Abstract

Acid-sensing ion channels (ASICs), which are members of the sodium-selective cation channels belonging to the epithelial sodium channel/degenerin (ENaC/DEG) family, act as membrane-bound receptors for extracellular protons as well as nonproton ligands. At least five ASIC subunits have been identified in mammalian neurons, which form both homotrimeric and heterotrimeric channels. The highly proton sensitive ASIC3 channels are predominantly distributed in peripheral sensory neurons, correlating with their roles in multimodal sensory perception, including nociception, mechanosensation, and chemosensation. Different from other ASIC subunit composing ion channels, ASIC3 channels can mediate a sustained window current in response to mild extracellular acidosis (pH 7.3−6.7), which often occurs accompanied by many sensory stimuli. Furthermore, recent evidence indicates that the sustained component of ASIC3 currents can be enhanced by nonproton ligands including the endogenous metabolite agmatine. In this review, we first summarize the growing body of evidence for the involvement of ASIC3 channels in multimodal sensory perception and then discuss the potential mechanisms underlying ASIC3 activation and mediation of sensory perception, with a special emphasis on its role in nociception. We conclude that ASIC3 activation and modulation by diverse sensory stimuli represent a new avenue for understanding the role of ASIC3 channels in sensory perception. Furthermore, the emerging implications of ASIC3 channels in multiple sensory dysfunctions including nociception allow the development of new pharmacotherapy.
Keywords: Acid-sensing ion channel, ASIC3, nonproton ligand, nociception, mechanosensation, chemosensation
Introduction
Three decades ago, acid-induced currents blocked by amiloride in sensory neurons as well as most neurons of the mammalian brain were observed (1−3). To date, functional cloning of the ion channels underlying these currents revealed four genes that give rise to at least six ASIC informs (ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3, and ASIC4) (4,5). ASICs are trimeric channel complexes (6,7) and share overall structural similarity with other members of the epithelial sodium channel/degenerin (ENaC/DEG) family. ASIC subunits are composed of cytosolic N and C termini, two transmembrane helices, and a disulfide-rich, multidomain extracellular region, and can associate into homo- or heterotrimers (4−7). ASICs are voltage-insensitive cationic channels that are activated by the reduction of extracellular pH (4,5,8). Protons trigger a transient inward current that desensitizes rapidly in all ASICs except for ASIC3, which mediates an extra sustained current that does not fully desensitize while the extracellular pH remains acidic (9−12). Moreover, ASIC3 channels open in a nondesensitizing manner (13) at more physiologically relevant pH values (pH 7.3−6.7). Recently, we characterized a novel nonproton ligand sensor domain in the ASIC3 channel responding to synthetic compounds such as 2-guanidine-4-methylquinazoline (GMQ) and an endogenous GMQ analogue, agmatine, at neutral pH in a nondesensitizing manner (13). ASIC3 is widely expressed in sensory neurons as well as in peripheral non-neuronal tissues (9−11) (Figure 1). Therefore, ASIC3 channels are well positioned to participate in multimodal sensory perception.
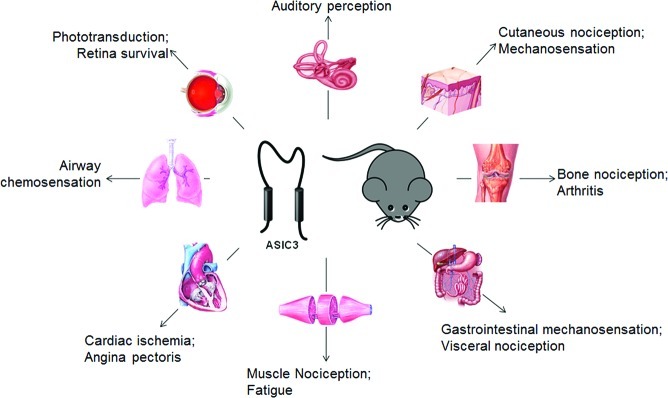
Figure 1.
Distribution of ASIC3 in peripheral tissues and its potential roles. In addition to its well-known roles in DRG neurons (see the text), ASIC3 has an unexpected widespread expression in a variety of non-neuronal tissues as indicated.
Properties and the Distribution of ASIC3
Distribution Profiles
In mammals, there is a defined, although not absolute, specificity of the distribution of various ASIC subtypes between the central nervous system (CNS) and peripheral nervous system (PNS). ASIC1a, ASIC2a, and ASIC2b are expressed in both PNS and CNS, while ASIC1b and ASIC3 are primarily expressed in sensory neurons (4,5). As the most sensitive ASIC subtype to extracellular pH, the widespread distribution of ASIC3 is consistent with its role as key receptors for extracellular protons in peripheral tissues. The actual distribution of ASIC3 in sensory neurons is not clear; however, ASIC3 is highly expressed in sensory neurons that govern the excitability of specific tissues (Figure 1), such as cardiac sensory neurons (14), cutaneous sensory neurons (15), metaboreceptive sensory neurons (16), dural afferent trigeminal sensory neurons (17), and so on. In the dorsal root ganglion (DRG) and nodose ganglia sensory neurons, ASIC3 subunits mainly form heteromultimeric channels with ASIC2a (18,19) or ASIC1 (20), or other ASIC subunits (21,22).
In addition to sensory neurons, ASIC3 has been demonstrated to be expressed in a large number of different peripheral tissues (Figure 1). In the respiratory system, ASIC3 is expressed in the apical membranes of lung epithelial cells and colocalizes with the cystic fibrosis transmembrane conductance regulator (CFTR) (23). Moreover, ASIC3 is shown to express in the prickle cells and muscular mucosa of the rat esophagus (24), in rat carotid body glomus cells (25), and in nucleus pulposus cells of the intervertebral disk (26) in addition to type B synoviocytes and chondrocytes (27,28) as well as their innervating sensory neurons (29).
Sustained Activation and Window Currents
Generally, when activated by extracellular protons, ASIC channels exhibit a transient (peak) current (Figure 2, panel b) that lasts hundreds of milliseconds to several seconds, followed by channel desensitization during the continued presence of acidic external solution (4,5). The rapid desensitization kinetics often calls into question the long-lasting physiological roles of ASICs (4,5). ASIC3, however, is well suited to play a long-lasting physiological role because it mediates a sustained current (Figure 2, panel c) that does not fully desensitize during the continued presence of acidic extracellular pH (9−12). Moreover, this sustained component of ASIC3 becomes markedly enhanced when the channel is activated in the presence of FMRFamide (Phe-Met-Arg-Phe amide) and related neuropeptides (30−32). FMRFamide is an invertebrate neurotransmitter, but its related neuropeptides, such as neuropeptide FF (NPFF, Phe-Leu-Phe-Gln-Pro-Gln-Arg-Phe amide), are expressed in the mammalian CNS (30), arguing for a physiological role of neuropeptide-enhanced ASIC3 activity in mammals (31,32). Indeed, the interaction between neuropeptides and ASICs in regulating inflammatory pain-like behaviors in rodents has been a topic of recent reviews (33,34). In addition to neuropeptides, there may be other small molecule modulators from the inflammatory soup that have similar stimulating effects on ASIC3 channels (4). Together, these ASIC3-enhancing effects are likely of physiological relevance in pain sensation (36). Consistent with this notion, inhibition of the sustained component of ASIC3 currents by a synthetic compound A-317567 resulted in analgesia (37,38). However, RF-amide related peptides (FMRFamide, NPFF, and so on) may also affect other unidentified molecular targets responsible for nociceptive signaling (39). Thus, both functional outputs for the enhanced sustained component of ASIC3 currents and the molecular targets of the algogenic effect of FRMFamide are still ambiguous.
Figure 2.
