Abstract
The ATP-binding cassette transporter 2 (ABCA2) is an endolysosomal protein expressed in oligodendrocytes and Schwann cells, prostate, ovary and macrophages. In cell cultures, ABCA2 over-expression has been linked with resistance to the anticancer agent, estramustine phosphate (EMP; a nor-nitrogen mustard conjugate of estradiol). The present study shows that Abca2 knockout (KO) mice have greater sensitivity to a variety of side effects induced by EMP treatment. Chronic EMP (12 × 100 mg/kg body weight) produced mortality in 36% of KO mice, but only 7% of age-matched wild type (WT). Side effects of the drug were also more prevalent in the KO mouse. For example, during the first week of EMP treatments, 67% of KO males (compared to 6% of WT males) responded with episodic erectile events. In WT mice, ABCA2 protein localized within pene corpuscles, (which rely on modified Schwann cells for amplification of tactile signals) suggesting that the transporter may function in the erectile process. Endothelial nitric oxide synthase (eNOS; a source of nitric oxide during erectile response) levels were similar in WT and KO male penile tissue. Treatment with 100 mg/kg EMP (once daily for four days) elevated serum estradiol and estrone in both WT and KO. However, the circulating levels of these estrogens were higher in KO mice implying a reduced plasma clearance of estrogens as a consequence of ABCA2 ablation. Consistent with the pro-convulsant effects of estrogens, KO mice also displayed an increased incidence of seizures following EMP (14% vs. 0%). Taken together, these data indicate that ABCA2 deficiency renders mice more sensitive to EMP treatment-induced effects implying that the transporter has a role in regulating EMP transport and/or metabolism.
Keywords: Estramustine, Estrogen, ABC transporter, Prostate, Drug toxicities
1. Introduction
ABCA2 is one member of the ATP-binding cassette transporter superfamily of proteins and is localized primarily to endolysosomal organelle membranes [1]. It is preferentially expressed in several mammalian tissues such as myelinating cells of the central (oligodendrocytes) and peripheral (Schwann cells) nervous systems [2,3], excitatory and inhibitory neurons [4], macrophages [2–6] and reproductive tissues (prostate, ovary, uterus) [7]. Increased levels of ABCA2 occur in some human cancer cell lines [1] and in biopsies of T-cell acute lymphoblastic leukemia [8] and human vestibular schwannoma [9]. Genetic ablation of ABCA2 generated KO that characteristically displayed a tremor without ataxia, hyperactivity and reduced body weight as compared to WT and HET littermates [10]. The latter two phenotypes were more exaggerated in females than males, implying some functional role for ABCA2 in hormone-dependent neurological and developmental pathways. Increased myelin sheath thickness was accompanied by a reduced myelin membrane periodicity (compaction) in both spinal cord and cerebrum of KO mice compared to WT, differences that contributed to the observed tremor [10]. In an independently constructed KO strain, elevated levels of gangliosides, cerebrosides and sulfatides and lower levels of sphingomyelin were found in 4 to 64 week-old mice, while older mice had accumulated cerebrosides and sulfatides [11]. In myelin fractions of whole brain, high levels of the ganglioside GM1 and low levels of sphingomyelin were detected [11]. Taken together, these differences suggested a role of ABCA2 in pathways of lipid metabolism, a principle that has received further support from indications that ABCA2 is also associated with the direct transport of cholesterol [12,13].
In normal mouse brain development, increased expression of ABCA2 was found to occur concurrently with myelin sheath-associated proteins [14,15]. Sterol-dependent up-regulation of ABCA2 also occurred during normal differentiation and development of monocytes into macrophages, [5]. In addition, altered expression patterns of ABCA2 have linked its function with cholesterol homeostasis [5,16], resistance to estrogen-based drugs [1,7,17,18,19] and response to oxidative stress [6]. In particular, selection of an ovarian cancer cell line resistant to estramustine produced gene amplification of a region of chromosome 9 that resulted in marked over-expression of ABCA2 [17].
In light of the growing body of evidence linking lipid/sterol metabolism with ABCA2 expression patterns, we sought to determine if ABCA2 KO mice showed any differential response to treatments with EMP. Our results show that certain side effects of the drug were exacerbated in the absence of ABCA2 expression in vivo.
2. Materials and methods
2.1. Animals
Abca2 KO [10] and WT mice were housed in micro-isolation cages and provided with standard rodent diet and tap water ad libitum. Animal experiments were conducted in accordance with the MUSC Institutional Animal Care and Use Committee.
