Abstract
While century old clinical reports document the periosteum's remarkable regenerative capacity, only in the past decade have scientists undertaken mechanistic investigations of periosteum's regenerative potential. Here we outline three presentations from a 2012 Orthopaedic Research Society Workshop which reviewed the current state of the art in molecular, cellular and tissue scale approaches to elucidate the mechanisms underlying the periosteum's regenerative potential and translational therapies as well as engineering solutions inspired by the periosteum's remarkable regenerative capacity. First, we highlight the development of the periosteum and its role in bone repair, noting that the entire population of osteoblasts within periosteum, and at endosteal and trabecular bone surfaces within the bone marrow, derive from the embryonic perichondrium. Secondly, we underscore the role of periosteum derived cells in postnatal bone healing and regeneration; cells of the periosteum contribute more to formation of cartilage and bone within the callus during fracture healing than do cells of the bone marrow or endosteum, which not migrate out of the bone marrow compartment. Furthermore, a current healing paradigm regards the activation, expansion and differentiation of periosteal stem/progenitor cells as an essential step in building a template for subsequent neovascularization, bone formation and remodeling. Thirdly, the periosteum comprises a complex, composite structure, providing a niche for pluripotent cells and a repository for molecular factors that modulate cell behavior. The periosteum's advanced, "smart" material properties change depending on the mechanical, chemical and biological state of the tissue. Understanding periosteum development, progenitor cell-driven initiation of periosteum's endogenous tissue building capacity, as well as the complex structure-function relationships of periosteum as an advanced material are important for harnessing and engineering ersatz materials to mimic the periosteum's remarkable regenerative capacity.
Keywords: periosteum, regenerative medicine, tissue engineering, bone biology, advanced materials
Introduction
At the 50th Orthopaedic Research Society Meeting held in San Franciscio (February 2012), a workshop, comprising three presentations by leading researchers in the field, was held to bring biomedical researchers up-to-date on the state of the art in the science of periosteum and its regenerative potential. While clinical reports of the periosteum's remarkable regenerative capacity can be found in century old scientific literature, mechanistic investigations of periosteum's regenerative potential have been published mostly in the past decade. The periosteum comprises a complex, composite structure that provides a niche for pluripotent cells and a repository for molecular factors that modulate cell behavior. In addition, periosteum exhibits advanced, smart material properties that change depending on the mechanical, chemical and biological state of the tissue. Hence, the aim of the ORS workshop was to review molecular, cellular and tissue scale approaches to elucidate the mechanisms underlying the periosteum's regenerative potential and to discuss these approaches in light of translational therapies and engineering solutions inspired by the periosteum's remarkable regenerative capacity. An interactive panel discussion at the end of the workshop highlighted current hurdles to advancement and clinical translation of these insights as well as current controversies in the field. This article summarizes the research and discussion presented by the workshop speakers, Drs. Céline Colnot, Xinping Zhang, and Melissa Knothe Tate.
A. Development of the periosteum and its role during bone repair
The periosteum is a thin layer of tissue lining the outer surface of bone. This tissue is highly vascularized and its preservation is crucial for normal bone repair. Periosteum is rich in osteoblasts that deposit the new bone matrix in the outer cortex, as well as in osteoblast precursors. Although bone marrow-derived pluripotent cells have been mostly exploited so far for regenerative medicine applications to facilitate healing of orthopaedic injuries, the periosteum is now recognized as an attractive source of cells [1–3]. Over the past decade, a number of animal models have been developed to assess the mechanisms of skeletal stem/progenitor cell recruitment during bone repair and to test the therapeutic effects of skeletal stem/progenitor cells. Several studies have revealed that the endogenous regenerative potential of periosteum is high compared to bone marrow and other cell sources.
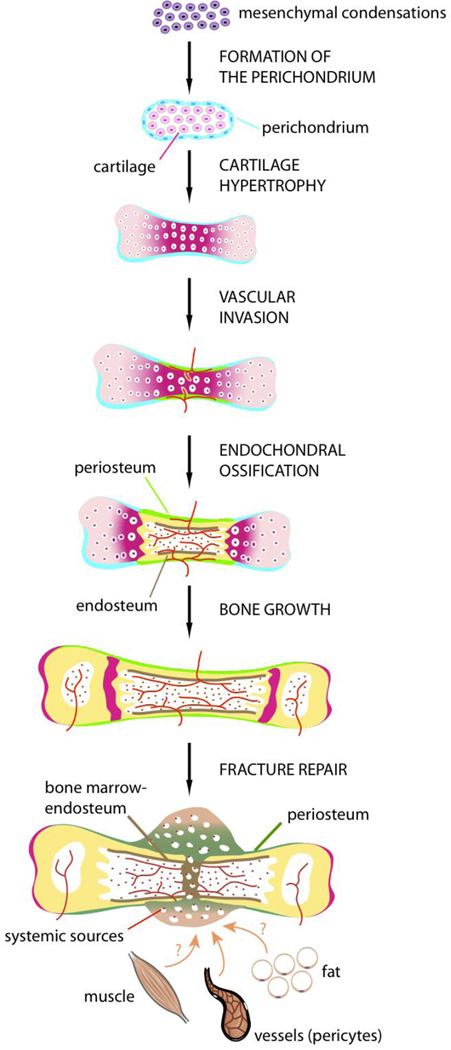
During long bone development, all the populations of osteoblasts within periosteum, as well as osteoblasts at the endosteal and trabecular bone surfaces within the bone marrow, are derived from the embryonic perichondrium [4]. Each skeletal element is derived from a mesenchymal condensation that gives rise to a cartilage template surrounded by the perichondrium (Fig. 1). Ossification of these skeletal elements begins with the vascular invasion of the perichondrium followed by the invasion of hypertrophic cartilage in the center of the cartilage template [5]. The initial vascular invasion is followed by rapid removal of the calcified cartilage matrix and its replacement by bone and bone marrow. This process of endochondral ossification is highly regulated by several signaling pathways, including Hedgehog, BMP, TGF-beta, PTH/PTHrP, FGF, Wnt, Notch, VEGF that act at the levels of chondrocytes, perichondrium and blood vessels, allowing the synchronization of cell differentiation in these adjacent tissues [6–11]. Although many cell types participate in this process, with some differentiating locally and others brought by blood vessels, lineage analyses show that osteoblasts all come from the perichondrium [4]. Osteoblasts precursors originating from the perichondrium migrate along with blood vessels to form the primary ossification center [12]. Thus, during development, osteoblasts within periosteum and bone marrow are derived locally from the initial mesenchymal condensations, and more specifically the perichondrium, without systemic contribution from invading blood vessels.
Figure 1. Development of the periosteum and its contribution to bone repair.
Stages of long bone development including formation of the initial mesenchymal condensations, followed by the segregation of cartilage (pink) and perichondrium (blue), vascular invasion and replacement of hypertrophic cartilage by bone and bone marrow. Osteoblasts within periosteum (green), bone marrow and endosteum (brown) are derived from the embryonic perichondrium. In the adult, after bone injury, cells that form cartilage and bone in the fracture callus are recruited locally from periosteum, bone marrow, blood vessels (pericytes) and potentially other adjacent tissues such as muscle and fat. Cellular contribution from systemic sources is minimal (red dots).
