Abstract
Niflumic acid, 2-{[3-(trifluoromethyl)phenyl]amino}pyridine-3-carboxylic acid (NFA), a nonsteroidal anti-inflammatory drug that blocks cyclooxygenase (COX), was shown previously to activate [Na+]i-regulated Slo2.1 channels. In this study, we report that other fenamates, including flufenamic acid, mefenamic acid, tolfenamic acid, meclofenamic acid, and a phenyl acetic acid derivative, diclofenac, also are low-potency (EC50 = 80 μM to 2.1 mM), partial agonists of human Slo2.1 channels heterologously expressed in Xenopus oocytes. Substituent analysis determined that N-phenylanthranilic acid was the minimal pharmacophore for fenamate activation of Slo2.1 channels. The effects of fenamates were biphasic, with an initial rapid activation phase followed by a slow phase of current inhibition. Ibuprofen, a structurally dissimilar COX inhibitor, did not activate Slo2.1. Preincubation of oocytes with ibuprofen did not significantly alter the effects of NFA, suggesting that neither channel activation nor inhibition is associated with COX activity. A point mutation (A278R) in the pore-lining S6 segment of Slo2.1 increased the sensitivity to activation and reduced the inhibition induced by NFA. Together, our results suggest that fenamates bind to two sites on Slo2.1 channels: an extracellular accessible site to activate and a cytoplasmic accessible site in the pore to inhibit currents.
Introduction
Intracellular Na+-activated potassium currents (IKNa) first were identified in guinea pig cardiomyocytes 28 years ago (Kameyama et al., 1984), yet their physiological and pathophysiological roles are not well characterized. Closed under normal physiological and ionic conditions, KNa channels are activated markedly upon the elevation of [Na+]i (Kameyama et al., 1984). In the heart, [Na+]i is increased during ischemia, and activation of KNa currents may serve a cardioprotective function by shortening the action potential duration and preventing Ca2+ overload (Kameyama et al., 1984; Wang et al., 1991).
Two types of KNa channels have been cloned, Slo2.1 (Bhattacharjee et al., 2003) and Slo2.2 (Yuan et al., 2003). Slo2.1 (also known as KCa4.2 or Slick) channels are encoded by the KCNT2 gene in humans and are expressed in the nervous system and the heart. In addition to intracellular Na+, Slo2.1 channels also are activated by intracellular Cl− and inhibited by intracellular ATP (Bhattacharjee et al., 2003). Low-potency and nonspecific blockers of Slo2.1 current (ISlo2.1) include quinidine (90% block at 1 mM) and tetraethylammonium (60% block at 20 mM). The protein kinase C activator phorbol 12-myristate 13-acetate inhibits ISlo2.1 indirectly with an IC50 value of 20 nM (Santi et al., 2006). We reported recently that the fenamates niflumic acid, 2-{[3-(trifluoromethyl)phenyl]amino}pyridine-3-carboxylic acid (NFA), and flufenamic acid, 2-[3-(trifluoromethyl)anilino]benzoic acid (FFA), can activate Slo2.1 with EC50 values of 2.1 and 1.4 mM, respectively, in the absence of a change in [Na+]i (Dai et al., 2010). Although NFA is the first compound shown to activate Slo2.1, its low potency and nonspecificity severely limit its usefulness as a chemical probe in physiological studies.
Fenamates are N-substituted anthranilic acid derivatives used clinically as nonsteroidal anti-inflammatory drugs (NSAIDs) for the treatment of fever, pain, and inflammation (Jiang et al., 2012). These small molecules exert their action by inhibiting cyclooxygenase (COX) enzyme and thereby reduce the biosynthesis of prostaglandins (Flower et al., 1972; Sanger and Bennett, 1979). Fenamates also are low-potency modulators of a diversity of ion channels and enzymes, exhibiting either activator or inhibitory effects. For example, fenamates inhibit Ca2+-activated Cl− channels (White and Aylwin, 1990; Greenwood and Large, 1995), ATP-sensitive potassium channels (Grover et al., 1994), and nonselective cation channels (Gogelein et al., 1990) but activate large-conductance Ca2+-activated K+ (Slo1) channels (Farrugia et al., 1993; Ottolia and Toro, 1994; Gribkoff et al., 1996), transient receptor potential ankyrin-1 channels (Hu et al., 2010), and brain liver intestine Na+ channels (Wiemuth and Grunder, 2011). Fenamates also have been reported to enhance or alter the gating of Kv4.2 and Kv4.3 channels (Wang et al., 1997), KCNQ1/KCNE1 channels (Busch et al., 1994), KCNQ2/3 channels (Peretz et al., 2005), and human ether-a-go-go-related gene-1–3 channels (Malykhina et al., 2002; Fernandez et al., 2008).
In the present study, we determined the effects of several fenamates on Slo2.1 channels heterologously expressed in Xenopus oocytes, including NFA, FFA, mefenamic acid, 2-(2,3-dimethylanilino)benzoic acid (MFA), tolfenamic acid, 2-[(3-chloro-2-methylphenyl)amino]benzoic acid (TFA), meclofenamic acid, 2-[(2,6-dichloro-3-methylphenyl)amino]benzoic acid (MCFA), and a phenyl acetic acid derivative, diclofenac, 2-[2-(2,6-dichloroanilino)phenyl]acetic acid. We show that NFA and other fenamates exhibit a mixed agonist behavior. At high concentrations, NFA application causes rapid activation of ISlo2.1 followed by partial inhibition. A limited structure-activity relationship study was performed to define the minimal pharmacophore requirement for fenamate-mediated activation of Slo2.1.
Materials and Methods
Solutions and Drugs.
