Abstract
We prospectively measured functional performances (WOMAC, SF-36, 2-minute walk test and timed get-up-and-go test) of patients who underwent total hip arthroplasty (THA) and had serum vitamin D levels tested during the preoperative evaluation. Of 219 patients, 102 patients (46.6%) had low vitamin D levels (25-hydroxyvitamin D < 30 ng/mL). Low vitamin D status did not adversely affect short-term function at 6 weeks after THA. In addition, there was no association between serum vitamin D levels and the within-patient changes of scores of each outcome measurement. Since this 6-week period is generally adequate to correct vitamin D deficiency, orthopaedic surgeons can safely perform THA without delay. Nevertheless, because vitamin D deficiency impairs bone quality, patients with low vitamin D levels should be treated once identified.
INTRODUCTION
The role of vitamin D in the musculoskeletal system has gained much interest. Recent data describe a surprisingly high prevalence of vitamin D deficiency, with the worldwide deficiency rate estimated at approximately one billion [1–3]. While vitamin D deficiency has emerged as a global health issue [4], the prevalence of vitamin D deficiency is relevant for the musculoskeletal system and the orthopaedic population [5].
Apart from the well-known effects on bone metabolism, vitamin D deficiency is also associated with muscle weakness, predominantly of the proximal muscle groups [6]. Evidence for the potential role of vitamin D in muscle function in older persons comes from the well-documented association between low vitamin D levels and decreased muscle strength, difficulty in performing activities of daily living, and increased risk of falls [7,8]. However, studies examining the relationship between vitamin D status and muscle strength and disability in older populations yielded conflicting results. Several cross-sectional studies showed a significant correlation between serum vitamin D and muscle strength in frail or hospitalized elderly patients [2,8–10]. Conversely, some studies that reported the effect of vitamin D on muscle function in a healthy, ambulatory elderly population, found no correlation between serum vitamin D level and muscle strength [11–13]. A comparison of results from these studies however is hampered by differences in patient demographics, study design and outcome variables. Nevertheless, vitamin D status is a critical factor for maintaining musculoskeletal health, and remains an important consideration in patients undergoing orthopaedic surgery [7,8,10].
Muscle injury from the surgical approach during total hip arthroplasty (THA) can lead to muscle deconditioning and a subsequent, slow recovery period [14,15]. It is therefore important to attempt all measures to minimize muscle injury to facilitate postoperative recovery after THA. The role of vitamin D on muscular and physical functioning after THA is not known. This is potentially important because vitamin D deficiency can be easily and safely corrected by six weeks of oral vitamin D supplementation [16–18]. For instance, if there were an association between low vitamin D levels and poorer functional outcomes, it would be prudent to pretreat these patients with oral vitamin D supplementation. Therefore, the objective of our study was to determine whether there is impairment in short-term functional outcomes after THA in patients with low serum vitamin D levels. In this study, we assessed postoperative functional outcomes from both self-report questionnaires and performance-based tests.
MATERIALS AND METHODS
The study was approved by the Institutional Review Board and informed consent was obtained from all patients. Patients of two arthroplasty surgeons scheduled for primary THA were prospectively investigated between March 2010 and June 2011. These two participating surgeons routinely measured serum 25-hydroxyvitamin D (25(OH)D) levels in all patients scheduled for THA as part of their preoperative laboratory assessment. Patients were eligible if they were between 40 and 100 years old and were medically stable. Patients were excluded if they had hip surgery within one year, did not return the self-report questionnaires, declined to perform the performance-based tests, or declined to participate in research. Patients were recruited and enrolled when they returned to the hospital to attend a preoperative educational class or for preadmission testing. Patients were asked to complete self-report questionnaires and perform the performance-based tests at preoperative and at six-week postoperative follow-up.
Self-report questionnaires
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) is a disease-specific questionnaire that is valid, reliable, and sensitive to change in health status in patients with hip and knee osteoarthritis [19]. The WOMAC is composed of 24 items grouped into three subscales: pain (5 items), stiffness (2 items), and physical function (17 items). In this study, we obtained the WOMAC from the Hip disability and Osteoarthritis Outcome Score (HOOS), a larger questionnaire used to evaluate symptoms and functional limitations related to the hip [20]. The HOOS contains all WOMAC (version 3.0) items in unchanged form. A subscale score is calculated ranging from 0 to 100, with higher scores indicating higher levels of function or well-being.
The Short Form-36 (SF-36) is a generic questionnaire consisting of 36 items grouped into eight subscales (physical function, physical role function, bodily pain, general health perception, vitality, social role function, emotional role function, and mental heath). A score for each subscale can be calculated, ranging from 0 to 100, with higher scores indicating better function or health status.
Performance-Based Tests
Functional evaluation was performed by using two performance-based tests: two-minute walk test and timed get-up-and-go test. The results of these two performance-based tests at both pre- and postoperative follow-up were recorded by one of the investigators who was blinded to the patients’ vitamin D levels. For the two-minute walk test, patients were asked to walk up and down a designated corridor for two minutes. Patients were instructed to walk at their normal pace and to turn around at the ends of the corridor without stopping. Patients were permitted to use walking aids if they wished. Standardized encouraging phrases, such as “You are doing well, keep up the good work” “You have 30 seconds left”, were provided at 30-second intervals during the test [21]. Results were recorded as total distance walked in meters.
