Abstract
Introduction
Cancer antigen 125 (CA-125), known as a biomarker for women genital tract malignancies, could be also useful in detecting and monitoring endometriosis. The aim of this study was to evaluate CA-125 in serum and peritoneal fluid (PF) as an indicator of endometriosis.
Material and methods
Fifty-six patients admitted to the First Department of Obstetrics and Gynaecology for diagnostic or therapeutic laparoscopy conducted for infertility, pelvic pain, suspected endometriosis or ovarian cysts entered the study. Those with laparoscopically confirmed endometriosis were assigned to group A, those without this condition to group B. Blood for CA-125 was taken prior to surgery, centrifuged and assayed in accordance with the manufacturer's instructions (VIDAS CA-125 II). Peritoneal fluid and an endometrial biopsy were taken during laparoscopy. Statistical comparisons were performed using Statistica 7.1.
Results
Group A consisted of 44 women with laparoscopically confirmed diagnosis; 15 patients served as a control group. The mean value of CA-125 concentration in the endometriosis group was 33.98 U/ml, vs. 9.3 U/ml in the control group. The mean value of CA-125 in peritoneal fluid was 1241.88 U/ml in the non-endometriosis group versus 2640.23 U/ml in the study group; both results were statistically significant (p < 0.05). There was a significant correlation between the stage of endometriosis and CA-125 plasma concentration (R = 0.5993, p < 0.001). Cancer antigen 125 concentration in serum was a moderate predictor to distinguish between patients with and without endometriosis (AUC 0.794; 95% CI 0.668-0.921; p = 0.001).
Conclusions
Cancer antigen 125 is a well-known biomarker for endometriosis and helpful in daily clinical practice when endometriosis is suspected. The cut-off value in serum suggesting endometriosis with 68% sensitivity is 11 U/ml. This value is normal range for Ca-125 concentration.
Keywords: pelvic pain, infertility, laparoscopy, endometriosis
Introduction
Cancer antigen 125 (CA-125) is a protein (cell surface antigen, member of the mucin family glycoproteins, encoded by the MUC 16 gene) known since the early 1980s [1]. It serves as a biomarker of ovarian cancer, especially for monitoring ovarian cancer therapy and early recurrences [2, 3]. Other cancers characterized by CA-125 increase are those originating in the endometrium, fallopian tubes, lungs, breast and gastrointestinal tract (i.e. pancreatic cancer). In normal women, plasma concentrations of CA-125 are increased slightly at ovulation and significantly during menstruation. A marked increase is observed during pregnancy [4]. Wilczak et al. described a pathological mass in the pelvis with highly elevated CA-125 in serum, which suggested a malignant process of the ovary. Laparotomy in this case revealed a large inflammatory tumour connecting the right adnexa and appendix vermiformis [5]. Cancer antigen 125 is commonly elevated in endometriosis, especially in its moderate and severe degree. The disease is found in women of reproductive age [6], mostly in those with fertility problems. The prevalence of endometriosis is really unknown. It affects 15% to 70% of women diagnosed with chronic pelvic pain syndrome, endometrial ovarian cysts or infertility [7–9]. A recent study conducted by Barbosa et al. revealed that 16.25% of fertile asymptomatic patients operated on for other reasons had minimal or mild endometriosis [10]. The degree of endometriosis can only be established by invasive procedures: laparoscopy or open surgery. The CA-125 test has a specificity of 89% in detecting moderate and severe endometriosis according to a meta-analysis of 23 studies [9]. Its usefulness for mild endometriosis is limited by unsatisfactory sensitivity and specificity. Timing of blood collection for CA-125 in relation to the menstrual cycle significantly affects this test with higher values during and shortly after menstruation [11]. Abrao et al. found that CA-125 was the marker presenting the highest levels during the menstrual phase, between the first and third day of the cycle compared to the luteal phase of the cycle [12].
Nowadays the main goal is to find a group of biomarkers for endometriosis. CA-125 plays an important role in nearly all statistical analyses conducted on this topic recently. Mihalyi et al. recently presented the results of a study on six biomarkers. He proved that plasma levels of IL-6, IL-8 and CA-125 were increased in all women with endometriosis compared with the control group. More important is the fact that the elevated concentration of mentioned cytokines and CA-125 was statistically significant in minimal-mild endometriosis compared with the control group [14].
