Abstract
Purpose
To examine the association between small for gestational age (SGA) in the first pregnancy and risk for infant mortality in the second pregnancy.
Methods
This is a population-based, retrospective cohort study in which we used the Missouri maternally linked cohort dataset for 1978–2005. Analyses were restricted to women who had two singleton pregnancies during the study period. The exposure was SGA in the first pregnancy, whereas the primary outcome was infant mortality in the second pregnancy. Kaplan-Meier Estimate and Cox proportional hazard regression were conducted.
Results
Infant mortality was significantly greater among mothers with previous SGA (P < .01). A persistent association of previous SGA with subsequent infant mortality was observed (adjusted hazard ratio [AHR] 1.35, 95% confidence interval [95% CI] 1.24–1.48). Race-specific data illustrated that black women with a previous SGA birth were 40% more likely to experience infant mortality (AHR 1.40, 95% CI 1.21–1.63) than their counterparts without a history of SGA, but white women with a previous SGA had an increased risk of 31% (AHR 1.31, 95% CI 1.17–1.46).
Conclusions
Women with previous SGA bear increased risks for subsequent infant mortality, which was greater among black mothers. Hence, SGA plays an important role in the black–white disparity in infant mortality. Women’s previous childbearing experiences could serve as important criterion in determining appropriate interconception strategies to improve infant health and survival.
Keywords: Small for gestational age, Infant mortality, Pregnancy, Birth outcomes
Introduction
The literature is rich with evidence that buttresses the importance of previous birth history as a predictor of subsequent pregnancy outcomes [1–10]. Previous history of delivering a small-for-gestational-age (SGA) infant not only increases the risk of having an SGA infant in the next pregnancy [5,11] but also increases the risk for subsequent stillbirth [1,9,12]. Consequently, a pregnancy that produces a SGA infant may result in lasting perinatal effects or predispositions that transmit or continue to the subsequent pregnancies, manifesting as a sibship aggregation of risks and adverse health outcomes. Nonetheless, it remains poorly understood whether subsequent siblings of SGA infants are also at increased risk of infant death. Furthermore, previous SGA history may play an unexplored role in the persistence of black–white disparity in infant mortality in the United States.
SGA (defined as a birth weight <10th population centile) is an important marker of fetal growth inhibition that often represents an underlying feto-placental pathology associated with pregnancy-related complications, such as preeclampsia, preterm labor, placental abruption, intrapartum complications, and stillbirth [13]. Infants born SGA experience an increased risk of death during infancy [14,15]. SGA also has been noted to contribute significantly to adult-onset disorders, including type 2 diabetes [16–19], hypertension [16,18–20], and coronary heart disease [16,19,21], as well as neurodevelopmental sequelae [22].
In the United States, infant mortality rates are complicated by a persistent black–white infant mortality gap [23,24]. Among infants born to non-Hispanic black mothers, the infant mortality rate is more than twice the rate of non-Hispanic whites (13.31 and 5.63 per 1000 live births, respectively) [25]. The mechanisms underlying this disparity are still under elucidation and likely involve a complex array of biologic and social ecological factors [26,27]. In a study conducted in the United Kingdom, investigators found that a history of SGA is a marker for subsequent sudden infant death syndrome [28], which points to the role of previous SGA as an important determinant of subsequent infant mortality, particularly post-neonatal mortality. Until now, the contribution of previous SGA history to the black–white disparity in infant mortality and its components has yet to be explored in the United States.
There is evidence of a black–white disparity in SGA, with black women having significantly greater risks for delivering a SGA infant [29]. Therefore, black women may disproportionately experience adverse outcomes associated with previously having a SGA infant. This issue highlights the need to explore history of SGA in the context of the black–white infant mortality gap, not only for identifying infants at risk of early mortality but also for the formulation of optimal prevention and educational strategies for pregnant women with a SGA child [30].
Furthermore, although it is understood that SGA infants are more likely to die [14,15], it remains poorly understood whether subsequent siblings of SGA infants also are at increased risk of infant death. Accordingly, we propose this study to examine the association between SGA and infant mortality in the first and second pregnancies, respectively. We hypothesize that subsequent siblings of infants born SGA experience a greater likelihood of mortality during infancy. We further hypothesize that the association between SGA in the first pregnancy and infant mortality in the subsequent pregnancy varies across racial/ethnic subgroups.
Methods
We conducted a population-based retrospective cohort using the Missouri maternally linked cohort data files from 1978 through 2005 (inclusive), with a total population of 2,278,952 births. This dataset links siblings to their biological mothers through the use of unique identifiers. A multistage algorithm was used to conduct the probabilistic linkage of the Missouri data files longitudinally. A computer program was used to compare a combination of variables from two files, giving a separate weight to each value that matches or mismatches. A weighted score for each possible pair of records reflecting the likelihood that they belong to the same person was calculated. Correct and incorrect match weights were assigned to each pair of records and summed.
A quality of agreement indicator, determined by the agreement of birth outcome, maternal first and family name, year and month of birth, and race, was then assigned to the remaining linkages. A priority indicator also was assigned, with the greatest priority given to records with a high linkage weight that matched exactly on month and year of birth, as well as maternal first and family names, and the lowest priority given to records with no agreement on maternal first and family names. Both quality of agreement and priority indicators were used to assess the validity of potential linkages. For multiple linkages, the most likely potential linkage was based on the weighted score and quality of agreement indicator. The methods and algorithm used in linking vital records information into sibling relationships and the process of validation have been described in detail previously [31,32].
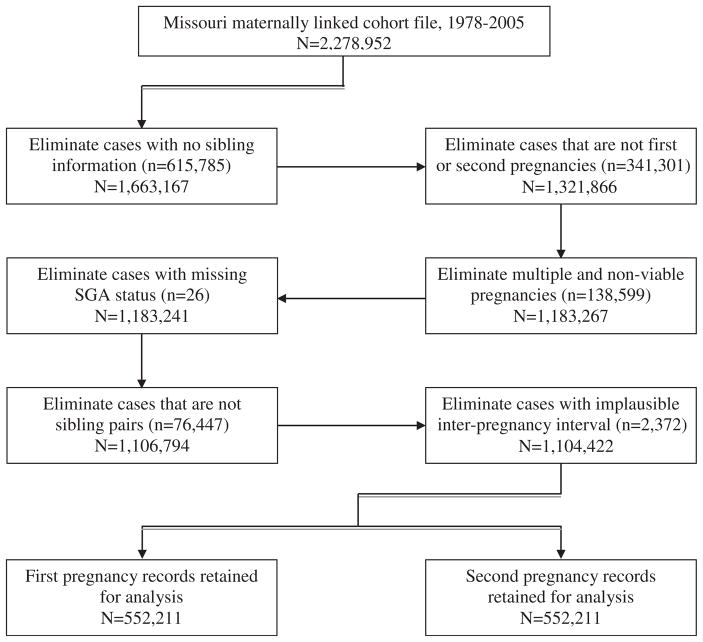
The Missouri vital record system is a reliable one that has been used as the gold standard to validate U.S. national datasets that involve matching and linking procedures [32,33]. The Missouri maternally linked cohort dataset contains information on both live births and fetal deaths for each sibling and provides a platform for a longitudinal study of birth outcomes for a given cohort of mothers. Because our objective was to examine the association between SGA in the first pregnancy and infant mortality in the second pregnancy, we excluded births to women who had only one pregnancy during the study period. After applying further inclusion and exclusion criteria for the study, we retained a total of 1,104,422 births (552,211 from each pregnancy) for this analysis. The step-by-step details of this elimination process are illustrated in a comprehensive manner in Figure 1.
