Abstract
Voluntary wheel running (RUN) prevents declines in insulin-mediated vasodilation, an important component of insulin-mediated glucose disposal, in rats prone to obesity and insulin resistance.
Objective
Determine whether RUN: 1) improves insulin-stimulated vasodilation after insulin resistance has been established, and 2) differentially affects arterioles from red and white muscle.
Methods
Insulin signaling and vasoreactivity to insulin (1–1000 μIU/mL), were assessed in second order arterioles (2A) from the white (Gw) and red (Gr) gastrocnemius of sedentary OLETF rats at 12 and 20 weeks of age (SED12; SED20) and those undergoing RUN (RUN20) or caloric restriction (CR20; to match body weight of RUN) from 12–20 weeks.
Results
Glucose and insulin responses to i.p. glucose were reduced in RUN20, elevated in SED20 (P<0.05 vs. SED12), and maintained in CR20. Insulin-stimulated vasodilation was greater in Gw, but not Gr, 2As of RUN20 (P<0.01 vs. all groups) and was improved by ET-1 receptor inhibition in Gw 2As from SED20 and CR20 (P<0.05). There were no differences in microvascular insulin signaling among groups or muscle beds.
Conclusions
RUN selectively improved insulin-mediated vasodilation in Gw 2As, in part through attenuated ET-1 sensitivity/production, an adaptation that was independent of changes in adiposity and may contribute to enhanced insulin-stimulated glucose disposal.
Keywords: type 2 diabetes, exercise, insulin, microvascular, endothelin-1
Introduction
Type 2 diabetes is often characterized by insensitivity to the metabolic effects of insulin, including suppression of hepatic glucose production and impaired stimulation of skeletal muscle glucose uptake(8). Deficits in skeletal muscle glucose uptake in type 2 diabetes are commonly attributed to defects in insulin signaling within skeletal muscle(10, 39). However, accumulating evidence indicates that abnormalities in vascular reactivity to insulin also play a role by limiting the perfusion, and consequently, delivery of glucose and insulin to target tissues(18, 37).
In the endothelium, insulin stimulates production of the vasodilator nitric oxide (NO) through the PI3K/Akt pathway and the vasoconstrictor endothelin-1 (ET-1) through the MAPK pathway(11, 38). In the absence of disease, the cumulative effect of insulin is vasodilation, which serves to redistribute blood flow, and, thus, glucose and insulin, to target tissues(3). Insulin-mediated increases in skeletal muscle blood flow account for as much as 40% of insulin-stimulated glucose uptake(2, 3). However, vasoreactivity to insulin is impaired in obesity and type 2 diabetes, and decrements in insulin-stimulated blood flow correlate strongly with reductions in insulin-mediated glucose uptake(3, 18, 36, 37).
Exercise training improves the metabolic actions of insulin (i.e., the ability of insulin to stimulate skeletal muscle glucose uptake) in patients with type 2 diabetes(9, 17). Furthermore, we have demonstrated that daily exercise effectively prevents impairments in insulin-mediated vasodilation in Otsuka Long Evans Tokushima Fatty (OLETF) rats, a rodent model of hyperphagia-induced obesity and type 2 diabetes(25). Evidence also suggests that exercise training improves bulk muscle blood flow during insulin infusion in patients with type 2 diabetes (9, 16). However, little work has been done to determine whether exercise training is effective in ameliorating or reversing impairments in microvascular insulin signaling and reactivity in skeletal muscle after insulin resistance has been established.
Additionally, it is unclear whether chronic exercise training differentially impacts insulin-stimulated vasodilation in vessels from different skeletal muscle musclebeds. The greatest gains in skeletal muscle insulin sensitivity are seen in muscles recruited during exercise(9). Likewise, vessels that experience increases in blood flow during exercise tend to exhibit improvements in endothelial function(20, 24). Mounting evidence indicates that the primary role for insulin in the vasculature is not simply to increase bulk blood flow, but to redistribute it to target tissues(35). Although little work has been done to examine the global hemodynamic effects of insulin, we speculate that insulin may assist in redistributing blood flow to white muscle, which is more reliant upon glucose metabolism and typically receives lower basal rates of blood flow than red muscle.
This study was designed to test the hypotheses that: 1) daily exercise is effective as a treatment to reverse or improve insulin signaling and insulin-mediated vasodilation in skeletal muscle arterioles of rodents with established insulin resistance, and 2) daily exercise training differentially affects insulin-mediated vasodilation in arterioles from red and white muscles.
Methods
Protocol approval
The experimental protocol was approved by the Animal Care and Use Committee at the University of Missouri.
Animals and housing
Four week old, male OLETF rats (N=80) rats were obtained from Tokushima Research Institute, Otsuka Pharmaceutical (Tokushima, Japan). Rats were individually housed in cages maintained in temperature controlled (21°C) animal quarters with 06.00–18.00 h light and 18.00-06.00 h dark cycles.
Experimental design
All rats were given ad libitum access to food and remained sedentary between four and twelve weeks of age. Under sedentary conditions, OLETF rats consistently develop insulin resistance by 12 weeks of age(30, 33). At 12 weeks, rats were randomized to one of four groups: 1) studied at 12 weeks; (SED12), 2) sedentary, weeks 12–20 (SED20), 3) sedentary with caloric restriction (fed ~70% of ad libitum fed SED animals to match the body weights of the RUN group), weeks 12–20 (CR20), or 4)voluntary wheel running and ad libitum fed, weeks 12–20(RUN20), n=20 per group.
Our rationale for this design was that direct comparisons between SED12 and SED20 reveal the effects of the worsening of insulin resistance between 12 and 20 weeks in OLETF rats (31). Comparisons between RUN20 and SED12 provide insight into the efficacy of daily voluntary wheel running on reversing disease, whereas comparisons between RUN20 and SED20 illuminate the efficacy of wheel running in preventing its progression. Calorie restriction attenuates gains in adiposity and mitigates the advancement of insulin resistance in OLETF rats (25, 32). Thus, direct comparisons between CR20 and SED20 provide insight into the effects of attenuating gains in adiposity, whereas comparisons between CR20 (lowered adiposity but sedentary) and RUN20 (lowered adiposity plus daily wheel running) allow us to evaluate the specific effects of daily voluntary wheel running, independent of changes in adiposity.