Our research on serum and peritoneal fluid concentration of CA-125 is part of a major experiment whose goal is to find a non-invasive procedure to diagnose endometriosis. Additionally we are evaluating vascular endothelial growth factor (VEGF), IL-1β and C-reactive protein (CRP) concentration in patients with endometriosis and after treatment of this disease with danazol. The end of this research is planned for 2012.
Material and methods
The study included 56 patients admitted to the First Department of Obstetrics and Gynaecology for diagnostic or therapeutic laparoscopy. The patients underwent laparoscopic surgery for infertility, pelvic pain, suspected endometriosis or ovarian cysts. Those with laparoscopically confirmed endometriosis were assigned to group A, those without this condition to group B (serving as a control group for the statistical analysis). The control group consisted of patients with tubal occlusion, benign ovarian cysts (except chocolate cysts) and polycystic ovary. Women without endometriosis in whom no other cause of infertility was found during laparoscopy were also assigned to group B. Women with any conditions known as influencing CA-125 concentration and with ovarian malignancy established by intraoperative histopathological examination as well as women in the luteal phase of the cycle were excluded from the study. The study protocol was approved by the Ethics Committee of the Medical University of Lodz. All subjects included in the study signed informed consent. Thereafter all women were asked to give blood and to complete the questionnaire about regularity and complaints of menstrual cycle. Blood samples were collected in sterile tubes, immediately transported to the laboratory and centrifuged (3000/min). Serum CA-125 levels were measured in accordance with the manufacturer's instructions (VIDAS CA-125 II). During the procedure peritoneal fluid and an endometrial biopsy were taken. Peritoneal fluid was aspirated by laparoscopic needle, immediately cooled and centrifuged in the same conditions as blood samples, diluted 11 times and assayed for CA-125 concentration. The dilution process was conducted using CA-125 diluent, a reagent included in the VIDAS kits. The biopsy of the endometrium was histopathologically examined to establish the phase of the menstrual cycle. The presence and extent of endometriosis were carefully assessed, in accordance with the rASRM 1996 (the Revised American Society for Reproductive Medicine) classification of endometriosis. In some cases a peritoneal biopsy or ovarian cyst excision was conducted. Specimens were fixed in a 10% formalin solution and sent for histopathological analysis.
Statistical analysis
Statistical comparisons were performed using Statistica 7.1 and the graphical analysis was conducted using SPSS 12.0. The null hypothesis was tested with Shapiro-Wilk test. If the distribution of tested parameters was not normal non-parametric Mann-Whitney U test was used for assessing whether two independent samples of observations had equally large values. For normal distribution Student's t-test was used to compare groups. Kruskal-Wallis test and Spearman's correlation – non-parametric measurement tools of statistical dependence between two variables – were used to assess the relationship between measured variables. Regardless of the statistical test, only p values ≤ 0.05 were considered significant.
Results
Endometriosis was confirmed laparoscopically in a group of 44 women; 15 women served as a control group. The differences in age and the menstruation length were not statistically significant between groups. Duration of the cycle was longer in the control group and was 37.3 days in comparison to 30.2 days in the endometriosis group and this difference was statistically significant. The percentage of smoking patients was higher in the control group (46.7% for n = 7 vs. 19.6% n = 8 in group A) and this difference was also statistically significant (p = 0.0422). The mean value of CA-125 concentrations in the endometriosis group was 33.98 U/ml, with the maximal value of 173.6 U/ml. The mean level of that protein concentration in the control group was 9.3 U/ml. The difference was statistically significant (p < 0.05). The same relation was observed in CA-125 concentration in perito-neal fluid. The mean value of 1241.88 U/ml in the non-endometriosis group was compared with 2640.23 U/ml in the study group and this difference was also found to be statistically significant.
The rASRM (1996) staging was applied for all patients with endometriosis. There were 27 patients in initial stages (I and II) and 22 patients in advanced stages (III and IV). The distribution of each stage is presented in Table I. Endometriosis of second stage was the most common diagnosis. The prevalence of endometriosis of stage I or II was 50%.
Table I.