Fig. 1.
Flow diagram of exclusion criteria for the study.
After the application of our inclusion and exclusion criteria, we then categorized the study population into an exposed group and an unexposed comparison group. The exposed group consisted of women who gave birth to an infant that was SGA in their first pregnancy, which accounted for 10.46% (n = 57,759) of the total population. The unexposed comparison group was composed of those who had a live birth of a non-SGA infant in their first pregnancy (89.54%; n = 494,452). SGA was defined as <10th percentile of birth weight for gestational age using population based national reference curve for singletons [34]. The women in this study were then followed to their second pregnancy and 1 year after delivery to document mortality status of their liveborn infants.
The main outcome of interest was infant mortality (death of the infant within the period from day 0 through 364) in the second pregnancy. We also computed secondary outcomes, including neonatal death (death of newborn within the first 28 days of life) and postneonatal death (death of the infant within the period from day 29 through 364). We further analyzed the study population to see the combined effect of infant mortality and SGA in the first pregnancy on infant mortality outcome on the second pregnancy. We compared mothers with previous SGA versus those who had non-SGA infants with respect to the following maternal characteristics at the time of the second delivery: race, age, marital status, educational level, cigarette smoking during pregnancy, body mass index (BMI), interpregnancy interval, and adequacy of prenatal care. Because of our focus on black–white differentials, maternal race was grouped into three categories: white, black, and other. Hispanics and other ethnicities were not considered as separate categories because of the very small proportion in the data (<3% of Missouri population) [35].
Maternal age was dichotomized as women who were of advanced age (ie, ≥35 years old) or <35 years old at the time of second delivery. Maternal marital status was grouped as either married or unmarried, with all persons divorced, widowed, or of unknown marital status classified as unmarried. Maternal educational level was categorized as those with at least a high school degree or its equivalent (≥12 years of education) and those without a high school degree (<12 years). Those mothers with missing and unknown educational information were grouped under the latter category (<12 years). Prepregnancy obesity was determined based on BMI, which was calculated as weight (kg)/height (m2) using height, as measured at the first prenatal visit of the second pregnancy, and prepregnancy weight, as reported at the first prenatal visit. Obese mothers were defined as those with a prepregnancy BMI of >30.
Adequacy of prenatal care was assessed using the Revised-Graduated Index (ie, R-GINDEX) algorithm. This index assesses the adequacy of care based on the trimester when prenatal care began, the number of visits, and the gestational age of the infant at birth [34]. In the present study, inadequate prenatal care use refers to women who either had missing prenatal care information, had prenatal care but the level was considered suboptimal (ie, fewer prenatal care visits as compared with the length of pregnancy), or had no prenatal care at all. The interval between the first day of the last menstrual period of the second pregnancy and the date of birth of the child in the first pregnancy was used to compute inter-pregnancy intervals in days.
We performed crude frequency comparisons for the presence of common obstetric and medical complications during the second pregnancy. These complications included anemia (defined as <30 cl/L hematocrit and/or <12 g/dL hemoglobin); insulin-dependent diabetes mellitus (defined as absolute deficiency of insulin secretion); other types of diabetes mellitus (defined as either gestational diabetes or adult-onset diabetes); chronic hypertension (defined as an increase in systolic or diastolic pre-existing blood pressure to a level of ≥140/90 mm Hg, respectively, before 20th week of gestation); pre-eclampsia (defined as pregnancy-induced high blood pressure at ≥140/90 mm Hg and excess protein in the urine after 20 weeks of pregnancy); eclampsia (defined as pregnancy-induced hypertension associated with convulsions); abruption placenta (defined as premature separation from the uterus of normally implanted placenta); placenta previa (defined as attachment of the placenta to the uterine wall close to or covering the cervix); cardiac diseases; and renal disease. We also constructed a composite variable indicating the presence of at least one of these conditions, which was used for the multivariate analyses.
To examine the impact of potential bias on our results attributable to missingness, we analyzed the data using two strategies [36,37]. First, we deleted cases with missing information from multivariable analysis. Second, we conducted multiple imputation for the missing data, assigning plausible values, and then analyzed the data. Both of these approaches yielded similar results; therefore, we reported the results based on available information.
Statistical analysis
The χ2 test was used to conduct crude frequency comparisons with respect to sociodemographic characteristics and pregnancy complications between the exposed and unexposed groups. The student sample t-test was used when the outcome was continuous (infant birth weight, gestational age, and interpregnancy interval). Infant, neonatal, and postneonatal mortality rates were computed by dividing the number of deaths by the total live births and multiplying by 1000. Infant death among mothers with previous SGA (exposed) and among those with no SGA (unexposed) was compared with the use of the Kaplan-Meier product-limit estimator, which calculates the cumulative probability of infant death for each group.
To determine whether the difference in neonatal death between the two groups was statistically significant, we applied the Wilcoxon statistic, instead of the more common Mantel log-rank test, because deaths were less common in the post-neonatal period. We used the Cox proportional hazards regression model to derive adjusted hazard ratios (AHR) after testing for nonviolation of the proportionality assumption in each case [38]. We confirmed this by plotting the log-negative-log of the Kaplan-Meier estimates of the survival function versus the log of time. The resulting curves were parallel. Adjusted hazard ratios were derived by loading all the variables that were considered to be potential confounders into the model. For this analysis, we estimated three models, first adjusting for selected sociodemographics, then adding pregnancy complications, and finally entering interpregnancy interval.
All hypothesis tests were two tailed, with a type 1 error rate fixed at 5%. SAS version 9.2 (SAS Institute, Cary, NC) was used to perform all analyses. This study was approved by the Institutional Review Board at the University of South Florida before initiation.
