Abstract
Background: Maternal care (MC) and dopamine modulate brain activity during emotion processing in inferior frontal gyrus (IFG), striatum and amygdala. Reuptake of dopamine from the synapse is performed by the dopamine transporter (DAT), whose abundance is predicted by variation in its gene (DAT 3′VNTR; 10 > 9-repeat alleles). Here, we investigated the interaction between perceived MC and DAT 3′VNTR genotype on brain activity during processing of aversive facial emotional stimuli.
Methods: Sixty-one healthy subjects were genotyped for DAT 3′VNTR and categorized in low and high MC individuals. They underwent functional magnetic resonance imaging while performing a task requiring gender discrimination of facial stimuli with angry, fearful or neutral expressions.
Results: An interaction between facial expression, DAT genotype and MC was found in left IFG, such that low MC and homozygosity for the 10-repeat allele are associated with greater activity during processing of fearful faces. This greater activity was also inversely correlated with a measure of emotion control as scored with the Big Five Questionnaire. Moreover, MC and DAT genotype described a double dissociation on functional connectivity between IFG and amygdala.
Conclusion: These findings suggest that perceived early parental bonding may interact with DAT 3′VNTR genotype in modulating brain activity during emotionally relevant inputs.
Keywords: emotion, dopamine, maternal care, fMRI, amygdala, inferior frontal gyrus
INTRODUCTION
Converging evidence has suggested that emotional behavior in animals and humans is modulated by genetic variation (Soliman et al., 2010). However, twin studies have also indicated that heritability of basic emotions is not always large (Bartels et al., 2010). For example, environmental stimuli like quality of the parent–child relationship are known to modulate understanding of emotions (Cassidy et al., 1992). In fact, several studies have demonstrated gene by environment interactions on emotional behavior supporting the relevance of the relationship between ‘nature and nurture’ (Francis et al., 1999; Caspi et al., 2003; Francis et al., 2003; Ishai et al., 2005; Badgaiyan et al., 2009; Domes et al., 2009; Fusar-Poli et al., 2009a; Fusar-Poli et al., 2009b; Trautmann et al., 2009). However, only a limited number of studies in humans have investigated gene by environment interactions on physiological correlates of specific behavioral phenotypes related to emotion processing (Gatt et al., 2010a; Gatt et al., 2010b).
A large body of literature has consistently indicated that emotional behavior is supported by activity of limbic and prefrontal areas (Phillips, 2003). More specifically, previous functional neuroimaging studies in humans have revealed that the amygdala, the prefrontal cortex and striatal regions are involved in processing of emotionally salient sensory cues (Adolphs et al., 1996; Phelps and LeDoux, 2005). Moreover, the ventral striatum may be selectively involved in processing of anger but not of other emotions (Calder et al., 2004). Within these brain areas, there is evidence for further selectivity. A recent meta-analysis strongly suggests laterality effects on emotional processing, with left-lateralized amygdala response during evaluation of aversive emotional stimuli (Fusar-Poli et al., 2009a). Several other studies also support lateralization of activity in the left inferior frontal gyrus (IFG) during processing of aversive stimuli (Badgaiyan et al., 2009; Fusar-Poli et al., 2009a; Trautmann et al., 2009). These studies suggest that processing of aversive stimuli is lateralized more robustly to left amygdala and IFG.
Dopamine (DA) signaling is an important modulator of activity in the emotion brain network (Grace and Rosenkranz, 2002; Menon et al., 2007; Blasi et al., 2009b). For example, studies in animal models have indicated that DA signaling biases affective responses by increasing excitatory sensory inputs and attenuating inhibitory prefrontal inputs to limbic regions, including the amygdala (Grace and Rosenkranz, 2002) and the medial striatum (Karreman and Moghaddam, 1996). Consistently, studies in humans have revealed that DA is released under stress and modulates activity in medial temporal and prefrontal regions during processing of emotionally relevant stimuli (LaBar and Cabeza, 2006; Panksepp, 2006; Oswald et al., 2007). Furthermore, a recent PET study in healthy humans has indicated that DA is released during emotional processing in amygdala and IFG, once again in the left hemisphere (Badgaiyan et al., 2009).
An important environmental variable baring a tight relationship with emotion processing and DA physiology is maternal care (MC). For instance, early maternal sensitivity has been positively associated with later aggressive responses, and early maternal depressive symptoms have been positively associated with children's negative attributions (Raikes and Thompson, 2008). Moreover, MC behavior appears to be tightly related with DA signaling in animals (Byrnes et al., 2002; Miller and Lonstein, 2005; Numan et al., 2005) and in humans (Lee et al., 2010), especially in dorsal and ventral striatal areas (Pruessner et al., 2004). All together, these data suggest that emotion processing, MC and DA signaling are strongly interconnected in modulating brain physiology.
One of the main modulators of DA signaling is the DA transporter (DAT). The DAT is a presynaptic neuronal membrane protein, which removes DA from the synaptic cleft (Sesack et al., 1998; Lewis et al., 2001). In the cortex, the DAT is mainly extrasynaptic, and thus, it seems better situated to regulating DA diffusion to the extrasynaptic space (Cragg and Rice, 2004). A functional variable number of tandem repeat (VNTR) polymorphism (rs28363170) in the 3′untranslated region of the DAT gene (SLC6A3, 5p15.3—DAT 3′VNTR) has been previously described (Vandenbergh et al., 1992). Alleles of this polymorphism range from 3 to 11 repeats, with the 9- and 10-repeat alleles by far the most common (Vandenbergh et al., 1992). The 10-repeat allele has been associated with greater levels of DAT expression (Heinz et al., 2000; Fuke et al., 2001; Mill et al., 2002; VanNess et al., 2005), although data in the opposite direction have also been reported (van Dyck et al., 2005). This polymorphism has also been associated with prefrontal and hippocampal activity during performance of cognitive tasks (Bertolino et al., 2006; Bertolino et al., 2008; Prata et al., 2009a; Prata et al., 2009b), with anxiety traits (Hunnerkopf et al., 2007) and with quality of parenting (Lee et al., 2010). More in detail, the DAT 3′VNTR 10 allele predicts greater scores for negative parenting in mothers when their offspring with ADHD symptoms present high child deviance (Lee et al., 2010). These previous results suggest that DAT 3′VNTR may have a relevant impact on behavioral phenotypes associated with emotion processing.