In the adult, the stages of bone repair recapitulate the well-defined stages of bone formation during embryogenesis, except for the inflammatory response, which is critical for bone regeneration [13–18]. During this initial phase of repair, skeletal stem cells are activated at the fracture site and differentiate into osteoblasts and chondrocytes that deposit the extracellular matrix necessary for bone bridging. Skeletal stem cells differentiate during the inflammatory phase of repair and are exposed to inflammatory cytokines, growth factors and mechanical signals [19–27]. How these various cellular, molecular and mechanical signals influence the recruitment of skeletal stem cells remains largely unknown. Histologically, it is clear however that cells within the periosteum respond rapidly to these signals as a periosteal reaction can be detected within 24 to 48 hours post-injury [28,29]. In the absence of stabilization, this periosteal reaction is particularly robust leading to the formation of a large callus and healing via endochondral ossification [28,29]. When fractures are rigidly stabilized, cells in the periosteum are not stimulated as efficiently and callus formation is minimal. In this mechanically stable environment healing occurs essentially via intramembranous ossification. These differences in cellular response to mechanical signals reside mostly within the periosteum, as shown by the up-regulation of the BMP pathway within the periosteum during the first stage of healing in non-stabilized fractures [31,32]. The periosteum contains skeletal progenitors that play an important role in bone repair, however their identity and relative contribution to healing compared to other cell sources are not well defined. Many sources of skeletal progenitors have been proposed to participate in adult bone repair including the local bone marrow, periosteum, soft tissues and blood vessel walls, as well as cells brought to the injury site via blood vessels. Several approaches have been used to determine the potential recruitment of cells from distant sites during fracture repair, including parabiosis, bone marrow transplantation and intravenous injection of cells in animal models [33–37]. These approaches suggest that cells recruited systemically are minimal contributors to cartilage and bone, but give rise mostly to inflammatory cells and osteoclasts [38,39]. The contribution of blood vessels themselves has been addressed using genetic lineage tracing. Endothelial cells do not appear to transdifferentiate into skeletal progenitors involved in fracture healing as cartilage and bone within the callus are not derived from Tie2-expressing cells [40]. Pericytes marked with smooth muscle actin have been shown, however, to coincide with a population of endogenous mesenchymal progenitors and can largely contribute to fracture healing [41]. The tissue origins of these mesenchymal progenitors are not determined, yet they are likely derived from the local blood vessels around the fracture site. Other populations of mesenchymal stem cells expressing Mx1 have been identified within the bone marrow cavity and have osteogenic potential during the repair of calvarial defects [42]. All of these studies provide new molecular tools to elucidate the mechanisms of skeletal stem cell recruitment during bone repair. In addition, lineage analyses based on bone grafting have revealed that periosteum largely contributes to cartilage and bone within the callus compared to bone marrow and endosteum [43–46]. Cells within local bone marrow and endosteum form bone mostly within the bone marrow cavity, and do not migrate out of the bone marrow compartment to form the callus [43]. These results show that not only local tissues, but perhaps most importantly, the periosteum are the key cellular contributors to bone repair (Fig. 1). The extent to which progenitors within bone marrow and periosteum exhibit distinct regenerative capacities remains to be established. The tissue location may play an important role as cells located in periosteum and bone marrow may not receive the same signals upon injury. Understanding these differences may help define competent cell sources and ways to stimulate the regenerative capacities of mesenchymal stem cells from other tissues such as muscle, fat, umbilical cord, etc. More research is underway to characterize the endogenous cell sources for bone repair, identify the molecular pathways controlling their recruitment and apply this knowledge to bone tissue engineering approaches [3,47–52].
B. New Approaches toward Understanding Skeletal Repair and Regeneration
Skeletal repair is a dynamic and well-orchestrated process that involves complex and spatiotemporally coordinated function of different cellular compartments and integrated molecular pathways. Understanding complex molecular and cellular interactions during skeletal healing represents a critical step toward developing effective treatment strategies for enhancing repair and reconstruction. Immediately following cortical bone injury, periosteum undergoes a series of changes to initiate endochondral and intramembraneous bone formation at the site of injury. Both endochondral and intramembranous bone formation begin with intensive proliferation of periosteal progenitor cells located within periosteum. Cells situated near the cortical bone junction differentiate into chondroprogenitors whereas cells at the periphery of the cortex furthest away from cortical bone junction adopt an osteogenic cell fate.
The contribution of the periosteal progenitors to initial callus formation was examined using a segmental bone graft transplantation model in mice [44,52]. By transplantation of a Rosa26A bone graft, the study demonstrated a predominant contribution of periosteal progenitors to both endochondral and intramembraneous bone formation at the initiation of repair. By tracking the LacZ+ve cell fate, the study further suggested a research paradigm in which activation, expansion and differentiation of periosteal stem/progenitor cells act as an essential step to build a template for subsequent neovascularization, bone formation and remodeling. Understanding this progenitor cell-driven initiation process not only provides mechanistic insight into endogenous regeneration capacity of periosteum, but could also offers necessary information for optimization of tissue engineering constructs for fabrication of a periosteum substitute for repair and reconstruction.
Using genetically modified mouse models, a number of molecular pathways have been highlighted to play a critical role in the initiation of periosteum-mediated regeneration. Among them, BMP-2 appears to be at the apex of the signaling cascade that initiates the cellular proliferation and differentiation of periosteal progenitors during repair and regeneration. Genetic deletion of BMP-2 gene via Prx-1-Cre in limb mesenchyme condensation is shown to be dispensable for development of long bones. However, long bone lacking BMP-2 expression develops spontaneous fractures in adult animals. Most strikingly, deletion of BMP-2 completely abolishes fracture callus formation, suggesting a critical role of BMP-2 in the initiation of repair [53]. In a similar study in which BMP-2 was knocked out at the initiation stage of healing in adult animals using a Tamoxifen inducible CreER mouse model [54,55], deletion of BMP-2 at the onset of healing completely abrogated both endochondral and intramembranous bone repair. Deletion of BMP-2 in periosteal progenitor cells not only blocked cellular differentiation, but also impaired proliferation and survival of the cells. Further tracking of the BMP-2 mutant cells in a chimeric periosteal callus showed that few mutant cells could differentiate into chondrocytes and osteoblasts, even when the mutant cells were placed in a wild type host injury environment, indicating an essential role of endogenous BMP-2 signaling in the initiation of periosteal callus formation. In addition to BMP-2, several key components of BMP family proteins and their corresponding receptors have been identified in the activated periosteum. These include BMP-2, 3, 4, 5, 8, noggin, BMPRIA, BMPRII, and pSmad 1/5/8 [32]. The differential role of BMP isoforms in repair has been examined using floxed mouse models that allow conditional deletion of BMP isoforms via Prx-1Cre. BMP-4 and 6 are dispensable for repair whereas BMP-3 is shown to play a negative role for early periosteum development and potentially in postnatal repair and regeneration [56–58]. Transgenic mice overexpressing BMP3 via the type I collagen promoter displayed spontaneous rib fractures as early as E17.0. The fractures were due to defects in differentiation of the periosteum and late hypertrophic chondrocytes resulting in thinner cortical bone with decreased mineralization.
