demonstrated. Further, a bisubstrate analogue was obtained by an alkylating agent covalently attached to a peptide substrate like an adenosine-derived mustard.270 Despite of its poor drug-likeness, it could be useful as a chemical tool to probe the mechanism of PRMTs.
E. Inhibitors of histone lysine demethylases
While there was some evidence that histones could become unmethylated,271 debates on the existence of histone demethylases had remained for quite a long time.76,271,272 In 2004, LSD-1, the first histone lysine demethylase that removes one or two methyl groups from histone H3 was reported by Shi and coworkers,273 providing direct evidence for a dynamic regulation of histone methylation. Histone lysine demethylases are implicated in cancer. For example, loss of trimethylation at Lys 20 and acetylation at Lys16 of histone H4 is a common hallmark of human cancer.274 Therefore, inhibitors of histone demethylases may be useful as anticancer agents. Till now, only a few inhibitors have been identified against histone demethylases. Further effort for the discovery of small molecules to regulate histone lysine demethylases are still on the way.
LSD1 inhibitors
Several potent inhibitors of LSD1 have already been reported as shown in Figure 17. In the early stage of inhibitor development for LSD1, some mechanism-based or suicide inactivators are identified by targeting FAD, the cofactor of the redox reaction for the demethylation by LSD1.262 Since LSD1 and monoamine oxidases (MAO) show homology in their catalytic sites,275-278 many of MAO inhibitors (e.g., pargyline, chlorgyline, deprenyl, tranylcypromine, phenelzine (PCPA), and nialamide) have been screened for their activity against LSD1. Results demonstrated that these MAO inhibitors showed inhibitory activity against recombinant LSD1 at rather high concentrations. Among them, two inhibitors are phenelzine and tranylcypromine with an IC50 value of 21 μM.40,277 Recently, based on the crystal structures of FAD-PCPA adduct and the FAD-N-propargyl lysine peptide adduct complex with LSD1279,280, Ueda and coworkers designed a series of PCPA-lysine hybrid compounds and evaluated their inhibitory activities targeting at LSD1 and MAO-A and MAO–B.281 Among the four synthesized compounds, the LSD1 inhibitory activities of compound 1 and 2 (Figure 17) were over 10-fold more potent than that of PCPA (IC50 values: PCPA = 32 μM; 1 = 2.5 μM; 2 = 1.9 μM). In particular, compound 1 and 2 specifically inhibit LSD1 in comparison to MAO-A and –B (the IC50 values against MAO-A are 230μM and 500μM; The IC50 against MAO-B: 290μM and >1mM). In addition to the application of MAO inhibitors for LSD1 inhibition, Cole and coworkers have designed, synthesized, and examined a series of novel H3 tail peptide analogues containing classical MAO warhead groups (e.g., propargyl, chlorovinyl, hydrazine, and cyclopropyl) as LSD1 inhibitors.282,283 These mechanism-based suicide inhibitors of LSD1 have the potential to be applied for proteomic analysis such as affinity labeling. Recently, Huang and coworkers reported that novel biguanide and bisguanidine polyamine analogs (compound 22 and 23) are also potent inhibitors against LSD1.244 These compounds inhibit LSD1 in human colon carcinoma cells and affect a reexpression of multiple, aberrantly silenced genes important in the development of colon cancer.
Figure 17.
Inhibitors of LSD1.
JMJD inhibitors
Except LSD1 and LSD2, several JMJD lysine demethylases have been identified, including KDM2(FBXL) family, KDM3(JMJD1) family, KDM4(JMD2) family, KDM5(JARID1) family and KDM6 family.80 A few inhibitors have been reported for JMJD demethylases that demethylate specific lysines in the H3 tail (Figure 18).237,284-286 The analog of α-ketoglutarate, oxalyglycine, has been reported to be a weak inhibitor against JMJD2C83 and the catalytic core of JMJD2A(c-JMJD2A).284 Recently, Rose and coworkers demonstrated that HDAC inhibitors and pyridine carboxylic acids such as compound 6a and 7 (Figure 18) act as micromolar inhibitors against JMJD2E (IC50: 1.4 μM for 6a and 6.6 μM for 7).237 By a MALDI TOF MS-based assay, Sekirnik and coworkers identified a series of Zn(II) ejectors such as pyridine-2,4-dicarboxylic acid, selenium derivatives (e.g., PhSeOOH, PhSeSePh, PhSeCl), and di-sulfiram derivatives (compound 1a, 1b and 16) as JMJD2A inhibitors (Figure 18).285 Many of these compounds showed inhibition potency in the micromolar range. Furthermore, by dynamic combinatorial chemistry coupled with MS and turnover assays, the same group identified a series of N-oxalyl-D-tyrosine derivatives as JMJD2 inhibitors.286 Among them, the most potent compound (compound 7f, shown in Figure 18) has an inhibitory activity at 5.4 μM. Notably, for pyridine carboxylic acids (6a), the crystal structure in complex with JMJD2A has also been released (PDB entry: 2VD7). These complex structures provide a platform for the structure-based design of more potent and selective inhibitors against JMJD demethylases. In addition, through a miniaturized screening, Sakurai et al.238 recently found that catechol and flavonoid compounds (e.g., myricetin and beta-lapachone) were able to inhibit JMJD2E catalysis in the micromolar range. These types of compounds are bidentate iron chelators and likely inhibit 2-oxoglutarate-dependent oxygenases in general, implicating that their in vivo activities may be correlated with pharmacologic intervention of multiple cellular targets.
Figure 18.
Inhibitors of JMJD demethylases.
7. CONCLUSIONS
Histone methylation represents an important regulatory mode for epigenetic control of DNA function. In this review we highlighted recent advances of using various chemical and biochemical approaches to probe mechanism and function of histone methylation. Since methylated lysines and arginines have no spectroscopic distinction from unmethylated residues, antibody-based immunoassays remain to play critical roles to provide molecular information for the function of methylation in response to extra- and intra-cellular signals. MS will clearly continue to be an important technique for identifying new methylation substrates and sites and for studying kinetics and dynamics of histone methylation under various physiological and pathological contexts. Bioorganic chemical approaches that facilitate site-specific labeling of histones at target methylation sites will be very useful for making the desired protein constructs for biochemical and functional investigation. Like other types of histone modifications, methylation is a dynamic process and can appear/disappear on chromatin within minutes of stimulus arriving at the cell surface.287 New chemical probes that can image or analyze histone methylation in the cell in a spatially and temporally resolved manner will be in great demand. Although quite a few histone methyltransferase inhibitors have been identified or synthesized, most of these inhibitors only have micromolar potency. Further effort is needed to obtain HKMT and PRMT inhibitors with potency at nano or pico-molar levels. Importantly, a systematic evaluation of the pharmacological impact of new methyltransferase and demethylase inhibitors in cancer cells and other disease models need to be performed in order to validate the therapeutic potential of these inhibitors. Notable progress in the protein methylation field is the ever growing evidence that methylation is found to occur broadly on a variety of nonhistone proteins, thus out of the realm of chromatin biology. Histone methylation on chromatin templates has been considered to be intimately associated with transcriptional activation and repression. However, current knowledge about the biological function of methylation in non-chromatin proteins is very limited. Since methylation does not change the overall charge on lysine and arginine residues, the addition of methyl groups is unlikely to cause a significant global structural change within the protein substrate.288 Instead, the methyl groups could block biomolecular interactions by rendering steric hindrance on Lys and Arg residues, or in the opposite manner, provide an epitopic motif for introducing physical interaction with downstream effector proteins. Excitingly, several protein domains, such as Tudor domain, Chromo domain and PWWP domain,289,290,291,292 have been identified to recognize specific methyl marks, which suggests that protein methylation could be extensively involved in mediating intra- and intermolecular protein-protein or protein-nucleic acid interactions and cell signaling.64 These and other hypotheses regarding the biological functions of protein methylation need be addressed and will be facilitated by newly developed chemical biology methods and tools.
ACKNOWLEDGEMENTS
We apologize to those colleagues whose important work could not be cited due to space limitations. The authors’ contributions are: KKL contributed to Chapters 1, 2 and 3. CL and JH contributed to Chapter 6. DW contributed to Chapter 4. YGZ contributed to Chapters 2, 5, and 6 and the overall organization. CL’s and HJ’s research projects are supported by the National Natural Science Foundation of China (20972174), Shanghai Science and Technology Innovation Program (08431900800) and the State Key Program of Basic Research of China grant (2009CB918502), National S&T Major Project (2009ZX09501-001). YGZ’s work is funded by Georgia Cancer Coalition Distinguished Cancer Scientist program and American Heart Association.
Biography
Keqin Kathy Li received her B.S. degree from Lanzhou University in 1994 and her Ph.D. degree from Beijing Basic Medical Institute in 2002. She received her postdoctoral training at the Wistar Institute from 2002 to 2006 and Emory University Medical School from 2006 to 2009. She currently is a professor in State Key Laboratory of Medical Genomics, Shanghai Institute of Hematology. Her current research interests focus on investigation of the epigenetic mechanisms in blood cancer diseases by using X-ray crystal structure, ChIP-on-chip and ChIP-seq bioinformatics technologies.
Cheng Luo got his B.S. degree in chemistry from Fuzhou University in 1994, his M.S in physical chemistry from Fudan University in 2001 and his Ph.D. in organic chemistry from Shanghai Institute of Materia Medica (SIMM), Chinese Academy of Sciences in 2004. He received his postdoctoral training from the Wistar Institute. He is currently an Associate Professor in the Drug Discovery and Design Center of SIMM. His current research interest resides in the identification of small molecules to regulate epigenetic related enzymes and the design of antibacterial drugs.
Dongxia Wang got his Ph.D. degree in biochemistry from Indiana University School of Medicine. He is currently an analytical chemist at Centers for Disease Control and Prevention (CDC). His current research focuses are on the application and development of mass spectrometry-based methods for rapid detection of protein toxins and characterization of biologically significant proteins and post-translational modifications.
Hualiang Jiang got his B.S. degree in chemistry from Nanjing University in 1987, his M.S in physical chemistry from East-China Normal University in 1992 and his Ph.D. in organic chemistry from SIMM, CAS in 1995. He is currently the deputy director and professor of SIMM. His current research interest resides in computer aided drug design, computational biology and structural biology.
Yujun George Zheng got his B.S. degree from Peking University in 1995 and his Ph.D. from University of Miami in 2002. He conducted his postdoc research at The Johns Hopkins University School of Medicine from 2002 to 2006. He is currently an Assistant Professor in the Dept of Chemistry at Georgia State University. His research interest includes investigation of function and inhibition of histone acetyltransferases and histone methyltransferases.