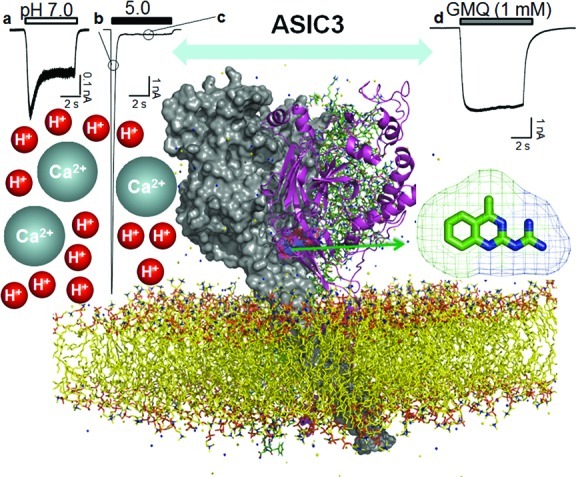
Structure and activation modes of ASIC3. The structural model of ASIC3, shown parallel to the membrane layer, is a homologue model based on the X-ray crystal structure of ASIC1 (chicken, PDB code: 2QTS) (7). One ASIC3 subunit is shown in cartoon view, while the other two ASIC1 subunits are shown in line view or surface view. As a proton-gated ion channel, ASIC3 channels manifest different kinds of channel activation, shown with different scale and kinetics, in response to extracellular acidosis. Mild acidosis (i.e., pH 7.0) induces a considerable peak current followed by a slow desensitizing current (panel a). Extreme acidosis (i.e., pH 5.0) induces a larger peak current (panel b) and a much smaller sustained current (panel c). In addition to acid-induced channel activation, nonproton ligands (i.e., GMQ) induce a sustained current (panel d) at physiologically normal pH (pH 7.4) (13). The arrow highlights the nonproton ligand sensor domain lined by residues around E423 and E79 of the extracellular palm domain of the ASIC3 channel (13). Different modes of ASIC3 activation may correspond to different sensory perception roles (see the text). The typical current traces show differential kinetics of channel activation responding to protons and GMQ, respectively. Note the scale difference of the current amplitude.
In addition to the pronounced sustained component induced under strong acidosis, ASIC3 channels manifest a marked slowly desensitizing current (40) in response to mild acidosis near physiological pH (pH 7.3−6.7) (Figure 2, panel a). The bell-shaped relationship between current amplitude and pH changes suggests that the overall current may result from the combined effects of steady-state activation and desensitization of ASIC3 (40), giving rise to a characteristic “window current” (15,40). Lactic acid increases the pH sensitivity of the window current (40), presumably through a mechanism of chelation of extracellular Ca2+41,42. Furthermore, newly identified nonproton ligands including an endogenous ligand agmatine (13) also significantly enhanced the window current, supporting a specific role of the sustained window current in pathophysiological processes. This property of ASIC3 matches the acid-gated current in cardiac ischemia-sensing neurons (14). Importantly, the modest pH changes (pH 7.3−6.7) for ASIC3 activation most likely occur during myocardial ischemia (40), which argues for an important role of ASIC3 in cardiac signaling. Similar pH changes enabling ASIC3 activation might occur under other conditions, such as inflammatory pain (15).
Nonproton Ligand Sensor Domain in ASIC3
Recently, a synthetic compound GMQ was shown to cause persistent activation of ASIC3 (Figure 2, panel d) at neutral pH (13). Using GMQ as a probe and combining mutagenesis and covalent modification analysis, we uncovered a novel nonproton ligand sensor motif lined by residues around E423 and E79 of the extracellular palm domain (6,7) of the ASIC3 channel (Figure 2). Moreover, we identified an endogenous ligand, agmatine, which also activated ASIC3 via the nonproton ligand sensor domain (13). Interestingly, the nonproton ligands also acted synergistically with mild acidosis or reduced extracellular Ca2+ to activate ASIC3 and to facilitate its window current (13). There may exit structural overlaps or interactions (Figure 2) among multifunctional domains that underlie ASIC3 activation by mild acidosis and the newly identified nonproton ligand (13), as well as its inhibition by Ca2+. Therefore, activation of ASIC3 independent of acidosis may open a new avenue for exploring the role of ASIC3 in sensory perception. Identification of yet unknown endogenous nonproton ligands and demonstration of ligand−ASIC3 interaction in the context of the sensory environment could represent a new direction for the field of ASIC3 and sensory perception.
Activation Modes of ASIC3
As the name indicated, ASICs are traditionally recognized as proton-gated ion channels (4,5,8). The discovery of nonproton ligands (Figure 2, panel d) for ASIC3 (13) suggests the potential for modular activation of ASIC3. As the simplest ligand, H+ could in principle gate a channel by titration of any single polar amino acid (43). Alternatively, ASIC3 has been proposed to be opened by catalyzing the relief of Ca2+ blockade (42). At the resting state, ASIC3 is stabilized by Ca2+ binding to specific sites (7,44), presumably with different affinities (Figure 2). Therefore, ASIC3 manifests different activation characteristics in response to different pH solutions (9−12), resulting from graded protonation states of the ASIC3 channel protein or relief from Ca2+ blockade. However, it is difficult to calculate the degree of acid-evoked channel activation at different pH values. Generally, the acidic pH can be categorized as mild and extreme acidosis, although there is no absolute cutoff point. Mild acidosis (pH 7.0, Figure 2, panel a) induces both a transient current and a sustained window current (40), while extreme acidosis (pH 5.0) induces a much larger transient current (Figure 2, panel b) followed by a much smaller sustained component (Figure 2, panel c) in the continued presence of the acidic pH solution (9−12). The two distinct activation modes (Figure 2, panels b and c) might correspond to different sensory stimuli under different physiological environments. For example, the rapidly desensitizing ASIC3 current argues that ASIC3 is best suited to respond to rapid pH fluctuations under certain physiological conditions (45). It is reasonable to hypothesize that touch stimuli activate ASIC3 channels through the rapid release of protons, generating a rapidly adapting response in the touch receptor. Accordingly, ASIC3 has been suggested to participate in touch perception (46) (see below). However, the sustained current evoked by mild acidosis, which can be amplified by and/or evoked independently by nonproton ligands, might underlie the long-lasting physiological roles of these channels under conditions such as inflammatory pain (15), cardiac ischemia, and angina pectoris (40). Furthermore, inflammatory or ischemic signals, such as hyperosmolarity, arachidonic acid, and lactic acid, all enhance the window current and thereby amplify the ASIC3 response (15) (Figure 3). These synergetic interactions on the sustained window current by inflammatory or ischemic cofactors emphasize the critical roles of ASIC3 in sensory perception.
Figure 3.
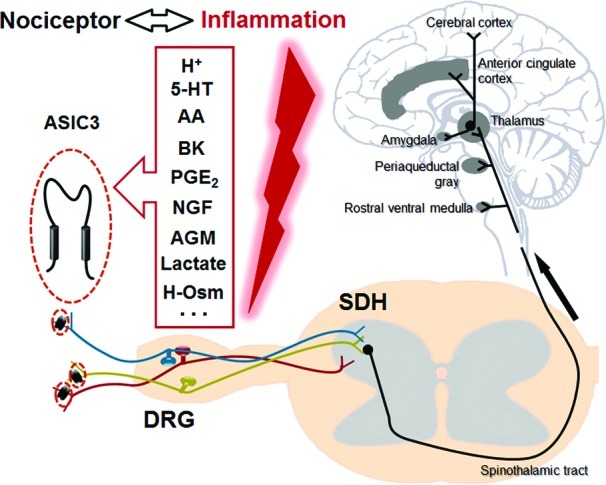
ASIC3-dependent nociceptive signaling pathway during inflammation. ASIC3 is expressed in nociceptors, which respond to many pro-inflammatory mediators, such as acidosis (H+), serotonin (5-HT), arachidonic acid (AA), bradykinin (BK), prostaglandin E2 (PGE2), nerve growth factor (NGF), and agmatine (AGM). Both H+ and AGM induce ASIC3 channel activation independently, although they are mutually interacting. AA and hyperosmolarity (H-Osm) indirectly facilitate ASIC3 activation. 5-HT, BK, PGE2, and NGF stimulate ASIC3 expression. The multicolor DRG neurons represent diverse sensory pathways relaying various nociceptive inputs dependent on ASIC3 channels to the spinal dorsal horn (SDH) under inflammation, although the exact distribution profiles of ASIC3 in nerve fibers are unclear. Stimulation of the nociceptor triggers a synaptic response in the SDH, and the noxious stimuli are then transmitted to the supraspinal structures as indicated.