Downstream of BMPs, the hedgehog pathway, in particular the Ihh pathway has long been suspected to play a role in periosteum-mediated endochondral bone repair [17,59–62]. Both Shh and Ihh have been implicated in early embryonic development that implicates mesenchymal progenitor cell differentiation and self-renewal [63]. Ihh has been shown to play a key role in the perichondrium development and collar bone formation. Embryonic deletion of Ihh disrupts collar bone formation and early osteoblast development [64–66]. Ihh is abundantly expressed in prehypertrophic and hypertrophic chondrocytes in fracture callus. Using in situ hybridization and Ptc-LacZ staining, a recent study from Zhang’s laboratory further showed that Ihh was expressed in the nascent cartilaginous tissues in periosteum callus adjacent to the bone surface at the initiation stage of healing. These hedgehog producing cells send out signals to the surrounding chondroprogenitors, osteoblast progenitors as well as cells associated with the early invading vessels [67]. To further determine the role of hedgehog pathway in postnatal periosteum-mediated repair and regeneration, Wang et al. isolated a unique population of mesenchymal progenitors from day 5 autograft periosteum. Characterization of these cells showed that these isolated periosteal cells expressed a number of mesenchymal progenitor cell markers namely SSEA4, CD105, CD29, CD140b, and ScaI. They could further give rise to osteoblasts, chondrocytes and adipocyte in vitro. Compared with mesenchymal progenitors isolated from other tissues such as adipose and bone marrow, these cells showed stronger responsiveness to both BMP-2 and hedgehog agonists. Overexpression of a hedgehog agonist, namely Shh N-terminal peptide in these cells induced robust ectopic bone formation in nude mice. Further deletion of Smoothened1, a receptor that transduces all hedgehog signaling using a Tamoxifen inducible CreER mouse model significantly reduced periosteal bone formation in the conditional knockout mice [67]. These studies indicate an important role of hedgehog pathway in periosteum-mediated repair and regeneration. Further studies are necessary to determine the potential use of hedgehog agonists in repair and in bone tissue engineering applications.
COX-2 is discovered as an inducible isoform of cyclooxygenase in the prostaglandin biosynthesis pathway. As an immediate early gene, COX-2 is induced by a variety of inflammatory cytokines and growth factors, including bone anabolic factors FGF, IGF, TGF β and BMP-2 [68–71]. The induction of COX-2 was localized in chondroprogenitors, proliferating chondrocytes and osteoblasts, concomitant with the initiation of endochondral and intramembraneous bone formation in periosteum [45]. Although deletion of COX-2 has no discernible effect on postnatal bone development, deletion of COX-2 globally or specifically in mesenchyme or cartilage significantly impairs periosteal progenitor cell proliferation and delays subsequent endochondral and intramembranous repair [13,45]. The critical role of COX-2 in repair was further illustrated in a study of fracture healing in aged mice. In comparison to the young mice, aged mice elicited a mitigated induction of COX-2 during early endochondral bone formation. Treatment of aged mice with an agonist of prostaglandin receptor type 4 receptor (EP4) rescued the delayed endochondral bone formation [72], suggesting a beneficial effect of targeting EP receptor for improved healing.
Wnt/β-catenin, a critical player in osteoblast differentiation and bone formation has recently emerged as a potential therapeutic target for bone repair and fracture healing [73,74]. Multiple Wnt proteins as well as their modulators were shown to be expressed in periosteum [75]. Delivery of a Wnt/β-catenin inhibitor DKK1 suppressed bone repair whereas administration of a DKK1 neutralizing antibody improved repair and regeneration [75–77]. Interestingly, several pathways known to stimulate fracture repair including BMP-2 and Hh pathways enhances Wnt/β-catenin pathway[67,78,79]. In addition, intermittent PTH treatment strongly stimulated fracture healing in part by inducing canonical Wnt signaling [80]. Prostaglandin E2, the major metabolite from COX-2 enzymatic activity, has also been shown to activate canonical Wnt signaling via EP2 and EP4 receptors [81]. How these pathways converge on Wnt/β-catenin to enhance repair has become the focus of intensive studies.
C. Surgical and Engineering Approaches to Elucidate and Unleash the Regenerative Power of the Periosteum
The periosteum is a composite tissue [82,83] that provides a niche for pluriopotent osteochondroprogenitor cells and exhibits a remarkable regenerative capacity to generate bone de novo within critical sized defects. Surgeons have harnessed this regenerative capacity for more than a century [84]. Recent studies have focused on the mechanisms underlying periosteum's remarkable regenerative capacity, and in particular the role of mechanical and mechanically modulated signals in this process.
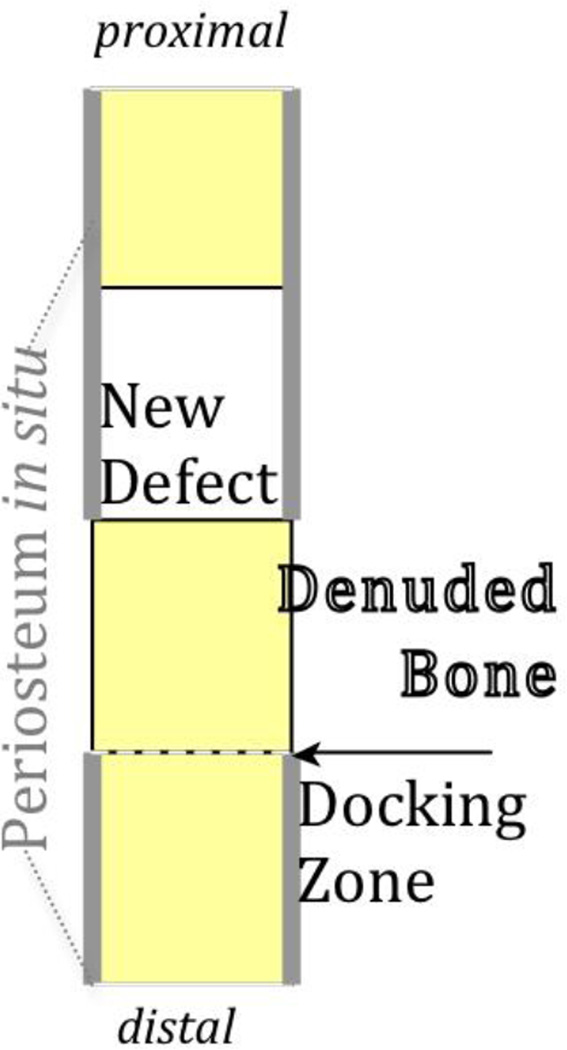
A recently described case study [85] demonstrating the capacity of the periosteum in situ to regenerate a several inch segment of resected fibula provided the inspiration for a one stage bone transport procedure [86] for treatment of critical sized long bone defects. In this procedure, a solid, reamed intramedullary nail fills the medullary cavity, stabilizing the femur. Proximal to the critical sized defect, the periosteum is carefully lifted and peeled back, maintaining the blood supply but disrupting the Sharpey's fibres that anchor the periosteum to the underlying bone. Osteotomy then produces a periosteum denuded bone segment, which is transported and docked distally to fill the original defect zone. The periosteum is then sutured closed, in situ, around the newly created defect zone, and to the denuded bone segment, forming a sleeve around the haematoma or autologous graft filled defect. [86]. When treated by enveloping the critical sized defect by periosteum in situ, de novo bone formation completely bridges the defect after 16 weeks, even in the absence of the medullary cavity which is filled by an intramedullary nail for mechanical stabilization. Interestingly, filling the periosteum enveloped defect with graft retards the time course of infilling by the periosteum [84,86]. On the one hand, the slowing of the healing response may be attributed to increased resistance to cellular egression and mass transport from the periosteum to the defect zone. On the other hand, the bone graft filling the defect must be resorbed prior to vasculogenesis and new bone apposition, requiring additional time for healing [84].