For two-microelectrode voltage-clamp experiments, the extracellular solution (KCM 211) contained the following: 98 mM NaCl, 2 mM KCl, 1 mM CaCl2, 1 mM MgCl2, and 5 mM HEPES (pH 7.6). NFA, FFA, MFA, TFA, MCFA, diclofenac sodium (DFS), N-phenylanthranilic acid (PAA), diphenylamine, anthranilic acid, N-methylanthranilic acid, biphenyl-2-carboxylic acid, and 2-benzyl benzoic acid were purchased from Sigma-Aldrich (St. Louis, MO). Ibuprofen was purchased from Cayman Chemical (Ann Arbor, MI). Concentrated stock solutions (100 mM to 1 M) of all of the compounds were prepared in dimethyl sulfoxide, except MCFA and DFS that were dissolved in deionized water, and stored at −20°C until used. Solutions containing the final concentration of the drug were freshly prepared each day by diluting stock solutions, and the pH was adjusted to 7.6 with 1 N NaOH.
Molecular Biology.
KCNT2 cDNA (provided by L. Kaczmarek, Yale University, New Haven, CT) was subcloned into the psGEM oocyte expression vector (Dai et al., 2010). A278R Slo2.1 was generated by using the QuikChange site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA) and confirmed by DNA sequencing. KCNT1 (hSlo2.2) cDNA in the pCR-XL-TOPO vector was obtained from GenBank. A MluI restriction site was introduced into the 5′ end, and the cDNA was excised from the vector using MluI and XhoI and subcloned into the pUNIV vector (Addgene, Cambridge, MA). Finally, an XbaI site was introduced into the vector region near the 3′ end of hSlo2.2. KCNMA1 (hSlo1) cDNA in the pcDNA3.1 (+) vector was provided by Jianmin Cui (Washington University, St. Louis, MO). Complementary RNAs (cRNAs) for all of the cDNAs were prepared by in vitro transcription with mMESSAGE mMACHINE T7 (Invitrogen, Carlsbad, CA) after the linearization of the plasmid with SfiI (KCNT2), XbaI (KCNT1), or PvuI (KCNMA1). The concentrations of cRNA were determined using the RiboGreen assay (Invitrogen).
Oocyte Isolation and cRNA Injection.
Protocols for oocyte isolation from Xenopus laevis were approved by the Institutional Animal Care and Use Committee of the University of Utah. Frogs were anesthetized with a 0.2% tricaine methanesulfonate solution before a small surgical incision was made to remove ovarian lobes. Oocytes were separated manually from the lobes using tweezers and digested with 1 mg/ml type II collagenase (Worthington Biochemicals, Freehold, NJ) for 60 min to remove the follicle cell layer. The collagenase solution was prepared using ND-96 Ca2+-free solution (pH 7.6) that contained 96 mM NaCl, 2 mM KCl, 1 mM MgCl2, and 5 mM HEPES.
For the characterization of ISlo2.1, stage IV and V oocytes were injected with 0.5 to 1 ng of wild-type (WT) cRNA or 0.2 to 0.5 ng of A278R mutant KCNT2 cRNA and incubated for 1 to 2 days at 18°C in Barth's saline solution (pH 7.4) that contained 88 mM NaCl, 1 mM KCl, 0.41 mM CaCl2, 0.33 mM Ca(NO3)2, 1 mM MgSO4, 2.4 mM NaHCO3, 10 mM HEPES, 1 mM pyruvate, and 50 mg/liter gentamycin. To record ISlo1, oocytes were injected with 0.04 to 1.0 ng of KCNMA1 cRNA, and currents were recorded 1 to 3 days later. Slo2.2 channels express poorly in oocytes. Therefore, to record ISlo2.2, oocytes were injected with 32 ng of KCNT1 cRNA, and currents were recorded after 4 to 7 days.
Voltage Clamp.
Whole-cell currents were recorded from oocytes using a standard two-microelectrode voltage-clamp technique (Stühmer, 1992; Dai et al., 2010). Pipettes were pulled from borosilicate glass and tip-filled with 1% agarose dissolved in 3 M KCl and then back-filled with 3 M KCl to fabricate agarose-cushion microelectrodes (Schreibmayer et al., 1994). All of the voltage-clamp recordings were performed at room temperature (23–25°C), and the recording chamber was perfused with the drug solutions at a rate of 1 ml/min. For time course and drug concentration-response studies, the holding potential was −80 mV, and step pulses of 300 ms in duration were applied to 0 mV with an interval of 30 s until a steady-state change in current magnitude was achieved. To determine current-voltage (I-V) relationships, test pulses to voltages between −140 and +80 mV were applied in 20-mV increments.
Data Analysis and Statistics.
Currents were analyzed using pClamp 8.2 (Molecular Devices, Sunnyvale, CA), Origin 8.5 (Originlab, Northampton, MA), and Excel (Microsoft, Redmond, WA) software. Results are expressed as mean ± S.E.M. (n = number of oocytes). For concentration-response curves, currents were normalized to the maximum response produced by each test compound. These data were fitted by nonlinear curve fitting (Origin 8.5) to the logistic equation to estimate the EC50 value and Hill coefficient, nH. Statistical significance was evaluated by Student's t test (p ≤ 0.05 was considered significant). Chemical structures were drawn using ChemSketch (Advanced Chemistry Development, Toronto, ON, Canada).
Results
Biphasic Action of NFA on Slo2.1 Channels.
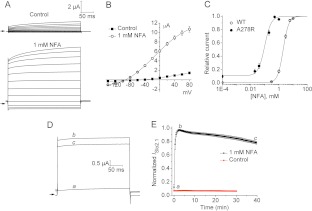
As reported previously (Dai et al., 2010), negligible currents were observed in oocytes injected with low amounts of KCNT2 cRNA under control conditions (Fig. 1A, top). However, application of 1 mM NFA induced a rapid and marked increase in ISlo2.1 amplitude (Fig. 1A, bottom). Currents were enhanced at all of the test potentials and activated rapidly (τact = 5.7 ± 0.1 ms at 0 mV). The I-V relationship for NFA-activated ISlo2.1 exhibited outward rectification with a reversal potential of −95 mV (Fig. 1B). Thus, when relatively small amounts of cRNA (<0.5 ng per oocyte) are injected, Slo2.1 channel activity is small or barely detectable but can be rapidly and robustly activated with NFA.
