Background: The mammalian antiviral response is regulated by virus-induced changes in cellular gene expression.
Results: Cellular miRNAs are induced by influenza virus to regulate antiviral response genes including IRAK1 and MAPK3.
Conclusion: Influenza virus infection alters the miRNA profile of human cells to regulate innate immune signaling.
Significance: The miRNA response to influenza virus identifies new pathways of cell regulation that modulate the mammalian innate antiviral response.
Keywords: Gene Regulation, Host-Pathogen Interactions, Influenza Virus, Innate Immunity, MicroRNA
Abstract
The cellular response to virus infection is initiated by recognition of the invading pathogen and subsequent changes in gene expression mediated by both transcriptional and translational mechanisms. In addition to well established means of regulating antiviral gene expression, it has been demonstrated that RNA interference (RNAi) can play an important role in antiviral responses. Virus-derived small interfering RNA (siRNA) is a primary antiviral response exploited by plants and invertebrate animals, and host-encoded microRNA (miRNA) species have been clearly implicated in the regulation of innate and adaptive immune responses in mammals and other vertebrates. Examination of miRNA abundance in human lung cell lines revealed endogenous miRNAs, including miR-7, miR-132, miR-146a, miR-187, miR-200c, and miR-1275, to specifically accumulate in response to infection with two influenza A virus strains, A/Udorn/72 and A/WSN/33. Known antiviral response pathways, including Toll-like receptor, RIG-I-like receptor, and direct interferon or cytokine stimulation did not alter the abundance of the tested miRNAs to the extent of influenza A virus infection, which initiates primary miRNA transcription via a secondary response pathway. Gene expression profiling identified 26 cellular mRNAs targeted by these miRNAs, including IRAK1, MAPK3, and other components of innate immune signaling systems.
Introduction
The cellular innate immune response is an important early line of defense against virus infection. Components of virions and virus replication intermediates are detected by several receptors, including Toll-like receptors (TLRs)3 and RIG-I-like receptors (RLRs), which signal through several intermediates to stimulate transcription factor activation and expression of an antiviral gene program (1). The protein products of these genes produce a broadly effective cellular resistance to virus infection referred to as the antiviral state. In addition to direct antiviral mediators, such as type I interferon (IFN), many virus infections also activate the secretion of proinflammatory cytokines, chemokines, and other immune stimulators that contribute to innate and adaptive defenses and comprise well established paradigms that control gene expression in response to virus infections.
It has become clearly established that small RNAs contribute to antiviral responses in plants and invertebrate animals via RNA interference involving virus-derived small interfering RNAs (siRNAs) (2). However, there is little evidence to support a similar role for siRNAs in mediating general or specific antiviral responses in mammalian hosts. Mounting evidence does suggest a role for miRNA modulated responses during infection (3).
Mature miRNAs are small, endogenously produced 17–24-nucleotide RNAs that regulate gene expression either by inhibiting mRNA translation through seed-region annealing to the 3′ untranslated regions (UTRs) of mRNAs or by inducing mRNA degradation. MicroRNAs are generated from primary miRNA transcripts (pri-miRNAs) that are produced in the nucleus by RNA polymerase II as independent transcriptional units or within intronic regions of mRNAs. Short hairpin RNAs, known as precursor miRNAs (pre-miRNAs), are excised from the pri-miRNA by the ribonuclease, DROSHA. The pre-miRNAs are exported to the cytoplasm and further processed by cleavage with DICER, resulting in a mature miRNA duplex that is incorporated into the RNA-induced silencing complex primed for translational inhibition (4–7). Translational inhibition can occur either by miRNA-induced inhibition of translation initiation, or by miRNA-induced mRNA degradation, or a combination of the two mechanisms. MicroRNAs, once associated with RNA-induced silencing complex, are able to bind their target and recruit deadenylation factors to remove the poly(A) tail of mRNA transcripts (8, 9). Once deadenylation occurs, decapping enzymes remove the m7G cap on the 5′ end of mRNA transcripts and promotes 5′ to 3′ mRNA decay (10). Mounting evidence indicates that target degradation is the predominant form of miRNA-mediated translational repression (11, 12).
Although miRNAs have been implicated as potentially important regulators of mammalian antiviral responses, their precise roles in general or specific antiviral systems have not yet been extensively reported (13–16). Recent studies have shown that miRNAs play subtle roles in the antiviral response of mammals. The abundance of several miRNAs is modulated in response to virus infections, and in some cases the regulated miRNAs have been linked to important innate immune signaling factors. For example, miR-146a has been described as a negative regulator of signaling from TLR and RLR pathways (14, 15). Viruses such as vesicular stomatitis virus (VSV) and Epstein-Barr virus stimulate expression of miR-146a and down-regulate signaling from these innate immune signaling pathways (14, 17, 18). Other cellular miRNAs have been reported to interact with viral genomes or mRNAs, resulting in both positive and negative effects on virus fitness (19–21).
Recent studies have suggested miRNA involvement in influenza A virus infection and replication (16, 22, 23). Influenza A virus is the causative agent of a contagious respiratory infection, which is well known to modulate the host antiviral defense systems (24, 25). In the present study, we identify cellular miRNAs that are induced in human lung cells after influenza A virus infection and demonstrate that miRNA accumulation is due to an increase in primary miRNA transcription in the infected cell. Analysis of mRNA and protein targets indicates a role for the induced miRNAs in the regulation of innate immune signaling systems.
EXPERIMENTAL PROCEDURES
Cell Culture, Viruses, Treatments, and Transfection
A549 cells (ATCC) were maintained in Ham's F-12 media with Kaign's modification (F12K, Invitrogen) with 10% cosmic calf serum (Hyclone) and 500 units/ml of penicillin and 500 μg/ml of streptomycin. BEAS-2B cells (ATCC) were maintained in BEGM media (Lonza, Walkersville, MD). Primary differentiated human airway epithelial (HAE) cells were purchased from Mattek Corporation (Ashland, MA) and maintained according to the manufacturer's instructions.
The A/Udorn/72 and A/WSN/33 strains of influenza virus (gift of R. A. Lamb, Northwestern University) were propagated and titered on Madin-Darby canine kidney cells. Encephalomyocarditis virus (EMCV) and parainfluenza virus 5 (PIV5) were propagated and titered on Vero cells. VSV was propagated and titered on CV1 cells. Sendai virus (Cantell strain) was propagated in embryonated chicken eggs and titered on Vero cells. Virus infections were performed in serum-free media (SFM) supplemented with 1% BSA. At 2 h post-infection, the inoculation media was replaced with medium containing 2% cosmic calf serum and cells were washed with serum-free media prior to RNA purification.
Cytokine treatments of A549 cells were as follows: 1000 units/ml of IFNα (Hoffman LaRoche), 500 ng/ml of IFNγ (PBL Interferon Source), 400 ng/ml of IL-6, and 500 ng/ml of soluble IL-6 receptor (Peprotech), or 10 ng/ml of TNFα (R&D Systems). Cells were incubated with cytokines for 10 h before RNA purification. For poly(I:C) transfections, synthetic dsRNA was transfected into A549 cells (5 μg/ml media) poly(I:C) (Amersham Biosciences), using Lipofectamine 2000 (Invitrogen).
For the GAS luciferase reporter gene assay, A549 cells were transfected with 800 ng of 4xM67 pTATA TK LUC plasmid and 20 ng of constitutively active Renilla luciferase (pRenilla null) plasmid. Twenty-four hours post-transfection, cells were left untreated or treated with IL-6 and soluble IL-6 receptor for 10 h, harvested, and assayed for luciferase activity with the dual luciferase assay system (Promega, Madison, WI). For miRNA mimic transfections, A549 cells were transfected with miR-7, miR-132, miR-146a, miR-187, miR-200c, miR-1275, and negative control miRNA mimics (Ambion pre-miR®) at 50 nm total concentration using Lipofectamine 2000 (Invitrogen) in F12K media lacking penicillin and streptomycin.