REFERENCES
- 1.Altucci L, Stunnenberg HG. Time for epigenetics. Int J Biochem Cell Biol. 2009;41(1):2–3. doi: 10.1016/j.biocel.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 2.Baylin SB, Schuebel KE. Genomic biology: the epigenomic era opens. Nature. 2007;448(7153):548–549. doi: 10.1038/448548a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldberg AD, Allis CD, Bernstein E. Epigenetics: a landscape takes shape. Cell. 2007;128(4):635–638. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Kim JK, Samaranayake M, Pradhan S. Epigenetic mechanisms in mammals. Cell Mol Life Sci. 2009;66(4):596–612. doi: 10.1007/s00018-008-8432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128(4):683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szyf M. Epigenetics, DNA methylation, and chromatin modifying drugs. Annu Rev Pharmacol Toxicol. 2009;49:243–263. doi: 10.1146/annurev-pharmtox-061008-103102. [DOI] [PubMed] [Google Scholar]
- 7.Hirst M, Marra MA. Epigenetics and human disease. Int J Biochem Cell Biol. 2009;41(1):136–146. doi: 10.1016/j.biocel.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Feinberg AP, Vogelstein B. Hypomethylation distinguishes genes of some human cancers from their normal counterparts. Nature. 1983;301(5895):89–92. doi: 10.1038/301089a0. [DOI] [PubMed] [Google Scholar]
- 9.Iacobuzio-Donahue CA. Epigenetic changes in cancer. Annu Rev Pathol. 2009;4:229–249. doi: 10.1146/annurev.pathol.3.121806.151442. [DOI] [PubMed] [Google Scholar]
- 10.Fraga MF, Ballestar E, Villar-Garea A, Boix-Chornet M, Espada J, Schotta G, Bonaldi T, Haydon C, Ropero S, Petrie K, Iyer NG, Perez-Rosado A, Calvo E, Lopez JA, Cano A, Calasanz MJ, Colomer D, Piris MA, Ahn N, Imhof A, Caldas C, Jenuwein T, Esteller M. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet. 2005;37(4):391–400. doi: 10.1038/ng1531. [DOI] [PubMed] [Google Scholar]
- 11.Ballestar E, Esteller M. Epigenetic gene regulation in cancer. Adv Genet. 2008;61:247–267. doi: 10.1016/S0065-2660(07)00009-0. [DOI] [PubMed] [Google Scholar]
- 12.Mai A, Altucci L. Epi-drugs to fight cancer: from chemistry to cancer treatment, the road ahead. Int J Biochem Cell Biol. 2009;41(1):199–213. doi: 10.1016/j.biocel.2008.08.020. [DOI] [PubMed] [Google Scholar]
- 13.Zheng YG, Wu J, Chen Z, Goodman M. Chemical regulation of epigenetic modifications: opportunities for new cancer therapy. Medicinal research reviews. 2008;28(5):645–687. doi: 10.1002/med.20120. [DOI] [PubMed] [Google Scholar]
- 14.Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389(6648):251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 15.Bernstein BE, Meissner A, Lander ES. The mammalian epigenome. Cell. 2007;128(4):669–681. doi: 10.1016/j.cell.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Zang C, Rosenfeld JA, Schones DE, Barski A, Cuddapah S, Cui K, Roh TY, Peng W, Zhang MQ, Zhao K. Combinatorial patterns of histone acetylations and methylations in the human genome. Nat Genet. 2008;40(7):897–903. doi: 10.1038/ng.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403(6765):41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 18.Wang GG, Allis CD, Chi P. Chromatin remodeling and cancer, Part I: Covalent histone modifications. Trends Mol Med. 2007;13(9):363–372. doi: 10.1016/j.molmed.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293(5532):1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 20.Taverna SD, Li H, Ruthenburg AJ, Allis CD, Patel DJ. How chromatin-binding modules interpret histone modifications: lessons from professional pocket pickers. Nat Struct Mol Biol. 2007;14(11):1025–1040. doi: 10.1038/nsmb1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marmorstein R, Trievel RC. Histone modifying enzymes: structures, mechanisms, and specificities. Biochim Biophys Acta. 2009;1789(1):58–68. doi: 10.1016/j.bbagrm.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang K, Dent SY. Histone modifying enzymes and cancer: going beyond histones. J Cell Biochem. 2005;96(6):1137–1148. doi: 10.1002/jcb.20615. [DOI] [PubMed] [Google Scholar]
- 23.Gibbons RJ. Histone modifying and chromatin remodelling enzymes in cancer and dysplastic syndromes. Hum Mol Genet. 2005;14(Spec No 1):R85–92. doi: 10.1093/hmg/ddi106. [DOI] [PubMed] [Google Scholar]
- 24.MacDonald VE, Howe LJ. Histone acetylation: where to go and how to get there. Epigenetics. 2009;4(3):139–143. doi: 10.4161/epi.4.3.8484. [DOI] [PubMed] [Google Scholar]
- 25.Cole PA. Chemical probes for histone-modifying enzymes. Nat Chem Biol. 2008;4(10):590–597. doi: 10.1038/nchembio.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng Y, Thompson PR, Cebrat M, Wang L, Devlin MK, Alani RM, Cole PA. Selective HAT inhibitors as mechanistic tools for protein acetylation. Methods Enzymol. 2004;376:188–199. doi: 10.1016/S0076-6879(03)76012-1. [DOI] [PubMed] [Google Scholar]
- 27.Nakayama J, Rice JC, Strahl BD, Allis CD, Grewal SI. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science. 2001;292(5514):110–113. doi: 10.1126/science.1060118. [DOI] [PubMed] [Google Scholar]
- 28.Litt MD, Simpson M, Gaszner M, Allis CD, Felsenfeld G. Correlation between histone lysine methylation and developmental changes at the chicken beta-globin locus. Science. 2001;293(5539):2453–2455. doi: 10.1126/science.1064413. [DOI] [PubMed] [Google Scholar]
- 29.Lu X, Simon MD, Chodaparambil JV, Hansen JC, Shokat KM, Luger K. The effect of H3K79 dimethylation and H4K20 trimethylation on nucleosome and chromatin structure. Nat Struct Mol Biol. 2008;15(10):1122–1124. doi: 10.1038/nsmb.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papait R, Monti E, Bonapace IM. Novel approaches on epigenetics. Current opinion in drug discovery & development. 2009;12(2):264–275. [PubMed] [Google Scholar]
- 31.Tian X, Fang J. Current perspectives on histone demethylases. Acta biochimica et biophysica Sinica. 2007;39(2):81–88. doi: 10.1111/j.1745-7270.2007.00272.x. [DOI] [PubMed] [Google Scholar]
- 32.Trojer P, Reinberg D. Histone lysine demethylases and their impact on epigenetics. Cell. 2006;125(2):213–217. doi: 10.1016/j.cell.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Zakrzewicz D, Eickelberg O. From arginine methylation to ADMA: a novel mechanism with therapeutic potential in chronic lung diseases. BMC Pulm Med. 2009;9:5. doi: 10.1186/1471-2466-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee YH, Stallcup MR. Minireview: protein arginine methylation of nonhistone proteins in transcriptional regulation. Mol Endocrinol. 2009;23(4):425–433. doi: 10.1210/me.2008-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lan F, Shi Y. Epigenetic regulation: methylation of histone and non-histone proteins. Sci China C Life Sci. 2009;52(4):311–322. doi: 10.1007/s11427-009-0054-z. [DOI] [PubMed] [Google Scholar]
- 36.Cheng X, Collins RE, Zhang X. Structural and sequence motifs of protein (histone) methylation enzymes. Annu Rev Biophys Biomol Struct. 2005;34:267–294. doi: 10.1146/annurev.biophys.34.040204.144452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bedford MT, Clarke SG. Protein arginine methylation in mammals: who, what, and why. Mol Cell. 2009;33(1):1–13. doi: 10.1016/j.molcel.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ng SS, Yue WW, Oppermann U, Klose RJ. Dynamic protein methylation in chromatin biology. Cell Mol Life Sci. 2009;66(3):407–422. doi: 10.1007/s00018-008-8303-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruthenburg AJ, Allis CD, Wysocka J. Methylation of lysine 4 on histone H3: intricacy of writing and reading a single epigenetic mark. Mol Cell. 2007;25(1):15–30. doi: 10.1016/j.molcel.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 40.Spannhoff A, Hauser AT, Heinke R, Sippl W, Jung M. The emerging therapeutic potential of histone methyltransferase and demethylase inhibitors. ChemMedChem. 2009;4(10):1568–1582. doi: 10.1002/cmdc.200900301. [DOI] [PubMed] [Google Scholar]
- 41.Jenuwein T. Re-SET-ting heterochromatin by histone methyltransferases. Trends Cell Biol. 2001;11(6):266–273. doi: 10.1016/s0962-8924(01)02001-3. [DOI] [PubMed] [Google Scholar]
- 42.Santos-Rosa H, Schneider R, Bannister AJ, Sherriff J, Bernstein BE, Emre NC, Schreiber SL, Mellor J, Kouzarides T. Active genes are tri-methylated at K4 of histone H3. Nature. 2002;419(6905):407–411. doi: 10.1038/nature01080. [DOI] [PubMed] [Google Scholar]
- 43.Krivtsov AV, Armstrong SA. MLL translocations, histone modifications and leukaemia stem-cell development. Nat Rev Cancer. 2007;7(11):823–833. doi: 10.1038/nrc2253. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Trojer P, Xu CF, Cheung P, Kuo A, Drury WJ, 3rd, Qiao Q, Neubert TA, Xu RM, Gozani O, Reinberg D. The target of the NSD family of histone lysine methyltransferases depends on the nature of the substrate. J Biol Chem. 2009;284(49):34283–34295. doi: 10.1074/jbc.M109.034462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun XJ, Wei J, Wu XY, Hu M, Wang L, Wang HH, Zhang QH, Chen SJ, Huang QH, Chen Z. Identification and characterization of a novel human histone H3 lysine 36-specific methyltransferase. J Biol Chem. 2005;280(42):35261–35271. doi: 10.1074/jbc.M504012200. [DOI] [PubMed] [Google Scholar]
- 46.Hu M, Sun XJ, Zhang YL, Kuang Y, Hu CQ, Wu WL, Shen SH, Du TT, Li H, He F, Xiao HS, Wang ZG, Liu TX, Lu H, Huang QH, Chen SJ, Chen Z. Histone H3 lysine 36 methyltransferase Hypb/Setd2 is required for embryonic vascular remodeling. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.0915033107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown MA, Sims RJ, 3rd, Gottlieb PD, Tucker PW. Identification and characterization of Smyd2: a split SET/MYND domain-containing histone H3 lysine 36-specific methyltransferase that interacts with the Sin3 histone deacetylase complex. Mol Cancer. 2006;5:26. doi: 10.1186/1476-4598-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson EC, Travers AA. A Drosophila Smyd4 homologue is a muscle-specific transcriptional modulator involved in development. PLoS One. 2008;3(8):e3008. doi: 10.1371/journal.pone.0003008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schotta G, Ebert A, Reuter G. SU(VAR)3-9 is a conserved key function in heterochromatic gene silencing. Genetica. 2003;117(2-3):149–158. doi: 10.1023/a:1022923508198. [DOI] [PubMed] [Google Scholar]