Roles in Nociception
The location and properties of ASIC3 make it an ideal candidate as a nociceptor sensing pH reduction associated with inflammation. It is well known that peripheral pH falls to <7 in inflammation, infection, ischemia, hematomas, and exercise (4). Moreover, such acidosis is well recognized to activate nociceptors and produce pain in humans that can be attenuated by the DEG/ENaC inhibitor amiloride (47−49). Additionally, inflammatory mediators, such as nerve growth factor (NGF), serotonin, interleukin-1, bradykinin, and brain-derived neurotrophic factor (BDNF) can stimulate ASICs transcription, including ASIC3, which perhaps contributes to the pain-enhancing effects of these mediators (50,51) (Figure 3).
Cutaneous Nociception
Although it seemed that studying gene-targeted mice would clarify the role of ASIC3 in nociception, reports to date give a mixed picture. Price et al. (46) showed that disrupting the mouse asic3 gene altered sensory transduction in specific and distinct ways. For example, in the isolated skin-nerve preparation, the response to a drop of pH to 5.0 was reduced in C fibers of ASIC3 null animals. The loss of ASIC3 increased the sensitivity of mechanoreceptors to light touch, but it reduced the sensitivity of a mechanoreceptor to respond to a noxious pinch and decreased the responses of acid- and noxious heat-sensitive nociceptors (46). In contrast, Chen et al. (52) found an increased sensitivity to thermal, mechanical, and acidic stimuli in ASIC3 knockout mice but detected no alterations in hypersensitivity after capsaicin or carrageenan treatment. In transgenic mice overexpressing a dominant-negative form of ASIC3, it has been shown that the response to thermal stimuli was normal, but the animals showed an increased response to intraperitoneal acid injection and mechanical hypersensitivity after inflammatory stimuli (53). There has been no satisfactory explanation for these discrepancies so far. Possible causes are the variability in genetic background or species, differences in testing paradigms, environmental variability, and other compensatory effects. For example, Mogil et al. (53) reported an elevated function of transient receptor potential vanilloid type 1 (TRPV1) channels in ASIC3-deficient mice. This increased TRPV1 activity may contribute to the observed hypersensitivity to painful stimuli in mice with impaired ASIC3 function. In addition, there might be species differences for the role of ASIC3 in cutaneous pain. Mice express relatively low levels of ASICs in their DRG neurons (54,55) as compared with other species such as rats (15).
Recently, it has been reported that activation of peripheral ASIC3 by acidosis and inflammatory mediators contributes to inflammatory pain in rats (15). About 60% of rat cutaneous sensory neurons display ASIC3-like currents. Native as well as recombinant ASIC3-containing channels respond synergistically to three different inflammatory signals, i.e., mild acidosis (∼pH 7.0), hyperosmolarity, and arachidonic acid. Moderate pH, alone or in combination with hyperosmolarity and arachidonic acid, increases nociceptor excitability (Figure 3) and causes pain-like behaviors suppressed by the toxin APETx2, a specific blocker of ASIC3. Both APETx2 treatment and the in vivo knockdown of ASIC3 with a specific siRNA have potent analgesic effects against primary inflammation-induced hyperalgesia in rats. Thus, peripheral ASIC3 channels are essential sensors of acidic pain and integrators of molecular signals produced during inflammation when they contribute to primary hyperalgesia in rats. More recently, Yen et al. (56) reinvestigated the role of ASIC3 in primary hyperalgesia through more comprehensive behavioral analysis and pathological evaluation in mice. These authors concluded that ASIC3 participated in the maintenance of subacute-phase primary hyperalgesia in subcutaneous inflammation in mice (56). Compared with the wild-type (WT) mice, asic3−/− mice showed normal thermal and mechanical hyperalgesia in acute (4-h) intraplantar complete Freund's adjuvant (CFA)- or carrageenan-induced inflammation, but the hyperalgesic effects in the subacute phase (1−2 days) were milder in all paradigms except for thermal hyperalgesia with CFA-induced inflammation. Interestingly, carrageenan-induced primary hyperalgesia was accompanied by an ASIC3-dependent Nav1.9 up-regulation and an increase of tetrodotoxin (TTX)-resistant sodium currents. Furthermore, asic3−/− mice showed attenuated pathological features such as less CFA-induced granulomas and milder carrageenan-evoked vasculitis as compared with the WT mice. Therefore, the role of ASIC3 in cutaneous nociception may depend on a specific phase of the inflammatory process. That ASIC3 participates in cutaneous nociception in both rats and mice is reminiscent of the findings that acid injection in humans activates nociceptors and produces pain that can be attenuated by the DEG/ENaC inhibitor amiloride (47−49). These data suggest that drugs targeting ASIC3 channels hold the potential to be effective analgesics in humans.
Muscle Nociception and Fatigue
In contrast to the conflicting results generated from studies of ASIC3 knockout or dominant-negative mutant mice concerning its roles in cutaneous pain, the results on the roles of ASIC3 in muscles are more consistent. Sluka et al. (57) showed that DRG neurons that innervate muscles express ASIC3 and respond to acidic pH with a fast inward current followed by a sustained component that resembles those mediated by ASIC3 channels. Repeated injection of acidic saline into the gastrocnemius muscle produced long-lasting mechanical hyperalgesia in WT but not asic3−/− mice (57). Moreover, mechanical hyperalgesia is prevented by prior treatment of the muscle with amiloride (57) or APETx2 (58), the nonselective ASIC antagonist or selective ASIC3 inhibitor, respectively, suggesting the involvement of ASIC3 channels. In contrast, asic1−/− mice developed hyperalgesia in a manner similar to that of their WT littermates (57), suggesting a specific role of ASIC3 in muscle pain. However, the heat but not mechanical hyperalgesia induced by muscular carrageenan injection remained even when the asic3 gene was disrupted (59). Injection of an ASIC3-encoding virus into muscle but not skin of asic3−/− mice resulted in the development of mechanical hyperalgesia similar to that observed in WT mice (59). Thus, ASIC3 in primary afferent fibers that innervate muscle is critical to the development of mechanical but not heat hyperalgesia associated with muscle inflammation. Furthermore, Walder et al. (60) found that asic3−/− mice showed deficits specifically in secondary hyperalgesia (increased response to noxious stimuli outside the site of injury) but not primary hyperalgesia (increased response to noxious stimuli at the site of injury). By contrast, asic1−/− mice did not develop primary muscle hyperalgesia but developed secondary paw hyperalgesia. Therefore, peripheral ASIC1 and ASIC3 appear to play differential but complementary roles (ASIC1 for primary vs ASIC3 for secondary) in the development of hyperalgesia after inflammatory muscle injury. Mechanistically, Gautam at al. (61) proposed that the increased response of muscle innervating sensory neurons to decreases in pH during muscle inflammation might underlie the role of ASIC3 in muscle pain.
Muscle fatigue is associated with a number of clinical diseases, including chronic pain conditions. Burnes et al. (62) examined the role of ASIC3 in the development of muscle fatigue. Using exercise protocols to measure exercise-induced muscle fatigue, they found that enhanced muscle fatigue occurs in male but not female asic3−/− mice in a task-dependent manner (62). Moreover, WT female mice that were ovariectomized and administered testosterone developed less muscle fatigue than control female mice and behaved similarly to WT male mice. However, testosterone was unable to rescue the muscle fatigue responses in ovariectomized asic3−/− mice. Also, the cytoplasmic level of testosterone from male asic3−/− mice was significantly lower than that in WT male mice and was similarly low in female WT mice. These data suggest that both ASIC3 and testosterone are necessary for protecting against muscle fatigue (62). Moreover, differences in the expression of ASIC3 and the development of exercise-induced fatigue could explain the female predominance in clinical syndromes of pain that include muscle fatigue (62). The underlying mechanisms are unclear but could involve decreases in extracellular pH, activation of ASIC3 channels, and subsequent muscle depolarization, which protects against fatigue or causes pain sensation. Moderate exercise increases the expression of asic3 mRNA in chronic fatigue syndrome patients but not in normal subjects (63). Given the urgent needs for clinical treatment of muscle pain and fatigue, it is conceivable that ASIC3 channels represent a novel molecular target for future drug therapy (64).