Prevalence of endometriosis
| Endometriosis (EEC) | Number of patients | % |
|---|---|---|
| Control group | 15 | 27.78 |
| EEC I | 10 | 18.52 |
| EEC II | 17 | 31.48 |
| EEC III | 11 | 20.37 |
| EEC IV | 1 | 1.85 |
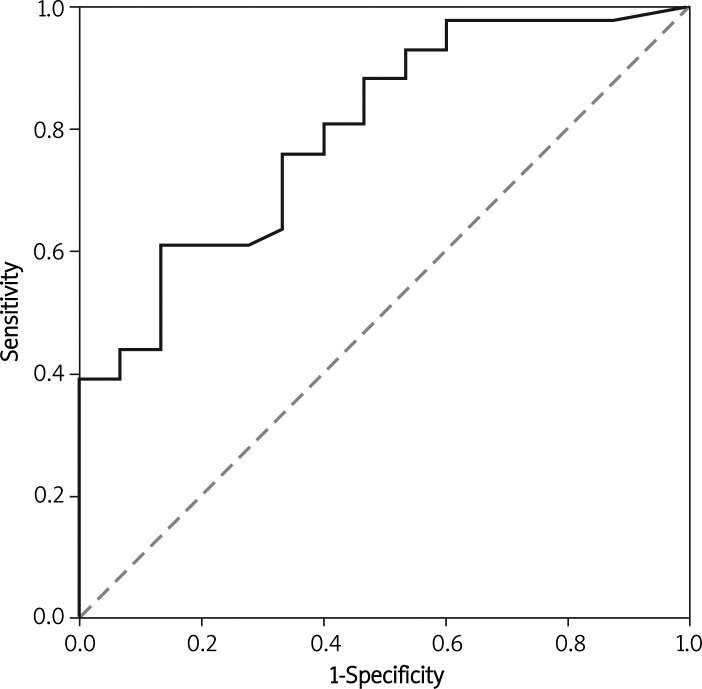
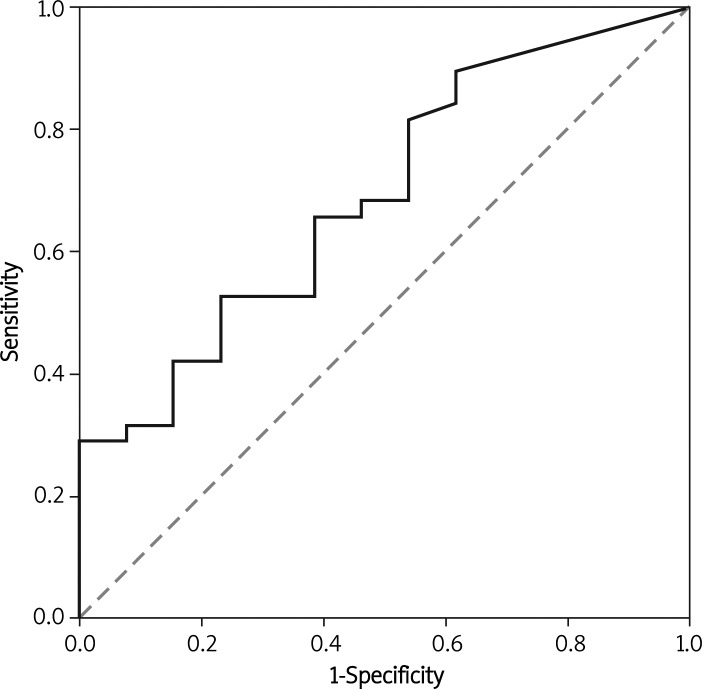
A receiver operating characteristic (ROC) curve is a graphical plot of the sensitivity versus false positive rate (two operating characteristics). In this case ROC analysis is related directly to making the best prognosis of endometriosis. The area under the ROC curve (AUC) estimates how good a predictor CA-125 could be for endometriosis (Figures I–II).
Figure 1.
ROC curve (marked thick line) and AUC (area under curve) for CA-125 concentration in plasma
Figure 2.