Results
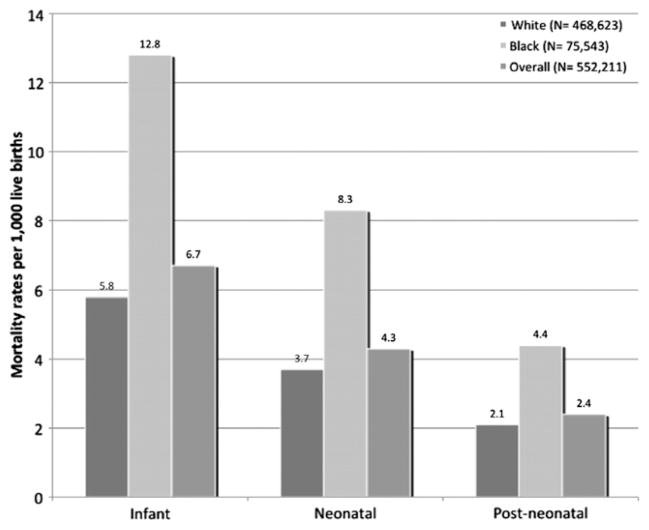
A total of 552,211 women had first and second consecutive singleton pregnancies in the study period. Of these, 57,759 women (10.46%) had experienced SGA in the first pregnancy (exposed group), whereas the remaining 494,452 women (89.54%) had a non-SGA infant (unexposed). Within the study population, a total of 3663 cases of infant death occurred in the second pregnancy, representing an infant mortality rate of 6.7 per 1000 (Fig. 2). Of these, 2355 cases (64.3%) occurred within the neonatal period, representing a neonatal mortality rate of 4.3 per 1000. Infant, neonatal, and postneonatal mortality rates among black subjects were more than twice the rates experienced in among white subjects.
Fig. 2.
Infant mortality rates among the study population at second pregnancy (Missouri: 1978–2005).
A comparison of selected maternal demographic characteristics and obstetric complications in the second pregnancy by exposure status is presented in Table 1. Women with SGA in the first pregnancy were more likely to be black, had a lower level of education (<12 years), and smoked during pregnancy (P < .01). These women also tended to have a greater level of adequate prenatal care (P < .01). On the other hand, women with a non-SGA birth in the first pregnancy were more likely to be white, of advanced age (≥35 years), married, and obese (P < .01). On average, the length of pregnancy in mothers with previous SGA was notably shorter than for those with a non-SGA infant (mean gestational age ± SD: 38.6 ± 2.8 weeks vs. 39.0 ± 2.3 weeks; P <.0001), whereas the inter-pregnancy interval was considerably longer among mothers who previously had a SGA infant (mean interpregnancy interval ± SD: 1,014.5 ± 916.8 days vs. 993.2 ± 856.7 days; P < .0001). Infants of mothers with previous SGA birth weighed on average 457.9 g less than those whose mothers did not have a history of SGA (mean birth weight ± SD: 2999.7 ± 544.2 gm vs. 3457.6 ± 540.9 g; P <.0001).
Table 1.
Distribution of selected maternal sociodemographic and pregnancy complications in the second pregnancy between women with SGA infants in the first pregnancy and those with non-SGA infants (Missouri: 1978–2005)
| Sociodemographic characteristics, N = 552,211 | SGA, n = 57,759, n (%) | No SGA, n = 494,452, n (%) | P |
|---|---|---|---|
| Race | <.01 | ||
| White | 43,453 (75.23) | 425,170 (85.99) | |
| Black | 13,293 (23.01) | 62,250 (12.59) | |
| Other | 1013 (1.75) | 7032 (1.42) | |
| Advance maternal age (≥35 years) | 4287 (7.42) | 42,814 (8.66) | <.01 |
| Maternal education (<12 years) | 14,693 (25.24) | 79,634 (16.11) | <.01 |
| Marital status (married) | 39,103 (67.70) | 394,752 (79.84) | <.01 |
| Maternal smoking | 21,641 (37.47) | 105,097 (21.26) | <.01 |
| Obesity (BMI > 30) | 6558 (11.35) | 69,868 (14.13) | <.01 |
| Adequate prenatal care | 32,002 (55.41) | 258,660 (52.31) | <.01 |
| Pregnancy complications | |||
| Anemia | 595 (1.03) | 4446 (0.90) | <.01 |
| Insulin-dependent diabetes mellitus | 210 (0.36) | 2467 (0.50) | <.01 |
| Other types of diabetes | 741 (1.28) | 7449 (1.51) | <.01 |
| Chronic hypertension | 545 (0.94) | 2788 (0.56) | <.01 |
| Preeclampsia | 1723 (2.98) | 8941 (1.81) | <.01 |
| Eclampsia | 38 (0.07) | 174 (0.04) | <.01 |
| Abruptio placenta | 422 (0.73) | 2205 (0.45) | <.01 |
| Placenta previa | 152 (0.26) | 1381 (0.28) | .38 |
| Renal disease | 115 (0.20) | 690 (0.14) | <.01 |
| Cardiac diseases | 237 (0.41) | 1882 (0.38) | .22 |
| Overall complications* | 2649 (4.59) | 20,724 (4.19) | <.01 |
BMI = body mass index; SGA = small for gestational age.
Overall complications is a composite variable indicating the presence of at least one of the listed pregnancy complications.
The incidence of pregnancy complications during the second pregnancy was 4.23% (23,373) in the overall study population. Pregnancy complications were greater among mothers who experienced SGA during their first pregnancy, as compared with those with non-SGA births (4.59% vs. 4.19%; P < .01). Using crude rates, mothers with previous SGA were approximately 1.5 times as likely to experience chronic hypertension, preeclampsia, eclampsia, abruptio placenta, and renal disease in their second pregnancy and were about 1.1 times as likely to present with anemia in their second pregnancy. Comparable rates of placenta previa and cardiac diseases were observed in both groups, whereas diabetes was observed to be greater in frequency in mothers with no previous SGA (Table 1).