For the timed get-up-and-go test, patients were instructed to rise from a high-seated chair, walk at a safe and comfortable pace to a mark three meters away, and return to a sitting position with their backs against the chair. Patients were permitted to use their arms when rising from and returning to a seated position. A stopwatch was used to measure the time to complete this activity to the nearest one tenth of a second. Patients were asked to perform this task three times and the average time was calculated.
Demographic data and clinical information were collected at enrollment. As a measure of comorbidity, we calculated a Charlson comorbidity index [22] and defined three comorbidity levels: a score of 0 (low), given to patients with no previously recorded disease categories included in the index; a score of 1 to 2 points (medium); and a score of > 3 points (high) [23]. Active back pain and major joint pain of the lower extremity were also recorded, including the contralateral hip, and the contralateral and ipsilateral knees and ankles, and reported as the total number of additional painful lower extremity joints for each patient. We also collected data on the use of a walking aid, categorized as none (walking without an assistive device), or requiring a cane, walker, or wheelchair. The primary outcome of our study was the within-patient change of scores of the WOMAC functional subscale. Our secondary outcomes included the within-patient change of WOMAC pain and stiffness subscale scores, SF-36 physical function score, two-minute walk test and timed get-up-and-go test. The within-patient change of each outcome was calculated by subtracting the preoperative value from the postoperative value.
All operations were performed with a minimally invasive surgical technique through a posterior approach. The prostheses used in this study were all of cementless design with a tantalum hemispherical monoblock acetabular component (Zimmer, Warsaw, Ind) and titanium femoral component with hydroxyapatite coated proximally (Novation; Exactech, Gainesville, Fla). Each patient was treated with patient-controlled epidural analgesia for 24 hours. Once the patient-controlled epidural analgesia was discontinued, pain was further controlled by oral pain medication. Pain medications included acetaminophen and short-acting opioids. The goal was to keep pain at 2 to 3 of 10 as measured by a visual analog scale. The patients were instructed to take pain medications as needed for approximately six weeks after surgery.
Each patient started physical therapy either on the day of surgery, or one day after the procedure, depending on the time of day that surgery was completed. Patients were discharged from the hospital either to their home or a rehabilitation facility once they had achieved their physical therapy goals. The physical therapy goals were adjusted for each individual depending on his/her preoperative ambulatory status and household environment. The criteria for patients being discharged home included the attainment of all three functional milestones: transferring in- and out of bed, ambulating with assisting device, and climbing up and down four steps of stairs [24,25]. If patients couldn’t attain all of these functional milestones, they were sent to a rehabilitation facility to receive further intensive in-patient physical therapy.
Laboratory Analyses
Non-fasting blood samples were obtained at the preoperative visit, and sent for analysis at the central laboratory at our institution. Serum calcium and albumin were obtained as part of the complete metabolic panel. Serum 25(OH)D level was measured using a radioreceptor assay, and intact parathyroid hormone was measured using a chemiluminescence technique. Normal serum vitamin D level was defined as serum 25(OH)D level ≥ 30 ng/mL [26]. Low serum vitamin D levels (25(OH)D < 30 ng/mL) can be subcategorized to insufficiency and deficiency based on 25(OH)D levels: insufficiency (25(OH)D = 20 ng/mL to < 30 ng/mL) and deficiency (25(OH)D < 20 ng/mL) [26]. We used the level of serum 25(OH)D as the main indicator for vitamin D status because it is the most abundant circulating metabolite and the most reliable indicator of vitamin D intake and stores.
Statistical Analysis
In order to account for the other covariates that could have a larger affect on the primary outcome [27], a power analysis was conducted for a linear regression using the change in WOMAC score as the dependent variable. Results from a power analysis of a multiple linear regression model showed us that a sample size of 146 patients achieved 80% power to detect an R2 change from 0.30 in the null model to an R2 of 0.35 with three independent variables (preoperative WOMAC function, gender, and preoperative comorbidities), an effect size of vitamin D estimated to be 0.05, and statistical significance set to alpha equal to 0.05.
Data are shown as mean ± SD for normally distributed variables and as median and interquartile ranges for variables that were not normally distributed. Categorical variables are presented as frequencies and percentages. Each variable was evaluated for normality. Following the descriptive analysis, differences between low- and normal vitamin D levels on the within-patient changes of WOMAC, SF-36 physical function subscale, and two-minute walk test were analyzed using independent samples t-tests, whereas difference between the two groups on the within-patient change of timed get-up-and-go test was analyzed using the Mann-Whitney U test. Within-patient changes of scores from both self-report questionnaires and performance-based tests from preoperative to six-week postoperative period were assessed with paired t-tests, whereas the within-patient changes in timed get-up-and-go test were assessed using Wilcoxon rank-sum test. Additional subgroup analyses of the outcomes based on vitamin D levels (normal, insufficiency, deficiency) were performed using one-way ANOVA for the within-patient changes of WOMAC, SF-36 and two-minute walk test and Kruskal–Wallis one-way analysis of variance for timed get-up-and-go test. Furthermore, correlations between serum vitamin D levels and the postoperative and the within-patient changes of scores from self-report questionnaires and performance-based tests were assessed with the Pearson’s correlation coefficient (r) for parametric data and with the Spearman’s rho (ρ) for nonparametric data. The degree of correlation was defined as low if the coefficient was less than 0.3, moderate if it was between 0.3 and 0.5, and strong if it was greater than 0.5 [28]. The Bonferroni technique was used to adjust critical p-values for all multiple comparisons. All analyses were conducted using SPSS® software (Version 17.0; SPSS Inc, Chicago, IL, USA).
