Abstract
Aging is characterized by a progressive decline in cardiac function, but endurance exercise training has been shown to retard a number of deleterious effects of aging. However, underlying mechanisms by which exercise training improves age-related decrements in myocardial contractile function are not well understood. The purpose of this study was to determine the effects of exercise training on power output properties in permeablized (skinned) myocytes of old rats. Thirty-month-old rats were divided into sedentary control (C) and groups undergoing 11 weeks of treadmill exercise training at moderate intensity (MI) and at high intensity (HI). Peak power output normalized to maximal force was significantly increased in MI but not in HI compared to C with significant increases in atrial myosin light chain 1 in ventricle. These results suggest that MI exercise training is beneficial as a significant increase was seen in the ability of the myocardium to do work, but this effect was not seen with HI training.
Key Words: Aging, Moderate-intensity exercise training, High-intensity exercise training, Force–velocity, force–power, myosin heavy chain, myosin light chain 1, Aging, Aging
Exercise training has been prescribed for the prevention of cardiac disease as well as age-associated cardiac dysfunction (1,2). Favorable effects of exercise are seen in several parameters of cardiac function with age (3–5). For example, age-related prolongation of isometric contraction duration (5,6) and slowed early left ventricular filling during diastole (3) were significantly attenuated after training. In addition, some studies (5,7), but not others (4), demonstrated that time-to-peak tension development in isolated papillary muscle or trabeculae was significantly decreased in response to exercise training.
Most studies have demonstrated that maximal isometric tension and the maximum rate of tension generation (+dP/dt) are unaffected either by aging (4,7–10) or exercise training (4,7,11). However, these properties of isometric tension development are not closely related to the ability of the ventricle to eject blood. At the cellular level, this ability to eject blood during systole is mainly regulated by the properties of shortening in the myocardium against load (loaded shortening velocity). These properties are described by the force–velocity relationship and by the force–power relationship in the myocardium. We previously demonstrated that power output is decreased in cardiac myocytes from 33-month-old Fisher 344 × Brown Norway (F344BN) F1 hybrid rats compared with 9 month young adults (12). In addition, we have previously demonstrated that, in young rats, exercise training was capable of altering the force–velocity properties and increasing power output of myocytes (13). However, it is not known if exercise training is capable of minimizing the decrease in power output in myocytes from aged rats. Thus, the objective of this study was to determine whether endurance exercise training can attenuate the age-associated decline in power output properties seen in the myocardium of old rats.
One major difficulty in investigating the mechanisms of exercise effects in old animals is determining the proper exercise training intensity. Previous studies demonstrate that the extent of cardiac adaptation often depends on the exercise intensity used. For example, high-intensity (HI) training yields substantially larger effects on maximal oxygen consumption (VO2max), cardiac hypertrophy, cardiomyocyte contractility, and Ca2+ handling than moderate-intensity (MI) treadmill training in young rats (14,15). It is known that exercise capacity decreases with advanced age, and thus older animals may not be able to sustain a similar exercise intensity as that used to induce training adaptations in young animals. Low-intensity exercise training has been shown to have specific benefits such as lowering mean blood pressure and attenuating hypertension (16) and delaying the onset of heart failure and improving survival in spontaneously hypertensive rats (17). Given the relative lack of information on adaptations to exercise in rats of this age and exercise intensity dependence of cardiac adaptations in aged rats, it was of interest to determine cellular adaptations to different exercise training stimuli in the hearts of rats of advanced age. We hypothesized that higher intensity training would induce greater exercise adaptations in the heart. Thus, we measured loaded shortening and power output properties in single myocytes from 33-month-old F344BN F1 hybrid rats after 11 weeks of treadmill exercise training at two different training intensities.
Methods
Animals
Thirty-month-old male F344BN F1 hybrid rats were obtained from the National Institute on Aging colony maintained by Harlan Sprague-Dawley (Indianapolis, IN) and studied after 11 weeks of treadmill exercise training. Animals were selected to represent senescent (ie, 33 month: ≈ 50% mortality) rats at the end of training study. The rats were individually housed in clear plastic cages in a temperature (21°C) controlled animal facility maintained on a 12:12-hour light/dark reverse cycle under the care of a full-time veterinarian. The rats were fed NIH31 diet (Teklad) and water ad libitum. Body weight was measured prior to the training for 5d/wk during the first 4 weeks and 2d/wk for the remaining of the study. Food intake was monitored for 5d/wk to determine the role of food intake in any exercise training effects.
Treadmill Exercise Training
Training was started 1 week after rats’ arrival to reduce the stress associated with shipping and acclimation to a new location. The rats were matched for body weight and assigned to a sedentary control (C) or an exercise training group (T). All rats (C and T) were acclimated to the treadmill for 1-week period. The rats were trained between 11:00 amand 1:00 pm., approximately 5 hours after dark cycle started. Starting the second week, the C rats were placed on the treadmill for 5min/d once or twice per week. This was done to reduce the handling stress for the C animals on the day of sacrifice, as well as to control for the effects of daily animal handling. Rats assigned to the T group were run on the treadmill 5d/wk for 11 weeks. All training sessions consisted of 5-minute warm-up at 8 m/min and were then followed by a given training intensity. The duration and intensity were initially 5min/d at a speed of 3 m/min at a 5% grade, and progressively increased during the first 7 weeks. At 7 weeks, the intensity, duration, and grade reached 13 m/min,35min/d, and 10% grade, respectively. At 8 weeks (32 months of age), the T group was divided into two groups and these two groups were trained at different intensities for the remainder of the study. The HI rats continued at the same workload (13 m/min for 35min/d at 10% grade), whereas the MI rats ran at a decreased exercise intensity (8 m/min for 20min/d at 10% grade) for the last 4 weeks. Three out of the original 19 rats were excluded from the study due to injury or growth of tumors. Therefore, 16 rats (MI: n = 5, HI: n = 5, and C; n = 6) were used for the results. Handling and euthanasia were carried out under the guidelines of University of Wisconsin-Madison Animal Use and Care Committee.