MicroRNA Microarray Analysis
Total RNA was isolated using the miRNeasy kit (Qiagen) and fractioned using flashPAGE (Ambion) to isolate RNAs smaller than 40 nucleotides in length. MicroRNA microarrays were performed at LC Sciences (Houston, TX) using the complete miRbase 11.0. MicroRNA microarrays were performed as a single biological replicate with at least 3 independent verifications by qRT-PCR using TaqMan microRNA assays.
Gene Expression Profiling and Pathway Analysis
Total RNA was purified from A549 cells that were mock infected, infected with A/WSN/33 at multiplicity of infection of 5 pfu/cell or A549 cells expressing miRNA mimics and infected with A/WSN/33 at a multiplicity of infection of 5 pfu/cell. Total RNA from three biological replicates was purified 10 h postinfection and hybridized to Illumina Bead Array whole genome expression microarrays (26, 27). Determination of potential miRNA targets was performed with TargetScan and MiR-Walk algorithms (28, 29). Pathway analysis to identify functions of miRNA target genes was performed using the Innate DB database (30). Interactome analysis was generated by literature search for interacting partners and visualized within Cytoscape with the Agilent Literature Search Software (Agilent Technologies, Santa Clara, CA) (31).
RNA Purification, Reverse Transcription, Real-time PCR, and miRNA Analysis
For mRNA analysis, total RNA was purified from 1 × 106 A549 or BEAS-2B cells using the miRNeasy RNA isolation kit (Qiagen), reverse transcribed using oligo(dT) primers and Superscript III reverse transcriptase (Invitrogen). Real-time PCR was performed using SYBR Green detection and primers specific for: IFNβ, forward, 5′-CATTACCTGAAGGCCAAGGA-3′; reverse, 5′-CAATTGTCCAGTCCCAGAGG-3′; IL-28a, forward, 5′-GGAGGGTCAGACACACAGGT-3′; reverse, 5′-GAGGCCTCTGTCACCTTCAA-3′; ISG15, forward, 5′-AATGCGACGAACCTCTGAAC-3′; reverse, 5-GAAGGTCAGCCAGAACAGGT-3′; CCL5, forward, 5′-CGCTGTCATCCTCATTGCTA-3′; reverse, 5′-GCACTTGCCACTGGTGTAGA-3′; PB1, forward, 5′-AATGTGCTAATTGGGCAAGG-3′; reverse, 5′-CGAATTCTTTTGGTCGCTGT-3′; GAPDH, forward, 5′-ACAGTCAGCCGCATCTTCTT-3′; reverse, 5′-ACGACCAAATCCGTTGACTC-3′.
Relative mRNA abundance was determined by normalizing the mRNA of interest to GAPDH. All real-time PCR data are presented as 2−ΔΔCt (32). For analysis of mature miRNAs, TaqMan miRNA assays (Applied Biosystems) were used. Total RNA was reverse transcribed using a miRNA-specific primer and Multiscribe reverse transcriptase (Applied Biosystems.) Real-time PCR was performed with a miRNA-specific probe according to the manufacturer's instructions (Applied Biosystems). Relative miRNA abundance was determined by normalizing the miRNA of interest to U6 small nuclear RNA. The following TaqMan miRNA assays were used: dme-miR-7, hsa-miR-16, hsa-miR-132, hsa-miR-132, mmu-mir-187, hsa-mir-200c, and hsa-miR-1275.
For detection of the primary miRNA transcripts, total RNA was reverse transcribed using random primers (Invitrogen) and the Superscript III first strand kit (Invitrogen). Real-time PCR was performed as above with TaqMan probes specific for hsa-primiR-132, hsa-primiR-146a, hsa-primiR-187, and hsa-primiR-200c (Applied Biosystems). Relative pri-miRNA abundance was determined by normalizing the pri-miRNA of interest to U6 small nuclear RNA. All real-time PCR was performed using MX3005p real-time PCR machines (Agilent Technologies, Santa Clara, CA).
Indirect Immunofluorescence
Cells were fixed in 3.7% formaldehyde, permeablized in ice-cold methanol, and stained with antibodies specific for the influenza A virus nucleoprotein, NP (Abcam), and a fluorescein-conjugated secondary antibody. Stained cells were visualized using a Nikon Eclipse Ti-U inverted fluorescence microscope.
Immunoblotting
A549 cells were washed in ice-cold phosphate-buffered saline and then lysed in whole cell extract buffer containing 50 mm Tris, pH 8.0, 280 mm NaCl, 0.5% IGEPAL, 0.2 mm EDTA, 2 mm EGTA, 10% glycerol, 1 mm DTT, 0.1 mm sodium vanadate, and protease inhibitor mixture. Forty μg of total protein was separated by SDS-PAGE. Protein was transferred to a nitrocellulose membrane, blocked in 5% nonfat milk in TBST, and detected by specific antibodies to influenza NP (Abcam), MAPK1 + MAPK3 (Abcam), IRAK1 (Santa Cruz Biotechnologies), and GAPDH (Santa Cruz Biotechnologies). Antibody detection was visualized by chemiluminescence (PerkinElmer Life Sciences).
RESULTS
MicroRNA Abundance Changes during Influenza A Virus Infection of Human Cells
To determine whether the abundance of cellular miRNAs was altered by infection, two common laboratory strains of influenza A virus, A/Udorn/72 and A/WSN/33, were used to infect two different respiratory-derived cell lines, the human alveolar epithelial cell line, A549, or the human bronchial epithelial cell line, BEAS-2B. Following infection with influenza virus, total RNA was prepared at 8, 12, or 24 h postinfection (hpi). In parallel, RNA samples from mock-infected cells were prepared for comparison. To verify the host cell response to infection, the level of antiviral mRNAs was examined. Total RNA was isolated, reverse transcribed with oligo(dT) primers, and subjected to real-time PCR with primers specific for the cellular mRNAs IFNβ, ISG15, IL28a, CCL5, IP10, and the virus encoded PB1 to verify infection (Fig. 1). All genes tested were significantly induced by influenza virus infection.
FIGURE 1.
Cellular response to influenza A virus infection. A549 and BEAS-2B cells were mock infected or infected with 1 pfu/cell A/Udorn/72 or A/WSN/33 for 8, 12, or 24 h. The relative abundance of antiviral mRNAs IFNβ, ISG15, CCL5, and the influenza virus mRNA PB1 was measured. p.i., postinfection.
To compare the abundance of cellular miRNAs in mock-infected samples with influenza virus-infected samples, a microarray representing 678 miRNAs was probed with cDNA derived from size-fractionated total RNA. MicroRNAs that exhibited persistent differences in abundance throughout the experimental time course with a comparative p value of <0.05 by Student's t test were chosen for further validation (Fig. 2). This focused set of miRNAs includes: miR-7, miR-132, miR-146a, miR-187, miR-200c, and miR-1275 (Table 1).
FIGURE 2.
The abundance of selected miRNAs increases in response to influenza virus infection. A, A549 and BEAS-2B cells (106 cells) were mock infected or infected with 1 pfu/cell A/Udorn/72 or A/WSN/33 for 8, 12, or 24 h. Heat maps indicate significant (p < 0.05) changes in expression of cellular microRNAs at all time points during influenza A virus infection in microarray experiments. B, A549 or BEAS-2B cells were treated as in A. Relative abundance of miRNAs miR-7, miR-132, miR-146a, miR-187, miR-200c, miR-1275, and miR-16 was measured.
TABLE 1.