All groups were fed standard chow (Formula b 5008, Purina Mills, St Louis, MO) comprised of roughly 26%, 18%, and 56% of energy was from protein, fat, and carbohydrate, respectively. Daily running distance was determined by equipping cages with running wheels connected to a Sigma Sport BC 800 bicycle computer (Cherry Creek Cyclery, Foster Falls, VA). Average running speed was estimated by dividing daily running distance by daily running time. Five hours prior to tissue collection, food was removed and running wheels were locked, as previously described (6, 25). Intraperitoneal (i.p.) administration of sodium pentobarbital (100 mg/kg) was used to anesthetize the rats, and after tissues were harvested, the animals were killed by exsanguination.
Intraperitoneal glucose tolerance test (IPGTT)
One week prior to experiments, an i.p. injection of 2 g glucose/kg body weight was administered after a 12 hour fast as previously described (6, 25). Blood was collected from a tail vein into EDTA tubes at 0, 15, 30, 45, 60, and 120 min post-injection and centrifuged at 3000 x g for 5 min. The plasma was removed, stored at −80°C, and later analyzed for glucose [glucose oxidase reagent kit (Sigma, St. Louis, MO)] and insulin [radioimmunoassay (Linco, St. Charles, MO)] (26). The glucose and insulin area under the curve (AUC0–120) were calculated by the trapezoidal method.
Body composition
On the day of experiments, the body mass of the rats was measured to the nearest 0.01 g and, following anesthetization, body composition was determined using dual energy x-ray absorptiometry (Hologic QDR-1000/w DEXA calibrated for rats). Epididymal, retroperitoneal and omental fat pads were then removed and weighed to the nearest 0.01 g.
Glycosylated hemoglobin (HbA1c)
Whole blood was collected into EDTA tubes for analysis of HbA1c by the boronate-affinity HPLC method (Primus Diagnostics, Kansas City, MO).
Arteriole isolation
The medial head of the gastrocnemius muscles were removed and placed in cold (4°C) MOPS-buffered physiological saline solution (PSS) containing, in mM, 145.0 NaCl, 4.7 KCl, 2.0 CaCl2, 1.17 MgSO4, 1.2 NaH2PO4, 5.0 glucose, 2.0 pyruvate, 0.02 EDTA, and 3.0 MOPS (pH 7.4) for dissection. Second and third order arterioles (2As and 3As respectively) were isolated from the white and red portions of the medial head of the gastrocnemius muscle (Gw and Gr, respectively).
Microvascular reactivity to insulin
The 2As from 12 animals per group were then cannulated, pressurized and mounted in MOPS-PSS (37 °C) on a microscope outfitted with a video camera for continuous measurement of intraluminal diameter, as previously described (1, 20). Vasoactive compounds were added directly to the MOPS-PSS to attain desired concentrations. Acetylcholine (ACh, 10−8-10−6 M) and KCl (80 mM) were used to verify endothelial and smooth muscle integrity. Vessels that did not respond to ACh or KCl were considered unviable and discarded. Phenylephrine (PE; 10−8-10−4 M) was used to pre-constrict vessels that did not develop spontaneous smooth muscle tone (<70% maximal diameter). Changes in intraluminal diameter were determined in response to four concentrations (1, 10, 100, and 1000 μIU/mL) of bovine insulin (Sigma, St. Louis, USA; dissolved in 0.01 N HCl and diluted 1:10 with PSS containing 1% BSA) alone. In order to determine the relative contributions of NO and ET-1 to microvascular reactivity to insulin, the experiments were repeated following incubation with the non-selective ET-1 receptor blocker, tezosentan (3 μM), or the NOS inhibitor, L- NG-nitro-L-arginine methyl ester (L-NAME; 0.1 mM). The vessels were then incubated in calcium-free PSS containing 30 μg/mL papaverine for >30 min in order to determine maximal vessel diameter.
In vitro insulin signaling
In order to obtain sufficient protein for immunoblotting, all 2As and 3As from the Gw and Gr were collected from both legs of eight animals per group. The vessels from one leg were incubated in MOPS-buffered PSS (37 °C) for 50 min, and vessels from the other leg were incubated in MOPS-buffered PSS for 50 min with the addition of insulin (100 μIU/mL) during the final 20 min of incubation. The samples were then centrifuged at 10,000 g for 2 min to pellet the arterioles. The buffer was removed, Laemmli buffer (62.5 mMTris, pH 6.8, 6 M urea, 160 mM 1,4-dithiothreitol, 2% SDS, and 0.0001% bromophenol blue) supplemented with Sigma phosphatase inhibitor cocktails 1 and 2(1:100) was added, and the vessels were flash frozen in liquid nitrogen and stored at −80°C until analysis. Western blotting was performed as previously described(14). Briefly, samples were boiled and sonicated, and protein was quantified using the QuantiT protein assay (Invitrogen, Carlsbad, CA). Samples were then diluted with Price-Laemmli buffer, and 12 ug protein of each sample was loaded onto polyacrylamide gels for separation by electrophoresis. Next, proteins were transferred to polyvinylidenediflouride (PVDF) membranes and probed with the antibodies Akt (1:500, Upstate Cell Signaling, Lake Placid, NY), phospho-specific Aktat Ser473 (1:250, Cell Signaling, Beverly, MA), eNOS (1:1000, BD Biosciences, Sparks, MD), phospho-specific eNOSat Ser1177 (1:250, BD Biosciences), p44/42-MAPK (1:500, Cell Signaling), and phospho-specific p44/42-MAPK at Thr202/Tyr204 (1:250, Cell Signaling). Total and phospho-specific densities were quantified, and the ratios of phospho specific to total density were calculated.
Western blot analysis was also used as previously described (21)to determine the content of cytochrome c, a marker of mitochondrial content, in heart, soleus, and red, white and mixed portions of the gastrocnemius muscles of six animals per group. Briefly, samples were flash frozen in liquid nitrogen, 20 mg of tissue was homogenized in ice-cold lysis buffer, and protein content was determined by BCA assay (Pierce Chemical Co., Rockford, IL). Samples were diluted with Laemmli buffer, separated by SDS-PAGE gel electrophoresis, transferred to PVDF membranes, probed for cytochrome c (1:100, Cell Signaling), and quantified by densitometry. No images contained bands with saturated pixels.