There was at least a 48-hour rest between the last exercise session and the sacrifice in order to avoid the influence of any acute exercise effect. The rats were anesthetized by inhalation of halothane, and the hearts were quickly excised. The heart was placed in ice-cold Ca2+-free relaxing solution, trimmed of atria, connective tissue, and vascular tissue. The ventricles were weighed and then separated into the left and right ventricle. Left ventricles were quickly frozen and stored at −80°C for subsequent analysis.
Cardiac Myocyte Preparation, Force–Velocity and Force–Power Measurement
The experimental apparatus and the solutions used for contractile measurements on skinned myocytes preparations have been described previously (18). The protocol for force–velocity and force–power measurements was modified from previous methods (13). Briefly, all mechanical measurements were done at 15°C. Sarcomere length was set to 2.3 µm in relaxing solution, and it was monitored throughout the experiment to determine that it did not change significantly during activation using video microscopy. The shortening velocity of skinned myocytes was determined at varied loads. The myocytes were transferred into activating solution (pCa 4.5) and steady tension was allowed to develop. The myocytes were rapidly stepped to a specified force, which was maintained for 250ms while changes in myocytes length were monitored (Figure 1A and B). Following the force clamp, the myocyte was rapidly slackened to reduce force close to zero and re-extended to its initial length. Ten to thirteen different force levels were carried out on given myocytes. Force was expressed normalized to the peak force generated by that cell during a given activation (P/P o). If the maximal force declined below 80% of the initial maximal force during the experimental protocol, then that cell was discarded and the data were not used.
Figure 1.

Force (A) and length (B) traces from three force-clamp experiments in a representative myocyte. After steady tension had developed in maximally activating solution (pCa 4.5), the servomotor was switched to force-control mode, and the force was stepped down to a preselected value, in this example, maximum isometric forces of 0.8, 0.5, and 0.3 of P o. Length changes during isotonic shortening at each load were fit by using linear regression, with the slope of the line taken as the velocity of shortening for that load. (C) Force–velocity curve in a single myocyte resulting from force-clamp experiments illustrated in A and B. A total of nine force-clamp measurements were done for this myocytes (•). Force and velocity data during the clamp were plotted and then these points were fit to the Hill equation (line).
Analysis of Myosin Heavy-Chain Isoform Content
The relative amount of myosin heavy-chain (MHC) isoform of ventricular homogenates was determined with sodium dodecyl sulfate–polyacrylamide gel electrophoresis technique as described previously (19).
Analysis of Myosin Light-Chain Isoform Content
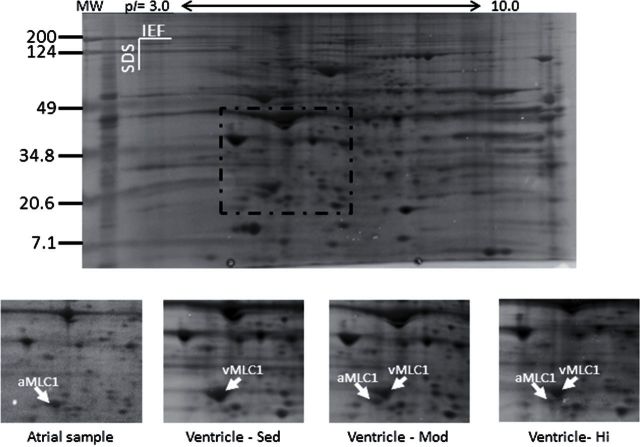
We analyzed the relative amount of atrial versus ventricular myosin light chain 1 (MLC1) in heart homogenates by using two-dimensional gel electrophoresis. This method has been used previously to separate the ventricular and atrial isoforms of MLC1 in human (20,21), porcine (22), and rat (23,24) myocardium. Frozen tissue was homogenized at 100mg/mL in sample buffer (8M urea, 2M thiourea, 75mM dithiothreitol, and 10mM Tris, pH 7.0). The protein concentration of this homogenate was determined by using a Bio-Rad protein assay kit with bovine serum albumin as the standard. Isoelectric focusing was performed by using Bio-Rad’s Protean IEF cell and 11-cm precast IPG (immobilized pH gradient) gel strips (pH 3–10). Three hundred micrograms of protein in sample buffer plus 1.85% CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate) and 0.185% carrier ampholytes were loaded onto the strips via 1 hour of passive rehydration and 12 hour of active loading at 50V and 20°C. The Bio-Rad IEF unit was programmed to rapidly ramp to 250V for the first 15 minutes, rapidly ramp to 6,700–7,000V for the next 2.5 hours (limited to 50 mA/strip), and hold at peak voltage and 50 mA/strip for 35,000 V-h. Strips were held at 500V at the conclusion of their run until removed from the power unit. Strips were incubated in equilibration buffers I (125mM Tris HCl, pH 6.8, 20% glycerol, 2% sodium dodecyl sulfate, 6M urea, and 2% dithiothreitol) and II (Tris HCl, pH 6.8, 20% glycerol, 2% sodium dodecyl sulfate, 6M urea, and 2.5% iodoacetamide) for 20 minutes each. After equilibration of the strips, second dimension polyacrylamide gel electrophoresis was performed by using 12.5% Bio-Rad Criterion precast gels with IPG 1 well combs, run at 20 mA/gel for 45 minutes and 30 mA/gel for the duration of the run (2.5 hour total). Gels were stained by using a zinc stain (Pierce) and digitized by using a Kodak image station 440CF. Sixteen exposures were summed to increase the signal-to-noise ratio. PDQuest software (Bio-Rad, version 6.0) was used for gel image analysis. Identification of spots corresponding to atrial myosin light chain 1 (aMLC1) and ventricular myosin light chain 1 (vMLC1) were based on the predicted isoelectric point and molecular weight information as well as by comparison with previously published two-dimensional gel analysis of these proteins (21) and our own previous results using these same methods (23,24). The predicted isoelectric point and molecular weight of rat aMLC1 is 4.97 and 21150.99 respectively, whereas the isoelectric point and molecular weight of vMLC1 is 5.03 and 22025.01 (http://us.expasy.org).