2.2. Drug treatments
EMP (Emcyt®, Pfizer) was dissolved in 0.9% saline at a concentration of 10 mg/mL and sterilized using a 0.22 μm filter unit (Millipore). Drug was stored at 4° C for no longer than 48 h (fresh drug was made on days 1 and 3 of treatment weeks). Treatment protocols for acute (2d, 4d) and chronic (4w, 6w) EMP administration are outlined in Fig. 1. Animals were injected i.p. at doses of 100 mg per kg body weight of EMP or sterile saline vehicle.
Fig. 1.
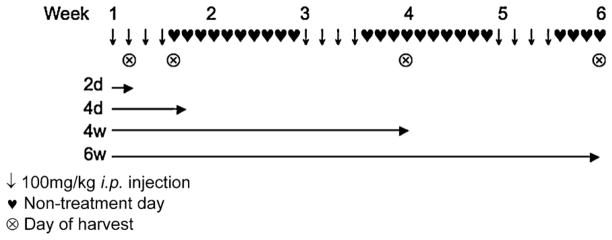
Estramustine phosphate treatment protocols.
Tissue from WT and KO mice treated with either: chronic 6 weeks; acute 4 days or 24 h EMP treatments were collected for histological examination or for RNA and/or protein expression studies. One hour prior to sacrifice, animals were treated with 50 mg/kg 5-bromo-2-deoxyuridine (BrdU). Tissues were fixed in 70% ethanol, for BrdU immunohistochemistry (Calbiochem), or 4% paraformaldehyde/PBS pH 7.4 for analysis by the TUNEL assay (Roche), using the manufacturer’s protocols.
Animals (treated with the chronic 6-week regimen of EMP or vehicle) were anesthetized with i.p. 2-2-2 tribromoethanol and sacrificed by cervical dislocation. Whole brain, heart, lung, liver, kidney, spleen, small intestine, trachea, axillary lymph node, thymus and adipose were excised and fixed overnight in 4% PBS (pH 7.4). Fixed tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin and sections were examined for histopathological differences at the MUSC Department of Pathology and Laboratory Medicine.
2.3. Immunohistochemistry
Tissue from WT and KO mice treated with either chronic 6w, acute 4d or 24 h EMP treatments were collected for histological examination or for RNA and/or protein expression studies. One hour prior to sacrifice, animals were treated with 50 mg/kg 5-bromo-2-deoxyuridine (BrdU). Tissues were fixed in 70% ethanol, for BrdU immunohistochemistry (Calbiochem), or 4% paraformaldehyde (PFA)/PBS pH 7.4 for analysis by the TUNEL assay (Roche), using the manufacturer’s protocols. ABCA2 immunohistochemistry was conducted with 4% PFA fixed, paraffin-embedded tissue sections. Tissues were de-waxed through a standard xylene/ ethanol series. Antigen retrieval was conducted in 10 mM sodium citrate, pH 6.0 and microwaved for 10 min (30% power). Sections were blocked in 0.1% H2O2 for 20 min, then in 10% goat serum, 1% bovine serum albumin, 0.3% TritonX-100 in PBS for 1 h. Primary antibody, polyclonal rabbit anti-ABCA2 (1:500 in blocking solution) was incubated with sections at 4° C overnight. Secondary antibody (biotin-conjugated anti-rabbit IgG; ABC kit, Calbiochem) was added per the manufacturer’s protocol. Tissue sections were then developed with DAB reagent (Calbiochem), subject to 10% hematoxylin counterstaining, dehydration through the ethanol/ xylene series and coverslip-mounted with Permount. Signal was visualized and captured by light microscopy (Nikon).
2.4. Blood chemistry profiling and complete blood counts
Whole blood was drawn from the submandibular site using a sterile 5 mm lancet (GoldenRod; MEDIpoint, Inc., Mineola, NY) and collected into heparin-coated tubes (B-D Biosciences, Franklin Lakes, NJ). Samples were analyzed for complete blood counts and blood chemistry profiles using the VetScan HMII Hematology Analyzer and Vet Scan, respectively, per the manufacturer’s protocols (Abaxis Veterinary Diagnostics, Union City, CA).