Source of Funding
No external funding was received in preparation of the manuscript.
RESULTS
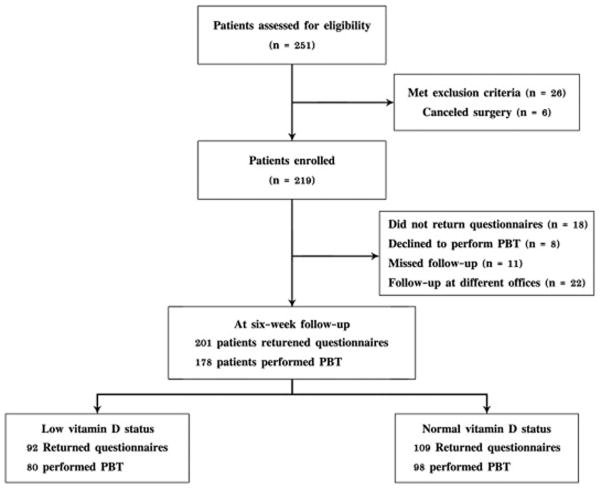
A total of 259 patients was assessed for eligibility in this study and 219 patients were enrolled. Reasons for non-enrollment were: prior hip surgery or bilateral one-stage THA (6); complex surgical procedure (9); postoperative revision due to infection, dislocation, or perioperative fracture (12); and declining to continue research at six weeks (7). Six patients canceled surgery due to personal reasons. Of the 219 patients, 102 patients (46.6%) had low vitamin D levels (< 30 ng/mL) while 117 patients (53.4%) had normal vitamin D levels (> 30 ng/mL). At the six-week postoperative follow-up, 18 patients did not return the questionnaires, 8 declined to perform the performance-based tests, 11 missed the postoperative appointment, and 22 followed-up at an outside institute. Thus, scores of self-report questionnaires and performance-based tests were available for final analysis in 201 and 178 patients, respectively (Fig. 1).
Fig. 1.
Diagram showing patient flow through the study. PBT indicates performance-based tests.
There were more women in the normal vitamin D group (77 women, 65.8%), whereas the low vitamin D group had more men (53 men, 52.0%) (p = 0.008). Patients with low vitamin D tend to be younger and have higher body mass index than those with normal vitamin D levels (p = 0.001). There were no differences in other demographic and clinical characteristics such as race, diagnosis, side, Charlson comorbidity index, number of additional painful lower extremity joints or back pain, and the use of a walking aid (Table 1). In both groups, the majority of patients were Caucasians, diagnosed with primary osteoarthritis, and were ambulatory without assistive walking devices. Aside from vitamin D levels, the two groups did not differ in laboratory findings including serum calcium, albumin and intact parathyroid hormone levels (Table 2).
Table I.
Demographic and Clinical Characteristics
| Variables | Low vitamin D < 30 ng/mL (N = 102) |
Normal vitamin D ≥ 30 ng/mL (N = 117) |
p-value |
|---|---|---|---|
|
| |||
| Age (years) | 65.0 ± 9.6 | 69.3 ± 9.3 | 0.001 |
|
| |||
| Female sex (N, %) | 49 (48.0%) | 77 (65.8%) | 0.008 |
|
| |||
| Race (N, %) | |||
| - Caucasian | 97 (95.1%) | 111 (94.9%) | 0.547 |
| - Latino | 0 (0%) | 2 (1.7%) | |
| - African-american | 3 (2.9%) | 2 (1.7%) | |
| - Asian | 2 (2.0%) | 2 (1.7%) | |
|
| |||
| Body mass index (kg/m2) | 28.8 ± 6.6 | 26.3 ± 4.8 | 0.001 |
|
| |||
| Diagnosis (N, %) | |||
| - Primary osteoarthritis | 56 (54.9%) | 66 (56.4%) | 0.909 |
| - Dysplastic hip | 29 (28.4%) | 34 (29.1%) | |
| - Others | 17 (16.7%) | 17 (14.5%) | |
|
| |||
| Right side (N, %) | 55 (53.9%) | 62 (53.0%) | 0.891 |
|
| |||
| Charlson comorbidity index (N,%) | |||
| - 0 | 69 (67.6%) | 82 (70.1%) | 0.600 |
| - 1–2 | 29 (28.4%) | 33 (28.2%) | |
| - ≥ 3 | 4 (3.9%) | 2 (1.7%) | |
|
| |||
| Number of other painful lower extremity joints (N, %) | |||
| - 0 | 22 (21.6%) | 18 (15.4%) | 0.879 |
| - 1 | 26 (25.5%) | 39 (33.3%) | |
| - 2 | 33 (32.4%) | 41 (35.0%) | |
| - 3 | 17 (16.7%) | 12 (10.2%) | |
| - 4 | 4 (3.9%) | 7 (6.0%) | |
|
| |||
| Active back pain (N, %) | 54 (52.9%) | 68 (58.1%) | 0.442 |
|
| |||
| The use of walking aid (N, %) | |||
| - None | 65 (63.7%) | 68 (58.1%) | 0.705 |
| - Cane | 31 (30.4%) | 42 (35.9%) | |
| - Walker | 3 (2.9%) | 5 (4.2%) | |
| - Wheelchair | 3 (2.9%) | 2 (1.7%) | |
Data was presented as mean ± SD for continuous variables and number (%) for categorical variables.
Table II.