Statistical Analysis
Due to the non-normal distribution of the data, the Kruskal-Wallis test was used to test for overall differences in body weight and composition among groups. One-way analysis of variance (ANOVA) was used to detect significant group effects for HbA1c, fasting insulin and glucose as well as the glucose and insulin AUC0–120. The proc MIXED procedure (Group X Drug) was used to detect group effects for changes in vessel diameter in response to insulin alone or in the presence of either tezosentan or L-NAME. The Kruskal-Wallis test was used to detect differences in insulin signaling. Where significant group effects were detected, univariate analyses with Tukey-Kramer post hoc comparisons were performed to detect specific between group differences. All analyses were performed using SAS Version 9.1 (SAS Institute, Cary, NC, USA). Data are presented as means ± SE, and significance was set at P < 0.05, unless otherwise indicated. Due to the large number of comparisons, significance was set at P < 0.01 for tests of vascular function and insulin signaling. Within each figure, groups not sharing the same letter are significantly different from one another.
Results
Voluntary wheel running
When given access to a running wheel, the OLETFs began running 1.8 ± 0.3 km/d at 12 weeks of age, increased to 8.7 ± 0.3 km/d at 16 weeks, and fell to 5.9 ± 0.3 km/d at 20 weeks (Figure 1A). The estimated average running speed followed a similar trend, starting at approximately 27 m/min at 12 weeks, peaking at 35 m/min at 16 weeks, and decreasing to 31 m/min at 20 weeks of age (Figure 1B).
Figure 1.
Daily running distance (km/d) (A) and speed (m/min) (B) of the RUN20 group.
Body Composition
Voluntary wheel running prevented significant gains in body weight, increased lean body mass, and reduced total fat mass between 12 and 20 weeks (Figure 2A; P < 0.05, RUN20 vs. SED12). Food restriction attenuated gains in fat mass (Figure 2A; P < 0.05, CR20 vs. SED12). Animals that remained sedentary between 12 and 20 weeks of age gained significantly more weight than those with access to wheels or restricted access to food, owing largely to gains in body fat (Figure 2A; P < 0.05, SED20 vs. SED12, CR20, and RUN20). Food intake was similar among all groups through 12 weeks of age (202±3 g/wk, equivalent to 0.47±0.04 g food/kg body weight). At 20 weeks of age, food intake was reduced to 154±0 g/wk (0.32±0.01 g/kg) in animals from the calorie restricted group, but increased in the ad libitum fed animals to 225±18 g/wk (0.37±0.02 g/kg) in the sedentary group and 244±15 g/wk (0.55±0.04 g/kg) in the group given access to a running wheel.
Figure 2.
(A) Total, fat and lean mass (g) and percent body fat (% Fat). (B) Epididymal, omental, and retroperitoneal fat mass (g). All data are expressed as means ± SE. Different letters denote statistically significant between-group differences, such that groups not sharing the same letter are significantly different from one another(P < 0.05).
Omental and retroperitoneal fat stores increased significantly in sedentary animals between 12 and 20 weeks (Figure 2B; P < 0.05, SED20 vs. SED12). Food restriction attenuated gains in omental and retroperitoneal fat mass (Figure 2B; P < 0.05 CR20 vs. SED20), and voluntary wheel running further reduced epididymal and retroperitoneal fat stores (Figure 2B; P < 0.05, RUN20 vs. CR20).
Glucose, insulin and hemoglobin A1c
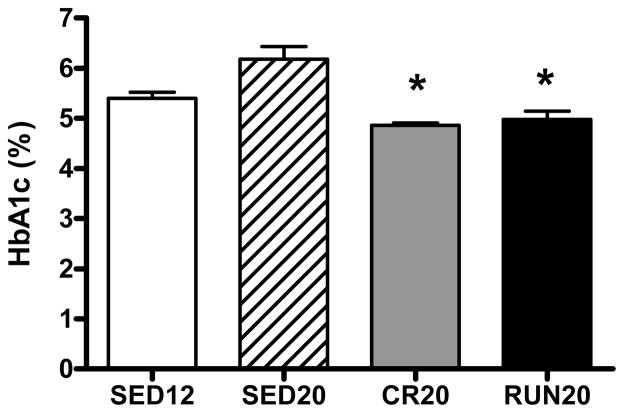
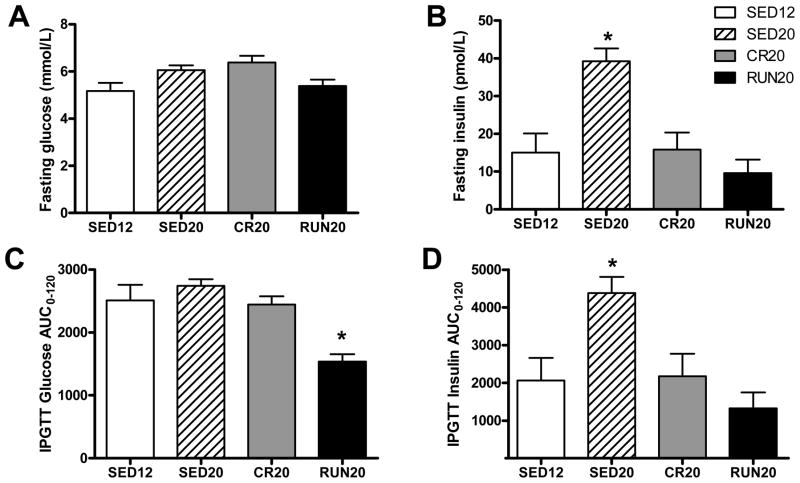
Food restriction and voluntary wheel running lowered hemoglobin A1c (HbA1c), an index of long-term glycemic control, to a similar degree (Figure 3; P < 0.05 vs. SED20). Fasting plasma glucose was similar among all four groups but was accompanied by significantly higher concentrations of plasma insulin in sedentary animals at 20 weeks (Figures 4A and 4B; P < 0.05, SED20 vs. SED12, CR20, and RUN20). Whereas the insulin response to the IPGTT was higher in sedentary animals at 20 weeks (Figure 4D; P < 0.05, SED20 vs. SED12, CR20, RUN20), the glucose response to the IPGTT was lower only in animals given access to a running wheel (Figure 4C; P < 0.05, RUN20 vs. SED12, SED20, CR20), indicating glucose tolerance was enhanced by daily physical activity, maintained by food restriction, and diminished by sedentary behavior between 12 and 20 weeks of age.
Figure 3.
