Abstract
Human islet amyloid polypeptide (hIAPP) is the source of the major component of the amyloid deposits found in the islets of Langerhans of around 95 per cent type 2 diabetic patients. The formation of aggregates and mature fibrils is thought to be responsible for the dysfunction and death of the insulin-producing pancreatic β-cells. Investigation on the conformation, orientation and self-assembly of the hIAPP at time zero could be beneficial for our understanding of its stability and aggregation process. To obtain these insights, the hIAPP at time zero was studied at the air–aqueous interface using the Langmuir monolayer technique. The properties of the hIAPP Langmuir monolayer at the air–aqueous interface on a NaCl subphase with pH 2.0, 5.6 and 9.0 were examined by surface pressure- and potential-area isotherms, UV–Vis absorption, fluorescence spectroscopy and Brewster angle microscopy. The conformational and orientational changes of the hIAPP Langmuir monolayer under different surface pressures were characterized by p-polarized infrared-reflection absorption spectroscopy, and the results did not show any prominent changes of conformation or orientation. The predominant secondary structure of the hIAPP at the air–aqueous interface was α-helix conformation, with a parallel orientation to the interface during compression. These results showed that the hIAPP Langmuir monolayer at the air–aqueous interface was stable, and no aggregate or domain of the hIAPP at the air–aqueous interface was observed during the time of experiments.
Keywords: protein, hIAPP, Langmuir monolayer, stability, orientation
1. Introduction
One of the major diseases of modern humanity which can be effectively managed but for which there is no permanent cure is diabetes mellitus. Owing to increases in the quality of human life, associated with decreases in physical activity and increases in obesity, diabetes mellitus has reached epidemic proportions [1]. For example, it was estimated in 2010 that 285 million people were affected by diabetes compared with 30 million in 1985 [1]. Type 2 diabetes mellitus, which is also known as non-insulin-dependent diabetes mellitus, is determined by having a high blood glucose level associated with cellular insulin resistance, and relative insulin deficiency. It is the most common type of diabetes, and accounts for more than 90 per cent of all diabetes cases. The age of onset of type 2 diabetes mellitus is also falling and it has become increasingly common among those under 30 [2,3]. The two major hormones of the endocrine pancreas involved in diabetes are human insulin and human islet amyloid polypeptide (hIAPP, also known as human amylin). While insulin has probably been the most studied hormone, the body of knowledge on the roles of hIAPP is much less.
hIAPP is a 37 amino acid residues peptide with a disulfide bridge and an amidated C-terminus. This hormone is co-synthesized and co-secreted to the blood circulation together with insulin in an approximately 1 : 100 (hIAPP : insulin) molar ratio from secretory granules of pancreatic β-cells [4]. The monomeric hIAPP is involved in the glycaemic downregulation in a way that it slows gastric emptying, and thereby prevents sudden spikes in blood glucose levels [5,6]. It is responsible for the satiation signal, and is hypothesized as one of the preventers of obesity [7,8]. Similar to the Alzheimer-related β-amyloid, this hormone also is a highly amyloidogenic peptide, and its amyloid deposits are observed in the islets of Langerhans of around 95 per cent type 2 diabetic patients [9,10], and in pancreatic cancer [11]. The interaction of hIAPP with the β-cell membrane is thought to play a crucial role in the dysfunction and death of β-cells [12]. Studies on both model membranes and cells show that this cytotoxicity is linked to peptide aggregation on the membrane surface by growing hIAPP fibrils or by toxic oligomers [13–15]. However, there is increasing evidence that hIAPP oligomers formed early during aggregation may be the most cytotoxic species, not the mature hIAPP amyloid fibrils [15–18].
The molecular mechanism for the cytotoxicity of hIAPP is still unclear, although several mechanisms have been proposed, such as membrane disruption, the formation of reactive oxygen species, endoplasmic reticulum stress and inflammatory response induced by amyloid formation [19,20]. Recent progress in the study of hIAPP and the phospholipid membrane model shows that the electrostatic interaction between the positively charged hIAPP and negatively charged phospholipid model accelerates the aggregation of hIAPP [13,16,21–26]. In the presence of such lipids, hIAPP fibrils form within a few minutes, whereas no fibrils are observed on a neutral phospholipid [22]. Besides membranes, it is known that other factors can play important roles in the aggregation process of hIAPP, such as pH, metal ions, ionic strength and other protein components (e.g. insulin, C-peptide, pro-insulin and pro-amylin) [27–31]. In the physiological range, acidic pH has been shown to inhibit fibrillation of hIAPP in vitro, whereas basic pH promotes the fibril formation [27,31–33]. Zinc, which naturally occurs in the secretory granules at millimolar concentrations and can bind to the histidine residue at position 18 of hIAPP, has been found to have a dual effect on the aggregation: a lower concentration inhibits aggregation, whereas higher concentrations have the opposite effect [34]. The role of insulin in the fibrillation of hIAPP is more complicated, as there are variations for fibril formation in the reported effects [31,35–37].
hIAPP is monomeric in its physiological state in healthy pancreas, but undergoes a multi-step process of aggregation in the disease state. Detailed structural information could facilitate the understanding of the mechanism and therapeutic intervention. However, the three-dimensional structure of hIAPP in different aggregation states remains elusive, although much progress regarding the structure has been made [38]. Several studies have shown that the structural changes of hIAPP accompany the changes from unstructured monomer to β-sheet amyloid aggregate in solution [39–41]. When bound to the negatively charged lipid membrane, hIAPP is found to adopt an α-helix conformation first before conversion to the β-sheet structure during aggregation [24]. In a micelle prepared from SDS, hIAPP adopts an overall kinked–helix motif, with residues 7–17 and 21–28 in a helical conformation [33].
The Langmuir monolayer technique is a two-dimensional method well accepted for the structure and property study of the amphiphilic biomacromolecules at the interface, such as proteins and membrane components [22,42–45]. The advantages of this method for protein study lie in the possibility of controlling both the intramolecular structure and intermolecular ordering of the amphiphilic biomacromolecules via controllable variables, such as the surface pressure, monolayer and subphase composition, temperature, pH and phase transition [44,46]. Furthermore, the structures and properties of proteins in the Langmuir monolayer could be quite different from those in bulk aqueous solution, as the degree of freedom of proteins in the organized Langmuir monolayer is largely decreased. Among these variables, surface pressure is the dominant factor for the intramolecular and intermolecular interactions of protein in the Langmuir monolayer, as it represents how closely packed the protein molecules are between each other in two dimensions. When the Langmuir monolayer of protein is compressed, the increasing surface pressure promotes the intramolecular and intermolecular interactions, and thus helps self-assembly of protein [47]. Furthermore, the protein–protein and protein–interface interactions become the main forces to determine the conformation, orientation and activity of proteins.
Investigation on the conformation, orientation and self-assembly of hIAPP at time zero could be beneficial for our understanding of its stability and aggregation process. To obtain these insights, the hIAPP in the present study was studied at the air–aqueous interface using the Langmuir monolayer technique with different experimental conditions, such as pH and ionic strength of the subphase. The compression–decompression cycles and stability measurements were employed to study the stability of the Langmuir monolayer, followed by the spectroscopic studies of UV–Vis absorption and fluorescence emission. The conformational and orientational specifics of the hIAPP Langmuir monolayer induced by surface pressure were characterized by p-polarized infrared-reflection absorption spectroscopy (IRRAS). The morphology of the hIAPP domains, if formed by self-assembly at the air–aqueous interface, was observed by Brewster angle microscopy (BAM).