Cardiac Ischemia and Angina Pectoris
Sensory neurons that innervate the heart sense ischemic injury and mediate angina pectoris. Benson et al. (65) first characterized acid-evoked currents in rat cardiac sensory neurons using fluorescent labeling and patch-clamp recording techniques. Acidic pH evoked extremely large depolarizing currents in almost all cardiac afferent neurons from DRG and nodose ganglia, implying a critical role for acid sensation associated with myocardial ischemia (65). The electrophysiological and pharmacological properties of acid-evoked currents in cardiac ischemia-sensing neurons resemble those of ASIC3 channels (14,66). Furthermore, Yagi et al. (40) found that in both cardiac sensory neurons and cell lines expressing rat ASIC3 channels, an acidic solution (pH 7.3 to 6.7) evoked sustained window currents that persisted over tens of minutes. Lactic acid as an anaerobic metabolite allows the currents to be evoked at slightly more basic pH, a pH condition often associated with myocardial ischemia. Moreover, the newly identified nonproton ligands could further facilitate the window current carried by ASIC3 channels (13). Although speculative, as a remarkable feature unique to ASIC3 channels, the sustained window current might be most relevant for triggering angina and other types of ischemic pain in rats and probably in humans.
In addition to the results from rats, the role of ASIC3 in cardiac sensory perception in mice has also been investigated (18). ASIC2a and ASIC3 heteromultimerized to form pH-sensitive channels in mouse cardiac sensory neurons (18). This was supported by studies in ASIC-null mice, where acid-evoked currents from asic3−/− cardiac afferents matched the properties of ASIC2a channels, while those from asic2−/− cardiac afferents matched the properties of ASIC3 channels. Since the ASIC2a/3 heteromers give much larger sustained currents than the ASIC3 homomers (40), a similar sustained window current mode for these ASIC3-containing channels may underlie cardiac ischemia and angina pectoris in mice as well. Because ASIC3 is widely distributed in cardiac sensory neurons, further genetic and functional studies are required to characterize the specific role of ASIC3 in cardiac signaling using cardiac ischemia and angina pectoris models.
Visceral Nociception
Members of the ASIC family are strong candidates for mechanical transducers in sensory function, including colonic mechanotransduction and visceral pain in the gastrointestinal system. Page et al. (67) systemically investigated the role of ASIC1, 2, and 3 in gastrointestinal mechanosensory function using asic gene-deficient mice. These authors reported that (1) the disruption of ASIC1 increased the mechanical sensitivity of splanchnic colonic afferents and vagal gastro-esophageal afferents (67,68); (2) in ASIC2 knockout mice, mechanosensitivity was increased in gastro-esophageal mucosal endings and colonic serosal endings but was decreased in gastro-esophageal tension receptors and remained unchanged in colonic mesenteric endings; and (3) the disruption of ASIC3 markedly reduced the mechanosensitivity of all afferent classes except gastro-esophageal mucosal receptors. Therefore, ASIC3 makes the critical positive contribution to mechanosensitivity in most of the visceral afferents, whereas ASIC1 appears to exert an inhibitory regulation to the ion channel complex (67). However, the role of ASIC2 differs widely across subclasses of afferents. As supporting evidence, the expression ASIC1, 2, and 3 mRNAs in mouse colonic sensory neurons within thoracolumbar DRG was reported (69). Furthermore, the inhibitory effect of benzamil on colonic afferent mechanosensitivity was markedly diminished in asic2−/− and asic3−/− mice but remained unchanged in asic1−/− mice (70), supporting the idea of using ASIC3 and ASIC2 as molecular targets for developing clinical drugs (i.e., benzamil) for the treatment of visceral pain.
Jones et al. (71) assessed the visceral nociception, modeled by the visceromotor response to colorectal distension and colon afferent fiber mechanosensitivity, in control mice and two congenic knockout mouse strains with deletions of either TRPV1 or ASIC3. They found that both TRPV1 and ASIC3 knockout mice were significantly less sensitive to distension, with an average response magnitude of only about 58% and 50% of the WT controls, respectively. The behavioral deficits observed in both strains of knockout mice were associated with a significant and selective reduction in afferent fiber sensitivity to the circumferential stretch of the colon. In addition, whereas stretch-evoked afferent fiber responses were enhanced by chemical inflammatory mediators in control mice, they were differentially impaired in both knockout mouse strains. These results demonstrate a peripheral mechanosensory role for TRPV1 and ASIC3 in the mouse colon that contributes to nociceptive behavior and possibly peripheral sensitization during tissue insult. Moreover, these authors (72) used a mouse model (i.e., intracolonic treatment with zymosan) that reproduces the major features of irritable bowel syndrome (long-lasting colon hypersensitivity without inflammation) to examine the role of TRPV1 and ASIC3 in the development of behavioral hypersensitivity and assessed the function of colon mechanoreceptors of hypersensitive mice. As expected, the behavioral hypersensitivity was partially dependent on both TRPV1 and ASIC3 since deletions of either of these genes blunted the zymosan effect, suggesting that ASIC3 and TRPV1 are important peripheral mediators of functional (i.e., noninflammatory) visceral hypersensitivity.
Bone Nociception
After being identified in the bone (27), the contribution of ASICs to joint inflammation has received increasing attention. Ikeuchi et al. (73) tested for the first time whether ASIC3 was necessary for the development of both primary and secondary mechanical hyperalgesia following joint inflammation. They reported that secondary mechanical hyperalgesia did not develop in asic3−/− mice but that primary mechanical hyperalgesia of the inflamed knee joint still remained (73). This mode is similar to the role of ASIC3 in muscle pain (60). Furthermore, the expression of ASIC3 was increased in an acute arthritic pain model of mice (29), supporting the idea that ASIC3 is a promising therapeutic target for pain control in arthritis (74).
Roles in Mechanosensation
Mechanosensory transduction refers to processes that convert mechanical forces into bioelectrical signals. Mechanotransduction is important for different physiological functions such as touch, hearing, or proprioception. Several members of the ENaC/DEG family of ion channels have been proposed to participate in mechanosensation, the most famous being the degenerins in Caenorhabditis elegans, where they play a critical role in touch sensation and proprioception (75). ASIC3 channels are expressed in mechanoreceptors, including specialized cutaneous mechanosensory structures such as Meissner corpuscles, Merkel nerve endings, free nerve endings, and palisades of lanceolate nerve endings surrounding the hair shaft (46). Mice with targeted deletion of ASIC3 were reported to display a subtle increase in normal light touch perception and painful cutaneous mechanical sensitivity (46) (see also the Cutaneous Nociception section above). A stomatin-domain protein essential for touch sensation in the mouse, the stomatin-like protein 3 (SLP3), has been shown to associate in vitro with ASIC3, but not TRPV1, a control sensory ion channel (76). SLP3 inhibits the endogenous proton-gated currents mediated by ASICs in sensory neurons (76), suggesting the involvement of ASIC3 in mechanosensory complexes. Recently, asic3−/− mice and their WT counterparts were exposed to a novel stroking stimulus to test sensitivity to dynamic mechanical stimulation (77). The asic3−/− mice were significantly more sensitive (77), which is consistent with the early report showing increased light-touch response in asic3−/− mice (46). These data strongly suggest the participation of ASIC3 in touch perception. However, whether ASIC3 plays a role as direct peripheral mechanoreceptors is unclear due to the lack of evidence for the mechanical gating of these channels (78).
The role of ASIC3 in visceral mechanosensation has been discussed in the previous section entitled Visceral Nociception. Auditory perception is a special type of mechanosensation. Both the ASIC3 transcript and protein were found to be enriched in the cochlea (79). ASIC3 expression was shown in cells and neural fibers of the spiral ganglion as well as cells of the organ of Corti (79). In addition to the normal mouse ASIC3 cDNA, an alternatively spliced transcript was elucidated by RT-PCR from the mouse inner ear (79). This transcript may represent a new isoform with an as yet unknown function. The asic3−/− mice were tested for a hearing loss phenotype and were found to have normal hearing at 2 months of age but appeared to develop hearing loss early in life, suggesting a contributing role of ASIC3 in hearing. As a piece of following evidence, ASIC3 was implicated in hearing perception thereafter to affect the social development of pups (80). This specific deficit further strengthens the role of ASIC3 in auditory perception.
