Abstract
The epsins are a conserved family of endocytic adaptors essential for cell viability in yeast and for embryo development in higher eukaryotes. Epsins function as adaptors by recognizing ubiquitinated cargo and as endocytic accessory proteins by contributing to endocytic network stability/regulation and membrane bending. Importantly, epsins play a critical role in signaling by contributing to epidermal growth factor receptor downregulation and the activation of notch and RhoGTPase pathways. In this review, we present an overview of the epsins and emphasize their functional importance as coordinators of endocytosis and signaling.
Keywords: endocytosis, epsin, notch, RhoGTPase, signaling
Introduction
Endocytosis is the process by which cells internalize portions of the plasma membrane to uptake nutrients and to control their membrane composition and surface area. In addition to cellular homeostasis, this fundamental cellular process is crucial for the regulation of multiple signaling pathways. It is well-known that endocytosis attenuates signals by internalizing activated ligand-receptor complexes from the cell surface. However, a growing body of evidence demonstrates that endocytosis also activates signaling, for example, by contributing to the assembly of signaling endosomes from which new signaling events can be generated. This review discusses the epsin family of endocytic proteins and their role in coordinating endocytosis and signaling. For a more detailed chronological perspective and discussion of topics, such as epsin regulation, we refer the reader to an excellent review by Beverly Wendland (1).
The epsins
Brief overview of the epsin protein family
The epsins are a family of endocytic adaptor proteins conserved across evolution with crucial physiological roles. Thus, while in metazoans epsins are required for proper embryo development (2 – 5), in yeast, they are essential for cell viability (6–8). The founding member of the family, rat epsin-1 (Eps15 interacting protein 1) was discovered by the DeCamilli lab (Yale University) as an interaction partner of the endocytic accessory protein Eps15 (9). Sequence analysis of epsin-1 revealed striking similarities with the mitotic phosphoprotein-90 from Xenopus laevis (10) and two Saccharomyces cerevisae proteins later identified as epsin homologs (6). Soon after, a second paralog, epsin-2 was discovered (11). Both, epsin-1 and -2 were characterized as plasma membrane-localized components of the clathrin-mediated endocytic machinery (9, 11). Although ubiquitously expressed, epsin-1 and -2 were found to be enriched in the brain (9, 11). In contrast, a third epsin paralog, epsin-3, was subsequently shown to have a very restricted expression profile that includes migrating keratinocytes (12), gastric parietal cells (13), and several carcinomas (12 – 15).
Saccharomyces cerevisiae and most vertebrates (including primates, rodents, and zebrafish) contain at least two epsin paralogs. In contrast, only one epsin gene is present in Schizosaccharomyces pombe, Caenorhabditis elegans, and Drosophila melanogaster. However, the single epsin gene in D. melanogaster produces two alternatively spliced forms (3, 16). In addition to these classical plasma membrane-localized epsins, there is a ubiquitous family of epsin-like proteins that display partial sequence similarities but possess several distinctive features. Specifically, these epsin-related (epsinR) proteins (also known as enthoprotin, clint, or epsin-4) have a different set of protein and lipid interaction partners, localize to the trans-golgi network/endosomes and participate in endosome to golgi transport (17–21). Therefore, a detailed description of this protein subfamily is beyond the scope of this review.
Domain organization of epsins
Domain organization of the epsins will be described from N- to C-terminus taking a generic mammalian epsin as a model (Figure 1). At the N-terminus, epsins bear a highly conserved, approximately 150 amino acid-long epsin N-terminal homology (ENTH) domain (22) [reviewed in (23)]. The structure of the mammalian ENTH domain has been solved by X-ray crystallography as well as by NMR spectroscopy (24–27), and the emerging structural consensus revealed a superhelical fold comprised of seven α helices followed by an eighth helix misaligned with the superhelical axis. Structurally, the ENTH domain is most similar to the VHS domain characteristic of the Vps27, Hrs, and STAM trafficking proteins, and it also contains armadillo and HEAT repeat segments found in β-catenin and karyopherin-β, respectively (24). The ENTH domain binds phosphatidylinositol 4,5-bisphosphate, a lipid enriched at different regions within the plasma membrane including endocytic sites (25, 27, 28) [reviewed in (29)]. Importantly, an elegant study from the McMahon lab demonstrated that this interaction triggers a dramatic conformational change that induces the formation of an additional N-terminal alpha-helix, helix0 (α0) (27). Owing to its amphipathic nature, α0 inserts into the cytoplasmic leaflet of the plasma membrane and promotes membrane curvature (27, 28, 30–32). This remarkable process is believed to be one of the initial steps of membrane invagination toward the formation of clathrin-coated pits (CCPs) and eventually of clathrin-coated vesicles [reviewed in (33–36)].
Figure 1.
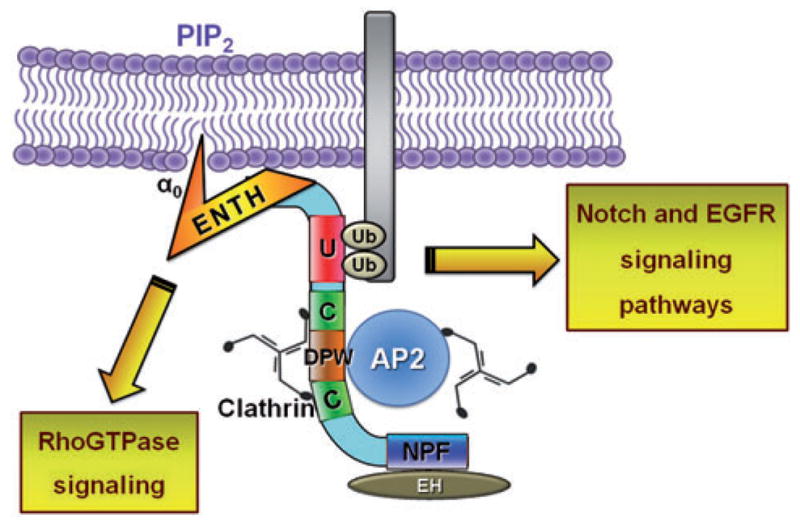
Schematic representation of a generic mammalian epsin. Cartoon represents typical epsin domain organization, known interaction partners, and signaling links. PIP2, phosphatidylinositol-4,5-bisphosphate; ENTH, epsin N-terminus homology domain; α0, helix 0; Ub, ubiquitin; U, ubiquitin-interacting motif; C, clathrin-binding motif; DPW (or DPF), AP2-binding aspartate-proline-tryptophan (or aspartate-proline-phenylalanine) motifs; NPF, asparagine-proline-phenylalanine motif that binds to EH domain-containing proteins, such as Eps15 and intersectin; EH, Eps15 homology domain; AP2, clathrin assembly or adaptor protein 2. Figure is not drawn to scale; not all possible interactions are depicted.
Following the discovery of ubiquitin interacting motifs (UIM) in a subunit of the 26S proteasome (37), the consensus UIM sequence was identified in several proteins involved in receptor-mediated endocytosis, including epsin (38). Most epsins contain two or three UIM copies that interact with ubiquitinated cargo and are located C-terminal to the ENTH domain (39–41) [reviewed in (1)]. Importantly, cooperative binding of the UIMs is required for high avidity and ubiquitin-dependent recruitment of the yeast epsins to biological membranes (41). Furthermore, it has been proposed that multiple monoubiquitination or limited polyubiquitination (up to approximately four ubiquitin units) of cargo is essential for achieving sufficient interaction avidity for epsin recruitment (42–45) [reviewed in (46)].
The region between the UIMs and the very C-terminus of epsin is mostly unstructured (47) and displays high interspecies sequence divergence. However, this region also includes several conserved short signature motifs for binding various components of the endocytic machinery (corresponding to 5–13% of this region’s sequence). Specifically, mammalian epsins possess two clathrin-binding motifs (CBM) that closely follow the consensus LøZøZ motif (where L is leucine, ø and Z are amino acids with bulky hydrophobic and polar side chains, respectively) and bind to the terminal domain of clathrin heavy chain (48, 49) [reviewed in (50)]. These clathrin-binding sequences flank a cluster of three to eight DP[W/F] (aspartate-proline-tryptophan/phenylalanine) repeats that bind to the adaptor complex AP2 (9, 51–54). In fact, the resulting avidity of these AP2-binding motifs is so high that overexpression of an epsin-1 fragment containing its DP[W/F] motifs leads to severe impairment of AP2-mediated internalization by a dominant-negative mechanism (9).