2. Experimental section
2.1. Material
hIAPP was obtained from MP Biomedicals (Solon, OH, USA) with a molecular weight of 3903 Da. The isoelectric point of hIAPP derived from its amino acid sequence is 8.9 [48]. NaCl was purchased from Sigma Aldrich (St Louis, MO, USA) with purity higher than 99.5 per cent. Hydrogen chloride and sodium hydroxide used for adjusting pH were from Pharmco (Brookfield, CT, USA) and MP Biomedicals, respectively. The solvents 1,1,1,3,3,3-hexafluoroisopropanol (HFIP), methanol and chloroform were obtained from MP Biomedicals. All chemicals were used without any further purification. The pure water used in the experiments was obtained from a Modulab 2020 Water purification system (Continental Water System Corp., San Antonio, TX, USA) with a resistivity of 18 MΩ cm, surface tension of 72.6 mN m−1, and pH 5.6 at 20.0 ± 0.5°C.
2.2. Methods
All the isotherm measurements and UV–Vis absorption, fluorescence and IRRAS spectroscopy were measured in a clean room (class 1000) with a constant temperature of 20.0 ± 0.5°C and humidity of 50 ± 1 per cent. A Kibron μ-trough S (Kibron Inc., Helsinki, Finland) with an area of 5.9 × 21.1 cm was used for the studies of surface pressure- and surface potential-area isotherms, compression–decompression cycles, stability, IRRAS and BAM. Surface pressure was monitored by the Wilhelmy method using an alloy wire probe with a sensitivity of ±0.01 mN m−1. Surface potential was measured with a Kelvin probe to an accuracy of 10 mV.
The UV–Vis absorption and fluorescence spectra of the hIAPP Langmuir monolayer were collected on the top of a KSV trough (KSV Instrument Ltd., Helsinki, Finland). The trough had an area of 7.5 × 30 cm and a quartz window in the middle. The UV–Vis absorption spectra of the hIAPP Langmuir monolayer were measured with a Hewlett–Packard 8452A spectrophotometer, whereas the fluorescence spectra were measured by an optical fibre detector connected to a FluoroLog 3 spectrofluorimeter (Horiba Scientific, Edison, NJ, USA). The slit widths in the spectrofluorimeter were set at 5 nm. UV–Vis and fluorescence spectra for the aqueous solutions of hIAPP were measured using a Lambda 900 UV/Vis/NIR spectrophotometer (Perkin-Elmer, Norwalk, CT, USA) and a FluoroLog 3 spectrofluorometer, respectively.
The IRRAS measurements of the hIAPP Langmuir monolayer at the air–aqueous interface were recorded with an EQUINOX 55 Fourier transform infrared spectrometer (Bruker Optics, Billerica, MA, USA), connected to an XA-511 external reflection accessory with a mercury–cadmium–telluride detector cooled by liquid nitrogen. The measurements were performed using p-polarized light on a Kibron μ-trough S. Each spectrum was acquired by the co-addition of 1200 scans with a resolution of 8 cm−1. The IRRAS spectra were used without any processing or baseline correction.
BAM was performed at the air–aqueous interface using an IElli-2000 imaging ellipsometer (Accurion, Menlo Park, CA, USA) with BAM2plus software. The standard laser of the BAM2plus was a frequency-doubled Nd:YAG laser with a wavelength of 532 nm. The laser had a power of 50 mW in a collimated beam. The angle of incidence was set at 53.65° as the Brewster angle of 1.0 M NaCl with pH 5.6.
2.3. Langmuir monolayer preparation
hIAPP was rendered in monomeric form by first dissolving in HFIP and stored under room temperature for 2 h [40,49]. Then, the solution was evaporated in a vacuum desiccator for 2 h to remove the solvent HFIP, followed by re-dissolving in deionized water (pH 5.6) at a concentration of 0.40 mg ml−1 (1.0 × 10−4 M). Different concentrations of NaCl solutions (0.1, 0.5, 1.0 and 2.0 M) were used as the subphase at pH 2.0, 5.6 and 9.0, respectively. The volume of the spreading hIAPP solution was 30 and 60 μl for Kibron and KSV troughs, respectively. The freshly prepared hIAPP solution was spread at the air–aqueous interface using a 100 μl syringe (Hamilton Co., Reno, NV, USA) by uniform droplet deposition over the subphase surface. A waiting time period of 20 min was taken for the hIAPP Langmuir monolayer to reach equilibrium. The monolayer was compressed at a rate of 50 Å2 per molecule. Triplicate experiments confirmed the reproducibility of the data.
3. Results and discussion
3.1. Surface pressure- and surface potential-area isotherms: the effect of sodium chloride concentration in the subphase
Pure water was first used as the subphase to form the hIAPP Langmuir monolayer. As shown in figure 1a, the surface pressure had a lifting point of around 350 Å2 per molecule on the pure water subphase. The extrapolation of the linear part of the plot to zero surface pressure gives a limiting molecular area of around 205 Å2 per molecule. During compression from 300 to 200 Å2 per molecule, the surface pressure increased steadily. The film was characterized by the liquid expanded phase at around 250 Å2 per molecule, and the coexistence of a liquid expanded–liquid condensed phase between 250 and 150 Å2 per molecule. The monolayer collapsed at a surface pressure of around 20 mN m−1.
Figure 1.
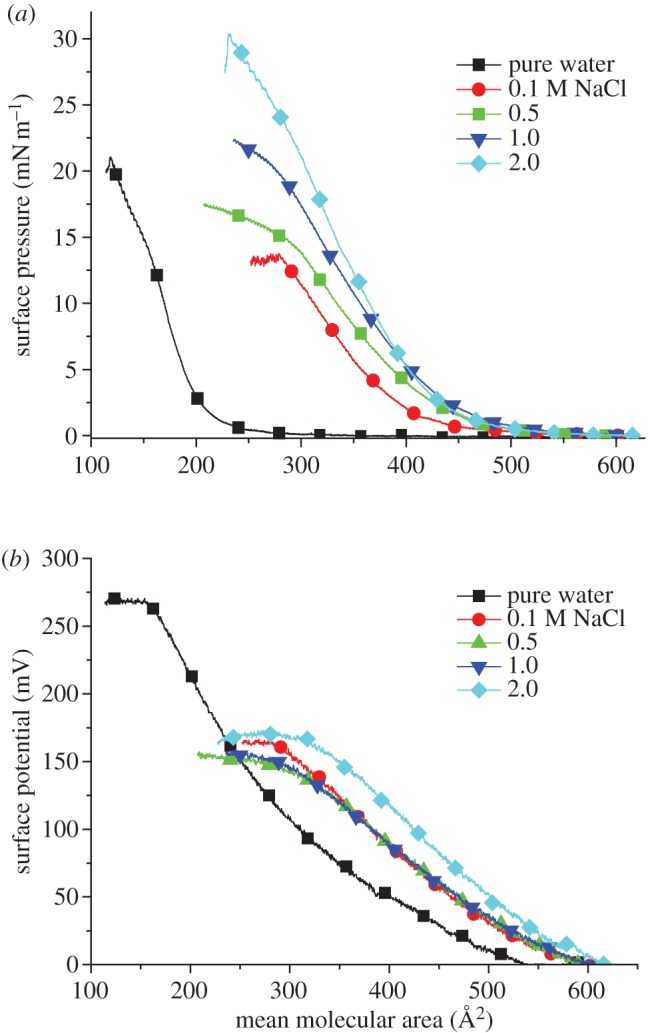
Surface pressure-area isotherm (a) and surface potential-area isotherm (b) of the hIAPP Langmuir monolayer on pure water and subphase solution with 0.1, 0.5, 1.0 and 2.0 M NaCl. (Online version in colour.)