Data Analysis
Length traces, force–velocity curves, and force–power curves were analyzed as previously described (13). Briefly, force and velocity data were fitted to the Hill equation (25)
 |
where P is the force at velocity V, P o is the peak isometric force, and a and b are constants with dimensions of force and velocity, respectively. The maximum shortening velocity was determined from the y-intercept of the best-fit line, extrapolating a force–velocity curve based on raw data (Figure 1C). The force–power curve was then constructed as the product of force value and velocity value at each load on the force–velocity curve. The force at which power output is optimal (F opt) was obtained by using the equation (26)
 |
Once F opt was determined for each cell, we calculated the velocity at that force and used that value as V opt (the velocity at which power is optimal). Data were fit to equations using commercial software (SigmaPlot, Jandel Scientific). Peak power output in each myocyte was taken from the highest power value on the fitted line. Power output in single myocyte was normalized to cell mass using the following formula based on an elongated elliptical shape of the myocytes: muscle mass volume (V) × density (D); V = L × W 2/2667 pL−1 µm3, where V is in picoliter, L (length) and W (width) are in µm; and D = 1.065 (27). Power output was also normalized for maximal force and this value (P/P o × ML/s) was given in Table 2.
Table 2.
Mechanical Properties of Myocytes Isolated from c, mi, and hi Rats
| Rats | Passive Force(µN) | Maximal Force(kN/m2) | V max (ML/s) | a/P o | Absolute PeakPower Output(µW/mg) | Peak Power Output(normalized to maximumforce; P/P o × ML/s) | F opt (P/P o) | V opt (V/V max) |
| C (n = 45) | 0.88±0.18 | 11.58±7.39 | 0.96±0.28 | 0.20±0.10 | 2.32±1.45 | 0.069±0.008 | 0.29±0.01 | 0.016±0.06 |
| MI (n = 51) | 0.76±0.218 | 12.59±5.67 | 1.01±0.33 | 0.31±0.07* | 2.46±1.29 | 0.136±0.015* | 0.33±0.01 | 0.020±0.07 |
| HI (n = 49) | 0.81±0.17 | 10.05±6.42 | 0.88±0.23 | 0.34±0.05* | 2.69±1.85 | 0.0915±0.011 | 0.31±0.01 | 0.019±0.08 |
Notes: All mechanical measurements were done at 15°C. Values are mean ± SEM. *—Significantly different at p < .05 from C. n = number of cells; Passive force was measured in pCa 9.0 solution. Maximal force was measured in pCa 4.5 solution. ML = muscle length (measured after attachment); V max = maximal unloaded shortening velocity; a/P o = measure of the curvature of force–velocity relationship with lower values indicating a greater curve; Peak power output was the highest power determined from best-fit line; P/P o = force relative to maximum isometric force; F opt = relative force at which power output was optimal; V opt = relative velocity at which power output was optimal; C = Control; MI = moderate intensity; HI = high intensity; SEM = standard errors of means.
Statistical Analysis
All data were expressed as mean and standard errors of means. A one-way analysis of variance was used for comparing among groups. Fisher’s least-significant difference procedures were used for multiple comparisons when analysis of variance test was significant. Differences were considered significant at p < .05. Tests were performed using SPSS version 11.
Results
The effect of exercise training on physical characteristics is presented in Table 1. There was no significant difference in body weight among groups prior to exercise training. However, rats from MI and HI groups decreased body weight significantly (p .05) after 11 weeks exercise training. Over the time course (from the age of 30 to 33 months) of this study, all three groups decreased body weight, but in trained groups (both MI and HI), the decline was much steeper than control. Significantly decreased body weight in both exercise groups are partially due to their lower food intake, especially during the period when the training intensity was gradually increased. However, food intake was not significantly different between MI and C during the last 4 weeks when the intensity of exercise was decreased in the MI group (data not shown). Exercise training had no effect on cardiac hypertrophy, indicated by absolute ventricular weight (VW), VW normalized by either body weight (VW/BW) or tibial length (VW/TL). Size of myocytes (length or width) used in contractile measurements was not different between groups (data not shown). Citrate synthase (CS) activity of plantaris muscle homogenates from HI (p .05), but not MI, was increased by 28% compared with C (MI and C did not significantly differ).
Table 1.