Baseline Laboratory Tests
| Variables | Low vitamin D < 30 ng/mL (N = 102) |
Normal vitamin D ≥ 30ng/mL (N = 117) |
p-value |
|---|---|---|---|
| Serum calcium (mg/dL) | 9.6 ± 0.4 | 9.5 ± 1.0 | 0.303 |
| Serum albumin (g/dL) | 4.2 ± 0.6 | 4.2 ± 0.4 | 0.838 |
| Serum 25(OH)D (ng/mL) | 40.2 ± 9.0 | <0.001 | |
| Serum PTH (pg/mL) | 73.0 ± 29.7 | 67.7 ± 44.9 | 0.313 |
23.6 ± 4.4
Data was presented as mean ± SD for continuous variables and number (%) for categorical variables. 25(OH)D = 25-hydroxyvitamin D; PTH = parathyroid hormone.
Of the 201 patients that returned both preoperative and postoperative questionnaires, 177 completed all three subscales of the WOMAC and 194 patients completed all eight subscales of the SF-36. The remaining patients had at least one missing subscale from the WOMAC or SF-36. The WOMAC scores (pain, stiffness, and function subscales) and the SF-36 physical function score were found to conform to normal, Gaussian-shaped distributions, and are reported as means and standard deviations. There were no differences in all three preoperative WOMAC subscales and SF-36 physical function domain between the two vitamin D groups (Table 3). At six weeks follow-up, all the mean outcome scores significantly improved when compared to baseline levels (p < 0.001); however, there were no differences in postoperative and within-patient change of scores from self-report questionnaires between the two groups (Table 3).
Table III.
Preoperative, Postoperative, and the Change in Scores of Self-Report Questionnaires
| Self-report questionnaires | Low vitamin D < 30 ng/mL (N = 102) |
Normal vitamin D ≥ 30ng/mL (N = 117) |
p-value | ||
|---|---|---|---|---|---|
|
| |||||
| n | Scores | n | Scores | ||
|
| |||||
| WOMAC pain | |||||
| Preoperative | 98 | 51.3 ± 20.4 | 117 | 53.4 ± 17.2 | 0.413 |
| Postoperative | 91 | 84.8 ± 16.4 | 107 | 86.8 ± 14.0 | 0.348 |
| Within-patient change | 87 | 34.4 ± 21.9* | 107 | 33.2 ± 20.5* | 0.683 |
|
| |||||
| WOMAC stiffness | |||||
| Preoperative | 102 | 41.5 ± 19.8 | 116 | 42.8 ±20.2 | 0.649 |
| Postoperative | 91 | 68.4 ± 18.9 | 109 | 69.5 ± 20.0 | 0.695 |
| Within-patient change | 91 | 27.5 ± 24.5* | 109 | 26.3 ± 23.6* | 0.722 |
|
| |||||
| WOMAC function | |||||
| Preoperative | 97 | 46.6 ± 19.9 | 115 | 48.4 ± 17.7 | 0.499 |
| Postoperative | 81 | 78.6 ± 17.1 | 96 | 78.1 ± 15.3 | 0.828 |
| Within-patient change | 77 | 32.3 ± 21.5* | 94 | 29.5 ± 18.1* | 0.354 |
|
| |||||
| SF-36 physical function | |||||
| Preoperative | 99 | 33.3 ± 24.6 | 107 | 32.8 ± 22.6 | 0.873 |
| Postoperative | 91 | 55.8 ± 27.6 | 108 | 55.5 ± 25.4 | 0.921 |
| Within-patient change | 88 | 22.1 ± 25.0* | 99 | 24.4 ± 24.3* | 0.513 |
Data was presented as mean ± SD.
p < 0.001 for within-patient change, measured by paired t-test. N = total number of patients in each group; n = number of patients with data available at the indicated time point; WOMAC = the Western Ontario and McMaster Universities Osteoarthritis Index; SF-36 = short form-36. A score for each subscale is calculated ranging from 0 to 100, with higher scores indicating higher levels of function or well-being.
Of the 178 patients with postoperative values on performance-based tests, five had severe functional limitations and could not perform both the timed get-up-and-go test and the two-minute walk test. All five patients were using a wheelchair, and it was considered unsafe for them to proceed with the performance-based tests. Three of these patients had low vitamin D levels, and two were in the normal vitamin D group. Results of the two-minute walk test followed a normal Gaussian-shaped distribution, while values of the timed get-up-and-go test were skewed and were reported as medians and interquartile ranges (Table 4). Similar to the results for the self-report questionnaires, there was a significant improvement for the two-minute walk test. The timed get-up-and-go test, however, was significantly improved only in the low vitamin D level group (p = 0.004 and 0.077 for low and normal vitamin D groups respectively). There were no differences for postoperative and within-patient changes for the performance-based tests between the two vitamin D groups (Table 4).
Table IV.
Pre- and Postoperative Performance-Based Tests
| Performance-based tests | Low vitamin D < 30 ng/mL (N = 102) |
Normal vitamin D ≥ 30ng/mL (N = 117) |
p-value | ||
|---|---|---|---|---|---|
| Two-minute walk test (meters) | n | Mean + SD | n | Mean + SD | |
| preoperative | 100 | 109.4 ± 38.3 | 115 | 101.7 ± 35.9 | 0.128 |
| postoperative | 80 | 119.7 ± 44.9 | 98 | 109.8 ± 34.3 | 0.095 |
| within-patient change | 78 | 9.2 ± 29.5* | 97 | 10.0 ± 31.4* | 0.863 |
| Timed get-up-and-go test (sec) | n | Median (interquartile ranges) | n | Median (interquartile ranges) | p-value |
| preoperative | 101 | 9.6 (8.1 – 12.5) | 116 | 10.3 (8.0 – 13.5) | 0.438 |
| postoperative | 80 | 9.0 (7.2 – 11.1) | 98 | 10.0 (7.7 – 11.7) | 0.115 |
| within-patient change | 79 | −0.5 (−2.5 – 0.8)* | 97 | −0.6 (−2.5 – 1.4) | 0.497 |
The within-patient change is statistically significant (p < 0.01), measured by paired t-test for parametric data, and Wilcoxon rank-sum test for nonparametric data. N = total number of patients in each group; n = number of patients with data available at the indicated time point.