Owing to the possible dissolution of protein molecules into the pure water subphase, the surface pressure and limiting molecular area of the obtained protein Langmuir monolayer may be smaller than its actual number [50]. Addition of NaCl in the subphase could be used to decrease the quantity of hIAPP molecules that dissolve or sink into the bulk subphase solution, resulting in the formation of a more closely packed hIAPP Langmuir monolayer. Different concentrations of NaCl (0.1, 0.5, 1.0 and 2.0 M) were introduced to the subphase solution in order to maximize the number of hIAPP molecules at the air–aqueous interface. As shown in figure 1a, all the four concentrations of NaCl present in the subphase show a higher lifting area at about 550 Å2 per molecule, indicating that more molecules are present at the air–aqueous interface. The limiting molecular area increases to around 400, 430, 445 and 440 Å2 per molecule for the 0.1, 0.5, 1.0 and 2.0 M NaCl subphases, respectively. An increase in collapse surface pressure was also observed as the NaCl concentration in the subphase increased. Therefore, the dissolution of hIAPP in the subphase solution decreases in the presence of the electrolyte, which is similar to other proteins, such as alcohol dehydrogenease [50] and acetylcholinesterase [51]. The decreasing solubility of hIAPP is due to the salting-out effects that come from dehydration of the protein by the added NaCl. The salt ions would have a stronger attraction with the highly polar water molecules than the less polar protein molecules [52]. Also, ion–protein dispersion potentials originating from the polarizabilities of ions and hIAPP could be involved in the salting-out effect [50].
The surface potential-area isotherm was also correlated with the surface pressure-area isotherm as shown in figure 1b. Because the surface potential measures the dipole–dipole interactions at much longer distances than the interactions measured by surface pressure (usually owing to van der Waals interactions), the surface potential-area isotherm showed an increase in the surface potential as soon as the compression of the monolayer was started [53]. The collapse surface potential on pure water was observed at about 270 mV, much higher than that on the subphase that contained NaCl. The reason might be that the presence of NaCl neutralized the charge of the protein, thus reducing the net surface potential.
On the basis of salt effect on the surface pressure- and surface potential-area isotherms, an ionic concentration of NaCl at 1.0 M in the subphase was selected for the pH effect.
3.2. The pH effect on the hIAPP Langmuir monolayer
hIAPP contains a single histidine residue at position 18 of its amino acid sequence with the pKa of this residue near 6.5 in the monomer state [31]. In healthy pancreas, the hIAPP is safely stored in the β-cell granules with pH around 5.5 and released into the extracellular environment with pH about 7.4. It is feasible to think that the pH has an effect on the aggregation of hIAPP. Indeed, studies have shown that the aggregation of hIAPP in solution at pH 4.0 is much slower than that at pH of 8.8 [27,32]. In order to study the pH effect on the self-assembly of hIAPP as the Langmuir monolayer, 1.0 M NaCl subphase solution with pH values 2.0, 5.6 and 9.0 was investigated, as shown in figure 2. Under these three pH values, the lifting areas were similar (around 550 Å2 per molecule). The limiting molecular areas for both pH 2.0 and 9.0 were smaller, around 410 Å2 per molecule, than 445 Å2 per molecule at pH 5.6.
Figure 2.

Surface pressure-area isotherms of the hIAPP Langmuir monolayer on 1.0 M NaCl subphase solution with pH 2.0, 5.6 and 9.0. (Online version in colour.)
Different surface pressure-area isotherms with a smaller limiting molecular area were expected for higher pH at 9.0, as they had been shown to promote aggregation of the hIAPP in solution. However, the pH did not seem to have a pronounced effect on the hIAPP Langmuir monolayer during the experiment. One possible reason for this observation could be that it might take a longer time for the hIAPP molecules to form aggregates than the time needed for the Langmuir monolayer experiment. It typically took a few hours to detect fibril formation by thioflavin T fluorescence in solution [27,32,34], but one set of Langmuir monolayer experiments needed only 30–40 min. Another possibility could be that some aggregation did form, but the surface pressure-area isotherm could not detect it. However, it could be excluded as the compression–decompression cycles, UV–Vis absorption, IRRAS and BAM in the following sections did not detect large aggregation or domain formation, although the existence of some oligomers could not be ruled out.
On the basis of effects of salt and pH, 1.0 M NaCl aqueous solution with pH 5.6 was chosen as the subphase for compression–decompression cycles and stability studies of the hIAPP Langmuir monolayer at the air–aqueous interface.
3.3. The compression–decompression cycles and stability measurements of the hIAPP Langmuir monolayer
Three compression–decompression cycles of the hIAPP Langmuir monolayers were examined for the stability of hIAPP the Langmuir monolayer at surface pressures of 5 and 15 mN m−1 on a 1.0 M NaCl subphase, as shown in figure 3. From the surface pressure-area isotherm shown in figure 1, the hIAPP Langmuir monolayer at both 5 and 15 mN m−1 was in a liquid-condensed phase. A hysteresis was observed at both surface pressures but the difference between the first compression and the last compression was relatively small, i.e. 8 and 12 per cent, respectively. Also, the surface pressure could return to 0 mN m−1 when the barriers moved to the maximum area position. These facts showed that the formation of the hIAPP Langmuir monolayer was reversible, suggesting the self-assembly or domain formation of the hIAPP at time zero did not happen.
Figure 3.
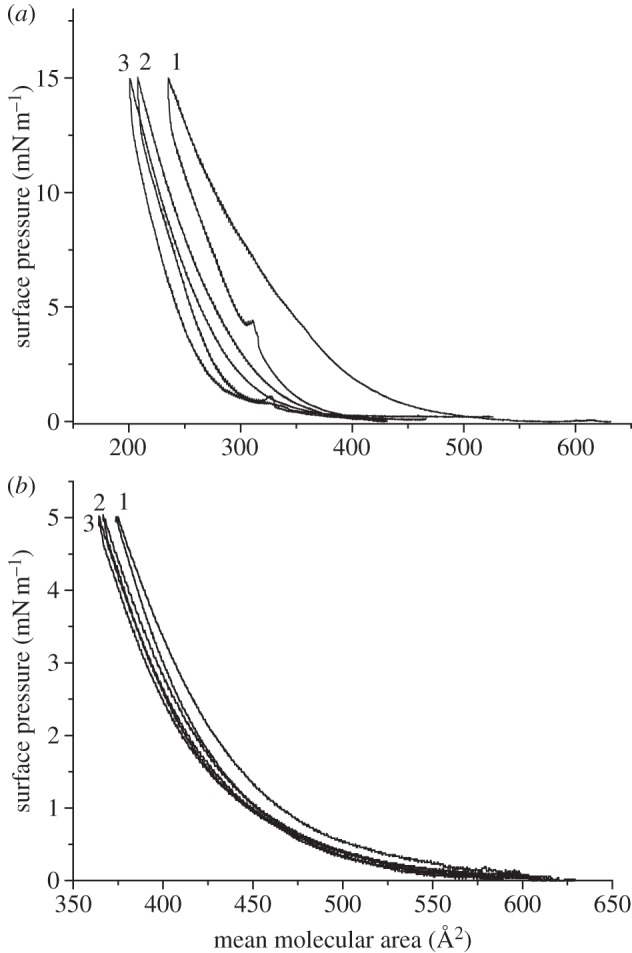
Compression–decompression cycles of the hIAPP Langmuir monolayer on 1.0 M NaCl subphase with pH 5.6 at surface pressure (a) 15 and (b) 5 mN m−1.