Effect of Exercise Training on Physical Characteristics in Aged Rats
| Physical Characteristics | C (n = 6) | MI (n = 5) | HI (n = 5) |
| BW (g)—Initial, 30 mo | 592.75±15.35 | 599.00±12.22 | 579.28±17.65 |
| BW (g)—Final, 33 mo | 560.85±12.45 | 506.98±13.11* | 470.38±15.00* |
| VW (g) | 1.30±0.03 | 1.35±0.08 | 1.20±0.04 |
| TL (mm) | 45.42±0.25 | 45.40±0.44 | 45.16±0.27 |
| VW/BW × 1000 | 2.32±0.05 | 2.69±0.21 | 2.56±0.09 |
| VW/TL × 1000 | 28.60±0.72 | 29.85±1.70 | 26.65±0.92 |
| Plantaris CS activity, (µmol/min/g wet wt) | 12. 60±0.67 | 12.15±0.86 | 16.10±1.02*,† |
Notes: Values are mean ± SEM. n = number of rats per group; C = Control; MI = moderate intensity; HI = high intensity; BW = body weight; VW = ventricular weight; TL = tibial length; CS = citrate synthase; SEM = standard errors of means. CS activity was assayed at 25°C. *p < .05, significantly different than C;. †p < .05, significantly different than MI group.
Table 2 and Figure 2 show mechanical properties for C, MI, and HI groups. We present two different ways of depicting the effects of training on the force–velocity and force–power relationship in skinned cardiac myocytes from C, MI, and HI (Table 2 and Figure 2) as described previously (12). First, data for each cell were fitted to the Hill equation as described in the Methods section. This analysis resulted in a value for maximal unloaded shortening velocity (V max) as well as a value for the term a/P o (a measure of the curvature of the force–velocity curve). Conversion of force–velocity values to power output resulted in values for absolute peak power output (normalized for cell mass), peak power output (normalized for maximal force), F opt (the relative force at which peak power was reached), and V opt (the relative velocity at which peak power was reached). Force–velocity and force–power data were characterized for 45 cells from C, 51 cells from MI, and 49 cells from HI. These data are presented in Table 2 along with maximal tension data. Myocytes from MI yielded 97% greater peak power output (normalized to maximal force, P/P o × ML/s) than C, but there were no significant differences in peak power output normalized to maximal force between HI versus C. Neither maximal unloaded shortening velocity (V max) nor maximal force or passive force was different between any of the groups, but a/P o was significantly greater in both trained groups compared with the sedentary groups.
Figure 2.

(A) Composite force–velocity curves from control (C), moderate-intensity (MI), and high-intensity (HI) myocytes. Data were compiled from 45 C, 51 MI, and 49 HI myocytes. Isotonic shortening velocity values at each load were averaged from all myocytes in each group. Data points indicate mean and error bars indicates standard errors of means for velocity. • and dashed line = C; o and solid line = MI, ▲ and and dotted line = HI. Lines are the best-fit regression line using the Hill equation as described in the Methods section.(B) Composite force–power curve constructed from force–velocity data. In each myocytes at each load, force values (expressed as P/P o) were multiplied times mean velocity values (expressed ML/s) to result in a value of power output for that load. Lines are the best-fit regression line using the Hill equation as described in the Methods section. Peak power output was taken from the highest point in the best-fit line. • and dashed line = C; o and solid line = MI, and ▲ and dotted line = HI.
Along with Table 2, which provides quantifiable data that can be summed between animals, we present composite force–velocity and force–power curve to illustrate cell-to-cell variability in response to exercise training. Figure 2A is composite force–velocity curve showing mean ± standard errors of means velocity at each relative force (P/P o) value for all of the HI (n = 49), MI (n = 51), and C (n = 45) myocytes. Mean force and velocity data were then fit with the Hill equation, shown by solid, dashed, and dotted lines. Figure 2B shows a composite force–power curve with mean ± standard errors of means values obtained by multiplying the velocity values times force values for each force clamp. For this analysis, peak power output normalized maximal force was 0.13 P/P o × ML/s in HI myocytes, 0.17 P/P o × ML/s in MI myocytes, and 0.11 P/P o × ML/s in C myocytes.
The effects of exercise training on cardiac MHC isoform content in ventricular homogenates from C, MI, and HI rats are presented in Figure 3A and × 3B. The percent × -MHC content was not different among groups in 33-month-old F344BN F1 hybrid rats. To determine changes in expression of aMLC1 compared with vMLC1 isoforms, we performed two-dimensional electrophoretic analysis of homogenates from hearts of C, MI and HI rats (n = 6/group) and from atria. A representative two-dimensional gel is shown in Figure 4A. The highlighted area was analyzed for the presence of aMLC1 and vMLC1 based on the predicted isoelectric point and molecular weight of these two isoforms. A magnified image of this region of the gel is shown in Figure 4B. Mean data for six animals from each group show that there was no detectable aMLC1 protein in C ventricles, whereas in MI animals, aMLC1 increased to 11.7±2.2% of the total MLC1, and in HI ventricles, aMLC1 increased to 7.9±2.8% of the total MLC1. This difference is statistically significant between MI and C and between MI and HI (p < .05).
Figure 3.

(A) Representative 6% sodium dodecyl sulfate–polyacrylamide gels showing the distribution of myosin heavy-chain (MHC) isoforms in ventricular homogenates from control (C), moderate-intensity (MI), and high-intensity (HI) rats. Lane 1 is from C, lane 2 is from HI, and lane 3 is MI. There is no significant difference among groups. (B) Bar graph represents mean with standard errors of means of the percent -MHC in ventricular homogenates from C, MI, and HI rats.