Fig. 1.
Effect of NFA on WT Slo2.1 channels. A, voltage-clamp protocol (top) and currents recorded from an oocyte expressing WT Slo2.1 channels before (middle, Control) and after treatment with 1 mM NFA (bottom). Oocytes were recorded after 3 days of injection with 0.4 ng of WT Slo2.1 cRNA. Arrows indicate 0 current. B, average I-V relationships for WT ISlo2.1 recorded before (Control) and after treatment with 1 mM NFA (n = 4).
The time course of NFA on ISlo2.1 was characterized by applying repetitive test pulses to 0 mV once every 30 s. The onset of ISlo2.1 activation by 1 mM NFA was rapid and reached maximal effect in 4 to 5 min. The effects of NFA were rapidly and completely reversible upon washout. However, after ∼5 min in the continued presence of NFA, currents slowly decreased in magnitude over the next 35 min until they decayed to 30% of its peak value (Fig. 2A). As described for kidney CLC-K chloride channels (Liantonio et al., 2006), the biphasic effects of NFA on Slo2.1 could result from drug binding to distinct activator and inhibitory sites on the channel. Activation of Slo2.1 channels by extracellular NFA in inside-out patches (Dai et al., 2010) and the more accessible potent activity of external NFA on Slo1 channels expressed in lipid bilayers (Ottolia and Toro, 1994) indicate that the activator effect is mediated by interaction with an extracellular domain of the channel. The delayed and slow decay of current magnitude that follows the initial channel activation could result from the time required for the acidic drug to cross the cell membrane and inhibit channels by a second mechanism, either causing a direct pore block or a consequence of inhibition of cytosolic COX activity (e.g., inhibition by elevated levels of arachidonic acid). To test for the latter possibility, oocytes expressing WT Slo2.1 were preincubated with 1 mM ibuprofen for 40 to 120 min before the coapplication of 1 mM NFA and 1 mM ibuprofen. Ibuprofen is structurally dissimilar to the fenamates and does not activate ISlo2.1 (P. Garg, unpublished observations) but should completely inhibit COX, because it inhibits human recombinant COX-1 and COX-2 with IC50 values of 2.6 and 1.53 μM, respectively (Barnett et al., 1994). Pretreatment of oocytes with ibuprofen did not alter the magnitude or time course of NFA-mediated current activation and inhibition (Fig. 2B). Thus, the NFA-mediated inhibition of Slo2.1 is independent of the COX-prostaglandin pathway.
Fig. 2.
Biphasic action of NFA on Slo2.1. A, effects of 1 mM NFA on ISlo2.1 recorded at a test pulse of 0 mV (left). a, current before the application of NFA. b, current at the peak of the activation response. c, current after 40 min of exposure to NFA. Time-dependent effect of 1 mM NFA on ISlo2.1 (right). Currents were normalized to the peak activation response for each oocyte (n = 11). B, time-dependent activity of 1 mM NFA with the coapplication of the nonselective COX inhibitor ibuprofen (IBP, 1 mM) on ISlo2.1 (left). a, current at 0 mV before the application of drugs. b, current at the peak of the activation response. c, current after 40 min of exposure to NFA + IBP. Time-dependent effect of NFA + IBP (blue square) and NFA alone (open circle) (right). Currents were normalized to the peak activation response for each oocyte. Average peak current for NFA treatment alone is 4.0 ± 0.4 μA (n = 6), and that for NFA + IBP is 4.9 ± 0.7 μA (n = 8). Data summarized in B were obtained from a single batch of oocytes.
A278R Mutant Channels Are More Sensitive to the Activator Effect but Less Sensitive to the Inhibitory Effect of NFA.
Mutations in Slo2.1 can alter constitutive channel activity and response to NFA (Dai et al., 2010). In the S6 segment, we found that mutation of Ala278 to arginine increased the basal activity of Slo2.1 and greatly increased its sensitivity to the activator action of NFA. The effect of 1 mM NFA on A278R channel currents is illustrated in Fig. 3A. Outward currents in the presence of NFA were nearly instantaneous and mostly time independent during the 300-ms test pulse, except at the most positive test potentials. This differs from WT channels, where the time-dependent component was more prominent (compare Figs. 1A and 3A). The I-V relationships measured before and after 1 mM NFA are plotted in Fig. 3B. The magnitude of NFA-activated inward currents of A278R channels was larger than that for WT channels, indicating a negative shift in the conductance-voltage relationship. Similar to our previous report (Dai et al., 2010), activation of WT channels by NFA was concentration dependent over the range of 1 to 10 mM with an EC50 value of 2.1 ± 0.1 mM and nH of 2.4 ± 0.1 (n = 9). A278R channels were 19-fold more sensitive to NFA with an EC50 value of 0.11 ± 0.01 mM and nH of 2.0 ± 0.2 (n = 13) (Fig. 3C).
Fig. 3.