C-terminal to the CBM-DPW cassette, two or three NPF (asparagine-proline-phenylalanine) tripeptide repeats are found. These motifs are recognized by the eps15-homology (EH) domain of proteins like eps15, intersectin, and POB1 (9, 55, 56).
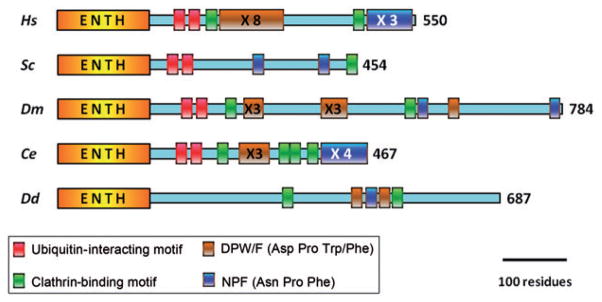
This general architecture (Figure 1) is mostly preserved in higher eukaryotes with minor variations in the number of some binding motifs. In contrast, yeast epsins display significant differences. For example, the AP2-binding DP[W/F] motifs are absent, and a single clathrin-binding domain is present at their very C-terminus (6) (see Figure 2). Other deviations from this general architecture are present in the epsin ortholog of Dictyostelium discoideum. This epsin lacks UIMs but interacts with clathrin and AP2 at the plasma membrane (57) (Figure 2). In Trypanosoma bruceii, the single ENTH domain-containing protein, TbEpsinR, displays features common to both epsin and epsinR. Similar to epsinR, TbEpsinR lacks UIMs, and a fraction is partially localized at endosomal membranes; however, unlike epsinR, a second population contributes to clathrin-dependent endocytosis at the plasma membrane (58).
Figure 2.
Comparison of domain architecture of epsins from different organisms.
Hs, Homo sapiens (NP_037465.2); Sc, S. cerevisiae (NP_010120); Dm, D. melanogaster (AAF05113); Ce, C. elegans (NP_510459); Dd, D. discoideum (XP_635269).
Epsin and endocytosis
Epsins are proteins capable of fulfilling different roles at nascent endocytic sites. On one hand, as these proteins bear UIMs, they act as endocytic adaptors by directly binding to ubiquitinated cargo. On the other hand, epsins also interact with other elements of the endocytic machinery including the hubs of the endocytic network, i.e., AP2 and clathrin. Therefore, epsins function as an accessory protein by consolidating and regulating the endocytic network.
Epsin-specific cargoes
Although yeast epsins are known to participate in the internalization of several membrane proteins, such as the alpha-factor receptor Ste2 (40, 59), they are not essential for their endocytosis, i.e., to date, no physiological cargo has been found to be epsin specific. In higher eukaryotes, epsin is required for endocytosis of notch ligands (4, 60–62), and a body of evidence strongly implicates this adaptor in epidermal growth factor receptor (63–66) and epithelial sodium channel (67–69) internalization.
Notch ligands
The notch signaling pathway is a conserved signaling module involved in cell fate determination [reviewed in (70)]. While genetic evidence obtained in D. melanogaster suggests that endocytosis is required in both signal-sending and -receiving cells for notch activation (71), other studies indicated that Drosophila epsin, liquid facets (Lqf) is only required in the signal-sending cell (60, 61). These observations imply that epsin contributes to notch activation by regulating/facilitating the endocytosis of the notch ligands delta/serrate/lag2 (DSL) (4, 60–62). Introduction of a tyrosine-based internalization signal (from the LDL receptor) in the cytoplasmic domain of delta (Dl) could bypass the need for epsin in notch signaling (61), supporting the notion that the endocytic adaptor function of epsin is critical for this pathway activation. Moreover, the endocytosis and signaling of Dl was found to be epsin specific, as disruption of AP2 had no significant impact (72). Dl and serrate accumulate at the plasma membrane in cells deficient in mindbomb or neuralized, suggesting that these E3 ligases ubiquitinate DSL ligands for epsin-mediated internalization (61, 62, 73–77). Although the exact mechanism by which epsin-mediated endocytosis triggers notch activation is not fully understood, it is clear that it requires ubiquitination, epsin binding, and internalization of notch ligands. Details on the signaling relevance of epsins for this pathway are described in the section on ‘Epsin controls notch signaling activation via endocytosis’.
Epidermal growth factor receptor (EGFR)
This receptor tyrosine kinase belongs to the erythroblastic leukemia viral oncogene homolog (ErbB) family consisting of four closely related members: ErbB1 (EGFR), ErbB2, ErbB3, and ErbB4 (also designated as HER1-4 for human epidermal growth factor receptor). This protein family, particularly ErbB1, has been the focus of extensive research due to its role in cancer. Indeed, EGFR expression is frequently upregulated in many solid tumors where it promotes cancer progression [recently reviewed in (78)], thus making the use of EGFR inhibitors a powerful therapeutic approach. Importantly, deficient internalization of EGFR also leads to receptor hypersignaling and, in consequence, to malignant transformation (79, 80) [reviewed in (81)]. Therefore, the study of EGFR endocytosis has captured considerable interest. Classically, binding of ligands to the extracellular domain of their receptors not only induces downstream signal transduction but also promotes receptor endocytosis [reviewed in (82, 83)]. The ubiquitin-ligase Cbl is believed to initiate the internalization of EGFR (63) by promoting mono, multi-, and polyubiquitination (63, 66, 84). However, whether ubiquitination is necessary and sufficient for internalization still remains to be fully established [reviewed in (85)].
Although there is some result variability, likely associated with knock-down efficiency and diversity of cell lines used, it is broadly accepted that internalization of ubiquitinated-EGFR is dependent on epsin and requires the integrity of its UIMs (43, 64–66). Whether this process is clathrin dependent or not has been a topic of debate. On one hand, it has been proposed that upon exposure to high (but still physiological) concentrations of ligand, EGFR undergoes extensive ubiquitination (64). Under these conditions, in addition to the classical clathrin-dependent internalization mechanism, a clathrin-independent, caveolae-, Eps15-, and epsin-dependent internalization route has been reported to occur for EGFR endocytosis (64). Interestingly, another report indicated that epsin binding to ubiquitin negatively affected its interaction with clathrin (86). Further, it was proposed that the clathrin-independent pathway of EGFR internalization leads to degradation of cargo (i.e., to signaling termination), whereas the clathrin-dependent route promotes persistent signaling as well as recycling of EGFR (64, 87). In contrast, other studies have indicated that epsins are able to interact with both clathrin and ubiquitin simultaneously (43, 63, 65, 66) and that EGFR does not significantly colocalize with caveolar structures (88). These seemingly contradictory results likely suggest that EGFR endocytosis is a more complex process than anticipated. In fact, other posttranslational modifications besides ubiquitination have recently been found to contribute to EGFR uptake (84), raising the possibility of multiple endocytic routes and additional adaptors besides epsin (yet to be identified) being involved in the process.
Epithelial sodium (Na) channel (ENaC)
This amiloride-sensitive sodium channel is primarily found in polarized epithelia in lung, kidney, colon, sweat ducts, and salivary glands. ENaC is a heteromer composed of two alpha, one beta, and one gamma subunit. This channel functions to maintain electrolyte balance and fluid movement across the epithelia [reviewed in (89)]. It is known that the ubiquitin-ligase neural precursor cell expressed, developmentally downregulated protein 4-2 (Nedd4-2) ubiquitinates and promotes ENaC internalization (90–93). In Liddle’s syndrome where ENaC recognition by Nedd4-2 is affected, the channel activity is increased resulting in hypertension (94, 95).
ENaC was found in clathrin-coated vesicles along with epsin, and these proteins efficiently co-immunoprecipitated together in an epsin UIM-dependent manner (68). Furthermore, coexpression of ENaC with Nedd4-2 or epsin decreased ENaC-dependent currents, in contrast to a dominant-negative dynamin mutant, which increased such currents (67). This evidence implicates epsin in the downregulation of this sodium channel. Interestingly, the same authors reported that epsin was not implicated in the endocytosis of the K+ channel (68), renal outer medullary potassium channel (ROMK), even when it is also known to be subjected to ubiquitination (96).