In the present study, we have used functional magnetic resonance imaging (fMRI) in healthy humans to investigate the putative effect of self-reported MC, of the DAT 3′VNTR polymorphism, and of their interaction on brain activity during processing of aversive emotional stimuli. Based on previous knowledge about the association between DAT 3′VNTR and MC with DA signaling and about the role of DA in emotion processing and behavior, we hypothesized that DAT 3′VNTR and MC would explain some of the variance in brain activity and connectivity of the emotion brain network. Given previous data indicating interaction between DA and emotion processing (Ishai et al., 2005; Badgaiyan et al., 2009; Domes et al., 2009; Fusar-Poli et al., 2009a; Fusar-Poli et al., 2009b; Trautmann et al., 2009) as well as between DA and perceived MC (Pruessner et al., 2004) on activity of the left amygdala, the left IFG and the striatum, we focused our investigation in these areas.
METHODS AND MATERIALS
Subjects
Sixty-one healthy subjects (19 males, age mean ± SD 23.7 ± 4.4 years, IQ 108.9 ± 11.9 [WAIS], handedness 0.7 ± 0.4 [Edinburgh Inventory]) who had undergone extensive clinical evaluation, were recruited for this study. Inclusion criteria were absence of any neurological or psychiatric disorder and of any other medical condition, absence of any pharmacological treatment that could influence cerebral metabolism or blood flow, history of significant drug or alcohol abuse (no active drug use in the past year). All subjects gave written informed consent to the study after the procedure had been fully explained. The protocol was approved by the local Institutional Review Board (IRB) at the University of Bari.
Genotyping
DNA was extracted from whole blood using standard procedures. rs28363170 genotypes were determined as reported previously (Bertolino et al., 2006, Supplementary Material; Bertolino et al., 2009). Previous studies have consistently demonstrated association of the rs4795541 (5-HTTLPR) functional polymorphism in the serotonin transporter gene (SLC6A4) with activity in the emotion network during incidental processing of aversive stimuli. Thus, we genotyped all subjects also for this genetic variant as previously reported (Bertolino et al., 2005) in order to control for potential biasing effects. With this aim, χ2 was performed on DAT 3′VNTR to investigate between groups distribution of the 5-HTTLPR variant.
Maternal care
All subjects completed the Parental Bonding Instrument (PBI) for assessment of the quality of parenting (Parker, 1979) (see Supplementary Material). This questionnaire uses a cut-off score of 27 to discriminate subjects with ‘high’ and ‘low’ MC (Parker, 1979; Parker and Lipscombe, 1979) and has been utilized as such in several previous studies (Pruessner et al., 2004; Buss et al., 2007; Engert et al., 2009; Pruessner et al., 2010). Moreover, in the original studies by Parker et al. (Parker and Brown, 1979; Parker, 1983, 1989, 1990) factor analytic strategies were used to identify PBI items that best defined and refined parental dimensions. Therefore, use of this questionnaire to derive a continuous score of MC is not recommended (Parker and Brown, 1979; Parker, 1983, 1989, 1990). To control for other potential confounding effects associated with aspects of personality and childhood trauma, subjects also completed the Big Five Questionnaire (BFQ) for the measurement of the Big Five Factor Model (which includes the factors Extraversion, Agreeableness or Friendliness, Conscientiousness, Emotional Stability or Neuroticism, and Intellect or Openness to Experience) (Caprara et al., 1993) and the Stressful Life Events (SLEs) (Caspi et al., 2003), with the aid of a life-history calendar (Freedman et al., 1988; Caspi et al., 1996), a structured interview to facilitate rapid and accurate recall of life events experienced by adults (See Supplementary Material).
Experimental paradigm
The event-related fMRI paradigm (Blasi et al., 2009a; Blasi et al., 2009b) consisted of the presentation of faces with angry, fearful, happy and neutral emotional expressions from a validated set of facial pictures (Tottenham et al., 2009). Subjects were asked to identify the gender of each face (implicit emotional processing). Each stimulus was presented for 500 ms, with an interstimulus interval (ISI) randomly jittered between 2 s and 7 s. The order of facial emotional stimuli was pseudo-randomized across the session (Friston et al., 1999). The stimuli used were pictures of faces with four different expressions. In particular, the same ∼ 40 faces were presented with each of the expressions used. The total number of stimuli was 144: 30 angry, 39 fearful, 37 happy and 38 neutral faces. A fixation cross hair was presented during ISI. Total duration of the task was 6 min and 8 s. In the present study, we focused our analysis on brain responses during processing of aversive facial stimuli (angry and fearful expressions). We focused on these stimuli because their processing is associated with robust and consistent responses of the emotion network (Ljungberg et al., 1992; Adolphs et al., 1996; Phelps and LeDoux, 2005) and it is supported by activity of the DA system (Pani et al., 2000; Panksepp, 2006; Oswald et al., 2007; Kienast et al., 2008).
fMRI
Blood Oxygen Level Dependent (BOLD) fMRI was performed on a GE Signa 3T scanner (gradient echo-planar imaging sequence, TR/TE = 2000/28 ms; 24 interleaved slices, thickness = 4 mm, gap = 1 mm; voxel size 3.75 × 3.75 × 5 mm; scan repetitions = 180; flip angle = 90°; field of view = 24 cm; matrix = 64 × 64) while subjects performed the task. The first four scans were discarded to allow for signal saturation. Stimuli were presented via a back-projection system and responses were recorded through a fiber optic response box which allowed measurement of behavioral data as percent of correct responses and reaction time (RT). fMRI responses were modeled using a canonical hemodynamic response function and temporally filtered using a high-pass filter of 128 Hz to minimize scanner drift.