A278R Slo2.1 channels are more sensitive to NFA. A, currents recorded from an oocyte expressing A278R mutant channels before (top, control) and after treatment with 1 mM NFA (bottom). Oocytes were recorded 1 day after the injection with 0.2 ng of cRNA. Vt was varied from −140 to +80 mV and applied in 20-mV increments from a holding potential of −80 mV. Arrows indicate 0 current. B, average I-V relationships for A278R ISlo2.1 recorded before (Control) and after the treatment with 1 mM NFA (n = 10). C, NFA concentration-response relationships for WT (n = 9) and A278R (n = 13) ISlo2.1 measured at 0 mV. Data were fitted with a logistic equation (smooth curve). For WT channels, EC50 = 2.1 ± 0.1, nH = 2.4 ± 0.06. For A278R channels, EC50 = 0.11 ± 0.01, nH = 2.0 ± 0.2. D, effects of 1 mM NFA on A278R ISlo2.1 recorded at a test pulse of 0 mV. a, current recorded at a test pulse of 0 mV before the application of NFA. b, current at the peak of the activation response. c, current after 40 min of exposure to NFA. E, constitutively active A278R ISlo2.1 is stable over 30 min of recording (Control, n = 7), whereas NFA-activated A278R ISlo2.1 exhibits a 20% decline over 40 min (n = 11).
At the same concentration used to characterize WT channels, 1 mM NFA only reduced the peak ISlo2.1 by 20% after 40 min (Fig. 3, D and E). No time-dependent reduction in constitutively active channel activity was observed in the absence of NFA (Fig. 3E), confirming that current inhibition was caused by NFA and was not due to channel rundown.
Structure-Activity Relationship for Fenamates.
The effects of several other fenamates, including FFA, MFA, TFA, and MCFA and a phenyl acetic acid derivative, diclofenac (Fig. 4A), on WT Slo2.1 channels were determined. The concentration-response relationships for all of the drugs are shown in Fig. 4B. MCFA was the most potent activator with an EC50 value of 0.08 ± 0.01 mM (n = 5). The rank order of potency of fenamates for the activation of Slo2.1 was MCFA > TFA > MFA > DFS > FFA > NFA (Table 1). Similar to NFA, the onset of current activation for all of the other fenamates was rapid and completely reversible after 6 to 9 min of washout of the drug.
Fig. 4.
Concentration-response relationships for fenamates and diclofenac. A, chemical structures of fenamates and diclofenac. B and C, concentration-response relationships for the compounds on WT (B) and A278R (C) ISlo2.1. For each compound, ISlo2.1 was measured at 0 mV and normalized to the peak response. Data were fitted with a logistic equation (smooth curve) to determine the EC50 values and nH as presented in Table 1.
TABLE 1.
Comparison of activation effects (EC50 values) of fenamates on WT and A278R Slo2.1 channels
| Compounds | WT hSlo2.1 |
A278R hSlo2.1 |
Ratio (EC50-WT/EC50-A278R) | ||||
|---|---|---|---|---|---|---|---|
| EC50 | nH | n | EC50 | nH | n | ||
| mM | mM | ||||||
| Niflumic acid | 2.09 ± 0.14 | 2.4 ± 0.06 | 9 | 0.11 ± 0.01 | 2.0 ± 0.2 | 13 | 19 |
| Flufenamic acid | 1.1 ± 0.09 | 2.2 ± 0.16 | 5 | 0.059 ± 0.008 | 2.0 ± 0.1 | 6 | 19 |
| Mefenamic acid | 0.366 ± 0.03 | 1.5 ± 0.1 | 13 | 0.051 ± 0.006 | 1.7 ± 0.06 | 6 | 7.2 |
| Tolfenamic acid | 0.30 ± 0.02 | 1.9 ± 0.09 | 8 | 0.047 ± 0.004 | 2.0 ± 0.09 | 5 | 6.4 |
| Meclofenamic acid | 0.079 ± 0.006 | 1.7 ± 0.07 | 5 | 0.017 ± 0.005 | 1.7 ± 0.2 | 6 | 5 |
| Diclofenac | 0.72 ± 0.07 | 2.4 ± 0.2 | 5 | 0.31 ± 0.02 | 2.3 ± 0.16 | 5 | 2.3 |
| N-Phenylanthranilic acid | 0.79 ± 0.03 | 1.5 ± 0.03 | 7 | 0.29 ± 0.02 | 1.5 ± 0.06 | 8 | 3 |
| Diphenylamine | – | – | – | – | – | – | – |
| Anthranilic acid | – | – | – | – | – | – | – |
| N-Methylanthranilic acid | – | – | – | – | – | – | – |
| Biphenyl-2-Carboxylic acid | – | – | – | – | – | – | – |
| Benzyl-benzoic acid | – | – | – | – | – | – | – |
–, No activation.
A278R mutant channels were used to estimate the activator effects of other fenamates in the relative absence of inhibitory activity. As summarized in Fig. 4C, A278R Slo2.1 channels were more sensitive than WT channels to fenamates. Identical to NFA, A278R channels were 19 times more sensitive to FFA (EC50 = 0.06 ± 0.01 mM). The leftward shifts of the concentration-response relationships were less for MFA, TFA, MCFA, and DFS, exhibiting approximately 3- to 7-fold reduction in EC50. The nH varied from 1.7 to 2.3, indicating similar positive cooperativity for A278R and WT channels. Evidently, A278R Slo2.1 channels are primed for gating modification by fenamates, presumably because of a reduced energy of activation induced by the mutation.
We further probed the structure-activity relationship of fenamates to determine the minimum structural requirement for the activation of Slo2.1. The effects of a series of commercially available compounds, including PAA, diphenylamine, anthranilic acid, N-methylanthranilic acid, biphenyl-2-carboxylic acid, and 2-benzyl benzoic acid (Fig. 5A), were determined. PAA is a planar molecule with a carboxylic acid, two six-membered aromatic rings (I and II), and a bridging imino group. The carboxylic group is attached to ring I at the ortho position to the imino nitrogen atom. Coplanarity between these groups is stabilized by resonance interactions and internal hydrogen bonding between the carboxylic group and the imino group (Dhanaraj and Vijayan, 1988). PAA is the parent moiety for all of the fenamates and lacks substituents on ring II. As shown in Fig. 5, B–D, PAA activates ISlo2.1 with an EC50 value of 0.79 ± 0.03 mM (nH = 1.5 ± 0.03; n = 7), indicating that the presence of substituents on ring II is not essential for activity.