In addition to the abovementioned cargoes, endocytosis of a number of other proteins seems to be dependent on epsins. For example, epsins play a role in the uptake of influenza virus bound to its receptor in mammalian cells (97). Epsin is also important for the clathrin-mediated internalization of the antigen-presenting MHC class I molecules following ubiquitination by a Kaposi’s sarcoma-associated herpesvirus ubiquitin ligase (45, 98). Furthermore, epsin is involved in the internalization of insulin receptor (42), and a large-scale RNA interference study revealed that epsin contributes to the internalization of dopamine transporter in neurons (99). Additionally, epsin was shown to mediate the internalization of ubiquitinated G protein-coupled receptor protease activated receptor 1 (PAR1) (100). The Drosophila epsin homolog Lqf was reported to be required for the receptor-mediated endocytosis of Drosophila larval serum proteins into larval fat body cells from the hemolymph (101).
Epsin as an accessory protein
Besides its role in the internalization of ubiquitinated cargo, epsins are believed to participate in the stabilization of the endocytic network. An interesting study from the Schmid laboratory (Scripps Institute) demonstrated that CCPs undergo maturation into functional clathrin-coated vesicles only if they successfully pass through an ‘endocytic checkpoint’ (102). The checkpoint verifies, via protein-protein interactions, a critical mass of cargo, endocytic components, and other factors (102). Failure to reach this critical mass leads to destabilization of the immature CCPs, which subsequently abort (102). This observation is in agreement with a previous study that demonstrated that stochastically initiated CCPs undergo rapid collapse if conditions for stabilization are not met (103). Epsin-1, along with CALM and SNX9, contributes to this endocytic checkpoint, and even a partial depletion of epsin-1 (by 40%) leads to an increase in the proportion of abortive CCPs (104). Epsins in yeast are also recruited during the early stages of clathrin coat formation (105) and participate in the assembly of clathrin-coated areas (106). Furthermore, the yeast epsins play a similar role in expanding and sustaining the endocytic network via protein-protein interactions mediated by their UIM and NPF motifs (59, 107). In addition, epsins might be involved in other regulatory mechanisms affecting the endocytic network, for example, in Dictyostelium, epsin has been implicated in facilitating phosphorylation of the actin-binding adaptor protein huntingtin-interacting protein 1 related (Hip1r) (108, 109).
Epsin and signaling
The process of endocytosis plays a dual role in signaling regulation. It not only desensitizes cells by downregulating activated receptors (e.g., EGFR) but is also required for the activation of other signaling pathways (e.g., via internalization of notch ligands). Importantly, epsins are functional in both the modes of signaling regulation. The role of epsins in signal termination is directly linked to their ability to support endocytosis of receptors (as described in the ‘Epsin and endocytosis’ section) targeted for lysosomal degradation. The function of epsins in signaling activation has been linked to the notch developmental pathway and RhoGTPase signaling.
Epsin controls notch signaling activation via endocytosis
Endocytosis is critical for the activation of the notch signaling pathway. It has been established that Lqf is essential for proper signaling by the DSL ligands (60, 61). lqf − clones showed no overall disruption of global endocytosis, but Dl was unable to signal to receiving cells (61). In agreement, epsins’ relevance for embryogenesis and development has been clearly demonstrated: Lqf null mutation is embryonic lethal (2), and flies overexpressing epsin or expressing mutant epsin alleles display severe eye, wing, and leg morphology defects (3, 110). Similarly, epsin mutations, in both D. melanogaster and C. elegans, led to defects in germline and heart development due to deficiencies in notch signaling (4). A detailed study on the phenotypes of double epsin-1/-2 knockout (KO) mice revealed that embryos did not survive beyond E10.5, while single epsin KO pups were healthy (5). However, embryonic fibroblasts taken from double epsin KO embryos did not show abnormalities in general clathrin-mediated endocytosis. The major cause for the lethality of the embryos was the disruption of the extraembryonic structures, defects in cardiovascular development, somitogenesis, and neural tube differentiation of the embryo (5). Interestingly, these phenotypes are highly comparable to those produced by impairment of the notch pathway. These studies suggest that although epsin is dispensable for general endocytosis, it may be critical for cargo-specific signaling functions.
Epsin contributes to RhoGTPase signaling activation
Cdc42 is a highly conserved member of the RhoGTPase family that acts as the master regulator of cell polarity [reviewed in (111)]. This signaling protein was first identified in yeast through mutants that led to a cell division arrest and loss of actin cytoskeleton polarization (112). Indeed, Cdc42 regulates the actin and microtubule cytoskeletons [reviewed in (111)] and modulates membrane trafficking [reviewed in (113)] among other functions. Complementary to their endocytic role, epsins are involved in the regulation of this key cell polarity protein (8). Mechanistically, the epsin’s ENTH domain interacts with and sequesters/inhibits yeast Cdc42 GTPase-activating proteins (Cdc42 GAPs) (8). This interaction has been proposed to relieve the GAP-induced Cdc42 inhibition and thereby to promote Cdc42 signaling activation. Interestingly, this function is analogous to the one described for the epsin-interacting protein intersectin that sequesters/ inhibits a Cdc42 GAP, CdGAP (114), and displays GEF activity toward Cdc42 (115).
In yeast, the ENTH domain is necessary and sufficient to sustain cell viability upon epsin deletion (6, 8). Furthermore, the specific residues required for this essential function of the ENTH domain were also identified. As expected, ENTH domain mutations in these residues (ENTHY100R and ENTHT104D) led to defects in Cdc42 activation, reduced viability, and actin cytoskeleton depolarization (8). In agreement, in Dictyostelium discoideum, T107, the residue analogous to the yeast T104, was essential for normal actin dynamics at the membrane (109) as well as for the development of normal spore morphology (57). Interestingly, yeast epsin Ent2 has also been found to have physical and genetic interactions with the only S. cerevisiae Cdc42 GEF (116, 117). In addition, the ENTH domain of Ent2 bears an additional activity in cell division signaling pathways (118) that is dependent on its ability to interact strongly with one of the yeast Cdc42 GAPs: bud emergence 3 (Bem3). Bem3 is also one of the key players in the assembly of the septin cytoskeleton, a family of scaffolding proteins essential for proper cell division (119, 120). In fact, overexpression of the ENTH domain of Ent2, but not of Ent1, induces severe abnormalities in septin organization (118).
Taken together, this evidence indicates that the endocytic protein epsin is involved in Cdc42 regulation, probably coordinating the processes of establishment of cell polarity and endocytosis in time and space.
Importantly, epsin involvement in Rho GTPase signaling function is conserved in mammals. Specifically, epsins via their ENTH domain were found to interact with the Cdc42/ Rac1 GAP and Ral effector, Ral-binding protein 1 (RalBP1) (121). Indeed, siRNA-mediated knockdown of epsins led to Rac1 activation deficiencies (121). Importantly, this interaction was shown to be essential for another polarity-dependent process: cell migration. Interestingly, epsins are enriched in the leading edge of migratory cells (121, 122), and epsin-3 is selectively expressed in migratory keratinocytes (12). Further, overexpresssion of epsins enhanced cell migration and invasion, with epsin-3 being the most potent paralog for induction of this behavior (121). Interestingly, upregulation of epsins has been reported in skin, breast, and lung cancers (12–14, 123). In addition, our lab also identified epsin-3 expression in mouse pancreatic cancer models and in human pancreatic cancer cell lines, such as BxPC-3 and Panc-1 (15, 121). Furthermore, overexpression of epsin-2 and -3 in Madin Darby canine kidney cells induced morphological changes characterized by extended lamellipodia that prompted the epsin-transfected cells to migrate out of epithelial colonies (15). Therefore, it is tempting to speculate that epsin upregulation can contribute to enhance cancer cell invasion in vivo by RhoGTPase (Rac1) and notch signaling hyperactivation, and it constitutes an interesting possibility to be investigated.
Nonclassical functions of epsins
Apart from its role in endocytosis and signaling, epsin seem to be important for autophagy in Drosophila, as larvae lacking Lqf are unable to form autophagosomes (101). Further, epsin has been reported to accumulate in the nucleus following inhibition of nuclear export and to be involved in regulation of transcription (24, 124). Additionally, the ability of epsin to induce membrane curvature is important for proper mitotic membrane organization (125). Interaction of epsin with microtubules has also been reported (126).
Perspectives
Epsin is a multifaceted protein that apart from its classical role in cargo recognition and endocytic network stabilization, plays a crucial role in signaling. Epsin is required for the activation of the notch pathway, and it affects RhoGTPase signaling. Therefore, we propose that epsins are proteins that coordinate endocytosis and signaling in time and space.