Clinical reports and recent experiments indicate that mechanical loading enhances the regenerative capacity of the periosteum. Specifically, in studies using the one stage bone transport procedure in an ovine critical sized femur defect model, prevailing mechanical loads and proximity to the periosteum were hypothesized to modulate early bone generation in the defect zone and late measures of healing and remodeling of autograft in the denuded bone transport segment [84,86,87]. Quantification of the area (a measure of bone quantity) and intensity (a measure of bone quality, as the intensity of fluorescence indicates the concentration of mineral) of calcein green fluorochrome, which chelates to bone formed in the first weeks after surgery, allowed for correlation of new bone formation to both loading histories during the first weeks after surgery as well as proximity to the periosteum. Namely, the major and minor centroidal axes of the long bone cross section indicate axes about which the bone is most and least resistant to bending loads, respectively. Further, using the laser confocal microscope as a spectrocope, it is possible to measure the intensity of the fluorochrome signal as a function of distance from the periosteum. Using these measures to compare groups treated with and without packed morcellized bone graft, the amount of early bone formation was shown to be significantly higher along the bone axis most resistant to bending loads (major centroidal axis), but the quality of early bone formed (density as measured by intensity or concentration of mineralized tissue) was higher along the bones axis least resistant to bending loads (minor centroidal axis). Finally, the spatial distribution of new bone formed in the first weeks after surgery was shown to correlate significantly with the distance from the periosteum as well as prevailing mechanical loads. [84] Interestingly, although the periosteum itself regenerates in the denuded bone segment after the one stage bone transport procedure [88], the thickness of the regenerated periosteum does not correlate significantly to prevailing mechanical loads.
In sum, retention of the periosteum in situ around a critical sized defect results in rapid infilling of the defect with woven bone, as rapidly as in two weeks in the case where the defect is not packed with morcellized cancellous bone graft [84,86,87]. This rapid infilling with bone occurs in absence of factors from the medullary cavity, which is filled by an intramedullary nail in the one stage bone transport studies. Finally, the process of de novo tissue formation and infilling is mechanically modulated [84,87,89]. Based on the aforementioned studies, factors inherent to the periosteum, including the ingression of pluripotent cells from the periosteum, drive the process. Although correlation does not equal causation, ongoing studies aim to elucidate mechanistic relationships between mechanical loading, transport of cells and molecular factors, and de novo generation of tissue in critical sized bone defects.
The regenerative capacity of the periosteum exhibits great clinical promise for treatment of nonunions and tissue defects occurring due to tumor resection, infection, trauma and congenital defects. The approach exemplified by the one stage bone transport procedure has been implemented successfully in limited clinical cases where other treatment modalities were not feasible [90]. Engineering of substitute periosteum is a promising area of research that benefits from both top down perspectives as well as bottom up approaches to recreate the multiscale structure-function relationships embodied by the smart material, using nature's engineering paradigms [91].
From a structural perspective, the periosteum is a bilayered membrane that bounds bones. The outer layer consists mostly of collagens, aligned with the longitudinal axis, and elastin [82,83,93], and is hypothesized to serve a mostly structural (mechanical) role [94]. The periosteum's innermost layer (closest to the bone) is made up mostly of progenitor cells, which constantly build [82] and repair bone [43,44,95]. As described above, a series of recent studies underscores the profound regenerative capacity of periosteum, where pluripotent cells egressing from the periosteum fill bone defects rapidly with bone [84,86,87], and this process is modulated by mechanical signals to which the tissue and its resident cells are exposed [84,86,87,27].
An understanding of periosteum's mechanical properties and the local mechanical environment of its progenitor cells is important to understand and harness periosteum's mechanobiology and regenerative capacity. In a series of recent studies, Knothe Tate's lab showed that periosteum is hypoosmolaric (swells in phosphate buffered saline), pre-stressed and anisotropic; periosteum shrinks twice as much in the axial than in the circumferential direction when released from the underlying bone, which is indicative of pre-stress in the tissue [92]. Furthermore, the elastic modulus is 10× greater in the axial than in the circumferential direction and exhibits strain stiffening at loading rates corresponding to orthopaedic trauma. These anisotropic material properties are expected to exhibit a profound influence on bone mechanobiology, during development, growth and healing, as well as in health and disease [92].
The multiscale permeability properties of periosteum are intriguing. Periosteum tissue exhibits barrier properties, such as swelling under isotonic conditions and physiological pH [92]. Furthermore, periosteum permeability is modulated by stress, and is directionally dependent as well as site specific. For example, the permeability of the ovine femur is significantly more permeable in the bone→muscle direction when pre-stress is maintained throughout testing. When periosteum pre-stress is not maintained during testing, it is more permeable in the muscle→bone direction. At a cell-molecular scale, recent Western blot experiments show that periosteum derived cells express proteins for ZO-1 (zona occludens 1, a tight junction protein) and N-cadherin (an adherens junction protein), both of which are necessary for the formation of tight junctions, which confer barrier properties to tissues [96].
Not only prestress but also prevailing stress appears to modulate periosteum behavior. Namely, high resolution optical strain mapping of the local mechanical milieu of cells within the periosteum show that bone generation in defects correlates to regions of native, intact periosteum experiencing the greatest net change in strain [89]. In addition, histomorphometric studies demonstrate that the region of bone most resistant to bending exhibits the greatest volume of new bone generated in the first weeks after surgery. In contrast, the region of bone least resistant to bending shows the highest density of new bone generated in the same period [84].
The spatial arrangement of cells and temporal presentation of other periosteal (biochemical) factors is critical to de novo bone tissue generation [15–19]. Two recent bottom up approaches to engineering substitute periosteum comprise replicating the periosteum and its anisotropic mechanical and transport properties as well as biochemical synthetic and cell biological approaches to engineer architectures emulating periosteum's cellular and molecular organization [96–98]. In the first, an implant cum delivery device was created and tested successfully in an ovine critical sized defect model, enabling controlled and directional delivery of periosteal factors to the defect zone [97]. Furthermore, implementation of directional delivery implants designed as periosteum substitutes show that periosteum-derived cells as well as other biologic factors intrinsic to periosteum play a key role for infilling of critical sized defects. In the second approach, solid supported lipid bilayers were engineered to provide a novel substrate for the culture of periosteum derived cells and as a novel platform for bottom-up engineering and synthesis of periosteum substitutes [96].
A number of mechanobiological factors facilitate de novo tissue building by the periosteum as well as surgical and engineering approaches to unleash the power of the periosteum for trauma and reconstruction surgery. While top down approaches to understanding the endogenous capacity of the periosteum to heal defects in situ and in vivo are important to understand nature's own healing and developmental paradigms, bottom up approaches to engineer substitute periosteum or smart materials mimicking the properties of periosteum may open up novel treatment modalities to address the currently intractable clinical problem of creating bone and/or other tissues where there is none.