Data analysis
Behavioral data
In order for the analyses of behavioral data to be consistent with those of functional imaging (see below), accuracy and RT were analyzed for relative differences using a ratio of a difference to a baseline value with the following formula:
The same procedure was used for RT data. Thus, a repeated measures ANOVA with MC and DAT as categorical predictors and normalized performance relative to each facial expression (angry and fearful) as the within-subjects factor was used to compare these behavioral measures. Fisher’s LSD (Least Significant Difference) and t-test for dependent samples were used for post-hoc comparisons as appropriate.
fMRI data
Whole-brain image analysis was performed using Statistical Parametric Mapping 5 (SPM5—http://www.fil.ion.ucl.ac.uk/spm; Wellcome Department of Imaging Neuroscience, London, UK). Images for each subject were realigned, spatially normalized into the Montreal Neurological Institute template (12 parameter affine model), and spatially smoothed (10 mm Gaussian filter). After realignment, data sets were also screened for high quality (scan stability) as demonstrated by small motion correction (<2 mm translation, <1.5° rotation). Emotional faces were used as regressors and convolved with a canonical Hemodynamic Response Function. BOLD responses relative to processing of happy faces were included as a separate regressor and not examined. Predetermined condition effects at each voxel were created using a t statistic, producing statistical images for BOLD responses to brain processing of each aversive relative to neutral facial expression (angry > neutral and fearful > neutral). Therefore, individual contrasts were entered in a second-level random effects ANOVA to investigate at the group level the effect of DAT 3′VNTR genotype, of MC, and of their interaction. In particular, we performed a factorial ANOVA with task condition as a repeated measure (no sphericity correction) and DAT 3′VNTR as well as MC as independent factors.
To evaluate change of functional connectivity within the circuitry associated with processing of aversive stimuli as a function of MC and DAT 3′VNTR genotype, we performed psychophysiological interaction (PPI) analysis (Friston et al., 1997) using as seed the cluster in left IFG where an interaction between Task, MC and DAT 3′VNTR was found (see Results). The first eigenvariate of individual raw activation time courses was extracted by using singular value decomposition method from a volume of interest (VOI) centered on the subject-specific peak cluster within the seed regions. These time courses were then mean centered, high-pass filtered and deconvolved. A general linear model was computed using three regressors: a physiological regressor (the time course response in the VOI), a psychological regressor (the presentation of emotional faces) and a PPI term, calculated as the cross-product of the previous two terms (for more specific details, see Supplementary Method).
Thus, subject-specific statistical PPI contrast images were entered in second-level random effects analyses to evaluate the effect of DAT 3′VNTR genotype, of MC, and of their interaction.
Brodmann’s areas were assigned to activated clusters using the Talairach Daemon (http://ric.uthscsa.edu/projects/talairachdaemon.html) after converting the MNI coordinates of the local maxima in the activated clusters to Talairach coordinates (http://www.mrc-cbu.cam.ac.uk/Imaging/Common/mnispace.shtml).
Based on previous literature which led to our specific regional hypotheses (Ishai et al., 2005; Badgaiyan et al., 2009; Domes et al., 2009; Fusar-Poli et al., 2009a; Fusar-Poli et al., 2009b; Trautmann et al., 2009), we used a region of interest (ROI) approach to investigate putative effects of DAT 3′VNTR genotype, of MC, and their interaction on responses of the left amygdala, left IFG and bilateral striatum. In particular, we based our approach on previous studies showing involvement of the left amygdala and left IFG in processing of aversive facial expressions and on their previously demonstrated modulation by DA during emotion processing (Ishai et al., 2005; Badgaiyan et al., 2009; Domes et al., 2009; Fusar-Poli et al., 2009a; Fusar-Poli et al., 2009b; Trautmann et al., 2009). Furthermore, we focused our analysis on ventral striatum, putamen and caudate based on previous data indicating their association with MC-associated behavior (Pruessner et al., 2004). ROIs were identified using the WFU Pickatlas software version 1.04 (http://www.rad.wfubmc.edu/fmri) (Maldjian et al., 2003).
All statistical maps were thresholded at P < 0.005, uncorrected, minimum cluster size of 3 voxel (Forman et al., 1995). On the resulting clusters in the predicted ROIs, we applied a Family Wise Error (FWE) correction at P < 0.05 (Worsley et al., 1996; Genovese et al., 2002). We used the same procedure for PPI analyses. Finally, BOLD responses and PPI values were extracted from significant clusters using MarsBar (http://marsbar.sourceforge.net/) to further explore the impact of MC, DAT 3′VNTR genotype and facial expressions on brain activity and connectivity.
Brain activity—behavior correlations
To further investigate the relationship between imaging findings and behavior, we performed Pearson’s tests to evaluate the potential correlation between BOLD responses in the IFG cluster differentiating responses in LMC 10-10 and HMC 10-10 individuals (see below, Results section) and an index of emotional behavior. For this purpose, we used the BFQ (Caprara et al., 1993), which measures personality traits including those relative to emotional behavior. In particular, we focused on the facet ‘emotion control’ within the ‘emotion stability’ dimension of the BFQ, which is defined as the capacity to cope adequately with one’s own anxiety and emotionality (Caprara et al., 1993). Our choice to focus on this parameter was based on the fact that we are evaluating brain responses to emotional stimuli and on the previously demonstrated relationship between emotion control scores and genetic modulation of dopaminergic signaling (Blasi et al., 2009b).
RESULTS
Demographics and behavioral performance
There were 24 DAT 3′VNTR 10-10 individuals, 26 9-10, 11 9-9. Genotypes displayed Hardy–Weinberg equilibrium (χ2 = 0.7, P = 0.4). Because of the relatively small number of 9-repeat homozygous subjects and consistent with earlier studies as well as with the known distribution of these two alleles in the population (Szekeres et al., 2004; Bertolino et al., 2006; Gilbert et al., 2006; Scott et al., 2006; Laucht et al., 2007; Bertolino et al., 2008; Bertolino et al., 2009), we grouped all subjects carrying at least one 9-repeat allele (9-repeat carriers—9-car—, N = 37) for further analyses.