Fig. 5.
PAA is the minimal structural requirement for the activation of Slo2.1 channels. A, chemical structures of PAA (rings I and II are indicated), DPA, ANA, MAA, BCA, and BBA. B, average I-V relationship for WT ISlo2.1 recorded before (Control) and after treatment with 3 mM PAA. C, traces of WT currents recorded at 0 mV before (Control) and after exposure of oocytes to the indicated concentrations of PAA. D, concentration-response relationship for PAA. EC50 = 0.79 ± 0.03 mM, nH = 1.5 ± 0.03 (n = 7). E–I, I-V relationships determined before (Control), after the treatment of oocytes with the indicated test compound, and then finally with 1 mM NFA (n = 3–5).
We dissected the requirement for each functional group in the PAA moiety by testing compounds lacking a specific functional group at their highest soluble concentrations. For compounds that showed no response, 1 mM NFA was applied to confirm that the oocytes tested had adequate channel expression. Diphenylamine, lacking the carboxylic acid of ring I, did not activate ISlo2.1 at 1 mM (Fig. 5E). Elimination of ring II yields anthranilic acid, which also did not activate currents at a concentration of 3 mM (Fig. 5F). We reasoned that ring II may endow essential hydrophobicity to the compounds; therefore, we tested N-methylanthranilic acid (10 mM) wherein ring II is replaced by a methyl group. This compound also failed to activate ISlo2.1 (Fig. 5G), indicating that this second aromatic group is indispensable for activity. Biphenyl-2-carboxylic acid lacks the ring-bridging imino group. The application of 3 mM biphenyl-2-carboxylic acid did not activate ISlo2.1 (Fig. 5H). Finally, replacement of the anilinic nitrogen with carbon in 2-benzyl benzoic acid also failed to activate Slo2.1 at 10 mM (Fig. 5I), indicating the possible importance of internal hydrogen bonding between the imino nitrogen atom and the carboxylic oxygen atom.
Relative Efficacies of Fenamates.
We evaluated the apparent efficacies of all of the fenamates on WT channels by comparing the peak outward ISlo2.1 measured at 0 mV for each compound with that measured for NFA at their previously determined EC90 values. The same batch of oocytes was used for each comparative experiment. The rank order of efficacy was NFA = TFA ≥ FFA > MCFA = MFA = DFS = PAA (Fig. 6). Even though NFA was the least potent, it exhibited greater maximal efficacy. In contrast, MCFA was the most potent activator of Slo2.1 but showed lower efficacy than NFA (p = 0.025). Thus, fenamates have variable efficacies that differ from the rank order of their potencies. The difference in potency versus efficacy of fenamate-mediated activation might be due to simultaneous activation and inhibition mediated by separate binding sites or an intrinsic property of a single receptor (i.e., the compounds are partial agonists).
Fig. 6.

Apparent efficacies of several fenamates compared with that of NFA. An equieffective concentration (∼EC90) of each fenamate was applied to oocytes expressing WT Slo2.1 (NFA, 5.4 mM; FFA, 2.9 mM; MFA, 1.65 mM; TFA, 0.98 mM; MCFA, 0.32 mM; PAA, 3.14 mM; DFS, 1.86 mM). The maximal response (Ipeak at 0 mV) for each compound (n = 6–7) was normalized relative to the activation measured with NFA from the same batch of oocytes. The efficacies of MFA, MCFA, PAA, and DFS were less than that of NFA (*, p < 0.05; **, p < 0.01).
PAA Does Not Inhibit Slo2.1.
In contrast to what we observed for NFA, 1 mM PAA inhibited WT channel currents <10% after 20 min before exhibiting a second slower phase of continuing activation (Fig. 7A), suggesting that the fenamate-induced inhibition is a characteristic of its more hydrophobic derivatives and not the parent moiety itself. The time course of MCFA, the most potent activator of Slo2.1, also was investigated to determine if its decreased efficacy could be explained by a faster than normal onset of inhibition. For this experiment, the approximate EC20 value for MCFA (0.03 mM) was used to match the EC20 value of NFA (1 mM) previously studied for its inhibitory effect. However, as shown in Fig. 7B, the onset of time-dependent inhibition exerted by MCFA was similar to that induced by NFA, suggesting that it is a partial agonist of the Slo2.1 channel.
Fig. 7.
Time-dependent effects of PAA and MCFA on ISlo2.1. A, effects of 1 mM PAA on ISlo2.1 recorded at a test pulse of 0 mV (left). a, current before the application of PAA. b, peak current response. c, current after 40 min of PAA. Time-dependent effect of 1 mM PAA on ISlo2.1 (right). Currents were normalized to the peak activation response for each oocyte (n = 10). B, effects of 0.03 mM MCFA on ISlo2.1 recorded at a test pulse of 0 mV (left). a, current before the application of MCFA. b, peak current response. c, current after 40 min of MCFA. Time-dependent effect of 0.03 mM MCFA on ISlo2.1 (right). Currents were normalized to the peak activation response for each oocyte (n = 9).
Fenamates Also Activate Slo2.2 and Slo1 Channels.