Conclusion
Bone repair is a dynamic process beginning with the recruitment of skeletal stem/progenitor cells during the inflammatory phase of repair, followed by cell differentiation, extracellular matrix deposition and remodeling. In humans, bone repair occurs spontaneously providing that the fractures are properly reduced. However, under certain conditions, due to extreme trauma, infection or the health status of the patient, healing may be impaired leading to delayed-union or non-union. Orthopaedic interventions to treat these skeletal repair defects aim to mechanically support repair or better stimulate the endogenous healing response and therefore mainly rely on the intrinsic regenerative capacities of bone. When these regenerative capacities are compromised, more efficient therapeutic approaches are needed and in particular cellular approaches. Although BMPs and other molecules can augment bone repair therapeutically, the demand for cell-based therapies and novel, advanced materials based implants to deliver cells and healing factors, is growing. The need for additional sources of cells is particularly evident in case of severe trauma, cancer treatment and reconstructive surgery. Skeletal developmental and degenerative diseases, such as osteogenesis imperfecta and osteoporosis, could also benefit from cell-based therapy. Many efforts are now focused on the design of new scaffolds and materials to create a biocompatible environment and means to modulate spatiotemporal delivery of skeletal stem/progenitor cells. To date, most of these engineering approaches take advantage of bone marrow-derived cells that are easily accessible and have been extensively described in the literature. This review highlights the potential of the periosteum and its resident cells as a new source of inspiration and raw material for novel regenerative medicine and tissue engineering approaches.
Figure 2. One stage bone transport model to elucidate and to harness the regenerative capacity of the periosteum.
Proximal to the defect zone, the periosteum is peeled back gently and the denuded bone underneath is osteotomized, transported and docked distally, filling the original defect zone and creating a new, more proximal defect. The periosteum which was peeled back is then sutured in place, in situ, forming a sleeve around the new, haematoma filled defect. The entire construct is stabilized by an interlocked intramedullary nail. Figure after [86].
Acknowledgements
The ORS Workshop, "Current Insights on the Regenerative Potential of the Periosteum: Molecular, Cellular and Endogenous Engineering Approaches" was organized by Dr. Melissa Knothe Tate and sponsored by the Women's Leadership Forum. CC's work is supported by Institut National de la Santé et de la Recherche Médicale, Sanofi, FP7 Marie Curie, Osteosynthesis and Trauma Care Foundation and NIH-NIAMS R01 AR053645. XZ's study is supported by grants from the Musculoskeletal Transplant Foundation (XPZ), NYSTEM N08G-495 (XPZ) and N09G346 (XPZ), and the National Institutes of Health (R21 DE021513 to XPZ, RC1AR058435 to XPZ, AR051469 to XPZ, and AR048681 to RJO and XPZ). MKT's research studies are supported through grants from the National Institutes of Health, National Science Foundation, AO Foundation, Alexander von Humboldt Foundation, the Coulter Case Translational Research Partnership and the Christopher Columbus Foundation - U.S. Chamber of Commerce.
References
- 1.Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. doi: 10.1002/jor.1100090504. [DOI] [PubMed] [Google Scholar]
- 2.Bianco P, Robey PG. Stem cells in tissue engineering. Nature. 2001;414:118–121. doi: 10.1038/35102181. [DOI] [PubMed] [Google Scholar]
- 3.Chang H, Knothe Tate ML. The periosteum: tapping into a reservoir of clinically useful progenitor cells. Stem Cells Translational Medicine. doi: 10.5966/sctm.2011-0056. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colnot C, Lu C, Hu D, Helms JA. Distinguishing the contributions of the perichondrium, cartilage, and vascular endothelium to skeletal development. Dev Biol. 2004;269:55–69. doi: 10.1016/j.ydbio.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Colnot CI, Helms JA. A molecular analysis of matrix remodeling and angiogenesis during long bone development. Mech Dev. 2001;100:245–250. doi: 10.1016/s0925-4773(00)00532-3. [DOI] [PubMed] [Google Scholar]
- 6.Colnot C. Cellular and molecular interactions regulating skeletogenesis. J Cell Biochem. 2005;95:688–697. doi: 10.1002/jcb.20449. [DOI] [PubMed] [Google Scholar]
- 7.Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev Cell. 2002;2:389–406. doi: 10.1016/s1534-5807(02)00157-0. [DOI] [PubMed] [Google Scholar]
- 8.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 9.Du X, Xie Y, Xian CJ, Chen L. Role of FGFs/FGFRs in skeletal development and bone regeneration. J Cell Physiol. 2012 doi: 10.1002/jcp.24083. [DOI] [PubMed] [Google Scholar]
- 10.Zanotti S, Canalis E. Notch regulation of bone development and remodeling and related skeletal disorders. Calcif Tissue Int. 2012;90:69–75. doi: 10.1007/s00223-011-9541-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zelzer E, Mamluk R, Ferrara N, Johnson RS, Schipani E, Olsen BR. VEGFA is necessary for chondrocyte survival during bone development. Development. 2004;131:2161–2171. doi: 10.1242/dev.01053. [DOI] [PubMed] [Google Scholar]
- 12.Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, Carmeliet G, Kronenberg HM. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19:329–344. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Schwarz EM, Young DA, Puzas JE, Rosier RN, O'Keefe RJ. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest. 2002;109:1405–1415. doi: 10.1172/JCI15681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xing Z, Lu C, Hu D, Yu YY, Wang X, Colnot C, Nakamura M, Wu Y, Miclau T, Marcucio RS. Multiple roles for CCR2 during fracture healing. Dis Model Mech. 2010;3:451–458. doi: 10.1242/dmm.003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexander KA, Chang MK, Maylin ER, Kohler T, Müller R, Wu AC, Van Rooijen N, Sweet MJ, Hume DA, Raggatt LJ, Pettit AR. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J Bone Miner Res. 2011;26:1517–1532. doi: 10.1002/jbmr.354. [DOI] [PubMed] [Google Scholar]
- 16.Vortkamp A, Pathi S, Peretti GM, Caruso EM, Zaleske DJ, Tabin CJ. Recapitulation of signals regulating embryonic bone formation during postnatal growth and in fracture repair. Mechanisms of Development. 1998;71:65–76. doi: 10.1016/s0925-4773(97)00203-7. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson C, Alpern E, Miclau T, Helms JA. Does adult fracture repair recapitulate embryonic skeletal formation? Mechanisms of Development. 1999;87:57–66. doi: 10.1016/s0925-4773(99)00142-2. [DOI] [PubMed] [Google Scholar]