- 50.Tachibana M, Ueda J, Fukuda M, Takeda N, Ohta T, Iwanari H, Sakihama T, Kodama T, Hamakubo T, Shinkai Y. Histone methyltransferases G9a and GLP form heteromeric complexes and are both crucial for methylation of euchromatin at H3-K9. Genes Dev. 2005;19(7):815–826. doi: 10.1101/gad.1284005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang H, An W, Cao R, Xia L, Erdjument-Bromage H, Chatton B, Tempst P, Roeder RG, Zhang Y. mAM facilitates conversion by ESET of dimethyl to trimethyl lysine 9 of histone H3 to cause transcriptional repression. Mol Cell. 2003;12(2):475–487. doi: 10.1016/j.molcel.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 52.Xu PF, Zhu KY, Jin Y, Chen Y, Sun XJ, Deng M, Chen SJ, Chen Z, Liu TX. Setdb2 restricts dorsal organizer territory and regulates left-right asymmetry through suppressing fgf8 activity. Proc Natl Acad Sci U S A. 107(6):2521–2526. doi: 10.1073/pnas.0914396107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hwang C, Giri VN, Wilkinson JC, Wright CW, Wilkinson AS, Cooney KA, Duckett CS. EZH2 regulates the transcription of estrogen-responsive genes through association with REA, an estrogen receptor corepressor. Breast cancer research and treatment. 2008;107(2):235–242. doi: 10.1007/s10549-007-9542-7. [DOI] [PubMed] [Google Scholar]
- 54.Bikoff EK, Morgan MA, Robertson EJ. An expanding job description for Blimp-1 PRDM1. Curr Opin Genet Dev. 2009;19(4):379–385. doi: 10.1016/j.gde.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 55.Rea S, Eisenhaber F, O’Carroll D, Strahl BD, Sun ZW, Schmid M, Opravil S, Mechtler K, Ponting CP, Allis CD, Jenuwein T. Regulation of chromatin structure by site-specific histone H3 methyltransferases. Nature. 2000;406(6796):593–599. doi: 10.1038/35020506. [DOI] [PubMed] [Google Scholar]
- 56.Kim KC, Huang S. Histone methyltransferases in tumor suppression. Cancer Biol Ther. 2003;2(5):491–499. doi: 10.4161/cbt.2.5.629. [DOI] [PubMed] [Google Scholar]
- 57.Yang H, Mizzen CA. The multiple facets of histone H4-lysine 20 methylation. Biochem Cell Biol. 2009;87(1):151–161. doi: 10.1139/O08-131. [DOI] [PubMed] [Google Scholar]
- 58.Singer MS, Kahana A, Wolf AJ, Meisinger LL, Peterson SE, Goggin C, Mahowald M, Gottschling DE. Identification of high-copy disruptors of telomeric silencing in Saccharomyces cerevisiae. Genetics. 1998;150(2):613–632. doi: 10.1093/genetics/150.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gary JD, Clarke S. RNA and protein interactions modulated by protein arginine methylation. Prog Nucleic Acid Res Mol Biol. 1998;61:65–131. doi: 10.1016/s0079-6603(08)60825-9. [DOI] [PubMed] [Google Scholar]
- 60.Pal S, Sif S. Interplay between chromatin remodelers and protein arginine methyltransferases. Journal of cellular physiology. 2007;213(2):306–315. doi: 10.1002/jcp.21180. [DOI] [PubMed] [Google Scholar]
- 61.Lee DY, Teyssier C, Strahl BD, Stallcup MR. Role of protein methylation in regulation of transcription. Endocrine reviews. 2005;26(2):147–170. doi: 10.1210/er.2004-0008. [DOI] [PubMed] [Google Scholar]
- 62.Krause CD, Yang ZH, Kim YS, Lee JH, Cook JR, Pestka S. Protein arginine methyltransferases: evolution and assessment of their pharmacological and therapeutic potential. Pharmacology & therapeutics. 2007;113(1):50–87. doi: 10.1016/j.pharmthera.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 63.Bedford MT, Richard S. Arginine methylation an emerging regulator of protein function. Mol Cell. 2005;18(3):263–272. doi: 10.1016/j.molcel.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 64.Cosgrove MS, Boeke JD, Wolberger C. Regulated nucleosome mobility and the histone code. Nature structural & molecular biology. 2004;11(11):1037–1043. doi: 10.1038/nsmb851. [DOI] [PubMed] [Google Scholar]
- 65.Heinke R, Spannhoff A, Meier R, Trojer P, Bauer I, Jung M, Sippl W. Virtual screening and biological characterization of novel histone arginine methyltransferase PRMT1 inhibitors. ChemMedChem. 2009;4(1):69–77. doi: 10.1002/cmdc.200800301. [DOI] [PubMed] [Google Scholar]
- 66.Cheung N, Chan LC, Thompson A, Cleary ML, So CW. Protein arginine methyltransferase-dependent oncogenesis. Nat Cell Biol. 2007;9(10):1208–1215. doi: 10.1038/ncb1642. [DOI] [PubMed] [Google Scholar]
- 67.Abramovich C, Yakobson B, Chebath J, Revel M. A protein-arginine methyltransferase binds to the intracytoplasmic domain of the IFNAR1 chain in the type I interferon receptor. EMBO J. 1997;16(2):260–266. doi: 10.1093/emboj/16.2.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pawlak MR, Scherer CA, Chen J, Roshon MJ, Ruley HE. Arginine N-methyltransferase 1 is required for early postimplantation mouse development, but cells deficient in the enzyme are viable. Mol Cell Biol. 2000;20(13):4859–4869. doi: 10.1128/mcb.20.13.4859-4869.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu W, Chen H, Du K, Asahara H, Tini M, Emerson BM, Montminy M, Evans RM. A transcriptional switch mediated by cofactor methylation. Science. 2001;294(5551):2507–2511. doi: 10.1126/science.1065961. [DOI] [PubMed] [Google Scholar]
- 70.Branscombe TL, Frankel A, Lee JH, Cook JR, Yang Z, Pestka S, Clarke S. PRMT5 (Janus kinase-binding protein 1) catalyzes the formation of symmetric dimethylarginine residues in proteins. J Biol Chem. 2001;276(35):32971–32976. doi: 10.1074/jbc.M105412200. [DOI] [PubMed] [Google Scholar]
- 71.Pal S, Vishwanath SN, Erdjument-Bromage H, Tempst P, Sif S. Human SWI/SNF-associated PRMT5 methylates histone H3 arginine 8 and negatively regulates expression of ST7 and NM23 tumor suppressor genes. Mol Cell Biol. 2004;24(21):9630–9645. doi: 10.1128/MCB.24.21.9630-9645.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boulanger MC, Liang C, Russell RS, Lin R, Bedford MT, Wainberg MA, Richard S. Methylation of Tat by PRMT6 regulates human immunodeficiency virus type 1 gene expression. J Virol. 2005;79(1):124–131. doi: 10.1128/JVI.79.1.124-131.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sgarra R, Lee J, Tessari MA, Altamura S, Spolaore B, Giancotti V, Bedford MT, Manfioletti G. The AT-hook of the chromatin architectural transcription factor high mobility group A1a is arginine-methylated by protein arginine methyltransferase-6. J Biol Chem. 2006;281(7):3764–3772. doi: 10.1074/jbc.M510231200. [DOI] [PubMed] [Google Scholar]
- 74.Gros L, Delaporte C, Frey S, Decesse J, de Saint-Vincent BR, Cavarec L, Dubart A, Gudkov AV, Jacquemin-Sablon A. Identification of new drug sensitivity genes using genetic suppressor elements: protein arginine N-methyltransferase mediates cell sensitivity to DNA-damaging agents. Cancer Res. 2003;63(1):164–171. [PubMed] [Google Scholar]
- 75.Lee J, Sayegh J, Daniel J, Clarke S, Bedford MT. PRMT8, a new membrane-bound tissue-specific member of the protein arginine methyltransferase family. J Biol Chem. 2005;280(38):32890–32896. doi: 10.1074/jbc.M506944200. [DOI] [PubMed] [Google Scholar]
- 76.Shi Y, Whetstine JR. Dynamic regulation of histone lysine methylation by demethylases. Mol Cell. 2007;25(1):1–14. doi: 10.1016/j.molcel.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 77.Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119(7):941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 78.Karytinos A, Forneris F, Profumo A, Ciossani G, Battaglioli E, Binda C, Mattevi A. A novel mammalian flavin-dependent histone demethylase. The Journal of biological chemistry. 2009;284(26):17775–17782. doi: 10.1074/jbc.M109.003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ciccone DN, Su H, Hevi S, Gay F, Lei H, Bajko J, Xu G, Li E, Chen T. KDM1B is a histone H3K4 demethylase required to establish maternal genomic imprints. Nature. 2009;461(7262):415–418. doi: 10.1038/nature08315. [DOI] [PubMed] [Google Scholar]
- 80.Tsukada Y, Fang J, Erdjument-Bromage H, Warren ME, Borchers CH, Tempst P, Zhang Y. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439(7078):811–816. doi: 10.1038/nature04433. [DOI] [PubMed] [Google Scholar]
- 81.Joshi AA, Struhl K. Eaf3 chromodomain interaction with methylated H3-K36 links histone deacetylation to Pol II elongation. Mol Cell. 2005;20(6):971–978. doi: 10.1016/j.molcel.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 82.Yamane K, Toumazou C, Tsukada Y, Erdjument-Bromage H, Tempst P, Wong J, Zhang Y. JHDM2A, a JmjC-containing H3K9 demethylase, facilitates transcription activation by androgen receptor. Cell. 2006;125(3):483–495. doi: 10.1016/j.cell.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 83.Cloos PA, Christensen J, Agger K, Maiolica A, Rappsilber J, Antal T, Hansen KH, Helin K. The putative oncogene GASC1 demethylates tri- and dimethylated lysine 9 on histone H3. Nature. 2006;442(7100):307–311. doi: 10.1038/nature04837. [DOI] [PubMed] [Google Scholar]
- 84.De Santa F, Totaro MG, Prosperini E, Notarbartolo S, Testa G, Natoli G. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell. 2007;130(6):1083–1094. doi: 10.1016/j.cell.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 85.Yamane K, Tateishi K, Klose RJ, Fang J, Fabrizio LA, Erdjument-Bromage H, Taylor-Papadimitriou J, Tempst P, Zhang Y. PLU-1 is an H3K4 demethylase involved in transcriptional repression and breast cancer cell proliferation. Mol Cell. 2007;25(6):801–812. doi: 10.1016/j.molcel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 86.Santos C, Rodriguez-Revenga L, Madrigal I, Badenas C, Pineda M, Mila M. A novel mutation in JARID1C gene associated with mental retardation. Eur J Hum Genet. 2006;14(5):583–586. doi: 10.1038/sj.ejhg.5201608. [DOI] [PubMed] [Google Scholar]
- 87.Lee Y, Song AJ, Baker R, Micales B, Conway SJ, Lyons GE. Jumonji, a nuclear protein that is necessary for normal heart development. Circ Res. 2000;86(9):932–938. doi: 10.1161/01.res.86.9.932. [DOI] [PubMed] [Google Scholar]