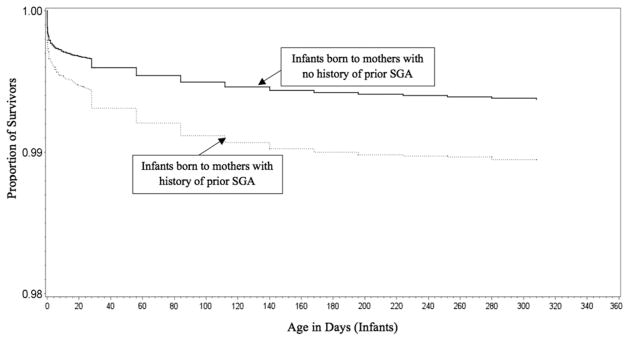
A comparison between mothers who had experienced previous SGA versus those with no previous SGA relative to the cumulative probability of survival at 365 days after birth is displayed using the Kaplan-Meier curve in Figure 3. This figure shows the rate of infant mortality to be significantly greater among mothers with previous SGA (P < .01). Table 2 provides AHR estimates for the association between the occurrence of SGA in the first pregnancy and infant death in the second pregnancy, stratified by racial groups. Model 1 provides hazard ratios adjusted for sociodemographic variables in the second pregnancy, whereas Model 2 provides estimates with further adjustment for pregnancy complications, and Model 3 comprises Model 2, plus adjustment for interpregnancy interval. In Model 1, women with previous SGA were 1.35 times as likely to experience infant death during the second pregnancy (AHR 1.35; 95% confidence interval [CI] 1.24–1.48), and this heightened risk of mortality remained during the neonatal period (AHR 1.42; 95% CI 1.27–1.58) and the postneonatal period (AHR 1.24; 95% CI 1.07–1.44). Black mothers with previous SGA demonstrated a 40% greater risk for infant mortality (AHR 1.40; 95% CI 1.21–1.63), which was slightly more increased when we assessed the mortality risk within the neonatal period (AHR 1.52; 95% CI 1.27–1.83), as compared with their non-SGA counterparts.
Fig. 3.
Kaplan-Meier curves comparing cumulative probability of infant death between infants born to mothers who had previous SGA and with those born to mothers with no previous SGA (Missouri: 1978–2005).
Table 2.
Risk of infant, neonatal, and postneonatal mortality among women who experienced SGA birth in the first pregnancy by race (Missouri: 1978–2005)
| N = 552,211 | Model 1*
|
Model 2†
|
Model 3‡
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Infant AHR (95% CI) | Neonatal AHR (95% CI) | Post-neonatal AHR (95% CI) | Infant AHR (95% CI) | Neonatal AHR (95% CI) | Post-neonatal AHR (95% CI) | Infant AHR (95% CI) | Neonatal AHR (95% CI) | Post-neonatal AHR (95% CI) | |
| Women with no previous SGA | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
|
| |||||||||
| Women with previous SGA | 1.35 (1.24–1.48)§ | 1.42 (1.27–1.58)§ | 1.24 (1.07–1.44)§ | 1.35 (1.23–1.47)§ | 1.41 (1.27–1.58)§ | 1.24 (1.07–1.44)§ | 1.35 (1.23–1.47)§ | 1.41 (1.27–1.58)§ | 1.24 (1.06–1.43)§ |
| White women with no previous SGA | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| White women with previous SGA | 1.31 (1.17–1.46)§ | 1.34 (1.16–1.54)§ | 1.27 (1.05–1.52)§ | 1.30 (1.16–1.45)§ | 1.33 (1.15–1.53)§ | 1.26 (1.05–1.51)§ | 1.30 (1.16–1.45)§ | 1.32 (1.15–1.52)§ | 1.26 (1.05–1.51)§ |
| Black women with no previous SGA | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| Black women with previous SGA | 1.40 (1.21–1.63)§ | 1.52 (1.27–1.83)§ | 1.21 (0.93–1.56) | 1.41 (1.21–1.63)§ | 1.52 (1.27–1.83)§ | 1.21 (0.93–1.56) | 1.41 (1.21–1.63)§ | 1.53 (1.27–1.83)§ | 1.21 (0.93–1.56) |
AHR = adjusted hazard ratio; CI = confidence interval; Ref = referent group; SGA = small for gestational age.
Model 1: Adjusted for race (only for the overall analysis comparing those with previous SGA and no previous SGA irrespective of race), maternal age, maternal education, maternal marital status, maternal smoking, obesity, gender of the child, year of birth, and prenatal care adequacy.
Model 2: Adjusted for Model 1 + overall complication. NB: Overall complications is a composite variable indicating the presence of at least one of the following: anemia, insulin dependent diabetes, other diabetes, chronic hypertension, cardiac diseases, abruption, and renal disease.
Model 3: adjusted for Model 2 + interpregnancy interval.
Significant values.
The risk among white women was slightly lower, with white mothers who previously had a SGA infant having a 31% increased likelihood of subsequent infant mortality (AHR 1.31; 95% CI 1.17–1.46). Similar increases among white women were observed for subsequent neonatal mortality (AHR 1.34; 95% CI 1.16–1.54) and postneonatal mortality (AHR 1.27; 95% CI 1.05–1.52). When we adjusted for pregnancy complications and interpregnancy interval in Models 2 and 3, we found similar results across all groups, with nonsignificant levels remaining for the postneonatal period in black women. However, there was no evidence for an interaction between previous SGA and race because the interaction term was not statistically significant.
To investigate the role of SGA in racial differences in infant mortality, we examined the effect estimate for race before and after adjusting for SGA in the second pregnancy. Adjustment for SGA in the second pregnancy reduced the risk of infant mortality by 37% in white women and by 20% in black women. The risk of infant mortality remained statistically significant in black women (AHR 1.20; 95% CI 1.03–1.40) but not in white women (AHR 0.93; 95% CI 0.82–1.05).
We also analyzed the likelihood of infant mortality in the second pregnancy based on the co-occurrence of infant mortality and SGA with the first pregnancy, and the results are presented in Table 3. This analysis showed that black mothers who lost their SGA infants at first pregnancy were at a more than fivefold greater risk of infant mortality during the second pregnancy (AHR 5.33; 95% CI 3.13–9.07), compared with white mothers with no exposure to both SGA and infant mortality, whereas white mothers with the same childbirth history of SGA and infant mortality were more than twice as likely to experience infant death in the second pregnancy (AHR 2.23; 95% CI 1.36–3.66). Compared with white mothers with no SGA and no infant mortality, black mothers who lost their non-SGA infant during the first pregnancy were than four times as likely to experience infant death in the subsequent pregnancy (AHR 4.09; 95% CI 2.64–6.33), and white mothers who lost their non-SGA infant during the first pregnancy were more than three times as likely to experience repeat infant death (AHR 3.39; 95% CI 2.62–4.39).
Table 3.