The stability measurements of the Langmuir monolayer were also obtained over a period of time at a constant surface pressure of 10 mN m−1, as shown in figure 4. Over 250 min, the approximate mean molecular area change was about 20 per cent. The reason could be the relaxation of the Langmuir monolayer, dissolution of hIAPP into the subphase, or a change of conformation of the protein over time. The hypothesis of the dissolution of hIAPP into the subphase can be ruled out based on the UV–Vis spectra discussed later.
Figure 4.
Stability measurement of the hIAPP Langmuir monolayer at surface pressure 10 mN m−1 on 1.0 M NaCl subphase with pH 5.6. Surface pressure, red; mean molecular area, black. (Online version in colour.)
3.4. UV–Vis absorption of hIAPP at the air–aqueous interface
The UV–Vis absorption of the hIAPP Langmuir monolayer on 1.0 M NaCl with pH 2.0, 5.6 and 9.0 was investigated under different surface pressures. As shown in figure 5, it did not have any significant absorbance except at 202 nm at pH 5.6, which corresponded to n − π* transition of C=O chromophore of the amide bonds in hIAPP. The inset shows the linear relationship between the absorbance at 202 nm and the surface pressure. The hIAPP Langmuir monolayer had the same absorption and linearity at 202 nm with pH 2.0 (data not shown). We did study the absorption at pH 9.0, but no good ‘zero’ background was gained probably due to the light scattering. The linearity of absorption at pH 2.0 and 5.6 is proof that during compression, the number of hIAPP molecules per unit area was increasing linearly, indicating that aggregation most likely did not happen, nor was there an effect on the solubility of the protein. If the self-assembly of the hIAPP Langmuir monolayer was forming aggregates or domains by the increasing surface pressure, the UV–Vis absorption bands would be red-shifted relative to the monomer spectrum.
Figure 5.
UV–Vis absorption spectra of the hIAPP Langmuir monolayer on 1.0 M NaCl subphase at pH 5.6. Inset: absorbance at 202 nm versus surface pressure. (Online version in colour.)
Compared with the absorption of hIAPP in the Langmuir monolayer, the aqueous solution of hIAPP had a weak broad band around 275 nm owing to the π–π* transition of the tyrosine residue in hIAPP, as shown in electronic supplementary material, figure S1a. The absence of this band for the hIAPP Langmuir monolayer can be explained by the fact that the amount of tyrosine residue per unit area was too low to be detected. Owing to the extremely strong absorption of peptide bonds in aqueous solution, the UV–Vis absorption bands of hIAPP below 225 nm could not be observed.
3.5. Fluorescence of the hIAPP Langmuir monolayer at the air–aqueous interface
The molecule of hIAPP contains one fluorescent tyrosine residue. The fluorescence emission wavelength of hIAPP in aqueous solution is 309 nm at the excitation wavelength of 270 nm, as shown in electronic supplementary material, figure S1b. However, no fluorescence of tyrosine in the hIAPP Langmuir monolayer was observed on 1.0 M NaCl subphase with pH 2.0, 5.6 or 9.0 owing to the small amount of hIAPP molecules per unit area and low quantum yield of fluorescence of tyrosine.
3.6. IRRAS of the hIAPP Langmuir monolayer at the air–aqueous interface
The hIAPP can appear in various aggregation states, i.e. monomer, oligomer or fibril, all with variations in secondary, tertiary and quaternary structure. The synthetically produced hIAPP monomer in aqueous solution exhibits typically a random coil structure from circular dichroism spectra [54]. By contrast, hIAPP treated with helix promoting solvent, such as trifluoroethanol and HFIP, predominantly adopts α-helical conformation [33,34,40,49,55]. Besides the structure information in solution, the interaction study between hIAPP and negatively charged lipid membrane suggests that the hIAPP adopts the α-helix conformation first before conversion to the β-sheet structure during aggregation [22,24,56].
Since Dluhy's research group in the mid-1980s first acquired molecular structure information from Langmuir lipid monolayers in situ at the air–aqueous interface using infrared spectroscopy [57], this technique has been widely expanded to investigate the conformational and orientational studies of the lipid, peptide or protein monolayer at the air–aqueous interface [58–61]. As one of the leading structural methods for the in situ characterization of the secondary structures of protein at the air–aqueous interface, IRRAS facilitates the spectroscopic analysis of the amide bands of proteins caused by possible conformational or orientational changes. For most proteins, α-helix predominated structures exhibit amide I (mainly the C=O stretching vibration of the peptide bond) and amide II (the N–H bending vibration and the C–N stretching vibration of the peptide bond) absorptions in the spectral range from 1650 to 1660 cm−1 and 1540 to 1550 cm−1, respectively. β-Sheets exhibit similar absorptions at 1620–1640 and 1520–1535 cm−1 [62,63].
p-Polarized IRRAS has been intensively applied to the investigation of secondary structure and orientation changes of the protein Langmuir monolayer at the air–aqueous interface. p-Polarized light probes the dipole moments parallel and perpendicular to the interface, whereas s-polarized light probes only the dipole moment components parallel to the interface [64]. For the p-polarized IRRAS, the measured signal can contain positive and negative bands, depending on the angles of incidence and orientation of the transition dipole moment with respect to the air–aqueous interface [24,65]. If the transition dipole moments preferentially parallel to the air–aqueous interface, the absorbance bands are initially negative and increase in intensity with an increase in the incident angle until the Brewster angle (e.g. 54.2° for the IR light at 2850 cm−1) is reached [66]. Above the Brewster angle, there is an inversion of the bands to positive values and the intensity decreases with a further increase in the incident angle. If the transition dipole moment is preferentially perpendicular to the interface, the opposite should be observed for both the sign and intensity of the bands when the angle of the incident light is varied [64,65,67].
The p-polarized IRRAS of the hIAPP Langmuir monolayer on 1.0 M NaCl subphase with pH 2.0, 5.6 and 9.0 was investigated for the information about the molecular orientation and conformation of the protein in the Langmuir monolayer. As shown in figure 6, the inversions of the peaks of the hIAPP Langmuir monolayer at 10 mN m−1 under various incident angles suggest that the transition dipole moments of the hIAPP Langmuir monolayer interacting with p-polarized IRRAS were preferentially paralleled to the air–aqueous interface. Figure 7 shows the p-polarized IRRAS spectra of the hAPP Langmuir monolayer at incident angle 65° (this angle was chosen based on its good signal-to-noise ratio; [46]) on 1.0 M NaCl subphase with pH 5.6 (IRRAS spectra with pH 2.0 and pH 9.0 are shown as electronic supplementary material, figure S2). The bands of approximately 1650 and approximately 1545 cm−1 were assigned to the α-helix conformation, corresponding to the vibrations of the amide I and II absorptions, respectively, whereas the bands at approximately 1525 cm−1 were the β-sheet conformation [63].
Figure 6.
p-Polarized IRRAS of the hIAPP Langmuir monolayer on 1.0 M NaCl subphase with pH 5.6 at surface pressure 10 mN m−1 at various incident angles. The spectra were shifted vertically for better visibility. (Online version in colour.)
Figure 7.
p-Polarized IRRAS of the hIAPP Langmuir monolayer on 1.0 M NaCl subphase with pH 5.6 at various surface pressures at the incident angle 65°. The spectra were shifted vertically for better visibility. (Online version in colour.)