Glycosylated hemoglobin (HbA1c, %). All data are expressed as means ± SE. (*) indicates that CR20 and RUN20 are lower than both sedentary groups (SED12 and SED20)(P < 0.05).
Figure 4.
Fasting (A) glucose and (B) insulin. (C) Glucose and (D) insulin responses to i.p. glucose tolerance test. AUC; area under the curve. All data are expressed as means ± SE. (*) denotes statistically different than all other groups(P < 0.05).
Microvascular reactivity to insulin
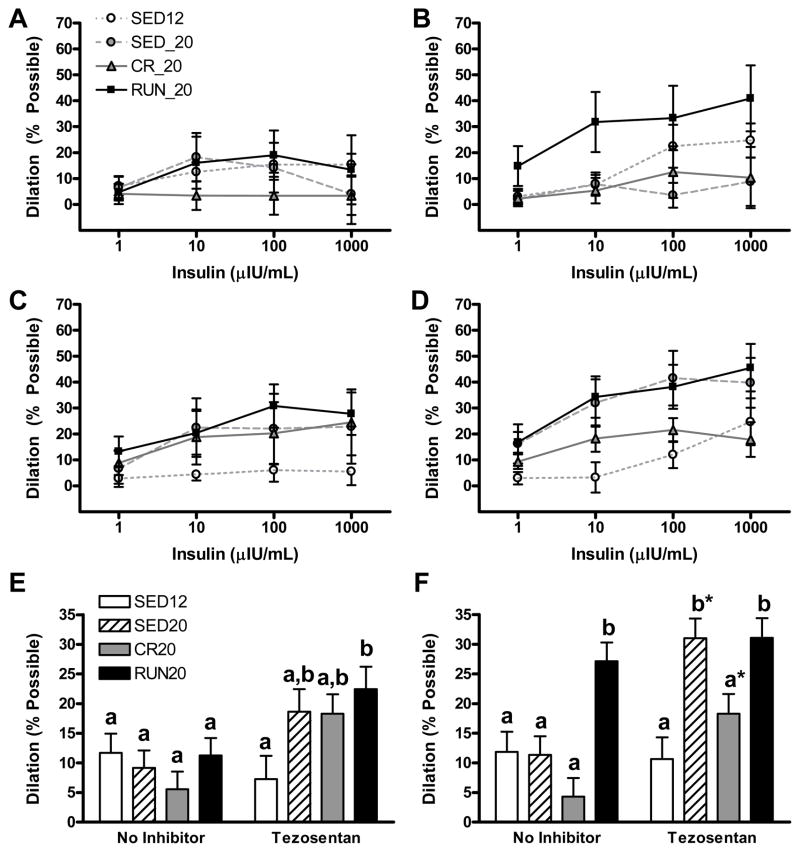
As shown in Figure 5A, B, C, and D, insulin produced a dose related dilation in arterioles of red and white gastrocnemius muscle. Our initial analysis of vasoreactivity to insulin evaluated the interaction between group assignment and insulin dose. However, none of the group by dose interactions were statistically significant (P > 0.50 in all cases). Thus, responses to insulin across all doses were pooled, and simple main effects models were used to examine group effects (Figure 5E and F).
Figure 5.
Vascular reactivity, to insulin alone or insulin in the presence of the non-selective ET-1 receptor tezosentan. Dose-response curves to graded doses of insulin alone (A, B) or insulin in the presence of tezosentan (C, D) in red portion of gastrocnemius (A, C) and white (B, D, F) portion of the gastrocnemius muscle are presented.. Statistical analysis revealed both a dose effect and a group effect on insulin responses but there was no group by dose interaction. Therefore we examined the group effect by pooling so that reactivity to insulin is expressed as mean percent possible dilation across all doses of insulin in second order arterioles of (E) red and (F) white portions of the gastrocnemius. All data are expressed as means ± SE. Different letters denote statistical significance, such that groups not sharing the same letter are significantly different from one another (P < 0.01). *Significantly different from no inhibitor, within group (P < 0.05).
Daily voluntary wheel running improved the vasodilatory response to insulin alone in 2As from white (Figure 5F; P < 0.01, RUN20 vs. SED12, SED20, CR20), but not red (Figure 5E), gastrocnemius muscles. Contrary to our hypothesis, vasoreactivity to insulin did not decline in 2As from red or white muscle in sedentary animals between 12 to 20 weeks of age as the rats became more insulin resistant. ET-1 receptor blockade with tezosentan produced sizable increases in the vasodilatory response to insulin in 2As of Gr that failed to reach statistical significance. Insulin-stimulated vasodilation in the presence of the ET-1 receptor blocker tezosentan was greater in Gr 2As of RUN20 than SED12 (Figure 5E; P < 0.05) and was greater in Gw 2As of RUN20 than SED12 or CR20 (Figure 5F; P<0.05). In 2As from Gw, tezosentan significantly augmented vasoreactivity to insulin in SED20 and CR20 groups (Figure 5F; P < 0.05). Similar results were obtained with the ET-1B receptor blocker, BQ-788 (data not shown).
Assessment of vasoreactivity to insulin in the presence of L-NAME was problematic in 12 and 20 wk OLETFs. In all groups, as expected, the addition of L-NAME initially caused vasoconstriction (0–20 min)by eliminating the contribution of NO to basal vascular tone. Typically, vessels will maintain this level of tone, or vessel diameter, until other treatments are applied or until the L-NAME is removed. However, 20 min after administering L-NAME, we observed gradual dilation until ~40 min later when the vessel returned to its initial diameter. This occurred both in the presence and absence of insulin administration. Similar results were obtained when L-NNA (1 mM) was used in place of L-NAME, when the dose of L-NAME was increased from 0.1 mM to 1 mM, and when L-NAME (0.1 mM) was co-administered with indomethacin (5 μM), catalase (100 U/mL), or sulfaphenazole (10 μM). We did not detect significant group or regional differences in these responses.
Insulin Signaling
As shown in Figure 6, insulin stimulated an increase in phospho-Akt in arterioles of red and white gastrocnemius muscle and increased phospho-MAPK in white muscle. However, there were no group differences in basal levels of phosphorylated or unphosphorylatede NOS, Akt or MAPK. Furthermore, the activation of eNOS, AKT, or MAPK in response to insulin was similar among the groups (Figures 12A–12F). Similarly, there were no differences in protein content or phosphorylation between arterioles from Gr or Gw, suggesting insulin signaling to NO and ET-1 was similar among the groups and vessels sampled.