Slo2.2 is closely related to Slo2.1 and also is activated by intracellular Na+. Slo2.2 channels express poorly in oocytes, requiring the injection of larger amounts of cRNA and longer periods of incubation compared with Slo2.1 to achieve functional expression. As expected, Slo2.2 also was activated by NFA (Fig. 8, A and B) with an EC50 value (2.7 ± 0.19 mM; Fig. 8C) similar to that determined for Slo2.1. Slo1 channels are large-conductance K+ channels activated by intracellular Ca2+ that have been reported previously to be activated by fenamates (Farrugia et al., 1993; Ottolia and Toro, 1994; Gribkoff et al., 1996). However, as shown in Fig. 9, fenamates are less potent activators of Slo1 channels than Slo2 channels. The effects of 1 mM NFA, MCFA, and PAA on ISlo1 are illustrated in Fig. 9, A–C. I-V relationships for ISlo1 under control conditions and after the activation of channels by several concentrations of these fenamates are plotted in Fig. 9, D–F. The EC50 value for ISlo1 activation was 0.68 ± 0.19 mM (n = 11) for MCFA (Fig. 9G), ∼9-fold less sensitive than that for Slo2.1 channels. Slo1 also was less sensitive to NFA and PAA, because only partial activation was achieved with a concentration of 10 mM (Fig. 9G). The EC50 values for these two compounds, 10.0 ± 0.9 mM (n = 5) for NFA and 13.4 ± 0.7 mM (n = 7) for PAA, were determined by the extrapolation of the concentration-response relationships (Fig. 9G) with the assumption that nH for these compounds was the same as that determined for Slo2.1 channels. Finally, we compared the efficacy of NFA to MCFA (Fig. 9H). Similar to our finding with Slo2.1 channels, MCFA was more potent but less efficacious than NFA in the activation of Slo1 channels.
Fig. 8.
NFA activates Slo2.2 channels. A, currents recorded from an oocyte expressing Slo2.2 channels before (top) and after treatment with 1 mM NFA (bottom). Oocytes were recorded after 6 days of injection with 32 ng of WT Slo2.2 cRNA. Arrows indicate 0 current. B, average I-V relationships for WT ISlo2.2 recorded before (Control) and after treatment with the indicated concentrations of NFA (n = 7). C, concentration-response relationships for NFA on ISlo2.2 measured at 0 mV and normalized to the peak response (n = 7). Data were fitted with a logistic equation to determine the EC50 value (2.7 ± 0.19 mM) and nH (2.0 ± 0.14).
Fig. 9.
Effects of fenamates on Slo1 channels. A–C, currents recorded from oocytes expressing Slo1 channels before (top) and after treatment with 1 mM of the indicated fenamate (bottom). Arrows indicate 0 current. D–F, concentration-dependent effects of NFA (n = 5), MCFA (n = 11), and PAA (n = 7) on averaged I-V relationships. Oocytes were recorded after 1 to 3 days of injection with 0.04 to 1.0 ng of WT Slo1 cRNA. G, concentration-response relationships for the indicated fenamate. Data were fitted with a logistic equation (smooth curves) to estimate the EC50 value. For MCFA, the EC50 value was 0.68 ± 0.19 (nH = 2.6 ± 0.4; n = 11). For NFA and PAA, nH was fixed at 2.4 and 1.5, respectively, to estimate EC50 values of 10.0 ± 0.9 mM (n = 5) for NFA and 13.4 ± 0.7 mM (n = 7) for PAA. For NFA, ISlo1 was measured at 0 mV, and responses in uninjected oocytes were used to correct for the activation of endogenous currents. For MCFA and PAA, ISlo1 was measured at +60 mV without correction, because the endogenous currents evoked by these compounds were very small. H, apparent efficacy of Slo2.2 channel activation for MCFA compared with that for NFA. The maximal response (Ipeak at 0 mV) for each compound (n = 5–11) was normalized relative to the activation measured with NFA from the same batch of oocytes. The efficacy of MCFA was less than that of NFA (*, p < 0.001).
Discussion
In the present study, we have shown that fenamate NSAIDs, including NFA, FFA, MFA, TFA, MCFA, and a phenyl acetic acid derivative, diclofenac, are low-potency modulators of Slo2.1 channels heterologously expressed in Xenopus oocytes. These agents exhibit a biphasic action on Slo2.1 with a rapid onset of activation and a relatively slow and delayed onset of inhibition. Fenamates are widely used clinically to inhibit COX activity. However, their activation and inhibitory effects on Slo2.1 are not associated with COX inhibition, because pretreatment of oocytes with 1 mM ibuprofen did not alter the magnitude or time course of ISlo2.1 modification by 1 mM NFA. The potency of NFA for the activation of intracellular Na+-modulated Slo2.2 channels (EC50 = 2.7 mM) was similar to that determined for Slo2.1 (EC50 = 2.1 mM); however, intracellular Ca2+-modulated Slo1 channels were 5-fold less sensitive to NFA (EC50 = ∼10 mM). Slo1 channels also were ∼9-fold and ∼17-fold less sensitive to activation by MCFA and PAA, respectively. Thus, although fenamates are known to block or modulate the gating of a plethora of ion channels, these compounds are more potent modulators of Slo2 than Slo1 channels.
The dual action of fenamates has been reported previously for kidney CLC-K Cl− channels (Liantonio et al., 2006, 2008). In our study, the activation effect of NSAIDs was rapid in onset and completely and quickly reversible on washout, suggesting that these drugs bind to a readily accessible extracellular domain of the channel protein as demonstrated previously for Slo1 channels. NFA was shown to be 5 times more potent in activation of Slo1 channels reconstituted into lipid bilayers when added to the extracellular side compared with the internal side of the channel (Ottolia and Toro, 1994). In contrast, the onset of fenamate-induced inhibition of ISlo2.1 was delayed and much slower to develop. Slow inhibition can be explained by the longer time required for these acidic compounds to cross the cell membrane and bind to an inhibitory site accessible only from the cytoplasm. Current inhibition could result from direct occlusion of the Slo2.1 channel pore. Consistent with a pore block mechanism was our finding that inhibition of A278R channels was much reduced compared with that of WT channels. Ala278 residues are located in each of the four S6 segments that line the central cavity of Slo2.1. Reduced sensitivity to inhibition suggests that substitution of alanine with arginine reduces the binding of fenamates to a pore-occluding binding site. A278R channels also exhibited an increased basal activity and enhanced sensitivity to activation by fenamates. Together, these mutant channel properties were useful for quantifying the activator effects of fenamates in the relative absence of inhibitory activity.