Figure 4.
Results of two-dimensional electrophoresis analysis of aMLC1 protein expression in trained and control ventricular tissue. (A) Whole-gel results of first-dimension isoelectric focusing using a pH 3–10 gradient and second-dimension sodium dodecyl sulfate–polyacrylamide gel electrophoresis with a 12.5% acrylamide gel. (B) Close-up of the aMLC1/vMLC1 region of gels (box in A) used for analysis. Shown are representative gels using homogenates from control atrial tissue, ventricular tissue from control (C), moderate-intensity (MI), and high-intensity (HI) animals. Identification of aMLC1 and vMLC1 is based on predicted isoelectric point and molecular weight values for these proteins as well as previously published two-dimensional electrophoretic analyses of these proteins.
Discussion
The novel finding of this study is that the contractile function in myocytes from hearts of aging rats, characterized by increased peak power output (normalized to maximal force), is increased by endurance exercise training, but this improvement in function depends on the intensity of the exercise training. We had previously shown that aging was associated with a decline in myocyte power output (12); but, in the present study, a more moderate training stimulus (MI group) significantly ameliorates the previously seen age-associated decline in power output. However, a higher level of training stimulus (HI group) had no significant effect on power output. To our knowledge, this is the first study to demonstrate that exercise training, initiated at an advanced age, is capable of improving the age-associated decrease in the capacity of the myocardium to perform external work.
We saw no effect of exercise training on either passive force or maximal force. We previously saw no age-related fall in myocyte maximal force (12), and the present study showed no effect of exercise on the maximal force. In contrast, we did observe that training, regardless of intensity, increased the term a/P o, a measure of the curvature of the force–velocity curve. We previously demonstrated an age-related decline in a/P o and exercise training apparently reduces this age-related effect. However, the a/P o term is usually related to both F opt (the relative force at which peak power is reached) and V opt (the relative velocity at which peak power is reached), and we saw no significant effect of training on these values.
Exercise Training in Old Animals
The longer lived F344BN F1 rat strain has improved the ability to study age-related changes in a rat model. However, the study of exercise training adaptations in these rats has proven challenging, due to the reduced exercise capacities of these very old rats. It seems clear that it is not feasible to merely impose upon aged rats a similar treadmill training protocol (eg, 26 m/min for 1h/d for 12 weeks) as those used in many younger rat studies (13). For example, Mace and colleagues (28) investigated the effect of exercise training on cardiac adaptation in F344BN F1 rats, but they reported the results with wide range of ages (27–31 months) and varied training period (8–11 weeks). Betik and colleagues (29) used interval treadmill exercise training in 29-month-old F344BN F1 rats, which consisted of 8 minutes at a base velocity followed by a 2-minute interval of higher velocity than basal velocity. When rats reached 31 months old (after 8 weeks of training), the exercise protocol was modified to 4d/wk to allow more recovery time to the senescent rats (29). Olfert and colleagues (30) stated that most of the older F344BN F1 rats (35 months of age) in their study were physically unable to move faster than 8 m/min, partially due to the mechanical inefficiencies associated with their locomotion. In our study, we modified our treadmill training protocol to provide a lower intensity training program for some of the rats to ensure that, if rats could not complete the HI program, there would at least be one group of animals that completed the training. Contrary to our hypothesis, it was this MI group that showed the greatest adaptation to the exercise.
Effects of Moderate-Intensity Versus High-Intensity Exercise
Exercise training studies in young animals generally show that higher intensity endurance training promotes beneficial cardiovascular adaptation. But, in our study, the more MI training stimulus increased myocyte power output. The reasons why HI exercise training failed to increase peak power output in old rats are not clear. The interpretation of this result is complicated by the way we conducted our training protocol, since the two groups had the same training protocol except for the last 4 weeks. Thus, it is not clear whether the cellular alterations occurred early in the training in both groups and then the MI rats were able to maintain that training effect while those alterations may have been minimized or reversed in the HI group during the higher intensity training. In any case, our results do indicate that cellular changes in the hearts of aging rats may vary with the training stimulus. There is some evidence from previous studies that, in aged animals, less intense exercise training has beneficial effects. For example, moderate intensity exercise training, using a protocol similar to our MI protocol, initiated at 31 month in F344BN F1 rats, was shown to ameliorate age-associated extracellular matrix remodeling and decreased fibrosis (31). Moreover, low intensity of swimming training in old rats improves endurance capacity with an increase in antioxidant defense system, whereas MI to HI exercise does not induce such protective effects (32). Thus, in aged animals, a lower intensity training program may be more effective at promoting positive myocardial adaptations.
It is interesting that, in our study, MI training elicited positive adaptations in myocyte function without accompanying changes in other indices of endurance exercise training. One of the hallmarks of endurance training has been thought to be an increase in skeletal muscle oxidative capacity, often represented by an increase in CS activity. In our study, we found that only rats from the HI group had a higher plantaris muscle CS activity (a 28% increase) compared with C, but MI training did not change CS activity. These results agree with a previous study showing that a low-intensity exercise training program delays the onset of decompensated heart failure without change in skeletal muscle CS activity (17), but a relatively HI exercise training increased CS activity in old rats (5). These earlier results, along with the results of the present study, suggest that, in some populations (such as aged), there is less coupling between exercise-induced increases in skeletal muscle oxidative capacity and exercise-induced cardiac adaptations.