Risk of infant mortality in the second pregnancy among women with a history of infant mortality/SGA, as well as combined SGA and infant mortality in the first pregnancy by race (Missouri: 1978–2005)
| First pregnancy outcome | Infant mortality at second pregnancy, AHR (95% CI)* |
|---|---|
| White: no SGA & no infant death | Referent |
| Black: no SGA & no infant death | 1.54 (1.39–1.70)* |
| White: SGA & infant death | 2.23 (1.36–3.66)* |
| Black: SGA & infant death | 5.33 (3.13–9.07)* |
| White: no SGA & infant death | 3.39 (2.62–4.39)* |
| Black: no SGA & infant death | 4.09 (2.64–6.33)* |
| White: SGA & no infant death | 1.33 (1.19–1.49)* |
| Black: SGA & no infant death | 2.07 (1.78–2.41)* |
AHR = adjusted hazard ratio; BMI = body mass index; CI = confidence interval.
Adjusted for education, marital status, maternal age, BMI, smoking, adequacy of prenatal care, gender of the child, year of birth, inter-pregnancy interval, and overall complication.
NB: Overall complication is a composite variable indicating the presence of at least one of the following: anemia, insulin-dependent diabetes, other diabetes, chronic hypertension, cardiac diseases, abruption, and renal disease.
Significant values.
Discussion
In this population-based study, we found that a history of SGA was associated with an increased likelihood of subsequent infant mortality. However, this relationship differed by race/ethnicity, with a heightened risk for black mothers compared with white mothers. These results are suggestive that SGA in the first pregnancy is predictive of infant mortality in a subsequent pregnancy, albeit with considerable variation by race. Our stratified analysis for the association between previous SGA and subsequent infant mortality should be interpreted with regards to the lack of evidence for significant interaction between previous SGA and race. Although our analysis did not reveal a significant interaction between race and previous SGA on subsequent infant mortality, we did observe some important relationships. As compared to their white counterparts with similar experience (AHR 2.23; 95% CI 1.36–3.66), black women with previous SGA and infant death (AHR 5.33; CI 3.13–9.07) had a 300% greater likelihood for subsequent infant mortality even after adjustment for other risk factors (Table 3). The present study confirmed the association between SGA in a first pregnancy and the risk for infant mortality in the subsequent pregnancy found in a study conducted by Smith and colleagues [28] in the United Kingdom. Furthermore, to the authors’ knowledge, this is the first study to assess this association in the context of ethnoracial disparities in the United States.
In this context, we found that a history of a previous SGA plays an important role in the black–white disparities in infant mortality in the United States. One reasonable explanation for this finding is that similar sociodemographic factors might impact both the occurrence of SGA in the first pregnancy and infant mortality in the subsequent pregnancy. It is well established that low socioeconomic status of the mother can serve as precursor of infant morbidity and mortality [39–42]. In particular, lower maternal education and income levels are associated with adverse maternal behaviors (such as cigarette smoking during pregnancy) that can negatively affect birth outcomes [42–47]. In our study, mothers with previous SGA were more likely to have a lower level of education (<12 years). However, after controlling for maternal education and other socio-demographic factors, the association of SGA with infant, neonatal, and postneonatal persisted, making the explanation of persistent maternal disadvantage less likely (although not completely excluded).
An alternative explanation is that the persistence of maternal complications from one pregnancy to the next could be the link between SGA and subsequent infant mortality. Previous SGA was associated with the occurrence of several complications in the second pregnancy, including anemia, chronic hypertension, pre-eclampsia/eclampsia, and abruptio placentae. However, the effects of SGA on subsequent infant mortality remained minimally influenced after controlling for pregnancy complications. This makes the explanation of pregnancy complications less likely of being the solely determinant of the relationship between previous SGA and subsequent infant mortality. The observation of an association between SGA in the first pregnancy and subsequent infant mortality that was not explained by sociodemographic factors, pregnancy complications, interpregnancy interval, or previous infant death supports an independent effect of SGA on subsequent infant mortality. The findings in this study should, therefore, be regarded as an impetus for more elaborate studies on SGA and its link to infant survival in subsequent births.
There are a number of potential limitations to note regarding the present study. First, as we used an existing dataset, we were restricted in considering the role of available variables potentially important in the association between SGA and subsequent infant mortality. However, the Missouri maternally linked cohort data files originate from a vital record system considered reliable and with a documented validation process in previous studies [31–33]. We made the decision to dichotomize all covariates included in multivariable analyses. Previous authors have recommended against dichotomizing continuous predictors in regression analyses [48,49]. It is possible that our decision to dichotomize may have influenced our findings in an unintended manner. Nevertheless, our categorization decisions were based on established clinical and epidemiological cut-off points that represent differential levels of risk or vulnerability. For instance, marital status was dichotomized in married and unmarried, based on the fact that unmarried is a risk factor associated to adverse pregnancy outcomes [50].
Similarly, less than 12 years of education has been found to significantly increase the risk for adverse health outcomes. Another limitation was imposed by the exclusion of other ethno-racial groups, such as Asian, Native Americans, and Hispanic/Latinas, which restrict the generalizability of our findings to only non-Hispanic whites and non-Hispanic blacks included in the study. However, the proportions of other ethnoracial groups within our study population were extremely low and, consequently, were excluded due to insufficient power in the sub-group analysis.
There were some unmeasured biopsychosocial factors that could have played a role but were not included in our model, including stress [51–54], perceptions of racism and discrimination [55,56], social support [57], intimate partner violence [58], paternal contributions [59,60], and neighborhood-level factors [61,62]. In this regard, black women are more likely to suffer the effects of racial discrimination as an added chronic stressor [63,64], which has been consistently implicated in adverse birth outcomes and could have explained black–white differentials. However, because of the limitation of our data, we were not able to assess the contribution of the added burden of chronic stress, racism, and discrimination. Further studies should explore the synergistic effects of stress, racism, and discrimination to clarify the association between SGA and subsequent adverse pregnancy outcome. Another important set of unmeasured covariates include paternal factors that may influence pregnancy outcomes, such as paternal involvement during pregnancy [65–69], and the effect of partner change on the risk of subsequent adverse pregnancy outcomes [59,60]. In this regard, the data set used in this study does not have any paternal-level information available for analysis. Future studies are warranted to uncover the potential role of paternal factors in subsequent adverse pregnancy outcomes.
Overall, a visible strength in this study is the large population size covered, which offers substantial power for detection of group differences in the analysis. Another merit of the analysis is that the results come from a population-wide database, which will, therefore, be minimally impacted by selectivity of individuals (eg, referrals, etc.), a potent source of selection bias. As a consequence, the results in this study are reasonably generalizable. A unique feature in this paper is the subanalysis by race/ethnicity, which offers more insights into black–white disparity with respect to infant mortality based on previous SGA experience. The observation that the risk conferred by previous SGA on subsequent infant mortality was considerably more elevated in blacks points to a persistent ethnoracial disadvantage with potential public health implications. Such ethnoracial disadvantage was also corroborated in a previous study by the authors [4] that explored the role of previous stillbirth on subsequent infant mortality. These findings warrant further studies to uncover the biopsychosocial mechanisms that can explain why previous SGA is an independent risk factor for infant mortality in the context of ethnoracial disparities.