- 18.Mountziaris PM, Spicer PP, Kasper FK, Mikos AG. Harnessing and modulating inflammation in strategies for bone regeneration. Tissue Eng Part B Rev. 2011;17:393–402. doi: 10.1089/ten.teb.2011.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Einhorn TA, Majeska RJ, Rush EB, Levine PM, Horowitz MC. The expression of cytokine activity by fracture callus. J Bone Miner Res. 1995;10:1272–1281. doi: 10.1002/jbmr.5650100818. [DOI] [PubMed] [Google Scholar]
- 20.Morgan EF, Mason ZD, Chien KB, Pfeiffer AJ, Barnes GL, Einhorn TA, Gerstenfeld LC. Micro-computed tomography assessment of fracture healing: relationships among callus structure, composition, and mechanical function. Bone. 2009;44:335–344. doi: 10.1016/j.bone.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Numerous articles in Russian by GA Ilizarov, dating as far back as the 1960s [Google Scholar]
- 22.Perren SM. Physical and biological aspects of fracture healing with special relevance to internal fixation. Clin Orthop Rel Res. 1979;138:175–196. [PubMed] [Google Scholar]
- 23.Prendergast PJ. Finite element models in tissue mechanics and orthopaedic implant design. Clin Biomech (Bristol, Avon) 1997;12:343–366. doi: 10.1016/s0268-0033(97)00018-1. [DOI] [PubMed] [Google Scholar]
- 24.Claes LE, Heigele CA. Magnitudes of local stress and strain along bony surfaces predict the course and type of fracture healing. J Biomech. 1999;32:255–266. doi: 10.1016/s0021-9290(98)00153-5. [DOI] [PubMed] [Google Scholar]
- 25.Gardner TN, Stoll T, Marks L, Mishra S, Knothe Tate M. The influence of mechanical stimulus on the pattern of tissue differentiation in a long bone fracture - an FEM study. J Biomech. 2000;33:415–425. doi: 10.1016/s0021-9290(99)00189-x. [DOI] [PubMed] [Google Scholar]
- 26.Smith-Adaline EA, Volkman SK, Ignelzi MA, Jr, Slade J, Platte S, Goldstein SA. Mechanical environment alters tissue formation patterns during fracture repair. J Orth Res. 2004;22:1079–1856. doi: 10.1016/j.orthres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Knothe Tate ML, Falls TD, McBride SH, Atit R, Knothe UR. Mechanical modulation of osteochondroprogenitor cell fate. Int J Biochem Cell Biol. 2008;40:2720–2738. doi: 10.1016/j.biocel.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colnot C, Thompson Z, Miclau T, Werb Z, Helms JA. Altered fracture repair in the absence of MMP9. Development. 2003;130:4123–4133. doi: 10.1242/dev.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu C, Miclau T, Hu D, Hansen E, Tsui K, Puttlitz C, Marcucio RS. Cellular basis for age-related changes in fracture repair. J Orthop Res. 2005;23:1300–1307. doi: 10.1016/j.orthres.2005.04.003.1100230610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson Z, Miclau T, Hu D, Helms JA. A model for intramembranous ossification during fracture healing. J Orthop Res. 2002;20:1091–1098. doi: 10.1016/S0736-0266(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 31.Yu YY, Lieu S, Lu C, Colnot C. Bone morphogenetic protein 2 stimulates endochondral ossification by regulating periosteal cell fate during bone repair. Bone. 2010;47:65–73. doi: 10.1016/j.bone.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu YY, Lieu S, Lu C, Miclau T, Marcucio RS, Colnot C. Immunolocalization of BMPs, BMP antagonists, receptors, and effectors during fracture repair. Bone. 2010;46:841–851. doi: 10.1016/j.bone.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Colnot C, Huang S, Helms J. Analyzing the cellular contribution of bone marrow to fracture healing using bone marrow transplantation in mice. Biochem Biophys Res Commun. 2006;350:557–561. doi: 10.1016/j.bbrc.2006.09.079. [DOI] [PubMed] [Google Scholar]
- 34.Kumagai K, Vasanji A, Drazba JA, Butler RS, Muschler GF. Circulating cells with osteogenic potential are physiologically mobilized into the fracture healing site in the parabiotic mice model. J Orthop Res. 2008;26(2):165–175. doi: 10.1002/jor.20477. 2008. [DOI] [PubMed] [Google Scholar]
- 35.Taguchi K, Ogawa R, Migita M, Hanawa H, Ito H, Orimo H. The role of bone marrow-derived cells in bone fracture repair in a green fluorescent protein chimeric mouse model. Biochem Biophys Res Commun. 2005;331:31–36. doi: 10.1016/j.bbrc.2005.03.119. [DOI] [PubMed] [Google Scholar]
- 36.Otsuru S, Tamai K, Yamazaki T, Yoshikawa H, Kaneda Y. Circulating bone marrow-derived osteoblast progenitor cells are recruited to the bone-forming site by the CXCR4/stromal cell-derived factor-1 pathway. Stem Cells. 2008;26:223–234. doi: 10.1634/stemcells.2007-0515. [DOI] [PubMed] [Google Scholar]
- 37.Granero-Moltó F, Weis JA, Miga MI, Landis B, Myers TJ, O'Rear L, Longobardi L, Jansen ED, Mortlock DP, Spagnoli A. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells. 2009;27:1887–1898. doi: 10.1002/stem.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dominici M, Marino R, Rasini V, Spano C, Paolucci P, Conte P, Hofmann TJ, Horwitz EM. Donor cell-derived osteopoiesis originates from a self-renewing stem cell with a limited regenerative contribution after transplantation. Blood. 2008;111:4386–4391. doi: 10.1182/blood-2007-10-115725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rieger K, Marinets O, Fietz T, Körper S, Sommer D, Mücke C, Reufi B, Blau WI, Thiel E, Knauf WU. Mesenchymal stem cells remain of host origin even a long time after allogeneic peripheral blood stem cell or bone marrow transplantation. Exp Hematol. 2005;33(5):605–611. doi: 10.1016/j.exphem.2005.02.004. 2005. [DOI] [PubMed] [Google Scholar]
- 40.Lu C, Marcucio R, Miclau T. Assessing angiogenesis during fracture healing. Iowa Orthop J. 2006;26:17–26. [PMC free article] [PubMed] [Google Scholar]