MicroRNA induction by influenza A virus infection
| MicroRNAa | A549b |
BEAS-2B |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A/Udorn/72 | A/WSN/33 | A/Udorn/72 | A/WSN/33 | |||||||||
| 8c | 12c | 24c | 8 | 12 | 24 | 8 | 12 | 24 | 8 | 12 | 24 | |
| miR-7 | 1.37 | 1.66 | 2.58 | 1.85 | 2.05 | 1.90 | 1.87 | 2.02 | 2.02 | 1.51 | 1.96 | 2.41 |
| miR-132 | 1.75 | 1.45 | 1.79 | 1.25 | 1.28 | 1.33 | 1.33 | 1.41 | 1.41 | 1.76 | 2.47 | 3.78 |
| miR-146a | 2.21 | 3.25 | 3.00 | 4.46 | 6.62 | 11.23 | 3.34 | 4.77 | 4.77 | 3.82 | 6.03 | 6.52 |
| miR-187 | 2.13 | 3.20 | 1.86 | 2.41 | 3.39 | 2.62 | 1.65 | 1.98 | 1.98 | 1.76 | 2.59 | 2.37 |
| miR-200c | 4.10 | 4.91 | 6.62 | 3.87 | 4.60 | 4.70 | 0.80 | 0.61 | 0.61 | 0.74 | 1.33 | 0.05 |
| miR-1275 | 6.42 | 7.35 | 7.04 | 4.02 | 5.06 | 3.85 | 1.60 | 2.10 | 2.10 | 1.92 | 2.77 | 2.86 |
| miR-16 | 1.00 | 0.92 | 0.61 | 0.94 | 1.05 | 0.83 | 0.85 | 0.91 | 0.91 | 0.98 | 0.98 | 0.73 |
a MicroRNAs displaying significant increases (p value < 0.05) in abundance throughout the time course of infection. Numbers indicate fold-change of miRNA abundance of infected sample compared to mock infected sample.
b A549 or BEAS-2B cells were infected with A/Udorn/72 or A/WSN/33 strains of influenza virus (1 pfu/cell).
c Hours postinfection.
To verify the changes in miRNA abundance identified by microarray profiling in influenza virus-infected cells, mature miRNAs were measured by TaqMan-based semi-quantitative reverse transcription real-time PCR (Fig. 2B). MicroRNAs miR-7, miR-132, and miR-146a were found to increase continuously over the course of infection in both cell types after infection with either A/Udorn/72 or A/WSN/33. MicroRNAs miR-187 and miR-1275 increased transiently during the course of infection, whereas miR-200c was observed to increase after infection of A549 cells but not BEAS-2B cells. Increased multiplicity of infection accentuated miRNA levels modestly (not shown). One tested miRNA, miR-16, displayed constant expression levels throughout the infection in both cell lines (Fig. 2B). These miRNA abundance changes illustrate both general and cell-specific virus-induced expression patterns and indicate that influenza virus infection has the capacity to induce selective increases in miRNA accumulation in human respiratory cell lines.
To determine whether induction of miRNAs by influenza A virus in these cell lines is also observed under more physiological conditions, similar miRNA analysis was conducted in differentiated primary HAE tissue cultures. These three-dimensional ciliated airway tissues are readily infected with influenza A/Udorn/72, although not as uniformly as homogenous monolayer cells such as A549 (Fig. 3C). The level of antiviral mRNAs for IFNβ, ISG15, IL-28a, and CCL5 and the influenza virus PB1 mRNA were examined at 8 and 24 hpi, and all genes were significantly induced (Fig. 3A). In parallel, mature miRNA levels were examined and were found to increase in abundance in the HAE cells at 8 hpi, and most of the miRNA increase was resolved to near baseline levels by 24 hpi (Fig. 3B). The magnitude of miRNA induction in HAE cells ranged from 3- to 6-fold and miRNA accumulation preceded mRNA accumulation (Fig. 3, A and B). Importantly, the level of a control miRNA, miR-21, was unchanged in HAE cells after infection with A/Udorn/72 (Fig. 3B).
FIGURE 3.
mRNA and miRNA abundance changes during influenza A virus infection in primary airway epithelial cells. HAE cells were mock infected or infected with 106 pfu/cell culture chamber of A/Udorn/72 for 8 or 24 h. A, relative abundance of antiviral mRNAs IFNβ, ISG15, IL-28a, and the influenza virus PB1 mRNA were measured. B, relative abundance of miR-16, miR-7, miR-132, miR-146a, miR-200c, miR-1275, and miR-21 was measured. C, immunofluorescence of HAE cells that were mock infected or infected as above for 24 h. Cells were immunostained for the influenza nucleoprotein (NP). p.i., postinfection.
Several of these miRNA changes were consistent across the three cell systems tested (miR-7, miR-132, miR-146a, miR-187, miR-1275) and others exhibited cell type differences (miR-16 and miR-200c). Together, these data support the conclusion that selected miRNA levels change as a result of influenza A virus infection, and that this is a property of both primary human airway cells and human respiratory-derived cell lines.
Influenza Virus Activates miRNA Induction
To determine whether the effect of influenza A virus infection on miRNA abundance is a general feature of RNA virus infection or is specific to influenza A virus, A549 cells were mock infected or infected with EMCV, Sendai virus (SeV), PIV5, or VSV in parallel with A/Udorn/72 and A/WSN/33. After 10 h, total RNA was prepared and the relative abundance of the miRNAs was evaluated by real-time PCR. As a control to ensure that all virus infections were generating an antiviral response, the transcriptional induction of CCL5 mRNA was measured by RT-PCR (Fig. 4). Although several of the miRNAs were found to accumulate in response to infection with other viruses (Fig. 4), the magnitude of the increase was greatest in response to influenza A virus infection, with the notable exception of PIV5, which also caused increased expression of miR-132. The abundance of miR-146a, miR-187, miR-200c, and miR-1275 was dramatically increased in the influenza A virus-infected cells when compared with cells infected with the other viruses tested. All viruses tested increased the abundance of miR-146a between 3- and 7-fold (Fig. 4, inset), and other minor fluctuations in miRNA abundance were observed, but influenza virus infection was consistently the most robust miRNA activator. These results suggest that miRNA induction is caused by a signal that is more strongly induced during influenza virus infection than during infection with the other viruses tested.
FIGURE 4.
Influenza virus is a more potent inducer of miRNAs. A549 cells were infected with 1 pfu/cell of the indicated virus. Total RNA was purified 10 h postinfection. Relative abundance of miR-146a, miR-187, miR-200c, miR-1275, miR-132, miR-7, miR-16, and CCL5 mRNA was measured. Inset graph shows smaller changes in miR-146a due to EMCV, SeV, PIV5, and VSV.
Influenza A Virus Infection Induces Primary miRNA Transcription
Alterations in miRNA abundance have been reported in many systems, and changes in mature miRNA levels may occur via enhancement of primary miRNA transcription, or through post-transcriptional mechanisms that regulate the processing of a pre-existing pool of pri-miRNAs or pre-miRNA (33–35). To determine whether the increase in miRNA abundance was due to increased pri-miRNA transcription or enhanced miRNA processing, miR-132, miR-146a, miR-187, and miR-200c were subjected to further analysis. In most cases, the pri-miRNA transcripts were found to increase detectably between 2 and 4 h after infection, and continued to increase throughout the time course. In all cases, the appearance of the pri-miRNA preceded the mature miRNA accumulation, suggesting that the observed induction of miRNAs is regulated at the level of pri-miRNA accumulation rather than enhanced processing of a pre-existing pool of pri-miRNAs or pre-miRNAs (Fig. 5A).
FIGURE 5.
Influenza virus induction of pri-miRNAs requires new protein synthesis. A–C, A549 cells were mock infected (M) or infected with 1 pfu/cell A/Udorn/72 for 2, 4, or 6 hpi in the absence or presence of cycloheximide (CHX, 100 mg/ml). A, relative abundance of primiR-146a, primiR-187, primiR-200c, and primiR-132 was determined. B, relative abundance of IL-28a, ISG15, CCL5, and PB1 mRNA was determined. C, relative abundance of mature miR-146a, miR-187, miR-200c, and miR-132 was determined. D, same as A–C, but infection was for 8 or 24 h, as indicated. Relative abundance of primiR-146a, primiR-187, primiR-200c, and primiR-132 and mature miR-146a, miR-187, miR-200c, and miR-132 was determined.