When evaluating the changes in self-report questionnaires and performance-based tests based on three vitamin D subgroups [normal (25(OH)D > 30 ng/mL), insufficient (25(OH)D 20 to < 30 ng/mL) and deficient (25(OH)D < 20 ng/mL)], there were no statistical differences in these outcome measured among the three vitamin D levels (Table 5). In addition, based on bivariate analysis, there were no significant correlations between serum vitamin D levels and postoperative scores for both self-report questionnaires and performance-based tests (r ranged from 0.004 to 0.084, p > 0.2). Similarly, there were no correlations between serum vitamin D levels and within-patient changes in scores (r ranged from 0.018 to 0.120, p > 0.1) (Table 6).
Table V.
Pre-, Postoperative, and the Within-Patient Change in Scores of Self-Report Questionnaires and Performance-Based Tests Based on Three Vitamin D Groups
| Functional performance | Vitamin D deficiency < 20 ng/mL (N = 19) |
Vitamin D insufficiency 20–30 ng/mL (N = 83) |
Vitamin D sufficiency > 30 ng/mL (N = 117) |
p-value | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| n | Scores | n | Scores | n | Scores | ||
|
| |||||||
| WOMAC function | |||||||
| Preoperative | 19 | 40.4 ± 20.1 | 78 | 47.9 ± 19.5 | 115 | 48.6 ± 17.8 | 0.209 |
| Postoperative | 12 | 75.3 ± 22.0 | 69 | 78.7 ± 16.3 | 96 | 78.4 ± 15.3 | 0.787 |
| Within-patient change | 12 | 35.0 ± 19.7** | 65 | 31.6 ± 21.6** | 94 | 29.6 ± 18.2** | 0.608 |
|
| |||||||
| SF-36 physical function | |||||||
| Preoperative | 17 | 32.1 ± 27.8 | 82 | 33.4 ± 23.9 | 107 | 32.9 ± 22.7 | 0.974 |
| Postoperative | 18 | 50.2 ± 31.2 | 73 | 56.5 ± 26.8 | 108 | 55.9 ± 25.4 | 0.662 |
| Within-patient change | 16 | 15.4 ± 26.0* | 72 | 23.4 ± 24.6** | 99 | 24.5 ± 24.4** | 0.392 |
|
| |||||||
| Two-minute walk test (meters) | |||||||
| Preoperative | 19 | 100.1 ± 36.4 | 82 | 111.6 ± 38.2 | 115 | 101.5 ± 36.2 | 0.138 |
| Postoperative | 17 | 105.3 ± 35.8 | 63 | 123.4 ± 43.5 | 98 | 109.6 ± 34.6 | 0.058 |
| Within-patient change | 17 | 1.7 ± 43.7 | 61 | 11.1 ± 23.8** | 97 | 10.1 ± 31.7** | 0.518 |
|
| |||||||
| Timed get-up-and-go test (sec)† | |||||||
| Preoperative | 19 | 10.7 (8.3 – 18.5) | 82 | 9.3 (7.8 – 12.3) | 116 | 10.4 (8.3 – 13.6) | 0.151 |
| Postoperative | 17 | 10.7 (7.8 – 13.5) | 63 | 8.8 (6.6 – 10.6) | 98 | 10.0 (7.7 – 11.8) | 0.018 |
| Within-patient change | 17 | 0.0 (−3.0 – 1.3) | 62 | −0.6 (−2.3 – 1.0)** | 97 | −0.7 (−2.5 – 1.4) | 0.733 |
Data was presented as mean ± SD.
Data was presented as median and interquartile ranges.
The within-patient change is statistically significant (p < 0.05).
The within-patient change is statistically significant (p < 0.01).
N = total number of patients in each group; n = number of patients with data available at the indicated time point; WOMAC = the Western Ontario and McMaster Universities Osteoarthritis Index; SF-36 = short form-36. A score for each subscale is calculated ranging from 0 to 100, with higher scores indicating higher levels of function or well-being.
Table VI.
The Correlations Between Serum Vitamin D Levels and Postoperative and Within-Patient Change of Scores of Self-Report Questionnaires and Performance-Based Tests
| Self-report questionnaires and performance-based tests | Vitamin D level
|
||
|---|---|---|---|
| Correlation coefficient* | p-value | ||
|
| |||
| Postoperative score | |||
| WOMAC pain | 0.067 | 0.351 | |
| WOMAC stiffness | 0.061 | 0.387 | |
| WOMAC function | −0.028 | 0.708 | |
| SF-36 physical function | 0.004 | 0.950 | |
| Two-minute walk test | −0.084 | 0.265 | |
| Timed get-up-and-go test | 0.060 | 0.424 | |
|
| |||
| Within-patient change | |||
| WOMAC pain | −0.087 | 0.227 | |
| WOMAC stiffness | 0.025 | 0.726 | |
| WOMAC function | −0.120 | 0.117 | |
| SF-36 physical function | 0.034 | 0.648 | |
| Two-minute walk test | 0.022 | 0.775 | |
| Timed get-up-and-go test | 0.018 | 0.811 | |
Pearson correlation coefficient (r) was used for all parametric data, whereas Spearman’s rho (ρ) was used for timed get-up-and-go test (nonparametric data). WOMAC = the Western Ontario and McMaster Universities Osteoarthritis Index; SF-36 = short form-36.