- 41.Grcevic D, Pejda S, Matthews BG, Repic D, Wang L, Li H, Kronenberg MS, Jiang X, Maye P, Adams DJ, Rowe DW, Aguila HL, Kalajzic I. In vivo fate mapping identifies mesenchymal progenitor cells. Stem Cells. 2012;30:187–196. doi: 10.1002/stem.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park D, Spencer JA, Koh BI, Kobayashi T, Fujisaki J, Clemens TL, Lin CP, Kronenberg HM, Scadden DT. Endogenous Bone Marrow MSCs Are Dynamic, Fate-Restricted Participants in Bone Maintenance and Regeneration. Cell Stem Cell. 2012;10:259–272. doi: 10.1016/j.stem.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res. 2009;24:274–282. doi: 10.1359/jbmr.081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang X, Xie C, Lin AS, Ito H, Awad H, Lieberman JR, Rubery PT, Schwarz EM, O'Keefe RJ, Guldberg RE. Periosteal progenitor cell fate in segmental cortical bone graft transplantations: implications for functional tissue engineering. J Bone Miner Res. 2005;20(12):2124–2137. doi: 10.1359/JBMR.050806. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie C, Liang B, Xue M, Lin AS, Loiselle A, Schwarz EM, Guldberg RE, O'Keefe RJ, Zhang X. Rescue of impaired fracture healing in COX-2−/− mice via activation of prostaglandin E2 receptor subtype 4. Am J Pathol. 2009;175(2):772–785. doi: 10.2353/ajpath.2009.081099. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xie C, Ming X, Wang Q, Schwarz EM, Guldberg RE, O'Keefe RJ, Zhang X. COX-2 from the injury milieu is critical for the initiation of periosteal progenitor cell mediated bone healing. Bone. 2008;43:1075–1083. doi: 10.1016/j.bone.2008.08.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Colnot C. Cell sources for bone tissue engineering: insights from basic science. Tissue Eng Part B Rev. 2011;17:449–457. doi: 10.1089/ten.teb.2011.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robey PG. Cell sources for bone regeneration: the good, the bad, and the ugly (but promising) Tissue Eng Part B Rev. 2011;17(6):423–430. doi: 10.1089/ten.teb.2011.0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alman BA, Kelley SP, Nam D. Heal thyself: using endogenous regeneration to repair bone. Tissue Eng Part B Rev. 2011;17:431–436. doi: 10.1089/ten.TEB.2011.0189. [DOI] [PubMed] [Google Scholar]
- 50.Rosen V. Harnessing the parathyroid hormone, Wnt, and bone morphogenetic protein signaling cascades for successful bone tissue engineering. Tissue Eng Part B Rev. 2011;17:475–479. doi: 10.1089/ten.TEB.2011.0265. [DOI] [PubMed] [Google Scholar]
- 51.Satija NK, Singh VK, Verma YK, Gupta P, Sharma S, Afrin F, Sharma M, Sharma P, Tripathi RP, Gurudutta GU. Mesenchymal stem cell-based therapy: a new paradigm in regenerative medicine. J Cell Mol Med. 2009;13:4385–4402. doi: 10.1111/j.1582-4934.2009.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang X, Awad HA, O'Keefe RJ, Guldberg RE, Schwarz EM. A perspective: engineering periosteum for structural bone graft healing. Clin Orthop Relat Res. 2008;466(8):1777–1787. doi: 10.1007/s11999-008-0312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38:1424–1429. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- 54.Xie C, Xue M, Wang Q, Schwarz EM, O'Keefe RJ, Zhang X. Tamoxifen-inducible CreER-mediated gene targeting in periosteum via bone-graft transplantation. J Bone Joint Surg Am. 2008;90:9–13. doi: 10.2106/JBJS.G.01212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Q, Huang C, Xue M, Zhang X. Expression of endogenous BMP-2 in periosteal progenitor cells is essential for bone healing. Bone. 2011;48:524–532. doi: 10.1016/j.bone.2010.10.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gamer LW, Cox K, Carlo JM, Rosen V. Overexpression of BMP3 in the developing skeleton alters endochondral bone formation resulting in spontaneous rib fractures. Dev Dyn, 2009. 2009;238(9):2374–2381. doi: 10.1002/dvdy.22048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lowery JW, Pazin D, Intini G, Kokabu S, Chappuis V, Capelo LP, Rosen V. The role of BMP2 signaling in the skeleton. Crit Rev Eukaryot Gene Expr. 2011;21(2):177–185. doi: 10.1615/critreveukargeneexpr.v21.i2.60. [DOI] [PubMed] [Google Scholar]
- 58.Tsuji K, Cox K, Bandyopadhyay A, Harfe BD, Tabin CJ, Rosen V. BMP4 is dispensable for skeletogenesis and fracture-healing in the limb. J Bone Joint Surg Am. 90:14–18. doi: 10.2106/JBJS.G.01109. [DOI] [PubMed] [Google Scholar]
- 59.Desai BJ, Meyer MH, Porter S, Kellam JF, Meyer RA., Jr The effect of age on gene expression in adult and juvenile rats following femoral fracture. J Orthop Trauma. 2003;17:689–698. doi: 10.1097/00005131-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 60.Miyaji T, Nakase T, Iwasaki M, Kuriyama K, Tamai N, Higuchi C, Myoui A, Tomita T, Yoshikawa H. Expression and distribution of transcripts for sonic hedgehog in the early phase of fracture repair. Histochem Cell Biol. 2003;119:233–237. doi: 10.1007/s00418-003-0501-z. [DOI] [PubMed] [Google Scholar]
- 61.Murakami S, Noda M. Expression of Indian hedgehog during fracture healing in adult rat femora. Calcif Tissue Int. 2000;66:272–276. doi: 10.1007/pl00005843. [DOI] [PubMed] [Google Scholar]
- 62.Vortkamp A, Pathi S, Peretti GM, Caruso EM, Zaleske DJ, Tabin CJ. Recapitulation of signals regulating embryonic bone formation during postnatal growth and in fracture repair. Mech Dev. 1998;71:65–76. doi: 10.1016/s0925-4773(97)00203-7. [DOI] [PubMed] [Google Scholar]
- 63.Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. doi: 10.1101/gad.938601. [DOI] [PubMed] [Google Scholar]
- 64.Long F, Chung UI, Ohba S, McMahon J, Kronenberg HM, McMahon AP. Ihh signaling is directly required for the osteoblast lineage in the endochondral skeleton. Development. 2004;131:1309–1318. doi: 10.1242/dev.01006. [DOI] [PubMed] [Google Scholar]
- 65.Long F, Zhang XM, Karp S, Yang Y, McMahon AP. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development. 2001;128:5099–5108. doi: 10.1242/dev.128.24.5099. [DOI] [PubMed] [Google Scholar]
- 66.St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Q, Huang C, Zeng F, Xue M, Zhang X. Activation of the Hh pathway in periosteum-derived mesenchymal stem cells induces bone formation in vivo: implication for postnatal bone repair. Am J Pathol. 2010;177:3100–3111. doi: 10.2353/ajpath.2010.100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chikazu D, Li X, Kawaguchi H, Sakuma Y, Voznesensky OS, Adams DJ, Xu M, Hoshio K, Katavic V, Herschman HR, Raisz LG, Pilbeam CC. Bone morphogenetic protein 2 induces cyclo-oxygenase 2 in osteoblasts via a Cbfal binding site: role in effects of bone morphogenetic protein 2 in vitro and in vivo. J Bone Miner Res. 2002;17:1430–1440. doi: 10.1359/jbmr.2002.17.8.1430. [DOI] [PubMed] [Google Scholar]
- 69.Tai H, Miyaura C, Pilbeam CC, Tamura T, Ohsugi Y, Koishihara Y, Kubodera N, Kawaguchi H, Raisz LG, Suda T. Transcriptional induction of cyclooxygenase-2 in osteoblasts is involved in interleukin-6-induced osteoclast formation. Endocrinology. 1997;138:2372–2379. doi: 10.1210/endo.138.6.5192. [DOI] [PubMed] [Google Scholar]
- 70.Kawaguchi H, Pilbeam CC, Gronowicz G, Abreu C, Fletcher BS, Herschman HR, Raisz LG, Hurley MM. Transcriptional induction of prostaglandin G/H synthase-2 by basic fibroblast growth factor. J Clin Invest. 1995;96:923–930. doi: 10.1172/JCI118140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Min YK, Rao Y, Okada Y, Raisz LG, Pilbeam CC. Regulation of prostaglandin G/H synthase-2 expression by interleukin-1 in human osteoblast-like cells. J Bone Miner Res. 1998;13:1066–1075. doi: 10.1359/jbmr.1998.13.7.1066. [DOI] [PubMed] [Google Scholar]