New Protein Synthesis Is Required for Influenza Virus-induced Pri-miRNA Increase
To investigate whether primary response pathways or secondary mechanisms lead to pri-miRNA transcription, the role of newly synthesized protein in pri-miRNA levels was examined. A549 cells were infected with A/Udorn/72 or mock infected in the presence or absence of cycloheximide (CHX, 100 μg/ml), and pri-miRNA levels were measured. As a control, the mRNA levels of two known primary responders in the innate immune response, IL-28a and CCL5, were analyzed. The accumulation of these mRNAs was not altered by CHX treatment. However, CHX did inhibit the production of a secondary immune responder mRNA, ISG15, and the viral mRNA encoding the polymerase protein, PB1 (Fig. 5B). Similar to the pattern observed for the secondary response genes, the infection-induced increase of both pri-miRNAs and mature miRNAs was abrogated by treatment with CHX (Fig. 5, A and C). Primary miRNAs continued to accumulate until 8 h postinfection, then declined by 24 h, concomitant with increases in mature miRNA levels, consistent with a precursor-product relationship (Fig. 5D). These data suggest that the influenza virus induced an increase in pri-miRNA abundance, and thereby the accumulation of mature miRNAs, observed following infection with influenza virus, and involves newly synthesized proteins. Examination of miRNA induction by expression of influenza virus mRNAs, either individually or in combination, was insufficient to induce the expression of the miRNAs (not shown), implying that a secondary cellular response pathway is responsible.
Antiviral Mediators Are Insufficient for miRNA Induction
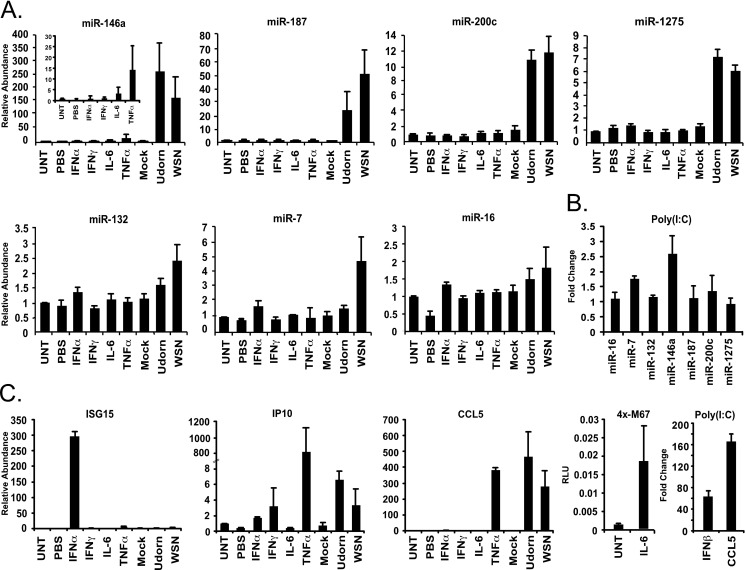
The production and secretion of cytokines, specifically interferons (IFN), interleukins (IL), and tumor necrosis factor α (TNFα) are important in antiviral responses, and have been implicated in miRNA regulation (15, 36). To investigate if signaling by these cytokines contributes to influenza virus induction of miRNAs, A549 cells were mock infected or infected with A/Udorn/72 or A/WSN/33 or treated with a panel of inducers: IFNα (1000 units/ml), IFNγ (500 ng/ml), IL-6 (400 plus 500 ng/ml of soluble IL-6 receptor), or TNFα (10 ng/ml) for 10 h. As a control, the abundance of ISG15 mRNA, IP10 mRNA, and CCL5 mRNA was measured to ensure cells were responding to IFNα, IFNγ, and TNFα, respectively. Additionally, a parallel sample was used to ensure IL-6 activation, using a STAT3-responsive m67-GAS luciferase reporter gene assay (Fig. 6C). The abundance of the influenza virus-induced miRNAs was determined by real-time PCR. Although most of the tested miRNAs remained unchanged by these cytokine treatments (Fig. 6A), the abundance of miR-146a was increased 14.3-fold after treatment with TNFα, in agreement with prior literature (15). However, this level of miR-146a induction was dramatically lower than that observed in influenza A virus-infected cells, which increased miR-146a by 150-fold (Fig. 6A). These results suggest that signaling by IFNs, IL-6, and TNFα are, by themselves, unable to account for the vast majority of influenza-induced miRNA accumulation.
FIGURE 6.
Antiviral mediators are insufficient for miRNA induction. A, A549 cells were either untreated (UNT), treated with 1000 units/ml of IFNα or 500 ng/ml of IFNγ, 400 ng/ml of IL-6 and 500 ng/ml of soluble IL-6 receptor, 10 ng/ml of NFα, mock infected (mock), or infected with 1 pfu/cell A/Udorn/72 or A/WSN/33 for 10 h. Relative abundance of miR-146a, miR187, miR-200c, miR-1275, miR-132, miR7, and miR-16 was determined, inset graph shows smaller changes in miR-146a due to cytokine treatments. B, A549 cells were either mock transfected, or transfected with 5 μg of poly(I:C) per ml of media for 12 h. Relative abundance of miR-16, miR-7, miR-132, miR-146a, miR-187, miR-200c, and miR-1275 was determined. C, control experiments to verify the cellular response to cytokine treatments or poly(I:C) transfections as indicated. Relative abundance of ISG15, CCL5 mRNA was determined to verify response to IFNα, IFNγ and TNFα. IL-6 activity was confirmed using a GAS luciferase assay (4x-M67). To verify response to poly(I:C) transfection relative abundance of IFNβ and CCL5 mRNA was determined.
Recognition of influenza virus by the innate immune system can occur through several pattern recognition pathways (37–39). The RLRs are the main pattern recognition receptors for viruses in A549 cells because these cells lack TLR7 and TLR8 expression (40). To determine whether the synthetic double-stranded RNA, poly(I:C), induced changes in miRNA expression, A549 cells were transfected with poly(I:C) prior to RNA isolation 12 h later (41). As a control, the level of IFNβ and CCL5 mRNA was measured to verify the efficacy of the poly(I:C) transfection in inducing RLR signaling (Fig. 6C). Under these conditions, miR-146a was induced 2.6-fold, but miR-7, miR-132, miR-187, miR-200c, and miR-1275 remained constant or below a 2-fold increase threshold (Fig. 6B). These results indicate that signaling induced by poly(I:C) may contribute to miR-146a induction, but is insufficient to account for other miRNAs that are increased in response to influenza A virus infection.
Influenza Virus-induced miRNAs Target Cell Signaling Pathways
As miRNAs are able to repress translation by inducing mRNA degradation, gene expression profiling was performed to identify miRNA-targeted mRNA transcripts that decrease in abundance during infection and contain miRNA seed matches within their 3′ UTR. Genomewide mRNA abundance was assessed in A549 cells that were mock infected or infected with A/WSN/33 in the presence or absence of miR-7, miR-132, miR-146a, miR-187, miR-200c, and miR-1275 mimics. Total RNA was purified from cells 10 h postinfection and mRNA was measured using an Illumina whole genome expression microarray.
In the absence of miRNA mimics, 1970 genes were differentially regulated by infection greater than 1.5-fold, with 1177 genes displaying decreased expression and 793 genes displaying increased expression. There are many known mechanisms that might account for the decreased mRNA expression during influenza virus infection including cap-snatching, inhibition of pre-mRNA formation, and degradation of RNA PolII, in addition to antiviral gene products such as RNase L that can randomly target cellular and viral mRNAs (42, 43). However, it is notable that 448 of the 1177 down-regulated genes (38%) contained seed matches for the tested miRNAs, as determined by 3 separate algorithms using the MiR-Walk software (29).
In the presence of miRNA mimics, most mRNA levels were identical to the control samples. However, data analysis identified 36 genes that were differentially expressed during infection in the presence of miRNA mimics: decreased expression was observed for 26 genes, whereas 10 genes increased expression (greater than 1.5-fold threshold, see Table 2). Of the 26 repressed genes, all but one contained at least one seed match to the specifically expressed miRNAs. At the top of this list is IRAK1, a previously identified target of miR-146a, affirming the efficacy of this mRNA expression profiling approach for identification of miRNA targets.