Based on the PBI cut-off score (Parker, 1979; Parker and Lipscombe, 1979), there were 38 high MC (HMC) and 23 low MC (LMC) individuals. This distribution allowed behavioral and imaging analyses on the following four groups: HMC 10-10 (N = 12), LMC 10-10 (N = 12), HMC 9-car (N = 26) and LMC 9-car (N = 11) (Table1). Groups were matched in terms of gender (χ2 = 3.36, P = 0.76), age, handedness, socio-economical status and IQ (all P > 0.4). Moreover, groups were matched for SLC6A4 5-HTTLPR distribution (χ2 = 7.3; P = 0.3), as well as for BFQ and in number of SLE scores (all P > 0.4, see Supplementary Table 1).
Table 1.
Demographics of the subjects included in the study
| HMC – DAT 10/10 | HMC – DAT 9car |
|---|---|
| n = 12 (3 males) | n = 26 (9 males) |
| age = 25.6 ± 5.2 years | age = 24 ± 5.2 years |
| Hollingshead = 40 ± 21.5 | Hollingshead = 38.7 ± 15.8 |
| IQ = 108.3 ± 10.8 | IQ = 109.3 ± 12.5 |
| HMC – DAT 10/10 | HMC – DAT 9car |
|---|---|
| n = 12 (2 males) | n = 11 (5 males) |
| age = 23 ± 2.8 years | age = 22.3 ± 2.05 years |
| Hollingshead = 45.3 ± 10.2 | Hollingshead = 31.8 ± 17.6 |
| IQ = 111.6 ± 7.4 | IQ = 105.8 ± 15.7 |
Accuracy data at the fMRI task did not indicate significant effects (all P > 0.3). RT data revealed a main effect of facial expression (slower responses to angry than fearful faces; F1,60 = 8.05; P = 0.006), no main effect of DAT 3′VNTR genotype, no main effect of MC (all P > 0.3), and an interaction between facial expression and MC (F1,59 = 4.56; P = 0.03). Post-hoc analysis with t-test revealed that only subjects with HMC are faster when processing fearful relative to angry faces (P = 0.002, Figure 1). No other significant interaction was found (all P > 0.3). In other words, the quality of facial aversive stimuli did not affect behavioral RT in subjects with LMC.
Fig. 1.
Means ± 0.95 confidence interval indicating the interaction between facial expression and MC on RTs. Although subjects with HMC have faster RTs during processing of fearful compared with angry faces, subjects with LMC do not present this difference. See text for statistics.
fMRI
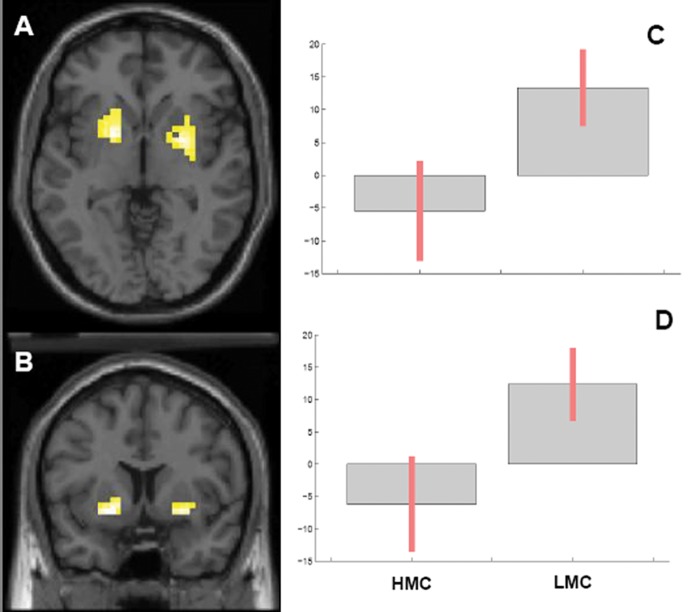
ANOVA indicated a main effect of facial expression in left amygdala, left IFG and bilateral striatum, with greater activity for angry relative to fearful faces. Furthermore, there was a main effect of MC in bilateral striatum, including the left putamen and bilateral caudate, with greater activity in subjects with LMC (Figure 2). No significant main effect of 3′VNTR DAT genotype was found in these brain regions. However, a three-way interaction between MC, DAT genotype and facial expression was identified in left IFG (Figure 3). Fisher’s post-hoc test on BOLD responses extracted from this cluster revealed that subjects with LMC have greater activity than those with HMC in the context of 10/10 genotype only and during processing of fearful faces only (Post-hoc Fisher’s P = 0.04, Figure 3). No other significant interaction was found. Tailairach coordinates are reported in Table 2. To further evaluate the specificity of the results for negative emotions, we performed follow-up analyses. ANOVA on IFG BOLD response adding happy faces as another repeated measure did not modify the results. Moreover, adding happy faces to the baseline in the SPM statistical model produced similar results, although with reduced statistical significance (See Supplementary Materials).
Fig. 2.
Main effect of MC on BOLD fMRI responses averaged between angry-neutral and fearful neutral contrast in bilateral caudate and left putamen, where subjects with LMC have greater activity. (A) Axial section; (B) Coronal section; (C) Parameter estimates extracted from the cluster with Talairach coordinates, x = −19, y = 7; z = −7; (D) Parameter estimates extracted from the cluster with Talairach coordinates, x = 26, y = 4; z = −7.
Fig. 3.
(A) Rendered image of the brain showing the cluster in left IFG with a three-way interaction between MC, DAT genotype and facial expression (BA 45; x = −56; y = 26; z = 6). See text for statistics. (B) BOLD response extracted from the IFG cluster depicted in (A). X-axis: DAT 3′ VNTR genotype for each emotional stimulus investigated (i.e. fearful and angry faces). The bars represent 0.95 confidence interval.
Table 2.