- 72.Naik AA, Xie C, Zuscik MJ, Kingsley P, Schwarz EM, Awad H, Guldberg R, Drissi H, Puzas JE, Boyce B, Zhang X, O'Keefe RJ. Reduced COX-2 expression in aged mice is associated with impaird fracture healing. J Bone Miner Res. 2009;24:251–264. doi: 10.1359/jbmr.081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Einhorn TA. The Wnt signaling pathway as a potential target for therapies to enhance bone repair. Sci Transl Med. 2010;2 doi: 10.1126/scitranslmed.3001149. 42ps36. [DOI] [PubMed] [Google Scholar]
- 74.Secreto FJ, Hoeppner LH, Westendorf JJ. Wnt signaling during fracture repair. Curr Osteoporos Rep. 2009;7:64–69. doi: 10.1007/s11914-009-0012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim JB, Leucht P, Lam K, Luppen C, Ten Berge D, Nusse R, Helms JA. Bone regeneration is regulated by wnt signaling. J Bone Miner Res. 2007;22:1913–1923. doi: 10.1359/jbmr.070802. [DOI] [PubMed] [Google Scholar]
- 76.Chen Y, Whetstone HC, Lin AC, Nadesan P, Wei Q, Poon R, Alman BA. Beta-catenin signaling plays a disparate role in different phases of fracture repair: implications for therapy to improve bone healing. PLoS Med. 2007;4:e249. doi: 10.1371/journal.pmed.0040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li X, Grisanti M, Fan W, Asuncion FJ, Tan HL, Dwyer D, Han CY, Yu L, Lee J, Lee E, Barrero M, Kurimoto P, Niu QT, Geng Z, Winters A, Horan T, Steavenson S, Jacobsen F, Chen Q, Haldankar R, Lavallee J, Tipton B, Daris M, Sheng J, Lu HS, Daris K, Deshpande R, Valente EG, Salimi-Moosavi H, Kostenuik PJ, Li J, Liu M, Li C, Lacey DL, Simonet WS, Ke HZ, Babij P, Stolina M, Ominsky MS, Richards WG. Dickkopf-1 regulates bone formation in young growing rodents and upon traumatic injury. J Bone Miner Res. 2011;26:2610–2621. doi: 10.1002/jbmr.472. [DOI] [PubMed] [Google Scholar]
- 78/71.Kamiya N. The role of BMPs in bone anabolism and their potential targets SOST and DKK1. Curr Mol Pharmacol. 2011 doi: 10.2174/1874467211205020153. [DOI] [PubMed] [Google Scholar]
- 79.Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development. 2005;132:49–60. doi: 10.1242/dev.01564. [DOI] [PubMed] [Google Scholar]
- 80.Kakar S, Einhorn TA, Vora S, Miara LJ, Hon G, Wigner NA, Toben D, Jacobsen KA, Al-Sebaei MO, Song M, Trackman PC, Morgan EF, Gerstenfeld LC, Barnes GL. Enhanced chondrogenesis and Wnt signaling in PTH-treated fractures. J Bone Miner Res. 22:1903–1912. doi: 10.1359/jbmr.070724. [DOI] [PubMed] [Google Scholar]
- 81.Banu SK, Lee J, Speights VO, Jr, Starzinski-Powitz A, Arosh JA. Selective inhibition of prostaglandin E2 receptors EP2 and EP4 induces apoptosis of human endometriotic cells through suppression of ERK1/2, AKT, NFkappaB, and beta-catenin pathways and activation of intrinsic apoptotic mechanisms. Mol Endocrinol. 2009;23:1291–1305. doi: 10.1210/me.2009-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Allen MR, Burr DB. Human femoral neck has less cellular periosteum, and more mineralized periosteum, than femoral diaphyseal bone. Bone. 2005;36:311–316. doi: 10.1016/j.bone.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 83.Allen MR, Hock JM, Burr DB. Periosteum: biology, regulation, and response to osteoporosis therapies. Bone. 2004;35:1003–1012. doi: 10.1016/j.bone.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 84.Knothe UR, Dolejs S, Matthew Miller R, Knothe Tate ML. Effects of mechanical loading patterns, bone graft, and proximity to periosteum on bone defect healing. J Biomech. 2010;43:2728–2737. doi: 10.1016/j.jbiomech.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 85.Knothe U, Springfield DSS. A novel surgical procedure for bridging of massive bone defects. World J Surg Oncol. 2005;3:7. doi: 10.1186/1477-7819-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Knothe Tate ML, Ritzman TF, Schneider E, Knothe UR. Testing of a new one-stage bone-transport surgical procedure exploiting the periosteum and bone transport for repair of long bone defects. J Bone Joint Surg Am. 2007;89:307–316. doi: 10.2106/JBJS.E.00512. [DOI] [PubMed] [Google Scholar]
- 87.Knothe Tate ML, Dolejs S, McBride SH, Matthew Miller R, Knothe UR. Multiscale Mechanobiology of De Novo Bone Generation as well as Remodeling & Adaptation of Autograft - An Integrative Review. J Mech Behav Biomed Mater. 2011;4:829–840. doi: 10.1016/j.jmbbm.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Merritt FJW, Erinc AM, Knothe Tate ML. Periosteum Regenerates on Periosteum-Denuded, Transported Bone Segment. Trans Orth Res Society. 2012;50:1570. [Google Scholar]
- 89.McBride SH, Dolejs S, Brianza S, Knothe U, Knothe Tate ML. Net Change in Periosteal Strain Correlates to Rapid De Novo Bone Generation in Critical Sized Defects. Ann Biomed Eng. 2011;39:1570–1581. doi: 10.1007/s10439-010-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sauser B. Faster healing for severe fractures. MIT Tech Rev. 2011 http://www.technologyreview.com/printer_friendly_article.aspx?id=24711. [Google Scholar]
- 91.Knothe Tate ML. Top down and bottom up engineering of bone. J Biomech. 2011;44:304–312. doi: 10.1016/j.jbiomech.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 92.McBride SH, Evans S, Knothe Tate ML. Anisotropic mechanical properties of ovine femoral periosteum and the effects of cryopreservation. J Biomech. 2011;44:1954–1959. doi: 10.1016/j.jbiomech.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Popowics TE, Zhu Z, Herring SW. Mechanical properties of the periosteum in the pig, Sus scrofa. Arch Oral Biol. 2002;47:733–741. doi: 10.1016/s0003-9969(02)00065-1. [DOI] [PubMed] [Google Scholar]
- 94.Yiannakopoulos CK, Kanellopoulos AD, Trovas GP, Dontas IA, Lyritis GP. The biomechanical capacity of the periosteum in intact long bones. Arch Orthop Trauma Surg. 2008;128:117–120. doi: 10.1007/s00402-007-0433-5. [DOI] [PubMed] [Google Scholar]
- 95.Ozaki A, Tsunoda M, Kinoshita S, Saura R. Role of fracture hematoma and periosteum during fracture healing in rats: interaction of fracture hematoma and the periosteum in the initial step of the healing process. J Orthop Sci. 2000;5:64–70. doi: 10.1007/s007760050010. [DOI] [PubMed] [Google Scholar]
- 96.Evans SF, Bernecker A, Docheva D, Richter RP, Knothe Tate ML. Junctional Adhesion Markers of Periosteum Cells as a Basis for Functionalization of Solid Supported Lipid Bilayers. Trans Orth Res Soc. 2012;50:1601. [Google Scholar]
- 97.Knothe Tate ML, Chang H, Moore SR, Knothe UR. Surgical Membranes as Directional Delivery Devices to Generate Tissue in Critical Sized Defects. PLoS One. 2011;6:e28702. doi: 10.1371/journal.pone.0028702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moore SR, Knothe U, Milz S, Knothe Tate ML. Analysis of Spatial Regeneration of Tissue Following Treatment with Artificial Periosteum. Trans Orth Res Soc. 2012;50:1600. [Google Scholar]