This coordination between endocytosis and signaling is of utmost importance because efficient functioning of one requires precise regulation by the other. For example, work in yeast has demonstrated that the polarized distribution of signaling molecules, such as Cdc42 regulates membrane trafficking, which in turn reinforces polarization (127, 128) [recently reviewed in (129)]. The establishment and maintenance of such polarized regions is crucial for eukaryotic cellular organization and impacts central functions, such as cell viability, cell fate specification, and cellular migration.
Thus, it is not surprising that epsin is required for cell viability (yeast), proper embryo development (flies and mice), as well as establishment of cell polarity and functions associated with it (e.g., cytokinesis, cell migration, and invasion). Unlike other endocytic proteins [reviewed in (130)], the epsins (particularly epsin-3) are found to be upregulated in cancer cells. Although a direct link to carcinogenesis remains to be established, the role of epsins in promoting cancer invasion is a promising direction to be explored.
Acknowledgments
We thank Drs. Henry Chang (Purdue University) and Steven Caplan (University of Nebraska, Omaha) for critical reading of the manuscript. We apologize to all authors whose original contributions we could not cite due to space limitations. For more complete listings please refer to the cited reviews and references therein. The Aguilar lab is supported by grants from the National Science Foundation MCB-1021377 and by the Center for Science of Information (CSoI), an NSF Science and Technology Center, under grant agreement CCF-0939370. K.M. is supported by a fellowship from the Cancer Prevention Interdisciplinary Program at Purdue University, NIH grant R25CA128770.
Biographies

Arpita Sen obtained a Bachelor of Science degree in Botany from the University of Calcutta in 2005. In 2007 she was granted a Master of Science in Biophysics and Molecular Biology from the same institution. In 2008 she joined the Department of Biological Sciences, Purdue University where she is pursuing doctoral studies. The focus of her research is on the regulation of cell signaling by the endocytic machinery. Arpita is the recipient of multiple awards and scholarships from the American Society for Cell Biology, Purdue University and the University of Calcutta.

Kayalvizhi Madhivanan received Honors Master of Science degrees both in Biological Sciences and Pharmacy from the Birla Institute of Technology and Science, Pilani, India, in 2009. Starting in the Fall 2009, Kayalvizhi joined the graduate program of the Department of Biological Sciences, Purdue University. Her thesis focuses on the signaling functions of the endocytic protein epsin in mammalian cells. She is the recipient of the 2011 Yeunkyung Woo Achieve Excellence Travel Fund (Purdue University) and the best presentation award by the Minority Affairs Committee from the American Society for Cell Biology during the 51st Annual meeting.

Debarati Mukherjee has a Master of Science degree (2006) in Biological Sciences from the Birla Institute of Technology and Science, Pilani, India. From 2007 to 2011, she joined the graduate school at the Department of Biological Sciences, Purdue University. Debarati obtained her PhD in Cell, Molecular and Developmental Biology with a focus in cell-signaling regulation by vesicle trafficking in 2011. In the Fall of the same year she accepted a Post-doctoral position at the National Center for Biological Sciences (NCBS), Bangalore. At NCBS she studies the neurobiology of autistic spectrum disorders under the supervision of Dr. Sumantra Chattarji. Debarati has multiple awards including the 2010 H. E. Umbarger Outstanding Graduate Student in Research, Purdue University and a Purdue Research Foundation Fellowship.

R. Claudio Aguilar obtained his PhD from the University of Buenos Aires in Immunochemistry and Molecular Endocrinology. In 1996, he moved to Bethesda, MD to pursue a Post-doctoral position in Cell Biology with a focus on vesicle trafficking at the National Institutes Health (NIH) under the supervision of Dr. Juan Bonifacino. Most of his work at the NIH was centered on the role of clathrin-associated adaptor protein complexes in protein sorting. From 2001 to 2005, Dr. Aguilar served as Associate Research Scientist in the Department of Biology, The Johns Hopkins University, in the laboratory of Dr. Beverly Wendland. During this period he studied different aspects of epsin function in Saccharomyces cerevisiae using biochemical, cell biological and genetic approaches. In 2005, Dr. Aguilar accepted a position as Assistant Professor, and currently serves as Associate Professor and Assistant Head, in the Department of Biological Sciences, Purdue University. The Aguilar lab is interested in the interplay between vesicle trafficking and signaling in health and disease.
Footnotes
Conflict of interest statement
The author stated that there are no conflicts of interest regarding the publication of this article.
References
- 1.Wendland B. Epsins: adaptors in endocytosis? Nat Rev Mol Cell Biol. 2002;3:971–7. doi: 10.1038/nrm970. [DOI] [PubMed] [Google Scholar]
- 2.Fischer JA, Leavell SK, Li QH. Mutagenesis screens for interacting genes reveal three roles for fat facets during Drosophila eye development. Dev Genet. 1997;21:167–74. doi: 10.1002/(SICI)1520-6408(1997)21:2<167::AID-DVG6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 3.Cadavid ALM, Ginzel A, Fischer JA. The function of the Drosophila fat facets deubiquitinating enzyme in limiting photoreceptor cell number is intimately associated with endocytosis. Development. 2000;127:1727–36. doi: 10.1242/dev.127.8.1727. [DOI] [PubMed] [Google Scholar]
- 4.Tian X, Hansen D, Schedl T, Skeath JB. Epsin potentiates notch pathway activity in Drosophila and C. elegans Development. 2004;131:5807–15. doi: 10.1242/dev.01459. [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Ko G, Zatti A, Di Giacomo G, Liu L, Raiteri E, Perucco E, Collesi C, Min W, Zeiss C, De Camilli P, Cremona O. Embryonic arrest at midgestation and disruption of notch signaling produced by the absence of both epsin 1 and epsin 2 in mice. Proc Natl Acad Sci USA. 2009;106:13838–43. doi: 10.1073/pnas.0907008106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wendland B, Steece KE, Emr SD. Yeast epsins contain an essential N-terminal ENTH domain, bind clathrin and are required for endocytosis. EMBO J. 1999;18:4383–93. doi: 10.1093/emboj/18.16.4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sakamoto C, Kawamoto C, Takeuchi K, Miyamoto I, Shuntoh H. Fission yeast epsin, Ent1p is required for endocytosis and involved in actin organization. Kobe J Med Sci. 2004;50:47–57. [PubMed] [Google Scholar]
- 8.Aguilar RC, Longhi SA, Shaw JD, Yeh L-Y, Kim S, Schon A, Freire E, Hsu A, McCormick WK, Watson HA, Wendland B. Epsin N-terminal homology domains perform an essential function regulating Cdc42 through binding Cdc42 GTPase-activating proteins. Proc Natl Acad Sci USA. 2006;103:4116–21. doi: 10.1073/pnas.0510513103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen H, Fre S, Slepnev VI, Capua MR, Takei K, Butler MH, Di Fiore PP, De Camilli P. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature. 1998;394:793–7. doi: 10.1038/29555. [DOI] [PubMed] [Google Scholar]