Osmoreception represents another type of mechanosensation. Systemic osmoregulation is a vital process whereby changes in plasma osmolality, detected by osmoreceptors, modulate ingestive behavior, sympathetic outflow, and renal function to stabilize the tonicity and volume of the extracellular fluid (81). It remains an open question whether ASIC3 is involved in osmoregulation. ASIC3 activation was potentiated by hyperosmolarity (15), suggesting that an osmosensor domain might relate to or overlap with the known proton sensors (15) and/or the newly identified nonproton sensor (13) residing in ASIC3 channel complexes. The physiological relevance of the osmosensitivity of ASIC3 was suggested by the finding that the enhancing effect of hyperosmolarity on pain-related behavior of rats was diminished by ASIC3 blocker APETx2 (15). However, the exact role of ASIC3 in systemic osmoregulation at the behavioral level awaits further experimentations. Interestingly, a recent statistical association analysis between genetic polymorphisms of the asic3 gene and blood pressure variations in Taiwanese suggested that genetic variation in the asic3 gene influences blood pressure levels (82). However, this effect may result from chemosensation (83) (see below) rather than osmoregulation.
Roles in Chemosensation
ASIC3 senses extracellular acidosis, Ca2+ levels, and some metabolites, thereby participating in many aspects of chemosensation. Molliver et al. (16) identified a class of sensory neurons which innervate blood vessels and express ASIC3. They suggested that these afferents might be muscle metaboreceptors. Therefore, when there is insufficient oxygen, neurons that sense the metabolic state of muscle can trigger pain, particularly when there is increased production of lactic acid, which enhances ASIC3 currents through chelation of Ca2+41. Moreover, Huang et al. (84) reported that ASIC3 was functionally expressed in adipose cells. The asic3−/− mice were protected against age-dependent glucose intolerance with enhanced insulin sensitivity. Acute administration of the ASIC3-selective blocker APETx2 improved glucose control and increased insulin sensitivity in older (25−27 weeks) WT mice. Therefore, ASIC3 signaling might be involved in the control of age-dependent glucose intolerance and insulin resistance (84). In support of this finding, a routine health examination among Taiwanese suggested a close association between certain ASIC3 gene variants and insulin resistance (85).
Carotid body chemoreceptors sense hypoxemia, hypercapnia, and acidosis, and play an important role in cardiorespiratory regulation. Tan et al. (25) characterized the contribution of ASICs to the transduction of extracellular acidosis in rat carotid body glomus cells and showed that ASIC3 was expressed in the rat carotid body and manifested an acidosis-evoked current, which was inhibited by the ASIC blocker amiloride and enhanced by either Ca2+-free buffer or the addition of lactic acid. These authors (25) proposed that ASIC3 may contribute to the chemotransduction of low pH by carotid body chemoreceptors and that extracellular acidosis directly activates carotid body chemoreceptors through ASIC3 activation. Additionally, it was demonstrated that chemoreceptor hypersensitivity and up-regulation of ASIC3 occurred before the onset of hypertension in spontaneously hypertensive rats (83). This observation established a novel molecular basis for increased chemotransduction that contributes to excessive sympathetic activity before the onset of hypertension. As other aspects of chemosensation, both CO2 chemosensing in the rat esophagus (24) and chemosensation in epithelial cells of the airway system (23), also involve ASIC3 activation. Apparently, more efforts are required before a clearer picture comes up with the exact role of ASIC3 in chemosensation.
Participation in Other Sensory Modalities
ASIC3 has a role in retinal function and cell survival. ASIC3 is present in the rod inner segment of photoreceptors and in horizontal and some amacrine cells (86). Inactivation of ASIC3 enhanced visual transduction at the age of 2 to 3 months but induced late-onset rod photoreceptor death in mice, suggesting an important role of ASIC3 in maintaining retinal integrity (86). ASIC3 was also reported to be expressed in the rat vestibular endorgans and ganglia (87), hypothalamus (88), and suprachiasmatic nucleus (89), in which the roles of ASIC3 have not been explored.
Concluding Remarks
ASIC3 has been shown to participate in a number of different sensory processes (Figure 1). Activation of ASIC3 channels in sensory neurons by acidosis-associated cardiac ischemia, inflammation, tumors, or injury has been proposed to contribute to the generation of pain. Moreover, the acidosis-dependent ASIC3 activation is potentiated by various inflammatory and nociceptive signals. Thus, ASIC3 acts as an integrator and works as a modular protein to sense multiple stimuli (Figures 2 and 3), including acidosis, lactate production, inflammatory mediators, hyperosmolarity, and agmatine. The role of ASIC3 in nociception is further complicated by its differential contribution to cutaneous nociception, muscle nociception and fatigue, visceral nociception, cardiac nociception, and bone nociception (Figure 1). Each type of nociception may correlate with specific sensory stimuli or environmental changes and use distinct mechanisms, implying the modular nature of the ASIC3 channels in multisensory perception. Exploring the structural basis for individual aspects of ASIC3 activation or desensitization represents a substantial future challenge. ASIC3 also participates in the mechanosensory function, but the molecular basis remains largely unknown. It is interesting to know whether such mechanotransduction is pH-dependent or independent. In this regard, the recent discovery of novel nonproton ligands (13) raises the possibility that ASIC3-dependent mechanosensation (46,67,71) may be mediated by as yet unknown ligands induced by mechanical stimuli.
ASIC3 has intrinsic osmosensitivity, which might underlie systematic osmoregulation. ASIC3 is clearly involved in chemosensation, sensing a variety of chemical signals including acidosis, reduced oxygen level, Ca2+ level, hyperosmolarity, and so on, either in neurons or at the carotid body, esophagus, or airway system. ASIC3 is present in the retina, inner ear, and vestibular endorgans. It is an important modulator of visual transduction and might have potential roles in equilibratory sensation and hearing.
Finally, Wu et al. (90) evaluated possible central alterations or compensatory changes in the CNS in asic3−/− mice but found no significant deficits in hippocampal synaptic plasticity, locomotion activities, fear memory, and spatial learning and memory. These authors showed that mice lacking ASIC3 displayed reduced anxiety-like behavior on the elevated plus maze and reduced aggression, two types of paradigms reflecting central processing of emotion. Because the expression level of ASIC3 is low and limited to the brainstem and hippocampus (91) of CNS in mice, Wu et al. (90) suggested that ASIC3-dependent sensory activities might be related to the central processing of emotion independent of ASIC3 activities in CNS. Since ASIC3 transgenic and knockout mice have deficits in pain perception (see above), which may also involve altered emotions, comprehensive electrophysiological examinations at the level of both PNS (i.e., DRG) and CNS (e.g., spinal cord dorsal horn, brain stem, thalamus, and cortices; see Figure 3) should be performed on these mice in future studies.
Acknowledgments
We thank Dr. James Celentano for reading the manuscript and Zhu-Dan Zhang for assistance in the preparation of figures.
Abbreviations
ASIC, acid-sensing ion channel; BDNF, brain-derived neurotrophic factor; CFA, complete Freund's adjuvant; CFTR, cystic fibrosis transmembrane conductance regulator; CNS, central nervous system; DRG, dorsal root ganglion; ENaC/DEG, epithelial sodium channel/degenerin; FMRFamide, Phe-Met-Arg-Phe amide; GMQ, 2-guanidine-4-methylquinazoline; NPFF, neuropeptide FF (Phe-Leu-Phe-Gln-Pro-Gln-Arg-Phe amide); NGF, nerve growth factor; PNS, peripheral nervous system; SLP3, stomatin-like protein 3; TRPV1, transient receptor potential vanilloid type 1; TTX, tetrodotoxin; WT, wild-type.
W.G.L. and T.L.X. wrote the manuscript.
This study was supported by grants from the National Natural Science Foundation of China (No. 30830035), the National Basic Research Program of China (No. 2011CB809000), and the Shanghai Municipal Government (09XD1404900).