DISCUSSION
There is increasing evidence that vitamin D plays an essential role in many tissues including skeletal muscle [29,30]. Muscle biopsies obtained in osteomalacic patients reveal an atrophy of type II muscle fibers with enlarged interfibrillar spaces and infiltration of fibrosis, glycogen granules and fat [31,32]. Fatty degeneration of the hip muscles was strongly correlated with lower performance on physical tests requiring these muscles [33]. However, histological changes in the muscle fiber are reversible with vitamin D supplementation [34]. During the past several decades, there is a growing number of clinical studies of the muscular effects of vitamin D supplementation and research on vitamin D muscle cell receptor. These studies have improved our understanding of the role and actions of vitamin D in muscle tissue and on physical performance [35–37]. Since muscle around the hip is an important determinant of postoperative recovery, it is important to evaluate whether there is an association between serum vitamin D levels and function after THA. In this study, we assessed short-term functional status using change in scores from self-report questionnaires and performance-based tests in patients who had THA.
Our study showed that there were no differences in short-term function after THA between low and normal serum vitamin D groups. In addition, there was no association between serum vitamin D levels and postoperative functional status, determined from both self-report questionnaires and performance-based tests. These findings were different from those of several cross-sectional studies, which found a direct association between vitamin D status and parameters of physical performance in community-dwelling elderly individuals, especially when 25(OH)D levels were < 30 ng/mL [38,39]. One possible explanation for our findings may be related to postoperative pain. Since postoperative pain has been identified as one of the major factors influencing functional performance [40,41], the impact of postoperative surgical pain during the early recovery period may overwhelm the effect of vitamin D in arthroplasty patients. This potentially could explain the lack of association between vitamin D status and functional outcomes in our study.
In another recent study evaluating the effect of serum 25(OH)D in patients undergoing THA, patients with vitamin D deficiency (defined as 25(OH)D < 16 ng/mL) had lower six-month postoperative Harris hip scores and were less likely to attain an excellent outcome from arthroplasty [42]. However, patients with vitamin D deficiency in that study also had lower preoperative Harris hip scores; and it is known that preoperative function is a major predictor of postoperative outcome. Thus, their results may just reflect the already known association between vitamin D levels and preoperative functional status.
Although the Institute of Medicine has concluded that a serum 25(OH)D level of 20 ng/ml (50 nmol/L) is “generally considered adequate for bone and overall health in healthy individuals” [43], many experts feel that these recommendations are insufficient and instead suggest maintaining levels above 30 ng/mL [17,26,44]. Consequently, in our study low serum vitamin D levels were subcategorized as insufficient (25(OH)D = 20 ng/mL to < 30 ng/mL) and deficient (25(OH)D < 20 ng/mL). When subanalyzing our outcomes based on these three groups (normal, insufficient and deficient), there were no statistical differences in the within-patient change of functional scores among the groups (p > 0.35, Table 5). However, the proportion of subjects with vitamin D deficiency was small; 19 (deficient) versus 83 (insufficient) and 117 (sufficient). When performing post-hoc analysis based on the effect size of the within-patient change of WOMAC and SF-36 subscales, our study was underpowered to detect a significant difference defined as five points for the WOMAC function and eight points for the SF-36 physical function subscales between the vitamin D deficient group and each of the other two groups. Therefore, these results should be interpreted cautiously given the chance that we may have failed to observe a difference when in truth there was one (Type II error). Since the effect of vitamin D deficiency on longer-term postoperative functional outcome and risk of periprosthetic fracture is not known, it is still prudent to keep serum vitamin D levels in a normal range. This may also maximize muscle strength postoperatively which can enhance postoperative rehabilitation.
The current study has several limitations. First, our follow-up period was only six weeks after arthorplasty. Clearly, a longer-term study to determine the effects of low vitamin D status on postoperative function is essential and should be carried out. However, there are potential ethical considerations in choosing not to treat patients that are known to have low vitamin D levels. Therefore, we first evaluated effects of serum vitamin D in the short-term period after THA. In light of our finding that there is no functional impairment in patients with low vitamin D levels during the first six weeks after THA, and considering that it requires four to six weeks to correct low vitamin D levels [16,18], orthopaedic surgeons do not need to delay surgery because of low vitamin D status. Another limitation is that we used self-report questionnaires that may not be appropriate to assess functional level during the early postoperative period. Specifically, since our postoperative precaution protocol was still applied to all patients during the first six weeks after THA, patients were advised not to perform certain activities that are assessed in the self-report questionnaires, such as squatting, running, and twisting/pivoting. Thus, scores obtained at the six-week postoperative follow-up may not truly reflect the actual functional status of the patients. Another limitation relates to the performance-based tests we used. We selected the two-minute walk test and the timed get-up-and-go test because both tests measure function of the lower extremity [45,46]. However, there are many performance-based tests available and it is not known which tests are most appropriate shortly after THA.