- 88.Horton JR, Upadhyay AK, Qi HH, Zhang X, Shi Y, Cheng X. Enzymatic and structural insights for substrate specificity of a family of jumonji histone lysine demethylases. Nat Struct Mol Biol. 17(1):38–43. doi: 10.1038/nsmb.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang C, Xiang Y, Wang Y, Li X, Xu L, Zhu Z, Zhang T, Zhu Q, Zhang K, Jing N, Chen CD. Dual-specificity histone demethylase KIAA1718 (KDM7A) regulates neural differentiation through FGF4. Cell Res. 20(2):154–165. doi: 10.1038/cr.2010.5. [DOI] [PubMed] [Google Scholar]
- 90.Chang B, Chen Y, Zhao Y, Bruick RK. JMJD6 is a histone arginine demethylase. Science. 2007;318(5849):444–447. doi: 10.1126/science.1145801. [DOI] [PubMed] [Google Scholar]
- 91.Webby CJ, Wolf A, Gromak N, Dreger M, Kramer H, Kessler B, Nielsen ML, Schmitz C, Butler DS, Yates JR, 3rd, Delahunty CM, Hahn P, Lengeling A, Mann M, Proudfoot NJ, Schofield CJ, Bottger A. Jmjd6 catalyses lysyl-hydroxylation of U2AF65, a protein associated with RNA splicing. Science. 2009;325(5936):90–93. doi: 10.1126/science.1175865. [DOI] [PubMed] [Google Scholar]
- 92.Bernstein BE, Humphrey EL, Erlich RL, Schneider R, Bouman P, Liu JS, Kouzarides T, Schreiber SL. Methylation of histone H3 Lys 4 in coding regions of active genes. Proc Natl Acad Sci U S A. 2002;99(13):8695–8700. doi: 10.1073/pnas.082249499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yuan GC, Liu YJ, Dion MF, Slack MD, Wu LF, Altschuler SJ, Rando OJ. Genome-scale identification of nucleosome positions in S. cerevisiae. Science. 2005;309(5734):626–630. doi: 10.1126/science.1112178. [DOI] [PubMed] [Google Scholar]
- 94.Liu CL, Kaplan T, Kim M, Buratowski S, Schreiber SL, Friedman N, Rando OJ. Single-nucleosome mapping of histone modifications in S. cerevisiae. PLoS Biol. 2005;3(10):e328. doi: 10.1371/journal.pbio.0030328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pokholok DK, Harbison CT, Levine S, Cole M, Hannett NM, Lee TI, Bell GW, Walker K, Rolfe PA, Herbolsheimer E, Zeitlinger J, Lewitter F, Gifford DK, Young RA. Genome-wide map of nucleosome acetylation and methylation in yeast. Cell. 2005;122(4):517–527. doi: 10.1016/j.cell.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 96.Umlauf D, Goto Y, Feil R. Site-specific analysis of histone methylation and acetylation. Methods Mol Biol. 2004;287:99–120. doi: 10.1385/1-59259-828-5:099. [DOI] [PubMed] [Google Scholar]
- 97.Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M. Genome-wide analysis of estrogen receptor binding sites. Nat Genet. 2006;38(11):1289–1297. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- 98.Bernstein BE. Methylation of histone H3 Lys 4 in coding regions of active genes. Proc Natl Acad Sci USA. 2002;99:8695–8700. doi: 10.1073/pnas.082249499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Robyr D. Microarray deacetylation maps determine genome-wide functions for yeast histone deacetylases. Cell. 2002;109:437–446. doi: 10.1016/s0092-8674(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 100.Schubeler D. The histone modification pattern of active genes revealed through genome-wide chromatin analysis of a higher eukaryote. Genes Dev. 2004;18:1263–1271. doi: 10.1101/gad.1198204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Koch CM. The landscape of histone modifications across 1% of the human genome in five human cell lines. Genome Res. 2007;17:691–707. doi: 10.1101/gr.5704207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barski A. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 103.Mikkelsen TS. Genome-wide maps of chromatin state in pluripotent and lineage-committed cells. Nature. 2007;448:553–560. doi: 10.1038/nature06008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mardis ER. ChIP-seq: welcome to the new frontier. Nat Methods. 2007;4(8):613–614. doi: 10.1038/nmeth0807-613. [DOI] [PubMed] [Google Scholar]
- 105.Liu XS. Getting started in tiling microarray analysis. PLoS Comput Biol. 2007;3:1842–1844. doi: 10.1371/journal.pcbi.0030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ji H, Wong WH. TileMap: create chromosomal map of tiling array hybridizations. Bioinformatics. 2005;21:3629–3636. doi: 10.1093/bioinformatics/bti593. [DOI] [PubMed] [Google Scholar]
- 107.Johnson WE. Model-based analysis of tiling-arrays for ChIP-chip. Proc Natl Acad Sci USA. 2006;103:12457–12462. doi: 10.1073/pnas.0601180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smith AD, Xuan Z, Zhang MQ. Using quality scores and longer reads improves accuracy of Solexa read mapping. BMC Bioinformatics. 2008;9:128. doi: 10.1186/1471-2105-9-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. The human genome browser at UCSC. Genome Res. 2002;12(6):996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ji X, Li W, Song J, Wei L, Liu XS. CEAS: cis-regulatory element annotation system. Nucleic Acids Res. 2006;34(Web Server issue):W551–554. doi: 10.1093/nar/gkl322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hodges E. Genome-wide in situ exon capture for selective resequencing. Nature Genet. 2007;39:1522–1527. doi: 10.1038/ng.2007.42. [DOI] [PubMed] [Google Scholar]
- 112.Freitas MA, Sklenar AR, Parthun MR. Application of mass spectrometry to the identification and quantification of histone post-translational modifications. Journal of cellular biochemistry. 2004;92(4):691–700. doi: 10.1002/jcb.20106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bonaldi T, Imhof A, Regula JT. A combination of different mass spectroscopic techniques for the analysis of dynamic changes of histone modifications. Proteomics. 2004;4(5):1382–1396. doi: 10.1002/pmic.200300743. [DOI] [PubMed] [Google Scholar]
- 114.Su XD, Ren C, Freitas MA. Mass spectrometry-based strategies for characterization of histones and their post-translational modifications. Expert review of proteomics. 2007;4(2):211–225. doi: 10.1586/14789450.4.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Garcia BA, Shabanowitz J, Hunt DF. Characterization of histones and their post-translational modifications by mass spectrometry. Curr Opin Chem Biol. 2007;11(1):66–73. doi: 10.1016/j.cbpa.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 116.Brumbaugh J, Phanstiel D, Coon JJ. Unraveling the histone’s potential: a proteomics perspective. Epigenetics. 2008;3(5):254–257. doi: 10.4161/epi.3.5.7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang K, Tang H, Huang L, Blankenship JW, Jones PR, Xiang F, Yau PM, Burlingame AL. Identification of acetylation and methylation sites of histone H3 from chicken erythrocytes by high-accuracy matrix-assisted laser desorption ionization-time-of-flight, matrix-assisted laser desorption ionization-postsource decay, and nanoelectrospray ionization tandem mass spectrometry. Anal Biochem. 2002;306(2):259–269. doi: 10.1006/abio.2002.5719. [DOI] [PubMed] [Google Scholar]
- 118.Bergmuller E, Gehrig PM, Gruissem W. Characterization of post-translational modifications of histone H2B-variants isolated from Arabidopsis thaliana. Journal of proteome research. 2007;6(9):3655–3668. doi: 10.1021/pr0702159. [DOI] [PubMed] [Google Scholar]
- 119.Johnson L, Mollah S, Garcia BA, Muratore TL, Shabanowitz J, Hunt DF, Jacobsen SE. Mass spectrometry analysis of Arabidopsis histone H3 reveals distinct combinations of post-translational modifications. Nucleic acids research. 2004;32(22):6511–6518. doi: 10.1093/nar/gkh992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang K, Williams KE, Huang L, Yau P, Siino JS, Bradbury EM, Jones PR, Minch MJ, Burlingame AL. Histone acetylation and deacetylation: identification of acetylation and methylation sites of HeLa histone H4 by mass spectrometry. Mol Cell Proteomics. 2002;1(7):500–508. doi: 10.1074/mcp.m200031-mcp200. [DOI] [PubMed] [Google Scholar]
- 121.Pesavento JJ, Kim YB, Taylor GK, Kelleher NL. Shotgun annotation of histone modifications: a new approach for streamlined characterization of proteins by top down mass spectrometry. J Am Chem Soc. 2004;126(11):3386–3387. doi: 10.1021/ja039748i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Thomas CE, Kelleher NL, Mizzen CA. Mass spectrometric characterization of human histone H3: a bird’s eye view. J Proteome Res. 2006;5(2):240–247. doi: 10.1021/pr050266a. [DOI] [PubMed] [Google Scholar]
- 123.Wells JM, McLuckey SA. Collision-induced dissociation (CID) of peptides and proteins. Methods Enzymol. 2005;402:148–185. doi: 10.1016/S0076-6879(05)02005-7. [DOI] [PubMed] [Google Scholar]
- 124.Sleno L, Volmer DA. Ion activation methods for tandem mass spectrometry. J Mass Spectrom. 2004;39(10):1091–1112. doi: 10.1002/jms.703. [DOI] [PubMed] [Google Scholar]
- 125.Zubarev RA, Horn DM, Fridriksson EK, Kelleher NL, Kruger NA, Lewis MA, Carpenter BK, McLafferty FW. Electron capture dissociation for structural characterization of multiply charged protein cations. Analytical chemistry. 2000;72(3):563–573. doi: 10.1021/ac990811p. [DOI] [PubMed] [Google Scholar]
- 126.Coon JJ, Ueberheide B, Syka JE, Dryhurst DD, Ausio J, Shabanowitz J, Hunt DF. Protein identification using sequential ion/ion reactions and tandem mass spectrometry. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(27):9463–9468. doi: 10.1073/pnas.0503189102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mikesh LM, Ueberheide B, Chi A, Coon JJ, Syka JE, Shabanowitz J, Hunt DF. The utility of ETD mass spectrometry in proteomic analysis. Biochimica et biophysica acta. 2006;1764(12):1811–1822. doi: 10.1016/j.bbapap.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Little DP, Speir JP, Senko MW, O’Connor PB, McLafferty FW. Infrared multiphoton dissociation of large multiply charged ions for biomolecule sequencing. Anal Chem. 1994;66(18):2809–2815. doi: 10.1021/ac00090a004. [DOI] [PubMed] [Google Scholar]
- 129.Cornish TJ, Cotter RJ. Tandem time-of-flight mass spectrometer. Anal Chem. 1993;65(8):1043–1047. doi: 10.1021/ac00056a017. [DOI] [PubMed] [Google Scholar]
- 130.Chu F, Nusinow DA, Chalkley RJ, Plath K, Panning B, Burlingame AL. Mapping post-translational modifications of the histone variant MacroH2A1 using tandem mass spectrometry. Mol Cell Proteomics. 2006;5(1):194–203. doi: 10.1074/mcp.M500285-MCP200. [DOI] [PubMed] [Google Scholar]