Figure 6.
Protein expression of phospho-specific (A, B) eNOS, (C, D) Akt, and (E, F) MAPK in second and third order arterioles from (A, C, E) red and (B, D, F) white portions of the gastrocnemius muscles of OLETF rats under basal (−Insulin) or insulin-stimulated conditions (+Insulin). Total and phospho-specific densities were quantified, and the ratios of phosphospecific to total density were calculated. Delta – difference between – Insulin and +Insulin; eNOS - endothelial nitric oxide synthase; MAPK – mitogen activated protein kinase. Data are expressed as means ± SE.
Mitochondrial content
Cytochrome c was significantly lower in Gw in all groups relative to SED12 (Figure 7; P < 0.05). Although cytochrome c was 75% higher in Gw of RUN20 relative to SED20, the difference was not statistically significant. Likewise, cytochrome c content was roughly 22%, 15% and 115% higher in the heart, Gr, and Gm of RUN20 relative to SED20, respectively. These differences also failed to reach statistical significance.
Figure 7.
Protein expression of cytochrome c, a marker of mitochondrial content, in the heart, red, mixed, and white gastrocnemius (Gr, Gm, and Gw, respectively), and soleus. Data are expressed as means ± SE. (*) denotes significantly higher in the SED12 group compared to other groups(P < 0.05).
Discussion
The primary finding from this study is that daily voluntary wheel running selectively improved insulin-mediated vasodilation in arterioles isolated from white, but not red, portions of the gastrocnemius muscle of insulin resistant OLETF rats. Similarly, ET-1 receptor inhibition improved insulin-stimulated vasodilation in arterioles from white muscle of sedentary and calorie restricted OLETFs at 20 weeks of age, but produced only modest, statistically insignificant increases in insulin-stimulated vasodilation of arterioles from red muscle of the same animals. Sedentary behavior worsened insulin resistance in hyperphagic OLETF rats between 12 and 20 weeks of age, whereas calorie restriction mitigated and chronic daily exercise reversed the progression of insulin resistance. Although calorie restriction attenuated the progression of insulin resistance and gains in adiposity, it did not improve microvascular reactivity to insulin, suggesting the effects of wheel running on insulin-mediated vasodilation are exercise specific and independent of changes in adiposity. Collectively, these data suggest that voluntary wheel running preferentially improves insulin-mediated vasodilation in the white portion of the gastrocnemius muscle, an effect which may be mediated by attenuated sensitivity to ET-1 and may contribute to more favorable glucose responses to the IPGTT.
Of interest is the finding that daily wheel running improved insulin-mediated vasodilation in arterioles from Gw even though markers of mitochondrial content and oxidative capacity (cytochrome c) did not increase significantly in the same muscle. Although classic adaptations to exercise in skeletal muscle vasculature (increased capillary density or increased vasodilatory responses) are often associated with evidence of a training effect in the muscle (e.g., increases in mitochondrial content)(20), this is not always the case (19). Furthermore, improvements in vascular function in response to exercise training have been reported in nonworking skeletal muscle, brain, viscera, and skin, suggesting that vascular adaptations to exercise training are not always specific to the muscles recruited during exercise and are not dependent upon changes in skeletal muscle mitochondrial content (28). In agreement with prior studies demonstrating that improvements in skeletal muscle insulin sensitivity may occur independent of changes in muscle oxidative capacity or body composition, as is the case with a single bout of exercise (34)or in response to short-term exercise training (seven days)(17), our data indicate that improvements in vascular insulin sensitivity may occur independent of significant mitochondrial adaptations within the skeletal muscle and independent of changes in body composition or adiposity.
ET-1 receptor inhibition enhanced vasodilator responses to insulin in arterioles from the Gw of SED20 and CR20 animals but not those from SED12, indicating that the contribution of ET-1-mediated vasoconstriction to the microvascular response to insulin increased in 2As from the Gw of sedentary animals between 12 and 20 weeks. The increase in ET-1-mediated vasoconstriction in response to insulin was ablated by voluntary wheel running but not by calorie restriction. Thus, the improvement in vascular reactivity to insulin in the Gw observed in RUN20 may be mediated, in part, through the attenuation of production of and/or sensitivity to ET-1. These data are in contrast to our findings in OLETFs at 40 weeks of age in which voluntary wheel running from 4 weeks of age through 40 weeks of age maintained or prevented impairments in insulin-stimulated vasodilation through increases in phospho-specific eNOS and NO-mediated dilation (25). Comparison of these previous findings 40 weeks of age OLETF rats to current results suggests the mechanisms by which daily voluntary wheel running prevents impairments in insulin-stimulated vasodilation may be different from those by which insulin-stimulated vasodilation is improved after insulin resistance has been established, as in the present study.
Although ET-1 receptor inhibition improved vasodilator responses to insulin in the Gw of some groups, it did not produce statistically significant changes in insulin-stimulated vasodilation in arterioles from the Gr of any group. We have previously reported greater sensitivity to ET-1 in feed arteries from the gastrocnemius than those from the soleus in OLETF rats at 20 weeks, indicating that responsiveness to ET-1 may be lower in vessels perfusing more oxidative tissues(5). Sensitivity to ET-1 was not associated with differences in ET-1A or ET-1B receptor density in the previous study (5), and we speculate that regional differences in vascular sensitivity to ET-1 may relate to skeletal muscle fiber type and/or skeletal muscle recruitment patterns during exercise.
In agreement with previous reports, NOS blockade appeared to remove inhibition of an alternate vasoactive substance, putatively an unidentified endothelium-derived hyperpolarizing factor (EDHF)(6, 27), revealing evidence of an NO-independent vasodilator in OLETFs at 12 and 20 weeks of age. Although we were not able to identify the substance, we were able to determine that it was not responsive to catalase, indomethacin, or sulphafenazole, indicating that the substance is not hydrogen peroxide, cyclooxygenase, or a product of the cytochrome P450 pathway blocked by sulfaphenazole. While the presence of the hyperpolarizing factor obscured attempts to assess NO-independent responses to insulin, the majority of the available evidence suggests that insulin-mediated vasodilation is NO-dependent(7, 11), and we speculate that the vascular responses to insulin observed in the presence of tezosentan are mediated primarily by NO.