Another hallmark of endurance exercise training is the induction of cardiac hypertrophy (33). However, 11 weeks of treadmill training in aged rats did not increase absolute ventricular weight (VW), VW normalized by either body weight (VW/BW) or tibial length(VW/TL), which normally used for indices of cardiac hypertrophy. Again, this may represent a clear difference between endurance exercise adaptations in aged animals compared with young animals. It is generally accepted that the aging heart undergoes a modest degree of hypertrophy (8,9), but other studies have also seen no additional increase in response to exercise training (3,5,6). This is contradictory finding from young rats that exercise training induces cardiac hypertrophy (13,34). The results from CS activity and physical characteristics of aged rats suggest that the mechanisms of adaptation to exercise in old animals are quite different compared with young animals.
Potential Molecular Mechanisms
To explore possible molecular mechanisms of the MI exercise-induced increased power output properties, we investigated MHC isoform content that has been previously shown to be associated with changes in power output properties (35). MHC isoform content appears to be highly correlated with cardiac function (36,37), and it is thought that increases in α-MHC can be beneficial in a setting of poor contractile function (38). With age, the normal rodent heart undergoes a shift in MHC isoform characterized by decreasing α-MHC and increasing β-MHC. Previous studies have suggested that the increase in β-MHC isoform content with aging is associated with decreases in maximal unloaded shortening velocity and the rate of shortening (39,40) without affecting the maximal tension generation ability (40,41). However, our results indicated that MHC isoform content was not altered by exercise training, which agrees with previous studies in old rats (42,43).
Previous studies have suggested that other modifications in regulatory proteins associated with exercise training might alter the myocyte power output in the absence of changes in the MHC isoform content. Phosphorylation of cTnI and cMyBP-C following treatment with protein kinase A has been shown to increase power output in rat skinned cardiac myocytes (44), and exercise training increases basal levels of protein kinase A-induced phosphorylation of myofibrillar proteins in porcine myocardium (45). We previously determined that, in young rats, exercise training is capable of increasing aMLC1 expression in ventricular myocardium (23,24). In the course of development, MLC expression changes in ventricular tissue from the atrial form to the ventricular isoform. However, aMLC1 expression has been shown to increase in pathological conditions (46) and following exercise training (23,24). In the present study, we found that MI exercise training induced a significant increase in aMLC1 content in ventricular myocardium, whereas HI training induced a smaller increase. As increases in aMLC1 have been correlated with increased Ca2+ sensitivity of tension (24), increased maximal shortening velocity (47), and increased power output (48), this increase in aMLC1 content may provide a mechanism for the increase in power output in the MI myocytes. However, it remains to be determined (a) why MI exercise training has a differential effect on aMLC1 expression compared with HI and (b) why the small increase in aMLC1 content in HI myocytes did not result in an increase in power output.
Implications for Whole-Heart Function
It is not clear what the implications of our findings of increased power output in the MI myocytes are for whole-heart or whole-animal function. The relationship between loaded shortening (and thus power output) in the myocardium and the ability of the heart to eject blood would suggest that MI exercise training in our study, but not the HI exercise, improved the whole-heart function. But there is no information about the effect of exercise training on intact heart function in these very old rats. In addition, there is little information about the effect of exercise training in these very old animals on indices of general cardiovascular function, such as VO2max. Previously, Olfert and his colleagues (30) measured peak oxygen consumption (VO2peak) of F344BN F1 rats and demonstrated that VO2peak decreases linearly with age. However, the workloads associated with VO2peak in Olfert and his colleagues (30) were much lower than the work intensity of both the MI and HI rats in the present study. Thus, there appears to be the need to characterize whole-body as well as whole-heart response to exercise training in these very old F344BN F1 rats.
In summary, the present study demonstrates that MI exercise, rather than the more commonly used HI exercise, is able to induce cellular adaptations that may improve functional capacity without eliciting an increase in common markers of endurance training (ie, CS activity) in old animals. MI increased peak power output normalized to maximal force, but HI did not improve power output properties in old rats. Increased peak power output induced by MI occurs in the absence of shift in MHC isoform content but may be related to an increase in aMLC1 expression.
Funding
This work was supported by the National Institutes of Health (NIH) (AG030423).