- 131.Wisniewski JR, Zougman A, Kruger S, Mann M. Mass spectrometric mapping of linker histone H1 variants reveals multiple acetylations, methylations, and phosphorylation as well as differences between cell culture and tissue. Mol Cell Proteomics. 2007;6(1):72–87. doi: 10.1074/mcp.M600255-MCP200. [DOI] [PubMed] [Google Scholar]
- 132.Lu A, Zougman A, Pudelko M, Bebenek M, Ziolkowski P, Mann M, Wisniewski JR. Mapping of lysine monomethylation of linker histones in human breast and its cancer. J Proteome Res. 2009;8(9):4207–4215. doi: 10.1021/pr9000652. [DOI] [PubMed] [Google Scholar]
- 133.Jung HR, Pasini D, Helin K, Jensen ON. Quantitative mass spectrometry of histone H3.2 and H3.3 in Suz12 deficient mouse ES cells reveals distinct, dynamic post-translational modifications at K27 and K36. Mol Cell Proteomics. doi: 10.1074/mcp.M900489-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wu T, Yuan TZ, Tsai SN, Wang CM, Sun SM, Lam HM, Ngai SM. Mass spectrometry analysis of the variants of histone H3 and H4 of soybean and their post-translational modifications. Bmc Plant Biology. 2009;9 doi: 10.1186/1471-2229-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dormeyer W, Ott M, Schnolzer M. Probing lysine acetylation in proteins: strategies, limitations, and pitfalls of in vitro acetyltransferase assays. Mol Cell Proteomics. 2005;4(9):1226–1239. doi: 10.1074/mcp.M500047-MCP200. [DOI] [PubMed] [Google Scholar]
- 136.Biemann K. Appendix 5. Nomenclature for peptide fragment ions (positive ions) Methods Enzymol. 1990;193:886–887. doi: 10.1016/0076-6879(90)93460-3. [DOI] [PubMed] [Google Scholar]
- 137.Zhang K, Yau PM, Chandrasekhar B, New R, Kondrat R, Imai BS, Bradbury ME. Differentiation between peptides containing acetylated or tri-methylated lysines by mass spectrometry: an application for determining lysine 9 acetylation and methylation of histone H3. Proteomics. 2004;4(1):1–10. doi: 10.1002/pmic.200300503. [DOI] [PubMed] [Google Scholar]
- 138.Trelle MB, Salcedo-Amaya AM, Cohen AM, Stunnenberg HG, Jensen ON. Global histone analysis by mass spectrometry reveals a high content of acetylated lysine residues in the malaria parasite Plasmodium falciparum. Journal of proteome research. 2009;8(7):3439–3450. doi: 10.1021/pr9000898. [DOI] [PubMed] [Google Scholar]
- 139.Wilm M, Neubauer G, Mann M. Parent ion scans of unseparated peptide mixtures. Anal Chem. 1996;68(3):527–533. doi: 10.1021/ac950875+. [DOI] [PubMed] [Google Scholar]
- 140.Couttas TA, Raftery MJ, Bernardini G, Wilkins MR. Immonium ion scanning for the discovery of post-translational modifications and its application to histones. J Proteome Res. 2008;7(7):2632–2641. doi: 10.1021/pr700644t. [DOI] [PubMed] [Google Scholar]
- 141.McLafferty FW, Kelleher NL, Begley TP, Fridriksson EK, Zubarev RA, Horn DM. Two-dimensional mass spectrometry of biomolecules at the subfemtomole level. Curr Opin Chem Biol. 1998;2(5):571–578. doi: 10.1016/s1367-5931(98)80085-9. [DOI] [PubMed] [Google Scholar]
- 142.Kelleher NL. Top-down proteomics. Anal Chem. 2004;76(11):197A–203A. [PubMed] [Google Scholar]
- 143.Siuti N, Kelleher NL. Decoding protein modifications using top-down mass spectrometry. Nat Methods. 2007;4(10):817–821. doi: 10.1038/nmeth1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chait BT. Chemistry. Mass spectrometry: bottom-up or top-down? Science. 2006;314(5796):65–66. doi: 10.1126/science.1133987. [DOI] [PubMed] [Google Scholar]
- 145.Meng F, Forbes AJ, Miller LM, Kelleher NL. Detection and localization of protein modifications by high resolution tandem mass spectrometry. Mass Spectrom Rev. 2005;24(2):126–134. doi: 10.1002/mas.20009. [DOI] [PubMed] [Google Scholar]
- 146.Garcia BA, Mollah S, Ueberheide BM, Busby SA, Muratore TL, Shabanowitz J, Hunt DF. Chemical derivatization of histones for facilitated analysis by mass spectrometry. Nat Protoc. 2007;2(4):933–938. doi: 10.1038/nprot.2007.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Alfageme CR, Zweidler A, Mahowald A, Cohen LH. Histones of Drosophila embryos. Electrophoretic isolation and structural studies. The Journal of biological chemistry. 1974;249(12):3729–3736. [PubMed] [Google Scholar]
- 148.Medzihradszky KF, Zhang X, Chalkley RJ, Guan S, McFarland MA, Chalmers MJ, Marshall AG, Diaz RL, Allis CD, Burlingame AL. Characterization of Tetrahymena histone H2B variants and posttranslational populations by electron capture dissociation (ECD) Fourier transform ion cyclotron mass spectrometry (FT-ICR MS) Mol Cell Proteomics. 2004;3(9):872–886. doi: 10.1074/mcp.M400041-MCP200. [DOI] [PubMed] [Google Scholar]
- 149.Pesavento JJ, Garcia BA, Streeky JA, Kelleher NL, Mizzen CA. Mild performic acid oxidation enhances chromatographic and top down mass spectrometric analyses of histones. Mol Cell Proteomics. 2007;6(9):1510–1526. doi: 10.1074/mcp.M600404-MCP200. [DOI] [PubMed] [Google Scholar]
- 150.Pesavento JJ, Yang H, Kelleher NL, Mizzen CA. Certain and progressive methylation of histone H4 at lysine 20 during the cell cycle. Mol Cell Biol. 2008;28(1):468–486. doi: 10.1128/MCB.01517-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Phanstiel D, Brumbaugh J, Berggren WT, Conard K, Feng X, Levenstein ME, McAlister GC, Thomson JA, Coon JJ. Mass spectrometry identifies and quantifies 74 unique histone H4 isoforms in differentiating human embryonic stem cells. Proc Natl Acad Sci U S A. 2008;105(11):4093–4098. doi: 10.1073/pnas.0710515105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Siuti N, Kelleher NL. Efficient readout of posttranslational codes on the 50-residue tail of histone H3 by high-resolution MS/MS. Anal Biochem. 396(2):180–187. doi: 10.1016/j.ab.2009.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nicklay JJ, Shechter D, Chitta RK, Garcia BA, Shabanowitz J, Allis CD, Hunt DF. Analysis of histones in Xenopus laevis. II. mass spectrometry reveals an index of cell type-specific modifications on H3 and H4. J Biol Chem. 2009;284(2):1075–1085. doi: 10.1074/jbc.M807274200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Garcia BA. What does the future hold for Top Down mass spectrometry? J Am Soc Mass Spectrom. 21(2):193–202. doi: 10.1016/j.jasms.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 155.Zhang L, Eugeni EE, Parthun MR, Freitas MA. Identification of novel histone post-translational modifications by peptide mass fingerprinting. Chromosoma. 2003;112(2):77–86. doi: 10.1007/s00412-003-0244-6. [DOI] [PubMed] [Google Scholar]
- 156.Syka JE, Marto JA, Bai DL, Horning S, Senko MW, Schwartz JC, Ueberheide B, Garcia B, Busby S, Muratore T, Shabanowitz J, Hunt DF. Novel linear quadrupole ion trap/FT mass spectrometer: performance characterization and use in the comparative analysis of histone H3 post-translational modifications. J Proteome Res. 2004;3(3):621–626. doi: 10.1021/pr0499794. [DOI] [PubMed] [Google Scholar]
- 157.Hake SB, Garcia BA, Duncan EM, Kauer M, Dellaire G, Shabanowitz J, Bazett-Jones DP, Allis CD, Hunt DF. Expression patterns and post-translational modifications associated with mammalian histone H3 variants. J Biol Chem. 2006;281(1):559–568. doi: 10.1074/jbc.M509266200. [DOI] [PubMed] [Google Scholar]
- 158.Smith CM, Gafken PR, Zhang Z, Gottschling DE, Smith JB, Smith DL. Mass spectrometric quantification of acetylation at specific lysines within the amino-terminal tail of histone H4. Anal Biochem. 2003;316(1):23–33. doi: 10.1016/s0003-2697(03)00032-0. [DOI] [PubMed] [Google Scholar]
- 159.Ong SE, Mittler G, Mann M. Identifying and quantifying in vivo methylation sites by heavy methyl SILAC. Nat Methods. 2004;1(2):119–126. doi: 10.1038/nmeth715. [DOI] [PubMed] [Google Scholar]
- 160.Yang L, Tu S, Ren C, Bulloch EM, Liao CL, Tsai MD, Freitas MA. Unambiguous determination of isobaric histone modifications by reversed-phase retention time and high-mass accuracy. Anal Biochem. 396(1):13–22. doi: 10.1016/j.ab.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Su X, Ren C, Freitas MA. Mass spectrometry-based strategies for characterization of histones and their post-translational modifications. Expert Rev Proteomics. 2007;4(2):211–225. doi: 10.1586/14789450.4.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Burlingame AL, Zhang X, Chalkley RJ. Mass spectrometric analysis of histone posttranslational modifications. Methods. 2005;36(4):383–394. doi: 10.1016/j.ymeth.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 163.Pesavento JJ, Bullock CR, LeDuc RD, Mizzen CA, Kelleher NL. Combinatorial modification of human histone H4 quantitated by two-dimensional liquid chromatography coupled with top down mass spectrometry. The Journal of biological chemistry. 2008;283(22):14927–14937. doi: 10.1074/jbc.M709796200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Johnson L, Mollah S, Garcia BA, Muratore TL, Shabanowitz J, Hunt DF, Jacobsen SE. Mass spectrometry analysis of Arabidopsis histone H3 reveals distinct combinations of post-translational modifications. Nucleic Acids Res. 2004;32(22):6511–6518. doi: 10.1093/nar/gkh992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Plazas-Mayorca MD, Zee BM, Young NL, Fingerman IM, LeRoy G, Briggs SD, Garcia BA. One-pot shotgun quantitative mass spectrometry characterization of histones. J Proteome Res. 2009;8(11):5367–5374. doi: 10.1021/pr900777e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, Mann M. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics. 2002;1(5):376–386. doi: 10.1074/mcp.m200025-mcp200. [DOI] [PubMed] [Google Scholar]
- 167.Zhu H, Pan S, Gu S, Bradbury EM, Chen X. Amino acid residue specific stable isotope labeling for quantitative proteomics. Rapid Commun Mass Spectrom. 2002;16(22):2115–2123. doi: 10.1002/rcm.831. [DOI] [PubMed] [Google Scholar]
- 168.Pimienta G, Chaerkady R, Pandey A. SILAC for global phosphoproteomic analysis. Methods Mol Biol. 2009;527:107–116. x. doi: 10.1007/978-1-60327-834-8_9. [DOI] [PubMed] [Google Scholar]