ROC curve (marked thick line) and AUC (area under curve) for CA-125 concentration in peritoneal fluid
Cancer antigen 125 concentration in serum was a moderate predictor to distinguish between patients with and without endometriosis (AUC 0.794; 95% CI 0.668-0.921; p = 0.001). The statistical power of CA-125 concentration in peritoneal fluid to differentiate between the two study groups was even worse than that of serum CA-125 (AUC 0.691; 95% CI 0.53-0.852; p = 0.041). If the cut-off value for CA-125 in serum is 11 U/ml, the respective sensitivity of the test – which measures the proportion of actual positives which are correctly identified as with endometriosis – is 68.29%. Specificity, which measures the proportion of negatives which are correctly identified (as without disease), is 66.67%. Positive predictive value (PPV) is 84.85% (the probability that a patient with positive test results is correctly diagnosed) and negative predictive value (NPV) (the probability that a patient with negative test results really does not have the disease) is 43.48%. For CA-125 tested in peritoneal fluid the cut-off point of 1295 U/ml was established from the ROC curve and the statistical analysis showed results as listed in Table II. AUC for CA-125 serum concentration in the control group and the group with the first stage of endometriosis I (EEC I) did not differ statistically significantly. AUC for this marker in the control and EEC II group was 0.798; 95% CI 0.645-0.951; p = 0.004. With the cut-off value for CA-125 ≥ 9.7 U/ml the sensitivity of the test for detecting EEC II was 82.35%. The statistical power of serum CA-125 concentration was the best for advanced stages of endometriosis (EEC III + EEC IV) in comparison to the control group – AUC 0.939; 95% CI 0.849-1.029; p < 0.001. The sensitivity and specificity for the cut-off point of CA-125 ≥ 14.7 U/ml established from the ROC curve are listed in Table III. AUC for peritoneal fluid concentration of CA-125 (for cut-off value of 1262.2 U/ml) in advanced stages of endometriosis is 0.654; 95% CI 0.418-0.890; p = 0.215.
Table II.
Statistical data for CA-125 = 1295 U/ml in peritoneal fluid
| Sensitivity | 60.53% |
| Specificity | 61.54% |
| Positive predictive value | 82.14% |
| Negative predictive value | 34.78% |
Table III.
Statistical data for CA-125 ≥ 14.7 U/ml in serum to diagnose advanced stages of endometriosis (EEC III + EEC IV)
| Sensitivity | 91.67% |
| Specificity | 86.67% |
| Positive predictive value | 84.62% |
| Negative predictive value | 92.86% |
We compared the frequency of occurrence of symptoms typical for endometriosis such as pain before menstrual bleeding, dysmenorrhoea and pain after intercourse. There was no significant difference in occurrence of listed symptoms between patients with and without endometriosis.
There is a significant correlation between the stage of endometriosis and CA-125 plasma concentration. The value of Spearman's rank correlation coefficient is R = 0.5993 (p < 0.001). It means that this is a positive correlation; however, a perfect Spearman correlation should be nearly +1 for variables that are a perfect monotone function of the other. There was no significant correlation between plasma and peritoneal concentration of CA-125.
Discussion
One percent of men and women have no reason for higher concentration of CA-125. This protein can be elevated in non-physiological conditions such as peritoneal infection, ascites, ovarian cysts, pancreatitis, heart or liver insufficiency and in patients after surgery of the gastrointestinal tract. The role of CA-125 in establishing the diagnosis of endometriosis is well known. However, the sensitivity of this biomarker alone is unsatisfactory. In our study the sensitivity of serum concentration of CA-125 in the diagnosis of disease was 68% reaching up to 91.67% for the diagnosis of advanced stages of endometriosis. Bedaiwy and Falcone reviewed the Medline database for studies about CA-125 performance in testing endometriosis. His meta-analysis showed that sensitivity of serum CA-125 varied in a wide range from 24% to 94%. The specificity reached in our study was only about 67%, but the cut-off point for CA-125 concentration was 11 U/ml. Most studies included in the meta-analysis accepted the value of 35 U/ml as a cut-off point for CA-125 serum concentration [15]. In one of the largest studies on CA-125 the authors proved that in the diagnosis of endometriosis without endometriomas, combined use of two cut-off values for CA-125, 20 U/ml and 30 U/ml, provides improved diagnostic performance [16]. There have been a lot of studies on the role of other biomarkers in endometriosis (e.g. TNF-α, IL-6, VEGF, CRP) conducted recently. The diagnostic accuracy of each marker alone was either similar or worse than that of CA-125. The concentrations of various cytokines, growth and angiogenic factors, metalloproteinases, peptides, subpopulations of leukocytes and expression of various genes were examined in endometriosis [17–19]. Nowadays researchers are trying to develop a statistical model based on three or four serum biomarkers which could have enough statistical power to diagnose endometriosis without the necessity of laparoscopy. Cancer antigen 125 measurement is used in nearly all studies that raise this issue.