References
- Krishtal O. A.; Pidoplichko V. I. (1980) A receptor for protons in the nerve cell membrane. Neuroscience 5, 2325–2327. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A.; Pidoplichko V. I. (1981) A receptor for protons in the membrane of sensory neurons may participate in nociception. Neuroscience 6, 2599–2601. [DOI] [PubMed] [Google Scholar]
- Krishtal O. A.; Pidoplichko V. I. (1981) Receptor for protons in the membrane of sensory neurons. Brain Res. 214, 150–154. [DOI] [PubMed] [Google Scholar]
- Wemmie J. A.; Price M. P.; Welsh M. J. (2006) Acid-sensing ion channels: advances, questions and therapeutic opportunities. Trends Neurosci. 29, 578–586. [DOI] [PubMed] [Google Scholar]
- Krishtal O. (2003) The ASICs: signaling molecules? Modulators?. Trends Neurosci 26, 477–483. [DOI] [PubMed] [Google Scholar]
- Gonzales E. B.; Kawate T.; Gouaux E. (2009) Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature 460, 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasti J.; Furukawa H.; Gonzales E. B.; Gouaux E. (2007) Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature 449, 316–323. [DOI] [PubMed] [Google Scholar]
- Waldmann R.; Champigny G.; Bassilana F.; Heurteaux C.; Lazdunski M. (1997) A proton-gated cation channel involved in acid-sensing. Nature 386, 173–177. [DOI] [PubMed] [Google Scholar]
- Babinski K.; Le K. T.; Seguela P. (1999) Molecular cloning and regional distribution of a human proton receptor subunit with biphasic functional properties. J. Neurochem. 72, 51–57. [DOI] [PubMed] [Google Scholar]
- de Weille J. R.; Bassilana F.; Lazdunski M.; Waldmann R. (1998) Identification, functional expression and chromosomal localisation of a sustained human proton-gated cation channel. FEBS Lett. 433, 257–260. [DOI] [PubMed] [Google Scholar]
- Waldmann R.; Bassilana F.; de Weille J.; Champigny G.; Heurteaux C.; Lazdunski M. (1997) Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J. Biol. Chem. 272, 20975–20978. [DOI] [PubMed] [Google Scholar]
- Salinas M.; Lazdunski M.; Lingueglia E. (2009) Structural elements for the generation of sustained currents by the acid pain sensor ASIC3. J. Biol. Chem. 284, 31851–31859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y.; Chen Z.; Li W. G.; Cao H.; Feng E. G.; Yu F.; Liu H.; Jiang H.; Xu T. L. (2010) A nonproton ligand sensor in the acid-sensing ion channel. Neuron 68, 61–72. [DOI] [PubMed] [Google Scholar]
- Sutherland S. P.; Benson C. J.; Adelman J. P.; McCleskey E. W. (2001) Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc. Natl. Acad. Sci. U.S.A. 98, 711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deval E.; Noel J.; Lay N.; Alloui A.; Diochot S.; Friend V.; Jodar M.; Lazdunski M.; Lingueglia E. (2008) ASIC3, a sensor of acidic and primary inflammatory pain. EMBO J. 27, 3047–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliver D. C.; Immke D. C.; Fierro L.; Pare M.; Rice F. L.; McCleskey E. W. (2005) ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol. Pain 1, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Edelmayer R. M., Wei X., Felice M. D., Porreca F., and Dussor G. (2010) Dural afferents express acid-sensing ion channels: A role for decreased meningeal pH in migraine headache. Pain [online early access], DOI: 10.1016/j.pain.2010.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T.; Chen J.; Harding A. M.; Price M. P.; Lu Y.; Abboud F. M.; Benson C. J. (2009) ASIC2a and ASIC3 heteromultimerize to form pH-sensitive channels in mouse cardiac dorsal root ganglia neurons. Circ. Res. 105, 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babinski K.; Catarsi S.; Biagini G.; Seguela P. (2000) Mammalian ASIC2a and ASIC3 subunits co-assemble into heteromeric proton-gated channels sensitive to Gd3+. J. Biol. Chem. 275, 28519–28525. [DOI] [PubMed] [Google Scholar]
- Xie J.; Price M. P.; Wemmie J. A.; Askwith C. C.; Welsh M. J. (2003) ASIC3 and ASIC1 mediate FMRFamide-related peptide enhancement of H+-gated currents in cultured dorsal root ganglion neurons. J. Neurophysiol. 89, 2459–2465. [DOI] [PubMed] [Google Scholar]
- Xie J.; Price M. P.; Berger A. L.; Welsh M. J. (2002) DRASIC contributes to pH-gated currents in large dorsal root ganglion sensory neurons by forming heteromultimeric channels. J. Neurophysiol. 87, 2835–2843. [DOI] [PubMed] [Google Scholar]
- Benson C. J.; Xie J.; Wemmie J. A.; Price M. P.; Henss J. M.; Welsh M. J.; Snyder P. M. (2002) Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc. Natl. Acad. Sci. U.S.A. 99, 2338–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X.; Li Q.; Shrestha K.; Cormet-Boyaka E.; Chen L.; Smith P. R.; Sorscher E. J.; Benos D. J.; Matalon S.; Ji H. L. (2006) Interregulation of proton-gated Na(+) channel 3 and cystic fibrosis transmembrane conductance regulator. J. Biol. Chem. 281, 36960–36968. [DOI] [PubMed] [Google Scholar]
- Akiba Y.; Mizumori M.; Kuo M.; Ham M.; Guth P. H.; Engel E.; Kaunitz J. D. (2008) CO2 chemosensing in rat oesophagus. Gut 57, 1654–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Z. Y.; Lu Y.; Whiteis C. A.; Benson C. J.; Chapleau M. W.; Abboud F. M. (2007) Acid-sensing ion channels contribute to transduction of extracellular acidosis in rat carotid body glomus cells. Circ. Res. 101, 1009–1019. [DOI] [PubMed] [Google Scholar]
- Uchiyama Y.; Cheng C. C.; Danielson K. G.; Mochida J.; Albert T. J.; Shapiro I. M.; Risbud M. V. (2007) Expression of acid-sensing ion channel 3 (ASIC3) in nucleus pulposus cells of the intervertebral disc is regulated by p75NTR and ERK signaling. J. Bone Miner. Res. 22, 1996–2006. [DOI] [PubMed] [Google Scholar]
- Jahr H.; van Driel M.; van Osch G. J.; Weinans H.; van Leeuwen J. P. (2005) Identification of acid-sensing ion channels in bone. Biochem. Biophys. Res. Commun. 337, 349–354. [DOI] [PubMed] [Google Scholar]
- Kolker S. J.; Walder R. Y.; Usachev Y.; Hillman J.; Boyle D. L.; Firestein G. S.; Sluka K. A. (2010) Acid-sensing ion channel 3 expressed in type B synoviocytes and chondrocytes modulates hyaluronan expression and release. Ann. Rheum. Dis. 69, 903–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi M.; Kolker S. J.; Sluka K. A. (2009) Acid-sensing ion channel 3 expression in mouse knee joint afferents and effects of carrageenan-induced arthritis. J. Pain 10, 336–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askwith C. C.; Cheng C.; Ikuma M.; Benson C.; Price M. P.; Welsh M. J. (2000) Neuropeptide FF and FMRFamide potentiate acid-evoked currents from sensory neurons and proton-gated DEG/ENaC channels. Neuron 26, 133–141. [DOI] [PubMed] [Google Scholar]
- Catarsi S.; Babinski K.; Seguela P. (2001) Selective modulation of heteromeric ASIC proton-gated channels by neuropeptide FF. Neuropharmacology 41, 592–600. [DOI] [PubMed] [Google Scholar]
- Chen X.; Paukert M.; Kadurin I.; Pusch M.; Grunder S. (2006) Strong modulation by RFamide neuropeptides of the ASIC1b/3 heteromer in competition with extracellular calcium. Neuropharmacology 50, 964–974. [DOI] [PubMed] [Google Scholar]