Table showing local maxima of the main effect of emotion (post-hoc analysis: angry > fearful), of MC (post-hoc analysis: LMC > HMC) and of the interaction between MC, DAT 3′VNTR genotype and emotion (post-hoc analysis LMC 10-10 individuals > HMC 10-10 individuals during processing of fearful faces)
| Brain region | BA | Coordinates |
k | Z | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Main effect of emotion(angry > fearful) | ||||||
| Left ifg | 46 | −53 | 44 | 5 | 60 | 3.22 |
| 47 | −45 | 47 | −6 | |||
| Left amygdala | −23 | 3 | −19 | 9 | 3.93 | |
| Left caudate body | −15 | 5 | 24 | 25 | 2.92 | |
| Right caudate body | 15 | −3 | 21 | 9 | 3.05 | |
| Main effect of mc (LMC > HMC) | ||||||
| Left putamen | −19 | 7 | −7 | 27 | 3.23 | |
| Left caudate body | −15 | 18 | −1 | 18 | 3.08 | |
| Right caudate body | 26 | 4 | −7 | 36 | 3.13 | |
| Emotion by DAT 3′VNTR by mc interaction | ||||||
| Left ifg | 45 | 56 | 26 | 6 | 6 | 3.08 |
Psychophysiological interaction
PPI was performed using as seed the left IFG cluster of activity in which the three-way interaction was identified. ANOVA indicated no effect of facial expression, MC or DAT 3′VNTR on IFG functional connectivity. However, there was a significant interaction between DAT 3′VNTR genotype and MC on functional connectivity between IFG and left amygdala (left amygdala: x = −30, y = −4, z = −22; Figure 4). Post-hoc analysis with Fisher’s test on functional connectivity values revealed greater IFG-amygdala functional connectivity in LMC 10-10 subjects relative to LMC 9-car (Fisher’s P = 0.007) and to HMC 10-10 subjects (Fisher’s P = 0.005) as well as in HMC 9-car relative to LMC 9-car (P = 0.015) and HMC 10-10 (P = 0.009) subjects (Figure 3). No other significant interaction was found.
Fig. 4.
Coronal (A) and axial (B) sections of the brain showing the cluster in left amygdala (x = −30, y = −4, z = −22) where an interaction between DAT 3′VNTR genotype and MC was found on PPI with the IFG. (C) PPI values extracted from the cluster in left amygdala shown in (A) and (B). The bars represent 0.95 confidence interval.
Correlations
There was a statistically significant negative correlation between emotion control scores and IFG BOLD responses during processing of fearful faces (r = −0.66, P = 0.04) in LMC 10-10 subjects (Figure 5). This correlation was not significant in HMC 10-10 individuals (r = −0.01, P = 0.98). Furthermore, a sign test indicated that there was a strong statistical trend for a difference between coefficients of these correlations (P = 0.07).
Fig. 5.
Scatterplot showing a negative correlation between BOLD signal change in IFG and BFQ emotion control scores in LMC subjects. See text for statistics.
DISCUSSION
The present results indicate that the interaction between self-reported MC scores and 3′VNTR DAT genotype is associated with differential brain activity and connectivity during implicit processing of aversive facial stimuli. More specifically, HMC subjects were faster when processing fearful relative to angry faces while no difference was evident in LMC subjects. LMC subjects had greater activity in striatum including its ventral portion relative to HMC individuals during emotion processing. Furthermore, the interaction between emotion processing, DAT 3′VNTR genotype and MC was associated with activity in left IFG, where HMC subjects homozygous for the 10-repeat allele have lower activity during processing of fearful faces. Finally, left IFG-left amygdala functional connectivity during emotional processing was also modulated by the interaction between MC and DAT 3′VNTR genotype demonstrating a clear double dissociation. All together, these results suggest that genetic and environmental factors such as DAT 3′VNTR genotype and MC interact in modulating behavior and brain physiology during emotion processing in healthy humans.
We found a specific effect of perceived MC on striatal activity. LMC subjects have greater BOLD responses in striatum including its ventral portion during evaluation of aversive emotional stimuli. Previous evidence suggests that the ventral striatum may be selectively involved in processing signals of anger (Calder et al., 2004) and that LMC is associated with greater DA release in basal ganglia during stressful situations (Pruessner et al., 2004). Furthermore, stress associated with perception of qualitatively and quantitatively different pain is positively correlated with DA levels in this brain region (Scott et al., 2006). Our findings of greater striatal activity in LMC subjects, possibly driven by greater DA signaling, are in line with these earlier studies and/or more generalized reactivity to these stimuli. However, a more parsimonious interpretation of the difference in striatal activity is that LMC subjects tend to over-react to emotional stimuli. This suggestion is consistent with a large body of literature suggesting that LMC is related to greater anxiety and reactivity to emotional stimuli (Liu et al., 1997; Francis et al., 1999; Meaney, 2001).
The interaction between genotype, MC and emotion processing on activity in IFG is also consistent with earlier studies (Huettel et al., 2005; Vickery and Jiang, 2008) that suggested the involvement of DA in processing of fear (Fadok et al., 2009) and with others indicating association of genetic modulation of DA signaling with brain responses to fearful faces (Williams et al., 2010). A possible explanation of this interaction is based on the relationship between both these genetic and environmental factors with DA (Vandenbergh et al., 1992; Pruessner et al., 2004; Bertolino et al., 2006; Bertolino et al., 2008). In particular, previous studies have indicated that greater MC in humans predicts lower DA release during stress (Pruessner et al., 2004). This effect may be more strongly elicited on a specific genetic background, i.e. the DAT 10 allele, which has been previously associated with greater expression of DAT relative to the 9 allele (Vandenbergh et al., 1992) thus providing greater reuptake and reduced extracellular levels of DA. Therefore, a possible interpretation of the present results is that lower left IFG activity during emotion processing in HMC 10-10 individuals is because of lower DA signaling associated with the interaction between these genetic and environmental factors. Moreover, BOLD responses in IFG of LMC 10-10 subjects correlates with emotional control scores suggesting that greater activity in these individuals during fearful faces reflects less efficient indices of emotional behavior. A potential caveat of this interpretation is that not all studies have associated the 10-repeat allele with greater DAT expression (Pinsonneault et al., 2011). A more detailed and specific explanation of these findings requires future studies to address the molecular mechanisms determining this interaction.