2.5. Plasma collection and estradiol determination
For blood collection, microcentrifuge tubes were coated with 10 μl heparin sodium salt (20 U/mL in PBS). Whole blood was collected from the submandibular site as described above and kept on ice for 30 min. Samples were centrifuged at 3000 × g for 10 min at 4° C. Plasma layers were removed and placed into a sterile microcentrifuge tubes and stored at −80° C until assayed at the Biomarkers Core Lab Yerkes National Primate Research Center at Emory University, Atlanta, GA. Plasma estradiol and estrone were determined by commercially available EIA (Cayman Chemical MI) and Elisa (DRG International NJ) kits respectively, while testosterone was determined by radioimmunoassay (RIA; Diagnostic Systems Laboratories, Webster, TX). In brief, 50 μl plasma samples were assayed for estradiol in duplicate using an EIA competitive assay. This assay is based on the competition between estradiol and an estradiol-acetylcholinesterase conjugate. After sample addition, the estradiol AChE tracer and estradiol EIA antisera were added followed by 1 h incubation at room temperature with shaking. Ellman’s reagent was used to develop the assay plate that was read at 420 nm on a Modulus plate reader (Turner Biosystems). Data were analyzed using a 4 Parameter Logistics curve fit and SPSS statistical software version 20. For the estrone Elisa, 50 ul of diluted plasma samples were assayed in duplicate. After sample addition, 100 μl of enzyme conjugate was added to each sample followed by 1 h incubation at room temperature. After washing, 150 μl of substrate solution was added to each well and allowed to incubate for 30 min. The absorbance was read at 450 nm on a Modulus plate reader (Turner Biosystems). Data were analyzed using a 4 Parameter Logistics curve fit and SPSS statistical software version 20.
3. Results
3.1. Knockout mice have reduced survival following chronic estramustine phosphate treatment
Using the administration protocol summarized in Fig. 1, chronic EMP treatments (12 × 100 mg/kg body weight) produced a mortality rate of 36% of KO mice and only 7% of age-matched WT (Table 1). A Kolmogorov-Smirnov test of the survival data (Fig. 2) confirmed that the difference in survival between the genotypes was significant (P = 0.001; D = 0.80).
Table 1.
Incidence of estramustine phosphate-induced effects in knockout and wild type mice.
| Treatment group | Effect | Incidence
|
|||
|---|---|---|---|---|---|
| WT-vehicle | WT-EMP | KO-vehicle | KO-EMP | ||
| 4d, 4w, 6wa | Erectile episode | 0/14 | 1/17 (6) | 0/8 | 8/12 (67) |
| Seizure | 0/23 | 0/32 | 0/16 | 3/22 (14) | |
| 4w, 6w | Death | 0/13 | 1/14 (7) | 0/7 | 4/11 (36) |
WT: wild type; EMP: estramustine phosphate; KO: knockout.
Erectile episodes and seizures reported for 4d, 4w and 6w treatment groups occurred only within first 4 days of treatment. Values in brackets are percent of total animals.
Fig. 2.
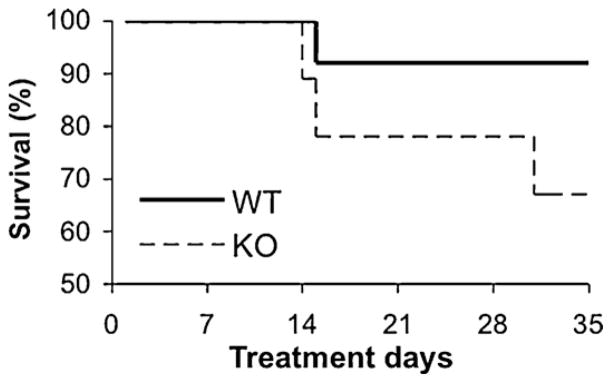
Reduced survival in knockout (KO) mice following chronic 6w estramustine phosphate (EMP) treatment. Kaplan-Meyer survival curves of wild type (WT) (solid line) and KO (dashed line) animals treated with 6w schedule of EMP (P = 0.001).