- 10.Stukenberg PT, Lustig KD, McGarry TJ, King RW, Kuang J, Kirschner MW. Systematic identification of mitotic phosphoproteins. Curr Biol. 1997;7:338–48. doi: 10.1016/s0960-9822(06)00157-6. [DOI] [PubMed] [Google Scholar]
- 11.Rosenthal JA, Chen H, Slepnev VI, Pellegrini L, Salcini AE, Di Fiore PP, De Camilli P. The epsins define a family of proteins that interact with components of the clathrin coat and contain a new protein module. J Biol Chem. 1999;274:33959–65. doi: 10.1074/jbc.274.48.33959. [DOI] [PubMed] [Google Scholar]
- 12.Spradling KD, McDaniel AE, Lohi J, Pilcher BK. Epsin 3 is a novel extracellular matrix-induced transcript specific to wounded epithelia. J Biol Chem. 2001;276:29257–67. doi: 10.1074/jbc.M101663200. [DOI] [PubMed] [Google Scholar]
- 13.Ko G, Paradise S, Chen H, Graham M, Vecchi M, Bianchi F, Cremona O, Di Fiore PP, De Camilli P. Selective high-level expression of epsin 3 in gastric parietal cells, where it is localized at endocytic sites of apical canaliculi. Proc Natl Acad Sci USA. 2010;107:21511–6. doi: 10.1073/pnas.1016390107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang YH, Dai ZY, Sadee WG, Hancock WS. A pharmacoproteomics study of the cancer cell line EKVX using capillary-LC/ MS/MS. Mol Pharm. 2006;3:566–78. doi: 10.1021/mp060002b. [DOI] [PubMed] [Google Scholar]
- 15.Coon BG, Direnzo DM, Konieczny SF, Aguilar RC. Epsins’ novel role in cancer cell invasion. Commun Integr Biol. 2011;4:95–7. doi: 10.4161/cib.4.1.14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen X, Zhang B, Fischer JA. A specific protein substrate for a deubiquitinating enzyme: liquid facets is the substrate of fat facets. Genes Dev. 2002;16:289–94. doi: 10.1101/gad.961502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wasiak S, Legendre-Guillemin V, Puertollano R, Blondeau F, Girard M, de Heuvel E, Boismenu D, Bell AW, Bonifacino JS, McPherson PS. Enthoprotin: a novel clathrin-associated protein identified through subcellular proteomics. J Cell Biol. 2002;158:855–62. doi: 10.1083/jcb.200205078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalthoff C, Groos S, Kohl R, Mahrhold S, Ungewickell EJ. Clint: a novel clathrin-binding ENTH-domain protein at the golgi. Mol Biol Cell. 2002;13:4060–73. doi: 10.1091/mbc.E02-03-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mills IG, Praefcke GJ, Vallis Y, Peter BJ, Olesen LE, Gallop JL, Butler PJ, Evans PR, McMahon HT. EpsinR: an AP1/clathrin interacting protein involved in vesicle trafficking. J Cell Biol. 2003;160:213–22. doi: 10.1083/jcb.200208023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirst J, Motley A, Harasaki K, Peak Chew SY, Robinson MS. EpsinR: an ENTH domain-containing protein that interacts with AP-1. Mol Biol Cell. 2003;14:625–41. doi: 10.1091/mbc.E02-09-0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duncan MC, Costaguta G, Payne GS. Yeast epsin-related proteins required for golgi-endosome traffic define a γ-adaptin ear-binding motif. Nat Cell Biol. 2003;5:77–81. doi: 10.1038/ncb901. [DOI] [PubMed] [Google Scholar]
- 22.Kay BK, Yamabhai M, Wendland B, Emr SD. Identification of a novel domain shared by putative components of the endocytic and cytoskeletal machinery. Protein Sci. 1999;8:435–8. doi: 10.1110/ps.8.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Camilli P, Chen H, Hyman J, Panepucci E, Bateman A, Brunger AT. The ENTH domain. FEBS Lett. 2002;513:11–8. doi: 10.1016/s0014-5793(01)03306-3. [DOI] [PubMed] [Google Scholar]
- 24.Hyman J, Chen H, Di Fiore PP, De Camilli P, Brunger AT. Epsin 1 undergoes nucleocytosolic shuttling and its eps15 interactor NH2-terminal homology (ENTH) domain, structurally similar to Armadillo and HEAT repeats, interacts with the transcription factor promyelocytic leukemia Zn2+ finger protein (PLZF) J Cell Biol. 2000;149:537–46. doi: 10.1083/jcb.149.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Itoh T, Koshiba S, Kigawa T, Kikuchi A, Yokoyama S, Takenawa T. Role of the ENTH domain in phosphatidylinositol-4,5-bisphosphate binding and endocytosis. Science. 2001;291:1047–51. doi: 10.1126/science.291.5506.1047. [DOI] [PubMed] [Google Scholar]
- 26.Koshiba S, Kigawa T, Kikuchi A, Yokoyama S. Solution structure of the epsin N-terminal homology (ENTH) domain of human epsin. J Struct Funct Genomics. 2002;2:1–8. doi: 10.1023/a:1011397007366. [DOI] [PubMed] [Google Scholar]
- 27.Ford MGJ, Mills IG, Peter BJ, Vallis Y, Praefcke GJK, Evans PR, McMahon HT. Curvature of clathrin-coated pits driven by epsin. Nature. 2002;419:361–6. doi: 10.1038/nature01020. [DOI] [PubMed] [Google Scholar]
- 28.Stahelin RV, Long F, Peter BJ, Murray D, De Camilli P, McMahon HT, Cho W. Contrasting membrane interaction mechanisms of AP180 N-terminal homology (ANTH) and epsin N-terminal homology (ENTH) domains. J Biol Chem. 2003;278:28993–9. doi: 10.1074/jbc.M302865200. [DOI] [PubMed] [Google Scholar]
- 29.Itoh T, Takenawa T. Regulation of endocytosis by phosphatidylinositol 4,5-bisphosphate and ENTH proteins. Curr Top Microbiol Immunol. 2004;282:31–47. doi: 10.1007/978-3-642-18805-3_2. [DOI] [PubMed] [Google Scholar]
- 30.Kweon D-H, Shin Y-K, Shin JY, Lee J-H, Lee J-B, Seo J-H, Kim YS. Membrane topology of helix 0 of the Epsin N-terminal homology domain. Mol Cells. 2006;21:428–35. [PubMed] [Google Scholar]
- 31.Yoon Y, Tong J, Lee PJ, Albanese A, Bhardwaj N, Källberg M, Digman MA, Lu H, Gratton E, Shin Y-K, Cho W. Molecular basis of the potent membrane-remodeling activity of the epsin 1 N-terminal homology domain. J Biol Chem. 2010;285:531–40. doi: 10.1074/jbc.M109.068015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Capraro BR, Yoon Y, Cho W, Baumgart T. Curvature sensing by the epsin N-terminal homology domain measured on cylindrical lipid membrane tethers. J Am Chem Soc. 2010;132:1200–1. doi: 10.1021/ja907936c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hurley JH, Wendland B. Endocytosis: driving membranes around the bend. Cell. 2002;111:143–6. doi: 10.1016/s0092-8674(02)01044-9. [DOI] [PubMed] [Google Scholar]
- 34.Nossal R, Zimmerberg J. Endocytosis: curvature to the ENTH degree. Curr Biol. 2002;12:R770–2. doi: 10.1016/s0960-9822(02)01289-7. [DOI] [PubMed] [Google Scholar]
- 35.Legendre-Guillemin V, Wasiak S, Hussain NK, Angers A, McPherson PS. ENTH/ANTH proteins and clathrin-mediated membrane budding. J Cell Sci. 2004;117(Pt 1):9–18. doi: 10.1242/jcs.00928. [DOI] [PubMed] [Google Scholar]
- 36.Horvath CA, Vanden Broeck D, Boulet GA, Bogers J, De Wolf MJ. Epsin: inducing membrane curvature. Int J Biochem Cell Biol. 2007;39:1765–70. doi: 10.1016/j.biocel.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 37.Young P, Deveraux Q, Beal RE, Pickart CM, Rechsteiner M. Characterization of two polyubiquitin binding sites in the 26 S protease subunit 5a. J Biol Chem. 1998;273:5461–7. doi: 10.1074/jbc.273.10.5461. [DOI] [PubMed] [Google Scholar]
- 38.Hofmann K, Falquet L. A ubiquitin-interacting motif conserved in components of the proteasomal and lysosomal protein degradation systems. Trends Biochem Sci. 2001;26:347–50. doi: 10.1016/s0968-0004(01)01835-7. [DOI] [PubMed] [Google Scholar]
- 39.Polo S, Sigismund S, Faretta M, Guidi M, Capua MR, Bossi G, Chen H, De Camilli P, Di Fiore PP. A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature. 2002;416:451–5. doi: 10.1038/416451a. [DOI] [PubMed] [Google Scholar]
- 40.Shih SC, Katzmann DJ, Schnell JD, Sutanto M, Emr SD, Hicke L. Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nat Cell Biol. 2002;4:389–93. doi: 10.1038/ncb790. [DOI] [PubMed] [Google Scholar]