Our study, based on population-based data, underscores the utility of a women’s childbirth history, since it can contribute to the identification of potential risks for subsequent adverse pregnancy outcomes and provide opportunities for the attenuation of maternal and infant risks. More specifically, our finding that women with a history of SGA experience greater risk for subsequent infant mortality could serve as an important criterion in determining appropriate interconception strategies to improve maternal and infant health. In this regard, the findings in this paper highlight the importance of maternal biopsychosocial risk assessment using a life course perspective [70,71] as a platform for targeted interconceptional interventions.
References
- 1.Surkan PJ, Stephansson O, Dickman PW, Cnattingius S. Previous preterm and small-for-gestational-age births and the subsequent risk of stillbirth. N Engl J Med. 2004;350(8):777–85. doi: 10.1056/NEJMoa031587. [DOI] [PubMed] [Google Scholar]
- 2.Ekwo E, Moawad A. The risk for recurrence of premature births to African-American and white women. J Assoc Acad Minor Phys. 1998;9(1):16–21. [PubMed] [Google Scholar]
- 3.Wikstrom AK, Stephansson O, Cnattingius S. Previous preeclampsia and risks of adverse outcomes in subsequent nonpreeclamptic pregnancies. Am J Obstet Gynecol. 2011;204(2):148.e1–6. doi: 10.1016/j.ajog.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 4.Salihu HM, August EM, Weldeselasse HE, Biroscak BJ, Mbah AK. Stillbirth as a risk factor for subsequent infant mortality. Early Hum Dev. 2011;87(9):641–6. doi: 10.1016/j.earlhumdev.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Bakewell JM, Stockbauer JW, Schramm WF. Factors associated with repetition of low birthweight: Missouri longitudinal study. Paediatr Perinat Epidemiol. 1997;11(Suppl 1):119–29. doi: 10.1046/j.1365-3016.11.s1.4.x. [DOI] [PubMed] [Google Scholar]
- 6.Mbah AK, Alio AP, Marty PJ, Bruder K, Wilson R, Salihu HM. Recurrent versus isolated pre-eclampsia and risk of feto-infant morbidity outcomes: racial/ethnic disparity. Eur J Obstet Gynecol Reprod Biol. 2011;156(1):23–8. doi: 10.1016/j.ejogrb.2010.12.036. [DOI] [PubMed] [Google Scholar]
- 7.Adams MM, Elam-Evans LD, Wilson HG, Gilbertz DA. Rates of and factors associated with recurrence of preterm delivery. JAMA. 2000;283(12):1591–6. doi: 10.1001/jama.283.12.1591. [DOI] [PubMed] [Google Scholar]
- 8.Lee T, Carpenter MW, Heber WW, Silver HM. Preterm premature rupture of membranes: risks of recurrent complications in the next pregnancy among a population-based sample of gravid women. Am J Obstet Gynecol. 2003;188(1):209–13. doi: 10.1067/mob.2003.115. [DOI] [PubMed] [Google Scholar]
- 9.Smith GC, Shah I, White IR, Pell JP, Dobbie R. Previous preeclampsia, preterm delivery, and delivery of a small for gestational age infant and the risk of unexplained stillbirth in the second pregnancy: a retrospective cohort study, Scotland, 1992–2001. Am J Epidemiol. 2007;165(2):194–202. doi: 10.1093/aje/kwj354. [DOI] [PubMed] [Google Scholar]
- 10.Lykke JA, Paidas MJ, Langhoff-Roos J. Recurring complications in second pregnancy. Obstet Gynecol. 2009;113(6):1217–24. doi: 10.1097/AOG.0b013e3181a66f2d. [DOI] [PubMed] [Google Scholar]
- 11.Kleijer ME, Dekker GA, Heard AR. Risk factors for intrauterine growth restriction in a socio-economically disadvantaged region. J Matern Fetal Neonatal Med. 2005;18(1):23–30. doi: 10.1080/14767050500127674. [DOI] [PubMed] [Google Scholar]
- 12.Salihu HM, Sharma PP, Aliyu MH, Kristensen S, Grimes-Dennis J, Kirby RS, et al. Is small for gestational age a marker of future fetal survival in utero? Obstet Gynecol. 2006;107(4):851–6. doi: 10.1097/01.AOG.0000206185.55324.5b. [DOI] [PubMed] [Google Scholar]
- 13.McCowan L, Horgan RP. Risk factors for small for gestational age infants. Best Pract Res Clin Obstet Gynaecol. 2009;23(6):779–93. doi: 10.1016/j.bpobgyn.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Kristensen S, Salihu HM, Keith LG, Kirby RS, Fowler KB, Pass MA. SGA subtypes and mortality risk among singleton births. Early Hum Dev. 2007;83(2):99–105. doi: 10.1016/j.earlhumdev.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Getahun D, Amre D, Rhoads GG, Demissie K. Maternal and obstetric risk factors for sudden infant death syndrome in the United States. Obstet Gynecol. 2004;103(4):646–52. doi: 10.1097/01.AOG.0000117081.50852.04. [DOI] [PubMed] [Google Scholar]
- 16.Morgan AR, Thompson JM, Murphy R, Black PN, Lam WJ, Ferguson LR, et al. Obesity and diabetes genes are associated with being born small for gestational age: results from the Auckland Birthweight Collaborative study. BMC Med Genet. 2010;11:125. doi: 10.1186/1471-2350-11-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forsen T, Eriksson J, Tuomilehto J, Reunanen A, Osmond C, Barker D. The fetal and childhood growth of persons who develop type 2 diabetes. Ann Intern Med. 2000;133(3):176–82. doi: 10.7326/0003-4819-133-3-200008010-00008. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson J, Forsen T, Tuomilehto J, Osmond C, Barker D. Fetal and childhood growth and hypertension in adult life. Hypertension. 2000;36(5):790–4. doi: 10.1161/01.hyp.36.5.790. [DOI] [PubMed] [Google Scholar]
- 19.Barker DJ, Eriksson JG, Forsen T, Osmond C. Fetal origins of adult disease: strength of effects and biological basis. Int J Epidemiol. 2002;31(6):1235–9. doi: 10.1093/ije/31.6.1235. [DOI] [PubMed] [Google Scholar]
- 20.Law CM, Shiell AW, Newsome CA, Syddall HE, Shinebourne EA, Fayers PM, et al. Fetal, infant, and childhood growth and adult blood pressure: a longitudinal study from birth to 22 years of age. Circulation. 2002;105(9):1088–92. doi: 10.1161/hc0902.104677. [DOI] [PubMed] [Google Scholar]