3.2. Knockout mice have compromised gastrointestinal mucosa recovery but no change in hematological effects following acute estramustine phosphate
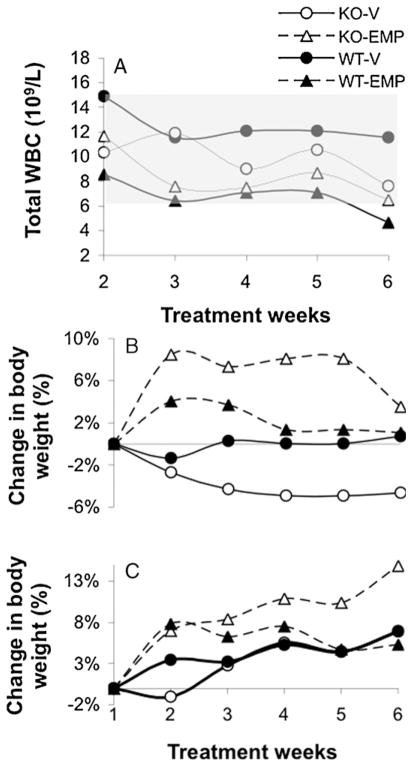
One of the dose-limiting toxicities of EMP in both man and rodent is intestinal mucositis. Using BrdU immunostaining of the ileum, a decrease in overall proliferative index was measured in female KO mice treated with EMP (Table 2) indicative of a delayed recovery of this tissue in these animals. However, these same mice showed no significant changes in hematological parameters following chronic EMP treatments. Complete blood counts were analyzed weekly during the 6w chronic EMP schedule (Fig. 3A) and no significant toxicities in either genotypic group were observed throughout the entire treatment course. Similarly, no significant changes in body weights were observed in either males or females (Fig. 3B, C). Systemic toxicities to liver and kidney were assessed by blood chemistry profiling following a 4d or 4w schedule and no significant changes were observed for ALB, ALP, ALT, AMY, TBIL, BUN Ca2+, PHOS, CRE, GLU, Na+, K+, TP or GLOB levels between WT and KO (P > 0.1).
Table 2.
Proliferative recovery of gastrointestinal epithelial cells following 4d estramustine phosphate is slightly reduced in female knockout mice.
| Sex | Genotype | Treatment | BrdU indexa | Mice (n) | P-value vs. vehicle |
|---|---|---|---|---|---|
| Female | WT | Vehicle | 93 ±20 | 6 | 0.47 |
| WT | 4d EMP | 86 ±16 | 8 | ||
| KO | Vehicle | 96 ±12 | 4 | 0.06 | |
| KO | 4d EMP | 67 ±22 | 4 | ||
| Males | WT | Vehicle | 66 ±11 | 5 | 0.25 |
| WT | 4d EMP | 79 ±21 | 8 | ||
| KO | Vehicle | 83 ±17 | 4 | 0.88 | |
| KO | 4d EMP | 81 ±9 | 4 |
BrdU: bromodeoxyuridine; WT: wild type; EMP: estramustine phosphate; KO: knockout.
Percentage of epithelial cells positive for BrdU immunostaining within ileal crypts (20 half-crypts per animal).
Fig. 3.
Lack of hematological toxicities in both wild type (WT) and knockout (KO) mice in response to chronic (6w) estramustine phosphate (EMP) treatment. (A) Total white blood cell counts remained within the normal range throughout the treatment weeks (shaded area indicates normal range for WBC count in mice). No significant changes in body weights were observed in males (B) or females (C). Closed shapes, WT; open shapes, KO. Circles, vehicle treated; triangles, EMP-treated.
3.3. Erectile episodes and seizures occur with greater frequency in estramustine phosphate-treated knockout mice compared to wild type littermates
During the course of the drug treatments detailed animal observations indicated two specific anomalies that were caused by EMP and distinguished the KO form the wild type animals. A majority of KO males (67%) responded with erectile episodes during the first week of treatment, while these incidences occurred only in 6% of WT males. For either group, these episodes only occurred when EMP-treated female mice from the same strain were in close proximity to the test group of males. In addition, there was an increased incidence of drug-induced seizures in KO mice, absent in WT, following EMP (14% vs. 0%; Table 1).
3.4. ATP-binding cassette transporter 2 protein is expressed in lamellar corpuscles and arteries of wild type mouse pene
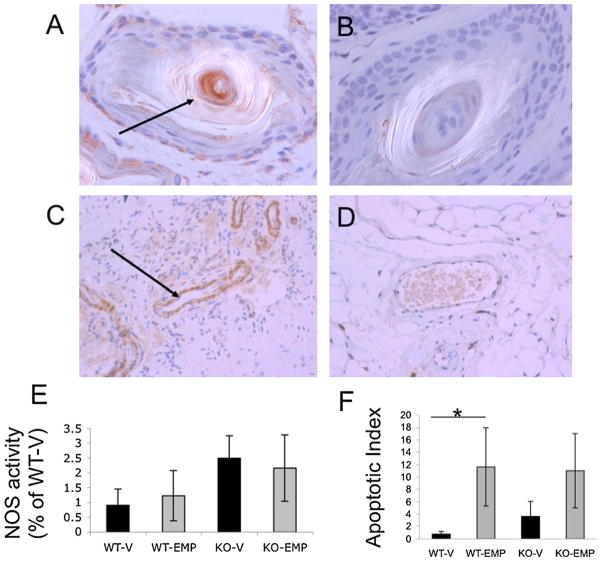
Since there is no previous report of ABCA2 expression in the mouse penis, immunohistochemistry was used to show that ABCA2 protein distribution in this organ. Staining localized within the lamellar corpuscles (modified Schwann cells) and helicine arteries of the corpus cavernosum of WT mouse pene (Fig. 4A, B). Each of these anatomical structures is known to be involved in mammalian erectile responses[20]KOtissuewas usedas acontrolforbackgroundstaining and corpuscles were negative for any ABCA2 staining.