The possibility of conformational or orientational changes induced by increasing surface pressure at the air–aqueous interface was examined by analysing band position and intensity of amide I and amide II of IRRAS [68]. As shown in figure 7 and electronic supplementary material, figure S2, the band positions of the hIAPP Langmuir monolayer on 1.0 M NaCl subphase with pH 2.0, 5.6 and 9.0 remained almost the same as the surface pressure increased, indicating that the hIAPP Langmuir monolayer did not undergo conformational changes upon compression during the time of experiment and retained mainly α-helical structure. This is probably due to the electrostatic repulsion of the positively charged hIAPP molecules. However, as previous studies have shown, the conformation of hIAPP changed from α-helix to β-sheet with the presence of negatively charged phospholipid, while no obvious structural changes were observed with the presence of neutral phospholipid [15,24,25]. This suggested that the attraction between the negatively charged phospholipid and positively charged hIAPP might play a critical role to accelerate or catalyse the aggregation of hIAPP. No notable intensity of amide I and amide II was observed on the subphase with each pH, indicating that the orientations of hIAPP molecules at the air–aqueous interface were almost parallel to the interface even before compression and remained the same orientation during the time of compression [69].
The secondary structure information gained here was consistent with the previous theoretical and experimental results. It has been shown using molecular simulation that hIAPP monomer could adopt an α-helical conformation with a short β-sheet near the C-terminus [40]. The adsorption study of the hIAPP at the air–aqueous interface also found that it adopted an α-helix structure before conversion to β-sheet-rich fibril [24]. A recent nuclear magnetic resonance (NMR) study in solution found that the structure of hIAPP was defined by a kinked–helix from residue 11 to residue 30. Significantly, this finding was a relatively close match to the crystalline structure of the hIAPP dimer fused to maltose-binding protein, indicating that the helix–kink–helix motif was probably an early on-pathway intermediate to aggregation [34]. Therefore, it was also very likely that the α-helical conformation obtained here at the air–aqueous interface was a relatively stable intermediate before conversion to aggregates.
3.7. Brewster angle microscopy of the hIAPP Langmuir monolayer
BAM was employed to see the changes of phase domain and morphology of hIAPP in the Langmuir monolayer at air–aqueous interface. As a reference, BAM of phase domains of the arachidic acid monolayer were obtained in the two-phase coexistence region of the isotherm at pH 12 under 25°C [70], as shown in figure 8a. Unfortunately, we could not see the BAM image of the hIAPP Langmuir monolayer on 1.0 M NaCl subphase with pH 2.0, 5.6 and 9.0 under different surface pressures. Figure 8b,c shows only the BAM images of the hIAPP Langmuir monolayer on 1.0 M NaCl subphase with pH 5.6 before compression (b) and at 10 mN m−1 (c). Microscopy ruled out the formation of domains from self-assembly of the protein at any surface pressure during the time of experiment.
Figure 8.
BAM image of phase domains of arachidic acid monolayer in the two-phase coexistence region of the isotherm with pH 12 at (a) 25°C. BAM image of the hIAPP Langmuir monolayer on 1.0 M NaCl subphase with pH 5.6 at (b) 20°C before compression and at (c) 10 mN m−1. Size: 768 × 572 μm2.
3.8. Human insulin promotes aggregation
Although the present study shows the relative stability of hIAPP without detection of aggregates or domain formation at time zero after deposition of the protein at the air–aqueous interface, one has observed an important fibrillation process for the mixed human insulin : hIAPP (1 : 1 molar ratio). The mixture system was incubated at 37°C at different time period intervals and their surface pressure-area isotherm was measured at these time intervals. At any surface pressure, the corresponding area per molecule diminished with the time of incubation. This observation clearly indicates that there is an interaction between hIAPP and human insulin to favour the fibrillation process although the nature of the interaction is still under investigation.
4. Conclusion
The conformation, orientation and self-assembly of the hIAPP at time zero were studied at the air–aqueous interface using the Langmuir monolayer technique. The limiting molecular area of hIAPP on 1.0 M NaCl subphase as the optimal condition was found to be around 445 Å2 per molecule. The pH of the subphase solution (2.0, 5.6 and 9.0) did not seem to have much effect on the isotherms. The compression–decompression cycles and stability studies of the hIAPP Langmuir monolayer showed that it was relatively stable and did not form aggregates or domains during the experiment. UV–Vis absorption of the hIAPP Langmuir monolayer on 1.0 M NaCl with pH 2.0 and 5.6 showed the linearity of absorbance of peptide bonds at 202 nm, confirming that no aggregate or domain was formed. p-Polarized IRRAS was employed to study the conformational and orientational changes of the hIAPP Langmuir monolayer on 1.0 M NaCl subphase with pH 2.0, 5.6 and 9.0. The spectra showed that the predominant secondary structures of the hIAPP Langmuir monolayer were α-helix conformation, independent of the pH of subphase and the induced surface pressure. It was also very likely that this α-helical conformation was a relatively stable intermediate before the conversion to aggregates. The orientation of the hIAPP Langmuir monolayer at the air–aqueous interface was parallel to the air–aqueous interface during compression. No BAM image of domains of the hIAPP Langmuir monolayer was observed on 1.0 M NaCl subphase with pH 2.0, 5.6 or 9.0, further confirming its stability during the Langmuir monolayer experiment. However, ongoing study did show that the process of aggregation of hIAPP could be promoted by human insulin.
Acknowledgments
R.M.L. is grateful for the financial support provided by the National Science Foundation (grant no. CBET-0944290), and Cooper Fellowships awarded by College of Arts and Sciences, the University of Miami.