- 21.Forsen T, Eriksson JG, Tuomilehto J, Osmond C, Barker DJ. Growth in utero and during childhood among women who develop coronary heart disease: longitudinal study. BMJ. 1999;319(7222):1403–7. doi: 10.1136/bmj.319.7222.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kiess W, Chernausek SD, Hokken-Koelega ACS, Karger S. Small for gestational age causes and consequences. Basel, New York: Karger; 2009. [Google Scholar]
- 23.Centers for Disease Control and Prevention. Infant mortality and low birth weight among black and white infants–United States, 1980–2000. MMWR Morb Mortal Wkly Rep. 2002;51(27):589–92. [PubMed] [Google Scholar]
- 24.Anachebe NF. Racial and ethnic disparities in infant and maternal mortality. Ethn Dis. 2006;16(2 Suppl 3):S3–71–6. [PubMed] [Google Scholar]
- 25.Mathews TJ, MacDorman MF. Infant mortality statistics from the 2007 period linked birth/infant death data set. Natl Vital Stat Rep. 2011;59(6):1–30. [PubMed] [Google Scholar]
- 26.Kramer MR, Hogue CR. What causes racial disparities in very preterm birth? A biosocial perspective Epidemiol Rev. 2009;31:84–98. doi: 10.1093/ajerev/mxp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alio AP, Richman AR, Clayton HB, Jeffers DF, Wathington DJ, Salihu HM. An ecological approach to understanding black-white disparities in perinatal mortality. Matern Child Health J. 2010;14(4):557–66. doi: 10.1007/s10995-009-0495-9. [DOI] [PubMed] [Google Scholar]
- 28.Smith GCS, Wood AM, Pell JP, Dobbie R. Sudden infant death syndrome and complications in other pregnancies. Lancet. 2005;366(9503):2107–11. doi: 10.1016/S0140-6736(05)67888-9. [DOI] [PubMed] [Google Scholar]
- 29.Salihu HM, Fitzpatrick L, Aliyu MH. Racial disparity in fetal growth inhibition among singletons and multiples. Am J Obstet Gynecol. 2005;193(2):467–74. doi: 10.1016/j.ajog.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Hessol NA, Fuentes-Afflick E. Ethnic differences in neonatal and postneonatal mortality. Pediatrics. 2005;115(1):e44–51. doi: 10.1542/peds.2004-0478. [DOI] [PubMed] [Google Scholar]
- 31.Herman AA, McCarthy BJ, Bakewell JM, Ward RH, Mueller BA, Maconochie NE, et al. Data linkage methods used in maternally-linked birth and infant death surveillance data sets from the United States (Georgia, Missouri, Utah and Washington State), Israel, Norway, Scotland and Western Australia. Paediatr Perinat Epidemiol. 1997;11(Suppl 1):5–22. doi: 10.1046/j.1365-3016.11.s1.11.x. [DOI] [PubMed] [Google Scholar]
- 32.Aliyu MH, Lynch O, Belogolovkin V, Zoorob R, Salihu HM. Maternal alcohol use and medically indicated vs. spontaneous preterm birth outcomes: a population-based study. Eur J Public Health. 2010;20(5):582–7. doi: 10.1093/eurpub/ckq036. [DOI] [PubMed] [Google Scholar]
- 33.Martin J, Curtin S, Saulnier M, Mousavi J. Development of the matched multiple birthfile, 1995–1998 matched multiple birth dataset NCHS CD-ROM series 21, no 13a. Hyattsville, MD: National Center for Health Statistics; 2003. [Google Scholar]
- 34.Alexander GR, Kotelchuck M. Quantifying the adequacy of prenatal care: a comparison of indices. Public Health Rep. 1996;111(5):408–18. discussion 419. [PMC free article] [PubMed] [Google Scholar]
- 35.The Kaiser Family Foundation. Data source: Missouri: Population Distribution by Race/Ethnicity, states (2009–2010) [accessed 18.07.2012];US Census Bureau’s March 2010 and 2011 Current Population Survey (CPS: Annual Social and Economic Supplements) 2011 http://www.statehealthfacts.orgl.
- 36.Rubin DB. Inference and missing data. Biometrika. 1976;63(3):581–92. [Google Scholar]
- 37.Rubin DB. Multiple imputation after 18+ years. J Am Stat Assoc. 1996;91(434):473–89. [Google Scholar]
- 38.Cox DR. Regression models and life-tables. J Roy Stat Soc Ser B (Methodological) 1972;34(2):187–220. [Google Scholar]
- 39.Arora NK, Paul VK, Singh M. Morbidity and mortality in term infants with intrauterine growth retardation. J Trop Pediatr. 1987;33(4):186–9. doi: 10.1093/tropej/33.4.186. [DOI] [PubMed] [Google Scholar]
- 40.Ashworth A. Effects of intrauterine growth retardation on mortality and morbidity in infants and young children. Eur J Clin Nutr. 1998;52(Suppl 1):S34–41. discussion S41–2. [PubMed] [Google Scholar]
- 41.Starfield B, Shapiro S, McCormick M, Bross D. Mortality and morbidity in infants with intrauterine growth retardation. J Pediatr. 1982;101(6):978–83. doi: 10.1016/s0022-3476(82)80025-5. [DOI] [PubMed] [Google Scholar]
- 42.Kramer MS, Seguin L, Lydon J, Goulet L. Socio-economic disparities in pregnancy outcome: why do the poor fare so poorly? Paediatr Perinatal Epidemiol. 2000;14(3):194–210. doi: 10.1046/j.1365-3016.2000.00266.x. [DOI] [PubMed] [Google Scholar]
- 43.Tong VT, Jones JR, Dietz PM, D’Angelo D, Bombard JM. Trends in smoking before, during, and after pregnancy–Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 31 sites, 2000–2005. MMWR Surveill Summ. 2009;58(4):1–29. [PubMed] [Google Scholar]
- 44.Shoham-Yakubovich I, Barell V. Maternal education as a modifier of the association between low birthweight and infant mortality. Int J Epidemiol. 1988;17(2):370–7. doi: 10.1093/ije/17.2.370. [DOI] [PubMed] [Google Scholar]
- 45.Nicolaidis C, Ko CW, Saha S, Koepsell TD. Racial discrepancies in the association between paternal vs. maternal educational level and risk of low birth-weight in Washington State. BMC Pregnancy Childbirth. 2004;4(1):10. doi: 10.1186/1471-2393-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen J, Millar WJ. Birth outcome, the social environment and child health. Health Rep. 1999;10(4):57–67. (Eng); 59–71(Fre) [PubMed] [Google Scholar]