Fig. 4.
ATP-binding cassette transporter 2 (ABCA2) protein expression and impact of estramustine phosphate (EMP) treatment within male reproductive tissues. Representative localization of ABCA2 in Pacinian corpuscles of (A) wild type (WT); or (B) knockout (KO) mouse pene. (C) ABCA2 expression (see arrow) in helicine arteries of corpus cavernosum of WT and (D) KO mice. KO mouse tissues in (B) and (D) are shown for background staining of ABCA2 polyclonal antibodies. (E) Nitric oxide synthase (NOS) activity is elevated in KO pene compared to WT, but falls short of statistical significance (P = 0.16). NOS activity data are the mean ± standard error of % conversion per μg total protein, shown as percentage of WT-V NOS activity. (F) Prostatic tissue in WT mice shows a significant elevation in apoptotic index following 4d EMP treatment (P < 0.05; defined as the number of TUNEL-positive nuclei per 10 high-powered fields from three to eight mice per group).
3.5. No impact of estramustine phosphate treatment on endothelial oxide synthase expression or total nitric oxide synthase activity
Because of the importance of nitric oxide in the erection response, activity and expression (mRNA and protein) of eNOS and PDE5A were determined and found to be similar in both WT and KO male penile tissue following an acute 2-day EMP treatment (data not shown). Total NOS activity was elevated in KO mice, but the values fell short of significance (P = 0.16, Fig. 4C).
3.6. Elevated apoptosis in prostate of both wild type and knockout mice treated with acute estramustine phosphate
Prostate tissue from mice treated with the 4d acute regimen of EMP was subject to TUNEL staining to determine levels of apoptotic induction. Both WT and KO mice showed elevated levels of TUNEL-positive nuclei (defined as the average number per high-powered field). However, only the WT–EMP group significantly rose above the vehicle cohort (14-fold; P < 0.05). Vehicle treated KO mice displayed an already elevated number of apoptotic cells in the prostate, which allowed for only a doubling of the index upon EMP treatment (P > 0.05), nor was it significantly higher than the WT-V group (Fig. 4D).
3.7. Elevated plasma estradiol and estrone levels in knockout mice following estramustine phosphate
Following a 4d EMP treatment both estradiol and estrone products of EMP metabolism [21,22] were higher in the KO animals than in the WT. Plasma collected 24 h following the fourth injection revealed that EMP-treated KO mice had elevated levels of both estradiol (9712 vs. 6423 pg/mL) and estrone (4497 vs. 5458 pg/mL), two in vivo metabolites of EMP. In these same mice, testosterone levels remained unchanged as a consequence of EMP treatment (data not shown) (Table 3).
Table 3.
Elevated metabolites of estramustine (estradiol and estrone) in knockout mouse plasma following estramustine phosphate administration.
| Genotype | Treatment | Estradiol (pg/mL) | Estrone (pg/mL) |
|---|---|---|---|
| WT | Vehicle | 39 ± 9 | 53 ± 76 |
| WT | EMP | 6423 ± 950 | 4497 ± 538 |
| KO | Vehicle | 33 ± 17 | 11 ± 0 |
| KO | EMP | 9712 ± 3194 | 5458 ± 1042 |
WT: wild type; EMP: estramustine phosphate; KO: knockout. Data shown are the mean ± SE of three to five male mice per group.