TABLE 2.
Abundance of miRNA targeted mRNAs during influenza A virus infection
| Gene IDa | Mockb | A/WSN/33b | A/WSN/33b + mimics | Fold-changec | 3′ UTR target sitesd |
|---|---|---|---|---|---|
| IRAK1 | 3.137 ± 0.286e | 3.066 ± 0.016 | 2.563 ± 0.039 | −3.178 | miR-146 (2),f miR-1275 |
| NAP1L1 | 3.049 ± 0.304 | 3.010 ± 0.037 | 2.517 ± 0.019 | −3.109 | miR-132 (3) miR-200c |
| EIF2S3 | 3.198 ± 0.130 | 2.934 ± 0.028 | 2.460 ± 0.013 | −2.979 | miR-7, miR-132 (3) |
| SDF2 | 2.855 ± 0.010 | 2.744 ± 0.035 | 2.373 ± 0.029 | −2.350 | miR-132 |
| NDUFB10 | 3.299 ± 0.067 | 3.324 ± 0.048 | 2.955 ± 0.021 | −2.338 | miR-132 |
| COPS8 | 2.606 ± 0.154 | 2.748 ± 0.009 | 2.424 ± 0.028 | −2.109 | miR-146a, miR-200c |
| DAZAP2 | 3.481 ± 0.059 | 3.472 ± 0.018 | 3.152 ± 0.039 | −2.087 | miR-7, miR-132 |
| TIMM17B | 2.481 ± 0.203 | 2.544 ± 0.037 | 2.255 ± 0.026 | −1.944 | miR-146a |
| ESYT2 | 2.972 ± 0.087 | 2.826 ± 0.026 | 2.541 ± 0.011 | −1.925 | miR-132, miR-146a (2), miR-200c, miR-1275 |
| BRI3 | 2.627 ± 0.062 | 2.705 ± 0.002 | 2.464 ± 0.020 | −1.741 | miR-132 |
| DCBLD2 | 3.541 ± 0.430 | 3.373 ± 0.012 | 3.135 ± 0.071 | −1.727 | miR-7, miR-132 (2), miR-200c (3), miR-1275 |
| TSPAN6 | 2.522 ± 0.155 | 2.770 ± 0.037 | 2.548 ± 0.023 | −1.668 | miR-132 |
| MAPK3 | 2.362 ± 0.015 | 2.536 ± 0.015 | 2.316 ± 0.016 | −1.660 | miR-132, miR-1275 |
| YWHAG | 2.770 ± 0.350 | 2.782 ± 0.040 | 2.563 ± 0.036 | −1.655 | miR-132 (2), miR-200c (2) |
| MMGT1 | 2.817 ± 0.305 | 2.718 ± 0.007 | 2.511 ± 0.034 | −1.612 | miR-132, miR-200c |
| CALU | 2.566 ± 0.098 | 2.617 ± 0.029 | 2.414 ± 0.024 | −1.595 | miR-7, miR-132 (2), miR = 146a (2), miR-200c, miR-1275 |
| TOR1A | 2.491 ± 0.150 | 2.429 ± 0.027 | 2.231 ± 0.005 | −1.578 | miR-146a |
| MTPN | 3.010 ± 0.189 | 3.086 ± 0.006 | 2.889 ± 0.063 | −1.573 | miR-7 |
| STMN1 | 2.535 ± 0.176 | 2.615 ± 0.014 | 2.423 ± 0.047 | −1.555 | miR-132 |
| MRPL43 | 2.839 ± 0.140 | 2.681 ± 0.023 | 2.492 ± 0.039 | −1.543 | N/A |
| PAIP2 | 2.931 ± 0.306 | 2.641 ± 0.019 | 2.457 ± 0.009 | −1.527 | miR-132 (2), miR-200c |
| PPP1R14B | 2.910 ± 0.297 | 3.082 ± 0.013 | 2.900 ± 0.044 | −1.521 | miR-1275 |
| DPY19L1 | 2.935 ± 0.073 | 2.936 ± 0.027 | 2.756 ± 0.013 | −1.515 | miR-132, miR-200c |
| AK3 | 2.781 ± 0.058 | 2.894 ± 0.008 | 2.714 ± 0.014 | −1.513 | miR-7 (2), miR-132, miR-1275 |
| DPYSL3 | 2.459 ± 0.175 | 2.558 ± 0.010 | 2.379 ± 0.041 | −1.512 | miR-7 (3), miR-132, miR-1275 (3) |
| TMEM136 | 2.380 ± 0.052 | 2.437 ± 0.016 | 2.260 ± 0.012 | −1.501 | miR-132 (2), miR-146a, miR-187 |
a Genes that display differential mRNA abundance (>1.5-fold) during infection of A549 cells with A/WSN/33 (multiplicity of infection 5 pfu/cell, 10 hpi) and infection during overexpression of influenza virus-induced miRNAs.
b A549 cells were either mock infected, infected with A/WSN/33 (multiplicity of infection 5 pfu/cell), or infected with A/WSN/33 in the presence of miR-7, miR-132, miR-146a, miR-187, miR-200c, and miR-1275 mimics.
c Fold-change indicated is difference between A/WSN/33 + mimics and A/WSN/33.
d Predicted binding sites of influenza-induced miRNAs within the 3′ UTR of indicated gene utilizing the TargetScan algorithm. Parentheses indicate number of target sites for a given miRNA.
e Data indicate average log10 signal of Illumina gene expression array ± S.D. of 3 biological replicates.
To identify functional pathways correlated with the gene expression changes observed due to miRNA expression, the Innate DB database was used for a systems biology pathway analysis (30). The identified miRNA-targeted genes were connected to 22 cellular innate response pathways including TLR, JAK-STAT, TNFα, insulin, IL-1, and IL-7 signal transduction systems (Table 3). To further analyze these targets, an interactome network was constructed based on literature search terms connected with the gene targets (Fig. 7A). Twelve of the target genes were clustered by this analysis, with a set of central interconnected nodes surrounding MAPK3, IRAK1, and STMN1 (Fig. 7B). Two of these targets, IRAK1 and MAPK3, connect diverse and interrelated pathways and signaling proteins known to be important for the regulation of cellular responses to infections, and these nodes are deeply rooted in TLR and cytokine signaling pathways (44, 45).
TABLE 3.
Pathway analysis of influenza virus-induced miRNA targets
| Pathway namea | Gene symbols |
|---|---|
| Neurotrophin signaling pathway | IRAK1; MAPK3; YWHAG |
| EGFR1 | DCBLD2; MAPK3; PPP1R14B |
| ERK cascade (insulin receptor signaling (mammal)) | MAPK3; YWHAG |
| ERK cascade (insulin receptor signaling) | MAPK3; YWHAG |
| IL1 | IRAK1; MAPK3 |
| Leishmaniasis | IRAK1; MAPK3 |
| Insulin receptor signaling (insulin receptor signaling) | MAPK3; YWHAG |
| Insulin receptor signaling (mammal) | MAPK3; YWHAG |
| Toll-like receptor signaling pathway | IRAK1; MAPK3 |
| Chagas disease (American trypanosomiasis) | IRAK1; MAPK3 |
| Prolactin | MAPK3; YWHAG |
| Oocyte meiosis | MAPK3; YWHAG |
| Toxoplasmosis | IRAK1; MAPK3 |
| Alzheimer disease | MAPK3; NDUFB10 |
| AKT(PKB)-Bad signaling ( EPO signaling pathway(JAK2 STAT1 STAT3 STAT5)) | IRAK1; MAPK3 |
| Positive regulation of (transcription of SOCS by STAT dimer) in JAK STAT pathway | IRAK1; MAPK3 |
| AKT(PKB)-Bad signaling (IL-7 signaling (JAK1 JAK3 STAT5)) | IRAK1; MAPK3 |
| IL-7 signaling pathway (JAK1 JAK3 STAT5) | IRAK1; MAPK3 |
| EPO signaling pathway (JAK2 STAT1 STAT3 STAT5) | IRAK1; MAPK3 |
| VEGF signaling pathway (VEGF signaling pathway) | IRAK1; MAPK3 |
| MAPK signaling pathway | MAPK3; STMN1 |
| TNFα | MAPK3; YWHAG |
| JAK-STAT pathway and regulation pathway | IRAK1; MAPK3 |
a Pathway enrichment determined by InnateDB database (30). Pathway analysis of influenza virus-induced miRNA targets indicated by down-regulated genes during infection of A549 cells by A/WSN/33 in the presence of overexpressed miR-7, miR-132, miR-146a, miR-187, miR-200c, and miR-1275 mimics.