The fenamates examined here exhibited a range of potencies and apparent efficacies for the activation of Slo2.1. For example, NFA exhibited high efficacy but low potency, whereas MCFA exhibited low efficacy but high potency. These attributes could result from differences in intrinsic activity (partial agonism) and/or the balance between binding to two distinct receptor sites that mediate activation and inhibitory effects on gating and/or ion permeation. A possible explanation for the low potency of NFA compared with those of other fenamates is that it has a nearly coplanar conformation (Dhanaraj and Vijayan, 1988). Other fenamates have nonplanar orientations of the two aromatic rings.
With the exception of NFA, all the other fenamates are N-aryl-substituted derivatives of anthranilic acid with different substituents on the phenyl ring. Starting from the parent structure PAA as the lead compound and dissecting all its functional moieties thereafter, the minimal structural requisite for the activation of Slo2.1 was established. Together, the acidic carboxylic group, two aromatic rings, and an imino moiety bridging the two rings constitute the minimal fenamate pharmacophore for Slo2.1 activation. Because fenamates are amphipathic molecules (Dhanaraj and Vijayan, 1988), the binding site of these drugs may comprise both hydrophilic and hydrophobic regions. The carboxylic group may interact with a polar region, whereas ring II could interact with a hydrophobic site on the protein. However, replacement of ring II with a methyl group (N-methylanthranilic acid) resulted in the loss of activity, indicating the requirement for either an aryl or a hydrophobic substituent larger than a methyl group at this position.
In summary, we have shown that fenamates are both partial agonists and antagonists of Slo2.1 and that these activities are likely mediated by distinct binding sites. PAA was established as the minimum structural requirement for the activation of Slo2.1 channels. Although the mechanisms of activation of the examined set of fenamates seem to be similar, relatively minor perturbations in the basic structure can lead to marked changes in their potencies and efficacies. These studies represent the first step in the search for selective and potent modulators of Slo2.1 channels that will be needed to pharmacologically probe the physiological and pathophysiological roles of these channels.
Acknowledgments
We are thankful to Dr. Li Dai for conducting preliminary experiments on A278R Slo2.1 channels.
This work was supported by the National Institutes of Health National Heart Lung and Blood Institute [Grant R01HL103877]; and the Nora Eccles Treadwell Foundation.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- IKNa
- intracellular Na+-activated potassium current
- COX
- cyclooxygenase
- cRNA
- complementary RNA
- DFS
- diclofenac sodium, 2-[2-(2,6-dichloroanilino)phenyl]acetic acid
- FFA
- flufenamic acid, 2-[3-(trifluoromethyl)anilino]benzoic acid
- IBP
- ibuprofen
- ISlo2.1
- Slo2.1 current
- MCFA
- meclofenamic acid, 2-[(2,6-dichloro-3-methylphenyl)amino]benzoic acid
- MFA
- mefenamic acid, 2-(2,3-dimethylanilino)benzoic acid
- NFA
- niflumic acid, 2-{[3-(trifluoromethyl)phenyl]amino}pyridine-3-carboxylic acid
- nH
- Hill coefficient
- NSAID
- nonsteroidal anti-inflammatory drug
- PAA
- N-phenylanthranilic acid
- Slo1
- large-conductance Ca2+-activated K+
- TFA
- tolfenamic acid, 2-[(3-chloro-2-methylphenyl)amino]benzoic acid
- WT
- wild-type.
Authorship Contributions
Participated in research design: Garg and Sanguinetti.
Conducted experiments: Garg and Sanguinetti.
Performed data analysis: Garg and Sanguinetti.
Wrote or contributed to the writing of the manuscript: Garg and Sanguinetti.
References
- Barnett J, Chow J, Ives D, Chiou M, Mackenzie R, Osen E, Nguyen B, Tsing S, Bach C, Freire J. (1994) Purification, characterization and selective inhibition of human prostaglandin G/H synthase 1 and 2 expressed in the baculovirus system. Biochim Biophys Acta 1209:130–139 [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A, Joiner WJ, Wu M, Yang Y, Sigworth FJ, Kaczmarek LK. (2003) Slick (Slo2.1), a rapidly-gating sodium-activated potassium channel inhibited by ATP. J Neurosci 23:11681–11691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch AE, Herzer T, Wagner CA, Schmidt F, Raber G, Waldegger S, Lang F. (1994) Positive regulation by chloride channel blockers of IsK channels expressed in Xenopus oocytes. Mol Pharmacol 46:750–753 [PubMed] [Google Scholar]
- Dai L, Garg V, Sanguinetti MC. (2010) Activation of Slo2.1 channels by niflumic acid. J Gen Physiol 135:275–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanaraj V, Vijayan M. (1988) Structural studies of analgesics and their interactions. XII. Structure and interactions of anti-inflammatory fenamates. A concerted crystallographic and theoretical conformational study. Acta Crystallogr B 44:406–412 [DOI] [PubMed] [Google Scholar]
- Farrugia G, Rae JL, Sarr MG, Szurszewski JH. (1993) Potassium current in circular smooth muscle of human jejunum activated by fenamates. Am J Physiol 265:G873–G879 [DOI] [PubMed] [Google Scholar]