4. Discussion
EMP has been widely used in the therapy of prostate cancer [23] where its anti-tumor mechanisms of action are both antimitotic and estrogenic. In this study, we considered whether the ABCA2 deficient mouse strain would exhibit differential responses to EMP treatment. Our data suggest that ABCA2 KO mice have deficiencies in the metabolism/clearance of EMP and its metabolites, estradiol and estrone, which has resulted in an overall increase in side effects from this agent. A chronic 6-week treatment with EMP resulted in a higher mortality rate among KO (36%) compared to WT mice (7%). Although the precise cause(s) of animal toxicity were unclear, one moribund KO mouse euthanized during week 3 of treatment had severe lethargy and apparent hind limb paralysis. Upon dissection, a high degree of peritoneal fibrosis, tissue necrosis and intestinal prolapse was observed. Other necropsies carried out during weeks 3 and 5 of the chronic regimen showed considerable tissue necrosis, but no easily attributable cause of death. Acute treatment of ABCA2 KO mice with EMP for 4 days caused an increase in EMP-induced seizures and erectile episodes compared to WT when males were concomitantly treated with EMP-treated females in the same holding room. However, the erectile episodes did not occur when only male cohorts were treated, suggesting that EMP initiated a hormone or pheromone-like effect in females that may induce erectile episodes in EMP-treated males.
We demonstrate for the first time that ABCA2 is expressed in mouse pene, particularly in Pacinian corpuscles, large encapsulated sensory receptors responsive to pressure and vibration. These consist of delicate capsules enclosing many concentric lamellae of flattened cells that are modified Schwann cells separated by interstitial fluid space and collagen fibers. Distortion of the Pacinina corpuscle produces an amplified mechanical stimulus in the core that is transduced into an action potential in the sensory neuron. Towards the center of the corpuscle, the lamellae become closely packed and the core contains a single large un-branched, non-myelinated nerve fiber with several club-like terminals that are myelinated beyond the corpusle. ABCA2 expression in these areas may be functionally linked with the transport of estrogenic metabolites or lipid components of the lamellae. Further, this transporter may be involved in hypersensitivity of penile tissue or spinal ejaculation centers, each of which is directly involved with premature ejaculation [24]. Since this phenomenon only occurred in the presence of EMP-treated females, the data are consistent with the effects being related to the response of males to pheromones released by females in estrous [25]. Taken together, these observations suggest that EMP treatment may impact both male and female reproductive systems.
Clinically, GI symptoms are dose-limiting toxicities of EMP treatment [26]. Indices of proliferation (BrdU IHC) and apoptosis (TUNEL) were examined in KO and WT ileum, areas susceptible to drug-induced mucositis. Data acquired for both WT and KO tissues followed similar patterns of decreased proliferation after 2- and 4-day EMP treatments. No significant changes were observed in apoptotic indices at this dose of EMP. Other non-life threatening side effects of EMP, including mild hematologic toxicity and flushing are attributable to release of the metabolites, estrone and estradiol [26,27]. While previous reports have linked ABCA2 transport properties with lipids, cholesterol and steroids, it remains unknown whether EMP is a specific substrate for transport. Because of the relatively high degree of homology between ABCA2 and ABCA1 compared to other ABCA subfamily members (54% similarity, 46% identity; [28]) there is likely some functional redundancy in vivo, perhaps explaining why the ABCA2 KO strains are viable and fertile [10,11]. Nevertheless, phenotypic characteristics of the KO mouse have implicated a functional role in myelin sheath development in the brain and spinal cord [10,11]. The mechanism(s) by which EMP-induced seizures in KO mice may be related to these effects. Expression levels of estrogen receptor (α and β) or glutamate- and GABA-receptors (factors that may be involved in erectile responses) in the KO brain do not show significant variation from those of WT mice (unpublished results). Further evaluation of peripheral and sensory nerves in this model may be warranted.
Overall, our results implicate ABCA2 in mediating the toxicities of EMP and its estrogenic metabolites and suggest a possible role for the transporter in erectile responses to physical and/or olfactory stimuli in mice.
Acknowledgments
This work was supported by grants from the National Institutes of Health (CA08660, CA117259, NCRR P20RR024485 - COBRE in Oxidants, Redox Balance and Stress Signaling) and support from the South Carolina Centers of Excellence program and was conducted in a facility constructed with the support from the National Institutes of Health, Grant Number C06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources. Supported in part by the Drug Metabolism and Clinical Pharmacology shared Resource, Hollings Cancer Center, Medical University of South Carolina.