References
- 1. Renlund DG, Gerstenblith G. Exercise and the aging heart Cardiol Clin 1987;5(2):331 336 [PubMed] [Google Scholar]
- 2. Shephard RJ, Balady GJ. Exercise as cardiovascular therapy Circulation 1999;99(7):963 972 [DOI] [PubMed] [Google Scholar]
- 3. Brenner DA, Apstein CS, Saupe KW. Exercise training attenuates age-associated diastolic dysfunction in rats Circulation 2001;104(2):221 226 [DOI] [PubMed] [Google Scholar]
- 4. Taffet GE, Michael LA, Tate CA. Exercise training improves lusitropy by isoproterenol in papillary muscles from aged rats J Appl Physiol. 1996;81(4):1488 1494 [DOI] [PubMed] [Google Scholar]
- 5. Tate CA, Taffet GE, Hudson EK, Blaylock SL, McBride RP, Michael LH. Enhanced calcium uptake of cardiac sarcoplasmic reticulum in exercise-trained old rats Am J Physiol Heart Circ Physiol. 1990;258(2):H431 H435 [DOI] [PubMed] [Google Scholar]
- 6. Gwathmey JK, Slawsky MT, Perreault CL, Briggs GM, Morgan JP, Wei JY. Effect of exercise conditioning on excitation-contraction coupling in aged rats J Appl Physiol. 1990;69(4):1366 13671 [DOI] [PubMed] [Google Scholar]
- 7. Spurgeon HA, Steinbach MF, Lakatta EG. Chronic exercise prevents characteristic age-related changes in rat cardiac contraction Am J Physiol Heart Circ Physiol. 1983;244(4):H513 H518 [DOI] [PubMed] [Google Scholar]
- 8. Lakatta EG, Yin FC. Myocardial aging: functional alterations and related cellular mechanisms Am J Physiol Heart Circ Physiol. 1982;242(6):H927 H941 [DOI] [PubMed] [Google Scholar]
- 9. Anversa P, Hiler B, Ricci R, Guideri G, Olivetti G. Myocyte cell loss and myocyte hypertrophy in the aging rat heart J Am Coll Cardiol. 1986;8(6):1441 1448 [DOI] [PubMed] [Google Scholar]
- 10. Capasso JM, Malhotra A, Remily RM, Scheuer J, Sonnenblick EH. Effects of age on mechanical and electrical performance of rat myocardium Am J Physiol Heart Circ Physiol. 1983;245(1):H72 H81 [DOI] [PubMed] [Google Scholar]
- 11. Li Y, Lincoln T, Mendelowitz D, Grossman W, Wei JY. Age-related differences in effect of exercise training on cardiac muscle function in rats Am J Physiol Heart Circ Physiol. 1986;251(1):H12 H18 [DOI] [PubMed] [Google Scholar]
- 12. Chung E, Diffee GM. Effect of aging on power output properties in rat skinned cardiac myocytes J Gerontol A Biol Sci Med Sci. 2011;66A(12):1267 1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diffee GM, Chung E. Altered single cell force-velocity and power properties in exercise-trained rat myocardium J Appl Physiol. 2003;94(5):1941 1948 [DOI] [PubMed] [Google Scholar]
- 14. Kemi OJ, Haram PM, Loennechen JP, et al. Moderate vs. high exercise intensity: Differential effects on aerobic fitness, cardiomyocyte contractility, and endothelial function Cardiovasc Res. 2005;67(1):161 172 [DOI] [PubMed] [Google Scholar]
- 15. Wisloff U, Helgerud J, Kemi OJ, Ellingsen O. Intensity-controlled treadmill running in rats: {V}O2 max and cardiac hypertrophy Am J Physiol Heart Circ Physiol. 2001;280(3):H1301 H1310 [DOI] [PubMed] [Google Scholar]
- 16. Veras-Silva AS, Mattos KC, Gava NS, Brum PC, Negrao CE, Krieger EM. Low-intensity exercise training decreases cardiac output and hypertension in spontaneously hypertensive rats Am J Physiol Heart Circ Physiol. 1997;273(6):H2627 H2631 [DOI] [PubMed] [Google Scholar]
- 17. Emter CA, McCune SA, Sparagna GC, Radin MJ, Moore RL. Low-intensity exercise training delays the onset of decompensated heart failure in the spontaneously hypertensive heart failure (SHHF) rat Am J Physiol Heart Circ Physiol. 2005;289(5):H2030 H2038 [DOI] [PubMed] [Google Scholar]
- 18. Diffee GM, Seversen EA, Titus MM. Exercise training increases the Ca2+ sensitivity of tension in rat cardiac myocytes J Appl Physiol. 2001;91:309 315 [DOI] [PubMed] [Google Scholar]
- 19. Warren CM, Greaser ML. Method for cardiac myosin heavy chain separation by sodium dodecyl sulfate gel electrophoresis Anal Biochem 2003;320(1):149 151 [DOI] [PubMed] [Google Scholar]
- 20. Pleissner KP, Regitz-Zagrosek V, Weise C, et al. Chamber-specific expression of human myocardial proteins detected by two-dimensional gel electrophoresis Electrophoresis. 1995;16(5):841 850 [DOI] [PubMed] [Google Scholar]
- 21. Morano I, Arndt H, Gartner C, Ruegg J. Skinned fibers of human atrium and ventricle: myosin isoenzymes and contractility Circ Res. 1988;62(3):632 639 [DOI] [PubMed] [Google Scholar]
- 22. Morano M, Boels P, Haworth SG, Haase H, Morano I. Expression and function of atrial myosin light chain 1 in the porcine right ventricle of normal and pulmonary hypertensive animals Adv Exp Med Biol. 1998;453:481 488 [DOI] [PubMed] [Google Scholar]
- 23. Diffee GM, Seversen EA, Stein TD, Johnson JA. Microarray expression analysis of effects of exercise training: increase in atrial MLC-1 in rat ventricles Am J Physiol Heart Circ Physiol. 2003;284:H830 H837 [DOI] [PubMed] [Google Scholar]
- 24. Diffee GM, Nagle DF. Regional differences in effects of exercise training on contractile and biochemical properties of rat cardiac myocytes J Appl Physiol. 2003;95(1):35 42 [DOI] [PubMed] [Google Scholar]
- 25. Hill AV. The heat of shortening and the dynamic constraints of muscle Proc Roy Soc B. 1938;126:136 195 [Google Scholar]