- Lingueglia E.; Deval E.; Lazdunski M. (2006) FMRFamide-gated sodium channel and ASIC channels: a new class of ionotropic receptors for FMRFamide and related peptides. Peptides 27, 1138–1152. [DOI] [PubMed] [Google Scholar]
- Deval E.; Gasull X.; Noel J.; Salinas M.; Baron A.; Diochot S.; Lingueglia E. (2010) Acid-sensing ion channels (ASICs): Pharmacology and implication in pain. Pharmacol. Ther. 128, 549–558. [DOI] [PubMed] [Google Scholar]
- Waldmann R.; Lazdunski M. (1998) H(+)-gated cation channels: neuronal acid sensors in the NaC/DEG family of ion channels. Curr. Opin. Neurobiol. 8, 418–424. [DOI] [PubMed] [Google Scholar]
- Dube G. R.; Elagoz A.; Mangat H. (2009) Acid sensing ion channels and acid nociception. Curr. Pharm. Des. 15, 1750–1766. [DOI] [PubMed] [Google Scholar]
- Dube G. R.; Lehto S. G.; Breese N. M.; Baker S. J.; Wang X.; Matulenko M. A.; Honore P.; Stewart A. O.; Moreland R. B.; Brioni J. D. (2005) Electrophysiological and in vivo characterization of A-317567, a novel blocker of acid sensing ion channels. Pain 117, 88–96. [DOI] [PubMed] [Google Scholar]
- Yudin Y. K.; Tamarova Z. A.; Ostrovskaya O. I.; Moroz L. L.; Krishtal O. A. (2004) RFa-related peptides are algogenic: evidence in vitro and in vivo. Eur. J. Neurosci. 20, 1419–1423. [DOI] [PubMed] [Google Scholar]
- Yagi J.; Wenk H. N.; Naves L. A.; McCleskey E. W. (2006) Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ. Res. 99, 501–509. [DOI] [PubMed] [Google Scholar]
- Immke D. C.; McCleskey E. W. (2001) Lactate enhances the acid-sensing Na+ channel on ischemia-sensing neurons. Nat. Neurosci. 4, 869–870. [DOI] [PubMed] [Google Scholar]
- Immke D. C.; McCleskey E. W. (2003) Protons open acid-sensing ion channels by catalyzing relief of Ca2+ blockade. Neuron 37, 75–84. [DOI] [PubMed] [Google Scholar]
- Paukert M.; Chen X.; Polleichtner G.; Schindelin H.; Grunder S. (2008) Candidate amino acids involved in H+ gating of acid-sensing ion channel 1a. J. Biol. Chem. 283, 572–581. [DOI] [PubMed] [Google Scholar]
- Shaikh S. A.; Tajkhorshid E. (2008) Potential cation and H+ binding sites in acid sensing ion channel-1. Biophys. J. 95, 5153–5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler M. (2003) Regulation and modulation of pH in the brain. Physiol. Rev. 83, 1183–1221. [DOI] [PubMed] [Google Scholar]
- Price M. P.; McIlwrath S. L.; Xie J.; Cheng C.; Qiao J.; Tarr D. E.; Sluka K. A.; Brennan T. J.; Lewin G. R.; Welsh M. J. (2001) The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron 32, 1071–1083. [DOI] [PubMed] [Google Scholar]
- Steen K. H.; Reeh P. W. (1993) Sustained graded pain and hyperalgesia from harmless experimental tissue acidosis in human skin. Neurosci. Lett. 154, 113–116. [DOI] [PubMed] [Google Scholar]
- Ugawa S.; Ueda T.; Ishida Y.; Nishigaki M.; Shibata Y.; Shimada S. (2002) Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J. Clin. Invest. 110, 1185–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. G.; Slater R.; Cadiou H.; McNaughton P.; McMahon S. B. (2004) Acid-induced pain and its modulation in humans. J. Neurosci. 24, 10974–10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamet J.; Baron A.; Lazdunski M.; Voilley N. (2002) Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J. Neurosci. 22, 10662–10670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voilley N.; de Weille J.; Mamet J.; Lazdunski M. (2001) Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J. Neurosci. 21, 8026–8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. C.; Zimmer A.; Sun W. H.; Hall J.; Brownstein M. J. (2002) A role for ASIC3 in the modulation of high-intensity pain stimuli. Proc. Natl. Acad. Sci. U.S.A. 99, 8992–8997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogil J. S.; Breese N. M.; Witty M. F.; Ritchie J.; Rainville M. L.; Ase A.; Abbadi N.; Stucky C. L.; Seguela P. (2005) Transgenic expression of a dominant-negative ASIC3 subunit leads to increased sensitivity to mechanical and inflammatory stimuli. J. Neurosci. 25, 9893–9901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler A.; Monter B.; Koltzenburg M. (2006) The role of the capsaicin receptor TRPV1 and acid-sensing ion channels (ASICS) in proton sensitivity of subpopulations of primary nociceptive neurons in rats and mice. Neuroscience 139, 699–709. [DOI] [PubMed] [Google Scholar]
- Lin Y. W.; Min M. Y.; Lin C. C.; Chen W. N.; Wu W. L.; Yu H. M.; Chen C. C. (2008) Identification and characterization of a subset of mouse sensory neurons that express acid-sensing ion channel 3. Neuroscience 151, 544–557. [DOI] [PubMed] [Google Scholar]
- Yen Y. T.; Tu P. H.; Chen C. J.; Lin Y. W.; Hsieh S. T.; Chen C. C. (2009) Role of acid-sensing ion channel 3 in sub-acute-phase inflammation. Mol. Pain 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka K. A.; Price M. P.; Breese N. M.; Stucky C. L.; Wemmie J. A.; Welsh M. J. (2003) Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain 106, 229–239. [DOI] [PubMed] [Google Scholar]
- Karczewski J.; Spencer R. H.; Garsky V. M.; Liang A.; Leitl M. D.; Cato M. J.; Cook S. P.; Kane S.; Urban M. O. (2010) Reversal of acid-induced and inflammatory pain by the selective ASIC3 inhibitor, APETx2. Br. J. Pharmacol. 161, 950–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka K. A.; Radhakrishnan R.; Benson C. J.; Eshcol J. O.; Price M. P.; Babinski K.; Audette K. M.; Yeomans D. C.; Wilson S. P. (2007) ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain 129, 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder R. Y.; Rasmussen L. A.; Rainier J. D.; Light A. R.; Wemmie J. A.; Sluka K. A. (2010) ASIC1 and ASIC3 play different roles in the development of Hyperalgesia after inflammatory muscle injury. J. Pain 11, 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam M.; Benson C. J.; Sluka K. A. (2010) Increased response of muscle sensory neurons to decreases in pH after muscle inflammation. Neuroscience 170, 893–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnes L. A.; Kolker S. J.; Danielson J. F.; Walder R. Y.; Sluka K. A. (2008) Enhanced muscle fatigue occurs in male but not female ASIC3−/− mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1347–R1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light A. R.; White A. T.; Hughen R. W.; Light K. C. (2009) Moderate exercise increases expression for sensory, adrenergic, and immune genes in chronic fatigue syndrome patients but not in normal subjects. J. Pain 10, 1099–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluka K. A.; Winter O. C.; Wemmie J. A. (2009) Acid-sensing ion channels: A new target for pain and CNS diseases. Curr. Opin. Drug Discovery Dev. 12, 693–704. [PMC free article] [PubMed] [Google Scholar]
- Benson C. J.; Eckert S. P.; McCleskey E. W. (1999) Acid-evoked currents in cardiac sensory neurons: A possible mediator of myocardial ischemic sensation. Circ. Res. 84, 921–928. [DOI] [PubMed] [Google Scholar]
- Benson C. J.; Sutherland S. P. (2001) Toward an understanding of the molecules that sense myocardial ischemia. Ann. N.Y. Acad. Sci. 940, 96–109. [DOI] [PubMed] [Google Scholar]