Current conjecture is that insulin-mediated blood flow is impaired in insulin resistance and type 2 diabetes as a result of aberrations in the PI3K/Akt/eNOS branch of the insulin signaling pathway concurrent with over stimulation of the MAPK/ET-1 branch, resulting in diminished production of NO and overproduction of ET-1 (12, 13, 15, 25, 29). However, not all data are in agreement(22, 23), and we did not see evidence of changes in insulin signaling to eNOS or of augmentation of insulin signaling to ET-1 in skeletal muscle 2As from Gr or Gw of sedentary OLETF rats as insulin resistance progressed between 12 and 20 weeks of age.
We did not observe differences in basal or insulin-stimulated concentrations of eNOS or phospho-eNOS in arterioles from the red or white gastrocnemius of OLETFs given access to a running wheel between 12 and 20 weeks of age, suggesting that the enhanced insulin-mediated vasodilation observed in the arterioles from the Gw of animals from RUN20 was not due to changes in eNOS content or activity. In contrast to our current findings, we have previously reported increases in phosphorylated eNOS in the white gastrocnemius of 40 week old OLETF rats given access to a running wheel at 4 weeks of age (25). It is not clear why eNOS expression was increased in arterioles of animals given access to a running between 4 and 40 weeks but not in those with access between 12 and 20 weeks, but may be related to the methodological differences (i.e., immunohistochemistry was used to measure eNOS and phospho-eNOS expression in muscle sections of 40 wk animals under basal conditions, whereas, protein expression was measured in isolated vessels (unpressurized and unmounted) under basal and insulin-stimulated conditions in the 12 and 20 wk animals). Furthermore, we have previously demonstrated that phospho-eNOS is higher in conduit arteries and arterioles of the Gw in OLETFs given access to a running wheel between 4 and 40 weeks of age(6, 25), but not those with access between 4 and 20 weeks of age or in sedentary animals at 20 or 40 weeks (6), suggesting the age of the animals, severity of insulin resistance, and/or duration of access to the running wheel may be important moderators of phospho-eNOS.
Insulin-mediated increases in blood flow appear to be a critical component of insulin-mediated glucose uptake(3, 4, 18). There is a strong correlation between leg blood flow and leg glucose uptake in healthy and insulin resistant individuals(18). Additionally, insulin-mediated blood flow has been shown to co-localize with insulin-stimulated glucose uptake in humans (35), and attenuating insulin-mediated blood flow via co-administration of L-NAME coordinately reduces insulin-mediated glucose uptake (3). We observed improvements in microvascular reactivity to insulin in 2As from the white portion of the gastrocnemius following eight weeks of daily voluntary wheel running. Furthermore, this coincided with improvements in whole-body glucose disposal, suggesting enhanced microvascular reactivity to insulin may have contributed to the reduced glucose response to the IPGTT observed in the OLETFs given access to a running wheel.
In summary, these data demonstrate that physical activity in the form of daily voluntary wheel running effectively reverses insulin resistance in OLETF rats and selectively improves insulin-mediated vasodilation in arterioles from the Gw. Furthermore, the efficacy of wheel running as a treatment for improving glucose tolerance and insulin-stimulated vasodilation appears to be largely independent of changes in adiposity (i.e., not observed in CR). These data also highlight the need for further investigation of regional differences in insulin-stimulated vasodilation and of the temporal effects of the development and progression of insulin resistance on insulin-mediated blood flow.
Perspectives
Insulin-mediated vasodilation typically accounts for up to 40% of insulin-stimulated glucose disposal but is reduced in insulin resistance and type 2 diabetes. The data presented here indicate that microvascular reactivity to insulin may be improved by daily physical activity after insulin resistance has been established. Furthermore, the effects of daily activity on microvascular responses to insulin differ among arterioles from distinct muscle beds, are independent of changes in body weight or composition, and may contribute to improvements in insulin-mediated glucose disposal.
Acknowledgments
Support
This work was supported by the College of Veterinary Medicine, Department of Internal Medicine, and Institute for Clinical and Translational Sciences at the University of Missouri, the VA (Career Development Award, JPT), the American Heart Association (CRM), and NIH grants T32 AR048523 (CRM), HL-36088 (MHL), and F32 DK-83182 (RSR).
The OLETF rats were a generous gift from the Tokushima Research Institute, Otsuka Pharmaceutical (Tokushima, Japan). The authors would like to thank Pam Thorne, Ann Melloh, and Leryn Boyle for their technical assistance. We also thank Dr. Richard Madsen for his skillful analysis of the data and the Diabetes Diagnostics Lab at the University of Missouri for performing hemoglobin A1c measurements. This work was supported with resources and the use of facilities at the Harry S Truman Memorial Veterans Hospital in Columbia, MO.