- 47.Millar WJ, Chen J. Maternal education and risk factors for small-for-gestational-age births. Health Rep. 1998;10(2):43–51. (Eng); 47–56 (Fre) [PubMed] [Google Scholar]
- 48.Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25(1):127–41. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- 49.Naggara O, Raymond J, Guilbert F, Roy D, Weill A, Altman DG. Analysis by categorizing or dichotomizing continuous variables is inadvisable: an example from the natural history of unruptured aneurysms. AJNR Am J Neuroradiol. 2011;32(3):437–40. doi: 10.3174/ajnr.A2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bennett T, Braveman P, Egerter S, Kiely JL. Maternal marital status as a risk factor for infant mortality. Fam Plann Perspect. 1994;26(6):252–6. 271. [PubMed] [Google Scholar]
- 51.Austin MP, Leader L. Maternal stress and obstetric and infant outcomes: epidemiological findings and neuroendocrine mechanisms. Aust N Z J Obstet Gynaecol. 2000;40(3):331–7. doi: 10.1111/j.1479-828x.2000.tb03344.x. [DOI] [PubMed] [Google Scholar]
- 52.Gennaro S, Hennessy MD. Psychological and physiological stress: impact on preterm birth. J Obstet Gynecol Neonatal Nurs. 2003;32(5):668–75. doi: 10.1177/0884217503257484. [DOI] [PubMed] [Google Scholar]
- 53.Gennaro S, Shults J, Garry DJ. Stress and preterm labor and birth in black women. J Obstet Gynecol Neonatal Nursing. 2008;37(5):538–45. doi: 10.1111/j.1552-6909.2008.00278.x. [DOI] [PubMed] [Google Scholar]
- 54.Latendresse G. The interaction between chronic stress and pregnancy: preterm birth from a biobehavioral perspective. J Midwifery Womens Health. 2009;54(1):8–17. doi: 10.1016/j.jmwh.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murrell NL. Stress, self-esteem, and racism: relationships with low birth weight and preterm delivery in African American women. J Natl Black Nurses Assoc. 1996;8(1):45–53. [PubMed] [Google Scholar]
- 56.Rich-Edwards J, Krieger N, Majzoub J, Zierler S, Lieberman E, Gillman M. Maternal experiences of racism and violence as predictors of preterm birth: rationale and study design. Paediatr Perinat Epidemiol. 2001;15(Suppl 2):124–35. doi: 10.1046/j.1365-3016.2001.00013.x. [DOI] [PubMed] [Google Scholar]
- 57.Collins NL, Dunkel-Schetter C, Lobel M, Scrimshaw SC. Social support in pregnancy: psychosocial correlates of birth outcomes and postpartum depression. J Pers Soc Psychol. 1993;65(6):1243–58. doi: 10.1037//0022-3514.65.6.1243. [DOI] [PubMed] [Google Scholar]
- 58.Boy A, Salihu HM. Intimate partner violence and birth outcomes: a systematic review. Int J Fertility Women’s Med. 2004;49(4):159–64. [PubMed] [Google Scholar]
- 59.Zhang J, Patel G. Partner change and perinatal outcomes: a systematic review. Paediatr Perinat Epidemiol. 2007;21(Suppl 1):46–57. doi: 10.1111/j.1365-3016.2007.00837.x. [DOI] [PubMed] [Google Scholar]
- 60.Vatten LJ, Skjaerven R. Effects on pregnancy outcome of changing partner between first two births: prospective population study. BMJ. 2003;327(7424):1138. doi: 10.1136/bmj.327.7424.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo ZC, Kierans WJ, Wilkins R, Liston RM, Mohamed J, Kramer MS. Disparities in birth outcomes by neighborhood income: temporal trends in rural and urban areas, British Columbia. Epidemiology. 2004;15(6):679–86. doi: 10.1097/01.ede.0000142149.34095.88. [DOI] [PubMed] [Google Scholar]
- 62.Janevic T, Stein CR, Savitz DA, Kaufman JS, Mason SM, Herring AH. Neighborhood deprivation and adverse birth outcomes among diverse ethnic groups. Ann Epidemiol. 2010;20(6):445–51. doi: 10.1016/j.annepidem.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Giurgescu C, McFarlin BL, Lomax J, Craddock C, Albrecht A. Racial discrimination and the black-white gap in adverse birth outcomes: a review. J Midwifery Womens Health. 2011;56(4):362–70. doi: 10.1111/j.1542-2011.2011.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dominguez TP. Adverse birth outcomes in African American women: the social context of persistent reproductive disadvantage. Soc Work Public Health. 2011;26(1):3–16. doi: 10.1080/10911350902986880. [DOI] [PubMed] [Google Scholar]
- 65.Alio AP, Mbah AK, Kornosky JL, Wathington D, Marty PJ, Salihu HM. Assessing the impact of paternal involvement on racial/ethnic disparities in infant mortality rates. J Community Health. 2011;36(1):63–8. doi: 10.1007/s10900-010-9280-3. [DOI] [PubMed] [Google Scholar]
- 66.Shah PS. Paternal factors and low birthweight, preterm, and small for gestational age births: a systematic review. Am J Obstet Gynecol. 2010;202(2):103–23. doi: 10.1016/j.ajog.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 67.Lu MC, Jones L, Bond MJ, Wright K, Pumpuang M, Maidenberg M, et al. Where is the F in MCH? Father involvement in African American families. Ethnicity Dis. 2010;20(1 Suppl 2):S2–49–61. [PubMed] [Google Scholar]
- 68.Alio AP, Salihu HM, Kornosky JL, Richman AM, Marty PJ. Feto-infant health and survival: does paternal involvement matter? Maternal and Child Health J. 2010;14(6):931–7. doi: 10.1007/s10995-009-0531-9. [DOI] [PubMed] [Google Scholar]
- 69.Alio AP, Kornosky JL, Mbah AK, Marty PJ, Salihu HM. The impact of paternal involvement on feto-infant morbidity among whites, blacks and Hispanics. Maternal Child Health J. 2010;14(5):735–41. doi: 10.1007/s10995-009-0482-1. [DOI] [PubMed] [Google Scholar]
- 70.Halfon N, Hochstein M. Life course health development: an integrated framework for developing health, policy, and research. Milbank Q. 2002;80(3):433–79. iii. doi: 10.1111/1468-0009.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu MC. We can do better: improving perinatal health in America. J Womens Health (Larchmt) 2010;19(3):569–74. doi: 10.1089/jwh.2009.1415. [DOI] [PubMed] [Google Scholar]