FIGURE 7.
Functional interactome analysis of miRNA targets. A, genes identified to be down-regulated in the presence of miR-7, miR-132, miR-146a, miR-187, miR-200c, and miR-1275 mimics were analyzed based on literature search terms. Twelve of the 26 down-regulated genes were functionally clustered. B, expanded view of the dense central interconnected node representing IRAK1, MAPK3, and STMN1.
To verify the mRNA microarray data at the protein level, MAPK3 and IRAK1 protein abundance was measured by immunoblotting before and after infection with influenza A/WSN/33, both in the absence and presence of specific miRNA mimics. For MAPK3, miR-132- and miR-1275-specific mimics were used, and for IRAK1, miR-146a and miR-1275 mimics were used as these miRNAs were identified to bind the 3′ UTR of respective mRNA (Table 2). At 10 h postinfection, whole cell extracts were generated, separated by SDS-PAGE, transferred to nitrocellulose membranes, and probed with antibodies that recognize MAPK1 + MAPK3, IRAK1, influenza NP, and GAPDH. Results indicate that both MAPK3 and IRAK1 protein levels decrease during influenza virus infection, and that this decrease is more pronounced in the presence of specific mimics, but not control mimics (Fig. 8). These conditions did not alter the levels of control proteins, MAPK1, GAPDH, or influenza NP. These data confirm the microarray findings and demonstrate that miR-132, miR-146a, and miR-1275 work in conjunction to negatively regulate MAPK3 and IRAK1 at both RNA and protein levels during influenza A virus infection.
FIGURE 8.
MAPK3 and IRAK1 protein levels are reduced during influenza virus infection. A549 cells were mock infected (M) or infected with A/WSN/33 (m.o.i. of 5 pfu/cell; 10 h) in the absence or presence of control (CTRL) or specific miRNA mimics for miR-132, miR-146a, or miR-1275 as indicated. 40 μg of total protein was separated by SDS-PAGE and probed with antisera for MAPK1 + MAPK3, IRAK1, influenza NP, and GAPDH.
DISCUSSION
Most of the cell-autonomous responses to RNA virus infections are mediated at the level of regulated gene expression. In addition to the alteration in mRNA expression profiles, protein expression is also modulated in response to infection through mechanisms that regulate mRNA translation, such as the action of the antiviral protein kinase, PKR, or pathways that control translation initiation downstream of IFN signaling (46). The present study adds a further dimension to the cellular response to virus infection, through the analysis of endogenous human miRNAs that are induced by infection with influenza A viruses. Specifically, experiments indicate that miR-7, miR-132, miR-146a, miR-187, miR-200c, and miR-1275 increase in abundance during influenza virus infection of human respiratory cells, causing down-regulation of antiviral proteins such as IRAK1 and MAPK3.
In addition to the influenza virus-induced human miRNAs identified in the present study, infection of chicken and mouse lungs with influenza A viruses also has been reported to change the levels of several miRNAs (22, 47). Despite the conceptual similarities among these studies, comparing the results reveals little or no commonality among the miRNAs reported to be induced by influenza A viruses in these diverse systems. For example, miR-21 was found to be elevated in infected mouse lungs, but remained constant in HAE cells (Fig. 3). A second miRNA identified here, miR-146a, was also identified as differentially expressed in influenza A virus-infected chicken lungs; however, in contrast with the dramatic increase in mature miR-146a observed in human cells, the chicken miR-146a level was strongly reduced in response to avian influenza virus infection (47). There are several potential explanations for the apparent discrepancies among the studies, including the use of different host species and virus strains for each experiment: for example, miR-21 is one of the most abundant miRNAs present in A549 cells at steady state, but this is not the case for other cells tested, and both strain-specific and temporal regulation of miRNA abundance was observed during infection of A549 cells with swine and avian origin influenza viruses (16). A further complication may arise from the analysis of heterogeneous endogenous tissue (such as whole lungs), in which only a small number of infected cells is likely to be present and therefore only partially contributing to differential miRNA expression. Not only would the contribution of infected cells to the total miRNA pool be diluted in tissue RNA preparations, but also non-cell autonomous effects such as cytokine or apoptotic signaling could cause differential miRNA expression in uninfected diverse cell types that are present in complex tissue samples.
Identification of influenza A virus-regulated miRNAs raises a question of their potential endogenous host mRNA targets. As most miRNA target prediction algorithms are based on 6–8-bp imperfect seed region matches, hundreds of potential targets can be computationally identified for each miRNA analyzed. None of the miRNAs that were characterized had the potential ability, as determined by seed sequence match, to target the influenza virus genome or mRNAs. Such a specific targeting mechanism has been demonstrated to be theoretically possible, as computationally-predicted miRNAs (miR-323, miR-491, and miR-654) with seed matches to influenza virus genomes were shown to control influenza virus replication when overexpressed in Madin-Darby canine kidney cells (23). However, this type of regulation by miRNAs is evolutionarily unfavorable, as variant viruses with disruptions to the seed sequences will be selected.
Considering that many miRNA-regulated targets are also subject to combinatorial recognition by several miRNAs, deciphering the exact target(s) for individual miRNAs represents a formidable challenge, especially in the context of changes induced during acute virus infections (48). Both known and novel miRNA targets were observed during infection in the absence or presence of miRNA mimics. The 3′ UTR of the 26 identified target mRNAs were analyzed to find target sites of miR-7, miR-132, miR-146a, miR-187, miR-200c, and miR-1275 to correlate mRNA expression with miRNA target sites. The data indicate that two genes involved in several innate immune processes, MAPK3 and IRAK1, are targeted for mRNA degradation and protein translation inhibition by the combinatorial effect of miR-132 and miR-1275, and miR-146a and miR-1275, respectively. This suggests that at least three of the six influenza virus-induced miRNAs tested have the ability to regulate innate immune signaling pathways. Interestingly, miR-132 has been shown to be regulated transcriptionally by a MAPK3 (ERK1) pathway and this study suggests that MAPK3 may use miR-132 to regulate its activity in a negative feedback loop (49, 50). The extent of pathways targeted by just a small number of influenza virus-induced miRNAs suggests that miRNA regulation of cellular responses is an ongoing process in the infected cell that can fine-tune the cellular response. Considering the potential for activation of even more miRNAs in the infected cell, we predict they will have a significant impact in regulating cellular mRNA and protein levels.
Several of the miRNAs identified in this study have been previously linked to biological responses related to cellular responses to virus infection. MicroRNA-132 has been shown to be induced in cells infected with Kaposi's sarcoma-associated herpesvirus, and was proposed to target the histone acetyltransferase protein, p300, which is required for the production of IFNβ (51). Our study indicates that influenza A virus is also capable of inducing miR-132 accumulation, although not to the same degree as reported for the Kaposi's sarcoma-associated herpesvirus study. Again, this discrepancy might be explained by variations in both the cell types and the viruses under investigation. Notably, within our gene expression analysis for targets of miR-132, CBP/p300 was not identified as differentially regulated at the mRNA level. Another well characterized miRNA, miR-146a, already shown to be induced by LPS, IL-1β, VSV, and LMP-1 of Epstein-Barr virus, is dramatically induced by influenza A virus infection (14, 15, 52). This miRNA has been shown to be capable of regulating the activation of NF-κB by targeting the expression of the upstream signaling molecules, IRAK1, IRAK2, and TRAF6 (14, 15). Our analysis indicates that miR-146a can target IRAK1, and that miR-1275 may also contribute to this effect. The present study is the first to link miR-7, miR-187, miR-200c, or miR-1275 with virus infections or cellular antiviral responses.