List of Abbreviations
- 2A
second order arteriole
- ACh
acetylcholine
- Akt
protein kinase B
- ANOVA
analysis of variance
- AUC
area under the curve
- BCA
bicinchoninic acid
- bpm
beats per minute
- C
Celcius
- CaCl2
calcium chloride
- CR
calorie restriction
- d
day
- DEXA
dual energy x-ray absorptiometry
- EDHF
endothelium derived hyperpolarizing factor
- EDTA
ethylenediaminetetraacetic acid
- eNOS
endothelial nitric oxide synthase
- ET-1
endothelin-1
- g
grams
- g
times gravity
- Gr
red gastrocnemius
- Gw
white gastrocnemius
- h
hour
- HbA1c
hemoglobin A1c
- HCl
hydrochloric acid
- HPLC
high pressure liquid chromatography
- i.p
intraperitoneal
- IPGTT
intraperitoneal glucose tolerance test
- KCl
potassium chloride
- kg
kilograms
- L-NAME
L-NG-Nitroarginine methyl ester
- L-NNA
NG-nitro-L-Arginine
- MAPK
mitogen activated protein kinase
- MgSO4
magnesium sulfate
- min
minute
- MOPS
3-(N-morpholino) propanesulfonic acid
- NaCl
sodium chloride
- NaH2PO4
sodium phosphate
- NO
nitric oxide
- OLETF
Otsuka Long Evans Tokushima Fatty rat
- PE
phenylephrine
- PI3K
phosphoinositide 3-kinase
- PSS
physiological saline solution
- PVDF
polyvinylidene fluoride
- RUN
voluntary wheel running
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SED
sedentary
- SE
standard error of the mean
- wk
week
References
- 1.Aaker A, Laughlin MH. Differential adenosine sensitivity of diaphragm and skeletal muscle arterioles. J Appl Physiol. 2002;93:848–856. doi: 10.1152/japplphysiol.00032.2002. [DOI] [PubMed] [Google Scholar]
- 2.Baron AD, Laakso M, Brechtel G, Hoit B, Watt C, Edelman SV. Reduced postprandial skeletal muscle blood flow contributes to glucose intolerance in human obesity. J Clin Endocrinol Metab. 1990;70:1525–1533. doi: 10.1210/jcem-70-6-1525. [DOI] [PubMed] [Google Scholar]
- 3.Baron AD, Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G. Insulin-mediated skeletal muscle vasodilation contributes to both insulin sensitivity and responsiveness in lean humans. The Journal of clinical investigation. 1995;96:786–792. doi: 10.1172/JCI118124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baron AD, Zhu JS, Marshall S, Irsula O, Brechtel G, Keech C. Insulin resistance after hypertension induced by the nitric oxide synthesis inhibitor L-NMMA in rats. Am J Physiol. 1995;269:E709–715. doi: 10.1152/ajpendo.1995.269.4.E709. [DOI] [PubMed] [Google Scholar]
- 5.Bender SB, Newcomer SC, Laughlin MH. Differential vulnerability of skeletal muscle feed arteries to dysfunction in insulin resistance: impact of fiber type and daily activity. Am J Physiol Heart Circ Physiol. 2011 doi: 10.1152/ajpheart.01093.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bunker AK, Arce-Esquivel AA, Rector RS, Booth FW, Ibdah JA, Laughlin MH. Physical activity maintains aortic endothelium-dependent relaxation in the obese type 2 diabetic OLETF rat. Am J Physiol Heart Circ Physiol. 2010;298:H1889–1901. doi: 10.1152/ajpheart.01252.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen YL, Messina EJ. Dilation of isolated skeletal muscle arterioles by insulin is endothelium dependent and nitric oxide mediated. Am J Physiol. 1996;270:H2120–2124. doi: 10.1152/ajpheart.1996.270.6.H2120. [DOI] [PubMed] [Google Scholar]
- 8.DeFronzo RA, Gunnarsson R, Bjorkman O, Olsson M, Wahren J. Effects of insulin on peripheral and splanchnic glucose metabolism in noninsulin- dependent (type II) diabetes mellitus. J Clin Invest. 1985;76:149–155. doi: 10.1172/JCI111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dela F, Larsen JJ, Mikines KJ, Ploug T, Petersen LN, Galbo H. Insulin-stimulated muscle glucose clearance in patients with NIDDM. Effects of one-legged physical training. Diabetes. 1995;44:1010–1020. doi: 10.2337/diab.44.9.1010. [DOI] [PubMed] [Google Scholar]
- 10.Dela F, Ploug T, Handberg A, Petersen LN, Larsen JJ, Mikines KJ, Galbo H. Physical training increases muscle GLUT4 protein and mRNA in patients with NIDDM. Diabetes. 1994;43:862–865. doi: 10.2337/diab.43.7.862. [DOI] [PubMed] [Google Scholar]
- 11.Eringa EC, Stehouwer CD, Merlijn T, Westerhof N, Sipkema P. Physiological concentrations of insulin induce endothelin-mediated vasoconstriction during inhibition of NOS or PI3-kinase in skeletal muscle arterioles. Cardiovasc Res. 2002;56:464–471. doi: 10.1016/s0008-6363(02)00593-x. [DOI] [PubMed] [Google Scholar]
- 12.Eringa EC, Stehouwer CD, Roos MH, Westerhof N, Sipkema P. Selective resistance to vasoactive effects of insulin in muscle resistance arteries of obese Zucker (fa/fa) rats. Am J Physiol Endocrinol Metab. 2007;293:E1134–1139. doi: 10.1152/ajpendo.00516.2006. [DOI] [PubMed] [Google Scholar]
- 13.Gogg S, Smith U, Jansson PA. Increased MAPK activation and impaired insulin signaling in subcutaneous microvascular endothelial cells in type 2 diabetes: the role of endothelin-1. Diabetes. 2009;58:2238–2245. doi: 10.2337/db08-0961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jasperse JL, Woodman CR, Price EM, Hasser EM, Laughlin MH. Hindlimb unweighting decreases ecNOS gene expression and endothelium-dependent dilation in rat soleus feed arteries. J Appl Physiol. 1999;87:1476–1482. doi: 10.1152/jappl.1999.87.4.1476. [DOI] [PubMed] [Google Scholar]
- 15.Jiang ZY, Lin YW, Clemont A, Feener EP, Hein KD, Igarashi M, Yamauchi T, White MF, King GL. Characterization of selective resistance to insulin signaling in the vasculature of obese Zucker (fa/fa) rats. The Journal of clinical investigation. 1999;104:447–457. doi: 10.1172/JCI5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Juel C, Holten MK, Dela F. Effects of strength training on muscle lactate release and MCT1 and MCT4 content in healthy and type 2 diabetic humans. J Physiol. 2004;556:297–304. doi: 10.1113/jphysiol.2003.058222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirwan JP, Solomon TP, Wojta DM, Staten MA, Holloszy JO. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2009;297:E151–156. doi: 10.1152/ajpendo.00210.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laakso M, Edelman SV, Brechtel G, Baron AD. Impaired insulin-mediated skeletal muscle blood flow in patients with NIDDM. Diabetes. 1992;41:1076–1083. doi: 10.2337/diab.41.9.1076. [DOI] [PubMed] [Google Scholar]