- 41.Aguilar RC, Watson HA, Wendland B. The yeast epsin Ent1 is recruited to membranes through multiple independent interactions. J Biol Chem. 2003;278:10737–43. doi: 10.1074/jbc.M211622200. [DOI] [PubMed] [Google Scholar]
- 42.Sugiyama S, Kishida S, Chayama K, Koyama S, Kikuchi A. Ubiquitin-interacting motifs of epsin are involved in the regulation of insulin-dependent endocytosis. J Biochem. 2005;137:355–64. doi: 10.1093/jb/mvi044. [DOI] [PubMed] [Google Scholar]
- 43.Hawryluk MJ, Keyel PA, Mishra SK, Watkins SC, Heuser JE, Traub LM. Epsin 1 is a polyubiquitin-selective clathrin-associated sorting protein. Traffic. 2006;7:262–81. doi: 10.1111/j.1600-0854.2006.00383.x. [DOI] [PubMed] [Google Scholar]
- 44.Barriere H, Nemes C, Lechardeur D, Khan-Mohammad M, Fruh K, Lukacs GL. Molecular basis of oligoubiquitin-dependent internalization of membrane proteins in mammalian cells. Traffic. 2006;7:282–97. doi: 10.1111/j.1600-0854.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 45.Duncan LM, Piper S, Dodd RB, Saville MK, Sanderson CM, Luzio JP, Lehner PJ. Lysine-63-linked ubiquitination is required for endolysosomal degradation of class I molecules. EMBO J. 2006;25:1635–45. doi: 10.1038/sj.emboj.7601056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madshus IH. Ubiquitin binding in endocytosis – how tight should it be and where does it happen? Traffic. 2006;7:258–61. doi: 10.1111/j.1600-0854.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- 47.Kalthoff C, Alves J, Urbanke C, Knorr R, Ungewickell EJ. Unusual structural organization of the endocytic proteins AP180 and epsin 1. J Biol Chem. 2002;277:8209–16. doi: 10.1074/jbc.M111587200. [DOI] [PubMed] [Google Scholar]
- 48.Drake MT, Downs MA, Traub LM. Epsin binds to clathrin by associating directly with the clathrin-terminal domain. Evidence for cooperative binding through two discrete sites. J Biol Chem. 2000;275:6479–89. doi: 10.1074/jbc.275.9.6479. [DOI] [PubMed] [Google Scholar]
- 49.Drake MT, Traub LM. Interaction of two structurally distinct sequence types with the clathrin terminal domain β-propeller. J Biol Chem. 2001;276:28700–9. doi: 10.1074/jbc.M104226200. [DOI] [PubMed] [Google Scholar]
- 50.Dell’Angelica EC. Clathrin-binding proteins: got a motif? Join the network! Trends Cell Biol. 2001;11:315–8. doi: 10.1016/s0962-8924(01)02043-8. [DOI] [PubMed] [Google Scholar]
- 51.Owen DJ, Vallis Y, Noble ME, Hunter JB, Dafforn TR, Evans PR, McMahon HT. A structural explanation for the binding of multiple ligands by the α-adaptin appendage domain. Cell. 1999;97:805–15. doi: 10.1016/s0092-8674(00)80791-6. [DOI] [PubMed] [Google Scholar]
- 52.Traub LM, Downs MA, Westrich JL, Fremont DH. Crystal structure of the alpha appendage of AP-2 reveals a recruitment platform for clathrin-coat assembly. Proc Natl Acad Sci USA. 1999;96:8907–12. doi: 10.1073/pnas.96.16.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Owen DJ, Vallis Y, Pearse BM, McMahon HT, Evans PR. The structure and function of the β 2-adaptin appendage domain. EMBO J. 2000;19:4216–27. doi: 10.1093/emboj/19.16.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brett TJ, Traub LM, Fremont DH. Accessory protein recruitment motifs in clathrin-mediated endocytosis. Structure. 2002;10:797–809. doi: 10.1016/s0969-2126(02)00784-0. [DOI] [PubMed] [Google Scholar]
- 55.Yamabhai M, Hoffman NG, Hardison NL, McPherson PS, Castagnoli L, Cesareni G, Kay BK. Intersectin, a novel adaptor protein with two Eps15 homology and five Src homology 3 domains. J Biol Chem. 1998;273:31401–7. doi: 10.1074/jbc.273.47.31401. [DOI] [PubMed] [Google Scholar]
- 56.Morinaka K, Koyama S, Nakashima S, Hinoi T, Okawa K, Iwamatsu A, Kikuchi A. Epsin binds to the EH domain of POB1 and regulates receptor-mediated endocytosis. Oncogene. 1999;18:5915–22. doi: 10.1038/sj.onc.1202974. [DOI] [PubMed] [Google Scholar]
- 57.Brady RJ, Wen Y, O’Halloran TJ. The ENTH and C-terminal domains of Dictyostelium epsin cooperate to regulate the dynamic interaction with clathrin-coated pits. J Cell Sci. 2008;121(Pt 20):3433–44. doi: 10.1242/jcs.032573. [DOI] [PubMed] [Google Scholar]
- 58.Gabernet-Castello C, Dacks JB, Field MC. The single ENTH-domain protein of trypanosomes; endocytic functions and evolutionary relationship with epsin. Traffic. 2009;10:894–911. doi: 10.1111/j.1600-0854.2009.00910.x. [DOI] [PubMed] [Google Scholar]
- 59.Dores MR, Schnell JD, Maldonado-Baez L, Wendland B, Hicke L. The function of yeast epsin and Ede1 ubiquitin-binding domains during receptor internalization. Traffic. 2010;11:151–60. doi: 10.1111/j.1600-0854.2009.01003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Overstreet E, Fitch E, Fischer JA. Fat facets and liquid facets promote delta endocytosis and delta signaling in the signaling cells. Development. 2004;131:5355–66. doi: 10.1242/dev.01434. [DOI] [PubMed] [Google Scholar]
- 61.Wang W, Struhl G. Drosophila epsin mediates a select endocytic pathway that DSL ligands must enter to activate notch. Development. 2004;131:5367–80. doi: 10.1242/dev.01413. [DOI] [PubMed] [Google Scholar]
- 62.Wang W, Struhl G. Distinct roles for mind bomb, neuralized and epsin in mediating DSL endocytosis and signaling in Drosophila. Development. 2005;132:2883–94. doi: 10.1242/dev.01860. [DOI] [PubMed] [Google Scholar]
- 63.Stang E, Blystad FD, Kazazic M, Bertelsen V, Brodahl T, Raiborg C, Stenmark H, Madshus IH. Cbl-dependent ubiquitination is required for progression of EGF receptors into clathrin-coated pits. Mol Biol Cell. 2004;15:3591–604. doi: 10.1091/mbc.E04-01-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sigismund S, Woelk T, Puri C, Maspero E, Tacchetti C, Transidico P, Di Fiore PP, Polo S. Clathrin-independent endocytosis of ubiquitinated cargos. Proc Natl Acad Sci USA. 2005;102:2760–5. doi: 10.1073/pnas.0409817102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kazazic M, Bertelsen V, Pedersen KW, Vuong TT, Grandal MV, Rødland MS, Traub LM, Stang E, Madshus IH. Epsin 1 is involved in recruitment of ubiquitinated EGF receptors into clathrin-coated pits. Traffic. 2009;10:235–45. doi: 10.1111/j.1600-0854.2008.00858.x. [DOI] [PubMed] [Google Scholar]
- 66.Bertelsen V, Sak MM, Breen K, Rødland MS, Johannessen LE, Traub LM, Stang E, Madshus IH. A chimeric pre-ubiquitinated EGF receptor is constitutively endocytosed in a clathrin-dependent, but kinase-independent manner. Traffic. 2011;12:507–20. doi: 10.1111/j.1600-0854.2011.01162.x. [DOI] [PubMed] [Google Scholar]
- 67.Staruschenko A, Pochynyuk O, Stockand JD. Regulation of epithelial Na+ channel activity by conserved serine/threonine switches within sorting signals. J Biol Chem. 2005;280:39161–7. doi: 10.1074/jbc.M509608200. [DOI] [PubMed] [Google Scholar]
- 68.Wang H, Traub LM, Weixel KM, Hawryluk MJ, Shah N, Edinger RS, Perry CJ, Kester L, Butterworth MB, Peters KW, Kleyman TR, Frizzell RA, Johnson JP. Clathrin-mediated endocytosis of the epithelial sodium channel. Role of epsin. J Biol Chem. 2006;281:14129–35. doi: 10.1074/jbc.M512511200. [DOI] [PubMed] [Google Scholar]
- 69.Weixel KM, Edinger RS, Kester L, Guerriero CJ, Wang H, Fang L, Kleyman TR, Welling PA, Weisz OA, Johnson JP. Phosphatidylinositol 4-phosphate 5-kinase reduces cell surface expression of the epithelial sodium channel (ENaC) in cultured collecting duct cells. J Biol Chem. 2007;282:36534–42. doi: 10.1074/jbc.M703970200. [DOI] [PubMed] [Google Scholar]