- Page A. J.; Brierley S. M.; Martin C. M.; Price M. P.; Symonds E.; Butler R.; Wemmie J. A.; Blackshaw L. A. (2005) Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut 54, 1408–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page A. J.; Brierley S. M.; Martin C. M.; Martinez-Salgado C.; Wemmie J. A.; Brennan T. J.; Symonds E.; Omari T.; Lewin G. R.; Welsh M. J.; Blackshaw L. A. (2004) The ion channel ASIC1 contributes to visceral but not cutaneous mechanoreceptor function. Gastroenterology 127, 1739–1747. [DOI] [PubMed] [Google Scholar]
- Hughes P. A.; Brierley S. M.; Young R. L.; Blackshaw L. A. (2007) Localization and comparative analysis of acid-sensing ion channel (ASIC1, 2, and 3) mRNA expression in mouse colonic sensory neurons within thoracolumbar dorsal root ganglia. J. Comp. Neurol. 500, 863–875. [DOI] [PubMed] [Google Scholar]
- Page A. J.; Brierley S. M.; Martin C. M.; Hughes P. A.; Blackshaw L. A. (2007) Acid sensing ion channels 2 and 3 are required for inhibition of visceral nociceptors by benzamil. Pain 133, 150–160. [DOI] [PubMed] [Google Scholar]
- Jones R. C. III; Xu L.; Gebhart G. F. (2005) The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J. Neurosci. 25, 10981–10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. C. III; Otsuka E.; Wagstrom E.; Jensen C. S.; Price M. P.; Gebhart G. F. (2007) Short-term sensitization of colon mechanoreceptors is associated with long-term hypersensitivity to colon distention in the mouse. Gastroenterology 133, 184–194. [DOI] [PubMed] [Google Scholar]
- Ikeuchi M.; Kolker S. J.; Burnes L. A.; Walder R. Y.; Sluka K. A. (2008) Role of ASIC3 in the primary and secondary hyperalgesia produced by joint inflammation in mice. Pain 137, 662–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan F. L.; Chen F. H.; Lu W. G.; Li X. (2010) Acid-sensing ion channels 3: a potential therapeutic target for pain treatment in arthritis. Mol. Biol. Rep. 37, 3233–3238. [DOI] [PubMed] [Google Scholar]
- Kellenberger S.; Schild L. (2002) Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol. Rev. 82, 735–767. [DOI] [PubMed] [Google Scholar]
- Wetzel C.; Hu J.; Riethmacher D.; Benckendorff A.; Harder L.; Eilers A.; Moshourab R.; Kozlenkov A.; Labuz D.; Caspani O.; Erdmann B.; Machelska H.; Heppenstall P. A.; Lewin G. R. (2007) A stomatin-domain protein essential for touch sensation in the mouse. Nature 445, 206–209. [DOI] [PubMed] [Google Scholar]
- Borzan J.; Zhao C.; Meyer R. A.; Raja S. N. (2010) A role for acid-sensing ion channel 3, but not acid-sensing ion channel 2, in sensing dynamic mechanical stimuli. Anesthesiology 113, 647–654. [DOI] [PubMed] [Google Scholar]
- Drew L. J.; Rohrer D. K.; Price M. P.; Blaver K. E.; Cockayne D. A.; Cesare P.; Wood J. N. (2004) Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones. J. Physiol. 556, 691–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand M. S.; de Silva M. G.; Klockars T.; Rose E.; Price M.; Smith R. J.; McGuirt W. T.; Christopoulos H.; Petit C.; Dahl H. H. (2004) Characterisation of DRASIC in the mouse inner ear. Hear. Res. 190, 149–160. [DOI] [PubMed] [Google Scholar]
- Wu W. L.; Wang C. H.; Huang E. Y.; Chen C. C. (2009) Asic3(−/−) female mice with hearing deficit affects social development of pups. PLoS One 4, e6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque C. W. (2008) Central mechanisms of osmosensation and systemic osmoregulation. Nat. Rev. Neurosci. 9, 519–531. [DOI] [PubMed] [Google Scholar]
- Ko Y. L.; Hsu L. A.; Wu S.; Teng M. S.; Chang H. H.; Chen C. C.; Cheng C. F. (2008) Genetic variation in the ASIC3 gene influences blood pressure levels in Taiwanese. J. Hypertens. 26, 2154–2160. [DOI] [PubMed] [Google Scholar]
- Tan Z. Y.; Lu Y.; Whiteis C. A.; Simms A. E.; Paton J. F.; Chapleau M. W.; Abboud F. M. (2010) Chemoreceptor hypersensitivity, sympathetic excitation, and overexpression of ASIC and TASK channels before the onset of hypertension in SHR. Circ. Res. 106, 536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. J.; Yang W. S.; Lin Y. W.; Wang H. C.; Chen C. C. (2008) Increase of insulin sensitivity and reversal of age-dependent glucose intolerance with inhibition of ASIC3. Biochem. Biophys. Res. Commun. 371, 729–734. [DOI] [PubMed] [Google Scholar]
- Wu S.; Hsu L. A.; Chou H. H.; Teng M. S.; Chang H. H.; Yeh K. H.; Chen C. C.; Chang P. Y.; Cheng C. F.; Ko Y. L. (2010) Association between an ASIC3 gene variant and insulin resistance in Taiwanese. Clin. Chim. Acta 411, 1132–1136. [DOI] [PubMed] [Google Scholar]
- Ettaiche M.; Deval E.; Pagnotta S.; Lazdunski M.; Lingueglia E. (2009) Acid-sensing ion channel 3 in retinal function and survival. Invest. Ophthalmol. Vis. Sci. 50, 2417–2426. [DOI] [PubMed] [Google Scholar]
- Mercado F.; Lopez I. A.; Acuna D.; Vega R.; Soto E. (2006) Acid-sensing ionic channels in the rat vestibular endorgans and ganglia. J. Neurophysiol. 96, 1615–1624. [DOI] [PubMed] [Google Scholar]
- Meng Q. Y.; Wang W.; Chen X. N.; Xu T. L.; Zhou J. N. (2009) Distribution of acid-sensing ion channel 3 in the rat hypothalamus. Neuroscience 159, 1126–1134. [DOI] [PubMed] [Google Scholar]
- Chen C. H.; Hsu Y. T.; Chen C. C.; Huang R. C. (2009) Acid-sensing ion channels in neurones of the rat suprachiasmatic nucleus. J. Physiol. 587, 1727–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W. L.; Lin Y. W.; Min M. Y.; Chen C. C. (2010) Mice lacking Asic3 show reduced anxiety-like behavior on the elevated plus maze and reduced aggression. Genes Brain Behav. 9, 603–614. [DOI] [PubMed] [Google Scholar]
- Lein E. S.; Hawrylycz M. J.; Ao N.; Ayres M.; Bensinger A.; Bernard A.; Boe A. F.; Boguski M. S.; Brockway K. S.; Byrnes E. J.; Chen L.; Chen T. M.; Chin M. C.; Chong J.; Crook B. E.; Czaplinska A.; Dang C. N.; Datta S.; Dee N. R.; Desaki A. L.; Desta T.; Diep E.; Dolbeare T. A.; Donelan M. J.; Dong H. W.; Dougherty J. G.; Duncan B. J.; Ebbert A. J.; Eichele G.; Estin L. K.; Faber C.; Facer B. A.; Fields R.; Fischer S. R.; Fliss T. P.; Frensley C.; Gates S. N.; Glattfelder K. J.; Halverson K. R.; Hart M. R.; Hohmann J. G.; Howell M. P.; Jeung D. P.; Johnson R. A.; Karr P. T.; Kawal R.; Kidney J. M.; Knapik R. H.; Kuan C. L.; Lake J. H.; Laramee A. R.; Larsen K. D.; Lau C.; Lemon T. A.; Liang A. J.; Liu Y.; Luong L. T.; Michaels J.; Morgan J. J.; Morgan R. J.; Mortrud M. T.; Mosqueda N. F.; Ng L. L.; Ng R.; Orta G. J.; Overly C. C.; Pak T. H.; Parry S. E.; Pathak S. D.; Pearson O. C.; Puchalski R. B.; Riley Z. L.; Rockett H. R.; Rowland S. A.; Royall J. J.; Ruiz M. J.; Sarno N. R.; Schaffnit K.; Shapovalova N. V.; Sivisay T.; Slaughterbeck C. R.; Smith S. C.; Smith K. A.; Smith B. I.; Sodt A. J.; Stewart N. N.; Stumpf K. R.; Sunkin S. M.; Sutram M.; Tam A.; Teemer C. D.; Thaller C.; Thompson C. L.; Varnam L. R.; Visel A.; Whitlock R. M.; Wohnoutka P. E.; Wolkey C. K.; Wong V. Y.; Wood M.; Yaylaoglu M. B.; Young R. C.; Youngstrom B. L.; Yuan X. F.; Zhang B.; Zwingman T. A.; Jones A. R. (2007) Genome-wide atlas of gene expression in the adult mouse brain. Nature 445, 168–176. [DOI] [PubMed] [Google Scholar]