Cancer antigen 125 concentration is usually analysed from a blood sample. It can also be measured in fluid from the chest or abdominal cavity. Assaying CA-125 in peritoneal fluid requires high sample dilutions or a modified immunoradiometric assay, and until now, its clinical value has been questionable. All the tests currently in use are based on the use of an antibody that is directed against the CA-125 protein (monoclonal antibody technique). Krasnicki proved that the sensitivity of the peritoneal fluid CA-125 test for endometriosis was higher than the respective serum test. He suggested that the measurement of CA-125 levels in peritoneal fluid could be useful in the detection of early stage endometriosis, which seems to be missed by the CA-125 serum test. In our opinion this dependence is not so clear. However, this study is not readily comparable with ours because of the different (luteal) menstrual cycle phase in which the study was conducted [20]. There are a lot of arguments that the CA-125 concentration is higher in the first cycle phase [4]. In our study an endometrial biopsy was taken from each patient to eliminate the differences between CA-125 concentration in the follicular and luteal phase of the menstrual cycle. Only women in the early follicular phase were included in the study.
The duration of the cycle in the control group in our study was probably influenced by other conditions such as polycystic ovary syndrome. Like other researchers we found no relationship between severity of symptoms which are believed to be typical for endometriosis and the extent of endometriotic lesions at laparoscopy. There were no differences between minimal and severe disease.
There are publications reporting a positive correlation between serum and peritoneal fluid values of CA-125 in women with and without endometriosis [21] but we did not find such a correlation in our study. Cancer antigen 125 levels are much higher in peritoneal fluid but to compare results between studies we should have information about the manufacturer of the CA-125 test and the way of preparing the test (e.g. dilution used).
In conclusion, serum CA-125 measurement is an inexpensive test whose role is to improve diagnostic accuracy for endometriosis. In our study CA-125 concentration in serum was a moderate predictor to distinguish between patients with and without this disease. There is a need for further studies about its role in statistical analysis as a marker that enhances statistical power for the group of non-invasive biomarkers for endometriosis.
Acknowledgments
The study was part of research supported by a grant: “The influence of danazol treatment on angiogenesis and inflammatory response in patients with endometriosis” no. 2431/B/P01/2009/37 financed by the Polish Ministry of Science and Higher Education.
References
- 1.Yin BW, Dnistrian A, Lloyd KO. Ovarian cancer antigen CA-125 is encoded by the MUC16 mucin gene. Int J Cancer. 2002;98:737–40. doi: 10.1002/ijc.10250. [DOI] [PubMed] [Google Scholar]
- 2.Nowak-Markwitz E, Michalak M, Spaczyński M. Prediction value of serum Ca 125 level in benefit secondary cytoreduction in advanced ovarian cancer patients. Współcz Onkol. 2003;7:662–7. [Google Scholar]
- 3.Berek JS, Taylor PT, Nicodemus CF. CA-125 velocity at relapse is a highly significant predictor of survival post relapse: results of a 5-year follow-up survey to a randomized placebo-controlled study of maintenance oregovomab immunotherapy in advanced ovarian cancer. J Immunother. 2008;31:207–14. doi: 10.1097/CJI.0b013e31816060ce. [DOI] [PubMed] [Google Scholar]