- 169.Zhang G, Neubert TA. Use of stable isotope labeling by amino acids in cell culture (SILAC) for phosphotyrosine protein identification and quantitation. Methods Mol Biol. 2009;527:79–92. xi. doi: 10.1007/978-1-60327-834-8_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Knapp AR, Ren C, Su X, Lucas DM, Byrd JC, Freitas MA, Parthun MR. Quantitative profiling of histone post-translational modifications by stable isotope labeling. Methods. 2007;41(3):312–319. doi: 10.1016/j.ymeth.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Scharf AN, Barth TK, Imhof A. Establishment of histone modifications after chromatin assembly. Nucleic Acids Res. 2009;37(15):5032–5040. doi: 10.1093/nar/gkp518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zee BM, Levin RS, Xu B, LeRoy G, Wingreen NS, Garcia BA. In vivo residue-specific histone methylation dynamics. J Biol Chem. 285(5):3341–3350. doi: 10.1074/jbc.M109.063784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bonenfant D, Towbin H, Coulot M, Schindler P, Mueller DR, van Oostrum J. Analysis of dynamic changes in post-translational modifications of human histones during cell cycle by mass spectrometry. Mol Cell Proteomics. 2007;6(11):1917–1932. doi: 10.1074/mcp.M700070-MCP200. [DOI] [PubMed] [Google Scholar]
- 174.Schwanhausser B, Gossen M, Dittmar G, Selbach M. Global analysis of cellular protein translation by pulsed SILAC. Proteomics. 2009;9(1):205–209. doi: 10.1002/pmic.200800275. [DOI] [PubMed] [Google Scholar]
- 175.Fodor BD, Kubicek S, Yonezawa M, O’Sullivan RJ, Sengupta R, Perez-Burgos L, Opravil S, Mechtler K, Schotta G, Jenuwein T. Jmjd2b antagonizes H3K9 trimethylation at pericentric heterochromatin in mammalian cells. Genes Dev. 2006;20(12):1557–1562. doi: 10.1101/gad.388206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Garcia BA, Thomas CE, Kelleher NL, Mizzen CA. Tissue-specific expression and post-translational modification of histone H3 variants. Journal of proteome research. 2008;7(10):4225–4236. doi: 10.1021/pr800044q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Garcia BA, Pesavento JJ, Mizzen CA, Kelleher NL. Pervasive combinatorial modification of histone H3 in human cells. Nature methods. 2007;4(6):487–489. doi: 10.1038/nmeth1052. [DOI] [PubMed] [Google Scholar]
- 178.Cocklin RR, Wang M. Identification of methylation and acetylation sites on mouse histone H3 using matrix-assisted laser desorption/ionization time-of-flight and nanoelectrospray ionization tandem mass spectrometry. Journal of protein chemistry. 2003;22(4):327–334. doi: 10.1023/a:1025334006014. [DOI] [PubMed] [Google Scholar]
- 179.Beck HC, Nielsen EC, Matthiesen R, Jensen LH, Sehested M, Finn P, Grauslund M, Hansen AM, Jensen ON. Quantitative proteomic analysis of post-translational modifications of human histones. Mol Cell Proteomics. 2006;5(7):1314–1325. doi: 10.1074/mcp.M600007-MCP200. [DOI] [PubMed] [Google Scholar]
- 180.Huyen Y, Zgheib O, Ditullio RA, Jr., Gorgoulis VG, Zacharatos P, Petty TJ, Sheston EA, Mellert HS, Stavridi ES, Halazonetis TD. Methylated lysine 79 of histone H3 targets 53BP1 to DNA double-strand breaks. Nature. 2004;432(7015):406–411. doi: 10.1038/nature03114. [DOI] [PubMed] [Google Scholar]
- 181.Robinson PJ, An W, Routh A, Martino F, Chapman L, Roeder RG, Rhodes D. 30 nm chromatin fibre decompaction requires both H4-K16 acetylation and linker histone eviction. Journal of molecular biology. 2008;381(4):816–825. doi: 10.1016/j.jmb.2008.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bhatia M, Thompson PR. Methyllysine analogs: rewriting the code. Nature chemical biology. 2007;3(5):249–250. doi: 10.1038/nchembio0507-249. [DOI] [PubMed] [Google Scholar]
- 183.Simon MD, Chu F, Racki LR, de la Cruz CC, Burlingame AL, Panning B, Narlikar GJ, Shokat KM. The site-specific installation of methyl-lysine analogs into recombinant histones. Cell. 2007;128(5):1003–1012. doi: 10.1016/j.cell.2006.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Guo J, Wang J, Lee JS, Schultz PG. Site-specific incorporation of methyl- and acetyl-lysine analogues into recombinant proteins. Angew Chem Int Ed Engl. 2008;47(34):6399–6401. doi: 10.1002/anie.200802336. [DOI] [PubMed] [Google Scholar]
- 185.Gellman SH. On the role of methionine residues in the sequence-independent recognition of nonpolar protein surfaces. Biochemistry. 1991;30(27):6633–6636. doi: 10.1021/bi00241a001. [DOI] [PubMed] [Google Scholar]
- 186.Gloss LM, Kirsch JF. Decreasing the basicity of the active site base, Lys-258, of Escherichia coli aspartate aminotransferase by replacement with gamma-thialysine. Biochemistry. 1995;34(12):3990–3998. doi: 10.1021/bi00012a017. [DOI] [PubMed] [Google Scholar]
- 187.Nguyen DP, Garcia Alai MM, Kapadnis PB, Neumann H, Chin JW. Genetically encoding N(epsilon)-methyl-L-lysine in recombinant histones. J Am Chem Soc. 2009;131(40):14194–14195. doi: 10.1021/ja906603s. [DOI] [PubMed] [Google Scholar]
- 188.Dawson PE, Muir TW, Clark-Lewis I, Kent SB. Synthesis of proteins by native chemical ligation. Science. 1994;266(5186):776–779. doi: 10.1126/science.7973629. [DOI] [PubMed] [Google Scholar]
- 189.He S, Bauman D, Davis JS, Loyola A, Nishioka K, Gronlund JL, Reinberg D, Meng F, Kelleher N, McCafferty DG. Facile synthesis of site-specifically acetylated and methylated histone proteins: reagents for evaluation of the histone code hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(21):12033–12038. doi: 10.1073/pnas.2035256100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shogren-Knaak M, Ishii H, Sun JM, Pazin MJ, Davie JR, Peterson CL. Histone H4-K16 acetylation controls chromatin structure and protein interactions. Science. 2006;311(5762):844–847. doi: 10.1126/science.1124000. [DOI] [PubMed] [Google Scholar]
- 191.Shogren-Knaak MA, Fry CJ, Peterson CL. A native peptide ligation strategy for deciphering nucleosomal histone modifications. The Journal of biological chemistry. 2003;278(18):15744–15748. doi: 10.1074/jbc.M301445200. [DOI] [PubMed] [Google Scholar]
- 192.Muir TW, Sondhi D, Cole PA. Expressed protein ligation: a general method for protein engineering. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(12):6705–6710. doi: 10.1073/pnas.95.12.6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Evans TC, Jr., Benner J, Xu MQ. Semisynthesis of cytotoxic proteins using a modified protein splicing element. Protein Sci. 1998;7(11):2256–2264. doi: 10.1002/pro.5560071103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Muralidharan V, Muir TW. Protein ligation: an enabling technology for the biophysical analysis of proteins. Nature methods. 2006;3(6):429–438. doi: 10.1038/nmeth886. [DOI] [PubMed] [Google Scholar]
- 195.Berrade L, Camarero JA. Expressed protein ligation: a resourceful tool to study protein structure and function. Cell Mol Life Sci. 2009;66(24):3909–3922. doi: 10.1007/s00018-009-0122-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Schurter BT, Koh SS, Chen D, Bunick GJ, Harp JM, Hanson BL, Henschen-Edman A, Mackay DR, Stallcup MR, Aswad DW. Methylation of histone H3 by coactivator-associated arginine methyltransferase 1. Biochemistry. 2001;40(19):5747–5756. doi: 10.1021/bi002631b. [DOI] [PubMed] [Google Scholar]
- 197.Manohar M, Mooney AM, North JA, Nakkula RJ, Picking JW, Edon A, Fishel R, Poirier MG, Ottesen JJ. Acetylation of histone H3 at the nucleosome dyad alters DNA-histone binding. The Journal of biological chemistry. 2009;284(35):23312–23321. doi: 10.1074/jbc.M109.003202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bang D, Chopra N, Kent SB. Total chemical synthesis of crambin. J Am Chem Soc. 2004;126(5):1377–1383. doi: 10.1021/ja0385078. [DOI] [PubMed] [Google Scholar]
- 199.Bang D, Makhatadze GI, Tereshko V, Kossiakoff AA, Kent SB. Total chemical synthesis and X-ray crystal structure of a protein diastereomer: [D-Gln 35]ubiquitin. Angew Chem Int Ed Engl. 2005;44(25):3852–3856. doi: 10.1002/anie.200463040. [DOI] [PubMed] [Google Scholar]
- 200.Johnson EC, Malito E, Shen Y, Rich D, Tang WJ, Kent SB. Modular total chemical synthesis of a human immunodeficiency virus type 1 protease. J Am Chem Soc. 2007;129(37):11480–11490. doi: 10.1021/ja072870n. [DOI] [PubMed] [Google Scholar]
- 201.Crich D, Banerjee A. Native chemical ligation at phenylalanine. J Am Chem Soc. 2007;129(33):10064–10065. doi: 10.1021/ja072804l. [DOI] [PubMed] [Google Scholar]
- 202.Marinzi C, Offer J, Longhi R, Dawson PE. An o-nitrobenzyl scaffold for peptide ligation: synthesis and applications. Bioorg Med Chem. 2004;12(10):2749–2757. doi: 10.1016/j.bmc.2004.02.039. [DOI] [PubMed] [Google Scholar]
- 203.Marinzi C, Bark SJ, Offer J, Dawson PE. A new scaffold for amide ligation. Bioorg Med Chem. 2001;9(9):2323–2328. doi: 10.1016/s0968-0896(01)00136-5. [DOI] [PubMed] [Google Scholar]
- 204.Yan LZ, Dawson PE. Synthesis of peptides and proteins without cysteine residues by native chemical ligation combined with desulfurization. J Am Chem Soc. 2001;123(4):526–533. doi: 10.1021/ja003265m. [DOI] [PubMed] [Google Scholar]
- 205.Wan Q, Danishefsky SJ. Free-radical-based, specific desulfurization of cysteine: a powerful advance in the synthesis of polypeptides and glycopolypeptides. Angew Chem Int Ed Engl. 2007;46(48):9248–9252. doi: 10.1002/anie.200704195. [DOI] [PubMed] [Google Scholar]
- 206.Neumann H, Hancock SM, Buning R, Routh A, Chapman L, Somers J, Owen-Hughes T, van Noort J, Rhodes D, Chin JW. A method for genetically installing site-specific acetylation in recombinant histones defines the effects of H3 K56 acetylation. Mol Cell. 2009;36(1):153–163. doi: 10.1016/j.molcel.2009.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Alvarez J, Shoichet B. Virtual Screening in Drug Discovery. CRC press 2005. [Google Scholar]
- 208.Merz KM, Ringe D, Reynolds CH. Drug Design: Structure- and Ligand-Based Approaches. Cambridge University Press; 2010. [Google Scholar]
- 209.Andricopulo AD. Structure- and Ligand-Based Drug Design: Advances and Perspectives. Current Topics in Medicinal Chemistry. 2009;9(9):754–754. doi: 10.2174/156802609789207073. [DOI] [PubMed] [Google Scholar]
- 210.Waszkowycz B. Structure-based approaches to drug design and virtual screening. Current Opinion in Drug Discovery & Development. 2002;5(3):407–413. [PubMed] [Google Scholar]
- 211.Taylor JB, Triggle DJ, Mason JS. Chapter 4. Computer-Assisted Drug Design. (Comprehensive medicinal chemistry II) 2007. p. 4.