- 70.Lai EC. Notch signaling: control of cell communication and cell fate. Development. 2004;131:965–73. doi: 10.1242/dev.01074. [DOI] [PubMed] [Google Scholar]
- 71.Seugnet L, Simpson P, Haenlin M. Requirement for dynamin during notch signaling in Drosophila neurogenesis. Dev Biol. 1997;192:585–98. doi: 10.1006/dbio.1997.8723. [DOI] [PubMed] [Google Scholar]
- 72.Windler SL, Bilder D. Endocytic internalization routes required for delta/notch signaling. Curr Biol. 2010;20:538–43. doi: 10.1016/j.cub.2010.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deblandre GA, Lai EC, Kintner C. Xenopus neuralized is a ubiquitin ligase that interacts with XDelta1 and regulates notch signaling. Dev Cell. 2001;1:795–806. doi: 10.1016/s1534-5807(01)00091-0. [DOI] [PubMed] [Google Scholar]
- 74.Lai EC, Deblandre GA, Kintner C, Rubin GM. Drosophila neuralized is a ubiquitin ligase that promotes the internalization and degradation of delta. Dev Cell. 2001;1:783–94. doi: 10.1016/s1534-5807(01)00092-2. [DOI] [PubMed] [Google Scholar]
- 75.Pavlopoulos E, Pitsouli C, Klueg KM, Muskavitch AT, Moschonas NK, Delidakis C. Neuralized encodes a peripheral membrane protein involved in Delta signaling and endocytosis. Dev Cell. 2001;1:807–16. doi: 10.1016/s1534-5807(01)00093-4. [DOI] [PubMed] [Google Scholar]
- 76.Yeh E, Dermer M, Commisso C, Zhou L, McGlade CJ, Boulianne GL. Neuralized functions as an E3 ubiquitin ligase during Drosophila development. Curr Biol. 2001;11:1675–9. doi: 10.1016/s0960-9822(01)00527-9. [DOI] [PubMed] [Google Scholar]
- 77.Itoh M, Kim CH, Palardy G, Oda T, Jiang YJ, Maust D, Yeo SY, Lorick K, Wright GJ, Ariza-McNaughton L, Weissman AM, Lewis J, Chandrasekharappa SC, Chitnis AB. Mind bomb is a ubiquitin ligase that is essential for efficient activation of notch signaling by Delta. Dev Cell. 2003;4:67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- 78.Mitsudomi T, Yatabe Y. Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. FEBS J. 2010;277:301–8. doi: 10.1111/j.1742-4658.2009.07448.x. [DOI] [PubMed] [Google Scholar]
- 79.Bao J, Gur G, Yarden Y. Src promotes destruction of c-Cbl: implications for oncogenic synergy between Src and growth factor receptors. Proc Natl Acad Sci USA. 2003;100:2438–43. doi: 10.1073/pnas.0437945100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shtiegman K, Kochupurakkal BS, Zwang Y, Pines G, Starr A, Vexler A, Citri A, Katz M, Lavi S, Ben-Basat Y, Benjamin S, Corso S, Gan J, Yosef RB, Giordano S, Yarden Y. Defective ubiquitinylation of EGFR mutants of lung cancer confers prolonged signaling. Oncogene. 2007;26:6968–78. doi: 10.1038/sj.onc.1210503. [DOI] [PubMed] [Google Scholar]
- 81.Roepstorff K, Grøvdal L, Grandal M, Lerdrup M, van Deurs B. Endocytic downregulation of ErbB receptors: mechanisms and relevance in cancer. Histochem Cell Biol. 2008;129:563–78. doi: 10.1007/s00418-008-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wells A. EGF receptor. Int J Biochem Cell Biol. 1999;31:637–43. doi: 10.1016/s1357-2725(99)00015-1. [DOI] [PubMed] [Google Scholar]
- 83.Sorkin A. Internalization of the epidermal growth factor receptor: role in signalling. Biochem Soc Trans. 2001;29(Pt 4):480–4. doi: 10.1042/bst0290480. [DOI] [PubMed] [Google Scholar]
- 84.Goh LK, Huang F, Kim W, Gygi S, Sorkin A. Multiple mechanisms collectively regulate clathrin-mediated endocytosis of the epidermal growth factor receptor. J Cell Biol. 2010;189:871–83. doi: 10.1083/jcb.201001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sorkin A, Goh LK. Endocytosis and intracellular trafficking of ErbBs. Exp Cell Res. 2008;314:3093–106. doi: 10.1016/j.yexcr.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen H, De Camilli P. The association of epsin with ubiquitinated cargo along the endocytic pathway is negatively regulated by its interaction with clathrin. Proc Natl Acad Sci USA. 2005;102:2766–71. doi: 10.1073/pnas.0409719102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sigismund S, Argenzio E, Tosoni D, Cavallaro E, Polo S, Di Fiore PP. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev Cell. 2008;15:209–19. doi: 10.1016/j.devcel.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 88.Kazazic M, Roepstorff K, Johannessen LE, Pedersen NM, van Deurs B, Stang E, Madshus IH. EGF-induced activation of the EGF receptor does not trigger mobilization of caveolae. Traffic. 2006;7:1518–27. doi: 10.1111/j.1600-0854.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- 89.Snyder PM. Minireview: regulation of epithelial Na+ channel trafficking. Endocrinology. 2005;146:5079–85. doi: 10.1210/en.2005-0894. [DOI] [PubMed] [Google Scholar]
- 90.Staub O, Abriel H, Plant P, Ishikawa T, Kanelis V, Saleki R, Horisberger JD, Schild L, Rotin D. Regulation of the epithelial Na+ channel by Nedd4 and ubiquitination. Kid Int. 2000;57:809–15. doi: 10.1046/j.1523-1755.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- 91.Kamynina E, Tauxe C, Staub O. Distinct characteristics of two human Nedd4 proteins with respect to epithelial Na+ channel regulation. Am J Physiol Renal Physiol. 2001;281:F469–77. doi: 10.1152/ajprenal.2001.281.3.F469. [DOI] [PubMed] [Google Scholar]
- 92.Snyder PM, Olson DR, Thomas BC. Serum and glucocorticoid-regulated kinase modulates Nedd4-2-mediated inhibition of the epithelial Na+ channel. J Biol Chem. 2002;277:5–8. doi: 10.1074/jbc.C100623200. [DOI] [PubMed] [Google Scholar]
- 93.Snyder PM, Steines JC, Olson DR. Relative contribution of Nedd4 and Nedd4-2 to ENaC regulation in epithelia determined by RNA interference. J Biol Chem. 2004;279:5042–6. doi: 10.1074/jbc.M312477200. [DOI] [PubMed] [Google Scholar]
- 94.Staub O, Dho S, Henry P, Correa J, Ishikawa T, McGlade J, Rotin D. WW domains of Nedd4 bind to the proline-rich PY motifs in the epithelial Na+ channel deleted in Liddle’s syndrome. EMBO J. 1996;15:2371–80. [PMC free article] [PubMed] [Google Scholar]
- 95.Snyder PM. Liddle’s syndrome mutations disrupt cAMP-mediated translocation of the epithelial Na+ channel to the cell surface. J Clin Invest. 2000;105:45–53. doi: 10.1172/JCI7869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin DH, Sterling H, Wang Z, Babilonia E, Yang B, Dong K, Hebert SC, Giebisch G, Wang WH. ROMK1 channel activity is regulated by monobuquitination. Proc Natl Acad Sci USA. 2005;102:4306–11. doi: 10.1073/pnas.0409767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen C, Zhuang X. Epsin 1 is a cargo-specific adaptor for the clathrin-mediated endocytosis of the influenza virus. Proc Natl Acad Sci USA. 2008;105:11790–5. doi: 10.1073/pnas.0803711105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goto E, Yamanaka Y, Ishikawa A, Aoki-Kawasumi M, Mito-Yoshida M, Ohmura-Hoshino M, Matsuki Y, Kajikawa M, Hirano H, Ishido S. Contribution of lysine 11-linked ubiquitination to MIR2-mediated major histocompatibility complex class I internalization. J Biol Chem. 2010;285:35311–9. doi: 10.1074/jbc.M110.112763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sorkina T, Miranda M, Dionne KR, Hoover BR, Zahniser NR, Sorkin A. RNA interference screen reveals an essential role of Nedd4-2 in dopamine transporter ubiquitination and endocytosis. J Neurosci. 2006;26:8195–205. doi: 10.1523/JNEUROSCI.1301-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chen B, Dores MR, Grimsey N, Canto I, Barker BL, Trejo J. Adaptor protein complex-2 (AP-2) and epsin-1 mediate protease-activated receptor-1 internalization via phosphorylation- and ubiquitination-dependent sorting signals. J Biol Chem. 2011;286:40760–70. doi: 10.1074/jbc.M111.299776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Csikós G, Lippai M, Lukácsovich T, Juhász G, Henn L, Erdélyi M, Maróy P, Sass M. A novel role for the Drosophila epsin (lqf): involvement in autophagy. Autophagy. 2009;5:636–48. doi: 10.4161/auto.5.5.8168. [DOI] [PubMed] [Google Scholar]