Abbreviations
- ABC
ATP-Binding Cassette Transporter
- BrdU
Bromodeoxyuridine
- EMP
Estramustine Phosphate
- HET
Heterozygous
- NOS
Nitric Oxide Synthase
- WT
Wild Type
- KO
Knockout
- GI
Gastrointestinal
- PBS
Paraformaldehyde-phosphate-Buffered Saline
- PFA
Paraformaldehyde
Footnotes
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
References
- 1.Vulevic B, Chen Z, Boyd JT, Davis W, Walsh ES, Jr, Belinsky MG, et al. Cloning and characterization of human adenosine 5′-triphosphate-binding cassette, subfamily A, transporter 2 (ABCA2) Cancer Res. 2001;61:3339–47. [PubMed] [Google Scholar]
- 2.Zhou CJ, Inagaki N, Pleasure SJ, Zhao LX, Kikuyama S, Shioda S. ATP-binding cassette transporter ABCA2 (ABC2) expression in the developing spinal cord and PNS during myelination. J Comp Neurol. 2002;451:334–45. doi: 10.1002/cne.10354. [DOI] [PubMed] [Google Scholar]
- 3.Kim WS, Guillemin GJ, Glaros EN, Lim CK, Garner B. Quantitation of ATP-binding cassette subfamily-A transporter gene expression in primary human brain cells. Neuroreport. 2006;17:891–6. doi: 10.1097/01.wnr.0000221833.41340.cd. [DOI] [PubMed] [Google Scholar]
- 4.Broccardo C, Nieoullon V, Amin R, Masmejean F, Carta S, Tassi S, et al. ABCA2 is a marker of neural progenitors and neuronal subsets in the adult rodent brain. J Neurochem. 2006;97:345–55. doi: 10.1111/j.1471-4159.2006.03714.x. [DOI] [PubMed] [Google Scholar]
- 5.Kaminski WE, Piehler A, Pullmann K, Porsch-Ozcurumez M, Duong C, Bared GM, et al. Complete coding sequence, promoter region, and genomic structure of the human ABCA2 gene and evidence for sterol-dependent regulation in macrophages. Biochem Biophys Res Commun. 2001;281:249–58. doi: 10.1006/bbrc.2001.4305. [DOI] [PubMed] [Google Scholar]
- 6.Chen ZJ, Vulevic B, Ile KE, Soulika A, Davis W, Reiner PB, Jr, et al. Association of ABCA2 expression with determinants of Alzheimer’s disease. Faseb J. 2004;18:1129–31. doi: 10.1096/fj.03-1490fje. [DOI] [PubMed] [Google Scholar]
- 7.Ile KE, Davis W, Boyd JT, Jr, Soulika AM, Tew KD. Identification of a novel first exon of the human ABCA2 transporter gene encoding a unique N-terminus. Biochim Biophys Acta. 2004;1678:22–32. doi: 10.1016/j.bbaexp.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Efferth T, Gillet JP, Sauerbrey A, Zintl F, Bertholet V, de Longueville F, et al. Expression profiling of ATP-binding cassette transporters in childhood T-cell acute lymphoblastic leukemia. Mol Cancer Ther. 2006;5:1986–94. doi: 10.1158/1535-7163.MCT-06-0086. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Yamada K, Tanaka Y, Ishikawa K, Inagaki N. Expression of ABCA2 protein in human vestibular schwannoma and peripheral nerve. J Neurol Sci. 2005;232:59–63. doi: 10.1016/j.jns.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 10.Mack JT, Beljanski V, Soulika AM, Townsend DM, Brown CB, Davis W, et al. Skittish Abca2 knockout mice display tremor, hyperactivity, and abnormal myelin ultrastructure in the central nervous system. Mol Cell Biol. 2007;27:44–53. doi: 10.1128/MCB.01824-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sakai H, Tanaka Y, Tanaka M, Ban N, Yamada K, Matsumura Y, et al. ABCA2 deficiency results in abnormal sphingolipid metabolism in mouse brain. J Biol Chem. 2007;282:19692–9. doi: 10.1074/jbc.M611056200. [DOI] [PubMed] [Google Scholar]
- 12.Davis W., Jr The ATP-binding cassette transporter-2 (ABCA2) regulates cholesterol homeostasis and low-density lipoprotein receptor metabolism in N2a neuroblastoma cells. Biochim Biophys Acta. 2011;1811:1152–64. doi: 10.1016/j.bbalip.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis W., Jr The ATP-binding cassette transporter-2 (ABCA2) increases endogenous amyloid precursor protein expression and Abeta fragment generation. Curr Alzheimer Res. 2010;7:566–77. doi: 10.2174/156720510793499002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tachikawa M, Watanabe M, Hori S, Fukaya M, Ohtsuki S, Asashima T, et al. Distinct spatiotemporal expression of ABCA and ABCG transporters in the developing and adult mouse brain. J Neurochem. 