In conclusion, our study showed that low serum vitamin D status was common before THA and did not adversely affect postoperative function at six weeks after surgery. Since this time period is generally adequate to clinically correct vitamin D deficiency, orthopaedic surgeons can still safely perform THA without delay despite low serum vitamin D levels. Nevertheless, due to an unknown long-term effect of low vitamin D and its association with poor bone quality, it is highly recommended to correct low vitamin D status once identified. Further investigations are warranted to evaluate the long-term effect of vitamin D on the risk of periprosthetic fracture and functional recovery after THA.
Footnotes
Investigation performed at Department of Orthopaedic Surgery, Hospital for Special Surgery, New York, NY, United States
References
- 1.Gloth FM, 3rd, Gundberg CM, Hollis BW, et al. Vitamin D deficiency in homebound elderly persons. JAMA. 1995;274:21. doi: 10.1001/jama.1995.03530210037027. [DOI] [PubMed] [Google Scholar]
- 2.Gloth FM, 3rd, Tobin JD. Vitamin D deficiency in older people. J Am Geriatr Soc. 1995;43:7. doi: 10.1111/j.1532-5415.1995.tb07059.x. [DOI] [PubMed] [Google Scholar]
- 3.Utiger RD. The need for more vitamin D. N Engl J Med. 1998;338:12. doi: 10.1056/NEJM199803193381209. [DOI] [PubMed] [Google Scholar]
- 4.Mithal A, Wahl DA, Bonjour JP, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20:11. doi: 10.1007/s00198-009-0954-6. [DOI] [PubMed] [Google Scholar]
- 5.Bogunovic L, Kim AD, Beamer BS, et al. Hypovitaminosis D in patients scheduled to undergo orthopaedic surgery: a single-center analysis. J Bone Joint Surg Am. 2010;92:13. doi: 10.2106/JBJS.I.01231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glerup H, Mikkelsen K, Poulsen L, et al. Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcif Tissue Int. 2000;66:6. doi: 10.1007/s002230010085. [DOI] [PubMed] [Google Scholar]
- 7.Bischoff HA, Stahelin HB, Urscheler N, et al. Muscle strength in the elderly: its relation to vitamin D metabolites. Arch Phys Med Rehabil. 1999;80:1. doi: 10.1016/s0003-9993(99)90307-6. [DOI] [PubMed] [Google Scholar]
- 8.Mowe M, Haug E, Bohmer T. Low serum calcidiol concentration in older adults with reduced muscular function. J Am Geriatr Soc. 1999;47:2. doi: 10.1111/j.1532-5415.1999.tb04581.x. [DOI] [PubMed] [Google Scholar]
- 9.Sorensen OH, Lund B, Saltin B, et al. Myopathy in bone loss of ageing: improvement by treatment with 1 alpha-hydroxycholecalciferol and calcium. Clin Sci (Lond) 1979;56:2. doi: 10.1042/cs0560157. [DOI] [PubMed] [Google Scholar]
- 10.Mets T. Calcium, vitamin D, and hip fractures. Incidence of falls may have decreased. BMJ. 1994;309:6948. doi: 10.1136/bmj.309.6948.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boonen S, Lysens R, Verbeke G, et al. Relationship between age-associated endocrine deficiencies and muscle function in elderly women: a cross-sectional study. Age Ageing. 1998;27:4. doi: 10.1093/ageing/27.4.449. [DOI] [PubMed] [Google Scholar]
- 12.Grady D, Halloran B, Cummings S, et al. 1,25-Dihydroxyvitamin D3 and muscle strength in the elderly: a randomized controlled trial. J Clin Endocrinol Metab. 1991;73:5. doi: 10.1210/jcem-73-5-1111. [DOI] [PubMed] [Google Scholar]
- 13.Verreault R, Semba RD, Volpato S, et al. Low serum vitamin d does not predict new disability or loss of muscle strength in older women. J Am Geriatr Soc. 2002;50:5. doi: 10.1046/j.1532-5415.2002.50219.x. [DOI] [PubMed] [Google Scholar]
- 14.Cohen RG, Katz JA, Skrepnik NV. The relationship between skeletal muscle serum markers and primary THA: a pilot study. Clin Orthop Relat Res. 2009;467:7. doi: 10.1007/s11999-009-0809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teratani T, Naito M, Shiramizu K. Intraoperative muscle damage in total hip arthroplasty. J Arthroplasty. 2010;25:6. doi: 10.1016/j.arth.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Bacon CJ, Gamble GD, Horne AM, et al. High-dose oral vitamin D3 supplementation in the elderly. Osteoporos Int. 2009;20:8. doi: 10.1007/s00198-008-0814-9. [DOI] [PubMed] [Google Scholar]
- 17.Holick MF. Optimal vitamin D status for the prevention and treatment of osteoporosis. Drugs Aging. 2007;24:12. doi: 10.2165/00002512-200724120-00005. [DOI] [PubMed] [Google Scholar]
- 18.Przybelski R, Agrawal S, Krueger D, et al. Rapid correction of low vitamin D status in nursing home residents. Osteoporos Int. 2008;19:11. doi: 10.1007/s00198-008-0619-x. [DOI] [PubMed] [Google Scholar]
- 19.Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:12. [PubMed] [Google Scholar]
- 20.Nilsdotter AK, Lohmander LS, Klassbo M, et al. Hip disability and osteoarthritis outcome score (HOOS)--validity and responsiveness in total hip replacement. BMC Musculoskelet Disord. 2003;4:10. doi: 10.1186/1471-2474-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mancuso CA, Choi TN, Westermann H, et al. Measuring physical activity in asthma patients: two-minute walk test, repeated chair rise test, and self-reported energy expenditure. J Asthma. 2007;44:4. doi: 10.1080/02770900701344413. [DOI] [PubMed] [Google Scholar]