- 4.Muyldermans M, Cornillie FJ, Koninckx PR. CA-125 and endometriosis. Hum Reprod Update. 1995;1:173–87. doi: 10.1093/humupd/1.2.173. [DOI] [PubMed] [Google Scholar]
- 5.Wilczak M, Rzymski P, Stryjakowska K, Stanek R, Opala T. Highly elevated levels of the antigen Ca-125 associated with inflammatory abdominal masses. Arch Med Sci. 2007;3:278–80. [Google Scholar]
- 6.Kennedy S, Bergqvist A, Charon C, et al. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum Reprod. 2005;20:2698–704. doi: 10.1093/humrep/dei135. [DOI] [PubMed] [Google Scholar]
- 7.Mahmood TA, Templeton A. Prevalence and genesis of endometriosis. Hum Reprod. 1991;6:544–9. doi: 10.1093/oxfordjournals.humrep.a137377. [DOI] [PubMed] [Google Scholar]
- 8.Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160:784–96. doi: 10.1093/aje/kwh275. [DOI] [PubMed] [Google Scholar]
- 9.Child TJ, Tan SL. Endometriosis: aetiology, pathogenesis and treatment. Drugs. 2001;61:1735–50. doi: 10.2165/00003495-200161120-00005. [DOI] [PubMed] [Google Scholar]
- 10.Barbosa CP, de Souza AM, Bianco B, Christofolini DM, Mafra FA, de Lima GR. OC-125 immunostaining in endometriotic lesion samples. Arch Gynecol Obstet. 2009;281:43–7. doi: 10.1007/s00404-009-1055-7. [DOI] [PubMed] [Google Scholar]
- 11.Spaczynski RZ, Duleba AJ. Diagnosis of endometriosis. Semin Reprod Med. 2003;21:193–208. doi: 10.1055/s-2003-41326. [DOI] [PubMed] [Google Scholar]
- 12.Mol BW, Bayram N, Lijmer JG, et al. The performance of CA-125 measurement in the detection of endometriosis: a meta-analysis. Fertil Steril. 1998;70:1101–8. doi: 10.1016/s0015-0282(98)00355-0. [DOI] [PubMed] [Google Scholar]
- 13.Abrão MS, Podgaec S, Filho BM, Ramos LO, Pinotti JA, de Oliveira RM. The use of biochemical markers in the diagnosis of pelvic endometriosis. Hum Reprod. 1997;12:2523–7. doi: 10.1093/humrep/12.11.2523. [DOI] [PubMed] [Google Scholar]
- 14.Mihalyi A, Gevaert O, Kyama CM, et al. Non-invasive diagnosis of endometriosis based on a combined analysis of six plasma biomarkers. Hum Reprod. 2010;25:654–64. doi: 10.1093/humrep/dep425. [DOI] [PubMed] [Google Scholar]
- 15.Bedaiwy MA, Falcone T. Laboratory testing for endome-triosis. Clin Chim Acta. 2004;340:41–56. doi: 10.1016/j.cccn.2003.10.021. [DOI] [PubMed] [Google Scholar]
- 16.Kitawaki J, Ishihara H, Koshiba H, et al. Usefulness and limits of CA-125 in diagnosis of endometriosis without associated ovarian endometriomas. Hum Reprod. 2005;20:1999–2003. doi: 10.1093/humrep/deh890. [DOI] [PubMed] [Google Scholar]
- 17.Górski J, Szyłło K, Banasik M, Lewkowicz P, Tchórzewski H. CD4+, CD8+ and CD4 + CD25+ T lymphocytes in peripheral blood and peritoneal fluid of women with endometriosis – preliminary report. Arch Med Sci. 2007;3:37–42. [Google Scholar]
- 18.Kajihara H, Yamada Y, Kanayama S, et al. New insights into the pathophysiology of endometriosis: from chronic inflammation to danger signal. Gynecol Endocrinol. 2011;2:73–9. doi: 10.3109/09513590.2010.507292. [DOI] [PubMed] [Google Scholar]
- 19.Velasco I, Acién P, Campos A, Acién MI, Ruiz-Maciá E. Interleukin-6 and other soluble factors in peritoneal fluid and endometriomas and their relation to pain and aromatase expression. J Reprod Immunol. 2010;84:199–205. doi: 10.1016/j.jri.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Kraśnicki D. Serum and peritoneal fluid CA-125 concentration in women with endometriosis. Ginekol Pol. 2001;72:1365–9. [PubMed] [Google Scholar]
- 21.Amaral VF, Ferriani RA, Sá MF, et al. Positive correlation between serum and peritoneal fluid CA-125 levels in women with pelvic endometriosis. Sao Paulo Med J. 2006;124:223–7. doi: 10.1590/S1516-31802006000400010. [DOI] [PMC free article] [PubMed] [Google Scholar]