2005;95:294–304. doi: 10.1111/j.1471-4159.2005.03369.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhou C, Zhao L, Inagaki N, Guan J, Nakajo S, Hirabayashi T, et al. Atp-binding cassette transporter ABC2/ABCA2 in the rat brain: a novel mammalian lysosome-associated membrane protein and a specific marker for oligodendrocytes but not for myelin sheaths. J Neurosci. 2001;21:849–57. doi: 10.1523/JNEUROSCI.21-03-00849.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis W, Boyd JT, Jr, Ile KE, Tew KD. Human ATP-binding cassette transporter-2 (ABCA2) positively regulates low-density lipoprotein receptor expression and negatively regulates cholesterol esterification in Chinese hamster ovary cells. Biochim Biophys Acta. 2004;1683:89–100. doi: 10.1016/j.bbalip.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 17.Laing NM, Belinsky MG, Kruh GD, Bell DW, Boyd JT, Barone L, et al. Amplification of the ATP-binding cassette 2 transporter gene is functionally linked with enhanced efflux of estramustine in ovarian carcinoma cells. Cancer Res. 1998;58:1332–7. [PubMed] [Google Scholar]
- 18.Hazlehurst LA, Enkemann SA, Beam CA, Argilagos RF, Painter J, Shain KH, et al. Genotypic and phenotypic comparisons of de novo and acquired melphalan resistance in an isogenic multiple myeloma cell line model. Cancer Res. 2003;63:7900–6. [PubMed] [Google Scholar]
- 19.Boonstra R, Timmer-Bosscha H, van Echten-Arends J, van der Kolk DM, van den Berg A, de Jong B, et al. Mitoxantrone resistance in a small cell lung cancer cell line is associated with ABCA2 up-regulation. Br J Cancer. 2004;90:2411–7. doi: 10.1038/sj.bjc.6601863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizusawa H, Hedlund P, Hakansson A, Alm P, Andersson KE. Morphological and functional in vitro and in vivo characterization of the mouse corpus cavernosum. Br J Pharmacol. 2001;132:1333–41. doi: 10.1038/sj.bjp.0703938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edman K, Svensson L, Eriksson B, Gunnarsson PO. Determination of estramustine phosphate and its metabolites estromustine, estramustine, estrone and estradiol in human plasma by liquid chromatography with fluorescence detection and gas chromatography with nitrogen-phosphorus and mass spectrometric detection. J Chromatogr B Biomed Sci Appl. 2000;738:267–79. doi: 10.1016/s0378-4347(99)00526-5. [DOI] [PubMed] [Google Scholar]
- 22.Breda M, Basileo G, James CA. Simultaneous determination of estramustine phosphate and its four metabolites in human plasma by liquid chromatography-ionspray mass spectrometry. Biomed Chromatogr. 2004;18:293–301. doi: 10.1002/bmc.319. [DOI] [PubMed] [Google Scholar]
- 23.Hudes GR, Greenberg R, Krigel RL, Fox S, Scher R, Litwin S, et al. Phase II study of estramustine and vinblastine, two microtubule inhibitors, in hormone-refractory prostate cancer. J Clin Oncol. 1992;10:1754–61. doi: 10.1200/JCO.1992.10.11.1754. [DOI] [PubMed] [Google Scholar]
- 24.Allard J, Truitt WA, McKenna KE, Coolen LM. Spinal cord control of ejaculation. World J Urol. 2005;23:119–26. doi: 10.1007/s00345-004-0494-9. [DOI] [PubMed] [Google Scholar]
- 25.Sachs BD. Erection evoked in male rats by airborne scent from estrous females. Physiol Behav. 1997;62:921–4. doi: 10.1016/s0031-9384(97)00307-7. [DOI] [PubMed] [Google Scholar]
- 26.Hudes G, Haas N, Yeslow G, Gillon T, Gunnarsson PO, Ellman M, et al. Hartley-Asp, Phase I clinical and pharmacologic trial of intravenous estramustine phosphate. J Clin Oncol. 2002;20:1115–27. doi: 10.1200/JCO.2002.20.4.1115. [DOI] [PubMed] [Google Scholar]
- 27.Tew KD, Glusker JP, Hartley-Asp B, Hudes G, Speicher LA. Preclinical and clinical perspectives on the use of estramustine as an antimitotic drug. Pharmacol Ther. 1992;56:323–39. doi: 10.1016/0163-7258(92)90023-s. [DOI] [PubMed] [Google Scholar]
- 28.Kaminski WE, Piehler A, Wenzel JJ. ABC A subfamily transporters: structure, function and disease. Biochim Biophys Acta. 2006;1762:510–24. doi: 10.1016/j.bbadis.2006.01.011. [DOI] [PubMed] [Google Scholar]