References
- 1.Shaw J. E., Sicree R. A., Zimmet P. Z. 2010. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 87, 4–14 10.1016/j.diabres.2009.10.007 (doi:10.1016/j.diabres.2009.10.007) [DOI] [PubMed] [Google Scholar]
- 2.Song S. H. 2008. Review. Early-onset type 2 diabetes mellitus: a condition with elevated cardiovascular risk? Br. J. Diabetes Vasc. Dis. 8, 61–65 10.1177/14746514080080020201 (doi:10.1177/14746514080080020201) [DOI] [Google Scholar]
- 3.Alberti G., Zimmet P., Shaw J., Bloomgarden Z., Kaufman F., Silink M. 2004. Type 2 diabetes in the young: the evolving epidemic. Diabetes Care 27, 1798–1811 10.2337/diacare.27.7.1798 (doi:10.2337/diacare.27.7.1798) [DOI] [PubMed] [Google Scholar]
- 4.Gedulin B., Cooper G. J. S., Young A. A. 1991. Amylin secretion from the perfused pancreas: Dissociation from insulin and abnormal elevation in insulin-resistant diabetic rats. Biochem. Biophys. Res. Commun. 180, 782–789 10.1016/S0006-291X(05)81133-7 (doi:10.1016/S0006-291X(05)81133-7) [DOI] [PubMed] [Google Scholar]
- 5.Westermark P., Li Z.-C., Westermark G. T., Leckström A., Steiner D. F. 1996. Effects of beta cell granule components on human islet amyloid polypeptide fibril formation. FEBS Lett. 379, 203–206 10.1016/0014-5793(95)01512-4 (doi:10.1016/0014-5793(95)01512-4) [DOI] [PubMed] [Google Scholar]
- 6.Gedulin B. R., Jodka C. M., Herrmann K., Young A. A. 2006. Role of endogenous amylin in glucagon secretion and gastric emptying in rats demonstrated with the selective antagonist, AC187. Regul. Peptides 137, 121–127 10.1016/j.regpep.2006.06.004 (doi:10.1016/j.regpep.2006.06.004) [DOI] [PubMed] [Google Scholar]
- 7.Reda T. K., Geliebter A., Pi-Sunyer F. X. 2002. Amylin, food intake, and obesity. Obesity 10, 1087–1091 10.1038/oby.2002.147 (doi:10.1038/oby.2002.147) [DOI] [PubMed] [Google Scholar]
- 8.Wielinga P. Y., Löwenstein C., Muff S., Munz M., Woods S. C., Lutz T. A. 2010. Central amylin acts as an adiposity signal to control body weight and energy expenditure. Physiol. Behav. 101, 45–52 10.1016/j.physbeh.2010.04.012 (doi:10.1016/j.physbeh.2010.04.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Westermark P., Wernstedt C., Wilander E., Hayden D. W., O'Brien T. D., Johnson K. H. 1987. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc. Natl Acad. Sci. USA 84, 3881–3885 10.1073/pnas.84.11.3881 (doi:10.1073/pnas.84.11.3881) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hull R. L., Westermark G. T., Westermark P., Kahn S. E. 2004. Islet amyloid: a critical entity in the pathogenesis of type 2 diabetes. J. Clin. Endocrinol. Metab. 89, 3629–3643 10.1210/jc.2004-0405 (doi:10.1210/jc.2004-0405) [DOI] [PubMed] [Google Scholar]
- 11.Permert J., Larsson J., Westermark G. T., Herrington M. K., Christmanson L., Pour P. M., Westermark P., Adrian T. E. 1994. Islet amyloid polypeptide in patients with pancreatic cancer and diabetes. N. Engl. J. Med. 330, 313–318 10.1056/NEJM199402033300503 (doi:10.1056/NEJM199402033300503) [DOI] [PubMed] [Google Scholar]
- 12.Höppener J. W. M., Ahrén B., Lips C. J. M. 2000. Islet amyloid and type 2 diabetes mellitus. N. Engl. J. Med. 343, 411–419 10.1056/NEJM200008103430607 (doi:10.1056/NEJM200008103430607) [DOI] [PubMed] [Google Scholar]
- 13.Engel M. F. M., Khemtémourian L., Kleijer C. C., Meeldijk H. J. D., Jacobs J., Verkleij A. J., de Kruijff B., Killian J. A., Höppener J. W. M. 2008. Membrane damage by human islet amyloid polypeptide through fibril growth at the membrane. Proc. Natl Acad. Sci. USA 105, 6033–6038 10.1073/pnas.0708354105 (doi:10.1073/pnas.0708354105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demuro A., Mina E., Kayed R., Milton S. C., Parker I., Glabe C. G. 2005. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J. Biol. Chem. 280, 17 294–17 300 10.1074/jbc.M500997200 (doi:10.1074/jbc.M500997200) [DOI] [PubMed] [Google Scholar]
- 15.Jayasinghe S. A., Langen R. 2007. Membrane interaction of islet amyloid polypeptide. Biochim. Biophys. Acta Biomembr. 1768, 2002–2009 10.1016/j.bbamem.2007.01.022 (doi:10.1016/j.bbamem.2007.01.022) [DOI] [PubMed] [Google Scholar]
- 16.Engel M. F. M. 2009. Membrane permeabilization by islet amyloid polypeptide. Chem. Phys. Lipids 160, 1–10 10.1016/j.chemphyslip.2009.03.008 (doi:10.1016/j.chemphyslip.2009.03.008) [DOI] [PubMed] [Google Scholar]
- 17.Gurlo T., Ryazantsev S., Huang C., Yeh M. W., Reber H. A., Hines O. J., O'Brien T. D., Glabe C. G., Butler P. C. 2010. Evidence for proteotoxicity in β cells in type 2 diabetes: toxic islet amyloid polypeptide oligomers form intracellularly in the secretory pathway. Am. J. Pathol. 176, 861–869 10.2353/ajpath.2010.090532 (doi:10.2353/ajpath.2010.090532) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brender J. R., Hartman K., Reid K. R., Kennedy R. T., Ramamoorthy A. 2008. A single mutation in the nonamyloidogenic region of islet amyloid polypeptide greatly reduces toxicity. Biochemistry 47, 12 680–12 688 10.1021/bi801427c (doi:10.1021/bi801427c) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westermark P., Andersson A., Westermark G. T. 2011. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol. Rev. 91, 795–826 10.1152/physrev.00042.2009 (doi:10.1152/physrev.00042.2009) [DOI] [PubMed] [Google Scholar]
- 20.Brender J. R., Salamekh S., Ramamoorthy A. 2012. Membrane disruption and early events in the aggregation of the diabetes related peptide IAPP from a molecular perspective. Acc. Chem. Res. 45, 454–462 10.1021/ar200189b (doi:10.1021/ar200189b) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jayasinghe S. A., Langen R. 2005. Lipid membranes modulate the structure of islet amyloid polypeptide. Biochemistry 44, 12 113–12 119 10.1021/bi050840w (doi:10.1021/bi050840w) [DOI] [PubMed] [Google Scholar]
- 22.Engel M. F. M., Yigittop H., Elgersma R. C., Rijkers D. T. S., Liskamp R. M. J., de Kruijff B., Höppener J. W. M., Killian J. A. 2006. Islet amyloid polypeptide inserts into phospholipid monolayers as monomer. J. Mol. Biol. 356, 783–789 10.1016/j.jmb.2005.12.020 (doi:10.1016/j.jmb.2005.12.020) [DOI] [PubMed] [Google Scholar]
- 23.Khemtémourian L., Lahoz Casarramona G., Suylen D. P. L., Hackeng T. M., Meeldijk J. D., de Kruijff B., Höppener J. W. M., Killian J. A. 2009. Impaired processing of human pro-islet amyloid polypeptide is not a causative factor for fibril formation or membrane damage in vitro. Biochemistry 48, 10 918–10 925. (doi:10.1021/bi901076d) [DOI] [PubMed] [Google Scholar]