- 26. Woledge RC, Curtin NA, Homsher E. Energetic Aspects of Muscle Contraction London: Academic Press; 1985:47 71 [PubMed] [Google Scholar]
- 27. Natali AJ, Wilson LA, Peckham M, Turner DL, Harrison SM, White E. Different regional effects of voluntary exercise on the mechanical and electrical properties of rat ventricular myocytes J Physiol (Lond). 2002;541(3):863 875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mace LC, Palmer BM, Brown DA, et al. Influence of age and run training on cardiac Na+/Ca2+ exchange J Appl Physiol. 2003;95(5):1994 2003 [DOI] [PubMed] [Google Scholar]
- 29. Betik AC, Thomas MM, Wright KJ, Riel CD, Hepple RT. Exercise training from late middle age until senescence does not attenuate the declines in skeletal muscle aerobic function Am J Physiol Regul Integr Comp Physiol. 2009;297(3):R744 R755 [DOI] [PubMed] [Google Scholar]
- 30. Olfert IM, Balouch J, Mathieu-Costello O. Oxygen consumption during maximal exercise in Fischer 344 x Brown Norway F1 hybrid rats J Gerontol A Biol Sci Med Sci 2004;59(8):B801 B808 [DOI] [PubMed] [Google Scholar]
- 31. Kwak H-B, Kim J-H, Joshi K, Yeh A, Martinez DA, Lawler JM. Exercise training reduces fibrosis and matrix metalloproteinase dysregulation in the aging rat heart FASEB J 2011;25(3):1106 1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ravi Kiran T, Subramanyam MVV, Asha Devi S. Swim exercise training and adaptations in the antioxidant defense system of myocardium of old rats: relationship to swim intensity and duration Comp Biochem Physiol B Biochem Mol Biol 2004;137(2):187 196 [DOI] [PubMed] [Google Scholar]
- 33. Woodiwiss AJ, Norton GR. Exercise-induced cardiac hypertrophy is associated with an increased myocardial compliance J Appl Physiol. 1995;78(4):1303 1311 [DOI] [PubMed] [Google Scholar]
- 34. Wisloff U, Loennechen JP, Falck G, et al. Increased contractility and calcium sensitivity in cardiac myocytes isolated from endurance trained rats Cardiovasc Res. 2001;50(3):495 508 [DOI] [PubMed] [Google Scholar]
- 35. Herron TJ, Korte FS, McDonald KS. Loaded shortening and power output in cardiac myocytes are dependent on myosin heavy chain isoform expression Am J Physiol Heart Circ Physiol. 2001;281(3):H1217 H1222 [DOI] [PubMed] [Google Scholar]
- 36. Jin H, Yang R, Li W, et al. Effects of exercise training on cardiac function, gene expression, and apoptosis in rats Am J Physiol Heart Circ Physiol. 2000;279(6):H2994 H3002 [DOI] [PubMed] [Google Scholar]
- 37. Zhang X-Q, Ng Y-C, Musch TI, Moore RL, Zelis R, Cheung JY. Sprint training attenuates myocyte hypertrophy and improves Ca2+ homeostasis in postinfarction myocytes J Appl Physiol. 1998;84(2):544 552 [DOI] [PubMed] [Google Scholar]
- 38. James J, Martin L, Krenz M, et al. Forced expression of {alpha}-myosin heavy chain in the rabbit ventricle results in cardioprotection under cardiomyopathic conditions Circulation 2005;111(18):2339 2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fitzsimons DP, Patel JR, Moss RL. Aging-dependent depression in the kinetics of force development in rat skinned myocardium Am J Physiol Heart Circ Physiol. 1999;276(5):H1511 H1519 [DOI] [PubMed] [Google Scholar]
- 40. Wahr PA, Michele DE, Metzger JM. Effects of aging on single cardiac myocyte function in Fischer 344 × Brown Norway rats Am J Physiol Heart Circ Physiol. 2000;279(2):H559 H565 [DOI] [PubMed] [Google Scholar]
- 41. Carnes CA, Geisbuhler TP, Reiser PJ. Age-dependent changes in contraction and regional myocardial myosin heavy chain isoform expression in rats J Appl Physiol. 2004;97(1):446 453 [DOI] [PubMed] [Google Scholar]
- 42. Farrar RP, Starnes JW, Cartee GD, Oh PY, Sweeney HL. Effects of exercise on cardiac myosin isozyme composition during the aging process J Appl Physiol. 1988;64(2):880 883 [DOI] [PubMed] [Google Scholar]
- 43. Machida S, Tsujimoto H, Suzuki H, Kasuga N, Kobayashi K, Narusawa M. Age-related differences in the effect of running training on cardiac myosin isozyme composition in Rats J Gerontol A Biol Sci Med Sci. 2002;57(9):B339 B343 [DOI] [PubMed] [Google Scholar]
- 44. Herron TJ, Korte FS, McDonald KS. Power output is increased after phosphorylation of myofibrillar proteins in rat skinned cardiac myocytes Circ Res. 2001;89(12):1184 1190 [DOI] [PubMed] [Google Scholar]
- 45. Hinken AC, Korte FS, McDonald KS. Porcine cardiac myocyte power output is increased after chronic exercise training J Appl Physiol. 2006;101(1):40 46 [DOI] [PubMed] [Google Scholar]
- 46. Morano M, Zacharzowski U, Maier M, et al. Regulation of human heart contractility by essential myosin light chain isoforms J Clin Invest. 1996;98(2):467 473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fewell JG, Hewett TE, Sanbe A, et al. Functional significance of cardiac myosin essential light chain isoform switching in transgenic mice J Clin Invest 1998;101(12):2630 2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sanbe A, Gulick J, Hayes E, et al. Myosin light chain replacement in the heart Am J Physiol Heart Circ Physiol. 2000;279(3):H1355 H1364 [DOI] [PubMed] [Google Scholar]