- 22.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:5. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 23.de Groot V, Beckerman H, Lankhorst GJ, et al. How to measure comorbidity. a critical review of available methods. J Clin Epidemiol. 2003;56:3. doi: 10.1016/s0895-4356(02)00585-1. [DOI] [PubMed] [Google Scholar]
- 24.Ganz SB, Wilson PD, Jr, Cioppa-Mosca J, et al. The day of discharge after total hip arthroplasty and the achievement of rehabilitation functional milestones: 11-year trends. J Arthroplasty. 2003;18:4. doi: 10.1016/s0883-5403(03)00070-6. [DOI] [PubMed] [Google Scholar]
- 25.Unnanuntana A, Rebolledo BJ, Gladnick BP, et al. Does Vitamin D Status Affect the Attainment of In-Hospital Functional Milestones After Total Hip Arthroplasty? J Arthroplasty. 2011 doi: 10.1016/j.arth.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 26.Heaney RP, Holick MF. Why the IOM recommendations for vitamin D are deficient. J Bone Miner Res. 2011;26:3. doi: 10.1002/jbmr.328. [DOI] [PubMed] [Google Scholar]
- 27.Wang W, Morrison TA, Geller JA, et al. Predicting short-term outcome of primary total hip arthroplasty:a prospective multivariate regression analysis of 12 independent factors. J Arthroplasty. 2010;25:6. doi: 10.1016/j.arth.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 28.Cohen J. Statistical power analysis for the behavioral sciences. L. Erlbaum Associates; Hillsdale, N.J: 1988. [Google Scholar]
- 29.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(Suppl):6. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 30.Ceglia L. Vitamin D and its role in skeletal muscle. Curr Opin Clin Nutr Metab Care. 2009;12:6. doi: 10.1097/MCO.0b013e328331c707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshikawa S, Nakamura T, Tanabe H, et al. Osteomalacic myopathy. Endocrinol Jpn. 1979;26(Suppl) doi: 10.1507/endocrj1954.26.supplement_65. [DOI] [PubMed] [Google Scholar]
- 32.Russell JA. Osteomalacic myopathy. Muscle Nerve. 1994;17:6. doi: 10.1002/mus.880170603. [DOI] [PubMed] [Google Scholar]
- 33.Daguet E, Jolivet E, Bousson V, et al. Fat content of hip muscles: an anteroposterior gradient. J Bone Joint Surg Am. 2011;93:20. doi: 10.2106/JBJS.J.00509. [DOI] [PubMed] [Google Scholar]
- 34.Ziambaras K, Dagogo-Jack S. Reversible muscle weakness in patients with vitamin D deficiency. West J Med. 1997;167:6. [PMC free article] [PubMed] [Google Scholar]
- 35.Pfeifer M, Begerow B, Minne HW. Vitamin D and muscle function. Osteoporos Int. 2002;13:3. doi: 10.1007/s001980200012. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki T, Kwon J, Kim H, et al. Low serum 25-hydroxyvitamin D levels associated with falls among Japanese community-dwelling elderly. J Bone Miner Res. 2008;23:8. doi: 10.1359/jbmr.080328. [DOI] [PubMed] [Google Scholar]
- 37.Wicherts IS, van Schoor NM, Boeke AJ, et al. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92:6. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 38.Okuno J, Tomura S, Yanagi H. Correlation between vitamin D and functional capacity, physical function among Japanese frail elderly living in the community. Nihon Ronen Igakkai Zasshi. 2007;44:5. doi: 10.3143/geriatrics.44.634. [DOI] [PubMed] [Google Scholar]
- 39.Dam TT, von Muhlen D, Barrett-Connor EL. Sex-specific association of serum vitamin D levels with physical function in older adults. Osteoporos Int. 2009;20:5. doi: 10.1007/s00198-008-0749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma V, Morgan PM, Cheng EY. Factors influencing early rehabilitation after THA: a systematic review. Clin Orthop Relat Res. 2009;467:6. doi: 10.1007/s11999-009-0750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holtzman J, Saleh K, Kane R. Effect of baseline functional status and pain on outcomes of total hip arthroplasty. J Bone Joint Surg Am. 2002;84-A:11. doi: 10.2106/00004623-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Nawabi DH, Chin KF, Keen RW, et al. Vitamin D deficiency in patients with osteoarthritis undergoing total hip replacement: a cause for concern? J Bone Joint Surg Br. 2010;92:4. doi: 10.1302/0301-620X.92B3.23535. [DOI] [PubMed] [Google Scholar]
- 43.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96:1. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hollis BW, Wagner CL. Normal serum vitamin D levels. N Engl J Med. 2005;352:5. doi: 10.1056/NEJM200502033520521. [DOI] [PubMed] [Google Scholar]
- 45.Gine-Garriga M, Guerra M, Manini TM, et al. Measuring balance, lower extremity strength and gait in the elderly: construct validation of an instrument. Arch Gerontol Geriatr. 2010;51:2. doi: 10.1016/j.archger.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 46.Ratchford JN, Shore W, Hammond ER, et al. A pilot study of functional electrical stimulation cycling in progressive multiple sclerosis. NeuroRehabilitation. 2010;27:2. doi: 10.3233/NRE-2010-0588. [DOI] [PubMed] [Google Scholar]