In line with previous studies on emotion processing indicating that DA is released in IFG and in amygdala (Badgaiyan et al., 2009) possibly modulating their functional connectivity (Rosenkranz and Grace, 2002; Drabant et al., 2006), the impact of MC and DAT genotype on DA signaling may also be invoked to interpret at least part of their association with differential functional connectivity between left IFG and left amygdala. In particular, LMC 10-10 individuals and HMC 9-car had greater functional connectivity between these brain regions relative to the other two groups of subjects demonstrating a clear double dissociation. These results are consistent with previous literature demonstrating DA-mediated regulation of neural processing in frontal cortex. In particular, several previous studies have indicated that optimal levels of DA signaling are associated with more efficient processing of environmental stimuli (Meyer-Lindenberg and Weinberger, 2006). DAT 3′VNTR genotype/MC configurations possibly associated with lower (HMC 10-10) or with greater (LMC 9-car) dopaminergic tone are those with lower PPI values. In these subjects, DA levels may be less than optimal, with associated reduced prefronto–amygdala functional connectivity.
A possible limitation of this study could be the use of perceived retrospective MC measures which could reflect the subjects' false memories or even their current interpretation of the type of care they received. However, previous literature has indicated that PBI scores are stable over time (Gotlib et al., 1988; Wilhelm et al., 2005), suggesting that they are a reliable measure of perceived MC, at least in terms of stability.
Despite this limitation about the interpretation, our findings suggest that genetic and environmental factors modulate brain activity and behavior during emotion processing in humans. Future studies addressing the contribution of the interaction between DAT 3′VNTR genotype and MC to the pathophysiology of brain disorders are warranted.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Acknowledgments
We thank Riccarda Lomuscio, BA, Rita Masellis, BA and Grazia Caforio, MD, for their help with data acquisition, and all people who participated to this study.
REFERENCES
- Adolphs R, Damasio H, Tranel D, Damasio AR. Cortical systems for the recognition of emotion in facial expressions. Journal of Neuroscience. 1996;16:7678–87. doi: 10.1523/JNEUROSCI.16-23-07678.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badgaiyan RD, Fischman AJ, Alpert NM. Dopamine release during human emotional processing. Neuroimage. 2009;47:2041–5. doi: 10.1016/j.neuroimage.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels M, Saviouk V, de Moor MH, et al. Heritability and genome-wide linkage scan of subjective happiness. Twin Research and Human Genetics. 2010;13:135–42. doi: 10.1375/twin.13.2.135. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Arciero G, Rubino V, et al. Variation of human amygdala response during threatening stimuli as a function of 5'HTTLPR genotype and personality style. Biological Psychiatry. 2005;57:1517–25. doi: 10.1016/j.biopsych.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Blasi G, Latorre V, et al. Additive effects of genetic variation in dopamine regulating genes on working memory cortical activity in human brain. Journal of Neuroscience. 2006;26:3918–22. doi: 10.1523/JNEUROSCI.4975-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Fazio L, Di Giorgio A, et al. Genetically determined interaction between the dopamine transporter and the D2 receptor on prefronto-striatal activity and volume in humans. Journal of Neuroscience. 2009;29:1224–34. doi: 10.1523/JNEUROSCI.4858-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Di Giorgio A, Blasi G, et al. Epistasis between dopamine regulating genes identifies a nonlinear response of the human hippocampus during memory tasks. Biological Psychiatry. 2008;64:226–34. doi: 10.1016/j.biopsych.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Blasi G, Hariri AR, Alce G, et al. Preferential amygdala reactivity to the negative assessment of neutral faces. Biological Psychiatry. 2009a;66:847–53. doi: 10.1016/j.biopsych.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi G, Lo Bianco L, Taurisano P, et al. Functional variation of the dopamine D2 receptor gene is associated with emotional control as well as brain activity and connectivity during emotion processing in humans. Journal of Neuroscience. 2009b;29:14812–9. doi: 10.1523/JNEUROSCI.3609-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Lord C, Wadiwalla M, et al. Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. Journal of Neuroscience. 2007;27:2592–5. doi: 10.1523/JNEUROSCI.3252-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes EM, Rigero BA, Bridges RS. Dopamine antagonists during parturition disrupt maternal care and the retention of maternal behavior in rats. Pharmacology Biochemistry and Behaviour. 2002;73:869–75. doi: 10.1016/s0091-3057(02)00941-3. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Keane J, Lawrence AD, Manes F. Impaired recognition of anger following damage to the ventral striatum. Brain. 2004;127:1958–69. doi: 10.1093/brain/awh214. [DOI] [PubMed] [Google Scholar]
- Caprara GV, Barbaranelli C, Borgogni L. BFQ Big Five Questionnaire. OS Firenze; 1993. [Google Scholar]
- Caspi A, Moffitt TE, Newman DL, Silva PA. Behavioral observations at age 3 years predict adult psychiatric disorders. Longitudinal evidence from a birth cohort. Archives of General Psychiatry. 1996;53:1033–9. doi: 10.1001/archpsyc.1996.01830110071009. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Cassidy J, Parke RD, Butkovsky L, Braungart JM. Family-peer connections: the roles of emotional expressiveness within the family and children's understanding of emotions. Child Development. 1992;63:603–18. doi: 10.1111/j.1467-8624.1992.tb01649.x. [DOI] [PubMed] [Google Scholar]
- Cragg SJ, Rice ME. DAncing past the DAT at a DA synapse. Trends in Neurosciences. 2004;27:270–7. doi: 10.1016/j.tins.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Domes G, Schulze L, Herpertz SC. Emotion recognition in borderline personality disorder-a review of the literature. Journal of Personality Disorders. 2009;23:6–19. doi: 10.1521/pedi.2009.23.1.6. [DOI] [PubMed] [Google Scholar]