- 102.Loerke D, Mettlen M, Yarar D, Jaqaman K, Jaqaman H, Danuser G, Schmid SL. Cargo and dynamin regulate clathrin-coated pit maturation. PLoS Biol. 2009;7:e57. doi: 10.1371/journal.pbio.1000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ehrlich M, Boll W, Van Oijen A, Hariharan R, Chandran K, Nibert ML, Kirchhausen T. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell. 2004;118:591–605. doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 104.Mettlen M, Stoeber M, Loerke D, Antonescu CN, Danuser G, Schmid SL. Endocytic accessory proteins are functionally distinguished by their differential effects on the maturation of clathrin-coated pits. Mol Biol Cell. 2009;20:3251–60. doi: 10.1091/mbc.E09-03-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Toret CP, Lee L, Sekiya-Kawasaki M, Drubin DG. Multiple pathways regulate endocytic coat disassembly in Saccharomyces cerevisiae for optimal downstream trafficking. Traffic. 2008;9:848–59. doi: 10.1111/j.1600-0854.2008.00726.x. [DOI] [PubMed] [Google Scholar]
- 106.Newpher TM, Smith RP, Lemmon V, Lemmon SK. In vivo dynamics of clathrin and its adaptor-dependent recruitment to the actin-based endocytic machinery in yeast. Dev Cell. 2005;9:87–98. doi: 10.1016/j.devcel.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 107.Maldonado-Baez L, Dores MR, Perkins EM, Drivas TG, Hicke L, Wendland B. Interaction between epsin/Yap180 adaptors and the scaffolds Ede1/Pan1 is required for endocytosis. Mol Biol Cell. 2008;19:2936–48. doi: 10.1091/mbc.E07-10-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Repass SL, Brady RJ, O’Halloran TJ. Dictyostelium Hip1r contributes to spore shape and requires epsin for phosphorylation and localization. J Cell Sci. 2007;120:3977–88. doi: 10.1242/jcs.011213. [DOI] [PubMed] [Google Scholar]
- 109.Brady RJ, Damer CK, Heuser JE, O’Halloran TJ. Regulation of Hip1r by epsin controls the temporal and spatial coupling of actin filaments to clathrin-coated pits. J Cell Sci. 2010;123:3652–61. doi: 10.1242/jcs.066852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Eun SH, Lea K, Overstreet E, Stevens S, Lee J-H, Fischer JA. Identification of genes that interact with Drosophila liquid facets. Genetics. 2007;175:1163–74. doi: 10.1534/genetics.106.067959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Etienne-Manneville S. Cdc42 – the centre of polarity. J Cell Sci. 2004;117:1291–300. doi: 10.1242/jcs.01115. [DOI] [PubMed] [Google Scholar]
- 112.Adams AEM, Johnson DI, Longnecker RM, Sloat BF, Pringle JR. Cdc42 and cdc43, 2 additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J Cell Biol. 1990;111:131–42. doi: 10.1083/jcb.111.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ridley AJ. Rho proteins: linking signaling with membrane trafficking. Traffic. 2001;2:303–10. doi: 10.1034/j.1600-0854.2001.002005303.x. [DOI] [PubMed] [Google Scholar]
- 114.Jenna S, Hussain NK, Danek EI, Triki I, Wasiak S, McPherson PS, Lamarche-Vane N. The activity of the GTPase-activating protein CdGAP is regulated by the endocytic protein intersectin. J Biol Chem. 2002;277:6366–73. doi: 10.1074/jbc.M105516200. [DOI] [PubMed] [Google Scholar]
- 115.Hussain NK, Jenna S, Glogauer M, Quinn CC, Wasiak S, Guipponi M, Antonarakis SE, Kay BK, Stossel TP, Lamarche-Vane N, McPherson PS. Endocytic protein intersectin-I regulates actin assembly via Cdc42 and N-WASP. Nat Cell Biol. 2001;3:927–32. doi: 10.1038/ncb1001-927. [DOI] [PubMed] [Google Scholar]
- 116.Drees BL, Sundin B, Brazeau E, Caviston JP, Chen GC, Guo W, Kozminski KG, Lau MW, Moskow JJ, Tong A, Schenkman LR, McKenzie A, 3rd, Brennwald P, Longtine M, Bi E, Chan C, Novick P, Boone C, Pringle JR, Davis TN, Fields S, Drubin DG. A protein interaction map for cell polarity development. J Cell Biol. 2001;154:549–71. doi: 10.1083/jcb.200104057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cole KC, Barbour J-ER, Midkiff JF, Marble BM, Johnson DI. Multiple proteins and phosphorylations regulate Saccharomyces cerevisiae Cdc24p localization. FEBS Lett. 2009;583:3339–43. doi: 10.1016/j.febslet.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 118.Mukherjee D, Coon BG, Edwards DF, 3rd, Hanna CB, Longhi SA, McCaffery JM, Wendland B, Retegui LA, Bi E, Aguilar RC. The yeast endocytic protein epsin 2 functions in a cell-division signaling pathway. J Cell Sci. 2009;122:2453–63. doi: 10.1242/jcs.041137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Smith GR, Givan SA, Cullen P, Sprague GF. GTPase-activating proteins for Cdc42. Eukary Cell. 2002;1:469–80. doi: 10.1128/EC.1.3.469-480.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Caviston JP, Longtine M, Pringle JR, Bi E. The role of Cdc42p GTPase-activating proteins in assembly of the septin ring in yeast. Mol Biol Cell. 2003;14:4051–66. doi: 10.1091/mbc.E03-04-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Coon BG, Burgner J, Camonis JH, Aguilar RC. The epsin family of endocytic adaptors promotes fibrosarcoma migration and invasion. J Biol Chem. 2010;285:33073–81. doi: 10.1074/jbc.M110.124123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang Y, Ding S-J, Wang W, Jacobs JM, Qian W-J, Moore RJ, Yang F, Camp DG, 2nd, Smith RD, Klemke RL. Profiling signaling polarity in chemotactic cells. Proc Natl Acad Sci USA. 2007;104:8328–33. doi: 10.1073/pnas.0701103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pawlowski KM, Krol M, Majewska A, Badowska-Kozakiewicz A, Mol JA, Malicka E, Motyl T. Comparison of cellular and tissue transcriptional profiles in canine mammary tumor. J Physiol Pharmacol. 2009;60 (Suppl 1):85–94. [PubMed] [Google Scholar]
- 124.Vecchi M, Polo S, Poupon V, van de Loo JW, Benmerah A, Di Fiore PP. Nucleocytoplasmic shuttling of endocytic proteins. J Cell Biol. 2001;153:1511–7. doi: 10.1083/jcb.153.7.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu Z, Zheng Y. A requirement for epsin in mitotic membrane and spindle organization. J Cell Biol. 2009;186:473–80. doi: 10.1083/jcb.200902071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hussain NK, Yamabhai M, Bhakar AL, Metzler M, Ferguson SS, Hayden MR, McPherson PS, Kay BK. A role for epsin N-terminal homology/AP180 N-terminal homology (ENTH/ ANTH) domains in tubulin binding. J Biol Chem. 2003;278:28823–30. doi: 10.1074/jbc.M300995200. [DOI] [PubMed] [Google Scholar]
- 127.Marco E, Wedlich-Soldner R, Li R, Altschuler SJ, Wu LF. Endocytosis optimizes the dynamic localization of membrane proteins that regulate cortical polarity. Cell. 2007;129:411–22. doi: 10.1016/j.cell.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Slaughter BD, Das A, Schwartz JW, Rubinstein B, Li R. Dual modes of cdc42 recycling fine-tune polarized morphogenesis. Dev Cell. 2009;17:823–35. doi: 10.1016/j.devcel.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Harris KP, Tepass U. Cdc42 and vesicle trafficking in polarized cells. Traffic. 2010;11:1272–9. doi: 10.1111/j.1600-0854.2010.01102.x. [DOI] [PubMed] [Google Scholar]
- 130.Mosesson Y, Mills GB, Yarden Y. Derailed endocytosis: an emerging feature of cancer. Nat Rev Cancer. 2008;8:835–50. doi: 10.1038/nrc2521. [DOI] [PubMed] [Google Scholar]