An interesting feature of the miRNAs described here is their unique regulation in response to influenza virus infection. Other RNA viruses tested, EMCV, Sendai virus, PIV5, and VSV, are among the most commonly used viruses for studying innate antiviral responses, yet they are unable to increase the abundance of these miRNAs to the same extent as influenza virus. Treatment of cells with several antiviral mediators, including IFNα, IFNγ, IL-6, and TNFα, or transfection with poly(I:C), did not induce changes in any miRNA to the degree observed with influenza virus infection. It is possible that additional untested mediators are fully responsible for some or all of the miRNA induction observed, or that combinations of these individual treatments might better imitate the milieu of influenza virus infection. TNFα was found to induce miR-146a accumulation, in agreement with prior studies, but influenza A virus infection was a much more effective inducer (15).
This apparent specificity and potency of activation might be explained by cumulative effects of multiple cellular pathways and signaling systems activated by influenza virus infection that could then converge synergistically on miRNA regulation. This model is consistent with our finding that miR-132, miR-146a, and miR-1275 may work in conjunction to negatively regulate MAPK3 and IRAK1 at both RNA and protein levels during influenza A virus infection. This regulatory event is achieved by one or more secondary response pathway(s) that can regulate miRNA accumulation during the time course of influenza virus infection. As this study demonstrates alteration of the abundance of a select group of miRNAs can dramatically influence mRNA targeting, it will be of great interest to expand the measurement of miRNA changes genome-wide using next-generation sequencing technology.
Acknowledgments
We are grateful to Bob Lamb and Jeremy Rossman (Northwestern University) for providing influenza virus strains and expertise in their propagation, the Northwestern Genomics Core for microarray and bioinformatics analysis, and to members of the Horvath lab for critical and helpful comments.
This work was supported, in whole or in part, by National Institutes of Health Grant U01AI082984 from the NIAID IMVC program.
- TLR
- Toll-like receptors
- RLR
- RIG-I-like receptors
- pri-miRNA
- primary microRNA
- pre-miRNA
- precursor miRNAs
- EMCV
- encephalomyocarditis virus
- hpi
- h postinfection
- HAE
- human airway epithelial
- PIV5
- parainfluenza virus 5
- CHX
- cycloheximide
- VSV
- vesicular stomatitis virus.
REFERENCES
- 1. Ishii K. J., Koyama S., Nakagawa A., Coban C., Akira S. (2008) Host innate immune receptors and beyond. Making sense of microbial infections. Cell Host Microbe 3, 352–363 [DOI] [PubMed] [Google Scholar]
- 2. Ding S. W. (2010) RNA-based antiviral immunity. Nat. Rev. Immunol. 10, 632–644 [DOI] [PubMed] [Google Scholar]
- 3. Umbach J. L., Cullen B. R. (2009) The role of RNAi and microRNAs in animal virus replication and antiviral immunity. Genes Dev. 23, 1151–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huntzinger E., Izaurralde E. (2011) Gene silencing by microRNAs. Contributions of translational repression and mRNA decay. Nat. Rev. Genet. 12, 99–110 [DOI] [PubMed] [Google Scholar]
- 5. Bartel D. P. (2004) MicroRNAs. Genomics, biogenesis, mechanism, and function. Cell 116, 281–297 [DOI] [PubMed] [Google Scholar]
- 6. Davis-Dusenbery B. N., Hata A. (2010) Mechanisms of control of microRNA biogenesis. J. Biochem. 148, 381–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miyoshi K., Uejima H., Nagami-Okada T., Siomi H., Siomi M. C. (2008) In vitro RNA cleavage assay for Argonaute-family proteins. Methods Mol. Biol. 442, 29–43 [DOI] [PubMed] [Google Scholar]
- 8. Wu L., Fan J., Belasco J. G. (2006) MicroRNAs direct rapid deadenylation of mRNA. Proc. Natl. Acad. Sci. U.S.A. 103, 4034–4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Giraldez A. J., Mishima Y., Rihel J., Grocock R. J., Van Dongen S., Inoue K., Enright A. J., Schier A. F. (2006) Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science 312, 75–79 [DOI] [PubMed] [Google Scholar]
- 10. Rehwinkel J., Behm-Ansmant I., Gatfield D., Izaurralde E. (2005) A crucial role for GW182 and the DCP1·DCP2 decapping complex in miRNA-mediated gene silencing. RNA 11, 1640–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guo H., Ingolia N. T., Weissman J. S., Bartel D. P. (2010) Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466, 835–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lim L. P., Lau N. C., Garrett-Engele P., Grimson A., Schelter J. M., Castle J., Bartel D. P., Linsley P. S., Johnson J. M. (2005) Microarray analysis shows that some microRNAs down-regulate large numbers of target mRNAs. Nature 433, 769–773 [DOI] [PubMed] [Google Scholar]
- 13. Parameswaran P., Sklan E., Wilkins C., Burgon T., Samuel M. A., Lu R., Ansel K. M., Heissmeyer V., Einav S., Jackson W., Doukas T., Paranjape S., Polacek C., dos Santos F. B., Jalili R., Babrzadeh F., Gharizadeh B., Grimm D., Kay M., Koike S., Sarnow P., Ronaghi M., Ding S. W., Harris E., Chow M., Diamond M. S., Kirkegaard K., Glenn J. S., Fire A. Z. (2010) Six RNA viruses and 41 hosts. Viral small RNAs and modulation of small RNA repertoires in vertebrate and invertebrate systems. PLoS Pathog. 6, e1000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hou J., Wang P., Lin L., Liu X., Ma F., An H., Wang Z., Cao X. (2009) MicroRNA-146a feedback inhibits RIG-I-dependent Type I IFN production in macrophages by targeting TRAF6, IRAK1, and IRAK2. J. Immunol. 183, 2150–2158 [DOI] [PubMed] [Google Scholar]
- 15. Taganov K. D., Boldin M. P., Chang K. J., Baltimore D. (2006) NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. U.S.A. 103, 12481–12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Loveday E. K., Svinti V., Diederich S., Pasick J., Jean F. (2012) Temporal- and strain-specific host microRNA molecular signatures associated with swine-origin H1N1 and avian-origin H7N7 influenza A virus infection. J. Virol. 86, 6109–6122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Motsch N., Pfuhl T., Mrazek J., Barth S., Grässer F. A. (2007) Epstein-Barr virus-encoded latent membrane protein 1 (LMP1) induces the expression of the cellular microRNA miR-146a. RNA Biol. 4, 131–137 [DOI] [PubMed] [Google Scholar]
- 18. Cameron J. E., Yin Q., Fewell C., Lacey M., McBride J., Wang X., Lin Z., Schaefer B. C., Flemington E. K. (2008) Epstein-Barr virus latent membrane protein 1 induces cellular microRNA miR-146a, a modulator of lymphocyte signaling pathways. J. Virol. 82, 1946–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang J., Wang F., Argyris E., Chen K., Liang Z., Tian H., Huang W., Squires K., Verlinghieri G., Zhang H. (2007) Cellular microRNAs contribute to HIV-1 latency in resting primary CD4+ T lymphocytes. Nat. Med. 13, 1241–1247 [DOI] [PubMed] [Google Scholar]
- 20. Pedersen I. M., Cheng G., Wieland S., Volinia S., Croce C. M., Chisari F. V., David M. (2007) Interferon modulation of cellular microRNAs as an antiviral mechanism. Nature 449, 919–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jopling C. L. (2008) Regulation of hepatitis C virus by microRNA-122. Biochem. Soc. Trans. 36, 1220–1223 [DOI] [PubMed] [Google Scholar]