- 212.Malorye AB. Finding the Perfect Fit. Bio-IT World. 2004;3(4):36–37. [Google Scholar]
- 213.Spannhoff A, Heinke R, Bauer I, Trojer P, Metzger E, Gust R, Schule R, Brosch G, Sippl W, Jung M. Target-based approach to inhibitors of histone arginine methyltransferases. Journal of medicinal chemistry. 2007;50(10):2319–2325. doi: 10.1021/jm061250e. [DOI] [PubMed] [Google Scholar]
- 214.Spannhoff A, Machmur R, Heinke R, Trojer P, Bauer I, Brosch G, Schule R, Hanefeld W, Sippl W, Jung M. A novel arginine methyltransferase inhibitor with cellular activity. Bioorg Med Chem Lett. 2007;17(15):4150–4153. doi: 10.1016/j.bmcl.2007.05.088. [DOI] [PubMed] [Google Scholar]
- 215.Garcia-Manero G, Issa JP. Histone deacetylase inhibitors: a review of their clinical status as antineoplastic agents. Cancer investigation. 2005;23(7):635–642. doi: 10.1080/07357900500283119. [DOI] [PubMed] [Google Scholar]
- 216.Cheng JC, Yoo CB, Weisenberger DJ, Chuang J, Wozniak C, Liang G, Marquez VE, Greer S, Orntoft TF, Thykjaer T, Jones PA. Preferential response of cancer cells to zebularine. Cancer cell. 2004;6(2):151–158. doi: 10.1016/j.ccr.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 217.Kubicek S, O’Sullivan RJ, August EM, Hickey ER, Zhang Q, Teodoro ML, Rea S, Mechtler K, Kowalski JA, Homon CA, Kelly TA, Jenuwein T. Reversal of H3K9me2 by a small-molecule inhibitor for the G9a histone methyltransferase. Molecular cell. 2007;25(3):473–481. doi: 10.1016/j.molcel.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 218.Cheng D, Yadav N, King RW, Swanson MS, Weinstein EJ, Bedford MT. Small molecule regulators of protein arginine methyltransferases. The Journal of biological chemistry. 2004;279(23):23892–23899. doi: 10.1074/jbc.M401853200. [DOI] [PubMed] [Google Scholar]
- 219.Gowher H, Zhang X, Cheng X, Jeltsch A. Avidin plate assay system for enzymatic characterization of a histone lysine methyltransferase. Anal Biochem. 2005;342(2):287–291. doi: 10.1016/j.ab.2005.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Berndsen CE, Denu JM. Assays for mechanistic investigations of protein/histone acetyltransferases. Methods. 2005;36(4):321–331. doi: 10.1016/j.ymeth.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 221.Dorgan KM, Wooderchak WL, Wynn DP, Karschner EL, Alfaro JF, Cui Y, Zhou ZS, Hevel JM. An enzyme-coupled continuous spectrophotometric assay for S-adenosylmethionine-dependent methyltransferases. Anal Biochem. 2006;350(2):249–255. doi: 10.1016/j.ab.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 222.Hendricks CL, Ross JR, Pichersky E, Noel JP, Zhou ZS. An enzyme-coupled colorimetric assay for S-adenosylmethionine-dependent methyltransferases. Anal Biochem. 2004;326(1):100–105. doi: 10.1016/j.ab.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 223.Riddles PW, Blakeley RL, Zerner B. Reassessment of Ellman’s reagent. Methods in enzymology. 1983;91:49–60. doi: 10.1016/s0076-6879(83)91010-8. [DOI] [PubMed] [Google Scholar]
- 224.Eyer P, Worek F, Kiderlen D, Sinko G, Stuglin A, Simeon-Rudolf V, Reiner E. Molar absorption coefficients for the reduced Ellman reagent: reassessment. Anal Biochem. 2003;312(2):224–227. doi: 10.1016/s0003-2697(02)00506-7. [DOI] [PubMed] [Google Scholar]
- 225.Biastoff S, Teuber M, Zhou ZS, Drager B. Colorimetric activity measurement of a recombinant putrescine N-methyltransferase from Datura stramonium. Planta medica. 2006;72(12):1136–1141. doi: 10.1055/s-2006-947191. [DOI] [PubMed] [Google Scholar]
- 226.Wang C, Leffler S, Thompson DH, Hrycyna CA. A general fluorescence-based coupled assay for S-adenosylmethionine-dependent methyltransferases. Biochem Biophys Res Commun. 2005;331(1):351–356. doi: 10.1016/j.bbrc.2005.03.170. [DOI] [PubMed] [Google Scholar]
- 227.Collazo E, Couture JF, Bulfer S, Trievel RC. A coupled fluorescent assay for histone methyltransferases. Anal Biochem. 2005;342(1):86–92. doi: 10.1016/j.ab.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 228.Wynne Aherne G, Rowlands MG, Stimson L, Workman P. Assays for the identification and evaluation of histone acetyltransferase inhibitors. Methods. 2002;26(3):245–253. doi: 10.1016/S1046-2023(02)00028-2. [DOI] [PubMed] [Google Scholar]
- 229.Feng Y, Xie N, Wu J, Yang C, Zheng YG. Inhibitory study of protein arginine methyltransferase 1 using a fluorescent approach. Biochemical and Biophysical Research Communications. 2009;379(2):567–572. doi: 10.1016/j.bbrc.2008.12.119. [DOI] [PubMed] [Google Scholar]
- 230.Nash T. The colorimetric estimation of formaldehyde by means of the Hantzsch reaction. The Biochemical journal. 1953;55(3):416–421. doi: 10.1042/bj0550416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kobayashi K, Yamamoto T, Taguchi M, Chiba K. High-performance liquid chromatography determination of N- and O-demethylase activities of chemicals in human liver microsomes: application of postcolumn fluorescence derivatization using Nash reagent. Anal Biochem. 2000;284(2):342–347. doi: 10.1006/abio.2000.4709. [DOI] [PubMed] [Google Scholar]
- 232.Anthon GE, Barrett DM. Comparison of three colorimetric reagents in the determination of methanol with alcohol oxidase. Application to the assay of pectin methylesterase. J Agric Food Chem. 2004;52(12):3749–3753. doi: 10.1021/jf035284w. [DOI] [PubMed] [Google Scholar]
- 233.Kleeberg U, Klinger W. Sensitive formaldehyde determination with Nash’s reagent and a ‘tryptophan reaction’. J Pharmacol Methods. 1982;8(1):19–31. doi: 10.1016/0160-5402(82)90004-3. [DOI] [PubMed] [Google Scholar]
- 234.Farrelly JG. A new assay for the microsomal metabolism of nitrosamines. Cancer Res. 1980;40(9):3241–3244. [PubMed] [Google Scholar]
- 235.Lizcano JM, Unzeta M, Tipton KF. A spectrophotometric method for determining the oxidative deamination of methylamine by the amine oxidases. Anal Biochem. 2000;286(1):75–79. doi: 10.1006/abio.2000.4782. [DOI] [PubMed] [Google Scholar]
- 236.Couture JF, Collazo E, Ortiz-Tello PA, Brunzelle JS, Trievel RC. Specificity and mechanism of JMJD2A, a trimethyllysine-specific histone demethylase. Nature structural & molecular biology. 2007;14(8):689–695. doi: 10.1038/nsmb1273. [DOI] [PubMed] [Google Scholar]
- 237.Rose NR, Ng SS, Mecinovic J, Lienard BM, Bello SH, Sun Z, McDonough MA, Oppermann U, Schofield CJ. Inhibitor scaffolds for 2-oxoglutarate-dependent histone lysine demethylases. J Med Chem. 2008;51(22):7053–7056. doi: 10.1021/jm800936s. [DOI] [PubMed] [Google Scholar]
- 238.Sakurai M, Rose NR, Schultz L, Quinn AM, Jadhav A, Ng SS, Oppermann U, Schofield CJ, Simeonov A. A miniaturized screen for inhibitors of Jumonji histone demethylases. Mol Biosyst. 6(2):357–364. doi: 10.1039/b912993f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Forneris F, Binda C, Vanoni MA, Mattevi A, Battaglioli E. Histone demethylation catalysed by LSD1 is a flavin-dependent oxidative process. FEBS Lett. 2005;579(10):2203–2207. doi: 10.1016/j.febslet.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 240.Holt A, Sharman DF, Baker GB, Palcic MM. A continuous spectrophotometric assay for monoamine oxidase and related enzymes in tissue homogenates. Anal Biochem. 1997;244(2):384–392. doi: 10.1006/abio.1996.9911. [DOI] [PubMed] [Google Scholar]
- 241.Holt A, Palcic MM. A peroxidase-coupled continuous absorbance plate-reader assay for flavin monoamine oxidases, copper-containing amine oxidases and related enzymes. Nature protocols. 2006;1(5):2498–2505. doi: 10.1038/nprot.2006.402. [DOI] [PubMed] [Google Scholar]
- 242.Szutowicz A, Kobes RD, Orsulak PJ. Colorimetric assay for monoamine oxidase in tissues using peroxidase and 2,2′-azinodi(3-ethylbenzthiazoline-6-sulfonic acid) as chromogen. Anal Biochem. 1984;138(1):86–94. doi: 10.1016/0003-2697(84)90773-5. [DOI] [PubMed] [Google Scholar]
- 243.Wang Y, Murray-Stewart T, Devereux W, Hacker A, Frydman B, Woster PM, Casero RA., Jr Properties of purified recombinant human polyamine oxidase, PAOh1/SMO. Biochem Biophys Res Commun. 2003;304(4):605–611. doi: 10.1016/s0006-291x(03)00636-3. [DOI] [PubMed] [Google Scholar]
- 244.Huang Y, Greene E, Murray Stewart T, Goodwin AC, Baylin SB, Woster PM, Casero RA., Jr. Inhibition of lysine-specific demethylase 1 by polyamine analogues results in reexpression of aberrantly silenced genes. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(19):8023–8028. doi: 10.1073/pnas.0700720104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Imhof A. Epigenetic regulators and histone modification. Briefings in Functional Genomics and Proteomics. 2006;5(3):222–227. doi: 10.1093/bfgp/ell030. [DOI] [PubMed] [Google Scholar]
- 246.Krueger KE, Srivastava S. Posttranslational protein modifications: current implications for cancer detection, prevention, and therapeutics. Mol Cell Proteomics. 2006;5(10):1799–1810. doi: 10.1074/mcp.R600009-MCP200. [DOI] [PubMed] [Google Scholar]
- 247.Kuendgen A, Lubbert M. Current status of epigenetic treatment in myelodysplastic syndromes. Ann Hematol. 2008;87(8):601–611. doi: 10.1007/s00277-008-0477-9. [DOI] [PubMed] [Google Scholar]
- 248.Huang S. Histone methyltransferases, diet nutrients and tumour suppressors. Nature reviews. 2002;2(6):469–476. doi: 10.1038/nrc819. [DOI] [PubMed] [Google Scholar]
- 249.Amur SG, Shanker G, Cochran JM, Ved HS, Pieringer RA. Correlation between inhibition of myelin basic protein (arginine) methyltransferase by sinefungin and lack of compact myelin formation in cultures of cerebral cells from embryonic mice. Journal of neuroscience research. 1986;16(2):367–376. doi: 10.1002/jnr.490160204. [DOI] [PubMed] [Google Scholar]
- 250.Hanzelka BL, Greenberg EP. Quorum sensing in Vibrio fischeri: evidence that S-adenosylmethionine is the amino acid substrate for autoinducer synthesis. Journal of bacteriology. 1996;178(17):5291–5294. doi: 10.1128/jb.178.17.5291-5294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Johnson BA, Najbauer J, Aswad DW. Accumulation of substrates for protein L-isoaspartyl methyltransferase in adenosine dialdehyde-treated PC12 cells. The Journal of biological chemistry. 1993;268(9):6174–6181. [PubMed] [Google Scholar]
- 252.Najbauer J, Johnson BA, Aswad DW. Analysis of stable protein methylation in cultured cells. Archives of biochemistry and biophysics. 1992;293(1):85–92. doi: 10.1016/0003-9861(92)90369-8. [DOI] [PubMed] [Google Scholar]
- 253.Mowen KA, Schurter BT, Fathman JW, David M, Glimcher LH. Arginine methylation of NIP45 modulates cytokine gene expression in effector T lymphocytes. Molecular cell. 2004;15(4):559–571. doi: 10.1016/j.molcel.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 254.Greiner D, Bonaldi T, Eskeland R, Roemer E, Imhof A. Identification of a specific inhibitor of the histone methyltransferase SU(VAR)3-9. Nature chemical biology. 2005;1(3):143–145. doi: 10.1038/nchembio721. [DOI] [PubMed] [Google Scholar]