- Drabant EM, Hariri AR, Meyer-Lindenberg A, et al. Catechol O-methyltransferase val158met genotype and neural mechanisms related to affective arousal and regulation. Archives of General Psychiatry. 2006;63:1396–406. doi: 10.1001/archpsyc.63.12.1396. [DOI] [PubMed] [Google Scholar]
- Engert V, Joober R, Meaney MJ, Hellhammer DH, Pruessner JC. Behavioral response to methylphenidate challenge: influence of early life parental care. Developmental Psychobiology. 2009;51:408–16. doi: 10.1002/dev.20380. [DOI] [PubMed] [Google Scholar]
- Fadok JP, Dickerson TM, Palmiter RD. Dopamine is necessary for cue-dependent fear conditioning. Journal of Neuroscience. 2009;29:11089–97. doi: 10.1523/JNEUROSCI.1616-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–8. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Francis DD, Szegda K, Campbell G, Martin WD, Insel TR. Epigenetic sources of behavioral differences in mice. Nature Neuroscience. 2003;6:445–6. doi: 10.1038/nn1038. [DOI] [PubMed] [Google Scholar]
- Freedman D, Thornton A, Camburn D, Alwin D, Young-demarco L. The life history calendar: a technique for collecting retrospective data. Sociological Methodology. 1988;18:37–68. [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Zarahn E, Josephs O, Henson RN, Dale AM. Stochastic designs in event-related fMRI. Neuroimage. 1999;10:607–19. doi: 10.1006/nimg.1999.0498. [DOI] [PubMed] [Google Scholar]
- Fuke S, Suo S, Takahashi N, Koike H, Sasagawa N, Ishiura S. The VNTR polymorphism of the human dopamine transporter (DAT1) gene affects gene expression. Pharmacogenomics Journal. 2001;1:152–6. doi: 10.1038/sj.tpj.6500026. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, et al. Laterality effect on emotional faces processing: ALE meta-analysis of evidence. Neuroscience Letters. 2009a;452:262–7. doi: 10.1016/j.neulet.2009.01.065. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, et al. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. Journal of Psychiatry and Neuroscience. 2009b;34:418–32. [PMC free article] [PubMed] [Google Scholar]
- Gatt JM, Nemeroff CB, Schofield PR, et al. Early life stress combined with serotonin 3A receptor and brain-derived neurotrophic factor valine 66 to methionine genotypes impacts emotional brain and arousal correlates of risk for depression. Biological Psychiatry. 2010a;68:818–824. doi: 10.1016/j.biopsych.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Gatt JM, Williams LM, Schofield PR, et al. Impact of the HTR3A gene with early life trauma on emotional brain networks and depressed mood. Depression and Anxiety. 2010b;27:752–9. doi: 10.1002/da.20726. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gilbert DL, Wang Z, Sallee FR, et al. Dopamine transporter genotype influences the physiological response to medication in ADHD. Brain. 2006;129:2038–46. doi: 10.1093/brain/awl147. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Mount JH, Cordy NI, Whiffen VE. Depression and perceptions of early parenting: a longitudinal investigation. British Journal of Psychiatry. 1988;152:24–27. doi: 10.1192/bjp.152.1.24. [DOI] [PubMed] [Google Scholar]
- Grace AA, Rosenkranz JA. Regulation of conditioned responses of basolateral amygdala neurons. Physiology and Behaviour. 2002;77:489–93. doi: 10.1016/s0031-9384(02)00909-5. [DOI] [PubMed] [Google Scholar]
- Heinz A, Goldman D, Jones DW, et al. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22:133–9. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Song AW, McCarthy G. Decisions under uncertainty: probabilistic context influences activation of prefrontal and parietal cortices. Journal of Neuroscience. 2005;25:3304–11. doi: 10.1523/JNEUROSCI.5070-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunnerkopf R, Strobel A, Gutknecht L, Brocke B, Lesch KP. Interaction between BDNF Val66Met and dopamine transporter gene variation influences anxiety-related traits. Neuropsychopharmacology. 2007;32:2552–60. doi: 10.1038/sj.npp.1301383. [DOI] [PubMed] [Google Scholar]
- Ishai A, Schmidt CF, Boesiger P. Face perception is mediated by a distributed cortical network. Brain Research Bulletin. 2005;67:87–93. doi: 10.1016/j.brainresbull.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Karreman M, Moghaddam B. The prefrontal cortex regulates the basal release of dopamine in the limbic striatum: an effect mediated by ventral tegmental area. Journal of Neurochemistry. 1996;66:589–98. doi: 10.1046/j.1471-4159.1996.66020589.x. [DOI] [PubMed] [Google Scholar]
- Kienast T, Hariri AR, Schlagenhauf F, et al. Dopamine in amygdala gates limbic processing of aversive stimuli in humans. Nature Neuroscience. 2008;11:1381–2. doi: 10.1038/nn.2222. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Laucht M, Skowronek MH, Becker K, et al. Interacting effects of the dopamine transporter gene and psychosocial adversity on attention-deficit/hyperactivity disorder symptoms among 15-year-olds from a high-risk community sample. Archives of General Psychiatry. 2007;64:585–90. doi: 10.1001/archpsyc.64.5.585. [DOI] [PubMed] [Google Scholar]
- Lee SS, Chronis-Tuscano A, Keenan K, et al. Association of maternal dopamine transporter genotype with negative parenting: evidence for gene x environment interaction with child disruptive behavior. Molecular Psychiatry. 2010;15:548–58. doi: 10.1038/mp.2008.102. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Melchitzky DS, Sesack SR, Whitehead RE, Auh S, Sampson A. Dopamine transporter immunoreactivity in monkey cerebral cortex: regional, laminar, and ultrastructural localization. Journal of Comparative Neurology. 2001;432:119–36. doi: 10.1002/cne.1092. [DOI] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–62. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Ljungberg T, Apicella P, Schultz W. Responses of monkey dopamine neurons during learning of behavioral reactions. Journal of Neurophysiology. 1992;67:145–63. doi: 10.1152/jn.1992.67.1.145. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Review of Neuroscience. 2001;24:1161–92. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Menon M, Jensen J, Vitcu I, et al. Temporal difference modeling of the blood-oxygen level dependent response during aversive conditioning in humans: effects of dopaminergic modulation. Biological Psychiatry. 2007;62:765–72. doi: 10.1016/j.biopsych.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nature Reviews Neuroscience. 2006;7:818–27. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Mill J, Asherson P, Browes C, D'Souza U, Craig I. Expression of the dopamine transporter gene is regulated by the 3' UTR VNTR: evidence from brain and lymphocytes using quantitative RT-PCR. American Journal of Medical Genetics. 2002;114:975–9. doi: 10.1002/ajmg.b.10948. [DOI] [PubMed] [Google Scholar]