- 19.Laughlin MH, Roseguini B. Mechanisms for exercise training-induced increases in skeletal muscle blood flow capacity: differences with interval sprint training versus aerobic endurance training. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society. 2008;59 (Suppl 7):71–88. [PMC free article] [PubMed] [Google Scholar]
- 20.Laughlin MH, Woodman CR, Schrage WG, Gute D, Price EM. Interval sprint training enhances endothelial function and eNOS content in some arteries that perfuse white gastrocnemius muscle. J Appl Physiol. 2004;96:233–244. doi: 10.1152/japplphysiol.00105.2003. [DOI] [PubMed] [Google Scholar]
- 21.Laye MJ, Rector RS, Borengasser SJ, Naples SP, Uptergrove GM, Ibdah JA, Booth FW, Thyfault JP. Cessation of daily wheel running differentially alters fat oxidation capacity in liver, muscle, and adipose tissue. J Appl Physiol. 2009;106:161–168. doi: 10.1152/japplphysiol.91186.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lteif AA, Fulford AD, Considine RV, Gelfand I, Baron AD, Mather KJ. Hyperinsulinemia fails to augment ET-1 action in the skeletal muscle vascular bed in vivo in humans. Am J Physiol Endocrinol Metab. 2008;295:E1510–1517. doi: 10.1152/ajpendo.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mather KJ, Lteif A, Steinberg HO, Baron AD. Interactions between endothelin and nitric oxide in the regulation of vascular tone in obesity and diabetes. Diabetes. 2004;53:2060–2066. doi: 10.2337/diabetes.53.8.2060. [DOI] [PubMed] [Google Scholar]
- 24.McAllister RM, Newcomer SC, Laughlin MH. Vascular nitric oxide: effects of exercise training in animals. Appl Physiol Nutr Metab. 2008;33:173–178. doi: 10.1139/H07-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mikus CR, Rector RS, Arce-Esquivel AA, Libla JL, Booth FW, Ibdah JA, Laughlin MH, Thyfault JP. Daily physical activity enhances reactivity to insulin in skeletal muscle arterioles of hyperphagic Otsuka Long-Evans Tokushima Fatty rats. J Appl Physiol. 2010;109:1203–1210. doi: 10.1152/japplphysiol.00064.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris RT, Laye MJ, Lees SJ, Rector RS, Thyfault JP, Booth FW. Exercise-induced attenuation of obesity, hyperinsulinemia, and skeletal muscle lipid peroxidation in the OLETF rat. J Appl Physiol. 2008;104:708–715. doi: 10.1152/japplphysiol.01034.2007. [DOI] [PubMed] [Google Scholar]
- 27.Nishikawa Y, Stepp DW, Chilian WM. Nitric oxide exerts feedback inhibition on EDHF-induced coronary arteriolar dilation in vivo. American journal of physiology Heart and circulatory physiology. 2000;279:H459–465. doi: 10.1152/ajpheart.2000.279.2.H459. [DOI] [PubMed] [Google Scholar]
- 28.Padilla J, Simmons GH, Bender SB, Arce-Esquivel AA, Whyte JJ, Laughlin MH. Vascular effects of exercise: endothelial adaptations beyond active muscle beds. Physiology (Bethesda) 2011;26:132–145. doi: 10.1152/physiol.00052.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Potenza MA, Marasciulo FL, Chieppa DM, Brigiani GS, Formoso G, Quon MJ, Montagnani M. Insulin resistance in spontaneously hypertensive rats is associated with endothelial dysfunction characterized by imbalance between NO and ET-1 production. American journal of physiology Heart and circulatory physiology. 2005;289:H813–822. doi: 10.1152/ajpheart.00092.2005. [DOI] [PubMed] [Google Scholar]
- 30.Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, Mikus CR, Laye MJ, Laughlin MH, Booth FW, Ibdah JA. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. J Hepatol. 2010;52:727–736. doi: 10.1016/j.jhep.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rector RS, Thyfault JP, Uptergrove GM, Morris EM, Naples SP, Borengasser SJ, Mikus CR, Laye MJ, Laughlin MH, Booth FW, Ibdah JA. Mitochondrial dysfunction precedes insulin resistance and hepatic steatosis and contributes to the natural history of non-alcoholic fatty liver disease in an obese rodent model. Journal of hepatology. 2010;52:727–736. doi: 10.1016/j.jhep.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rector RS, Uptergrove GM, Morris EM, Borengasser SJ, Laughlin MH, Booth FW, Thyfault JP, Ibdah JA. Daily exercise vs. caloric restriction for prevention of nonalcoholic fatty liver disease in the OLETF rat model. Am J Physiol Gastrointest Liver Physiol. 2011;300:G874–883. doi: 10.1152/ajpgi.00510.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato T, Asahi Y, Toide K, Nakayama N. Insulin resistance in skeletal muscle of the male Otsuka Long-Evans Tokushima Fatty rat, a new model of NIDDM. Diab tologia. 1995;38:1033–1041. doi: 10.1007/BF00402172. [DOI] [PubMed] [Google Scholar]
- 34.Thyfault JP. Setting the stage: possible mechanisms by which acute contraction restores insulin sensitivity in muscle. American journal of physiology Regulatory, integrative and comparative physiology. 2008;294:R1103–1110. doi: 10.1152/ajpregu.00924.2007. [DOI] [PubMed] [Google Scholar]
- 35.Utriainen T, Nuutila P, Takala T, Vicini P, Ruotsalainen U, Ronnemaa T, Tolvanen T, Raitakari M, Haaparanta M, Kirvela O, Cobelli C, Yki-Jarvinen H. Intact insulin stimulation of skeletal muscle blood flow, its heterogeneity and redistribution, but not of glucose uptake in non-insulin-dependent diabetes mellitus. The Journal of clinical investigation. 1997;100:777–785. doi: 10.1172/JCI119591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vincent MA, Barrett EJ, Lindner JR, Clark MG, Rattigan S. Inhibiting NOS blocks microvascular recruitment and blunts muscle glucose uptake in response to insulin. Am J Physiol Endocrinol Metab. 2003;285:E123–129. doi: 10.1152/ajpendo.00021.2003. [DOI] [PubMed] [Google Scholar]
- 37.Wallis MG, Wheatley CM, Rattigan S, Barrett EJ, Clark AD, Clark MG. Insulin-mediated hemodynamic changes are impaired in muscle of Zucker obese rats. Diabetes. 2002;51:3492–3498. doi: 10.2337/diabetes.51.12.3492. [DOI] [PubMed] [Google Scholar]
- 38.Zeng G, Quon MJ. Insulin-stimulated production of nitric oxide is inhibited by wortmannin. Direct measurement in vascular endothelial cells. J Clin Invest. 1996;98:894–898. doi: 10.1172/JCI118871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zierath JR, He L, Guma A, Odegoard Wahlstrom E, Klip A, Wallberg-Henriksson H. Insulin action on glucose transport and plasma membrane GLUT4 content in skeletal muscle from patients with NIDDM. Diabetologia. 1996;39:1180–1189. doi: 10.1007/BF02658504. [DOI] [PubMed] [Google Scholar]