- 24.Lopes D. H. J., Meister A., Gohlke A., Hauser A., Blume A., Winter R. 2007. Mechanism of islet amyloid polypeptide fibrillation at lipid interfaces studied by infrared reflection absorption spectroscopy. Biophys. J. 93, 3132–3141 10.1529/biophysj.107.110635 (doi:10.1529/biophysj.107.110635) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evers F., Jeworrek C., Tiemeyer S., Weise K., Sellin D., Paulus M., Struth B., Tolan M., Winter R. 2009. Elucidating the mechanism of lipid membrane-induced iapp fibrillogenesis and its inhibition by the red wine compound resveratrol: a synchrotron X-ray reflectivity study. J. Am. Chem. Soc. 131, 9516–9521 10.1021/ja8097417 (doi:10.1021/ja8097417) [DOI] [PubMed] [Google Scholar]
- 26.Knight J. D., Miranker A. D. 2004. Phospholipid catalysis of diabetic amyloid assembly. J. Mol. Biol. 341, 1175–1187 10.1016/j.jmb.2004.06.086 (doi:10.1016/j.jmb.2004.06.086) [DOI] [PubMed] [Google Scholar]
- 27.Khemtémourian L., Doménech E., Doux J. P. F., Koorengevel M. C., Killian J. A. 2011. Low pH acts as inhibitor of membrane damage induced by human islet amyloid polypeptide. J. Am. Chem. Soc. 133, 15 598–15 604 10.1021/ja205007j (doi:10.1021/ja205007j) [DOI] [PubMed] [Google Scholar]
- 28.Liu P., Zhang S., Chen M., Liu Q., Wang C., Wang C., Li Y., Besenbacher F., Dong M. 2012. Co-assembly of human islet amyloid polypeptide (hIAPP)/insulin. Chem. Commun. 48, 191–193 10.1039/C1CC14285B (doi:10.1039/C1CC14285B) [DOI] [PubMed] [Google Scholar]
- 29.Jha S., Sellin D., Seidel R., Winter R. 2009. Amyloidogenic propensities and conformational properties of proiapp and iapp in the presence of lipid bilayer membranes. J. Mol. Biol. 389, 907–920 10.1016/j.jmb.2009.04.077 (doi:10.1016/j.jmb.2009.04.077) [DOI] [PubMed] [Google Scholar]
- 30.Salamekh S., Brender J. R., Hyung S.-J., Nanga R. P. R., Vivekanandan S., Ruotolo B. T., Ramamoorthy A. 2011. A two-site mechanism for the inhibition of IAPP amyloidogenesis by zinc. J. Mol. Biol. 410, 294–306 10.1016/j.jmb.2011.05.015 (doi:10.1016/j.jmb.2011.05.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeToma A. S., Salamekh S., Ramamoorthy A., Lim M. H. 2012. Misfolded proteins in Alzheimer's disease and type II diabetes. Chem. Soc. Rev. 41, 608–621 10.1039/C1CS15112F (doi:10.1039/C1CS15112F) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abedini A., Raleigh D. P. 2005. The role of His-18 in amyloid formation by human islet amyloid polypeptide. Biochemistry 44, 16 284–16 291 10.1021/bi051432v (doi:10.1021/bi051432v) [DOI] [PubMed] [Google Scholar]
- 33.Nanga R. P. R., Brender J. R., Vivekanandan S., Ramamoorthy A. 2011. Structure and membrane orientation of IAPP in its natively amidated form at physiological pH in a membrane environment. Biochim. Biophys. Acta Biomembr. 1808, 2337–2342 10.1016/j.bbamem.2011.06.012 (doi:10.1016/j.bbamem.2011.06.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brender J. R., Hartman K., Nanga R. P. R., Popovych N., de la Salud Bea R., Vivekanandan S., Marsh E. N. G., Ramamoorthy A. 2010. Role of zinc in human islet amyloid polypeptide aggregation. J. Am. Chem. Soc. 132, 8973–8983 10.1021/ja1007867 (doi:10.1021/ja1007867) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Larson J. L., Miranker A. D. 2004. The mechanism of insulin action on islet amyloid polypeptide fiber formation. J. Mol. Biol. 335, 221–231 10.1016/j.jmb.2003.10.045 (doi:10.1016/j.jmb.2003.10.045) [DOI] [PubMed] [Google Scholar]
- 36.Brender J. R., Lee E. L., Hartman K., Wong P. T., Ramamoorthy A., Steel D. G., Gafni A. 2011. Biphasic effects of insulin on islet amyloid polypeptide membrane disruption. Biophys. J. 100, 685–692 10.1016/j.bpj.2010.09.070 (doi:10.1016/j.bpj.2010.09.070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui W., Ma J., Lei P., Wu W., Yu Y., Xiang Y., Tong A., Zhao Y., Li Y. 2009. Insulin is a kinetic but not a thermodynamic inhibitor of amylin aggregation. FEBS J. 276, 3365–3371 10.1111/j.1742-4658.2009.07061.x (doi:10.1111/j.1742-4658.2009.07061.x) [DOI] [PubMed] [Google Scholar]
- 38.Bedrood S., Li Y., Isas J. M., Hegde B. G., Baxa U., Haworth I. S., Langen R. 2012. Fibril structure of human islet amyloid polypeptide. J. Biol. Chem. 287, 5235–5241 10.1074/jbc.M111.327817jbc.M111.327817 (doi:10.1074/jbc.M111.327817jbc.M111.327817) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mishra R., Geyer M., Winter R. 2009. NMR spectroscopic investigation of early events in IAPP amyloid fibril formation. Chem. Bio. Chem. 10, 1769–1772 10.1002/cbic.200900237 (doi:10.1002/cbic.200900237) [DOI] [PubMed] [Google Scholar]
- 40.Reddy A. S., Wang L., Singh S., Ling Y. L., Buchanan L., Zanni M. T., Skinner J. L., de Pablo J. J. 2010. Stable and metastable states of human amylin in solution. Biophys. J. 99, 2208–2216 10.1016/j.bpj.2010.07.014 (doi:10.1016/j.bpj.2010.07.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Padrick S. B., Miranker A. D. 2001. Islet amyloid polypeptide: identification of long-range contacts and local order on the fibrillogenesis pathway. J. Mol. Biol. 308, 783–794 10.1006/jmbi.2001.4608 (doi:10.1006/jmbi.2001.4608) [DOI] [PubMed] [Google Scholar]
- 42.Pavinatto F. J., Caseli L., Pavinatto A., dos Santos D. S., Nobre T. M., Zaniquelli M. E. D., Silva H. S., Miranda P. B., de Oliveira O. N. 2007. Probing chitosan and phospholipid interactions using Langmuir and Langmuir−Blodgett films as cell membrane models. Langmuir 23, 7666–7671 10.1021/la700856a (doi:10.1021/la700856a) [DOI] [PubMed] [Google Scholar]
- 43.Dabkowska A. P., Barlow D. J., Hughes A. V., Campbell R. A., Quinn P. J., Lawrence M. J. 2011. The effect of neutral helper lipids on the structure of cationic lipid monolayers. J. R. Soc. Interface 9, 548–561 10.1098/rsif.2011.0356 (doi:10.1098/rsif.2011.0356) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leblanc R. M. 2006. Molecular recognition at Langmuir monolayers. Curr. Opin. Chem. Biol. 10, 529–536 10.1016/j.cbpa.2006.09.010 (doi:10.1016/j.cbpa.2006.09.010) [DOI] [PubMed] [Google Scholar]
- 45.Du X., Hlady V., Britt D. 2005. Langmuir monolayer approaches to protein recognition through molecular imprinting. Biosens. Bioelectron. 20, 2053–2060 10.1016/j.bios.2004.08.044 (doi:10.1016/j.bios.2004.08.044) [DOI] [PubMed] [Google Scholar]
- 46.Mendelsohn R., Mao G., Flach C. R. 2010. Infrared reflection–absorption spectroscopy: principles and applications to lipid–protein interaction in Langmuir films. Biochim. Biophys. Acta Biomembr. 1798, 788–800 10.1016/j.bbamem.2009.11.024 (doi:10.1016/j.bbamem.2009.11.024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liao Z., Lampe J. W., Ayyaswamy P. S., Eckmann D. M., Dmochowski I. J. 2011. Protein assembly at the air–water interface studied by fluorescence microscopy. Langmuir 27, 12 775–12 781 10.1021/la203053g (doi:10.1021/la203053g) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bjellqvist B., Hughes G. J., Pasquali C., Paquet N., Ravier F., Sanchez J.-C., Frutiger S., Hochstrasser D. 1993. The focusing positions of polypeptides in immobilized pH gradients can be predicted from their amino acid sequences. Electrophoresis 14, 1023–1031 10.1002/elps.11501401163 (doi:10.1002/elps.11501401163) [DOI] [PubMed] [Google Scholar]