- Miller SM, Lonstein JS. Dopamine D1 and D2 receptor antagonism in the preoptic area produces different effects on maternal behavior in lactating rats. Behavioral Neuroscience. 2005;119:1072–83. doi: 10.1037/0735-7044.119.4.1072. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Pliakou N, et al. The effects of D1 or D2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats. Behavioral Neuroscience. 2005;119:1588–1604. doi: 10.1037/0735-7044.119.6.1588. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wong DF, Zhou Y, et al. Impulsivity and chronic stress are associated with amphetamine-induced striatal dopamine release. Neuroimage. 2007;36:153–66. doi: 10.1016/j.neuroimage.2007.01.055. [DOI] [PubMed] [Google Scholar]
- Pani L, Porcella A, Gessa GL. The role of stress in the pathophysiology of the dopaminergic system. Molecular Psychiatry. 2000;5:14–21. doi: 10.1038/sj.mp.4000589. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Emotional endophenotypes in evolutionary psychiatry. Progress in Neuro-Psychopharmacology Biological Psychiatry. 2006;30:774–84. doi: 10.1016/j.pnpbp.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Parker G, editor. Parental Overprotection: A Risk Factor in Psychosocial Development. New York: Grune & Stratton; 1983. [Google Scholar]
- Parker G. The Parental Bonding Instrument: psychometric properties reviewed. Psychiatric Developments. 1989;7:317–35. [PubMed] [Google Scholar]
- Parker G. The Parental Bonding Instrument. A decade of research. Social Psychiatry and Psychiatric Epidemiology. 1990;25:281–2. doi: 10.1007/BF00782881. [DOI] [PubMed] [Google Scholar]
- Parker G, Lipscombe P. Parental characteristics of Jews and Greeks in Australia. Australian and New Zealand Journal of Psychiatry. 1979;13:225–9. doi: 10.3109/00048677909159140. [DOI] [PubMed] [Google Scholar]
- Parker G, Tupling H, Brown LB. A parental bonding instrument. British Journal of Medical Psychology. 1979;52:1–10. [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48:175–87. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Phillips ML. Understanding the neurobiology of emotion perception: implications for psychiatry. British Journal of Psychiatry. 2003;182:190–2. doi: 10.1192/bjp.182.3.190. [DOI] [PubMed] [Google Scholar]
- Pinsonneault JK, Han DD, Burdick KE, Kataki M, et al. Dopamine transporter gene variant affecting expression in human brain is associated with bipolar disorder. Neuropsychopharmacology. 2011;36:1644–55. doi: 10.1038/npp.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prata DP, Mechelli A, Fu CH, et al. Epistasis between the DAT 3' UTR VNTR and the COMT Val158Met SNP on cortical function in healthy subjects and patients with schizophrenia. Proceedings of the National Acadamy of Sciences of the United States of America. 2009a;106:13600–5. doi: 10.1073/pnas.0903007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prata DP, Mechelli A, Picchioni MM, et al. Altered effect of dopamine transporter 3'UTR VNTR genotype on prefrontal and striatal function in schizophrenia. Archives of General Psychiatry. 2009b;66:1162–72. doi: 10.1001/archgenpsychiatry.2009.147. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [11C]raclopride. Journal of Neuroscience. 2004;24:2825–31. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Pruessner M, et al. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations - 2008 Curt Richter Award Winner. Psychoneuroendocrinology. 2010;35:179–91. doi: 10.1016/j.psyneuen.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Raikes HA, Thompson RA. Attachment security and parenting quality predict children's problem-solving, attributions, and loneliness with peers. Attachment and Human Development. 2008;10:319–44. doi: 10.1080/14616730802113620. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning. Nature. 2002;417:282–7. doi: 10.1038/417282a. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Heitzeg MM, Koeppe RA, Stohler CS, Zubieta JK. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. Journal of Neuroscience. 2006;26:10789–95. doi: 10.1523/JNEUROSCI.2577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. Journal of Neuroscience. 1998;18:2697–708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–6. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres G, Keri S, Juhasz A, et al. Role of dopamine D3 receptor (DRD3) and dopamine transporter (DAT) polymorphism in cognitive dysfunctions and therapeutic response to atypical antipsychotics in patients with schizophrenia. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2004;124B:1–5. doi: 10.1002/ajmg.b.20045. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168:242–9. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautmann SA, Fehr T, Herrmann M. Emotions in motion: dynamic compared to static facial expressions of disgust and happiness reveal more widespread emotion-specific activations. Brain Research. 2009;1284:100–15. doi: 10.1016/j.brainres.2009.05.075. [DOI] [PubMed] [Google Scholar]
- van Dyck CH, Malison RT, Jacobsen LK, et al. Increased dopamine transporter availability associated with the 9-repeat allele of the SLC6A3 gene. Journal of Nuclear Medicine. 2005;46:745–51. [PubMed] [Google Scholar]
- Vandenbergh DJ, Persico AM, Hawkins AL, et al. Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics. 1992;14:1104–6. doi: 10.1016/s0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- VanNess SH, Owens MJ, Kilts CD. The variable number of tandem repeats element in DAT1 regulates in vitro dopamine transporter density. BMC Genetics. 2005;6:55. doi: 10.1186/1471-2156-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickery TJ, Jiang YV. Inferior parietal lobule supports decision making under uncertainty in humans. Cerebral Cortex. 2009;19:916–25. doi: 10.1093/cercor/bhn140. [DOI] [PubMed] [Google Scholar]
- Wilhelm K, Niven H, Parker G, Hadzi-Pavlovic D. The stability of the Parental Bonding Instrument over a 20-year period. Psychological Medicine. 2005;35:387–93. doi: 10.1017/s0033291704003538. [DOI] [PubMed] [Google Scholar]
- Williams LM, Gatt JM, Grieve SM, et al. COMT Val(108/158)Met polymorphism effects on emotional brain function and negativity bias. Neuroimage. 2010;53:918–25. doi: 10.1016/j.neuroimage.2010.01.084. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]