- 22. Li Y., Chan E. Y., Li J., Ni C., Peng X., Rosenzweig E., Tumpey T. M., Katze M. G. (2010) MicroRNA expression and virulence in pandemic influenza virus-infected mice. J. Virol. 84, 3023–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Song L., Liu H., Gao S., Jiang W., Huang W. (2010) Cellular microRNAs inhibit replication of the H1N1 influenza A virus in infected cells. J. Virol. 84, 8849–8860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hale B. G., Randall R. E., Ortín J., Jackson D. (2008) The multifunctional NS1 protein of influenza A viruses. J. Gen. Virol. 89, 2359–2376 [DOI] [PubMed] [Google Scholar]
- 25. Gack M. U., Albrecht R. A., Urano T., Inn K. S., Huang I. C., Carnero E., Farzan M., Inoue S., Jung J. U., García-Sastre A. (2009) Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell Host Microbe 5, 439–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Du P., Kibbe W. A., Lin S. M. (2008) Lumi, a pipeline for processing Illumina microarray. Bioinformatics 24, 1547–1548 [DOI] [PubMed] [Google Scholar]
- 27. Bolstad B. M., Irizarry R. A., Astrand M., Speed T. P. (2003) A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19, 185–193 [DOI] [PubMed] [Google Scholar]
- 28. Lewis B. P., Burge C. B., Bartel D. P. (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120, 15–20 [DOI] [PubMed] [Google Scholar]
- 29. Dweep H., Sticht C., Pandey P., Gretz N. (2011) miRWalk database. Prediction of possible miRNA binding sites by “walking” the genes of three genomes. J. Biomed. Inform. 44, 839–847 [DOI] [PubMed] [Google Scholar]
- 30. Lynn D. J., Winsor G. L., Chan C., Richard N., Laird M. R., Barsky A., Gardy J. L., Roche F. M., Chan T. H., Shah N., Lo R., Naseer M., Que J., Yau M., Acab M., Tulpan D., Whiteside M. D., Chikatamarla A., Mah B., Munzner T., Hokamp K., Hancock R. E., Brinkman F. S. (2008) InnateDB. Facilitating systems level analyses of the mammalian innate immune response. Mol. Syst. Biol. 4, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shannon P., Markiel A., Ozier O., Baliga N. S., Wang J. T., Ramage D., Amin N., Schwikowski B., Ideker T. (2003) Cytoscape. A software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔδCT method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 33. Wulczyn F. G., Smirnova L., Rybak A., Brandt C., Kwidzinski E., Ninnemann O., Strehle M., Seiler A., Schumacher S., Nitsch R. (2007) Post-transcriptional regulation of the let-7 microRNA during neural cell specification. FASEB J. 21, 415–426 [DOI] [PubMed] [Google Scholar]
- 34. Obernosterer G., Leuschner P. J., Alenius M., Martinez J. (2006) Post-transcriptional regulation of microRNA expression. RNA 12, 1161–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thomson J. M., Newman M., Parker J. S., Morin-Kensicki E. M., Wright T., Hammond S. M. (2006) Extensive post-transcriptional regulation of microRNAs and its implications for cancer. Genes Dev. 20, 2202–2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perry M. M., Williams A. E., Tsitsiou E., Larner-Svensson H. M., Lindsay M. A. (2009) Divergent intracellular pathways regulate interleukin-1β-induced miR-146a and miR-146b expression and chemokine release in human alveolar epithelial cells. FEBS Lett. 583, 3349–3355 [DOI] [PubMed] [Google Scholar]
- 37. Diebold S. S., Kaisho T., Hemmi H., Akira S., Reis e Sousa C. (2004) Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303, 1529–1531 [DOI] [PubMed] [Google Scholar]
- 38. Kato H., Takeuchi O., Sato S., Yoneyama M., Yamamoto M., Matsui K., Uematsu S., Jung A., Kawai T., Ishii K. J., Yamaguchi O., Otsu K., Tsujimura T., Koh C. S., Reis e Sousa C., Matsuura Y., Fujita T., Akira S. (2006) Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441, 101–105 [DOI] [PubMed] [Google Scholar]
- 39. Sirén J., Imaizumi T., Sarkar D., Pietilä T., Noah D. L., Lin R., Hiscott J., Krug R. M., Fisher P. B., Julkunen I., Matikainen S. (2006) Retinoic acid inducible gene-I and mda-5 are involved in influenza A virus-induced expression of antiviral cytokines. Microbes Infect 8, 2013–2020 [DOI] [PubMed] [Google Scholar]
- 40. Tissari J., Sirén J., Meri S., Julkunen I., Matikainen S. (2005) IFN-α enhances TLR3-mediated antiviral cytokine expression in human endothelial and epithelial cells by up-regulating TLR3 expression. J. Immunol. 174, 4289–4294 [DOI] [PubMed] [Google Scholar]
- 41. Bamming D., Horvath C. M. (2009) Regulation of signal transduction by enzymatically inactive antiviral RNA helicase proteins MDA5, RIG-I, and LGP2. J. Biol. Chem. 284, 9700–9712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rodriguez A., Perez-Gonzalez A., Nieto A. (2007) Influenza virus infection causes specific degradation of the largest subunit of cellular RNA polymerase II. J. Virol. 81, 5315–5324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Krug R. M., Broni B. A., Bouloy M. (1979) Are the 5′ ends of influenza viral mRNAs synthesized in vivo donated by host mRNAs? Cell 18, 329–334 [DOI] [PubMed] [Google Scholar]
- 44. Gottipati S., Rao N. L., Fung-Leung W. P. (2008) IRAK1. a critical signaling mediator of innate immunity. Cell Signal. 20, 269–276 [DOI] [PubMed] [Google Scholar]
- 45. Joshi S., Kaur S., Kroczynska B., Platanias L. C. (2010) Mechanisms of mRNA translation of interferon stimulated genes. Cytokine 52, 123–127 [DOI] [PubMed] [Google Scholar]
- 46. Platanias L. C. (2005) Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 5, 375–386 [DOI] [PubMed] [Google Scholar]
- 47. Wang Y., Brahmakshatriya V., Zhu H., Lupiani B., Reddy S. M., Yoon B. J., Gunaratne P. H., Kim J. H., Chen R., Wang J., Zhou H. (2009) Identification of differentially expressed miRNAs in chicken lung and trachea with avian influenza virus infection by a deep sequencing approach. BMC Genomics 10, 512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Selbach M., Schwanhäusser B., Thierfelder N., Fang Z., Khanin R., Rajewsky N. (2008) Widespread changes in protein synthesis induced by microRNAs. Nature 455, 58–63 [DOI] [PubMed] [Google Scholar]
- 49. Remenyi J., Hunter C. J., Cole C., Ando H., Impey S., Monk C. E., Martin K. J., Barton G. J., Hutvagner G., Arthur J. S. (2010) Regulation of the miR-212/132 locus by MSK1 and CREB in response to neurotrophins. Biochem. J. 428, 281–291 [DOI] [PubMed] [Google Scholar]
- 50. Kawashima H., Numakawa T., Kumamaru E., Adachi N., Mizuno H., Ninomiya M., Kunugi H., Hashido K. (2010) Glucocorticoid attenuates brain-derived neurotrophic factor-dependent up-regulation of glutamate receptors via the suppression of microRNA-132 expression. Neuroscience 165, 1301–1311 [DOI] [PubMed] [Google Scholar]
- 51. Lagos D., Pollara G., Henderson S., Gratrix F., Fabani M., Milne R. S., Gotch F., Boshoff C. (2010) miR-132 regulates antiviral innate immunity through suppression of the p300 transcriptional co-activator. Nat. Cell Biol. 12, 513–519 [DOI] [PubMed] [Google Scholar]
- 52. Tang Y., Luo X., Cui H., Ni X., Yuan M., Guo Y., Huang X., Zhou H., de Vries N., Tak P. P., Chen S., Shen N. (2009) MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum. 60, 1065–1075 [DOI] [PubMed] [Google Scholar]