- Fernandez D, Sargent J, Sachse FB, Sanguinetti MC. (2008) Structural basis for ether-a-go-go-related gene K+ channel subtype-dependent activation by niflumic acid. Mol Pharmacol 73:1159–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower R, Gryglewski R, Herbaczyńska-Cedro K, Vane JR. (1972) Effects of anti-inflammatory drugs on prostaglandin biosynthesis. Nat New Biol 238:104–106 [DOI] [PubMed] [Google Scholar]
- Gögelein H, Dahlem D, Englert HC, Lang HJ. (1990) Flufenamic acid, mefenamic acid and niflumic acid inhibit single nonselective cation channels in the rat exocrine pancreas. FEBS Lett 268:79–82 [DOI] [PubMed] [Google Scholar]
- Greenwood IA, Large WA. (1995) Comparison of the effects of fenamates on Ca-activated chloride and potassium currents in rabbit portal vein smooth muscle cells. Br J Pharmacol 116:2939–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribkoff VK, Lum-Ragan JT, Boissard CG, Post-Munson DJ, Meanwell NA, Starrett JE, Jr, Kozlowski ES, Romine JL, Trojnacki JT, Mckay MC, et al. (1996) Effects of channel modulators on cloned large-conductance calcium-activated potassium channels. Mol Pharmacol 50:206–217 [PubMed] [Google Scholar]
- Grover GJ, D'Alonzo AJ, Sleph PG, Dzwonczyk S, Hess TA, Darbenzio RB. (1994) The cardioprotective and electrophysiological effects of cromakalim are attenuated by meclofenamate through a cyclooxygenase-independent mechanism. J Pharmacol Exp Ther 269:536–540 [PubMed] [Google Scholar]
- Hu H, Tian J, Zhu Y, Wang C, Xiao R, Herz JM, Wood JD, Zhu MX. (2010) Activation of TRPA1 channels by fenamate nonsteroidal anti-inflammatory drugs. Pflugers Arch 459:579–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Zeng B, Chen GL, Bot D, Eastmond S, Elsenussi SE, Atkin SL, Boa AN, Xu SZ. (2012) Effect of non-steroidal anti-inflammatory drugs and new fenamate analogues on TRPC4 and TRPC5 channels. Biochem Pharmacol 83:923–931 [DOI] [PubMed] [Google Scholar]
- Kameyama M, Kakei M, Sato R, Shibasaki T, Matsuda H, Irisawa H. (1984) Intracellular Na+ activates a K+ channel in mammalian cardiac cells. Nature 309:354–356 [DOI] [PubMed] [Google Scholar]
- Liantonio A, Picollo A, Babini E, Carbonara G, Fracchiolla G, Loiodice F, Tortorella V, Pusch M, Camerino DC. (2006) Activation and inhibition of kidney CLC-K chloride channels by fenamates. Mol Pharmacol 69:165–173 [DOI] [PubMed] [Google Scholar]
- Liantonio A, Picollo A, Carbonara G, Fracchiolla G, Tortorella P, Loiodice F, Laghezza A, Babini E, Zifarelli G, Pusch M, et al. (2008) Molecular switch for CLC-K Cl− channel block/activation: optimal pharmacophoric requirements towards high-affinity ligands. Proc Natl Acad Sci USA 105:1369–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malykhina AP, Shoeb F, Akbarali HI. (2002) Fenamate-induced enhancement of heterologously expressed HERG currents in Xenopus oocytes. Eur J Pharmacol 452:269–277 [DOI] [PubMed] [Google Scholar]
- Ottolia M, Toro L. (1994) Potentiation of large conductance KCa channels by niflumic, flufenamic, and mefenamic acids. Biophys J 67:2272–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peretz A, Degani N, Nachman R, Uziyel Y, Gibor G, Shabat D, Attali B. (2005) Meclofenamic acid and diclofenac, novel templates of KCNQ2/Q3 potassium channel openers, depress cortical neuron activity and exhibit anticonvulsant properties. Mol Pharmacol 67:1053–1066 [DOI] [PubMed] [Google Scholar]
- Sanger GJ, Bennett A. (1979) Fenamates may antagonize the actions of prostaglandin endoperoxides in human myometrium. Br J Clin Pharmacol 8:479–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi CM, Ferreira G, Yang B, Gazula VR, Butler A, Wei A, Kaczmarek LK, Salkoff L. (2006) Opposite regulation of Slick and Slack K+ channels by neuromodulators. J Neurosci 26:5059–5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreibmayer W, Lester HA, Dascal N. (1994) Voltage clamping of Xenopus laevis oocytes utilizing agarose-cushion electrodes. Pflugers Arch 426:453–458 [DOI] [PubMed] [Google Scholar]
- Stühmer W. (1992) Electrophysiological recording from Xenopus oocytes. Methods Enzymol 207:319–339 [DOI] [PubMed] [Google Scholar]
- Wang HS, Dixon JE, McKinnon D. (1997) Unexpected and differential effects of Cl− channel blockers on the Kv4.3 and Kv4.2 K+ channels. Implications for the study of the I(to2) current. Circ Res 81:711–718 [DOI] [PubMed] [Google Scholar]
- Wang Z, Kimitsuki T, Noma A. (1991) Conductance properties of the Na(+)-activated K+ channel in guinea-pig ventricular cells. J Physiol 433:241–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MM, Aylwin M. (1990) Niflumic and flufenamic acids are potent reversible blockers of Ca2+-activated Cl− channels in Xenopus oocytes. Mol Pharmacol 37:720–724 [PubMed] [Google Scholar]
- Wiemuth D, Gründer S. (2011) The pharmacological profile of brain liver intestine Na+ channel: inhibition by diarylamidines and activation by fenamates. Mol Pharmacol 80:911–919 [DOI] [PubMed] [Google Scholar]
- Yuan A, Santi CM, Wei A, Wang ZW, Pollak K, Nonet M, Kaczmarek L, Crowder CM, Salkoff L. (2003) The sodium-activated potassium channel is encoded by a member of the Slo gene family. Neuron 37:765–773 [DOI] [PubMed] [Google Scholar]