- 49.Cort J., Liu Z. H., Lee G., Harris S. M., Prickett K. S., Gaeta L. S. L., Andersen N. H. 1994. β-Structure in human amylin and 2 designer β-peptides: CD and NMR spectroscopic comparisons suggest soluble β-oligomers and the absence of significant populations of β-strand dimers. Biochem. Biophys. Res. Commun. 204, 1088–1095 10.1006/bbrc.1994.2574 (doi:10.1006/bbrc.1994.2574) [DOI] [PubMed] [Google Scholar]
- 50.Kamilya T., Pal P., Mahato M., Talapatra G. B. 2009. Effect of salt on the formation of alcohol-dehydrogenease monolayer: a study by the Langmuir−Blodgett technique. J. Phys. Chem. B 113, 5128–5135 10.1021/jp9001059 (doi:10.1021/jp9001059) [DOI] [PubMed] [Google Scholar]
- 51.Dziri L., Puppala K., Leblanc R. M. 1997. Surface and spectroscopic properties of acetylcholinesterase monolayer at the air/water interface. J. Colloid Interface Sci. 194, 37–43 10.1006/jcis.1997.5069 (doi:10.1006/jcis.1997.5069) [DOI] [PubMed] [Google Scholar]
- 52.Zhou H. 2005. Interactions of macromolecules with salt ions: an electrostatic theory for the Hofmeister effect. Proteins: Struct. Funct. Bioinf. 61, 69–78 10.1002/prot.20500 (doi:10.1002/prot.20500) [DOI] [PubMed] [Google Scholar]
- 53.Orbulescu J., Micic M., Ensor M., Trajkovic S., Daunert S., Leblanc R. M. 2009. Human cardiac troponin I: a Langmuir monolayer study. Langmuir 26, 3268–3274 10.1021/la903033x (doi:10.1021/la903033x) [DOI] [PubMed] [Google Scholar]
- 54.Higham C. E., Jaikaran E. T. A. S., Fraser P. E., Gross M., Clark A. 2000. Preparation of synthetic human islet amyloid polypeptide (IAPP) in a stable conformation to enable study of conversion to amyloid-like fibrils. FEBS Lett. 470, 55–60 10.1016/S0014-5793(00)01287-4 (doi:10.1016/S0014-5793(00)01287-4) [DOI] [PubMed] [Google Scholar]
- 55.McLean L. R., Balasubramaniam A. 1992. Promotion of β-structure by interaction of diabetes associated polypeptide (amylin) with phosphatidylcholine. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1122, 317–320 10.1016/0167-4838(92)90411-6 (doi:10.1016/0167-4838(92)90411-6) [DOI] [PubMed] [Google Scholar]
- 56.Apostolidou M., Jayasinghe S. A., Langen R. 2008. Structure of α-helical membrane-bound human islet amyloid polypeptide and its implications for membrane-mediated misfolding. J. Biol. Chem. 283, 17 205–17 210 10.1074/jbc.M801383200 (doi:10.1074/jbc.M801383200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dluhy R. A. 1986. Quantitative external reflection infrared spectroscopic analysis of insoluble monolayers spread at the air-water interface. J. Phys. Chem. 90, 1373–1379 10.1021/j100398a033 (doi:10.1021/j100398a033) [DOI] [Google Scholar]
- 58.Thakur G., Wang C., Leblanc R. M. 2008. Surface chemistry and in situ spectroscopy of a lysozyme Langmuir monolayer. Langmuir 24, 4888–4893 10.1021/la703893m (doi:10.1021/la703893m) [DOI] [PubMed] [Google Scholar]
- 59.Xu Z., Brauner J. W., Flach C. R., Mendelsohn R. 2004. Orientation of peptides in aqueous monolayer films. Infrared reflection−absorption spectroscopy studies of a synthetic amphipathic β-sheet. Langmuir 20, 3730–3733 10.1021/la0304316 (doi:10.1021/la0304316) [DOI] [PubMed] [Google Scholar]
- 60.Hussain H., Kerth A., Blume A., Kressler J. 2004. Amphiphilic block copolymers of poly(ethylene oxide) and poly(perfluorohexylethyl methacrylate) at the water surface and their penetration into the lipid monolayer. J. Phys. Chem. B 108, 9962–9969 10.1021/jp0495702 (doi:10.1021/jp0495702) [DOI] [Google Scholar]
- 61.Baekmark T. R., Wiesenthal T., Kuhn P., Bayerl T. M., Nuyken O., Merkel R. 1997. New insights into the phase behavior of lipopolymer monolayers at the air/water interface. IRRAS study of a polyoxazoline lipopolymer. Langmuir 13, 5521–5523 10.1021/la970610l (doi:10.1021/la970610l) [DOI] [Google Scholar]
- 62.Surewicz W. K., Mantsch H. H., Chapman D. 1993. Determination of protein secondary structure by Fourier transform infrared spectroscopy: a critical assessment. Biochemistry 32, 389–394 10.1021/bi00053a001 (doi:10.1021/bi00053a001) [DOI] [PubMed] [Google Scholar]
- 63.Pelton J. T., McLean L. R. 2000. Spectroscopic methods for analysis of protein secondary structure. Anal. Biochem. 277, 167–176 10.1006/abio.1999.4320 (doi:10.1006/abio.1999.4320) [DOI] [PubMed] [Google Scholar]
- 64.Meister A., Nicolini C., Waldmann H., Kuhlmann J., Kerth A., Winter R., Blume A. 2006. Insertion of lipidated ras proteins into lipid monolayers studied by infrared reflection absorption spectroscopy (IRRAS). Biophys. J. 91, 1388–1401 10.1529/biophysj.106.084624 (doi:10.1529/biophysj.106.084624) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang C., Zheng J., Oliveira O. N., Leblanc R. M. 2007. Nature of the interaction between a peptidolipid Langmuir monolayer and paraoxon in the subphase. J. Phys. Chem. C 111, 7826–7833 10.1021/jp071000p (doi:10.1021/jp071000p) [DOI] [Google Scholar]
- 66.Dluhy R. A., Cornell D. G. 1985. In situ measurement of the infrared spectra of insoluble monolayers at the air-water interface. J. Phys. Chem. 89, 3195–3197 10.1021/j100261a006 (doi:10.1021/j100261a006) [DOI] [Google Scholar]
- 67.Ren Y., Kato T. 2002. Polarized infrared external reflection spectroscopy of a uniaxial monolayer at the air/water interface. Langmuir 18, 6699–6705 10.1021/la020185i (doi:10.1021/la020185i) [DOI] [Google Scholar]
- 68.Golczak M., Kirilenko A., Bandorowicz-Pikula J., Desbat B., Pikula S. 2004. Structure of human annexin A6 at the air-water interface and in a membrane-bound state. Biophys. J. 87, 1215–1226 10.1529/biophysj.103.038240 (doi:10.1529/biophysj.103.038240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yassine W., Milochau A., Buchoux S., Lang J., Desbat B., Oda R. 2010. Effect of monolayer lipid charges on the structure and orientation of protein VAMP1 at the air–water interface. Biochim. Biophys. Acta Biomembr. 1798, 928–937 10.1016/j.bbamem.2010.01.009 (doi:10.1016/j.bbamem.2010.01.009) [DOI] [PubMed] [Google Scholar]
- 70.Fainerman V. B., Vollhardt D., Johann R. 2000. Arachidic acid monolayers at high pH of the aqueous subphase: studies of counterion bonding. Langmuir 16, 7731–7736 10.1021/la0003903 (doi:10.1021/la0003903) [DOI] [Google Scholar]