- 255.Liu F, Chen X, Allali-Hassani A, Quinn AM, Wasney GA, Dong A, Barsyte D, Kozieradzki I, Senisterra G, Chau I, Siarheyeva A, Kireev DB, Jadhav A, Herold JM, Frye SV, Arrowsmith CH, Brown PJ, Simeonov A, Vedadi M, Jin J. Discovery of a 2,4-Diamino-7-aminoalkoxyquinazoline as a Potent and Selective Inhibitor of Histone Lysine Methyltransferase G9a. Journal of medicinal chemistry. 2009 doi: 10.1021/jm901543m. DOI: 10.1021/jm901543m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Miranda TB, Cortez CC, Yoo CB, Liang G, Abe M, Kelly TK, Marquez VE, Jones PA. DZNep is a global histone methylation inhibitor that reactivates developmental genes not silenced by DNA methylation. Molecular cancer therapeutics. 2009;8(6):1579–1588. doi: 10.1158/1535-7163.MCT-09-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Tan J, Yang X, Zhuang L, Jiang X, Chen W, Lee PL, Karuturi RK, Tan PB, Liu ET, Yu Q. Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes & development. 2007;21(9):1050–1063. doi: 10.1101/gad.1524107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ragno R, Simeoni S, Castellano S, Vicidomini C, Mai A, Caroli A, Tramontano A, Bonaccini C, Trojer P, Bauer I, Brosch G, Sbardella G. Small molecule inhibitors of histone arginine methyltransferases: homology modeling, molecular docking, binding mode analysis, and biological evaluations. Journal of medicinal chemistry. 2007;50(6):1241–1253. doi: 10.1021/jm061213n. [DOI] [PubMed] [Google Scholar]
- 259.Balasubramanyam K, Varier RA, Altaf M, Swaminathan V, Siddappa NB, Ranga U, Kundu TK. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. The Journal of biological chemistry. 2004;279(49):51163–51171. doi: 10.1074/jbc.M409024200. [DOI] [PubMed] [Google Scholar]
- 260.Mai A, Cheng D, Bedford MT, Valente S, Nebbioso A, Perrone A, Brosch G, Sbardella G, De Bellis F, Miceli M, Altucci L. epigenetic multiple ligands: mixed histone/protein methyltransferase, acetyltransferase, and class III deacetylase (sirtuin) inhibitors. Journal of medicinal chemistry. 2008;51(7):2279–2290. doi: 10.1021/jm701595q. [DOI] [PubMed] [Google Scholar]
- 261.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Del Rev. 1997;23:3–25. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 262.Sippl WJM, editor. Epigenetic Targets in Drug Discovery. Wiley-VCH; 2009. [Google Scholar]
- 263.Spannhoff A, Machmur R, Heinke R, Trojer P, Bauer I, Brosch G, Schule R, Hanefeld W, Sippl W, Jung M. A novel arginine methyltransferase inhibitor with cellular activity. Bioorganic & Medicinal Chemistry Letters. 2007;17(15):4150–4153. doi: 10.1016/j.bmcl.2007.05.088. [DOI] [PubMed] [Google Scholar]
- 264.Chen D, Ma H, Hong H, Koh SS, Huang SM, Schurter BT, Aswad DW, Stallcup MR. Regulation of transcription by a protein methyltransferase. Science. 1999;284(5423):2174–2177. doi: 10.1126/science.284.5423.2174. [DOI] [PubMed] [Google Scholar]
- 265.Hong H, Kao C, Jeng MH, Eble JN, Koch MO, Gardner TA, Zhang S, Li L, Pan CX, Hu Z, MacLennan GT, Cheng L. Aberrant expression of CARM1, a transcriptional coactivator of androgen receptor, in the development of prostate carcinoma and androgen-independent status. Cancer. 2004;101(1):83–89. doi: 10.1002/cncr.20327. [DOI] [PubMed] [Google Scholar]
- 266.Majumder S, Liu Y, Ford OH, 3rd, Mohler JL, Whang YE. Involvement of arginine methyltransferase CARM1 in androgen receptor function and prostate cancer cell viability. Prostate. 2006;66(12):1292–1301. doi: 10.1002/pros.20438. [DOI] [PubMed] [Google Scholar]
- 267.Frietze S, Lupien M, Silver PA, Brown M. CARM1 regulates estrogen-stimulated breast cancer growth through up-regulation of E2F1. Cancer Res. 2008;68(1):301–306. doi: 10.1158/0008-5472.CAN-07-1983. [DOI] [PubMed] [Google Scholar]
- 268.El Messaoudi S, Fabbrizio E, Rodriguez C, Chuchana P, Fauquier L, Cheng D, Theillet C, Vandel L, Bedford MT, Sardet C. Coactivator-associated arginine methyltransferase 1 (CARM1) is a positive regulator of the Cyclin E1 gene. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(36):13351–13356. doi: 10.1073/pnas.0605692103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Purandare AV, Chen Z, Huynh T, Pang S, Geng J, Vaccaro W, Poss MA, Oconnell J, Nowak K, Jayaraman L. Pyrazole inhibitors of coactivator associated arginine methyltransferase 1 (CARM1) Bioorganic & Medicinal Chemistry Letters. 2008;18(15):4438–4441. doi: 10.1016/j.bmcl.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 270.Osborne T, Roska RL, Rajski SR, Thompson PR. In situ generation of a bisubstrate analogue for protein arginine methyltransferase 1. J Am Chem Soc. 2008;130(14):4574–4575. doi: 10.1021/ja077104v. [DOI] [PubMed] [Google Scholar]
- 271.Bannister AJ, Schneider R, Kouzarides T. Histone methylation: dynamic or static? Cell. 2002;109(7):801–806. doi: 10.1016/s0092-8674(02)00798-5. [DOI] [PubMed] [Google Scholar]
- 272.Shi Y. Histone lysine demethylases: emerging roles in development, physiology and disease. Nat Rev Genet. 2007;8(11):829–833. doi: 10.1038/nrg2218. [DOI] [PubMed] [Google Scholar]
- 273.Shi YJ, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y. Histone demethylation mediated by the nuclear arnine oxidase homolog LSD1. Cell. 2004;119(7):941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 274.Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, Heine-Suner D, Cigudosa JC, Urioste M, Benitez J, Boix-Chornet M, Sanchez-Aguilera A, Ling C, Carlsson E, Poulsen P, Vaag A, Stephan Z, Spector TD, Wu YZ, Plass C, Esteller M. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A. 2005;102(30):10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Metzger E, Wissmann M, Yin N, Muller JM, Schneider R, Peters AH, Gunther T, Buettner R, Schule R. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature. 2005;437(7057):436–439. doi: 10.1038/nature04020. [DOI] [PubMed] [Google Scholar]
- 276.Yang MJ, Culhane JC, Szewczuk LM, Jalili P, Ball HL, Machius M, Cole PA, Yu HT. Structural basis for the inhibition of the LSD1 histone demethylase by the antidepressant trans-2-phenylcyclopropylamine. Biochemistry. 2007;46(27):8058–8065. doi: 10.1021/bi700664y. [DOI] [PubMed] [Google Scholar]
- 277.Schmidt DM, McCafferty DG. trans-2-Phenylcyclopropylamine is a mechanism-based inactivator of the histone demethylase LSD1. Biochemistry. 2007;46(14):4408–4416. doi: 10.1021/bi0618621. [DOI] [PubMed] [Google Scholar]
- 278.Lee MG, Wynder C, Schmidt DM, McCafferty DG, Shiekhattar R. Histone H3 lysine 4 demethylation is a target of nonselective antidepressive medications. Chem Biol. 2006;13(6):563–567. doi: 10.1016/j.chembiol.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 279.Yang M, Culhane JC, Szewczuk LM, Jalili P, Ball HL, Machius M, Cole PA, Yu H. Structural basis for the inhibition of the LSD1 histone demethylase by the antidepressant trans-2-phenylcyclopropylamine. Biochemistry. 2007;46(27):8058–8065. doi: 10.1021/bi700664y. [DOI] [PubMed] [Google Scholar]
- 280.Yang M, Culhane JC, Szewczuk LM, Gocke CB, Brautigam CA, Tomchick DR, Machius M, Cole PA, Yu H. Structural basis of histone demethylation by LSD1 revealed by suicide inactivation. Nat Struct Mol Biol. 2007;14(6):535–539. doi: 10.1038/nsmb1255. [DOI] [PubMed] [Google Scholar]
- 281.Ueda R, Suzuki T, Mino K, Tsumoto H, Nakagawa H, Hasegawa M, Sasaki R, Mizukami T, Miyata N. Identification of Cell-Active Lysine Specific Demethylase 1-Selective Inhibitors. Journal of the American Chemical Society. 2009;131(48):17536–17537. doi: 10.1021/ja907055q. [DOI] [PubMed] [Google Scholar]
- 282.Culhane JC, Szewczuk LM, Liu X, Da G, Marmorstein R, Cole PA. A mechanism-based inactivator for histone demethylase LSD1. J Am Chem Soc. 2006;128(14):4536–4537. doi: 10.1021/ja0602748. [DOI] [PubMed] [Google Scholar]
- 283.Culhane JC, Wang D, Yen PM, Cole PA. Comparative analysis of small molecules and histone substrate analogues as LSD1 lysine demethylase inhibitors. J Am Chem Soc. 132(9):3164–3176. doi: 10.1021/ja909996p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Chen Z, Zang J, Kappler J, Hong X, Crawford F, Wang Q, Lan F, Jiang C, Whetstine J, Dai S, Hansen K, Shi Y, Zhang G. Structural basis of the recognition of a methylated histone tail by JMJD2A. Proc Natl Acad Sci U S A. 2007;104(26):10818–10823. doi: 10.1073/pnas.0704525104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Sekirnik R, Rose NR, Thalhammer A, Seden PT, Mecinovic J, Schofield CJ. Inhibition of the histone lysine demethylase JMJD2A by ejection of structural Zn(II) Chem Commun (Camb) 2009;(42):6376–6378. doi: 10.1039/b916357c. [DOI] [PubMed] [Google Scholar]
- 286.Rose NR, Woon ECY, Kingham GL, King ONF, Mecinovi J, Clifton IJ, Ng SS, Talib-Hardy J, Oppermann U, McDonough MA, Schofield CJ. Selective Inhibitors of the JMJD2 Histone Demethylases: Combined Nondenaturing Mass Spectrometric Screening and Crystallographic Approaches. Journal of Medicinal Chemistry. 2010 doi: 10.1021/jm901680b. Article ASAP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 288.Aletta JM, Cimato TR, Ettinger MJ. Protein methylation: a signal event in post-translational modification. Trends Biochem Sci. 1998;23(3):89–91. doi: 10.1016/s0968-0004(98)01185-2. [DOI] [PubMed] [Google Scholar]
- 289.Cote J, Richard S. Tudor domains bind symmetrical dimethylated arginines. The Journal of biological chemistry. 2005;280(31):28476–28483. doi: 10.1074/jbc.M414328200. [DOI] [PubMed] [Google Scholar]
- 290.Wang Y, Reddy B, Thompson J, Wang H, Noma K, Yates JR, 3rd, Jia S. Regulation of Set9-mediated H4K20 methylation by a PWWP domain protein. Mol Cell. 2009;33(4):428–437. doi: 10.1016/j.molcel.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Akhtar A, Zink D, Becker PB. Chromodomains are protein-RNA interaction modules. Nature. 2000;407(6802):405–409. doi: 10.1038/35030169. [DOI] [PubMed] [Google Scholar]
- 292.Min J, Zhang Y, Xu RM. Structural basis for specific binding of Polycomb chromodomain to histone H3 methylated at Lys 27. Genes & development. 2003;17(15):1823–1828. doi: 10.1101/gad.269603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Hirota J, Satomi Y, Yoshikawa K, Takao T. Epsilon -N,N,N-trimethyllysine-specific ions in matrix-assisted laser desorption/ionization-tandem mass spectrometry. Rapid Commun Mass Spectrom. 2003;17(5):371–376. doi: 10.1002/rcm.924. [DOI] [PubMed] [Google Scholar]
- 294.Gehrig PM, Hunziker PE, Zahariev S, Pongor S. Fragmentation pathways of N(G)-methylated and unmodified arginine residues in peptides studied by ESI-MS/MS and MALDI-MS. Journal of the American Society for Mass Spectrometry. 2004;15(2):142–149. doi: 10.1016/j.jasms.2003.10.002. [DOI] [PubMed] [Google Scholar]